Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mechanochemical Fischer indolisation: an eco-friendly design for a timeless reaction†
Andrea
Porcheddu
 a,
Rita
Mocci
a,
Rita
Mocci
 *a,
Margherita
Brindisi
*a,
Margherita
Brindisi
 *b,
Federico
Cuccu
*b,
Federico
Cuccu
 a,
Claudia
Fattuoni
a,
Claudia
Fattuoni
 a,
Francesco
Delogu
a,
Francesco
Delogu
 c,
Evelina
Colacino
c,
Evelina
Colacino
 d and
Maria Valeria
D'Auria
d and
Maria Valeria
D'Auria
 b
b
aDipartimento di Scienze Chimiche e Geologiche, Università degli Studi di Cagliari, Cittadella Universitaria, 09042 Cagliari, Italy. E-mail: rita.mocci@unica.it
bDipartimento di Farmacia, Università degli Studi di Napoli ‘Federico II’, Via Domenico Montesano 49, 80131, Napoli, Italy. E-mail: margherita.brindisi@unina.it
cDipartimento di Ingegneria Meccanica, Chimica, e dei Materiali, Università degli Studi di Cagliari, via Marengo 2, 09123 Cagliari, Italy
dICGM, Univ Montpellier CNRS, ENSCM, Montpellier, France
First published on 11th May 2022
Abstract
We developed an environmentally friendly mechanochemical protocol to induce an effective Fischer indolisation to synthesize indoles and indolines taking advantage of oxalic acid and dimethylurea. The solvent-free procedure shows versatility and wide scope; it applies to a broad range of arylhydrazines and carbonyl compounds, leading to variously decorated indoles and indolenines. Upon suitable modification, the methodology can also allow preparing compounds of pharmaceutical interest.
Introduction
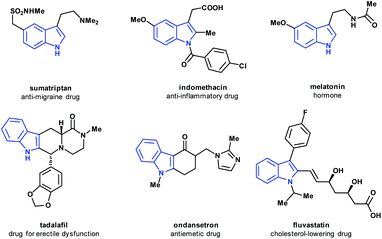
Nitrogen-containing heterocycles have crucial importance in medicinal chemistry and drug discovery, as demonstrated by their striking predominance in marketed drugs.1–4 Owing to their variety of biological activities, indole- and indoline-based structures have always deserved particular importance, being broadly found in medicinally relevant compounds of natural5–8 and synthetic origins.9–11The indole scaffold has been referred to as a “privileged scaffold”12–14 owing to its capacity to interact with a diverse range of receptors, such as dopamine, serotonin and sigma receptors with high affinity.15–18 Moreover, indole-based marketed drugs cover a broad set of indications, including anti-inflammatory, antihypertensive, anti-tumor, antiemetic, and antimigraine activities, among others (Fig. 1).19
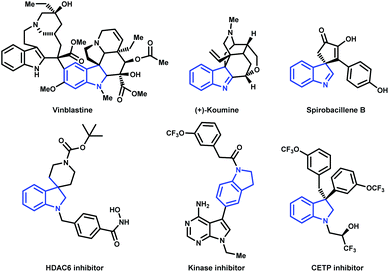
Despite indole primacy within the family, indolenines and their reduced counterparts (i.e. indolines) also represent recurrent substructures inherently incorporated in numerous alkaloids as well as medicinally relevant compounds (Fig. 2).20–26
 | ||
| Fig. 2 Structures of indol(en)ine-containing alkaloids and another synthetic medicinally relevant compounds. | ||
In general, the central relevance of this class of compounds significantly fostered synthetic research efforts towards the achievement of indole-based complex molecules and simpler architectures for medicinal chemistry applications.27,28
Fisher indolisation and its interrupted variation still represent the preeminent method for synthesizing the indole and indolenine cores, respectively (Scheme 1, pathways a and b). This timeless reaction was first discovered in 1883 by Emil Fischer and found a broad array of applications in industry and academia over the years.29–34 Instead, an indolenine ring is generated in the case of α,α′-disubstituted aldehydes and ketones (namely, the interrupted Fischer variation, Scheme 1, pathway b).29–32 The metastable indolenines quickly undergo 1,2-migration under acid conditions to give the thermodynamically more stable indole (Scheme 1, pathway c). This is a well-known phenomenon that readily occurs in indolenine chemistry.33,34
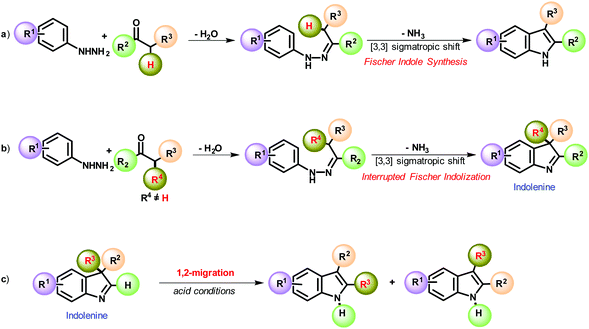 | ||
| Scheme 1 General Fischer indole synthesis (pathway a) and its interrupted variation (pathway b). Acid promoted 1,2-alkyl migration of 3,3′ alkylindolenine (pathway c). | ||
Effective generation of indole-based compounds employing Fischer reaction usually requires strong acids in organic solvents (toluene, xylene, DMF, THF, etc.) at elevated temperatures (up to 200 °C). Typical acidic catalysts used for Fischer-type reactions include Brønsted acids (HCl, H2SO4, TFA, p-TsOH, PPA, AcOH),34–39 and Lewis acids (BF3, ZnCl2, AlCl3, TiCl4, PCl3).40–42
As a result, the Fischer indolisation is an energy-demanding process generating considerable waste, mainly because of the large amount of strong acid catalysts needed to boost the reaction towards the complete conversion of the reactants. Many of the reported methods suffer from drawbacks such as harsh or sensitive reaction conditions (ethyl diazoacetate, NIS, hazardous reagents: Zn(CN)2, BF3·OEt2, N-nitrosoanilines, vinyl azides) or limited substrate scope.43–48
Implementation of Fischer protocols in a greener and more sustainable mode has attracted significant attention in the past few years. Newly proposed methodologies have focused on heterogeneous and recyclable solid acid catalysts (e.g. zeolites, montmorillonite clays, amberlyst).49–51 Still, their practical application is often anyway limited by restricted accessibility and/or rapid deactivation. The Fischer reaction was also developed in environmentally benign solvents such as ethanol and water.35,52,53 However, the latter procedures are promoted by SO3H-functionalized Brønsted acidic ionic liquids. The preparation of these imidazolium-based catalysts requires additional synthetic steps and strong acids (H2SO4, HCl, H3PO4etc., at water reflux) for the protonation of the final zwitterions, thus undermining the validity of the initial assumptions, and questioning about the sustainability of the process considering the toxicity of ionic liquids.54,55 In addition, complete conversion is achieved at temperatures above 80 °C, which are often inconsistent with the use of temperature-sensitive functional substrates.
