 Open Access Article
Open Access ArticleHealth associations of liver enzymes and inflammatory scores with urinary citrus flavonoid metabolites†
Vanessa
Bullón-Vela
 *abc,
Yifan
Xu
c,
Cristina
Razquin
abd,
Itziar
Abete
*abc,
Yifan
Xu
c,
Cristina
Razquin
abd,
Itziar
Abete
 bdef,
Maria Angeles
Zulet
bdef,
Maria Angeles
Zulet
 bdef,
Miguel A.
Martínez-González
abdg,
Pilar
Buil-Corsiales
ab,
Facundo
Vitelli-Storelli
h,
Vicente
Martín Sánchez
bdef,
Miguel A.
Martínez-González
abdg,
Pilar
Buil-Corsiales
ab,
Facundo
Vitelli-Storelli
h,
Vicente
Martín Sánchez
 hi,
Zenaida
Vazquez-Ruíz
hi,
Zenaida
Vazquez-Ruíz
 ad,
Carmen
Sayón-Orea
abd,
Maite
Domínguez-Fernández
ad,
Carmen
Sayón-Orea
abd,
Maite
Domínguez-Fernández
 ef,
Concepción
Cid
bef,
Ramon
Estruch
dj,
Rosa María
Lamuela-Raventós
ef,
Concepción
Cid
bef,
Ramon
Estruch
dj,
Rosa María
Lamuela-Raventós
 dk,
Montserrat
Fitó
dl,
Gemma
Blanchart
l,
Nancy
Babio
dk,
Montserrat
Fitó
dl,
Gemma
Blanchart
l,
Nancy
Babio
 dmn,
Jordi
Salas-Salvadó
dmn,
Francisco J.
Tinahones
do,
Josep A.
Tur
dmn,
Jordi
Salas-Salvadó
dmn,
Francisco J.
Tinahones
do,
Josep A.
Tur
 dp,
Dora
Romaguera
dq,
Jadwiga
Konieczna
dq,
Xavier
Pintó
dr,
Lidia
Daimiel
s,
Ana
Rodriguez-Mateos‡
dp,
Dora
Romaguera
dq,
Jadwiga
Konieczna
dq,
Xavier
Pintó
dr,
Lidia
Daimiel
s,
Ana
Rodriguez-Mateos‡
 *c and
José Alfredo
Martínez‡
*c and
José Alfredo
Martínez‡
 bdeft
bdeft
aDepartment of Preventive Medicine and Public Health, University of Navarra, Pamplona, Spain. E-mail: mbullon@alumni.unav.es
bNavarra Institute for Health Research (IdiSNA), Pamplona, Spain
cDepartment of Nutritional Sciences, School of Life Course and Population Sciences, Faculty of Life Sciences and Medicine, King's College London, London, United Kingdom. E-mail: ana.rodriguez-mateos@kcl.ac.uk
dConsorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERObn), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
eDepartment of Nutrition, Food Science and Physiology, University of Navarra, Pamplona, Spain
fCenter for Nutrition Research, University of Navarra, Pamplona, Spain
gDepartment of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, USA
hInstitute of Biomedicine (IBIOMED), University of León, León, Spain
iCentro de Investigación Biomédica en Red de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
jDepartment of Internal Medicine, IDIBAPS, Hospital Clinic, University of Barcelona, Barcelona, Spain
kDepartment of Nutrition, Food Sciences and Gastronomy, XaRTA, INSA-UB, School of Pharmacy and Food Sciences, Nutrition and Food Safety Research Institute, University of Barcelona, Barcelona, Spain
lCardiovascular Risk and Nutrition Research Group (CARIN), Hospital del Mar Research Institute (IMIM), Barcelona, Spain
mUniversitat Rovira i Virgili, Departament de Bioquímica i Biotecnologia, Unitat de Nutrició Humana, Reus, Tarragona, Spain
nInstitut d'Investigació Pere Virgili (IISPV), Hospital Universitari Sant Joan de Reus, Reus, Spain
oDepartment of Endocrinology, Instituto de Investigación Biomédica de Málaga-IBIMA, University of Málaga, Virgen de la Victoria Hospital, Málaga, Spain
pResearch Group on Community Nutrition & Oxidative Stress, University of Balearic Islands-IUNICS, Palma de Mallorca, Spain
qResearch Group on Nutritional Epidemiology & Cardiovascular Physiopathology (NUTRECOR), Health Research Institute of the Balearic Islands (IdISBa), University Hospital Son Espases (HUSE), Palma de Mallorca, Spain
rLipids and Vascular Risk Unit, Internal Medicine, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain
sNutritional Control of the Epigenome Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM+ CSIC, Madrid, Spain
tCardiometabolic Nutrition Group, Precision Nutrition and Cardiovascular Health Program, IMDEA Food, CEI UAM+ CSIC, Madrid, Spain
First published on 23rd December 2022
Abstract
Background: Dietary flavonoid intake is associated with a reduced risk of some cardiometabolic disorders, attributed in part to their claimed anti-inflammatory activity. Our aim was to investigate the potential association between specific urine flavonoid metabolites, liver enzymes, and inflammatory status in individuals with metabolic syndrome (MetS). Methods: In this cross-sectional study, clinical and dietary data from 267 participants, aged 55 to 75 years, participating in the PREDIMED Plus study (PREvención con DIeta MEDiterránea) were analyzed. At the baseline, spot urine samples were collected and seven urinary flavonoid metabolites were quantified using ultra-performance liquid chromatography coupled to triple quadrupole mass spectrometry (UPLC-Q-q-Q MS). Liver enzymes, inflammatory scores, and urinary flavonoid concentrations were inverse normally transformed. Results: Adjusted linear regression models showed an inverse association between urinary citrus flavanone concentrations and gamma-glutamyl transferase (GGT) (all p-values <0.05). Naringenin 7′-GlcUA was significantly associated with a lower aggregate index of systemic inflammation (AISI) (Bper 1SD = −0.14; 95% CI: −0.27 to −0.02; p-value = 0.025) and systemic inflammation index (SII) (Bper 1SD = −0.14; 95% CI: −0.27 to −0.02; p-value = 0.028). To investigate the relationship between flavanone subclasses and GGT levels, we fitted a score of citrus-flavanones, and subjects were stratified into quartiles. The highest values of the citrus-flavanone score (per 1-SD increase) were associated with lower GGT levels (Bper 1SD = −0.41; 95% CI: −0.74 to −0.07), exhibiting a linear trend across quartiles (p-trend = 0.015). Conclusion: This cross-sectional study showed that higher urinary excretion of citrus-flavanone metabolites was associated with lower GGT levels in subjects diagnosed with MetS and obesity.
Introduction
Metabolic syndrome (MetS) is a health condition, which includes a cluster of metabolic risk factors for developing cardiovascular disease (CVD), type 2 diabetes (T2D), and non-alcoholic fatty liver disease (NAFLD).1–3 NAFLD encompasses a wide range of diseases, ranging from simple steatosis to cirrhosis, leading to hepatic carcinoma in ending stages, in the absence of excessive alcohol intake.1 Growing pieces of evidence suggest that NAFLD is the hepatic representation of MetS and it would be one of the main contributors to its pathogenesis.3,4 MetS is closely related to a low-grade inflammatory status leading to oxidative stress, which can promote the activation of multiple inflammatory pathways, increasing the risk of liver dysfunction and CVD.5,6 These hepatic and extrahepatic dysfunctions are marked by the increase in liver enzymes and hematologic disorders linked to MetS.7–12In recent years, there has been considerable interest in healthy dietary patterns characterized by a higher intake of plant-based foods and their benefits on health outcomes.13 In this sense, some studies have suggested that the Mediterranean diet (MedDiet), rich in fruits, vegetables, olive oil, and whole-grain cereals, could prevent and manage most diet-related noncommunicable diseases.14,15 In human and in vitro studies, plant-derived (poly)phenolic compounds showed anti-inflammatory properties and the ability to inhibit oxidative stress.16–20 However, the results from epidemiological studies are inconsistent and scarce;16,21 probably due to the fact that evaluating accurately nutrient intake such as (poly)phenols from food frequency questionnaires (FFQ) is complex.22 In this context, the use of dietary biomarkers for assessing directly the dietary intake/status could avoid the bias of the dietary self-reported data.22,23 Experimental and human studies have evidenced that flavonoids might exert hepatoprotective properties,24 cardiovascular benefits,18,25,26 anti-metastatic activity, and improvement in the metabolic profile.19 Flavonoids are widely present in many plant-based foods, such as berry fruits, red wine, citrus fruits, grapes, apples, tea, soybeans, and others.27 These (poly)phenolic compounds consist of a diphenyl propane carbon skeleton, containing two benzene rings linked by a linear three-carbon chain (C6–C3–C6).28 They can be categorized according to the degree of oxidation and saturation in the heterocyclic C-ring into flavones, flavanones, flavonols, flavan-3-ols, anthocyanins, and isoflavones.28 Flavanones represent a major part of the total flavonoid intake in European countries, hesperetin and naringenin being the most abundant ones. They are mainly excreted in urine as phase II conjugates that are recognized as biomarkers of citrus-derived food consumption.23
Citrus fruits contain phenolic acids and more than 60 types of flavonoids, but they are also rich in vitamin C, folic acids, potassium, dietary fiber, and carotenoids.29 Several animal studies suggested that citrus-flavanones have anti-inflammatory systemic effects and they protect against liver damage and vascular endothelial dysfunction.19,30–32 Nevertheless, few human studies have evaluated the impact of flavonoids on health in individuals with MetS. We hypothesized that higher urinary excretion of these specific urinary flavonoid metabolites is associated with liver health and inflammatory status. Therefore, our primary objective was to investigate the interplay between selected urinary flavonoid metabolites, liver enzymes and inflammatory scores in subjects with MetS. The secondary aim was to evaluate the association between (poly)phenol-rich food intake and urinary flavonoid metabolite concentrations.
