Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Solvent design for catalyst recycling of rhodium/amine catalysts via scCO2 extraction in the reductive hydroformylation of alpha olefins†
T.
Rösler
 a,
J.
Betting
a,
J.
Betting
 a,
S.
Püschel
a,
S.
Püschel
 a,
A. J.
Vorholt
a,
A. J.
Vorholt
 *a and
W.
Leitner
*a and
W.
Leitner
 *ab
*ab
aMax Planck Institute for Chemical Energy Conversion, Stiftstraße 34, 45470 Mülheim an der Ruhr, Germany. E-mail: j.vorholt@cec.mpg.de
bRWTH Aachen University, Worringerweg 2, 52074 Aachen, Germany
First published on 30th July 2022
Abstract
Efficient transformation protocols to directly convert olefins to alcohols are highly sought after. Ethylene glycol based solvents have proved to support the reductive hydroformylation of linear alpha olefins to alcohols using [Rh(acac)(CO)2] in combination with tertiary amines as catalysts. Incorporation of the amine functionality into the solvent using 2-[2-(dimethylamino)ethoxy]ethanol allowed simplification of the reaction system to three components and a catalytic activity with a TOF of 280 h−1 for the reductive hydroformylation of 1-octene to be achieved. To immobilize the rhodium catalyst in a recycling approach using scCO2 as the extracting solvent for product alcohols, amine functionalized PEG derivatives have been synthesized as the stationary catalyst phase. Amide condensation of PEG600-diacid with trimethyldiaminoethane resulted in a diaminated PEG600 in which the amine group is linked via an amide bridge to the PEG600 backbone. During nine consecutive runs, in which this PEG600-diamine was used as the stationary catalyst phase and product alcohols have been extracted with scCO2, no loss in activity or selectivity was observed. Leaching of the stationary phase was ≤3 wt% (≤0.5 wt% after the first two runs) of the extracted mass per run and rhodium leaching was determined to be 0.1% of the initial rhodium over all nine runs combined.
Introduction
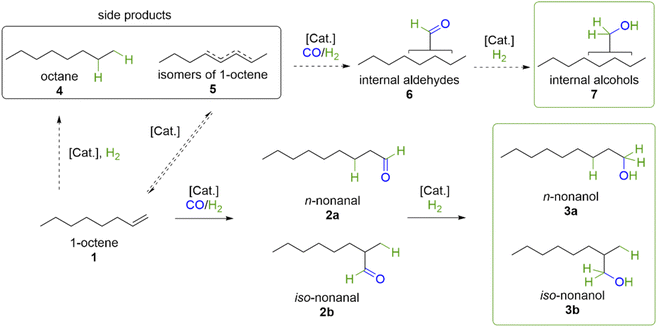
Alcohols are important products in the current chemical value chain with direct application as solvents, disinfectants or as intermediates for a variety of chemicals like detergents or polymers.1 Additionally, the high interest in alcohols as fuel additives in bio-synthetic fuels, e.g. in PtX and BtL concepts,2–4 during recent years makes highly efficient protocols for their production pressing. With the Fischer–Tropsch synthesis being a centre piece in a lot of those concepts, the transformation of middle to long chain olefins to alcohols is of special interest.5–7 Currently, middle to long chain alcohols are commonly produced from olefins via a sequence of hydroformylation and hydrogenation reactions (Fig. 1, upper pathway).A beneficial alternative from an economic and ecological point of view would be the tandem catalysed reductive hydroformylation to directly yield alcohols from olefins (Fig. 1, lower pathway), thus making the intermediate separation and purification steps obsolete. Since the energy intensive cobalt catalysed processes going back to the 1960s,8,9 no one-step process which can compete with the current two step processes has made it to application though.10
An alternative to the classic phosphine based catalytic systems is the combination of rhodium and tertiary amines as a catalyst.11,12 Although known since the 1970s, new interest in these types of catalysts has sparked during recent years.13–17 In 2021, we reported about a highly robust catalytic system which utilizes rhodium as the catalyst in combination with tertiary amines as additives for the reductive hydroformylation of 1-octene. Tertiary amines supposedly bind to rhodium as ligands during the catalytic cycle.18 Even though the catalytic system proved to be active, stable against oxygen and, with tertiary amines being resistant towards most decomposition pathways known to other ligand classes,19 concepts for the recycling and separation of those catalysts from alcohols have not been reported until just recently. Because of their surfactant like nature and their strong interaction via hydrogen bonding, separation of alcohols from product mixtures is oftentimes challenging and resource intensive.20
In 2022, our group reported about the catalyst recycling in a liquid/liquid biphasic reaction system for the reductive hydroformylation of linear olefins and olefinic mixtures in which the rhodium catalyst is immobilized in a water phase by employing water-soluble alkanolamines.21 Although the concept proved to be feasible, careful adjustment and control of the mixture composition are necessary and leaching of the alkanolamines into the product phase should be reduced. More recently, we also described the use of a CO2 switchable rhodium/tertiary amine catalyst, in which the separation of the catalyst was achieved using an additional feature of alkanolamines – the ability to bind CO2 and the resulting polarity change, inducing a second liquid phase that contained the products. In this case, the formation of carbamates and therefore the catalyst leaching is closely linked to the CO2 concentration. It was found that the catalyst is deactivated at higher CO2 concentrations and a trade-off between catalytic activity and rhodium leaching has to be made.22
In order to develop processes for the one-step reductive hydroformylation relevant for application, not only active catalytic systems but efficient strategies for the recycling of the homogeneous catalyst are necessary which allow for minimal leaching of the rhodium catalyst in combination with highly pure product streams. In particular, with the rising importance of alcohols as fuels from bio-synthetic value chains, future processes need to be particularly resource efficient to be economically feasible.