We herein report the results of our extensive experimentation aimed at developing a mechanochemical Fischer-type protocol of broad usefulness to synthesize indole derivatives. The mechanochemical approach is gaining increasing attention due to its ability of imparting existing methodologies with higher efficiency and sustainability.56–67
Among the advantages, mechanochemical activation helps circumventing the challenges associated with solvent selection and reagent solubility.65,68,69 This aspect is particularly relevant when setting up ‘greener’ protocols and multistep sequences. Furthermore, reduced energy costs and minimum operator's exposure to chemicals render the process more cost-effective.56,58,70 In this context, implementing a sustainable mechanochemical Fisher indolisation protocol prone to broad application shows undeniable appeal.
A recently published article71 on the Fischer indole synthesis by mechanochemical activation highlights the relevance of this topic and the usefulness of ball-milling to ensure more sustainable access to this class of compounds. However, despite the significant improvement, this procedure still has some shortcomings that severely limit its utility. In particular, the scope of this newly developed methodology is limited to the preparation of tetrahydrocarbazole frameworks in the presence of silica as a grinding additive (720 mg mmol−1 of ketone).
We started our investigation on the mechanochemical Fischer synthesis prompted by the need for wide-scope, operationally eco-compatible and straightforward protocols. p-Tolylhydrazine hydrochloride 1a and propiophenone 2a were selected as the model substrates to set up the appropriate conditions for developing a more comprehensive strategy for the adequate preparation of indole- and indoline-based templates (Table 1).
| Entry | Additive (mg) | Acid (mg) | 3a (%) |
|---|---|---|---|
a
1a (158.6 mg, 1.0 mmol), 2a (147.6 mg, 1.1 mmol) and additive were ball-milled in a 15 mL ZrO2 milling jar with 2 milling balls (∅ = 8 mm, mtot = 6.5 g overall) of the same material.
b Selectivity ratio 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a >99%.
c Determined by GC-MS analysis.
d Catalytic amounts of MeOH were used (η = 0.1 μL mg−1).
e Catalytic amounts of CH3CN were used (η = 0.1 μL mg−1). 4a >99%.
c Determined by GC-MS analysis.
d Catalytic amounts of MeOH were used (η = 0.1 μL mg−1).
e Catalytic amounts of CH3CN were used (η = 0.1 μL mg−1).
|
|||
| 1 | SiO2 (300) | — | 14 |
| 2 | SiO 2 (300) | NaHSO 4 (120) | 46 |
| 3 | SiO2 (150) | NaHSO4 (120) | 20 |
| 4d | SiO2 (300) | NaHSO4 (120) | 34 |
| 5e | SiO2 (300) | NaHSO4 (120) | 35 |
| 6 | — | NaHSO4 (120) | — |
Aldehydes and ketones are predominantly liquid, so the reaction needs a solid grinding auxiliary/adsorbent to be performed effectively. Initially, we reasoned that silica gel could be the best choice since it was already successfully used for the mechanosynthesis of tetrahydrocarbazole derivatives. Related experiments have been performed in ZrO2 jars (15 mL) with two balls (∅ = 8 mm, mtot: 6.5 g), where p-tolylhydrazine hydrochloride 1a (159 mg, 1.0 mmol) and propiophenone 2a (147 mg, 1.1 mmol) were ball-milled in the presence of silica (300 mg) with a mixer mill (30 Hz) for 100 minutes.
Under these conditions, as previously highlighted,71,72 silica becomes an even stronger solid acid by absorbing HCl on its surface, thus promoting the subsequent acid-catalyzed Fischer reaction. To our surprise, in these first attempts to promote the indolisation of in situ generated hydrazone 4a,73 we detected only the formation of small amounts of the desired indole 3a accompanied by the unreacted ketone 2a (Table 1, entry 1, 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a = 99
4a = 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
At this point, knowing that Fischer indole synthesis is an acid promoted reaction, we increased the acidity of the resulting solid mixture by adding NaHSO4 (120 mg) to promote the [3 + 3] sigmatropic rearrangement under ball-milling conditions (Table 1, entry 2).74 The increased acidity resulted in a significant rise in indole yield (46%),75–77 highlighting that the operating acidity of the reactive medium is a crucial design parameter controlling the mechanochemical hydrazone-indole conversion. This result prompted us to increase the ratio of the sulfate acid salt relating to silica gel. However, this was detrimental on the indolisation process (Table 1, entry 3).
We observed similar outcomes by adding a catalytic amount of solvent (MeOH, CH3CN; LAG, η = 0.1 μL mg−1; Table 1, entries 4 and 5). On the other hand, NaHSO4 (120 mg) in the absence of silica gel did not promote the transformation denoting a synergistic effect of the two components (Table 1, entry 6). Under these reaction conditions, even extending the milling time (300 min) did not significantly improve the result. Since acid catalysis proved crucial for boosting reaction, we turned our attention to other solid acids such as tetrabutylammonium bisulfate, p-toluensulfonic acid (PTSA), and tartaric acid (Table 2 and Table S1 in ESI†). However, they showed modest efficiency in promoting the Fischer indole synthesis, thus providing indole 3a in low yields.
| Entrya | Catalysts (mmol) | 3a (%) | Ratiob (%) of 3a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4a 4a |
|---|---|---|---|
| a 1a (158.6 mg, 1.0 mmol), 2a (147.6 mg, 1.1 mmol), and catalysts were ball-milled in a 15 mL ZrO2 milling jar with 2 milling balls (∅ = 8 mm, 6.5 g overall) of the same material. b Determined by GC-MS. c 1a (158.6 mg, 1.0 mmol), 2a (147.6 mg, 1.1 mmol) and imidazole (68.1 mg, 1.0 mmol) were milled for 60 minutes, afterward PTSA (344.4 mg, 2.0 mmol) was added, and the mixture was ball-milled for additional 100 minutes. d The jar was loaded with ZrO2 balls (20, ∅ = 3 mm, mtot = 6.5 g). e The mixture was ball milled for 300 minutes. | |||
| 1 | PTSA (1.5) | 36 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 2 | OA (1.5) | 32 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 3 | Tartaric acid (1.5) | 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
| 4 | Imidazole (1) | — | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 99 99 |
| 5c | PTSA/imidazole (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
25 | 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| 6 | DMU/PTSA (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
43 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| 7 | DMU/OA (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
40 | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 29 |
| 8d | DMU/OA (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
56 | 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 31 31 |
| 9d,e | DMU/OA (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3) 3) |
50 | 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 27 |
| 10d,e | DMU/OA (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7) 7) |
58 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 11d | DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5) 3.5) |
60 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 12d,e | DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5) 3.5) |
68 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 13d,e | DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5), MeOH (η = 0.1 μL mg−1) 3.5), MeOH (η = 0.1 μL mg−1) |
55 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 14 , |
DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 3.5), CH
3
COOH (η = 0.1 μL mg
−1
) 3.5), CH
3
COOH (η = 0.1 μL mg
−1
)
|
76 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1 1
|
| 15d,e | DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5), 3-pentanol (η = 0.1 μL mg−1) 3.5), 3-pentanol (η = 0.1 μL mg−1) |
77 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| 16d,e | DMU/OA (1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5), DMF (η = 0.1 μL mg−1) 3.5), DMF (η = 0.1 μL mg−1) |
79 | 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
Assuming that the inefficient formation of phenylhydrazone 4a is a consequence of our working conditions, we decided to use a basic scavenger for hydrochloric acid, thus favoring the nucleophilic attack from the acid-free phenylhydrazine.
