Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Life cycle assessment of enzymatic poly(ethylene terephthalate) recycling†
Taylor
Uekert
 ab,
Jason S.
DesVeaux
ab,
Jason S.
DesVeaux
 bc,
Avantika
Singh
bc,
Scott R.
Nicholson
ab,
Patrick
Lamers
bc,
Avantika
Singh
bc,
Scott R.
Nicholson
ab,
Patrick
Lamers
 a,
Tapajyoti
Ghosh
a,
John E.
McGeehan
d,
Alberta C.
Carpenter
ab and
Gregg T.
Beckham
a,
Tapajyoti
Ghosh
a,
John E.
McGeehan
d,
Alberta C.
Carpenter
ab and
Gregg T.
Beckham
 *be
*be
aStrategic Energy Analysis Center, National Renewable Energy Laboratory, Golden, CO, USA
bBOTTLE Consortium, Golden, CO, USA
cCatalytic Carbon Transformation and Scale-Up Center, National Renewable Energy Laboratory, Golden, CO, USA. E-mail: gregg.beckham@nrel.gov
dCentre for Enzyme Innovation, University of Portsmouth, Portsmouth, UK
eRenewable Resources and Enabling Sciences Center, National Renewable Energy Laboratory, Golden, CO, USA
First published on 2nd August 2022
Abstract
Enzymatic hydrolysis of poly(ethylene terephthalate) (PET) is a chemical recycling approach intended to enable a circular economy for polyesters. To quantitatively compare this technology to other recycling and synthesis approaches for PET, it is critical to conduct rigorous and transparent process analyses. We recently reported a detailed process model that was used to conduct economic, energy, and greenhouse gas emissions analyses for enzymatic PET recycling. Here, we expand upon this previous work by conducting a process-based life cycle assessment (LCA) of the same enzymatic hydrolysis system, to produce both terephthalic acid (TPA) and ethylene glycol (EG) for use in a closed-loop PET recycling scheme. This LCA shows that enzymatic hydrolysis currently performs 1.2 to 17 times worse than virgin TPA and PET production across most impact categories, excepting ecotoxicity and fossil fuel depletion. The top contributors to these impacts include post-consumer PET collection and processing, sodium hydroxide, and electricity. Sensitivity analysis shows that improving yields throughout the recycling process and eliminating certain process steps, such as amorphization pre-treatment and reaction pH control, can reduce the overall environmental impacts of enzymatic PET recycling to levels statistically equivalent to virgin TPA and PET production, thereby highlighting crucial areas for further research and innovation.
Introduction
Enzymatic hydrolysis of poly(ethylene terephthalate) (PET) is one of several strategies being pursued to increase recycling rates of this ubiquitous polyester, which has many applications including single-use beverage bottles, carpets, textiles, and food packaging.1–6 The basis of enzymatic PET recycling is the use of hydrolytic enzymes to cleave the ester linkages in PET, ultimately producing the constituent monomers terephthalic acid (TPA) and ethylene glycol (EG). The development of enzymatic hydrolysis approaches capable of complete PET conversion has been accelerated by recent reports on the discovery, engineering, and evolution of thermophilic hydrolase enzymes, including the leaf compost cutinase (LCC), PHL7, several enzymes from the genus Thermobifida, and the PETase enzyme from Ideonella sakaiensis.7–21 Enzymatic hydrolysis has the potential to complement existing mechanical recycling processes by using undesirable contaminated or colored PET feedstocks to produce high-quality plastic.4To understand the critical drivers for realizing enzymatic PET recycling at scale, we recently reported a process model and techno-economic analysis (TEA).22 The model first treated PET flake by extrusion and cryo-grinding to produce amorphous PET powder from post-consumer waste, which was subsequently enzymatically depolymerized according to the conditions demonstrated by Tournier et al. in 2020.19 The recycled monomers rTPA and rEG were recovered by downstream processing including crystallization at low pH (rTPA), precipitation (generating a sodium sulfate co-product), and distillation (rEG). The process economics in the base case predicted a minimum selling price (MSP) for rTPA of $1.93 kg−1, which was dominated by feedstock cost (assuming a PET flake price of $0.66 kg−1 (ref. 23 and 24)). We next employed the Materials Flows through Industry (MFI) tool25 to estimate the process energy and greenhouse gas (GHG) emissions for the enzymatic process in comparison to virgin TPA (vTPA), vEG, and vPET manufacturing.26 The base case results indicated that enzymatic hydrolysis enables up to an 83% reduction in energy use and up to a 43% reduction in GHG emissions relative to vTPA. We also used the Bio-based carbon economy Environmentally-extended Input–Output Model (BEIOM), which examines life cycle and socio-economic impacts on a U.S. economy-wide basis.27 This work demonstrated that enzymatic PET recycling has the potential to improve nearly every assessed socio-economic and life cycle impact category, with the exception of a 3-fold increase in water use. Overall, our previous work highlighted key cost, energy, and GHG emissions drivers to guide researchers towards the development of less energy intensive pre-treatments and separations processes as well as identification and engineering of enzymes able to digest crystalline PET, and away from less critical factors such as depolymerization residence time and enzyme cost.
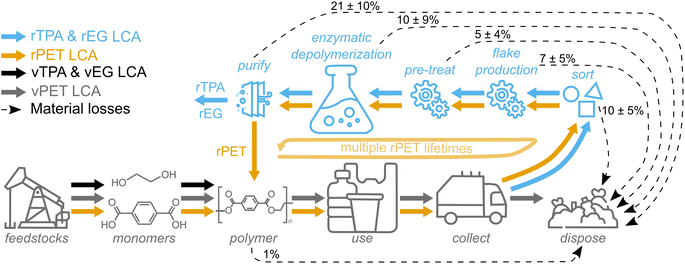
While this prior assessment was comprehensive, it used somewhat disjointed datasets, scales, and system boundaries, and lacked a high resolution, process-based life cycle assessment (LCA) directly linked to the TEA model. Here, we utilize SimaPro software, ecoinvent 3.3 background data, and the Tool for the Reduction and Assessment of Chemical and other environmental Impacts (TRACI) and Available WAter REmaining (AWARE) methods to conduct an LCA of enzymatic PET recycling. We include two systems: cradle-to-gate rTPA from PET waste collection to rTPA recovery (functional unit of 1 kg TPA) and cradle-to-grave rPET that includes the initial vPET production through to rPET repolymerization and reuse, all normalized across the multiple potential lifetimes of the re-recyclable material (functional unit of 1 kg PET, Fig. 1). We assess a full suite of life cycle impacts for both systems, identify the top process contributors in each category, and compare the results to those for vTPA and vPET manufacturing (functional units of 1 kg TPA and PET, respectively). We subsequently perform sensitivity analysis on various aspects of the enzymatic process, including PET pre-treatment, depolymerization, and the product recovery steps, as well as plant size and electricity sources. The LCA shows that enzymatic PET recycling as modeled exhibits environmental impacts higher than the virgin polymer, but that there are several areas for potential improvement and innovation.
