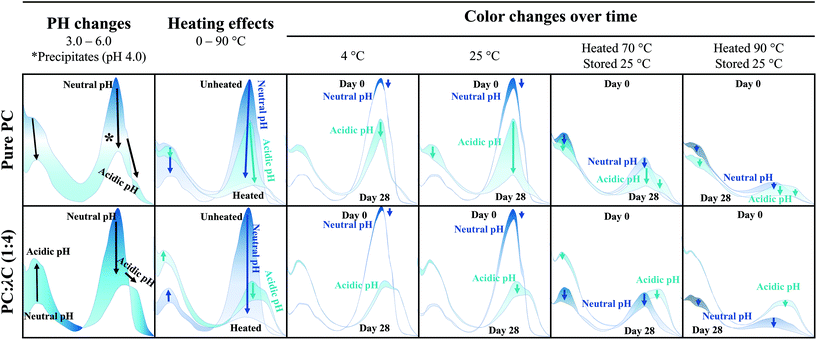
Influence of storage temperature on the stability of heat treated phycocyanin-λ-carrageenan complexes in liquid formulations†
Stephan
Buecker
 a,
Lutz
Grossmann
ab,
Myriam
Loeffler
c,
Elena
Leeb
d and
Jochen
Weiss
a,
Lutz
Grossmann
ab,
Myriam
Loeffler
c,
Elena
Leeb
d and
Jochen
Weiss
 *a
*a
aDepartment of Food Material Science, Institute of Food Science and Biotechnology, University of Hohenheim, Garbenstrasse 21, 70599 Stuttgart, Germany. E-mail: j.weiss@uni-hohenheim.de; Tel: +49 711 459 24415
bDepartment of Food Science, University of Massachusetts Amherst, Amherst, MA 01003, USA
cDepartment of Microbial and Molecular Systems, Leuven Food Science and Nutrition Research Centre, KU Leuven - Ghent Technology Campus, Gebroeders de Smetstraat 1, 9000 Ghent, Belgium
dGNT Europa GmbH, 52072 Aachen, Kackertstrasse 22, Germany
First published on 6th May 2022
Abstract
The protein chromophore complex present in cyanobacteria like Arthrospira platensis can be utilized as a natural food colorant, but the appearance is prone to changes under environmental conditions that alter the protein-chromophore interactions. The study investigates the color stability of phycocyanin stabilized by λ-carrageenan during storage at 4 and 25 °C under different heating and pH conditions. Phycocyanin solutions and combinations with λ-carrageenan (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) were prepared at a pH of 2.5–6.0, with and without thermal treatment at 70 °C and 90 °C. Visual appearance, absorption spectra, zeta potential, and the diameter of the structures formed were observed over a period of 28 days. Considerable color losses were determined for pure phycocyanin at a pH of 2.5 to 4.5 when the samples were stored at 25 °C, which was improved by the formation of soluble complexes with λ-carrageenan in associative conditions. Interestingly, soluble complexes at a pH < pI were found to be more resistant at high treatment temperatures with a higher storage stability than co-soluble polymers at a pH > pI, but soluble structures formed at a pH of 2.5 had a significantly larger diameter (871 ± 185 nm) that increased in size during storage (2025 ± 1012 nm after 14 d at 25 °C) than at a pH of 3.0 (200 ± 5 nm) when heated to 90 °C. These results were related to changes in protein structure and mass transport phenomena and might be of high importance for low-viscous food formulations.
4) were prepared at a pH of 2.5–6.0, with and without thermal treatment at 70 °C and 90 °C. Visual appearance, absorption spectra, zeta potential, and the diameter of the structures formed were observed over a period of 28 days. Considerable color losses were determined for pure phycocyanin at a pH of 2.5 to 4.5 when the samples were stored at 25 °C, which was improved by the formation of soluble complexes with λ-carrageenan in associative conditions. Interestingly, soluble complexes at a pH < pI were found to be more resistant at high treatment temperatures with a higher storage stability than co-soluble polymers at a pH > pI, but soluble structures formed at a pH of 2.5 had a significantly larger diameter (871 ± 185 nm) that increased in size during storage (2025 ± 1012 nm after 14 d at 25 °C) than at a pH of 3.0 (200 ± 5 nm) when heated to 90 °C. These results were related to changes in protein structure and mass transport phenomena and might be of high importance for low-viscous food formulations.
1 Introduction
The use of coloring foods in food formulations instead of synthetic colors is compelling. Their plant origin and exclusively physical processing make them not only a natural but also a renewable and vegan source that is very attractive to consumers.1–4 When the Southampton Study declared, in 2007, that six synthetic dyes may increase hyperactivity in children, a radical drop in their commercial use was noticed.5 Nonetheless, colorants are typically added to food formulations to make them more appealing to consumers, to account for natural color losses during production and differences in raw material quality, or color fading over time. A convincing alternative to synthetic dyes is the use of food colorants that are derived from color-intensive food-grade raw materials including vegetables, fruits, or cyanobacteria. These foods are exclusively processed by physical processing methods and H2O extraction to obtain a concentrated extract that is rich in the desired pigment.6 Their origin and minimal processing make them not only a natural but also a renewable and vegan source that is very attractive to consumers.1–4 However, these extracts are often less stable than their synthetic counterparts, which presents a challenge for producing stable foods with a long shelf life, thus minimizing food losses.Up to now, phycocyanin (PC) present in eukaryote algae and cyanobacteria such as Arthrospira platensis (also known as Spirulina) is the only authorized food coloring in the European Union with a blue appearance, which is a major challenge to replace synthetic blue dyes in many food formulations.7 PC consists of the more blueish green-appearing allophycocyanin (aPC) and the royal blue-appearing C-phycocyanin (CPC). Both aPC and CPC are alike in their tertiary structure, but differ in amino acid sequence, number of chromophores (phycocyanobilins), and molecular weight.8 Each holds an α- and β-subunit with globular folding. In aPC, both subunits bind one chromophore, whereas in CPC, the β-subunit attaches two chromophores. The chromophores are covalently bound to the apoprotein by thioether bonds.8–10 In solution, PC is present as a complex mixture of monomers, trimers, and hexamers. Monomers are the predominant form at acidic pH values. They assemble into hexamers around their isoelectric point (pI) of a pH of 4.6.11 A further increase of the pH leads to dissociation into trimers.8
A major drawback of utilizing chromophores that are stabilized by proteins is the sensitivity of protein structures to denaturation. This influences the blue appearance of PC-containing products.12 However, many food formulations undergo thermal, pH, and ionic changes during production and storage. For example, a lot of beverages have a pH of 3.0, which is challenging in terms of PC storage stability due to an increased number of unstable PC monomers.8,13 Additionally, pasteurization takes place at <100 °C and is one of the most common treatments to preserve foods, but alters the structure of proteins.12 It is already known that adverse temperatures and pH levels affect PC stability and, thus, color intensity.14
Previous studies have shown that PC can be stabilized by complexation with different carrageenans, whereas λ- and ι-carrageenan proved to efficiently stabilize PC against thermal- and acidic-degradation, such as precipitation and color loss.12,15,16 Carrageenans consist of repeating disaccharide units of 3-linked β-D-galactopyranose and 4-linked α-galactopyranose or 3,6-anhydro-α-galactopyranose, have a high molecular weight (200–800 kDa) and are commonly found in red algae (Rhodophyta). With three sulfate groups per disaccharide unit, λ-carrageenan carries a strong negative charge, which seemed to be beneficial for the complexation with PC as the complexes are primarily formed by electrostatic interactions.15,17,18 Additionally, hydrophobic interactions and hydrogen bonds affect the complex structure.18
While carrageenan was found to be promising to stabilize PC against heat treatments, far too little attention has been paid to the influence of storage time and storage temperature on these complexes. However, this is of significant importance, since color stability throughout the shelf life is of high relevance in food formulations. For this reason, this study aims to elucidate the effect of pH and temperature treatment on the color stability of PC and its mixtures with λ-carrageenan during 28 days of storage at different storage conditions.
2 Materials and methods
2.1 Materials
An Arthrospira platensis protein concentrate rich in PC (henceforth referred to as PC powder) obtained by spray drying was kindly supplied by GNT International B.V. (Mierlo, Netherlands) and was chosen to enhance the pertinence of this study for the food and beverage industry. The λ-carrageenan (λC) powder was obtained from TIC Gums Inc. Both source materials were chosen to be comparable to previous studies. (White Marsh, USA). Hydrochloric acid (HCl), sodium hydroxide (NaOH), and sodium azide (NaN3) were purchased from Carl Roth GmbH & Co. KG (Karlsruhe, Germany).2.2 Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC ratio of 1
λC ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 while being stirred for another 5 minutes.18 To exclude turbidity effects of microbial growth, 0.05% (w/w) NaN3 was added to the samples. Hereinafter, the 0.1% (w/w) PC powder solutions, which yield in a final PC concentration of 0.0369% (w/w), are referred to as 0.04% (w/w) PC solutions.
4 while being stirred for another 5 minutes.18 To exclude turbidity effects of microbial growth, 0.05% (w/w) NaN3 was added to the samples. Hereinafter, the 0.1% (w/w) PC powder solutions, which yield in a final PC concentration of 0.0369% (w/w), are referred to as 0.04% (w/w) PC solutions.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions with an initial pH of 6.7 were adjusted to a pH of 6.0, 5.0, 4.5, 4.0, 3.5, 3.0, and 2.5 (±0.02) using either HCl or NaOH (0.1–3.0 M) and solutions were kept overnight at 4 °C. Before the heat treatment, the pH values of all samples were checked and readjusted if necessary. The pH maintained stable throughout the observed storage period.
λC solutions with an initial pH of 6.7 were adjusted to a pH of 6.0, 5.0, 4.5, 4.0, 3.5, 3.0, and 2.5 (±0.02) using either HCl or NaOH (0.1–3.0 M) and solutions were kept overnight at 4 °C. Before the heat treatment, the pH values of all samples were checked and readjusted if necessary. The pH maintained stable throughout the observed storage period.
2.3 Analyses
In order to investigate the influence of storage time and different storage temperatures on color shifts and intensity, pure PC and PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) mixtures were analyzed during the storage period on day 0, 3, 6, 14, and 28.
4) mixtures were analyzed during the storage period on day 0, 3, 6, 14, and 28.
| cC-phycocyanin = 0.162A620 − 0.098A650 (g L−1) | (1) |
| callophycocyanin = 0.180A650 − 0.042A620 (g L−1) | (2) |
Average colors of the solutions were extracted by GIMP version 2.10.24 (available at: https://www.gimp.org/).
2.4 Modeling and calculations
2.5 Statistics
Each experiment was conducted twice and all measurements were repeated at least two times. Means and standard deviations were determined with Excel (Microsoft Inc., Redmond, USA). SPSS Statistics 27 (IBM Inc., Armonk, USA) was used to statistically evaluate significant differences between results gained from PC concentration calculations and size measurements using a one-way ANOVA followed by a post-hoc Tukey test (α = 0.05).3 Results and discussion
In a previous study, it was shown that pure PC was unstable around its pI due to decreased electrostatic repulsion among PC and that heat treatment has a profound impact on the color stability of pure PC.14,18 Initially, the protein and PC content in the powder were analyzed, and based on these results, the storage stability was investigated.3.1 Protein content
The protein content of the PC powder was determined as 89.83 ± 0.25%, which is considerably higher than that of untreated biomass with a dry matter protein content of typically 46–63%.21 This is attributed to the production of PC powder, which involves cell and cell wall disruption, separation of insoluble compounds, and filtration steps, such as ion-exchange filtration.22,23,23 Additionally, the protein concentration of cyanobacteria can be optimized by modifying the growth conditions, e.g., luminosity, temperature, stirring speed, dissolved solids, pH, water quality, and macro as well as micronutrients.24 According to the manufacturer, the protein content was 88.75% (Appendix Table 4) and was determined via the Kjeldahl method. This is about 1.2% less than our result, but corresponds to the protein values of the Dumas method, which are usually up to 1.4% higher.25According to the calculations from eqn (1) and (2), the amount of color active PC was 36.9%. Thus, around 41% of the protein in PC powder can be classified as color active PC. The CPC concentration was determined to be 31.3% (w/w) and the aPC content 5.6% (w/w). This is in alignment with the literature, which proposed that aPC is a minor component of Arthrospira platensis.26
3.2 Color fading of pure PC solutions
The color stability of PC-containing solutions during storage is a key quality parameter that is important to ensure a consistent color intensity and so minimize food waste. Therefore, the color stability of unheated and heated (70/90 °C) pure PC and mixed biopolymer solutions (PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC; 1
λC; 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was investigated at a pH of 2.5–6.0 over a period of 28 days at 4 °C and 25 °C. These two temperatures were selected to reflect typical storage conditions at room temperature or under refrigeration conditions. Measurements were conducted on days 0, 3, 6, 14, and 28, while only stable (precipitated solutions were excluded) solutions were considered in the analysis.
4) was investigated at a pH of 2.5–6.0 over a period of 28 days at 4 °C and 25 °C. These two temperatures were selected to reflect typical storage conditions at room temperature or under refrigeration conditions. Measurements were conducted on days 0, 3, 6, 14, and 28, while only stable (precipitated solutions were excluded) solutions were considered in the analysis.
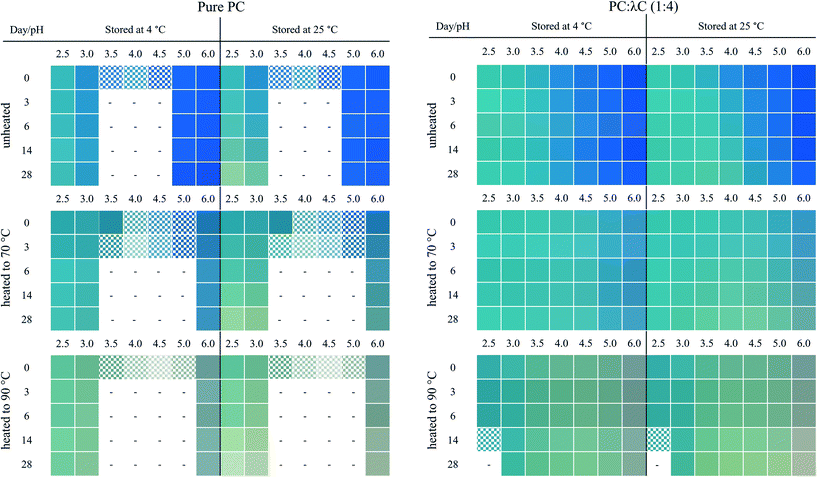
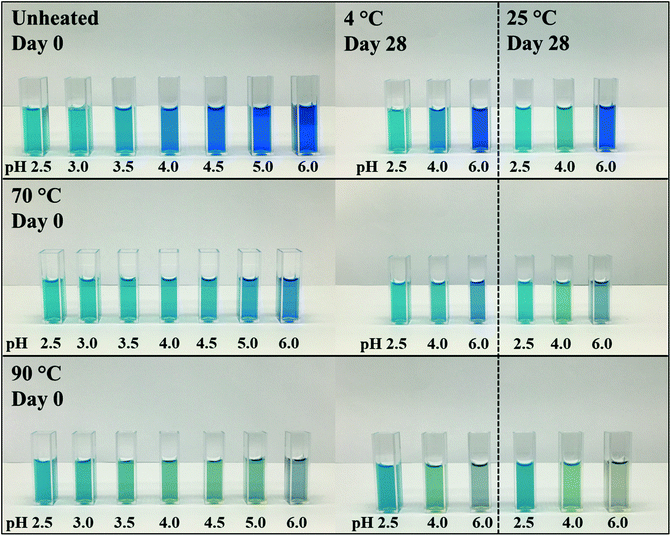
Fig. 1 shows the unheated and heat-treated 0.04% (w/w) PC solutions at day 0 and selected samples (pH 2.5, 3.0, and 6.0) after 28 days of storage at 4 and 25 °C. These pH values were chosen because solutions at pH values of 3.5, 4.0, 4.5 precipitated. Further, the pH values of 2.5, as well as 3.0, reflect the pH values of most beverage formulations. A pH of 6.0 was selected as pH > pI.13
 | ||
| Fig. 1 Visual color appearance of 0.04% (w/w) PC solutions unheated and subjected to thermal treatments at 70 and 90 °C at pH 2.5–6.0 at day 0 and after 28 days of storage at 4 and 25 °C. | ||
PC was found to precipitate at around a pH of 4.0 on day 0, which can be related to the pI of PC (pH ∼ 4.6).8,11 The apparent pIs of the solutions were calculated from the zeta potential measurements (Appendix Table 3) to be 4.05, 4.28, and 4.37 for unheated solutions, and solutions heated to 70 or 90 °C, respectively. The change of pI might be attributed to protein unfolding upon thermal denaturation.12
Unheated PC solutions with a pH of 2.5, 3.0, 5.0, or 6.0 (pH 5 data not shown) were found to be visually stable against precipitation for the entire 28 days of storage, independent of the storage temperature used, whereas precipitates could also be detected in heated PC solutions having a pH of 5.0 (Fig. 2). This could be related to the aforementioned pI shift at higher temperatures leading to a reduced electrostatic repulsion of PC at pH 5.0. Small changes in the protein structure by thermal treatments were already reported to cause a significant color loss which is also in alignment with our findings.10,14
In contrast to the stability of the solution to precipitation, differences in color stability were found depending on the storage temperatures used. All color losses at 4 and 25 °C were discussed in turn. The color of PC solutions that were not heat treated or heated to a core temperature of 70 °C was found to be visually stable when stored at 4 °C over the course of 28 days (Fig. 1). Minor color losses at 4 °C were reported by Kannaujiya & Sinha (2016).27 Solutions heated to 90 °C, with subsequent storage at 4 °C, appeared to undergo a color shift from blue to a dark grey at a pH of 6.0 and to greenish turquoise at a pH of 2.5 and 3.0, respectively. A green color can be related to a further dissociation of PC into monomers over time.8,27 Recently it was shown that the planarity of the chromophore significantly impacts the color appearemce.28 The grey color was described as color fading and might be related to aggregation of PC particles.6
The color shift and fading were more pronounced at 25 °C storage temperature (Fig. 1). For instance, at a pH of 2.5 and 3.0, the color shift towards a greenish turquoise was observed for both the unheated PC solutions and the solutions heated to 70 °C. A loss of color intensity could be noticed for almost all solutions stored at 25 °C for 28 days. These observations can be related to the extreme temperature sensitivity of PC.27 At a pH of 6.0, the color of the unheated solutions remained stable at 25 °C, with only small visual changes. This could be due to PC stacks being organized as hexamers, the predominant PC structure at a pH of 6.0, which was reported to be more stable than monomeric PC.14
| Heat treatment | Day | c PC in mg mL−1 at pH 3 | c PC in mg mL−1 at pH 6 | ||
|---|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | ||
| Indices with small letters indicate significant differences among solutions prepared with the same heat treatment in one column (α < 0.05). Indices with capital letters indicate a difference between a pH of 3.0 and 6.0 within the same row determined by t-test (α < 0.05). | |||||
| None | 0 | 0.24 ± 0.01aA (63.2%) | 0.38 ± 0.01aB (100%) | ||
| 3 | 0.22 ± 0.01b (57.9%) | 0.19 ± 0.01b (50.0%) | 0.38 ± 0.01ab (100%) | 0.38 ± 0.01a (100%) | |
| 6 | 0.21 ± 0.01b (55.3%) | 0.17 ± 0.01c (44.7%) | 0.38 ± 0.01ab (100%) | 0.37 ± 0.01b (97.4%) | |
| 14 | 0.20 ± 0.01c (52.6%) | 0.13 ± 0.01d (34.2%) | 0.37 ± 0.01ab (97.4%) | 0.36 ± 0.01c (94.7%) | |
| 28 | 0.19 ± 0.01c (50.0%) | 0.09 ± 0.01e (23.7%) | 0.37 ± 0.01b (97.4%) | 0.35 ± 0.01d (92.1%) | |
| 70 °C | 0 | 0.14 ± 0.01aA (36.8%) | 0.15 ± 0.01aB (39.5%) | ||
| 3 | 0.14 ± 0.01a (36.8%) | 0.13 ± 0.01b (34.2%) | 0.15 ± 0.01a (39.5%) | 0.15 ± 0.02a (39.5%) | |
| 6 | 0.14 ± 0.01ab (36.8%) | 0.11 ± 0.01c (28.9%) | 0.15 ± 0.01a (39.5%) | 0.14 ± 0.02a (36.8%) | |
| 14 | 0.13 ± 0.01b (34.2%) | 0.09 ± 0.01d (23.7%) | 0.15 ± 0.01ab (39.5%) | 0.13 ± 0.02a (34.2%) | |
| 28 | 0.13 ± 0.01c (34.2%) | 0.07 ± 0.01e (18.4%) | 0.14 ± 0.01b (36.8%) | 0.11 ± 0.02a (28.9%) | |
| 90 °C | 0 | 0.07 ± 0.01aA (18.4%) | 0.07 ± 0.02aA (18.4%) | ||
| 3 | 0.07 ± 0.01a (18.4%) | 0.06 ± 0.01b (15.8%) | 0.07 ± 0.01a (18.4%) | 0.07 ± 0.01a (18.4%) | |
| 6 | 0.07 ± 0.01a (18.4%) | 0.06 ± 0.01c (15.8%) | 0.07 ± 0.01a (18.4%) | 0.07 ± 0.01a (18.4%) | |
| 14 | 0.06 ± 0.01a (15.8%) | 0.04 ± 0.01d (10.5%) | 0.07 ± 0.01a (18.4%) | 0.06 ± 0.01a (15.8%) | |
| 28 | 0.06 ± 0.01a (15.8%) | 0.03 ± 0.01e (7.9%) | 0.07 ± 0.01a (18.4%) | 0.05 ± 0.01a (13.2%) | |
A pronounced PC degradation was facilitated by heating the solutions to either 70 or 90 °C. At a pH of 3.0, the initial PC concentration of unheated solutions and solutions heated to 70 °C was significantly lower than at a pH of 6.0. A possible explanation for this might be that, for acidic pH values, PC is primarily present as a monomer that has a lower absorption than PC hexamers.8 Furthermore, PC was reported to be more temperature resistant when assembled as hexamer.14,29 However, in PC solutions heated to 90 °C, the initial PC concentration on day 0 was equal at a pH of 3.0 and 6.0, but the overall PC concentration was lower compared to untreated solutions and solutions heated to 70 °C. This can be attributed to the 6th order degradation kinetics that phycocyanin follows.30 Thus, denaturation of highly concentrated PC occurs much faster. Since the initial concentration of the PC solution at pH 3.0 was lower, both values converge with time.
There was a tendency that PC degradation at the same storage temperature was stronger at pH 3.0 than at pH 6.0, which could be due to increased stability of PC hexamers.14 This was especially prevalent at 25 °C storage temperature. Solutions stored at 4 °C having a pH of 6.0 showed a higher PC stability, while the strongest decrease in PC concentration was observed for untreated solutions prepared at a pH of 3.0. This is in alignment with the findings of Kannaujiya & Sinha (2016) and can be related to the temperature sensitivity of the PC protein structure.27 Unheated solutions exhibited the greatest PC color loss over time (Table 1). These solutions had the highest initial concentration, whereas the lowest PC concentration was observed in solutions heated to 90 °C. These relationships may partly be explained by the fact that PC denaturation slows down significantly at low concentrations.30
3.3 Color fading of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) λC complex solutions
λC complex solutions
As pointed out in the previous chapter, pure PC solutions precipitated at a pH of 3.5–5.0 and were unstable against thermal treatments. Further, a color shift from blue, at a pH of 6.0, to turquoise, at a pH of 2.5, was observed. It was related to a change in the PC monomer, trimer, and hexamer concentration and an increasing chromophore planarity.28 Together, the results showed that PC was less stable under acidic pH values or high storage temperatures (25 °C). This study aimed to improve the storage stability of PC by complexation with λC. In the upcoming section, the present findings are compared to the appearance and absorption measurements of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) solutions. The PC concentration in the complexes was identical to the pure PC solutions and all measurements were performed equally.
4) solutions. The PC concentration in the complexes was identical to the pure PC solutions and all measurements were performed equally.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
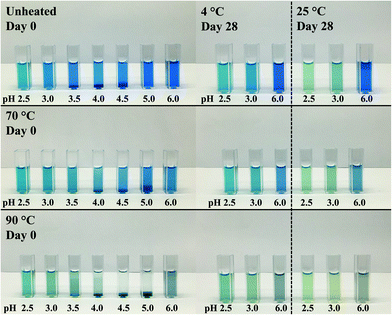
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC mixtures, the solutions were again visually analyzed. Fig. 2 illustrates the average colors of the pictures from 0.04% (w/w) PC solutions and PC
λC mixtures, the solutions were again visually analyzed. Fig. 2 illustrates the average colors of the pictures from 0.04% (w/w) PC solutions and PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) solutions unheated and heated (70 or 90 °C) on day 0, 3, 6, 14, 28 stored at either 4 °C or 25 °C.
4) solutions unheated and heated (70 or 90 °C) on day 0, 3, 6, 14, 28 stored at either 4 °C or 25 °C.
First, the effects of pH were evaluated. All biopolymer complex solutions were stable against precipitation on day 0 (Fig. 2 and Appendix Fig. 7), which was not the case for PC solutions, especially not at a pH of 4 (close to the pI) (Fig. 1). Moreover, all solutions, except for solutions prepared at a pH of 2.5 and heated to 90 °C, were stable against precipitation over the storage period of 28 days. The improvement against pH-dependent precipitation around the isoelectric point can be related to an increase in bulk viscosity, steric interactions among polysaccharide residues, and electrostatic repulsion, which was described for carrageenan-whey protein complexes.31
The effects of storage on color were described below. Unheated solutions showed almost no color loss over time, independent of the pH used. The unheated PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions stored at a pH of 4.0 showed a shift towards turquoise when stored at 25 °C, while the same solutions stored at 4 °C had a more bluish color after 28 days of storage. This indicates an altering protein structure with a simultaneous modification of the phycocyanobilin structure which was accelerated by the increased storage temperature.32 Structural changes could be due to hydrophobic interactions between PC proteins that evolve over time.33
λC solutions stored at a pH of 4.0 showed a shift towards turquoise when stored at 25 °C, while the same solutions stored at 4 °C had a more bluish color after 28 days of storage. This indicates an altering protein structure with a simultaneous modification of the phycocyanobilin structure which was accelerated by the increased storage temperature.32 Structural changes could be due to hydrophobic interactions between PC proteins that evolve over time.33
Next, the heating effects were specified. The color loss caused by thermal denaturation appeared to be stronger at higher pH values, whereas an intense turquoise color was maintained at a pH of 2.5 and 3.0, especially when the solutions were treated at 90 °C. A possible explanation for this might be that PC is stabilized by λC at acidic pH values below the pI of ∼pH 4.6 by electrostatic complexation between the anionic λC and the positively charged PC, as previously reported. These interactions do not occur, or to a far lesser extent, at pH values above the pI, as for solutions prepared at a pH of 6.
From here on, the various effects of storage time and temperature, heat treatment and pH were considered in combination. At a pH of 2.5, PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions that were heated to 90 °C precipitated (Appendix Fig. 8), which could be due to the formation of large and dense complexes. These structures are fostered by the aggregation of PC by hydrophobic interactions and simultaneous complexation of PC and λC by electrostatic interactions.12,33 However, the sedimentation occurred slowly and is hence not visible in Fig. 2.
λC solutions that were heated to 90 °C precipitated (Appendix Fig. 8), which could be due to the formation of large and dense complexes. These structures are fostered by the aggregation of PC by hydrophobic interactions and simultaneous complexation of PC and λC by electrostatic interactions.12,33 However, the sedimentation occurred slowly and is hence not visible in Fig. 2.
Over the observed storage time, a temperature of 4 °C had only a minor influence on the appearance of those solutions that have been heated to 70 °C and adjusted to a pH of 2.5–4.5. The intensity of the blue color at a pH of 5.0–6.0 was slightly less compared to the unheated solutions. As suggested previously, this is due to electrostatic interactions at lower pH values. At a pH of 4.5, which is above the pI, λC might attach to some positively charged patches on the PC surface.18,34
However, increasing the storage temperature from 4 to 25 °C had a remarkable effect on the color appearance and intensity of the heated solutions. Mixed biopolymer solutions heated to 70 °C showed a color shift from turquoise towards a greyish green at a pH of 3.5–4.5, and to a light grey at a pH of 5.0 to 6.0. The shift towards green can be related to PC dissociation causing a color shift from 620 to 346–360 nm, while the grey color might be associated with protein aggregation causing diffuse light scattering.6,8 The effects were even more pronounced for PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions that have been heated to 90 °C and could already be observed under cold storage conditions. One explanation could be that, due to the strong thermal treatment, hydrophobic patches which were previously hidden inside the PC structure became exposed and resulted in PC aggregation overt time.12,33 However, independent of the storage time and temperature, all the mixed biopolymer solutions were found to maintain a turquoise color at a pH 2.5 of and 3.0.
λC solutions that have been heated to 90 °C and could already be observed under cold storage conditions. One explanation could be that, due to the strong thermal treatment, hydrophobic patches which were previously hidden inside the PC structure became exposed and resulted in PC aggregation overt time.12,33 However, independent of the storage time and temperature, all the mixed biopolymer solutions were found to maintain a turquoise color at a pH 2.5 of and 3.0.
The absorption of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions (1
λC solutions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
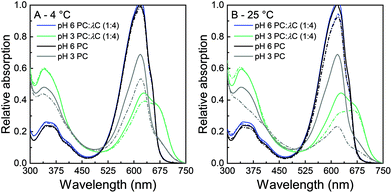
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) was measured to quantify the color shift and reveal changes in the spectrum with pH change, heat treatment, as well as storage time and temperature. The absorption of solutions is directly linked to the visual appearance of the solutions and is subsequently presented for unheated (Fig. 3) and heated (Fig. 4) solutions at a pH of 3.0 and a pH of 6.0 that have been stored for 0 and 28 days at either 4 °C (A) or 25 °C (B), respectively. Solutions with a pH of 3.0 show a high stability of PC
4) was measured to quantify the color shift and reveal changes in the spectrum with pH change, heat treatment, as well as storage time and temperature. The absorption of solutions is directly linked to the visual appearance of the solutions and is subsequently presented for unheated (Fig. 3) and heated (Fig. 4) solutions at a pH of 3.0 and a pH of 6.0 that have been stored for 0 and 28 days at either 4 °C (A) or 25 °C (B), respectively. Solutions with a pH of 3.0 show a high stability of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions against precipitation and color degradation (see above) even at high treatment temperatures. A pH of 6.0 served as a standard reference for solutions with a pH > pI. The latter solutions showed poorer color stabilization.
λC solutions against precipitation and color degradation (see above) even at high treatment temperatures. A pH of 6.0 served as a standard reference for solutions with a pH > pI. The latter solutions showed poorer color stabilization.
Unheated solutions (Fig. 3) had a lower absorption at a pH of 3.0 as compared to a pH of 6.0 which can be attributed to PC hexamers dissociating to monomers at low pH values.8 During storage, solutions that were stored at 25 °C had a greater decrease in absorption intensity compared to those stored at 4 °C, but overall losses were smaller compared to pure unheated PC solutions. Similar to the prevention of the pI-dependent formation of PC aggregates without the presence of λC, this effect might be related to an increased viscosity, steric interactions among polysaccharide residues, and electrostatic repulsion.18,31
The second major difference observed in the absorption of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions is the peak at 660 nm. Individual PC solutions had a maximal absorption at 620 nm while decreasing the pH in the biopolymer mixture led to an increasing shift towards the red region. It is well known that anionic surfactants and urea can cause protein dissociation.9 In addition the separation of the PC subunits by urea was reported to cause an absorption shift from 620 to 662 nm.9,36 Thus, the previous results are likely to be related to a rearrangement or dissociation of the α- and β-subunits due to the complexation with λC coming with planarity changes of the phycocyanobilin.18
λC solutions is the peak at 660 nm. Individual PC solutions had a maximal absorption at 620 nm while decreasing the pH in the biopolymer mixture led to an increasing shift towards the red region. It is well known that anionic surfactants and urea can cause protein dissociation.9 In addition the separation of the PC subunits by urea was reported to cause an absorption shift from 620 to 662 nm.9,36 Thus, the previous results are likely to be related to a rearrangement or dissociation of the α- and β-subunits due to the complexation with λC coming with planarity changes of the phycocyanobilin.18
Three findings from the absorption measurements are of particular interest. First, the pH 3.0 PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions had a higher absorption at 620 nm as compared to solutions prepared at a pH of 6.0. Second, A620 decreased significantly over time at 4 °C for PC
λC solutions had a higher absorption at 620 nm as compared to solutions prepared at a pH of 6.0. Second, A620 decreased significantly over time at 4 °C for PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions adjusted to a pH of 6.0 and heated to 90 °C. At a 25 °C storage temperature, all PC
λC solutions adjusted to a pH of 6.0 and heated to 90 °C. At a 25 °C storage temperature, all PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions having a pH of 6.0 significantly decreased in A620 over time, whereas PC
λC solutions having a pH of 6.0 significantly decreased in A620 over time, whereas PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions at a pH of 3.0, heated to 90 °C, remained stable over the period of 28 days. Third, PC
λC solutions at a pH of 3.0, heated to 90 °C, remained stable over the period of 28 days. Third, PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions adjusted to a pH of 3.0 were found to be more heat resistant than solutions adjusted to a pH of 6.0. The latter finding is contrary to the results we found for pure heated PC solutions.
λC solutions adjusted to a pH of 3.0 were found to be more heat resistant than solutions adjusted to a pH of 6.0. The latter finding is contrary to the results we found for pure heated PC solutions.
The results pinpoint the stability improvement against storage-related color fading in acidic pH values due to electrostatic interactions of λC and PC, especially after thermal treatments (Fig. 4). Thus, the color might additionally be stabilized by hydrophobic interactions which build up during the heat treatment.12,37
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) complex solutions unheated and heated to 70 or 90 °C at pH 2.5 and 3.0 and stored over 28 days at either 4 or 25 °C. PC
4) complex solutions unheated and heated to 70 or 90 °C at pH 2.5 and 3.0 and stored over 28 days at either 4 or 25 °C. PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) complex solutions contained 0.15% (w/w) λC and 0.04% (w/w) PC
4) complex solutions contained 0.15% (w/w) λC and 0.04% (w/w) PC
| Heat treatment | Day | d in nm at pH 2.5 | d in nm at pH 3.0 | ||
|---|---|---|---|---|---|
| 4 °C | 25 °C | 4 °C | 25 °C | ||
| Indices with small letters indicate differences within one column of solutions prepared with the same heat treatment (α = 0.05). Indices with capital letters indicate a difference between a pH of 2.5 and 3.0 within one row calculated from a t-test (α = 0.05).a Were excluded because of precipitation. | |||||
| None | 0 | 890 ± 155aA | 907 ± 14aA | ||
| 3 | 868 ± 126a | 526 ± 31b | 901 ± 9a | 698 ± 80b | |
| 6 | 770 ± 82a | 476 ± 31b | 835 ± 23ab | 562 ± 41bc | |
| 14 | 715 ± 60a | 360 ± 17b | 780 ± 52bc | 420 ± 25cd | |
| 28 | 547 ± 12a | 316 ± 7b | 694 ± 11c | 354 ± 10d | |
| 70 °C | 0 | 484 ± 57aA | 646 ± 59aA | ||
| 3 | 464 ± 62a | 416 ± 29ab | 614 ± 79a | 554 ± 10ab | |
| 6 | 436 ± 92a | 386 ± 5abc | 545 ± 90a | 509 ± 50ab | |
| 14 | 448 ± 57a | 336 ± 20bc | 603 ± 62a | 410 ± 3bc | |
| 28 | 424 ± 26a | 291 ± 4c | 532 ± 8a | 360 ± 29c | |
| 90 °C | 0 | 871 ± 185aA | 200 ± 5aB | ||
| 3 | 1355 ± 960a | 1661 ± 938a | 202 ± 10a | 202 ± 11a | |
| 6 | 1305 ± 950a | 1734 ± 904a | 200 ± 9a | 202 ± 7a | |
| 14 | 1382 ± 1068a | 2025 ± 1012a | 199 ± 12a | 202 ± 14a | |
| 28 | 201 ± 9a | 210 ± 5a | |||
On day 0, the unheated biopolymer mixtures had a similar diameter of about 900 nm at both pH values. With increasing storage time, the complex diameter decreased. The electrostatic attraction of both biopolymers might slowly cause association of the biopolymers, resulting in an increase in density. According to Veis, Aranyi (1960) the structural rearrangement could be driven by an electrostatic entropy gain caused by detached counter ions.38
The rearrangement of the complexes was accelerated by a higher storage temperature, which could be due to an increased Brownian motion and a decreased solution viscosity at higher temperatures, both influencing the movement of biopolymers in solutions. Also, hydrophobic interactions might develop at higher temperatures.33 Moreover, unheated PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC complexes at a pH of 2.5 were found to be smaller than the respective solutions at a pH of 3.0, which could be a result of an increased electrostatic attraction at lower pH values. This is in agreement with the transmittance measurements. At acidic pH values, the transmittance of complex solutions decreased, which was shown to be a result of strong protein-polysaccharide associations39 (Appendix Fig. 8).
λC complexes at a pH of 2.5 were found to be smaller than the respective solutions at a pH of 3.0, which could be a result of an increased electrostatic attraction at lower pH values. This is in agreement with the transmittance measurements. At acidic pH values, the transmittance of complex solutions decreased, which was shown to be a result of strong protein-polysaccharide associations39 (Appendix Fig. 8).
Heating the complexes to 70 °C led to smaller complexes compared to the unheated mixtures, which could either be a result of an accelerated chemical reaction between the biopolymers or hydrophobic interactions among PC due to the thermal treatment favoring association interactions.12,40 Further, positively charged amino acid residues could be exposed due to structural rearrangements which promote electrostatic interactions.41 The complexes were again similar in diameter at a pH of 2.5 and 3.0 when heated to 70 °C on day 0. With prolonged storage, it was evident that complexes formed at a pH of 2.5 possessed smaller diameters than at a pH of 3.0. Independent of the pH, a storage temperature of 25 °C led again to smaller complexes as compared to cold storage. The rearrangement might be related to carrageenans’ backbone flexibility and increased hydrophobic interactions at higher temperatures.31,37,42
Heating the solutions to 90 °C during preparation caused an entirely different effect. At a pH of 2.5, the complexes had a significantly larger diameter than at a pH of 3.0 and the solutions became very inhomogeneous in size, which might be related to aggregation. In principle, secondary aggregation of protein–carbohydrate complexes is reduced by steric interactions and electrostatic repulsion. However, the PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC solutions showed a significantly decreased magnitude in zeta potential from a pH of 6.0 to 2.5, when the solutions were heated to 90 °C (Appendix Table 3). This could either be related to charge neutralization or dissociation of λC which has a pKa of around 2.0.43 Thus, complex-complex association might be a result of decreased electrostatic repulsion. Further, the increasing aggregation of PC might be related to hydrophobic interactions. As the solutions precipitated after 14 days, an ongoing rearrangement and aggregation of the complexes over time led to dense and big particles that tend to precicipitate. Zeeb et al. (2018) reported that biopolymer complexes of whey protein isolate and pectin showed an Ostwald ripening-like dependence on the size after thermal treatments due to increasing hydrophobic and decreasing electrostatic interactions.44 To the contrary, stable solutions could be produced at a pH of 3.0, which might be linked to the higher magnitude of the zeta potential and thereby stronger electrostatic repulsion (Appendix Table 3).
λC solutions showed a significantly decreased magnitude in zeta potential from a pH of 6.0 to 2.5, when the solutions were heated to 90 °C (Appendix Table 3). This could either be related to charge neutralization or dissociation of λC which has a pKa of around 2.0.43 Thus, complex-complex association might be a result of decreased electrostatic repulsion. Further, the increasing aggregation of PC might be related to hydrophobic interactions. As the solutions precipitated after 14 days, an ongoing rearrangement and aggregation of the complexes over time led to dense and big particles that tend to precicipitate. Zeeb et al. (2018) reported that biopolymer complexes of whey protein isolate and pectin showed an Ostwald ripening-like dependence on the size after thermal treatments due to increasing hydrophobic and decreasing electrostatic interactions.44 To the contrary, stable solutions could be produced at a pH of 3.0, which might be linked to the higher magnitude of the zeta potential and thereby stronger electrostatic repulsion (Appendix Table 3).
