Open Access Article
Open Access ArticleProtective effect of traditional Korean fermented soybean foods (doenjang) on a dextran sulfate sodium-induced colitis mouse model
Hee-Jong
Yang
a,
Su-Ji
Jeong
a,
Myeong Seon
Ryu
a,
Gwangsu
Ha
a,
Do-Youn
Jeong
a,
Young Mi
Park
b,
Hak Yong
Lee
b and
Jun Sang
Bae
 *c
*c
aMicrobial Institute for Fermentation Industry (MIFI), Sunchang, Jeonbuk 56048, Korea
bINVIVO Co. Ltd., Deahak-ro, 121, Nonsan, Chungnam 32992, Korea
cDepartment of Pathology, College of Korean Medicine, Wonkwang University, 460, Iksan, Jeonbuk 54538, Korea. E-mail: jsbae78@wku.ac.kr; Fax: +82-63-856-6843; Tel: +82-63-850-7359
First published on 27th July 2022
Abstract
Objective: The cause of ulcerative colitis (UC) is unknown, and the use of anti-inflammatory and immunosuppressive drugs with certain side effects is currently replacing treatment. Therefore, it is important to find new healthy foods or ingredients that exhibit potential protective and anti-inflammatory effects on UC. This study investigated the potential protective effect of doenjang on dextran sulfate sodium (DSS)-induced colitis in a mouse model. Materials and methods: Four doenjang samples (TCD21-51-1, TCD21-55-1, TMD21-16-1, and TFD21-1-1) were used. To examine the effects of the four doenjang samples on UC caused by DSS in a mouse model, the clinical symptoms of UC, such as body weight, disease activity index (DAI), and colon macroscopic damage index (CMDI) were analyzed. Moreover, immune-related blood cell counts, serum levels and protein expression of tumor necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), and nitric oxide (NO) production were measured in DSS-induced UC in mice for analysis. Results: The four doenjang samples increased the colon length shortened by DSS, reduced DAI (diarrhea and hemoccult), CMDI (ulceration, inflammation, and hemorrhage) and the content of immune-related cells in the blood. Moreover, the levels of TNF-α, IL-6, and NO increased by DSS were decreased by doenjang, and tissue damage was significantly reduced. Conclusions: These findings confirmed that doenjang exerts protective effects against UC, suggesting its possible use in developing therapeutic strategies or functional products.
1. Introduction
Inflammatory bowel disease (IBD) is characterized by chronic bowel inflammation. The cause of IBD is not clearly known, and it is thought that autoimmune, infectious, environmental, and genetic factors are involved. Inflammatory bowel diseases are largely divided into ulcerative colitis (UC) and Crohn's disease (CD).1,2 UC affects only the rectum and colon, and the lesions are more homogeneous and continuous. In contrast, CD affects all parts of the gastrointestinal tract with characteristic skipping lesions, with UC being more frequently reported as a causative factor for colorectal cancer than CD.3,4UC is characterized by diarrhea, fever, weight loss, and abdominal pain as the main symptoms. In severe cases, stools with several episodes of bleeding, weight loss, anemia, and dehydration may occur. Additionally, when a long period elapses, inflammatory ulcers occur and the colon is atrophied, with shortening of its length and loss of its shape.5,6 Since the cause of UC is unknown, it is difficult to treat it with fundamental drug therapy. Currently, using anti-inflammatory drugs and immunosuppressants, which have certain side effects, is replacing treatment. The main drugs used to maintain and improve the condition are 5-aminosalicylate (5-ASA) and sulfasalazine (SF).6–8 Moreover, a recent study reported that a healthy diet plays an important role in alleviating UC symptoms.9 Therefore, it is important to find new healthy foods or ingredients that can have potential protective and anti-inflammatory effects in UC.
Fermented food contains beneficial enzymes and nutrients that raw materials do not have, and are widely consumed because of its taste and easy storage. Particularly, fermented soybean products and kimchi, which are traditional Korean fermented foods, are considered the best health foods worldwide.10 Traditional fermented Korean soybean foods are also known as doenjang. Soybeans contain a variety of biological functional components that can be classified as isoflavones, soyasaponins, lignans, cinnamic acid derivatives, terpenes and sterols.11 The main phytochemicals in doenjang are reported to be free isoflavones, non-2,3-dihydro-2,5-dihydroxy-6-methyl-4H-pyran-4-one (DDMP)-conjugated soyasaponins, peptides, amino acids, and kojic acid.11Doenjang has been suggested to exert various biological effects, such as antioxidant,12 anti-obesity,13 anti-tumorigenic,14 anti-hypertensive,15 anti-sarcopenic,16 immune modulation,17 and anti-diabetic18 activities.
Recent studies have shown that doenjang has an anti-inflammatory effect on biological activity.19–21 However, there is still a lack of research showing that doenjang may help relieve the symptoms of UC through its anti-inflammatory activities. Therefore, this study investigated the potential protective and anti-inflammatory effects of doenjang on dextran sulfate sodium (DSS)-induced colitis in a mouse model.
