Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cocoa flavanol consumption improves lower extremity endothelial function in healthy individuals and people with type 2 diabetes
Mariam
Bapir†
a,
Gavrielle R.
Untracht†
 bc,
Debbie
Cooke
d,
John H.
McVey
bc,
Debbie
Cooke
d,
John H.
McVey
 e,
Simon S.
Skene
e,
Simon S.
Skene
 a,
Paola
Campagnolo
a,
Paola
Campagnolo
 e,
Martin B.
Whyte
e,
Martin B.
Whyte
 a,
Nikolaos
Dikaios
f,
Ana
Rodriguez-Mateos
a,
Nikolaos
Dikaios
f,
Ana
Rodriguez-Mateos
 g,
David D.
Sampson
g,
David D.
Sampson
 c,
Danuta M.
Sampson†
c,
Danuta M.
Sampson†
 ahi and
Christian
Heiss†
ahi and
Christian
Heiss†
 *aj
*aj
aDepartment of Clinical and Experimental Medicine, School of Bioscience & Medicine, University of Surrey, Guildford, United Kingdom. E-mail: c.heiss@surrey.ac.uk; Web: https://twitter.com/heissheissheiss
bOptical+Biomedical Engineering Laboratory, School of Electrical, Electronic and Computer Engineering, The University of Western Australia, Perth, Australia
cSurrey Biophotonics, Advanced Technology Institute, School of Physics and School of Biosciences and Medicine, University of Surrey, Guildford, United Kingdom
dSchool of Health Sciences, Faculty of Health & Medical Sciences, University of Surrey, Guildford, United Kingdom
eDepartment of Biochemical Sciences, School of Bioscience & Medicine, University of Surrey, Guildford, United Kingdom
fMathematics Research Center, Academy of Athens, Athens, Greece
gDepartment of Nutritional Sciences, School of Life Course and Population Sciences, Faculty of Life Science and Medicine, King's College London, London, United Kingdom
hSurrey Biophotonics, Centre for Vision, Speech and Signal Processing and School of Biosciences and Medicine, The University of Surrey, Guildford, United Kingdom
iUniversity College London, Institute of Ophthalmology, London, United Kingdom
jSurrey and Sussex NHS Healthcare Trust, Redhill, United Kingdom
First published on 20th September 2022
Abstract
Background: diabetes and age are major risk factors for the development of lower extremity peripheral artery disease (PAD). Cocoa flavanol (CF) consumption is associated with lower risk for PAD and improves brachial artery (BA) endothelial function. Objectives: to assess if femoral artery (FA) endothelial function and dermal microcirculation are impaired in individuals with type 2 diabetes mellitus (T2DM) and evaluate the acute effect of CF consumption on FA endothelial function. Methods: in a randomised, controlled, double-blind, cross-over study, 22 individuals (n = 11 healthy, n = 11 T2DM) without cardiovascular disease were recruited. Participants received either 1350 mg CF or placebo capsules on 2 separate days in random order. Endothelial function was measured as flow-mediated dilation (FMD) using ultrasound of the common FA and the BA before and 2 hours after interventions. The cutaneous microvasculature was assessed using optical coherence tomography angiography. Results: baseline FA-FMD and BA-FMD were significantly lower in T2DM (FA: 3.2 ± 1.1% [SD], BA: 4.8 ± 0.8%) compared to healthy (FA: 5.5 ± 0.7%, BA: 6.0 ± 0.8%); each p < 0.001. Whereas in healthy individuals FA-FMD did not significantly differ from BA-FMD (p = 0.144), FA-FMD was significantly lower than BA-FMD in T2DM (p = 0.003) indicating pronounced and additional endothelial dysfunction of lower limb arteries (FA-FMD/BA-FMD: 94 ± 14% [healthy] vs. 68 ± 22% [T2DM], p = 0.007). The baseline FA blood flow rate (0.42 ± 0.23 vs. 0.73 ± 0.35 l min−1, p = 0.037) and microvascular dilation in response to occlusion in hands and feet were significantly lower in T2DM subjects than in healthy ones. CF increased both FA- and BA-FMD at 2 hours, compared to placebo, in both healthy and T2DM subgroups (FA-FMD effect: 2.9 ± 1.4%, BA-FMD effect 3.0 ± 3.5%, each pintervention< 0.001). In parallel, baseline FA blood flow and microvascular diameter significantly increased in feet (3.5 ± 3.5 μm, pintervention< 0.001) but not hands. Systolic blood pressure and pulse wave velocity significantly decreased after CF in both subgroups (−7.2 ± 9.6 mmHg, pintervention = 0.004; −1.3 ± 1.3 m s−1, pintervention = 0.002). Conclusions: individuals with T2DM exhibit decreased endothelial function that is more pronounced in the femoral than in the brachial artery. CFs increase endothelial function not only in the BA but also the FA both in healthy individuals and in those with T2DM who are at increased risk of developing lower extremity PAD and foot ulcers.
Introduction
Lower extremity peripheral arterial disease (PAD) is characterised by atherosclerosis of the arteries that supply the legs leading to reduced blood flow and limiting the delivery of oxygenated blood and nutrients. PAD may be seen in up to 50% of people with diabetes,1,2 and increases the risk of non-healing wounds (ulcers), amputation,3,4 and mortality.5 The mortality after diabetes-related amputations exceeds 70% at 5 years.6 Globally, the number of people with PAD increased from 202 million in 2010, to 237 million in 2015,7 likely as a consequence of the diabetes epidemic.2 Hospital admissions for ulcer care or amputation are higher than those for congestive heart failure, renal disease, depression, and most forms of cancer.8 In the UK, the cost of health care for ulceration and amputation in diabetes in 2014–2015 was estimated at £837–£962 million; 0.8–0.9% of the National Health Service budget.9 Best management of risk factors including smoking cessation can decrease the risk by 40% and timely revascularization promotes wound healing and prevents amputation.10 However, specific therapies to target the development of PAD, in particular, in people with diabetes are not available.Endothelial dysfunction is the key pathophysiological event that initiates and drives atherosclerosis.11 Diabetes is associated with impaired brachial artery (BA) endothelial function and forearm blood flow, indicating both micro- and macrovascular dysfunction.12
There are significant associations between diabetes mellitus, oxidative stress, and endothelial dysfunction.13 Oxidative stress is increased by chronic hyperglycaemia and by acute glucose fluctuations – induced by postprandial hyperglycaemia in patients with diabetes mellitus. In addition, selective insulin resistance in the phosphoinositide 3-kinase/Akt/endothelial nitric oxide (NO) synthase pathway in endothelial cells leads to decreased NO production and increased endothelin-1 production, resulting in endothelial dysfunction.13 Most studies investigating vascular function in the context of diabetes have focused on the association between endothelial dysfunction as measured by flow-mediated dilation (FMD) in the BA and events related to the coronary circulation. Whether a direct link exists between leg artery endothelial dysfunction and PAD development in patients with diabetes has not been investigated. In fact, it is unknown whether endothelial function is impaired in leg arteries of people with diabetes, which is clinically important as PAD and ulcers almost exclusively occur in the lower extremities.
