Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
The sustainability of phytomass-derived materials: thermodynamical aspects, life cycle analysis and research perspectives
B.
Duchemin

Normandie Univ, UNILEHAVRE, CNRS, LOMC, 76600 Le Havre, France. E-mail: benoit.duchemin@univ-lehavre.fr; Tel: +33 2 35 21 71 54
First published on 18th March 2022
Abstract
Cellulose in particular and phytomass in general are at the heart of our food system. They are also a central energy vector and a vital source of materials. In this article, a multiscale approach to the complex issue of lignocellulose sustainability is developed. Global thermodynamic concepts help to place current biomass exploitation in a global energetic context. In particular, the notion of entropy appears pivotal to understand energy and material fluxes at the scale of the planet and the limits of biomass production. Entropy is, however, best described at the microscopic scale, despite its large-scale consequences. Recent advances in entropy-driven colloid assembly parallel nature's choices and lignocellulose assembly at the nanometric scale. The functional concept of exergy is then developed and a few examples of its concrete use in photosynthesis and biorefinery research are given. In a subsequent part, an evaluation of the relative importance of biomass is performed with respect to non-renewable materials. This discussion helps to explain the interdependence of resources, including ores and fossil fuels. This interdependence has important consequences for current and future biomass uses. Some of these dependences are then quantitatively discussed using life cycle analysis (LCA) results from the literature. These results are of importance to different technological fields such as paper, biobased insulation, construction wood, information and communication technologies, and biobased textiles. A conclusion is then drawn that exposes the research tracks that are the most likely to be sustainable, including self-assembly, exergetically favourable options and low tech solutions.
1. Introduction
It is scientifically crucial to analyse the use of resources in the long run for ecological reasons. Indeed, human activities cause unsustainable global geochemical redistributions, climate change, soil exhaustion and ore depletion. The extent of modifications to the planet is such that geologists consider this a new geological era, often named the Anthropocene.1,2 The consequences of mankind on Earth are well documented by the International Panel on Climate Change (IPCC) and in many synthetic academic works.2–5 For a number of geochemical, biochemical or biological indicators, planetary boundaries have already been reached.2 Planetary boundaries are defined as limits within which certain ecological indicators (ocean acidification, biogeochemical flows, freshwater use, biodiversity, etc.) can evolve within a “safe operating space”, without irreversibly affecting the Earth system.2,6,7 Crossing planetary boundaries does not necessarily mean than the tipping point is surpassed, even though changes can be abrupt once this boundary is crossed. Rapid climate change and new types of pollution with deleterious consequences for mankind are deeply interconnected with the question of chemicals or materials in general, and biomaterials in particular.262–264 It is therefore important to assess the sustainability of phytomass-derived materials.So far, most modern experimental developments in materials have been driven by short-term performance, such as mechanical performance, electrical conductivity, or data storage capacity, to name a few examples. This has produced some undiscussable technological advances. Nevertheless, the possibility to use the same technology for thousands of years has systematically been out of scope. One might wonder why materials scientists have largely failed to prioritize sustainability in their daily routine. Shouldn't the role of the materials scientist also be to integrate the future drawbacks of their findings, as did early foresters?8–10 A common approach is to deem research work as being “green” for internal, but self-consistent, reasons. For instance, there is the underlying assumption that lignocellulose-derived materials are environmentally preferable to fossil-based materials because fossil-based materials are not natural nor renewable. Another assumption would be that the amelioration of a transformation step in a given multi-step process would enhance the whole process, without looking at the entire process in its physical context. But these are only ad hoc arguments. They clearly need closer scrutiny because they do not acknowledge the complexity of materials’ life cycles. These life-cycle aspects include the growth of biomass, the global energetic context, the reliance on non-renewable materials, end-of-life scenarios and sometimes sociological and economic considerations. Therefore, the question should rather be whether materials scientists can prioritize sustainability in their daily routine at all, since sustainability concerns expand far beyond the laboratory gates.
A simple definition of sustainability is the quality of supporting long-term ecological balance. A more thorough definition should acknowledge the tight and dynamic interplay between materials, energy, ecosystems and socio-political aspects. For instance, sustainability can be understood as a hierarchical inclusion of subsets: the industrial economy is a heterotrophic activity included in a human and social subset; this pre-existing social subset is itself included a larger multispecies environmental set.11 This multispecies set comprises plants, animals and bacteria and there are specific interactions between each of these subgroups and humans in general. As a consequence, sustainability is a goal that can be reached only with a deep understanding of the role taken by other species and the reliance of mankind on “multispecies entanglement”, or ecosystem dynamics.11,12 Another functional observation is that sustainability goals are emerging locally and their propagation at the global scale can occur along four different paths: aggregation (local concerns add up at the global scale), compensation (local issues are offset from one region to another), learning (lessons learnt in one place are learnt globally) and contagion (one local event, good or bad, diffuses over the globe).13 As a consequence of these mechanisms, local sustainability can differ but somehow interact due to other local goals or global considerations. Sustainability is therefore an important concept, but also a complex and multiscale one. The understanding of this complexity makes it possible to work out analytical methodologies to decipher what sustainability means in practical contexts, such as that of materials science. A macroscopic understanding can be provided by thermodynamics, and precise case studies can be analysed using exergy analysis or life cycle analysis.
Overall, this article is an attempt to examine when the exploitation of natural materials is the most sustainable and whether it will come up against other physical limits, both theoretically and in practice. This article is also an attempt to sketch the directions taken by the vast materials science community dealing with lignocellulose, as part of a bigger picture involving thermodynamic and ecological aspects. Because the notion of sustainability is intricate and complex, a specific scientific method is developed therein (Fig. 1). Global considerations are best described by thermodynamics, because this discipline seems to encompass most energy and material fluxes due to its inherent statistical nature. These global considerations will be completed by important figures on the interdependency between renewables and non-renewables. Ecological indicators extracted from life cycle analysis (LCA) or other studies will illustrate the ecological footprints of some technologically important materials derived from the phytomass. Case studies on wood, biopolymers, textile fibres, biobased composites, building materials and paper will be presented. This approach will bring a nuanced answer to the question of lignocellulosic sustainability. It will also highlight research perspectives characterized by the most promising sustainability profiles.
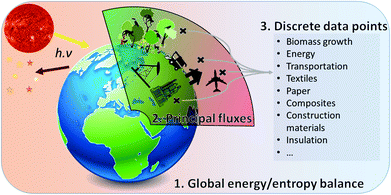 | ||
| Fig. 1 Conceptual view of the method developed in this article: macro-concepts (1) necessarily contain subsets (2) for which discrete data points from LCA results can be extracted (3). | ||
2. Thermodynamic standpoint: the singularity of the phytomass
The Earth is a system in equilibrium with deep space and the energy received from the sun. The energy balance is explained by several factors: Earth captures some of this radiation at its surface (47% of the incoming radiation), after it has gone through the atmosphere and the clouds (absorbing respectively 19 and 4% of the incoming radiation).14 The clouds reflect 23% of the incoming radiation. The surface of the Earth also reflects some incident sunlight (7%) and this is called albedo. Part of the reflected sunlight is sent back again towards the Earth's surface by clouds in particular. Because the Earth's surface is heated by sunlight, it emits energy in the form of infrared radiation. That is where greenhouse gases (GHG) come into play: they are heated by this infrared radiation and warm up the atmosphere. In the global balance, the Earth sends back slightly more energy than it takes from the sun.15 The thermal equilibrium and near constant temperature of the Earth (15 °C on average) are explained by the Earth's atmosphere, its steady rotation and the fact that the Earth's core also produces heat due to slow radioactive decay.15 As is well-known, the current excess of GHG (including CO2 and CH4) due to humans burning fossil fuels intensifies heating of the atmosphere, which provokes “global warming”, or “climate change”. Biomass plays a role in maintaining the thermal balance of the Earth by fine-tuning the atmospheric CO2 content and the albedo (broadleaf forests, coniferous forests, the seas, deserts or the savannahs have different albedos). Therefore, its exploitation has a very special impact on global thermodynamics.2.1. An introduction to entropy
The notion of entropy is not often associated with discussions on materials’ sustainability, but the concept of entropy can shed a different light on the evaluation of the sustainability of materials, and plant-based materials in particular. The notion of entropy was developed in the first half of the 19th century by Lazare and Sadi Carnot, but it was formalized in its currently used form by Rudolph Clausius in the second half of the same century. The term “entropy” was proposed in 1865 by Clausius to mean that irreversible heat losses are inherent to the system. The use of entropy is associated with the second law of thermodynamics, which is an inequality stating that the entropy of an isolated system never decreases. If the process is reversible, the entropy is constant. The second law of thermodynamics is therefore simply written as:| ΔS ≥ 0 |
A perhaps more functional and mechanistic definition of entropy was given by Ludwig Boltzmann in 1877. This definition is very powerful when backed up by the concept of a phase space.† If 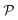 is this phase space, then the entropy of a state x contained in a box
is this phase space, then the entropy of a state x contained in a box 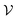 of volume V can be written as:
of volume V can be written as:
S = k![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) V V | (1) |
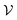 corresponds to a subdivision of
corresponds to a subdivision of 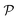 in which states are macroscopically indistinguishable from each other. The subdivision of
in which states are macroscopically indistinguishable from each other. The subdivision of 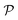 in
in  spaces is somewhat arbitrary and corresponds to an approach called coarse graining, which is developed in the relevant literature.15 In ordinary situations such as that of a gas in a container, the box
spaces is somewhat arbitrary and corresponds to an approach called coarse graining, which is developed in the relevant literature.15 In ordinary situations such as that of a gas in a container, the box  will be almost the same size as the whole phase space
will be almost the same size as the whole phase space 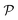 , a situation in which thermal equilibrium is reached and entropy is maximised. Due to the use of a logarithm in the Boltzmann definition, the fact that this box is slightly smaller than the whole phase space makes these two states indistinguishable. A more precise Boltzmann's equation can be given for gas systems and this approach gives rise to the field of statistical physics. Other definitions of entropy have been given and they are expressed (in a simplified manner) as a probability of undergoing a process divided by the probability of undergoing the reverse process.17–19 It is, however, important to remember that energy will naturally tend to spread out evenly between particles, and this is what entropy measures: energy dissipation.
, a situation in which thermal equilibrium is reached and entropy is maximised. Due to the use of a logarithm in the Boltzmann definition, the fact that this box is slightly smaller than the whole phase space makes these two states indistinguishable. A more precise Boltzmann's equation can be given for gas systems and this approach gives rise to the field of statistical physics. Other definitions of entropy have been given and they are expressed (in a simplified manner) as a probability of undergoing a process divided by the probability of undergoing the reverse process.17–19 It is, however, important to remember that energy will naturally tend to spread out evenly between particles, and this is what entropy measures: energy dissipation.
2.2. Local entropy: self-organisation of colloidal building blocks
Entropy is often considered to be a measure of disorder: the more disorder, the higher the entropy of the system. This definition is largely imparted to practical uses of the Boltzmann definition of entropy and its application in classical thermodynamics for gases and liquids. However, entropy needs to be understood differently when the particles are confined or subjected to an external force field, such as a gravitational force field.15,20–22 Indeed, a system with fewer degrees of freedom will have a smaller phase space, and therefore a lower entropy.15,20,22 Counterintuitively, entropy can thus contribute to the emergence and design of organised colloidal structures, such as packed tetrahedra or hard rods, when atoms, molecules or nanoparticles are confined and self-assembled.20,21,23–26 This can easily be explained by the higher number of possible configurations provided by some organised structures when confinement is prevalent, in comparison with disorganised structures: when packing occurs, the maximum volume fraction of a disorganised state (Vdisorderpacking) is always lower than that of an organised state (Vorderpacking). Therefore, there exists an “organisational concentration window” between Vdisorderpacking and Vorderpacking in which organised particles have more degrees of freedom, a larger phase space, and therefore a higher entropy. Since higher entropy will always be preferred, it becomes thermodynamically favourable to organise a confined colloid so that particles can keep “wiggling” around their mean position instead of percolating against each other and becoming immobile. This explains why elongated polyhedral particles tend to align and form liquid crystal-like phases, such as nematic or smectic.23,24So entropy means that, in a fixed colloidal system or in biological systems, rearrangement, biomorphogenesis and growth can occur spontaneously, which means that energy use is optimized.19,28,29 Entropy-driven processes have been suggested to drive a plethora of local phenomena associated with life or not, including RNA self-replication, protein folding, temperature-dependence of contact angles or enzyme selectivity; at a larger scale, entropy can be used to describe the interactions between ecosystems or economics.10,20,28,30–34 The Nobel laureate Ilya Prigogine introduced the term “self-organisation” to describe these phenomena. Since entropy maximisation can lead to the formation of structures without external work or heat, entropy can form the basis of passive, or energy-efficient, processes. This point is important if one is to draw inspiration from natural biological materials that draw energy from their surroundings, as opposed to heavily processed materials, which need a lot of external energy sources in the form of heat, mechanical pressure or chemical energy.
There are limited studies dedicated to the importance of entropy in the colloidal assembly of lignocellulosics.265 Among them, only a few are focused on cellulose itself. In one study dedicated to understanding the temperature dependence of the axial Young's modulus of cellulose I, the crystallite stiffness was found to be influenced by entropy.17 In the same study, entropy was calculated by determining the relative fluctuations of the atoms around their mean positions, as conceptualised above. This result is interesting in that it shows that, in at least one situation with a hydrophilic organic crystal, the temperature-dependence of the Young's modulus is affected by entropy rather than by internal energy and supposedly weakened hydrogen bonds. Against expectations, these hydrogen bonds account for a relatively small portion of the bonded energy (5–10%) and stiffness (20%). In another recent study, it was found that free volume entropy could favour the formation of kinks (local folds) in nanofibrils placed in slits, an effect attributed solely to geometrical confinement.35 This effect is interesting because, rather than bending, it seems like the nanofibrils prefer to break along a relatively straight line and this could have applications in biomass fractionation.
Other studies focus on the interactions between polysaccharides in the cell wall. It has been shown that the adsorption of xyloglucan (XG) on cellulose surfaces was endothermic, and therefore entropic: the entropy of the whole system (including solvating water molecules) increases if water molecules are freed from the cellulose surface and replaced with portions of XG chains.36,37 The underlying reason is that the polymer state-conformation in solution is restricted by covalent monomer–monomer bonds, compared with that of small water molecules with unrestricted conformational space. It is therefore entropically advantageous to replace cellulose–water interactions with cellulose–XG ones. This non-electrostatic adsorption has been demonstrated by molecular dynamics simulations and also experimentally by neutron reflectivity, AFM and surface plasmon resonance spectroscopy.36–38 As a consequence, polysaccharide adsorption in this case is driven by entropy rather than chemical bonding (hydrogen bonds or van der Waals forces), as previously assumed.37,265 A practical outcome could be the slower and weaker aggregation of hemicelluloses on cellulose at low temperatures, which could have consequences for biomass separation and perhaps biomass growth rates at different latitudes.
There are still relatively few studies related to entropically-driven self-assemblies of cellulose nanocrystals (CNC), but the field of entropically-driven self-assembly in general is rapidly growing and it should gain momentum in the upcoming years.20,25,26,39 In particular, this field allows one to reverse-engineer colloidal crystals of various shapes, including rods, fibres, and tetrahedra.23,25 When dealing with macromolecules, this field still faces complex topological issues such as those of geometric frustration and network entanglement, two effects that also shape polymer assembly.40,41 Nevertheless, CNC can self-organise in the form of tactoids or films over several length scales, an effect that is kinetically and thermodynamically dependent on their concentration, ionic strength, size, aspect ratio and polydispersity.42–46 The organisation of suspensions seems to be independent of temperature.24,42 Organisation can also be templated by letting the CNC self-organise in nanogrooves, or in long capillaries (Fig. 2).39,47 This type of approach can have applications in fields such as papermaking, electronics or textiles, to name a few.
