Open Access Article
Open Access ArticleOrganic waste valorisation towards circular and sustainable biocomposites
Erlantz
Lizundia
 ab,
Francesca
Luzi
c and
Debora
Puglia
ab,
Francesca
Luzi
c and
Debora
Puglia
 *d
*d
aLife Cycle Thinking Group, Department of Graphic Design and Engineering Projects, Faculty of Engineering in Bilbao, University of the Basque Country (UPV/EHU), Bilbao 48013, Spain. E-mail: erlantz.liizundia@ehu.eus
bBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, 48940 Leioa, Spain
cDepartment of Materials, Environmental Sciences and Urban Planning (SIMAU), Polytechnic University of Marche, Via Brecce Bianche 12, 60131 Ancona, Italy. E-mail: f.luzi@staff.univpm.it
dCivil and Environmental Engineering Department, University of Perugia, Strada di Pentima 4, 05100, Terni, Italy. E-mail: debora.puglia@unipg.it
First published on 16th June 2022
Abstract
The adoption of circular production and consumption patterns that counteract the current issues related to the depletion of natural resources, global warming, and environmental pollution is one of the most pressing global challenges that faces our society. Considering the potential of organic waste and residue streams to be transformed into valuable products, much effort is now being directed to foster circular bio-economy strategies. The valorisation of organic waste reduces the pressure on non-renewable resources and avoids the generation of waste. Organic waste valorisation has attracted much attention from fundamental and applied fields, given its wide availability and versatility. This review aims to provide an insight into valorisation of organic waste of aquatic, agricultural, forestry and animal origin to polymeric matrices, bionanoparticles and their combination. An introductory analysis dealing with state-of-the-art circular bioeconomy, recycling and upcycling is provided. Then, a literature review in the context of biopolymers and derived nanoparticles is provided, emphasizing toxicity and biodegradability aspects. The environmental impacts of valorisation processes are analyzed according to life cycle assessment. The establishment of organic waste conversion routes will lead to innovative bio-based industries, opening new market opportunities for bio-based products and achieving efficient resource utilisation. However, the social, economic and political barriers still encountered must be overcome.
1. Introduction to waste valorisation, bioeconomy and the circular economy
The establishment of responsible consumption and production patterns lies at the heart of the targets for a sustainable society aimed to counteract the depletion of natural resources, global warming, and environmental pollution. The intense efforts dedicated to the extraction of primary materials and their subsequent accumulation once their end-of-life (EoL) has been reached is considered a foremost environmental issue, polymers being a clear example. Most conventional plastics are based on fossil hydrocarbons, which together with their massive production (407 Mt primary plastic production in 2015), requires large amounts of non-renewable resources.1 Additionally, the stability of plastics, which has been a key property promoting their use during the 20th century, limits their degradability. Under the current linear “take, make and dispose” model, the plastics industry is far from being environmentally sustainable.2,3A possible solution to overcome the environmental pressures of plastics is the use of materials having a renewable origin, which are also generally biodegradable. This means that they can be degraded through natural processes such as enzymatic or hydrolytic degradation.4,5 However, the degradation rate of biodegradable plastics under natural environments is slower than under laboratory conditions.6 For example, polylactide (PLA) is readily biodegradable under industrial composting and anaerobic digesting conditions, but it is hardly biodegradable in soil and aquatic environments.6 In this scenario, waste valorisation can bypass the environmental burdens associated with the production and subsequent disposal of either non-biodegradable or biodegradable waste. This may represent a step forward in reaching the Sustainable Development Goals of the United Nations “Life below water” and “Life on land”.
With a current estimated amount of 1.1 teratonnes on a dry-weight basis, biomass provides a nearly unlimited amount of resources.7 The valorisation of underutilized organic waste by transformation into high-value materials in the form of polymeric matrices or (nano)particles can reduce the pressure on natural resources arising from non-renewable resources. The use of not only waste but also renewable resources combines the benefits originating from the circular economy and bioeconomy, and is considered as “the biological motor of a future circular economy, which is based on optimal use of resources and the production of primary raw materials from renewably sourced feedstock”.8 As a sustainability-oriented approach, bioeconomy could transform the current production and consumption linear mode into an efficient waste-using circular economy.9 In addition, organic waste collection and conversion is simple and energy-efficient in comparison with the deep drilling, mining and extensive refining/purification needed for non-renewable materials (petroleum, minerals).
Organic waste feedstocks of aquatic origin, both freshwater and marine ecosystems (algae, shrimps…), of agricultural and forestry origin (fruit pomaces, husks, lignocellulose…) or of terrestrial animal origin (egg shells, feathers…) are especially suitable for valorisation. The United Nations Environmental Programme estimates an annual global biomass waste production of 140 billion metric tons from agricultural activities.10 As agricultural productivity is projected to grow 60% by 2050 (regarding 2005/2007), it makes sense to think that the amount of waste will be increased.11 Concerning forest resources, nearly 20% of the global production of wood-derived biomass (∼4.6 Gt annually) is lost during production as waste. Similarly, organic waste accounts for 75 million tons (34% of the total) of the municipal waste created every year across the EU27 (2019 data).12 This waste remains underutilized, as 69% of its total is subjected to incineration, landfilling or composting.12 Considering that the European Commission has proposed a 65% reuse and recycling target for municipal solid waste by 2030,12 novel alternatives are urgently required. Waste valorisation can also lower the global warming associated with lost resources, as post-consumer organic landfilled waste generates ∼12% of global CH4 emissions.13 This impact is such that if food waste (1.3 billion tonnes per year) were a country, it would be the third-highest greenhouse gas emitter in the world with ∼6% of global emissions.13,14
Putting the concept of waste as a resource at the centre, waste valorisation involves the processing of residues/by-products into raw materials, use of discarded products as raw materials or energy sources, application of waste materials in manufacturing processes, or addition of waste materials to finished products.15 Recycling, defined as the process through which waste is recovered and reprocessed into new useful products, is of the most commonly followed waste valorisation activities.16 The conversion process often involves a downgrading into raw inputs that are used in a new process. Some of the physico–mechanical properties are typically lost, yielding applications of lower functionalities than the original purpose. This process is known as downcycling.17 On the contrary, upcycling implies the reuse of waste so that the new product or material presents a higher quality, value or function than the original one.18,19 Therefore, upcycling is preferred for the valorisation of waste.
For example, organic waste upcycling yields products replacing critical raw materials (CRMs) that present notable environmental sustainability and supply chain security issues.20,21 Providing alternative choices to CRMs may lessen dependency on the often toxic and polluting materials, as the biomass feedstock originates from a wide range of local resources. For example, pyrolysis (temperatures above 400 °C, atmospheric pressure, under nitrogen, argon or air atmosphere) and/or hydrothermal carbonization (temperatures below 300 °C, high pressures in aqueous media) of biomass waste can be followed to obtain advanced carbon structures with potential application in energy storage or catalysis,22–24 boosting the transition towards a greener and more circular economy.
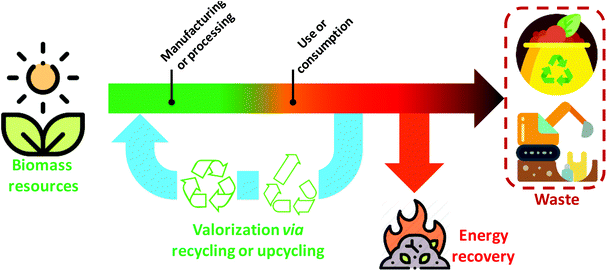
Waste-to-energy conversion is a common valorisation approach to generate a variety of bioenergy resources from biomass residues.25 Relevant examples are thermochemical including gasification, liquefaction, or pyrolysis methods, or biochemical conversion techniques based on anaerobic digestion, alcoholic fermentation, or photobiological hydrogen production.26,27 However, waste-to-energy conversion is in conflict with circular economy principles, which seek a “regenerative system in which resource input and waste, emission, and energy leakage are minimized by slowing, closing, and narrowing material and energy loops. This can be achieved through long-lasting design, maintenance, repair, reuse, remanufacturing, refurbishing, and recycling”.28 As shown in Fig. 1, the waste streams ending in composting or landfill scenarios can be limited through alternative EoL solutions that close material and energy loops.29 In this sense, waste valorisation via recycling and upcycling approaches better retains the value of the materials.28,30
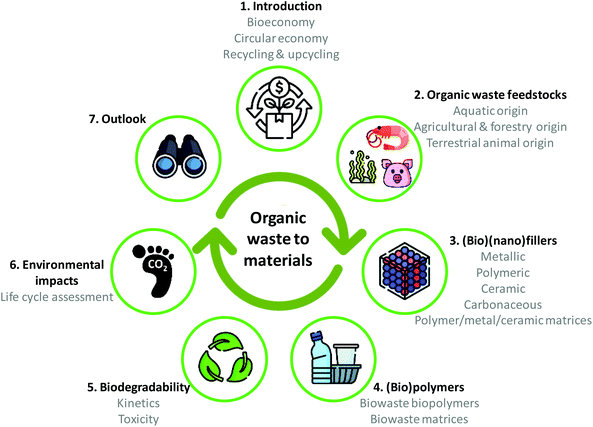
Organic waste valorisation is attracting increasing attention from fundamental and applied fields given the wide material availability and potential application areas. Although recent years have witnessed elegant review works on the application of biowaste,31–39 the literature lacks a life cycle approach connecting the different available resources, the synthetic methods leading to different properties and applications, their end of life (biodegradability and toxicity) and their environmental impact. As highlighted in Fig. 2, this work turns attention to biowaste feedstock valorisation into polymeric matrices or nanoparticles from a circular materials economy perspective. Feedstocks of aquatic origin, of agricultural and forestry origin and of terrestrial animal origin are considered as representative examples. Prospects on the potential application of the added-value materials in the fields of packaging, energy storage, sensing, wastewater treatment or biomedicine are given. The biodegradability aspects leading to environmentally closed circular ecosystems are also discussed, and toxicity issues are considered. To provide a full picture of the environmental performance, environmental metrics based on life cycle assessment (LCA) are provided, especially regarding global warming impact. We expect this outlook could serve researchers, industry and policymakers to implement circular approaches in the field of materials. This is an inevitably urgent task considering that as of 2021, only 8.6% of our global economy was circular.40
 | ||
| Fig. 2 Summary of organic waste valorisation into polymeric matrices or nanoparticles towards circular bionanocomposites. Certain figures are reproduced from Flaticon with permission. | ||
2. Organic waste feedstock
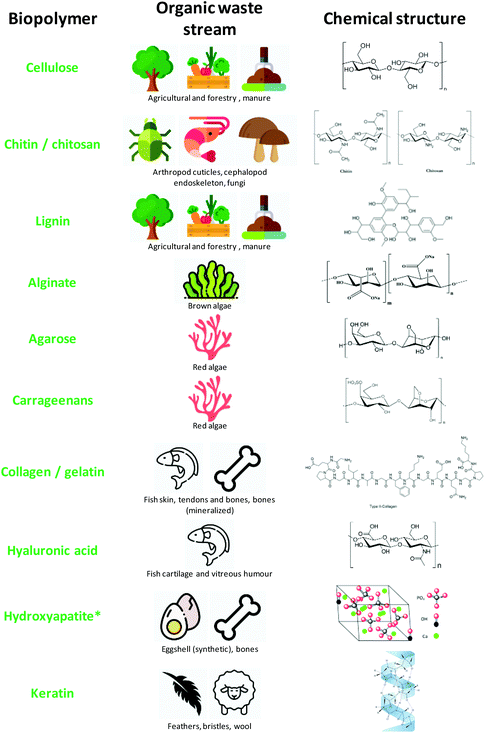
This section summarizes the most widely used and promising organic waste types, although it is far from being a complete and exhaustive list. However, there may be further sources of waste not being taken into account, as certain organic feedstock treatments (such as sugarcane) need extensive processing, yielding secondary and tertiary waste streams.41Fig. 3 summarizes the most commonly valorised biopolymers from waste streams, while Table 1 classifies the biopolymers into those of aquatic origin, agricultural/forestry origin and terrestrial animal origin.| Source | Biowaste | Compositiona | Special features/application |
|---|---|---|---|
| a Approximate values are provided given the batch-to-batch variation. Reported values are on a dry basis when not otherwise stated. | |||
| Aquatic origin, both freshwater and marine | Marine-arthropod cuticles | α-Chitin nanofibrils wrapped with diverse proteins, pigments and 14 wt% CaCO3; chitin content: 13–15% in crabs; 14–27% in shrimps | Chiral nematic structure useful for photonic materials and load-bearing functions |
| Cephalopods endoskeleton | 31–49 wt% β-chitin, proteins, lipids, 0.03–1.9 wt% mineralization | Chitin prone to solvation; higher molecular weights; neutral biopolymer | |
| Chitin | Chitosan obtained upon chitin deacetylation | Polycationic | |
| Brown algae | Alginate in the cell walls | Gelation with Ca2+; polyanionic | |
| Red algae | Agarose in the cell walls | Temperature-induced gelation; neutral biopolymer | |
| Red algae | Carrageenans in the cell walls, sulphation degrees ranging from 20 to 41% | Thickening/gelation additive; polyanionic | |
| Fishes | Collagen (from skin); gelatin upon its denaturation | Thickening/gelation additive; biomedical applications; polyanionic | |
| Fishes | Hyaluronic acid; from cartilage and vitreous humour | Thickening/gelation additive; polyanionic | |
| Agricultural and forestry origin | Agricultural | Cereals: corn, rice, wheat, oats, rye, barley, millet; Fruits: orange, grape, banana, apple, coffee, peach, apricot, mango, pineapple, kiwifruit; Vegetables: tomato, carrots, onions, olive husk, red beet, potato; Legumes: vetch, lupins, cow pea, lentils, beans chickpea, cellulose, hemicelluloses and lignin | Edible biomass, source for cellulose and lignin in the form of polymeric matrices and nanoparticles |
| Forestry | Grass, hardwood, softwood, sawdust; cellulose, hemicelluloses and lignin | Non-edible biomass, complex cell wall structure. Source for cellulose and lignin in the form of polymeric matrices and nanoparticles | |
| Fungi | 50–60% glucans, 1–20% chitin, 30–50% glycoproteins, melanin. Some species also contain chitosan. | Chitin extraction from fungi preferable over crustacean resources | |
| Terrestrial animal origin | Eggshell | 94% CaCO3, 4% organic matter, 1% MgCO3, 1% Ca3(PO4)2 | Patterning agent for hydroxyapatite |
| Bones | Natural hydroxyapatite/Mineralized collagen fibres | Improved tissue regeneration | |
| Feathers | β-Keratin fibres; porous honeycomb architecture | Mechanical strength; thermal/acoustic insulation | |
| Bristles (non-feather) | Multi-layer cuticle with internal cortex; α-keratin macrofibrils + sulphur-rich matrix | Resistance to bioconversion; sulphur and carbon source | |
| Wool | 60% α-keratin fibres, 15% moisture, 10% fat, 10% sheep sweat, 5% impurities. Stabilized through hydrogen and hydrophobic interactions together with disulfide linkages | Thermal and sound insulation; binding to water pollutants | |
| Manure | 55% carbon, 31% oxygen, 7.5% hydrogen, 5.1% nitrogen, 1.7% sulphur | Hydrochar synthesis; energy and resource-efficient nanocellulose extraction | |
| Terrestrial-arthropod exoskeletons | Chitin fibres wrapped with proteins organized into a Bouligand structure | Chitin nanofibres; chitin derivatives (including chitosan) | |
| Animal-derived structural proteins | Silk, collagen and its partial derivative, gelatin | Similarities with extracellular matrix; tailored mechanical properties | |
2.1. Aquatic origin (freshwater and marine ecosystems)
Among the vast diversity of aquatic organisms, algae and crustaceans represent the most widely exploited organic wastes. The large quantity of marine algae reaching our coast and the unacceptable amount of discarded marine food offer enormous possibilities to obtain diverse polysaccharides. Fisheries discard 9.1 million tonnes annually,42 while seafood accounts for 31% of the consumer-level food losses in the USA.43Many marine-arthropod cuticles show chirally arranged biomacromolecules with hierarchically ordered structures at the nano- and micro-scale levels. This twisted plywood organization, known as the Bouligand structure,44,45 is the origin of photonic responses originating from the selective interaction of light with layers, also found in many cellulosic materials.46 Accordingly, shrimps, king crabs, snow crabs or lobsters could be used in the extraction of chitin nanofibrils (∅ = 10–50 nm; L 50–1000 nm) through acid-catalyzed hydrolysis processes, which also remove mineral and protein phases.24 Worldwide annual chitin production is estimated between 2.8 × 107 and 1.3 × 109 tonnes,47 while yields of 13–15% for crabs and 14–27% from shrimps are achieved.48 The obtained α-chitin (chains antiparallely aligned with strong intra- and inter-sheets hydrogen bonds) is insoluble in ordinary solvents. An additional deacetylation process with strong bases yields chitosan nanofibrils,49 which can be solubilised under weak acidic conditions (diluted acetic or formic acid). Chitin and chitosan nanofibrils from marine waste are used in optical sensors,50 materials with load-bearing functions,51 or energy storage after carbonization.52 A chitin prone to solvation is extracted from cephalopods (squid pens), which are composed of 31–49 wt% β-chitin (chains parallel aligned with weak intra- and inter-sheets hydrogen bonds).53 The low mineralization of 0.03–1.9 wt of squid pens as compared with the ∼14 wt% of shrimp shells makes the chitin β-chitin isolation easier (molecular weights of 5300 to 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 680 g mol−1 for β-chitin; 650 to 1036 g mol−1 for α-chitin).54
680 g mol−1 for β-chitin; 650 to 1036 g mol−1 for α-chitin).54
The anionic and water-soluble alginate can be extracted from brown seaweeds such as Laminaria hyperborean via alkali treatment. Alginate comprises the α-L-guluronic acid and β-D-mannuronic acid monomers and is used for hydrogel fabrication after a Ca2+-induced gelation process.55 Red seaweed provides agarose, a neutral polysaccharide consisting of β-D-galactopyranosyl and 3,6-anhydro-α-L-galactopyranosyl units. Agarose has an increased stability to biodegradation and develops gels via hydrogen bonds upon temperature decrease.55 Carrageenans are another relevant group of water-soluble polysaccharides extracted from red seaweed and have a chain structure similar to agarose.56 The main difference is that the unit A is in the D-conformation, while in the agar unit it is found in the L-conformation.57 With a linear chain structure, carrageenans with different sulphation degrees can be achieved: kappa (20%), iota (33%), and lambda (41%).58 These groups impart a negative charge to the material.
