Open Access Article
Open Access ArticleA hemicellulose and lignin-first process for corn stover valorization catalyzed by aluminum sulfate in γ-butyrolactone/water co-solvent†
Yiping
Luo
a,
Min
Wei
a,
Bin
Jiang
a,
Mingyi
Zhang
a,
Qian
Miao
a,
Hongquan
Fu
 *c,
James H.
Clark
*c,
James H.
Clark
 b and
Jiajun
Fan
b and
Jiajun
Fan
 *b
*b
aCollege of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu, Sichuan 610059, PR China
bGreen Chemistry Centre of Excellence, Department of Chemistry, University of York, York, YO10 5DD, UK. E-mail: alice.fan@york.ac.uk
cCollege of Chemistry and Chemical Engineering, Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, China West Normal University, Nanchong, Sichuan 637000, China. E-mail: fubestone@163.com
First published on 5th August 2022
Abstract
A novel hemicellulose and lignin-first process for corn stover valorization catalyzed by Al2(SO4)3 in GBL/H2O co-solvent was developed in this study. In 25% GBL/H2O at 160 °C, Al2(SO4)3 assisted H2O in breaking down intermolecular linkages of corn stover. The hydrolysis of Al2(SO4)3 could produce H+, [Al(OH)2(H2O)x]+ and SO42−. With the solvation of GBL, the H+ and [Al(OH)2(H2O)x]+ from Al2(SO4)3 hydrolysis increased the co-conversion and dissolution of hemicellulose (96.9 wt%) and lignin (68.0 wt%), while the AlHSO4(OH)2H2O species formed by combining with [Al(OH)2(H2O)x]+ and SO42− was found to inhibit cellulose conversion (88.7 wt% kept). Expecting for H+ from Al2(SO4)3 hydrolysis, the formed AlHSO4(OH)2H2O was found to be the catalytic active species for the cleavage of glycosidic bonds in hemicellulose to produce xylose, and also acted as a “stabilizer” to prevent the further degradation of xylose to improve its yield (87.6%, based on the weight of hemicellulose). Al2(SO4)3 selectively promoted lignin depolymerization to lower Mw oligomers, as well as monophenols (11.20%) with high selectivity of 70.0% to VG and VP via Cα–OH dehydration and –Cγ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH decarboxylation reaction. The obtained cellulose-rich residues show great potential for further use within many industrial processes. The developed process is recyclable, giving important insights to design new approaches for corn stover valorization.
O)–OH decarboxylation reaction. The obtained cellulose-rich residues show great potential for further use within many industrial processes. The developed process is recyclable, giving important insights to design new approaches for corn stover valorization.
1. Introduction
The over-dependence on fossil fuels for the production of fuels and chemicals has resulted in significant energy shortages and environmental issues.1,2 As a renewable and abundant feedstock, lignocellulosic biomass is a promising candidate to replace fossil fuels.3,4 Furthermore, lignocellulosic biomass can adsorb and use CO2 from the atmosphere via photosynthesis, resulting in a nearly “closed carbon balance”.5,6 Corn stover is one of the major agricultural waste biomass in China with approximately 300 million tons produced per year, and its yield has steadily increased year after year.7,8 Due to a lack of effective utilization modes, farmers have no other choice but to burn corn stover openly, resulting in severe air pollution and the loss of valuable resources. Therefore, it is necessary to accelerate the effective utilization of corn stover. To compete with the current petroleum refineries, it is critical to maximize the utilization of corn stover in a cost-effective and environmentally sustainable manner.1,3 Together with the development of sustainable green processes, it will further increase the availability of corn stover and offer considerable promise for the supply of renewable carbon.3 However, corn stover valorization presents a significant challenge due to its inherent recalcitrance and heterogeneity.Corn stover mainly consists of three major components: cellulose, hemicellulose, and lignin. Currently, a major challenge is the development of efficient pretreatment methods for the complete and efficient utilization of the three main components in corn stover.9 The sustainable biorefinery encouraged a prior fractionation of biomass into its components, such as hemicellulose, cellulose and lignin, reducing the complexity of downstream processes and maximizing biomass utilization.10 Hemicellulose is more unstable and has a lower degree of polymerization than cellulose and lignin, making it more susceptible to degradation during the pretreatment process.11 As a result, most lignocellulosic biomass fractionations start with the removal of hemicellulose, leaving as much lignin and cellulose as possible in solid residues.11,12 It was reported that a hydrothermal catalytic method could remove more than 90% hemicellulose from biomass, but the associated delignification of 10–30% was difficult to be avoided.10 In lignocellulosic biomass, hemicellulose and lignin are crosslinked with each other by intermolecular linkages, forming a networked structure that wraps the cellulose.13 Several studies have already been performed to show the potential of cellulose-rich solid residues for enzymatic saccharification or chemocatalytic conversion.14,15 Recently, “lignin-first” strategy is reported to be promising to convert biomass efficiently, which refers to the fractionation and valorization of lignin prior to cellulose valorization.16,17 It was found that hemicellulose was easily extracted with lignin. Therefore, borrowing the concept of “lignin-first” strategy, the first simultaneous removal of hemicellulose and lignin while leaving cellulose in the solid residues is noted as “hemicellulose and lignin-first” strategy, which is a promising process for the effective utilization of biomass. According to the work of Mellmer et al.,18 the use of organic solvents could obtain high biomass conversion and product selectivity. In recent years, γ-valerolactone /water (GVL/H2O) co-solvent has been used to improve the simultaneous removal of hemicellulose and lignin from biomass, since GVL is a safe, recyclable, and green biomass-derived solvent.19,20 GBL (γ-butyrolactone), which has similar properties as GVL, can be also produced from biomass.21–23 Zhang et al. discovered that GBL/H2O co-solvent was very effective for dissolving lignin from hemicellulose-free corncob residues.21 Furthermore, GBL has great potential as a solvent in industrial applications because it is less expensive than GVL. Therefore, GBL/H2O as a solvent for biomass transformation is worth for further investigation.