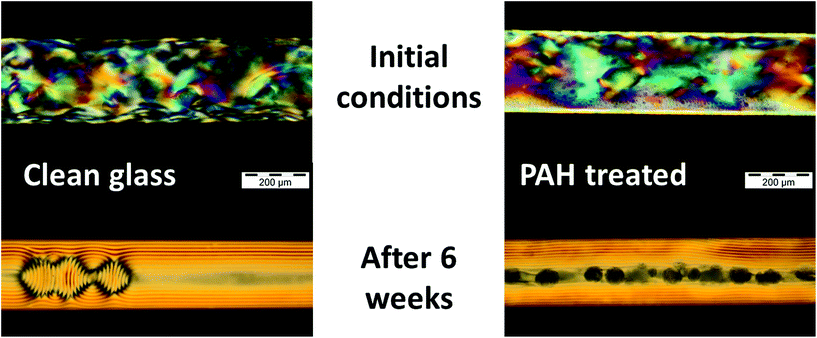 | ||
Fig. 2 Illustration of autonomous, entropy-driven, colloidal self-organisation. The systems were sealed and observed for 6 weeks at T = 22 °C. A cholesteric aqueous CNC suspension (6.41 wt%, surface charges 0.128 e nm−2, ζ = −63.7 ± 2.6 mV, Le Maine University)27 was injected into two glass capillaries (5 μl NMR tubes, ∅internal ∼ 286 μm). Before injection, both capillaries were cleaned with Piranha solution (H2O2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2SO4 1 H2SO4 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to produce a negatively charged surface from the activated silanol groups. The capillary on the right-hand side was further functionalized with a 0.4 wt % aqueous solution of poly(allylamine hydrochloride) (PAH) in order to confer positive surface charges to the glass wall. Both capillaries were thoroughly cleaned and dried under a flow of dry N2 before being filled with the CNC suspension. Note how the long-range organisation is affected by the boundary conditions of a molecularly thick layer: the cholesteric phase formed large transverse disclinations permitted (lower left) by a repulsive wall and inhibited (lower right) by an attractive wall. Inhibition resulted in small tactoids being formed at the centre of the tube (unpublished results). 1) to produce a negatively charged surface from the activated silanol groups. The capillary on the right-hand side was further functionalized with a 0.4 wt % aqueous solution of poly(allylamine hydrochloride) (PAH) in order to confer positive surface charges to the glass wall. Both capillaries were thoroughly cleaned and dried under a flow of dry N2 before being filled with the CNC suspension. Note how the long-range organisation is affected by the boundary conditions of a molecularly thick layer: the cholesteric phase formed large transverse disclinations permitted (lower left) by a repulsive wall and inhibited (lower right) by an attractive wall. Inhibition resulted in small tactoids being formed at the centre of the tube (unpublished results). | ||
Other works have been dedicated to understanding the thermodynamical interactions of nanocelluloses with different species in solution, a field that has possible applications in water depollution, medical assays, bioethanol production, nanostructured sensors, etc. These measurements usually rely on isothermal titration calorimetry or specific reduced excess determination in batch experiments. In some instances, proof was obtained by molecular modelling. It has for instance been shown that the adsorption of divalent ions to negatively charged CNC was entropy-driven (ΔS > 0) to compensate for unfavourable endothermic enthalpy (ΔH > 0).48 This behaviour is explained by the overall entropy gain due to the replacement of surface water by cations, and by the supplementary degrees of freedom gained by the freed water molecules. It was demonstrated than an enthalpy–entropy compensation occurred for the adsorption of a range of moieties on nanocelluloses (Fig. 3).49,50 These moieties included a large range of cellulose binding modules, bio-macromolecules (expansin, albumin, xyloglucan, lipid bilayers), ions of opposite charges, drugs, dyes and common chemicals such as urea.51 This compensation meant that the Gibbs free energy of adsorption, which is the force for binding, remained essentially constant. The question of adsorption entropy is of course fairly universal and extends far beyond nanocelluloses since it is of relevance to the fields of heavy metal depollution or oil/alkane adsorption by cell wall components.50,52–57 In fact, micron-sized lignocellulosic fragments have often been found to be very efficient for these applications because of their entropic contribution (Fig. 3). This contribution is associated with the release of bound water in order to establish a cellulose–guest assembly. This is also associated with their more pronounced hydrophobic character at higher temperatures, leading to stronger interactions with all classes of surfactants.58 In this framework, the important porosity and hygroscopicity of lignocellulose is a clear advantage, leading to spontaneous adsorption, in the absence of external inputs.
 | ||
| Fig. 3 The adsorption of various moieties on nanocelluloses follows enthalpy–entropy compensation (CBM stands for cellulose binding module). In most cases with ions and proteins (but also with oils in general), the adsorption enthalpy is unfavourable (ΔH > 0) and the successful adsorption of the moiety is therefore driven by entropy. Figure adapted from refs. 49, 50, 56 and 57. | ||
2.3. Global entropy: geochemical cycles and the central role of biomass
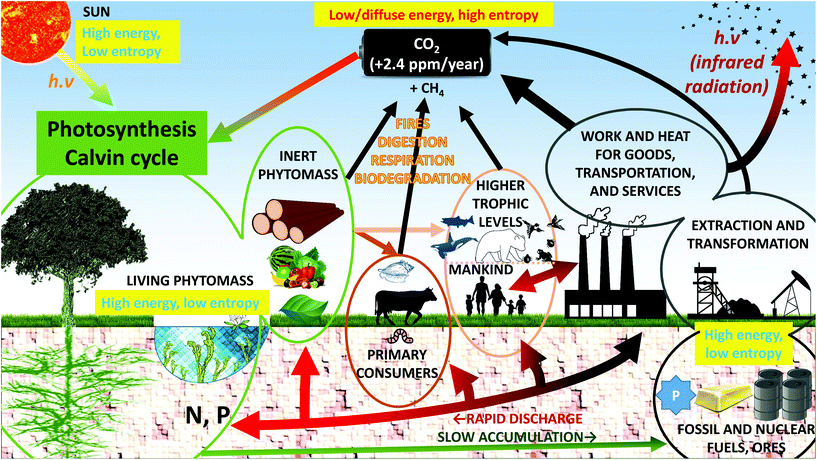
As seen in the previous part, living materials and materials in general show a tendency to structure themselves or to respond to exterior stimuli due to entropy-driven processes. In the photosynthesis process, a high entropy resource, atmospheric carbon dioxide, is converted into low entropy materials (glucose, and assembled polymers in the form of starch, cellulose or other lignocellulosic compounds) through photosynthesis.59,60 This would contradict the second law of thermodynamics unless one considers that plants draw their entropy from the sun's rays.15,59–61 The incoming energy is made of high-frequency photons (yellow, visible light) and the outgoing thermal energy leaves the Earth in the form of low-frequency, infrared, radiation.15 Max Planck's formula E = hν shows that the incoming energy is made up of high-energy photons, whereas the outgoing energy is made up of low-energy photons. Because of the total energy balance, number compensation must occur and the incoming photons must come in lower numbers that the outgoing ones.15,59 As a result, the incoming photons must have a smaller entropy (as well as degrees of freedom and phase space) than the “exchanged” infrared radiation: the solar energy is absorbed and dissipated. The metabolic pathways involved can be clarified by considering that all living organisms are open thermodynamic systems in an out-of-equilibrium state.30,31 Despite the local reduction in entropy associated with the conversion of CO2 into organic matter (sometimes called negative entropy, or negentropy), the entropy of the universe is increased by the growth process and the second law of thermodynamics is respected.Biodiversity is essential, but plants are specific in that they are by far the most abundant autotrophic‡ primary producers. Plants represent about 82.5% of biomass.62 They are the primary producers of food for all the other trophic levels. Because plants use light energy, they are called photoautotrophic. The most abundant biomass on Earth has thus adapted to convert, by photosynthesis, the only inexhaustible low entropy and high energy source (sunlight) into the organic matter that all heterotrophic organisms rely on.15,59 It is estimated that 100 J of radiative sun energy is required to produce 1 J of chemical energy in the form of lignocellulose.12 This has far-reaching consequences since the vast amounts of organic matter that have accumulated and sedimented over geological times have produced low entropy fossil fuel reserves.
The vast burning of fossil fuels and the associated availability of cheap energy has disturbed normal geochemical cycles (Fig. 4). As pointed out by the economist Georgescu-Roegen, fossil fuels constitute an enormous amount of low entropy material that is rapidly (and quasi-irreversibly) converted into high entropy products (CO2, SO2, NOx) by combustion.10,63 This combustion has for the most part taken place in the last two centuries and its origin is anthropogenic. It has resulted in two major products: (i) as far as matter is concerned, the main product is diffuse, high entropy CO2 massively (and quasi-irreversibly) released into the atmosphere despite land and ocean capture and (ii) as far as energy is concerned, heat being lost into space by infrared radiation. Thanks to cheap energy, three low entropy material resources have been rapidly dissipated or disseminated: non-renewable fossil fuels themselves (self-facilitating their own extraction), non-renewable ores and renewable biomass. These three sources are essentially making the most of the chemical potential of the Earth and they sustain life and humanity in its present form. The reduction of these sources means that the chemical potential is depleting.4 At the current discharge rate, only 1000 years of energetic potential would remain to feed the world's population; because of accelerating resource consumption, this potential is alarmingly decreasing by 200 years every 10 years.4 In other words, the current distribution of living species cannot be sustained chemically by the Earth in the long run and species become extinct. Since the “base” trophic level contains plants for the most part, the sustainability of phytomass-derived materials strongly depends on how and why lignocelluloses are used.
Whereas the case of fossil fuel combustion and biomass depletion is relatively straightforward to understand in an “Earth versus universe” battery schematic, the diffusion of condensed chemical elements on the planet seems more relevant when dealt with in terms of inter-regional patterns. Good examples are those of nitrogen and phosphorus. Common food and textile crops need both nitrogen and phosphorus to grow effectively, since both elements are essential for photosynthesis.2,64 Phosphorus cannot be found in the atmosphere but only in the hydrosphere or in the lithosphere. It is generally extracted from non-renewable reservoirs. These P reserves are low-entropy P. When P is extracted and irreversibly scattered on arable land, planetary P becomes of higher entropy since it is mixed with other elements and diffused in the lithosphere and in the hydrosphere, which increases its phase space volume. These P reserves are limited and peak phosphorus could soon be reached (Fig. 6).65,66 In contrast, N can be fixed by legumes from the atmosphere to the lithosphere. In most situations, however, N is brought in the form of synthetic fertilizers, which are produced using fossil fuels. Geological quantities of nitrogen-containing moieties such as NH3 and NOx are therefore released into the environment through the use of fertilizers produced by the Haber–Bosch process (∼120 Mt N per year) or by combustion of fossil fuels (∼40 Mt N per year).67,68 This diffusion of nitrogen fertilizers into the environment is estimated to sustain about half of the food production worldwide and it promotes carbon sequestration. Yet, it has deleterious consequences for the climate (nitrous oxide, N2O, released from the lithosphere and the hydrosphere is a GHG), for biomass (through eutrophication of the hydrosphere and acid rain) and for human health, because ozone production from NOx increases the oxidizing ability of the atmosphere, and because of aerosols. The coupling of the nitrogen cycle and the carbon cycle is extremely strong and current atmospheric N2O levels are at levels never seen before.67,68 Phosphorus is currently estimated to be the limiting factor in the N![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P ratio that enables optimal biomass growth. Its diffusion in a non-usable (high entropy) form therefore means that artificial biomass growth acceleration will be limited in time.69 Similarly to N and P, the metabolic processes of plants rely on freshwater; on a number of other chemical elements, including S, K, Ca, and Mg; and on micronutrients, such as Cl, Fe, B, Mn, Zn, Cu, Mo and Ni. The spatial distribution of these elements also requires close monitoring because their phytoavailability is important with respect to plant growth and human health, especially in the case of food crops.70,71 However, other elements are toxic and their confinement in low entropy, high concentration forms is desired.262 For instance, P fertilization comes with trace amounts of heavy metals, such as cadmium, that have deleterious consequences in the long term due to accumulation in soil, and migration to food. Consequently, the high levels of cadmium in French or Canadian durum wheat needs to be monitored.72 The example of cadmium shows how toxic elements that are concentrated locally (low entropy) can be scattered to levels that are unsuitable for the health of humans, other animals and plants. The same reasoning applies to nuclear wastes (actinides, depleted uranium, etc.), plastics (microplastics, environmental release of hormone-like moieties such as bisphenols) or other heavy metals. The issues of groundwater depletion and salination are also pivotal.73,74
P ratio that enables optimal biomass growth. Its diffusion in a non-usable (high entropy) form therefore means that artificial biomass growth acceleration will be limited in time.69 Similarly to N and P, the metabolic processes of plants rely on freshwater; on a number of other chemical elements, including S, K, Ca, and Mg; and on micronutrients, such as Cl, Fe, B, Mn, Zn, Cu, Mo and Ni. The spatial distribution of these elements also requires close monitoring because their phytoavailability is important with respect to plant growth and human health, especially in the case of food crops.70,71 However, other elements are toxic and their confinement in low entropy, high concentration forms is desired.262 For instance, P fertilization comes with trace amounts of heavy metals, such as cadmium, that have deleterious consequences in the long term due to accumulation in soil, and migration to food. Consequently, the high levels of cadmium in French or Canadian durum wheat needs to be monitored.72 The example of cadmium shows how toxic elements that are concentrated locally (low entropy) can be scattered to levels that are unsuitable for the health of humans, other animals and plants. The same reasoning applies to nuclear wastes (actinides, depleted uranium, etc.), plastics (microplastics, environmental release of hormone-like moieties such as bisphenols) or other heavy metals. The issues of groundwater depletion and salination are also pivotal.73,74
 | ||
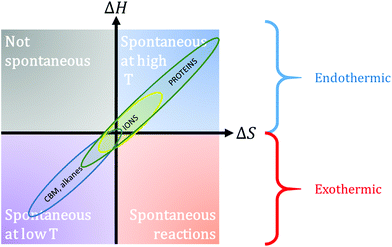
| Fig. 5 Left: Global amount of phytomass (black dots) and the amount of phytomass per capita (orange dots); data adapted from Smil (2011).100 Right: Primary food production (black dots), including cereals, root vegetables, sugar, dry legumes, nuts, fruits, vegetables and oil seeds, and primary textile production (orange dots) between 1961 and 2019; data from FAO.101 Annual viscose production is not represented, but it has been fluctuating between 0.5 and 0.8 kg per capita since 1950, and it currently amounts to ∼0.75 kg per capita.103 | ||
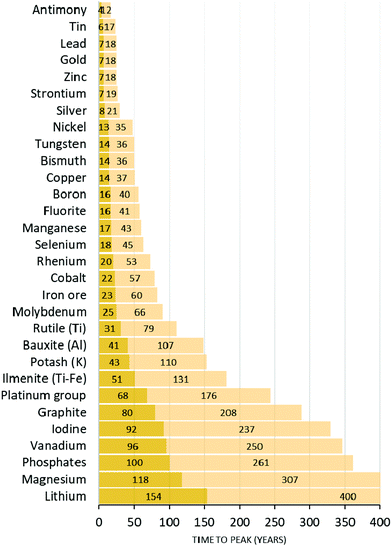 | ||
| Fig. 6 Projection of the basic availability of chemical elements (essentially metals and common rare earths) according to current demand (light colour) and with a projected boom (10% per year) in demand (dark colour). Figure adapted from ref. 266. | ||
The danger is that of a planet in a “post-modern” state with an irreversibly depleted low-entropy potential. This potential is constituted of highly usable biomass in addition to non-renewables, either in the form of fossil fuels or minerals of interest. In this post-modern state, the only low entropy, highly exploitable energy sources are the remaining biomass and sunlight. Slowing down this unsustainable cycle involves quickly limiting the use of fossil fuels, minerals and ores, and elaborating on conservation or restoration strategies to optimize CO2 capture by biomass.5,75–78 It also involves conservation strategies to use biomass at a rate that is entropically compatible with the health preservation of some ecosystems and the restoration of other ecosystems.
2.4. Entropy in conversion processes: exergy
Entropy, in the engineering sense, can precisely be used to tell how much energy is not available in a conversion system.79 The concept of exergy combines the first and the second laws of thermodynamics in that it couples energetic considerations with inevitable energy dissipation: exergy is therefore the optimal work that can be produced by a system.80 This optimum point is reached when the system is at equilibrium with the environment. In fact, a very simple definition of exergy (unit: J) can be given for an irreversible process occurring in a reference environment (sink) with a temperature T0 and it states that the total exergy B available is:§| B = H − T0Sgen |
| I = T0Sgen |
By definition, an ideal reversible process consumes zero exergy. Whereas energy is conserved (first law of thermodynamics), exergy is consumed due to process irreversibility. An important aspect lies in the consideration that the system operates in an infinite and stable atmosphere with a sink temperature T0. It is not affected by the process.81,82 Exergy is therefore a “real world” measure of energy quality (or potential), distinct from energy, which is a measure of quantity: exergy analysis shows how much energy can be utilized in a given context.80 Exergy has also been called the availability function, availability, available work or essergy.63,81,83 More precise definitions of exergy have been given for chemical reactions, adiabatic flows (no heat reservoir external to the cycle), sunlight absorption, electron transport chains, evaporation, condensation or kinetic processes.63,81,82
This tool is, according to some thermodynamicists, the only relevant unit to assess the sustainability (or “thermo-ecological cost”) of technological solutions.63,80,84 Let's explore the example of a combustion engine. Exergy analysis takes into account the fact that combustion of high exergy fossil fuels produces two main streams, other than high exergy work. First, there are the low exergy combustion residues that are chemical in nature (CO2, NOx, soot, etc.). Secondly, there is the low exergy heat dissipated to the surroundings. The exergy balance is quite different from the energy balance because it shows that 90 to 95% of the exergy input is wasted. Indeed, the input energy is transformed without loss: according to the first principle of thermodynamics, the fuel yields the mechanical work, but also heat and the energy for the chemical reactions involved in the production of exhaust fumes. The output energy comprises low grade energy in the sense that low temperature heat and combustion wastes are poorly usable once diffused in the environment (high entropy, low exergy). Generally speaking, exergy analysis can be a powerful tool to quantify the loss of energy quality, and a high exergy efficiency will mean both higher sustainability and lower environmental impacts, because less energy will go to waste.63,80,83,85
It is thus beneficial to express process efficiencies both in terms of energy efficiencies (output energy/input energy) and exergy efficiencies (output exergy/input exergy). Exergy efficiency is always lower than energy efficiency in the actions of fuel or electric heating, or electric cooling, due to thermodynamic irreversibility.80,83 One can therefore immediately see the specific role that biobased materials can play in sustainable development in terms of thermal insulation or any use of these materials for energy co-generation: direct incineration with energy recovery, by-product incineration in a biorefinery context, or more advanced energy conversion via biomethanation or hydrogenation.