Materials and methods
Study population
For the present study, baseline data from the Navarra-Nutrition Centre were analysed in the framework of the PREDIMED-Plus study (https://www.isrctn.com/ISRCTN89898870). The study design of this clinical trial has been described elsewhere.33 In brief, PREDIMED-Plus is a parallel-group multi-centre, controlled, randomized trial conducted in Spain (https://www.predimedplus.com/).33 The PREDIMED-Plus study was designed to evaluate a weight-loss intervention based on an energy-reduced MedDiet, physical activity promotion, and behavioural support, in comparison to a non-restrictive Mediterranean diet pattern plus usual care intervention, for CVD events as a primary event.33 Eligible participants were men (55–75 years old) and women (60–75 years old) with a body mass index (BMI) ≥ 27 kg m−2 to <40 kg m−2 who were diagnosed with MetS at enrolment according to the guidelines from the International Diabetes Federation/National Heart, Lung and Blood Institute/American Heart Association. For the study, participants were excluded if they had several medical conditions (i.e. history of previous CVD, including angina, cancer or history of malignancy, cirrhosis or liver dysfunction; alcohol or drug abuse; individuals with acute infection or inflammation; treatment with immunosuppressive, cytotoxic agents, and systemic corticosteroids).33 The study protocol was approved by the Research Ethics Committee for clinical investigations at the University of Navarra (053/2013), according to the ethical standards of the Declaration of Helsinki. All participants provided written informed consent. Two hundred sixty six participants with available urine samples were included in this cross-sectional study.Dietary assessment
Dietary data during the last year were collected using a validated semiquantitative food frequency questionnaire (FFQ), including 143 items commonly consumed in Spain.34 Frequencies of consumption of the food items were registered at 9 levels: never or seldom, 1–3 times per month, once per week, 2–4 times per week, 5–6 times per week, once per day, 2–3 times per day, 4–6 times per day, and >6 times per day. Reported frequencies of food intake were converted into frequencies per day and multiplied by the weight of the typical portion size indicated to obtain the intake information in g d−1.34 To evaluate dietary consumption and urinary flavonoid metabolites, we focused on flavonoid-rich foods such as vegetables, fruits, whole grains, and coffee. This FFQ was performed by trained dietitians in a face-to-face interview.33 Adherence to the energy-reduced MedDiet was assessed using a 17-item questionnaire,35 a version of the 14-point Mediterranean Diet Adherence Screener (MEDAS) score validated in the PREDIMED study.36 The 17-item questionnaire has a more restrictive threshold for some caloric-dense foods and additional items aimed to reduce the caloric intake.35Assessment of liver function and inflammatory status
After an overnight fast, the first spot urine and blood samples were collected. Aliquots of serum, plasma, and urine were collected, coded and stored at −80 °C.33 Liver function was evaluated by hepatic enzymes, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and GGT. All enzymes were measured using standardized and validated analytical procedures in accredited laboratories for clinical analysis. Blood cell counts were used to calculate inflammatory indexes, such as the aggregate index of systemic inflammation (AISI: neutrophils × platelets × monocytes/lymphocytes) and the systemic inflammatory index (SII: neutrophils × platelets/lymphocytes).37Covariable assessment
At the baseline, sociodemographic, clinical (personal medical history and use of medications), physical activity, lifestyle (alcohol intake and smoking status), and anthropometric data were collected by personnel of the study according to the study protocol.33 On each visit, the waist circumference (cm), body weight (kg) and height (cm) were measured using standardized procedures and calibrated equipment. BMI was calculated as weight (kg) divided by the square of the height (m). Blood pressure was measured three times using a validated semi-automatic oscillometer (OmronHEM-705C, the Netherlands) after 5 minutes of rest while the participants were in a seated position. Physical activity (Metabolic Equivalent of Task (MET)-minute per week) was evaluated using the short Registre Gironi del Core validated questionnaire.38,39 T2D was ascertained by the previous diagnosis or if they met one of the following criteria: (1) glycated haemoglobin (HbA1c) ≥6.5%, (2) use of antidiabetic medication or (3) fasting glucose ≥126 mg dl−1 considering the American Diabetes Association guidelines.40 Biochemical analyses, including glucose, insulin, triglycerides, and total cholesterol levels, were conducted by standard enzymatic assays, allowing for laboratory protocols.33Urine flavonoid metabolite assessment
The target flavonoid metabolites in spot urine were first extracted by micro-Solid-Phase Extraction (μ-SPE) and then measured by ultra-performance liquid chromatography and triple-quadrupole mass spectrometry (UPLC-Q-q-Q MS) with a validated method with minor modifications.41 Firstly, the spot urine was diluted (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 1
v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10) with HPLC water (Sigma-Aldrich, Steinheim, Germany). The diluted urine was acidified with 4% phosphoric acid (v
10) with HPLC water (Sigma-Aldrich, Steinheim, Germany). The diluted urine was acidified with 4% phosphoric acid (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v 1
v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and 600 μl of the mixture was loaded to the μ-SPE plate (Oasis 96-well reversed-phase HLB sorbent plate, Waters, Eschborn, Germany). The isotope internal standards (±)-catechin-2,3,4-13C3 (0.54 mg ml−1, Sigma-Aldrich, Steinheim, Germany) and ferulic acid-1,2,3-13C3 (0.99 mg ml−1, Sigma-Aldrich, Steinheim, Germany) were added to the samples before SPE for the calculation of the recovery rate. The loaded samples were washed with HPLC water (200 μl), 0.2% acetic acid (200 μl) and then eluted with 60 μl methanol.
1) and 600 μl of the mixture was loaded to the μ-SPE plate (Oasis 96-well reversed-phase HLB sorbent plate, Waters, Eschborn, Germany). The isotope internal standards (±)-catechin-2,3,4-13C3 (0.54 mg ml−1, Sigma-Aldrich, Steinheim, Germany) and ferulic acid-1,2,3-13C3 (0.99 mg ml−1, Sigma-Aldrich, Steinheim, Germany) were added to the samples before SPE for the calculation of the recovery rate. The loaded samples were washed with HPLC water (200 μl), 0.2% acetic acid (200 μl) and then eluted with 60 μl methanol.
The flavonoid metabolites were identified and quantified with authentic standards. 3′-Methoxy-(−)-epicatechin (3′-ME-EC) was obtained from GERBU Biotechnik (Heidelberg, Germany). Naringenin-4′-glucuronide (naringenin 4′-GlcUA), naringenin-7′-glucuronide (naringenin 7′-GlcUA) and hesperetin-3′-glucuronide (hesperetin 3′-GlcUA) were obtained from Toronto Research Chemicals (Toronto, ON, Canada). Kaempferol-3-glucuronide (kaempferol-3-GlcUA) was obtained from Extrasynthese (Genay, France). Myricetin and isorhamnetin were obtained from Sigma-Aldrich (Steinheim, Germany). The analysis was performed on a Shimadzu LCMS 8060 (Shimadzu, Kyoto, Japan). Briefly, 5 μl of eluted samples was injected through a Raptor Biphenyl column 2.1 × 50 mm, 1.8 μm (Restek, Bellefonte, USA) coupled with a guard cartridge 5 × 2.1 mm, 2.7 μm (Restek, Bellefonte, USA). The UPLC gradient included a 14-minute run joined with a 2-minute equilibration and the mobile phases were water and acetonitrile (Sigma-Aldrich, Steinheim, Germany) both acidified with 0.1% formic acid. The run was performed at 0.5 ml min−1 flow rate at 30 °C. The details of the analysis parameters were previously described.41 The targeted compounds were identified and quantified with 1 to 3 transitions in multiple reaction monitoring (MRM) mode in negative ion mode. Data acquisition and analysis were performed on LabSolutions software (SHIMADZU, Kyoto, Japan). The concentration of flavonoid metabolites was adjusted for urine creatinine levels42 before data analysis.
Statistical analysis
Descriptive variables are expressed as mean (SD). Categorical variables are shown as number (n) and percentage (%). Baseline comparison between men and women was applied using the Pearson chi-square test (χ2) for categorical variables or Student's t-test. The normality of the continuous markers was evaluated by the Shapiro–Wilk test. Liver enzymes, inflammatory scores, and the concentrations of urinary flavonoid metabolites were normalized and scaled to multiples of 1 SD with the rank-based inverse normal transformation, according to Blom's method.43 Spearman's correlation analyses assessed the strength of relationships between urinary flavonoid metabolites, flavonoid-rich foods, liver enzymes, and inflammatory scores.Linear regression models were applied to explore the association between transformed urinary flavonoid metabolites, liver enzymes, and inflammation scores (as outcomes). Results are presented as unstandardized B-coefficients and a 95% confidence interval (CI). All linear regression models were adjusted for several potential confounders, including sex, age (continuous, years), smoking status (never, former, current), marital status (single, married, widow, divorced or separated), educational level (primary education, secondary education, or academic/graduate), physical activity (continuous, MET-min per week), sleeping hours (continuous, h per day), energy intake (continuous, kcal d−1), intake of vitamins (yes/no) and alcohol consumption (continuous, g d−1). To account for multiple testing, we corrected p-values of the linear regression adjusted associations between a 1-SD increase in urine (poly)phenol metabolite levels and markers, using the Simes false discovery rate (FDR) procedure. An FDR-p-value <0.05 was considered to be statistically significant.
To evaluate the potential association between GGT and flavanones, we calculated the flavanone score by combining all the urine citrus-flavanone metabolites (naringenin 4′-GlcUA, naringenin 7′-GlcUA and hesperetin 3′-GlcUA) weighted with their respective individual coefficients from the fitted linear regression model. The flavanone score was calculated by summing all the weighted citrus-flavanones. This score was stratified into quartiles in the linear regression model adjusted for the same confounders as aforementioned. To assess the linear p-trend across quartiles, we calculated the median metabolite concentration within each quartile, which was included in the linear regression model as a continuous variable. All statistical tests were 2-sided, and p-values of less than 0.05 were considered statistically significant. All statistical analyses were conducted with Stata version 16.0 (StataCorp LP, College Station, TX, USA).