In this regard, the use of supercritical CO2 (scCO2) as the extraction agent for product alcohols is a promising approach to recycle the catalyst. By using scCO2 for the extraction of reaction products, a non-toxic, non-flammable and abundant resource is used which can be separated from the product stream afterwards simply by degassing and returning CO2 into its gaseous form.23,24 Thus, it allows for a highly efficient separation protocol which makes further downstream processing unnecessary as long as leaching of the polar phase is circumvented. Supercritical scCO2 as the extraction agent is already being applied with big success for the extraction of commonly non-polar components from a polar stationary phase on an industrial level, e.g. in the decaffeination of coffee beans.25,26 Furthermore, recycling of homogeneous catalysts using scCO2 phases is described for several reactions such as hydrogenation,27–35 oxidation,36 hydrovinylation,37 metathesis38,39 and hydroformylation40–46 reactions. An impressive example of the potential of this recycling approach was given by D. J. Cole Hamilton and his group in the hydroformylation of 1-octene using a sulfonated phosphorus catalyst immobilized in an ionic liquid phase. In a continuous setup in which the product aldehydes have been extracted under scCO2 flow, 30 h of steady operation with <1 ppm of rhodium leaching has been achieved.40,41 Besides ionic liquids and water, polyethyleneglycol (PEG) is often used as stationary phase in combination with scCO2. Polyethyleneglycol shows good solubility for most catalysts and gases but, depending on the chain length, limited solubility with scCO2. For example, in an innovative approach by our group, phosphorus ligands, used in combination with rhodium for the hydroformylation of various olefins, have been covalently bound to PEG750 and used as the stationary catalyst phase for the extraction of product aldehydes using scCO2. Using this approach, nine consecutive runs with a total-turnover-number (TTON) of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 have been accomplished.42 Although the extraction of aldehydes is described, to the best of our knowledge the use of supercritical scCO2 for the extraction of alcohols in the reductive hydroformylation reaction has not been investigated so far.
000 have been accomplished.42 Although the extraction of aldehydes is described, to the best of our knowledge the use of supercritical scCO2 for the extraction of alcohols in the reductive hydroformylation reaction has not been investigated so far.
Herein we describe the functionalization of various high molecular polyethylene glycol (PEG) derivatives with tertiary amine groups. Those novel PEG-amines fulfil the bifunctional role of ligand and solvent at the same time and serve as the stationary phase for the said separation technique of product alcohols from the rhodium/amine catalyst by extraction with supercritical CO2.
Results and discussion
Previously we reported about a highly active and selective catalytic system based on a rhodium catalyst in combination with various low molecular tertiary organic alkylamines as additives in the reductive hydroformylation of 1-octene.18Fig. 2 shows the reaction sequence and possible side products for the reductive hydroformylation of 1-octene as an example. In particular, the low selectivity towards the concurrent hydrogenation of initial olefin (4) makes the reaction system highly selective towards alcohols. The main by-products have been found to be isomeric alkenes (5) which are ultimately converted to branched alcohols (7).Acetonitrile and triethylene glycol (TEG) have been used as solvents for the reaction in our previous works. In this work we elaborate on those findings with an extensive screening of solvents with a focus on high molecular weight and ethylene glycol based solvents. The aim of this study was to investigate the effect of different functional groups in the solvent and to find environmentally benign solvents for possible catalyst recycling strategies. To test for this, each solvent was applied in the reductive hydroformylation of 1-pentene catalysed by [Rh(acac)(CO)2] in combination with methyldiethylamine, which proved to be a highly active catalytic system during previous investigations.18 Additionally, the Hansen solubility parameters (HSP = dispersion forces (δD); dipole–dipole interactions (δP); hydrogen-bonding-contribution (δH)) as a more quantitative way to describe solvent properties have been determined for each solvent. In Fig. 3, the total alcohol yield (sum of linear and branched alcohols), as a measure to display the catalytic activity, for important findings during this extensive solvent screening is displayed. The full screening for the total of 23 solvents, their corresponding Hansen solubility parameters and the resulting 3D-Hansen-space can be found in the ESI (Table S1 and Fig. S1†).