Exergy analyses have been undertaken to better understand the efficiency of plant metabolism and photosynthesis in particular. Photosynthesis is a complex two-part process that is well-described in modern textbooks or in relevant publications.12,82 The question of photosynthesis efficiency is central and the chemical reactions involved use 32% of the light captured by the chlorophyll, which has an extremely high efficiency, near quantum limits.12,86–88 It has also been found that the theoretical energy efficiency of photosynthesis as a whole is ∼35–37% when the definition considers the Gibbs free energy of one mole of glucose divided by the energy contained in photons.12,82,89 Yet, experimental measures usually point to much lower energy efficiencies, in the 1–4% range for the whole plant. Detailed analyses of the photosynthesis sub-processes have shown that exergy is lost at several steps of the process and explain the observed low efficiency.82,89 In particular, light absorption is restricted to a narrow photoactive region (400–700 nm) and part of the radiation of interest is reflected by the leaf. Another major loss of efficiency is attributed to the electron transfer chains, which are the channels through which the highly excited electrons are transferred away from their nucleus of origin. These studies shed light on a limiting factor of plant growth, and therefore biomass availability.
Exergy is also a natural candidate for comparing technologies in which low grade heat can be drawn from the environment or re-used within a process. It has for instance been used to compare different heat pumps.90 Another obvious application could be the study of phase change materials (PCM) used for latent heat storage since low temperature heat is extremely valuable in terms of exergy.91 Biobased solutions exist: cellulosic materials such as wood, spun filaments or cellulose nanofibres can be used as scaffolds for PCM stabilization and PCM materials such as myristic or palmitic acid enable the production of fully biobased composites for heat storage.92–95 Closer to the thermodynamics of industrial processes, exergy analysis has been used to characterize the exergy efficiency of biomass conversion in several biorefinery contexts. This type of study can help to identify the most exergy deficient organs of the biorefinery to improve its global efficiency.85,96 The example of a sugarcane mill producing simultaneously lactic acid, steam and electricity was investigated; in this case, a steam boiler was the most deleterious to exergy because the fast thermochemical reactions occurring within the boiler were a source of irreversibility.85 In another study, a whole-crop safflower biorefinery was studied and the exergetic efficiency of the process leading to ethanol and biodiesel production was found to reach ∼73%.97 This result demonstrates the important reduction in waste generation due to the biorefinery concept, with the ability in particular to recover metabolic CO2 from saccharification and fermentation. This CO2 recovery maximises the process exergetically while minimizing pollution. In this study, the largest exergy destruction rate was that of the water treatment unit, a unit characterized by the high irreversibility of its subunits (oxygen consumption by aerobic processes, desalination, electrodialysis and reverse osmosis); this unit alone accounted for ∼70% of the process's irreversibility and future efforts should therefore be aimed at improving this unit.
The analysis of processes using exergy is thus very useful to quantify the depletion of energy quality and the consumption of resources. Nevertheless, it is relatively ill-suited to the analysis of materials due to the complexity of the sink definition: the use of resources that need mining and heavy extraction processes are difficult to deal with.63,98 For instance, chlorine is used for paper fibre bleaching and it is disseminated into the environment. This dissemination is problematic for exergy analysis of the pulp and paper industries since the ground state (or zero state) of natural chlorine presence is not easily defined: the three most important environmental sinks (the atmosphere, the hydrosphere and the Earth's crust) thermodynamically balance each other, away from equilibrium.63 A more thorough exergy analysis would require the inclusion of the exergy cost of the other life cycle aspects, such as transportation, mining, harvesting, pre-treatments, use and disposal. This holistic approach has not been performed in exergy analysis because of the complexity problem. A holistic vision can nevertheless be provided by semi-quantitative approaches and by LCA.
3. Phytomass: availability and environmental context
3.1. The availability of biomass and phytomass-derived materials
Plants represent about 82.5% of biomass; other taxa are bacteria (∼12.8%), fungi (∼2.2%), archaea (∼1.3%), protists¶ (∼0.7%), animals (∼0.4%) and viruses (<0.1%).62 Among plants, only a small fraction (∼0.2%) is found in marine environments (green algae and seagrass). Forests cover 31% of terrestrial land and they amount to 60 to 75% of continental biogenic carbon.99 How much phytomass can be used? Phytomass has been divided in two since the advent of agriculture and it is currently estimated that mankind consumes biomass at a rate that is much higher than biomass can sustain (Fig. 5).4,77,100 In particular, one notices that the amount of phytomass per capita has been decreasing exponentially as it amounted to ∼70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t per capita in 5000 BCE, ∼800 t per capita in 1800, ∼400 t per capita in 1900 and to ∼71 t per capita in 2020 (Fig. 5). In contrast, modern agriculture has been able to provide more food than ever and production has increased by ∼50% over the last 40 years, despite crop productivity reductions (Fig. 5).101,102 As of today, it is estimated that the amount of food produced annually is ∼1.27 t per capita. Nevertheless, this increased food production is exogenic and anthropic as it is maintained by the use of fossil fuels, for mechanization, nitrogen production or phosphorus extraction. This increase has thus only been possible thanks to the additional use of non-renewable low entropy sources (fossil and nuclear fuels, ores) or to the exploitation of slowly built-up low entropy sources (massive deforestation). The other side of the coin is the low (< 2.9%) proportion of ecologically “intact habitats”, and the fast disappearance of wilderness areas in general.75,78 In contrast to food production, the production of textiles can hardly cope with the demand worldwide and it has been steadily decreasing over the last 60 years (Fig. 5). A rough estimate shows that ∼1.5 kg per capita of biobased textile fibres are currently produced annually, if viscose is added to the production of primary textile fibres (cotton, flax, jute, etc.).101,103–105 Several factors can explain this trend and the production of cotton fibres is a good example. This production accounts for roughly 1/3 of the global natural textiles but it is threatened by several environmental limitations such as high water consumption and a high vulnerability to pests.106,107 Furthermore, this cultivar is strongly sensitive to climate change; the threat is particularly strong in rain-dependent regions such as some parts of India. These figures show how little phytomass is currently available when compared to the past and they also demonstrate the growing importance of essential food crops in phytomass use, which should be a warning to the overuse of lignocelluloses in the form of non-essential items with planned or built-in obsolescence. Relatively modest textile production is a reminder that these crops in the form of biocomposites will probably not be able to replace steel or wood for large-scale structural applications.
000 t per capita in 5000 BCE, ∼800 t per capita in 1800, ∼400 t per capita in 1900 and to ∼71 t per capita in 2020 (Fig. 5). In contrast, modern agriculture has been able to provide more food than ever and production has increased by ∼50% over the last 40 years, despite crop productivity reductions (Fig. 5).101,102 As of today, it is estimated that the amount of food produced annually is ∼1.27 t per capita. Nevertheless, this increased food production is exogenic and anthropic as it is maintained by the use of fossil fuels, for mechanization, nitrogen production or phosphorus extraction. This increase has thus only been possible thanks to the additional use of non-renewable low entropy sources (fossil and nuclear fuels, ores) or to the exploitation of slowly built-up low entropy sources (massive deforestation). The other side of the coin is the low (< 2.9%) proportion of ecologically “intact habitats”, and the fast disappearance of wilderness areas in general.75,78 In contrast to food production, the production of textiles can hardly cope with the demand worldwide and it has been steadily decreasing over the last 60 years (Fig. 5). A rough estimate shows that ∼1.5 kg per capita of biobased textile fibres are currently produced annually, if viscose is added to the production of primary textile fibres (cotton, flax, jute, etc.).101,103–105 Several factors can explain this trend and the production of cotton fibres is a good example. This production accounts for roughly 1/3 of the global natural textiles but it is threatened by several environmental limitations such as high water consumption and a high vulnerability to pests.106,107 Furthermore, this cultivar is strongly sensitive to climate change; the threat is particularly strong in rain-dependent regions such as some parts of India. These figures show how little phytomass is currently available when compared to the past and they also demonstrate the growing importance of essential food crops in phytomass use, which should be a warning to the overuse of lignocelluloses in the form of non-essential items with planned or built-in obsolescence. Relatively modest textile production is a reminder that these crops in the form of biocomposites will probably not be able to replace steel or wood for large-scale structural applications.
Looking towards the future, it is difficult to extrapolate how climate change and mankind will further affect biomass availability.5,62,77,100 The effects of climate change can already be felt through price and yield instabilities of common crops, as well as increasing wildfires.108–110 While biomass could respond favourably to increased CO2 levels provided that N and P were available, the effect of heat stress is negatively impacting on plant metabolism by (i) decreasing the activity of photosynthetic enzymes, (ii) increasing evapotranspiration and decreasing irrigation and/or (iii) decreasing CO2 capture by stomatal conductance reduction.67,110–112 Heat stresses lower crop yields. It is therefore not a surprise to learn that climate change has decreased crop productivity by 21% between 1961 and 2021.102 Structural choices also threaten some cultivars and biodiversity, such as the hevea used for natural rubber or palm tree monocultures for palm oil.113,114 In this context, man has an important role to play in land care and biodiversity preservation.5,8,9,76,115 Novel agricultural practices, such as agroforestry, intercropping, the use of perennials, organic farming or permaculture, are attempts to address durability issues.76 Beyond the quantitative availability question, one certitude remains: biomass will remain readily available and universal in the long term.
3.2. Energetic considerations
What is the relative importance of biomass in comparison with crude oil for energy needs? With an annual production amounting to 4 to 5.5 Gt (cumulating dry wood and crops such as cereals, oils and sugar) and a total use of fossils amounting to 15 Gt (including coal), the replacement of fossil fuels with phytomass seems very unlikely.76,116 The addition to the mix of 3.3 Gt of grazed dry biomass used for livestock does not change the imbalance.76 Furthermore, 3 t of biomass from wood or agricultural residues only contains as much energy as ∼1 t of oil. Therefore, attempts to produce energy from lignocellulose will inherently be limited by the biomass growth rate.117–119 Biobased energy can be obtained by direct combustion or conversion into CH4 (biogas), H2 (in a pure form or mixed in syngas), ethanol, biodiesel (transesterified oils or fats) or electricity. The case of biofuels is well-documented in the scientific literature and debated in terms of environmental effects.76,120,121 The conversion of lignocellulose into H2 is the subject of active research worldwide due to political incentives to replace fossil fuels with H2. It can be performed by pyrolysis followed by steam reforming, gasification in supercritical water or in hot compressed water, or direct photocatalysis.118,122–125 However, H2 is already mass-produced, generating 2.5% of global CO2 emissions; it is currently an inefficient energy vector (reservoir leakages, limited yields) and an important source of GHG.63,122,123,126,127 About half of the total H2 production is used for ammonia production.127 The limits to biomass availability and H2 production yields mean that it will be a limited answer to the global energy demand; comparisons should also be made with more mature techniques that are less energy demanding, such as direct incineration or methanation in bioreactors with methanogenic archaea (landfill gas). Looking back in history, the quasi-abandonment of wood-gas generators in the mid-20th century is an interesting case study. It is largely attributed to an insufficient wood supply with respect to demand from the transportation sector for countries like France. Other disadvantages such as high maintenance, fire risks, long starting times or widespread carbon monoxide poisoning led to the abandonment of the technology in countries like Finland and Sweden.1283.3. Carbon storage potential and the importance of forest management
Biomass growth is an important step because wood, algae or industrial crops emanate from very different environments (namely, forests, sea and agricultural land) with different carbon storage potentials. While forests store 45% of organic carbon in their tissues and in the soil, the complete conversion of all existing forests to grassland or cropland would increase atmospheric CO2 by 130–290 ppm.115 To establish a comparison, the current CO2 amount in the atmosphere is ∼410 ppm (280 ppm in 1750, 315 ppm in 1958), with a current annual increase of ∼2.4 ppm. At the current rate, it is estimated that the combustion of fossil fuels yields 5–6 Gt C per year whereas phytomass captures about 2 Gt C per year (Fig. 4). The carbon mitigation potential of phytomass is therefore very limited as long as fossil fuels are as widely used as they are today. Current estimates show that large scale forest plantations could reduce atmospheric carbon by 15–60 ppm, but this storage capacity would take about 100 years to be reached due to the time needed for the forests to reach maturity.115 At longer timescales, from centuries to millennia, trees will enrich the soil carbon content through organic matter transfer in the rhizosphere and via litterfall; this is how atmospheric carbon can be mitigated in the long term. Litterfall means a strong interaction with an incredibly rich soil ecosystem composed of xylophage fungi (rots), other fungi, shrubs, xylophage and non-xylophage insects, earthworms, etc. (some of these organisms are working symbiotically). In this context, one understands the beneficial role that forests could play in carbon mitigation and the ambiguous part of intensive agriculture for annual crop production.Biomass stored in the permafrost could play a dramatic role in atmospheric CO2 release. Rapid permafrost thaw is currently considered a high threat to climate goals (such as the Paris Agreement) because it could cause massive release of CO2 and CH4 into the atmosphere (and mercury into the oceans) in a few decades.129–131 These two greenhouse gases are either stored in the ice or produced by decomposing biomass that was previously stored in the ice; the resulting warming effect is not currently included in models but this permafrost carbon feedback could lead to temperature increases 10–40% higher than estimated.131 Methane has a GWP 84 times that of CO2 over the first 20 years in the atmosphere, 25 times over a 100-year period and 7.6 times over a 500-year period.3,132 The limited lifetime of methane is explained by its photodissociation under UV radiation.
The complexity of natural forests means that their carbon storage capacity is higher than that of plantation forests.115 Yet, plantation forests can be beneficial and they are most efficient carbon sinks in tropical regions; nevertheless, afforestation can have a negative effect at high latitudes, on peatlands or in grassland or savannahs.115 For existing plantation forests, it is generally agreed that sustainable forest management, in contrast to abandoned plantations, can better mitigate carbon emissions.133,134 For instance, prescribed fires are recognized as beneficial treatments that increase the resilience of stands to wildfires and the carbon storage capacity of the forest.135–137 The carbon storage capacity of forests also depends on the use of the forest products. In terms of net CO2 emissions, a scenario in which forests are left untouched is less interesting than a scenario in which wood products are used to replace fossil fuels: untouched forests are not an optimal carbon sink as long as fossil resources are burnt.133,138 Some authors state that the substitution of fossil fuels and “fossil-intensive” materials by wood may be the best single opportunity for carbon mitigation.133
Paradoxically, it is often suspected that forestry operations are ecologically detrimental and cause long-term soil fertility losses due to old tree cuts and intensive management for pulp or biomass use.133,139,140 This perception is not senseless in the context where 99% of new plantation forests over last fifty years are monocultures, and where lots of them require the heavy use of fertilizers and pesticides, a little-known fact.115,141–144 To provide an example related to soil fertility, it has been estimated that heavily utilized stands with 15-year to 30-year rotations could induce important soil organic matter and nutrient losses; these losses translate, in the case of Douglas-fir stands on Vancouver island (Canada), to recovery times lasting up to 8 centuries.140 These suspicions should be considered carefully and they can be addressed with selection forestry and other sustainable management practices.8,9,115,139 For instance, it is recommended to plant mixtures of species.115 Nevertheless, intensive practices can sometimes have benefits: it has been shown that soil fertilization in nitrogen-deficient soils could increase the biomass yield and carbon storage capacity of European forests and heathlands.68,133,145
3.4. The interdependence of biomass and non-renewable resources
It has recently been estimated that man-made materials, including steel, concrete, glass, plastics, paper, wood and textiles, have outweighed the whole living biomass in 2020 (±6 years), reaching about 1.1 Tt.77 These colossal amounts of anthropogenic materials were produced because energy and ores were cheap and abundant. One might, however, question this trend. Indeed, it is now generally agreed that we are on the brink of peak oil (its annual production in 2020 was ∼4.5 Gt). Peak coal is now almost a decade ago and peak gas is still to come. The offset of cheap energy abundance will cause a price increase and rarefaction of all materials, since the prices of extraction, processing and transportation will be cascaded and increased themselves. This will apply to biomass-based materials (construction wood, paper, etc.) as well.The rarefaction of metals, and therefore rare earths, is well established.63,146,147,266 This rarefaction is expected to come rather soon for zinc, silver, nickel and copper (Fig. 6). Many of these strategic materials are currently listed by the EU as being critical for the EU's economy.114 In a similar manner to peak oil, these “metal peaks” will mark the time point after which the rate of extraction will decline due to diminishing ore grades and identifiable reserves. For instance, the approach of a “copper peak” is a threat to the wide-scale and long term deployment of microelectronics and large scale power installations. Semi-conductors are particularly threatened as they are used everywhere today in information and communication technologies. Metal rarefaction in conjunction with fossil fuel depletion is therefore expected to result in a change to the landscape with respect to materials in general, and electronics in particular, over the next centuries.146,148 What's more, these metals are omnipresent in the current technological context and serve all stages of biomass transformation to useful materials.80 This anthropogenic transformation inevitably brings a certain amount of non-renewable resources to their life-cycle.