Results
Study population characteristics and flavonoid-based urinary metabolites
The baseline characteristics of study participants are shown in Table 1. Two hundred sixty-six participants were evaluated (153 men, 64.5 ± 5.4 years and 114 women, 67.5 ± 3.9 years). Women were older (men = 64.6 ± 5.4 years; women = 67.5 ± 3.9 years, p < 0.001) and had higher BMI (men = 31.8 ± 3.1 kg m−2; women = 32.8 ± 3.7 kg m−2, p = 0.021) than men. On the other hand, men were more active and more probably smokers, showing higher energy (p = 0.004), coffee (p = 0.040), red wine (p < 0.001) and alcohol intake (p < 0.001) compared to women. In contrast, women showed higher intake of vegetables (men = 318.2 ± 123.0 g d−1; women = 354.1 ± 124.0 g d−1, p = 0.020) than men. No differences between men and women were found in the intake of total fruit, whole wheat bread, citrus fruit, and total nuts. The mean intake of other dietary components is presented in the electronic supplemetary information (ESI) (Table 1S†). Adherence to MedDiet did not show differences according to sex. Regarding clinical parameters, men had higher levels of ALT (p = 0.004) and AST (p = 0.043) than women (Table 1). On the other hand, women had higher insulin (p = 0.025) and total cholesterol levels (p < 0.001) compared with men.| All (n = 267) | Men (n = 153) | Women (n = 114) | p-Valuea | |
|---|---|---|---|---|
| Data were calculated by chi-square or Student's t-test as appropriate. Results are expressed as n (%) or mean (standard deviation).a p for differences between sexes.b Mediterranean dietary score from 0 to 17 points. Abbreviations: BMI, body mass index; HOMA-IR, homeostatic model assessment for insulin resistance; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; AISI, aggregate index of systemic inflammation; SII, systemic inflammation index; MET, metabolic equivalent; MedDiet, Mediterranean diet. AISI index = ((neutrophils × monocytes × platelets)/lymphocytes); SII index = ((neutrophils × platelets)/lymphocytes). | ||||
| Age (years) | 65.8 (5.1) | 64.6 (5.4) | 67.5 (3.9) | <0.001 |
| BMI (kg m−2) | 32.2 (3.4) | 31.8 (3.1) | 32.8 (3.7) | 0.021 |
| Hypertension (%) | 252 (94.4) | 144 (94.1) | 108 (94.7) | 0.828 |
| Type 2 diabetes (%) | 100 (37.5) | 61 (39.9) | 39 (34.2) | 0.345 |
| Glucose (mg dL−1) | 119.3 (32.7) | 120.9 (35.0) | 117.3 (29.4) | 0.385 |
| Insulin (mIU L−1) | 14.0 (9.0) | 13.0 (7.1) | 15.5 (10.9) | 0.025 |
| HOMA-IR | 4.2 (3.2) | 3.9 (2.4) | 4.6 (4.2) | 0.065 |
| Total cholesterol (mg dL−1) | 200.3 (36.5) | 192.2 (34.2) | 211.3 (36.7) | <0.001 |
| Triglycerides (mg dL−1) | 148.0 (61.9) | 151.1 (69.0) | 144.0 (50.9) | 0.355 |
| ALT (U L−1) | 28.0 (20.6) | 31.2 (24.5) | 23.8 (12.3) | 0.004 |
| AST (U L−1) | 23.7 (13.4) | 25.2 (16.3) | 21.8 (7.3) | 0.043 |
| GGT (U L−1) | 42.0 (41.0) | 44.9 (41.5) | 38.3 (40.2) | 0.193 |
| AISI index | 270.8 (385.5) | 249.8 (270.3) | 299.1 (500.6) | 0.306 |
| SII index | 381.0 (182.3) | 374.8 (180.7) | 389.3 (184.9) | 0.523 |
| Alcohol intake (g d−1) | 12.8 (17.6) | 19.7 (19.8) | 3.6 (7.2) | <0.001 |
| Leisure time physical activity (MET min per week) | 3104 (2753) | 3690 (3101) | 2316 (1953) | <0.001 |
| Total energy intake (g d−1) | 2609 (538) | 2689 (535) | 2500 (526) | 0.004 |
| Adherence to MedDietb | 8.9 (2.5) | 8.8 (2.4) | 9.0 (2.5) | 0.448 |
| Total fruits (g d−1) | 425.5 (221.6) | 407.3 (221.6) | 450.0 (220.4) | 0.120 |
| Total vegetables (g d−1) | 333.6 (124.5) | 318.2 (123.0) | 354.1 (124.0) | 0.020 |
| Citrus fruit (g d−1) | 151.4 (125.5) | 144.1 (127.2) | 161.2 (123.0) | 0.269 |
| Whole wheat bread (g d−1) | 55.2 (92.2) | 50.6 (97.3) | 61.4 (85.0) | 0.345 |
| Total nuts (g d−1) | 15.0 (18.1) | 15.6 (18.1) | 14.3 (18.2) | 0.549 |
| Coffee intake (g d−1) | 37.2 (51.4) | 42.8 (52.0) | 29.7 (49.7) | 0.040 |
| Total red wine (g d−1) | 61.2 (105.7) | 91.3 (120.8) | 20.7 (61.8) | <0.001 |
| Medications, n (%) | ||||
| Cholesterol-lowering treatment (%) | 117 (43.8) | 67 (43.8) | 50 (43.9) | 0.991 |
| Any anti-diabetic treatment (%) | 69 (25.8) | 43 (28.1) | 26 (22.8) | 0.328 |
| Smoking habits (%) | <0.001 | |||
| Never smoker | 105 (39.3) | 29 (19.0) | 77(66.7) | |
| Former smoker | 125 (46.8) | 97 (63.4) | 28 (24.6) | |
| Current smoker | 37 (13.9) | 27 (17.6) | 10 (8.8) | |
Urinary flavonoid excretions are presented in Table 2. Stratification by sex was also conducted due to the significant differences seen in dietary intake between men and women. In general, women presented significantly higher excretion of flavonoid metabolites compared to men. Nevertheless, there was no difference in the urinary excretion of kaempferol-3-GlcUA (men = 0.1 ± 0.2; women = 0.2 ± 0.3 nmol g−1 creatinine; p = 0.051) when comparing men and women. Regarding the mean excretion of flavonoids in the total sample, the highest excretion was for 3′-ME-EC (1.2 ± 1.1 nmol g−1 creatinine), followed by flavanone metabolites, 3′-GlcUA (19.3 ± 38.2 nmol g−1 creatinine), naringenin 4′-GlcUA (18.0 ± 38.3 nmol g−1 creatinine) and naringenin 7′-GlcUA (11.0 ± 25.9 nmol g−1 creatinine). Meanwhile, flavonols showed the lowest urinary excretion: the mean urinary excretion for myricetin was (5.2 ± 9.2 nmol g−1 creatinine), isorhamnetin (0.5 ± 0.4 nmol g−1 creatinine), and kaempferol-3-GlcUA (0.1 ± 0.2 nmol g−1 creatinine).
| Urinary metabolites nmol g−1 creatinine) | All (n = 267) | Men (n = 153) | Women (n = 114) | p-Valuea |
|---|---|---|---|---|
| Data were calculated by Student's t-test. Results are expressed as mean (standard deviation).a p for differences between sexes. Abbreviations: 3′-ME-EC, 3′-methoxy-(−)-epicatechin. | ||||
| Flavanol | ||||
| 3′-ME-EC | 1.2 (1.1) | 1.0 (0.6) | 1.5 (1.5) | <0.001 |
| Flavanones | ||||
| Naringenin 4′-GlcUA | 18.0 (38.3) | 13.5 (34.5) | 24.0 (42.2) | 0.027 |
| Naringenin 7′-GlcUA | 11.0 (25.9) | 7.9 (22.8) | 15.2 (29.2) | 0.022 |
| Hesperetin 3′-GlcUA | 19.3 (38.2) | 13.7 (25.1) | 26.7 (49.8) | 0.006 |
| Flavonols | ||||
| Kaempferol-3-GlcUA | 0.1 (0.2) | 0.1 (0.2) | 0.2 (0.3) | 0.051 |
| Myricetin | 5.2 (9.2) | 3.7 (3.6) | 7.3 (13.2) | 0.001 |
| Isorhamnetin | 0.5 (0.4) | 0.4 (0.3) | 0.7 (0.5) | <0.001 |
Relationship between dietary intake, liver enzymes and inflammatory indexes
The correlations between the dietary intake of selected flavonoid-rich foods estimated from FFQ, liver enzymes and inflammatory scores are presented in Table 3. Inverse associations among liver enzymes, fruit, and vegetable intake (rho values of −0.134 to −0.178) were found. For example, we observed that GGT levels had significant inverse correlations with the intake of total fruit (rho = −0.166), fruits and vegetables (rho = −0.178), and the groups composed of fruits, vegetables and cereals (rho = −0.161). Concerning inflammatory scores, the AISI showed an inverse correlation with citrus fruit intake (rho = −0.138). In addition, a positive association between onion intake and SII was found (rho = 0.202).| Markers | Watermelon | Citrus | Onion | Total fruit | Total vegetables | Fruits and vegetables | Fruit, vegetables and cereals |
|---|---|---|---|---|---|---|---|
| Data were expressed as Spearman's rho values. Liver and inflammatory markers were inverse normally transformed. Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; AISI, aggregate index of systemic inflammation index; SII, systemic inflammation index. p < 0.05*; p < 0.01**. | |||||||
| ALT | −0.160** | −0.098 | −0.144* | −0.152* | −0.018 | −0.123* | −0.114 |
| AST | −0.101 | −0.050 | −0.134* | −0.086 | 0.023 | −0.057 | −0.048 |
| GGT | −0.110 | −0.112 | −0.110 | −0.166** | −0.111 | −0.178** | −0.161** |
| AISI index | −0.017 | −0.138* | 0.138* | −0.088 | 0.070 | −0.018 | 0.011 |
| SII index | 0.023 | −0.057 | 0.202** | 0.024 | 0.105 | 0.086 | 0.117 |
Correlations between dietary intake and urinary flavonoid excretion
Considering the potential correlations between dietary consumption and outcomes (liver enzymes and inflammatory scores), we evaluated the association between dietary intake, selected urinary flavonoid excretion, and flavonoid-rich food from main vegetables, fruit sub-groups, and whole grain cereals in our sample. Table 4 shows the correlations between urinary flavonoid excretion and the dietary intake of key plant-based food groups. Of note is that urinary excretion of 3′-ME-EC exhibited significant correlations with apples (rho = 0.157), chard (rho = 0.143), total fruits (rho = 0.165), and vegetables (rho = 0.200). Regarding urine flavanone excretion, naringenin 4′-GlcUA was moderately correlated with citrus, olive, walnuts, and nuts (rho = 0.202 to 0.286). Similarly, naringenin 7′-GlcUA was positively correlated with olives, walnuts, nuts, total fruit, other vegetables, and whole wheat bread (rho = 0.137 to 0.181). A strong positive correlation was also observed between citrus fruit intake and naringenin 7′-GlcUA (rho = 0.348). Moreover, a moderate to strong correlation was identified between hesperetin 3′-GlcUA, total fruit (rho = 0.298) and citrus fruit (rho = 0.447). Weaker correlations were observed between urinary excretion of flavonols such as kaempferol-3-GlcUA and apple (rho = 0.148), walnut (rho = 0.191), and nut (rho = 0.179) consumption. Myricetin was correlated with apple, total fruit, and vegetable intake (rho = 0.126 to 0.139). On the other hand, isorhamnetin excretion was correlated with citrus fruit (rho = 0.129), chard (rho = 0.175), and total vegetable (rho = 0.138) intake.| Flavanol | Flavanones | Flavonols | |||||