2. Materials and methods
2.1. Samples
The four traditional Korean fermented soybean (doenjang) samples (TCD21-51-1, TCD21-55-1, TMD21-16-1, and TFD21-1-1) used in this study were provided by the Microbial Institute for Fermentation Industry (Sunchang, Jeonbuk, Korea). Dextran sulfate sodium (DSS) and sulfasalazine (SF) (≥98% purity) were purchased from Sigma-Aldrich (St Louis, MO, USA). The four doenjang samples were used for the experiment after dilution with distilled water and the prepared samples were refrigerated.2.2. Animals and experimental design
Specific-pathogen free (SPF) five-week-old male 70 C57BL/6 mice (Orient Bio, Inc., Seongnam, Gyeonggi, Korea) were acclimatized for seven days. During the acclimatization period, general solid feed (Purina Lab Rodent Chow #38057, Purina Co., Seoul, Korea) was used as the experimental feed, and filtered drinking water was changed daily so that it could be freely consumed. During the experimental period, the mice were kept under a 12 h light/dark cycle with controlled temperature (22 ± 3 °C), humidity (50 ± 5%), and illumination (150–300 lx). After the adaptation period, the body weights were measured, and the mean values between groups were used to equally divide the mice into seven groups (10 animals per group) according to a randomized block design: normal control group (normal), 3% DSS group (control), 3% DSS + TCD21-51-1 group (TCD21-51-1), 3% DSS + TCD21-55-1 group (TCD21-55-1), 3% DSS + TMD21-16-1 group (TMD21-16-1), 3% DSS + TFD21-1-1 group (TFD21-1-1), and 3% DSS + sulfasalazine (SF) group (SF, positive control). The four doenjang samples and SF were prepared at concentrations of 200 and 60 mg kg−1, respectively, and administered orally for 13 days. From the 8th day of oral administration, colitis was induced for 6 days in the control, TCD21-51-1, TCD21-55-1, TMD21-16-1, TFD21-1-1, and SF groups through free access to drinking water with a DSS concentration of 3%. The body weight, food intake, and drinking water intake were measured once a week before DSS administration and once a day after DSS administration at a certain time. After completion of the examination, an autopsy was performed under inhalation anesthesia (isoflurane, USP), and the colon tissue was removed and washed after blood collection. The extracted colons were used for tissue analysis after measuring their length and analyzing the clinical lesions. All animal experiments were approved by the Institutional Animal Care and Use Committee of INVIVO Co., Ltd (IV-RB-13-2109-25-R).2.3. Disease activity index (DAI) and colon macroscopic damage index (CMDI)
To assess the colitis severity, the disease activity index (DAI) was evaluated by checking stool consistency and bloody stools daily. The DAI was scored as 0 (normal stool), 1 (only loose stool), 2 (loose stool and hemoccult positive), 3 (diarrhea and hemoccult positive), and 4 (diarrhea and bleeding around the rectum). The colon macroscopic damage index (CMDI) was evaluated as previously described.22 Briefly, open colonic samples were thoroughly washed with cold normal saline, and blind assessment was performed via the CMDI, which included the severity of inflammation and the presence of ulcers. The CMDI was scored as 0 (normal), 1 (focal hypermia and no ulcers), 2 (ulceration without hypermia or bowel wall thickening), 3 (ulceration with inflammation at one site), 4 (two or more sites of inflammation and ulceration), 5 (major site of damage extending 1 cm along the length of the colon), and 6 (when the area of damage extended 2 cm along the length of the colon, the score increased by 1 for each additional cm of damage).2.4. Complete blood cell (CBC) count
For complete blood count analysis, the collected blood was placed in an EDTA tube (DB Caribe, Ltd, USA) and rotated in a roll mixer for 30 min, and the number of total white blood cells (WBCs), granulocytes, lymphocytes, and mid-range absolute counts (Mid) were measured using a Hemavet 950 counter (Drew Scientific Group, Dallas, TX, USA).2.5. Enzyme-linked immunosorbent assay (ELISA)
For cytokine analysis, after the blood was coagulated at room temperature for 30 min, the serum was separated by centrifugation at 3000 rpm for 10 min. The separated serum was analyzed for tumor necrosis factor-α (TNF-α; Thermo Fisher Scientific, Waltham, MA, USA) and interleukin-6 (IL-6; R&D Systems, Minneapolis, MN, USA) using an ELISA kit. To determine the content of nitric oxide (NO) in the colonic tissue, the extracted tissues were quantified equally at 10 mg followed by homogenization using a homogenizer. The homogenized tissues were analyzed for NO (Abcam, Cambridge, MA, USA) using an ELISA kit.2.6. Western blotting analysis
Briefly, the colon tissues were homogenized and extracted using PRO-PREP Protein Extraction Solution (iNtRON Biotechnology, Seongnam, Korea). Lysates were separated by centrifugation at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, at 4 °C for 10 min. The supernatant was quantified with the same amount of lysates using Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed with primary antibodies for TNF-α (Abcam), IL-6 (Abcam), and β-actin (Sigma-Aldrich). The membranes were washed with Tris-buffered saline and 0.1% Tween 20 (TBS-T), followed by treatment with secondary antibodies containing horseradish peroxidase (HRP) for 1 h. Membranes were washed with TBS-T, treated with an enhanced chemiluminescence (ECL) solution (EZ-Western Lumi Pico, DoGen, Korea), and detected using a C-Digit western scanner (LI-COR, Lincoln, NE, USA).