 | ||
| Fig. 3 Screening of benign solvents in the reductive hydroformylation of 1-pentene. Yalc.: combined yield of linear and branched alcohols. | ||
During the screening a variety of different ethylene glycol based solvents have been tested (Fig. 3, 1–6). Ethylene glycol based solvents are non-toxic, non-flammable and promising downstream products from renewables and therefore ideal candidates for environmentally sound high molecular weight solvents.47 Similar to triethylene glycol (1, Yalc. = 60%), the higher molecular weight polyethylene glycol mixture of PEG300 (2) (average MW = 300 g mol−1) resulted in high alcohol yields of 59%. While the functionalization of the terminal alcohols with ester groups (5, Yalc. = 55%) does not significantly reduce the hydrogenation activity, the stepwise addition of nonpolar groups in the examples of tripropylene glycol (3, Yalc. = 48%) and diethylene glycol monohexyl ether (4, Yalc. = 39%) leads to a decrease in the catalytic hydrogenation activity. Overall ethylene glycol based solvents seem to support a high hydrogenation activity for a broad range of molecular weight distributions and functional groups. Besides ethylene glycol based solvents, various other functional groups have been tested. Promising results have been achieved in the case of nitriles (6, Yalc. = 74%), phthalates (7, Yalc. = 63%) and other alcohols (ESI S1†). Overall, the incorporation of polar functional groups especially seems to play a crucial role for the hydrogenation step. This is supported by the results of various nonpolar solvents showing low to no hydrogenation activity (ESI S1†). The formation of polar rhodium cluster species in the presence of amines for the reductive hydroformylation of 1-octene has been reported by our group previously.18 It can be assumed that polar functional groups are necessary to stabilize those ionic species which are supposedly important intermediates for the active hydrogenation species. The promising results and acceptance of functional groups for ethylene glycol based solvents gave us the idea to test commercially available amine functionalized 2-[2-(dimethylamino)ethoxy]ethanol (DEG-amine) (9) as the solvent and without the addition of another amine source during the reaction. Also, in this case a high hydrogenation activity and selectivity towards alcohols with a yield of 60% was achieved (Fig. 3).
By incorporation of the amine functionality into the solvent at the example of 2-[2-(dimethylamino)ethoxyethanol (DEG-amine) the reaction system was reduced to only three initial components with [Rh(acac)(CO)2], the amine/solvent and the olefin. This not only reduces the complexity of the reaction system but also simplifies possible catalyst recycling strategies and downstream processing by inherent immobilization of the amine ligand and possibly immobilization of the rhodium catalyst by coordinative or ionic binding to the amine. After a brief investigation of the reaction system using DEG-amine as the solvent/ligand, it was possible to increase the substrate concentration from 5 wt% to 40 wt% without any loss of catalytic activity (ESI S2†). Investigation of the reaction time profile during the conversion of 1-octene (Fig. 4) showed that after 30 minutes 73% alcohols are generated which corresponds to a catalytic activity with a turnover frequency (TOF) of 280 h−1. Aldehyde intermediates in significant amounts can only be observed even within the first 20 minutes of the reaction, illustrating the high hydrogenation activity of the system. After 30 minutes, the full conversion (>99%) of the initial 1-octene is achieved. At this point, besides alcohol products, only octene isomers (20%), formed by the β-hydride transfer of 1-octene, and octane (2%) derived from hydrogenation of 1-octene are observed. The conversion of internal double bonds proceeds with a lower activity. Hence, octene isomers are converted at a much slower pace afterwards. After 3 hours, an alcohol yield of 92% is achieved with 4% of unconverted octene isomers left.
The high catalytic activity when using a combination of a tertiary amine group with the polar ethylene glycol backbone in the example of DEG-amine as the solvent for the reaction made us think about catalyst recycling strategies. Incorporation of the amine functionality into polar solvents makes an effective immobilization of the rhodium catalyst possible. Ethylene glycol based solvents are known to be near insoluble with supercritical CO2 (scCO2). Hence, we decided to start investigating the extraction of product alcohols with scCO2 from amine functionalized (poly)ethylene glycol derivatives.
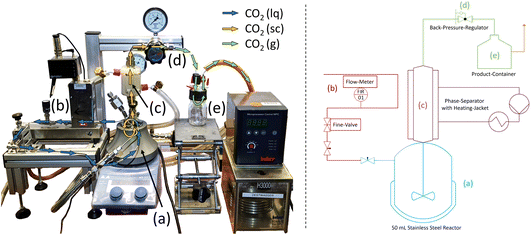
To test this, a setup which allows for a continuous scCO2 flow at a high pressure and temperature was developed. The setup consists of a high-pressure stainless-steel reactor with a volume of 50 mL (a). To the reactor a liquid CO2 line equipped with a flow meter (b) is connected (Fig. 5). During the extraction, a pressure of 95 bars is held at a temperature of 50 °C within the reactor to ensure the supercritical state of CO2. At the backend of the reactor, a 1/2-inch pipe with a volume of 24 cm3 and a 3D-printed heating jacket is used as phase settler unit. The phase settler is heated using a hot water pump to ensure full phase separation of scCO2 and the stationary phase (c). Finally, scCO2 is released at a back-pressure-regulator (d). At this point phase transition of scCO2 to its gaseous form occurs and all substances dissolved in the scCO2 phase are released to the product container (e).