Methods
Scope
The LCA utilizes an enzymatic PET hydrolysis process model that was developed in Aspen Plus for our previous study (Fig. S1†).22 Some key assumptions for the base case are as follows: the recycling facility is modeled at a scale of 150 metric tons per day (tpd) and utilizes clean PET flakes containing ∼30% colored flakes and 5% contamination. After mechanical pre-treatment by extrusion and cryo-grinding, the resulting lower crystallinity PET powder is fed into a reactor at pH 8 and 60 °C with a solids loading of 15% and an enzyme loading of 5 mg gPET−1 and allowed to reach 90% depolymerization to rTPA and rEG. Subsequently, 90% of rTPA (purity >98%) is captured by acidic precipitation, crystallization, and drying, whereas 50% of rEG (purity 99%) is recovered through membrane concentration and distillation, with sodium sulfate (Na2SO4) as a side product. Both Na2SO4 and rEG were considered co-products and therefore reduce impacts via negative credits.The LCA reported here incorporates additional process steps for two different system boundaries (Fig. 1). For comparison between rTPA and vTPA (blue and black arrows in Fig. 1, respectively), the existing enzymatic hydrolysis model was coupled with life cycle inventories for PET waste collection, sorting to bales, and initial shredding to flakes (Tables S1–S3†).28
For comparison between rPET and vPET (orange and gray arrows in Fig. 1, respectively), the system was further extended to include the repolymerization of the enzymatically recycled monomers to rPET and processing of rPET into bottles. In this case, only Na2SO4 was treated as a co-product since rEG was utilized to repolymerize rPET. Normalized life cycle impacts for the entire rPET system – including production of the original PET feedstock – over multiple lifetimes were calculated according to a system expansion approach.29 First, the number of product lifetimes (L) that can be obtained from rPET were calculated with eqn (1):
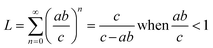 | (1) |
The normalized impact of the entire system (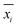 ) for impact category i was subsequently calculated with eqn (2):
) for impact category i was subsequently calculated with eqn (2):
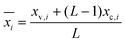 | (2) |
The following assumptions were made for the additional process steps. vPET and rPET polymerization proceeds at a yield of 99%,31 and the resulting material is manufactured into bottles by stretch blow molding. For comparison, scenarios in which vPET and rPET are manufactured into fibers, solid resins, and film according to American26 and global31 average consumption percentages were also calculated (Fig. S4†). It is assumed that rPET has the same material quality as vPET32 and thus can be directly substituted into the manufacturing stream. After post-consumer PET collection (Table S1†), the material is sorted into bales with an initial mass loss of 10% (Table S2†).28 The resulting bale contains 88.5 wt% PET (the average of American Grade A and Grade B bale qualities33), and is washed and shredded to flake (7% mass loss to obtain 95% purity, Table S3†). Any solid waste generated during enzymatic hydrolysis or other process steps is landfilled (80%) or incinerated for energy recovery (20%, credits given for electricity generation according to the 2016 U.S. grid mix), which is the ratio for plastics disposal in 2018 reported by the U.S. Environmental Protection Agency.34 Waste management decisions can have a significant impact on overall LCA results; switching from an 80/20 landfill/incineration ratio to 100% incineration or 100% landfill would change impacts by up to +30% and −5%, respectively.
LCA
Ecoinvent version 3.3 was used to supply most life cycle inventories (allocation, cut-off by classification – unit).35 U.S. specific inventories were utilized when available, with global data as a stand-in. Additional inventories were obtained from the literature and are available in the ESI,† including PET collection (Table S1†),28 sorting (Table S2†)28 and processing into flake (Table S3†)28 – which were adapted to meet the 95% PET flake purity assumption – as well as enzymatic hydrolysis (Tables S4 and S5†)22 and PET hydrolase enzyme production (Table S6†).36The LCA was conducted using SimaPro software and the TRACI 2.1 U.S. 200837 and AWARE38 methods. TRACI is a U.S. oriented method that uses a midpoint approach to quantify acidification (kg sulfur dioxide equivalent, or kg SO2 eq.), carcinogenics (comparative toxic unit for human, CTUh), ecotoxicity (comparative toxic unit for aquatic ecotoxicity, CTUe), eutrophication (kg nitrogen eq., kg N eq.), fossil fuel depletion (MJ surplus energy), global warming (kg carbon dioxide eq., kg CO2 eq.), non-carcinogenics (CTUh), ozone depletion (kg trichloro-fluoromethane eq., kg CFC-11 eq.), particulates exposure (kg fine particulate matter eq., kg PM2.5 eq.), and smog (kg ozone eq., kg O3 eq.). AWARE is a midpoint method for determining water use (m3) and incorporates the potential for water depletion averaged over one year (the inverse of water availability minus water demand). While many of these metrics were previously assessed using the BEIOM model,22 the resulting data provided economy-wide information rather than the process-specific LCA results reported here. Environmental impacts calculated by the ReCiPe method for base case enzymatic hydrolysis, which include additional metrics such as mineral resource scarcity, land use, and ionizing radiation, are available in Table S7.†
Sensitivity cases, which include variations in process design and assumptions, are depicted using combined scores. These scores were calculated by first normalizing all impact categories against the corresponding base case impact categories. The normalized impacts were then summed with equal weightings to obtain the natural environment (acidification, ecotoxicity, eutrophication, global warming, and ozone depletion), natural resources (fossil fuel depletion and water use), and human health (carcinogenics, non-carcinogenics, particulates exposure, and smog) scores. The data for individual impacts related to these sensitivity analyses are available in the ESI.†
Uncertainty analysis
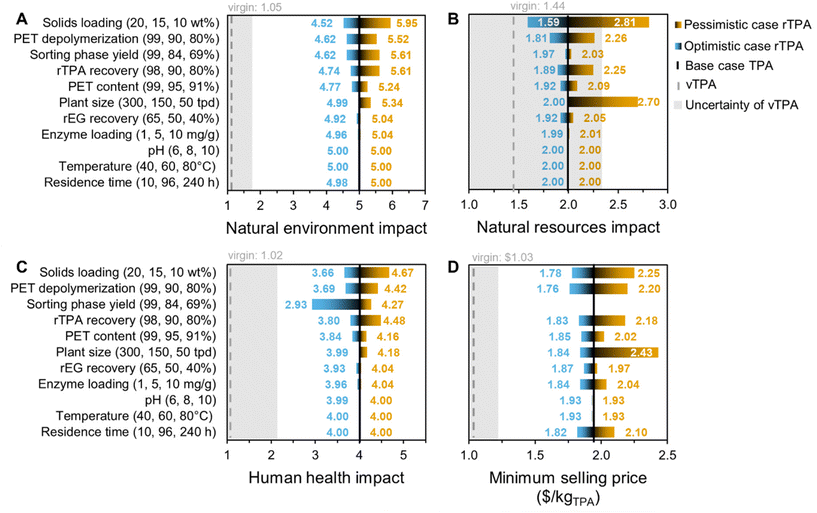
The uncertainty of the enzymatic hydrolysis inventory was estimated using a semi-quantitative pedigree approach.39 This method is relatively common in the LCA field and is crucial for verifying the reliability (or unreliability) of point estimates.39 Each item in the life cycle inventory was given reliability, completeness, temporal correlation, geographical correlation, and further technological correlation scores (scale of 1–5), according to the rubric in Table S8.† Low scores indicate higher technology readiness levels and lower data uncertainty. The sum of these scores was assigned a certain variability, the range of which was set to encompass known uncertainties around process yield; from a literature survey,19,22,40,41 we estimate that the average material mass yield from enzymatic hydrolysis is 0.77 ± 0.11, meaning standard deviation, σ, is ±14%. Scores of 5 to 9 were varied by ±5%, 10 to 14 by ±10%, 15 to 19 by ±15%, 20 to 24 by ±20%, and 25 by ±25%. Material and energy flows fall within the ±15% category, while process emissions have a conservative ±20% (Table S8†). These variabilities determine the low and high values of symmetric triangular distributions for each inventory item. The uncertainties of background processes were provided according to log–normal distributions in the ecoinvent database. Using these distributions, a Monte Carlo analysis was performed with 1000 iterations for each case study, giving mean and standard deviation values.Error propagation for adding/subtracting or multiplying/dividing values was calculated with eqn (3) and (4), respectively.