In this regard, previous work78 has proven that imidazole can act as a mild base, allowing to overcome drawbacks related to the low conversion of the reagents into phenylhydrazone 4a. Imidazole was very effective in promoting the complete formation of phenylhydrazone 4a; on the contrary, it significantly inhibited the subsequent [3 + 3] rearrangement to the indole ring (Table 2, entry 4).
The indolisation process was split in two distinct steps to fine-tune the reaction parameters. The first one was targeting the formation of phenylhydrazone 4a in the presence of imidazole, while in the second, PTSA (2 equiv.) was added to the same jar to promote indole ring generation. However, even in this case, only small amounts of the desired indole 3a were obtained, accompanied by a considerable percentage of unreacted starting materials and the simultaneous formation of aniline as a side-product (Table 2, entry 5). Such a result suggested the occurrence of a competing path arising from the cleavage of the N–N phenylhydrazine bond under our working conditions. At this stage, we envisioned the possibility of screening different binary mixtures of hydrogen bond acceptors and donors, wondering if such a mixture, under mechanochemical processing conditions, could act as an efficient catalyst for the Fischer indolisation (see Table 2 and Table S1 in ESI†).
Gratifyingly, the oxalic acid (OA) and dimethylurea (DMU) mixture revealed remarkable efficiency. Indeed, OA displays marked acidity that could be beneficial in promoting the Fischer reaction (Table 2, entries 6 and 7). Regarding DMU, its presence significantly increased the conversion rate, while playing a minor role in the process selectivity.
In these first studies, the mechanochemical processes were carried out in the presence of an excess of the two solid components, specifically DMU (7 equiv., 617 mg) and OA (3 equiv., 270 mg).39
Moreover, it was observed a slight increment in the efficacy of the process, switching from 2 (∅ = 8 mm) to 20 (∅ = 3 mm) zirconia balls, keeping the overall final weight unchanged (mtot: 6.5 g, Table 2, entry 8).
These reaction conditions proved valuable in the mechanochemical Fischer indole synthesis, with the desired indole 3a obtained in 56% of yield after 100 minutes of milling (Table 2, entry 8).
An increase in reaction time up to 250 minutes did not significantly improve the results in terms of reaction yields (Table 2, entry 9). In contrast, a change in the ratio composition of DMU and OA resulted in an increased selectivity (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, Table 2 entry 10). Additionally, comparable results were obtained halving the amount of auxiliary grinding mixture (DMU and OA), thus reducing the reaction waste (Table 2, entry 11). Under these reaction conditions, we observed that ball milling over longer times enhanced conversion without affecting the process selectivity (Table 2, entry 12).
1, Table 2 entry 10). Additionally, comparable results were obtained halving the amount of auxiliary grinding mixture (DMU and OA), thus reducing the reaction waste (Table 2, entry 11). Under these reaction conditions, we observed that ball milling over longer times enhanced conversion without affecting the process selectivity (Table 2, entry 12).
The procedure performed even better under liquid-assisted grinding conditions, in the presence of a small amount of solvent (η = 0.1, μL mg−1). We observed that higher boiling point solvents provided indole 3a with improved and comparable yields (Table 2, entries 14–16). However, for the scope of this procedure, we identified acetic acid as the most suitable additive, offering a good balance between efficacy, cost of production, and environmental impact. Moreover, acetic acid can be easily removed during the final purification step. Once the reaction was accomplished, the crude was indeed purified by trituration with water. Indole 3a was recovered by filtration without using a single drop of organic solvent, emphasizing the green character of this mechanochemical protocol.
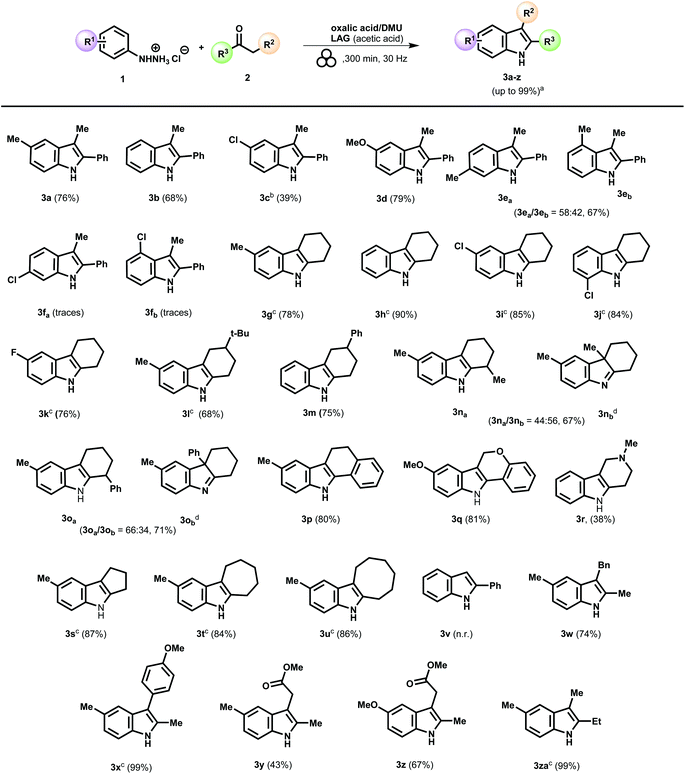
Once identified the optimal conditions, the reaction scope was broadened by reacting a set of different phenylhydrazines 1a–1i and 5a and ketones 2a–2q (as specified in the ESI†), in order to validate the generality and efficiency of this mechanochemical protocol. The results were mostly consistent with the general trend observed for the Fischer indole synthesis in solution, which is considerably influenced by the steric and electronic nature of the substituent groups (Scheme 2).
Going into more detail about the structure–reactivity relationship, we observed that 4-methyl- and phenylhydrazine provided indoles 3a and 3b, respectively, in comparable amounts (Scheme 2). However, introducing a chlorine substituent on the para position of the aromatic ring in the phenylhydrazine generally has a detrimental effect on the reaction outcome (Scheme 2, indole 3c). Low conversion of phenylhydrazone to the indole 3c was observed even after prolonged reaction time (400 minutes). Conversely, the reaction of 4-methoxy phenylhydrazine with the propiophenone proceeded more slightly, providing the corresponding indole in 79% yield (Scheme 2, indole 3d).
