Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanoenzymatic reactions with whole cell transaminases: shaken, not stirred†
Eve M.
Carter
 a,
Esther
Ambrose-Dempster
a,
John M.
Ward
a,
Esther
Ambrose-Dempster
a,
John M.
Ward
 b,
Tom D.
Sheppard
b,
Tom D.
Sheppard
 a and
Helen C.
Hailes
a and
Helen C.
Hailes
 *a
*a
aDepartment of Chemistry, University College London, 20 Gordon Street, London, WC1H 0AJ, UK. E-mail: h.c.hailes@ucl.ac.uk
bDepartment of Biochemical Engineering, University College London, Bernard Katz Building, Gower Street, London WC1E 6BT, UK
First published on 11th April 2022
Abstract
Mechanochemical reactions have emerged in recent years as a green synthetic method because reactions can be performed more rapidly and using less solvent than traditional synthetic approaches. To date, very few mechanoenzymatic reactions have been described. For the first time, transaminases, which are widely used for the amination of aldehydes and ketones, have been used here under mechanoenzymatic conditions to produce amines using significantly less aqueous medium than conventional biocatalytic reactions. The direct use of whole cells was also possible and shorter reaction times could be used to provide amines efficiently with high yields and stereoselectivities.
Introduction
Over the last decade, mechanochemical reactions have gained interest as a green method of performing organic synthetic reactions. IUPAC describes them as a ‘chemical reaction that is induced by the direct absorption of mechanical energy’.1 In practice, this involves grinding or milling a reaction using balls in a jar (Fig. 1) with little or no solvent. The advantages of mechanochemical methods over traditional reactions in solution include more rapid reactions, often with higher yields, solvent free reactions, and no requirement for external heating. Sometimes increased or modified selectivities are observed, and reactions with insoluble components can be achieved effectively.2,3 Many chemical reactions have been realised under these conditions, such as cycloadditions, asymmetric aldol reactions, peptide syntheses and metal catalysed coupling reactions, and large scale transformations have been achieved.2–7 Multistep reactions have also been performed under mechanochemical conditions in the synthesis of active pharmaceuticals such as axitinib8 and procainamide.6Biocatalysis, which involves using enzymes as renewable catalysts, is an important and expanding green synthetic method. Biocatalysts can perform highly stereoselective transformations under mild reaction conditions.9,10 Mechanoenzymatic reactions, enzyme-mediated reactions under mechanochemical conditions, are a recent advancement,3 first reported in 2016 with the use of immobilised Candida antarctica lipase B (CALB) for the kinetic resolution of alcohols.11 In a subsequent report the same enzyme was employed for the deracemisation of amines.12 These methods have been utilised in the kinetic resolution of (±)-ketorolac, where (S)-ketorolac is an anti-inflammatory and (R)-ketorolac is a potential drug for ovarian cancer management,13 and in the synthesis of (R)-rasagiline, used in the treatment of Parkinson's disease.12
In addition to lipases, the protease papain has been used under mechanoenzymatic conditions, where it was applied to the oligomerisation of amino acids.14 For the mechanoenzymatic degradation of poorly soluble materials, chitinases have been used to depolymerise chitin,15 and the hydrolysis of cellulose was realised by cellulases.16,17 These were all performed under milling conditions with non-immobilised enzymes that were commercially available as purified or unpurified reagents. There have also been several studies of enzyme stability (both immobilised and non-immobilised) under mechanoenzymatic conditions. Higher yields were often achieved when milling was carried out in cycles of grinding with rest periods in between (known as reactive aging, RAging).16 This static incubation period allows for cooling within the jars, which often have raised temperatures during grinding due to collisions between the balls and the walls of the jar. The cooling periods can thus prevent the vessel reaching high temperatures which might denature the enzyme. This resulted in higher yields compared to constant milling and also benefits from decreased energy consumption.18In vitro reactions with enzymes typically involve the use of dilute aqueous conditions. Using mechanoenzymatic methods, it is typically possible to use high substrate concentrations, resulting in more facile reaction work-ups and greater efficiencies.3,5
Transaminases (TAms) are used to reversibly transform a ketone or aldehyde group into an amine moiety using an amine donor and pyridoxal 5′-phosphate (PLP) as the cofactor. When using prochiral ketones, the products can be single enantiomers and it is possible to access either by using (S)- or (R)-selective TAms.19 Chiral amines are commonly found in pharmaceuticals and agrochemicals. Transaminases have been used in numerous industrial applications, enabling the stereoselective amine functionalisation of complex molecules.20–24 To the best of our knowledge, reported mechanoenzymatic reactions to date all involve hydrolases (EC 3).25–27 This work describes the novel use of TAms (EC 2.6.1) under mechanoenzymatic conditions (Fig. 1B) for the first time.