Advanced phytomass-derived materials exist with applications ranging from partly bio-based batteries to “tougher-than-steel” composites and lab on chips or medical assays (paper microfluidics) and they have a role to play in the future of materials science.149–152 However, forthcoming developments will need to be mindful of the interdependence of resources. To be sustainable, these developments have to minimise the use of non-renewables in the life-cycle of these advanced “green” materials. This also forces us to design materials that integrate a frugal circular bioeconomy to decrease the pressure on biomass production for material needs, so that biomass can be further exploited for energy and food.76
4. Life cycle analysis and relevant quantifications
The previous parts helped to provide a global view of material and energy availability based on thermodynamics and data indices. In this part, life cycle analysis will be introduced to get a better insight into precise environmental aspects of phytomass use.4.1. A succinct introduction to LCA
Life cycle analysis (LCA), sometimes referred to as “life cycle assessment”, is a set of tools aimed at quantifying the total impact that a given material, technological implementation or service has on the environment.148 LCA emerged in the 1960s due to a rise in environmental concerns. In practice, the results are used to rank or evaluate services or processes in terms of their environmental footprint. The results can be used to reduce the environmental cost, to compare two solutions, to inspire technological developments, to modify a product's design or to phase out outdated practices.148 LCA can also be used to document environmental impact reports. LCA can reveal positive outcomes in terms of health, ecosystems or employment. To do so, a LCA integrates the physical fluxes (matter and energy) embodied in the different life stages of a product. There are thus three important aspects that define LCA: energy fluxes, matter fluxes and life stages. Firstly, the energy fluxes should accurately reflect the type of energy and the amounts that were used. In general, the energy comes from three different sources: fossil fuels (coal plants, gas turbines, petrol and diesel generators or combustion engines), uranium (nuclear energy) or renewable energy (biomass, hydroelectric power, solar panels, wind energy). These three sources have very different environmental footprints in terms of resource consumption, greenhouse gas emission and water footprint. In some cases (sawmills, pulp and paper plants), the energy comes directly from biomass by-products (sawdust, lignin) through incineration. Regardless, the energy used at each stage of manufacture is highly dependent on the location of the plant and so are the modes of transportation (trucks or container ships, air freight, trains, cars, etc.). Secondly, the fluxes of matter take into account steps such as mining used for ore extraction, pulping for cellulosic materials, oil extraction for synthetic polymers, refining, transformations, synthesis, enrichment, land management, etc. Thirdly, the description of the different life stages (and their boundaries) is paramount in the description of the working hypothesis. These stages are commonly fractionated into manufacturing steps, transportation, use and disposal scenarios; the study can be narrowed down to any of these subsets. Therefore, LCA can make a global “cradle to grave” assessment but it can also serve to estimate pollution produced by different life stages of a given item or by the item in different life scenarios (for instance, by adjusting the primary energy source or by envisaging different end-of-life scenarios). Various categories of truncated (or partial) LCA have been identified in the literature: the “fence line approach” (product use on site), the “cradle to gate” (from the resource to the factory gate), the “cradle to cradle” in the context of recycling, the “gate to gate” when only one manufacturing/modification step is concerned, the “well to wheel” in the context of transportation or the “field to fork” in the context of food production.148 LCA has been normalized through a set of norms ranging from ISO 14040 to ISO 14049. These harmonized practices are important for interoperability reasons, since each subpart of an LCA has its own set of scenarios that might have been assessed using independent LCA and included in databases. The use of these databases permits the construction of complex scenarios and emphasizes the importance of LCA normalization and interoperability. In practice, LCA has been employed in various situations to assess how beneficial it would be to use electric powered vehicles instead of diesel engine vehicles, to insulate houses, to recycle construction wood instead of putting it into landfill, to recycle paper instead of burning it for energy recovery, to travel by train instead of travelling by air, to recycle batteries instead of manufacturing new ones, etc.76,148,153 LCA fundamentally questions the design of technological items, industrial processes and even political choices. Many counterintuitive results have shown that “common sense” is not an appropriate approach to evaluate environmental policies. Moreover, modern LCA can evaluate the social dimension of a technological system through relevant impact categories and provide information in terms of health and safety (work-related injuries or cancers, psychological stress), discrimination (salary fairness, forced sterilization), restrictions of freedom and associations (child or forced labour), security of living conditions, delocalization, corruption, social benefits, etc.154–156 These are very relevant categories when dealing with agricultural resources shared on a global market.In general, the LCA is built in a four stage process.157 This four step process is a direct implementation of the ISO14000 norm and it differs from earlier methodologies with a narrower focus on improvement analysis.139,148 In the first stage, the objectives and boundaries of the study need to be declared. This declaration will first and foremost include the use of the study (ecoconception, comparative assessment, environmental impact) and the functional unit (a car bonnet, a shopping bag, a kilogram of polymer, etc.). The calculation methods, data sourcing and the boundaries of the study (geographical validity, temporality, raw materials, energy sources, manufacturing, formulation, processing, transportation, distribution, use, reuse, maintenance, recycling and waste management, cases not included) are also indicated at this stage. The second stage is named the life cycle inventory (LCI) and its goal is to make an exhaustive report of the quantitative fluxes associated with the case study. The fluxes can conveniently be expressed through a flow diagram. These fluxes will be expressed in the same physical unit(s); they can be calculated from first principles or extracted from the relevant scientific literature, from public or free databases (US DOE, ADEME, USDA, lcadatabase.com, agribalyse), from restricted databases (Idea, ALCIG, DATASMART LCI package, etc.) or from database aggregators (OpenLCA Nexus, for instance). LCIs include, if relevant, inputs (raw material supplies, energy consumption, fossil fuels, etc.) and outputs such as atmospheric, land and/or water emissions (CO2 equivalent, CH4, NOx emissions, other greenhouse gases, PO4 diffused in water, cumulative radiative forcing, etc.). Interestingly, it is possible to integrate thermodynamic aspects (such as exergy) in conventional LCA.80 The output streams are often classified in various categories such as human health (organic or inorganic carcinogens, ozone layer, etc.) or ecosystem quality (eutrophication, acidification, land use, ecotoxicity, water footprint, etc.). Experimental data and actual on-site measurements can be inaccessible and therefore missing from the LCI. In the third phase of the analysis, the impacts will be evaluated by convoluting life scenarios and the LCI. In the fourth stage, the results will be interpreted and discussed in the light of restrictions (boundary conditions) imposed at the start of the study. In particular, potential biases and incompleteness, various limitations due to restricted data availability and methodological sensitivity should be discussed, as it is not uncommon to find contradictory results (positive or negative footprints, values one order of magnitude different, etc.).132,133 This last step is critical because the complexity of fluxes involved in any LCA is such that the analysis will inevitably be incomplete, as discussed in the introduction of this part.
Subsequently, a critical review can be made by certified experts to check its validity with respect to the norm.158 The experts should be recognized for their competency in both the field of study and LCA in general; their task will be to verify the different stages of the analysis in terms of methodology (adequacy with the norm, possible conflicts of interest, scientific and technological plausibility) and in terms of data sourcing and calculations. They will also verify the interpretations (coherency with the hypothesis and limits of the study, likelihood of the extrapolations) and transparency of the study.
4.2. LCA and other environmental indicators of biobased materials
This subchapter will report some interesting results from the literature that are related to biobased materials. In general, these studies are used to make comparisons between different life scenarios or between biobased materials and non-renewable ones in the context of a life service. The case of materials used in medicine will not be treated because the services rendered are invaluable. When relevant, LCA data are completed with data from the literature. For instance, supplementary figures related to carbon emissions are given.| Product | Impact analysis | Interpretation or additional information |
|---|---|---|
| PLA from corn163 | GWP: 3.1 kg CO2 eq. per kg granule; eutrophication: 0.024 kg N eq.; acidification: 0.62 mol H + eq.; OD: 3.5 × 10−7 kg CFC-11 eq.; HT carcinogens: 1.5 × 10−7 CTUh | A meta-analysis showed that whereas on average the GWP was around 0.7 kg CO2 eq., it could increase to 3.3 kg CO2 eq. when the end of life was included |
| PHA163 | GWP: 0.4 kg CO2 eq. per kg granule (meta-analysis) | A meta-analysis showed the GWP could increase to 3.1 kg CO2 eq. when the end of life was included |
| Thermoplastic starch163 | GWP: 2.0 kg CO2 eq. per kg granule; eutrophication: 0.009 kg N eq.; acidification: 0.53 mol H + eq.; OD: 2.9 × 10−7 kg CFC-11 eq.; human health carcinogens: 8 × 10−8 CTUh | A meta-analysis showed that whereas on average the GWP was around 1.1 kg CO2 eq., it could increase to 1.3 kg CO2 eq. when the end of life was included |
| Fossil-based HDPE163,166 | GWP: 1.9 kg CO2 eq. per kg granule; eutrophication: 4 × 10−4 kg N eq.; acidification: 0.33 mol H + eq.; OD: 0 kg CFC-11 eq.; human health carcinogens: 6 × 10−8 CTUh | Synthetic HDPE used as benchmark |
| PLA from sugarcane166 | GHG emission: 0.5 to 0.8 kg CO2 eq. per kg granule depending on the energy mix; acidification : 2.1 × 10−2 kg SO2 eq.; Eut: 5 × 10−3 PO43− eq.; ozone creation: 3.4 × 10−3 kg C2H4 eq.; HT: 8.5 × 10−3 kg DCB eq. | If PLA is incinerated, its GWP would reach 2.33 kg CO2 eq. per kg of granules. In the same study, HDPE is considered less favourable in terms of GWP, but more favourable in terms of acidification, eutrophication, ozone creation or human toxicity |
| Biobased HDPE from sugar beet169 | GWP: 1.6 kg; acidification 10.6 mmol H+ eq.; TE: 38.6 mmol N eq.; FE: 2.61 g P eq.; fossil fuel depletion: 520 g oil eq.; for ethylene production only: OD: 3.3 × 10−4 g CFC-11 eq.; land use: 2.37 × 104 g C deficit; mineral depletion: 60.19 g Fe eq. | |
| Biobased HDPE from wheat169 | GWP: 1550 g; acidification 16.5 mmol H+ eq.; TE: 62.2 mmol N eq.; FE: 0.75 g P eq.; fossil fuel depletion: 460 g oil eq.; for ethylene production only: OD: 3.4 × 10−4 g CFC-11 eq.; land use: 8.28 × 104 g C deficit; mineral depletion: 100.11 g Fe eq. | |
| Fossil-based HDPE169 | GWP: 4050 g; acidification 3.2 mmol H+ eq.; TE: 7.5 mmol N eq.; FE: −1.2 g P eq.; fossil fuel depletion: 1280 g oil eq.; for ethylene production only: OD: 2.9 × 10−7 g CFC-11 eq.; land use: 5.82 g C deficit; mineral depletion: 0.64 g Fe eq. | |
| Biobased PET from woody biomass168 | For many categories (climate change CO2 eq., acidification kg SO2 eq., TE mole N eq., human health particulate PM 2.5, smog kg 03 eq., ET CTUe, OD kg CFC-11 eq.), the impact increased in the following order: fossil based TA < wood-based TA < corn stover-based TA; for each subset, the impact increased according to fossil EG ≪ corn EG < wheat straw EG ≪ switchgrass EG; only fossil fuel depletion was generally lower for all biobased alternatives (up to 22%) | If carbon sequestration is hypothesized due to biogenic carbon stored in biobased PET, then biobased alternatives could have 21% lower GWP |
| Biobased epoxy (super entropy)162 | GWP: 4079 g CO2 eq.; abiotic depletion: 0.01 g Sb eq.; acidification 25 g SO2 eq.; Eut: 6.9 g PO4 eq.; freshwater aquatic ET 66 g DCB eq.; terrestrial ET: 229 g DCB eq.; cumulative energy demand: 1.90 kJ eq.; OD: 0 g CFC-11 eq.; HT: 545 g DCB eq. | Comparison with a petroleum-based epoxy resin: GWP: 6663 kg CO2 eq.; abiotic depletion: 59.4 kg Sb eq.; acidification 40 kg SO2 eq.; eutrophication : 6.6 kg PO4 eq.; freshwater aquatic ET 246 kg DCB eq.; terrestrial ET : 29 kg DCB eq.; cumulative energy demand: 2.16 MJ eq.; OD: 1.26 × 10−6 kg CFC-11 eq.; human toxicity : 490 kg DCB eq. |
| Biobased polyamide Vestamid® Terra DS174 | GWP: 4 kg CO2 eq.; abiotic depletion: 0.0639 kg Sb eq.; acidification 0.0748 kg SO2 eq.; Eut: 0.0848 kg PO4 eq.; freshwater aquatic ET 0.0342 kg DCB eq.; marine aquatic ET 4030 kg DCB eq.; terrestrial ET l: 0.0132 kg DCB eq.; primary energy demand: 231 MJ; OD: 2.58 × 10−7 kg CFC-11 eq.; HT: 1.12 kg DCB eq. | This polyamide is 100% based on castor oil |
| Cellulose acetate from corncob from a green approach175 | GWP: 176 kg CO2 eq.; abiotic depletion-fossil: 2.56 × 104 MJ eq.; terrestrial acidification 10.1 mol H+ eq.; TE: 17.1 mol N eq.; FE: 0.411 kg P eq.; marine Eut: 1.61 kg N eq.; OD: 8.46 × 10−3 kg CFC-11 eq.; terrestrial ET: 0.0132 kg DCB eq.; freshwater ET: 32.4 CTU; HT non-cancer: 2.27 × 10−4 CTU; HT cancer: 6.05 × 10−5 CTU |
PLA is often cited as an example of a biobased polymer. It is repeatedly claimed to be biodegradable, whereas it is in fact biodegradable only in industrial composting facilities.164 Being produced from corn or sugarcane, PLA is a typical example of a biobased material in competition with food resources.162 In a study dedicated to the cradle to gate analysis of PLA from sugarcane in Thailand, it was found that this polymer resulted in 500–800 kg CO2 eq. emissions per ton, about half of that of PP or PET.166 In this context, the primary energy demand is similar to that of comparable synthetic polymers. Since PLA is based on an agricultural system, it has trade-off contributions to acidification, photochemical ozone creation, eutrophication and farmland use. Its human toxicity potential was found to be similar to that of PE, PET, PP or PS. These results were later confirmed and similar trends in comparison with their fossil-based equivalents were found for other biobased polymers such as PHA, thermoplastic starch, biobased HDPE or biobased PET. Therefore, the advantage of biobased polymers in terms of global warming potential (GWP) and their disadvantages in terms of eutrophication, acidification or ozone depletion seem rather universal, since this trend is reported in a wide range of peer-reviewed articles or book chapters, including literature reviews.160–163,167–170 The mentioned drawbacks largely stem from the use of nitrogen-rich fertilizers in intensive agriculture. Regarding different end-of-life scenarios, it was assessed that the compostability of biobased polymers was not necessarily advantageous when compared to recycling of traditional polymers, because recycling strongly mitigates the GWP associated with the production of new plastics, as well as fossil fuel depletion.161 Furthermore, there are lots of uncertainties regarding methane emissions associated with end of life scenarios of PHA and PLA when they are sent to landfill or composted; these uncertainties can translate to a greatly increased GWP.161,163,166 The other main reproach that could be addressed for these analyses is their tendency to minimize the environmental burden associated with the “stochastic” fate of polymers in the environment: polymers in general cannot go through a strictly controlled end-of-life. Some accidently end-up in the environment and generate microplastic pollution in soils, animals or water; others will be burnt in an uncontrolled manner, generating toxic fumes. Both outcomes suggest very different impacts than initially predicted by LCAs. They underline the need for continuous research on true biodegradability, a domain that could benefit from advanced techniques such as isotope-ratio mass spectrometry or FTIR microspectroscopy.171,172 Overall, there still are uncertainties about the environmental benefits of biobased polymers with respect to fossil-based polymers, and there is room for more comparative studies with consistent methodologies. Furthermore, this domain will certainly benefit from more thorough social life-cycle assessments.173
Shen and coworkers have proposed a “cradle-to-factory gate” LCA of man-made cellulose fibres in comparison with three commonly used textile fibres: cotton, PET and PP fibres.183 Whereas Austrian viscose or modal plants make use of the by-products (black liquor and bark) for energy needs, similar representative factories located in Asia rely on fossil fuels or electricity from the local grid. This difference is due to the integration of dissolving-grade pulp preparation in the Austrian plants, as opposed to pulp import. Therefore, these plants are good examples of integrated processes in which biomass is used both for material and energy needs. In comparison with the viscose process, the NMMO process has the benefits of functioning in a nearly closed-loop cycle, thereby greatly reducing the amount of residual chemicals to be treated or recycled.184,185 A level of uncertainty in these results is induced by the non-inclusion of potentially deleterious forestry practices and their potential impacts on the ecosystem (rotation period, soil nutrient depletion, use of pesticides, etc.). Nevertheless, the LCA demonstrated that the primary energy requirements for Tencel and Austrian viscose were of the same order of magnitude as that of cotton (2.0 CO2 eq. per t fibre, the least energy intensive fibre in this study), with a lower amount of non-reusable energy (oil, gas, coal or uranium) needed. Older Tencel processes or Asian viscose required more primary energy than more controlled and recent alternatives, in large part because of the non-integrated pulp production and inherent transportation costs; nevertheless, all man-made fibres relying on renewable energy (often biomass) for their processing were emitting ∼100 times less CO2 per t of staple fibre. Furthermore, man-made cellulose fibres use 100–500 times less water than cotton if cooling water is ignored, and 10–20 times less water if cooling water is taken into account.183 Because irrigation water is often pumped underground (70% estimate), its intensive use for cotton growth can come in conflict with freshwater for basic human needs.105,183 Furthermore, cotton has an estimated freshwater and terrestrial ecotoxicity about 100 times higher than any other man-made fibres, including PET and PP, and this is due to heavy pesticide use. Eutrophication and land use also disfavoured this cultivar. Asian viscose, PET and PP fibres had the highest GWP, due their reliance on fossil fuels. Data of man-made cellulose fibres in terms of CO2 emissions and low water consumption were also confirmed in a metastudy by other authors.186 In a subsequent study, Shen et al. compared PET fibres, biobased PET fibres, recycled PET fibres, recycled PET, biobased PET fibres and PLA.187 They found out that the GHG emissions were ranked according to: PET > bio-based PET > PLA > recycled PET > recycled and biobased PET > man-made cellulose fibres.