|---|---|---|---|---|---|---|---|
| Foods | 3′-ME-EC | Naringenin 4′-GlcUA | Naringenin 7′-GlcUA | Hesperetin 3′-GlcUA | Kaempferol-3-GlcUA | Myricetin | Isorhamnetin |
| Data were expressed as Spearman's rho values. P < 0.05*; p < 0.01**; p < 0.001***. Abbreviations: 3′-ME-EC, 3′-methoxy-(−)-epicatechin. | |||||||
| Fruits | |||||||
| Citrus | 0.119 | 0.286*** | 0.348*** | 0.447*** | 0.114 | 0.093 | 0.129* |
| Apple | 0.157* | 0.066 | 0.069 | 0.081 | 0.148* | 0.126* | 0.092 |
| Watermelon | 0.104 | −0.005 | 0.009 | 0.091 | −0.054 | 0.059 | −0.001 |
| Olive | −0.014 | 0.204*** | 0.181** | 0.154* | 0.088 | −0.005 | −0.086 |
| Walnuts | 0.022 | 0.202*** | 0.154* | 0.174** | 0.191** | −0.022 | 0.037 |
| Total nuts | 0.015 | 0.222*** | 0.169** | 0.184** | 0.179** | −0.001 | 0.009 |
| Total fruits | 0.165** | 0.137* | 0.181** | 0.298*** | 0.039 | 0.139* | 0.094 |
| Vegetables | |||||||
| Chard | 0.143* | 0.083 | 0.051 | 0.157* | 0.061 | 0.048 | 0.175** |
| Onion | 0.115 | 0.012 | 0.013 | 0.050 | −0.012 | 0.054 | −0.031 |
| Other vegetables | 0.004 | 0.094 | 0.137* | 0.157* | 0.076 | 0.078 | 0.148* |
| Total vegetables | 0.200** | 0.011 | 0.024 | 0.087 | 0.014 | 0.138* | 0.138* |
| Cereals | |||||||
| Whole wheat bread | 0.116 | 0.130* | 0.149* | 0.116 | 0.056 | 0.010 | 0.037 |
| Whole grains | 0.092 | −0.046 | −0.030 | −0.007 | 0.014 | 0.045 | 0.068 |
Associations between urinary flavonoid metabolites, liver enzymes, and inflammatory scores
Table 5 presents the association between urinary flavonoid metabolites and liver enzymes. The adjusted models showed that specific citrus-flavanones: naringenin 4′-GlcUA (Bper 1SD = −0.15; 95%CI: −0.26 to −0.03; p-value = 0.014), naringenin 7′-GlcUA (Bper 1SD = −0.12; 95%CI: −0.24 to −0.01; p-value = 0.038), and hesperetin 3′-GlcUA (Bper 1SD = −0.12; 95%CI: −0.24 to −0.002; p-value = 0.047) were inversely associated with GGT levels. Nevertheless, there were no significant associations between other urinary flavonoids (3′-ME-EC, kaempferol-3-GlcUA, myricetin, and isorhamnetin) and hepatic enzymes. Related to inflammatory scores, as shown in Table 6, only a significant relationship was found between urinary excretion of naringenin 7′-GlcUA and AISI (Bper 1SD = −0.14; 95%CI: −0.27 to −0.02; p-value = 0.025) and SII (Bper 1SD = −0.14; 95%CI: −0.27 to −0.02; p-value = 0.028). However, p-values after the FDR adjustment did not reach statistical significance (p < 0.05) in any model. Because of the significant relationship between citrus-flavanone urinary metabolites and GGT levels, we explored the association between GGT levels and the weighted score merging citrus-flavanone metabolites (naringenin 4′-GlcUA, naringenin 7′-GlcUA, and hesperetin 3′-GlcUA) stratified into quartiles in Fig. 1. The linear regression model adjusted for the same confounders indicated that individuals with higher values of the citrus-flavanone score were associated with lower GGT levels (Bper 1SD increase: −0.41; 95%CI: −0.74 to −0.07), showing a linear trend across quartiles (p-trend = 0.015). Also, we observed that the mean of citrus-flavanone urinary excretion increased across quartiles of the score. For Q1 = 1.64 (nmol g−1 creatinine), Q2 = 3.98 (nmol g−1 creatinine), Q3 = 22.76 (nmol g−1 creatinine), and Q4 = 166.48 (nmol g−1 creatinine).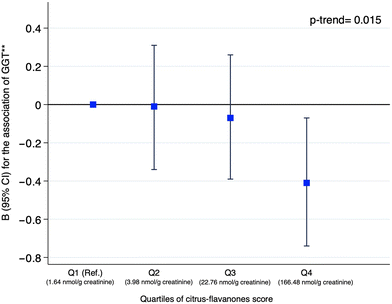 | ||
| Fig. 1 The graph represented the association between GGT **(inverse normally transformed) and quartiles of the citrus-flavanone score (per 1-SD increase). Fig. 1 shows the B (95% CI) from the linear regression model. The model was adjusted for sex, age, smoking status, marital status, educational level, physical activity, sleeping hours, energy intake, intake of vitamins and alcohol consumption. The p for trend in the analysis was calculated for the association across quartiles. The mean of citrus-flavanone urinary excretion (nmol g−1 creatinine) for Q1 = 1.64; Q2 = 3.98; Q3 = 22.76; Q4 = 166.48 (these values are expressed in crude). | ||
| ALT | AST | GGT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transformed urinary metabolites | B coefficient | 95% CI | p-Value | FDR-adjusted p-value | B coefficient | 95% CI | p-Value | FDR-adjusted p-value | B coefficient | 95% CI | p-Value | FDR-adjusted p-value |
| Models were adjusted for sex, age, smoking status (never, former, current), marital status (single, married, widow, divorced or separated), educational level (primary education, secondary education, or academic/graduate), physical activity (MET-min per week), sleeping hours (h per day), energy intake (kcal/d), intake of vitamins and alcohol consumption (g/d). FDR (false discovery rate) controlling adjustments were conducted by applying the method of Simes. Liver markers were inverse normally transformed. Abbreviations: CI, confidence interval; 3′-ME-EC, 3′-methoxy-(−)-epicatechin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; FDR, false discovery rate. | ||||||||||||
| Flavanol | ||||||||||||
| 3′-ME-EC | −0.01 | (−0.14, 0.11) | 0.819 | 0.967 | 0.003 | (−0.13, 0.13) | 0.969 | 0.969 | −0.02 | (−0.14, 0.11) | 0.785 | 0.967 |
| Flavanones | ||||||||||||
| Naringenin 4′-GlcUA | −0.03 | (−0.15, 0.09) | 0.662 | 0.927 | −0.01 | (−0.13, 0.12) | 0.914 | 0.936 | −0.15 | (−0.26, −0.03) | 0.014 | 0.327 |
| Naringenin 7′-GlcUA | −0.04 | (−0.16, 0.08) | 0.549 | 0.915 | 0.02 | (−0.10, 0.14) | 0.746 | 0.922 | −0.12 | (−0.24, −0.01) | 0.038 | 0.329 |
| Hesperetin 3′-GlcUA | −0.08 | (−0.20, 0.04) | 0.184 | 0.627 | −0.03 | (−0.16, 0.09) | 0.593 | 0.899 | −0.12 | (−0.24, −0.002) | 0.047 | 0.329 |
| Flavonols | ||||||||||||
| Kaempferol-3-GlcUA | −0.07 | (−0.18, 0.05) | 0.270 | 0.727 | −0.05 | (−0.17, 0.07) | 0.413 | 0.899 | −0.04 | (−0.15, 0.08) | 0.522 | 0.914 |
| Myricetin | 0.005 | (−0.12, 0.13) | 0.939 | 0.967 | −0.04 | (−0.16, 0.08) | 0.517 | 0.899 | −0.01 | (−0.13, 0.11) | 0.890 | 0.967 |
| Isorhamnetin | −0.01 | (−0.14, 0.12) | 0.908 | 0.967 | 0.01 | (−0.12, 0.14) | 0.880 | 0.936 | 0.03 | (−0.09, 0.16) | 0.629 | 0.927 |
| AISI index | SII index | |||||||
|---|---|---|---|---|---|---|---|---|
| Transformed urinary metabolites | B coefficient | 95% CI | p-Value | FDR-adjusted p-value | B coefficient | 95% CI | p-Value | FDR-adjusted p-value |
| Models were adjusted for sex, age, smoking status (never, former, current), marital status (single, married, widow, divorced or separated), educational level (primary education, secondary education, or academic/graduate), physical activity (MET-min per week), sleeping hours (h per day), energy intake (kcal d−1), intake of vitamins and alcohol consumption (g d−1). Inflammatory markers were inverse normally transformed. Abbreviations: CI, confidence interval; 3′-ME-EC, 3′-methoxy-(−)-epicatechin; AISI, aggregate index of systemic inflammation; SII, systemic inflammation index; FDR, false discovery rate. | ||||||||
| Flavanol | ||||||||
| 3′-ME-EC | −0.06 | (−0.19, 0.07) | 0.381 | 0.852 | −0.05 | (−0.19, 0.08) | 0.414 | 0.852 |
| Flavanones | ||||||||
| Naringenin 4′-GlcUA | −0.12 | (−0.24, 0.01) | 0.073 | 0.359 | −0.08 | (−0.20, 0.05) | 0.241 | 0.703 |
| Naringenin 7′-GlcUA | −0.14 | (−0.27, −0.02) | 0.025 | 0.327 | −0.14 | (−0.27, −0.02) | 0.028 | 0.327 |
| Hesperetin 3′-GlcUA | −0.11 | (−0.24, 0.01) | 0.082 | 0.359 | −0.10 | (−0.23, 0.03) | 0.121 | 0.471 |
| Flavonols | ||||||||
| Kaempferol-3-GlcUA | −0.06 | (−0.19, 0.06) | 0.337 | 0.843 | −0.03 | (−0.15, 0.10) | 0.655 | 0.927 |
| Myricetin | −0.08 | (−0.21, 0.04) | 0.197 | 0.627 | −0.05 | (−0.18, 0.08) | 0.453 | 0.881 |
| Isorhamnetin | −0.02 | (−0.15, 0.12) | 0.775 | 0.967 | −0.12 | (−0.25, 0.01) | 0.076 | 0.359 |
Discussion
