Open Access Article
Open Access ArticleNovel Emblica officinalis extract containing β-glucogallin vs. metformin: a randomized, open-label, comparative efficacy study in newly diagnosed type 2 diabetes mellitus patients with dyslipidemia†
Muhammed
Majeed
 ab,
Lakshmi
Mundkur
ab,
Lakshmi
Mundkur
 a,
Shaji
Paulose
a,
Shaji
Paulose
 a and
Kalyanam
Nagabhushanam
a and
Kalyanam
Nagabhushanam
 *b
*b
aSami-Sabinsa Group Limited, 19/1 & 19/2, I Main, II Phase, Peenya Industrial Area, Bangalore- 560 058, Karnataka, India
bSabinsa Corporation, 20 Lake Drive, East Windsor, NJ 08520, USA. E-mail: kalyanam@sabinsa.com
First published on 17th August 2022
Abstract
The efficacy of Emblica officinalis extract (EOE) containing 10% β-glucogallin was compared against metformin in newly diagnosed subjects with diabetic dyslipidemia which is a significant factor in cardiovascular disease. Daily administration with EOE-1 g, EOE-2 g, or metformin 500 mg for 90 days significantly decreased fasting blood sugar and postprandial blood sugar (FBS and PPBS), hemoglobin A1c (HbA1c) and lipid levels in all three treatment groups. The FBS, PPBS and HbA1c were significantly lower in the EOE-2 g group compared with metformin and EOE-1 g groups. The reductions in LDL and TC in the EOE-2 g group were also significantly higher than in the EOE-1 g group and were comparable to the metformin group. No serious adverse effects were observed in any study participants. EOE-1 g and 2 g day−1 are safe and potent antidiabetic agents, with comparable efficacy to the pharmaceutical drug, metformin. Supplementation with EOE-2 g day−1 showed greater efficacy than metformin in reducing circulating glucose levels.
Introduction
Type 2 diabetes mellitus (T2DM) is a metabolic disease characterized by hyperglycemia, caused mainly by insulin resistance or inadequate insulin production.1 Insulin deficiency along with metabolic disturbances causes chronic hyperglycemia in T2DM. As per the International Diabetes Federation Atlas guideline report, 463 million people in the age group of 20–79 years were diagnosed with diabetes in 2019, which is predicted to increase to 700 million by 2045.2T2DM with dyslipidemia is one of the most critical risk factors for cardiovascular diseases (CVD) in diabetic patients. Elevated triglycerides (TG), excess of small, very-low-density lipoprotein (VLDL), remnant lipoproteins, postprandial hyperlipidemia, and reduced high-density lipoprotein (HDL) are the characteristic features of diabetic dyslipidemia, which is more atherogenic than general dyslipidemia.3 Patients with diabetic dyslipidemia are at an increased risk of developing microvascular complications like diabetic nephropathy, neuropathy, and retinopathy due to inadequate glycemic control. Coronary artery disease, peripheral arterial disease, and stroke are the macrovascular complications associated with these patients.1,4
Emblica officinalis (Indian gooseberry or Amla), belonging to the Phyllanthaceae family, is an important medicinal plant in the Ayurveda, Unani, Sri Lankan, Chinese, and other traditional systems of medicine.5 Amla is also a well-known functional food with various presentations in the market. The dried and dehydrated amla fruits, mixed with sugar to suppress its natural sour and astringent taste, are used as snacks. In addition, amla fruits have been used in pickles and jams. Also, the amla juice prepared with water after straining out the pulp with the addition of extraneous honey is a popular drink. Amla fruit pastes with proper spices are also used in several food preparations. There are also bread preparations using amla fruit powder.6 Amla in traditional medicine is considered a potent rejuvenator and immunomodulator with its effect on digestion, cough, asthma, heart diseases, hair growth, eye health, and overall body and intellect.7 Its application in the management of diabetes, dyslipidemia, obesity, numerous types of cancer, liver disorders, arthritis, gingivitis, and wound healing have been reported in various scientific studies.8 Amla fruit contains numerous phytoconstituents, including polyphenols like tannins, gallic acid, ellagic acid, amino acids, vitamins, minerals, fixed oils, and flavonoids.9 Polyphenols, majorly tannins, and flavonoids play a key role in most of the bioactivities of E. officinalis.8,10
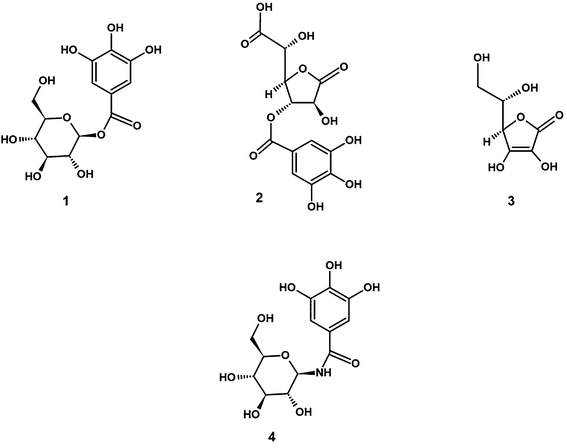
The standardization of amla extracts has been quite contentious. Very early investigations claimed the extensive presence of vitamin C in amla fruits which were later proven to be erroneous.11–13 The presence of actives such as emblicanin A and B11 was also discounted by two different research groups.12,13 However the aqueous extracts of amla fruit was found to contain good amounts of β-glucogallin (BGG) (1) that was proven beyond doubt by spectral means.12 In addition, amla fruit extracts contain copious amounts of mucic acid gallates (2), representative of hydrolysable tannins,14 which strongly resemble vitamin C (3) in their structural characteristics (Fig. 1). The fresh fruit extract of E. officinalis standardized for 10% BGG along with hydrolysable tannins, henceforth termed as EOE, is an unique extract available as a dietary supplement. Although the E. officinalis plant is reported for its antidiabetic activity, the biological properties of extracts may differ based on the extraction methods and the biomarkers standardized in the final product. We have earlier reported the antidiabetic activity of EOE, evaluated by in vitro methods.15