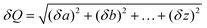 | (3) |
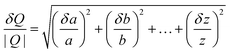 | (4) |
Base design case results
Fig. 2A depicts the base design case LCA results for enzymatically recycled rTPA compared to vTPA production (Table S9†). rTPA has 3–17× higher impacts than virgin production in most categories, excepting fossil fuel depletion (which is 1.4× lower than vTPA production). Impacts are even higher when assessed from the perspective of rEG (with rTPA treated as a co-product with negative credits) due to the lower quantity of generated rEG and lower baseline impacts associated with vEG (Fig. S5 and Table S10†). However, several categories have overlapping error bars that prevent ranking, particularly carcinogenics, non-carcinogenics, and water use. The majority of this error originates from uncertainty in background data rather than the foreground enzymatic process inventory. Water use has a particularly high standard deviation, which can be attributed to uncertainty associated with precipitation (and therefore water availability) and variations between hydrological models.42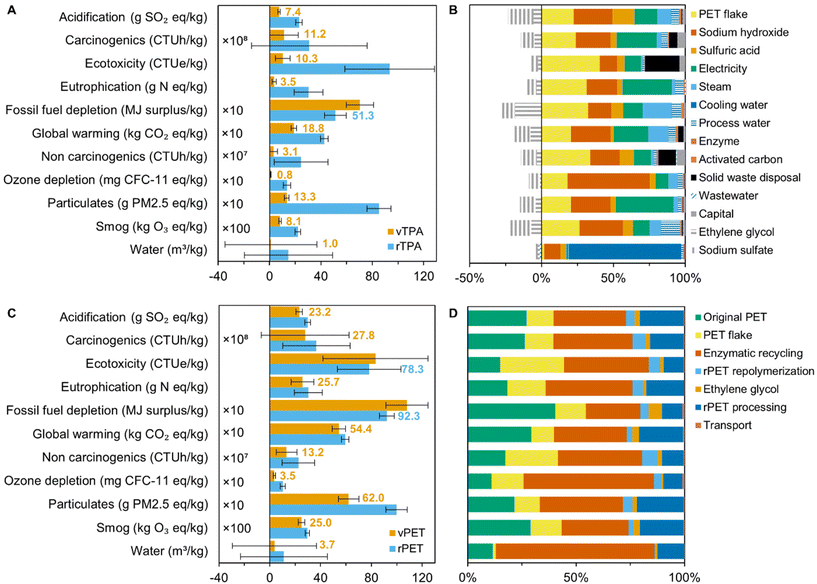 | ||
| Fig. 2 (A) Life cycle impacts of rTPA (blue) and vTPA (orange). (B) Contribution of different recycling process components to the environmental impacts of rTPA. (C) Life cycle impacts of rPET (blue) and vPET (orange). The rPET values are lifetime normalized according to the procedure described in the Methods section. (D) Contribution of different recycling process components to the environmental impacts of rPET. Some impact categories in (A) and (C) are multiplied by a conversion factor listed on the left sides of the graphs for visual clarity. The lowest values for each impact category are labelled. Corresponding data are available in Tables S9, S11, S14, and S16.† | ||
To determine the origin of the life cycle impacts for rTPA, Fig. 2B shows the normalized contributions of various process components to the overall impacts of enzymatic hydrolysis (Table S11†). High impact components include sodium hydroxide (NaOH), which is the first or second highest contributor to eight metrics (11–57% of totals), collection and shredding of post-consumer PET to produce flake, which is the first or second highest contributor to seven metrics (18–40% of totals), and electricity, which is the first or second highest contributor to four metrics (24–40% of totals). NaOH is produced by chlor-alkali electrolysis, an electricity-intensive process that releases CO2 and chlorine airborne emissions. It is used in the enzymatic process for pH control (0.55 kg kg−1 rTPA), resulting in correspondingly high contributions to the LCA. The impacts of PET flake are related to electricity and natural gas use to power the shredding process, as well as transportation for collection and movement between the material recovery facility (MRF), flake production facility, and enzymatic PET recycling plant (this category does not include impacts associated vPET manufacturing). PET flake has a particularly high effect on the rTPA LCA since 1.6 kg of flake is required to produce 1 kg of rTPA due to losses throughout the enzymatic recycling process. For electricity, we assumed a 2016 U.S. grid mix, with most environmental impacts associated with coal- and natural gas-based generation routes (63% of total electricity production43). Enzymatic hydrolysis consumes 1.76 kW h electricity per kg rTPA, the majority of which originates from mechanical pre-treatment to convert the PET flake feedstock into powder.
Additional contributors include water use in the form of cooling water (78%) and sulfuric acid (H2SO4, 4%). Waste disposal of unconverted plastic and other process losses constitute the second highest contribution to ecotoxicity (24%) and the third highest to non-carcinogenics (12%), while steam (assumed to be generated from natural gas) is the second highest contributor to fossil fuel depletion at 20%. The remaining process components – including enzymes, activated carbon, process water, capital construction, and wastewater treatment – account for less than 10% of all assessed impact categories. These results contrast markedly with the trends observed in TEA, where PET flake was similarly a top contributor to MSP (47%) but NaOH accounted for only 4.0% and electricity for 3.6%.22 MFI analysis identified impactful components similar to those in the current LCA, with a particular emphasis on electricity use.22 These results highlight the necessity of holistic analysis to identify process trade-offs and opportunities for innovation.
Fig. 2C expands the system boundaries to explore the LCA impacts of rPET produced from enzymatic hydrolysis as well as of vPET production (Tables S12–S14†). As depicted in Fig. 1 (orange arrows), the rPET system includes the original PET production stages, all collection, sorting, and enzymatic hydrolysis steps specified for the rTPA system, and repolymerization and reprocessing of the rTPA and rEG into rPET bottles. The overall impacts of this rPET system are normalized over the number of lifetimes (L) obtainable through the enzymatic hydrolysis process, as calculated with eqn (1) in the Methods section; L for this base case is set as 2.81 (Table S15†). This value is lower than that used in our previous analysis (L = 3.6),22 as we have now incorporated losses from the sorting and flaking process (16.3% PET mass loss). A complete cradle-to-grave approach was used as the vPET baseline and includes impacts from monomer production, polymerization, stretch blow molding into bottles, collection, and disposal by landfill (80%) and incineration (20%); use phase impacts are not considered. This system expansion method is crucial for fairly assessing closed-loop recycling technologies in which the polymer can be reused multiple times.
For most metrics, our results show that rPET has life cycle impacts 1.2–3.0× higher than vPET, excepting lower ecotoxicity (−6%) and fossil fuel depletion (−15%). However, these point estimate differences are in many cases obscured by the error bars, indicating that rPET and vPET have statistically similar environmental impacts. This overlap is larger than the previously assessed rTPA and vTPA, which can be attributed to the different system boundaries. The impacts of vPET are dominated by polymerization (top contributor to 7 metrics, accounting for 49–84% of total), with fossil feedstocks for vTPA and vEG monomer production having the highest environmental impacts of this phase. Disposal of vPET waste only contributes significantly to ecotoxicity and eutrophication at 60% and 37% of their totals, respectively. The narrowing of the impacts gap between rPET and vPET can therefore be primarily attributed to a reduction in virgin monomer demand, rather than diversion of waste PET from landfill or incineration.
