Open Access Article
Open Access ArticleShort-term serum and urinary changes in sex hormones of healthy pre-pubertal children after the consumption of commercially available whole milk powder: a randomized, two-level, controlled-intervention trial in China†
Jieshu
Wu
 a,
Xi
Shi
a,
Man
Zhang
a,
Xi
Shi
a,
Man
Zhang
 b,
Xiaolong
Lu
a,
Rui
Qin
a,
Manli
Hu
a and
Zhixu
Wang
b,
Xiaolong
Lu
a,
Rui
Qin
a,
Manli
Hu
a and
Zhixu
Wang
 *a
*a
aDepartment of Maternal, Child and Adolescent Health, School of Public Health, Nanjing Medical University, Nanjing 211166, China. E-mail: zhixu_wang@163.com
bDepartment of Nutrition and Food Hygiene, School of Public Health, Peking University, Beijing 100871, China
First published on 4th October 2022
Abstract
Currently, commercial milk may contain abundant pregnancy-related hormones, the regular consumption of which puts children at a risk of precocious puberty and sex-hormone-associated tumors in adulthood. In this intervention trial, 51 healthy prepubescent children were randomly assigned to the intervention or control arms at a ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to receive 250 or 600 mL m−2 (body surface area) of milk intervention or matching equienergetic sugar water as the control. On testing cow's milk, progesterone was detected, while estrone, estradiol (E2), and testosterone (T2) were not. Cow's milk ingestion did not significantly influence the serum FSH, E2, PRL, LH, and T2 levels (P > 0.05) of pre-pubertal children 3 h after the intervention, while it increased their serum progesterone levels (P < 0.05) when compared with that in the control arm. Regarding the urinary hormone levels, cow's milk ingestion increased the urinary pregnanediol level within 4 h (P < 0.05), but not significantly when compared with that of the control (P > 0.05). The level of pregnanediol and E2 in the morning urine for three consecutive days showed no significant difference between the two arms (P > 0.05). Drinking commercial milk with progesterone influenced the progesterone levels of pre-pubertal children in hours but not days and did not affect other sex hormone levels of pre-pubertal children.
1 to receive 250 or 600 mL m−2 (body surface area) of milk intervention or matching equienergetic sugar water as the control. On testing cow's milk, progesterone was detected, while estrone, estradiol (E2), and testosterone (T2) were not. Cow's milk ingestion did not significantly influence the serum FSH, E2, PRL, LH, and T2 levels (P > 0.05) of pre-pubertal children 3 h after the intervention, while it increased their serum progesterone levels (P < 0.05) when compared with that in the control arm. Regarding the urinary hormone levels, cow's milk ingestion increased the urinary pregnanediol level within 4 h (P < 0.05), but not significantly when compared with that of the control (P > 0.05). The level of pregnanediol and E2 in the morning urine for three consecutive days showed no significant difference between the two arms (P > 0.05). Drinking commercial milk with progesterone influenced the progesterone levels of pre-pubertal children in hours but not days and did not affect other sex hormone levels of pre-pubertal children.
Introduction
Human beings began to drink cow's milk thousands of years ago. With the increasing insight into the complex nutrients present in milk (including calcium, vitamin D, proteins with high biological value, and other bioactive substances), its health benefits on growth and development, and the reduced risk of disease (such as dental caries, fractures, protein-deficiency malnutrition), milk consumption is increasingly encouraged in several countries and has become of immense importance in the human diet.1 However, the natural reproductive cycle severely restrains milk harvesting, as lactation follows birth, which suppresses a cow's ovulation and conception. This results in a long interval with no milk production. To accommodate the ever-increasing human demand for milk, substantial changes in cow's milk production practices were introduced in the 1960s and 1970s. Through genetic improvement and artificial insemination, dairy cows, such as the Holstein, Jersey,2 and Norwegian dairy herds,3 could be kept pregnant while lactating continuously. This extends the milk production time to more than 300 days a year,3,4 which in turn increases pregnancy-related sex hormone levels (such as estrogens and progesterone) in milk for human consumption. It was estimated that approximately 75–80% of commercially-available milk is obtained from pregnant lactating cattle,2,4 and these natural hormones are not affected by food processing, such as filtration, pasteurization, homogenization, and fermentation.2,5 In addition, the concentrations of estrogens and progesterone in whole milk are usually higher than those in semi-skimmed milk (for example, 9.65 ± 0.89 ng mL−1vs. 4.56 ± 0.48 ng mL−1 of total progesterone).6,7 Thus, there is ongoing interest in the status of pregnancy-related sex hormones in current commercial milk (especially in whole milk) and their impacts on consumers.Evidence from cohort studies worldwide and meta-analysis has supported the positive association between milk or dairy intake and hormone-sensitive prostate cancer,8,9 endometrial cancer,10 and breast cancer.11 The latest results of the prospective China Kadoorie Biobank study showed that dairy consumption is positively associated with risks of total cancer, liver cancer, and female breast cancer in Chinese adults with the adjusted hazard ratios per 50 g day−1 usual consumption being 1.07 (95% CI 1.04–1.10), 1.12 (1.02–1.22), and 1.19 (1.01–1.41), respectively.12 More importantly, a population-based cohort study in Iceland with a 24.3-year follow-up reported a positive correlation between milk consumption in adolescence and prostate cancer.13 The endogenous sex hormone levels are low in children,14 and pre-pubertal children's hypothalamus–pituitary–genital axis (HPG) is particularly sensitive to exogenous sex hormones.15 Early and precocious puberty may occur when children continuously ingest naturally occurring sex hormones from commercial food.16,17 Moreover, researchers have found that an earlier thelarche (the age when breast growth begins) of girls increased the risk of breast cancer,18 and sexual maturation of boys at a younger age has a positive correlation with a higher risk of prostate cancer.19 Meanwhile, out of great concern for children's health, precocious puberty conditions, especially those caused by food, often raise great concern in the society and among the public,20 including in China. Therefore, research is urgently needed to demonstrate the impact of milk consumption on pre-adolescent children.
However, to date, the impact of bovine milk and dairy products on the sex hormones of pre-pubertal children has undergone very little investigation. Only one study involving six Japanese pre-pubertal children reported almost three times increased concentrations of estrone (E1), estradiol (E2), estriol (E3), and pregnanediol (P2) (a metabolite of progesterone) in urine after milk intake.4 However, no control was set, no serum samples were obtained, and no hormonal information on the milk was reported in that study. Therefore, to contribute to the body of evidence, we conducted a randomized controlled intervention trial to evaluate the changes in six sex hormones in the serum and urine of healthy Chinese pre-pubertal children, after the ingestion of commercially available whole milk powder, which had been tested for its hormonal content. These sex hormones, including E2, progesterone, follicle-stimulating hormone (FSH), luteinizing hormone (LH), prolactin (PRL), testosterone, and P2, are commonly used in clinics to understand sexual development. In addition, we only observed short-term changes in serum and urinary hormone levels of pre-pubertal children for ethical considerations.