In this cross-sectional study conducted in a subsample of the PREDIMED-Plus trial, we found that specific citrus flavanone urinary metabolites (naringenin 4′-GlcUA, naringenin 7′-GlcUA, and hesperetin 3′-GlcUA) are associated with lower GGT levels in individuals diagnosed with MetS. Individually, we observed an inverse association between naringenin 7′-GlcUA and inflammatory scores (AISI and SII), but those associations were not significant after FDR correction. Despite this, biologically, the hypothesis that the metabolites derived from citrus fruit consumption could have beneficial effects on liver health and MetS components is justified.24,30,31,44 For example, several animal studies indicated that naringenin could decrease the levels and expression of liver enzymes and pro-inflammatory cytokines, which can prevent hepatic damage produced by exposure to ethanol.45,46 A recent study evaluated the effect of MedDiet intervention on plasma citrus flavonoids (glycoside and aglycone) and their associations with biomarkers of inflammation and oxidative stress in individuals diagnosed with type 2 diabetes.47 After twelve weeks of intervention, the participants showed increased plasma levels of citrus-derived metabolites and reduced levels of the pro-inflammatory cytokine interleukin-6 in the MedDiet group compared to the control group.47 It should be kept in mind that oxidative stress and inflammation are the key drivers for liver diseases, leading to metabolic alterations in lipid and hepatic/extra-hepatic glucose homeostasis.5,6,48In recent years, flavonoids have attracted interest in scientific research focused on CVD.49 In a prospective cohort study among 69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 women from the Nurses’ Health Study, citrus fruits/juices were the main dietary flavanone, but also the highest intake of flavanones was associated with a reduced relative risk of ischemic stroke compared to the lowest consumption.21 Consistent with this result, a meta-analysis of prospective cohort studies evidenced that the intake of flavanones was inversely associated with the risk of CVD.49 It has to be mentioned that our population presented MetS and a high risk of CVD. This connection is critical considering that cardiometabolic disorders are linked to impaired hepatic and glucose metabolism in subjects diagnosed with MetS.50,51
622 women from the Nurses’ Health Study, citrus fruits/juices were the main dietary flavanone, but also the highest intake of flavanones was associated with a reduced relative risk of ischemic stroke compared to the lowest consumption.21 Consistent with this result, a meta-analysis of prospective cohort studies evidenced that the intake of flavanones was inversely associated with the risk of CVD.49 It has to be mentioned that our population presented MetS and a high risk of CVD. This connection is critical considering that cardiometabolic disorders are linked to impaired hepatic and glucose metabolism in subjects diagnosed with MetS.50,51
Thus, these findings suggest that citrus-flavanone metabolites appear to have prominent results on cardiometabolic risk factors in humans and could be important to reduce the liver disease burden in subjects diagnosed with MetS. The beneficial effects of citrus flavanones (e.g., radical scavenging, anti-inflammatory activity, and microbiota modulation properties) on liver diseases and associated complications may be due to the biochemical structures (the number and specific position of the hydroxyl groups in the A and B rings) and the presence of the sugar moiety.52
A higher intake of plant-based flavonoid-rich foods is a feature of the Mediterranean dietary pattern.53–55 Interestingly, we observed that urinary excretion of some flavanone metabolites such as naringenin 4′-GlcUA, naringenin 7′-GlcUA, and hesperetin 3′-GlcUA was positively correlated with citrus fruit consumption. It is recognized that citrus fruits are the main dietary source of flavanones and their concentrations are higher in fruit tissue compared to juice.56 Proline, betaine and flavanone glucuronides, such as hesperetin and naringenin, have been proposed as biomarkers of citrus intake.57 Our findings are in line with the studies on the evaluation of specific metabolites that reflect the consumption of citrus fruit. Tomás-Navarro et al. found that the main flavanones detected in urine were phase II conjugated derivatives of naringenin and hesperetin after ingesting 400 ml of citrus fruit (orange and mandarin).23 Previous studies from the European Prospective Investigation into Cancer (EPIC)53,58 and PREDIMED-Plus showed that flavonoids were the main contributors to total (poly)phenol intake in the Spanish population.54,55 On average, the consumption of citrus fruit was 151 g per day (around 35% of the total mean fruit intake) in our population. These findings are relevant, considering that a serving of orange juice (250 ml) has 25–65 mg of flavanones (as aglycones), and the flesh of orange (200 g) has a range of 125–375 mg.56 However, flavonoid composition of citrus fruit and juices is heterogeneous due to the use of different methods for quantifying and the different units of measurement.56 Also, the flavonoid content of citrus fruit can depend on many factors, including the variety, the cultivar, the geographical origin, harvesting techniques or weather.56 In our study, despite no differences in citrus intake among sexes, differences in urinary flavanone excretion were statistically significant. It is well documented that the bioavailability of (poly)phenols can be influenced by several factors.59 The interindividual variation in the bioavailability of flavonoids depends upon the form in which they are ingested. On the other hand, the transformations suffered during gastrointestinal digestion and metabolism (phases I and II) depend on genetic polymorphisms, sex, age, and the diversity of the colonic microbiota.59,60 The gut microbiota composition influences the flavanone bioavailability, but also a bidirectional interaction exists between citrus flavanones and gut microbiota.52 It is suggested that (poly)phenols could modulate the gut barrier and improve intestinal microbiota homeostasis in metabolic disease.52,61
By identifying the selected flavanones (naringenin 4′-GlcUA, naringenin 7′-GlcUA, and hesperetin 3′-GlcUA) as biomarkers that reflect citrus fruit consumption, we evaluated the relationship between citrus flavanones and liver enzymes, combining these urinary citrus-derived flavanones in weighted scores. Our results showed that higher values of the flavanone score (Q4) were associated with lower GGT levels in a dose–response manner. In the same line, a study evaluated the relationship between flavonoid intake and the risk of NAFLD measured by the fatty liver index (FLI). In a cohort study including 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 685 participants from the National Health and Nutrition Examination Survey (2005–2010), the authors showed that the lowest intake of flavonoids was associated with higher FLI, C-reactive protein (CRP) and liver enzyme levels. Meanwhile, higher flavonoid intake was associated with less likelihood of NAFLD [third tertile vs. first tertile; OR = 0.81 (95% CI: 0.78, 0.86)].62 As is known, the beneficial effects of citrus flavanones depend on their bioavailability.52 Citrus flavanone glycosides such as hesperidin and naringin are resistant to enzymatic breakdown in the stomach and small intestine, so they are mainly metabolized in the colon, releasing aglycone forms (hesperetin and naringenin) that are further metabolized into phase II metabolites with an intact structure and also catabolized into several ring-fission products.52,63
685 participants from the National Health and Nutrition Examination Survey (2005–2010), the authors showed that the lowest intake of flavonoids was associated with higher FLI, C-reactive protein (CRP) and liver enzyme levels. Meanwhile, higher flavonoid intake was associated with less likelihood of NAFLD [third tertile vs. first tertile; OR = 0.81 (95% CI: 0.78, 0.86)].62 As is known, the beneficial effects of citrus flavanones depend on their bioavailability.52 Citrus flavanone glycosides such as hesperidin and naringin are resistant to enzymatic breakdown in the stomach and small intestine, so they are mainly metabolized in the colon, releasing aglycone forms (hesperetin and naringenin) that are further metabolized into phase II metabolites with an intact structure and also catabolized into several ring-fission products.52,63
In our study, we only evaluated urinary structurally related flavanone phase II metabolites. Being gut microbial metabolites, the Tmax of the flavanone conjugates is around 5–7 hours post-consumption, therefore longer than that for other structurally related flavonoid metabolites that are usually absorbed in the small intestine and have a Tmax of 1–2 hours.60,63 Thus, this could be a reason why these flavanone metabolites moderately correlated with citrus consumption in the FFQ data and may be good biomarkers of habitual citrus consumption.