Fig. 2D showcases the top contributors to the rPET life cycle (Table S16†). These include enzymatic hydrolysis to produce rTPA and rEG (top contributor to 10 metrics, accounting for 31–73% of totals), the initial vPET lifetime (first- or second-highest contributor to 6 metrics, 18–41% of totals), and post-consumer PET collection, sorting, and shredding to flake (encompassed by the “PET flake” label, second-highest contributor to 5 metrics, 22–38% of total). The remaining steps combined – repolymerization of rTPA and rEG into rPET, additional vEG for the repolymerization step, processing of the rPET into bottles, and transportation – account for less than 30% of all life cycle impacts. The impacts of the enzymatic hydrolysis phase are linked to the process components discussed in Fig. 2B, while those of the original vPET production stage can be largely attributed to fossil feedstocks for monomer production as well as electricity use for stretch blow molding of vPET into bottles. Opportunities for advancing the enzymatic hydrolysis process by addressing some of these key environmental contribution areas will be discussed in the Research recommendations section.
Comparison to previous work
Considering global warming impact as a case study, the LCA results (rTPA impacts 2.3× higher than vTPA) differ from those we previously reported with the MFI tool (rTPA impacts 17% lower than vTPA).22 Environmental impacts are typically higher in LCA than MFI due to its broader scope (such as the incorporation of waste streams and emissions), but this cannot account for the observed change in the overall trend between rTPA and vTPA. We have identified several reasons for this discrepancy. First, GHG emissions from PET flake production are higher in LCA (0.67 kg CO2 eq. per kg) than MFI (0.38 kg CO2 eq. per kg) due to the incorporation of the collection and sorting phases (22.6% of the LCA GHG impact of PET flake) and waste disposal (1.8%), which are not included in MFI (see Fig. S6† for system boundaries). Including these post-consumer PET processing steps are crucial for a fair comparison between rTPA/rPET and vTPA/vPET, as waste collection for recycling is akin to fossil fuel extraction for virgin polymer production. While there are differences in GHG emissions from other process components – NaOH, Na2SO4, H2SO4, EG, process water, and steam (Table S17†) – they mostly negate one another overall, leaving the PET flake production process as the main outlier. Second, the MFI tool does not consider emissions associated with the disposal of waste streams. Given that the enzymatic process generates ∼5 kg of wastewater and ∼0.3 kg of solid waste per kg rTPA (versus ∼0.006 kg solid waste per kg vTPA, according to ecoinvent data), this accounts for a 3.9% contribution to global warming potential in the LCA. These emissions are primarily associated with transportation and processing of the waste, rather than its decomposition. Lastly, the baseline GHG emissions associated with vTPA are lower in LCA (1.88 kg CO2 eq. per kg) than MFI (3.27 kg CO2 eq. per kg), likely due to the use of global average data in the LCA tool (U.S. vTPA inventory was unavailable) relative to the U.S. industry-specific data in MFI. The combination of higher PET flake impacts and lower vTPA impacts leads to a switching of the results observed in MFI (rTPA better than vTPA) to those reported here (vTPA better than rTPA). Adjusting the LCA parameters for these discrepancies yields a global warming result in which rTPA emits 7% fewer greenhouse gases than vTPA, which approaches the 17% reported previously with MFI. There is therefore a core consistency between the LCA and MFI methods, but any changes in system boundaries, background data, and assumptions results in significantly different conclusions.Our previous analysis with BEIOM, which was used to scale the TEA model to a US economy-wide industry, resulted in lower environmental impacts in the acidification, ecotoxicity, eutrophication, carcinogenics and non-carcinogenics, ozone depletion, particulates exposure and smog formation categories for rTPA in comparison to vTPA.22 The focus of BEIOM is on economy-wide effects rather than process-specific modelling, and three key areas of discrepancy were identified between the environmentally-extended input–output analysis and this LCA. First is the PET feedstock: BEIOM only included sorting of post-consumer PET at a MRF, not its collection and shredding to flake. PET feedstock therefore contributes between 0.1–7% to the assessed impact categories for rTPA in BEIOM, in contrast to the 18–40% seen in this LCA analysis. Second, BEIOM applies a substitution method for co-products (rEG and Na2SO4) and assesses their replacement credit via economic value. Lastly, contributions from electricity and the remaining enzymatic hydrolysis supply chain are slightly lower in BEIOM than LCA due to different background data (e.g., 2012 U.S. grid mix used in BEIOM versus 2016 in LCA) and system boundaries. Comparison between LCA and BEIOM also highlights the possibility that environmental impacts that may be high at a single plant scale could be reduced at an economy-wide scale in which further material and process substitutions and tradeoffs are incorporated.
Sensitivity analysis
Having established the base case results for rTPA and rPET and determined the top process contributors to their life cycle impacts, we next assessed a series of univariate sensitivity cases to identify key future areas for innovation. These sensitivity cases were previously developed for the TEA22 and investigate optimistic and pessimistic scenarios for different steps of the enzymatic hydrolysis process, including feedstock pre-treatment (post-consumer sorting/flaking yield, PET content in the incoming flake), depolymerization reactor conditions (PET and enzyme loadings, pH, temperature, residence time, and depolymerization extent), product purification (rTPA and rEG recovery), and plant size (Table S18†).The results are given as combined life cycle impact scores: natural environment incorporates acidification, ecotoxicity, eutrophication, global warming, and ozone depletion; natural resources include fossil fuel depletion and water use; and human health combines carcinogenics, non-carcinogenics, particulates, and smog (equal weightings, as described in the Methods section). The tornado plots in Fig. 3 and 4 show the effect of different sensitivity cases on these scores, with our previous economic results included in Fig. 3D for comparison. The black vertical lines in these figures indicate the base case results (5 for natural environment, 2 for natural resources, 4 for human health, and $1.93 for MSP), the blue and orange bars correspond to the optimistic and pessimistic sensitivity cases, respectively, as defined in the y-axis labels, the dashed gray vertical lines give the virgin material value for comparison, and the transparent gray overlay indicates the uncertainty of those virgin material values. Non-aggregated results showcasing the effect of each sensitivity case on acidification, carcinogenics, ecotoxicity, eutrophication, fossil fuel depletion, global warming, non-carcinogenics, ozone depletion, particulates, smog, and water use are available in Tables S9 and S14.†
 | ||
| Fig. 3 (A–C) Sensitivity analysis on the overall life cycle impact scores of rTPA. (D) Techno-economic sensitivity analysis for rTPA; data reproduced from our previous work.22 Blue and orange represent the optimistic and pessimistic cases, respectively, while the gray dashed lines and transparent gray overlay indicate the virgin material (vTPA) impact or price and their standard deviations for comparison. The numbers listed in parentheses on the y-axis correspond to the left (blue), middle (black) and right (orange) points of the graph. See the ESI for corresponding data (Table S19†) and non-aggregated data (Table S9†). | ||
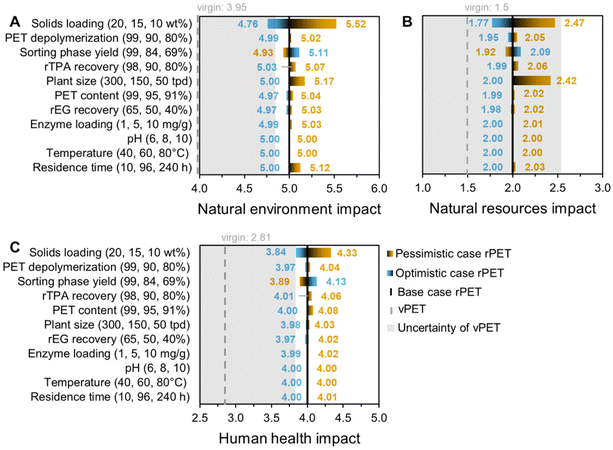 | ||