Preliminary tests of the extraction setup during the extraction of 1-hexanol dissolved in DEG-amine, showed that in this case no efficient separation of scCO2 and DEG-amine is achieved and alcohols as well as the solvent are extracted in their corresponding composition (ESI S3†). Further preliminary tests in which hexanol was extracted from a higher molecular glycol, PEG200, using scCO2 showed that hexanol can be extracted with basically no solvent leaching into the scCO2 phase (ESI S3†). This shows that the setup is suited for the general concept but successful catalyst recycling is only possible using higher molecular mass analogues compared to DEG-amine. In general, the solubility in scCO2 decreases with increasing molecularity and/or polarity.
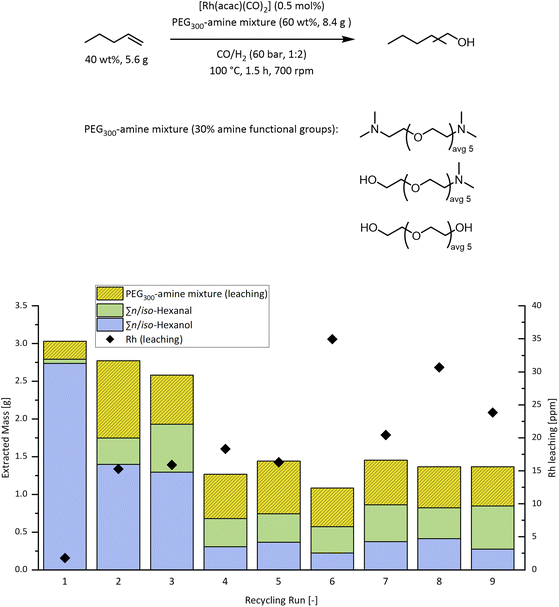
In an attempt to synthesize high molecular mass analogues to DEG-amine, different strategies for the amination of PEG300 have been investigated (detailed experimental procedures are given in the ESI S4†). Alcohol amination, using a ruthenium catalyst with dimethylammonium dimethylcarbamate as the amination agent, as reported by Seidensticker et al.,48 resulted in isolated yields of 50% with a mixture of mono- and di-aminated PEG300-dimethylamine (Fig. 6). The degree of amination can be effectively controlled from 30% after 24 h to approximately full amination after 48 h as shown by atmospheric-pressure-chemical-ionization mass-spectrometry (APCI-MS) of the product mixtures (ESI S5†). However, even when using prolonged reaction times PEG-amine moieties with monomethylated amine groups have been found as side products (<5%). This happens most likely due to the alkyl shift reactions between dimethylamine and PEG300-dimethylamine. Using this strategy, amine derivatives of PEG300 with an amination degree of 30% and full amination have been synthesized.
 | ||
| Fig. 6 Synthesis of dimethylamine functionalized PEG300-amine mixtures via alcohol amination as reported by Seidensticker et al.48 | ||
To investigate the catalytic activity when using those novel solvents, they have been applied in the reductive hydroformylation of 1-octene first on a small scale and under optimized conditions (Fig. 7). For comparison, the reactivity of the previously investigated low molecular weight DEG-amine is displayed (Fig. 7, (IV)). When PEG300 with a degree of amination >98% is used (Fig. 7, (I)), the hydrogenation activity of the system is significantly decreased. In comparison with DEG-amine (Fig. 7, (IV)), the alcohol yield drops from 79% to 20%. The main products in this case are intermediate aldehydes (62%), which proves that the overall hydroformylation activity is not affected but rather the hydrogenation step is inhibited. In this case, the lack of terminal alcohol groups seems to significantly affect the hydrogenation activity. This was confirmed in experiments in which unmodified PEG300 was added to PEG300-diamine (Fig. 7 (II)) or in which PEG300 with a lower amination degree of 30% is used as solvent (Fig. 7 (III)). In this case, the hydrogenation activity was restored and alcohol yields of 78% and 82% respectively are achieved.
These results, again, highlight the necessity of polar functional groups in the solvent to preserve the hydrogenation activity. Consequently, because of the high catalytic activity achieved when using the PEG300 mixture with an amination degree of 30%, it was chosen as the stationary catalyst phase in a first attempt to recycle the rhodium catalyst.
Using the setup described above (Fig. 5), a consecutive series of nine recycling runs has been accomplished for the reductive hydroformylation of 1-pentene using the 30% aminated PEG300 mixture as the stationary phase catalyst phase. A simplified scheme for the execution of the experiments is displayed in Fig. 8.