Results and discussion
Biocatalysts are typically used as purified enzymes (which can be costly) or cell lysates; in both cases preparation from the whole cell biocatalyst is required (ESI Fig. 1†).28 To realise the advantages of a mechanoenzymatic system, which can lyse cells and release active enzyme via the milling action, whole cell biocatalysts were used directly in our reactions. This simplifies the procedure, eliminating the need for enzyme lysate preparation or purification steps. The TAm from Chromobacterium violaceum (Cv-TAm)29 was selected as a representative enzyme as it is well documented to accept a wide range of substrates and amine donors, including isopropylamine (IPA).30,31 Lyophilised whole cells of Cv-TAm (in E. coli) were used to ensure reproducibility in the reaction screens.Initial investigations focussed on using benzaldehyde 1a, which is readily accepted by Cv-TAm, as a substrate in a mixer mill. The amine donors L-alanine, (S)-α-methylbenzylamine ((S)-MBA) and isopropylamine hydrochloride salt (IPA·HCl) were screened in the reaction at various concentrations. The salt IPA·HCl was used as pH adjustments are normally required when using the free amine. There are several parameters to be optimised in mechanoenzymatic reactions, such as the frequency of milling (e.g. 30 Hz), milling time (e.g. 5 min to 3 h) and aging period (e.g. 5 min to several days).13,14
To identify the best amine donor and equivalents with whole cell Cv-TAm, 30 Hz shaking was initially used with 3 cycles of 20 min milling and 20 min aging. The mechanoenzymatic reactions were successful and benzylamine 1b formed in ∼10–50% yields (by HPLC against standards) with L-alanine as an amine donor (Fig. 2). The highest yields were with 8 equiv. L-alanine and it was apparent that the amount of bulk aqueous solvent present had an effect, with 1000 μL total volume giving the highest yields. Of note is that reasonable yields could be achieved without using additional enzymes to recycle or remove the side-product pyruvate.32 When using (S)-MBA and IPA·HCl, again the total aqueous volume of 1000 μL led to higher yields. As lyophilised whole cells are used, this is presumably important to retain enzyme stability when lysed in the bead mill.19 In addition, higher equivalents of (S)-MBA resulted in lower yields; this has been noted in other reactions with Cv-TAm.32 As the use of IPA·HCl (75 equiv.) clearly gave the highest yield of 1b, ∼80%, this was used in further experiments.
 | ||
| Fig. 2 Yields of benzylamine 1b when reacting benzaldehyde 1a (0.1 mmol), PLP (0.001 mmol), lyophilised whole cell Cv-TAm (10 mg–0.05 mg TAm per mg whole cell enzyme) and varying equivalents of different amine donors in potassium phosphate (KPi) buffer (pH 8.0, 100 mM, 100–1000 μL total volume) in 10 mL stainless steel jars with 2 × 5 mm stainless steel balls for 3 cycles of 20 min shaking at 30 Hz then 20 min aging at rt. Benzylamine 1b yields were measured by HPLC against standards. The three ball symbol below the reaction arrow denotes that this is a mechanochemical reaction.33 | ||
Further optimisation was performed to establish the amount of Cv-TAm to be used, total volume of the reaction, frequency of milling, RAging periods and number of cycles. This established that the following conditions were optimal here: 5 mL jars and 2 × 5 mm balls; 10 mg lyophilised whole cell Cv-TAm in a 2 mL total volume with 50 mM benzaldehyde 1a and 3.75 M of IPA·HCl; shaking at 25 Hz for 2 cycles of 30 min and 30 min aging (2 h total reaction time). These conditions gave benzylamine 1b in almost quantitative yield (96% by HPLC analysis), with the best compromise between a high yield and a short reaction time and were therefore used in future reactions (Fig. 3). It was also noted that the concentration of benzaldehyde 1a could be doubled to 100 mM (in 2 mL) with twice as much enzyme to give benzylamine 1b in a similar yield (93%).