Discarded fish skins, tendons and bones offer a collagen source. Collagen shows a triple helix formed by three extended proteins wrapping around each other (molar masses of ∼300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1).59 Collagen has intra- and inter-molecular covalent cross-linking among the residual amino acids present in the short N- and C-terminal regions of the α-chains.38 Acidic, alkaline, or neutral solubilisation or enzymatic treatments are used to extract collagen. A heat denaturation process partially hydrolyses collagen into gelatin, which has a single chain structure and smaller molecular masses of 2000–200
000 g mol−1).59 Collagen has intra- and inter-molecular covalent cross-linking among the residual amino acids present in the short N- and C-terminal regions of the α-chains.38 Acidic, alkaline, or neutral solubilisation or enzymatic treatments are used to extract collagen. A heat denaturation process partially hydrolyses collagen into gelatin, which has a single chain structure and smaller molecular masses of 2000–200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1. The collagen and gelatin from fish skins and marine sponges (Chondrosia reniformis) have a low risk of pathogens compared with their bovine-derived counterparts. Fish scraps (cartilage and the vitreous humour) contain hyaluronic acid,60 a linear and negatively charged heteropolysaccharide composed of alternating chain disaccharide units of N-acetyl-D-glucosamine and D-glucuronic linked by β-(1 → 3) and β-(1 → 4) glycosidic bonds.57
000 g mol−1. The collagen and gelatin from fish skins and marine sponges (Chondrosia reniformis) have a low risk of pathogens compared with their bovine-derived counterparts. Fish scraps (cartilage and the vitreous humour) contain hyaluronic acid,60 a linear and negatively charged heteropolysaccharide composed of alternating chain disaccharide units of N-acetyl-D-glucosamine and D-glucuronic linked by β-(1 → 3) and β-(1 → 4) glycosidic bonds.57
2.2. Agricultural and forestry origin
Forestry-derived waste (grass, hardwood, softwood, sawdust) does not compete with global food needs and offers large lignocellulosic biomasses at low cost. Considering that every cubic metre of logged material generates an additional cubic metre of waste in the forest,61 ∼80 million tonnes of forestry residues are annually produced in the EU.62 As opposed to agricultural waste, forestry-lignocellulosic waste needs intensive physico–chemical treatments given the more complex cell wall structure.63 Hydrolysis or thermochemical processes are applied to extract valuable materials.Agricultural and forestry residues are a major contributor to CO2 emissions and generate significant amounts of fine particular matter due to the more than 2 Gt of crop residues that are openly burned in fields.64 Although the composition of biomass from forestry and agricultural origins varies depending on the plant species and the cell type, this biomass is generally composed of 35–50% cellulose, 20–35% hemicellulose, 15–20% lignin, plus other minor components (ash, protein, minerals).65 Some highly specialized plants/cell types are found; cotton stem trichomes are composed of almost pure cellulose,66 while Fenugreek and Psyllium plants produce seeds mostly composed of hemicelluloses and Pine trees are rich in lignin.65
With 139 million tonnes of agricultural residues predicted annually in the EU by 2030,62 edible agricultural biomass waste is a major resource-waste given the amounts of fertilizer, land, water and cropland areas needed. These residues contain high levels of cellulose, hemicelluloses, starch, proteins, and lipids, and predominantly comprise crop stalks, leaves, roots, fruits (pomace and peels) and seed/nut shells.11 Some 66% of global plant biomass waste originates from cereal straw (stem, leaf and sheath material). As the major global crops, wheat, maize, rice, soybean, barley, rapeseed, sugarcane and sugar beet produce over 3.3 Gt of residues every year.11 Pectin with remarkable gelling properties can be obtained from fruits such as apple.41 Vegetables can also be used as a source to extract valuable materials, where tomato, carrots, onions, olive husk including skins and stones, red beet, and potato are especially relevant.41 Legumes such as vetch, lupins, cow pea, lentils, beans or chickpea can be also found.
Agricultural and forestry wastes are a source of biobased polymeric matrices and nanoparticles, giving water-soluble cellulosic derivatives such as carboxymethyl cellulose,67 cellulose nanocrystals (CNCs) through acid-catalyzed hydrolysis,68 or cellulose nanofibres (CNFs) via mechanical destructuring processes.69 Similarly, lignins70 and lignin nanoparticles can be also extracted.71
Fungi are a source of γ-chitin (a mixture of antiparallel and parallel chains), which is found at the fungal cell wall to protect the contents of the cell, give rigidity and define the cellular structure.72 Chitin represents only 1–2% of the dry weight of yeast cell wall, while filamentous fungi contain up to 10–20% chitin.73 Some classes of Basidiomycetes, Ascomycetes or Zygomycetes also contain chitosan. The absence of allergenic substances, the lower amount of inorganic materials, the simpler extraction process, and lower waste production make fungi a preferable source for chitin,48 which has been already used to fabricate sustainable alternatives to synthetic leather.74
2.3. Terrestrial animal origin
With an estimated global egg production of 90 million tons by 2030,75 eggshell represents a classical example of terrestrial animal waste (with serious pathogen propagation risks) with parts still utilisable after disposal. Eggshell is a source of hydroxyapatite (HA) after calcination and subsequent reaction with salts such as Ca3(PO4)2.76,77 Porcine or bovine bones also offer good access to HA. After residual protein removal via alkaline treatments and high-temperature calcination, yields of ∼ 65 wt% are obtained.78 Contrary to synthetic HA, bioderived HA presents traces of Mg2+, Na+, K+, Zn2+, Sr2+, Al3+, Cl−, F−, CO32− and SO42−, which are beneficial for promoting cell proliferation fuctions.79 Bovine bones can be used for mineralized collagen fibres (∅ = 1 μm) extraction.80The valorisation of feathers from chicken, goose, duck, and ostrich into β-keratin is being studied to reduce the annual 8.5 billion tons of feather-waste generated worldwide.81 This fibrous protein has a tensile strength of 60–250 MPa,82,83 comparable to polyamides. Therefore, feathers are applied for reinforcing polymers and obtaining light weight and biodegradable composites for semi-structural applications.84,85 Feathers are highly hydrophobic and thermally insulating thanks to their porous honeycomb architecture. Feathers are washed, sterilized and chopped/milled to facilitate their processing. Another organic waste composed of keratin (α-keratin) is pig bristle.86 Bristles are a low-value biowaste with considerable processing issues given their resistance to chemical and enzymatic digestion.87 Bristle-waste has potential as a sulphur and carbon source for photocatalytic materials.88 Sheep wool is another illustrative example of keratinous organic waste. Wool is composed of α-keratin, which is further stabilized through hydrogen and hydrophobic interactions together with disulfide linkages. Consequently, it is highly resistant to biodegradation.89 The good thermal and sound insulation properties of sheep wool (thermal conductivity of 0.037 W m−1 K−1 and a sound absorption coefficient of 0.77 at 60 mm) with low flammability makes this material useful for construction uses. It is also used for active air and water filtration given its effective binding to heavy metals.90–92
The abundant nutrients in manure (phosphorus, nitrogen, potassium, sulphur)93 make this biowaste a water and solid contaminant when inappropriately managed. Therefore, it is necessary to provide efficient recycling or upcycling approaches to profit from the 73 million tons of manure produced each year in the United States alone.94 Swine manure has potential for hydrochars via hydrothermal carbonization.93 These carbon structures are used for energy storage or soil/water remediation purposes. The high cellulose content of (elephant) manure could be exploited to obtain nanocellulose through an energy-efficient approach profiting from the cellulose already attacked by animal acid and enzymes.95
Similarly to aquatic arthropods, certain insects have exoskeletons composed of chirally arranged chitin fibers.96 These sources are exploited to extract chitin nanofibres or chitin derivatives.97 In addition to chitin and chitosan, several structural proteins such as collagen or silk can be obtained, whose properties notably depend on the amino acid blocks. Due to their similarities with the extracellular matrix and tailored mechanical properties, these materials are used for tissue engineering.98
3. Processed feedstocks to biopolymers and polymeric biocomposites
The circular economy is emerging as a regenerative model that minimizes emissions, relies on renewable energy, and eliminates/reduces waste based on the design of closed-loop systems and the reuse of resources.99 The implementation of circular economy practices in resource-consuming agricultural systems is essential for reducing the environmental ramifications of the currently linear systems. A key problem with the current linear approach for plastics is, in fact, that it leads to low resource efficiency and high material and economic value losses, with a continuous input of virgin materials derived from the Earth's finite resources, as reported for the case of PP and PET.100,101As the renewable segment of the circular economy, bioeconomy facilitates the production of renewable biological resources (i.e., biomass) that transform into nutrients, bio-based products, and bioenergy. The use of traditional petroleum-based plastics is considered an important and emerging problem not only as a consequence of global pollution related to greenhouse gas (GHG) emissions dangerous for the environment, but also in relation to the use of important energy sources for the industrial/energy sector. Additionally, conventional plastics, when discarded in the environment, are believed to remain for hundreds of years.102 In this context, bioplastics and the revalorization of biowastes to produce green polymeric matrices are being proposed as safer alternatives to reduce the dependence on fossil resources.
Bioplastics are bio-based and/or biodegradable materials, typically derived from renewable sources. Food waste as feedstock symbolizes one of the new and contemporary applications in the research field of bioplastics production.103 The Food and Agriculture Organization (FAO) estimates that every year 1.3 billion tons of food is wasted globally from all stages of the food supply chain including post-production, handling/storage, manufacturing, wholesale/retail, and consumption. Food waste landfilling has negative aspects related to the increase of GHG emissions and groundwater contamination induced by the presence of large volume of wastes in the environment. Valorisation through the development of bioplastic systems offers the possibility to limit and reduce the disposal problem through renewable sustainable processes.104
In the quest to achieve a circular bioeconomy, biowastes and bioresources as recycled streams and/or renewables are considered for their eco-friendly utilization along with strategies for recirculation and/or end-of-life disposal. Bioplastics obtained from biomass are characterized by interesting properties and as viable opportunities that can meet functions and the demand of product manufacturing.105 Enormous quantities of biomass are generated from agro-industrial processes and consumption; consequently, due to the high carbon content of these wastes, global loss of food, as an example, generates 4.4 Gt of CO2, which significantly contributes to global warming.106
According to the estimations from Camia et al.,107 1466 Mt of dry matter of biomass are produced annually by the land-based sectors of the EU (agriculture 956 Mt and forestry 510 Mt). In agriculture, 46% of the production corresponds to residues out of which about one-fourth is collected. In the forestry sector, about two-thirds of the net annual increment of the forests are harvested as EU average, with marked differences among countries. The marine-based sectors (fisheries and aquaculture, algae) supply slightly less than 2 Mt of dry matter annually. In the case of food losses, in the EU around 88 million tonnes of food waste are produced annually with related costs estimated at 143 billion euros: the FAO's Food Loss Index (FLI) estimates that globally, around 14 percent of all food produced is lost from the post-harvest stage up to, but excluding, the retail stage.108 In a recent contribution from Bedoic,109 the authors gave an overview of the technical potential of agricultural co- and by-products generated from the top EU28 commodities in the agricultural value chain, showing that countries with less available land area, a noteworthy number of industrial zones and high population density were the biggest producers of agricultural wastes, co-products and by-products in the animal and vegetable sector (Belgium, France, Germany, Ireland and the Netherlands). On the other hand, South European countries, with lots of land area and mild weather conditions, were shown to be more dominant in the quantities of generated fruit wastes. The overall biomass flows, represented using Sankey diagrams, show that more than 60% is used in the feed and food sector, followed by bioenergy (19.1%) and biomaterials (18.8%).
Often food loss and waste can be utilized, for instance, as nutrients for livestock; however, the associated economic and environmental costs remain as important barriers for such use, for example, considering feed quality control, stream management, and others. The transformation into “green” materials is an emerging possibility that uses residual biomass and streams in the food supply chain. To date, several biowastes have been utilized as natural raw sources for the realization of bioplastics, mostly including fruit and vegetable wastes. The conversion of biowastes into biobased polymeric matrices can occur through some processes.102
Agri-food biowaste reuse and by-products also show great potentialities in the construction industry. Life cycle methodologies underpin circular economy strategies but also highlight some weaknesses,110 which can be overcome through the proper use of multi-criteria approaches. Recent studies, in fact, have demonstrated that multi-criteria approaches are useful and effective decision-aiding support tools to assess the potential of new sustainable construction materials.111
3.1. Biowaste sources as a matrix for biopolymers
In this section, an overview about the revalorization of biowaste as a natural source for the development of biopolymers is provided. As introduced in the previous paragraphs, biopolymers can be economically obtained from natural sources by considering specific pre-treatments and technologies adapted to the selected biomass.112 Accordingly, a review of bioplastics’ production methodology should be done to definitively give an idea of the complexity and sustainability of the specific routes for selected biowastes. Low-value or underutilized biomass, biocolloids, water-soluble biopolymers, polymerisable monomers, and nutrients can be also introduced as feasible building blocks for biotechnological conversion into bioplastics (Fig. 4).103 They can be incorporated into multifunctional packaging, biomedical devices, sensors, actuators, and energy conversion and storage devices,113 contributing to the valorisation efforts within the future circular bioeconomy. Specific strategies need, however, to be introduced to effectively synthesize, deconstruct and reassemble or engineer biowaste-derived monomeric, polymeric, and colloidal building blocks.114 On the other hand, current inefficiencies in waste processing, and lack of current waste standardization for quality and composition, represent obstacles to finding practical uses in advanced materials. All the collected wastes (agricultural residues, animal sources)115 use chemical and enzymatic routes for their treatments, whose parameters should be necessarily tuned according to the specificity of the biowaste.112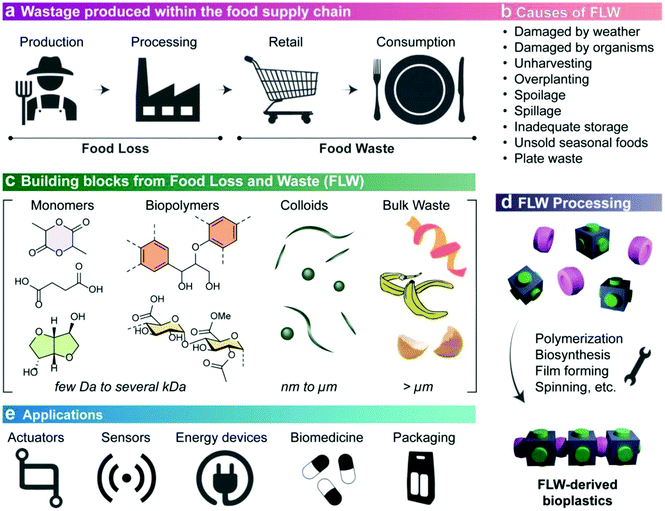 | ||
| Fig. 4 Illustrative landscape covering the utilization of food losses and waste (FLW) to produce bioplastics aimed to fulfil advanced applications. Reproduced from ref. 103 with permission from Wiley, copyright 2021. | ||
It needs to be underlined that, before any conversion, the quality of the collected bio-waste needs to be upgraded by removing foreign bodies or even specific categories of food waste, or by adding bio-waste from other, more specialized, sources (e.g. bakery residues) to produce compostable bio-plastics through an optimal synthesis process.116
Collecting and sorting from the source of waste generation can effectively reduce the cost of the subsequent steps to offer a strategic means for maximizing yield and profit, to reduce environmental burden, and to improve the reuse efficiency of material.104 However, plastic contamination, other physical (particulate) contaminants, and heavy metals that represent the main problems when food wastes are considered for energy (feedstock to methane),117 composting, or soil amendment, are still a serious problem to be mitigated when wastes are converted to biocomposites, so essential manual screening (de-packaging, picking) and energy-costly decontamination procedures need to be put in place. It should also be taken into account that suitable pre-treatment methods that may improve the conversion of substrates, such as physical (e.g., mechanical and thermal), chemical (alkaline treatments), and biological (bacterial and fungal) ones,118 should always be considered and adapted to the specific biomass: in the case of lignocellulosic, chemical delignification, steam explosion, organic/water solvent mixtures, ionic liquids, solid-state fermentation are the main adopted and preferred routes,119 while food waste pre-treatment is achieved by means of chemical and biological procedures.114 In this context, Fig. 5 shows generic strategies to be adopted for conversion of biowaste to bioplastics.
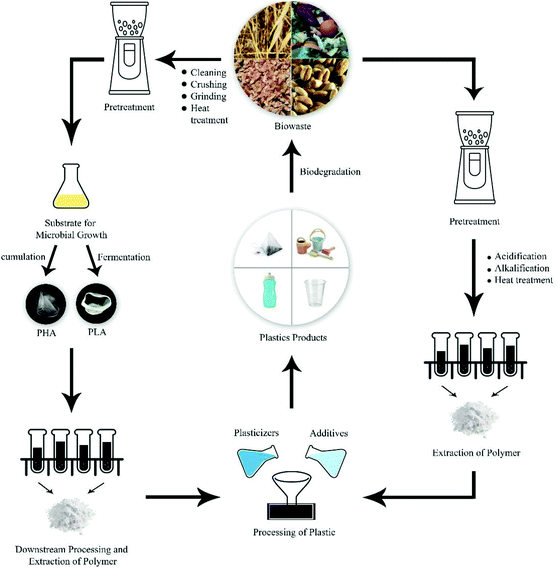 | ||
| Fig. 5 Bioplastic production from renewable biowaste. Reproduced from ref. 120 with permission from Applied Biotechnology Research Center, Baqiyatallah University of Medical Sciences (BMSU), copyright 2021. | ||
Other useful bio-sources are represented by vegetable oils, collected from oil industries, rich in triglycerides, such as sunflower, corn, soybean, castor, safflower, jojoba, rapeseed, linseed, fish oil and meadow foam, that can also synergistically act as potential monomeric units in the presence of suitable catalysts and co-monomers to improve the quality of the transformed biowastes.121 In the framework of starch-based polymeric products, it is clear that the use of wheat flours to realize biobased plastics is also considered an energetically and economically cheap alternative to purified starch.122 With this aim, Dominici and co-authors proposed the plasticization of wheat flour to realize thermoplastic systems.123 In this work, the effect of different bran content (refined flour with negligible bran fibre content, whole grain flour (20 wt% bran), higher bran amount (50 wt%)) on the overall thermomechanical behaviour of plasticized wheat flours was investigated. The results evidenced that, within the framework of different prospective EoL solutions, obtained thermoplastic-based systems disintegrated in accelerated composting conditions within 21 days. The germination test determined on compost extract taken 40 and 60 days after the incubation show an absence of any phytotoxic event. The results confirmed an efficient and eco-suitable use of the proposed material.123
To name and report a few of the overall modifications for biowaste to biopolymers reported in the literature, marine waste is of special interest. Almanza and co-authors proposed different deacetylation percentages of chitosan available in sand crab carapace:124 separate treatments were applied to the carapace, and the results in terms of ash, humidity and insoluble matter percentage confirmed the quality of chitosan for potential biomedical applications.124 Furthermore, marine-derived biowaste was valorised to develop chitin/fish gelatin porous materials used as bioactive carriers and moisture scavengers. Chitin was extracted from squid pens, an abundant and available biowaste from the fishery industry:125 in this case, demineralization and discoloration processes, required when crustacean shells are used, bring economic and environmental benefits. After extraction, chitin was employed as a reinforcing agent in porous gelatin: the results confirmed that the incorporation of chitin influenced the moisture and swelling behaviour of gelatin samples by inducing a more defined porosity, useful for bioactive delivery of drugs.