Then, the reactivity of meta-substituted phenylhydrazines was explored to investigate whether a possible effect on the selectivity could be detected. In solution, meta-substituted phenylhydrazines usually give a mixture of 4- and 6-substituted indoles. However, the relative amounts vary to only a slight degree based on the electronic nature of the substituents, being the 6-substituted indole the leading product in the presence of an electron-donating group and the 4-substituted indole with an electron-withdrawing group.79
According to this general trend, the ball milling of 3-methylphenylhydrazine with propiophenone gave a mixture of two regioisomers showing modest regioselectivity for the indole 3ea (3ea/3eb: 58/42, Scheme 2). On the other hand, 4- and 6-substituted chloro derivatives 3fa and 3fb were detected only in traces.
Subsequently, we focused on preparing an array of tetrahydrocarbazole products using as starting carbonyl components symmetric cyclic aliphatic ketones.
The optimized process conditions afforded tetrahydrocarbazoles 3g–oa in high yields and purity (Scheme 2). In this case, the presence of halogen-substituents on the aromatic ring was compelling and well-tolerated (entries 3i-k). Moreover, the presence of bulky or pendant (phenyl) groups on the cyclohexanone ring did not appear to dramatically perturb the performance of this Fischer indole synthesis (Scheme 2, indoles 3l and 3m). The investigation was next extended to the reaction between 2-substituted cyclohexanone and 4-methylphenylhydrazine (Scheme 2).
Previous studies79,80 in solution revealed that the selectivity of the process was influenced by both the nature of the acid catalyst and the electronic and steric properties of the 2-substituent, driving the reaction toward the preferential formation of the indole product (i.e., 3na) and/or the alternative indolenine 3nb, (Scheme 2). Usually, when the process is performed in the presence of glacial acetic acid, the indolenine derivative 3nb results in the major component. In contrast, under strongly acidic conditions, such as aqueous sulfuric acid, the formation of the less substituted ene-hydrazine is favored, leading to the preferential formation of the corresponding indole 3na. In addition, the nature of the substituents on the carbon in α-position to the carbonyl group significantly affects the regioselectivity.79,80 Under the optimized mechanochemical conditions, we examined the behavior of two cyclohexanone derivatives bearing a pendant at the alpha position, namely 2-methyl- and 2-phenyl-cyclohexanone.
Furthermore, to avoid any possible decomposition of the indolenine during the purification steps, after the completion of the conversion of the ketone to indolenine 3nb, 3ob or indole 3na, 3oa, the reaction mixture was reduced in situ (see ESI,† and Scheme 4), resulting in the transformation of the indolenine to the corresponding indoline. In the case of the 2-methyl cyclohexanone, the reaction led to a mixture of two possible products with a slight preference for the indolenine 3nb (Scheme 2, 3na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3nb = 44
3nb = 44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56), in line with previous studies in solution.79 The ratio indole/indolenine was reversed by using 2-phenyl-cyclohexanone. The presence of a phenyl group in the α-position to the carbonyl group favors the formation of the indole 3oa over the indolenine 3ob (Scheme 2, 3oa
56), in line with previous studies in solution.79 The ratio indole/indolenine was reversed by using 2-phenyl-cyclohexanone. The presence of a phenyl group in the α-position to the carbonyl group favors the formation of the indole 3oa over the indolenine 3ob (Scheme 2, 3oa![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3ob = 66
3ob = 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 34, Scheme 2).
34, Scheme 2).
A constrained cyclohexanone system, such as the α-tetralone 2e, was subjected to the reaction conditions leading to the corresponding indole (3p) in high yield (Scheme 2). At the same time, the cromanone 2l was coupled with 4-methoxy-phenylhydrazine providing the corresponding indole 3q in a very satisfying yield (Scheme 2). Remarkably, the present methodology was successfully applied to the synthesis of the indole 3r which represent a crucial precursor for the synthesis of Tubastatin A, a selective HDAC6 inhibitor.81
To examine the ring size effect of cyclic ketones, cyclopentanone, cycloheptanone, and cyclooctanone were milled in the presence of 4-methylphenylhydrazine, resulting in the target indoles 3s–3u, in good to excellent yields. The mechanochemical Fischer indole synthesis, performed using non-symmetric methyl ketone substrates, represents another interesting example of a substrate-controlled regioisomeric reaction.
Unfortunately, when acetophenone was submitted to the reaction conditions, the corresponding 2-phenylindole was detectable not even in trace amounts and after prolonged reaction time (Scheme 2, indole 3v). Although this may appear a significant limitation of our mechanochemical protocol, it has been exploited to selectively synthesize a series of 2-methyl indoles (Scheme 2: 3w–z). For example, the reaction of phenylhydrazine 1a with methyl ketones 2o and 2p (Table S2 in ESI†) gave only one of two possible regioisomers, affording the indoles nucleus decorated with a benzyl or a p-methoxy-phenyl pendant in 3-position in 74 and 99% isolated yields, respectively (Scheme 2, indoles 3w and 3x). Furthermore, the procedure proved to be compatible with the ester moiety, providing both the indoles 3y and 3z in 43% and 67% yields and opening a way for further derivatization through the carboxylic function (Scheme 2).
Finally, when a symmetric ketone with a linear alkyl chain was used, the indolisation proceeded smoothly (Scheme 2, indole 3za). In general, the developed mechanochemical protocol provided good results, in terms of yields and selectivity, for preparing indoles 3a–3za.
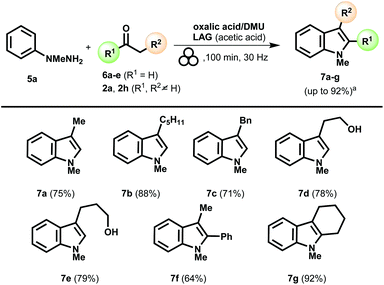
At this point, we addressed our effort toward the synthesis of 2-unsubstituted indoles starting from the corresponding aldehydes. The previously optimized mechanochemical protocol was extended to a set of different aldehydes, such as propionaldehyde, 3-phenyl-propionaldehyde, and heptanal in the presence of the p-tolylhydrazine 1a. Unfortunately, in all the experiments, the reaction provided a complex mixture from which it has not been possible to isolate the desired product. This issue could be overcome by adopting 1-methyl-1-phenylhydrazine 5a (Scheme 3). A methyl substituent on the nitrogen atom (N1) played a crucial role in the process, allowing for 7a–7e indole products (Scheme 3). Besides, indole derivatives 7f and 7g, using propiophenone and cyclohexanone, respectively, were successfully prepared (Scheme 3).