000 rpm, at 4 °C for 10 min. The supernatant was quantified with the same amount of lysates using Bradford reagent (Bio-Rad, Hercules, CA, USA). Proteins were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were probed with primary antibodies for TNF-α (Abcam), IL-6 (Abcam), and β-actin (Sigma-Aldrich). The membranes were washed with Tris-buffered saline and 0.1% Tween 20 (TBS-T), followed by treatment with secondary antibodies containing horseradish peroxidase (HRP) for 1 h. Membranes were washed with TBS-T, treated with an enhanced chemiluminescence (ECL) solution (EZ-Western Lumi Pico, DoGen, Korea), and detected using a C-Digit western scanner (LI-COR, Lincoln, NE, USA).
2.7. Histological analysis
The extracted colon tissue was refixed by trimming the sample fixed in 10% formalin solution, embedded in paraffin, and sliced into 4 μm sections. For hematoxylin–eosin staining, paraffin was removed from xylene and dehydrated, followed by staining with hematoxylin for 4 min and eosin for 2 min. The sectioned tissues were observed and photographed using an optical microscope (BX50 F4; Olympus, Fukuoka, Japan).2.8. Statistical analysis
All experimental results were calculated as mean ± standard error (mean ± SE) using a statistical program (SPSS ver.12.0, SPSS Inc., Chicago, IL, USA). One-way analysis of variance (ANOVA) and Duncan's test were performed for statistical analysis according to the statistical significance test for each experimental group. P-Values < 0.05 were considered statistically significant.3. Results
3.1. Effects of doenjang on DSS-induced ulcerative colitis symptoms
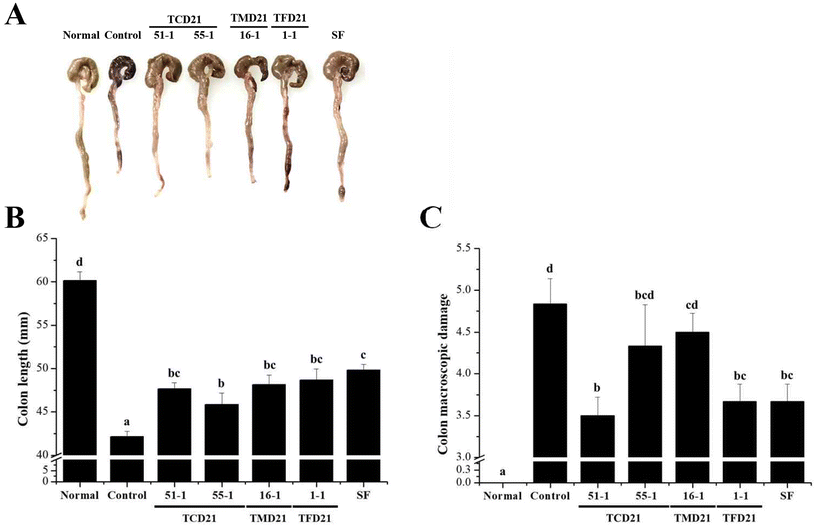
As the fermentation degree, soaking ratio, and maturation period of Meju (fermented soybean) vary depending on the manufacturer, there are differences in the quality and biological activity of doenjang products.23 Therefore, we used four doenjang samples (TCD21-51-1, TCD21-55-1, TMD21-16-1, and TFD21-1-1) in this study. To examine the effects of the four doenjang samples on ulcerative colitis (UC) caused by dextran sulfate sodium (DSS) in a mouse model, biomarkers such as body weight and disease activity index (DAI) were analyzed. In previous studies, DSS-induced UC models exhibited weight loss and colonic contraction in the early stages of colitis.24,25 Therefore, in this study, UC symptoms were assessed based on factors, such as body weight, DAI, and colon-related clinical characteristics. By measuring the dietary and drinking water intake of the four doenjang groups in a DSS-induced colitis mouse model, it was confirmed that the dietary and drinking water intake was reduced by DSS compared to the normal group (Fig. 1A and B). After DSS administration, the control group, the four doenjang groups, and the sulfasalazine (SF) group showed significantly lower body weights than the normal group, whereas the body weights of the four doenjang groups did not show a significant difference compared with the control group (Fig. 1C). On supplying 3% DSS, the symptoms of diarrhea and bloody stools were observed for 6 days, after which the DAI was measured. The analysis showed that the symptoms began to appear on the 2nd day of DSS treatment, and on the 6th day, the control group score was significantly higher than that of the normal group. Compared to the normal group, the DAI score was significantly higher in all experimental groups. Among the experimental groups, it was confirmed that the DAI score decreased in the order of the TMD21-16-1 group (2.61 ± 0.13) < TCD21-51-1 group (2.61 ± 0.16) < TFD21-1-1 group (2.81 ± 0.16) < sulfasalazine group (2.97 ± 0.09) < control group (3.00 ± 0.07) < TCD21-55-1 group (3.08 ± 0.12) (Fig. 1D). However, these results showed no difference among the four doenjang groups.3.2. Effects of doenjang on DSS-induced colon damage
In this study, we investigated the effect of doenjang on the colon length and colon macroscopic damage index (CMDI) in a DSS-induced UC model. The colon length was significantly decreased in the control group compared to that in the normal group, and each experimental group showed a significant increase compared to the control group (Fig. 2A). Among the experimental groups, it was confirmed that the colon length increased in the order of the sulfasalazine (SF) group (49.83 ± 0.65 mm) > TFD21-1-1 group (48.67 ± 1.31 mm) > TMD21-16-1 group (48.17 ± 1.08 mm) > TCD21-51-1 group (47.67 ± 0.71 mm) > TCD21-55-1 group (45.83 ± 1.35 mm) > control group (42.17 ± 0.60 mm) (Fig. 2B). On the 6th day of colitis induction, the colon was removed, and the damage (ulceration, inflammation, and bleeding) was visually observed and evaluated. By measuring the inflammatory index of the colon, it was found that the colitis-induced control group showed significantly higher levels than the normal group, but both the