Highly efficient oxidation of plant oils to C18 trihydroxy fatty acids by Escherichia coli co-expressing lipoxygenase and epoxide hydrolase†
Abstract
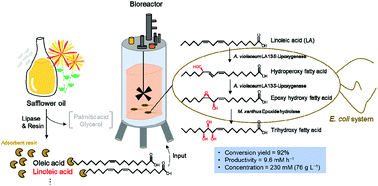
C18 trihydroxy fatty acids (THFAs), plant oxylipins, are used as antifungal agents and vaccine adjuvants. The chemical synthesis of THFAs has several disadvantages, including low yields and environmental pollution. Microorganisms and plants also produce THFAs in vivo; however, the productivity is low. Here, we reported a recombinant Escherichia coli co-expressing bacterial linoleic acid (LA) 13-lipoxygenase with high isomerization activity and epoxide hydrolase that converted 200 mM polyunsaturated fatty acids, including LA, α-linolenic acid, and γ-linolenic acid (GLA), into THFAs via epoxy hydroxy fatty acids with high conversion yields (>60%) in a flask. Among the products, GLA-derived 12S,13S-epoxy-11R-hydroxyoctadecadienoic acid and 11R,12R,13S-trihydroxyoctadecadienoic acid were new compounds. For the efficient biotransformation of safflower oil into THFA, the LA content in the safflower oil hydrolyzate was increased by the addition of an adsorbent resin with lipase to safflower oil. The resin bound unsaturated fatty acids, thereby removing unbound impurities such as palmitic acid and glycerol. In a 3 L-bioreactor, the recombinant cells converted 250 mM (70 g L−1) LA in resin-treated safflower oil hydrolyzate, which was derived from safflower oil (93 g L−1), into 230 mM (76 g L−1) 11R,12R,13S-trihydroxyoctadecenoic acid in 24 h, with a conversion yield of 92% and a productivity of 9.6 mM h−1. The product was isolated with a purity of 94% and an isolated yield of 75%. We successfully developed an efficient, cost-effective, and eco-friendly process for the biotransformation of safflower oil into THFAs.


 Please wait while we load your content...
Please wait while we load your content...