The presence of microvascular disease is independently associated with risk of limb amputation, in particular, in diabetes,14 hence the microcirculation presents an important therapeutic target, even prior to overt PAD. Dysfunction of the microcirculation typically occurs before the development of atherosclerotic symptoms and is associated with PAD, type 2 diabetes mellitus (T2DM), and other vascular diseases.15 However, it often remains undetected and the investigation of specific treatments have been hampered by the lack of applicable methodology to capture it.16 The introduction of optical coherence tomography angiography (OCTA) has enabled the direct visualization and quantitative characterization of microvascular structure and function.17,18 In contrast to other angiography modalities, OCTA does not require exogenous contrast agents and can therefore visualize the microvascular network directly with few micron-scale resolution – this makes it an ideal tool for clinical measurements of microvascular function and the efficacy of interventions.
A recent epidemiological study has shown that a higher intake of individual dietary polyphenol subclasses, including flavanols, is associated with lower risk for PAD.19 We have previously demonstrated that cocoa flavanols (CF) can improve BA endothelial function in patients with diabetes.20 Whether CF can also increase endothelial function in the clinically relevant arteries supplying the legs in people with diabetes is unknown.
The primary aims of the current proof-of-concept study were to assess if femoral artery (FA) endothelial function is impaired in individuals with T2DM and evaluate the acute effect of CF intervention on endothelial function. FMD is the primary endpoint. Secondary endpoints include blood flow rate, microvascular diameter and vessel area density, blood pressure, pulse wave velocity (PWV), and ankle brachial pressure index (ABPI).
Materials & methods
Study subjects
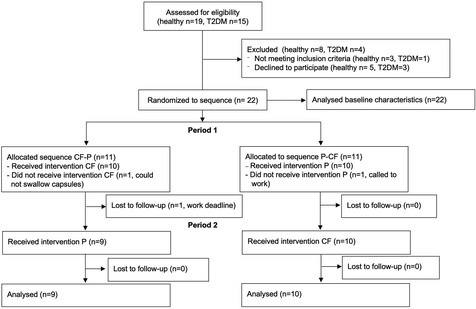
Twenty-two individuals (n = 11 healthy and n = 11 with T2DM) were recruited at the University of Surrey (May 2021) and measurements performed at the Clinical Trials Unit. See Fig. 1 for CONSORT study flow. Inclusion criteria for participation in the study were as follows: <75 years of age, and signed consent form. Exclusion criteria were clinical signs or symptoms of manifest cardiovascular disease (coronary artery disease [CAD], PAD, cerebrovascular disease), ankle brachial pressure index (ABPI) <0.9 or >1.4, heart rhythm other than sinus rhythm, sensitivity to the methylxanthines, caffeine and theobromine.Study design and protocol
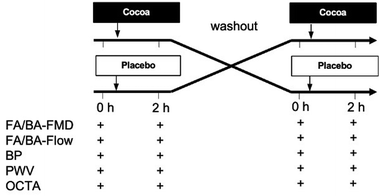
A 2-armed randomized controlled, double-masked, cross-over, dietary intervention trial was performed in healthy and T2DM individuals with a pre-specified comparison of baseline parameters between healthy and T2DM.After informed consent was obtained and screening for eligibility, the study participants were randomly assigned to one of two sequences of interventions; either CF at visit/phase 1 followed by placebo at visit/phase 2 or placebo at phase 1 followed by CF at visit/phase 2. The randomized assignment list was generated with a software freely available at https://www.graphpad.com/quickcalcs/randomize2/ (GraphPad Software, Inc., La Jolla, USA). During each study visit, measurements were taken at baseline before and at 1.5–2 h after consumption of the intervention. Compliance was assessed by supervised consumption of the interventions.
At each timepoint, we first measured blood pressure and pulse wave velocity (PWV) with a cuff on the upper arm after 10 min of supine rest in an air-conditioned room. We then performed FMD measurements on the BA and the FA. Together with FMD measurements, blood flow was assessed, and measurements of cutaneous microcirculation taken in parallel by a second investigator at rest and during post-occlusive reactive hyperaemia (PORH) with OCTA at the hand and foot, respectively. Finally, the perfusion pressure of the legs was measured on the ankle arteries with a cuff placed around the lower leg following a standard protocol1 allowing ABPI calculation together with brachial systolic pressure.
All researchers involved in conducting the study or assessing outcomes were masked with regard to the identity of the test products. The study was conducted according to the guidelines of the Declaration of Helsinki. All procedures were approved by the University of Surrey Ethics Committee and written informed consent was obtained from all study participants.