Mechanisms underlying the potential hepatoprotective effects of naringenin and hesperetin in humans are still poorly understood. In in vivo and in vitro studies, naringenin and hesperetin metabolites could reduce membrane phospholipid damage, thus preventing lipid peroxidation.24,64 These effects may be due to their higher lipophilicity, decreasing the membrane fluidity and reducing the interaction between free radicals and lipids.24,64 Also, citrus flavanones showed a great capacity to decrease the free radical scavenging activity, increase the expression of peroxisome proliferator-activated receptor α genes, and mitigate the activation of inflammatory pathways, avoiding liver damage, which are crucial approaches for NAFLD prevention.24,30,64,65 Despite the noteworthy results of experimental studies of citrus flavanone effects on the liver, randomized control trials (RCT) in humans that established safe and tolerable dosage ranges are lacking. A randomized cross-over trial evaluated the effect of 600 ml d−1 orange juice intake on twenty-nine men over 50 years with hypercholesterolemia for 4 weeks.66 The participants presented improvements in antioxidant parameters and endothelial function.66 Another RCT tested the intake of a (poly)phenolic extract from citrus fruits that contained around 20% naringenin in ninety-five participants with overweight. After twelve weeks of intervention, the participants showed significant improvements in body composition, inflammatory markers (CRP and fibrinogen), and oxidative status.67
Most of the data concerning flavonoids’ benefits on health outcomes have been collected from prospective studies and have some limitations regarding the quantification of flavonoids using FFQ in most studies.68 These discrepancies could be partially explained by the incomplete representation of flavonoid food sources on FFQs, and the misclassification of the dietary flavonoid content of foods due to the lack of good and reliable (poly)phenol composition databases, which cannot avoid potential bias.68 In this context, the development of sensitive, robust and accurate analytical methods to quantify (poly)phenol intake in biofluids to improve currently available (poly)phenol composition databases is needed.68
The strength of the present study is the large sample size of patients with relevant data. Furthermore, we applied a validated analytical method to accurately identify and measure flavonoid metabolites with authentic standards. Nevertheless, our study has some limitations. First, this study has a cross-sectional design and our findings cannot establish causation. Second, this Mediterranean cohort was older in age and had MetS. Thus, our results cannot be generalized to other ethnic, younger and/or healthier individuals. However, it is plausible that plant-based foods have beneficial effects on health outcomes.13 Third, the flavonoid metabolites evaluated in this study were limited and therefore influence our conclusions, but the flavanone metabolites used in the present study are well correlated with citrus fruit intake, and they have been suggested as biomarkers of citrus consumption.23,55,57
Conclusion
Our results suggest that higher urinary excretion of citrus-flavanone metabolites, such as naringenin 4′-GlcUA, naringenin 7′-GlcUA, and hesperetin 3′-GlcUA, might be associated with reduced levels of GGT. Further well-designed randomized trials are still needed to establish these associations and the amount of consumption of citrus foods that is required to protect against liver damage in individuals diagnosed with MetS.Author contributions
Conceptualization, V.B.-V., I.A., M.A.Z., A.R.-M., and J.A.M; formal analysis, V.B.-V., C.R., Y.X., I.A., M.A.Z., A.R.-M., and J.A.M.; investigation, V.B.-V., Y.X., C.R., I.A., M.A.Z., M.A.M.-G., P.B.-C, F.V.-S, V.M.S, Z.V.-R, C.S.-O., M.D.-F., C.C., R.E., R.M.L.-R., M.F., G.B., N.B., J.S.-S, F.J.T., J.A.T., D.R., J.K., X.P., L.D., A.R.-M., J.A.M; writing—original draft, V.B.-V., Y.X., C.R., I.A., M.A.Z., A.R.-M., F.V.-S, V.M.S, and J.A.M.; writing—review and editing, V.B.-V., Y.X., C.R., I.A., M.A.Z., M.A.M.-G., P.B.-C, F.V.-S, V.M.S, Z.V.-R, C.S.-O., M.D.-F., C.C., R.E., R.M.L.-R., M.F., G.B., N.B., J.S.-S, F.J.T., J.A.T., D.R., J.K., X.P., L.D., A.R.-M., J.A.M.; supervision, C.R., I.A., M.A.Z., A.R.-M., J.A.M. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The PREDIMED-Plus trial was supported by the European Research Council (Advanced Research grant 2014–2019; agreement #340918; granted to M.A. Martínez-González); the Official Spanish Institutions for Funding Scientific Biomedical Research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBERobn) and Instituto de Salud Carlos III (ISCIII) through the Fondo de Investigación para la Salud (FIS) that is co-funded by the European Regional Development Fund (coordinated FIS projects led by J. Salas-Salvadó and Vidal, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, PI20/01158), and the Especial Action Project “Implementación y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus” (J. Salas-Salvadó); the Recercaixa (grant number 2013ACUP00194) (J. Salas-Salvadó). Moreover, J. Salas-Salvadó gratefully acknowledges the financial support by ICREA under the ICREA Academia program; the SEMERGEN grant; International Nut and Dried Fruit Council–FESNAD (long-term effects of an energy-restricted Mediterranean diet on mortality and cardiovascular disease 2014–2015; No. 201302) (M.A. Martínez-González); Department of Health of the Government of Navarra (61/2015), the Fundació La Marató de TV (Ref. 201630.10); grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013; PS0358/2016; PI0137/2018), the PROMETEO/2017/017 grant from the Generalitat Valenciana, the SEMERGEN grant; grant of support to research groups 35/2011 (Balearic Islands Gov; FEDER funds) (Tur and Bouz). J. K. is supported by the Juan de la Cierva-Incorporación research grant (IJC2019–042420-I) of the Spanish Ministry of Economy, Industry and Competitiveness and European Social Funds. Y. X. is supported by the joint scholarship of King's College London and China Scholarship Council (K-CSC). The authors are especially grateful to all the participants for their collaboration, the staff of the PREDIMED-Plus study for their exceptional contribution, and the personnel of primary care centres for their support.References
- N. Chalasani, Z. Younossi, J. E. Lavine, M. Charlton, K. Cusi, M. Rinella, S. A. Harrison, E. M. Brunt and A. J. Sanyal, The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases, Hepatology, 2018, 67, 328–357 CrossRef PubMed.
- E. Kassi, P. Pervanidou, G. Kaltsas and G. Chrousos, Metabolic syndrome : definitions and controversies, 2011, 1–13 Search PubMed.
- D. Kim, A. Touros and W. R. Kim, Nonalcoholic Fatty Liver Disease and Metabolic Syndrome, Clin. Liver Dis., 2018, 22, 133–140 CrossRef PubMed.
- T. G. Cotter and M. Rinella, Nonalcoholic Fatty Liver Disease 2020: The State of the Disease, Gastroenterology, 2020, 158, 1851–1864 CrossRef CAS PubMed.
- Z. Chen, R. Tian, Z. She, J. Cai and H. Li, Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease, Free Radicals Biol. Med., 2020, 152, 116–141 CrossRef CAS PubMed.
- M. V. Bullón-Vela, I. Abete, J. A. Martínez and M. A. Zulet, Obesity and Nonalcoholic Fatty Liver Disease: Role of Oxidative Stress, in Obesity: Oxidative Stress and Dietary Antioxidants, ed. A. M. d. Moral and M. A. C. García, Academic Press, London, UK, 2018, pp. 111–133. ISBN 9780128125045 Search PubMed.
- C. F. Liu, W. N. Zhou, Z. Lu, X. T. Wang and Z. H. Qiu, The associations between liver enzymes and the risk of metabolic syndrome in the elderly, Exp. Gerontol., 2018, 106, 132–136 CrossRef CAS PubMed.
- J.-Y. Chen, Y.-H. Chen, Y.-C. Lee and M.-T. Tsou, The Association Between White Blood Cell Count and Insulin Resistance in Community-Dwelling Middle-Aged and Older Populations in Taiwan: A Community-Based Cross-Sectional Study, Front. Med., 2022, 326 CAS.
- J. K. Dyson, Q. M. Anstee and S. McPherson, Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging, Frontline Gastroenterol., 2014, 5, 211–218 CrossRef CAS PubMed.
- M. Franzini, I. Fornaciari, V. Fierabracci, H. A. Elawadi, V. Bolognesi, S. Maltinti, A. Ricchiuti, N. De Bortoli, S. Marchi, A. Pompella, C. Passino, M. Emdin and A. Paolicchi, Accuracy of b-GGT fraction for the diagnosis of non-alcoholic fatty liver disease, Liver Int., 2012, 32, 629–634 CrossRef CAS PubMed.
- F. Hadizadeh, E. Faghihimani and P. Adibi, Nonalcoholic fatty liver disease: Diagnostic biomarkers, World J. Gastrointest. Pathophysiol., 2017, 8, 11 CrossRef PubMed.
- F. Zhong, L. Guan, H. Lin, M. Zhao, Y. Qin, Q. Li, Z. Yuan, G. Yang, L. Gao and J. Zhao, Red Blood Cell Count: An Unrecognized Risk Factor for Nonalcoholic Fatty Liver Disease, Front. Endocrinol., 2021, 12, 1603 Search PubMed.
- E. C. Hemler and F. B. Hu, Plant-Based Diets for Personal, Population, and Planetary Health, Adv. Nutr., 2019, 10, S275–S283 CrossRef PubMed.
- M. A. Martínez-González, A. Gea and M. Ruiz-Canela, The Mediterranean Diet and Cardiovascular Health., Circ. Res., 2019, 124, 779–798 CrossRef PubMed.
- M. A. Martínez-González and G. Bastarrika, Mediterranean diet as the ideal model for preventing non-alcoholic fatty liver disease (NAFLD), Hepatobiliary Surg. Nutr., 2020, 9, 379–381 CrossRef PubMed.
- W. Aoi, M. Iwasa and Y. Marunaka, Metabolic functions of flavonoids: From human epidemiology to molecular mechanism, Neuropeptides, 2021, 88, 102163 CrossRef CAS PubMed.
- X. Wang, Y. Y. Ouyang, J. Liu and G. Zhao, Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies, Br. J. Nutr., 2014, 111, 1–11 CrossRef PubMed.
- J. A. Giménez-Bastida, A. González-Sarrías, F. Vallejo, J. C. Espín and F. A. Tomás-Barberán, Hesperetin and its sulfate and glucuronide metabolites inhibit TNF-α induced human aortic endothelial cell migration and decrease plasminogen activator inhibitor-1 (PAI-1) levels, Food Funct., 2016, 7, 118–126 RSC.
- D. Zhou, Z. Bai, T. Guo, J. Li, Y. Li, Y. Hou, G. Chen and N. Li, Dietary flavonoids and human top-ranked diseases: The perspective of in vivo bioactivity and bioavailability, Trends Food Sci. Technol., 2022, 120, 374–386 CrossRef CAS.
- V. Bullón-Vela, I. Abete, M. A. Zulet, Y. Xu, M. A. Martínez-González, C. Sayón-Orea, M. Ruiz-Canela, E. Toledo, V. M. Sánchez, R. Estruch, R. M. Lamuela-Raventós, E. Almanza-Aguilera, M. Fitó, J. Salas-Salvadó, A. Díaz-López, F. J. Tinahones, J. A. Tur, D. Romaguera, J. Konieczna, X. Pintó, L. Daimiel, A. Rodriguez-Mateos and J. Alfredo Martínez, Urinary Resveratrol Metabolites Output: Differential Associations with Cardiometabolic Markers and Liver Enzymes in House-Dwelling Subjects Featuring Metabolic Syndrome, Molecules, 2020, 25, 4340 CrossRef PubMed.
- A. Cassidy, E. B. Rimm, É. J. O'Reilly, G. Logroscino, C. Kay, S. E. Chiuve and K. M. Rexrode, Dietary flavonoids and risk of stroke in women, Stroke, 2012, 43, 946–951 CrossRef CAS PubMed.
- V. E. Hedrick, A. M. Dietrich, P. A. Estabrooks, J. Savla, E. Serrano and B. M. Davy, Dietary biomarkers: advances, limitations and future directions, Nutr. J., 2012, 11, 109 CrossRef PubMed.
- M. Tomás-Navarro, J. L. Navarro, F. Vallejo and F. A. Tomás-Barberán, Novel Urinary Biomarkers of Orange Juice Consumption, Interindividual Variability, and Differences with Processing Methods, J. Agric. Food Chem., 2021, 69, 4006–4017 CrossRef PubMed.
- E. Hernández-Aquino and P. Muriel, Beneficial effects of naringenin in liver diseases: Molecular mechanisms, World J. Gastroenterol., 2018, 24, 1679 CrossRef PubMed.