Materials and methods
Participants and study design
We conducted this randomized, two-level, controlled intervention trial in a primary school in Linyi City, Shandong Province, in May 2012. A total of 182 normal, pre-pubertal children aged 7–10 years (grade 1 to grade 3) were recruited from six school classes. After physical examination, including height, weight, blood pressure, and pulse, 52 of them were finally included in this study. The inclusion and exclusion criteria were as follows: (1) healthy children with parental informed consent and no clinically diagnosed precocious puberty or serious disease (leukemia, diabetes, heart disease, etc.) were included; and (2) children with a history of milk allergy or lactose intolerance were excluded.References to changes in hormone levels from drinking milk are rare. The sample size in this study was determined mainly based on a literature report in 2010.4 According to the urinary excretion volume of estrogen and P2 after milk intake in pre-pubertal children, P2, which had the smallest effect size, was used as the reference to calculate the sample size, using the formula: n = (Zα + Zβ)2 × 2σ2/δ2. In our study, we set α at 0.05 (Zα = 1.96) and β at 0.10 (Zβ = 1.28). By substituting δ = 2.5 μg and σ = 2.3 μg in the formula, it was determined that 18 individuals in each intervention group is enough to detect the smallest difference. The recruitment and implementation of intervention trials in children are difficult; both earlier and later milk-drinking trials used self-control (comparison before and after intervention).4,6 According to the statistical principle, when the sample sizes of the intervention and the control groups are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4–4
4–4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the test efficiency for the difference between groups will be the highest. Therefore, we assigned 52 participants to the control and intervention arms at a ratio of 1
1, the test efficiency for the difference between groups will be the highest. Therefore, we assigned 52 participants to the control and intervention arms at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
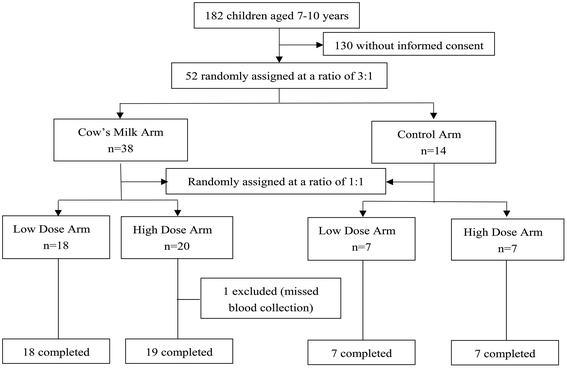
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. We met the sample size requirements simultaneously with 7 and 7 in the low-dose, high-dose sugar control arms and 18 and 20 in the low-dose, high-dose milk intervention arms. Finally, except for one child in the high-dose milk intervention group, who was excluded because no blood sample was available before the intervention, all pre-pubertal children completed the whole trial. The participant flowchart is shown in Fig. 1. Written informed consent was obtained from the legal guardians of all the children before participation. The trial was approved by the Ethics Committee of Nanjing Medical University and registered at https://www.chictr.org.cn/index.aspx (ChiCTR2000040525) (accessed on 1 December 2020) retrospectively.
3. We met the sample size requirements simultaneously with 7 and 7 in the low-dose, high-dose sugar control arms and 18 and 20 in the low-dose, high-dose milk intervention arms. Finally, except for one child in the high-dose milk intervention group, who was excluded because no blood sample was available before the intervention, all pre-pubertal children completed the whole trial. The participant flowchart is shown in Fig. 1. Written informed consent was obtained from the legal guardians of all the children before participation. The trial was approved by the Ethics Committee of Nanjing Medical University and registered at https://www.chictr.org.cn/index.aspx (ChiCTR2000040525) (accessed on 1 December 2020) retrospectively.
Samples for intervention and control
The test milk was a whole milk powder of a certain brand, randomly purchased from the market and diluted to form liquid milk by adding water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 before use. The nutrient contents of the milk powder were as follows: moisture, 3.0 g per 100 g; protein, 23.6 g per 100 g; fat, 28.1 g per 100 g; carbohydrate, 42.0 g per 100 g (see detailed information on the milk powder in the ESI Table 1†). Both earlier4 and latest6 studies on the changes in hormone levels after drinking milk were self-control study designs. To study the role of hormones in milk, we tried to find hormone-free commercial food with the same composition as milk as the control but failed due to its complexity. It has been reported in the literature that insulin secretion caused by food ingestion could interfere with the endocrine network.21 Therefore, to balance the influence of energy ingestion, after carefully considering the similar properties and eating methods to the intervention milk powder, and acceptability to the children, we used sugar as the control in this study. Sugar was also purchased from the same market, the main component of which is sucrose, with a purity of >99.0%.
6 before use. The nutrient contents of the milk powder were as follows: moisture, 3.0 g per 100 g; protein, 23.6 g per 100 g; fat, 28.1 g per 100 g; carbohydrate, 42.0 g per 100 g (see detailed information on the milk powder in the ESI Table 1†). Both earlier4 and latest6 studies on the changes in hormone levels after drinking milk were self-control study designs. To study the role of hormones in milk, we tried to find hormone-free commercial food with the same composition as milk as the control but failed due to its complexity. It has been reported in the literature that insulin secretion caused by food ingestion could interfere with the endocrine network.21 Therefore, to balance the influence of energy ingestion, after carefully considering the similar properties and eating methods to the intervention milk powder, and acceptability to the children, we used sugar as the control in this study. Sugar was also purchased from the same market, the main component of which is sucrose, with a purity of >99.0%.
Referring to the literature,4 the participants were given 250 or 600 mL m−2 (body surface area) milk for the low-dose or high-dose group, respectively, or the corresponding equienergetic sugar water. The body surface area (m2) of the children was calculated using the following formula: S = weight (kg) × 0.035 + 0.1 (weight < 30 kg); S = weight (kg) × 0.02 + 0.45 (weight > 30 kg). Ultimately, the milk intakes were 259.5 ± 35.4 and 636.7 ± 82.1 mL in the low-dose and high-dose groups, respectively. Furthermore, the sugar intakes for the equal energy sugar water control were 45.4 ± 6.9 g and 111.0 ± 17.3 g for the low-dose and high-dose groups, respectively.
Intervention process and quality control
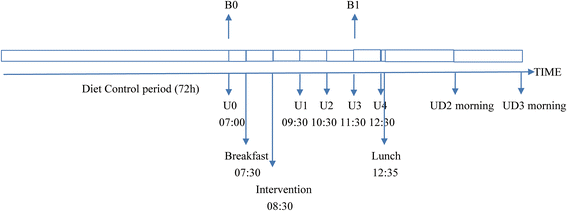
All participants were asked to abstain from all milk or dairy products for three days before the study and were forbidden from taking any medicines or supplements containing estrogens or progesterone. Due to ethical constraints, we did not forbid all foods that may contain hormones or their analogs, including almost all animal foods (meat, poultry, eggs, fish, seafood, animal fats, etc.) and soybean and its products, before the intervention. The children arrived at school in the early morning of the trial day after an overnight fast. Fasting venous blood and urine samples were collected between 7:00 am, and 8:00 am, followed by a uniform breakfast. The pre-pubertal children were then asked to drink the test milk or sugar water within 10 min at 1 h after breakfast to minimize the effect of eating on sex hormone levels. Venous blood samples were collected 3 h after drinking the milk or sugar water, and urine samples were collected at 1 h, 2 h, 3 h, and 4 h, respectively (see detailed information in Fig. 2). All the children drank the designated volume of milk or sugar water. The time difference in milk or sugar water drinking between all pre-pubertal children was no more than half an hour. Children were permitted to drink water and move freely during the trial but not eat. A staff member accompanied every 6–7 children to perform some light activities or reading during their idle time to complete the entire trial process with ease and pleasure. Morning urine samples of the children on the second and third days were collected between 7:00 am, and 8:00 am under the same dietary and medicine restrictions as before the intervention.A qualified doctor was responsible for the blood and urine collection and supervision of the entire intervention process. One child consuming the low-dose sugar and one in low-dose milk arm each passed no urine by 3 h after the consumption. Blood and urine samples at each time point (except the above two urine samples) of 51 children were collected without omission, and also no adverse events were reported. Trained volunteers recorded the time of each blood and urine sample collection, the breakfast time for each child, obtained the urine samples, and recorded the volume. The samples were labeled in a timely manner, it was checked that they were from the same child, and they were stored at 4 °C. In the end, blood samples in 5 mL serum separator tubes (BD Vacutainer; Becton Dickinson) were transported to the laboratory and centrifuged at 1500g for 10 min at 4 °C to obtain serum samples for sex hormone tests. Both the serums in the Eppendorf tubes and urine samples in polyethylene plastic containers were frozen at −70 °C until analysis.