Ionic liquids (ILs) have been an intensive research topic in the last 20 years. These liquids can be tailor-designed and some of them have proved to be very efficient cellulose solvents. Imidazolium-based solvents have been particularly scrutinized.188–190 In a study aiming to compare the potential use of the IL BmimCl with NMMO (Lyocell process), it was found that BmimCl was less favourable in terms of GWP, abiotic resource depletion, acidification potential, photochemical ozone creation potential, freshwater aquatoxicity potential and volatile organic compound emissions.191 Nevertheless, the developmental toxicity of ionic liquids has been assessed and the results of this LCA could benefit from available toxicity data.192–195 It is also well-known that the purity of ILs is a sensitive issue and that “real world” industrial ILs could depart from “academically perfect” laboratory-grade quantities. This study shows how hard it is to use LCA in a prospective way because of the unknowns persisting when a process has not been upscaled, including the EU REACH registration of the chemicals themselves.262 The development of biobased deep eutectic solvents could potentially improve the health track of ILs without compromising performance.196,197
The case of natural fibres other than cotton should be mentioned. There seems to be no consensus on the overall CO2 emissions of flax, hemp or jute fibres, but these fibres require much less irrigation than cotton, if any.105,186 The CO2 footprint of wool varies greatly across studies and it can amount from 1/2 to as much as 20 times that of cotton, because sheep are ruminants that emit methane.186 If sheep's wool is considered a by-product of meat production, the carbon footprint can be negative since wool production is a way to avoid waste generation.186
As of today, the textile industry remains heavily polluting.105,198 Post-finishing treatments (bleaching, scouring, dying and water-repellent, flame-retardant or antibacterial treatments) also contribute to their environmental impact. One ton of textile pollutes 200 t of water and the textile industry is responsible for ∼8–10% of global CO2 emissions.105,179 In this context, it is important to prioritize, in descending order, actual textile use (30% of garments are not even sold or worn), durability, reuse and recycling.105,132,186,199 Incineration and landfill are not desirable and reuse is relevant only if the environmental cost of it (advertising, internet sale, transportation, etc.) does not exceed the cost of production and distribution of equivalent garments.105,132,200 Other options, such as textile renting, updating or repairing, are of course expected to be eco-efficient. Researchers also explore textile recycling and the separation of polyester blended fabrics by selective dissolution of cellulose or wool using ionic liquids, or by selective degradation with keratinases to separate wool from polyester.199,201–203 These approaches have the advantage of dealing with biodegradable/non-biodegradable blends. These blends could otherwise not be recycled as textiles.
It has long been suspected that the replacement of glass fibre-reinforced polymers with natural fibres such as hemp fibres could lower CO2 emissions and decrease crude oil consumption because natural fibres have outstanding mechanical properties along the fibre axis.207 In a recent study, the use of jute fibres instead of glass fibres was studied for buggy bonnets.208 This study revealed that the use of jute had moderate environmental benefits that were essentially due to weight savings and to the production stage. A reduction of ∼10% in fossil fuel use was achieved. However, jute had large social advantages since jute cultivation promoted local farming and limited exodus from rural areas. In a more general study, it was found that natural fibre reinforced composites generally had a lower environmental impact when compared with glass fibre automotive parts for four main reasons: (i) their production pollutes less and acts as temporary carbon storage, (ii) the fibre volume fraction is higher when natural fibres are used, which means that less of the more polluting resins are needed, (iii) lighter weight means a better fuel efficiency of the vehicles during use and (iv) incineration of these parts permits energy recovery.209 However, the use of agricultural crops can have disadvantages too, as the use of fertilizers is known to induce water pollution (acidification and eutrophication).209 It is worthwhile to note that conventional surface treatments performed on natural fibres do not significantly weigh on their LCA outcome: they are much less energy intensive than the processes used for nanofibre production.208 Since the matrix is contributing a significant part of the environmental footprint, another idea would be to replace “virgin” polymers with recycled ones for automotive parts. In this case, LCA has proved the absence of environmental benefits due to lower mechanical performances of the matrix, meaning no weight gain.210 Very different results are obtained in the context of larger vehicles (buses or trucks) where larger structural steel parts can be replaced with pultruded glass fibre composites.211 In this context, the energy savings in use (i.e. during the service life of the vehicles) are very significant due to induced weight savings and fuel efficiency; however, it is not clear whether pultruded glass fibres can compete against aluminium, because aluminium can be recycled while providing similar weight savings.211
Generally speaking, natural fibres can result in 10 to 20% lower environmental load for automotive parts due to their strength and stiffness. One would think that the opportunity provided by nanocelluloses in terms of mechanical performance could be even greater for the LCA.212 Nevertheless, energy demanding manufacturing processes can tip the balance backwards. Despite their excellent mechanical properties, both bacterial cellulose and nanofibrillated cellulose (NFC) suffer from the high energy cost and low yield of their production stage.212–214 This burden weighs heavily on their life cycle. As a result, these technological solutions are presently less favourable than that of glass-fibre/polypropylene composites or neat PLA in an automotive context.215 In a gate-to-gate approach solely taking into account the manufacturing process, the production of cellulose nanowhiskers can be equivalent to or better than carbon nanofibres in terms of GWP or human toxicity (DCB).214 However, these results ignore the lifetime uses of the products and the end-of-life scenarios, but the expansion of nanocelluloses should be accompanied with an updated LCA.
It is demonstrated using dynamic LCA that biobased walls insulated with straw, a very frugal technology, are preferred over conventional insulating materials (such as glass wool or expanded polystyrene insulation) because no matter how efficient these modern materials are, they always contribute a net CO2 emission and ever-increasing radiative forcing.216,218,219 Since CO2 emissions are purely cumulative (as opposed to CH4), the climate impacts of these synthetic materials measured in terms of radiative forcing are most detrimental in the long term. Furthermore, the use of fast-growing lignocellulosic crops such as flax, barley, oat, wheat, corn, or hemp can yield interesting by-products (shives, straw) that can directly be integrated into wall insulation, which offers the chance to rapidly sequester CO2 in building materials.216,218 A scenario in which building facades are extensively renovated in 28 European countries with straw shows that ∼500 to 700 Mt CO2 eq. can be stored in the next 200 years and carbon neutrality would be almost immediate.218 In contrast, insulation with timber and glass wool would take about 150 years to reach carbon neutrality and a classical expanded polystyrene solution would yield 300–400 Mt CO2 eq. by the 2220 horizon. The net difference between these two material choices is about 1 Gt CO2 eq.; as a comparison, it was estimated that the GHG emissions of the oil industry due to gas flaring (such as methane leaks) and thermal extraction of heavy crude oil was ∼1.7 Gt CO2 eq. in 2015 alone.221 The total GHG emissions due to combustion are about 20 times higher (the contribution of methane is important).
Another idea would be to green-up the building envelope because this practice could be beneficial in terms of ecological diversity, air quality, psychological well-being, sound reduction and thermal insulation. Green facades (a.k.a. “vertical gardens”) are known to trap a layer of air against the facade, which helps to insulate the building in windy conditions, but they also provide some cooling effects in warmer conditions because they protect the facade from direct sunlight and help to cool it by evapotranspiration.222 In a LCA performed on different types of technical solutions, direct greening (natural growth of Hedera helix, commonly named ivy) had a negligible environmental footprint in its implementation, while providing significant savings for cooling in a Mediterranean climate. More advanced solutions, such as living wall systems with planter boxes or felt supports, provided better insulation for both cooling or warming, in temperate (Netherlands) or Mediterranean climates. Unfortunately, their environmental benefits in terms of insulation were largely offset by the energy required to produce the materials used for structural supports (steel profiles, HDPE boxes, PE fleece, etc.), the irrigation system and the use of tap water and nutrients to maintain these walls.222
A lot of research is devoted to the manufacturing of lightweight cellulose materials, such as nanocellulose foams, for thermal insulation or sound absorbance.223 However, these materials often rely on supercritical drying or freeze-drying, two energy intensive processes that are too long and costly to replace mass-production techniques. These remain laboratory achievements that cannot yet be transposed in an environmentally desirable way to commercial uses, but future enhancements are expected to solve the challenge of drying under ambient conditions.
There exists a strong connection between printing paper, information storage and the digital world. Information and communication technologies (ICT) have a huge ecological footprint that is largely ignored because the material aspect of ICT is hidden away from the user. Data consumption is out of control, as it was estimated that each individual on Earth was associated with ∼1.7 Mo per s in 2020.227 ICT used ∼3–4% of primary energy and emitted 3–4% of GHG in 2017; the use rate was increasing ∼9% per year by early 2019.228–230 It is projected that ICT could contribute 20% and as much as 51% of electricity demand in 2030.229 In the worst case scenario, ICT would account for as much as 23% of GHG emissions in 2030. Post-pandemic projections cannot yet be established with clarity.230 As a comparison, airplanes, which are often blamed for being heavy polluters, accounted for 2% GHG and the rate of flight commutes was estimated to increase by 5% per annum in 2017. Digital services in general, and data storage in particular, are therefore considerable GHG emitters and cannot be ignored when discussing sustainability.
Is it more sustainable to use interconnected, digital objects, rather than printed paper? Conflicting estimates show that decisive conclusions can hardly be made due to the fast evolution of ICT.231 Other results show that the environmental burden of paper is higher in terms of GHG or energy use when paper is not recycled, but falls in the same range as that of pure digital alternatives when paper recycling is included in the scenario.232,233 It has been found in other studies that the environmental impact of e-readers depended on the content read: they were less disadvantageous when replacing “long term prints” such as books, in contrast with newspapers. Nevertheless, these studies ignore at least part of the digital equipment production in their inventory, whereas this production phase is usually dominant in these LCAs since they include the important energy consumption and environmental burdens induced by rare earth or metal extraction.234–236 For instance, the environmental footprint (eCO2) of smartphones is 80–90% associated with the extraction, production and transport phases.235 It has been estimated in a separate study that the carbon footprint of a book was ∼1.3–7 kg eCO2 whereas that of the most common tablets or e-readers was in the 130–170 kg eCO2 range, meaning that between 19 and 130 books could have the same environmental footprint as the necessary hardware used for reading them electronically.236 Moving beyond GHG, the manufacturing of these devices depletes rare earths and metals and results in high human toxicity.235
However, there might be some domains where progress in paper science can be applied to introduce more sustainable alternatives to digital storage. Historically, paper was used in the form of punch cards to store data. Modern laser or printing techniques could mean printing data at 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 DPI (meaning one dot is 0.25 μm). If each dot is a bit of information (black and white scenario), then an A4 paper sheet could contain at least 60 Go of information. With 16-bit encoding, 1 To of information could be held on one cellulosic substrate the size of a standard paper sheet. Current experimental efforts have produced impressive results since nanocelluloses ∼3 nm in diameter mean very low roughness substrates. Existing nanolithography techniques or nano-embossing have achieved well-defined pillars about 7.5 μm in diameter spaced 4.5 μm apart, virtually resulting in an estimated information density of 54 Mo per A4 paper sheet.237 Cellulose nanocrystals cross-linked with citric acid have been imprinted with features as small as 140 nm (lines) or hole patterns with a periodicity as small as 800 nm. In the latter case, the information density would reach 12 Go per A4 paper sheet (the equivalent of 8000 scientific journal articles in pdf format, each 1.5 Mo on average).238 Patterns with features above ∼100 nm could easily be read at high rates using modern optical devices.239 One needs to remember that cellulose acetate has long been used for TEM imaging too, with the ability to replicate much smaller features. Therefore, cellulose could be used for long term passive storage. Cellulose 250 million years old has been found intact in halite crystals, giving an idea of the durability of cellulose when preserved under the right conditions and what a long-lasting passive storage it could be.240
000 DPI (meaning one dot is 0.25 μm). If each dot is a bit of information (black and white scenario), then an A4 paper sheet could contain at least 60 Go of information. With 16-bit encoding, 1 To of information could be held on one cellulosic substrate the size of a standard paper sheet. Current experimental efforts have produced impressive results since nanocelluloses ∼3 nm in diameter mean very low roughness substrates. Existing nanolithography techniques or nano-embossing have achieved well-defined pillars about 7.5 μm in diameter spaced 4.5 μm apart, virtually resulting in an estimated information density of 54 Mo per A4 paper sheet.237 Cellulose nanocrystals cross-linked with citric acid have been imprinted with features as small as 140 nm (lines) or hole patterns with a periodicity as small as 800 nm. In the latter case, the information density would reach 12 Go per A4 paper sheet (the equivalent of 8000 scientific journal articles in pdf format, each 1.5 Mo on average).238 Patterns with features above ∼100 nm could easily be read at high rates using modern optical devices.239 One needs to remember that cellulose acetate has long been used for TEM imaging too, with the ability to replicate much smaller features. Therefore, cellulose could be used for long term passive storage. Cellulose 250 million years old has been found intact in halite crystals, giving an idea of the durability of cellulose when preserved under the right conditions and what a long-lasting passive storage it could be.240
5. Perspectives
There are many things to conclude from this review. Perhaps, first and foremost, fundamental thermodynamic considerations should be placed back at the centre of the discussion on sustainability. It is evident from a rough study of entropy levels on Earth that photosynthetic organisms and their sophisticated machinery have extracted high entropy CO2 from the atmosphere over geological periods and turned it into low entropy resources in the form of fossil fuels and the entire biomass, including phytomass. This constitutes the chemical battery of the Earth. This chemical battery is the root of our biological existence, but also that of our current economy. The second law of thermodynamics shows that resource depletions are equivalent to a battery discharging, which translates into quasi-irreversible atmospheric chemical changes (CO2 levels). The carbon mitigation potential of phytomass-derived materials is modest (at best) in comparison with our unreasonable use of fossil fuels: if storing carbon in buildings by insulating Europe for 200 years with straw could lead to the storage of 1 Gt CO2 eq., a gigantic 1.7 Gt CO2 eq. was emitted by the oil industry in 2015 through flaring alone. Without massively reducing the burning of fossil fuels, the carbon mitigation power of phytomass will be insignificant. Phytomass will not be the miracle remedy to CO2 emissions. The biomass capital is also declining due to overexploitation and climate change; it is currently at its lowest since 5000 BCE. Even worse, when biomass and crops are impacted by climate, consequences can be bleak. It is estimated that recent social conflicts in the Middle East or in Africa have been triggered by unusually warm and dry summers, or by abundant rainfall.241,242 Economic pressures due to lumber shortages on the wood market are currently felt worldwide due to a higher demand for wood in construction. Therefore, it is very important that technological developments do not alter the phytomass budget any further.Technological developments should also be mindful of non-renewable resources, such as freshwater, and basic chemicals used for fertilization, such as phosphorus and nitrogen. Water, nitrogen and phosphorus are planetary boundary indicators in a critical state; furthermore, the global nitrogen geochemical cycle is out-of-balance due to massive and inefficient use of fertilizers.2,4,68 Nitrogen availability, and synthetic fertilizers containing nitrogen in particular, are known to boost food production. Yet, they are currently present in excess due to favourable fossil fuel availability, a situation that cannot be sustained indefinitely and that will be critical to address with respect to global food demand.2,64 The use of phytomass for food first will become paramount. Consequently, biomass needs to be taken care of in the long term through better agricultural practices, such as agroforestry or permaculture. These practices can have social benefits: the development of local crops in rural areas can put a brake on rural exodus and promote wealth, education and safety. Looking further at the interdependence of resources, other worrying facts emerge. In particular, the depletion of non-renewable resources means that there is no backup plan for a wide range of technologies (including agriculture) once peak oil, peak coal, peak metals or peak rare earths are reached. That is where renewables from biomass have a particular role to play, along with more abundant geological resources such as calcium carbonate, quartz or clays. The exponentially increasing consumption of materials and energy over the last two centuries hides a more optimistic result: that of efficiency. Indeed, whereas technological progress has enabled man to use more and more resources at a higher rate, technological progress has also enabled a much higher efficiency. As a result, fossil fuel consumption per dollar of gross domestic product has been halved between 1965 and 2015.5 The increase in global consumption is therefore attributed to two things: the increase of the world's population and that of its global well-being, thanks to the rebound effect||. The rebound effect is spectacular in the digital world in which remarkable innovations in energy efficiency or data storage have led to more mass-production.243,244
Nevertheless, the entropic aspect of biomass production is in itself inspiring to produce more efficient technologies. The quantum exergy efficiency of photosynthesis is remarkable and it has long been recognized in academic works.82,86–88 Consequently, it is doubtful whether atmospheric carbon capture technologies will ever achieve better light-to-chemical energy conversion in such an environmentally innocuous way.245,246 Bioinspired processes should therefore follow the same path in trying to use solar energy and entropic paths. Closer to feasible research subjects, low-grade and diffuse energy sources should be used as much as possible, as shown in the exergy subchapter. This approach will give processes a net advantage in energy efficiency and sustainability from the ground up. These energy sources can be chemical (H2O, CO2, NOx, etc.), kinetic (wind, river or ocean currents) or thermal (low grade infrared, convective heat in water streams). Examples therefore include the use of biomass in techniques such as windmills, phase change materials or passive desalination membranes.92,247 Another interesting lesson from this thermodynamic study is that entropy can actually be a driving force for materials self-assembly, meaning autonomous or low-energy processes, potentially with minimal environmental footprints. Existing examples in the domain of cellulose science include xyloglucan/cellulose self-assembly as well as spontaneous adsorption of a vast array of chemicals on lignocellulosic substrates.