Homogeneous metal salts are particularly interesting due to their excellent properties as Lewis/Brønsted acids and salting-in(out)agents.6 Aluminum sulfate (Al2(SO4)3) is an inexpensive, efficient, stable, and low toxicity metal-salt catalyst, which was found to be effective for the conversion of sugars and biomass-derived oligomers.24–27 In these catalytic reactions, Lewis acid species [Al(OH)2(aq)]+ and Brønsted acid species [H+] from Al2(SO4)3 hydrolysis were found to have a synergistic effect, promoting the hydrolysis, isomerization and dehydration reaction. It is worth mentioning, however, that the transformation mechanism of raw biomass was more complex than pure xylan. Previous work showed that the synergistic effects of H2O and Al2(SO4)3 facilitated the selective conversion of hemicellulose from corn stover to 85.1 wt% xylose, where the aluminum species [Al(OH)2(H2O)x]+ and H+ from Al2(SO4)3 hydrolysis were found to promote the conversion of hemicellulose.28 While the function of SO42− from Al2(SO4)3 on the conversion of the three main components was ignored. Furthermore, the role of Al2(SO4)3 in the organosolv fractionation of raw biomass was unknown. The use of Al2(SO4)3 with GBL/H2O for corn stover valorization could be an interesting route, which needed to be investigated further.
Herein a novel process for the hemicellulose-lignin first fractionation from corn stover catalyzed by Al2(SO4)3 in GBL/H2O co-solvent is reported. With the cellulose mostly intact, Al2(SO4)3 promoted the hemicellulose depolymerization to xylose, as well as lignin depolymerization to produce monophenols and oligomers in 25% GBL/H2O. The cellulose-rich solid residues could be further used to produce value-added chemicals and materials. This enabled to achieve the use of corn stover to its fullest. This is the first example to discuss the unique functions of Al2(SO4)3 in GBL/H2O for corn stover valorization. Additionally, the recyclability of GBL and Al2(SO4)3 from the developed process was studied. This gives important insights to design new pretreatment methods for maximizing the value of waste biomass.
2. Materials and methods
2.1 Materials
Corn stover (CS) was collected from Shuangliu district of Chengdu, Sichuan province, China, which contained 17.1 wt% hemicellulose, 35.2 wt% cellulose, 19.2 wt% lignin, 6.4 wt% ash, 9.8 wt% moisture, 11.2 wt% extractives, and 1.1 wt% others. Before use, it was ground into a 40 meshes powder and dried overnight in an oven at 105 °C. Gama-butyrolactone (GBL, AR, 99%, Adamas), ethanol (EA, AR, 95%, Greagent), 2-methyltetrahydrofuran (2-MeTHF, AR, 99%, Adamas), aluminum sulfate octadecahydrate (Al2(SO4)3·18H2O, AR, 99%, Greagent), hydrochloric acid (HCl, AR, 36%-38%, Chengdu Kelong Chemicals Co., LTD), sulfuric acid (H2SO4, AR, 95%-98%, Chengdu Kelong Chemicals Co., LTD), and various metal salts (sulfate salts and chloride salts) were purchased from commercial sources and utilized without purification.2.2 The process for corn stover conversion
Corn stover conversion was carried out in a 150 mL sealed autoclave with mechanical agitation (Beijing Century Senlong experimental apparatus Co., Ltd). In a typical run, 3 g corn stover powder and 60 mL co-solvent (e.g. GBL/H2O, EA/H2O and 2-MeTHF/H2O) with various organic solvent contents (0, 25%, 50%, 75% and 100%) were added into the autoclave and sealed. With an initial N2 pressure of 1.0 MPa, the reactor was heated from room temperature to the designed value. When the reactor reached the desired temperature, the reaction time began to be recorded. The reaction was stopped when it reached the desired time, and flow water was used to cool it down to room temperature. The mixture of liquid fraction and solid residues were collected, and filtered by pre-weighed filter paper. The collected liquid fraction and solid residues were used for further analysis. To investigate the roles of Al2(SO4)3 in GBL/H2O for corn stover conversion, a certain amount of metal salts (e.g. sulfate salts and chloride salts) and mineral acids (H2SO4 and HCl) were added and performed using the same procedure described above.2.3 Characterization of corn stover and reaction residues
The amounts of hemicellulose, cellulose and lignin in corn stover and reaction residues were determined using the Van Soest chemical titration method.29 The surface functional groups of solid samples were determined using a Fourier transform infrared spectroscopy (FTIR, Bruker invenio r). The FT-IR spectra were collected with a resolution of 4 cm−1 and 16 scans. The crystalline structures of solid samples were studied using an X-ray diffractometer (XRD, Rigaku Ultima IV, Japan). The crystallinity index (CI) of samples was calculated using the Segal formula.30 A scanning electron microscope (SEM, Phenom Pharos 20-135X) was used to observe the surface images of solid samples. Prior to analysis, the solid samples were gold coated.2.4 Characterization of the liquid fraction
The contents of small molecular products mainly from hemicellulose in liquid fraction were analyzed by high performance liquid chromatography (HPLC, SHIMADZU LC-20, Japan). The HPLC was equipped with a Bio-Rad Aminex HPX-87 column, SPD-20 UV/vis detector (UV) and RID-10A refractive index detector (RI). The measurement conditions were set according to previous work.28 Before analysis, the liquid fraction obtained after treatment with GBL/H2O co-solvent was diluted five times with water. The yield of small molecular products mainly from hemicellulose was calculated using the following equations: | (1) |
 | (2) |
Qualitative and quantitative analysis of monophenol contents derived from lignin in liquid fraction were performed on Gas chromatography equipped with a flame ionization detector (GC-FID, PERKINELMER Clarus 580), according to the standard curves of monophenol mixture. The measurement conditions were set according to previous work.31 Before analysis, the liquid fraction obtained after treatment with GBL/H2O co-solvent was subjected to a rotary evaporator to remove water at 50 °C.21 The contents of monophenols were quantified using benzyl alcohol (0.5 mg mL−1) as an internal standard. The yield of monophenols was based on the weight of lignin in corn stover, as shown in the equation below:
 | (3) |