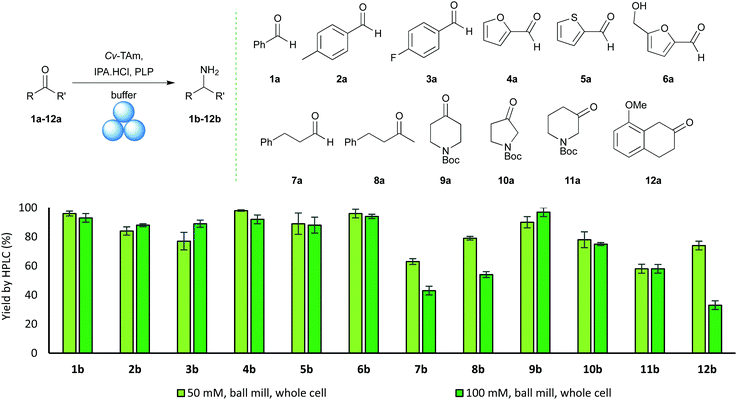
A range of aldehyde and ketone substrates (1a–12a) were then used in mechanoenzymatic reactions to give the corresponding amines 1b–12b in good to excellent yields (Fig. 3). In agreement with previous studies,31 it was observed that aldehydes generally reacted more readily than ketones. Aldehydes 1a–6a provided the corresponding amines 1b–6b in 80–95% yields at both 50 mM and 100 mM substrate concentrations. In most cases, 10 mg of whole cell Cv-TAm was used with 50 mM substrate and 20 mg for 100 mM. Some substrates, particularly ketones, required increased amounts of Cv-TAm, with up to 60 mg lyophilised whole cell Cv-TAm required for 100 mM reactions with 8a (Fig. 3). At 50 mM scale reactions, the ketone-derived products 8b–12b were formed in 58–90% yields after a 2 h total reaction time, incorporating 2 × 30 min of milling.
The enantiomeric excesses (ees) were confirmed for 8b, 10b, 11b and 12bvia the use of racemic standards and chiral HPLC/GC, with all mechanoenzymatic reaction products found to be a single (S)-enantiomer (see ESI†).34–36
Reactions were also performed to compare mechanoenzymatic whole cell Cv-TAm reactions to reactions under typical enzymatic conditions (with whole cell Cv-TAm), i.e. in a thermomixer. In nearly all cases this resulted in significantly higher yields when milled (Fig. 4). Furthermore, reactions were performed with an equivalent amount of enzyme as clarified lysate and yields were higher in the ball mill (Fig. 4). Indeed, for several substrates significant yield enhancements were noted. This highlighted that the mechanical grinding appears to assist the reactions by both lysing the cells and the mechanoenzymatic effect.
 | ||
| Fig. 4 Yields of amines 1b–12b when reacting aldehydes and ketones 1a–12a (50 mM), PLP (0.5 mM), Cv-TAm and IPA·HCl (3.75 M) in KPi buffer (pH 8.0, 100 mM, 2 mL total volume). Ball mill (green bars): these were performed in 5 mL stainless steel jars with 2 × 5 mm stainless steel balls, Cv-TAm (10–40 mg – see ESI Table 1† – 0.05 mg TAm per mg whole cell enzyme), with shaking at 25 Hz for 2 cycles of 30 min shaking, then 30 min aging at rt. Control reactions were carried out using whole cell empty vector BL21 which gave no amine products in all cases. No milling (whole cell – orange bars; clarified lysate – blue bars): reactions were performed in Eppendorf tubes using the same quantities of lyophilised whole cell or clarified lysate Cv-TAm in a thermomixer at rt for 2 h (2 cycles of 30 min mixing and 30 min standing). Yields were determined by HPLC against product standards. | ||
Conclusions
In summary, here we report for the first time mechanoenzymatic-mediated transaminase reactions using whole cell biocatalysts. Reaction conditions were optimised using benzaldehyde 1a as a substrate, where the periods for milling and aging were considered as well as milling frequency, amount of aqueous solvent present, substrate concentration and enzyme loading. These were then applied to a range of aldehydes and ketones, with high yields and good enantioselectivities observed throughout. The reactions were also compared to whole cell enzymatic reactions and those with lysates, with higher yields observed in the ball mill in comparison to both of these approaches. This is a valuable transformation to add to the repertoire of mechanoenzymatic reactions and highlights the value of ball-milling using whole cell biocatalysts, rather than more traditional biocatalytic reactions using prepared enzyme lysates or purified-enzymes in stirred-vessels with larger solvent volumes. Indeed, ‘shaken, not stirred’ reaction scenarios are worth considering more widely within the field of biocatalysis.Author contributions
E. M. C. investigated the mechanochemical enzymatic reactions and E. M. C. and E. A.-D. developed the methodologies. The project conceptualisation was by all authors and supervised by T. D. S., J. M. W. and H. C. H. The original draft of the manuscript was written by E. M. C. and H. C. H. The manuscript has been reviewed and edited by all contributing authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Engineering and Physical Sciences Research Council (EPSRC) for funding E. M. C. (EP/N509577/1) and E. A.-D. (EP/R513143/1). Additionally, we gratefully thank the UCL Mass Spectrometry and NMR Facilities in the Department of Chemistry UCL, 700 MHz NMR equipment support by EPSRC (EP/P020410/1) and NERC NE/V010735/1 for purchase of the ball mill.Notes and references
- A. D. McNaught and A. Wilkinson, IUPAC. Compendium of Chemical Terminology, 2nd edn, 1997 (the ‘Gold Book’) Search PubMed.
- A. Stolle, B. Ondruschka, A. Krebs and C. Bolm, in Innovative Catalysis in Organic Synthesis, 2012, pp. 327–349 Search PubMed.
- K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed.
- V. Declerck, P. Nun, J. Martinez and F. Lamaty, Angew. Chem., Int. Ed., 2009, 48, 9318–9321 CrossRef CAS PubMed.
- C. Bolm and J. G. Hernández, ChemSusChem, 2018, 11, 1410–1420 CrossRef CAS PubMed.
- T. Portada, D. Margetić and V. Štrukil, Molecules, 2018, 23, 3163 CrossRef PubMed.
- A. Stolle, R. Schmidt and K. Jacob, Faraday Discuss., 2014, 170, 267–286 RSC.
- J. Yu, Z. Hong, X. Yang, Y. Jiang, J. Zhijiang and W. Su, Beilstein J. Org. Chem., 2018, 14, 786–795 CrossRef CAS PubMed.
- R. A. Sheldon and J. M. Woodley, Chem. Rev., 2018, 118, 801–838 CrossRef CAS PubMed.
- J. P. Adams, M. J. B. Brown, A. Diaz-Rodriguez, R. C. Lloyd and G. D. Roiban, Adv. Synth. Catal., 2019, 361, 2421–2432 CAS.
- J. G. Hernández, M. Frings and C. Bolm, ChemCatChem, 2016, 8, 1769–1772 CrossRef.
- M. Pérez-Venegas and E. Juaristi, Tetrahedron, 2018, 74, 6453–6458 CrossRef.
- M. Pérez-Venegas, A. M. Rodríguez-Treviño and E. Juaristi, ChemCatChem, 2020, 12, 1782–1788 CrossRef.
- K. J. Ardila-Fierro, D. E. Crawford, A. Körner, S. L. James, C. Bolm and J. G. Hernández, Green Chem., 2018, 20, 1262–1269 RSC.