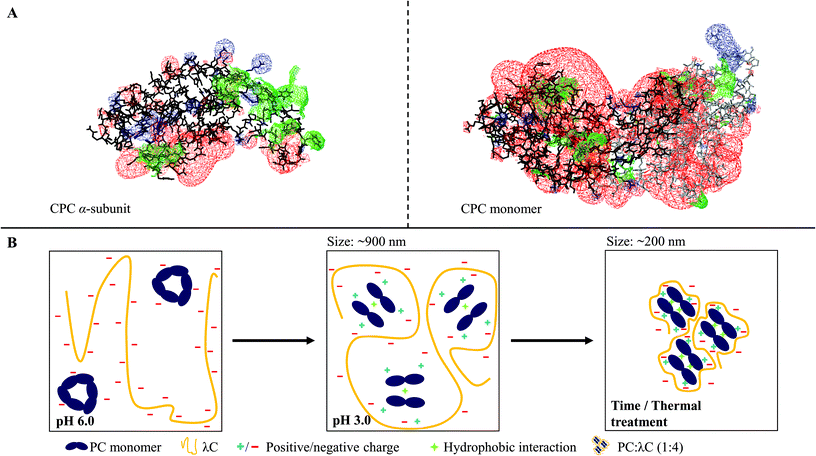
3.4 Mechanistic insights
Summarizing the results of previous studies and the present investigations, a hypothetical scheme is proposed in this study, which is summarized in Fig. 5.Phycocyanin carries differently charged patches on its surface (Fig. 5A). At a pH of 6.0, PC trimers and carrageenan coexist in solution but do not attract each other (Fig. 5B). Lowering the pH to 3.0 causes the surface charge of PC to become more positive leading to the association of the polymers, which form stable soluble complexes. The PC structure shifts to monomers and the absorption shift towards red, which might indicate a further dissociation into α- and β-subunits caused by the complex formation (Fig. 5B). Ongoing interactions and rearrangements of hydrophobic nature cause smaller or denser PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC complexes over time, which is favored by higher storage temperatures (Fig. 5B). Moreover, heat treatments during sample preparation lead to smaller or denser complexes as a result of increased hydrophobic interactions between PC trapped within the complex and accelerated electrostatic complexation.
λC complexes over time, which is favored by higher storage temperatures (Fig. 5B). Moreover, heat treatments during sample preparation lead to smaller or denser complexes as a result of increased hydrophobic interactions between PC trapped within the complex and accelerated electrostatic complexation.
4 Conclusion
Fig. 6 compiles our key findings on the effects of storage time and temperature on heated and unheated PC solutions and PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC complex solutions in a schematic illustration.
λC complex solutions in a schematic illustration.
The color intensity of pure PC decreased over time especially at low pH values (pH 2.5 to 4.5), high heating (90 °C), and storage (25 °C) temperatures. However, electrostatic complexation with λC considerably increased the color stability at pH < pI and facilitated a stabilization in color over the 28-day storage period especially at a pH of 3.0 even when heated to 90 °C and stored at 25 °C. These results might be of great interest for low-viscous food applications such as smoothies or soft drinks that are stored either at refrigerated or room temperature and formulated with natural colorants.
Conflicts of interest
There are no conflicts to declare.Appendix
See Fig. 7 and 8 and Tables 3 and 4.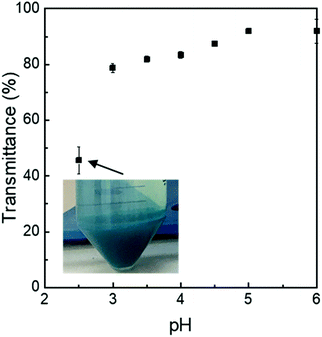 | ||
Fig. 8 Relative transmittance of PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1 λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) complexes (∼0.04% (w/w) PC) prepared at 90 °C and stored for 14 days at 25 °C plotted over pH. 4) complexes (∼0.04% (w/w) PC) prepared at 90 °C and stored for 14 days at 25 °C plotted over pH. | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1
λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) complex solutions unheated and subjected to heat treatments at 70 or 90 °C at a pH of 2.5–6.0
4) complex solutions unheated and subjected to heat treatments at 70 or 90 °C at a pH of 2.5–6.0
| Heat treatment | pH | ||||||
|---|---|---|---|---|---|---|---|
| 2.5 | 3.0 | 3.5 | 4.0 | 4.5 | 5.0 | 6.0 | |
| 0.04% PC solutions | |||||||
| None | 23.1 ± 0.4 | 24.0 ± 0.4 | 16.7 ± 0.1 | 1.7 ± 1.2 | −16.4 ± 3.0 | −20.1 ± 0.4 | −12.4 ± 0.1 |
| 70 °C | 22.9 ± 0.9 | 22.9 ± 1.3 | 21.0 ± 0.7 | 14.1 ± 1.2 | −10.9 ± 2.2 | −25.3 ± 2.3 | −26.6 ± 0.4 |
| 90 °C | 22.3 ± 1.6 | 23.3 ± 0.3 | 20.7 ± 1.0 | 15.9 ± 3.0 | −5.4 ± 4.4 | −21.7 ± 3.4 | −24.3 ± 2.4 |
PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) λC (1 λC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) complex solutions 4) complex solutions |
|||||||
| None | −59.5 ± 2.3 | −58.3 ± 1.0 | −57.1 ± 1.3 | −56.9 ± 1.8 | −59.2 ± 0.1 | −57.8 ± 1.0 | −56.3 ± 1.8 |
| 70 °C | −56.7 ± 0.7 | −54.6 ± 1.9 | −55.3 ± 1.4 | −58.1 ± 0.8 | −56.0 ± 1.4 | −57.2 ± 1.9 | −55.8 ± 0.1 |
| 90 °C | −36.7 ± 1.4 | −45.6 ± 0.1 | −50.5 ± 1.2 | −55.0 ± 0.6 | −56.3 ± 1.2 | −55.6 ± 1.3 | −57.2 ± 0.8 |
| Arthrospira platensis extract | λ-Carrageenan | |
|---|---|---|
| Value (unit) | ||
| Energy | 364 (kcal per 100 g) | 272 (kcal per 100 g) |
| Protein | 87.5 (g per 100 g) | 1.0 (g per 100 g) |
| Carbohydrates | 1.2 (g per 100 g) | 67.00 (g per 100 g) |
| Sugar | 1.17 (g per 100 g) | 0.00 (g per 100 g) |
| Fat | 0.60 (g per 100 g) | 0.00 (g per 100 g) |
| Saturated fatty acids | 0.36 (g per 100 g) | 0.00 (g per 100 g) |
| Dietary fiber | 0.5 (g per 100 g) | 67 (g per 100 g) |
| Common salt | 1.51 (g per 100 g) | 17.2 (g per 100 g) |
Acknowledgements
This project was partly funded by GNT Europa GmbH.References
- D. L. Zink, Emerging Infect. Dis., 1997, 3, 467–469 CrossRef CAS PubMed.
- C. Spence, Int. J. Gastron. Food Sci., 2019, 17, 1–10 Search PubMed.
- L. Jespersen, L. D. Strmdahl, K. Olsen and L. H. Skibsted, Eur. Food Res. Technol., 2005, 220, 261–266 CrossRef CAS.
- T. T. L. Cheung, A. F. Junghans, G. B. Dijksterhuis, F. Kroese, P. Johansson, L. Hall and D. T. D. de Ridder, Appetite, 2016, 106, 2–12 CrossRef CAS PubMed.
- B. Weiss, Environ. Health Perspect., 2008, 116, 240–241 CrossRef PubMed.
- Handbook on natural pigments in food and beverages. Industrial applications for improving food color, ed. R. Carle and R. M. Schweiggert, Woodhead Publishing, Duxford, UK, 2016, number 295 Search PubMed.
- European Parliament and European Council Regulation (EC) No 1333/2008 in OJEU L 354/16, 2008, pp. 1–18.
- D. S. Berns and R. MacColl, Chem. Rev., 1989, 89, 807–825 CrossRef CAS.
- K. L. Thoren, K. B. Connell, T. E. Robinson, D. D. Shellhamer, M. S. Tammaro and Y. M. Gindt, Biochemistry, 2006, 45, 12050–12059 CrossRef CAS PubMed.
- N. Adir, R. Vainer and N. Lerner, Biochim. Biophys. Acta, Bioenerg., 2002, 1556, 168–174 CrossRef CAS.
- Z. Zhang, S. Cho, Y. Dadmohammadi, Y. Li and A. Abbaspourrad, Food Hydrocoll., 2021, 110, 106055 CrossRef CAS.
- G. O. Phillips and P. A. Williams, Handbook of food proteins, Woodhead Publishing, Cambridge UK, Philadelphia, 2011, no. 222 Search PubMed.
- A. Reddy, D. F. Norris, S. S. Momeni, B. Waldo and J. D. Ruby, J. Am. Dent. Assoc., JADA, 2016, 147, 255–263 CrossRef PubMed.
- R. Chaiklahan, N. Chirasuwan and B. Bunnag, Process Biochem., 2012, 47, 659–664 CrossRef CAS.
- Y. Li, Z. Zhang and A. Abbaspourrad, Food Hydrocoll., 2021, 119, 106852 CrossRef CAS.
- J. L. MacDonald and E. Leeb, EP3692804A1, 2020-08-12.
- W. R. Blakemore, Reference Module in Food Science, Elsevier, 2016 Search PubMed.
- S. Buecker, L. Grossmann, M. Loeffler, E. Leeb and J. Weiss, Food Chem., 2022, 380, 132157 CrossRef CAS PubMed.
- (a) C. Safi, M. Charton, O. Pignolet, F. Silvestre, C. Vaca-Garcia and P.-Y. Pontalier, J. Appl. Phycol., 2013, 25, 523–529 CrossRef CAS; (b) R. Matissek, M. Fischer and G. Steiner, Lebensmittelanalytik, Springer Berlin Heidelberg, Berlin, Heidelberg, 2018 CrossRef.
- N. Yoshikawa and A. Belay, J. AOAC Int., 2008, 91, 524–529 CrossRef CAS PubMed.
- E. W. Becker, Biotechnol. Adv., 2007, 25, 207–210 CrossRef CAS PubMed.
- (a) S. T. Silveira, L. K. d. M. Quines, C. A. V. Burkert and S. J. Kalil, Bioprocess Biosyst. Eng., 2008, 31, 477–482 CrossRef CAS PubMed; (b) CN101942014B.
- 秦松,刘冰,闫鸣艳,林甜甜,衣悦涛, CN101942014B, 2010-08-23.
- (a) S. Benelhadj, A. Gharsallaoui, P. Degraeve, H. Attia and D. Ghorbel, Food Chem., 2016, 194, 1056–1063 CrossRef CAS PubMed; (b) A. Vonshak, Spirulina platensis (Arthrospira). Physiology, cell-biology, and biotechnology, Taylor & Francis, London, Bristol PA, 1997 CrossRef.
- M. Thompson, L. Owen, K. Wilkinson, R. Wood and A. Damant, Analyst, 2002, 127, 1666–1668 RSC.
- (a) H.-N. Su, B.-B. Xie, X.-L. Chen, J.-X. Wang, X.-Y. Zhang, B.-C. Zhou and Y.-Z. Zhang, J. Appl. Phycol., 2010, 22, 65–70 CrossRef CAS; (b) M. Morançais, J.-L. Mouget and J. Dumay, Microalgae in Health and Disease Prevention, Elsevier, 2018, pp. 145–175 Search PubMed.
- V. K. Kannaujiya and R. P. Sinha, J. Appl. Phycol., 2016, 28, 1063–1070 CrossRef CAS.
- N. Soulier and D. A. Bryant, Photosynth. Res., 2021, 147, 11–26 CrossRef CAS PubMed.
- L. Böcker, T. Hostettler, M. Diener, S. Eder, T. Demuth, J. Adamcik, K. Reineke, E. Leeb, L. Nyström and A. Mathys, Food Chem., 2020, 316, 126374 CrossRef PubMed.
- L. Böcker, S. Ortmann, J. Surber, E. Leeb, K. Reineke and A. Mathys, Innovative Food Sci. Emerging Technol., 2019, 52, 116–121 CrossRef.
- G. O. Phillips and P. A. Williams, Handbook of Hydrocolloids, Woodhead Pub, Cambridge, 2nd edn, 2009 Search PubMed.
- R. MacColl, J. Struct. Biol., 1998, 124, 311–334 CrossRef CAS PubMed.
- X. Li, L. Li, Y. Ma, R. Wang, Y. Gu and L. Day, Food Biosci., 2020, 34, 100530 CrossRef CAS.
- C. K. W. Koo, C. Chung, J.-T. R. Fu, A. Sher, P. Rousset and D. J. McClements, Food Res. Int., 2019, 123, 779–789 CrossRef CAS PubMed.
- (a) R. MacColl, L. E. Eisele and A. Menikh, Biopolymers, 2003, 72, 352–365 CrossRef CAS PubMed; (b) O. Kao, D. S. Berns and R. MacColl, Eur. J. Biochem., 1971, 19, 595–599 CrossRef CAS PubMed.
- R. MacColl and D. Guard-Friar, Phycobiliproteins, CRC Press, Boca Raton Fla., 1987 Search PubMed.
- C. Tanford, The hydrophobic effect. Formation of micelles and biological membranes, Wiley, New York, 2nd edn, 1980 Search PubMed.
- A. Veis and C. Aranyi, J. Phys. Chem., 1960, 64, 1203–1210 CrossRef CAS.
- (a) F. Weinbreck, R. de Vries, P. Schrooyen and C. G. de Kruif, Biomacromolecules, 2003, 4, 293–303 CrossRef CAS PubMed; (b) S. Kim, J. Huang, Y. Lee, S. Dutta, H. Y. Yoo, Y. M. Jung, Y. Jho, H. Zeng and D. S. Hwang, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E847–E853 CrossRef CAS PubMed.
- G. Job and R. Rüffler, Physical Chemistry from a Different Angle, Springer International Publishing, Cham, 2016 Search PubMed.
- G. Saelensminde, Ø. Halskau and I. Jonassen, Extremophiles, 2009, 13, 11–20 CrossRef CAS PubMed.
- (a) X. Lu, S. Xie, L. Wang, H. Xie, Q. Lei and W. Fang, Chem. Phys., 2020, 538, 110910 CrossRef CAS; (b) G. Berth, J. Vukovic and M. D. Lechner, J. Appl. Polym. Sci., 2008, 110, 3508–3524 CrossRef CAS.
- N. Al-Zebari, S. M. Best and R. E. Cameron, J. Phys. Mater., 2019, 2, 15003 CrossRef CAS.
- B. Zeeb, L. Mi-Yeon, M. Gibis and J. Weiss, Food Hydrocoll., 2018, 74, 53–61 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc00809b |
| This journal is © The Royal Society of Chemistry 2022 |