The oligomers in liquid fraction were analyzed by gel permeation chromatography (GPC, Waters), two dimensional heteronuclear single quantum coherence (2D HSQC NMR, BRUKER ADVANCE 400 MHz spectrometer) and electrospray ionization-mass spectrometry (ESI-MS, Shimadzu). For 2D HSQC NMR analysis, the liquid fraction was firstly precipitated by adding a large amount of H2O (VH2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Vliquid = 8–10) to obtain powder samples that mainly contained lignin-derived oligomers.19,20 After centrifugation, the obtained powder samples were then fully dissolved in DMSO-d6 for 2D HSQC NMR analysis. The measurement methods were the same as in the previous work.31
Vliquid = 8–10) to obtain powder samples that mainly contained lignin-derived oligomers.19,20 After centrifugation, the obtained powder samples were then fully dissolved in DMSO-d6 for 2D HSQC NMR analysis. The measurement methods were the same as in the previous work.31
2.5 Quantum chemical calculation
All density functional theory (DFT) calculations were carried out with the Gaussian 09 programs.32 The PBE0 functional method33,34 was employed with the 6-311++G(d, p) basis set for C, H, O, and S atoms35 and the aug-cc-pvtz basis set for Al atoms.36 Solvent effect was considered in optimization employing the self-consistent reaction field (SCRF) method based on the universal solvation model SMD.37 Geometry optimizations were run to locate all the stationary points. Unless otherwise specified, the relative Gibbs free energies (ΔG, kcal mol−1) were obtained at the level of PBE0/6-311++G(d, p), aug-cc-pvtz at 433.15 K with the pressure of 10 atm. In aqueous solution (25% GBL/H2O), the formed Gibbs free energy (ΔG) of each [Al(HSO4)p(OH)m(H2O)n]3−m−p species starting from three independent species (HSO4−, OH− and H2O) with Al3+ for five coordination was computed following the reaction: Al3+ + p HSO4− + m OH− + n H2O → [Al(HSO4)p(OH)m(H2O)n]3−m−p, and using the equation: ΔG = G[Al(HSO4)p(OH)m(H2O)n]3−m−p − GAl3+ − pGHSO4− − mGOH− − nGH2O.3. Results and discussion
3.1 Performance of GBL/H2O co-solvent on the transformation of corn stover
As a kind of readily available raw material in China, the achievement of corn stover valorization is a two-win strategy that benefits both the economic and environment. Currently, the effective utilization of the three main components in corn stover, that is, hemicellulose, cellulose and lignin is a hot topic. Here, “hemicellulose and lignin-first” strategy is provided to achieve corn stover valorization and its goal is to first simultaneous fractionation of hemicellulose and lignin, while keeping as much cellulose as possible in solid residues that can be further used. According to previous research,38,39 water tends to dissolve hemicellulose, while organic solvents have a higher affinity for lignin. As a result, three types of green solvents, EA, 2-MeTHF and GBL, were mixed with water to make co-solvents for corn stover transformation at 160 °C for 2 h, respectively (Fig. 1 and Fig. S1†). In the three pure organic solvent systems, the transformation of corn stover were all less than 30%, indicating that pure organic solvent was not beneficial for corn stover transformation. In pure H2O, the transformation of corn stover was 44.4%. For EA/H2O co-solvent system, the transformation of corn stover gradually decreased from 44.4% to 32.1% with increasing EA concentrations to 75% (Fig. S1(A)†). In 2-MeTHF/H2O co-solvent system, the corn stover transformation increased gradually, peaking at 47.1% when 2-MeTHF content reached 50% (Fig. S1(B)†). With further increasing 2-MeTHF content to 75%, the transformation of corn stover reduced. The transformation of corn stover in GBL/H2O co-solvent exhibited a similar trend as that in 2-MeTHF/H2O co-solvent system, reaching a maximum of 56.2% at 50% GBL/H2O (Fig. 1(A)). GVL is also a biomass-derived green solvent that has similar properties as GBL. In the previous work,31 GVL contents in GVL/H2O system showed a significant effect on the transformation of corn stover, and reached a maximum of 46.4% in 25% GVL/H2O. In terms of corn stover transformation, GBL/H2O outperformed the above three co-solvent systems.The amount of organic solvent in co-solvent was thought to be the most important factor influencing the behavior of the three main components in corn stover.38 The efficient conversion of biomass in EA/H2O and 2-MeTHF/H2O generally required higher temperatures (>200 °C) or acid catalysts.21,40 After being treated with pure H2O, the co-conversion and dissolution of hemicellulose and lignin were 69.4 and 53.6 wt%, respectively (Fig. 1(B)). With the change of organic solvent concentrations at a lower temperature of 160 °C for 2 h, EA/H2O and 2-MeTHF/H2O exhibited a lower co-conversion and dissolution of hemicellulose and lignin than pure H2O system (Fig. S1(C) and (D)†). The change of GBL or GVL contents in solvent systems, on the other hand, increased the co-conversion and dissolution of hemicellulose and lignin. It was previously reported that 25% GVL /H2O gave notably higher hemicellulose (74.4%) and lignin (51.4%) removal, while 89.5% cellulose was kept intact at 160 °C for 2 h.31 Compared to GVL, GBL exhibited better performance on the simultaneous removal of hemicellulose and lignin (Fig. 1(B)). With the treatment of 25% GBL/H2O, the maximum hemicellulose removal of 91.2 wt% was obtained, and the dissolution of lignin was 65.3 wt%. The maximum lignin removal of 78.9 wt% was obtained after being treated with 50% GBL/H2O, but the removal of hemicellulose was only 67.2 wt%. At any GBL/H2O proportion, the dissolution of cellulose in corn stover was less than 30 wt% at 160 °C for 2 h. XRD analysis of solid residues obtained after GBL/H2O co-solvent treatment revealed higher CI values than raw corn stover (Fig. 2(A)). The CI values of solid residues obtained at 25% GBL/H2O (CI = 79.7%) and 50% GBL/H2O (CI = 81.5%) were significantly higher than those obtained at other proportions of GBL/H2O co-solvents, indicating that the relative content of cellulose in the solid residues was higher. While compared to 50% GBL/H2O, 25% GBL/H2O required less organic content and yielded significantly higher co-conversion and dissolution of hemicellulose and lignin, hence 25% GBL/H2O was chosen for further research.