| Fig. 4 Sensitivity analysis for the overall life cycle impact scores of rPET. Blue and orange represent the optimistic and pessimistic cases, respectively, while the gray dashed lines and transparent gray overlay indicate the virgin material (vPET) impacts and their standard deviations for comparison. The numbers listed in parentheses on the y-axis correspond to the left (blue), middle (black) and right (orange) points of the graph. See the ESI for corresponding data (Table S20†) and non-aggregated data (Tables S14 and S15†). | ||
For the rTPA system (Fig. 3, Tables S9 and S19†), the optimistic cases with impact scores closest to vTPA are 20% solids loading, 99% depolymerization extent, 99% yields from the sorting and flaking process, 98% rTPA recovery, 99% PET content, 300 tpd plant size, and 65% rEG recovery. High solids loading significantly reduces several impactful utilities, including steam consumption (from 4.03 to 2.32 kg kgrTPA−1) and cooling, process, and chiller water, and slightly increases Na2SO4 recovery. In contrast, a smaller plant size suffers from less efficient utility usage. The effects of the depolymerization extent, rTPA recovery, PET content, rEG recovery, and sorting yield scenarios are primarily linked to changes in overall product yield and waste generation. Changes in enzyme loading, pH, temperature, and residence time have minimal effects. These results correlate well with the TEA sensitivity analysis, with residence time and enzyme loading as the only outliers (Fig. 3D).22 There are several trade-offs between the environmental impacts and MSP; for instance, choosing to increase enzyme loading given its minimal effect on environmental impacts (but potential enhancement of depolymerization rates) could result in a cost increase from $1.93 kgrTPA−1 to $2.04 kgrTPA−1. In general, the different sensitivity cases bring rTPA impacts within a statistically equivalent range to those of vTPA for the natural resources category, which can be attributed to the low fossil fuel depletion of rTPA in comparison to vTPA. However, the natural environment and human health scores for rTPA remain significantly (and statistically) higher than those of the virgin material. MSP is similarly higher for rTPA than vTPA across all sensitivity cases, although previous work showed that lower PET flake feedstock costs could enable cost competitiveness.22
The sensitivity analysis of the rPET system shows similar trends (Fig. 4, Tables S14, S15 and S20†). As expected, the overall spreads of the optimistic and pessimistic cases are smaller than for rTPA since enzymatic hydrolysis accounts for only one step of several across the rPET life cycle. High solids loading brings the natural environment and human health impacts of rPET within the standard deviation range of vPET. For natural resources, all sensitivity cases fall within the vPET uncertainty range. Several scenarios linked to process yield – particularly rTPA recovery and sorting phase yield – have inverted results in this figure because their calculated lifetimes (e.g., 99% and 69% sorting yield have L = 4.20 and 2.12, respectively) differ from that of the base case (L = 2.81). When compared across the raw data (pre-systems expansion), 99% sorting phase yield results in natural environment, natural resources, and human health scores of 4.71, 1.99, and 3.88, respectively, which closely match the rTPA results. Note that there is a tradeoff between energy use and sorting yields and reaching levels beyond 99% would likely increase environmental impacts due to decreased energy efficiency (i.e., increasingly complex sorting steps for minimal material gain).
Finally, a series of additional process design cases were analyzed for the rTPA (Fig. 5A) and rPET (Fig. 5B) systems. All cases include error bars depicting their standard deviations, which were calculated by error propagation. Many of these cases have overlapping error bars indicating statistical equivalence; statistically distinct cases are highlighted in the following text.
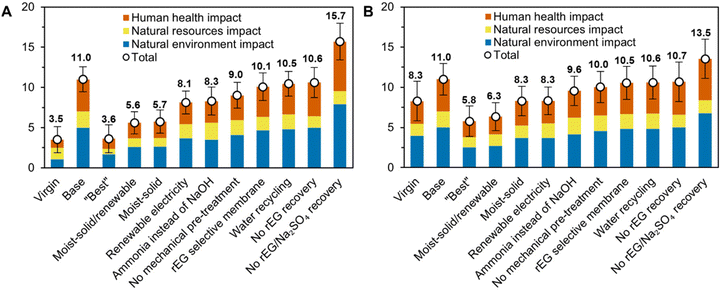 | ||
| Fig. 5 Overall life cycle impact scores for different enzymatic hydrolysis process design cases for (A) TPA and (B) PET. These scores incorporate human health impact (carcinogenics, non-carcinogenics, particulates exposure, and smog), natural resources impact (fossil fuel depletion and water use), and natural environment impact (acidification, ecotoxicity, eutrophication, global warming, and ozone depletion), with each metric normalized on a 0–1 scale relative to the base case and summed with equal weighting. Error bars indicate the standard deviations of the total scores. Corresponding data are available in Tables S19 and S20.† | ||
These design cases were developed in response to the high environmental impact areas highlighted above. They examine the process variables associated with pre-treatment (no extrusion or cryo-grinding of the PET flake feed), enzymatic hydrolysis (replacement of NaOH with ammonia), and product purification steps (no rEG recovery, no rEG or Na2SO4 recovery, rEG separation via selective membrane without distillation, and process water recycling), as well as market-driven alterations (100% electricity from renewable sources). We also propose a “best” case scenario, which includes 99% sorting phase yield, no mechanical pre-treatment, renewable electricity, 99% PET content, 1 mg g−1 enzyme loading, 20% solids loading, 99% depolymerization, 98% rTPA recovery, and 90% rEG recovery with the selective membrane. Furthermore, we develop an alternative, optimistic process model based on the “moist-solid” system reported by Kaabel et al. (details, process schematic, and inventory in Fig. S7 and Table S21†).32 This case study directly utilizes PET flake without mechanical pre-treatment in the enzymatic hydrolysis reactor at a solids loading of 40% at pH 7, 55 °C, and an enzymatic loading of 30 mg g−1 PET. rTPA precipitates under these conditions and therefore no NaOH is required for pH control (solubilized rTPA is what causes acidification in the base case). rEG is subsequently clarified and recovered using the same processes described in the base case, although higher concentrations facilitate more efficient recovery and therefore higher yields of 73%. rTPA is solubilized in dimethyl sulfoxide (DMSO) at 80 °C and recovered by crystallization (90% yield).44 The DMSO is recycled by low-pressure distillation, and solid contaminants are removed by filtration and sent to disposal.
Case studies in which rEG and Na2SO4 are not recovered increase the environmental impacts of rTPA and rPET from a base case overall score of 11 to 15.7 ± 2.3 and 13.5 ± 2.5, respectively, due to the loss of the credits associated with co-product generation. For rTPA, this increase relative to the base case is statistically significant. Avoiding only rEG recovery, on the other hand, marginally decreases environmental impacts, as the steam savings outweigh the rEG co-credits. Recovering rEG by membrane separations rather than distillation improves the overall environmental scores to 10.1 ± 1.7 for rTPA and 10.5 ± 2.1 for rPET due to a 17% decrease in steam use and an increase in rEG recovery from 50% to ∼90%. Recycling process water back through the depolymerization reactor instead of sending it to waste disposal also decreases impacts to 10.5 ± 1.5 and 10.6 ± 2.1 for rTPA and rPET, respectively. This is due to a reduction in steam (−6%), process water (−69%), and wastewater, as well as a slight increase in rEG and Na2SO4 recovery. Utilizing a different base for pH control (ammonia rather than NaOH) reduces impacts to 8.3 ± 1.7 for rTPA and 9.6 ± 1.8 for rPET. The lower environmental effects of ammonia, lower quantity of ammonia required to neutralize the reactor (in comparison to NaOH on a weight basis), and higher credits from the ammonium sulfate co-product counteract the higher steam and cooling water usage (1.6× and 1.2× base case values) required for this process design.