doenjang and sulfasalazine groups showed lower levels than the control group. The occult blood index in the colon was significantly higher in the control group than in the normal group, and intracolonal bleeding was observed. However, the doenjang group had significantly lower scores than the control group. The CMDI score, which combines these indices, was significantly higher in the control group than in the normal group. The CMDI score increased in the order of the TCD21-51-1 group (3.50 ± 0.22) < TFD21-1-1 group (3.67 ± 0.21) < sulfasalazine group (3.67 ± 0.21) < TCD21-55-1 group (4.33 ± 0.49) < TMD21-16-1 group (4.50 ± 0.22) < control group (4.83 ± 0.31). In particular, the levels in the TCD21-51-1, TFD21-1-1, and sulfasalazine groups were significantly lower than those in the control group (Fig. 2C). These results suggest that doenjang can alleviate the symptoms of UC caused by DSS.To investigate the effect of doenjang on the pathological changes in colonic tissue caused by colitis, we performed H&E staining. In the control group, ulceration was observed, mucosal goblet cells and crypts were lost, and the epithelial barrier was disrupted. Inflammatory cells over-infiltrated the submucosal tissue, and submucosal edema was severe. In contrast, the TCD21-51-1 experimental group showed relief of glandular ulcers and submucosal edema observed in the control group and showed favorable levels of epithelial tissue collapse, implying that tissue damage was the most alleviated among the four doenjang groups. For TCD21-55-1, TMD21-16-1, and TFD21-1-1 groups, submucosal edema was found only at a localized site, so tissue lesions were significantly reduced compared to that in the control. Only minor inflammatory cell infiltration and ulceration were observed, indicating a relatively good tissue condition. The sulfasalazine group showed only local infiltration of inflammatory cells (Fig. 3). Taken together, these results showed that doenjang could effectively prevent damage to colonic tissue.
3.3. Effects of doenjang on inflammatory factors in DSS-induced UC
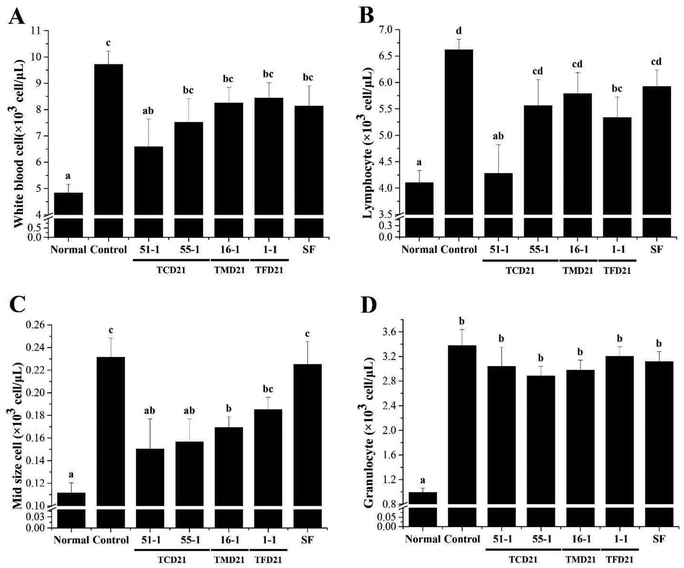
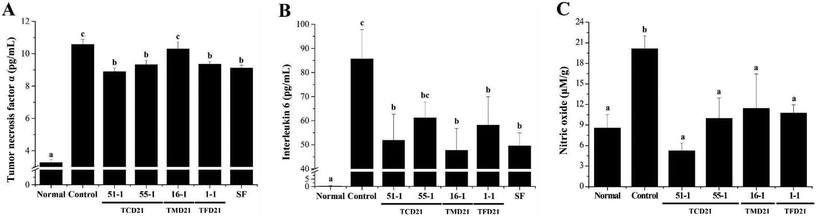
In UC, ulcers are formed owing to the infiltration of inflammatory cells and an increase in pro-inflammatory cytokines. Therefore, to confirm the improvement effect of doenjang on UC, blood was collected on the 6th day after UC induction, and the number of total white blood cells (WBCs), granulocytes, lymphocytes, and mid-range absolute counts (Mid) was measured. The number of total WBCs, granulocytes, lymphocytes, and Mid in the blood was significantly higher in the control groups than in the normal group. The four doenjang groups showed significantly decreased total WBCs, lymphocytes, and Mid counts compared to the control groups (Fig. 4A–C). However, the four doenjang groups showed no difference in granulocyte counts compared to the control group (Fig. 4D). Next, to determine the anti-inflammatory effect of doenjang, the serum levels of TNF-α and IL-6 were measured. The serum TNF-α and IL-6 levels were significantly higher in the control group than in the normal group. However, the three doenjang groups, except for the TMD21-16-1 group, showed a significant decrease in TNF-α levels compared with the control group (Fig. 5A). In addition, the four doenjang groups showed a significant decrease in IL-6 levels compared to the control group (Fig. 5B). Finally, increased nitric oxide (NO) content is associated with the severity of colitis in patients with UC and in DSS-induced UC animal models.26 By measuring NO in the colon tissue of DSS-induced UC, it was found that the NO level of the control group increased compared to the normal group, and the administered four doenjang groups significantly decreased (Fig. 5C). Altogether, these findings indicate that doenjang treatment inhibited inflammatory factors, such as immune cells, pro-inflammatory cytokines, and NO in the DSS-induced UC mouse model.3.4. Expression of TNF-α and IL-6 protein with doenjang use in DSS-induced UC tissues
Intestinal immune cells and epithelial cells produce many pro-inflammatory cytokines such as TNF-α and IL-6 in DSS-induced UC mice.27 The expression of the pro-inflammatory proteins TNF-α and IL-6 in UC tissues was analyzed by western blotting. The expression of inflammation-related proteins TNF-α and IL-6 in the colon tissue was higher in the control group than in the normal group, and the four doenjang groups showed decreased expression levels of TNF-α and IL-6 compared to the control group (Fig. 6A). The expression of each protein was normalized to that of actin and the relative expression levels in the normal group were analyzed. We confirmed that TNF-α and IL-6 protein levels in the doenjang groups were lower than those in the control group (Fig. 6B).4. Discussion