3.2 Al2(SO4)3 improved the fractionation of hemicellulose and lignin from corn stover in GBL/H2O co-solvent
FT-IR analysis of the samples obtained after various treatments were carried out (Fig. 2(B)). After being treated with pure H2O, the FT-IR peak at 1635 cm−1 (assigned to a hydrogen bond between hemicellulose and lignin) disappeared.41 The two peaks at 1115 and 1062 cm−1 shifted to 1107 and 1057 cm−1, respectively, which was related to the disruption of interactions among the three main components.39,42 When 25% GBL was added in pure H2O, the two peaks were almost not significantly changed. With the addition of Al2(SO4)3 into 25% GBL/H2O, the peak at 1115 cm−1 was completely disappeared and the peak at 1062 cm−1 greatly increased. Therefore, Al2(SO4)3 aided H2O in the cleavage of the intermolecular linkages among the three main components in corn stover, while GBL did not show any effect. When 25% GBL was added in pure H2O, the absorption peaks at 1735 cm−1 (assigned to carboxylic acid in hemicellulose) greatly reduced.41 With the addition of Al2(SO4)3 in 25% GBL/H2O, the peak nearly disappeared. This indicated that GBL and Al2(SO4)3 aided the co-conversion and dissolution of hemicellulose. After being treated with 25% GBL/H2O, the characteristic absorption peaks of lignin at 1038 cm−1 mainly assigned to aromatic C–H in-plane deformation of G unit in lignin showed a blue shift.42 The absorption peak at 1267 cm−1, which corresponds to the C–O stretching vibration of G unit in lignin,39 showed a slight decrease. With the addition of Al2(SO4)3 in 25% GBL/H2O, the peak at 1038 cm−1 significantly shifted, and the peak at 1267 cm−1 was completely disappeared. This suggested that Al2(SO4)3 could further enhance the conversion-dissolution of G unit in lignin with the help of GBL. After being treated by Al2(SO4)3 in 25% GBL/H2O system, the relative content of cellulose in the solid residues increased due to the removal of hemicellulose and lignin, thus the absorbance peaks of cellulose at 1425, 1372, 1327, 1163 and 897 cm−139 slightly increased compared to raw corn stover (Fig. 2(B)). According to FT-IR and chemical titration results, Al2(SO4)3 assisted H2O in breaking down the intermolecular linkages among the three main components in corn stover. With the help of GBL solvation, Al2(SO4)3 promoted the co-conversion and dissolution of hemicellulose and lignin, keeping most cellulose intact. The hydrolysis of Al2(SO4)3 could produce aluminum species [Al(OH)2(H2O)x]+, H+ and SO42−.26,28 The following research was to study the roles of these species in the organosolv fractionation of corn stover.
When different sulfate salts and chloride salts with equal molar concentrations of cations were added in 25% GBL/H2O, respectively, it showed that the added sulfate salts greatly inhibited the conversion of cellulose in corn stover (Fig. 1(C) and (D)). For example, the conversion of cellulose were 17.8 and 13.4 wt% with the addition of Al2(SO4)3 and Fe2(SO4)3, respectively, while those were 29.8 and 39.6 wt% with adding AlCl3 and FeCl3. When H2SO4 was added in GBL/H2O co-solvent, the cellulose removal reduced from 30.1 to 22.6 wt%. In previous work,28 it was discovered that the H2SO4 produced by Al2(SO4)3 hydrolysis promoted cellulose removal from corn stover in pure H2O. However, this work found that H2SO4 produced by Al2(SO4)3 hydrolysis could inhibit cellulose conversion in 25% GBL/H2O. Li et al. reported that GBL/H2O could mediate the distribution of catalyst around reactants and products.43 This could be due to the solvation of GBL/H2O that resulted in the catalytic performance of H2SO4 from Al2(SO4)3 hydrolysis being different, preventing cellulose removal. It is also worth noting that, despite the fact that the H+ concentrations of H2SO4 and HCl are close, H2SO4 achieves significantly lower cellulose conversion (Fig. 1(C) and (D)). Furthermore, the ion strength of H2SO4 (0.06 mol kg−1) was close to that of HCl (0.09 mol kg−1), indicating that ion strength was not related to the inhibition of cellulose conversion (Table S1†). This suggested that the role of H2SO4 from Al2(SO4)3 hydrolysis in inhibiting cellulose conversion might be also related to SO42− rather than H+.
Therefore, with help of GBL/H2O, it was speculated that the H+ and [Al(OH)2(H2O)x]+ species from Al2(SO4)3 hydrolysis increased the co-conversion and dissolution of hemicellulose and lignin. The formed AlHSO4(OH)2H2O by combining with SO42− and [Al(OH)2(H2O)x]+ was found to be the active species to inhibit cellulose conversion.
3.3 Catalytic performance of Al2(SO4)3 on the depolymerization of hemicellulose and lignin in GBL/H2O co-solvent
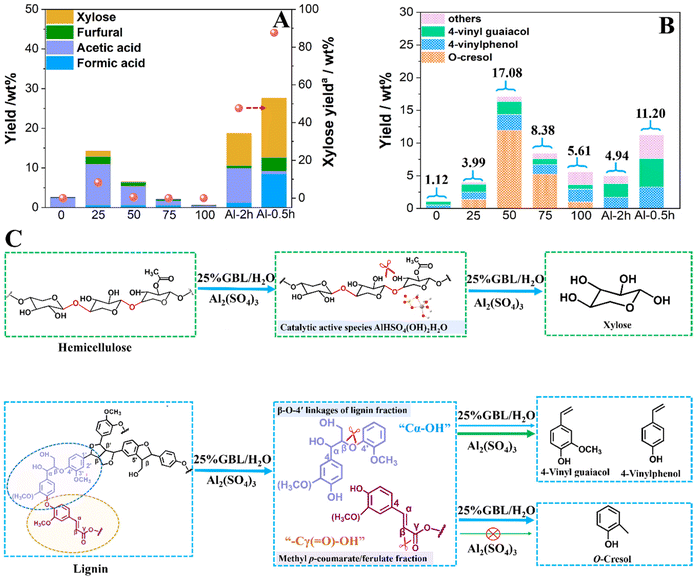
In 25% GBL/H2O system, the effects of sulfate salts and chloride salts on the distribution of small molecular products at 160 °C for 2 h were investigated (Fig. S5†). It was also found that ion strength had no significant effects on the hemicellulose depolymerization. With the addition of chloride salts in 25% GBL/H2O, the main products were acetic acid and furfural, and the yield of xylose was around 0.1–3.7%. While in addition to acetic acid and furfural, 1.4–8.3% xylose was produced with the addition of sulfate salts in 25% GBL/H2O. Al2(SO4)3 produced more xylose than the other sulfate salts, suggesting that xylose production was also related to aluminum species ([Al(OH)2(H2O)x]+). Interestingly, adding AlCl3 only resulted in 0.1% xylose, but it did promote the production of furfural. When compared to HCl with the close concentration of H+, H2SO4 promoted the production of xylose (8.1%). It is possible that the catalytic performance of H2SO4 derived from Al2(SO4)3 hydrolysis in the depolymerization of hemicellulose to xylose is not solely dependent on H+ in 25% GBL/H2O. Therefore, the synergistic actions of aluminum species [Al(OH)2(H2O)x]+ and H2SO4 (H+ and SO42−) produced by Al2(SO4)3 hydrolysis promoted the depolymerization of hemicellulose to xylose, while inhibiting furfural production by dehydration reaction. According to DFT calculation, AlHSO4(OH)2H2O species was found to be the most stable form by combined with [Al(OH)2(H2O)x]+, H+ and SO42− from Al2(SO4)3 hydrolysis, which was the active catalytic species to promote the production of xylose (Fig. 3(C) and Fig. S4†).
To further investigate the catalytic mechanism of AlHSO4(OH)2H2O in the depolymerization of hemicellulose to xylose with 25% GBL/H2O as solvent, DFT calculation was performed with xylobiose (XB) as the model compound. The reaction energy diagram for the depolymerization pathway of XB on AlHSO4(OH)2H2O species was shown in Fig. 5. It was found that the XB can first adsorb on AlHSO4(OH)2H2O species by hydrogen bond, forming an intermediate of XB-AlHSO4(OH)2H2O-1. The H+ from AlHSO4(OH)2H2O species can migrate to the bridge oxygen on XB, promoting the cleavage of glycosidic bonds and the formation of the stable intermediate XB-AlHSO4(OH)2H2O-2 (−36.9 kcal mol−1) with xylose moiety. Next, the –OH group on the AlHSO4(OH)2H2O species will then migrate to the xylose moiety, forming the intermediate XB-AlHSO4(OH)2H2O-3 with −25.1 kcal mol−1. Finally, the formation of two molecular xylose requires an energy of −38.4 kcal mol−1, indicating that this process being thermodynamically favorable. These findings indicated that the H+ from AlHSO4(OH)2H2O species interacted with glycosidic bonds in hemicellulose via hydrogen bonds, followed by glycosidic bonds cleavage and –OH group migration of catalytic species to form xylose. In the traditional acid hydrolysis reaction, H+ can promote the cleavage of glycosidic bonds in hemicellulose to produce xylose. However, less xylose was obtained using HCl as the catalyst in 25% GBL/H2O. Therefore, the formed AlHSO4(OH)2H2O species also acted as a “stabilizer” to prevent the further degradation of xylose to improve its yield from hemicellulose depolymerization.
GPC analysis of liquid fractions obtained from three kinds of systems including pure H2O, 25% GBL/H2O and 25% GBL/H2O–Al2(SO4)3 at 160 °C for 0.5 h were studied (Fig. 4). Compared to pure H2O, the molar mass distribution curve of liquid fraction obtained from 25% GBL/H2O shifted to a lower molecular weight (Fig. 4(A)). The addition of Al2(SO4)3 could enhance this shift. After being treated with pure H2O, the Mw and number-average molecular weight (Mn) of liquid fraction were 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 28
200 and 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 Da, respectively. The value of Đ was 1.29. Compared to pure H2O, the liquid fraction had a lower Mw (14
700 Da, respectively. The value of Đ was 1.29. Compared to pure H2O, the liquid fraction had a lower Mw (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 Da) and Mn (10
800 Da) and Mn (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 Da) after being treated with 25% GBL/H2O. The value of Đ slightly increased to 1.39. With the treatment by adding Al2(SO4)3 into 25% GBL/H2O, the Mw and Mn of liquid fraction significantly decreased to 7400 and 5300 Da, respectively. As shown in Fig. 4(B), more than 95% liquid products with Mw greater than 10
600 Da) after being treated with 25% GBL/H2O. The value of Đ slightly increased to 1.39. With the treatment by adding Al2(SO4)3 into 25% GBL/H2O, the Mw and Mn of liquid fraction significantly decreased to 7400 and 5300 Da, respectively. As shown in Fig. 4(B), more than 95% liquid products with Mw greater than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da were observed after being treated with pure H2O. In 25% GBL/H2O co-solvent, the liquid products with Mw greater than 10
000 Da were observed after being treated with pure H2O. In 25% GBL/H2O co-solvent, the liquid products with Mw greater than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da decreased to 52.9%, and 45.4% liquid products with Mw of 5000–10
000 Da decreased to 52.9%, and 45.4% liquid products with Mw of 5000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 were obtained. When Al2(SO4)3 was added into 25% GBL/H2O, only 18.4% liquid products with Mw were greater than 10
000 were obtained. When Al2(SO4)3 was added into 25% GBL/H2O, only 18.4% liquid products with Mw were greater than 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Da compared to that obtained from 25% GBL/H2O (52.9%) and pure H2O (97.9%). The liquid products with Mw of 1000–5000 Da significantly increased from 1.7% to 35.8% with the addition of Al2(SO4)3 in 25% GBL/H2O. In the above mentioned three kinds of systems, hemicellulose and lignin were both dissolved. So the results suggested that the co-existence of GBL and H2O was beneficial for the depolymerization of oligomers derived from hemicellulose and lignin, and Al2(SO4)3 significantly aided in the depolymerization.
000 Da compared to that obtained from 25% GBL/H2O (52.9%) and pure H2O (97.9%). The liquid products with Mw of 1000–5000 Da significantly increased from 1.7% to 35.8% with the addition of Al2(SO4)3 in 25% GBL/H2O. In the above mentioned three kinds of systems, hemicellulose and lignin were both dissolved. So the results suggested that the co-existence of GBL and H2O was beneficial for the depolymerization of oligomers derived from hemicellulose and lignin, and Al2(SO4)3 significantly aided in the depolymerization.
To further study the roles of Al2(SO4)3 in the depolymerization of lignin-derived oligomers, 2D HSQC NMR and ESI-MS analysis of liquid fractions obtained before and after adding Al2(SO4)3 in 25% GBL/H2O were performed (Fig. 4). 2D HSQC NMR analysis showed that the main signal of side chain region (δC/δH 50–90/2.5–5.0 ppm) assigned to Cγ–Hγ in β-O-4′ structure (Aγ) was weakened with the addition of Al2(SO4)3 in 25% GBL/H2O. The main signals of aromatic region (δC/δH 100–150/6.0–8.0 ppm) assigned to C2,6–H2,6 in oxidized(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) (H′2,6) or non-oxidized p-hydroxyphenyl units (H2,6), and C2,6-H2,6 in syringyl units(S2,6) increased (Fig. 4(G) and (H)). This suggested that Al2(SO4)3 promoted the cleavage of β-O-4′ structure in lignin-derived oligomers. From ESI-MS analysis results (Fig. 4(C–F) and Table S5†), it was observed that the peaks assigned to lignin-derived oligomers containing β-O-4′ linkages fraction (m/z = 261.13, 283.16, 309.18, 325.15, 347.16 and 365.16) and methyl p-coumarate/ferulate structure (m/z = 217.11, 182.06, 166.09) decreased with the addition of Al2(SO4)3 in 25% GBL/H2O. Ma et al. reported that the lignin underwent a dehydration-hydrogenolysis reaction to break the β-O-4 ether bonds to form lignin monomers such as methyl p-coumarate and methyl ferulate.14 And the production of VG and VP was from methyl p-coumarate and methyl ferulate via decarboxylation reaction. In accordance with the work of Liu et al.,45 previous work also found that the cleavage of β-O-4′ linkages from lignin followed by the dehydration of Cα-OH could produce VG and VP.31 According to GC-FID, 2D HSQC NMR and ESI-MS results, Al2(SO4)3 promoted the cleavage of β-O-4′ linkages followed by Cα-OH dehydration and –Cγ(
O) (H′2,6) or non-oxidized p-hydroxyphenyl units (H2,6), and C2,6-H2,6 in syringyl units(S2,6) increased (Fig. 4(G) and (H)). This suggested that Al2(SO4)3 promoted the cleavage of β-O-4′ structure in lignin-derived oligomers. From ESI-MS analysis results (Fig. 4(C–F) and Table S5†), it was observed that the peaks assigned to lignin-derived oligomers containing β-O-4′ linkages fraction (m/z = 261.13, 283.16, 309.18, 325.15, 347.16 and 365.16) and methyl p-coumarate/ferulate structure (m/z = 217.11, 182.06, 166.09) decreased with the addition of Al2(SO4)3 in 25% GBL/H2O. Ma et al. reported that the lignin underwent a dehydration-hydrogenolysis reaction to break the β-O-4 ether bonds to form lignin monomers such as methyl p-coumarate and methyl ferulate.14 And the production of VG and VP was from methyl p-coumarate and methyl ferulate via decarboxylation reaction. In accordance with the work of Liu et al.,45 previous work also found that the cleavage of β-O-4′ linkages from lignin followed by the dehydration of Cα-OH could produce VG and VP.31 According to GC-FID, 2D HSQC NMR and ESI-MS results, Al2(SO4)3 promoted the cleavage of β-O-4′ linkages followed by Cα-OH dehydration and –Cγ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH decarboxylation reaction from lignin-derived oligomers to produce VG and VP (Fig. 3(C)). Wang et al. reported the production of o-cresol from the pyrolysis of organsolv lignin after pretreated by H2O.46 Verma et al. reported that the formation of o-cresol was produced from a guaiacyl lignin-derived model compound via hydrodeoxygenation.47 Therefore, as shown in Fig. 3(C), it was speculated that the depolymerization of guaiacyl lignin-derived oligomers to produce o-cresol was inhibited with addition of Al2(SO4)3 in 25% GBL/H2O. While the mechanism of o-cresol production needs further investigation. Simultaneously, the dehydration reaction of Cα–OH and the decarboxylation reaction of –Cγ(
O)–OH decarboxylation reaction from lignin-derived oligomers to produce VG and VP (Fig. 3(C)). Wang et al. reported the production of o-cresol from the pyrolysis of organsolv lignin after pretreated by H2O.46 Verma et al. reported that the formation of o-cresol was produced from a guaiacyl lignin-derived model compound via hydrodeoxygenation.47 Therefore, as shown in Fig. 3(C), it was speculated that the depolymerization of guaiacyl lignin-derived oligomers to produce o-cresol was inhibited with addition of Al2(SO4)3 in 25% GBL/H2O. While the mechanism of o-cresol production needs further investigation. Simultaneously, the dehydration reaction of Cα–OH and the decarboxylation reaction of –Cγ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH in lignin were promoted, thus the yield of VG and VP increased.
O)–OH in lignin were promoted, thus the yield of VG and VP increased.
3.4 Recyclability of GBL and Al2(SO4)3 towards corn stover fractionation
Finally, the recyclability of GBL and Al2(SO4)3 towards corn stover fractionation were carried out, as shown in Fig. 6. GBL has excellent thermal stability with a high boiling point of 206 °C, and Al2(SO4)3 is more soluble in water than pure GBL. According to their properties, vacuum distillation is regarded as a promising method to recover GBL and Al2(SO4)3 by removing water at a lower temperature of 50 °C. After removing water from the liquid fraction obtained with the treatment of 25% GBL/H2O–Al2(SO4)3 at 160 °C for 0.5 h, white particles (aluminum sulfate) and GBL were left. The recovered Al2(SO4)3 and GBL were then mixed with fresh water to create a 25% GBL/H2O–Al2(SO4)3 system, which was used for a new cycle of corn stover fractionation. After three cycles, corn stover conversion remained around 45%. The removal of hemicellulose and lignin was kept around 95% and 70%, respectively, while cellulose conversion was significantly inhibited (∼16.9%). This implied that GBL and Al2(SO4)3 showed good recyclability towards corn stover fractionation. Additionally, the obtained cellulose-rich solid residues could be further used as starting materials to prepare materials and value-added chemicals within many industrial processes. Xylose from hemicellulose depolymerization mainly existed in water, which can be recovered with the removal of water via vacuum distillation. However, it cannot completely separate liquid products from solvent via vacuum distillation, especially those have a high affinity for GBL. As reported in literature,27,48 the products especially monophenols accumulated at the beginning in the recycling process, and then decreased due to repolymerization after multiple reactions. The effective separation of monophenols and lignin-derived oligomers from liquid fractions to recover high purity GBL needed further investigation. In addition to separation, the products in the GBL can be also used directly to prepare chemicals and fuels by the development of efficient catalysts. However, such impurity did not have a significant effect on the fractionation of corn stover. This study clearly demonstrated that the novel 25% GBL/H2O–Al2(SO4)3 system is promising to be recycled in corn stover-based biorefineries.4. Conclusions
This study developed a novel hemicellulose and lignin-first process catalyzed by Al2(SO4)3 in GBL/H2O for corn stover valorization. In 25% GBL/H2O, the H+ and [Al(OH)2(H2O)x]+ from Al2(SO4)3 hydrolysis increased the co-conversion and dissolution of hemicellulose and lignin, while the formed AlHSO4(OH)2H2O species inhibited cellulose conversion. AlHSO4(OH)2H2O was also found to be the catalytic species for hemicellulose depolymerization to xylose, which acted as a “stabilizer” to prevent the further degradation of xylose to improve its yield. Al2(SO4)3 selectively promoted lignin depolymerization to produce lower Mw oligomers, VG and VP under mild reaction conditions. The cellulose-rich solid residues can be further used to produce value-added chemicals and materials. This enabled to achieve the use of corn stover to its fullest. The developed process is green and recyclable, indicating a promising future to develop a sustainable corn stover-based biorefinery.Author contributions
Yiping Luo: Methodology, investigation, formal analysis, data curation, wring–original draft and funding acquisition; Min Wei, Bin Jiang, Mingyi Zhang and Qian Miao: investigation, formal analysis and data curation; Hongquan Fu, James H. Clark and Jiajun Fan: writing–review & editing and supervision; All authors approved the final version of the manuscript and contributed to the scientific discussion.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is financially supported by Natural Science Foundation of Sichuan Province of China (2022NSFSC1258), National Key Research and Development of China (2019YFD1100603) and Chengdu University of Technology Teachers Development Research Fund (10912-KYQD2019_08215).References
- D. M. Alonso, S. H. Hakim, S. Zhou, W. Won, O. Hosseinaei, J. Tao, V. Garcia-Negron, A. H. Motagamwala, M. A. Mellmer, K. Huang, C. J. Houtman, N. Labbe, D. P. Harper, C. T. Maravelias, T. Runge and J. A. Dumesic, Sci. Adv., 2017, 3, e1603301 CrossRef PubMed.
- C. O. Tuck, E. Pérez, I. T. Horváth, R. A. Sheldon and M. Poliakoff, Science, 2012, 337, 695 CrossRef CAS PubMed.
- Y. H. Liao, S. F. Koelewijn, G. V. d. Bossche, J. V. Aelst, S. V. d. Bosch, T. T. Renders, K. Navare, T. Nicolaï, K. V. Aelst, M. Maesen, H. H. Matsushima, J. M. Thevelein, K. V. Acker, B. Lagrain, D. Verboekend and B. F. Sels, Science, 2020, 367, 1385 CrossRef CAS PubMed.
- J. J. Bozell, Science, 2010, 329, 522 CrossRef CAS PubMed.
- X. Zhao, H. Zhou, V. S. Sikarwar, M. Zhao, A. H. A. Park, P. S. Fennell, L. Shen and L. S. Fan, Energy Environ. Sci., 2017, 10, 1885 RSC.
- N. R. Quiroz, A. M. Norton, H. Nguyen, E. Vasileiadou and D. G. Vlachos, ACS Catal., 2019, 9, 9923 CrossRef.
- Y. P. Luo, L. S. Zeng, Y. H. Zhao, Z. C. Zhao, M. Wei, B. Jiang, J. J. Fan and D. Li, J. Water Process. Eng., 2022, 47, 102743 CrossRef.
- S. Pan, G. Wang, H. Chen, S. Zhang, Y. Li, M. Guo, F. Ni and G. Chen, Biomass Convers. Biorefinery, 2021 DOI:10.1007/s13399-021-01792-4.
- S. Van den Bosch, T. Renders, S. Kennis, S. F. Koelewijn, G. Van den Bossche, T. Vangeel, A. Deneyer, D. Depuydt, C. M. Courtin, J. M. Thevelein, W. Schutyser and B. F. Sels, Green Chem., 2017, 19, 3313 RSC.
- S. Van den Bosch, W. Schutyser, R. Vanholme, T. Driessen, S. F. Koelewijn, T. Renders, B. De Meester, W. J. J. Huijgen, W. Dehaen, C. M. Courtin, B. Lagrain, W. Boerjan and B. F. Sels, Energy Environ. Sci., 2015, 8, 1748 RSC.
- Y. P. Luo, Z. Li, X. L. Li, X. F. Liu, J. J. Fan, J. H. Clark and C. W. Hu, Catal. Today, 2019, 319, 14 CrossRef CAS.
- T. Parsell, S. Yohe, J. Degenstein, T. Jarrell, I. Klein, E. Gencer, B. Hewetson, M. Hurt, J. I. Kim, H. Choudhari, B. Saha, R. Meilan, N. Mosier, F. Ribeiro, W. N. Delgass, C. Chapple, H. I. Kenttämaa, R. Agrawal and M. M. Abu-Omar, Green Chem., 2015, 17, 1492 RSC.
- X. K. Li, Z. Fang, J. Luo and T. C. Su, ACS Sustainable Chem. Eng., 2016, 4, 5804 CrossRef CAS.
- Z. Ma, S. Kasipandi, Z. Wen, L. Yu, K. Cui, H. Chen and Y. Li, Appl. Catal., B, 2021, 284, 119731 CrossRef CAS.
- R. M. Trevorah, T. Huynh, T. Vancov and M. Z. Othman, Bioresour. Technol., 2018, 250, 673 CrossRef CAS PubMed.
- X. D. Liu, F. P. Bouxin, J. J. Fan, V. L. Budarin, C. W. Hu and J. H. Clark, ChemSusChem, 2020, 13, 4296 CrossRef CAS PubMed.
- T. Renders, S. V. d. Bosch, S. F. Koelewijn, W. Schutyser and B. F. Sels, Energy Environ. Sci., 2017, 10, 1551 RSC.
- M. A. Mellmer, C. Sanpitakseree, B. Demir, P. Bai, K. Ma, M. Neurock and J. A. Dumesic, Nat. Catal., 2018, 1, 199 CrossRef CAS.
- H. Q. Lê, Y. Ma, M. Borrega and H. Sixta, Green Chem., 2016, 18, 5466 RSC.
- S. X. Li, M. F. Li, P. Yu, Y. M. Fan, J. N. Shou and R. C. Sun, Bioresour. Technol., 2017, 230, 90 CrossRef CAS PubMed.
- H. Zhang, X. D. Liu, J. M. Li, Z. C. Jiang and C. W. Hu, ChemSusChem, 2018, 11, 1494 CrossRef CAS PubMed.
- R. Zhu, G. Zhou, J. N. Teng, W. Liang, X. Li and Y. Fu, Green Chem., 2021, 23, 1758 RSC.
- R. Li, Q. X. Lin, Y. X. Wang, W. R. Yang, X. X. Liu, W. Y. Li, X. H. Wang, X. Y. Wang, C. F. Liu and J. L. Ren, Appl. Catal., B, 2021, 286, 119862 CrossRef CAS.
- G. T. Jeong and S. K. Kim, Biomass Bioenergy, 2021, 148, 106053 CrossRef CAS.
- L. P. Zhou, H. J. Zou, J. X. Nan, L. Wu, X. M. Yang, Y. L. Su, T. L. Lu and J. Xu, Catal. Commun., 2014, 50, 13 CrossRef CAS.
- T. Yang, Y. H. Zhou, S. Z. Zhu, H. Pan and Y. B. Huang, ChemSusChem, 2017, 10, 4066 CrossRef CAS PubMed.
- C. Liu, L. S. Wei, X. Y. Yin, M. Wei, J. M. Xu, J. C. Jiang and K. Wang, Ind. Crops Prod., 2020, 147, 112248 CrossRef CAS.
- Y. P. Luo, D. Li, R. L. Li, Z. Li, C. W. Hu and X. F. Liu, Renewable Sustainable Energy Rev., 2020, 122, 109724 CrossRef CAS.
- L. B. Hu, Y. P. Luo, B. Cai, J. M. Li, D. M. Tong and C. W. Hu, Green Chem., 2014, 16, 3107 RSC.
- Y. P. Luo, L. B. Hu, D. M. Tong and C. W. Hu, RSC Adv., 2014, 4, 24194 RSC.
- Y. P. Luo, Z. C. Zhao, B. Jiang, M. Wei, Z. Zhang, L. S. Zeng, J. H. Clark and J. J. Fan, Green Chem., 2022, 24, 1515 RSC.
- M. J. T. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, et al., Gaussian 09. Revision C.01 2010, Gaussian, Inc, Wallingford, CT, 2010 Search PubMed.
- Y. Zhao and D. G. Truhlar, The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals, Theor. Chem. Acc., 2008, 120, 215 Search PubMed.
- Y. Zhao and D. G. Truhlar, Density Functionals with Broad Applicability in Chemistry, Acc. Chem. Res., 2008, 41, 157 CrossRef CAS PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650 CrossRef CAS.
- W. A. de. Jong, R. J. Harrison and D. A. Dixon, J. Chem. Phys., 2001, 114, 48 CrossRef.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, J. Phys. Chem. B, 2009, 113, 6378 CrossRef CAS PubMed.
- Y. P. Luo, Z. Li, Y. N. Zuo, Z. S. Su and C. W. Hu, ACS Sustainable Chem. Eng., 2017, 5, 8137–8147 CrossRef CAS.
- Y. P. Luo, Z. Li, Y. N. Zuo, Z. S. Su and C. W. Hu, J. Agric. Food Chem., 2018, 66, 6094 CrossRef CAS PubMed.
- K. Tekin, N. Hao, S. Karagoz and A. J. Ragauskas, ChemSusChem, 2018, 11, 3559 CrossRef CAS PubMed.
- Z. C. Jiang, J. Yi, J. M. Li, T. He and C. W. Hu, ChemSusChem, 2015, 8, 1901 CrossRef CAS PubMed.
- X. Wu, J. J. Peng, Y. M. Dong, J. H. Pang and X. M. Zhang, Ind. Crops Prod., 2021, 172, 114013 CrossRef.
- R. Li, Q. X. Lin, J. L. Ren, Z. H. Guo, Y. X. Wang, X. B. Yang and X. J. Wang, Chem. Eng. J., 2022, 442, 136224 CrossRef CAS.
- H. W. Zhu, B. Y. Du, Y. T. Bai, Z. Pan, Y. Sun, X. Wang and J. H. Zhou, Biomass Convers. Biorefin., 2022 DOI:10.1007/s13399-021-02190-6.
- X. D. Liu, Z. C. Jiang, S. S. Feng, H. Zhang, J. M. Li and C. W. Hu, Fuel, 2019, 244, 247 CrossRef CAS.
- W. L. Wang, Y. C. Liu, Y. Wang, L. F. Liu and C. W. Hu, Processes, 2021, 9, 23 CrossRef CAS.
- A. M. Verma and N. Kishore, R. Soc. Open Sci., 2017, 4, 170650 CrossRef PubMed.
- H. Q. Le, J. P. Pokki, M. Borrega, P. Uusi-Kyyny, V. Alopaeus and H. Sixta, Ind. Eng. Chem. Res., 2018, 57, 15147 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc01692c |
| This journal is © The Royal Society of Chemistry 2022 |