Utilization of renewable sources for electricity can play a major role given that enzymatic hydrolysis is a relatively electricity-intensive process (1.76 kW h kgrTPA−1). Exchanging the current electricity grid mix with a 100% renewables option decreases the impacts of rTPA and rPET by 24–26%; the same switch yields a 9% decrease in vTPA impacts and 28% for vPET. Over 90% of process electricity requirements originate from mechanical pre-treatment of the PET flake to produce amorphized powder. Bypassing this step thus reduces the overall impact scores of rTPA and rPET to 9.0 ± 1.6 and 10.0 ± 1.9, respectively. For the rTPA system boundary, these scores remain statistically higher than that of vTPA. Upon expansion to the entire rPET life cycle, however, the scores become statistically equivalent to that of vPET, suggesting that enzymatic hydrolysis could already be a competitive alternative to virgin polymer production.
The “best” case scenario offers the most substantial improvement, with overall scores of 3.6 ± 1.7 for rTPA and 5.8 ± 1.9 for rPET in comparison to 3.5 ± 1.6 for vTPA and 8.3 ± 2.5 for vPET (i.e., the virgin materials and “best” recycled materials are statistically equivalent). It is thus essential to incorporate improvements at all stages of the recycling process, from feedstock preparation to enzymatic hydrolysis itself to product recovery, which will be discussed in the following section.
There are also opportunities to develop enzymatic processes that fundamentally diverge from our base case. The “moist-solid” system offers overall scores of 5.7 ± 1.4 and 8.3 ± 1.8 for rTPA and rPET, respectively; these values decrease slightly to 5.6 ± 1.4 and 6.3 ± 1.7 when renewable electricity is used (statistically equivalent to vTPA and vPET). The reduction in environmental impacts for the “moist-solid” system in comparison to the base case – which ranges from a minimum of −13% for ecotoxicity to a maximum of −68% for particulates exposure (Fig. 6, Table S22†) – is due to several interconnected factors. First, electricity is reduced to 0.12 kW h kg−1 rTPA through the removal of mechanical pre-treatment, contributing less than 8% to all impact metrics. Second, the high ratio of PET to water leads to precipitation of rTPA, which eliminates NaOH use for pH control and facilitates rTPA extraction with (recyclable) DMSO (<6% contribution to all impacts). The low water loading also results in a concentrated rEG stream, which can be more efficiently distilled for higher rEG recovery (73%) with lower steam consumption (−12%). Tradeoffs in this case include high enzyme loadings (30 mg g−1 PET, 6× higher than the base case, 3–20% contribution to all impacts) and the potential for lower extents of PET depolymerization in line with the study from Kaabel et al.32 The high enzyme loading also results in an increased MSP of $2.18 kgrTPA−1 in comparison to the base case at $1.93 kgrTPA−1 (Tables S23 and S24†).
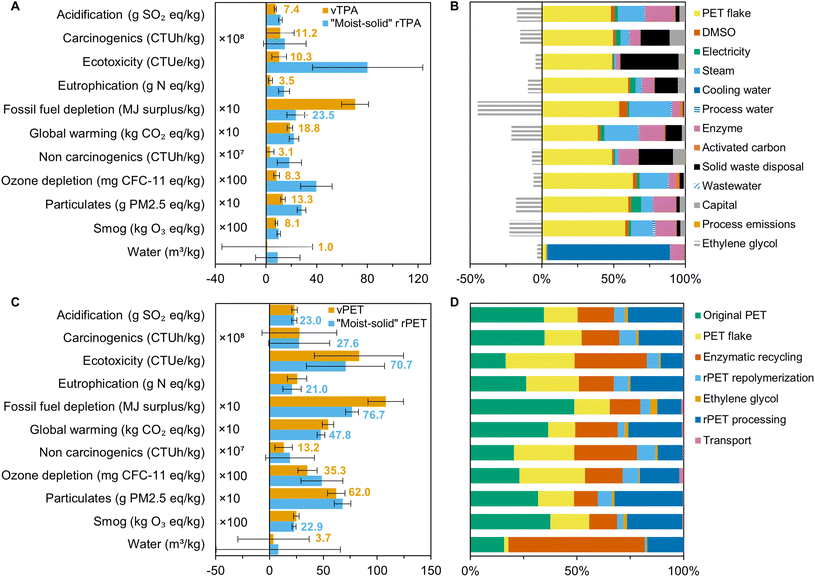 | ||
| Fig. 6 (A) Life cycle impacts of “moist-solid” rTPA (blue) and vTPA (orange). (B) Contribution of different process components to the environmental impacts of moist-solid rTPA. (C) Life cycle impacts of "moist-solid" rPET (blue) and vPET (orange). The rPET values are lifetime normalized according to the procedure described in the Methods section. (D) Contribution of different process components to the environmental impacts of moist-solid rPET. Some impact categories in (A) and (C) are multiplied by a conversion factor listed on the left sides of the graphs for visual clarity. The lowest values for each impact category are labelled. Corresponding data are available in Tables S9, S13, S14, and S22.† | ||
Research recommendations
Based on this LCA, we propose a series of areas and targets for researchers interested in developing more environmentally friendly enzymatic PET hydrolysis processes (Fig. 7).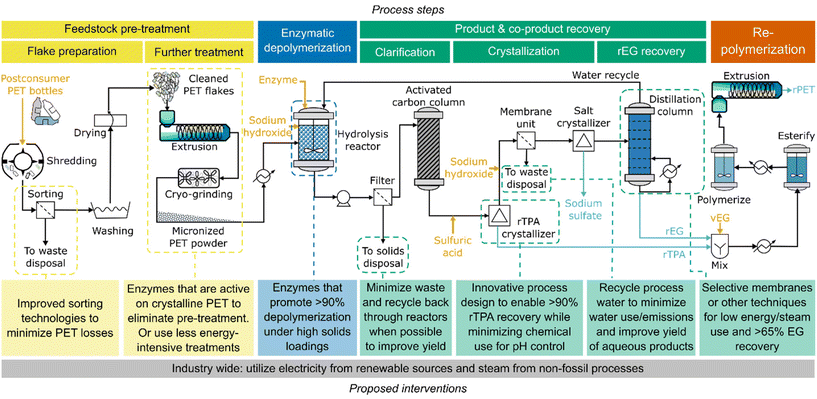 | ||
| Fig. 7 Potential improvements to the modelled enzymatic PET recycling process and the stages at which they could be implemented. | ||
First, process yields (quantity of product per quantity of post-consumer PET) must be improved. We estimate that a baseline target of >70% should enable overall environmental impacts of rPET to be lower than those of vPET. The sensitivity analyses in Fig. 3–5 – as well as in our previous TEA study22 – showed that parameters associated with material loss (sorting yield, feedstock purity, PET solids loading, PET depolymerization extent, and rEG and rTPA recovery) have a major role in reducing environmental impacts by both lowering the utilities required per unit of product and reducing the quantity of process waste for disposal. Research on this topic should focus not only on improving the efficiency of the enzymatic hydrolysis phase, but also on that of the essential surrounding systems for feedstock preparation and product recovery. For example, the rPET base case scenario studied here has a yield of 56% (calculated by multiplying 90% sorting yield, 93% flake, 95% pre-treatment, 90% depolymerization, 79% overall monomer, and 99% repolymerization). Enhancing depolymerization efficiency to 99% results in an overall rPET yield of 62%. Incorporating further adjustments into the pre-treatment and product recovery stages, such as in the “best” case scenario, can increase this value to 93%, which significantly decreases the overall environmental impacts of the technology (compare to vPET synthesis yield of ∼99%).31 Improved sorting technologies – which would benefit the waste management system in general – as well as novel monomer recovery techniques – selective membranes rather than energy-intensive distillation for EG or in situ product recovery for TPA – will be crucial for meeting these goals. A holistic view of process efficiency would also be beneficial, for instance, by immobilizing enzymes on a support for facile reuse, or recycling wastewater back through the depolymerization process to lower water consumption and minimize waste production.