Test materials
All test materials were purchased from Amazon. The CF interventions were 6 CocoaVia Capsules (Mars Inc, McLean, USA) containing 1350 mg total cocoa flavanols, 255 mg (−)-epicatechin, 60 mg caffeine and 15 kcal (Table 1). Empty identical placebo capsules were filled with 3.9 g of brown sugar delivering 16 kcal. The CF and placebo capsules were provided to participants in identical black opaque ziplock plastic bags each labelled with an alphanumeric code. Each participant consumed 6 capsules under the supervision of the investigators together with a standardized small breakfast consisting of 200 ml water, 2 slices of rye crisp bread (20 g, Dark Rye Crunchy Rye Bread, Ryvita, purchased at Tesco, Guildford, UK), a cheddar cheese snack (20 g, Tesco) and a cashew nut snack (60 g, Tesco).| Cocoa flavanols | Placebo | |
|---|---|---|
| Number of capsules (n) | 6 | 6 |
| Cocoa extract (mg) | 3330 | 0 |
| Cocoa flavanols (mg) | 1350 | 0 |
| (–)-Epicatechin (mg) | 240–255 | 0 |
| Caffeine (mg) | 60–90 | 0 |
| Theobromine (mg) | 180 | 0 |
| Energy (kcal) | 15 | 16 |
| Carbohydrates (g) | 3 | 3.9 |
FMD, blood flow and power analysis of primary endpoint
FMD was measured in the right common femoral artery first (FA-FMD), followed by BA-FMD using 5 min calf and forearm occlusion with a fitted cuff, respectively, by hand-held ultrasound (Vivid I, 12 MHz linear array, GE Healthcare, Chalfont St Giles, UK) in combination with a digital video frame grabber and semi-automated analysis system (Brachial Analyzer, MIA, Iowa City, IA, USA). Briefly, the femoral or brachial artery, respectively, was scanned above the femur head, or proximal to the elbow longitudinally, to obtain a clear anterior and posterior vessel wall-lumen interface. In ultrasound duplex mode, the Doppler spectrum showing blood flow velocity over time was visualised in parallel with the B-mode scan. After optimization of settings, a video clip of 10 s duration was recorded for assessment of baseline pre-inflation diameter. Then an appropriately sized blood pressure cuff around the proximal calf or forearm was inflated to 200 mmHg for 5 min. Starting shortly before deflation of the cuff, the recording of a 2 min video of the continuous duplex scan was started. FMD was calculated as maximal relative diastolic diameter increase during PORH as compared to baseline diameter: (diametermaximal − diameterbaseline)/diameterbaseline × 100. Lower limb and forearm blood flow were calculated as π × (diameter/2)2 × mean velocity. Peak shear rate was calculated as 8 × mean flow velocity/diameter at the onset of PORH.34The intra- and inter-individual variability for FMD measurements in FA and BA established in our laboratory are 0.9% and 0.6% (standard deviation of difference between repeated FMD measurements in n = 10 healthy subjects, unpublished) and 0.5% and 0.6% (standard deviation within a group of healthy subjects), respectively. A difference in change of FA-FMD between placebo and CF was defined as the primary outcome of the RCT. Based on previous intervention studies with CFs, a change in FMD by 1.3% was anticipated.21 Assuming a standard deviation of difference between repeated FMD of 0.9%, repeated measurements in 10 subjects would provide sufficient power to detect an absolute change in FMD of 0.9% (two sided alpha of 0.05%, power = 0.80) within group using a Bonferroni correction in pairwise comparisons.22
Blood pressure, pulse wave velocity and ABPI measurements
Office blood pressure and PWV were measured using a sphygmomanometer device (Arteriograph24, Tensiomed, Budapest, Hungary) at the upper right arm in a supine position, after 10 min of supine rest in a quiet room with the arm resting at heart level. At each timepoint 3 blood pressure measurements were taken. As the first blood pressure reading may be higher and not representative of true resting value, the first reading was discarded and the remaining two were used to calculate an average which was used for further analyses.23 ABPI was measured following the standard protocol described in clinical practice guidelines for the diagnosis of PAD.1 Briefly, we measured the systolic pressure of the dorsalis pedis and posterior tibial artery at the ankle level using Doppler with a cuff placed around the lower leg. The higher systolic value was divided by the average systolic pressure measured on the arm.Imaging of subcutaneous microcirculation with OCTA