Because resources are finite, “low tech” processes have an important role to play. They display inherent frugality because they rely on simple technologies and do not require external energy sources (such as electricity or fossil fuels) to function; instead, they rely on passive or low-grade sources such as solar energy, wind power or human power. Examples include the washing line or the shower bag, borrowing books from the library instead of downloading ebooks on a tablet, or more recently as seen during the COVID-19 pandemic, personal protective equipment in the form of home-made face masks/shields, or long exposure to natural light for fabric disinfection.236,243,248 Low tech processes contrast with those that are high tech (hydrogen production, hybrid vehicles, solar panels, etc.) in that they do not follow a high consumption lifestyle and growth-oriented macroeconomics; growth-oriented consumption is increasingly pointed out by modern economists as being unsustainable, both in terms of social and physical limits.10,244,249 Ideally, low tech solutions should have the advantage of being easy to implement in all areas, including isolated ones, with limited transportation involved. In the COVID-19 case, staying home was the most efficient option selected by most governments worldwide, and this change in attitude is a reminder that solutions to modern issues are not always technological. Nevertheless, the danger with low tech solutions, as well as environmental innovations in general, is once again the rebound effect. The rebound effect can translate into exacerbated resource consumption, an outcome in opposition to biomass availability. A bias would be to ignore that some low-tech realizations are scientifically challenging, as illustrated by hygromorphic materials (cladding, for instance) that can be used in passive responsive architecture.250–253 Since the sustainability of bio-based materials is fundamentally dependent on transformation processes, one solution could be to focus on low-tech transformation processes as well. Field retting is such an example in which wind, rain and naturally occurring soil microorganisms help to fractionate stem fibres prior to further processing and defibrillation.
Life cycle analysis and exergy analysis both produce some very interesting results on precise aspects of materials or processes. For instance, they show how polluting the textile industry is, but also which parts of this industry need to be addressed and how they can be addressed. They also show that the LCA of biopolymers or biofuels usually suffer from the footprint of intensive farming: intensive farming serves to produce dedicated crops, but also emits large amounts of NOx, contributes to freshwater depletion and leads to the acidification and eutrophication of water streams. In that sense, LCA demonstrates that sustainability is not obtained through the use of phytomass-derived resources alone. Dynamic LCAs are of particular relevance when dealing with carbon storage, a track that seems relevant in the building industry with phytomass-derived structural and insulating materials. LCA also serves to evaluate the environmental cost of recycling, an option that is generally favourable in the paper industry, for instance. A specific mention should be made of nanocelluloses since these materials are the subject of intense research efforts worldwide. Their production is currently rather process-intensive and a lot of work still remains to cleanly and efficiently deconstruct biomass into nanocelluloses.254 Perhaps it is not a surprise to see a shift in the nanocellulose community to top down approaches, rather than bottom-up ones.149,255,256 There will be some advantages in dealing with relatively bulky entities such as energy savings due to the absence of heavy refining. However, as far as delignification, densification or bleaching are required, the treatment of thick samples will be limited by heat and liquid diffusion kinetics; these two limitations will translate to other energy costs that are not as important for heavily refined samples with much smaller dimensions.257 Alternative techniques such as microwave ovens instead of conventional convective ovens, or an understanding of bulk chemistry for biomass functionalization will play a decisive role in the optimization of these processing paths.257,258 The scientific orientations and possibilities are extremely numerous, and a recent article by Jinwu Wang et al. echoes the 1999 article by Josef Schurz that has opened many avenues, many of them still to explore.259,260 In this manuscript, a rapid assessment of current nanolithography techniques shows that cellulosics are extremely promising for passive data storage. Sustainable data storage will without a doubt be a major challenge in the upcoming decades and important reductions of CO2 emissions could be obtained while temporarily storing carbon.
As a final conclusion, the question of materials sustainability, and ecological economics in general, will not have a single answer.261 In the context of lignocellulose, the absence of a single answer means that it does not suffice to use biobased materials to contribute sustainability. Regardless of the chosen strategies, the use of biobased materials will have to be relevant and unwasteful; the materials produced will need to be durable, re-usable, multifunctional, ideally upcyclable or recyclable. In particular, it is generally agreed that recycling, reuse and efforts in energy efficiency contribute to reductions in material and energy usage.76,225,244 There is therefore no difference from the sustainability standpoint between biobased materials and non-renewable materials such as ores, metals or synthetic organics: both are intertwined in the same technological framework. Whereas these conclusions will come as no surprise, the scale of the task is colossal if society is to comply with sustainability goals such as those defined by the Paris Agreement on Climate Change or the European Union and United Nations common goals for a sustainable future.
Abbreviations
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author would like to thank Joël Bréard (ABTE, UCN) for having introduced him to the field of exergy, Anne Pantet, Abdellah Alem and Nasre-Dine Ahfir (LOMC, ULHN) for having introduced him to the theme of bioremediation by plant fibres, and Jean-Christophe Avice (EVA, UCN) for our discussions on cadmium pollution linked with phosphate uses.Notes and references
- P. J. Crutzen, Nature, 2002, 415, 23–23 CrossRef CAS PubMed.
- W. Steffen, K. Richardson, J. Rockström, S. E. Cornell, I. Fetzer, E. M. Bennett, R. Biggs, S. R. Carpenter, W. de Vries, C. A. de Wit, C. Folke, D. Gerten, J. Heinke, G. M. Mace, L. M. Persson, V. Ramanathan, B. Reyers and S. Sörlin, Planetary boundaries: Guiding human development on a changing planet, Science, 2015, 347(6223), 1259855 CrossRef PubMed.
- S. Solomon, M. Manning, M. Marquis and D. Qin, Climate change 2007-the physical science basis: Working group I contribution to the fourth assessment report of the IPCC, Cambridge university press, 2007, vol. 4 Search PubMed.
- J. R. Schramski, D. K. Gattie and J. H. Brown, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 9511–9517 CrossRef CAS PubMed.
- M. G. Burgess and S. D. Gaines, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6328–6330 CrossRef CAS PubMed.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. I. Chapin, E. Lambin, T. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. Corell, V. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. Foley, Planetary Boundaries: Exploring the Safe Operating Space for Humanity, Ecol. Soc., 2009, 14(2) DOI:10.5751/ES-03180-140232.
- J. Rockström, W. Steffen, K. Noone, Å. Persson, F. S. Chapin, E. F. Lambin, T. M. Lenton, M. Scheffer, C. Folke, H. J. Schellnhuber, B. Nykvist, C. A. de Wit, T. Hughes, S. van der Leeuw, H. Rodhe, S. Sörlin, P. K. Snyder, R. Costanza, U. Svedin, M. Falkenmark, L. Karlberg, R. W. Corell, V. J. Fabry, J. Hansen, B. Walker, D. Liverman, K. Richardson, P. Crutzen and J. A. Foley, Nature, 2009, 461, 472–475 CrossRef PubMed.
- K. F. Wiersum, Environ. Manage., 1995, 19, 321–329 CrossRef.
- X. Rochel, Cultural heritage and sustainable forest management: the role of traditional knowledge, 2006, p. 85 Search PubMed.
- N. Georgescu-Roegen, East. Econ. J., 1986, 12, 3–25 Search PubMed.
- C. D. D. Rupprecht, J. Vervoort, C. Berthelsen, A. Mangnus, N. Osborne, K. Thompson, A. Y. F. Urushima, M. Kóvskaya, M. Spiegelberg, S. Cristiano, J. Springett, B. Marschütz, E. J. Flies, S. R. McGreevy, L. Droz, M. F. Breed, J. Gan, R. Shinkai and A. Kawai, Multispecies sustainability, Global Sustainability, 2020, 3 DOI:10.1017/sus.2020.28.
- M. Nabors, Biologie végétale: Structures, fonctionnement, écologie et biotechnologies, Benjamin Cummings, 2008 Search PubMed.
- E. M. Bennett, R. Biggs, G. D. Peterson and L. J. Gordon, One Earth, 2021, 4, 172–176 CrossRef.
- N. US Department of Commerce, The Earth-Atmosphere Energy Balance, https://www.weather.gov/jetstream/energy, (accessed June 1, 2021).
- R. Penrose, The Road to Reality: A Complete Guide to the Physical Unverse, BCA, The Random House Group Limited., 2004 Search PubMed.
- A. Gefter, How to Rewrite the Laws of Physics in the Language of Impossibility, https://www.quantamagazine.org/with-constructor-theory-chiara-marletto-invokes-the-impossible-20210429/, (accessed June 1, 2021).
- J. Wohlert, M. Bergenstråhle-Wohlert and L. A. Berglund, Cellulose, 2012, 19, 1821–1836 CrossRef CAS.
- G. E. Crooks, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1999, 60, 2721–2726 CrossRef CAS PubMed.
- N. Wolchover, A New Thermodynamics Theory of the Origin of Life | Quanta Magazine, https://www.quantamagazine.org/a-new-thermodynamics-theory-of-the-origin-of-life-20140122/, (accessed April 1, 2021).
- V. N. Manoharan, Science, 2015, 349, 1253751 CrossRef PubMed.
- A. Haji-Akbari, M. Engel, A. S. Keys, X. Zheng, R. G. Petschek, P. Palffy-Muhoray and S. C. Glotzer, Nature, 2009, 462, 773–777 CrossRef CAS PubMed.
- D. Frenkel, Phys. A, 1999, 263, 26–38 CrossRef CAS.
- P. F. Damasceno, M. Engel and S. C. Glotzer, Science, 2012, 337, 453–457 CrossRef CAS PubMed.
- L. Onsager, Ann. N. Y. Acad. Sci., 1949, 51, 627–659 CrossRef CAS.
- Y. Geng, G. van Anders, P. M. Dodd, J. Dshemuchadse and S. C. Glotzer, Sci. Adv., 2019, 5, eaaw0514 CrossRef CAS PubMed.
- B. C. Rocha, S. Paul and H. Vashisth, Entropy, 2020, 22, 877 CrossRef CAS PubMed.
- D. Lourdin, J. Peixinho, J. Bréard, B. Cathala, E. Leroy and B. Duchemin, Cellulose, 2016, 23, 529–543 CrossRef CAS.
- P. C. W. Davies, E. Rieper and J. A. Tuszynski, BioSystems, 2013, 111, 1–10 CrossRef CAS PubMed.
- T. Bäuerle, A. Fischer, T. Speck and C. Bechinger, Nat. Commun., 2018, 9, 1–8 CrossRef PubMed.
- R. C. Dewar, Philos. Trans. R. Soc., B, 2010, 365, 1429–1435 CrossRef CAS PubMed.
- J. L. England, Statistical physics of self-replication, J. Chem. Phys., 2013, 139(12), 09B623_1 CrossRef PubMed.
- A. D. Kirwan, Entropy, 2008, 10, 58–70 CrossRef.
- B. Duchemin, G. Cazaux, M. Gomina and J. Bréard, J. Colloid Interface Sci., 2021, 592, 215–226 CrossRef CAS PubMed.
- J. Lyklema, Colloids Surf., A, 1999, 156, 413–421 CrossRef CAS.
- K. B. Smith, J.-N. Tisserant, S. Assenza, M. Arcari, G. Nyström and R. Mezzenga, Adv. Sci., 2019, 6, 1801540 CrossRef PubMed.
- S. Kishani, T. Benselfelt, L. Wågberg and J. Wohlert, J. Colloid Interface Sci., 2021, 588, 485–493 CrossRef CAS PubMed.
- T. Benselfelt, E. D. Cranston, S. Ondaral, E. Johansson, H. Brumer, M. W. Rutland and L. Wågberg, Biomacromolecules, 2016, 17, 2801–2811 CrossRef CAS PubMed.
- B. Jean, L. Heux, F. Dubreuil, G. Chambat and F. Cousin, Langmuir, 2009, 25, 3920–3923 CrossRef CAS PubMed.
- A. Momeni, C. M. Walters, Y.-T. Xu, W. Y. Hamad and M. J. MacLachlan, Nanoscale Adv., 2021, 3, 5111–5121 RSC.
- D. M. Hall and G. M. Grason, Interface Focus, 2017, 7, 20160140 CrossRef PubMed.
- Y. Liu, N. Dehmamy and A.-L. Barabási, Nat. Phys., 2020, 1–7 Search PubMed.
- G. Nyström, M. Arcari, J. Adamcik, I. Usov and R. Mezzenga, ACS Nano, 2018, 12(6), 5141–5148 CrossRef PubMed.
- C. Schütz, M. Agthe, A. B. Fall, K. Gordeyeva, V. Guccini, M. Salajková, T. S. Plivelic, J. P. F. Lagerwall, G. Salazar-Alvarez and L. Bergström, Langmuir, 2015, 31, 6507–6513 CrossRef PubMed.
- C. Honorato-Rios and J. P. F. Lagerwall, Commun. Mater., 2020, 1, 1–11 CrossRef.
- C. Honorato-Rios, A. Kuhnhold, J. R. Bruckner, R. Dannert, T. Schilling and J. P. F. Lagerwall, Front. Mater., 2016, 21 Search PubMed.
- M. A. Hubbe and O. J. Rojas, BioResources, 2008, 3, 1419–1491 Search PubMed.
- G. Nyström, A. B. Fall, L. Carlsson and L. Wågberg, Cellulose, 2014, 21, 1591–1599 CrossRef.
- S. Lombardo, A. Gençer, C. Schütz, J. Van Rie, S. Eyley and W. Thielemans, Biomacromolecules, 2019, 20, 3181–3190 CrossRef CAS PubMed.
- S. Lombardo and W. Thielemans, Cellulose, 2019, 26, 249–279 CrossRef CAS.
- A. Ramesh, D. J. Lee and J. W. C. Wong, J. Colloid Interface Sci., 2005, 291, 588–592 CrossRef CAS PubMed.
- P. Chen, Y. Nishiyama, J. Wohlert, A. Lu, K. Mazeau and A. E. Ismail, J. Phys. Chem. B, 2017, 121, 2244–2251 CrossRef CAS PubMed.
- A. A. Beni and A. Esmaeili, Environ. Technol. Innovation, 2020, 17, 100503 CrossRef.
- X. Liu and D.-J. Lee, Bioresour. Technol., 2014, 160, 24–31 CrossRef CAS PubMed.
- Z. N. Garba, I. Lawan, W. Zhou, M. Zhang, L. Wang and Z. Yuan, Sci. Total Environ., 2020, 717, 135070 CrossRef CAS PubMed.
- B. Abbar, A. Alem, A. Pantet, S. Marcotte, N.-D. Ahfir, H. Wang, T. Ouahbi, B. Duchemin and D. Duriatti, Environ. Technol., 2018, 1–26 Search PubMed.
- G. M. Dorris and D. G. Gray, J. Colloid Interface Sci., 1980, 77, 353–362 CrossRef CAS.
- N. Gurnagul and D. G. Gray, Can. J. Chem., 1987, 65, 1935–1939 CrossRef CAS.
- B. Lindman and G. Karlström, C. R. Chim., 2009, 12, 121–128 CrossRef CAS.
- W. Brittin and G. Gamow, Proc. Natl. Acad. Sci. U. S. A., 1961, 47, 724 CrossRef CAS PubMed.
- R. C. Jennings, E. Engelmann, F. Garlaschi, A. P. Casazza and G. Zucchelli, Biochim. Biophys. Acta, Bioenerg., 2005, 1709, 251–255 CrossRef CAS PubMed.
- E. Schrodinger, What is life? The physical aspect of the living cell, At the University Press, 1951 Search PubMed.
- Y. M. Bar-On, R. Phillips and R. Milo, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 6506–6511 CrossRef CAS PubMed.
- R. U. Ayres, L. W. Ayres and K. Martinas, 1996. Eco-thermodynamics: exergy and life cycle analysis. INSEAD, Fontainebleau (France). Report No. 96/19/EPS. https://www.researchgate.net/profile/Katalin-Martinas/publication/246004207_Eco-thermo-dynamics_exergy_and_life_cycle_analysis/links/56af1abb08ae19a385171b9f/Eco-thermo-dynamics-exergy-and-life-cycle-analysis.pdf (accessed 14.02.2022) Search PubMed.
- P. Barbieri, S. Pellerin, V. Seufert, L. Smith, N. Ramankutty and T. Nesme, Nat. Food, 2021, 2, 363–372 CrossRef.
- D. Cordell and S. White, Sustainability, 2011, 3, 2027–2049 CrossRef.
- C. J. Rhodes, Sci. Prog., 2013, 96, 109–152 CrossRef CAS PubMed.
- N. Gruber and J. N. Galloway, Nature, 2008, 451, 293–296 CrossRef CAS PubMed.
- D. Fowler, M. Coyle, U. Skiba, M. A. Sutton, J. N. Cape, S. Reis, L. J. Sheppard, A. Jenkins, B. Grizzetti, J. N. Galloway, P. Vitousek, A. Leach, A. F. Bouwman, K. Butterbach-Bahl, F. Dentener, D. Stevenson, M. Amann and M. Voss, Philos. Trans. R. Soc., B, 2013, 368(1621), 20130164 CrossRef PubMed.
- Y. Peng, Z. Peng, X. Zeng and J. H. Houx, Plant Soil, 2019, 436, 245–252 CrossRef CAS.
- A. Kabata-Pendias, Geoderma, 2004, 122, 143–149 CrossRef CAS.
- X. Wei, M. Hao, M. Shao and W. J. Gale, Soil Tillage Res., 2006, 91, 120–130 CrossRef.
- C. Nguyen, E. Gourdain, G. Grignon, B. Barrier-Guillot and B. Meleard, in ICOBTE 2017, Zurich, Switzerland, 2017, p. 1 Search PubMed.
- L. Brown, World on the edge: how to prevent environmental and economic collapse, Earth Policy Institute, 2011 Search PubMed.
- R. Connor, The United Nations world water development report 2015: water for a sustainable world, UNESCO publishing, 2015, vol. 1 Search PubMed.
- J. E. M. Watson, D. F. Shanahan, M. Di Marco, J. Allan, W. F. Laurance, E. W. Sanderson, B. Mackey and O. Venter, Curr. Biol., 2016, 26, 2929–2934 CrossRef CAS PubMed.
- H. L. Bos and J. Broeze, Biofuels, Bioprod. Biorefin., 2020, 14, 187–197 CrossRef CAS.
- E. Elhacham, L. Ben-Uri, J. Grozovski, Y. M. Bar-On and R. Milo, Nature, 2020, 1–3 Search PubMed.
- A. J. Plumptre, D. Baisero, R. T. Belote, E. Vázquez-Domínguez, S. Faurby, W. JÈ©drzejewski, H. Kiara, H. Kühl, A. Benítez-López, C. Luna-Aranguré, M. Voigt, S. Wich, W. Wint, J. Gallego-Zamorano and C. Boyd, Front. For. Global Change, 2021, 4, 26 Search PubMed.
- J. Swinburne, Nature, 1943, 151, 335–336 CrossRef.
- I. Dincer and M. A. Rosen, Renewable Sustainable Energy Rev., 2005, 9, 169–189 CrossRef.
- J. H. Keenan, Br. J. Appl. Phys., 1951, 2, 183–192 CrossRef CAS.
- C. S. Silva, W. D. Seider and N. Lior, Chem. Eng. Sci., 2015, 130, 151–171 CrossRef CAS.
- K. H. Denbigh, Chem. Eng. Sci., 1956, 6, 1–9 CrossRef CAS.
- J. Szargut , The Magazine of Polish Academy of Sciences, 2005, 3, 31–33.
- M. Aghbashlo, M. Mandegari, M. Tabatabaei, S. Farzad, M. M. Soufiyan and J. F. Görgens, Energy, 2018, 149, 623–638 CrossRef CAS.
- O. Warburg, D. Burk, V. Schocken and S. B. Hendricks, Biochim. Biophys. Acta, 1950, 4, 335–348 CrossRef CAS.
- H. Frost-Christensen and K. Sand-Jensen, Oecologia, 1992, 91, 377–384 CrossRef CAS PubMed.
- J. Cao, R. J. Cogdell, D. F. Coker, H.-G. Duan, J. Hauer, U. Kleinekathöfer, T. L. C. Jansen, T. Mančal, R. J. D. Miller, J. P. Ogilvie, V. I. Prokhorenko, T. Renger, H.-S. Tan, R. Tempelaar, M. Thorwart, E. Thyrhaug, S. Westenhoff and D. Zigmantas, Sci. Adv., 2020, 6, eaaz4888 CrossRef CAS PubMed.
- R. Petela, Sol. Energy, 2008, 82, 311–328 CrossRef CAS.
- P. Poredoš, B. Vidrih and A. Poredoš, Entropy, 2021, 23, 47 CrossRef PubMed.
- Y.-M. Chu, N. H. Abu-Hamdeh, B. Ben-Beya, M. R. Hajizadeh, Z. Li and Q.-V. Bach, J. Mol. Liq., 2020, 320, 114457 CrossRef CAS.
- M. R. Yazdani, R. Ajdary, A. Kankkunen, O. J. Rojas and A. Seppälä, ACS Appl. Mater. Interfaces, 2021, 13, 6188–6200 CrossRef CAS PubMed.
- H. Yang, S. Wang, X. Wang, W. Chao, N. Wang, X. Ding, F. Liu, Q. Yu, T. Yang, Z. Yang, J. Li, C. Wang and G. Li, Appl. Energy, 2020, 261, 114481 CrossRef CAS.
- Y. Qian, N. Han, Z. Zhang, R. Cao, L. Tan, W. Li and X. Zhang, ACS Appl. Mater. Interfaces, 2019, 11, 45832–45843 CrossRef CAS PubMed.
- Y.-H. Ahn, S. J. A. DeWitt, S. McGuire and R. P. Lively, Ind. Eng. Chem. Res., 2021, 60, 3374–3384 CrossRef CAS.
- M. Aghbashlo, M. Tabatabaei, M. H. Nadian, S. Soltanian, H. Ghasemkhani, A. Shafizadeh and S. S. Lam, J. Cleaner Prod., 2020, 277, 124089 CrossRef CAS.
- Z. Khounani, H. Hosseinzadeh-Bandbafha, F. Nazemi, M. Shaeifi, K. Karimi, M. Tabatabaei, M. Aghbashlo and S. S. Lam, J. Environ. Manage., 2021, 279, 111822 CrossRef CAS PubMed.
- D. R. Morris and J. Szargut, Energy, 1986, 11, 733–755 CrossRef CAS.
- A. Roux, A. Colin, J.-F. Dhôte and B. Schmitt, Filière forêt-bois et atténuation du changement climatique: Entre séquestration du carbone en forêt et développement de la bioéconomie, Quae, Versailles, 2020 Search PubMed.
- V. Smil, Popul. Dev. Rev., 2011, 37, 613–636 CrossRef PubMed.
- FAOSTAT, http://www.fao.org/faostat/fr/#compare, (accessed June 17, 2021).
- A. Ortiz-Bobea, T. R. Ault, C. M. Carrillo, R. G. Chambers and D. B. Lobell, Nat. Clim. Change, 2021, 11, 306–312 CrossRef.
- C. Woodings, Regenerated Cellulose Fibres, Woodhead publishing limited, CRC, 2001 Search PubMed.
- J. Dyer and G. C. Daul, Ind. Eng. Chem. Prod. Res. Dev., 1981, 20, 222–230 CrossRef CAS.
- K. Niinimäki, G. Peters, H. Dahlbo, P. Perry, T. Rissanen and A. Gwilt, Nat. Rev. Earth Environ., 2020, 1, 189–200 CrossRef.
- OECD and Food and Agriculture Organization of the United Nations, in OECD-FAO Agricultural Outlook 2020–2029, OECD, 2020 Search PubMed.
- M. Mollaee, A. Mobli, N. K. Mutti, S. Manalil and B. S. Chauhan, in Cotton Production, John Wiley & Sons, Ltd, 2019, pp. 371–390 Search PubMed.
- Y. Liu, L. Tang, X. Qiu, B. Liu, X. Chang, L. Liu, X. Zhang, W. Cao and Y. Zhu, Agric. For. Meteorol., 2020, 284, 107900 CrossRef.
- D. C. Uprety and V. R. Reddy, Crop responses to global warming, Springer, 2016 Search PubMed.
- Q. Sun, C. Miao, M. Hanel, A. G. Borthwick, Q. Duan, D. Ji and H. Li, Environ. Int., 2019, 128, 125–136 CrossRef PubMed.
- C. E. Moore, K. Meacham-Hensold, P. Lemonnier, R. A. Slattery, C. Benjamin, C. J. Bernacchi, T. Lawson and A. P. Cavanagh, J. Exp. Bot., 2021, 72, 2822–2844 CrossRef CAS PubMed.
- M. Kovenock and A. L. S. Swann, Global Biogeochem. Cycles, 2018, 32, 1437–1448 CrossRef CAS.
- Swain, Franck, 2021.
- COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS Critical Raw Materials Resilience: Charting a Path towards greater Security and Sustainability, https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0474, (accessed June 7, 2021).
- B. Waring, M. Neumann, I. C. Prentice, M. Adams, P. Smith and M. Siegert, Front. For. Global Change, 2020, 3, 58 CrossRef.
- D. Lachenal, Presented in part at the Journées Scientifiques du GDR Sciences du bois, Grenoble, November, 2020.
- Z. Ren, T. E. Ward and J. M. Regan, Environ. Sci. Technol., 2007, 41, 4781–4786 CrossRef CAS PubMed.
- T. Minowa and T. Ogi, Catal. Today, 1998, 45, 411–416 CrossRef CAS.
- A. Jeihanipour, K. Karimi and M. J. Taherzadeh, Biotechnol. Bioeng., 2010, 105(3), 469–476 CrossRef CAS PubMed.
- P. J. Crutzen, A. R. Mosier, K. A. Smith and W. Winiwarter, in Paul J. Crutzen: A pioneer on atmospheric chemistry and climate change in the anthropocene, Springer, 2016, pp. 227–238 Search PubMed.
- H. K. Jeswani, A. Chilvers and A. Azapagic, Proc. R. Soc. A, 2020, 476, 20200351 CrossRef PubMed.
- C. Béguin, Une chronique de l'hydrogène: histoire des méthodes de production et des applications, Presses Polytechniques et Universitaires Romandes, 2016 Search PubMed.
- N. Armaroli and V. Balzani, ChemSusChem, 2011, 4, 21–36 CrossRef CAS PubMed.
- M. Watanabe, H. Inomata and K. Arai, Biomass Bioenergy, 2002, 22, 405–410 CrossRef CAS.
- D. W. Wakerley, M. F. Kuehnel, K. L. Orchard, K. H. Ly, T. E. Rosser and E. Reisner, Nat. Energy, 2017, 2, 1–9 Search PubMed.
- J. Armijo, L′hydrogène, https://lvsl.fr/lhydrogene-quel-role-dans-la-transition-energetique/, (accessed March 23, 2021).
- N. A. Burton, R. V. Padilla, A. Rose and H. Habibullah, Renewable Sustainable Energy Rev., 2021, 135, 110255 CrossRef CAS.
- T. Myllyntaus, Icon, 2010, 16, 101–122 Search PubMed.
- S. M. Natali, J. P. Holdren, B. M. Rogers, R. Treharne, P. B. Duffy, R. Pomerance and E. MacDonald, Proc. Natl. Acad. Sci. U. S. A., 2021, 118(21) DOI:10.1073/pnas.2100163118.
- M. R. Turetsky, B. W. Abbott, M. C. Jones, K. W. Anthony, D. Olefeldt, E. A. G. Schuur, G. Grosse, P. Kuhry, G. Hugelius, C. Koven, D. M. Lawrence, C. Gibson, A. B. K. Sannel and A. D. McGuire, Nat. Geosci., 2020, 13, 138–143 CrossRef CAS.
- K. A. Crichton, N. Bouttes, D. M. Roche, J. Chappellaz and G. Krinner, Nat. Geosci., 2016, 9, 683–686 CrossRef CAS.
- G. Sandin and G. M. Peters, J. Cleaner Prod., 2018, 184, 353–365 CrossRef CAS.
- B. Lippke, E. Oneil, R. Harrison, K. Skog, L. Gustavsson and R. Sathre, Carbon Manage., 2011, 2, 303–333 CrossRef.
- P. M. Fernandes, N. Guiomar and C. G. Rossa, Sci. Total Environ., 2019, 666, 79–88 CrossRef CAS PubMed.
- H. S. J. Zald and C. J. Dunn, Ecol. Appl., 2018, 28, 1068–1080 CrossRef PubMed.
- M. P. North, J. T. Stevens, D. F. Greene, M. Coppoletta, E. E. Knapp, A. M. Latimer, C. M. Restaino, R. E. Tompkins, K. R. Welch, R. A. York, D. J. N. Young, J. N. Axelson, T. N. Buckley, B. L. Estes, R. N. Hager, J. W. Long, M. D. Meyer, S. M. Ostoja, H. D. Safford, K. L. Shive, C. L. Tubbesing, H. Vice, D. Walsh, C. M. Werner and P. Wyrsch, For. Ecol. Manage., 2019, 432, 209–224 CrossRef.
- S. L. Stephens, A. L. Westerling, M. D. Hurteau, M. Z. Peery, C. A. Schultz and S. Thompson, Front. Ecol. Environ.t, 2020, 18, 354–360 CrossRef.
- P. Börjesson and L. Gustavsson, Energy Policy, 2000, 28, 575–588 CrossRef.
- M. E. Puettmann and J. B. Wilson, Wood Fiber Sci., 2005, 37, 18–29 CAS.
- J. A. Blanco, Sci. Total Environ., 2012, 437, 91–103 CrossRef CAS PubMed.
- T. Behrens, E. Lynge, I. Cree, J.-M. Lutz, M. Eriksson, P. Guénel, F. Merletti, M. Morales-Suarez-Varela, N. Afonso, A. Stengrevics, J. Févotte, S. Sabroe, A. Llopis-González, G. Gorini, L. Hardell, A. Stang and W. Ahrens, Cancer, Causes Control, 2012, 23, 141–151 CrossRef PubMed.
- M. Gorman Ng, E. Stjernberg, M. Koehoorn, P. A. Demers and H. W. Davies, Ann. Occup. Hyg., 2011, 55(7), 752–763 CAS.
- N. J. Payne, Crop Prot., 1998, 17, 171–180 CrossRef.
- G. d'Allens, Main basse sur nos forêts, C.E.R.A.S, La Plaine Saint-Denis, Seuil, 2019 Search PubMed.
- W. de Vries, S. Solberg, M. Dobbertin, H. Sterba, D. Laubhann, M. Van Oijen, C. Evans, P. Gundersen, J. Kros and G. W. W. Wamelink, For. Ecol. Manage., 2009, 258, 1814–1823 CrossRef.
- G. Pitron, La guerre des métaux rares: la face cachée de la transition énergétique et numérique, Éditions Les Liens qui libèrent, 2018.
- J. Wang, M. Guo, M. Liu and X. Wei, Resour. Policy, 2020, 65, 101569 CrossRef.
- D. F. Ciambrone, Environmental life cycle analysis, CRC Press, 1997 Search PubMed.
- Z. Zhu, G. Xiao, J. Chen and S. Fu, Cellulose, 2020, 27, 8513–8526 CrossRef CAS.
- M. Henriksson, L. A. Berglund, P. Isaksson, T. Lindström and T. Nishino, Biomacromolecules, 2008, 9, 1579–1585 CrossRef CAS PubMed.
- Q. Fu, K. Tu, C. Goldhahn, T. Keplinger, M. Adobes-Vidal, M. Sorieul and I. Burgert, ACS Nano, 2020, 14, 13775–13783 CrossRef CAS PubMed.
- T. Akyazi, L. Basabe-Desmonts and F. Benito-Lopez, Anal. Chim. Acta, 2018, 1001, 1–17 CrossRef CAS PubMed.
- M. Rinne, H. Elomaa, A. Porvali and M. Lundström, Resour., Conserv. Recycl., 2021, 170, 105586 CrossRef.
- C. Mair-Bauernfeind, M. Zimek, R. Asada, D. Bauernfeind, R. J. Baumgartner and T. Stern, Int. J. Life Cycle Assess., 2020, 25, 2027–2049 CrossRef.
- L. C. Dreyer, M. Z. Hauschild and J. Schierbeck, Int. J. Life Cycle Assess., 2010, 15, 247–259 CrossRef CAS.
- C. Du, C. Ugaya, F. Freire, L. C. Dias and R. Clift, Int. J. Life Cycle Assess., 2019, 24, 781–793 CrossRef.
- Comment réalise-t-on une ACV ?, https://www.ademe.fr/expertises/consommer-autrement/passer-a-laction/dossier/lanalyse-cycle-vie/comment-realise-t-acv, (accessed March 22, 2021).
- Faire une revue critique, https://www.ademe.fr/expertises/consommer-autrement/passer-a-laction/dossier/lanalyse-cycle-vie/faire-revue-critique-pourquoi-comment, (accessed March 22, 2021).
- M. Patel, in Biodegradable Polymers and Plastics, ed. E. Chiellini and R. Solaro, Springer US, Boston, MA, 2003, pp. 83–102 Search PubMed.
- M. R. Yates and C. Y. Barlow, Resour., Conserv. Recycl., 2013, 78, 54–66 CrossRef.
- T. A. Hottle, M. M. Bilec and A. E. Landis, Resour., Conserv. Recycl., 2017, 122, 295–306 CrossRef.
- A. D. La Rosa, in Biopolymers and Biotech Admixtures for Eco-Efficient Construction Materials, ed. F. Pacheco-Torgal, V. Ivanov, N. Karak and H. Jonkers, Woodhead Publishing, 2016, pp. 57–78 Search PubMed.
- T. A. Hottle, M. M. Bilec and A. E. Landis, Polym. Degrad. Stab., 2013, 98, 1898–1907 CrossRef CAS.
- E. Kabir, R. Kaur, J. Lee, K.-H. Kim and E. E. Kwon, J. Cleaner Prod., 2020, 258, 120536 CrossRef CAS.
- M. Guo, Life Cycle Assessment (LCA) of Light-Weight Eco-composites, PhD thesis, Imperial College, London, 2013 Search PubMed.
- W. J. Groot and T. Borén, Int. J. Life Cycle Assess., 2010, 15, 970–984 CrossRef CAS.
- D. Ita-Nagy, I. Vázquez-Rowe, R. Kahhat, G. Chinga-Carrasco and I. Quispe, Int. J. Life Cycle Assess., 2020, 25, 2169–2189 CrossRef CAS.
- L. Chen, R. E. O. Pelton and T. M. Smith, J. Cleaner Prod., 2016, 137, 667–676 CrossRef CAS.
- S. Belboom and A. Léonard, Biomass Bioenergy, 2016, 85, 159–167 CrossRef CAS.
- L. Schebek, in Renewable Raw Materials, John Wiley & Sons, Ltd, 2011, ch. 9, pp. 187–216 Search PubMed.
