Catalyst-free dynamic transesterification towards a high-performance and fire-safe epoxy vitrimer and its carbon fiber composite†
Abstract
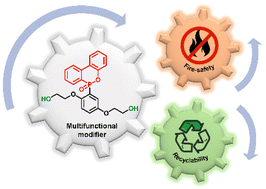
The preparation of fire-safe and high-performance carbon fiber reinforced composites with reprocessable resin matrix and recyclable reinforced fibers is of great importance and particularly urgent to solve the current overreliance on petrochemical resources for composite raw materials. Herein, we propose an effective strategy to simultaneously solve the flammability and nonrecyclability of the epoxy resin-based composites, in the form of fire-safe and catalyst-free dynamic transesterification networks by introducing a phosphaphenanthrene-derived diol as a multifunctional modifier for transesterification without using any toxic catalysts. In this strategy, the phosphaphenanthrene moieties enhanced the fire safety; while the hydroxy groups promoted the construction of catalyst-free dynamic transesterification networks. To our delight, the generated epoxy resin and its composite exhibit excellent mechanical properties, high thermal stability, fire safety, fast repairability and malleability. Furthermore, the resin matrix can be dissolved as the low-mass diol molecules participate in bond exchange reactions to achieve CFs with nearly 100% recyclability.
- This article is part of the themed collection: Sustainable Composites


 Please wait while we load your content...
Please wait while we load your content...