OCTA images were acquired using the multi-beam VivoSight Dx (Michelson Diagnostics Ltd, Kent, UK). For all participants, images were acquired of the skin on the dorsum of the right hand between the base of the thumb and index finger and on the dorsum of the right hallux (big toe). The handheld OCT probe was positioned for imaging on the skin through a plastic cap to reduce motion artefacts and maintain a constant distance between the imaging probe and the skin. Volumetric images were acquired over a 5 mm by 5 mm square area. A black spot was drawn with a permanent marker on the skin to ensure repeated imaging of the same location within each imaging session. OCTA images were acquired concurrently with the ultrasound FMD measurements. Five images were acquired sequentially in baseline conditions at the same location without adjusting the placement of the handheld probe. One image was acquired shortly before the deflation of the blood pressure cuff followed by six sequential images upon the deflation of the cuff. Each OCTA scan required a duration of approximately 30 seconds to acquire with 15 seconds saving time between scans.Two-dimensional (2D) en face OCTA angiograms of the superficial plexus were generated in MATLAB 2020a (The MathWorks, Inc., Natick, Massachusetts, USA) based on maximum intensity projection and the procedure outlined in Untracht et al.17 Each final en face OCTA image was inspected before undertaking the analysis, and images with significant motion artefacts were removed. Mean vessel diameter and vessel area density were calculated using the OCTAVA (Optical Coherence Tomography Angiography Vascular Analyser) software.17 The relative change between the two measurements was calculated using the formula: ΔMetric = (PORH − baseline)/baseline × 100.
Statistical analysis
The baseline characteristics of the study population are expressed as mean values ± standard deviation (SD). Characteristics were compared between healthy and T2DM with independent sample t-tests. The primary test for an effect in the randomized controlled trial was a repeated measurements ANOVA with within-subject factor ‘2 h change’ (CF vs. Placebo) and between subject factor ‘disease’ (T2DM vs. healthy). Estimated effects of CF are expressed as mean values ± standard deviation (SD). Analyses were computed with SPSS 28 (IBM, Armonk, USA).Results
We recruited a total of 22 participant (n = 11 healthy and n = 11 T2DM). The baseline characteristics are detailed in Table 2. While all subjects were allocated to a random sequence of treatments, only 9 healthy and 10 with T2DM completed both arms of the trial and were included in the analysis of the endpoints in the randomized controlled trial (Table 3). One participant could not swallow the capsule and 2 cancelled appointments for work related reasons (see Fig. 1 CONSORT flow diagram and Fig. 2 for study protocol). The test capsules were well tolerated, and no adverse events were reported in the context of this study.| Healthy | Type 2 diabetes | p | |
|---|---|---|---|
| Values are mean and standard deviation and p values are from independent t-tests comparing subgroups. BA = brachial artery, FA = femoral artery, BL = baseline, MV = microvascular, nd = not determined, PORH=post occlusive reactive hyperaemia, VAD = vessel area density. Significant p values in bold. | |||
| N | 11 | 11 | |
| Sex (male/female) | 3/8 | 8/3 | |
| Age (years) | 51 ± 10 | 59 ± 10 | 0.095 |
| Weight (kg) | 70 ± 12 | 93 ± 22 | 0.010 |
| Height (kg) | 1.72 ± 0.10 | 1.75 ± 0.13 | 0.531 |
| Body mass index (kg m−2) | 23.8 ± 3.8 | 29.9 ± 5.1 | 0.005 |
| Current smoker (n) | 0 | 0 | |
| Ex-smoker | 2 | 4 | |
| HbA1c (mmol mol−1) | nd | 47 ± 4 | |
| Metformin (n) | 0 | 5 | |
| Sulphonylurea (n) | 0 | 2 | |
| ACE/ARB (n) | 0 | 6 | |
| Statin (n) | 0 | 4 | |
| Ezetimibe (n) | 0 | 2 | |
| Beta blocker (n) | 0 | 3 | |
| Alphablocker (n) | 0 | 3 | |
| Ankle brachial pressure index | 1.00 ± 0.05 | 1.09 ± 0.09 | 0.012 |
| FA diameter BL (mm) | 8.12 ± 1.69 | 7.87 ± 1.92 | 0.748 |
| FA flow BL (l min−1) | 0.73 ± 0.35 | 0.42 ± 0.24 | 0.037 |
| FA peak flow rate PORH (l min−1) | 1.79 ± 0.63 | 1.66 ± 1.27 | 0.760 |
| FA peak shear rate (s−1) | 480 ± 184 | 577 ± 263 | 0.344 |
| FA FMD (mm) | 8.58 ± 1.78 | 8.12 ± 1.99 | 0.580 |
| FA FMD (%) | 5.6 ± 0.8 | 3.2 ± 1.1 | 0.000 |
| BA diameter BL (mm) | 4.41 ± 0.71 | 4.56 ± 0.63 | 0.600 |
| BA flow rate BL (l min−1) | 0.12 ± 0.06 | 0.13 ± 0.07 | 0.872 |
| BA peak flow rate PORH (l min−1) | 0.63 ± 0.30 | 0.68 ± 0.34 | 0.621 |
| BA peak shear rate (/s) | 1200 ± 382 | 1254 ± 525 | 0.788 |
| BA FMD (mm) | 4.68 ± 0.76 | 4.78 ± 0.67 | 0.729 |
| BA FMD (%) | 6.0 ± 0.7 | 4.8 ± 0.8 | 0.001 |
| FA FMD/BA FMD (%) | 95 ± 15 | 68 ± 22 | 0.004 |
| Systolic blood pressure (mmHg) | 114 ± 12 | 132 ± 19 | 0.021 |
| Diastolic blood pressure (mmHg) | 72 ± 10 | 78 ± 11 | 0.177 |
| Pulse wave velocity (m s−1) | 9.3 ± 2.4 | 10.0 ± 1.8 | 0.424 |
| Foot median MV diameter BL (μm) | 43 ± 4 | 45 ± 3 | 0.289 |
| Foot median MV diameter PORH (μm) | 52 ± 8 | 46 ± 4 | 0.049 |
| Foot median MV diameter PORH (% increase) | 21 ± 13 | 4 ± 10 | 0.002 |
| Hand median MV diameter BL (μm) | 50 ± 6 | 46 ± 4 | 0.110 |
| Hand median MV diameter PORH (μm) | 58 ± 5 | 53 ± 5 | 0.021 |
| Hand median MV diameter PORH (% increase) | 18 ± 13 | 15 ± 10 | 0.619 |
| Foot VAD BL (%) | 38 ± 9 | 40 ± 8 | 0.744 |
| Foot VAD PORH (%) | 50 ± 8 | 49 ± 8 | 0.753 |
| Foot VAD PORH (% increase) | 31 ± 13 | 25 ± 30 | 0.580 |
| Hand VAD BL (%) | 44 ± 6 | 32 ± 6 | 0.001 |
| Hand VAD PORH (%) | 62 ± 9 | 49 ± 10 | 0.010 |
| Hand VAD PORH (% Increase) | 42 ± 17 | 59 ± 41 | 0.265 |
| CF | Placebo | 2 h CF effect | p intervention | p disease | p disease×intervention | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 h | Delta 2 h | Baseline | 2 h | Delta 2 h | |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| BA = brachial artery, BL = baseline, CF = cocoa flavanols, DBP = diastolic blood pressure, FA = femoral artery, MV = microvascular, PORH = post occlusive reactive hyperaemia, PWV = pulse wave velocity, SBP = systolic blood pressure, SD = standard deviation, p values are from RM-ANOVA with within-subject factor ‘2 h change’ (CF vs. Placebo) and between subject factor ‘disease’ (diabetes vs. healthy). Significant p values in bold. | ||||||||||
| FA FMD (%) | 4.3 (1.3) | 7.1 (1.3) | 2.8 (1.3) | 4.2 (1.3) | 4.1 (1.3) | −0.1 (0.9) | 2.9 (1.4) | <0.001 | 0.062 | 0.374 |
| BA FMD (%) | 5.2 (0.9) | 8.7 (2.6) | 3.6 (3.1) | 5.4 (0.9) | 4.9 (1.3) | −0.5 (0.9) | 3.0 (3.5) | <0.001 | 0.081 | 0.107 |
| FA flow BL (l min−1) | 0.55 (0.33) | 0.70 (0.48) | 0.14 (0.22) | 0.53 (0.31) | 0.52 (0.37) | −0.01 (0.17) | 0.16 (0.29) | 0.029 | 0.022 | 0.917 |
| FA flow PORH (l min−1) | 1.58 (1.02) | 1.68 (0.74) | 0.09 (1.05) | 1.71 (1.02) | 1.46 (0.68) | −0.18 (0.79) | 0.27 (0.85) | 0.183 | 0.420 | 0.883 |
| BA flow BL (l min−1) | 0.13 (0.07) | 0.16 (0.12) | 0.03 (0.11) | 0.14 (0.07) | 0.12 (0.07) | −0.02 (0.07) | 0.05 (0.13) | 0.087 | 0.707 | 0.469 |
| BA flow PORH (l min−1) | 0.73 (0.32) | 0.75 (0.31) | 0.02 (0.27) | 0.69 (0.36) | 0.59 (0.25) | −0.10 (0.31) | 0.11 (0.59) | 0.251 | 0.528 | 0.916 |
| FA diameter (mm) | 8.2 (1.3) | 8.0 (1.3) | −0.2 (0.9) | 8.1 (1.3) | 8.1 (1.3) | 0.1 (0.4) | −0.1 (0.4) | 0.154 | 0.524 | 0.338 |
| BA diameter (mm) | 4.6 (0.4) | 4.5 (0.4) | −0.1 (0.4) | 4.4 (0.4) | 4.4 (0.4) | 0.0 (0.4) | 0.0 (0.9) | 0.688 | 0.163 | 0.776 |
| SBP (mmHg) | 123 (13) | 118 (13) | −4.1 (5.2) | 122 (13) | 125 (13) | 3.4 (9.2) | −7.2 (9.6) | 0.004 | 0.636 | 0.183 |
| DBP (mmHg) | 75 (9) | 72 (9) | −2.7 (5.7) | 75 (9) | 72 (9) | −3.3 (5.2) | 1.2 (11.8) | 0.573 | 0.488 | 0.924 |
| PWV (m s−1) | 10.0 (2.2) | 8.8 (1.7) | −1.2 (1.3) | 9.4 (1.7) | 9.5 (1.7) | 0.0 (0.4) | −1.3 (1.3) | 0.002 | 0.048 | 0.942 |
| Foot MV diameter BL (μm) | 44 (4) | 44 (4) | 0.3 (2.2) | 46 (4) | 43 (4) | −3.0 (3.5) | 3.5 (3.5) | <0.001 | 0.601 | 0.026 |
| Foot MV diameter PORH (μm) | 51 (9) | 50 (4) | −0.9 (6.5) | 57 (4) | 49 (9) | −0.2 (5.7) | −0.6 (8.3) | 0.751 | 0.446 | 0.511 |
| Hand MV diameter BL (μm) | 48 (4) | 48 (4) | 0.1 (4.4) | 49 (4) | 47 (4) | −1.6 (3.9) | 1.4 (6.5) | 0.371 | 0.304 | 0.924 |
| Hand MV diameter PORH (μm) | 57 (4) | 57 (4) | 0 (3.5) | 57 (4) | 55 (4) | −1.8 (4.4) | −1.8 (4.8) | 0.120 | 0.941 | 0.785 |
| Foot VAD BL (%) | 35 (9) | 35 (9) | −0.8 (7.8) | 40 (9) | 38 (9) | −2.2 (8.3) | 1.5 (11.3) | 0.581 | 0.898 | 0.591 |
| Foot VAD PORH (%) | 47 (9) | 47 (9) | −0.6 (9.1) | 49 (9) | 47 (9) | −2.2 (8.3) | 1.6 (10.5) | 0.506 | 0.860 | 0.761 |
| Hand VAD BL (%) | 38 (9) | 39 (9) | 0.2 (6.5) | 37 (9) | 38 (9) | 0.2 (6.5) | 0.0 (9.1) | 0.983 | 0.195 | 0.972 |
| Hand VAD PORH (%) | 59 (9) | 58 (9) | −0.2 (7.4) | 55 (3) | 56 (9) | 0.6 (8.7) | −0.8 (7.0) | 0.622 | 0.626 | 0.902 |
Lower limb endothelial dysfunction and decreased resting blood flow to the legs in T2DM
While the age was comparable between the healthy and T2DM subgroups, body weight and BMI were greater in T2DM (Table 2). Diabetes was well controlled (mean HbA1c 47 mmol mol−1 [range 41–52 mmol mol−1]). All ABPI values were within reference limits but were significantly higher in T2DM (1.0 ± 0.1 vs. 1.1 ± 0.1, p = 0.012). Also, systolic blood pressure (114 ± 12 mmHg vs. 132 ± 19 mmHg, p = 0.021) but not PWV was higher in T2DM.At baseline and under fasting conditions, both FA-FMD and BA-FMD were significantly lower in T2DM (FA: 3.2 ± 1.1%[SD], BA: 4.8 ± 0.8%) as compared to healthy (FA: 5.5 ± 0.7%, BA: 6.0 ± 0.8%; each p < 0.001 vs. T2DM). In healthy subjects FA-FMD did not significantly differ from BA-FMD (p = 0.144), whereas FA-FMD was significantly lower than BA-FMD in T2DM subjects (p = 0.003) indicating pronounced and additional endothelial dysfunction of lower limb arteries (FA-FMD/BA-FMD 94 ± 14% [healthy] vs. 68 ± 22% [T2DM], p = 0.007). The baseline diameters of both BA and FA did not differ between healthy and T2DM.
The resting baseline blood flow in the FA was significantly higher in healthy as compared to T2DM (0.73 ± 0.35 l min−1vs. 0.42 ± 0.24 l min−1, p = 0.037). The maximal blood flow during PORH did not significantly differ between healthy and T2DM (1.79 ± 0.63 vs. 1.66 ± 1.27 l min−1, p = 0.760). No difference between healthy and T2DM in terms of BA baseline or PORH blood flow or peak shear rate were observed. Interstingly though, shear rate was significantly lower in the FA as compared to BA in both cohorts (healthy: p = 0.004, T2DM: p = 0.001).
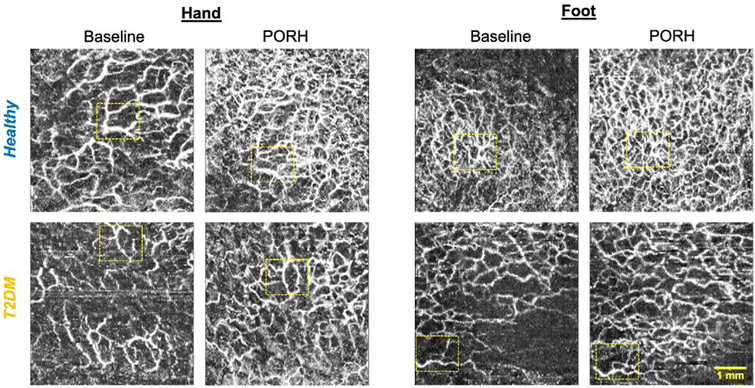
Imaging of the cutaneous microcirculation showed that there was a lower maximal diameter during PORH in the hands and feet of T2DM (hand: 53 ± 5 μm, feet: 46 ± 4 μm) as compared to healthy (feet: 58 ± 5 μm [p = 0.049 vs. T2DM], hands: 52 ± 8 μm [p = 0.021 vs. T2DM]; Fig. 3). Vessel area density was significantly lower in the hands of T2DM as compared to healthy (BL: 32 ± 6% vs. 43 ± 6% p = 0.010; PORH: 62 ± 9% vs. 49 ± 10%, p = 0.010) but not the feet.