Second, more versatile enzymes and enzyme cocktails would be advantageous. Removing mechanical pre-treatment and increasing solids loading can markedly lower the environmental impacts of enzymatic PET hydrolysis but implementing these process changes requires research into enzyme substrate selectivity and performance. Enzymes that are able to tolerate high substrate loading and operate efficiently on crystalline, flake-sized PET would be a major enabling breakthrough.4,32,45,46 Under this scenario, it may be possible to eliminate certain sorting or flaking steps, further reducing environmental impacts. The ability of enzymes to tolerate contaminated PET feedstocks should therefore be more thoroughly characterized as well.
There is also room for additional enabling innovations. For example, processes would benefit from a significant reduction in base consumption, which when modeled as NaOH accounts for 11–57% of the impacts in eight of the eleven assessed categories. NaOH is used primarily to regulate the pH of the depolymerization reactor, which is acidified as rTPA is released from PET. Reducing the operating pH to 5 only decreases NaOH consumption by 4% due to the pKas of TPA (3.51 and 4.82). Decreasing the pH further results in the precipitation of rTPA, which can be challenging to separate from unreacted PET powder and enzyme. Addressing this issue requires several changes to the overall system, as highlighted in the “moist-solid” case study. The “moist-solid” case utilized high PET flake loadings and operating conditions that facilitated rTPA precipitation, thereby removing the need for pH control while also enabling facile rTPA recovery and purification in DMSO. While we do assume higher monomer yields than reported in the publication (90% vs. 49% rTPA),32 this target should be obtainable through enzyme selection and engineering. Our analysis suggests that this system offers one route towards more environmentally friendly enzymatic PET recycling. Other alternatives include replacing NaOH with more environmentally benign (e.g., ammonia) or recoverable bases, as well as using enzymes that operate effectively without pH control or can tolerate organic solvents that enable in situ product recovery.
While many of these research suggestions overlap with those previously proposed to lower the MSP of rTPA, this LCA highlights the need to minimize consumables (water, steam, electricity, NaOH) and waste streams (wastewater, solid waste) that are relatively inexpensive from an economic perspective but costly from an environmental standpoint. A combination of process innovation (enzyme design, yields, and product separations) and industry-wide improvements (renewable electricity sources and steam from bio-methane rather than fossil fuels) will undoubtedly be beneficial. It should also be noted that our analysis does not include the learning-by-doing effect, through which efficiencies would be expected to improve organically over time.
Conclusions
LCA is a valuable tool for estimating the environmental impacts of emerging technologies and identifying key areas for improvement prior to their implementation at-scale. Here, we utilize our previously developed process model22 to investigate the life cycle impacts of an enzymatic hydrolysis technology for recycling PET. The LCA shows that enzymatic hydrolysis is currently outperformed by virgin PET across most of the assessed natural environment, natural resources, and human health categories. However, there is significant opportunity for improvement. We identified sodium hydroxide, electricity, and shredding of PET into flake as the top contributors to each impact category and utilized this information to design a series of sensitivity cases focused on different aspects of the enzymatic hydrolysis process. Decreasing material losses across all process phases, as well as bypassing mechanical pre-treatment, minimizing waste streams, employing alternative product recovery techniques, and utilizing renewable electricity sources were all shown to be crucial steps towards reducing the life cycle impacts of the technology. Combining several of these suggestions in the “best” case scenario or the recently proposed “moist-solid” process32 offers a potential route towards improved environmental impacts and simultaneous promotion of polymer circularity. This study highlights that while enzymatic PET recycling is not currently more environmentally friendly than virgin polyester, there are significant opportunities for research innovation that could improve its feasibility for application at scale.Conflicts of interest
G.T.B. and J.E.M. have submitted patent applications on enzymes for PET recycling.Acknowledgements
Funding was provided by the U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office (AMO) and Bioenergy Technologies Office (BETO). This work was performed as part of the BOTTLE™ Consortium and was supported by AMO and BETO under contract no. DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC. The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes. We thank Erika Erickson, Yimin Zhang, and Andre Fernandes Tomon Avelino for their feedback on this work.References
- V. Sinha, M. R. Patel and J. V. Patel, J. Polym. Environ., 2010, 18, 8–25 CrossRef CAS.
- R. Wei and W. Zimmermann, Microbiol. Biotechnol., 2017, 10, 1302–1307 CrossRef CAS PubMed.
- I. Taniguchi, S. Yoshida, K. Hiraga, K. Miyamoto, Y. Kimura and K. Oda, ACS Catal., 2019, 9, 4089–4105 CrossRef CAS.
- A. Carniel, V. de A. Waldow and A. M. de Castro, Biotechnol. Adv., 2021, 52, 107811 CrossRef CAS PubMed.
- L. D. Ellis, N. A. Rorrer, K. P. Sullivan, M. Otto, J. E. McGeehan, Y. Román-Leshkov, N. Wierckx and G. T. Beckham, Nat. Catal., 2021, 4, 539–556 CrossRef CAS.
- A. J. Martín, C. Mondelli, S. D. Jaydev and J. Pérez-Ramírez, Chem, 2021, 7, 1487–1533 Search PubMed.
- R. J. Müller, H. Schrader, J. Profe, K. Dresler and W. D. Deckwer, Macromol. Rapid Commun., 2005, 26, 1400–1405 CrossRef.
- Å. M. Ronkvist, W. Xie, W. Lu and R. A. Gross, Macromolecules, 2009, 42, 5128–5138 CrossRef.
- C. Silva, S. Da, N. Silva, T. Matamá, R. Araújo, M. Martins, S. Chen, J. Chen, J. Wu, M. Casal and A. Cavaco-Paulo, Biotechnol. J., 2011, 6, 1230–1239 CrossRef CAS PubMed.
- D. Ribitsch, E. H. Acero, K. Greimel, I. Eiteljoerg, E. Trotscha, G. Freddi, H. Schwab and G. M. Guebitz, Biocatal. Biotransform., 2012, 30, 2–9 CrossRef CAS.
- S. Sulaiman, S. Yamato, E. Kanaya, J. J. Kim, Y. Koga, K. Takano and S. Kanaya, Appl. Environ. Microbiol., 2012, 78, 1556–1562 CrossRef CAS PubMed.
- E. Herrero Acero, D. Ribitsch, A. Dellacher, S. Zitzenbacher, A. Marold, G. Steinkellner, K. Gruber, H. Schwab and G. M. Guebitz, Biotechnol. Bioeng., 2013, 110, 2581–2590 CrossRef CAS PubMed.
- C. Roth, R. Wei, T. Oeser, J. Then, C. Föllner, W. Zimmermann and N. Sträter, Appl. Microbiol. Biotechnol., 2014, 98, 7815–7823 CrossRef CAS PubMed.