Lignocellulosic wastes have been also reviewed as suitable wastes for bioplastic production (Fig. 6). Raj et al.126 demonstrated that renewable monomeric chemicals produced from lignocellulosic biomass can be converted into biodegradable and recyclable plastics, and these methods have the distinct advantage of selectively fractionating a specific portion for use in value-added applications. However, the structural variability, heterogeneity, and complexity of this biowaste are significant critical aspects that require technological advancements in commercial research and development. This issue may be remediated by developing strategies to enhance substrate conversion efficiency, which would greatly improve commercial viability.
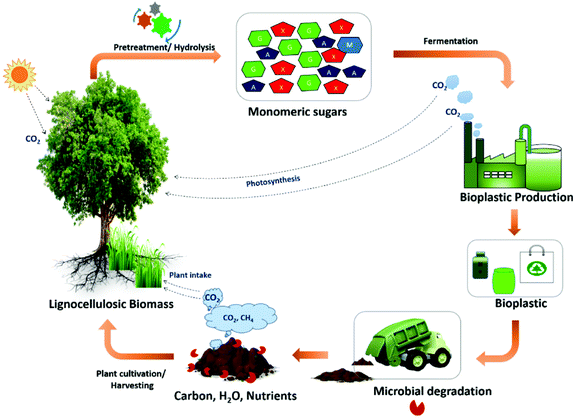 | ||
| Fig. 6 Closed loop biorefinery approach for bioplastic production from lignocellulosic wastes. Reproduced from ref. 126 with permission from Elsevier, copyright 2022. | ||
In summarizing and trying to give an overall critical fingerprint to the processing of accumulated biowastes, it has to be recognized that biopolymer production is, indeed, valuable from an industrial point of view; in addition to reducing greenhouse gas emissions, products from food wastes also compensate for the production cost of biopolymers, owing to the fractional raw material costs incurred compared with fresh raw material. In recent years, different techno-economic assessment studies have been performed, by using LCA procedures, to investigate the advantages of waste-to-high-value product conversion in a biorefinery concept. In general, for biorefineries and bio-based products to be commercially viable, several factors need to work together. This includes increasing the yield and decreasing the cost of biomass production, and developing efficient technologies that can utilize the biomass to produce various products.127
These biopolymers have major applications in industries such as biomedical fields, food industries, electrical and electronic products, agricultural products, automation products, cosmetics preparation, wastewater treatments, biocatalysts casings, and the entertainment industry.114 Therefore, future researchers are advised to explore more the scalability of these lab-scale processes to pilot plant and industrial scales, investigate process modelling aspects, and develop experiments employing green techniques and green solvents.
3.2 Biopolymeric composites from biowaste-derived matrices
The continuous research of new functional materials combining both advanced properties and increased environmental and cost sustainability has dramatically increased in the last decades. Instead of searching for new solutions, composites (formed by a combination of well-developed materials) are the subject of different studies due to their capability of merging the advantages of their components.128 A variety of biopolymers, reported to be used as matrices derived from biowastes, suffer in general from a lack in intrinsic properties (i.e., mechanical, thermal, optical properties and so on). So, in order to improve the properties of these biopolymeric matrices and maintain a fully green approach, either organic or biologically synthesized fillers derived from different wastes can be used.From a different perspective, the same biowaste can be also considered for biopolymer precursor and for polymer composite production. This is the case for spent coffee grounds, rich in carbohydrates, lipids, proteins, and minerals. It has been shown that carbohydrates (polysaccharides) can be extracted and fermented to produce lactic acid, succinic acid, or polyhydroxyalkanoate (PHA) and, in parallel, it is possible to successfully use it as a filler for composite production using the same matrix (or different biobased polymers).129 Biopolymeric composite performance is also undoubtedly correlated to compositions and phase morphologies, which need to be optimized by using compatibilisation methods or a nanocomposite approach.130 Naturally derived materials with hierarchical organization are also attractive candidates for high-performance and functional bionanocomposites because of their renewability, biocompatibility, biodegradability, flexibility, and availability of multiple reactive sites for introducing novel functionalities. Complementary to these inherent properties, the synergistic combination of biological and synthetic components can substantially enhance the structural performance and facilitate added functionalities of these bio-enabled materials.131
From this viewpoint, the development of biodegradable nanocomposite systems by incorporating, into gelatin biopolymer, silver nanoparticles biologically synthesized using industrial food waste was proposed (Fig. 7, Panel A), namely using cassava tuber peels.132 It was demonstrated that AgNPs enhanced the UV-shield capability of nanocomposite films, preventing lipid oxidation and showing significant antimicrobial activity, with an excellent notable increase in the shelf life of sapodilla fruits (Fig. 7, Panel B), confirming that the gelatin–AgNPs nanocomposite films are ideal for the food packaging industry. Ideally, any research must prioritize the development of useful products for society, and biocomposites made from waste fillers are no exception. In the current scenario, the advancement of waste-based biocomposite manufacturing technology will result in a rise in its applications.133 Nanotechnology is the key able to open new windows of innovation, and its combination with the “green-based” ideology has resulted in invaluable opportunities. Moreover, the use of bionanocomposites has acquired substantial interest due to their cost-effectiveness and high selectivity. However, with the current improvements in nanotechnology, there is a crucial need to assess its impact on the ecosystem and human health. In the following section 4, the integration of nanotechnology with naturally derived products to outline the tremendous contribution of nanobased bioproducts will be described.
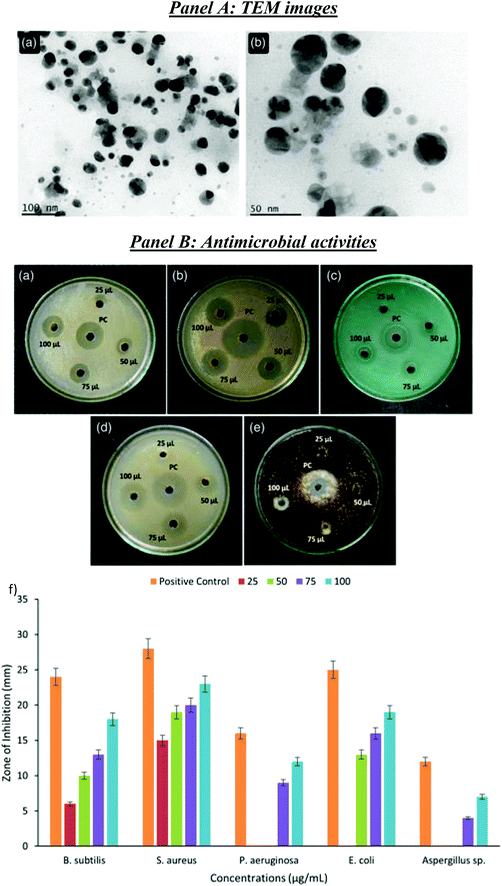 | ||
| Fig. 7 Panel A: Characterization of AgNPs. (a and b) HR-TEM images. Panel B: Antimicrobial activity of AgNPs. (a) B. subtilis, (b) S. aureus, (c) P. aeruginosa, (d) E. coli, (e) Aspergillus sp., and PC, positive control and (f) Zone of inhibition (ZOI). Reproduced from ref. 132 with permission from Hindawi, copyright 2021. | ||
4. Biowaste feedstocks for nanofillers
Nanoparticles’ synthesis includes several inputs, such as highly sophisticated instruments and considerable amounts of energy, most of which include drastic conditions, such as elevated temperature, voltage, etc. If we incorporate green chemistry procedures and biobased materials into the synthesis of nanoparticles, the problems that can cause damage to the environment could be invalidated, and non-toxicity, easy reproducibility and cost effectiveness could be achieved.134 Towards the future of green, sustainable and renewable products, interest in the idea of using different bio-wastes in the coming decades has increased.135 Biowaste and nanoparticle synthesis sounds like an improbable recipe, but recent investigations in the literature have shown that naturally occurring biomolecules present in waste have the potential to create nanoparticles with unique medicinal and pharmaceutical properties, linked to the possibility of collecting materials having diverse shapes and sizes (Fig. 8).136 Nature acts like a large “bio-laboratory” comprising biomolecules that can play a dynamic role in the formation of nanoparticles, thereby acting as a driving force for the design of greener, safe and environmentally benign protocols for the synthesis of NPs.31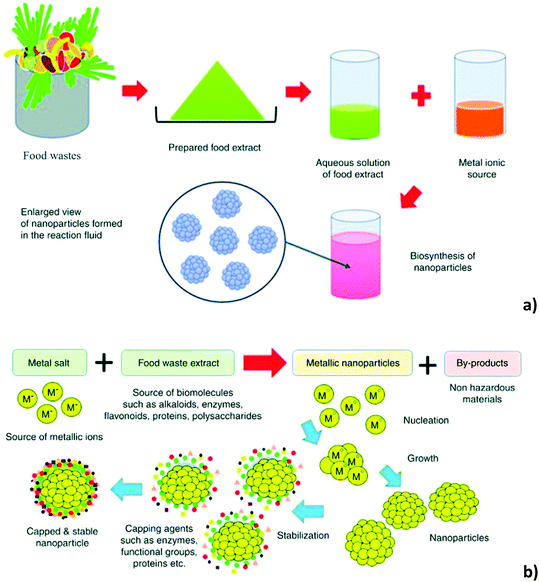 | ||
| Fig. 8 Schematic representation of the biogenic synthesis of nanoparticles using food waste extracts (a) and schematic diagram of the mechanism behind the biogenic synthesis of metallic nanoparticles, with food waste biomolecules acting as natural surfactants (capping agents) (b). Reproduced from ref. 148 with permission from Elsevier, copyright 2018. | ||
Biowaste contains beneficial biomolecules and compounds that can play actively in reducing precursor metal ions in aqueous solutions, as modelling agents for particle growth in particular orientations, or as capping agents to prevent nanoparticle agglomeration.137,138 Bio-wastes are definitely an extraordinary source of chemical richness whose valorisation is not only feasible and crucial to preserve life on our planet, but which also drives the creation of business, new technologies, livelihoods, and jobs.139 The abundance and diversity of agrowastes can successfully be exploited on wider scale for green synthesis of different nanoparticles; this approach is cost-effective and may also create some other useful products by nanotechnology. Utilization of agrowaste is of huge advantage, since it is the most efficient waste management process that constitutes high-value product formation from low-cost materials.140
As in the case of nanocarbons, hybrids, and nanocomposites, that are excellent materials for their versatility and potential use in the fabrication of a variety of high-value devices, biodegradable wastes that have high C-content represent an increasingly popular source for the preparation of such nanosized architectures.141 The literature reported that biowastes have variable mean C content: nearly 35% for cereal straws, sugarcane bagasse, pitch, lower for seaweed (16%) and approximately 50 wt% for fruit shells.142 A comprehensive study on various bio-based sources for conductive carbon has been recently made by Zia et al.,143 including agricultural crops, energy crops, crop residue, forest, forestry residue, aquatic crops, municipal waste and industrial waste, confirming the high potential application of these materials in sectors for energy storage and harvesting. Elemental composition in terms of nitrogen content also confirmed values of 1–5% in agricultural144 and food wastes.145
It has also been demonstrated that green mode-based synthesized nanoparticles exert antibacterial, antioxidant, and antifungal activity against a plethora of pathogens for food packaging purposes, facilitating a simple, alternative, interactive and reliable technology with positive feedback for the food industries and packaging markets.146 Even though research on bio-inspired nanomaterials is actually at a really early stage, there is however a niche of opportunity for it to be scaled at industrial levels, although the difficulty in reproducing them should necessarily consider the environmental factors affecting and altering the main specific properties of biobased wastes.147
Additionally, the lack of clear design guidelines for biosynthesis, the requirement for extensive fundamental research and coordination between the industrial and research communities, the lack of toxicity analysis protocols, higher regulatory barriers compared with nanoparticles synthesized via conventional approaches, and unclear end-market demands are the other challenges that must be addressed in the future to utilize biosynthesis as a potential synthesis approach for large-scale nanoparticle production.149 In the following paragraphs, the synthesis of different nanoparticles from biowaste feedstock is reviewed, taking into consideration the technical difficulties and overall advantages of using this kind of material as a precursor of nanosized fillers.
4.1 Biowaste for the synthesis of metal and metal oxide nanoparticles
The use of waste extracts for metal (and metal-based) hybrid nanoparticle synthesis is currently a brand of new research focus that has gained wide acceptance (see Fig. 9 and Table 2).150–152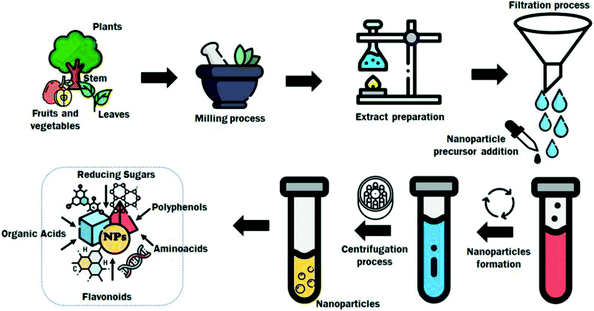 | ||
| Fig. 9 Schematic procedure for the biosynthesis of metal nanoparticles. Reproduced ion from ref. 153 with permission from Elsevier, copyright 2021. | ||
| Biowaste | Metal/metal oxide nanoparticles | Special features/applications | Ref. |
|---|---|---|---|
| Chitin/chitosan | Bi, Zr, Au; Ag, Pd; CuO | Anti-pathogenic/plant growth – Agrochemicals; antibacterial/filters; drug delivery/phototherapy, catalysis; antibacterial/antioxidant | 166–170 and 171 |
| Agarose Carrageenan | Ag, Au; CeO | Bacterial infections/non-spherical nanoparticles; regenerative medicine, | 172–176 |
| Collagen | ZnO | Antimicrobial/anticancer | 177 |
| Cellulose, hemicelluloses and lignin | Me and MeO (Me = Zn, Ti, Fe, Au, Pt, Pd) | UV protection, wastewater treatment, catalysis | 178–180 |
| Keratin | Au, Pt, Ag, Co, Pd | Catalysis, dye degradation, electro- and photocatalytic reactions, biosensing, imaging | 181 |
| Silk | Au | Diagnostics and bioimaging | 182 and 183 |
Recent review reports suggest that different plant biowastes such as banana, kiwi, orange, lemon, pomegranate, mango peels, eggshell membrane, rice husk, and wheat straw can be utilized for generation of metal NPs.154 Fruit peels contain abundant bioactive compounds including phenols, flavonoids, tannins, carotenoids, anthocyanins and essential oils, with substantial health benefits, anti-bacterial and antioxidant properties, generally discarded as by-products or waste by the fruit processing industry. NPs synthesized using bioactive compounds from fruit peel have futuristic applications for an unrealized market potential for nutraceutical and pharmaceutical delivery.155 Numerous studies have been conducted on the biosynthesis of metallic NPs such as silver, gold, iron, copper, palladium and titanium using fruit peel extract. AgNPs have been synthesized by starting from aqueous extract of cassava132 or onion peels.156 Various biogenic molecules present in plant materials, such as in leaves, roots, flowers and fruit, have been also utilized for the synthesis of NPs.157 Recently, conifer extracts have been found to be effective in the synthesis of metallic nanoparticles through a highly regulated process.158
In most cases, extracts from these wastes are used as reducing and stabilizing agents, with diverse activities ranging from antimicrobial, antioxidant, and catalytic to cytotoxicity against cancer cells. Generally, the extracts containing active biomolecules that catalyze the formation of nanoparticles can be obtained through a simple procedure of hot water extraction of dried and ground agro-waste materials. Metallic nanoparticles from those biowastes can also be considered as effective and environmentally friendly supports for heterogeneous catalysis, and may be the future alternative method for clean manufacturing technology.159
Another potential point in green synthesis is the possibility of infinite combinations of extracts to manipulate the structure and morphology to prepare metal oxide nanoparticles.160 Like metals, metal oxides have been widely synthesized via plant extracts and their synthesis is easier compared with metal nanoparticles, as the biomolecules in the extract can act as an oxygen donor, along with their capping, stabilizing, and metal-reducing properties,161–163 controlling particle shape and size in the synthesized material, since the phytochemical composition and selected plant part can influence the efficiency of the extract in forming the desired nanoparticles. Morphological properties of the nanoparticles are also influenced by several aspects, such as the pH, synthesis temperature, extract concentration and volume. The review by Akintelu et al. showed, as an example, how the type of extract used in the synthesis, as well as its concentration, influenced the synthesis of copper oxide, obtaining different moulds and applications.164 Analogously, Devatha et al. found that the greater the availability of these phytochemicals present in the medium, the faster the nanoparticle formation rate.165
Another opportunity in the synthesis of metal and metal oxide NPs is given by biobased industrial co-products, such as lignin and cellulose from agricultural by-products: the combination of organic nanoparticles, such as the ones derived from biomass feedstock, to realize nanohybrids may be a suitable strategy to obtain new interesting materials which show the advantages of the distinct components, as widely reported in the literature.184,185
Amino, carboxyl, carbonyl and aldehyde functional groups available from animal waste act as reducing agents for the synthesis of metal oxide nanoparticles. As an example, noble metal nanomaterials (Ag, Au) NPs and metal oxide nanomaterials, such as ZnO, Co3O4, PbO, Mn3O4, TiO2, have been successfully synthesized using egg shell membrane (ESM) as a template, which made the synthesis facile and under control.186
Limits of the presented approach are related to the need to explore commercial, economic and eco-friendly methodologies. Moreover, reproducibility of NPs in a high amount also poses a challenge in the green synthesis of metal and metal oxide nanoparticles. Substantial variation in the composition of extracts, variability in size and shape due to the interaction with metal ions, reaction time or temperature, and the variability of biowaste properties according to seasonality is information that must be considered essential for the reproducibility of the process and to understand the mechanisms culminating in the formation of metal-based NPs. Furthermore, there is a need to identify the biomolecules responsible for the synthesis of metallic nanoparticles, and to develop a single-step method to surpass the above-discussed challenges and pave the way for new opportunities for green chemistry to create eco-friendly metallic nanoparticles.