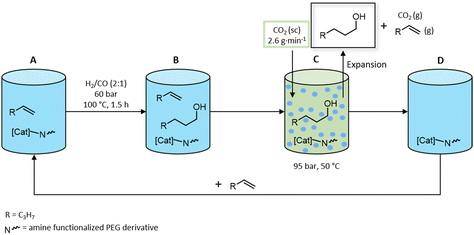 | ||
| Fig. 8 Conceptual scheme for recycling experiments. (A) Initial reaction mixture, (B) post reaction solution, (C) scCO2 extraction, (D) addition of fresh 1-pentene. | ||
Each reaction was conducted under the optimized conditions in a 50 mL autoclave reactor. After each reaction, the autoclave reactor was connected to the scCO2 extraction setup (Fig. 5) and the extraction of product alcohols with a continuous scCO2 flow of 2.6 g min−1 at T = 50 °C and p = 95 bar was conducted until 200 g of scCO2 (measured by a flow meter) have passed the reactor. After the extraction, fresh 1-pentene is added for the next reaction. Because sampling of the catalyst phase would alter all following runs, only the extracted mixture has been analysed for total weight, composition (determined via quantitative proton nuclear-magnetic-resonance spectroscopy (1H-qNMR)) and rhodium leaching (determined via inductively coupled- plasma mass spectrometry (ICP-MS)) (Fig. 9). Because of the low boiling point of 1-pentene, all non-converted olefins evaporate during the extraction process and are not condensed in the product container.
After the extraction of the initial run, a total mass of 3.03 g with an alcohol concentration of 91 wt%, corresponding to 27.0 mmol of the reaction product is obtained. Compared to the 79.9 mmol of substrate (1-pentene), this corresponds to a theoretical alcohol yield of 36%. Intermediate aldehydes are found in traces (<1 wt%), suggesting a high hydrogenation activity. The remaining 8 wt% of the mixture was found to consist of PEG300-amine mixture. Analysis of the extracted PEG300 fractions using APCI-MS (ESI S5 and S6†) showed that the extracted fractions are almost identical to the initial composition and no preference for molecules with a lower molecular weight or less polar molecules with a higher degree of amination is observed. This is especially interesting since during preliminary experiments, in which 1-hexanol was extracted from PEG200, almost no leaching (<1 wt%) of the stationary phase was observed at all (ESI S3†). It seems that the average lower polarity of the stationary phase due to exchange of hydroxy with tertiary amine groups allows to pull even di-hydroxylated PEG300 molecules into the scCO2 phase which have been proved to be basically insoluble in scCO2 before. Despite the slight leaching of the stationary PEG300-amine (30% amination) phase, ICP-MS measurements showed a low rhodium leaching of 1.8 ppm (0.01% of initial [Rh]) for the first run.
After the second run, the total amount of the extract decreases slightly (2.78 g) but an analysis of the composition revealed a significant drop in the hydrogenation activity, together with an about three-fold increase in the leaching of the stationary PEG300-amine phase. During the third run only 50% of the extracted compounds can be attributed to target alcohols, while 13 wt% aldehydes and >37 wt% leaching of the PEG300-amine mixture is observed. The drop in the hydrogenation activity can be partially attributed to the leaching of the stationary phase, but also suggests a deactivating mechanism for the catalytic species responsible for the hydrogenation step after the first run. In accordance with the increased leaching of the stationary phase, an increased leaching of the rhodium catalyst >15 ppm (0.1% of initial [Rh]) is observed.
From run four to run eight, the activity remains constant, although only about 25 wt% of the product mixture consists of target alcohols and the leaching of the PEG300-amine mixture is still high (45 wt%–50 wt%). In the last run, a further drop in the hydrogenation activity was observed and only 0.26 g of alcohols was extracted, suggesting that the constant leaching of stationary phase will ultimately abolish the reaction. After the ninth run, only ∼3.6 g of the initial 8.4 g PEG300-amine mixture was found left in the reactor. This first recycling attempt proves that the exchange of hydroxy with tertiary amine groups significantly increases the solubility of PEG derivatives in scCO2. Even though, the low rhodium leaching (<1% of initial [Rh] accumulated over nine runs) and the still active catalyst until the last run show that the recycling concept is generally feasible and no continuous deactivation or precipitation of the [Rh] catalyst occurs.
From this first attempt we concluded that the decreasing polarity due to tertiary amine functional groups requires further modifications of the aminated solvent/ligand. Based on the general trends to decrease the solubility in scCO2 and improve the retention of the stationary catalyst phase, potential modifications for the aminated polyethylene glycol must be
(1) a higher molecular weight and
(2) a higher overall polarity by the incorporation of polar groups.