CF increased FMD in both BA and FA of healthy and people with diabetes
CF increased both FA and BA-FMD at 2 hours as compared to placebo in both healthy and T2DM (FA: 2.9 ± 1.4%, BA 3.0 ± 3.5%, each pintervention< 0.001). The individual data plot provided in Fig. 4 shows that all participants exhibited a positive response to CF. In parallel, baseline FA blood flow significantly increased after CF (0.16 ± 0.29 l min−1, pintervention = 0.029) and this was accompanied by a greater increase in microvascular median diameter in the skin of the feet (3.5 ± 3.5 μm, pintervention< 0.001). Maximal blood flow during PORH and BA blood flow at baseline and during PORH as well as other microvascular parameters were not affected by CF. SBP and PWV significantly decreased by −7.2 ± 9.6 mmHg (pintervention = 0.004) and −1.3 ± 1.3 m s−1 (pintervention = 0.002), respectively.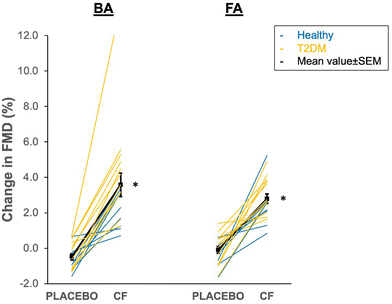 | ||
| Fig. 4 Acute 1.5–2 h effect of cocoa flavanol (CF) intervention and placebo in brachial artery (BA) and femoral artery (FA). Individual data of healthy (blue) and type 2 diabetes mellitus (T2DM, orange) participants. Black symbols reflect mean values and standard error of the mean. Note that the p values for interaction between disease state and intervention were 0.374 and 0.107, respectively, indicating that the response to CF did not differ between healthy and T2DM. *pintervention< 0.05 vs. placebo (see Table 3 for analysis). | ||
Discussion
The key findings of the present study are that individuals with T2DM exhibit decreased FMD that is more pronounced in the femoral as compared to brachial arteries. T2DM was associated with decreased baseline but not hyperaemia blood flow in femoral arteries. CF acutely increased FMD in both arteries together with baseline femoral blood flow and microvascular diameter in the foot and decreased SBP and PWV in both the healthy and T2DM subgroups.Individuals with diabetes are at increased risk of developing vascular disease and the risk to develop PAD is particularly high. Up to 50% people with diabetes have PAD.2 Why diabetes predisposes to atherosclerosis specifically in the lower extremity, causing PAD, is unclear. This is the first study to show that T2DM is associated not only with endothelial dysfunction as measured in the upper extremity brachial artery,12 but more pronounced endothelial dysfunction in arteries that supply the legs. One other study has shown a trend towards lower FMD in femoral arteries of people with diabetes that was not statistically significant.24 One explanation of lower FMD in femoral arteries versus brachial arteries could be that leg arteries are exposed to higher blood pressure due to the upright posture of humans and lower shear rate that may be due to larger artery diameter or microvascular dysfunction. Given the known interaction between diabetes and hypertension on atherosclerosis development,25 the leg arteries of people with diabetes will be more likely to manifest such site-specific pathology. We have previously shown that in patients with PAD, FMD is lower in the femoral artery as compared to the brachial artery.26 While the study26 was compatible with the idea that lower extremity endothelial dysfunction may play a role in PAD, it was limited by the fact that the patients had significant stenoses of leg arteries and therefore, the lower FMD may be secondary to blunted hyperaemic blood flow. The current study adds the important new finding that femoral FMD is lowered in people at risk for PAD but without flow limiting stenoses. Further studies are needed to investigate why endothelial function is more liable to be impaired in leg arteries of people with diabetes and whether FA-FMD could serve as a tissue specific biomarker or surrogate endpoint of PAD development.
Recent epidemiological studies have shown that the risk of PAD is lower in people with higher dietary polyphenol intake overall and flavanols in particular with greater effect observed in smokers and people with diabetes.19 In support of these findings, our current study shows for the first time that CFs increase endothelial function of leg arteries in healthy people and also normalise endothelial dysfunction in T2DM, offering a feasible explanation for how CFs can counteract the development and progression of lower limb atherosclerosis. We cautiously speculate that this may be mediated by increased bioactivity of nitric oxide as was previously shown in the brachial artery.27 Our data confirm previous studies that have shown significant improvements in brachial artery FMD in T2DM20 and extend them to a vascular segment with direct clinical relevance. Another interesting finding was that the baseline femoral blood flow rate was lower in T2DM as compared to controls and that CF increased it. The fact that hyperaemic blood flow did not differ points towards a functional rather than fixed structural impairment of leg blood flow. Previous studies have indicated that lower leg blood flow in T2DM may be due to enhanced activity of the endothelin-1A receptor antagonizing insulin and nitric oxide dependent vasodilation.28 In fact, there are several lines of evidence that support a role of endothelin-1 in diabetes associated complications, hypertension and endothelial dysfunction.29,30 Previous studies have indicated that epicatechin metabolites may decrease endothelin-1 expression in endothelial cells via epigenetic changes.31 However, the study31 does not directly relate to the present study as gene expression changes would take longer than 2 hours. Taken together, future investigations to elucidate if CFs interact with the endothelin-1 pathway hold the promise of further understanding the mechanisms by which CFs mediate health benefits. Whether micro- and macrovascular effects are mediated via the same mechanisms is another important question. In addition, CF may be an interesting intervention to increase leg blood flow to facilitate wound-healing in patients with critical limb ischaemia and diabetic foot ulcers. Supporting this, one study has already shown that CF can improve walking performance in PAD patients and increase capillary density.32
Only few studies have investigated microvascular structure and function of hands and legs with OCTA. Our data show that T2DM individuals had lower responsiveness of the subcutaneous microcirculation during PORH. This aligns with two previous publications from the same group that showed a trend towards lower microvascular diameter increase during PORH in people with diabetes.24,33 Surprisingly, the response to CF was rather small and the degree of reactive hyperaemia was not affected, which aligns with the unchanged blood flow response measured with ultrasound. This finding indicates that the improvement of FMD in the upstream brachial and femoral arteries and the decrease in systolic blood pressure and PWV were not predominantly driven by functional improvements in the microcirculation. Whether long-term exposure to CFs together with associated lowering of blood pressure will affect microvascular structure remains to be investigated.
The limitations of the current proof-of-concept study include the small sample size which limits generalizability. This is true particularly in terms of OCTA-derived microvascular metrics. While our preliminary results indicate differences in microvasculature function in T2DM in accordance with the literature,16,24,33 some image artefacts, image misalignment (see Fig. 3) and heterogeneity of diabetes phenotypes lead to large variability and will require a larger sample size to tease out effects of diabetes and CF on the microvasculature. In addition, the current OCTA microvascular measurements are restricted to the superficial cutaneous plexus and do not include capillaries which may be affected by T2DM and respond to CFs independently from the arterioles. The data gathered in the current study will help to adequately power future studies of the microcirculation. In addition, OCTA only captures the subcutaneous microcirculation. Whether the status of the subcutaneous microcirculation reflects the microvasculature in deeper skeletal muscle is not clear. Further potential limitations include that endothelium-independent vasodilation and CF metabolites in plasma were not measured in the present study. In addition, the FMD protocol used in the present study deviates from the protocol outlined in the FMD guideline document.34 We recorded 10 s (30 s recommended) for baseline pre-inflation measurements and 2 min (3 min recommended) post-deflation. This is unlikely to affect the reported outcome of our study as all participants’ arterial diameters had almost reached baseline values at 2 min and are most likely correctly captured in this time frame.