4.2 Biowaste for the synthesis of (bio)polymeric nanoparticles
Biopolymeric nanoparticles offer numerous advantages, which consist of easy preparation from well-studied biodegradable polymers and great stability in biological fluids and during storage.187 In the present analysis, the case of nanoparticles’ synthesis by raw natural biopolymers is not considered, while attention is given to biopolymeric sources from waste materials: potato bio-products, fish scale, and shrimp waste are natural sources for some useful biopolymers, such as starch, chitin, collagen, that can be employed for synthesizing nanoparticles of the same nature.188 Examples are represented by the work of Hasanin et al.,188 where the authors considered the extraction of starch nanoparticles (StNPs) from zero-value waste (potato peel waste) via simple alkali extraction followed by ultrasonic treatment; alternative and diverse sources are also represented by exudates or extracts from seeds, mucilages, fruits, peels, bark, or even leaves. Balde et al.189 considered the preparation of chitosan nanoparticles (CSNPs) loaded with diclofenac, with chitosan extracted and purified from Carinosquilla multicarinata shells, confirming that marine scrap can be used economically for the synthesis of specific drug-incorporated nanoparticles to cure various anti-inflammatory diseases. The same approach has been adopted for the extraction of keratin nanoparticles from poultry waste at various temperatures,190–192 and nanoparticles based on albumin and gelatin:187 among the colloidal systems, those based on proteins are very favourable, since they are biodegradable and non-toxic; they have superior strength in vivo and during storage, are rather easy to formulate, and their production can be scaled up.193 In addition, the protein-based nanoparticles offer several possibilities for surface modification and covalent drug functionalization.Collagen is another biomaterial valuable for its excellent biocompatibility, biodegradation and availability.194 Additionally, it is easily modifiable, opening the way to a number of applications in nanoparticle fabrication.195 Those modifications include adding other proteins, such as elastin, fibronectin and glycosaminoglycans, with the main aim of improving its physicochemical and biological properties as well as to regulate biodegradability. Biodegradable collagen-based nanoparticles are thermally stable, sterilisable and allow improved uptake of drug molecules into cells.
A number of publications are available on the extraction of lignin and cellulose nanoparticles from agricultural and forestry biowastes, reviewed in very recent papers as summarized in Fig. 10.36,196 In both cases, to reach large-scale production implementation, technological constraints and economic concerns must be solved. Thus, the development of high-value-added lignin and cellulose-based materials can not only conserve energy, but can also address several persistent ecological issues while also supporting economic development.
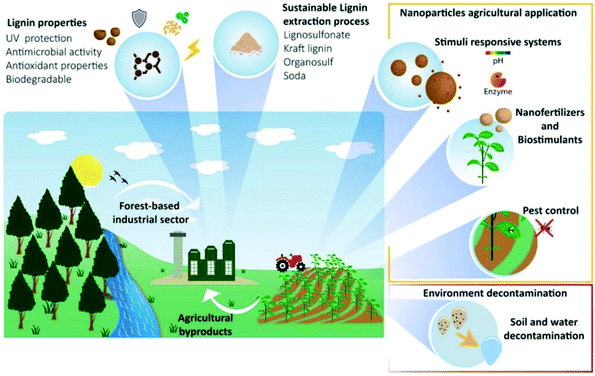 | ||
| Fig. 10 Lignin nanoparticles properties, sustainable extraction processes from forest and agricultural by-products and their agricultural applications. Reproduced from ref. 196 with permission from Elsevier, copyright 2022. | ||
4.3 Biowaste for the synthesis of carbonaceous nanoparticles
Biomass waste is a widespread natural carbon source which mainly contains cellulose, hemicellulose, lignin, chitin, ash, and proteins, and thus it is particularly suitable to be used as precursor to prepare high-value carbon-based materials stimulating a sustainable approach (Fig. 11).197–200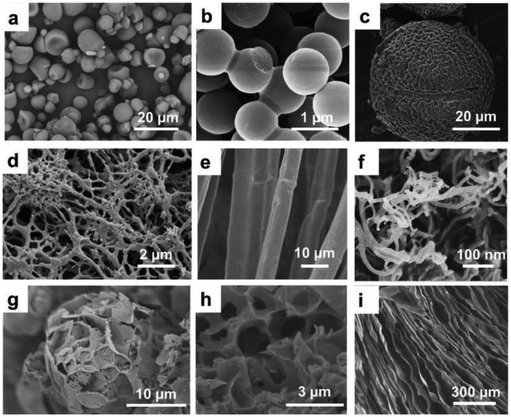 | ||
| Fig. 11 Scanning electron microscopy (SEM) images of carbon materials obtained from various biomass precursors with different morphologies and structures. (a) Cassava-starch-derived carbon particles. (b) Glucose-derived hydrothermal carbons. (c) Lotus pollen grains-derived NiO/C composite microsphere. (d) Wheat-starch-derived nanoporous reticular aerogels. (e) Ramie-derived carbon fibres. (f) Cellulose-derived nanoribbons; (g) D-fructose-derived carbons with flower-like structure. (h) Corn-husk-derived nanoporous foamy aerogels. (i) Gelatin-derived plate-like carbon cryogel. Reproduced from ref. 200 with permission from Wiley, copyright 2021. | ||
Plant-derived precursors can yield carbon nanomaterials of similar, and sometime even better, quality than one would normally get starting with fossil-fuel materials and petroleum.202 Most studies employed a hydrothermal carbonization process, which requires catalysts and high temperatures and pressures, i.e. subcritical conditions, at 80–260 °C in a closed system and 20–100 bar.203 Therefore, a sustainable green approach to synthesize carbonaceous nano/microstructures from biomass in a single-step process under ambient conditions would be of great interest.
One of the suitable routes to convert biomass into amorphous carbon is through pyrolysis or combustion;204 amorphous carbon is then further converted into carbon nanomaterials by means of catalytic graphitization, mechanical activation or chemical activation. Carbonaceous nanoparticles can also be obtained from biomass through a solid–vapour–solid process in which hydrocarbons and their derivatives are the precursors.205 Activated carbon-based nanoparticles (ACNPs) are also emerging as effective antimicrobial agents due to their anti-microbial properties; the development of activated carbon nanoparticles, especially from biowaste-derived carbon precursors, is a recent forthcoming technology which is easy available, economically viable, low cost and characterized by easy methods of production.202 The ever-increasing demand for green and clean energy has also moved research activities towards the development of cheap and efficient electrode materials for supercapacitors.206,207 Quite recently,201 a review on the subject underlined how sustainable carbon materials exhibit a potential advancement in various applications, focusing on diverse sustainable strategies. Composition complexity and batch stability of the carbon source are significant for the practical application of sustainable carbon materials (Table 3). Therefore, special attention should be paid to the production of sustainable carbon from industrial by-products. However, it is necessary to expand the production scale of emerging sustainable carbon materials from the laboratory stage to a practical industrial level, and further investigation and development of facile and greener manufacturing processes to obtain low-cost and environmentally friendly carbon materials are also urgently required.
| Biowaste | Routes/carbon | Application | Ref. |
|---|---|---|---|
| Chitin/chitosan | Dots | Bioimaging, wastewater treatment, cellular imaging and drug delivery, electrocatalysis and photocatalysis | 208–211 |
| Agarose Carrageenan | Dots | Environmental remediation, sensors, antitumoral/antiviral | 212–215 |
| Collagen | Sponges/dots | Battery electrodes, supercapacitors | 216–218 |
| Cellulose, lignin | Nanofibers, nanosheets, spheres | Battery electrodes, supercapacitors | 219–222 |
| Keratin | Dots | Heavy metal detection, supercapacitors | 223 and 224 |
| Silk | Membranes | Biological electronic devices for cell manipulation, cell culture, and cellular metabolism | 225 |
4.4 (Bio)(nano) fillers in polymers, metals and ceramic-based matrices
Naturally sourced particulates (agro-waste) has been discovered to be a very good reinforcement constituent, owing to the availability and immensely low cost of acquiring the recommended agricultural waste. So, many researchers have worked extensively on several natural wastes from agriculture, and have found them to rich in useful elemental oxides that are present in their ash content.226,227 The advent of the importance of agricultural waste in particulate form as a reinforcement for metallic matrix composites (MMCs) is not just of added advantage to our manufacturing industries because of its availability and low cost, but it also reduces the rate of environmental pollution, by converting such waste from agro-processes into useful raw materials for engineering purposes.113 The addition of agro waste ash as reinforcement in MMCs, indeed, improves specific strength, yield strength and ultimate tensile strength, and hardness, with satisfactory levels of corrosion resistance. In their review, Baharami et al.227 thoroughly investigated how composite performance strongly depends not only on its origin, chemical composition, and morphology, but also on the choice of processing route (chemical or mechanical). Although agricultural waste materials cannot be used in their original form in MMCs, choosing the most efficient procedure can yield ceramic phases with unique and valuable structures.The same approach and considerations can be extended to the preparation of ceramic matrix composites (CMCs). In this sense, the feasibility of utilizing bio-waste as a potential filler material for cement was also considered: different amounts of eggshell powder (0, 5%, 10% and 15% by weight) were adopted to replace cement, and the authors found that calcium carbonate in the biowaste can react with the aluminium phase in the cement to produce monocarboaluminate; they also concluded that the replacement level of 5% provides the best performance and reduces environmental pollution.228,229 Biowaste can also be thermally converted into char by pyrolysis treatment and subsequently introduced into cementitious matrix-forming cement-based composites (Fig. 12).
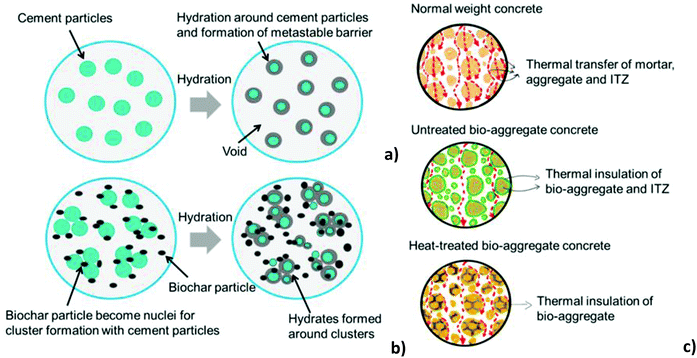 | ||
| Fig. 12 Schematics showing hydration product formation in (a) cement-only system and (b) system containing cement and biochar. (c) Thermal insulation mechanism of bio-based concrete (ITZ = interfacial transition zone). Reproduced from ref. 229 with permission from MDPI, copyright 2021. | ||
According to Gupta et al.,230 after adding biochar to a cementitious mixture, micropores and voids between the cement and sand grains are filled; due to the presence of hydroxyl functional groups, biochar attracts cement particles and forms a cluster around them. Clustering results in nucleation, which improves hydration by attracting more biochar particles. This causes hydration products to precipitate on the surfaces of the biochar clusters. As a result, biochar evolves into an active filler material that increases densification in the cementitious matrix. Highly porous biochars in the concrete act also as thermal insulators, preventing heat transfer within the matrix. Heat transfer in reference mortar occurs through the matrix, the aggregate, and the interfacial zone, while heat transfer in normal biochar-added concrete occurs only through the matrix. The absence of thermally insulating material (i.e., biochar) in a reference concrete caused direct heat transfer within the matrix, resulting in increased thermal conductivity.
The analysis of the mechanical properties of these materials also evidenced that, as an example, chitosan-derived char-containing composites showed an incipient fracture toughness capability, very appealing for possible structural applications.231,232 The possibility of improving the mechanical performance of cement and concrete (in particular the fracture toughness) by means of nanoscopic and/or microscopic fillers is an interesting research field with promising application in the creation of earthquake-resistant and monitoring-enabling cementitious materials.
Among the different types of fillers, carbonaceous ones are certainly widely investigated, due to the possibility of introducing novel advanced properties. Moreover, the introduction into cement of bio-based renewable materials and/or natural fibres derived from animal, vegetal and mineral sources attests itself as a real sustainable alternative choice encouraged by the building construction industry.232 Another possibility of biowaste reuse in the field of ceramic (and glass-based) materials is linked to the mineral content of organic waste that, although it actually varies widely depending on the type of plant, also provides the ability to batch a variety of glass and glass–ceramic compositions, allowing significant flexibility in producing these engineered materials.233
5. Biodegradability issues
Nowadays, with the help of research and technological advancements, waste can be converted into valuable products which will not only give results in terms of environmental sustainability, but also enhance the economy. The transformation of waste to wealth comprises reuse and recycling of resources, helping to limit the lack in resource supply homogeneity, cost of discarding waste, and environmental load, and resulting in sustainable exploitation of feedstock.234 Although they are environmentally friendly, the process of production is sometimes complex, reducing its economic feasibility. This problem can be resolved to a great extent by using organic wastes of biological origin as raw material for the production of bioplastics that have different possibilities for biodegradation.235 Despite earlier hopes, biodegradation is non-trivial, as the rate of biodegradation is highly dependent on a polymer's chemical structure, an easy destructuring or depolymerizing procedure, stabilizing additives, and surrounding environment.If we limit the biodegradability issue to the converted biowaste and not to the biowaste itself, and assume that biowaste can be considered biodegradable in its nature, all these conditions could be partially fulfilled and biodegradability could be partially achieved.236 The use of renewable resources and the production of bioplastics are no longer a guarantee for minimal environmental impact, and the production process, as well as their technical performance and their ultimate disposal, have to be carefully considered.237 Biodegradation can be classified by considering that we can have subsequent organic recovery of the bio-wastes, or the biodegradation process can modify the environment: in both cases, the introduction of toxic elements, for example heavy metals, or halogenated or aromatic hydrocarbons to make the biowaste a plastic, could contaminate the compost.238 It should be then taken into account that replacing conventional plastics with bioplastics does not necessarily solve the issues of resource depletion and plastic waste accumulation, regardless of their biodegradability. So, in order to come to a truly sustainable plastics economy, the growing bioplastics production must be paralleled with effective end-of-life strategies for bioplastic waste, such as mechanical, chemical and enzymatic recycling.239 Instead of complete biodegradation (composting), microorganisms and their hydrolysing enzymes can indeed be used to depolymerise condensation polymers into monomers, instead of CO2, similar to chemical recycling, aiming ideally to reuse the bioplastics and the original biowaste.
6. Environmental impacts
6.1. Life cycle assessment
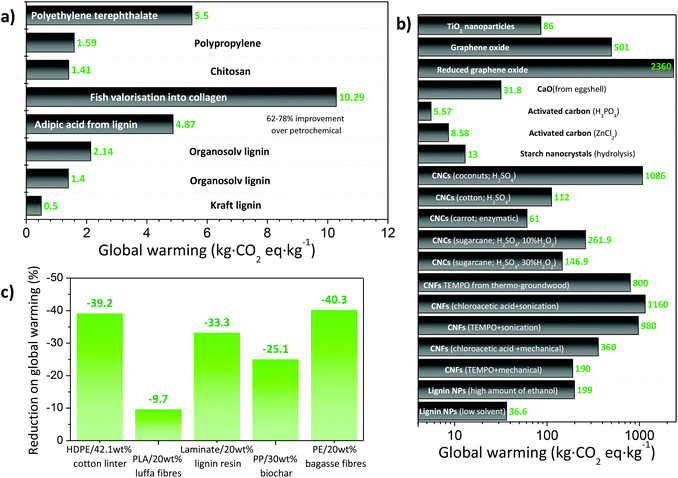
The potential of organic waste valorisation to obtain functional bionanocomposites for different applications has been highlighted through this review. These materials, besides being renewable and degradable, limit the uncontrolled accumulation of waste in aquatic and land environments. Avoiding landfilling or composting may encompass additional environmental benefits, as notable CO2, CH4, ammonia, nitrogen oxides, etc., emissions are generated during these EoL stages. Although CO2 emissions can be considered as neutral due to their biogenic nature, CH4 has a global warming equivalence 25 times higher than CO2. Additionally, between 130 and 160 kW h are required to compost 1 tonne of organic fraction of municipal solid waste. Altogether, it is estimated that composting encompasses 60 kg CO2 eq. per t, with additional notable acidification potential, photochemical oxidation potential, eutrophication, human toxicity and ozone layer depletion impacts.240 Nonetheless, waste valorisation approaches should be accompanied by accurate environmental performance metrics to unambiguously determine their environmental benefits. A key factor, for instance, is the transport of the waste into the recycling plant, especially when large volumes and masses are involved. As a representative example in which bulky and heavy waste is treated, Di Maria et al. analyzed the environmental impacts of the recycling of construction and demolition waste, and concluded that transport was the main contributor to the environmental burdens (accounting for more than 50%).240In this framework, the life cycle assessment (LCA) methodology provides a means for the evaluation of the environmental impacts of a product or service.241,242 LCA studies can be performed focusing on the whole life cycle, from the raw material extraction to its EoL (cradle-to-cradle), or can solely focus on the organic waste valorisation/conversion process (cradle-to-gate). While the former accounts for the impacts throughout the entire life cycle, the latter provides information on the valorisation process itself, so more accurate decisions on the environmentally preferred process can be made. One of the difficulties when performing the LCA of organic waste valorisation is the multi-output character. As the impacts need to be standardized for a given material (functional unit, FU), this process becomes difficult when different products are obtained. The non-mature technology for waste valorisation, the data scarcity and the often lab-scale studies are other bottlenecks for the accurate determination of environmental impacts. LCA allows determination of not only the equivalent CO2 emissions but also additional relevant impact categories including fossil resource scarcity, freshwater and terrestrial ecotoxicity/eutrophication, human toxicity, land use, or water consumption.243 However, for the sake of comparison most works mainly focus their efforts on the determination of global warming (measured in CO2 emission equivalents) and cumulative energy demand (CED, accounting for the direct and indirect energy use including the energy consumed during the extraction/manufacturing/disposal of the raw and auxiliary materials).244 In this section we summarize the recent reports in the field. To enable cross-comparison, mass-allocation is used, with 1 kg of material as a FU (when not directly available, our own extrapolations were made). Results are summarized in Fig. 13.