In the present study, we evaluated the effect of EOE in reducing hyperglycemia and dyslipidemia in comparison with metformin, the first-line treatment for newly diagnosed T2DM patients as per the American Diabetes Association's guidelines.16 In addition to antihyperglycemic activity, metformin possesses cardioprotective, antihyperlipidemic, and probable anti-cancer activity.17 Gastrointestinal intolerance and lactic acidosis are the most frequently observed contraindications associated with metformin, which is observed in one-fourth of patients prescribed the drug.18
Materials and methods
Emblica officinalis extract (EOE)
EOE is a formulated juice powder extracted from the fruits of E. officinalis fruits (amla). The fruits were crushed in a multi mill and squeezed to remove the separated juice using an industrial centrifuge and further concentrated at low temperature using an evaporator. The resultant thick syrup was formulated with suitable quantity of maltodextrin to obtain a powder with BGG 10–12% in the final product (termed as EOE). EOE is totally soluble in water. It was free of pesticides, aflatoxins, heavy metals and microbes when tested as per standard or US pharmacopeial methods. BGG in EOE was quantified by HPLC using a C18 BDS (Thermo), 4.6 × 250 mm with 5 μ particle size, under gradient of two mobile phases namely 0.1% formic acid in water and 100% methanol. Under these conditions, BGG eluted with a retention time of 9.8 min which was detected (λmax = 272 nm) and quantified to be 10–12% w/w. A typical HPLC chromatogram of EOE is provided in ESI Fig. 1.†Clinical study design
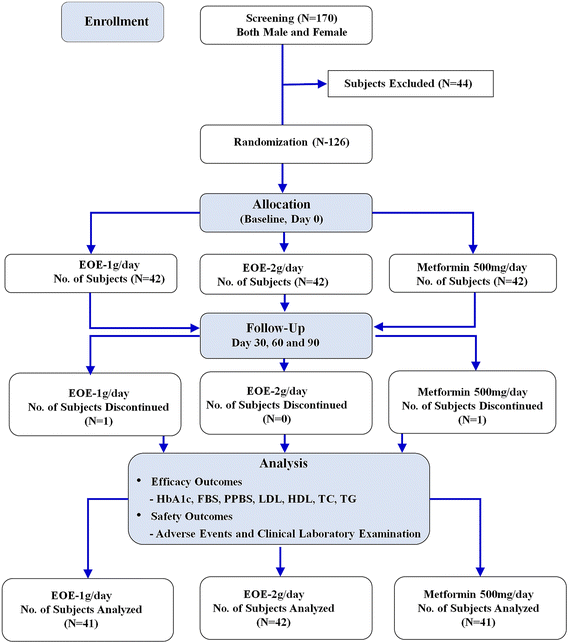
This randomized open-label, three-arm, comparative, multicenter study was conducted in newly diagnosed diabetes patients from Apollo Hospital (Chennai), Vijaya Super Specialty Hospital (Nellore), and BGS Global Institute of Medical Sciences (Bangalore). Patient recruitment started in January 2020 and was completed by February 2021. A total of 126 newly diagnosed diabetes patients with dyslipidemia aged 30–65 years who meet all the inclusion criteria were enrolled in this study. The subjects were randomly divided to receive EOE-1 g day−1 EOE-2 g day−1 or metformin 500 mg day−1 for 90 days. Two subjects were lost to follow up and 124 patients completed the study (Fig. 2).Patient details and ethics
Newly diagnosed type 2 diabetes patients in the age group of 30 to 65 years with Body Mass Index (BMI) ranging from 27 to 35 kg m−2, FBS 130 to 150 mg dL−1, PPBS 210 to 300 mg dL−1, hemoglobin A1 c (HbA1c) 6.5 to 9.0%, Total Cholesterol (TC) 200–300 mg dL−1, low density lipoprotein (LDL) 140 to 299 mg dL−1, and TG 160 to 300 mg dL−1 were included in the study. In addition, all the study participants agreed to do physical activity for a minimum of 30 minutes, five days a week. None of the subjects were taking any concomitant medication at the time of enrollment. Female subjects of reproductive potential who agreed to use a barrier method of contraception throughout the study period were included in the study.Exclusion criteria included subjects having a history of smoking and alcohol intake, type 1 diabetes, chronic gastrointestinal diseases, severe immune deficiency, lactose intolerance, clinically significant thyroid disorder, cardiovascular, hematological, hepatic, renal, respiratory, active malignancy, or genitourinary abnormalities or diseases, pregnant and lactating females, subjects on lipid-lowering therapies and antihypertensive medication, history of anaphylactic reactions and hypersensitivity of investigational products.
Written informed consent was taken from all the subjects before enrollment in the study. The trial was conducted in accordance with the Declaration of Helsinki, ICH-GCP, and applicable local regulations. The study was approved by the Institutional Ethics Committee of Apollo Hospital (Chennai), Vijaya Super Specialty Hospital (Nellore), and BGS Global Institute of Medical Sciences (Bangalore) and registered prospectively on the Clinical Trial Registry of India (CTRI) with the registration number CTRI/2019/11/021992.
Randomization
At baseline, seven days after screening visit, all the eligible subjects were randomized into three groups in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio on the day of randomization (Fig. 2). The randomization sequence was prepared using SAS version 9.4 by an independent statistician, who had no role in the conduct of the study. The sample size was calculated with an alpha error of 0.05 and a power of 90% based on the proportion of patients with an effective response at the end of the treatment period.