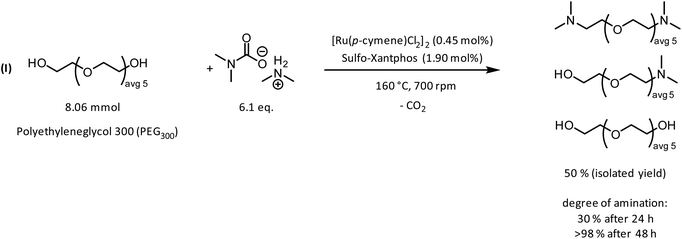
To achieve this, synthetic strategies for the functionalization of PEG600 have been considered. PEG600 offers a two-fold increase of the average molecular weight but is still a liquid at room temperature. Additionally, to add polar functional groups, amide condensation of PEG600-diacid with trimethyldiaminoethane resulted in an amine modified PEG600-derivative containing polar amide functional groups in the PEG backbone (Fig. 10). The reaction proved to be highly efficient and quantitative yields can be obtained after 48 h with a degree of amination >98% as proved by 1H-NMR. Since no catalyst or additional solvent is needed during the reaction, the only purification step is the removal of water, which is formed during the reaction, and excess trimethyldiaminoethane under reduced pressure.
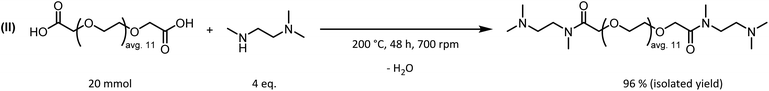 | ||
| Fig. 10 Procedure for the synthesis of dimethylamine functionalized PEG600 derivatives via amide condensation containing incorporated amide functionalities. | ||
Investigation of the diaminated PEG600-amide in the reductive hydroformylation of 1-octene (Fig. 7 (V)) showed that excellent hydrogenation activity is achieved. After 1.5 hours, 75% yield of alcohols is generated and no intermediate aldehydes in significant amounts have been found. Comparing this to the low hydrogenation activity when using the diaminated PEG300 as solvent (Fig. 7 (I)), this shows that in case of the PEG600-diamine as solvent no additional hydroxy groups are necessary to achieve a high hydrogenation activity, likely due to the incorporation of polar amide functional groups. After a reassuringly high activity for the reductive hydroformylation reaction, the diamine functionalized PEG600-amide was tested as the stationary catalyst phase during nine consecutive recycling runs for the reductive hydroformylation of 1-pentene following the same procedure described previously (Fig. 11). As only alterations, the product concentration was decreased from 40% to 30% to account for the increased molecular weight of the stationary phase and to keep a high ratio of the substrate to amine groups. Additionally, the total amount of CO2 used for the extraction was lowered from 200 g to 150 g. Tracking the amount of CO2 consumed during the extraction for the first recycling run and comparing it with the extracted mass shows that the amount of extract gained per gram of consumed CO2 drops off drastically with increasing CO2 consumption (ESI S7†). After 150 g of CO2 are consumed, the extraction efficiency dropped to 1/5th of the initial value. Since the efficiency with which products get extracted from the reaction solution is linked to the product concentration it can be assumed that at this point the majority of the products have been extracted already.
This second approach proved to be highly successful. During all nine consecutive runs, the total weight of the extract was found to be between 1.6 g and 3.1 g. Analysis of the collected mixture proves that a high hydrogenation activity is maintained during all nine runs with alcohols contributing between 75 wt% and 92 wt%, on average 83 wt%, to the extracted mixture (corresponding to alcohol yields between 25% and 45% under the assumption of complete extraction). Aldehyde intermediates make up for almost all of the rest of the extracted compounds (8 wt%–24 wt% of the extract). Gas chromatography of the exhaust gas stream of the product container shows that full condensation of alcohols and aldehydes is achieved and no reaction products are lost within the exhaust gas (ESI S8†). The consistently high hydroformylation and hydrogenation activity proves that no deactivation of the catalyst occurred over the course of the experiment. Leaching of the stationary PEG600-diamine phase was found to be ≤3 wt% of the extracted mass in the first two runs and ≤0.5 wt% in all consecutive runs. Rhodium leaching was found to be <6 ppm (<0.02% of initial [Rh] in all runs with the exception of run five) with an average of ≈3 ppm. Over the course of all nine runs, 0.1% of the initial rhodium catalyst has been transferred out of the reactor.
All in all, the PEG600-diamine proved to be a highly effective stationary phase for the extraction of alcohols using scCO2. Over all nine runs a total turnover number (TTON = ∑nproduct/ncatalyst) of 700 was achieved. Comparison with the recycling approach using the PEG300-amine mixture shows that a high molecular weight but more importantly incorporation of polar functional groups are key to achieving a high hydrogenation activity during the reaction and insolubility in scCO2 at the same time. It was shown that the herein presented high molecular weight amines propose highly stable, non-volatile, non-flammable and easily accessible reaction media for rhodium based reductive hydroformylation catalysts. During the extraction of product alcohols and aldehydes, abundant CO2 is used as an extraction medium and the only “chemical” which can be separated easily from the products by simply degassing as shown during the recycling experiments. Hence, no further, and likely energy intense, downstream processing is necessary.