The valorisation of marine and terrestrial animal waste resources into materials offers notable environmental benefits. Although not directly applied to obtaining polymeric materials, Lopes et al.249 studied the environmental impacts associated with the valorisation of fish by-products (fishmeal and oil production for animal feed) against different waste management scenarios. The valorisation scenario provided a global warming value of 5.1 kg CO2 eq. per tonne in comparison with the 256.8 kg CO2 eq. per tonne of the composting scenario. Similarly, García-Santiago et al. recently calculated the gate-to-gate impacts of fish biorefinery, and concluded that fish valorisation into collagen (and other by-products including fishmeal, fish oil and fish protein hydrolysates) could be carried out at 10.29 kg CO2 eq. per kg with a total electricity consumption of 276.8 kW h,250 which was more eco-efficient than the established process of fishmeal production. Regarding waste-derived polymers, Leceta et al. studied the impacts associated with chitosan-films considering the material extraction, film manufacture, and end of life (FU: 1 m2 of film).251,252 A 11% increase in global warming value was obtained (over polypropylene), which was accompanied by an increase in respiratory inorganics and minerals (due to the HCl for raw material extraction and acetic acid for film manufacture) and an increase of over one order of magnitude in land use (due to glycerine). However, improvements in carcinogens and fossil fuel categories were achieved. The same group also evaluated the environmental impacts of bio-based films based on soy protein from the soy oil industry, chitosan from the skeleton of crustaceans, and agar from marine seaweeds (FU: 1 m2 of film).253 Obtained total global warming values were similar to those of polypropylene (1.22–1.37 × 10−6vs. 1.18 × 10−6 of polypropylene), mostly driven by the manufacturing stage which scored an order of magnitude higher for waste-derived films. However, raw material extraction and EoL stages were markedly improved, underlying the potential for waste-derived materials to lower environmental impacts if mature processing technologies are provided. A possible strategy to further improve the environmental profile of chitin and chitosan from marine waste would be the optimization of the raw material extraction; currently 1 kg chitin requires 33 kg of shrimp shells, 8 kg HCl 32%, 1.3 kg NaOH, 1.3 kW h electricity and 167 L freshwater; and 1 kg of chitosan requires 1.4 kg chitin, 5.18 kg NaOH, 1.06 kW h, 31 MJ wood fuel and 250 L water.254
Li et al.257 studied the environmental impacts of CNF fabrication in 2013 and reported global warming values ranging from 190 to 1160 kg CO2 eq. per kg.258 The impacts were dominated by fossil fuels for energy (CED of 3470 to 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 610 MJ). The use of the high-pressure homogenizer reduced the CO2 emissions by half in comparison with the mechanical disintegration process by sonication. Similarly, the use of TEMPO-oxidation limits the CO2 emissions by half over chemical modification involving chloroacetic acid etherification. The impacts of CNF production via enzymatic pretreatment, carboxymethylation pretreatment, and direct production with no pretreatment have been also compared, with CED values of 87, 180 and 240 MJ kg−1 and global warming values of 0.79, 99 and 1.2 CO2 eq. per kg, respectively. Carboxymethylation required ∼30 kg kg−1 CNF of the reagents (ethanol, isopropanol, and methanol). Very recently, Turk et al.259 used thermo-groundwood from a local paper manufacturer to synthesize TEMPO-oxidized CNFs, obtaining a global warming value of 800 kg CO2 eq. per kg.260 The primary energy consumption of 19
610 MJ). The use of the high-pressure homogenizer reduced the CO2 emissions by half in comparison with the mechanical disintegration process by sonication. Similarly, the use of TEMPO-oxidation limits the CO2 emissions by half over chemical modification involving chloroacetic acid etherification. The impacts of CNF production via enzymatic pretreatment, carboxymethylation pretreatment, and direct production with no pretreatment have been also compared, with CED values of 87, 180 and 240 MJ kg−1 and global warming values of 0.79, 99 and 1.2 CO2 eq. per kg, respectively. Carboxymethylation required ∼30 kg kg−1 CNF of the reagents (ethanol, isopropanol, and methanol). Very recently, Turk et al.259 used thermo-groundwood from a local paper manufacturer to synthesize TEMPO-oxidized CNFs, obtaining a global warming value of 800 kg CO2 eq. per kg.260 The primary energy consumption of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MJ kg−1 could be lowered to 10
000 MJ kg−1 could be lowered to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 MJ kg−1 if lignin was considered as a co-product (∼100, 75 and ∼200 MJ kg−1 for polypropylene, titanium and aluminium, respectively).261,262 The purification process contributed up to 95% of the whole environmental footprint. In another effort to use organic waste as the source material, Piccinno et al. obtained CNFs from carrot-waste using an aqueous enzymatic treatment. Due to the 87 wt% water content of carrots, 15 kg of carrot waste was required to obtain 150 g of CNF. Interestingly, the environmental impacts of CNF production at industrial scale via the enzymatic approach are predicted to be reduced by a factor of 6.5 in comparison with lab-scale production (50 kg vs. 10 g).263 CNCs are another representative form of bio-based nanoparticles. In this sense, Katakojwala reported that with a global warming value of 146.9 to 261.9 kg CO2 eq. per kg, electricity accounted up to 141.1 and 254.1 CO2 eq. per kg during the production of CNCs, confirming that chemical inputs (HNO3, H2SO4, NaOH, NaClO, CH3COOH and H2O2) had a negligible contribution to the global warming value.264 However, the wastewater generated had a strong impact on aquatic toxicity as estimations account that 2.19 × 105 to 3.42 × 107 L kg−1 CNC are required.265 In another study, global warming values of 61 kg CO2 eq. per kg were reported (CED of 2280 MJ kg−1),266 which remain well below the 112 CO2 eq. per kg for the CNCs extracted from cotton and especially the 1086 CO2 eq. per kg for the CNCs extracted from unripe coconuts (CNCs obtained in both cases via a H2SO4-induced acid hydrolysis; Brazilian energy mix; CED of 1800 and 15
100 MJ kg−1 if lignin was considered as a co-product (∼100, 75 and ∼200 MJ kg−1 for polypropylene, titanium and aluminium, respectively).261,262 The purification process contributed up to 95% of the whole environmental footprint. In another effort to use organic waste as the source material, Piccinno et al. obtained CNFs from carrot-waste using an aqueous enzymatic treatment. Due to the 87 wt% water content of carrots, 15 kg of carrot waste was required to obtain 150 g of CNF. Interestingly, the environmental impacts of CNF production at industrial scale via the enzymatic approach are predicted to be reduced by a factor of 6.5 in comparison with lab-scale production (50 kg vs. 10 g).263 CNCs are another representative form of bio-based nanoparticles. In this sense, Katakojwala reported that with a global warming value of 146.9 to 261.9 kg CO2 eq. per kg, electricity accounted up to 141.1 and 254.1 CO2 eq. per kg during the production of CNCs, confirming that chemical inputs (HNO3, H2SO4, NaOH, NaClO, CH3COOH and H2O2) had a negligible contribution to the global warming value.264 However, the wastewater generated had a strong impact on aquatic toxicity as estimations account that 2.19 × 105 to 3.42 × 107 L kg−1 CNC are required.265 In another study, global warming values of 61 kg CO2 eq. per kg were reported (CED of 2280 MJ kg−1),266 which remain well below the 112 CO2 eq. per kg for the CNCs extracted from cotton and especially the 1086 CO2 eq. per kg for the CNCs extracted from unripe coconuts (CNCs obtained in both cases via a H2SO4-induced acid hydrolysis; Brazilian energy mix; CED of 1800 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 944 MJ kg−1, respectively).267 Starch nanocrystals encompass notably lower impacts as these materials, when produced via hydrolysis with H2SO4, present improved values of 7.9–13 kg CO2 eq. per kg.268 However, marked acidification impacts were found.
944 MJ kg−1, respectively).267 Starch nanocrystals encompass notably lower impacts as these materials, when produced via hydrolysis with H2SO4, present improved values of 7.9–13 kg CO2 eq. per kg.268 However, marked acidification impacts were found.
In spite of the relatively large CO2 emissions during organic waste-derived nanoparticle production in comparison with organic waste-derived polymeric matrices, bioparticles are environmentally preferred over other nanoparticles such as carbonaceous nanoparticles, whose synthesis involves a large CED (1 to 900 GJ per 1 kg of carbon nanofibres, carbon nanotubes or fullerene).261 In this sense, global warming values of 1060–2360 kg CO2 eq. per kg for reduced graphene oxide or 217–501 kg CO2 eq. per kg for graphene oxide obtained via the Hummers and Marcano methods have been reported.269 However, it should be considered that other inorganic nanoparticles with long and well-established fabrication methods such as TiO2 offer lower CO2 emissions, as highlighted by the 12 to 86 kg CO2 eq. per kg obtained using titanium oxysulphate and titanium bis(ammonium-lactato)dihydroxide precursors, respectively (CED of 149 and 1952 MJ kg−1).270
Because of their relatively high C-content, organic waste is an increasingly popular precursor for the preparation of carbon (nano)materials. Activated carbon from eucalyptus wastes using slow pyrolysis with ZnCl2 and H3PO4 activation showed a global warming value of 8.58 and 5.57 CO2 eq. per kg, respectively, which remains below the 11.1 eq. per kg reported for the activated carbon obtained from olive-waste cake.271 Marine aquatic ecotoxicity and terrestrial acidification were major environmental loads of the system due to the activating reagents used and the fossil fuel. Interestingly, a CED of 118.6 to 153.8 MJ kg−1 was obtained (11.8–16.2% renewable share), which was 36% lower than that of the commercial activated carbon. A calcination approach can also be taken to valorise eggshells and convert their CaCO3 phase into CaO (with potential construction applications).272 A value of 31.8 kg CO2 eq. per kg was obtained, with additional remarkable contributions in marine ecotoxicity, freshwater ecotoxicity, freshwater eutrophication and human toxicity also observed. Due to the energy-intensive character of the calcination process, the avoided raw CaO use of landfill accounted solely for 0.3–4.7% of the environmental benefits.
Larger impacts have been also reported for specific categories. For example, lower cradle-to-gate impacts in global warming, ozone formation, terrestrial acidification and fossil resource scarcity are observed after the incorporation of bagasse fibres into sugarcane-polyethylene (2.01 kg CO2 eq. per kg for fossil polyethylene, 1.38 kg CO2 eq. per kg for bio-polyethylene and 1.20 CO2 eq. per kg for the biocomposite). However, ozone formation, freshwater eutrophication, and terrestrial acidification are increased.278 Likewise, with 1.7 kg CO2 eq. per 1 metric Sabin (sound absorption unit), chicken feather nonwoven fabrics for acoustic applications have lower global warming values than other acoustic insulation materials such as rice husk, cork scraps or end-life granulated tires. However, ozone layer depletion, ecotoxicity and eutrophication impacts are larger than those of stone wool.279
Finally, it should be considered that the introduction of organic waste nanoparticles into petroleum-based polymers to replace other particles such as glass fibres does not directly result in lower CO2 emissions. With cradle-to-gate global warming of 4.6 kg CO2 eq. for PLA, 4.9 kg CO2 eq. for glass fibre/polypropylene and 8.6 kg CO2 eq. for epoxy/CNF (FU based on tensile stiffness), it is necessary to expand to the whole life-cycle to obtain the environmental benefits (cradle-to-grave GWP of 26.9 kg CO2 eq. for PLA, 18.9 kg CO2 eq. for glass fibre/polypropylene and 19.9 kg CO2 eq. for epoxy/CNF).280 On the other side, valorised olive pomace incorporated into polypropylene or polyethylene (for building purposes) yields global warming values of 20.25 to 20.52 CO2 eq. per m2 of lath, avoiding the 141.5 kg CO2 eq. that the composting of the same olive pomace quantity would liberate.281 These results emphasize the need to consider the use phase and the end-of-life of nanocellulose-reinforced epoxy composites to exploit the “green credentials” of biowaste-derived particles.
Generally, organic waste valorisation has the potential to reduce non-renewable primary material use, occupied landfill area and the carbon footprint over other EoL scenarios such as incineration or landfill.285 Additionally, circular biocomposites can replace less sustainable materials, contributing to more responsible consumption and production through resource efficiency. For instance, up to 23 kg CO2 eq. per kg could be saved by replacing cotton with lignin.246 Overall, the implementation of green chemistry principles to (nano)particle synthesis from organic waste is encouraged in the near future.286 Apart from guiding researchers and industry towards the sustainable valorisation of waste by identifying the driving environmental factors, these LCA results can also empower policymakers to adopt efficient measures to boost the implementation of sustainable policies.
6.2. Toxicity
Even if raw biowaste is unanimously considered not toxic, chemical additives used to convert it into matrices and nanoparticles, such sorbed environmental pollutants during application, could strongly modify this characteristic. Nevertheless, biopolymer toxicity is an emerging issue, since adverse effects of biodegradation products must be taken into account.287 It has been considered that, actually, bio-based/biodegradable materials and conventional plastics have similar toxicity characteristics: the results indicated that the majority (67%) of bioplastics and plant-based products contain toxic chemicals as well as a large number and diversity of compounds (>1000 chemical features each in 80% of the samples).288 In order to develop bio-based/biodegradable materials that do indeed outperform conventional plastics, sustainability and chemical safety aspects must be addressed alike. One way to promote this is to integrate, as an example, chemical toxicity into the life cycle assessment of materials, or by using green chemistry to “design out” toxicity during the development of new bio-based and biodegradable materials. To this aim, it needs to be considered that although the main components in biomass wastes are biocompatible and nontoxic, there are still certain amounts of “impurities” or contaminants.289 Moreover, various nanoparticles are usually incorporated to improve the performance of biowaste-derived composite materials, which also cause some worries. Therefore, it is necessary to study case-by-case the outcome of these minorities in materials and their potential safety issues.A negative aspect is based on the incorporation of toxic elements such as heavy metals obtained from natural sources in the development of new polymeric system. As an example, Astolfi and co-authors289 proposed the realization of polyhydroxyalkanoate (PHA) with organic waste as the feedstock and analyzed the polluting elements.290 The potential impurities that could transfer from bio-waste to a PHA include inorganic elements such as heavy metals, as represented in the schematic illustration shown in Fig. 14. The total content of certain elements in PHA ranged between 0.0001 (Be) and 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 mg kg−1 (Na). The amounts of some alkaline (Na and K) and alkaline earth (Ca and Mg) metals were highest, which are of little environmental concern. These authors observed that the number of heavy metals in PHA realized from fruit waste or crops were inferior to those in the PHA realized from the mixture of sludge from wastewater treatment and the organic fraction of municipal waste. The PHA values obtained by extraction from wet biomass (acid storage) with aqueous phase extraction reagents and commercial PHA were below the migration limits required by the current regulation on plastics and articles intended to come into contact with food under refrigerated and frozen conditions.
500 mg kg−1 (Na). The amounts of some alkaline (Na and K) and alkaline earth (Ca and Mg) metals were highest, which are of little environmental concern. These authors observed that the number of heavy metals in PHA realized from fruit waste or crops were inferior to those in the PHA realized from the mixture of sludge from wastewater treatment and the organic fraction of municipal waste. The PHA values obtained by extraction from wet biomass (acid storage) with aqueous phase extraction reagents and commercial PHA were below the migration limits required by the current regulation on plastics and articles intended to come into contact with food under refrigerated and frozen conditions.
 | ||
| Fig. 14 Boundaries (inputs and outputs) of the system under the circular economy framework in polymeric processes. Schematic representation of polyhydroxyalkanoate production. Reproduced from ref. 289 with permission from Elsevier, copyright 2020. | ||
Another opportunity to limit the toxicity of biowaste is conversion, via pyrolysis into biochar, that could effectively deactivate toxic heavy metals: pyrolysed biowastes could be a clean and affordable input to improve soil and recycle nutrients in agriculture, delivering considerable economic and environmental benefits.291 Yet, how pyrolysed biowastes may affect soil food webs and biological diversity conservation as well as ecosystem processes should be further explored in more field studies.
7. Future outlook and conclusions
Produced annually on a remarkable scale of billions of kilos, biowastes should be redesigned considering circular economy principles (Fig. 15). Accordingly, sustainable growth should be ideally implemented through a closed loop for recycling and transformation, without damaging and/or diminishing the natural sources. Biowastes can be fundamentally transformed, via physical, chemical or biological methods into wide-ranging examples of end-use products and materials. However, the highly heterogeneous nature of biowastes makes the identification of adequate valorisation challenging. Accordingly, this review focuses on the conversion methods of biowaste to derived nanomaterials, namely biopolymers and nanocomposites, starting from a variety of largely available sources such as collagen, chitin, chitosan, hydroxyapatites, cellulose, lignin, and other C-based feedstocks for metal, metal oxide nanoparticles, biopolymeric and nanocarbon structures. Outlooks for the applications of these materials range from drug and agricultural delivery, to environmental remediation, catalysis, energy storage, etc.; nonetheless, many such studies can be still considered to be at an early stage and require an in-depth analysis of both technical and socio-ecological aspects, including energy balance and costs, environmental emissions, toxicity and biodegradability issues. Upcoming research is undeniably required to promote everyday applications, passing through proper quality control to determine compositions and molecular structure of targeted natural polymers and assure constant final properties of the converted materials. | ||
| Fig. 15 Schematic representation highlighting the life cycle aspects of biowaste valorization and the future opportunities challenges envisioned toward practical implementation. | ||
Ongoing research that looks at valorising bio-waste as bio-products (matrices and nanofillers) still has many challenges to be tackled to reach scaled production and commercialization, so further technical development is needed for their use at industrial level.12 Several factors hamper their adoption, which includes reproducibility of the properties and complexity/varying nature of bio-waste that demand pricy separation processes and purification.292 Accordingly, high costs to bring the products to higher scale are expected.293 The limited output volume of converted biomass is also another limiting factor to commercialization, since the required controlled compositions of biomass wastes to ensure the desirable properties of the final products certainly hinder growth to the market.
Looking at the functionality of converted biowastes, concise studies are essential to first tune the properties, design and control structures, so early commercial success can be reached if technological requirements for higher volume are easily overcome. Help in reaching this objective is offered, for example, at European level, by the Bio Base Europe Pilot Plant, that successfully conducted over 450 bilateral (private) projects with more than 150 small, medium and large-sized companies in more than 50 public project consortia, from 2013 to 2020, to finalize the construction of process lines and facilities and help in the market positioning of different processed biowastes new products on the market.294
To the best of the authors’ knowledge, chitosan biowastes surpassed these barriers and reached commercialization,295 with shrimp shell source chitosan occupying almost 80% of the total chitosan market (fungal-based chitosan products are still limited in market).296 However, the product is intended to be used for food/beverage and tissue engineering applications, while no indications for use and filler or matrix could be found. Cellulose and lignin conversion to biopolymers also reached Technology readiness levels (TRLs) higher than 5,235,297 and carrageenans in hydrogels also represent an interesting alternative to an integral valorisation of disposals, currently only used as fertilizing purposes.298 A variety of sustainable sources of collagen and keratin and the beneficial multiform application of these two proteins have been also extensively reviewed. In all the cited cases, we should, however, keep in mind that production is economically viable and cost-competitive in the market only if, in parallel, and regardless of the easy scaling of the procedures and the yield in marketable products, it represents an industrial activity with no considerable environmental impacts.
Author contributions
The manuscript was written through the contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
E.L. is grateful for the financial support from the “2021 Euskampus Missions 1.0. Programme” granted by Euskampus Fundazioa.References
- R. Geyer, J. R. Jambeck and K. L. Law, Sci. Adv., 2017, 3, e1700782 CrossRef PubMed.
- E. MacArthur, Science, 2017, 358, 843–843 CrossRef PubMed.
- A.-C. Albertsson and M. Hakkarainen, Science, 2017, 358, 872 LP-873 CrossRef PubMed.
- T. F. Garrison, A. Murawski and R. L. Quirino, Polymers, 2016, 8(7), 262 CrossRef PubMed.
- S. Lambert and M. Wagner, Chem. Soc. Rev., 2017, 46, 6855–6871 RSC.
- S. Choe, Y. Kim, Y. Won and J. Myung, Front. Chem., 2021, 9, 334 Search PubMed.
- E. Elhacham, L. Ben-Uri, J. Grozovski, Y. M. Bar-On and R. Milo, Nature, 2020, 588, 442–444 CrossRef CAS PubMed.
- M. Kardung, K. Cingiz, O. Costenoble, R. Delahaye, W. Heijman, M. Lovrić and M. van Leeuwen, et al. , Sustainability, 2021, 13(1), 413 CrossRef.
- D. D'Amato, S. Veijonaho and A. Toppinen, For. Policy Econ., 2020, 110, 101848 CrossRef.
- United Nations Environmental Programme, Converting Waste Agricultural Biomass into a Resource, 2009 Search PubMed.
- N. Tripathi, C. D. Hills, R. S. Singh and C. J. Atkinson, npj Clim. Atmos. Sci., 2019, 2, 35 CrossRef.
- European Environment Agency, Bio-waste in Europe—turning challenges into opportunities, 2020 Search PubMed.
- United Nations Environment Programme, Terminal evaluation of the UNEP GEF project: Land degradation assessment in drylands (LADA), 2011 Search PubMed.
- J. Poore and T. Nemecek, Science, 2018, 360, 987–992 CrossRef CAS PubMed.
- J. D. Kabongo, Waste Valorization, ed. S. O. Idowu, N. Capaldi, L. Zu and A. Das Gupta, Springer Berlin Heidelberg, Berlin, Heidelberg, 2013, pp. 2701–2706 Search PubMed.
- A. Merrington, Recycling of plastics, Applied Plastics Engineering Handbook, in Plastics Design Library, ed. M. Kutz, William Andrew Publishing, 2017, pp. 167–189 Search PubMed.
- J. M. Allwood, Squaring the Circular Economy: The Role of Recycling within a Hierarchy of Material Management Strategies, in Handbook of Recycling, ed. E. Worrell and M. A. Reuter, Elsevier, Boston, 2014, pp. 445–477 Search PubMed.
- F. Adıgüzel and C. Donato, J. Bus. Res., 2021, 130, 137–146 CrossRef.
- A. Lauria and E. Lizundia, J. Cleaner Prod., 2020, 262, 121288 CrossRef CAS.
- European Commission, Critical Raw Materials for Strategic Technologies and Sectors in the EU, A Foresight Study, 2020 Search PubMed.
- European Commission, Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability, 2020 Search PubMed.
- J. L. Gómez-Urbano, G. Moreno-Fernández, M. Arnaiz, J. Ajuria, T. Rojo and D. Carriazo, Carbon, 2020, 162, 273–282 CrossRef.
- S. A. Nicolae, H. Au, P. Modugno, H. Luo, A. E. Szego, M. Qiao, L. Li, W. Yin, H. J. Heeres, N. Berge and M.-M. Titirici, Green Chem., 2020, 22, 4747–4800 RSC.
- E. Lizundia, T.-D. Nguyen, R. J. Winnick and M. J. MacLachlan, J. Mater. Chem. C, 2021, 9, 796–817 RSC.
- W. Foster, U. Azimov, P. Gauthier-Maradei, L. C. Molano, M. Combrinck, J. Munoz, J. J. Esteves and L. Patino, Renewable Sustainable Energy Rev., 2021, 135, 110226 CrossRef CAS.
- S. Y. Lee, R. Sankaran, K. W. Chew, C. H. Tan, R. Krishnamoorthy, D.-T. Chu and P.-L. Show, BMC Energy, 2019, 1, 4 CrossRef.
- L. B. van Leeuwen, H. J. Cappon and K. J. Keesman, Biomass Bioenergy, 2021, 145, 105931 CrossRef.
- M. Geissdoerfer, P. Savaget, N. M. P. Bocken and E. J. Hultink, J. Cleaner Prod., 2017, 143, 757–768 CrossRef.
- E. MacArthur, Beyond plastic waste, Science, 2017, 358(6365), 843–843 CrossRef PubMed.
- P. Ghisellini, C. Cialani and S. Ulgiati, J. Cleaner Prod., 2016, 114, 11–32 CrossRef.
- C. Xu, M. Nasrollahzadeh, M. Selva, Z. Issaabadi and R. Luque, Chem. Soc. Rev., 2019, 48, 4791–4822 RSC.
- R. Balart, D. Garcia-Garcia, V. Fombuena, L. Quiles-Carrillo and M. P. Arrieta, Polymers, 2021, 13(15), 2532 CrossRef CAS PubMed.
- E. B. Vea, D. Romeo and M. Thomsen, Procedia CIRP, 2018, 69, 591–596 CrossRef.
- B. Ramírez-Pulido, C. Bas-Bellver, N. Betoret, C. Barrera and L. Seguí, Front. Sustainable Food Syst., 2021, 5, 654313 CrossRef.
- G. Capson-Tojo, M. Rouez, M. Crest, J.-P. Steyer, J.-P. Delgenès and R. Escudié, Rev. Environ. Sci. Bio/Technol., 2016, 15, 499–547 CrossRef CAS.
- A. S. Sodhi, N. Sharma, S. Bhatia, A. Verma, S. Soni and N. Batra, Chemosphere, 2022, 286, 131623 CrossRef CAS PubMed.
- A. Tassoni, T. Tedeschi, C. Zurlini, I. M. Cigognini, J.-I. Petrusan, Ó. Rodríguez, S. Neri, A. Celli, L. Sisti, P. Cinelli, F. Signori, G. Tsatsos, M. Bondi, S. Verstringe, G. Bruggerman and P. F. X. Corvini, Molecules, 2020, 25(6), 1383 CrossRef CAS PubMed.
- T. Maschmeyer, R. Luque and M. Selva, Chem. Soc. Rev., 2020, 49, 4527–4563 RSC.
- C. R. Lohri, S. Diener, I. Zabaleta, A. Mertenat and C. Zurbrügg, Rev. Environ. Sci. Bio/Technol., 2017, 16, 81–130 CrossRef.
- L. Haigh, M. de Wit, C. von Daniels, A. Colloricchio, J. Hoogzaad, M. Fraser and A. Heidtmann, The Circularity Gap Report 2021, 2021, Acknowledgments: This paper is part of research activities under the grant (304. PTEKIND. 6501034. D119) Search PubMed.
- A. Schieber, F. C. Stintzing and R. Carle, Trends Food Sci. Technol., 2001, 12, 401–413 CrossRef CAS.
- E. Gilman, A. Perez Roda, T. Huntington, S. J. Kennelly, P. Suuronen, M. Chaloupka and P. A. H. Medley, Sci. Rep., 2020, 10, 14017 CrossRef CAS PubMed.
- D. Gunders and J. Bloom, Wasted: How America is losing up to 40 percent of its food from farm to fork to landfill, Natural Resources Defense Council, 2017 Search PubMed.
- M. M. Giraud, J. Castanet, F. J. Meunier and Y. Bouligand, Tissue Cell, 1978, 10, 671–686 CrossRef CAS PubMed.
- Y. Bouligand, Tissue Cell, 1972, 4, 189–217 CrossRef CAS PubMed.
- T. D. Nguyen, E. Sierra, H. Eguiraun and E. Lizundia, Eur. J. Phys., 2018, 39(4), 045803 CrossRef.
- S. Kaur and G. S. Dhillon, Crit. Rev. Biotechnol., 2015, 35, 44–61 CrossRef CAS PubMed.
- M. M. Abo Elsoud and E. M. El Kady, Bull. Natl. Res. Cent., 2019, 43, 59 CrossRef.
- T.-D. Nguyen, B. U. Peres, R. M. Carvalho and M. J. MacLachlan, Adv. Funct. Mater., 2016, 26, 2875–2881 CrossRef CAS.
- T. D. Nguyen and M. J. MacLachlan, Adv. Opt. Mater., 2014, 2, 1031–1037 CrossRef CAS.
- D. X. Oh, Y. J. Cha, H. L. Nguyen, H. H. Je, Y. S. Jho, D. S. Hwang and D. K. Yoon, Sci. Rep., 2016, 6, 23245 CrossRef CAS PubMed.
- T. T. N. Dang, T. D. Nguyen, E. Lizundia, Q. T. Le and M. J. MacLachlan, ChemistrySelect, 2020, 5, 8207–8217 CrossRef.
- J. Jin, D. Lee, H.-G. Im, Y. C. Han, E. G. Jeong, M. Rolandi, K. C. Choi and B.-S. Bae, Adv. Mater., 2016, 28, 5169–5175 CrossRef CAS PubMed.
- H. N. Cuong, N. C. Minh, N. Van Hoa and T. S. Trung, Int. J. Biol. Macromol., 2016, 93, 442–447 CrossRef CAS PubMed.
- J. L. Wilson and T. C. McDevitt, Biofunctional hydrogels for Three-dimensional stem cell culture, ed. A. Vishwakarma and J. M. Karp, Academic Press, Boston, 2017, pp. 345–362 Search PubMed.
- V. L. Campo, D. F. Kawano, D. B. da Silva and I. Carvalho, Carbohydr. Polym., 2009, 77, 167–180 CrossRef CAS.
- M. J. Cardoso, R. R. Costa and J. F. Mano, Mar. Drugs, 2016, 14(2), 34 CrossRef PubMed.
- T. Karbowiak, H. Hervet, L. Léger, D. Champion, F. Debeaufort and A. Voilley, Biomacromolecules, 2006, 7, 2011–2019 CrossRef CAS PubMed.
- T. H. Silva, A. Alves, B. M. Ferreira, J. M. Oliveira, L. L. Reys, R. J. F. Ferreira, R. A. Sousa, S. S. Silva, J. F. Mano and R. L. Reis, Int. Mater. Rev., 2012, 57, 276–306 CrossRef CAS.
- M. A. Murado, M. I. Montemayor, M. L. Cabo, J. A. Vázquez and M. P. González, Food Bioprod. Process., 2012, 90, 491–498 CrossRef CAS.
- A. Koopmans and J. Koppejan, Agricultural and forest residues - generation, utilization and availability, Regional consultation on modern applications of biomass energy, 1997, 6, 10 Search PubMed.
- P. Harrison, C. Malins, S. Searle, A. Baral, D. Turley and L. Hopwood, Wasted: Europe's untapped resource, European Climate Foundation, Brussels, Belgium, 2014, 29pp Search PubMed.
- K. C. Badgujar and B. M. Bhanage, Dedicated and waste feedstocks for biorefinery: an approach to develop a sustainable society, in Waste Biorefinery, ed. T. Bhaskar, A. Pandey, S. V. Mohan, D.-J. Lee and S. K. B. T.-W. B. Khanal, Elsevier, 2018, pp. 3–38 Search PubMed.
- S. Devi, C. Gupta, S. L. Jat and M. S. Parmar, Open Agric., 2017, 2, 486–494 CrossRef.
- M. Pauly and K. Keegstra, Curr. Opin. Plant Biol., 2010, 13, 304–311 CrossRef PubMed.
- H. J. Kim and B. A. Triplett, Plant Physiol., 2001, 127, 1361–1366 CrossRef CAS PubMed.
- R. Suriyatem, N. Noikang, T. Kankam, K. Jantanasakulwong, N. Leksawasdi, Y. Phimolsiripol, C. Insomphun, P. Seesuriyachan, T. Chaiyaso, P. Jantrawut, S. R. Sommano, T. M. P. Ngo and P. Rachtanapun, Polymers., 2020, 12, 1505 CrossRef CAS PubMed.
- T.-D. Nguyen, E. Lizundia, M. Niederberger, W. Y. Hamad and M. J. MacLachlan, Chem. Mater., 2019, 31, 2174–2181 CrossRef CAS.
- E. Espinosa, F. Rol, J. Bras and A. Rodríguez, Cellulose, 2020, 27, 10689–10705 CrossRef CAS.
- J. Fernández-Rodríguez, O. Gordobil, E. Robles, M. González-Alriols and J. Labidi, J. Cleaner Prod., 2017, 142, 2609–2617 CrossRef.
- E. Lizundia, M. H. Sipponen, L. G. Greca, M. Balakshin, B. L. Tardy, O. J. Rojas and D. Puglia, Green Chem., 2021, 23(18), 6698–6760 RSC.
- M. D. Lenardon, C. A. Munro and N. A. R. Gow, Curr. Opin. Microbiol., 2010, 13, 416–423 CrossRef CAS PubMed.
- R. Garcia-Rubio, H. C. de Oliveira, J. Rivera and N. Trevijano-Contador, Front. Microbiol., 2020, 10, 2993 CrossRef PubMed.
- M. Jones, A. Gandia, S. John and A. Bismarck, Nat. Sustainability, 2021, 4, 9–16 CrossRef.
- Food and Agriculture Organization of the United Nations, Statistics Division (FAOSTAT) Production: Livestock Primary: Eggs Primary, 2019 Search PubMed.
- S.-C. Wu, H.-C. Hsu, S.-K. Hsu, Y.-C. Chang and W.-F. Ho, J. Asian Ceram. Soc., 2016, 4, 85–90 CrossRef.
- S. Mignardi, L. Archilletti, L. Medeghini and C. De Vito, Sci. Rep., 2020, 10, 2436 CrossRef CAS PubMed.
- N. A. M. Barakat, M. S. Khil, A. M. Omran, F. A. Sheikh and H. Y. Kim, J. Mater. Process. Technol., 2009, 209, 3408–3415 CrossRef CAS.
- P. Shi, M. Liu, F. Fan, C. Yu, W. Lu and M. Du, Mater. Sci. Eng., C, 2018, 90, 706–712 CrossRef CAS PubMed.
- F. A. Sheikh, M. A. Kanjwal, J. Macossay, M. A. Muhammad, T. Cantu, I. S. Chronakis, N. A. M. Barakat and H. K. Yong, J. Biomater. Tissue Eng., 2011, 1, 194–197 CrossRef PubMed.
- R. Dinu and A. Mija, Industrial feather waste valorisation for sustainable keratin-based materials, in Abstracts Of Papers Of The American Chemical Society, 2019, vol. 257, pp. 1155 Search PubMed.
- I. M. Weiss and H. O. K. Kirchner, J. Exp. Zool., Part A: Ecol. Genet. Physiol., 2010, 313A, 690–703 CrossRef PubMed.
- R. H. Bonser and C. Dawson, J. Mater. Sci. Lett., 1999, 18, 1769–1770 CrossRef CAS.
- M. F. Ali, M. S. Hossain, T. S. Moin, S. Ahmed and A. M. S. Chowdhury, Clean Eng. Technol., 2021, 4, 100190 CrossRef.
- V. Vilchez, E. Dieckmann, T. Tammelin, C. Cheeseman and K.-Y. Lee, ACS Sustainable Chem. Eng., 2020, 8, 14263–14267 CrossRef CAS.
- D. Hallam, C. Calpe and A. Abbassian, Food Outlook – Biannual Report on Global Food Markets, FAO, 2013 Search PubMed.
- W. Łaba, D. Chorążyk, A. Pudło, J. Trojan-Piegza, M. Piegza, A. Kancelista, A. Kurzawa, I. Żuk and W. Kopeć, Waste Biomass Valorization, 2017, 8, 527–537 CrossRef.
- A. Zuliani, M. J. Muñoz-Batista and R. Luque, Green Chem., 2018, 20, 3001–3007 RSC.
- N. Fakhfakh, N. Ktari, R. Siala and M. Nasri, J. Appl. Microbiol., 2013, 115, 424–433 CrossRef CAS PubMed.
- A. Patnaik, M. Mvubu, S. Muniyasamy, A. Botha and R. D. Anandjiwala, Energy Build., 2015, 92, 161–169 CrossRef.
- J. Zach, A. Korjenic, V. Petránek, J. Hroudová and T. Bednar, Energy Build., 2012, 49, 246–253 CrossRef.
- R. Naik, G. Wen, D. MS, S. Hureau, A. Uedono, X. Wang, X. Liu, P. G. Cookson and S. V. Smith, J. Appl. Polym. Sci., 2010, 115, 1642–1650 CrossRef CAS.
- Z. Lentz, P. Kolar and J. J. Classen, Process, 2019, 7(9), 560 CrossRef CAS.
- N. Kellogg, R. Moffitt and D. C. Gollehon, Estimates of Recoverable and Non-recoverable Manure Nutrients Based on the Census of Agriculture, United States Department of Agriculture Natural Resources Conservation Service, 2012 Search PubMed.
- M. Jones, K. Weiland, M. Kujundzic, J. Theiner, H. Kählig, E. Kontturi and A. Mautner, Biomacromolecules, 2019, 20, 3513–3523 CrossRef CAS PubMed.
- V. Sharma, M. Crne, J. O. Park and M. Srinivasarao, Science, 2009, 325, 449–451 CrossRef CAS PubMed.
- M. Triunfo, E. Tafi, A. Guarnieri, C. Scieuzo, T. Hahn, S. Zibek, R. Salvia and P. Falabella, Cosmetics, 2021, 8(2), 40 CrossRef CAS.
- Q. Li, S. Xu, Q. Feng, Q. Dai, L. Yao, Y. Zhang, H. Gao, H. Dong, D. Chen and X. Cao, Bioact. Mater., 2021, 6, 3396–3410 CrossRef CAS PubMed.
- E. Rodias, E. Aivazidou, C. Achillas, D. Aidonis and D. Bochtis, Energies, 2021, 14(1), 159 CrossRef.
- V. Shanmugam, O. Das, R. E. Neisiany, K. Babu, S. Singh, M. S. Hedenqvist, F. Berto and S. Ramakrishna, Mater. Circ. Econ., 2020, 2, 11 CrossRef.
- R. R. Bora, R. Wang and F. You, ACS Sustainable Chem. Eng., 2020, 8, 16350–16363 CrossRef CAS.
- M. A. Acquavia, R. Pascale, G. Martelli, M. Bondoni and G. Bianco, Polymers, 2021, 13(1), 158 CrossRef CAS PubMed.
- C. G. Otoni, H. M. C. Azeredo, B. D. Mattos, M. Beaumont, D. S. Correa and O. J. Rojas, Adv. Mater., 2021, 33, 2102520 CrossRef CAS PubMed.