Doenjang, a representative fermented soybean food in Korea, has been found to exhibit biological activities, including anti-inflammatory, antioxidant, anti-cancer, and anti-diabetic activities.12–19 The biological activity of doenjang has been reported to be mediated by isoflavone, non-DDMP-conjugated soyasaponins, peptides, amino acids, and kojic acids.11,19 However, research is still lacking to determine if doenjang may help relieve the symptoms of ulcerative colitis (UC) through its anti-inflammatory properties. Therefore, this study investigated the potential protective effect of doenjang on colitis caused by dextran sulfate sodium (DSS) in mice.DSS administration in rodents induces UC and exhibits clinical and histopathological characteristics similar to those of human UC, such as weight loss, colon shortening, bloody stool, diarrhea, inflammatory cell infiltration, epithelial damage, and mucosal disruption.5,28 Therefore, DSS-induced experimental animals are a widely accepted model for studying UC pathogenesis. Conventional drugs used to treat UC are anti-inflammatory and immunosuppressive, including corticosteroids, 5-aminosalicylic acid (5-ASA), and sulfasalazine (SF). However, these drugs can cause side effects.6–8 Alternative treatment strategies are currently used to compensate for these shortcomings. Dietary ingredients, natural products, and herbs have been shown to have therapeutic effects in colitis.9,29 Therefore, it is important to develop new health foods or ingredients that may have potential anti-inflammatory and immune-enhancing effects in UC.
In this study, DSS-treated mice exhibited greater weight loss, higher DAI scores, shorter colon length, increased CMDI scores, and morphological tissue damage, such as ulceration, mucosal and epithelial disruption, and inflammatory cell infiltration, compared to the normal group. Compared with the DSS-treated group, the four doenjang groups suppressed the clinical symptoms of UC and histological damage to the colon, such as ulceration and epithelial and mucosal damage. Doenjang groups showed a similar or lower DAI score than the positive control SF group. These results were consistent with previous studies showing that doenjang and phytochemicals from soybeans improved UC.20,30–34 These results suggest that doenjang effectively suppresses the symptoms of UC.
Cytokines perform important functions including lymphocyte differentiation, regulation of inflammation, cell survival, apoptosis, and immune responses.35 Among the diverse immune cells, T lymphocytes are essential regulatory cells of the adaptive immune system.36 The various cytokines secreted by the T cell subtypes Th1 and Th2 are important determinants of cellular function. Th1 and Th2 cells are mainly involved in cell-mediated and humoral responses, respectively, and promote the secretion of IL-2, TNF-α, IFN-γ and IL-4, IL-6, IL-10, respectively.37,38 Previous studies have demonstrated increased activity of inflammation-related cytokines, including TNF-α, IL-6, and IL-1β, in serum and colon tissues in several models of colitis due to colonic inflammation.20,25,26 Our results showed that the serum levels and protein expression of TNF-α and IL-6 were reduced in DSS-induced mice in the four doenjang groups compared to the control group. These results were consistent with previous studies showing that doenjang and phytochemicals from soybeans reduced the mRNA level and activity of inflammation-related cytokines, including TNF-α, IL-6, and IL-1β, in serum and colon tissues in a colitis mouse model.14,20,30–34 Thus, these compounds appeared to reduce inflammatory cytokines.14,39 Nitric oxide (NO) is an important physiological substance in many biological systems, including immune, nervous, and cardiovascular tissues.40 During inflammation, significant amounts of NO and prostaglandin E2 (PGE2) are produced by inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), respectively. High NO production causes DNA damage and mutations during inflammation and infection. Therefore, NO overexpression may be implicated in the pathogenesis of many diseases, such as colitis, colon cancer, neurodegenerative diseases, and cardiac infarction.19 Our results also showed that the serum levels of NO were decreased in the four doenjang groups compared to the control group. In previous studies, phytochemicals from soybeans have been revealed to be anti-inflammatory compounds with an inhibitory effect on NO production in chemically induced intestinal inflammation models and macrophages.39–43 Moreover, doenjang administration showed an anti-inflammatory effect on DSS-induced colon tissue by decreasing the mRNA level of iNOS, which produces NO.20,30 Altogether, our findings suggest that doenjang may alleviate UC by inhibiting the inflammatory factors TNF-α, IL-6, and NO.