- M. Rubín-García, F. Vitelli-Storelli, E. Toledo, S. Castro-Barquero, A. Tresserra-Rimbau, M. Á. Martínez-González, J. Salas-Salvadó, D. Corella, Á. Hernáez, J. A. Martínez, Á. M. Alonso-Gómez, J. Wärnberg, J. Vioque, D. Romaguera, J. López-Miranda, R. Estruch, M. R. Bernal-López, J. Lapetra, L. Serra-Majem, A. Bueno-Cavanillas, J. A. Tur, L. Álvarez-Álvarez, X. Pintó, J. J. Gaforio, P. Matía-Martín, J. Vidal, C. Vázquez, L. Daimiel, E. Ros, A. Gea, J. M. Manzanares, J. V. Sorlí, H. Schröder, I. Abete, L. Tojal-Sierra, E. Crespo-Oliva, A. González-Botella, E. Rayó, A. García-Rios, A. M. Gómez-Pérez, J. M. Santos-Lozano, R. Bartolomé Resano, M. M. Murphy, C. Ortega-Azorin, C. Medrano, M. Á. Zulet, C. Sorto-Sanchez, N. Babio, M. Fitó, R. M. Lamuela-Raventós and V. Martín-Sánchez, Polyphenol intake and cardiovascular risk in the PREDIMED-Plus trial. A comparison of different risk equations, Rev. Esp. Cardiol., 2022, 75, 401–411 CrossRef.
- L. Testai and V. Calderone, Nutraceutical Value of Citrus Flavanones and Their Implications in Cardiovascular Disease, Nutrients, 2017, 9, 502 CrossRef PubMed.
- J. A. Ross and C. M. Kasum, DIETARY FLAVONOIDS: Bioavailability, Metabolic Effects, and Safety, Annu. Rev. Nutr., 2002, 22, 19–34 CrossRef CAS PubMed.
- J. Viskupicova, M. Ondorejovic and E. Sturdik, Bioavailability and metabolism of flavonoids, J. Food Nutr. Res., 2008, 4, 151–162 Search PubMed.
- A. C. Matheyambath, P. Padmanabhan and G. Paliyath, in Encyclopedia of Food and Health, Elsevier, 2016, pp. 136–140 Search PubMed.
- F. Naeini, Z. Namkhah, A. Ostadrahimi, H. Tutunchi and M. J. Hosseinzadeh-Attar, A Comprehensive Systematic Review of the Effects of Naringenin, a Citrus-Derived Flavonoid, on Risk Factors for Nonalcoholic Fatty Liver Disease, Adv. Nutr., 2021, 12, 413–428 CrossRef PubMed.
- M. A. Alam, N. Subhan, M. M. Rahman, S. J. Uddin, H. M. Reza and S. D. Sarker, Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action, Adv. Nutr., 2014, 5, 404 CrossRef CAS PubMed.
- E. Hernández-Aquino and P. Muriel, Beneficial effects of naringenin in liver diseases: Molecular mechanisms, World J. Gastroenterol., 2018, 24, 1679–1707 CrossRef PubMed.
- M. A. Martínez-González, P. Buil-Cosiales, D. Corella, M. Bulló, M. Fitó, J. Vioque, D. Romaguera, J. A. Martínez, J. Wärnberg, J. López-Miranda, R. Estruch, A. Bueno-Cavanillas, F. Arós, J. A. Tur, F. Tinahones, L. Serra-Majem, V. Martín, J. Lapetra, C. Vázquez, X. Pintó, J. Vidal, L. Daimiel, M. Delgado-Rodríguez, P. Matía, E. Ros, F. Fernández-Aranda, C. Botella, M. P. Portillo, R. M. Lamuela-Raventós, A. Marcos, G. Sáez, E. Gómez-Gracia, M. Ruiz-Canela, E. Toledo, I. Alvarez-Alvarez, J. Díez-Espino, J. V Sorlí, J. Basora, O. Castañer, H. Schröder, E. M. Navarrete-Muñoz, M. A. Zulet, A. García-Rios and J. Salas-Salvadó, Cohort Profile: Design and methods of the PREDIMED-Plus randomized trial, Int. J. Epidemiol., 2019, 48, 387–388 CrossRef PubMed.
- J. D. Fernández-Ballart, J. L. Piñol, I. Zazpe, D. Corella, P. Carrasco, E. Toledo, M. Perez-Bauer, M. Á. Martínez-González, J. Salas-Salvadó and J. M. Martín-Moreno, Relative validity of a semi-quantitative food-frequency questionnaire in an elderly Mediterranean population of Spain, Br. J. Nutr., 2010, 103, 1808–1816 CrossRef PubMed.
- C. Sayón-Orea, C. Razquin, M. Bulló, D. Corella, M. Fitó, D. Romaguera, J. Vioque, Á. M. Alonso-Gómez, J. Wärnberg, J. A. Martínez, L. Serra-Majem, R. Estruch, F. J. Tinahones, J. Lapetra, X. Pintó, J. A. Tur, J. López-Miranda, A. Bueno-Cavanillas, M. Delgado-Rodríguez, P. Matía-Martín, L. Daimiel, V. M. Sánchez, J. Vidal, C. Vázquez, E. Ros, M. Ruiz-Canela, J. V. Sorlí, O. Castañer, M. Fiol, E. M. Navarrete-Muñoz, F. Arós, E. Gómez-Gracia, M. A. Zulet, A. Sánchez-Villegas, R. Casas, R. Bernal-López, J. M. Santos-Lozano, E. Corbella, C. Bouzas, A. García-Arellano, J. Basora, E. M. Asensio, H. Schröder, M. Moñino, M. García de la Hera, L. Tojal-Sierra, E. Toledo, A. Díaz-López, A. Goday, J. Salas-Salvadó and M. A. Martínez-González, Effect of a Nutritional and Behavioral Intervention on Energy-Reduced Mediterranean Diet Adherence Among Patients With Metabolic Syndrome, J. Am. Med. Assoc., 2019, 322, 1486 CrossRef PubMed.
- H. Schröder, M. Fitó, R. Estruch, M. A. Martínez-González, D. Corella, J. Salas-Salvadó, R. Lamuela-Raventós, E. Ros, I. Salaverría, M. Fiol, J. Lapetra, E. Vinyoles, E. Gómez-Gracia, C. Lahoz, L. Serra-Majem, X. Pintó, V. Ruiz-Gutierrez and M. Covas, A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women, J. Nutr., 2011, 141, 1140–1145 CrossRef PubMed.
- A. G. Fois, P. Paliogiannis, V. Scano, S. Cau, S. Babudieri, R. Perra, G. Ruzzittu, E. Zinellu, P. Pirina, C. Carru, L. B. Arru, A. Fancellu, M. Mondoni, A. A. Mangoni and A. Zinellu, The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients, Molecules, 2020, 25, 5725 CrossRef CAS PubMed.
- L. Molina, M. Sarmiento, J. Peñafiel, D. Donaire, J. Garcia-Aymerich, M. Gomez, M. Ble, S. Ruiz, A. Frances, H. Schröder, J. Marrugat and R. Elosua, Validation of the Regicor Short Physical Activity Questionnaire for the Adult Population., PLoS One, 2017, 12, e0168148 CrossRef PubMed.
- N. Rosique-Esteban, N. Babio, A. Díaz-López, D. Romaguera, J. Alfredo Martínez, V. M. Sanchez, H. Schröder, R. Estruch, J. Vidal, P. Buil-Cosiales, J. Konieczna, I. Abete and J. Salas-Salvadó, Leisure-time physical activity at moderate and high intensity is associated with parameters of body composition, muscle strength and sarcopenia in aged adults with obesity and metabolic syndrome from the PREDIMED-Plus study, Clin. Nutr., 2019, 38, 1324–1331 CrossRef PubMed.
- S. A. Atroshenko, I. A. Korolyov and N. Didenko, Standards of Medical Care in Diabetes- 2014, Diabetes Care, 2014, 37, S14–S80 CrossRef PubMed.
- M. Domínguez-Fernández, Y. Xu, P. Young Tie Yang, W. Alotaibi, R. Gibson, W. L. Hall, L. Barron, I. A. Ludwig, C. Cid and A. Rodriguez-Mateos, Quantitative Assessment of Dietary (Poly)phenol Intake: A High-Throughput Targeted Metabolomics Method for Blood and Urine Samples, J. Agric. Food Chem., 2021, 69, 537–554 CrossRef PubMed.
- T. Cook, Y. Ma and S. Gamagedara, Evaluation of statistical techniques to normalize mass spectrometry-based urinary metabolomics data, J. Pharm. Biomed. Anal., 2020, 177, 112854 CrossRef CAS PubMed.
- G. Blom, Statistical estimates and transformed beta-variables, New York, John Wiley & Sons A/S, 1958.
- M. H. Farzaei, A. K. Singh, R. Kumar, C. R. Croley, A. K. Pandey, E. Coy-Barrera, J. K. Patra, G. Das, R. G. Kerry, G. Annunziata, G. C. Tenore, H. Khan, M. Micucci, R. Budriesi, S. Momtaz, S. M. Nabavi and A. Bishayee, Targeting Inflammation by Flavonoids: Novel Therapeutic Strategy for Metabolic Disorders, Int. J. Mol. Sci., 2019, 20, 4957 CrossRef CAS PubMed.
- J. Jayaraman, V. A. S. Jesudoss, V. P. Menon and N. Namasivayam, Anti-inflammatory role of naringenin in rats with ethanol induced liver injury, Toxicol. Mech. Methods, 2012, 22, 568–576 CrossRef CAS PubMed.
- L. Zhao, N. Zhang, D. Yang, M. Yang, X. Guo, J. He, W. Wu, B. Ji, Q. Cheng and F. Zhou, Protective Effects of Five Structurally Diverse Flavonoid Subgroups against Chronic Alcohol-Induced Hepatic Damage in a Mouse Model, Nutrients, 2018, 10, 1754 CrossRef PubMed.
- H. A. Al-Aubaidy, A. Dayan, M. A. Deseo, C. Itsiopoulos, D. Jamil, N. R. Hadi and C. J. Thomas, Twelve-Week Mediterranean Diet Intervention Increases Citrus Bioflavonoid Levels and Reduces Inflammation in People with Type 2 Diabetes Mellitus, Nutrients, 2021, 13, 1133 CrossRef CAS PubMed.
- J. Yang, M. Fernández-Galilea, L. Martínez-Fernández, P. González-Muniesa, A. Pérez-Chávez, J. A. Martínez and M. J. Moreno-Aliaga, Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation, Nutrients, 2019, 11, 872 CrossRef CAS PubMed.
- X. Wang, Y. Y. Ouyang, J. Liu and G. Zhao, Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies, Br. J. Nutr., 2014, 111, 1–11 CrossRef PubMed.
- V. Micó, R. San-Cristobal, R. Martín, M. Á. Martínez-González, J. Salas-Salvadó, D. Corella, M. Fitó, Á. M. Alonso-Gómez, J. Wärnberg, J. Vioque, D. Romaguera, J. López-Miranda, R. Estruch, F. J. Tinahones, J. Lapetra, J. L. Serra-Majem, A. Bueno-Cavanillas, J. A. Tur, V. Martín Sánchez, X. Pintó, M. Delgado-Rodríguez, P. Matía-Martín, J. Vidal, C. Vázquez, A. García-Arellano, S. Pertusa-Martinez, A. Chaplin, A. Garcia-Rios, C. Muñoz Bravo, H. Schröder, N. Babio, J. V. Sorli, J. I. Gonzalez, D. Martinez-Urbistondo, E. Toledo, V. Bullón, M. Ruiz-Canela, M. P. Portillo, M. Macías-González, N. Perez-Diaz-del-Campo, J. García-Gavilán, L. Daimiel and J. A. Martínez, Morbid liver manifestations are intrinsically bound to metabolic syndrome and nutrient intake based on a machine-learning cluster analysis, Front. Endocrinol., 2022, 13, 936956 CrossRef PubMed.
- V. Bullón-Vela, I. Abete, J. A. Tur, J. Konieczna, D. Romaguera, X. Pintó, E. Corbella, M. A. Martínez-González, C. Sayón-Orea, E. Toledo, D. Corella, M. Macías-Gonzalez, F. J. Tinahones, M. Fitó, R. Estruch, E. Ros, J. Salas-Salvadó, L. Daimiel, C. M. Mascaró, M. A. Zulet and J. A. Martínez, Relationship of visceral adipose tissue with surrogate insulin resistance and liver markers in individuals with metabolic syndrome chronic complications, Ther. Adv. Endocrinol. Metab., 2020, 11, 204201882095829 CrossRef PubMed.
- Y. Stevens, E. Van Rymenant, C. Grootaert, J. Van Camp, S. Possemiers, A. Masclee and D. Jonkers, The Intestinal Fate of Citrus Flavanones and Their Effects on Gastrointestinal Health, Nutrients, 2019, 11, 1464 CrossRef CAS PubMed.
- R. Zamora-Ros, C. Andres-Lacueva, R. M. Lamuela-Raventós, T. Berenguer, P. Jakszyn, A. Barricarte, E. Ardanaz, P. Amiano, M. Dorronsoro, N. Larrañaga, C. Martínez, M. J. Sánchez, C. Navarro, M. D. Chirlaque, M. J. Tormo, J. R. Quirós and C. A. González, Estimation of Dietary Sources and Flavonoid Intake in a Spanish Adult Population (EPIC-Spain), J. Am. Diet. Assoc., 2010, 110, 390–398 CrossRef CAS PubMed.
- A. Tresserra-Rimbau, S. Castro-Barquero, N. Becerra-Tomás, N. Babio, M. Á. Martínez-González, D. Corella, M. Fitó, D. Romaguera, J. Vioque, A. M. Alonso-Gomez, J. Wärnberg, J. A. Martínez, L. Serra-Majem, R. Estruch, F. J. Tinahones, J. Lapetra, X. Pintó, J. A. Tur, J. López-Miranda, N. Cano-Ibáñez, M. Delgado-Rodríguez, P. Matía-Martín, L. Daimiel, V. Martín Sánchez, J. Vidal, C. Vázquez, E. Ros, F. J. Basterra, M. Fernández de la Puente, E. M. Asensio, O. Castañer, V. Bullón-Vela, L. Tojal-Sierra, E. Gómez-Gracia, E. Cases-Pérez, J. Konieczna, A. García-Ríos, T. Casañas-Quintana, M. R. Bernal-Lopez, J. M. Santos-Lozano, V. Esteve-Luque, C. Bouzas, Z. Vázquez-Ruiz, A. Palau-Galindo, R. Barragan, M. López Grau, C. Razquín, L. Goicolea-Güemez, E. Toledo, M. V. Vergaz, R. M. Lamuela-Raventós and J. Salas-Salvadó, Adopting a High-Polyphenolic Diet Is Associated with an Improved Glucose Profile: Prospective Analysis within the PREDIMED-Plus Trial, Antioxidants, 2022, 11, 316 CrossRef CAS PubMed.
- L. López-González, N. Becerra-Tomás, N. Babio, M. Á. Martínez-González, A. Díaz-López, D. Corella, A. Goday, D. Romaguera, J. Vioque, Á. M. Alonso-Gómez, J. Wärnberg, J. A. Martínez, L. Serra-Majem, R. Estruch, F. Tinahones, J. Lapetra, X. Pintó, J. A. Tur, J. López-Miranda, A. Bueno-Cavanillas, M. Delgado-Rodríguez, P. Matía-Martín, L. Daimiel, L. Álvarez-Álvarez, J. Vidal, C. Vázquez, E. Ros, Z. Vázquez-Ruiz, S. Canudas, R. F. Carrión, O. Castañer, M. Á. Zulet, L. T. Sierra, M. J. Ajejas Bazán, C. M. López García, M. Martín, A. García-Ríos, R. Casas, A. M. Gómez-Pérez, J. M. Santos-Lozano, E. Goñi, P. Guillem-Saiz, C. Lassale, I. Abete, I. S. Lete, S. Eguaras, H. Schröder and J. Salas-Salvadó, Variety in fruits and vegetables, diet quality and lifestyle in an older adult mediterranean population, Clin. Nutr., 2021, 40, 1510–1518 CrossRef PubMed.
- F. A. Tomás-Barberán and M. N. Clifford, Flavanones, chalcones and dihydrochalcones - nature, occurrence and dietary burden, J. Sci. Food Agric., 2000, 80, 1073–1080 CrossRef.
- E. Pujos-Guillot, J. Hubert, J.-F. Martin, B. Lyan, M. Quintana, S. Claude, B. Chabanas, J. A. Rothwell, C. Bennetau-Pelissero, A. Scalbert, B. Comte, S. Hercberg, C. Morand, P. Galan and C. Manach, Mass Spectrometry-based Metabolomics for the Discovery of Biomarkers of Fruit and Vegetable Intake: Citrus Fruit as a Case Study, J. Proteome Res., 2013, 12, 1645–1659 CrossRef CAS PubMed.
- R. Zamora-Ros, D. Achaintre, J. A. Rothwell, S. Rinaldi, N. Assi, P. Ferrari, M. Leitzmann, M. C. Boutron-Ruault, G. Fagherazzi, A. Auffret, T. Kühn, V. Katzke, H. Boeing, A. Trichopoulou, A. Naska, E. Vasilopoulou, D. Palli, S. Grioni, A. Mattiello, R. Tumino, F. Ricceri, N. Slimani, I. Romieu and A. Scalbert, Urinary excretions of 34 dietary polyphenols and their associations with lifestyle factors in the EPIC cohort study, Sci. Rep., 2016, 6, 1–9 CrossRef PubMed.
- M. Tomás-Navarro, F. Vallejo, E. Sentandreu, J. L. Navarro and F. A. Tomás-Barberán, Volunteer stratification is more relevant than technological treatment in orange juice flavanone bioavailability, J. Agric. Food Chem., 2014, 62, 24–27 CrossRef PubMed.
- F. Vallejo, M. Larrosa, E. Escudero, M. P. Zafrilla, B. Cerdá, J. Boza, M. T. García-Conesa, J. C. Espín and F. A. Tomás-Barberán, Concentration and solubility of flavanones in orange beverages affect their bioavailability in humans, J. Agric. Food Chem., 2010, 58, 6516–6524 CrossRef CAS PubMed.
- I. Milton-Laskibar, A. Cuevas-Sierra, M. P. Portillo and J. A. Martínez, Effects of Resveratrol Administration in Liver Injury Prevention as Induced by an Obesogenic Diet: Role of Ruminococcaceae, Biomedicines, 2022, 10, 8 CrossRef PubMed.
- M. Mazidi, N. Katsiki and M. Banach, A higher flavonoid intake is associated with less likelihood of nonalcoholic fatty liver disease: results from a multiethnic study, J. Nutr. Biochem., 2019, 65, 66–71 CrossRef CAS PubMed.
- J. K. Aschoff, K. M. Riedl, J. L. Cooperstone, J. Högel, A. Bosy-Westphal, S. J. Schwartz, R. Carle and R. M. Schweiggert, Urinary excretion of Citrus flavanones and their major catabolites after consumption of fresh oranges and pasteurized orange juice: A randomized cross-over study, Mol. Nutr. Food Res., 2016, 60, 2602–2610 CrossRef CAS PubMed.
- J. Li, T. Wang, P. Liu, F. Yang, X. Wang, W. Zheng and W. Sun, Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD, Food Funct., 2021, 12, 3898–3918 RSC.
- D. Martinez-Urbistondo, D. D'Avola, D. Navarro-González, L. Sanchez-Iñigo, A. Fernandez-Montero, N. Perez-Diaz-del-Campo, E. Bugianesi, J. A. Martinez and J. C. Pastrana, Interactive Role of Surrogate Liver Fibrosis Assessment and Insulin Resistance on the Incidence of Major Cardiovascular Events, J. Clin. Med., 2022, 11, 17–5190 Search PubMed.
- J. Constans, C. Bennetau-Pelissero, J.-F. Martin, E. Rock, A. Mazur, A. Bedel, C. Morand and A. M. Bérard, Marked antioxidant effect of orange juice intake and its phytomicronutrients in a preliminary randomized cross-over trial on mild hypercholesterolemic men, Clin. Nutr., 2015, 34, 1093–1100 CrossRef CAS PubMed.
- C. Dallas, A. Gerbi, Y. Elbez, P. Caillard, N. Zamaria and M. Cloarec, Clinical Study to Assess the Efficacy and Safety of a Citrus Polyphenolic Extract of Red Orange, Grapefruit, and Orange (Sinetrol-XPur) on Weight Management and Metabolic Parameters in Healthy Overweight Individuals, Phyther. Res., 2014, 28, 212–218 CrossRef CAS PubMed.
- Y. Xu, M. Le Sayec, C. Roberts, S. Hein, A. Rodriguez-Mateos and R. Gibson, Dietary Assessment Methods to Estimate (Poly)phenol Intake in Epidemiological Studies: A Systematic Review, Adv. Nutr., 2021, 5, 1781–1801 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02846h |
| ‡ Co-senior authors. |
| This journal is © The Royal Society of Chemistry 2023 |