Of note, in order to prevent gastrointestinal discomfort caused by milk consumption and meet their essential energy requirements, we prepared a simple breakfast for the pre-pubertal children, which was composed of staple foods, vegetables, and fruits, excluding animal foods (meat, poultry, eggs, fish, seafood, animal fats, etc.) and soybean and its products. The breakfast included 100 g of steamed buns, 30 g of pickled mustard, and 50 g of cherry tomatoes, which provided 12.9% of the energy, 89.1% of the carbohydrate, and 18.1% of the protein in the daily requirements for children in this age group (see detailed information in the ESI Table 2†). Most of the students ate all the food, and individual children left a small amount of steamed buns (not recorded). After the whole trial, a free hearty lunch was arranged to replenish the nutrients that the children needed.
Measurement of sex steroid hormones (E1, E2, progesterone, and testosterone) in the studied milk by liquid chromatography–mass spectrometry (LC-MS)
E1 is the main form of estrogen in milk, followed by E2.15 The major sex hormones (E1, E2, progesterone, and testosterone) of cow's milk were detected using LC-MS, which is a standardized method to detect steroid hormones in food, including milk.22 Sex steroid hormones exist in both conjugated and unconjugated forms in milk. According to the National Standards of the People's Republic of China (GB/T21981-2008),22 to determine the total concentration of steroid hormones in animal foods, milk samples were spiked with β-glucuronidase/arylsulfatase first and incubated at 37 °C and pH = 2.5 for 12 h for enzymatic hydrolysis. Then, the hormones were extracted twice using methanol (Merck, Darmstadt, Germany). Internal standards, including progesterone-d9, estradiol-3,4-13C2, estrone-2,4-d2, and testosterone-3,4-13C2 (CDN Isotopes, Pointe-Claire, QC, Canada), were used to determine the extraction efficiency. After extraction, the upper layer was purified using ENVI-Carb SPE cartridges and eluted with dichloro-methane–methanol (7/3, v/v), evaporated to dryness with nitrogen, and then redissolved in 1 mL methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1/1, v/v) for detection via LC-MS by a third-party testing company (SGS China, Tongbiao Standard Technical Service Co. Ltd, Shanghai, China), as described previously.22 The method had an intra-assay coefficient of variation (CV) of 5%. The limits of quantification (LOQ) of the analytes were 2, 5, 2, and 2 μg kg−1 for E1, E2, progesterone, and testosterone, respectively. The final detected progesterone concentration in the whole milk powder was 40.6 μg kg−1; E1, E2, and testosterone were undetected.
water (1/1, v/v) for detection via LC-MS by a third-party testing company (SGS China, Tongbiao Standard Technical Service Co. Ltd, Shanghai, China), as described previously.22 The method had an intra-assay coefficient of variation (CV) of 5%. The limits of quantification (LOQ) of the analytes were 2, 5, 2, and 2 μg kg−1 for E1, E2, progesterone, and testosterone, respectively. The final detected progesterone concentration in the whole milk powder was 40.6 μg kg−1; E1, E2, and testosterone were undetected.
Measurement of the sex hormones in the serum and urine samples by enzyme-linked immunosorbent assay (ELISA)
Both LC-MS and ELISA analyses are reliable and approved methods for assessing sex hormone levels in human body fluids.6 According to the literature, pre-pubescent children have extremely low endogenous levels of hormones. For example, their 17β-E2 concentration is <2 pg mL−1.23 ELISA has a high reproducibility with an optimal recovery and has great advantages of simple operation, low cost, and low LOQ. Thus, it is better suited for automated platforms and population studies,24 and has been widely used25–27 in clinical testing and scientific research; it is particularly suitable for detecting low hormone levels. Therefore, we used a quantitative test kit based on solid-phase ELISA to measure the concentrations of sex hormones in the serum and urine samples. The kit was purchased from Nanjing Jiancheng Bioengineering Inc. (Nanjing, China). The minimum detection concentrations of E2, progesterone, FSH, LH, PRL, testosterone, and P2 in this study were lower than 1.0 pmol L−1, 0.1 nmol L−1, 0.1 mIU mL−1, 1.0 mIU mL−1, 1.0 mIU L−1, 0.1 nmol L−1, and 0.1 μmol L−1, respectively. The intra-plate and inter-plate CVs were <10% and <13%, respectively, which indicated that the reproducibility of the kit was acceptable. The response linearity (correlation coefficient, R2) of this study was not less than 0.99 for all analytes. A standardized protocol was adopted for the testing process. All samples from each time point were ordered randomly and with a standard curve in each panel to ensure the reliability of the comparison results.Statistical analysis
Normally distributed continuous variables were expressed as the mean and standard deviation (SD), while non-normally distributed data were expressed as the median and interquartile ranges (IQR) (25th percentile, 75th percentile) A paired t-test was used to compare pre- and post-intervention differences in each arm. A general linear model without a random intercept term was adopted to analyze the fluctuation of hormones before and after milk or sugar consumption (low dose and high dose for both). The difference in the repeated measurements was taken as the dependent variable, which provided estimates of the mean difference induced by the milk or sugar intervention. A general linear model with a random intercept term was used to evaluate the effect of the milk intervention relative to that of the control sugar. The general linear model for repeated measurements with random intercept terms was used to summarize the overall change of E2 and P2 in urine within 4 hours and three days in each arm, and to compare the difference between the milk and control sugar intervention groups. Bonferroni correction was used in pairwise comparisons. All statistical analyses were performed using the IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA). Statistical significance was set at P < 0.05.Results