- J. P. D. Therien, F. Hammerer, T. Friščić and K. Auclair, ChemSusChem, 2019, 12, 3481–3490 CrossRef CAS PubMed.
- F. Hammerer, L. Loots, J.-L. Do, J. P. D. Therien, C. W. Nickels, T. Friščić and K. Auclair, Angew. Chem., Int. Ed., 2018, 57, 2621–2624 CrossRef CAS PubMed.
- J. Arciszewski and K. Auclair, ChemSusChem, 2022, 15, e202102084 CrossRef CAS PubMed.
- M. Pérez-Venegas and E. Juaristi, ChemSusChem, 2021, 14, 2682–2688 CrossRef PubMed.
- F. Guo and P. Berglund, Green Chem., 2017, 19, 333–360 RSC.
- C. K. Savile, J. M. Janey, E. C. Mundorff, J. C. Moore, S. Tam, W. R. Jarvis, J. C. Colbeck, A. Krebber, F. J. Fleitz, J. Brands, P. N. Devine, G. W. Huisman and G. J. Hughes, Science, 2010, 329, 305–309 CrossRef CAS PubMed.
- C. S. Fuchs, R. C. Simon, W. Riethorst, F. Zepeck and W. Kroutil, Bioorg. Med. Chem., 2014, 22, 5558–5562 CrossRef CAS PubMed.
- S. A. Kelly, S. Pohle, S. Wharry, S. Mix, C. C. R. Allen, T. S. Moody and B. F. Gilmore, Chem. Rev., 2018, 118, 349–367 CrossRef CAS PubMed.
- J. Ward and R. Wohlgemuth, Curr. Org. Chem., 2010, 14, 1914–1927 CrossRef CAS.
- T. Sehl, H. C. Hailes, J. M. Ward, R. Wardenga, E. Von Lieres, H. Offermann, R. Westphal, M. Pohl and D. Rother, Angew. Chem., Int. Ed., 2013, 52, 6772–6775 CrossRef CAS PubMed.
- S. Kaabel, T. Friščić and K. Auclair, ChemBioChem, 2020, 21, 742–758 CrossRef CAS PubMed.
- M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8, 8881–8893 CrossRef.
- C. G. Avila-Ortiz, M. Pérez-Venegas, J. Vargas-Caporali and E. Juaristi, Tetrahedron Lett., 2019, 60, 1749–1757 CrossRef CAS.
- J. Wachtmeister and D. Rother, Curr. Opin. Biotechnol., 2016, 42, 169–177 CrossRef CAS PubMed.
- U. Kaulmann, K. Smithies, M. E. B. Smith, H. C. Hailes and J. M. Ward, Enzyme Microb. Technol., 2007, 41, 628–637 CrossRef CAS.
- I. Slabu, J. L. Galman, R. C. Lloyd and N. J. Turner, ACS Catal., 2017, 7, 8263–8284 CrossRef CAS.
- A. Dunbabin, F. Subrizi, J. M. Ward, T. D. Sheppard and H. C. Hailes, Green Chem., 2017, 19, 397–404 RSC.
- N. Richter, R. C. Simon, H. Lechner, W. Kroutil, J. M. Ward and H. C. Hailes, Org. Biomol. Chem., 2015, 13, 8843–8851 RSC.
- N. R. Rightmire and T. P. Hanusa, Dalton Trans., 2016, 45, 2352–2362 RSC.
- L. Leipold, D. Dobrijevic, J. W. E. Jeffries, M. Bawn, T. S. Moody, J. M. Ward and H. C. Hailes, Green Chem., 2019, 21, 75–86 RSC.
- A. Dunbabin, PhD thesis, University College London, 2018, https://discovery.ucl.ac.uk/id/eprint/10049818.
- D. Baud, N. Tappertzhofen, T. S. Moody, J. M. Ward and H. C. Hailes, Adv. Synth. Catal., 2022 DOI:10.1002/adsc.202200146.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and NMR spectra. See DOI: https://doi.org/10.1039/d2gc01006b |
| This journal is © The Royal Society of Chemistry 2022 |