1 ratio on the day of randomization (Fig. 2). The randomization sequence was prepared using SAS version 9.4 by an independent statistician, who had no role in the conduct of the study. The sample size was calculated with an alpha error of 0.05 and a power of 90% based on the proportion of patients with an effective response at the end of the treatment period.
Intervention and compliance
EOE is the fresh fruit extract of E. officinalis standardized for 10% BGG along with hydrolysable tannins (Saberry®), is a novel extract available as a dietary supplement. Two tablets of EOE 500 mg once a day after breakfast (1 g day−1), two tablets of EOE 500 mg twice a day for after breakfast & dinner (2 g day−1), and one tablet of metformin 500 mg once a day with breakfast was given for 90 days respectively. Follow-up visits were conducted at day 30, day 60, and day 90, after the administration of actives and subjects were followed up telephonically after 15 days of the final visit for overall wellbeing. FBS, PPBS, and Lipid profiles were assessed at screening, days 30, 60, and 90. HbA1c, hematology, renal function, hepatic function, thyroid function, and urine analysis tests were carried out at screening and final visit. Adverse events were recorded throughout the study period. Electrocardiogram (ECG) was recorded as the screening parameter. The principal investigator and the study team made a constant follow-up to meet the patient compliance as per the protocol requirements.The principal investigator/study site team kept records of the study medications which included quantity dispensed, subject identity and, the date of dispensing in a master file. Medications that had been dispensed but not used by the patient were retained. After completion of the study, a final reconciliation of drug consumed and drug remaining was done. This reconciliation was logged on the drug reconciliation form, signed and dated.
Outcome measures
The primary outcome measures recorded were mean changes in HbA1c, FBS, and PPBS from screening to final visit. The secondary outcome measures were mean changes in TG, LDL, VLDL, HDL, and TC from screening to the final visit, comparative mean changes in glucose and lipid profiles with 1 and 2 g day−1 of EOE, changes in body weight and BMI.Safety assessments
As a safety outcome, the occurrence of adverse events, including changes in vitals, and laboratory parameters were considered. Demographic data were recorded at screening and at the end of the study. Physical examination, and vital signs, were recorded at screening, and days 30, 60 and 90. Hematology, hepatic function, renal function, thyroid function, and urine analysis, were examined at screening and end of the study. Adverse events, if any, were recorded at every visit. All the subjects were followed up for 15 days after the last visit to assess their overall wellbeing and to record any post-treatment adverse effects.Statistical analysis
The statistical analysis was performed for all the subjects by STATA Software version 16.0. The data gathered was considered as either continuous or categorical variables. One-way analysis of variance (ANOVA) was used to compare the means of the three groups. Continuous variables and normally distributed data within the group were compared using paired t-test, and data were presented as mean difference and p-value. The non-normally distributed data within the group were analyzed by the Wilcoxon Signed-Rank test. The frequency and percentage of the population are presented for categorical variables and were compared using the Chi-square test. For the comparative analysis between treatment groups, an unpaired t-test was used. The level of statistical significance was defined as p < 0.05. A descriptive comparison is provided to differentiate the treatment effect between the treatment groups and within treatment groups.Results
Demographics
A total number of 170 subjects were screened, out of which 126 (75 males and 51 females) were found eligible and were randomized to metformin 500 mg (N = 42), EOE-1 g (N = 42), and EOE-2 g (N = 42). One subject each from metformin 500 mg and EOE-1 g groups were lost to follow-up, and 124 subjects [metformin 500 mg (N = 41), EOE-1 g (N = 41), and EOE-2 g (N = 42)] completed the study (Fig. 2). The average age was 47 ± 9.90 years in overall subjects, 48.12 ± 10.42, 46.17 ± 9.61 and 46.71 ± 9.77 years respectively in metformin 500 mg, EOE-1 g, and EOE-2 g groups. None of the subjects had a history of smoking, alcohol, and drug abuse (ESI Table 1†) and no other concomitant medications were used by the patients throughout the study.Glucose profile
FBS reduced significantly in all the study groups. The reduction was from 140.39 ± 7.39 mg dL−1 and 115.66 ± 10.55 mg dL−1 in metformin 500 mg group (p < 0.01), 140.56 ± 5.30 mg dL−1 to 119.93 ± 13.35 mg dL−1 in EOE-1 g group (p < 0.01), and 140.24 ± 4.57 mg dL−1 to 109.69 ± 16.38 mg dL−1 in EOE-2 g (p < 0.01) respectively at the end of the study. The mean reduction in FBS was significantly higher in EOE-2 g group than EOE-1 g (p < 0.01) and metformin 500 mg (p = 0.05) treatment (Table 1).| Parameters | Visits | metformin (N = 41) | EOE-1 g (N = 41) | EOE-2 g (N = 42) |
|---|---|---|---|---|
| Blood glucose levels at the screening visit and the follow-up visits conducted on days 30, 60, and 90; data represented as mean ± standard deviation. * p < 0.01 within the group compared to screening visit. # p < 0.05 ## p < 0.01 compared to EOE-2 g; HbA1c significantly reduced at day 90 compared to screening visit. | ||||
| FBS (mg dL−1) | Screening | 140.39 ± 7.39 | 140.56 ± 5.30 | 140.24 ± 4.57 |
| Day 30 | 131.51± 10.82* | 133.83 ± 6.77* | 134.26 ± 8.45* | |
| Day 60 | 124.1 ± 10.07* | 128.29 ± 9.78*## | 120.31 ± 14.25* | |
| Day 90 | 115.66 ± 10.55*# | 119.93 ± 13.35*## | 109.69 ± 16.38* | |
| PPBS (mg dL−1) | Screening | 247.41 ± 26.39 | 245.66 ± 28.91 | 245.31 ± 22.71 |
| Day 30 | 237.95 ± 27.8* | 239.44 ± 23.81* | 241.31 ± 21.92* | |
| Day 60 | 230.1 ± 29.51* | 232.05 ± 20.58* | 223.74 ± 18.62* | |
| Day 90 | 220.22 ± 20.72*## | 221.73 ± 23.56*## | 205.57 ± 20.15* | |
| HbA1c (mg dL−1) | Screening | 167.56 ± 15.35 | 163.34 ± 14.09 | 163.74 ± 12.97 |
| Day 90 | 135.86 ± 35.52* | 141.78 ± 20.49*# | 126.45 ± 25.47* | |
PPBS reduced from 247.41 ± 26.39 mg dL−1 to 220.22 ± 20.72 mg dL−1 in metformin 500 mg (p < 0.01), 245.66 ± 26.39 mg dL−1 to 221.73 ± 23.56 mg dL−1 in EOE-1 g group (p < 0.01), 245.31 ± 22.71 mg dL−1 to 205.57 ± 20.15 mg dL−1 in EOE-2 g group (p < 0.01) after 90 days of treatment. When mean changes were compared between groups, EOE-2 g was significantly higher than EOE-1 g and metformin 500 mg group (p < 0.01) (Table 1).