The characteristics of the participants in this study are provided in Table 1. Overall, 51/52 (98.07%) pre-pubertal children completed the whole trial. The age of the intervention population was 8.4 ± 1.1 years with a body mass index (BMI) of 17.7 kg m−2 (IQR: 14.9, 20.5); 22 (43.1%) of the study participants were boys. There were no significant differences in age, sex, and body weight among the four arms (low-dose control, low-dose cow's milk, high-dose control, and high-dose cow's milk) (P > 0.05), which means that they were comparable.| Characteristics | Screening (n = 131) | Intervention (n = 51) | Trial participants | P | |||
|---|---|---|---|---|---|---|---|
| Low-dose arm | High-dose arm | ||||||
| Control sugar (n = 7) | Cow's milk (n = 18) | Control sugar (n = 7) | Cow's milk (n = 19) | ||||
| a The data distribution that satisfies the normal distribution is expressed as the mean ± SD; otherwise, it is expressed as the median (IQR). | |||||||
| Boys, n (%) | 39 (29.8) | 22 (43.1) | 3 (42.9) | 8 (44.4) | 4 (57.1) | 7 (36.8) | 0.604 |
| Age, years | 8.6 ± 1.1 | 8.4 ± 1.1 | 8.3 ± 1.0 | 8.1 ± 1.1 | 8.4 ± 1.0 | 8.7 ± 1.2 | 0.826 |
| Height, cm | 125.0 (123.0, 132.0) | 128.0 (120.4, 135.6) | 125.6 (120.5, 130.7) | 126.9 (119.4, 134.4) | 129.9 (119.9, 139.9) | 129.1 (121.4, 136.8) | 0.567 |
| Weight, kg | 25.1 (21.9, 29.4) | 29.2 (22.3, 36.1) | 29.3 (21.3, 37.3) | 28.8 (22.1, 35.5) | 30.7 (22.0, 39.4) | 29.0 (22.5, 35.5) | 0.96 |
| BMI, kg m−2 | 15.6 (14.6, 17.5) | 17.7 (14.9, 20.5) | 18.4 (14.4, 22.4) | 17.7 (15.1, 20.3) | 17.8 (15.4, 20.2) | 17.3 (14.6, 20.0) | 0.887 |
Changes in the serum hormone levels
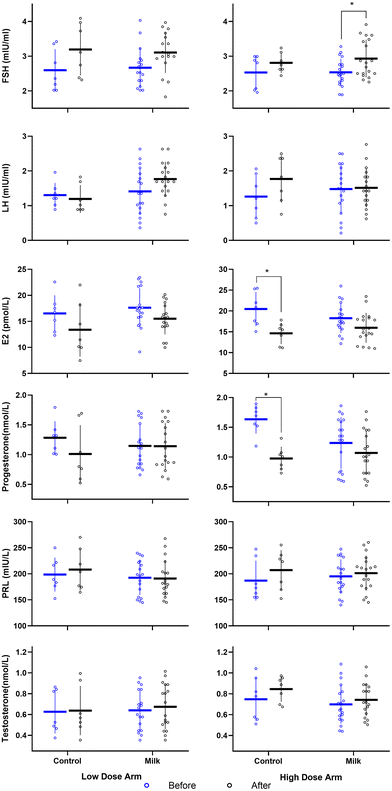
A graphical presentation of the serum concentrations of the sex hormones before and 3 h after of intervention in the four arms is shown in Fig. 3. The shift resulting from the milk or control sugar water intervention appears to be insignificant in the low-dose arm for E2, progesterone, FSH, LH, PRL, and testosterone levels in the serum of pre-pubertal children (P > 0.05). In the high-dose arm, the increase in FSH (P = 0.021) in the milk intervention arm and the decrease in E2 (P = 0.045) and progesterone (P = 0.003) in the control sugar water arm were statistically significant.The results of the corresponding general linear model are presented in Table 2. The beta values show the mean difference in the hormone concentrations between the baseline and after the intervention, and of the change between the milk and control sugar arms. After cow's milk intake, the level of FSH increased by 0.41 mIU mL−1 (95% CI: 0.15, 0.68; P = 0.003) and the level of E2 decreased by 2.20 pmol L−1 (95% CI, −4.05, −0.35; P = 0.021) from the baseline. Meanwhile after drinking sugar water, the FSH level increased by 0.44 mIU ml−1 (95% CI: 0.01, 0.86; P = 0.044), and E2 decreased by 4.49 pmol L−1 (95% CI, −7.50, −1.48; P = 0.004). There was no significant difference in the changes in FSH and E2 between the two groups (P < 0.05). For progesterone, the concentration in the serum of the control arm decreased from 1.46 nmol L−1 before the intervention to 0.99 nmol L−1 after the intervention, i.e. it decreased by 0.47 nmol L−1 (95% CI, −0.78, −0.15; P = 0.005), while that in the milk intervention arm showed no significant change, from 1.19 nmol L−1 before the intervention to 1.10 nmol L−1 after the intervention. Compared with the sugar water control, the milk intervention significantly increased the progesterone levels in the serum samples by 0.38 nmol L−1 (95% CI: 0.01, 0.75; P = 0.047).
| Control sugar (after vs. before) | Cow's milk (after vs. before) | Cow's milk vs. control sugar | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baselinea | β (95% CI)d | P | Baselineb | β (95% CI)d | P | Baselinec | β (95% CI)e | P | |
| FSH, follicle-stimulating hormone; LH, luteinizing hormone; E2, estradiol; PRL, pituitary prolactin.a Baseline values represent the average levels of serum hormone before taking sugar water.b Baseline values represent the average levels of serum hormone before taking cow's milk.c Baseline values represent the mean differences in serum hormones before and after intervention in the control arm.d The beta value indicates the difference between the study population's mean value for hormone level post-intervention and the study population's mean value at the baseline. A general linear model was used, with repeated measurements considered without a random intercept term.e The beta value indicates the difference between the study population's mean difference after milk consumption and the study population's mean difference after control sugar water consumption. A general linear model was used, with repeated measurements considered by a random intercept term. | |||||||||
| FSH (mIU ml−1) | 2.56 | 0.44 (0.01, 0.86) | 0.044 | 2.60 | 0.41 (0.15, 0.68) | 0.003 | 0.44 | −0.02 (−0.53, 0.48) | 0.926 |
| LH (mIU ml−1) | 1.28 | 0.20 (−0.29, 0.68) | 0.413 | 1.44 | 0.20 (−0.11, 0.50) | 0.199 | 0.20 | −0.003 (−0.57, 0.57) | 0.991 |
| E2 (pmol L−1) | 18.50 | −4.49 (−7.50, −1.48) | 0.004 | 17.94 | −2.20 (−4.05, −0.35) | 0.021 | −4.49 | 2.29 (−1.25, 5.82) | 0.200 |
| Progesterone (nmol L−1) | 1.46 | −0.47 (−0.78, −0.15) | 0.005 | 1.19 | −0.09 (−0.28, 0.11) | 0.365 | −0.47 | 0.38 (0.01, 0.75) | 0.047 |
| PRL (mIU L−1) | 192.73 | 14.81 (−11.23, 40.84) | 0.259 | 193.76 | 2.48 (−13.53, 18.50) | 0.757 | 14.81 | −12.32 (−42.89, 18.24) | 0.422 |
| Testosterone (nmol L−1) | 0.69 | 0.05 (−0.08, 0.19) | 0.410 | 0.67 | 0.04 (−0.04, 0.12) | 0.341 | 0.05 | −0.16 (−0.17, 0.14) | 0.842 |
We further analyzed the changes in serum progesterone in the children of two groups after the intervention. Among the 14 children with the sugar water intervention, 10 (71.5%) exhibited a decrease in the progesterone level (from 1.58 nmol L−1 before to 0.82 nmol L−1 after the intervention, with 0.76 nmol L−1 difference), 1 (7.1%) had no change (with less than 5% change), and 3 (21.4%) had a progesterone increase (from 1.20 nmol L−1 before to 1.56 nmol L−1 after the intervention, with 0.35 nmol L−1 difference). And among 37 children in the milk intervention group, 16 (43.2%) exhibited a decrease in the progesterone level (from 1.53 nmol L−1 before to 0.87 nmol L−1 after the intervention, with 0.66 nmol L−1 difference), 5 (13.5%) exhibited no change (with less than 5% change), and 16 (43.2%) exhibited an increase in the progesterone level (from 0.85 nmol L−1 before to 1.31 nmol L−1 after intervention, with 0.46 nmol L−1 difference). Compared with the sugar water control group, the proportion of the children with a decreased progesterone level was lower, and that with an increased progesterone level was higher after the commercial cow's milk intervention.
Change in urinary P2 and E2 levels in 4 hours
Except for one child in the low-dose sugar arm and one of the low-dose milk arms (with a missing urine sample), a total of 49 participants were included in the analysis of change in urinary P2 and E2 levels in 4 hours. Table 3 shows the concentrations of E2 and P2 in urine before and 1, 2, 3, and 4 h after the milk or control sugar intervention. Neither P2 levels (P = 0.323) nor E2 levels (P = 0.056) in urine, 1–4 h after the ingestion of the control sugar, changed significantly. In contrast, the P2 level in urine 1–4 h after the ingestion of milk increased significantly (P < 0.001), while the E2 level did not (P = 0.081) (Table 3). Further pairwise comparison showed that, 1, 2, 3, and 4 h after the ingestion of the milk, the P2 level increased by 0.50 (95% CI, 0.04, 0.95), 0.99 (95% CI, 0.56, 1.42), 0.57 (95% CI, 0.24, 0.90), and 0.97 (95% CI, 0.63, 1.32) ng mg−1 respectively. However, compared with the control arm, the change in the P2 level in urine after the milk intervention was not significant (P = 0.128).| P2 (ng mg−1 crea) | E2 (ng mg−1 crea) | |||||
|---|---|---|---|---|---|---|
| Control sugar n = 12 | Cow's milk n = 37 | Cow's milk vs. control sugar β (95% CI) | Control sugar n = 12 | Cow's milk n = 37 | Cow's milk vs. control sugar β (95% CI) | |
| Hormone levels in urine samples were expressed as the median (IQR). P2, pregnanediol; E2, estradiol.a Compared with the level of urine before intervention, the difference was significant. | ||||||
| 0 h | 1.26 (0.88, 1.61) | 1.13 (0.92, 1.35) | 34.75 (25.91, 40.17) | 32.00 (27.27, 36.73) | ||
| 1 h | 1.59 (0.94, 2.41) | 1.52 (1.19, 2.14)a | 36.57 (34.08, 40.88) | 34.68 (31.73, 38.62) | ||
| 2 h | 1.74 (1.26, 2.45) | 2.17 (1.90, 2.48)a | 0.166 (−0.05, 0.38) | 27.72 (20.07, 32.41) | 33.70 (30.01, 37.71) | 0.067 (−2.35, 2.49) |
| 3 h | 1.78 (1.24, 2.33) | 1.71 (1.41, 2.02)a | 31.16 (26.35, 35.96) | 37.18 (34.18, 40.18) | ||
| 4 h | 1.29 (0.97, 1.74) | 2.18 (1.94, 2.52)a | 37.55 (31.65, 42.19) | 31.58 (25.23, 36.08) | ||
| P | 0.323 | <0.001 | 0.128 | 0.056 | 0.081 | 0.956 |
Change in urinary P2 and E2 levels in 3 days
Data from the above 49 participants were included in the analysis of change in urinary P2 and E2 levels in 3 days. Table 4 shows the concentrations of E2 and P2 in the morning urine before and 24 and 48 h after the milk or control sugar intervention. The levels of P2 and E2 in the morning urine for three consecutive days in both the milk intervention and sugar control arms showed no significant change in each arm and no difference between the two arms was observed (P > 0.05) (Table 4).| P2 (ng mg−1 crea) | E2 (ng mg−1 crea) | |||||
|---|---|---|---|---|---|---|
| Control sugar n = 12 | Cow's milk n = 37 | Cow's milk vs. control sugar β (95% CI) | Control sugar n = 12 | Cow's milk n = 37 | Cow's milk vs. control sugar β (95% CI) | |
| Hormone levels in urine samples are expressed as the median (IQR). P2, pregnanediol; E2, estradiol. | ||||||
| 0 h | 1.26 (0.88, 1.61) | 1.13 (0.92, 1.35) | 34.75 (25.91, 40.17) | 32.00 (27.27, 36.73) | ||
| 24 h | 0.90 (0.57, 1.43) | 1.06 (0.66, 1.49) | −0.02 (−0.18, 0.14) | 33.24 (29.53, 40.43) | 35.64 (29.90, 40.55) | −0.14 (−3.78, 3.49) |
| 48 h | 1.06 (0.69, 1.50) | 0.95 (0.58, 1.38) | 34.06 (30.84, 39.85) | 34.47 (27.80, 42.37) | ||
| P | 0.417 | 0.304 | 0.793 | 0.661 | 0.258 | 0.937 |
Discussion
To the best of our knowledge, this is the first study to evaluate the changes in six sexual development-related hormone levels in the serum and urine samples of pre-pubertal children after ingesting milk with tested hormone content. In this randomized controlled intervention study, after consuming commercial milk with progesterone, the progesterone concentration in the serum samples after 3 h and urine samples after 4 h increased, while it did not significantly change in the levels of P2 in the morning urine for three consecutive days. There were no significant differences between the milk intervention and control arms for changes in FSH, LH, E2, PRL, and testosterone levels in serum samples and E2 in urine samples.Before evaluating the changes in sex hormone levels caused by milk consumption, it is necessary to discuss their milk contents. Estrogen and progesterone have been used in pregnancy diagnosis in cows since the 1990s and were found to increase with the increase over pregnancy months. For example, the plasma E1 concentrations in the first, second, and third trimesters reported by Pape-Zambito et al. were 0.8, 16.9, and 41.8 pg mL−1, respectively.28 It was estimated that the concentrations of hormones in commercially used processed milk were between those of a first- and a second-trimester cow.29 Earlier studies focused on estrogen in the milk. The reported mean range of total estrogen concentration in whole milk was 187 ng L−1 to 450 μg L−1 in Spain,30 the Netherlands,31 and France,32 whereas the concentration ranges of E1 and E2 were 6.2–1266 ng L−1 and 12.8–373 ng L−1, respectively. The earlier the report, the higher the estrogen content;29 notably, the levels of progesterone and other sex hormones in milk were not mentioned in all of the above studies. However, more comprehensive hormone information in commercial milk has been reported in recent years.6,33 Michels et al.6 measured progesterone (9.65 ± 0.89 ng mL−1), E1 (148.9 ± 36.7 pg mL−1), E2 (45.1 ± 5.5 pg mL−1), and testosterone (127.2 ± 13.3 pg mL−1) levels in whole milk, which is widely consumed in Germany. Qu et al. in 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33 measured the concentrations of 18 sex steroid hormones in raw milk in Tangshan City, Hebei Province, China, by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). They found progesterone in 85.9% of 195 samples, with a mean value of 5.12 μg kg−1 (2.12–9.04 μg kg−1); other sex hormones, including estrogen, progesterone and testosterone, were undetected.33 The absolute hormone contents depend on the source of milk and the detection methods used, including radioimmunoassay (RIA),4 ELISA,25 LC-MS,5 and gas chromatography–mass spectrometry (GC-MS).34 Meanwhile, assessing sex hormone levels in milk is still very challenging, because part of E2, progesterone, and testosterone combines with protein. After de-conjugation, using the LC-MS method, we detected the total contents of hormones in milk (including conjugated and unconjugated forms), and found that the progesterone concentration in our tested milk was 40.6 μg kg−1 (equivalent to 5.8 ng mL−1 in liquid milk), similar to the range in German (8.74–10.54 ng mL−1) and Chinese (N.D. to 9.4 ng mL−1) milk aforementioned,6,33 and the estrogen (E1 and E2) and testosterone levels were not detected, which was lower than those in the German milk6 and consistent with the results reported by Qu et al. for Chinese milk.33 Among the milk powders purchased from the same market, the content of progesterone in the test milk was typical (ESI Table 3†).
33 measured the concentrations of 18 sex steroid hormones in raw milk in Tangshan City, Hebei Province, China, by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS). They found progesterone in 85.9% of 195 samples, with a mean value of 5.12 μg kg−1 (2.12–9.04 μg kg−1); other sex hormones, including estrogen, progesterone and testosterone, were undetected.33 The absolute hormone contents depend on the source of milk and the detection methods used, including radioimmunoassay (RIA),4 ELISA,25 LC-MS,5 and gas chromatography–mass spectrometry (GC-MS).34 Meanwhile, assessing sex hormone levels in milk is still very challenging, because part of E2, progesterone, and testosterone combines with protein. After de-conjugation, using the LC-MS method, we detected the total contents of hormones in milk (including conjugated and unconjugated forms), and found that the progesterone concentration in our tested milk was 40.6 μg kg−1 (equivalent to 5.8 ng mL−1 in liquid milk), similar to the range in German (8.74–10.54 ng mL−1) and Chinese (N.D. to 9.4 ng mL−1) milk aforementioned,6,33 and the estrogen (E1 and E2) and testosterone levels were not detected, which was lower than those in the German milk6 and consistent with the results reported by Qu et al. for Chinese milk.33 Among the milk powders purchased from the same market, the content of progesterone in the test milk was typical (ESI Table 3†).
Up to now, only one study with six participants4 has discussed the profile of sex hormone levels in children after commercial milk consumption. Only urine samples were obtained; the urinary concentration/excretion of E1, E2, E3, and P2 within 4 h was significantly increased. The same intervention in 7 men showed that apart from the same change in urine as in pre-pubertal children, the concentrations of estrogen and progesterone in the serum significantly increased from 102.3 to 128.9 pg mL−1 and from 0.66 to 0.75 ng mL−1 within 2 h, respectively. However, the concentration of E2 did not change significantly (from 31 to 32 pg mL−1).4 According to the literature, the metabolic time for progesterone in the body was approximately 3 h,35 and the concentration of estrogen and progesterone in the urine reached a peak at 1–3 h after milk intake.4 In the present study, the progesterone level in serum 3 h after the cow's milk intervention significantly increased compared to the control, and that in urine samples at 4 h also increased, but was not significant when compared with the control. Our results are consistent with those reported in literatures. Progesterone levels in milk increased with an increase in the fat content. Goodson reported that the progesterone levels in saliva increased by 30–100% after the consumption of high-fat dairy products composed of butter, cheese, and ice cream in 17 healthy males.36 Different biological samples and progesterone contents in food may explain the changes in the progesterone levels after food ingestion. Furthermore, indicators, including progesterone, showed a greater variation in saliva or urine than in serum.37 Therefore, larger sample sizes are needed to observe significant changes in progesterone in urine, and information from multiple samples may be conducive to obtaining more reliable conclusions.
Not only P2 levels in the 4-hour urine samples, but also those in the morning urine for three consecutive days were observed in the present study. Our results showed no significant difference between the two groups, which was supported by Michael's research, showing that the intake of whole milk with approximately 10 ng mL−1 progesterone for four days had no significant effects on urinary P2 glucuronide excretion. Presently, it is generally believed that the bioavailability of oral progesterone is low and it can be quickly excreted in urine.38–40 Therefore, a single intake will not affect the level of progesterone in 24 h and 48 h urine samples.
Human hormone levels fluctuate daily with great intra- and inter-day variation under the influence of many factors, including diet. Research shows that the ingestion of foods induces insulin secretion to interfere with the endocrine hormones.21 In the present study, we observed that the serum progesterone level decreased 3 h after the control sugar intervention, which not only happened after the sugar ingestion, but also after an equienergetic cereal-based diet intervention in the supplement trial (ESI Table 4†). Moreover, in a rat model, lactose treatment also decreased the progesterone level.41 Therefore, the lack of a significant change in serum progesterone levels after the milk ingestion may be an offset between the intake of progesterone through milk and a decrease caused by the food/milk ingestion. Further studies are needed to confirm this hypothesis. Moreover, natural hormones contained in other foods also may obscure the change in the progesterone level induced by milk. Due to ethical constraints, we avoided providing all animal foods and soybeans and their products on the intervention day, while we only forbade milk or dairy products on the following two days. Taken together, commercial milk ingestion changed the progesterone level of pre-pubertal children within a few hours under the conditions of fasting while not influencing those on days with a normal diet. The WHO established that the acceptable daily intake (ADI) of progesterone is 30 μg per kg body weight on account of the lowest observed adverse effect level (LOAEL) on changes in the uterus.42 Accordingly, progesterone ingested through commercial milk is supposed to be acceptable to the human body.
Regarding estrogen, in our study, the concentration of E2 in both the serum and urine did not increase significantly compared with the control group, which was consistent with Michaels’ research.6 In the two previous studies changes in the estrogen levels were observed after short-term (a few hours or days) milk consumption, reporting a significant elevation in E1, but this was not included in our measurement. However, the non-detection of E1 and E2 in our test milk was considered the main reason for the insignificant difference in the change in E2 between our two groups. Furthermore, fewer studies have reported the short-term variation of other sex hormones (including FSH, LH, PRL, and testosterone) after the ingestion of milk. In our study, no significant changes in the serum LH, PRL, and testosterone after the milk ingestion were observed, which is in line with the results of another long-term observational study in healthy premenopausal women.43
In addition, we observed an elevation of serum FSH in milk intervention groups, whereas the E2 level in the serum was decreased after the milk ingestion in the present study. Normally, the decrease in E2 will feed back on the increase in the FSH level. This reverse correlation between changes in the estrogen and FSH after milk intake also existed in six Japanese men.4 Furthermore, in a population study it was observed that every additional serving of dairy food intake was associated with a ∼5% reduction in serum E2 concentration after adjusting for all covariates, including other hormones,43 which supported the decline of the serum E2 level in our study. Moreover, the ingestion of sugar water in our study also decreased the concentrations of E2 and increased the concentration of FSH in serum significantly among pre-pubertal children. Sugar can be digested into glucose and fructose. Our observations are in line with the results of the intervention with monosaccharides (glucose, fructose, and galactose) in rats41,44 and sheep.21 In research on a rat model it was found that the concentration of E2 was lowered and those of FSH and LH were elevated after rats were fed galactose, a breakdown component of lactose.45 Analyzing all the outcomes from the above studies, we speculated that the decline of E2 and the elevation of FSH in the serum in our study 3 h after the milk intervention are not related to the hormones in milk, but due to the effect of monosaccharides or dietary intake. The possible underlying mechanism of the sex hormone changes in the aforementioned studies was attributed to the interference of insulin secretion with the endocrine system induced by monosaccharide stimulation.21
This work marks the beginning of feeding experiments conducted in China. For the first time, the impact of commercially available milk consumption on sex hormones of pre-pubertal children in both serum and urine was explored, and the hormone contents of milk were detected simultaneously. Only progesterone was found with a content of 40.6 μg kg−1 (equivalent to 5.8 ng mL−1 in liquid milk), similar to values previously reported,6,33 while other hormones were not detected. It is unclear whether cattle feeding practices have changed or new technology has been used to remove the estrogen in milk. Our results proved that there is no significant impact on short-term hormone levels in pre-pubertal children after the ingestion of this milk, which provides important proof and significance for the safety of children drinking commercial milk.
This study has some limitations. First, estrogen, progesterone, and their metabolites take many forms in body fluids. We only monitored the main bioactive forms and main metabolites of them; therefore this study could not comprehensively reflect the changes in hormone profiles induced by milk consumption. Second, the selection of controls for milk intervention studies is challenging. Different control samples may affect the interpretation of the results. A mixture of multiple commercial milk powders is probably a better choice. Third, although the intervention trial in pre-adolescent children is not easy, a larger sample size, multiple time points, and long-term observation are still needed to confirm the change in hormones in body fluids caused by drinking milk and its possible effect on pre-pubertal children. Also, the adoption of a cross-over design would be better.
Conclusions
In summary, in this two-level, randomized controlled intervention trial, we found that drinking commercial milk containing progesterone had no significant influence on the hormone levels of pre-pubertal children over days. In addition, further attention also needs to be drawn to the long-term effect of consuming progesterone-containing commercial milk on the sex hormone levels and sexual development of pre-pubertal children.Author contributions
Conceptualization and supervision of the trial, Z. X. Wang and J. S. Wu; investigation and project administration, M. Zhang, R. Qin, M. L. Hu; formal analysis, validation and visualization, X. Shi and X. L. Lu; methodology, data curation, software and writing – original draft, J. S. Wu and X. Shi; writing – review & editing, resource and funding acquisition, Z. X. Wang. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by grants from the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (Public Health and Preventive Medicine).References
- A. Haug, A. T. Hostmark and O. M. Harstad, Bovine milk in human nutrition—A review, Lipids Health Dis., 2007, 6, 25 CrossRef PubMed.
- L. Qin, P. Wang, T. Kaneko, K. Hoshi and A. Sato, Estrogen: One of the risk factors in milk for prostate cancer, Med. Hypotheses, 2004, 62, 133–142 CrossRef CAS PubMed.
- K. S. Storli, G. Klemetsdal, H. Volden and R. Salte, The relationship between Norwegian Red heifer growth and their first-lactation test-day milk yield: A field study, J. Dairy Sci., 2017, 100, 7602–7612 CrossRef CAS PubMed.
- K. Maruyama, T. Oshima and K. Ohyama, Exposure to exogenous estrogen through intake of commercial milk produced from pregnant cows, Pediatr. Int., 2010, 52, 33–38 CrossRef CAS PubMed.
- D. W. Farlow, X. Xu and T. D. Veenstra, Quantitative measurement of endogenous estrogen metabolites, risk-factors for development of breast cancer, in commercial milk products by LC-MS/MS, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2009, 877, 1327–1334 CrossRef CAS PubMed.
- K. B. Michels, N. Binder, F. Courant, A. A. Franke and A. Osterhues, Urinary excretion of sex steroid hormone metabolites after consumption of cow milk: A randomized crossover intervention trial, Am. J. Clin. Nutr., 2019, 109, 402–410 CrossRef PubMed.
- A. R. Gilman, W. Buckett, W. Y. Son, J. Lefebvre, A. M. Mahfoudh and M. H. Dahan, The relationship between fat and progesterone, estradiol, and chorionic gonadotropin levels in Quebec cow's milk, J. Assist. Reprod. Genet., 2017, 34, 1567–1569 CrossRef CAS PubMed.
- D. Aune, D. A. N. Rosenblatt, D. S. M. Chan, A. R. Vieira, R. Vieira, D. C. Greenwood, L. J. Vatten and T. Norat, Dairy products, calcium, and prostate cancer risk: A systematic review and meta-analysis of cohort studies, Am. J. Clin. Nutr., 2015, 101, 87–117 CrossRef CAS PubMed.
- L.-Q. Qin, H.-Y. Xu, P.-Y. Wang, H. Tong and K. Hoshi, Milk consumption is a risk factor for prostate cancer in Western countries: Evidence from cohort studies, Asia Pac. J. Clin. Nutr., 2007, 16, 467–476 Search PubMed.
- D. Ganmaa, X. Cui, D. Feskanich, S. E. Hankinson and W. C. Willett, Milk, dairy intake and risk of endometrial cancer: A 26-year follow-up, Int. J. Cancer, 2012, 130, 2664–2671 CrossRef CAS PubMed.
- E. Y. Cho, D. Spiegelinan, D. J. Hunter, W. Y. Chen, M. J. Stampfer, G. A. Colditz and W. C. Willett, Premenopausal fat intake and risk of breast cancer, J. Natl. Cancer Inst., 2003, 95, 1079–1085 CrossRef PubMed.
- M. G. Kakkoura, H. Du, Y. Guo, C. Yu, L. Yang, P. Pei, Y. Chen, S. Sansome, W. C. Chan, X. Yang, L. Fan, J. Lv, J. Chen, L. Li, T. J. Key, Z. Chen and G. China Kadoorie Biobank Collaborative, Dairy consumption and risks of total and site-specific cancers in Chinese adults: An 11-year prospective study of 0.5 million people, BMC Med., 2022, 20, 134 CrossRef PubMed.
- J. E. Torfadottir, L. Steingrimsdottir, L. Mucci, T. Aspelund, J. L. Kasperzyk, O. Olafsson, K. Fall, L. Tryggvadottir, T. B. Harris, L. Launer, E. Jonsson, H. Tulinius, M. Stampfer, H. O. Adami, V. Gudnason and U. A. Valdimarsdottir, Milk intake in early life and risk of advanced prostate cancer, Am. J. Epidemiol., 2012, 175, 144–153 CrossRef PubMed.
- E. I. Felner and P. C. White, Prepubertal gynecomastia: Indirect exposure to estrogen cream, Pediatrics, 2000, 105, E55 CrossRef CAS PubMed.
- L. Aksglaede, A. Juul, H. Leffers, N. E. Skakkebaek and A. M. Andersson, The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens, Hum. Reprod. Update, 2006, 12, 341–349 CrossRef CAS PubMed.
- E. A. Eugster, Update on precocious puberty in girls, J. Pediatr. Adolesc. Gynecol., 2019, 32, 455–459 CrossRef PubMed.
- C. J. Partsch and W. G. Sippell, Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens, Hum. Reprod. Update, 2001, 7, 292–302 CrossRef CAS PubMed.
- D. H. Bodicoat, M. J. Schoemaker, M. E. Jones, E. McFadden, J. Griffin, A. Ashworth and A. J. Swerdlow, Timing of pubertal stages and breast cancer risk: the Breakthrough Generations Study, Breast Cancer Res., 2014, 16, R18 CrossRef PubMed.
- C. Bonilla, S. J. Lewis, R. M. Martin, J. L. Donovan, F. C. Hamdy, D. E. Neal, R. Eeles, D. Easton, Z. Kote-Jarai, A. A. Al Olama, S. Benlloch, K. Muir, G. G. Giles, F. Wiklund, H. Gronberg, C. A. Haiman, J. Schleutker, B. G. Nordestgaard, R. C. Travis, N. Pashayan, K. T. Khaw, J. L. Stanford, W. J. Blot, S. Thibodeau, C. Maier, A. S. Kibel, C. Cybulski, L. Cannon-Albright, H. Brenner, J. Park, R. Kaneva, J. Batra, M. R. Teixeira, H. Pandha, M. Lathrop, G. Davey Smith and P. Consortium, Pubertal development and prostate cancer risk: Mendelian randomization study in a population-based cohort, BMC Med., 2016, 14, 66 CrossRef PubMed.
- G. M. Fara, G. Del Corvo, S. Bernuzzi, A. Bigatello, C. Di Pietro, S. Scaglioni and G. Chiumello, Epidemic of breast enlargement in an Italian school, Lancet, 1979, 2, 295–297 CrossRef CAS.
- B. K. Campbell, N. R. Kendall, V. Onions and R. J. Scaramuzzi, The effect of systemic and ovarian infusion of glucose, galactose and fructose on ovarian function in sheep, Reproduction, 2010, 140, 721–732 CAS.
- C. MOPH, Determination of Hormone Multiresidues in Foodstuffs of Animal Origin – LC-MS/MS Method: GB/T 21981-2008, China Standards Press, Beijing, 2008, 32 Search PubMed.
- K. O. Klein, J. Baron, M. J. Colli, D. P. McDonnell and G. B. Cutler, Jr., Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay, J. Clin. Invest., 1994, 94, 2475–2480 CrossRef CAS PubMed.
- R. M. Lequin, Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA), Clin. Chem., 2005, 51, 2415–2418 CrossRef CAS PubMed.
- C. J. Munro, G. H. Stabenfeldt, J. R. Cragun, L. A. Addiego, J. W. Overstreet and B. L. Lasley, Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay, Clin. Chem., 1991, 37, 838–844 CrossRef CAS.
- M. J. De Souza, R. J. Toombs, J. L. Scheid, E. O'Donnell, S. L. West and N. I. Williams, High prevalence of subtle and severe menstrual disturbances in exercising women: Confirmation using daily hormone measures, Hum. Reprod., 2010, 25, 491–503 CrossRef CAS PubMed.
- K. A. O'Connor, E. Brindle, D. J. Holman, N. A. Klein, M. R. Soules, K. L. Campbell, F. Kohen, C. J. Munro, J. B. Shofer, B. L. Lasley and J. W. Wood, Urinary estrone conjugate and pregnanediol 3-glucuronide enzyme immunoassays for population research, Clin. Chem., 2003, 49, 1139–1148 CrossRef PubMed.
- D. A. Pape-Zambito, A. L. Magliaro and R. S. Kensinger, 17 beta-estradiol and estrone concentrations in plasma and milk during bovine pregnancy, J. Dairy Sci., 2008, 91, 127–135 CrossRef CAS PubMed.
- T. Snoj and G. Majdic, Mechanisms In Endocrinology: Estrogens in consumer milk: Is there a risk to human reproductive health?, Eur. J. Endocrinol., 2018, 179, R275–R286 CAS.
- X. Remesar, V. Tang, E. Ferrer, C. Torregrosa, J. Virgili, R. M. Masanes, J. A. Fernandez-Lopez and M. Alemany, Estrone in food: A factor influencing the development of obesity?, Eur. J. Nutr., 1999, 38, 247–253 CrossRef CAS.
- H. Malekinejad, P. Scherpenisse and A. Bergwerff, Naturally occurring estrogens in processed milk and in raw milk (from gestated cows), J. Agric. Food Chem., 2006, 54, 9785–9791 CrossRef CAS PubMed.
- F. Courant, J. Antignac, J. Laille, F. Monteau, F. Andre and B. Le Bizec, Exposure assessment of prepubertal children to steroid endocrine disruptors. 2. Determination of steroid hormones in milk, egg, and meat samples, J. Agric. Food Chem., 2008, 56, 3176–3184 CrossRef CAS PubMed.
- X. Qu, C. Su, N. Zheng, S. Li, L. Meng and J. Wang, A survey of naturally-occurring steroid hormones in raw milk and the associated health risks in Tangshan City, Hebei Province, China, Int. J. Environ. Res. Public Health, 2017, 15, 38 CrossRef.
- A. A. Franke, L. J. Custer, Y. Morimoto, F. J. Nordt and G. Maskarinec, Analysis of urinary estrogens, their oxidized metabolites, and other endogenous steroids by benchtop Orbitrap LCMS versus traditional quadrupole GCMS, Anal. Bioanal. Chem., 2011, 401, 1319–1330 CrossRef CAS PubMed.
- M. L. Padwick, J. Endacott, C. Matson and M. I. Whitehead, Absorption and metabolism of oral progesterone when administered twice daily, Fertil. Steril., 1986, 46, 402–407 CrossRef CAS PubMed.
- W. H. Goodson, P. HandagamaD. H. Moore and S. Dairkee, Milk products are a source of dietary progesterone, 30th Annual San Antonio Breast Cancer Symposium (SABCS)., San Antonio, TX, 2007106S93–S94.
- P. Wood, Salivary steroid assays – Research or routine?, Ann. Clin. Biochem., 2009, 46, 183–196 CrossRef CAS PubMed.
- A. Diemert, J. Goletzke, C. Barkmann, R. Jung, K. Hecher and P. Arck, Maternal progesterone levels are modulated by maternal BMI and predict birth weight sex—specifically in human pregnancies, J. Reprod. Immunol., 2017, 121, 49–55 CrossRef CAS.
- D. Ganmaa and A. Sato, The possible role of female sex hormones in milk from pregnant cows in the development of breast, ovarian and corpus uteri cancers, Med. Hypotheses, 2005, 65, 1028–1037 CrossRef CAS PubMed.
- J. L. Carwile, W. C. Willett and K. B. Michels, Consumption of low-fat dairy products may delay natural menopause, J. Nutr., 2013, 143, 1642–1650 CrossRef CAS PubMed.
- G. Liu, F. Shi, U. Blas-Machado, Q. Duong, V. L. Davis, W. G. Foster and C. L. Hughes, Ovarian effects of a high lactose diet in the female rat, Reprod., Nutr., Dev., 2005, 45, 185–192 CrossRef PubMed.
- A. JFWE, Evaluation of certain vaterinary drug residue in food: Fifty-second report of the Joint FAO/WHO Expert Committee on Food Additives, World Health Organ Tech Rep Ser., 1999, 888, 1–95 Search PubMed.
- K. Kim, J. Wactawski-Wende, K. A. Michels, T. C. Plowden, E. N. Chaljub, L. A. Sjaarda and S. L. Mumford, Dairy food intake is associated with reproductive hormones and sporadic anovulation among healthy premenopausal women, J. Nutr., 2017, 147, 218–226 CrossRef CAS.
- E. Munetsuna, H. Yamada, M. Yamazaki, Y. Ando, G. Mizuno, T. Ota, Y. Hattori, N. Sadamoto, K. Suzuki, H. Ishikawa, S. Hashimoto and K. Ohashi, Maternal fructose intake disturbs ovarian estradiol synthesis in rats, Life Sci., 2018, 202, 117–123 CrossRef CAS PubMed.
- S. Bandyopadhyay, J. Chakrabarti, S. Banerjee, A. K. Pal, S. K. Goswami, B. N. Chakravarty and S. N. Kabir, Galactose toxicity in the rat as a model for premature ovarian failure: an experimental approach readdressed, Hum. Reprod., 2003, 18, 2031–2038 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo02321k |
| This journal is © The Royal Society of Chemistry 2022 |