Blood, one of the most important indicators of the inflammatory response and immune function, defends the body in various ways, including clotting during bleeding and phagocytosis during bacterial invasion.44 Furthermore, immune cells such as T and B lymphocytes, monocytes, and macrophages play an important role in regulating immune and inflammatory responses.45 A previous study reveals that DSS-induced UC affects the immune system and causes a severe inflammatory response in the colon, increasing the number of total WBCs, lymphocytes, and Mid. It is also known to reduce hemoglobin (Hb), albumin, and triglyceride (TG) levels. However, ZnONP administration significantly induced a decrease in the number of total WBCs, lymphocytes, and Mid and induced an increase in Hb, albumin, and TG levels.46 In our results, the four doenjang groups significantly reduced in the number of total WBCs, lymphocytes and Mid in comparison with the DSS-treated group. These results show that doenjang may enhance anti-inflammatory activity and immune responses in DSS-induced colitis in mice.
Taken together, these results suggest protective and anti-colitic effects of doenjang on DSS-induced colitis in mice. Although our current study focused on the bioactive efficacy of doenjang intake to prevent UC rather than signaling pathways, further studies on the signaling pathways of these inflammatory factors are required to identify the precise anti-inflammatory mechanisms.
5. Conclusions
In conclusion, doenjang suppressed the clinical symptoms of UC and histological damage to the colon. Additionally, doenjang reduced the serum levels and protein expression of pro-inflammatory cytokines TNF-α and IL-6, and NO production and immune cells in DSS-induced UC mice. Therefore, these findings suggest that doenjang effectively protects against UC and may be useful in the development of therapeutic strategies or functional products.Author contributions
Conceptualization: HY, YMP, HYL, and JSB; data curation: HY, SJ, MSR, YMP, HYL, and JSB; formal analysis: HY, SJ, GH, DJ, YMP, and JSB; funding acquisition: HY and YMP; investigation: HY, SJ, MSR, YMP, and JSB; methodology: HY, SJ, GH, DJ, YMP, HYL, and JSB; project administration: HY, DJ, YMP, HYL, and JSB; resources: HYL and JSB; software: n/a; supervision: YMP and JSB; validation: HY, YMP, HYL, and JSB; visualization: HY, SJ, MSR, YMP, and JSB; writing – original draft: JSB; writing – review & editing: YMP, HYL, and JSB.Data availability
The data used to support the findings of this study have been included in this article.Conflicts of interest
The authors declare no conflicts of interest regarding the publication of this article.Acknowledgements
This work was supported by the “functional research of fermented soybean food (safety monitoring)” under the Ministry of Agriculture, Food and Rural Affairs and partly by Korea Agro-Fisheries and Food trade corporation.References
- H. Li, L. M. Christman, R. Li and L. Gu, Synergic interactions between polyphenols and gut microbiota in mitigating inflammatory bowel diseases, Food Funct., 2020, 11, 4878–4891 RSC.
- J. Kikut, N. Konecka, M. Ziętek, D. Kulpa and M. Szczuko, Diet supporting therapy for inflammatory bowel diseases, Eur. J. Nutr., 2021, 60, 2275–2291 CrossRef CAS PubMed.
- I. Loddo and C. Romano, Inflammatory Bowel Disease: Genetics, Epigenetics, and Pathogenesis, Front. Immunol., 2015, 6, 551 Search PubMed.
- N. N. Andersen and T. Jess, Has the risk of colorectal cancer in inflammatory bowel disease decreased?, World J. Gastroenterol., 2013, 19, 7561–7568 CrossRef CAS PubMed.
- S. Akiyama, A. Nesumi, M. Maeda-Yamamoto, M. Uehara and A. Murakami, Effects of anthocyanin-rich tea “Sunrouge” on dextran sodium sulfate-induced colitis in mice, BioFactors, 2012, 38, 226–233 CrossRef CAS PubMed.
- L. Peyrin-Biroulet, P. Desreumaux, W. J. Sandborn and J. F. Colombel, Crohn's disease: beyond antagonists of tumour necrosis factor, Lancet, 2008, 372, 67–81 CrossRef CAS.
- S. B. Hanauer, Inflammatory bowel disease, N. Engl. J. Med., 1996, 334, 841–848 CrossRef CAS PubMed.
- C. T. Xu, S. Y. Meng and B. R. Pan, Drug therapy for ulcerative colitis, World J. Gastroenterol., 2004, 10, 2311–2317 CrossRef CAS PubMed.
- M. A. Conlon and A. R. Bird, The impact of diet and lifestyle on gut microbiota and human health, Nutrients, 2014, 7, 17–44 CrossRef PubMed.
- H. K. Chung, H. J. Yang, D. Shin and K. R. Chung, Aesthetics of Korean foods: The symbol of Korean culture, J. Ethn. Foods, 2016, 3, 178–188 CrossRef.