In conclusion, T2DM is associated with pronounced impairment of femoral endothelial function and blood flow. Furthermore, CFs increase endothelial function not only in the BA but also in the FA both in healthy people and people with T2DM who are at increased risk of developing lower extremity PAD and foot ulcers.
Author contributions
Conceptualization: Christian Heiss, Danuta Sampson; resources: Christian Heiss, John McVey, Debbie Cooke, Paola Campagnolo, David Sampson; data curation: Danuta Sampson, Mariam Bapir, Gavrielle Untracht, Christian Heiss; formal analysis: Christian Heiss, Simon Skene, Mariam Bapir, Gavrielle Untracht, Nikolaos Dikaios; supervision: Christian Heiss, David Sampson, Danuta Sampson, Paola Campagnolo; funding acquisition: Christian Heiss, Danuta Sampson; project administration: Christian Heiss; writing – original draft: Christian Heiss; writing – review & editing: all authors; methodology: Christian Heiss, Danuta Sampson, Mariam Bapir, Gavrielle Untracht; investigation: Danuta Sampson, Mariam Bapir, Gavrielle Untracht, Christian Heiss; visualisation: Christian Heiss; software: Gavrielle Untracht.Conflicts of interest
The authors declare no conflict of interest other than the acknowledged funding sources.Acknowledgements
This study was funded by the University of Surrey (HEIF Industrial Strategy Internal Funding Accelerating disease detection using digital health technologies) and the Economic and Social Research Council (ES/V001744/1).References
- U. Frank, S. Nikol, J. Belch, V. Boc, M. Brodmann, P. H. Carpentier, A. Chraim, C. Canning, E. Dimakakos, A. Gottsater, C. Heiss, L. Mazzolai, J. Madaric, D. M. Olinic, Z. Pecsvarady, P. Poredos, I. Quere, K. Roztocil, A. Stanek, D. Vasic, A. Visona, J. C. Wautrecht, M. Bulvas, M. P. Colgan, W. Dorigo, G. Houston, T. Kahan, H. Lawall, I. Lindstedt, G. Mahe, R. Martini, G. Pernod, S. Przywara, M. Righini, O. Schlager and P. Terlecki, ESVM Guideline on peripheral arterial disease, Vasa, 2019, 48, 1–79 Search PubMed.
- K. Stoberock, M. Kaschwich, S. S. Nicolay, N. Mahmoud, F. Heidemann, H. C. Rieß, E. S. Debus and C.-A. Behrendt, The interrelationship between diabetes mellitus and peripheral arterial disease – a systematic review, Vasa, 2021, 50, 323–330 CrossRef PubMed.
- J. L. Mills Sr, M. S. Conte, D. G. Armstrong, F. B. Pomposelli, A. Schanzer, A. N. Sidawy and G. Andros, The Society for Vascular Surgery lower extremity threatened limb classification system: risk stratification based on wound, ischemia, and foot infection (WIfI), J. Vasc. Surg., 2014, 59, 220–234 CrossRef PubMed.
- R. Ward, J. Dunn, L. Clavijo, D. Shavelle, V. Rowe and K. Woo, Outcomes of critical limb ischemia in an urban, safety net hospital population with high WIfI amputation scores, Ann. Vasc. Surg., 2017, 38, 84–89 CrossRef PubMed.
- G. Agnelli, J. J. F. Belch, I. Baumgartner, P. Giovas and U. Hoffmann, Morbidity and mortality associated with atherosclerotic peripheral artery disease: A systematic review, Atherosclerosis, 2020, 293, 94–100 CrossRef CAS PubMed.
- L. A. Lavery, N. A. Hunt, A. Ndip, D. C. Lavery, W. Van Houtum and A. J. Boulton, Impact of chronic kidney disease on survival after amputation in individuals with diabetes, Diabetes Care, 2010, 33, 2365–2369 CrossRef PubMed.
- P. Song, D. Rudan, Y. Zhu, F. J. I. Fowkes, K. Rahimi, F. G. R. Fowkes and I. Rudan, Global, regional, and national prevalence and risk factors for peripheral artery disease in 2015: an updated systematic review and analysis, Lancet Glob. Health, 2019, 7, e1020–e1030 CrossRef.
- G. H. Skrepnek, J. L. Mills Sr, L. A. Lavery and D. G. Armstrong, Health care service and outcomes among an estimated 6.7 million ambulatory care diabetic foot cases in the U.S, Diabetes Care, 2017, 40, 936–942 CrossRef PubMed.
- M. Kerr, E. Barron, P. Chadwick, T. Evans, W. M. Kong, G. Rayman, M. Sutton-Smith, G. Todd, B. Young and W. J. Jeffcoate, The cost of diabetic foot ulcers and amputations to the National Health Service in England, Diabetic Med., 2019, 36, 995–1002 CrossRef CAS.
- R. J. Hinchliffe, J. R. Brownrigg, G. Andros, J. Apelqvist, E. J. Boyko, R. Fitridge, J. L. Mills, J. Reekers, C. P. Shearman, R. E. Zierler, N. C. Schaper and F. International, Working Group on the Diabetic, Effectiveness of revascularization of the ulcerated foot in patients with diabetes and peripheral artery disease: a systematic review, Diabetes/Metab. Res. Rev., 2016, 32(Suppl 1), 136–144 CrossRef PubMed.
- C. Heiss, A. Rodriguez-Mateos and M. Kelm, Central role of eNOS in the maintenance of endothelial homeostasis, Antioxid. Redox Signal., 2015, 22, 1230–1242 CrossRef CAS PubMed.
- S. Keymel, Y. Heinen, J. Balzer, T. Rassaf, M. Kelm, T. Lauer and C. Heiss, Characterization of macro-and microvascular function and structure in patients with type 2 diabetes mellitus, Am. J. Cardiovasc. Dis., 2011, 1, 68–75 CAS.
- T. Maruhashi and Y. Higashi, Pathophysiological association between diabetes mellitus and endothelial dysfunction, Antioxidants, 2021, 10, 1306 CrossRef CAS PubMed.
- J. A. Beckman, M. S. Duncan, S. M. Damrauer, Q. S. Wells, J. V. Barnett, D. H. Wasserman, R. J. Bedimo, A. A. Butt, V. C. Marconi, J. J. Sico, H. A. Tindle, M. P. Bonaca, A. W. Aday and M. S. Freiberg, Microvascular disease, peripheral artery disease, and amputation, Circulation, 2019, 140, 449–458 CrossRef.
- C. J. Abularrage, A. N. Sidawy, G. Aidinian, N. Singh, J. M. Weiswasser and S. Arora, Evaluation of the microcirculation in vascular disease, J. Vasc. Surg., 2005, 42, 574–581 CrossRef PubMed.