Conclusion
We herein described the successful synthesis of amine functionalized polyethylene glycol derivatives and their application as a combination of solvent and ligand for the rhodium catalysed reductive hydroformylation of linear alpha olefins. It was found that 2-[2-(dimethylamino)ethoxy]ethanol can be used as a combination of solvent and amine (ligand) in the reductive hydroformylation of 1-alkenes resulting in a highly active catalytic system. Using 2-[2-(dimethylamino)ethoxy]ethanol as an inspiration, higher molecular weight and amine modified poly ethylene glycol derivatives have been synthesized as the stationary phase for a recycling strategy based on the extraction of product alcohols with scCO2. While an amine modified PEG300-mixture did not allow for a successful separation of the product and catalyst phase, the application of a dimethylamine functionalized PEG600 derivative, containing polar amide groups in its backbone, proved to be much more successful as the stationary phase during this recycling approach. Minimal leaching of the stationary PEG-amine phase and the rhodium catalyst (0.1% for all runs) allowed the recycling of the catalyst over nine consecutive runs with no deactivation or change in selectivity. All in all, a powerful and robust catalytic system with a promising recycling strategy is presented comprising just two components, a rhodium catalyst and an amine functionalized PEG derivative as the solvent/ligand.Author Contributions
T. Rösler: investigation; methodology; writing – original draft; J. Betting: investigation; methodology; S. Püschel: investigation, methodology; A. J. Vorholt: methodology, supervision, writing – review and editing; W. Leitner: conceptualization, supervision, writing – review and editing.Conflicts of interest
The authors hereby confirm that there is no conflict of interest to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under Grant Agreement no. 817612. Open Access funding provided by the Max Planck Society.References
- S. Gehrmann and N. Tenhumberg, Chem. Ing. Tech., 2020, 92, 1444–1458 CrossRef CAS.
- B. Rajesh Kumar and S. Saravanan, Renewable Sustainable Energy Rev., 2016, 60, 84–115 CrossRef CAS.
- L. Cai, Y. Uygun, C. Togbé, H. Pitsch, H. Olivier, P. Dagaut and S. M. Sarathy, Proc. Combust. Inst., 2015, 35, 419–427 CrossRef CAS.
- B. Kerschgens, L. Cai, H. Pitsch, B. Heuser and S. Pischinger, Combust. Flame, 2016, 163, 66–78 CrossRef CAS.
- V. Dieterich, A. Buttler, A. Hanel, H. Spliethoff and S. Fendt, Energy Environ. Sci., 2020, 13, 3207–3252 RSC.
- J. Hu, F. Yu and Y. Lu, Catalysts, 2012, 2, 303–326 CrossRef CAS.
- B. Heuser, A. Vorholt, G. Prieto, B. Graziano, S. Schönfeld, M. Messagie, G. Cardellini, S. Tuomi, N. Sittinger and R. Hermanns, in transport Research Arena 2020, Helsinki, Finland, 2020 Search PubMed.
- F. E. Paulik, Catal. Rev., 1972, 6, 49–84 CrossRef CAS.
- J. L. Van Winkle, S. Lorenzo, R. C. Morris and R. F. Mason, US3440291, 1969.
- G. M. Torres, R. Frauenlob, R. Franke and A. Börner, Catal. Sci. Technol., 2015, 5, 34–54 RSC.
- B. Fell and A. Geurts, Chem. Ing. Tech., 1972, 44, 708–712 CrossRef CAS.
- A. T. Jurewicz, L. D. Rollmann and D. D. Whitehurst, in American Chemical Society Advances in Chemistry, 1974, pp. 240–251 Search PubMed.
- S. Fuchs, D. Lichte, M. Dittmar, G. Meier, H. Strutz, A. Behr and A. J. Vorholt, ChemCatChem, 2017, 9, 1436–1441 CrossRef CAS.
- L. L. W. Cheung, G. Vasapollo and H. Alper, Adv. Synth. Catal., 2012, 354, 2019–2022 CrossRef CAS.
- T. Vanbésien, A. Sayede, E. Monflier and F. Hapiot, Catal. Sci. Technol., 2016, 6, 3064–3073 RSC.
- C. Becquet, F. Berche, H. Bricout, E. Monflier and S. Tilloy, ACS Sustainable Chem. Eng., 2021, 9, 9444–9454 CrossRef CAS.
- T. Vanbésien, E. Monflier and F. Hapiot, Green Chem., 2016, 18, 6687–6694 RSC.
- T. Rösler, K. R. Ehmann, K. Köhnke, M. Leutzsch, N. Wessel, A. J. Vorholt and W. Leitner, J. Catal., 2021, 400, 234–243 CrossRef.
- R. H. Crabtree, Chem. Rev., 2015, 115, 127–150 CrossRef CAS PubMed.
- N. Abdehagh, F. H. Tezel and J. Thibault, Biomass Bioenergy, 2014, 60, 222–246 CrossRef CAS.
- S. Püschel, E. Hammami, T. Rösler, K. R. Ehmann, A. J. Vorholt and W. Leitner, Catal. Sci. Technol., 2022, 12, 728–736 RSC.
- S. Püschel, J. Sadowski, T. Rösler, K. R. Ehmann, A. J. Vorholt and W. Leitner, ACS Sustainable Chem. Eng., 2022, 10, 3749–3756 CrossRef PubMed.
- W. Leitner, Acc. Chem. Res., 2002, 35, 746–756 CrossRef CAS PubMed.
- U. Hintermair, G. Franciò and W. Leitner, Chem. Commun., 2011, 47, 3691 RSC.
- G. L. Zabot, in Green Sustainable Process for Chemical and Environmental Engineering and Science, ed. Inamuddin, A. M. Asiri and A. M. Isloor, Elsevier, 2020, pp. 255–278 Search PubMed.
- J. M. del Valle, J. Supercrit. Fluids, 2015, 96, 180–199 CrossRef CAS.
- G. B. Jacobson, C. T. Lee, K. P. Johnston and W. Tumas, J. Am. Chem. Soc., 1999, 121, 11902–11903 CrossRef CAS.
- K. Burgemeister, G. Franciò, V. H. Gego, L. Greiner, H. Hugl and W. Leitner, Chem. – Eur. J., 2007, 13, 2798–2804 CrossRef CAS PubMed.
- D. J. Heldebrant and P. G. Jessop, J. Am. Chem. Soc., 2003, 125, 5600–5601 CrossRef CAS PubMed.
- D. J. Heldebrant, H. N. Witt, S. M. Walsh, T. Ellis, J. Rauscher and P. G. Jessop, Green Chem., 2006, 8, 807 RSC.
- R. A. Brown, P. Pollet, E. McKoon, C. A. Eckert, C. L. Liotta and P. G. Jessop, J. Am. Chem. Soc., 2001, 123, 1254–1255 CrossRef CAS PubMed.
- M. Schmitkamp, D. Chen, W. Leitner, J. Klankermayer and G. Franciò, Chem. Commun., 2007, 4012 RSC.
- U. Hintermair, T. Höfener, T. Pullmann, G. Franciò and W. Leitner, ChemCatChem, 2010, 2, 150–154 CrossRef CAS.
- J. Theuerkauf, G. Franciò and W. Leitner, Adv. Synth. Catal., 2013, 355, 209–219 CrossRef CAS.
- U. Hintermair, G. Franciò and W. Leitner, Chem. – Eur. J., 2013, 19, 4538–4547 CrossRef CAS PubMed.
- Z. Hou, B. Han, L. Gao, T. Jiang, Z. Liu, Y. Chang, X. Zhang and J. He, New J. Chem., 2002, 26, 1246–1248 RSC.
- E. Janssen, A. Bösmann, G. Francio, M. Solinas, W. Leitner and P. Wasserscheid, Angew. Chem., Int. Ed., 2001, 2, 2697–2699 Search PubMed.
- A. Fürstner, D. Koch, K. Langemann, W. Leitner and C. Six, Angew. Chem., Int. Ed. Engl., 1997, 36, 2466–2469 CrossRef.
- R. Duque, E. Öchsner, H. Clavier, F. Caijo, S. P. Nolan, M. Mauduit and D. J. Cole-Hamilton, Green Chem., 2011, 13, 1187 RSC.
- M. F. Sellin, P. B. Webb and D. J. Cole-Hamilton, Chem. Commun., 2001, 781–782 RSC.
- P. B. Webb, T. E. Kunene and D. J. Cole-Hamilton, Green Chem., 2005, 7, 373 RSC.
- M. Solinas, J. Jiang, O. Stelzer and W. Leitner, Angew. Chem., Int. Ed., 2005, 44, 2291–2295 CrossRef CAS PubMed.
- T. E. Kunene, P. B. Webb and D. J. Cole-Hamilton, Green Chem., 2011, 13, 1476 RSC.
- U. Hintermair, G. Zhao, C. C. Santini, M. J. Muldoon and D. J. Cole-Hamilton, Chem. Commun., 2007, 1462 RSC.
- D. Koch and W. Leitner, J. Am. Chem. Soc., 1998, 120, 13398–13404 CrossRef CAS.
- G. Franciò, K. Wittmann and W. Leitner, J. Organomet. Chem., 2001, 621, 130–142 CrossRef.
- S. Kandasamy, S. P. Samudrala and S. Bhattacharya, Catal. Sci. Technol., 2019, 9, 567–577 RSC.
- M. Terhorst, C. Heider, A. Vorholt, D. Vogt and T. Seidensticker, ACS Sustainable Chem. Eng., 2020, 8, 9962–9967 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc01252a |
| This journal is © The Royal Society of Chemistry 2022 |