- M. G. Mazzotta, C. M. Reddy and C. P. Ward, Environ. Sci. Technol. Lett., 2021, 9(1), 37–41 CrossRef.
- R. C. Hale, M. E. Seeley, M. J. La Guardia, L. Mai and E. Y. Zeng, J. Geophys. Res.: Oceans, 2020, 125(1) DOI:10.1029/2018JC014719.
- S. Spierling, E. Knüpffer, H. Behnsen, M. Mudersbach, H. Krieg, S. Springer, S. Albrecht, C. Herrmann and H.-J. Endres, J. Cleaner Prod., 2018, 185, 476–491 CrossRef.
- Life Cycle Assessment of biobased polyamides VESTAMID® Terra, https://www.vestamid.com/product/peek-industrial/downloads/vestamid-terra-life-cycle-analysis-en.pdf, (accessed November 10, 2021).
- D. Araújo, M. C. R. Castro, A. Figueiredo, M. Vilarinho and A. Machado, J. Cleaner Prod., 2020, 260, 120865 CrossRef.
- B. Duchemin, Ph.D. Thesis, University of Canterbury, 2008.
- P. Marichalar, J. Public Health Policy, 2017, 38, 509–514 CrossRef.
- P. Blanc, in Occupational health and public health (Lessons from the past - Challenges for the Future), National Institute for Working Life, Stockholm, Sweden, Arbetlivinstitutet, 2006, p. 87 Search PubMed.
- E. Perrin and G. Bovon, Fast fashion - Les dessous de la mode à bas prix, ARTE, 2020 Search PubMed.
- H. Frumkin, Environ. Health Perspect., 1998, 106, 611–613 CrossRef CAS PubMed.
- C. Pinney, Contrib. Indian Sociol., 1999, 33, 77–106 CrossRef.
- Cutting Out Textile Pollution, https://cen.acs.org/articles/93/i41/Cutting-Textile-Pollution.html, (accessed May 10, 2021).
- L. Shen, E. Worrell and M. K. Patel, Resour., Conserv. Recycl., 2010, 55, 260–274 CrossRef.
- H.-P. Fink, P. Weigel, H. J. Purz and J. Ganster, Prog. Polym. Sci., 2001, 26, 1473–1524 CrossRef CAS.
- T. Rosenau and A. D. French, Cellulose, 2021, 28, 5985–5990 CrossRef CAS.
- G. Sandin, S. Roos and M. Johansson, Environmental impact of textile fibers–what we know and what we don't know: Fiber Bible part 2, 2019 Search PubMed.
- L. Shen, E. Worrell and M. K. Patel, Biofuels, Bioprod. Biorefin., 2012, 6, 625–639 CrossRef CAS.
- J. Schuermann, T. Huber, D. LeCorre, G. Mortha, M. Sellier, B. Duchemin and M. P. Staiger, Cellulose, 2016, 23, 1043–1050 CrossRef CAS.
- B. J. C. Duchemin, A. P. Mathew and K. Oksman, Composites, Part A, 2009, 40, 2031–2037 CrossRef.
- A. Pinkert, K. N. Marsh, S. Pang and M. P. Staiger, Chem. rev., 2009, 109(12), 6712–6728 CrossRef CAS PubMed.
- S. Righi, A. Morfino, P. Galletti, C. Samorì, A. Tugnoli and C. Stramigioli, Green Chem., 2011, 13, 367 RSC.
- M. M. Bailey, M. B. Townsend, P. L. Jernigan, J. Sturdivant, W. L. Hough-Troutman, J. F. Rasco, R. P. Swatloski, R. D. Rogers and R. D. Hood, Green Chem., 2008, 10, 1213–1217 RSC.
- B. Peric, E. Martí, J. Sierra, R. Cruañas, M. Iglesias and M. A. Garau, Environ. Toxicol. Chem., 2011, 30, 2802–2809 CrossRef CAS PubMed.
- T. P. T. Pham, C.-W. Cho and Y.-S. Yun, Water Res., 2010, 44, 352–372 CrossRef CAS PubMed.
- J. S. Torrecilla, J. García, E. Rojo and F. Rodríguez, J. Hazard. Mater., 2009, 164, 182–194 CrossRef CAS PubMed.
- D. A. Alonso, A. Baeza, R. Chinchilla, G. Guillena, I. M. Pastor and D. J. Ramón, Eur. J. Org. Chem., 2016, 612–632 CrossRef CAS.
- Y.-L. Chen, X. Zhang, T.-T. You and F. Xu, Cellulose, 2019, 26, 205–213 CrossRef CAS.
- A. Fotostock, Nat. Clim. Change, 2018, 8, 1 CrossRef.
- X. Sun, X. Wang, F. Su, M. Tian, L. Qu, P. Perry, H. Ovens and X. Liu, Adv. Mater., 2021, 33, 2105174 CrossRef CAS PubMed.
- H. Dahlbo, K. Aalto, H. Eskelinen and H. Salmenperä, Sustainable Prod.Consumption, 2017, 9, 44–57 CrossRef.
- R. D. Silva, X. Wang and N. Byrne, RSC Adv., 2014, 4, 29094–29098 RSC.
- L. Navone, K. Moffitt, K.-A. Hansen, J. Blinco, A. Payne and R. Speight, Waste Manage., 2020, 102, 149–160 CrossRef CAS PubMed.
- S. Haslinger, M. Hummel, A. Anghelescu-Hakala, M. Määttänen and H. Sixta, Waste Manage., 2019, 97, 88–96 CrossRef CAS PubMed.
- S. Das, Int. J. Life Cycle Assess., 2011, 16, 268–282 CrossRef CAS.
- F. Hesser, Composites, Part B, 2015, 79, 197–203 CrossRef CAS.
- X. Xu, K. Jayaraman, C. Morin and N. Pecqueux, J. Mater. Process. Technol., 2008, 198, 168–177 CrossRef CAS.
- M. Pervaiz and M. M. Sain, Resour., Conserv. Recycl., 2003, 39, 325–340 CrossRef.
- C. Alves, P. M. C. Ferrão, A. J. Silva, L. G. Reis, M. Freitas, L. B. Rodrigues and D. E. Alves, J. Cleaner Prod., 2010, 18, 313–327 CrossRef CAS.
- S. V. Joshi, L. T. Drzal, A. K. Mohanty and S. Arora, Composites, Part A, 2004, 35, 371–376 CrossRef.
- S. Rajendran, L. Scelsi, A. Hodzic, C. Soutis and M. A. Al-Maadeed, Resour., Conserv. Recycl., 2012, 60, 131–139 CrossRef.
- Y. S. Song, J. R. Youn and T. G. Gutowski, Composites, Part A, 2009, 40, 1257–1265 CrossRef.
- F. Foroughi, E. Rezvani Ghomi, F. Morshedi Dehaghi, R. Borayek and S. Ramakrishna, Materials, 2021, 14, 714 CrossRef CAS PubMed.
- N. Lavoine, I. Desloges, A. Dufresne and J. Bras, Carbohydr. Polym., 2012, 90, 735–764 CrossRef CAS PubMed.
- M. C. B. de Figueirêdo, M. de Freitas Rosa, C. M. L. Ugaya, M. D. S. M. de Souza, A. C. C. da Silva Braid and L. F. L. de Melo, J. Cleaner Prod., 2012, 35, 130–139 CrossRef.
- M. Hervy, S. Evangelisti, P. Lettieri and K.-Y. Lee, Compos. Sci. Technol., 2015, 118, 154–162 CrossRef CAS.
- F. Pittau, F. Krause, G. Lumia and G. Habert, Build. Environ., 2018, 129, 117–129 CrossRef.
- A. Levasseur, P. Lesage, M. Margni and R. Samson, J. Ind. Ecol., 2013, 17, 117–128 CrossRef CAS.
- F. Pittau, G. Lumia, N. Heeren, G. Iannaccone and G. Habert, J. Cleaner Prod., 2019, 214, 365–376 CrossRef.
- V. Zieger, T. Lecompte and A. Hellouin de Menibus, Build. Environ., 2020, 185, 107210 CrossRef.
- F. Røyne, D. Peñaloza, G. Sandin, J. Berlin and M. Svanström, J. Cleaner Prod., 2016, 116, 90–99 CrossRef.
- M. S. Masnadi, H. M. El-Houjeiri, D. Schunack, Y. Li, J. G. Englander, A. Badahdah, J.-C. Monfort, J. E. Anderson, T. J. Wallington and J. A. Bergerson, Science, 2018, 361, 851–853 CrossRef CAS PubMed.
- M. Ottelé, K. Perini, A. L. A. Fraaij, E. M. Haas and R. Raiteri, Energy Build., 2011, 43, 3419–3429 CrossRef.
- E. S. Ferreira, C. A. Rezende and E. D. Cranston, Green Chem., 2021, 23, 3542–3568 RSC.
- H. Lewis, K. Verghese and L. Fitzpatrick, Packag. Technol. Sci., 2010, 23, 145–160 CrossRef.
- J. Laurijssen, M. Marsidi, A. Westenbroek, E. Worrell and A. Faaij, Resour., Conserv. Recycl., 2010, 54, 1208–1218 CrossRef.
- T. Ekvall, J. Cleaner Prod., 1999, 7, 281–294 CrossRef.
- N. Papageorgiou, Bacterial nanopores open the future of data storage, https://actu.epfl.ch/news/bacterial-nanopores-open-the-future-of-data-stor-6/, (accessed June 2, 2021).
- H. Ferreboeuf, Lean ICT, https://theshiftproject.org/, (accessed June 2, 2021).
- A. S. G. Andrae and T. Edler, Challenges, 2015, 6, 117–157 CrossRef.
- C. Freitag, M. Berners-Lee, K. Widdicks, B. Knowles, G. S. Blair and A. Friday, Patterns, 2021, 2, 100340 CrossRef PubMed.
- J. G. Bull and R. A. Kozak, Environ. Impact Assess. Rev., 2014, 45, 10–18 CrossRef.
- J. Kim, Y. Kim, S. Oh, T. Kim and D. Lee, Int. J. of Sustainable Building Tech. and Urban Dev., n.d., 2021, 44–60 Search PubMed.
- K. Tahara, H. Shimizu, K. Nakazawa, H. Nakamura and K. Yamagishi, J. Cleaner Prod., 2018, 189, 59–66 CrossRef.
- Å. Moberg, C. Borggren and G. Finnveden, Int. J. Life Cycle Assess., 2011, 16, 238–246 CrossRef.
- J. Suckling and J. Lee, Int. J. Life Cycle Assess., 2015, 20, 1181–1196 CrossRef CAS.
- A.-A. Durand, http://www.metrofrance.com, 2011, 6.
- T. Mäkelä, M. Kainlauri, P. Willberg-Keyriläinen, T. Tammelin and U. Forsström, Microelectron. Eng., 2016, 163, 1–6 CrossRef.
- Y. Zhou, Y. Li, F. Dundar, K. R. Carter and J. J. Watkins, Cellulose, 2018, 25, 5185–5194 CrossRef CAS.
- Y. U. Lee, J. Zhao, Q. Ma, L. K. Khorashad, C. Posner, G. Li, G. B. M. Wisna, Z. Burns, J. Zhang and Z. Liu, Nat. Commun., 2021, 12, 1559 CrossRef CAS PubMed.
- J. D. Griffith, S. Willcox, D. W. Powers, R. Nelson and B. K. Baxter, Astrobiology, 2008, 8, 215–228 CrossRef CAS PubMed.
- P. H. Gleick, Weather, Clim., Soc., 2014, 6, 331–340 CrossRef.
- C. S. Hendrix and I. Salehyan, J. Peace Res., 2012, 49(1), 35–50 CrossRef.
- S. Alexander and P. Yacoumis, J. Cleaner Prod., 2018, 197, 1840–1848 CrossRef.
- T. Wendler, Environ. Resour. Econ., 2019, 74, 1383–1423 CrossRef.
- M. Z. Jacobson, Energy Environ. Sci., 2019, 12, 3567–3574 RSC.
- A. Gambhir and M. Tavoni, One Earth, 2019, 1, 405–409 CrossRef.
- X. Chen, X. Zhu, S. He, L. Hu and Z. J. Ren, Adv. Mater., 2021, 33, 2001240 CrossRef CAS PubMed.
- A. M. Armani, D. E. Hurt, D. Hwang, M. C. McCarthy and A. Scholtz, Nat. Rev. Mater., 2020, 5, 403–406 CrossRef CAS PubMed.
- J. Morgan, Ecol. Econ., 2017, 136, 169–177 CrossRef.
- A. Holstov, B. Bridgens and G. Farmer, Constr. Build. Mater., 2015, 98, 570–582 CrossRef.
- A. Le Duigou, G. Chabaud, F. Scarpa and M. Castro, Adv. Funct. Mater., 2019, 29, 1903280 CrossRef.
- A. Le Duigou, M. Castro, R. Bevan and N. Martin, Mater. Des., 2016, 96, 106–114 CrossRef.
- M. Eder, W. Schäffner, I. Burgert and P. Fratzl, Adv. Mater., 2021, 33, 2001412 CrossRef CAS PubMed.
- R. D. A. e Silva, A. I. Santa Brígida, M. de Freitas Rosa, R. M. da Silva Neto, W. A. Spinosa, E. B. de Sá Filho and M. C. B. de Figueirêdo, J. Cleaner Prod., 2020, 269, 122245 CrossRef.
- J. Garemark, X. Yang, X. Sheng, O. Cheung, L. Sun, L. A. Berglund and Y. Li, ACS Nano, 2020, 14, 7111–7120 CrossRef CAS PubMed.
- M. Frey, G. Biffi, M. Adobes-Vidal, M. Zirkelbach, Y. Wang, K. Tu, A. M. Hirt, K. Masania, I. Burgert and T. Keplinger, Adv. Sci., 2019, 6, 1802190 CrossRef.
- E. I. Akpan, B. Wetzel and K. Friedrich, Green Chem., 2021, 23, 2198–2232 RSC.
- M. Beaumont, S. Winklehner, S. Veigel, N. Mundigler, W. Gindl-Altmutter, A. Potthast and T. Rosenau, Green Chem., 2020, 22, 5605–5609 RSC.
- J. Wang, L. Wang, D. J. Gardner, S. M. Shaler and Z. Cai, Cellulose, 2021, 28, 4511–4543 CrossRef.
- J. Schurz, Prog. Polym. Sci., 1999, 24, 481–483 CrossRef CAS.
- P. Söderbaum, Ecol. Econ., 2015, 119, 420–423 CrossRef.
- L. Persson, B. M. Carney Almroth, C. D. Collins, S. Cornell, C. A. de Wit, M. L. Diamond, P. Fantke, M. Hassellöv, M. MacLeod, M. W. Ryberg, P. Søgaard Jørgensen, P. Villarrubia-Gómez, Z. Wang and M. Zwicky Hauschild, Outside the Safe Operating Space of the Planetary Boundary for Novel Entities, Env. Sci. Tech., 2022 DOI:10.1021/acs.est.1c04158.
- T. D. Nielsen, J. Hasselbalch, K. Holmberg and J. Stripple, Politics and the plastic crisis: A review throughout the plastic life cycle, WIREs Energy Environ., 2019, 9(1) DOI:10.1002/wene.360.
- V. Tulus, J. Pérez-Ramírez and G. Guillén-Gosálbez, Planetary metrics for the absolute environmental sustainability assessment of chemicals, Green Chem., 2021, 23(24), 9881–9893 RSC.
- M. Wohlert, T. Benselfelt, L. Wågberg, I. Furó, L. A. Berglund and J. Wohlert, Cellulose and the role of hydrogen bonds: not in charge of everything, Cellulose, 2021, 29(1), 1–23 CrossRef.
- M. Delamarche, De surprenantes matières premières, L’usine nouvelle, 2017, 3524–3525 Search PubMed.
Footnotes |
† A quick definition is given by Roger Penrose: “For a classical system of n featureless particles, (a phase space is) a space 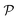 of 6n dimensions, each of those points represents the entire family of positions and momenta of all n particles”.15 The three positions can be the coordinates of 6n dimensions, each of those points represents the entire family of positions and momenta of all n particles”.15 The three positions can be the coordinates  in a Cartesian coordinate system and the momenta are in a Cartesian coordinate system and the momenta are  , where m is the particle's mass. , where m is the particle's mass. |
| ‡ Autotrophic organisms only require carbon dioxide or carbonates as a source of carbon and simple inorganic compounds (such as nitrogen) for metabolic synthesis of organic molecules (such as glucose). Other than plants, there are some other autotrophic organisms, such as some populations of archaea, that live on chemical energy sources. |
| § For the sake of simplicity, this functional definition omits potential exergy sources, such as kinetic exergy, electro-magnetic or gravitational potential exergies. This definition is largely operational since it can safely be assumed that all the transformations of relevance for us occur on Earth at constant altitude, at zero velocity relative to the planet and in the absence of a strong electro-magnetic field. |
| ¶ Protists are predominantly one-celled eukaryotic (cells with a nucleus) organisms that are not animals, plants or fungi. The protist domain includes protozoa, some algae, and slime mold. |
| || The rebound effect, also known as the Jevons paradox, states that sometimes consumption increases due to a weakening of the factors limiting the use of a given technology. As a result, the energy or materials economies that result from improved technology are partially or fully compensated for by a behavioural change in society with respect to this technology. For instance, when cell phones become easier to produce thanks to progress in the semi-conductor industry, their price drops and more people can afford them. |
| This journal is © The Royal Society of Chemistry 2022 |