Glycosylated hemoglobin percentage (HbA1c changed from 7.87 ± 0.81% to 7.18 ± 0.85%, 7.79 ± 0.87% to 7.25 ± 0.82%, and 7.90 ± 0.81% to 6.87 ± 0.67% and respectively in metformin 500 mg, EOE-1 g, and EOE-2 g treatment groups from screening to final visit. HbA1c reduction was higher in EOE-2 g than EOE-1 g and metformin (p < 0.01) (Table 1).
Lipid profile
The TG, LDL, VLDL, HDL, and total cholesterol changed significantly at the end of the study in all groups in comparison with their respective screening visit values. The reduction in TG was 20.32, 17.73, and 21.43 mg dL−1, and that for TC was 30.58, 26.41, and 35.33 mg dL−1 in metformin, EOE-1 g and EOE-2 g respectively (p < 0.01, for all). However, there were no significant differences between groups (Table 2).| Parameters | Visits | metformin (N = 41) | EOE-1 g (N = 41) | EOE-2 g (N = 42) |
|---|---|---|---|---|
| Lipid levels at the screening visit, and the follow-up visits conducted on days 30, 60, and 90; data represented as mean ± standard deviation. Data represented as mean ± standard deviation. * p < 0.01 within the group compared to screening visit. # p < 0.05 ## p < 0.01 compared to EOE-2 g. | ||||
| TC (mg dL−1) | Screening | 243.56 ± 19.56 | 246.20 ± 17.30 | 236.40 ± 16.99 |
| Day 30 | 233.37 ± 21.04 | 236.78 ± 16.81 | 228.21 ± 18.41 | |
| Day 60 | 217.41 ± 24.37 | 225.22 ± 15.64 | 209.31 ± 18.21 | |
| Day 90 | 212.98 ± 31.15* | 219.79 ± 16.87*# | 201.07 ± 26.82* | |
| TG (mg dL−1) | Screening | 173.54 ± 13.11 | 182.63 ± 40.21 | 176.62 ± 16.77 |
| Day 30 | 160.68 ± 19.02* | 167.05 ± 46.25* | 163.05 ± 22.28* | |
| Day 60 | 150.76 ± 16.88* | 158.51 ± 55.59* | 150.21 ± 24.22* | |
| Day 90 | 153.22 ± 19.16* | 164.90 ± 52.61* | 155.14 ± 23.38* | |
| LDL (mg dL−1) | Screening | 167.56 ± 15.35 | 163.34 ± 14.09 | 163.74 ± 12.97 |
| Day 30 | 156.65 ± 24.19* | 160.13 ± 17.99# | 150.69 ± 23.61* | |
| Day 60 | 140.87 ± 29.28* | 148.69 ± 18.83*## | 134.02 ± 22.19* | |
| Day 90 | 135.86 ± 35.52* | 141.78 ± 20.49*## | 126.45 ± 25.47* | |
| VLDL (mg dL−1) | Screening | 38.17 ± 20.24 | 38.60 ± 25.92 | 39.59 ± 25.70 |
| Day 30 | 34.8 ± 18.36* | 35.67 ± 23.68* | 36.01 ± 22.05* | |
| Day 60 | 32.59 ± 16.24* | 33.97 ± 26.24* | 33.52 ± 23.96* | |
| Day 90 | 33.44 ± 19.19* | 35.36 ± 26.19* | 34.67 ± 25.30* | |
| HDL (mg dL−1) | Screening | 46.10 ± 11.30 | 44.49 ± 7.71 | 43.10 ± 6.82 |
| Day 30 | 45.98 ± 11.56 | 44.46 ± 7.85 | 43.62 ± 6.63 | |
| Day 60 | 46.63 ± 11.54 | 45.56 ± 8.11* | 44.69 ± 6.47* | |
| Day 90 | 47.39 ± 11.18* | 45.61 ± 8.03* | 45.07 ± 6.67* | |
The LDL and VLDL reduction followed a similar pattern. The reduction in LDL was 31.7, 21.56, and 37.29 mg dL−1 in metformin, EOE-1 g, and EOE-2 g, respectively (p < 0.01, for all), resulting in 18.9%, 13.2%, and 22.7% decrease from the baseline value. There were no significant differences between EOE-1 g, EOE-2 g in comparison to metformin. However, reduction in EOE-2 g was significantly better than EOE-1 g (p < 0.01) (Table 2).
The VLDL reduction was 12.4% for metformin and EOE-2 g, while it was slightly lower at 8.4% for EOE-1 g treatment. While all three treatments were able to reduce VLDL from baseline in 90 days significantly, there was no significant difference between the treatment groups (Table 2). HDL values increased by 1.29, 1.12 and 1.97 mg dL−1 in metformin, EOE-1 g (2.5%) and EOE-2 g (4.5%) treatment groups respectively resulting in 2.8%, 2.5% and 4.5% (Table 2). Although these changes were significant within the groups from baseline to end of the study, there was no significant difference between the three treatment groups.
A sub-analysis of the lipid parameters performed for male and female subjects is presented as ESI Table 2.† The results indicate that there is no significant difference between the lipid parameters between males and females. In addition, the effect of EOE on HDL levels was essentially the same in both males and females.
Body weight, BMI and blood pressure
There was no significant change in body weight and BMI in any of the EOE treatment or reference group (metformin) at the end of the study compared to their respective screening visit values. Also, there were no significant differences between groups for both body weight and BMI. Systolic blood pressure (SBP) showed a significant reduction in the EOE group from screening to 90 days, although the values were in the normal range (ESI Table 3†).Safety outcomes
The safety parameters did not show any significant changes from baseline to 90 days. The hematological parameters, renal function test, hepatic function test, and urine analysis were in the normal range in all the three treatment groups at the end of the study (ESI Table 4†). Blood and vital parameters, including pulse rate, body temperature, respiratory rate, and ECG, were normal in all three groups throughout the study. One subject in metformin 500 mg and EOE-2 g group had fever as an adverse event (AE). In EOE-1 g group, three subjects had headache, and one subject had drowsiness. All AEs were mild, single event and resolved during the study period and they were considered not related to the investigational product. No subject discontinued the study due to AE.Discussion
The present study assessed the efficacy of two doses of EOE (1 g and 2 g) per day compared against metformin 500 mg in newly diagnosed diabetes subjects associated with dyslipidemia. Both doses of EOE and metformin effectively reduced the circulating levels of glucose and glycosylated hemoglobin after 90 days of treatment. At the 2 g day−1 dose, EOE clearly showed superior efficacy in reducing FBS and PPBS compared to EOE-1 g and metformin 500 mg. The reduction in HbA1c was higher in the 2 g day−1 dose than the 1 g day−1 dose of EOE. Lipid management showed equivalent efficacy for both doses of EOE and metformin treatment.Diabetes and dyslipidemia are well-known independent risk factors for atherosclerotic cardiovascular diseases.19 In diabetic patients, dyslipidemia is a major risk factor for macrovascular complications.5 Further, the presence of insulin resistance with dyslipidemia increases the risk for myocardial infarction.20 Improved glycemic control reduces cholesterol and TG levels by increasing LDL catabolism, while cholesterol-lowering therapy can significantly reduce the CVD risk in diabetic patients.21,22 Metformin, the recommended first-line therapy for patients with T2DM, controls the blood glucose levels by lowering insulin resistance and decreasing the intestinal absorption of glucose.23 Several clinical trials demonstrate the therapeutic potential of metformin in reducing cardiovascular mortality and morbidity in diabetic patients.24 Therefore, in the present study, we compared the efficacy of a standardized extract of E. officinalis with metformin in diabetic patients with dyslipidemia.
EOE have been studied earlier for the management of diabetes, its related complications, and dyslipidemia in experimental animals and clinical studies.8,25,26 The fruit extract was reported to enhance insulin function, reduce insulin resistance, activate insulin signaling pathways, protect β-cells, scavenging free radicals, control inflammation and reduce the accumulation of advanced glycation end products.27
A clinical study comparing the efficacy and safety of E. officinalis (500 mg day−1) with simvastatin (20 mg day−1) showed a significant reduction of TC, LDL, TG, and VLDL, and increase in HDL with both treatments, while the blood pressure reduction was higher with EOE.28 EOE at doses of 1 g, 2 g, and 3 g was shown to reduce fasting and postprandial blood sugar and LDL, TG, total lipids, total cholesterol, and increase in HDL in T2DM patients.29 All these studies were carried out with EOE that were not standardized for any specific marker compound. Fatima et al. have claimed the existence of emblicanin A and emblicanin B as active components in the EOE.30 The occurrence and identity of emblicanin A and emblicanin B have since been disputed at least by two different research groups,12,13 adducing convincing evidence that these two compounds are not present in the EOE. The presence of ascorbic acid has been implicated in the biological activity of extracts. However, few other definitive studies have indicated that ascorbic acid may be present only in trace amounts in E. officinalis,12 ruling out the possibility of attributing the observed effects to ascorbic acid. We had earlier established the presence of BGG in amla and shown further that it has superior antioxidant activity compared to ascorbic acid.13,31 The abundant amount of BGG in EOE was conclusively identified by other researchers also that serve as an ideal molecule for delineating the activity of amla extracts.32 BGG has also been reported to enhance skin barrier function paving the way for its applications in cosmetics.33
This study is perhaps the first to evaluate the clinical efficacy and safety of a standardized extract containing 10% BGG along with hydrolyzable tannins. BGG was reported to be the major component isolated from E. officinalis fruits showing potent inhibition of human aldose reductase implicated in the development of secondary complications of diabetes.32 In addition, BGG prevented lipopolysaccharide (LPS)-induced activation of protein kinases and lowered reactive oxygen species (ROS) levels through inhibiting aldose reductase in murine macrophages, a potential mechanism of its anti-inflammatory activity.34 The outstanding ability of BGG to counteract methylglyoxal induced oxidative stress in lens epithelial cells was recently demonstrated35 by pre-treatment of the cells with BGG prior to methylglyoxal exposure. BGG was not only able to recuperate the antioxidant enzymes such as GPx and GSH but also moderated the levels of several inflammatory cytokines such as COX-2, CXCR4, IL-6 and IL-8, MCP-1 and ICAM-1 in addition to NF-κB. BGG was also found to be quite effective in an experimental in vitro model of glaucoma.36 BGG also was shown to have neuroprotective properties conferring cells the ability to survive β-amyloid assault.37,38 Oral bioavailability of BGG was demonstrated by Takemoto and Davies (2011),39 who showed the excretion of unmodified BGG in the rat urine showing its systemic availability and its possible impact on various organs. Furthermore, the promising activities of naturally occurring BGG inspired synthetic analogs such as β-glucogallin amide (4) wherein the ester bond is replaced by an amide bond with higher metabolic stability (Fig. 1).40 Earlier, it was shown that EOE standardized for 10% BGG along with hydrolysable tannins inhibits the activity of pancreatic α-amylase, and salivary α-amylases, α-glucosidase, and DPP-4 enzyme in vitro, suggesting the possible mechanism of antidiabetic activity.15 Hyperglycemia activates signal transduction pathways involving mitochondrial respiratory chain enzymes, culminating in ROS, which has been suggested as the “dangerous metabolic route in diabetes”.41 The 10% BGG extract showed significant protection against ROS in vitro.15,31
The antioxidant effects of the gallic acid esters of mucic acid have been noted by several authors, especially that of mucic acid 1,4-lactone 3-O-gallate (2). Luo et al. (2011)42 reported that mucic acid (2) possessed antioxidant and antiproliferative activities against breast cancer MCF-7 cells. The compound (2) was especially displaying ability to chelate iron(II) preventing the formation of superoxide anion radicals and hydroxyl radicals by preventing Fenton type reactions. In addition, compound (2) was also suppressing linoleic acid peroxidation presumably attributed again to its complexation of iron(II) ions. Gallic acid present in small quantities in EOE also has similar effects.42
The various compounds constituting EOE have excellent ROS inhibiting activity, thus, resulting in the reduction of oxidative stress, and consequently attenuate diabetic complications.
In the present study, EOE reduced FBS levels by 14.6% and 21.8% in 1 g and 2 g day−1 doses, respectively, compared to 17.6% by metformin. A similar trend was observed for both PPBS and HbA1c, suggesting the improved efficacy of EOE-2 g day−1 over metformin. The TC, LDL, VLDL, and TG were reduced with all three treatments, while the increase in HDL was consistently improved with EOE-2 g day−1. Interestingly, EOE was found to reduce SBP during the treatment period, suggesting other properties of the extract, which could be explored in the future.
EOE was found to be safe and well-tolerated for the study duration of 90 days. There were no major adverse events, and observed adverse events were mild in nature and were not related to the treatment.
A few limitations of the study could be the relatively small number of subjects in each group and the inclusion of only newly diagnosed diabetic patients. The present clinical study with EOE was also directed to explore the dose ranges and the safety aspects of the extract. Future studies can be planned in larger populations with different stages of the disease to confirm the benefits of EOE in diabetic complications.
Conclusion
The present data provide strong evidence that EOE (standardized for 10% BGG) at 1 g and 2 g day−1 is safe, well tolerated and exerted effective antidiabetic and lipid-lowering activities in diabetic dyslipidemia patients. More importantly, EOE at a dose of 2 g day−1 showed superior antidiabetic and anti-dyslipidemia activities compared to the pharmaceutical drug, metformin. Natural products such as standardized extract of E. officinalis is an attractive lead as a potential supplement for the management of T2DM with associated dyslipidemia since their low toxicity allows them to be used as long-term prophylactics.Abbreviations
| AE | Adverse event |
| BGG | β-Glucogallin |
| BMI | Body mass index |
| COX-2 | Cyclooxygenase-2 |
| CVD | Cardiovascular disease |
| CXCR4 | Chemokine receptor 4 |
| DBP | Diastolic blood pressure |
| EOE | Emblica officinalis extract |
| FBS | Fasting blood sugar |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HbA1c | Glycosylated hemoglobin |
| HDL | High-density lipoprotein |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| LDL | Low density lipoprotein |
| LPS | Lipopolysaccharide |
| MCP-1 | Monocyte chemoattractant protein-1 |
| NF-κB | Nuclear factor kappa B |
| PPBS | Postprandial blood sugar |
| ROS | Reactive oxygen species |
| SBP | Systolic blood pressure |
| T2DM | Type 2 diabetes mellitus |
| TC | Total cholesterol |
| TG | Triglycerides |
| VLDL | Very-low-density lipoprotein |
Conflicts of interest
All the authors are associated with Sami-Sabinsa group of companies that manufactures and sells amla-based extracts.Acknowledgements
No external funding was received for this research.References
- American Diabetes Association, Standards of medical care in diabetes–2009, Diabetes Care, 2009, 32(Suppl 1), S13–S61 CrossRef PubMed.
- P. Saeedi, I. Petersohn, P. Salpea, B. Malanda, S. Karuranga, N. Unwin, S. Colagiuri, L. Guariguata, A. A. Motala and K. Ogurtsova, Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, Diabetes Res. Clin. Pract., 2019, 157, 107843 CrossRef PubMed.
- G. F. Lewis, C. Xiao and R. A. Hegele, Hypertriglyceridemia in the genomic era: a new paradigm, Endocr. Rev., 2015, 36, 131–147 CrossRef CAS PubMed.
- S. H. Saydah, J. Fradkin and C. C. Cowie, Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes, J. Am. Med. Assoc., 2004, 291, 335–342 CrossRef CAS PubMed.
- B. P. Gaire and L. Subedi, Phytochemistry, pharmacology and medicinal properties of Phyllanthus emblica Linn, Chin. J. Integr. Med., 2014 DOI:10.1007/s11655-014-1984-2.
- D. Alkandari, H. Sarfraz and J. S. Sidhu, Development of a functional food (pan bread) using amla fruit powder, J. Food Sci. Technol., 2019, 56, 2287–2295 CrossRef CAS PubMed.
- S. S. Yadav, M. K. Singh, P. K. Singh and V. Kumar, Traditional knowledge to clinical trials: A review on therapeutic actions of Emblica officinalis, Biomed. Pharmacother., 2017, 93, 1292–1302 CrossRef PubMed.
- J. J. D'souza, P. P. D'souza, F. Fazal, A. Kumar, H. P. Bhat and M. S. Baliga, Anti-diabetic effects of the Indian indigenous fruit Emblica officinalis Gaertn: Active constituents and modes of action, Food Funct., 2014, 5, 635–644 RSC.
- B. C. Variya, A. K. Bakrania and S. S. Patel, Emblica officinalis (amla): A review for its phytochemistry, ethnomedicinal uses and medicinal potentials with respect to molecular mechanisms, Pharmacol. Res., 2016, 111, 180–200 CrossRef CAS PubMed.
- B. Yang and P. Liu, Composition and biological activities of hydrolyzable tannins of fruits of Phyllanthus emblica, J. Agric. Food Chem., 2014, 62, 529–541 CrossRef CAS PubMed.
- S. Ghosal, Active constituents of Emblica officinalis: Part I. The chemistry and antioxidative effects of two new hydrolysable tannins, emblicanin A and B, Indian J. Chem., 1996, 35, 941–948 Search PubMed.
- M. Majeed, B. Bhat, A. N. Jadhav, J. S. Srivastava and K. Nagabhushanam, Ascorbic acid and tannins from Emblica officinalis Gaertn. Fruits–a revisit, J. Agric. Food Chem., 2009, 57, 220–225 CrossRef CAS PubMed.
- D. Olennikov, N. Kashchenko, H. Schwabl, C. Vennos and C. Loepfe, New mucic acid gallates from Phyllanthus emblica, Chem. Nat. Compd., 2015, 51, 666–670 CrossRef CAS.
- Y. J. Zhang, T. Tanaka, C. R. Yang and I. Kouno, New phenolic constituents from the fruit juice of Phyllanthus emblica, Chem. Pharm. Bull., 2001, 49, 537–540 CrossRef CAS PubMed.
- M. Majeed, S. Majeed, L. Mundkur, K. Nagabhushanam, S. Arumugam, K. Beede and F. Ali, Standardized Emblica officinalis fruit extract inhibited the activities of α-amylase, α-glucosidase, and dipeptidyl peptidase-4 and displayed antioxidant potential, J. Sci. Food Agric., 2020, 100, 509–516 CrossRef CAS PubMed.
- S. H. Lin, P. C. Cheng, S. T. Tu, S. R. Hsu, Y. C. Cheng and Y. H. Liu, Effect of metformin monotherapy on serum lipid profile in statin-naive individuals with newly diagnosed type 2 diabetes mellitus: a cohort study, PeerJ, 2018, 6, e4578 CrossRef PubMed.
- M. P. Wrobel, B. Marek, D. Kajdaniuk, D. Rokicka, A. Szymborska-Kajanek and K. Strojek, metformin - a new old drug, Endokrynol. Pol., 2017, 68, 482–496 CrossRef CAS PubMed.
- A. M. Emslie-Smith, D. I. Boyle, J. M. Evans, F. Sullivan and A. D. Morris, Contraindications to metformin therapy in patients with Type 2 diabetes–a population-based study of adherence to prescribing guidelines, Diabetic Med., 2001, 18, 483–488 CrossRef CAS PubMed.
- J. D. Schofield, Y. Liu, P. Rao-Balakrishna, R. A. Malik and H. Soran, Diabetes Dyslipidemia, Diabetes Ther., 2016, 7, 203–219 CrossRef CAS PubMed.
- M. B. Mortensen, I. Kulenovic and E. Falk, Statin use and cardiovascular risk factors in diabetic patients developing a first myocardial infarction, Cardiovasc. Diabetol., 2016, 15, 81 CrossRef PubMed.
- P. M. Kearney, L. Blackwell, R. Collins, A. Keech, J. Simes, R. Peto, J. Armitage and C. Baigent, Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis, Lancet, 2008, 371, 117–125 CrossRef CAS.
- K. K. Ray, S. R. Seshasai, S. Wijesuriya, R. Sivakumaran, S. Nethercott, D. Preiss, S. Erqou and N. Sattar, Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: A meta-analysis of randomised controlled trials, Lancet, 2009, 373, 1765–1772 CrossRef CAS.
- G. Rena, D. G. Hardie and E. R. Pearson, The mechanisms of action of metformin, Diabetologia, 2017, 60, 1577–1585 CrossRef CAS PubMed.
- N. M. Maruthur, E. Tseng, S. Hutfless, L. M. Wilson, C. Suarez-Cuervo, Z. Berger, Y. Chu, E. Iyoha, J. B. Segal and S. Bolen, Diabetes medications as monotherapy or metformin-based combination therapy for type 2 diabetes: A systematic review and meta-analysis, Ann. Intern. Med., 2016, 164, 740–751 CrossRef PubMed.
- A. Jacob, M. Pandey, S. Kapoor and R. Saroja, Effect of the Indian gooseberry (amla) on serum cholesterol levels in men aged 35–55 years, Eur. J. Clin. Nutr., 1988, 42, 939–944 CAS.
- B. Salehi, A. Ata, N. V. Anil Kumar, F. Sharopov, K. Ramírez-Alarcón, A. Ruiz-Ortega, S. A. Ayatollahi, P. V. Tsouh Fokou, F. Kobarfard, Z. A. Zakaria, M. Iriti, Y. Taheri, M. Martorell, A. Sureda, W. N. Setzer, A. Durazzo, M. Lucarini, A. Santini, R. Capasso, E. A. Ostrander, Atta-ur-Rahman, M. I. Choudhary, W. C. Cho and J. Sharifi-Rad, Antidiabetic Potential of Medicinal Plants and Their Active Components, Biomolecules, 2019, 9, 551 CrossRef PubMed.
- H. Z. Huang, M. Qiu, J. Z. Lin, M. Q. Li, X. T. Ma, F. Ran, C. H. Luo, X. C. Wei, R. C. Xu, P. Tan, S. H. Fan, M. Yang, L. Han and D. K. Zhang, Potential effect of tropical fruits Phyllanthus emblica L. for the prevention and management of type 2 diabetic complications: a systematic review of recent advances, Eur. J. Nutr., 2021, 60, 3525–3542 CrossRef PubMed.
- B. Gopa, J. Bhatt and K. G. Hemavathi, A comparative clinical study of hypolipidemic efficacy of amla (Emblica officinalis) with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitor simvastatin, Indian J. Pharmacol., 2012, 44, 238–242 CrossRef PubMed.
- M. S. Akhtar, A. Ramzan, A. Ali and M. Ahmad, Effect of amla fruit (Emblica officinalis Gaertn.) on blood glucose and lipid profile of normal subjects and type 2 diabetic patients, Int. J. Food Sci. Nutr., 2011, 62, 609–616 CrossRef CAS PubMed.
- N. Fatima, U. Pingali and N. Muralidhar, Study of pharmacodynamic interaction of Phyllanthus emblica extract with clopidogrel and ecosprin in patients with type II diabetes mellitus, Phytomedicine, 2014, 21, 579–585 CrossRef CAS PubMed.
- M. Majeed, B. Bhat and T. S. S. Anand, Inhibition of UV induced adversaries by b-glucogallin from amla (Emblic officinalis Gaertn.) fruits, Indian J. Nat. Prod. Resour., 2010, 1, 462–465 Search PubMed.
- M. Puppala, J. Ponder, P. Suryanarayana, G. B. Reddy, J. M. Petrash and D. V. LaBarbera, The isolation and characterization of β-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis, PLoS One, 2012, 7, e31399 CrossRef CAS PubMed.
- H.-G. Kim, K. S. Kim, M. Kim, S.-H. Shin, Y.-G. Lee, M.-H. Bang, D.-G. Lee and N.-I. Baek, β-Glucogallin isolated from Fusidium coccineum and its enhancement of skin barrier effects, Appl. Biol. Chem., 2020, 63, 77 CrossRef CAS.
- K. C. Chang, B. Laffin, J. Ponder, A. Enzsöly, J. Németh, D. V. LaBarbera and J. M. Petrash, Beta-glucogallin reduces the expression of lipopolysaccharide-induced inflammatory markers by inhibition of aldose reductase in murine macrophages and ocular tissues, Chem.–Biol. Interact., 2013, 202, 283–287 CrossRef CAS PubMed.
- Y. Ma, F. Liu and Y. Xu, Protective Effect of β-Glucogallin on Damaged Cataract Against Methylglyoxal Induced Oxidative Stress in Cultured Lens Epithelial Cells, Med. Sci. Monit., 2019, 25, 9310–9318 CrossRef CAS PubMed.
- T. Cao, J. Wang, Y. Wu, L. Wang and H. Zhang, Antiglaucoma Potential of β-Glucogallin Is Mediated by Modulating Mitochondrial Responses in Experimentally Induced Glaucoma, NeuroImmunoModulation, 2020, 27, 142–151 CrossRef CAS PubMed.
- I. Mook-Jung, H. Kim, W. Fan, Y. Tezuka, S. Kadota, H. Nishijo and M. W. Jung, Neuroprotective effects of constituents of the oriental crude drugs, Rhodiola sacra, R. sachalinensis and Tokaku-joki-to, against beta-amyloid toxicity, oxidative stress and apoptosis, Biol. Pharm. Bull., 2002, 25, 1101–1104 CrossRef CAS PubMed.
- T. Sylla, L. Pouységu, G. Da Costa, D. Deffieux, J. P. Monti and S. Quideau, Gallotannins and Tannic Acid: First Chemical Syntheses and In Vitro Inhibitory Activity on Alzheimer's Amyloid β-Peptide Aggregation, Angew. Chem., Int. Ed., 2015, 54, 8217–8221 CrossRef CAS PubMed.
- J. K. Takemoto and N. M. Davies, Method development for β-glucogallin and gallic acid analysis: application to urinary pharmacokinetic studies, J. Pharm. Biomed. Anal., 2011, 54, 812–816 CrossRef CAS PubMed.
- L. Li, K. C. Chang, Y. Zhou, B. Shieh, J. Ponder, A. D. Abraham, H. Ali, A. Snow, J. M. Petrash and D. V. LaBarbera, Design of an amide N-glycoside derivative of β-glucogallin: a stable, potent, and specific inhibitor of aldose reductase, J. Med. Chem., 2014, 57, 71–77 CrossRef CAS PubMed.
- J. A. Nogueira-Machado and M. M. Chaves, From hyperglycemia to AGE-RAGE interaction on the cell surface: a dangerous metabolic route for diabetic patients, Expert Opin. Ther. Targets, 2008, 12, 871–882 CrossRef CAS PubMed.
- W. Luo, M. Zhao, B. Yang, J. Ren, G. Shen and G. Rao, Antioxidant and antiproliferative capacities of phenolics purified from Phyllanthus emblica L. fruit, Food Chem., 2011, 126, 277–282 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo01862d |
| This journal is © The Royal Society of Chemistry 2022 |