- Y. F. Tsang, V. Kumar, P. Samadar, Y. Yang, J. Lee, Y. S. Ok, H. Song, K.-H. Kim, E. E. Kwon and Y. J. Jeon, Environ. Int., 2019, 127, 625–644 CrossRef CAS PubMed.
- T. Li, C. Chen, A. H. Brozena, J. Y. Zhu, L. Xu, C. Driemeier, J. Dai, O. J. Rojas, A. Isogai, L. Wågberg and L. Hu, Nature, 2021, 590, 47–56 CrossRef CAS PubMed.
- M. Crippa, E. Solazzo, D. Guizzardi, F. Monforti-Ferrario, F. N. Tubiello and A. Leip, Nat. Food, 2021, 2, 198–209 CrossRef CAS.
- A. Camia, N. Robert, K. Jonsson, R. Pilli, S. Garcia Condado, R. Lopez Lozano, M. Van Der Velde, T. Ronzon, P. Gurria Albusac, R. Mbarek, S. Tamosiunas, G. Fiore, R. Dos Santos Fernandes De Araujo, N. Hoepffner, L. Marelli and J. Giuntoli, Publ. Off. Eur. Union, 2018, JRC109869 Search PubMed.
- FAO, The state of Food and Agriculture, Moving forwards on food loss and waste reduction, 2019 Search PubMed.
- R. Bedoić, B. Ćosić and N. Duić, Sci. Total Environ., 2019, 686, 568–579 CrossRef PubMed.
- C. Peña, B. Civit, A. Gallego-Schmid, A. Druckman, A. C. Pires, B. Weidema, E. Mieras, F. Wang, J. Fava, L. M. i. Canals, M. Cordella, P. Arbuckle, S. Valdivia, S. Fallaha and W. Motta, Int. J. Life Cycle Assess., 2021, 26, 215–220 CrossRef.
- L. Moretti, P. Di Mascio and S. Bellagamba, Int. J. Environ. Res. Public Health, 2017, 14(6), 645 CrossRef PubMed.
- V. V. Devadas, K. S. Khoo, W. Y. Chia, K. W. Chew, H. S. H. Munawaroh, M.-K. Lam, J.-W. Lim, Y.-C. Ho, K. T. Lee and P. L. Show, Bioresour. Technol., 2021, 325, 124702 CrossRef CAS PubMed.
- A. A. Adeleke, P. P. Ikubanni, T. A. Orhadahwe, C. T. Christopher, J. M. Akano, O. O. Agboola, S. O. Adegoke, A. O. Balogun and R. A. Ibikunle, Heliyon, 2021, 7, e08025 CrossRef CAS PubMed.
- S. Ranganathan, S. Dutta, J. A. Moses and C. Anandharamakrishnan, Heliyon, 2020, 6, e04891 CrossRef PubMed.
- M. Kaya, T. Baran and M. Karaarslan, Nat. Prod. Res., 2015, 29, 1477–1480 CrossRef CAS PubMed.
- S. K. Bhatia, S. V. Otari, J.-M. Jeon, R. Gurav, Y.-K. Choi, R. K. Bhatia, A. Pugazhendhi, V. Kumar, J. Rajesh Banu, J.-J. Yoon, K.-Y. Choi and Y.-H. Yang, Bioresour. Technol., 2021, 326, 124733 CrossRef CAS PubMed.
- A. Thakali and J. D. MacRae, Environ. Res., 2021, 194, 110635 CrossRef CAS PubMed.
- D. A. Peguero, M. Gold, D. Vandeweyer, C. Zurbrügg and A. Mathys, Front. Sustainable Food Syst., 2022, 5, 745894 CrossRef.
- Y. Zhang, S. Ni, R. Wu, Y. Fu, M. Qin, S. Willför and C. Xu, Ind. Crops Prod., 2022, 177, 114451 CrossRef CAS.
- N. George, A. Debroy, S. Bhat, S. Bindal and S. Singh, J. Appl. Biotechnol. Rep., 2021, 8, 221–233 CAS.
- V. Sharma and P. P. Kundu, Prog. Polym. Sci., 2006, 31, 983–1008 CrossRef CAS.
- M. Soccio, F. Dominici, S. Quattrosoldi, F. Luzi, A. Munari, L. Torre, N. Lotti and D. Puglia, Biomacromolecules, 2020, 21, 3254–3269 CrossRef CAS PubMed.
- F. Dominici, F. Luzi, P. Benincasa, L. Torre and D. Puglia, Polymers, 2020, 12(10), 2248 CrossRef CAS PubMed.
- E. Águila-Almanza, S. S. Low, H. Hernández-Cocoletzi, A. Atonal-Sandoval, E. Rubio-Rosas, J. Violante-González and P. L. Show, J. Environ. Chem. Eng., 2021, 9, 105229 CrossRef.
- J. Uranga, A. Etxabide, S. Cabezudo, K. de la Caba and P. Guerrero, Sci. Total Environ., 2020, 706, 135747 CrossRef CAS PubMed.
- T. Raj, K. Chandrasekhar, A. Naresh Kumar and S.-H. Kim, Renewable Sustainable Energy Rev., 2022, 158, 112130 CrossRef CAS.
- K. Dhamodharan, S. Ahlawat, M. Kaushal and K. Rajendran, Economics and cost analysis of waste biorefineries, ed. R. Praveen Kumar, E. Gnansounou, J. Kenthorai Raman and G. Baskar, Academic Press, 2020, pp. 545–565 Search PubMed.
- S. Zhou, T. Khan, K. Jin, J. Lee and M. J. Buehler, Adv. Funct. Mater., 2022, 32, 2109881 CrossRef CAS.
- A. S. Bomfim, D. M. Oliveira, H. J. Voorwald, K. C. Benini, M.-J. Dumont and D. Rodrigue, Polymers, 2022, 14(3), 437 CrossRef CAS PubMed.
- F. Luzi, L. Torre, J. M. Kenny and D. Puglia, Materials, 2019, 11(12), 1999 CAS.
- R. Xiong, A. M. Grant, R. Ma, S. Zhang and V. V. Tsukruk, Mater. Sci. Eng., R, 2018, 125, 1–41 CrossRef.
- E. Kowsalya, K. MosaChristas, P. Balashanmugam, V. Manivasagan, T. Devasena and C. R. I. Jaquline, J. Food Process Eng., 2021, 44, e13641 CAS.
- O. Das, K. Babu, V. Shanmugam, K. Sykam, M. Tebyetekerwa, R. E. Neisiany, M. Försth, G. Sas, J. Gonzalez-Libreros, A. J. Capezza, M. S. Hedenqvist, F. Berto and S. Ramakrishna, Renewable Sustainable Energy Rev., 2022, 158, 112054 CrossRef CAS.
- A. García-Quintero and M. Palencia, Sci. Total Environ., 2021, 793, 148524 CrossRef PubMed.
- K. K. Brar, S. Magdouli, A. Othmani, J. Ghanei, V. Narisetty, R. Sindhu, P. Binod, A. Pugazhendhi, M. K. Awasthi and A. Pandey, Environ. Res., 2022, 207, 112202 CrossRef CAS PubMed.
- S. M. Abdelbasir, K. M. McCourt, C. M. Lee and D. C. Vanegas, Front. Chem., 2020, 8, 782 CrossRef CAS PubMed.
- P. R. Ghosh, D. Fawcett, S. B. Sharma and G. E. J. Poinern, Materials, 2017, 10(8), 852 CrossRef PubMed.
- P. Samaddar, Y. S. Ok, K.-H. Kim, E. E. Kwon and D. C. W. Tsang, J. Cleaner Prod., 2018, 197, 1190–1209 CrossRef CAS.
- J. Esteban and M. Ladero, Int. J. Food Sci. Technol., 2018, 53, 1095–1108 CrossRef CAS.
- D. Thangadurai, J. Naik, J. Sangeetha, A. Rahman Mohammad Said Al-Tawaha, C. O. Adetunji, S. Islam, M. David, A. K. Shettar and J. B. Adetunji, Nanomaterials from Agrowastes: Past, Present, and the Future, ed. O. V. Kharissova, L. M. T. Martínez and B. I. Kharisov, Springer International Publishing, Cham, 2020, pp. 1–17 Search PubMed.
- M. Selva, Curr. Opin. Green Sustainable Chem., 2020, 24, 38–41 CrossRef.
- K. Mensah-Darkwa, C. Zequine, P. K. Kahol and R. K. Gupta, Sustainability, 2019, 11(2), 414 CrossRef CAS.
- U. Zia, H. Iram, H. Z. Haider, F. Ameen, M. Abrar and M. Atif, ECS J. Solid State Sci. Technol., 2022, 11, 21001 CrossRef.
- B. Vaish, V. Srivastava, P. Kumar Singh, P. Singh and R. Pratap Singh, Environ. Eng. Res., 2020, 25, 623–637 CrossRef.
- Y.-E. Lee, D.-C. Shin, Y. Jeong, I.-T. Kim and Y.-S. Yoo, Energies, 2019, 12(23), 4528 CrossRef.
- A. Kumar, A. Choudhary, H. Kaur, S. Mehta and A. Husen, J. Nanobiotechnol., 2021, 19, 256 CrossRef PubMed.
- B. A. Omran and K.-H. Baek, J. Environ. Manage., 2022, 311, 114806 CrossRef CAS PubMed.
- A. Rastogi, P. Singh, F. A. Haraz and A. Barhoum, in Micro and Nano Technologies, ed. A. Barhoum and A. S. Hamdy Makhlouf, Elsevier, 2018, pp. 571–604 Search PubMed.
- J. Jeevanandam, S. F. Kiew, S. Boakye-Ansah, S. Y. Lau, A. Barhoum, M. K. Danquah and J. Rodrigues, Nanoscale, 2022, 14, 2534–2571 RSC.
- B. H. J. Gowda, M. G. Ahmed, S. Chinnam, K. Paul, M. Ashrafuzzaman, M. Chavali, R. Gahtori, S. Pandit, K. K. Kesari and P. K. Gupta, J. Drug Delivery Sci. Technol., 2022, 71, 103305 CrossRef CAS.
- O. V. Kharissova, B. I. Kharisov, C. M. Oliva González, Y. P. Méndez and I. López, R. Soc. Open Sci., 2022, 6, 191378 CrossRef PubMed.
- G. Ashrafi, M. Nasrollahzadeh, B. Jaleh, M. Sajjadi and H. Ghafuri, Adv. Colloid Interface Sci., 2022, 301, 102599 CrossRef CAS PubMed.
- K. Torres-Rivero, J. Bastos-Arrieta, N. Fiol and A. Florido, in Biosynthesized Nanomaterials, ed. S. K. Verma and A. K. B. T.-C. A. C. Das, Elsevier, 2021, vol. 94, pp. 433–469 Search PubMed.
- R. Suhag, R. Kumar, A. Dhiman, A. Sharma, P. K. Prabhakar, K. Gopalakrishnan, R. Kumar and A. Singh, Crit. Rev. Food Sci. Nutr., 2022, 1–20 CrossRef PubMed.
- M. M. Ahmed, M. T. Badawy, F. K. Ahmed, A. Kalia and K. A. Abd-Elsalam, in Nanobiotechnology for Plant Protection, ed. K. A. Abd-Elsalam, R. Periakaruppan and S. Rajeshkumar, Elsevier, 2022, pp. 231–257 Search PubMed.
- A. Santhosh, V. Theertha, P. Prakash and S. Chandran, Mater. Today: Proc., 2021, 46, 4460–4463 Search PubMed.
- S. Ali, X. Chen, M. Ajmal Shah, M. Ali, M. Zareef, M. Arslan, S. Ahmad, T. Jiao, H. Li and Q. Chen, Food Chem., 2021, 359, 129912 CrossRef CAS PubMed.
- K. Bhardwaj, D. S. Dhanjal, A. Sharma, E. Nepovimova, A. Kalia, S. Thakur, S. Bhardwaj, C. Chopra, R. Singh, R. Verma, D. Kumar, P. Bhardwaj and K. Kuča, Int. J. Mol. Sci., 2020, 21(23), 9028 CrossRef CAS PubMed.
- A. B. Mahanthesh, S. Haldar and S. Banerjee, Biotechnol. Zero Waste, 2022, 361–368 Search PubMed.
- M. B. da Costa Rocha, T. R. de Araújo, R. L. B. A. Medeiros, M. M. Oliveira and G. P. de Figueredo, Res. Soc. Dev., 2021, 11(8), 1003 Search PubMed.
- J. Singh, T. Dutta, K.-H. Kim, M. Rawat, P. Samddar and P. Kumar, J. Nanobiotechnol., 2018, 16, 84 CrossRef CAS PubMed.
- A. M. El Shafey, Green Process. Synth., 2020, 9, 304–339 Search PubMed.
- L. Soltys, O. Olkhovyy, T. Tatarchuk and M. Naushad, Magnetochemistry, 2021, 7(11), 145 CrossRef CAS.
- S. A. Akintelu, A. S. Folorunso, F. A. Folorunso and A. K. Oyebamiji, Heliyon, 2020, 6, e04508 CrossRef PubMed.
- C. P. Devatha, J. K and M. Patil, Environ. Nanotechnol., Monit. Manage., 2018, 9, 85–94 Search PubMed.
- Q. Zhu, W. Zhang, J. Cai, J. Li, L. Zhong, S. Pu and A. Li, Colloids Surf., A, 2022, 640, 128471 CrossRef CAS.
- T. T. Phan, D. T. Phan, X. T. Cao, T.-C. Huynh and J. Oh, Nanomaterials, 2021, 11(2), 273 CrossRef CAS PubMed.
- M. I. Hidayat, M. Adlim, I. Maulana, S. Suhartono, Z. Hayati and N. H. H. A. Bakar, Arabian J. Chem., 2022, 15, 103596 CrossRef CAS.
- D. Chouhan and P. Mandal, Int. J. Biol. Macromol., 2021, 166, 1554–1569 CrossRef CAS PubMed.
- O. Ejeromedoghene, A. Oladipo, O. Oderinde, E. S. Okeke, S. A. Amolegbe, T. M. Obuotor, C. A. Akinremi, S. Adewuyi and G. Fu, ACS Agric. Sci. Technol., 2021, 1, 664–673 CrossRef CAS.
- A. Roselyn Maheo, B. Scholastica Mary Vithiya, T. Augustine Arul Prasad, P. Tamizhdurai and V. L. Mangesh, Arabian J. Chem., 2022, 15, 103661 CrossRef CAS.
- E. Nourmohammadi, R. Kazemi Oskuee, L. Hasanzadeh, M. Mohajeri, A. Hashemzadeh, M. Rezayi and M. Darroudi, Ceram. Int., 2018, 44, 19570–19575 CrossRef CAS.
- M. K. Shukla, R. P. Singh, C. R. K. Reddy and B. Jha, Bioresour. Technol., 2012, 107, 295–300 CrossRef CAS PubMed.
- D. A. Lomelí-Rosales, A. Zamudio-Ojeda, S. A. Cortes-Llamas and G. Velázquez-Juárez, Sci. Rep., 2019, 9, 19327 CrossRef PubMed.
- R. F. Elsupikhe, K. Shameli, M. B. Ahmad, N. A. Ibrahim and N. Zainudin, Nanoscale Res. Lett., 2015, 10, 302 CrossRef PubMed.
- X. Chen, X. Zhao, Y. Gao, J. Yin, M. Bai and F. Wang, Mar. Drugs, 2018, 16(8), 277 CrossRef PubMed.
- S. Vijayakumar and B. Vaseeharan, Adv. Powder Technol., 2018, 29, 2331–2345 CrossRef CAS.
- S. Kamel and T. A. Khattab, Cellulose, 2021, 28, 4545–4574 CrossRef CAS.
- T. Li, S. Lü, Z. Wang, M. Huang, J. Yan and M. Liu, Sci. Total Environ., 2021, 765, 142745 CrossRef CAS PubMed.
- R. Kaur, S. K. Bhardwaj, S. Chandna, K.-H. Kim and J. Bhaumik, J. Cleaner Prod., 2021, 317, 128300 CrossRef CAS.
- Y. Huang, I. Mosleh and A. Abbaspourrad, Nano-Struct. Nano-Objects, 2022, 30, 100864 CrossRef CAS.
- M. Shin, S. Yang, H. W. Kwak and K. H. Lee, Eur. Polym. J., 2022, 164, 110960 CrossRef CAS.
- S. Xu, L. Yong and P. Wu, ACS Appl. Mater. Interfaces, 2013, 5, 654–662 CrossRef CAS PubMed.
- E. Lizundia, D. Puglia, T.-D. Nguyen and I. Armentano, Prog. Mater. Sci., 2020, 112, 100668 CrossRef CAS.
- M. Baláž, E. V. Boldyreva, D. Rybin, S. Pavlović, D. Rodríguez-Padrón, T. Mudrinić and R. Luque, Front. Bioeng. Biotechnol., 2021, 8, 612567 CrossRef PubMed.
- S. Sundar, J. Kundu and S. C. Kundu, Sci. Technol. Adv. Mater., 2010, 11, 14104 CrossRef PubMed.
- M. Yadav, N. Pareek and V. Vivekanand, in Nanobiotechnology for Plant Protection, ed. K. A. Abd-Elsalam, R. Periakaruppan and S. Rajeshkumar, Elsevier, 2022, pp. 259–280 Search PubMed.
- M. S. Hasanin, Starch – Stärke, 2021, 73, 2100055 CrossRef CAS.
- A. Balde, A. Hasan, I. Joshi and R. A. Nazeer, J. Air Waste Manage. Assoc., 2020, 70, 1227–1235 CrossRef CAS PubMed.
- C. Ferroni and G. Varchi, Appl. Sci., 2021, 11(20), 9417 CrossRef.
- G. V. Sree, P. Rajasekaran, O. Bazaka, I. Levchenko, K. Bazaka and M. Mandhakini, Carbon Trends, 2021, 5, 100083 CrossRef.
- I. Hassanin and A. Elzoghby, Cancer Drug Resist., 2020, 3, 930–946 CAS.
- M. Meyer, Biomed. Eng. Online, 2019, 18, 24 CrossRef PubMed.
- I. Sundaramurthy, G. Thiyagarajan, C. R. Panda and S. Sankar, Curr. Mater. Sci., 2021, 14, 80–92 CrossRef CAS.
- R. Reshmy, E. Philip, A. Madhavan, A. Tarfdar, R. Sindhu, P. Binod, R. Sirohi, M. Kumar Awasthi and A. Pandey, Fuel, 2022, 311, 122575 CrossRef CAS.
- A. do Espirito Santo Pereira, J. L. de Oliveira, S. M. Savassa, C. B. Rogério, G. Araujo de Medeiros and L. F. Fraceto, J. Cleaner Prod., 2022, 345, 131145 CrossRef.