As stated in the introduction, the indolenine/indoline core is highly relevant for constructing biologically active compounds. Despite several reports on eco-friendly Fisher indole syntheses, sporadic investigations have been performed on its interrupted variation.82,83 This is especially true for 3,3′-disubstituted indolenine frameworks associated with additional challenges.
Interrupted Fischer indolisation is still the most widely used approach to prepare the indolenine nucleus. However, as a rule, the synthesis of this heterocycle is developed in acetic acid, which plays the dual role of solvent and acid catalyst, needed to promote ring closure. The reaction requires an input of heat from an external source (>80 °C), and often the same experimental conditions needed to trigger the interrupted Fischer indolisation promote the migration of one of the two side chains on the 3,3′-disubstituted indolenines, which generates the most thermodynamically stable indole ring (Scheme 1, pathway C).34,84,85 Moreover, the acetic acid, used as a solvent in the synthesis of the indolenine core, is often incompatible with the reducing agents used in the next step to reduce the indolenines to indolines and, therefore, has to be distilled away. Solvent removal is energy demanding and could be a significant obstacle, mainly working at larger scale.
As extensively emphasized in the literature, working in this context is challenging. Finding innovative solutions that are entirely in line with the requirements of green chemistry becomes a complex puzzle hard to solve. Removing the solvent and working at room temperature are, at least from a theoretical point of view, the most straightforward solution to overcome the issues, combining the requirements of synthesis with those of green chemistry.
Once again, mechanochemistry is emerging as a green technology that can provide the right solutions to these complex problems, avoiding the propensity of the metastable indolenines to undergo 1,2-migration. For this reason, we decided to explore the feasibility of a Fischer interrupted reaction followed by the in situ reduction of the resulting indolenine and spiroindolenine.
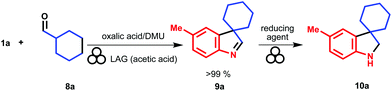
At first, we established the feasibility of the indolenine formation by reacting the 4-methyl-phenyl hydrazine and cyclohexane carboxaldehyde under the optimized reaction conditions. Then, we were pleased to observe an almost quantitative formation of the indolenine nucleus (Table 3, compound 9a) by GC-MS and 1H-NMR analysis on the reaction crude. Remarkably, the interrupted Fischer indolisation reaction proceeds smoothly using commercially available arylhydrazine hydrochlorides, which directly react in situ without any preliminary acid–base treatments to afford free hydrazine derivatives.
| Entrya | Reducing agent | Equiv. | 10b |
|---|---|---|---|
| a 1a (158.6 mg, 1 mmol), 8a (123.4 mg, 1.1 mmol), oxalic acid (315.10 mg, 3.5 mmol), dimethylurea (132.16 mg, 1.5 mmol) and acetic acid (η = 0.1 μL mg−1) were ball-milled in a 15 mL ZrO2 milling jar with 20 milling balls (∅ = 3 mm, mtot = 6.5 g) of the same material for 100 minutes, afterwards the jar was opened, the reducing agent was added and the reaction mixture was ball-milled for additional 60 minutes. b Determined by GC-MS. c The reaction mixture was ball milled in the presence of SiO2 (175 mg) and MeOH (η = 0.25 μL mg−1). d The reaction was performed in the presence of 3-pentanol instead of acetic acid (η = 0.1 μL mg−1). | |||
| 1c | Pd/NH4HCOOH | 5 (mol%)/3 | n.r. |
| 2 | NaBH(OAc)3 | n.r. | |
| 3 | NaBH4 | 4 | 90% |
| 4 | NaBH 4 | 2 | 91% |
| 5 | NaBH4 | 1 | 50% |
| 6d | NaBH4 | 1.5 | 90% |
| 7d | NaBH4 | 1 | 70% |
In addition, the desired indolenine was obtained selectively concerning the 1,2-migration product under the developed conditions. Based on previous reports on hydrogenation reactions under mechanochemical processing conditions,86 the in situ reduction of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond in the indolenine core was attempted. Our efforts to obtain the product using Pd catalyst and ammonium formate under transfer hydrogenation conditions led to a mixture of unidentified products (Table 3, entry 1).87 Moreover, when NaBH(OAc)3 was used, no conversion was observed (Table 3, entry 2). On the other hand, excellent results were achieved in the presence of 4 equivalents of NaBH4 (Table 3, entry 3).
N double bond in the indolenine core was attempted. Our efforts to obtain the product using Pd catalyst and ammonium formate under transfer hydrogenation conditions led to a mixture of unidentified products (Table 3, entry 1).87 Moreover, when NaBH(OAc)3 was used, no conversion was observed (Table 3, entry 2). On the other hand, excellent results were achieved in the presence of 4 equivalents of NaBH4 (Table 3, entry 3).
Finally, halving the amount of the reducing agent (2 matches) allowed complete conversion toward the desired product 10a (Table 3, compare entries 4 and 5).
Interestingly, 3-pentanol (LAG, η = 0.1 μL mg−1) allows to decrease the amount of sodium borohydride required to complete the reduction down to 1.5 equivalents (Table 3, compare entries 4 and 6). Nevertheless, green metrics based on the use of 3-pentanol did not show a significant improvement on the overall greenness of the methodology (see ESI, Scheme S4†). According to the above considerations, we adopted once again the acetic acid as liquid assistant of grinding.
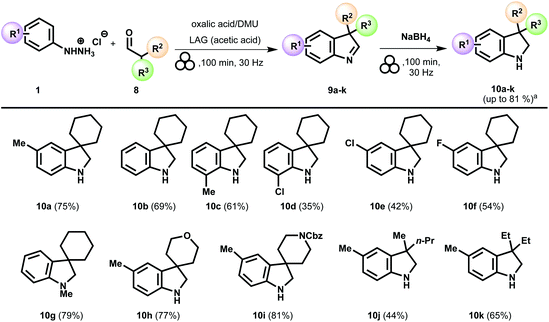
The scope of the reaction was studied, and the strategy showed to be effective for a variety of 2-disubstituted aldehydes (Scheme 4, indolines 10a–10k). Indoline derivative 10a was isolated in 75% yield over two reaction steps by a short pad of silica gel. Reasonably good results were obtained using the unsubstituted phenylhydrazine (Scheme 4, indoline 10b). In this case, halogens-functionalized phenylhydrazine proved to be a more challenging substrate even though the reaction proceeded significantly (Scheme 4, indolines 10d–10f). However, an outstanding outcome could be performed when using the 1-methyl-1-phenylhydrazine (Scheme 4, indoline 10g). Finally, 2,2-disubstituted aldehyde derivatives bearing a heteroatom as a part of the ring were well tolerated. More specifically, reacting 4-formyltetrahydropyran with 4-methyl-phenylhydrazine led to indoline derivative 10h in 77% yield. Besides, it was possible to efficiently prepare the indoline derivative 10i with a Cbz-protected nitrogen atom in high yield (Scheme 4, 81% yield). Similar responses were recorded using the racemic 2-methylpentanal (Scheme 4, indoline 10j, 44%) and 2-ethylbutanal (Scheme 4, indoline 10k) as carbonylic partners.
Finally, green metrics were calculated to assess the environmental impact, effectiveness, and sustainability of the developed procedures (Table 4, for details, see the ESI†). The evaluation of the findings reported in the table pointed out and further demonstrated the advantages provided by the mechanochemical promoted Fischer (and interrupted Fischer) process, both in terms of E-factor88–90 and Eco scale91 values. Going beyond the interesting numerical results of the green metrics, it is worth emphasizing that this procedure does not require heating and proceeds at room temperature, preventing any potential degradation of heat-labile compounds. The provided methodology allowed to avoid using large amounts of liquid acids, replaced by the easy-to-handle solid mixture. This feature is also beneficial in terms of the purification steps, which are remarkably simplified since there is no need for solvent switching in the different reaction stages and the OA-DMU mixture can be removed by water treatment.
| Compound | In solutiona | Mechanochemistry | ||
|---|---|---|---|---|
| E-Factor | Eco-scale | E-Factor | Eco-scale | |
| a For the Fischer and interrupted Fischer synthesis of indoles and indolines (in solution), two representative procedures were chosen for comparison: (i) in the presence of acidic clay as the catalyst for the indole compounds,51 (ii)using acetic acid and sodium triacetoxyborohydride as a reducing agent for the synthesis of indolines.85 | ||||
| 3h | 79.9 | 64.0 | 42.9 | 74.0 |
| 10b | 278.4 | 45.0 | 214.3 | 52.5 |
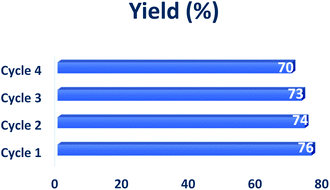
Finally, we have investigated the recycling of solid mixture containing OA and DMU in order to improve the environmental performance of the whole process. The experiments carried out on the model reaction highlighted that the recycled solid grinding components could be used four times, maintaining their efficiency (Table 5). However, it is worth pointing out that the water's evaporation is a high energy demand process that should be accurately assessed concerning the actual advantages provided by the recycling steps.
| a Reaction conditions: 1a (1.0 mmol), 2a (1.1 mmol) and acetic acid (η = 0.1 μL mg−1) were ball-milled with the recycled mixture in a 15 mL ZrO2 milling jar with 20 milling balls (∅ = 3 mm, mtot = 6.5 g) of the same material for the given time. |
|---|
|
Conclusions
In summary, we developed an effective mechanochemical protocol for generating a wide variety of indole- and indoline-based templates in short times and with high yield using a mixture of solid oxalic acid and dimethylurea. The number of examples and the variability of the nature of aldehydes, ketones and phenylhydrazines successfully converted demonstrated the broad scope and utility of the proposed method. Moreover, the newly developed protocol displays the potential to turn it into a practical coupling point for additional modification leading to compounds of pharmaceutical interest.Author contributions
A. P. conceptualization, data curation, manuscript editing; R. M.: conceptualization and initial draft preparation; M. B.: conceptualization and initial draft preparation; E. C. data curation and manuscript editing; FC: experimental data analysis. C. F.: GC-MS analysis, manuscript editing; F. D.: manuscript editing and funding; M. V. D. supervision, manuscript editing, funding.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by MIUR Italy PRIN 2017 project (2017A95NCJ) “Stolen molecules—Stealing natural products from the depot and reselling them as new drug candidate”. M. B. and M. V. D. would like to thank the Department of Excellence of Pharmacy (2018–2022), University of Naples Federico II. The MIUR National Project PRIN “MultIFunctional poLymer cOmposites based on groWn matERials (MIFLOWER)” (grant number: 2017B7MMJ5_001) is kindly acknowledged. This work is a contribution to the COST Action CA1811292–94 supported by COST (European Cooperation on Science and Technology).95 COST (European Cooperation in Science and Technology) is a funding agency for research and innovation networks. Our Actions help connect research initiatives across Europe and enable scientists to grow their ideas by sharing them with their peers. This boosts their research, career and innovation. E.C. is grateful to Région Occitanie (France) for the Pre-Maturation 2020 – MECH-API grant (ESR_PREMAT – 00262).Notes and references
- E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed.
- N. Kerru, L. Gummidi, S. Maddila, K. K. Gangu and S. B. Jonnalagadda, Molecules, 2020, 25, 1909 CrossRef CAS PubMed.
- M. M. Heravi and V. Zadsirjan, RSC Adv., 2020, 10, 44247–44311 RSC.
- A. Mermer, T. Keles and Y. Sirin, Bioorg. Chem., 2021, 114, 105076 CrossRef CAS PubMed.
- A. W. Schammel, G. Chiou and N. K. Garg, Org. Lett., 2012, 14, 4556–4559 CrossRef CAS PubMed.
- L. Zu, B. W. Boal and N. K. Garg, J. Am. Chem. Soc., 2011, 133, 8877–8879 CrossRef CAS PubMed.
- A. Nishida, R. Tsuji and M. Nakagawa, Heterocycles, 2002, 58, 587–593 CrossRef.
- J. H. Rigby and S. Sidique, Org. Lett., 2007, 9, 1219–1221 CrossRef CAS PubMed.
- S. Dadashpour and S. Emami, Eur. J. Med. Chem., 2018, 150, 9–29 CrossRef CAS PubMed.
- A. Thakur, A. Singh, N. Kaur, R. Ojha and K. Nepali, Bioorg. Chem., 2020, 94, 103436 CrossRef CAS PubMed.
- Y. Li, Y. Xiong, G. Zhang, L. Zhang, W. Yang, J. Yang, L. Huang, Z. Qiao, Z. Miao, G. Lin, Q. Sun, T. Niu, L. Chen, D. Niu, L. Li and S. Yang, J. Med. Chem., 2018, 61, 11398–11414 CrossRef CAS PubMed.
- S. Oh, G. W. Go, E. Mylonakis and Y. Kim, J. Appl. Microbiol., 2012, 113, 622–628 CrossRef CAS PubMed.
- S.-F. Fu, J.-Y. Wei, H.-W. Chen, Y.-Y. Liu, H.-Y. Lu and J.-Y. Chou, Plant Signaling Behav., 2015, 10, e1048052 CrossRef PubMed.
- Indoles are among the most critical bioactive heterocyclic structures, naturally present in tryptophan and the related neurotransmitter serotonin. Indole alkaloids also represent one of the largest classes of nitrogen-containing secondary metabolites, containing indole and/or indoline substructures. They are widely found in plants, bacteria, fungi and animals.
- A. Kumari and R. K. Singh, Bioorg. Chem., 2019, 89, 103021 CrossRef CAS PubMed.
- T. V. Sravanthi and S. L. Manju, Eur. J. Pharm. Sci., 2016, 91, 1–10 CrossRef CAS PubMed.