- U. T. Bornscheuer, Science, 2016, 351, 1154–1155 CrossRef CAS PubMed.
- S. Yoshida, K. Hiraga, T. Takehana, I. Taniguchi, H. Yamaji, Y. Maeda, K. Toyohara, K. Miyamoto, Y. Kimura and K. Oda, Science, 2016, 351, 1196–1199 CrossRef CAS PubMed.
- A. N. Shirke, C. White, J. A. Englaender, A. Zwarycz, G. L. Butterfoss, R. J. Linhardt and R. A. Gross, Biochemistry, 2018, 57, 1190–1200 CrossRef CAS PubMed.
- M. Furukawa, N. Kawakami, A. Tomizawa and K. Miyamoto, Sci. Rep., 2019, 9, 16038 CrossRef PubMed.
- R. Wei, D. Breite, C. Song, D. Gräsing, T. Ploss, P. Hille, R. Schwerdtfeger, J. Matysik, A. Schulze, W. Zimmermann, R. Wei, P. Hille, W. Zimmermann, D. Breite, A. Schulze, C. Song, D. Gräsing, J. Matysik, T. Ploss and R. Schwerdtfeger, Adv. Sci., 2019, 6, 1900491 CrossRef PubMed.
- V. Tournier, C. M. Topham, A. Gilles, B. David, C. Folgoas, E. Moya-Leclair, E. Kamionka, M. L. Desrousseaux, H. Texier, S. Gavalda, M. Cot, E. Guémard, M. Dalibey, J. Nomme, G. Cioci, S. Barbe, M. Chateau, I. André, S. Duquesne and A. Marty, Nature, 2020, 580, 216–219 CrossRef CAS PubMed.
- Y. Cui, Y. Chen, X. Liu, S. Dong, Y. Tian, Y. Qiao, R. Mitra, J. Han, C. Li, X. Han, W. Liu, Q. Chen, W. Wei, X. Wang, W. Du, S. Tang, H. Xiang, H. Liu, Y. Liang, K. N. Houk and B. Wu, ACS Catal., 2021, 11, 1340–1350 CrossRef CAS.
- C. Sonnendecker, J. Oeser, P. Konstantin Richter, P. Hille, Z. Zhao, C. Fischer, H. Lippold, P. Blázquez-Sánchez, F. Engelberger, C. A. Ramírez-Sarmiento, T. Oeser, Y. Lihanova, R. Frank, H.-G. Jahnke, S. Billig, B. Abel, N. Sträter, J. Matysik, W. Zimmermann, P. Konstantin Richter, C. Sonnendecker, Z. Zhao, Y. Lihanova, S. Billig, J. Matysik, W. Zimmermann, J. Oeser, P. Hille, T. Oeser, P. K. Richter, N. Sträter, P. Blázquez-Sánchez, F. Engelberger, A. Ramírez-Sarmiento, R. Frank, H. Jahnke and B. Abel, ChemSusChem, 2021, e202101062 Search PubMed.
- A. Singh, N. A. Rorrer, S. R. Nicholson, E. Erickson, J. S. DesVeaux, A. F. T. Avelino, P. Lamers, A. Bhatt, Y. Zhang, G. Avery, L. Tao, A. R. Pickford, A. C. Carpenter, J. E. McGeehan and G. T. Beckham, Joule, 2021, 5, 2479–2503 CrossRef CAS.
- Closed Loop Partners, Cleaning the rPET Stream: How we scale post-consumer recycled PET in the US, 2020 Search PubMed.
- Recycling Markets, Secondary materials pricing, 2021, https://www.recyclingmarkets.net/, (accessed 23 March 2022) Search PubMed.
- R. J. Hanes and A. Carpenter, Environ. Syst. Decis., 2017, 37, 6–12 CrossRef.
- S. R. Nicholson, N. A. Rorrer, A. C. Carpenter and G. T. Beckham, Joule, 2021, 5, 673–686 CrossRef CAS.
- P. Lamers, A. F. T. Avelino, Y. Zhang, E. C. D. Tan, B. Young, J. Vendries and H. Chum, Environ. Sci. Technol., 2021, 55, 5496–5505 CrossRef CAS PubMed.
- Franklin Associates, Life Cycle Impacts for Postconsumer Recycled Resins: PET, HDPE, and PP, 2018 Search PubMed.
- S. R. Nicholson, J. E. Rorrer, A. Singh, M. O. Konev, N. A. Rorrer, A. C. Carpenter, A. J. Jacobsen, Y. Roman-Leshkov and G. T. Beckham, Annu. Rev. Chem. Biomol. Eng., 2022, 13, 301–324 CrossRef PubMed.
- NAPCOR, 2020 PET Recycling Report, (National Association for PET Container Resources (NAPCOR)), Charlotte, 2021 Search PubMed.
- A.-S. Bescond and A. Pujari, PET Polymer, in Chemical Economics Handbook, IHS Markit, 2020 Search PubMed.
- S. Kaabel, J. P. D. Therien, C. E. Deschênes, D. Duncan, T. Friščić and K. Auclair, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2026452118 CrossRef CAS PubMed.
- The Association of Plastics Recyclers, Model Bale Specification: PET Bottles, 2021 Search PubMed.
- USEPA, Advancing Sustainable Materials Management: 2018 Tables and Figures, 2020 Search PubMed.
- G. Wernet, C. Bauer, B. Steubing, J. Reinhard, E. Moreno-Ruiz and B. Weidema, Int. J. Life Cycle Assess., 2016, 21, 1218–1230 CrossRef.
- National Renewable Energy Laboratory, U.S. Life Cycle Inventory Database: Enzyme, Cellulase, Novozyme Celluclast https://www.lcacommons.gov/lca-collaboration/National_Renewable_Energy_Laboratory/USLCI/dataset/PROCESS/3dd59224-ecba-3fb1-a2c9-9c01947b08b1, (accessed 10 November 2021).
- J. Bare, Clean Technol. Environ. Policy, 2011, 13, 687–696 CrossRef CAS.
- AWARE (Available WAter REmaining) Mission and Goals - WULCA https://wulca-waterlca.org/aware/, (accessed 21 March 2022).
- S. Muller, P. Lesage, A. Ciroth, C. Mutel, B. P. Weidema and R. Samson, Int. J. Life Cycle Assess., 2016, 21, 1327–1337 CrossRef.
- D. H. Kim, D. O. Han, K. In Shim, J. K. Kim, J. G. Pelton, M. H. Ryu, J. C. Joo, J. W. Han, H. T. Kim and K. H. Kim, ACS Catal., 2021, 11, 3996–4008 CrossRef CAS.
- AMI International, The purity drive in mechanical and chemical recycling of PET, Bristol, 2020 Search PubMed.
- A. M. Boulay, P. Lesage, B. Amor and S. Pfister, J. Ind. Ecol., 2021, 25, 1588–1601 CrossRef.
- U.S. Energy Information Administration, Annual Energy Outlook 2018, Washington, D.C., 2018 Search PubMed.
- H. L. Lee, C. W. Chiu and T. Lee, Chem. Eng. J. Adv., 2021, 5, 100079 CrossRef CAS.
- A. Maurya, A. Bhattacharya and S. K. Khare, Front. Bioeng. Biotechnol., 2020, 8, 1332 Search PubMed.
- N. A. Samak, Y. Jia, M. M. Sharshar, T. Mu, M. Yang, S. Peh and J. Xing, Environ. Int., 2020, 145, 106144 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc02162e |
| This journal is © The Royal Society of Chemistry 2022 |