- S. Mubarik, N. Qureshi, Z. Sattar, A. Shaheen, A. Kalsoom, M. Imran and F. Hanif, Nanomanufacturing, 2021, 1(3), 109–159 CrossRef.
- R. Del Sole, G. Mele, E. Bloise and L. Mergola, Polymers, 2021, 13(5), 2430 CrossRef CAS PubMed.
- B. H. Thippeswamy, A. S. Maligi and G. Hegde, Catalysis, 2021, 11(12), 1485 CAS.
- G. Lan, J. Yang, R.-P. Ye, Y. Boyjoo, J. Liang, X. Liu, Y. Li, J. Liu and K. Qian, Small Methods, 2021, 5, 2001250 CrossRef CAS PubMed.
- S. D. Lakshmi, P. K. Avti and G. Hegde, Nano-Struct. Nano-Objects, 2018, 16, 306–321 CrossRef CAS.
- L. Wang, Y. Guo, Y. Zhu, Y. Li, Y. Qu, C. Rong, X. Ma and Z. Wang, Bioresour. Technol., 2010, 101, 9807–9810 CrossRef CAS PubMed.
- M. T. Safian, U. S. Haron and M. N. M. Ibrahim, BioResources, 2020, 15, 9756–9785 Search PubMed.
- Z. Wang, D. Shen, C. Wu and S. Gu, Green Chem., 2018, 20, 5031–5057 RSC.
- G. Tatrari, M. Karakoti, C. Tewari, S. Pandey, B. S. Bohra, A. Dandapat and N. G. Sahoo, Mater. Adv., 2021, 2, 1454–1484 RSC.
- S. Sundriyal, V. Shrivastav, H. D. Pham, S. Mishra, A. Deep and D. P. Dubal, Resour., Conserv. Recycl., 2021, 169, 105548 CrossRef.
- Y. Yang, J. Cui, M. Zheng, C. Hu, S. Tan, Y. Xiao, Q. Yang and Y. Liu, Chem. Commun., 2012, 48, 380–382 RSC.
- P. Wang, L. Li, X. Pang, Y. Zhang, Y. Zhang, W.-F. Dong and R. Yan, RSC Adv., 2021, 11, 12015–12021 RSC.
- X. Liu, J. Pang, F. Xu and X. Zhang, Sci. Rep., 2016, 6, 31100 CrossRef CAS PubMed.
- A. Khan, M. Goepel, J. C. Colmenares and R. Gläser, ACS Sustainable Chem. Eng., 2020, 8, 4708–4727 CrossRef CAS.
- S. Liu, X. Wang, H. Zhao and W. Cai, Colloids Surf., A, 2015, 484, 386–393 CrossRef CAS.
- P. Chauhan, J. Saini and S. Chaudhary, Nano-Struct. Nano-Objects, 2020, 24, 100585 CrossRef CAS.
- H. E. Emam and H. B. Ahmed, Int. J. Biol. Macromol., 2021, 170, 688–700 CrossRef CAS PubMed.
- Y. Wang, Y. Liu, L. Zhao, L. Sun, X. Zhao and Y. Xia, J. Mater. Sci., 2021, 56, 1272–1285 CrossRef CAS.
- K. Subhani, X. Jin, N. Hameed, A. K. Lau, J. A. M. Ramshaw, V. Glattauer and N. V. Salim, Mater. Today Sustainability, 2022, 18, 100152 CrossRef.
- M. Ashokkumar, N. T. Narayanan, A. L. Mohana Reddy, B. K. Gupta, B. Chandrasekaran, S. Talapatra, P. M. Ajayan and P. Thanikaivelan, Green Chem., 2012, 14, 1689–1695 RSC.
- M. Ashokkumar, A. C. Chipara, N. T. Narayanan, A. Anumary, R. Sruthi, P. Thanikaivelan, R. Vajtai, S. A. Mani and P. M. Ajayan, ACS Appl. Mater. Interfaces, 2016, 8, 14836–14844 CrossRef PubMed.
- H. Wang, F. Fu, M. Huang, Y. Feng, D. Han, Y. Xi, W. Xiong, D. Yang and L. Niu, Nano Mater. Sci., 2022 DOI:10.1016/j.nanoms.2022.01.002.
- S. M. Ji and A. Kumar, Polymers, 2022, 14(1), 169 CrossRef CAS PubMed.
- W.-J. Chen, C.-X. Zhao, B.-Q. Li, T.-Q. Yuan and Q. Zhang, Green Chem., 2022, 24, 565–584 RSC.
- G. H. Lim, J.-W. Lee, J.-H. Choi, Y. C. Kang and K. C. Roh, Mater. Chem. Phys., 2022, 284, 126073 CrossRef CAS.
- Y. Song, N. Qi, K. Li, D. Cheng, D. Wang and Y. Li, RSC Adv., 2022, 12, 8108–8118 RSC.
- P. Sinha, A. Yadav, A. Tyagi, P. Paik, H. Yokoi, A. K. Naskar, T. Kuila and K. K. Kar, Carbon, 2020, 168, 419–438 CrossRef CAS.
- Z. Yang, T. Huang, P. Cao, Y. Cui, J. Nie, T. Chen, H. Yang, F. Wang and L. Sun, ACS Appl. Mater. Interfaces, 2022, 14, 18110–18119 CrossRef CAS PubMed.
- S. Seetharaman, J. Subramanian, R. A. Singh, W. L. Wong, M. L. Nai and M. Gupta, Technology, 2022, 10, 32 Search PubMed.
- O. O. Joseph and K. O. Babaremu, Fibers, 2019, 7(4), 33 CrossRef CAS.
- A. Bahrami, N. Soltani, M. I. Pech-Canul and C. A. Gutiérrez, Crit. Rev. Environ. Sci. Technol., 2016, 46, 143–208 CrossRef.
- Y. K. Chen, Y. Sun, K. Q. Wang, W. Y. Kuang, S. R. Yan, Z. H. Wang and H. S. Lee, Constr. Build. Mater., 2022, 325, 126220 CrossRef CAS.
- R. A. Mensah, V. Shanmugam, S. Narayanan, S. M. Razavi, A. Ulfberg, T. Blanksvärd, F. Sayahi, P. Simonsson, B. Reinke, M. Försth, G. Sas, D. Sas and O. Das, Sustainability, 2021, 13, 9336 CrossRef CAS.
- S. Gupta, S. Muthukrishnan and H. W. Kua, Constr. Build. Mater., 2021, 268, 121142 CrossRef CAS.
- R. Nisticò, L. Lavagna, D. Versaci, P. Ivanchenko and P. Benzi, Bol. Soc. Esp. Ceram. Vidrio, 2020, 59, 186–192 CrossRef.
- I. A. Cornejo, S. Ramalingam and J. S. Fish, Am. Ceram. Soc. Bull., 2014, 93, 24–27 CAS.
- R. Kothari, A. Singh, A. K. Pandey, V. V. Tyagi, D. Egamberdieva, S. D. Bellingrath-Kimura and N. K. Arora, Environ. Sustainability, 2021, 4, 199–200 CrossRef.
- J.-G. Rosenboom, R. Langer and G. Traverso, Nat. Rev. Mater., 2022, 7, 117–137 CrossRef PubMed.
- S. Agarwal, Macromol. Chem. Phys., 2020, 221, 2000017 CrossRef CAS.
- V. Siracusa, Polymers, 2019, 11, 1066 CrossRef CAS PubMed.
- S. De Gisi, G. Gadaleta, G. Gorrasi, F. P. La Mantia, M. Notarnicola and A. Sorrentino, J. Environ. Manage., 2022, 310, 114769 CrossRef CAS PubMed.
- G. Fredi and A. Dorigato, Adv. Ind. Eng. Polym. Res., 2021, 4, 159–177 CAS.
- E. Cadena, J. Colón, A. Artola, A. Sánchez and X. Font, Int. J. Life Cycle Assess., 2009, 14, 401–410 CrossRef CAS.
- A. Di Maria, J. Eyckmans and K. Van Acker, Waste Manage., 2018, 75, 3–21 CrossRef PubMed.
- J. B. Guinée, R. Heijungs, G. Huppes, A. Zamagni, P. Masoni, R. Buonamici, T. Ekvall and T. Rydberg, Environ. Sci. Technol., 2011, 45, 90–96 CrossRef PubMed.
- M. Iturrondobeitia, O. Akizu-Gardoki, R. Minguez and E. Lizundia, ACS Sustainable Chem. Eng., 2021, 9, 7139–7153 CrossRef CAS.
- M. A. J. Huijbregts, S. Hellweg, R. Frischknecht, H. W. M. Hendriks, K. Hungerbühler and A. J. Hendriks, Environ. Sci. Technol., 2010, 44, 2189–2196 CrossRef CAS PubMed.
- A. Corona, M. J. Biddy, D. R. Vardon, M. Birkved, M. Z. Hauschild and G. T. Beckham, Green Chem., 2018, 20, 3857–3866 RSC.
- F. Hermansson, M. Janssen and M. Svanström, Int. J. Life Cycle Assess., 2020, 25, 1620–1632 CrossRef.
- P. Yadav, D. Athanassiadis, I. Antonopoulou, U. Rova, P. Christakopoulos, M. Tysklind and L. Matsakas, J. Cleaner Prod., 2021, 279, 123515 CrossRef CAS.
- A. Alsabri and S. G. Al-Ghamdi, Energy Rep., 2020, 6, 364–370 CrossRef.
- K. W. Meereboer, M. Misra and A. K. Mohanty, Green Chem., 2020, 22, 5519–5558 RSC.
- C. Lopes, L. T. Antelo, A. Franco-Uría, A. A. Alonso and R. Pérez-Martín, Waste Manage., 2015, 46, 103–112 CrossRef CAS PubMed.
- X. García-Santiago, A. Franco-Uría, L. T. Antelo, J. A. Vázquez, R. Pérez-Martín, M. T. Moreira and G. Feijoo, J. Ind. Ecol., 2021, 25, 789–801 CrossRef.
- I. Leceta, P. Guerrero, S. Cabezudo and K. de la Caba, J. Cleaner Prod., 2013, 41, 312–318 CrossRef CAS.
- I. Leceta, A. Etxabide, S. Cabezudo, K. de la Caba and P. Guerrero, J. Cleaner Prod., 2014, 64, 218–227 CrossRef CAS.
- I. Muñoz, C. Rodríguez, D. Gillet and B. M. Moerschbacher, Int. J. Life Cycle Assess., 2018, 23, 1151–1160 CrossRef.
- M. L. M. Broeren, S. N. C. Dellaert, B. Cok, M. K. Patel, E. Worrell and L. Shen, J. Cleaner Prod., 2017, 149, 818–827 CrossRef.
- D. Koch, M. Paul, S. Beisl, A. Friedl and B. Mihalyi, J. Cleaner Prod., 2020, 245, 118760 CrossRef CAS.
- L. Sillero, A. Morales, R. Fernández-Marín, F. Hernández-Ramos, I. Dávila, X. Erdocia and J. Labidi, Sustainable Prod. Consum., 2021, 28, 749–759 CrossRef.
- Q. Li, S. McGinnis, C. Sydnor, A. Wong and S. Renneckar, ACS Sustainable Chem. Eng., 2013, 1, 919–928 CrossRef CAS.
- R. Arvidsson, D. Nguyen and M. Svanström, Environ. Sci. Technol., 2015, 49, 6881–6890 CrossRef CAS PubMed.
- J. Turk, P. Oven, I. Poljanšek, A. Lešek, F. Knez and K. Malovrh Rebec, J. Cleaner Prod., 2020, 247, 119107 CrossRef CAS.
- H. C. Kim and V. Fthenakis, J. Ind. Ecol., 2013, 17, 528–541 CrossRef CAS.
- F. Wu, Z. Zhou and A. L. Hicks, Environ. Sci. Technol., 2019, 53, 4078–4087 CrossRef CAS PubMed.
- F. Piccinno, R. Hischier, S. Seeger and C. Som, J. Cleaner Prod., 2018, 174, 283–295 CrossRef CAS.
- R. Katakojwala and S. V. Mohan, J. Cleaner Prod., 2020, 249, 119342 CrossRef CAS.
- R. M. Leão, P. C. Miléo, J. M. L. L. Maia and S. M. Luz, Carbohydr. Polym., 2017, 175, 518–529 CrossRef PubMed.
- F. Piccinno, R. Hischier, S. Seeger and C. Som, ACS Sustainable Chem. Eng., 2015, 3, 1047–1055 CrossRef CAS.
- M. C. B. de Figueirêdo, M. de F. Rosa, C. M. L. Ugaya, M. de S. M. de Souza Filho, A. C. C. da Silva Braid and L. F. L. de Melo, J. Cleaner Prod., 2012, 35, 130–139 CrossRef.
- D. LeCorre, C. Hohenthal, A. Dufresne and J. Bras, J. Polym. Environ., 2013, 21, 71–80 CrossRef CAS.
- L. Serrano-Luján, S. Víctor-Román, C. Toledo, O. Sanahuja-Parejo, A. E. Mansour, J. Abad, A. Amassian, A. M. Benito, W. K. Maser and A. Urbina, SN Appl. Sci., 2019, 1, 179 CrossRef.
- P. Caramazana-González, P. W. Dunne, M. Gimeno-Fabra, M. Zilka, M. Ticha, B. Stieberova, F. Freiberg, J. McKechnie and E. H. Lester, Green Chem., 2017, 19, 1536–1547 RSC.
- A. Heidari, E. Khaki, H. Younesi and H. R. Lu, J. Cleaner Prod., 2019, 241, 118394 CrossRef CAS.
- M. Lee, W.-S. Tsai and S.-T. Chen, J. Cleaner Prod., 2020, 265, 121845 CrossRef CAS.
- R. Vidal, P. Martínez and D. Garraín, Int. J. Life Cycle Assess., 2009, 14, 73–82 CrossRef CAS.
- X. Guo, Y. Yao, H. Zhao, C. Chi, F. Zeng, F. Qian, Z. Liu, L. Huo and Y. Lv, Sci. Total Environ., 2021, 788, 147852 CrossRef CAS PubMed.
- J. Hildebrandt, M. Budzinski, R. Nitzsche, A. Weber, A. Krombholz, D. Thrän and A. Bezama, Resour., Conserv. Recycl., 2019, 141, 455–464 CrossRef.
- G. Molins, M. D. Álvarez, N. Garrido, J. Macanás and F. Carrillo, J. Polym. Environ., 2018, 26, 873–884 CrossRef CAS.
- D. Tadele, P. Roy, F. Defersha, M. Misra and A. K. Mohanty, Clean Technol. Environ. Policy, 2020, 22, 639–649 CrossRef CAS.
- D. Ita-Nagy, I. Vázquez-Rowe, R. Kahhat, I. Quispe, G. Chinga-Carrasco, N. M. Clauser and M. C. Area, Sci. Total Environ., 2020, 720, 137586 CrossRef CAS PubMed.
- M. Casadesús, M. D. Álvarez, N. Garrido, G. Molins, J. Macanás, X. Colom, J. Cañavate and F. Carrillo, Resour., Conserv. Recycl., 2019, 149, 489–499 CrossRef.
- M. Hervy, S. Evangelisti, P. Lettieri and K.-Y. Lee, Compos. Sci. Technol., 2015, 118, 154–162 CrossRef CAS.
- G. Espadas-Aldana, P. Guaygua-Amaguaña, C. Vialle, J.-P. Belaud, P. Evon and C. Sablayrolles, Coatings, 2021, 11(5), 525 CrossRef CAS.
- A. Nawirska and M. Kwaśniewska, Food Chem., 2005, 91, 221–225 CrossRef CAS.
- K. C. Teh, C. Y. Tan and I. M. L. Chew, J. Environ. Chem. Eng., 2021, 9, 105381 CrossRef CAS.
- Y. Liu, Y. Nie, X. Lu, X. Zhang, H. He, F. Pan, L. Zhou, X. Liu, X. Ji and S. Zhang, Green Chem., 2019, 21, 3499–3535 RSC.
- S. Cobo, A. Dominguez-Ramos and A. Irabien, ACS Sustainable Chem. Eng., 2018, 6, 3493–3501 CrossRef CAS.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC.
- O. Alonso-López, S. López-Ibáñez and R. Beiras, Polymers, 2021, 13, 3742 CrossRef PubMed.
- L. Zimmermann, A. Dombrowski, C. Völker and M. Wagner, Environ. Int., 2020, 145, 106066 CrossRef CAS PubMed.
- C. Zhou and Y. Wang, Sci. Technol. Adv. Mater., 2020, 21, 787–804 CrossRef CAS PubMed.
- M. L. Astolfi, E. Marconi, L. Lorini, F. Valentino, F. Silva, B. S. Ferreira, S. Canepari and M. Majone, Chemosphere, 2020, 259, 127472 CrossRef CAS PubMed.
- L. Zhi, R. Zhipeng, L. Minglong, B. Rongjun, L. Xiaoyu, L. Haifei, C. Kun, Z. Xuhui, Z. Jufeng, L. Lianqing, D. Marios, J. Stephen, I. Natarjan and P. Genxing, J. Cleaner Prod., 2020, 276, 124208 CrossRef CAS PubMed.
- A. Schüch, G. Morscheck and M. Nelles, Technological Options for Biogenic Waste and Residues—Overview of Current Solutions and DevelopmentsSpringer Singapore, ed. S. K. Ghosh, Singapore, 2019, pp. 307–322 Search PubMed.
- F. Fava, G. Totaro, L. Diels, M. Reis, J. Duarte, O. B. Carioca, H. M. Poggi-Varaldo and B. S. Ferreira, New Biotechnol., 2015, 32, 100–108 CrossRef CAS PubMed.
- https://www.bbeu.org/what-we-can-achieve-together/testimonials-and-collaboration-results .
- T. Huq, A. Khan, D. Brown, N. Dhayagude, Z. He and Y. Ni, J. Bioresour. Bioprod., 2022, 7, 85–98 CrossRef CAS.
- V. Rudovica, et al. , Front. Mar. Sci., 2021, 8, 723333 CrossRef.
- E. Montoneri, Municipal Waste Treatment, Technological Scale up and Commercial Exploitation: The Case of Bio-waste Lignin to Soluble Lignin-like Polymers, ed. P. Morone, F. Papendiek and V. E. Tartiu, Springer International Publishing, Cham, 2017, pp. 79–120 Search PubMed.
- A. Bianchi, V. Sanz, H. Domínguez and M. D. Torres, Food Hydrocolloids, 2022, 122, 107070 CrossRef CAS.
- S. Timorshina, E. Popova and A. Osmolovskiy, Polymers, 2022, 14(8), 1601 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |