Metal-free photo-induced heteroarylations of C–H and C–C bonds of alcohols by flow chemistry†
Abstract
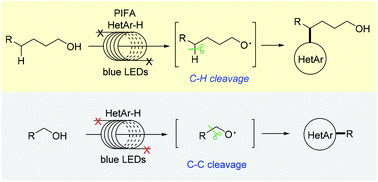
Practical, metal-free heteroarylations of C–H and C–C bonds of unprotected aliphatic alcohols are accomplished in a ‘stop-flow’ micro-tubing reactor. The reactions proceed through alkoxy radical-triggered hydrogen atom transfer and β-C(sp3)–C(sp3) bond rupture under mild photochemical conditions, leading to a plethora of valuable functionalized heteroarenes. The reaction is amenable for late-stage functionalization of complex heteroarenes, and can be readily scaled up by a continuous-flow method. The protocol features many advantages including good regioselectivity, broad functional group compatibility, and easy operation, and may find potential use in medicinal chemistry.
- This article is part of the themed collection: 2022 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...