- W. D. Strain and P. M. Paldanius, Diabetes, cardiovascular disease and the microcirculation, Cardiovasc. Diabetol., 2018, 17, 57 CrossRef CAS PubMed.
- G. R. Untracht, R. S. Matos, N. Dikaios, M. Bapir, A. K. Durrani, T. Butsabong, P. Campagnolo, D. D. Sampson, C. Heiss and D. M. Sampson, OCTAVA: An open-source toolbox for quantitative analysis of optical coherence tomography angiography images, PLoS One, 2021, 16, e0261052 CrossRef CAS PubMed.
- C. L. Chen and R. K. Wang, Optical coherence tomography based angiography [Invited], Biomed. Opt. Express, 2017, 8, 1056–1082 CrossRef PubMed.
- N. P. Bondonno, K. Murray, A. Cassidy, C. P. Bondonno, J. R. Lewis, K. D. Croft, C. Kyro, G. Gislason, C. Torp-Pedersen, A. Scalbert, A. Tjonneland, J. M. Hodgson and F. Dalgaard, Higher habitual flavonoid intakes are associated with a lower risk of peripheral artery disease hospitalizations, Am. J. Clin. Nutr., 2020, 113, 187–199 CrossRef PubMed.
- J. Balzer, T. Rassaf, C. Heiss, P. Kleinbongard, T. Lauer, M. Merx, N. Heussen, H. B. Gross, C. L. Keen, H. Schroeter and M. Kelm, Sustained benefits in vascular function through flavanol-containing cocoa in medicated diabetic patients a double-masked, randomized, controlled trial, J. Am. Coll. Cardiol., 2008, 51, 2141–2149 CrossRef CAS PubMed.
- L. Hooper, C. Kay, A. Abdelhamid, P. A. Kroon, J. S. Cohn, E. B. Rimm and A. Cassidy, Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: a systematic review and meta-analysis of randomized trials, Am. J. Clin. Nutr., 2012, 95, 740–751 CrossRef CAS PubMed.
- W. D. Dupont and W. D. Plummer, PS power and sample size program available for free on the Internet, Controlled Clin. Trials, 1990, 11, 116–128 CrossRef CAS PubMed.
- M. R. Salazar, W. G. Espeche, M. Aizpurua, C. E. Sisnieguez, B. C. Sisnieguez, C. A. Dulbecco, C. E. March, R. N. Stavile, E. H. Ferrari, M. Correa, P. M. Maciel, E. Balbin and H. A. Carbajal, Should the first blood pressure reading be discarded?, J. Hum. Hypertens., 2015, 29, 373–378 CAS.
- R. Argarini, R. A. McLaughlin, S. Z. Joseph, L. H. Naylor, H. H. Carter, A. Haynes, C. E. Marsh, B. B. Yeap, S. J. Jansen and D. J. Green, Visualizing and quantifying cutaneous microvascular reactivity in humans by use of optical coherence tomography: impaired dilator function in diabetes, Am. J. Physiol. Endocrinol. Metab., 2020, 319, E923–E931 CrossRef CAS PubMed.
- J. R. Petrie, T. J. Guzik and R. M. Touyz, Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms, Can. J. Cardiol., 2018, 34, 575–584 CrossRef PubMed.
- Y. Heinen, E. Stegemann, R. Sansone, K. Benedens, R. Wagstaff, J. Balzer, T. Rassaf, T. Lauer, M. Kelm and C. Heiss, Local association between endothelial dysfunction and intimal hyperplasia: relevance in peripheral artery disease, J. Am. Heart Assoc., 2015, 4, e001472 CrossRef PubMed.
- H. Schroeter, C. Heiss, J. Balzer, P. Kleinbongard, C. L. Keen, N. K. Hollenberg, H. Sies, C. Kwik-Uribe, H. H. Schmitz and M. Kelm, (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 1024–1029 CrossRef CAS PubMed.
- B. E. Young, J. Padilla, S. H. Finsen, P. J. Fadel and S. P. Mortensen, Role of Endothelin-1 Receptors in Limiting Leg Blood Flow and Glucose Uptake During Hyperinsulinemia in Type 2 Diabetes, Endocrinology, 2022, 163, bqac008 CrossRef PubMed.
- A. Ergul, Endothelin-1 and diabetic complications: focus on the vasculature, Pharmacol. Res., 2011, 63, 477–482 CrossRef CAS PubMed.
- J. G. Schneider, N. Tilly, T. Hierl, U. Sommer, A. Hamann, K. Dugi, G. Leidig-Bruckner and C. Kasperk, Elevated plasma endothelin-1 levels in diabetes mellitus, Am. J. Hypertens., 2002, 15, 967–972 CrossRef CAS PubMed.
- D. Milenkovic, K. Declerck, Y. Guttman, Z. Kerem, S. Claude, A. R. Weseler, A. Bast, H. Schroeter, C. Morand and W. Vanden Berghe, (-)-Epicatechin metabolites promote vascular health through epigenetic reprogramming of endothelial-immune cell signaling and reversing systemic low-grade inflammation, Biochem. Pharmacol., 2020, 173, 113699 CrossRef CAS PubMed.
- M. M. McDermott, M. H. Criqui, K. Domanchuk, L. Ferrucci, J. M. Guralnik, M. R. Kibbe, K. Kosmac, C. M. Kramer, C. Leeuwenburgh, L. Li, D. Lloyd-Jones, C. A. Peterson, T. S. Polonsky, J. H. Stein, R. Sufit, L. Van Horn, F. Villarreal, D. Zhang, L. Zhao and L. Tian, Cocoa to improve walking performance in older people with peripheral artery disease: the COCOA-PAD pilot pandomized clinical trial, Circ. Res., 2020, 126, 589–599 CrossRef CAS PubMed.
- R. Argarini, R. A. McLaughlin, S. Z. Joseph, L. H. Naylor, H. H. Carter, B. B. Yeap, S. J. Jansen and D. J. Green, Optical coherence tomography: a novel imaging approach to visualize and quantify cutaneous microvascular structure and function in patients with diabetes, BMJ Open Diabetes Res. Care, 2020, 8, e001479 CrossRef PubMed.
- D. H. J. Thijssen, R. M. Bruno, A. van Mil, S. M. Holder, F. Faita, A. Greyling, P. L. Zock, S. Taddei, J. E. Deanfield, T. Luscher, D. J. Green and L. Ghiadoni, Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans, Eur. Heart J., 2019, 40, 2534–2547 CrossRef PubMed.
Footnote |
| † These authors are equally contributed to this work. |
| This journal is © The Royal Society of Chemistry 2022 |