- V. Sharma, P. Kumar and D. Pathak, J. Heterocycl. Chem., 2010, 491–502 CAS.
- S. Lal and T. J. Snape, Curr. Med. Chem., 2012, 19, 4828–4837 CrossRef CAS PubMed.
- N. Chadha and O. Silakari, Eur. J. Med. Chem., 2017, 134, 159–184 CrossRef CAS PubMed.
- M. Brindisi, J. Senger, C. Cavella, A. Grillo, G. Chemi, S. Gemma, D. M. Cucinella, S. Lamponi, F. Sarno, C. Iside, A. Nebbioso, E. Novellino, T. B. Shaik, C. Romier, D. Herp, M. Jung, S. Butini, G. Campiani, L. Altucci and S. Brogi, Eur. J. Med. Chem., 2018, 157, 127–138 CrossRef CAS PubMed.
- D. Bates and A. Eastman, Br. J. Clin. Pharmacol., 2017, 83, 255–268 CrossRef CAS PubMed.
- J. T. Ndongo, J. N. Mbing, M. F. Tala, A. Monteillier, D. E. Pegnyemb, M. Cuendet and H. Laatsch, Phytochemistry, 2017, 144, 189–196 CrossRef CAS PubMed.
- K.-H. Lim, O. Hiraku, K. Komiyama and T.-S. Kam, J. Nat. Prod., 2008, 71, 1591–1594 CrossRef CAS PubMed.
- Y. Wang, F. Xie, B. Lin, M. Cheng and Y. Liu, Chem. – Eur. J., 2018, 24, 14302–14315 CrossRef CAS PubMed.
- S. Zeeli, T. Weill, E. Finkin-Groner, C. Bejar, M. Melamed, S. Furman, M. Zhenin, A. Nudelman and M. Weinstock, J. Med. Chem., 2018, 61, 4004–4019 CrossRef CAS PubMed.
- J. E. Wilson, R. Kurukulasuriya, M. Reibarkh, M. Reiter, A. Zwicker, K. Zhao, F. Zhang, R. Anand, V. J. Colandrea, A.-M. Cumiskey, A. Crespo, R. A. Duffy, B. Ann Murphy, K. Mitra, D. G. Johns, J. L. Duffy and P. Vachal, ACS Med. Chem. Lett., 2016, 7, 261–265 CrossRef CAS PubMed.
- F. De Moliner, L. Banfi, R. Riva and A. Basso, Comb. Chem. High Throughput Screening, 2011, 14, 782–810 CrossRef CAS PubMed.
- D. Mondal, P. L. Kalar, S. Kori, S. Gayen and K. Das, Curr. Org. Chem., 2020, 24, 2665–2693 CrossRef CAS.
- G. W. Gribble, Indole Ring Synthesis: From Natural Products to Drugs Discovery, Wiley-VCH, Chichester, UK, 2016 Search PubMed.
- G. K. Hughes and F. Lions, J. Proc. R. Soc. N. S. W., 1938, 71, 494–502 CAS.
- B. Witkop and J. B. Patrick, J. Am. Chem. Soc., 1951, 73, 1558–1564 CrossRef CAS.
- G. Jones and T. S. Stevens, J. Chem. Soc., 1953, 2344–2350 RSC.
- M. J. James, P. O'Brien, R. J. K. Taylor and W. P. Unsworth, Chem. – Eur. J., 2016, 22, 2856–2881 CrossRef CAS PubMed.
- K. G. Liu, A. J. Robichaud, J. R. Lo, J. F. Mattes and Y. Cai, Org. Lett., 2006, 8, 5769–5771 CrossRef CAS PubMed.
- Y.-K. Lim and C.-G. Cho, Tetrahedron Lett., 2004, 45, 1857–1859 CrossRef CAS.
- E. Yasui, M. Wada and N. Takamura, Tetrahedron Lett., 2006, 47, 743–746 CrossRef CAS.
- A. Guy, J.-P. Guetté and G. Lang, Synthesis, 1980, 222–223 CrossRef CAS.
- Y. Murakami, Y. Yokoyama, T. Miura, H. Hirasawa, Y. Kamimura and M. Izaki, Heterocycles, 1984, 22, 1211–1216 CrossRef CAS.
- S. Gore, S. Baskaran and B. König, Org. Lett., 2012, 14, 4568–4571 CrossRef CAS PubMed.
- J. L. Rutherford, M. P. Rainka and S. L. Buchwald, J. Am. Chem. Soc., 2002, 124, 15168–15169 CrossRef CAS PubMed.
- H. R. Snyder and C. W. Smith, J. Am. Chem. Soc., 2002, 65, 2452–2454 CrossRef.
- G. L. Rebeiro and B. M. Khadilkar, Synthesis, 2001, 370–372 CrossRef CAS.
- B. J. Stokes, H. Dong, B. E. Leslie, A. L. Pumphrey and T. G. Driver, J. Am. Chem. Soc., 2007, 129, 7500–7501 CrossRef CAS PubMed.
- B. Liu, C. Song, C. Sun, S. Zhou and J. Zhu, J. Am. Chem. Soc., 2013, 135, 16625–16631 CrossRef CAS PubMed.
- Y.-L. Li, J. Li, A.-L. Ma, Y.-N. Huang and J. Deng, J. Org. Chem., 2015, 80, 3841–3851 CrossRef CAS PubMed.
- S.-L. Cui, J. Wang and Y.-G. Wang, J. Am. Chem. Soc., 2008, 130, 13526–13527 CrossRef CAS PubMed.
- N. Zeidan, S. Bognar, S. Lee and M. Lautens, Org. Lett., 2017, 19, 5058–5061 CrossRef CAS PubMed.
- M. Sayyad, Y. Nanaji and M. K. Ghorai, J. Org. Chem., 2015, 80, 12659–12667 CrossRef CAS PubMed.
- D. Bhattacharya, D. W. Gammon and E. van Steen, Catal. Lett., 1999, 61, 93–97 CrossRef CAS.
- S. B. Mhaske and N. P. Argade, Tetrahedron, 2004, 60, 3417–3420 CrossRef CAS.
- A. Dhakshinamoorthy and K. Pitchumani, Appl. Catal., A, 2005, 292, 305–311 CrossRef CAS.
- R. A. Duval and J. R. Lever, Green Chem., 2010, 12, 304–309 RSC.
- D. Q. Xu, J. Wu, S. P. Luo, J. X. Zhang, J. Y. Wu, X. H. Du and Z. Y. Xu, Green Chem., 2009, 11, 1239–1246 RSC.
- S. P. M. Ventura, A. M. M. Gonçalves, T. Sintra, J. L. Pereira, F. Gonçalves and J. A. P. Coutinho, Ecotoxicology, 2013, 22, 1–12 CrossRef CAS PubMed.
- T. P. Thuy Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef PubMed.
- K. J. Ardila-Fierro and J. G. Hernández, ChemSusChem, 2021, 14, 2145–2162 CrossRef CAS PubMed.
- S. Mateti, M. Mathesh, Z. Liu, T. Tao, T. Ramireddy, A. M. Glushenkov, W. Yang and Y. I. Chen, Chem. Commun., 2021, 57, 1080–1092 RSC.
- E. Colacino, V. Isoni, D. Crawford and F. García, Trends Chem., 2021, 3, 335–339 CrossRef CAS.
- F. Puccetti, S. Lukin, K. Užarević, E. Colacino, I. Halasz, C. Bolm and J. G. Hernández, Chem. – Eur. J., 2022, 28, e202104409 CrossRef CAS PubMed.
- J. Andersen and J. Mack, Green Chem., 2018, 20, 1435–1443 RSC.
- A. Porcheddu, E. Colacino, L. De Luca and F. Delogu, ACS Catal., 2020, 10, 8344–8394 CrossRef CAS.
- M. Pérez-Venegas and E. Juaristi, ACS Sustainable Chem. Eng., 2020, 8, 8881–8893 CrossRef.
- J. L. Howard, Q. Cao and D. L. Browne, Chem. Sci., 2018, 9, 3080–3094 RSC.
- J. G. Hernández and C. Bolm, J. Org. Chem., 2017, 82, 4007–4019 CrossRef PubMed.
- E. Colacino, A. Porcheddu, C. Charnay and F. Delogu, React. Chem. Eng., 2019, 4, 1179–1188 RSC.
- S. L. James, C. J. Adams, C. Bolm, D. Braga, P. Collier, T. Friščić, F. Grepioni, K. D. M. Harris, G. Hyett, W. Jones, A. Krebs, J. Mack, L. Maini, A. G. Orpen, I. P. Parkin, W. C. Shearouse, J. W. Steed and D. C. Waddell, Chem. Soc. Rev., 2012, 41, 413–447 RSC.
- W. Pickhardt, S. Grätz and L. Borchardt, Chem. – Eur. J., 2020, 26, 12903–12911 CrossRef CAS PubMed.
- T. Friščić, C. Mottillo and H. M. Titi, Angew. Chem., Int. Ed., 2020, 59, 1018–1029 CrossRef PubMed.
- D. Virieux, F. Delogu, A. Porcheddu, F. García and E. Colacino, J. Org. Chem., 2021, 86, 13885–13894 CrossRef CAS PubMed.
- O. Galant, G. Cerfeda, A. S. McCalmont, S. L. James, A. Porcheddu, F. Delogu, D. E. Crawford, E. Colacino and S. Spatari, ACS Sustainable Chem. Eng., 2022, 10(4), 1430–1439 CrossRef CAS.
- Y. Qiu, K. Te Puni, C. C. Duplan, A. C. Lindsay and J. Sperry, Tetrahedron Lett., 2021, 72, 153068 CrossRef CAS.
- F. Krauskopf, K.-N. Truong, K. Rissanen and C. Bolm, Org. Lett., 2021, 23, 2699–2703 CrossRef PubMed.
- For some examples on hydrazones synthesis by mechanochemistry, in batch and in continuous, please refer to: (a) E. Colacino, A. Porcheddu, I. Halasz, C. Charnay, F. Delogu, R. Guerra and J. Fullenwarth, Green Chem., 2018, 20, 12230–12238 RSC; (b) D. E. Crawford, A. Porcheddu, A. S. McCalmont, F. Delogu, S. L. James and E. Colacino, ACS Sustainable Chem. Eng., 2020, 8, 12230–12238 CrossRef CAS.
- For other mechanochemical rearrangements please refer to ref. 59 and 69.
- G. W. Breton, J. Org. Chem., 1997, 62, 8952–8954 CrossRef CAS.
- T. Nishiguchi, K. Kawamine and T. Ohtsuka, J. Org. Chem., 1992, 57, 312–316 CrossRef CAS.
- K. K. Kapoor, B. A. Ganai, S. Kumar and C. S. Andotra, Synth. Commun., 2006, 36, 2727–2735 CrossRef CAS.
- R. Mocci, E. Colacino, L. De Luca, C. Fattuoni, A. Porcheddu and F. Delogu, ACS Sustainable Chem. Eng., 2021, 9, 2100–2114 CrossRef CAS.
- D. L. Hughes, Org. Prep. Proced. Int., 1993, 25, 607–632 CrossRef CAS.
- B. Robinson, Chem. Rev., 1963, 63, 373–401 CrossRef.
- K. V. Butler, J. Kalin, C. Brochier, G. Vistoli, B. Langley and A. P. Kozikowski, J. Am. Chem. Soc., 2010, 132, 10842–10846 CrossRef CAS PubMed.
- A. I. Alfano, A. Zampella, E. Novellino, M. Brindisi and H. Lange, React. Chem. Eng., 2020, 5, 2091–2100 RSC.
- A. I. Alfano, M. Brindisi and H. Lange, Green Chem., 2021, 23, 2233–2292 RSC.
- Q.-F. Wu, C. Zheng and S.-L. You, Angew. Chem., Int. Ed., 2012, 51, 1680–1683 CrossRef CAS PubMed.
- K. G. Liu, J. R. Lo and A. J. Robichaud, Tetrahedron, 2010, 66, 573–577 CrossRef CAS.
- B. G. Fiss, A. J. Richard, T. Friščić and A. Moores, Can. J. Chem., 2021, 99, 93–112 CrossRef CAS.
- T. Portada, D. Margetić and V. Štrukil, Molecules, 2018, 23, 3163 CrossRef PubMed.
- R. A. Sheldon, in Organic synthesis; past, present and future, Chem. Ind., 1992, pp. 903–906 Search PubMed.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC.
- R. A. Sheldon, ACS Sustainable Chem. Eng., 2018, 6, 32–48 CrossRef CAS.
- K. Van Aken, L. Strekowski and L. Patiny, Beilstein J. Org. Chem., 2006, 2(3) DOI:10.1186/1860-5397-2-3.
- For more information on COST Action CA18112 ‘Mechanochemistry for Sustainable Industry’: https://www.mechsustind.eu/ (accessed Febrauary 18, 2022).
- M. Baláž, L. Vella-Zarb, J. Hernandez, I. Halasz, D. E. Crawford, M. Krupička, V. André, A. Niidu, F. Garcia, L. Maini and E. Colacino, Chem. Today, 2019, 37, 32–34 Search PubMed.
- J. G. Hernández, I. Halasz, D. E. Crawford, M. Krupicka, M. Baláž, V. André, L. Vella-Zarb, A. Niidu, F. García, L. Maini and E. Colacino, Eur. J. Org. Chem., 2020, 8–9 CrossRef.
- For more information: https://www.cost.eu/ (accessed Febrauary 18, 2022).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2gc00724j |
| This journal is © The Royal Society of Chemistry 2022 |