- C. H. Jang, J. Oh, J. S. Lim, H. J. Kim and J. S. Kim, Fermented Soy Products: Beneficial Potential in Neurodegenerative Diseases, Foods, 2021, 10, 636 CrossRef CAS PubMed.
- N. Watanabe, K. Fujimoto and H. Aoki, Antioxidant activities of the water-soluble fraction in tempeh-like fermented soybean (GABA-tempeh), Int. J. Food Sci. Nutr., 2007, 58, 577–587 CrossRef CAS PubMed.
- R. Okouchi, Y. Sakanoi and T. Tsuduki, Miso (Fermented Soybean Paste) Suppresses Visceral Fat Accumulation in Mice, Especially in Combination with Exercise, Nutrients, 2019, 11, 560 CrossRef CAS PubMed.
- L. Coward, N. C. Barnes, K. D. R. Setchell and S. Barnes, Genistein, Daidzein, and Their Beta-Glycoside Conjugates—Antitumor Isoflavones in Soybean Foods from American and Asian Diets, J. Agric. Food Chem., 1993, 41, 1961–1967 CrossRef CAS.
- K. Ito, Review of the health benefits of habitual consumption of miso soup: focus on the effects on sympathetic nerve activity, blood pressure, and heart rate, Environ. Health Prev. Med., 2020, 25, 45 CrossRef PubMed.
- F. Takahashi, Y. Hashimoto, A. Kaji, R. Sakai, Y. Kawate, T. Okamura, N. Kitagawa, H. Okada, N. Nakanishi, S. Majima, T. Senmaru, E. Ushigome, M. Hamaguchi, M. Asano, M. Yamazaki and M. Fukui, Habitual Miso (Fermented Soybean Paste) Consumption Is Associated with a Low Prevalence of Sarcopenia in Patients with Type 2 Diabetes: A Cross-Sectional Study, Nutrients, 2020, 13, 72 CrossRef PubMed.
- T. Kumazawa, A. Nishimura, N. Asai and T. Adachi, Isolation of immune-regulatory Tetragenococcus halophilus from miso, PLoS One, 2018, 13, e0208821 CrossRef PubMed.
- D. Y. Kwon, J. W. Daily 3rd, H. J. Kim and S. Park, Antidiabetic effects of fermented soybean products on type 2 diabetes, Nutr. Res., 2010, 30, 1–13 CrossRef CAS PubMed.
- C. S. Kwak, D. Son, Y. S. Chung and Y. H. Kwon, Antioxidant activity and anti-inflammatory activity of ethanol extract and fractions of Doenjang in LPS-stimulated RAW 264.7 macrophages, Nutr. Res. Pract., 2015, 9, 569–578 CrossRef PubMed.
- J. K. Jeong, H. K. Chang and K. Y. Park, Doenjang prepared with mixed starter cultures attenuates azoxymethane and dextran sulfate sodium-induced colitis-associated colon carcinogenesis in mice, J. Carcinog., 2014, 13, 9 CrossRef PubMed.
- Y. R. Nam, S. B. Won, Y. S. Chung, C. S. Kwak and Y. H. Kwon, Inhibitory effects of Doenjang, Korean traditional fermented soybean paste, on oxidative stress and inflammation in adipose tissue of mice fed a high-fat diet, Nutr. Res. Pract., 2015, 9, 235–241 CrossRef CAS PubMed.
- Y. F. Huang, Q. P. Li, Y. X. Dou, T. T. Wang, C. Qu, J. L. Liang, Z. X. Lin, X. Q. Huang, Z. R. Su, J. N. Chen and Y. L. Xie, Therapeutic effect of Brucea javanica oil emulsion on experimental Crohn's disease in rats: Involvement of TLR4/NF-κB signaling pathway, Biomed. Pharmacother., 2019, 114, 108766 CrossRef CAS PubMed.
- T. W. Kim, J. H. Lee, S. E. Kim, M. H. Park, H. C. Chang and H. Y. Kim, Analysis of microbial communities in doenjang, a Korean fermented soybean paste, using nested PCR-denaturing gradient gel electrophoresis, Int. J. Food Microbiol., 2009, 131, 265–271 CrossRef CAS PubMed.
- B. A. Hendrickson, R. Gokhale and J. H. Cho, Clinical aspects and pathophysiology of inflammatory bowel disease, Clin. Microbiol. Rev., 2002, 15, 79–94 CrossRef PubMed.
- J. C. Jang, K. M. Lee and S. G. Ko, Angelica acutiloba Kitagawa Extract Attenuates DSS-Induced Murine Colitis, Mediators Inflammation, 2016, 2016, 9275083 Search PubMed.
- S. Shastri, T. Shinde, S. S. Sohal, N. Gueven and R. Eri, Idebenone Protects against Acute Murine Colitis via Antioxidant and Anti-Inflammatory Mechanisms, Int. J. Mol. Sci., 2020, 21, 484 CrossRef CAS PubMed.
- W. A. Rose 2nd, K. Sakamoto and C. A. Leifer, Multifunctional role of dextran sulfate sodium for in vivo modeling of intestinal diseases, BMC Immunol., 2012, 13, 41 CrossRef PubMed.
- K. Wang, X. Jin, M. You, W. Tian, R. K. Le Leu, D. L. Topping, M. A. Conlon, L. Wu and F. Hu, Dietary Propolis Ameliorates Dextran Sulfate Sodium-Induced Colitis and Modulates the Gut Microbiota in Rats Fed a Western Diet, Nutrients, 2017, 9, 875 CrossRef PubMed.
- F. Algieri, A. Rodriguez-Nogales, M. E. Rodriguez-Cabezas, S. Risco, M. A. Ocete and J. Galvez, Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review, Mediators Inflammation, 2015, 2015, 179616 Search PubMed.
- E. S. Park, E. J. Park, J. L. Song, I. S. Kim and K. Y. Park, Increased Anticolitic Effects in C57BL/6 Mice Based on Functional Ingredients of Ramyeon Noodles and Soup, J. Med. Food., 2018, 21, 1070–1074 CrossRef CAS PubMed.
- K. A. Kim, S. E. Jang, J. J. Jeong, D. H. Yu, M. J. Han and D. H. Kim, Doenjang, a korean soybean paste, ameliorates TNBS-induced colitis in mice by suppressing gut microbial lipopolysaccharide production and NF-κB activation, J. Funct. Foods, 2014, 11, 417–427 CrossRef CAS.
- R. Zhang, J. Xu, J. Zhao and Y. Chen, Genistein improves inflammatory response and colonic function through NF-κB signal in DSS-induced colonic injury, Oncotarget, 2017, 8, 61385–61392 CrossRef PubMed.
- J. Shen, N. Li and X. Zhang, Daidzein Ameliorates Dextran Sulfate Sodium-Induced Experimental Colitis in Mice by Regulating NF-κB Signaling, J. Environ. Pathol. Toxicol. Oncol., 2019, 38, 29–39 CrossRef PubMed.
- M. Morimoto, T. Watanabe, M. Yamori, M. Takebe and Y. Wakatsuki, Isoflavones regulate innate immunity and inhibit experimental colitis, J. Gastroenterol. Hepatol., 2009, 24, 1123–1129 CrossRef CAS PubMed.
- P. Lacy and J. L. Stow, Cytokine release from innate immune cells: association with diverse membrane trafficking pathways, Blood, 2011, 118, 9–18 CrossRef CAS PubMed.
- S. L. Constant and K. Bottomly, Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches, Annu. Rev. Immunol., 1997, 15, 297–322 CrossRef CAS PubMed.
- Y. Zhou, X. Chen, R. Yi, G. Li, P. Sun, Y. Qian and X. Zhao, Immunomodulatory Effect of Tremella Polysaccharides against Cyclophosphamide-Induced Immunosuppression in Mice, Molecules, 2018, 23, 239 CrossRef PubMed.
- M. S. Hayden, A. P. West and S. Ghosh, NF-kappaB and the immune response, Oncogene, 2006, 25, 6758–6780 CrossRef CAS PubMed.
- M. Hämäläinen, R. Nieminen, P. Vuorela, M. Heinonen and E. Moilanen, Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages, Mediators Inflammation, 2007, 2007, 45673 Search PubMed.
- Y. H. Sung, H. K. Chang, S. E. Kim, Y. M. Kim, J. H. Seo, M. C. Shin, M. S. Shin, J. W. Yi, D. H. Shin, H. Kim and C. J. Kim, Anti-inflammatory and analgesic effects of the aqueous extract of corni fructus in murine RAW 264.7 macrophage cells, J. Med. Food, 2009, 12, 788–795 CrossRef CAS PubMed.
- A. R. Basson, S. Ahmed, R. Almutairi, B. Seo and F. Cominelli, Regulation of Intestinal Inflammation by Soybean and Soy-Derived Compounds, Foods, 2021, 10, 774 CrossRef CAS PubMed.
- J. H. Kang, M. K. Sung, T. Kawada, H. Yoo, Y. K. Kim, J. S. Kim and R. Yu, Soybean saponins suppress the release of proinflammatory mediators by LPS-stimulated peritoneal macrophages, Cancer Lett., 2005, 230, 219–227 CrossRef CAS PubMed.
- S. Jeong, J. Oh, J. S. Lim, S. Kim, D. Jeong, S. R. Kim and J. S. Kim, Inhibitory Effect of Steamed Soybean Wastewater Against DSS-Induced Intestinal Inflammation in Mice, Foods, 2020, 9, 954 CrossRef CAS PubMed.
- G. R. Gandhi, M. T. S. L. Neta, R. G. Sathiyabama, J. S. S. Quintans, A. M. de Oliveira E Silva, A. A. S. Araújo, N. Narain, L. J. Q. Júnior and R. Q. Gurgel, Flavonoids as Th1/Th2 cytokines immunomodulators: A systematic review of studies on animal models, Phytomedicine, 2018, 44, 74–84 CrossRef CAS PubMed.
- O. Boyman and J. Sprent, The role of interleukin-2 during homeostasis and activation of the immune system, Nat. Rev. Immunol., 2012, 12, 180–190 CrossRef CAS PubMed.
- J. Li, H. Chen, B. Wang, C. Cai, X. Yang, Z. Chai and W. Feng, ZnO nanoparticles act as supportive therapy in DSS-induced ulcerative colitis in mice by maintaining gut homeostasis and activating Nrf2 signaling, Sci. Rep., 2017, 7, 43126 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |