Open Access Article
Open Access ArticlePsychobiotic supplementation of HK-PS23 improves anxiety in highly stressed clinical nurses: a double-blind randomized placebo-controlled study
Shu-I
Wu
 *ab,
Chien-Chen
Wu
c,
Li-Hao
Cheng
c,
Samuel W.
Noble
c,
Chih-Ju
Liu
d,
Yu-Hsia
Lee
d,
Chen-Ju
Lin
b,
Chih-Chieh
Hsu
c,
Wan-Lin
Chen
e,
Pei-Joung
Tsai
c,
Po-Hsiu
Kuo
fg and
Ying-Chieh
Tsai
h
*ab,
Chien-Chen
Wu
c,
Li-Hao
Cheng
c,
Samuel W.
Noble
c,
Chih-Ju
Liu
d,
Yu-Hsia
Lee
d,
Chen-Ju
Lin
b,
Chih-Chieh
Hsu
c,
Wan-Lin
Chen
e,
Pei-Joung
Tsai
c,
Po-Hsiu
Kuo
fg and
Ying-Chieh
Tsai
h
aDepartment of Medicine, MacKay Medical College, New Taipei City, Taiwan. E-mail: shuiwu@g.ntu.edu.tw
bSection of Psychiatry and Suicide Prevention Center, MacKay Memorial Hospital, Taipei, Taiwan
cBened Biomedical Co., Ltd., Taipei, Taiwan
dDepartment of Nursing, Mackay Memorial Hospital, Taipei, Taiwan
eDepartment of Medical Research, Mackay Memorial Hospital, Taipei, Taiwan
fDepartment of Public Health & Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University, Taipei, Taiwan
gDepartment of Psychiatry, National Taiwan University Hospital, Taipei, Taiwan
hInstitute of Biochemistry and Molecular Biology, National Yang Ming Chiao Tung University, Taipei, Taiwan
First published on 4th August 2022
Abstract
Nurses often experience adverse health effects associated with increasing levels of work-related stress. Stress may induce systemic effects through the HPA axis, glucocorticoid responses, and inflammatory cascades. Psychobiotics may help alleviate stress through associations of the microbiota, anti-inflammation factors, and the gut-brain axis. We aimed to investigate whether interventions with a psychobiotic, heat-killed (HK)-PS23 cells, may help improve perceived stress, anxiety, and related biological markers among highly stressed clinical nurses. This double-blind, randomized, placebo-controlled study included seventy clinical nurses from a medical center in Northern Taiwan who scored 27 or higher on the 10-item version of the Perceived Stress Scale (PSS), and participants were randomized into either taking HK-PS23 or a placebo for 8 weeks. Baseline and endpoint results of the PSS, Job Stress Scale, State and Trait Anxiety Index (STAI), emotional questionnaires, gastrointestinal severity questionnaires, Trails Marking Tests, blood biological markers, and sleep data were analyzed. While both groups demonstrated improvements in most measures over time, only the blood cortisol measure demonstrated significant group differences after the 8-week trial. Further analyses of the subgroup with higher anxiety (nurses with STAI ≥ 103) revealed that anxiety states had improved significantly in the HK-PS23 group but not in the placebo group. In summary, this placebo-controlled trial found significant reduction in the level of blood cortisol after 8 weeks of HK-PS23 use. The distinctive anxiolytic effects of HK-PS23 may be beneficial in improving perceived anxiety and stress hormone levels in female nurses under pressure. Clinical trial registration: https://clinicaltrials.gov/, identifier: NCT04452253-sub-project 1.
Introduction
The daily workload of nurses can be heavy and fast-paced, at times compounded by intense emergency situations. Full concentration, carefully directed attention, and quick decision-making are crucial.1 In addition, due to the needs of patients who may require 24-hour continuous care, hospital nurses often work night shifts that are out of sync with their natural circadian rhythms. Studies have shown that the combination of job stress and shift work pose a risk of various health disorders, such as an increased risk of mental illness,2,3 metabolic disease, or gastrointestinal disease.4,5 Work- or client-related burnout among clinical nurses6 may also contribute to fatigue,7 medical errors,2,8 and even patient harm.9There are multiple ways by which job pressures may contribute to these negative outcomes. These occupational stressors can influence the hypothalamus–pituitary–adrenal (HPA) axis and affect overall glucose metabolism.10 Regulation of such metabolic or inflammatory changes through microorganisms and their metabolites have recently spurred much discussion.11,12 The gut microbiome has been shown to play a role in modulating inflammation or anxiety-like behaviors under stress in animal models.12–14 Research has shown that germ-free mice were found to have fewer anxiety-like behaviors and lower levels of neurotrophins than mice with normal microbiota.15 Another study also described that microbial colonization in mice during early life may be associated with anxiety-like behaviors through the regulation of the hippocampal serotonergic system.14
Stress may induce systemic effects through the HPA axis, glucocorticoid responses, and inflammatory cascades. Emerging evidence suggests that stress-related depression or anxiety may be reduced by taking probiotics or psychobiotics, particularly the Bifidobacterium or Lactobacillus, through restoring the integrity of gut-barrier, modulating the effects of neurotransmitters including GABA or the BDNF, and stimulating the vagal nervous system to exert anti-inflammatory effects for brain protection via the gut-brain axis.16,17 Mohammadi et al. described significant improvements in general health, depression, anxiety, and stress in petrochemical workers who received 6 weeks of probiotic capsules or probiotic yogurt, but not in those who received conventional yogurt.18 Akkasheh et al. also reported significant decreases in depression, serum insulin levels, insulin resistance, and serum high-sensitivity C-reactive protein concentrations in patients with major depressive disorder after the administration of probiotics Bifidobacterium bifidum, Lactobacillus acidophilus, and Lactobacillus casei as compared to a placebo after 8 weeks of intervention.19 In healthy human volunteers, Wu et al. reported significant improvements in self-perceived stress, mood states, and positive and negative emotions, as well as decreased cortisol levels in highly stressed technology specialists who took Lactobacillus plantarum PS128 (PS128) for 8 weeks.20 In animal experiments, both live Lactobacillus paracasei PS23 (PS23) and heat-killed PS23 (HK-PS23), as well as PS128, demonstrated improvements in depression- and anxiety-like behaviors in mice with early life stress,21,22 senescence-accelerated mice,23 and mice exhibiting anxiety-like behaviors induced by chronic corticosterone injection.13 These results indicated that psychobiotics could potentially regulate mood and play a significant role in improving psychological well-being.
Research investigating the effects of psychobiotics on mental health conditions is scarce. No randomized placebo-controlled trials have yet been conducted to test the possible anti-stress or anti-anxiety effects of the psychobiotic HK-PS23. We designed this double-blind randomized controlled trial to examine whether an 8-week intervention of HK-PS23 may help improve perceived stress, anxiety, mood states, and stress- or oxidation-related blood biological markers among highly stressed clinical nurses.
Materials and methods
Study design and participants
This study was part of a larger study (clinical registration NCT04452253) consisting of two trial designs to test psychiatric symptoms in highly stressed employees before and after taking probiotics for an 8-week period. Sub-project 2, an open-label trial of technology specialists, was published previously.20 This study was approved by the institutional review board of the Mackay Memorial Hospital (IRB no: 19CT013be).In this trial, sub-project 1, we tested the efficacy of psychobiotic HK-PS23 versus a placebo. Our study subjects were full-time registered nurses aged 20–60 years, currently working in hospital wards or in special units in a medical center in Northern Taiwan. Participants were invited to participate in this 8-week trial and signed consent forms. They then completed the 10-item Perceived Stress Scale (PSS), and those who met the inclusion criteria of PSS ≥ 27 at the screening time point (V0) were included. These subjects were randomly allocated into either the treatment or control group according to a computer-generated list. Randomization was generated by the study statistician, who did not have contacts with the subjects. Blood samples were obtained, and participants underwent further stress and mood state evaluations (baseline, V1). Participants, samplers, the principal investigator, data collector, outcome adjudicators, and another statistical specialist handling the data analysis were all blind to the randomization of treatment allocation. We also provided a fitness tracker smart watch (Fitbit) and instructed participants to wear it in order to monitor their sleep conditions during the trial. After the evaluation at V1 was completed, the subjects were directed to take two test capsules once daily. A second assessment 8 weeks after V1 (endpoint, V2) was also performed, at which time participants were requested to return the remaining capsules. Participants’ blood samples were collected at the same time of the day at V1 and V2. Work schedules during the trial period were obtained to analyze any possible influence of shift time.
We excluded those (1) who had taken or had received antibiotics within 1 month prior to the study, (2) who had used probiotics in powder, capsule, or tablet form (except for yogurt, fermented milk, and other related foods) within 2 weeks prior to the study, (3) had hepatobiliary or gastrointestinal tract issues or who had previously undergone surgery; (4) who had past or present inflammatory bowel disease; (5) who had a history of cancer; (6) who were allergic to lactic acid bacteria; (7) who were currently pregnant or breastfeeding; or (8) who were currently taking medication to treat acute or chronic psychiatric diseases or sleep disorders. Participants were free to withdraw from the study if they experienced adverse reactions (e.g., abdominal fullness or diarrhea) or no longer wished to continue.
In this study, the primary outcome measures were the change in Perceived Stress Scale (PSS) score after the 8-week intervention. The secondary outcome measures were the differences in the State and Trait Anxiety Index (STAI), Questionnaire for Emotional Trait and State, Patient Health Questionnaire (PHQ)-9, Insomnia Severity Index (ISI), Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (QLESQ-SF), Job Stress Scale (JSS), Visual Analog Scale (VAS) of Gastrointestinal Symptoms, VAS of Occupational Stress, Trail-Making Test (TMT), Fitbit, and blood biomarkers.
Materials
The HK-PS23 treatment capsule contained 300 mg of powdered heat-killed L. paracasei PS23, equivalent to 10 billion HK-PS23 cells. The Taiwan Food and Drug Administration has approved L. paracasei as a food supplement with no serious adverse effects, and L. paracasei was included in the safety list notified by the European Food Safety Authority.20,24,25 The placebo capsule contained only the MCC. Neither type of capsule contained lactose, and thus could be safely taken by people with lactose intolerance. The placebo capsule was matched to the HK-PS23 treatment capsule for size, color, and taste. All subjects were administered two capsules per day for 8 weeks. In this study, no adverse events related to intervention were found.Perceived stress scale
We used the 10-item PSS as a screening tool to identify eligible trial subjects. The 14-item full PSS was used to compare whether there was perceived stress changes between V1 and V2 or differences between the HK-PS23 and placebo groups. Previous studies have shown the validity and reliability of the PSS (and its Mandarin version) to evaluate subjective feelings of perceived stress within the past month,26,27 and the scale has previously been applied in studies of stress levels of registered nurses.28 Higher scores on the PSS were associated with poor health outcomes or risk behaviors in nurses,29 increased anxiety and depression in older adults,30 and burnout or inability to cope in athletes.31The state and trait anxiety index
The State and Trait Anxiety Index (STAI) was used to compare anxiety level changes between V1 and V2, and differences between the HK-PS23 and placebo groups. The index consists of two subscales measuring the level of current “state” anxiety (transient autonomic nervous system arousal to a particular situation) and “trait” anxiety (susceptibility to feelings of stress or discomfort with less regard to specific situations). STAI scores have been found to be positively correlated with anxiety, stress, or hopelessness in healthcare workers.32,33 The Chinese-language version of the STAI has been found to be highly reliable.34Questionnaire for emotional trait and state
The 36-item Questionnaire for Emotional Trait and State was used to compare changes between V1 and V2, as well as differences between the two groups, in four emotion domains, including happiness, sadness/worry, anger, and hopefulness. Good construct validity and internal consistency of the questionnaire have been reported previously.35Patient health questionnaire-9
The Patient Health Questionnaire (PHQ)-9 was designed to detect depressive symptoms. At a cutoff score of ≥10, the Mandarin version of the PHQ-9 was found to have good internal consistency and validity among Chinese primary care patients.36Insomnia severity index
We used the Insomnia Severity Index (ISI) to compare the levels of sleep difficulties between both groups at V1 and V2. Good validity (0.87) had previously been demonstrated at a score of 14 or higher, which indicated the possibility of insomnia.37Quality of life enjoyment and satisfaction questionnaire-short form
The Quality of Life Enjoyment and Satisfaction Questionnaire-Short Form (QLESQ-SF) is used to measure patients’ subjective feelings regarding their general well-being, work or school functions, leisure activities, or social relations during the past week.38 Higher scores on the QLESQ-SF indicated a higher level of satisfaction with life.Job stress scale
The Job Stress Scale (JSS) used was the version translated by the Ministry of Labor in Taiwan. It is a self-rated scale with items addressing overall job stress, job burden, interpersonal relationships, satisfaction, and psychological and general well-being. We used the JSS to measure whether nurses’ overall job burden changed from V1 to V2.Visual analog scale of gastrointestinal symptoms
We used a 10-point visual analog scale (VAS) to compare whether there were differences between the groups or changes between the two time points in gastrointestinal areas of discomfort, including diarrhea, flatulence, difficulty swallowing, decreased appetite, dry mouth, nausea or vomiting, constipation, gastralgia, and upper or lower abdominal pain.39VAS of occupational stress
Both groups were asked to give an overall job-related stress score on another 10-point VAS at baseline and at the endpoint. A VAS score of ≥7 when assessing occupational stress has been shown to have a high level of sensitivity and specificity in detecting elevated job stress.40Trail-making test
The Trail-Making Test (TMT) consists of the TMT Part A (participants are asked to draw lines to connect numbers 1–25 in ascending order; their ability to do so is associated with visuomotor or graphical processing speed), and the TMT Part B (participants are asked to alternate between circling numbers and letters in ascending orders [1-A-2-B-3-C] as quickly as possible; their ability to do so is related to inhibition control and working memory). Each part of the test is measured three times, with numbers or letters shown at different locations each time. In this study, the TMT was primarily used to measure whether there were improvements or differences in executive function related to changes in stress or anxiety.41Fitbit
We asked the participants to wear the Fitbit, a fitness-tracking smart watch, during their sleep to monitor changes in sleep patterns and stages during the trial. Results of the Fitbit compared to those of standard overnight polysomnography have previously demonstrated high inter-device reliability and high sensitivity in identifying light, deep, or rapid eye movement (REM) sleep stages in healthy adults42 and in patients with depression.43 Total sleep time (TST), wake time after sleep onset (WASO), sleep onset latency (SOL), latency to REM sleep, stages of light, deep, or REM sleep, and sleep efficiency (SE) were also recorded.Blood biomarkers
We measured several blood inflammatory or antioxidative biomarkers, including cortisol, high-sensitivity C-reactive protein (Hs-CRP), total antioxidant capacity (TAC), and serum dehydroepiandrosterone sulfate (DHEA-S). Blood samples (10 mL) were collected not in a fasted state and at the same time of day at V1 and V2 to minimize the impact of circadian rhythms. The serum Hs-CRP level was determined by a turbidimetric immunoassay.44 Plasma TAC was measured using a colorimetric assay kit (BioVision Incorporated, Milpitas, CA, USA). Serum DHEA-S levels were measured using a chemiluminescent enzyme immunometric assay.45 Serum cortisol levels were determined using an electrochemiluminescence immunoassay kit (Elecsys Cortisol, Roche Diagnostics, Germany). All procedures were performed according to the manufacturer instructions.Statistical analysis
Our analyses of efficacy were performed on an intention-to-treat population and with the last observation carried forward. Baseline characteristics of HK-PS23 versus the placebo group were first compared using the chi-square test for categorical variables and the t-test for continuous variables. Repeated-measures analysis of variance from general linear models was then used to compare within-group changes (baseline to endpoint) and between-group differences (HK-PS23 versus placebo) of results from the TMT, blood markers and psychological measures. Linear mixed models were used to compare within- or between-group differences in different sleep stages and parameters obtained from 56 days of continuous Fitbit monitoring. Subgroup analyses distinguishing participants with PSS ≥ 33 or STAI ≥ 103 (based on median values, balanced and sufficient numbers in both groups, and median PSS and STAI scores obtained in our previous study regarding psychobiotic use in highly stressed information technology specialists20) were also performed to compare the possible effects of HK-PS23 vs. placebo in subgroups with relatively higher levels of stress or anxiety.At an anticipated mean PSS reduced by at least 20% (estimated from our previous study20), an α error of 0.05, and a power (1-β) of 0.80, the sample size required was 35 for each group.46 Considering a dropout rate of 8% from our previous study, we planned to recruit 38 subjects in each group. For subgroup analysis, with the same standard of an α error of 0.05, and a power of 0.80, the smallest sample size required was 10 for each group. Statistical significance was set at a p-value of <0.05.
Results
Baseline characteristics
Fig. 1 shows the flowchart of the study. Seventy registered nurses who met the inclusion criteria agreed to participate and signed an informed consent form. These participants were randomly allocated to the HK-PS23 and placebo groups. All except one participant in the HK-PS23 group were female, and 80% of the participants in the HK-PS23 group and 74.3% of that in the Placebo group were shift workers. All baseline characteristics were similar between the groups (Table 1). The health products the participants taking included multi-vitamin, vitamin B-complex, or calcium supplements.| HK-PS23 group (n = 35) | Placebo group (n = 35) | ||||
|---|---|---|---|---|---|
| n | % | n | % | p-Value | |
| Gender | |||||
| Female | 34 | 97.1 | 35 | 100 | 0.314 |
| Shift worker | 28 | 80.0 | 26 | 74.3 | 0.569 |
| Education | 0.730 | ||||
| Senior high + vocational school | 12 | 34.3 | 9 | 25.7 | |
| College degree | 21 | 60.0 | 24 | 68.6 | |
| Master degree | 2 | 5.7 | 2 | 5.7 | |
| Taking other health productsa | 6 | 17.1 | 9 | 25.7 | 0.382 |
| Mean | SD | Mean | SD | p-Value | |
|---|---|---|---|---|---|
| a Multivitamins, vitamin B-complex, or calcium supplementation. SD: standard deviation. | |||||
| Age | 35.39 | 11.19 | 36.24 | 9.20 | 0.729 |
| Job experiences (years) | 4.63 | 2.33 | 5.09 | 2.32 | 0.413 |
| Years of education | 15.83 | 1.70 | 15.89 | 1.69 | 0.889 |
| Baseline systolic blood pressure | 111.80 | 12.72 | 110.14 | 14.57 | 0.614 |
| Baseline diastolic blood pressure | 72.46 | 9.12 | 70.46 | 8.77 | 0.353 |
| Baseline BMI | 22.21 | 4.71 | 22.08 | 3.95 | 0.903 |
Outcomes of stress, mood, and sleep
Table 2 shows comparisons of intra- (time effects) and inter-group (group effects) differences on stress, mood, and insomnia measures during this 8-week trial. Participants in both groups demonstrated significant reductions in perceived stress, anxiety, perceived job-related stress, job burden, sleep disturbances, negative emotions, gastralgia, and overall stress measured by the VAS at 8 weeks as compared to baseline. Significant increases over time in scores regarding positive emotions, job satisfaction, energy level, psychological or general health, and quality of life were also found in both groups. Although mean differences in PSS scores between baseline and the endpoint were 8.60 (SD 9.47, p < 0.001) in the HK-PS23 group and 6.86 (SD 7.38, p < 0.001) in the placebo group, the difference was not significant (p = 0.915). Scores of the STAI, ISI, PHQ-9, QLESQ, and emotional trait and state questionnaire also followed a similar pattern, showing differences over time in both groups but not between groups. Changes in body weight, blood pressure, and gastrointestinal complaints did not differ significantly in the HK-PS23 and placebo groups before or after the intervention.| HK-PS23 group (n = 33) | Placebo group (n = 32) | Group effects | Time effects | Group × time effects | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | F | F | |
| PSS: perceived stress scale; STAI: state trait anxiety index; JSS: job stress scale; ISI: insomnia severity index; PHQ: patient health questionnaire; QLESQ: quality of life enjoyment and satisfaction questionnaire; GSI: gastrointestinal severity index. *P < 0.05; **P < 0.01; ***P < 0.001. SD: standard deviation. | |||||||
| PSS total | 0.01 | 58.04*** | 0.74 | ||||
| Baseline | 32.29 | 6.03 | 31.29 | 5.25 | |||
| 8 weeks | 23.69 | 6.40 | 24.43 | 8.24 | |||
| STAI | 1.04 | 25.69*** | 0.93 | ||||
| Baseline | 103.35 | 12.35 | 103.94 | 12.30 | |||
| 8 weeks | 91.21 | 14.83 | 95.68 | 12.96 | |||
| STAI-State | 1.42 | 28.27*** | 0.897 | ||||
| Baseline | 50.32 | 7.43 | 50.97 | 7.65 | |||
| 8 weeks | 42.71 | 8.91 | 45.66 | 8.15 | |||
| STAI-Trait | 0.68 | 15.85*** | 0.40 | ||||
| Baseline | 52.71 | 6.48 | 53.12 | 5.89 | |||
| 8 weeks | 48.46 | 6.92 | 50.03 | 5.78 | |||
| JSS | |||||||
| Job stress | 2.91 | 18.64*** | 0.33 | ||||
| Baseline | 67.43 | 17.55 | 72.00 | 13.89 | |||
| 8 weeks | 57.71 | 15.92 | 64.57 | 17.55 | |||
| Control over job | 1.16 | 1.27 | 0.74 | ||||
| Baseline | 69.55 | 9.19 | 66.61 | 8.36 | |||
| 8 weeks | 69.85 | 9.99 | 68.87 | 7.79 | |||
| Job burden | 0.05 | 5.75* | 0.06 | ||||
| Baseline | 75.25 | 12.38 | 74.82 | 12.64 | |||
| 8 weeks | 73.41 | 12.45 | 72.56 | 12.94 | |||
| Interpersonal relationships | 0.04 | 0.31 | 1.26 | ||||
| Baseline | 72.70 | 8.92 | 74.38 | 8.77 | |||
| 8 weeks | 74.54 | 12.12 | 73.75 | 11.77 | |||
| Job satisfaction | 2.28 | 6.36* | 1.08 | ||||
| Baseline | 64.00 | 15.94 | 61.14 | 15.30 | |||
| 8 weeks | 70.85 | 18.37 | 64.00 | 12.65 | |||
| Psychological health | 0.36 | 13.05** | 0.20 | ||||
| Baseline | 50.29 | 11.57 | 49.49 | 12.95 | |||
| 8 weeks | 57.49 | 13.83 | 55.09 | 15.13 | |||
| Energy level | 0.001 | 28.11*** | 0.02 | ||||
| Baseline | 37.57 | 14.62 | 37.86 | 13.74 | |||
| 8 weeks | 47.00 | 17.50 | 46.86 | 14.40 | |||
| General health | 0.04 | 9.57** | 1.06 | ||||
| Baseline | 62.74 | 13.33 | 64.91 | 15.49 | |||
| 8 weeks | 69.14 | 14.01 | 68.11 | 12.52 | |||
| ISI | 0.15 | 8.59** | 0.43 | ||||
| Baseline | 12.34 | 6.19 | 12.29 | 6.24 | |||
| 8 weeks | 9.69 | 4.96 | 10.60 | 4.75 | |||
| PHQ | 1.37 | 17.26*** | 0.04 | ||||
| Baseline | 9.52 | 4.25 | 10.40 | 4.10 | |||
| 8 weeks | 7.18 | 3.79 | 8.29 | 4.38 | |||
| QLESQ | 2.00 | 23.97*** | 0.10 | ||||
| Baseline | 48.06 | 7.22 | 46.29 | 5.80 | |||
| 8 weeks | 52.79 | 7.99 | 50.44 | 6.89 | |||
| The questionnaire of emotional trait and state | |||||||
| Total | 1.10 | 23.06*** | 0.29 | ||||
| Baseline | 93.29 | 14.83 | 91.09 | 13.02 | |||
| 8 weeks | 102.66 | 14.76 | 98.57 | 15.44 | |||
| Happy and acceptance | 0.68 | 23.19*** | 0.08 | ||||
| Baseline | 29.20 | 5.52 | 28.52 | 5.33 | |||
| 8 weeks | 32.66 | 5.25 | 31.60 | 4.92 | |||
| Sad and scared | 1.55 | 11.52** | 0.24 | ||||
| Baseline | 23.69 | 5.06 | 24.60 | 4.07 | |||
| 8 weeks | 21.20 | 5.07 | 22.74 | 5.10 | |||
| Angry and disgust | 0.55 | 15.50*** | 0.31 | ||||
| Baseline | 22.00 | 4.87 | 22.49 | 4.74 | |||
| 8 weeks | 19.57 | 4.69 | 20.66 | 5.53 | |||
| Look forward to | 0.28 | 23.62*** | 0.11 | ||||
| Baseline | 12.00 | 2.29 | 11.86 | 1.96 | |||
| 8 weeks | 13.37 | 2.16 | 12.89 | 2.10 | |||
| GSI | |||||||
| Total | 0.31 | 2.81 | 0.66 | ||||
| Baseline | 18.23 | 12.01 | 18.54 | 13.32 | |||
| 8 weeks | 14.69 | 11.74 | 17.31 | 13.26 | |||
| Dry mouth | 0.14 | 2.05 | 0.56 | ||||
| Baseline | 3.54 | 2.37 | 3.14 | 2.89 | |||
| 8 weeks | 3.00 | 2.53 | 2.97 | 2.66 | |||
| Difficulty swallowing | 0.25 | 0.01 | 3.96 | ||||
| Baseline | 0.51 | 1.22 | 0.17 | 0.45 | |||
| 8 weeks | 0.29 | 0.96 | 0.43 | 1.12 | |||
| Decreased appetite | 0.01 | 0.92 | 1.94 | ||||
| Baseline | 2.06 | 2.62 | 1.54 | 2.39 | |||
| 8 weeks | 1.29 | 2.18 | 1.69 | 2.49 | |||
| Nausea or vomiting | 0.86 | 0.001 | 0.01 | ||||
| Baseline | 0.51 | 1.76 | 0.80 | 1.55 | |||
| 8 weeks | 0.54 | 1.38 | 0.77 | 1.57 | |||
| Flatulence | 0.03 | 0.003 | 0.80 | ||||
| Baseline | 3.14 | 2.68 | 2.80 | 2.71 | |||
| 8 weeks | 2.89 | 2.75 | 3.03 | 2.98 | |||
| Gastralgia | 1.45 | 6.03** | 0.03 | ||||
| Baseline | 2.74 | 2.96 | 3.46 | 3.37 | |||
| 8 weeks | 1.89 | 2.78 | 2.71 | 2.89 | |||
| Upper abdominal pain | 0.16 | 0.37 | 0.13 | ||||
| Baseline | 1.09 | 2.09 | 1.34 | 2.13 | |||
| 8 weeks | 1.03 | 2.19 | 1.11 | 1.71 | |||
| Lower abdominal pain | 2.33 | 0.18 | 0.50 | ||||
| Baseline | 0.77 | 1.35 | 1.17 | 1.86 | |||
| 8 weeks | 0.71 | 1.27 | 1.40 | 2.20 | |||
| Constipation | 0.55 | 3.63 | 0.67 | ||||
| Baseline | 2.57 | 3.15 | 3.26 | 3.17 | |||
| 8 weeks | 2.29 | 2.70 | 2.54 | 2.45 | |||
| Diarrhea | 0.62 | 2.35 | 0.46 | ||||
| Baseline | 1.29 | 2.46 | 0.86 | 1.42 | |||
| 8 weeks | 0.77 | 1.63 | 0.66 | 1.21 | |||
| Visual analogue scale of stress | 2.61 | 39.84*** | 0.34 | ||||
| Baseline | 6.11 | 1.61 | 6.54 | 1.31 | |||
| 8 weeks | 4.60 | 2.00 | 5.29 | 1.84 | |||
Outcomes related to the TMT, fitbit, and blood biomarkers
Table 3 compares the results of TMT between the HK-PS23 and placebo groups and their time effects before and after the intervention. Only the time of completing question # 1 in the TMT Part B was significantly different when comparing the endpoint and the baseline in both groups. No significant between-group differences were observed for the other measures, including the Fitbit data.| HK- PS23 group (n = 33) | Placebo group (n = 32) | Group effects | Time effects | Group × time effects | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | F | F | |
| TMT: trails making tests; TMT-A#1: indicating the time needed to complete the first test in TMT part A (connect numbers 1–25 in ascending order); TMT part A#2: indicating the time needed to complete the second test in TMT Part A; TMT part B#3: indicating the time needed to complete the third test in TMT Part B (to circle and alternate between numbers and letters, e.g. 1-A-2-B-3-C); Sec: second. *P < 0.05; SD: standard deviation. | |||||||
| TMT part A#1 (sec) | 1.66 | 3.79 | 1.13 | ||||
| Baseline | 35.19 | 9.35 | 38.91 | 11.44 | |||
| 8 weeks | 34.05 | 8.40 | 35.03 | 7.79 | |||
| TMT part A#2 (sec) | 0.96 | 0.20 | 0.32 | ||||
| Baseline | 34.73 | 8.14 | 36.06 | 8.68 | |||
| 8 weeks | 33.68 | 9.64 | 36.19 | 10.52 | |||
| TMT part A#3 (sec) | 0.38 | 0.05 | 0.13 | ||||
| Baseline | 32.53 | 8.04 | 33.90 | 7.97 | |||
| 8 weeks | 33.00 | 7.54 | 33.79 | 8.80 | |||
| TMT part B#1 (sec) | 1.04 | 5.26* | 1.56 | ||||
| Baseline | 52.50 | 12.40 | 57.85 | 15.48 | |||
| 8 weeks | 50.71 | 15.74 | 51.80 | 16.13 | |||
| TMT part B#2 (sec) | 0.03 | 0.08 | 0.22 | ||||
| Baseline | 53.06 | 14.79 | 52.92 | 12.45 | |||
| 8 weeks | 51.98 | 13.01 | 53.21 | 16.06 | |||
| TMT part B#3 (sec) | 0.16 | 1.36 | 2.49 | ||||
| Baseline | 51.10 | 12.28 | 52.23 | 11.10 | |||
| 8 weeks | 51.68 | 16.07 | 48.36 | 11.06 | |||
A comparison of the blood stress biomarkers before and after the trial is shown in Fig. 2. Cortisol levels decreased significantly after the 8-week intervention period in the group-by-time interaction (p = 0.043). No other stress- or anxiety-related biomarkers showed significant differences after the intervention.
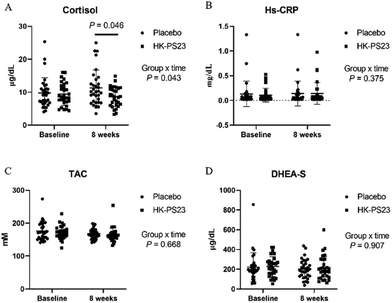 | ||
| Fig. 2 The effect of HK-PS23 intervention on blood stress biomarkers. Levels of cortisol (A), Hs-CRP (B), TAC (C), and DHEA-S (D) were determined at baseline and after eight weeks of intervention. | ||
Outcomes of sub-analyses
Tables 4 and 5 describe the results of the participant subgroups who, compared with the rest of the study subjects, had higher stress or anxiety scores at baseline. Among participants with higher baseline stress levels (PSS ≥ 33), significant between-group differences over time were found in levels of cortisol and job satisfaction (group-by-time interactions in Fig. 3A and Table 4). Although significant differences emerged between baseline and endpoint on STAI (p = 0.001), job-related stress (p = 0.007), job satisfaction (p = 0.008), psychological health (p = 0.014), and positive emotions of happiness and acceptance (p = 0.014) in the HK-PS23 group, and not in the placebo group, results from repeated measures did not show group-by-time interactions.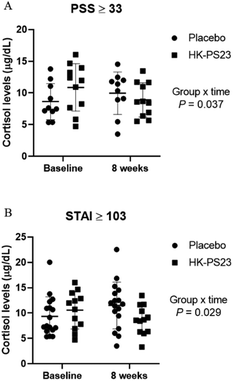 | ||
| Fig. 3 The effect of HK-PS23 intervention on blood level of cortisol among the subgroup of nurses with PSS ≥ 33 (A) and STAI ≥ 103 (B). | ||
| HK- PS23 group (n = 15) | Placebo group (n = 12) | Group effects | Time effects | Group × time effects | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | F | F | |
| STAI | 4.72* | 13.56** | 1.67 | ||||
| Baseline | 108.40 | 14.22 | 111.58 | 13.91 | |||
| 8 weeks | 88.80 | 13.99 | 102.17 | 14.50 | |||
| STAI-state | 6.37* | 15.06** | 1.78 | ||||
| Baseline | 52.93 | 8.37 | 55.83 | 7.58 | |||
| 8 weeks | 41.33 | 7.02 | 50.17 | 9.90 | |||
| JSS | |||||||
| Job stress | 8.62** | 2.80 | 4.63 | ||||
| Baseline | 68.00 | 18.21 | 75.00 | 12.43 | |||
| 8 weeks | 54.67 | 14.07 | 76.67 | 16.70 | |||
| Job satisfaction | 8.43** | 6.50** | 6.50** | ||||
| Baseline | 64.29 | 13.72 | 60.00 | 16.00 | |||
| 8 weeks | 67.86 | 17.50 | 61.54 | 12.55 | |||
| Psychological health | 7.05* | 6.52* | 1.59 | ||||
| Baseline | 48.00 | 14.18 | 41.00 | 14.49 | |||
| 8 weeks | 60.80 | 13.87 | 45.33 | 13.14 | |||
| The questionnaire of emotional trait and state | |||||||
| Total | 4.27* | 11.73** | 1.42 | ||||
| Baseline | 89.13 | 13.95 | 84.50 | 12.70 | |||
| 8 weeks | 105.00 | 14.22 | 92.17 | 15.27 | |||
| Happy and acceptance | 5.44* | 19.21*** | 1.43 | ||||
| Baseline | 27.13 | 5.34 | 25.33 | 4.66 | |||
| 8 weeks | 34.13 | 4.47 | 29.33 | 5.00 | |||
| Fitbit average during the 8-week trial (mixed linear model) | Mean difference | Standard error | p-Value | ||||
|---|---|---|---|---|---|---|---|
| PSS: perceived stress scale; STAI: state trait anxiety index; JSS: job stress scale; ISI: insomnia severity index; PHQ: patient health questionnaire; QLESQ: quality of life enjoyment and satisfaction questionnaire; GSI: gastrointestinal severity index. *P < 0.05; **P < 0.01; ***P < 0.001. SD: standard deviation. | |||||||
| Total sleep time (min) | 348.29 | 9.32 | 398.54 | 11.26 | −50.24 | 14.50 | 0.002 |
| REM (%) | 16.28 | 0.68 | 19.41 | 0.81 | −3.12 | 1.05 | 0.007 |
| HK-PS23 group (n = 17) | Placebo group (n = 19) | Group effects | Time effects | Group × time effects | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | F | F | F | |
| STAI-state | 4.21* | 45.09*** | 4.32* | ||||
| Baseline | 55.24 | 6.91 | 55.68 | 5.84 | |||
| 8 weeks | 41.76 | 6.61 | 48.58 | 8.39 | |||
| Visual analogue scale of stress | 5.92* | 20.94*** | 1.80 | ||||
| Baseline | 6.24 | 1.56 | 6.79 | 1.27 | |||
| 8 weeks | 4.12 | 1.73 | 5.63 | 2.01 | |||
| Fitbit average during the trial (mixed linear model) | Mean difference | Standard error | p-Value | ||||
|---|---|---|---|---|---|---|---|
| STAI: state trait anxiety index; WASO: wake up after sleep onset; *P < 0.05; **P < 0.01; ***P < 0.001. SD: standard deviation. | |||||||
| 8-Week WASO (min) | 177.95 | 53.81 | 256.17 | 51.68 | −78.22 | 24.27 | 0.005 |
| 8-Week light sleep (min) | 185.80 | 56.47 | 263.22 | 55.52 | −77.42 | 25.74 | 0.008 |
As for data from Fitbit, significant differences were found in mean total sleep time (minutes per day) and the mean percentage of rapid eye movement (REM) stage between the HK-PS23 and placebo groups during the 8-week observation period (Table 4).
Table 5 and Fig. 3B describes the significant results from the subgroup of participants with higher anxiety (STAI ≥ 103). Similarly, in this subcohort, significant group-by-time interactions were found in changes of cortisol and STAI-state. Although significant time effects were found for the PSS, STAI, job stress or burden, psychological or general health, energy level, PHQ, QLESQ, and positive and negative emotions at the endpoint, no significant differences in the changes over time between the HK-PS23 and placebo groups were detected. Furthermore, in this subgroup of individuals with higher levels of anxiety, significant differences were found in the average duration of wake up after sleep onset (WASO) (minutes per day) and the stage of light sleep between the HK-PS23 and the placebo groups during the eight-week observation (Table 5).
Discussion
This is the first double-blind randomized placebo-controlled study that compared effects of HK-PS23 intake with a placebo on improvements of perceived stress, mood states, or related blood biological markers among highly stressed registered nurses working in a clinical setting. The level of serum cortisol in the HK-PS23 group was significantly decreased as compared to that in the placebo group after the 8-week intervention.Although differences were found in almost all measures when comparing endpoint with baseline results, the overall differences in score changes between the HK-PS23 and placebo groups were not statistically significant. Further analyses on subgroups exhibiting higher initial stress or anxiety revealed significant improvements derived from taking HK-PS23 (but not from taking placebo) in levels of cortisol and job satisfaction or anxiety states, respectively, over the 8-week duration of the trial. Such outcomes indicate that HK-PS23 may have distinctive advantages in relieving anxiety symptoms in anxious individuals working under high pressure.
We found reductions in cortisol levels associated with HK-PS23 use among clinical nurses and subgroups with higher stress or anxiety than the rest of the participants after the 8-week trial. Such findings were consistent with previous literature describing decreased levels of corticosterone in mice given HK-PS23 after suffering from the stress of maternal separation in early life,21 and decreased levels of cortisol in information technology engineers after 8-week's intake of psychobiotic PS128.20 Chronic psychological stress may trigger the HPA axis, activate the autonomic nervous system, increase the blood level of corticosterone, and initiate inflammation.47 Research has demonstrated anti-inflammatory effects after the administration of oral probiotics in healthy or subjects with metabolic or liver diseases.47 The gut microbiota diversity has been shown to be related to saliva cortisol stress response.48 Although we were unable to examine levels of other pro- or anti-inflammatory markers or cytokines, our result was partly supported by literature describing how HK-PS23 may help decrease the level of serum corticosterone accompanied by the increase in anti-inflammatory interleukin-10.21 Such changes in levels of cortisol or cytokines were found to be associated with decreased anxiety- or depression-like behaviors in mice after HK-PS23 administration. Huang et al. also reported that after senescence-accelerated mice received PS23, reductions in pro-inflammatory cytokines were correlated with decreases in severity of inflammation, the progression of aging, and anxiety-like behaviors.23 They concluded that PS23 may be neuroprotective through the modulation of the microbiota-gut-brain communications. Further investigations are still needed to examine whether the supplement of psychobiotics may decrease inflammatory responses manifested by a decrease in serum cortisol and can regulate mental health through the linkages of microbiota, the gut, and the central nervous system.
We found that significant reduction in state anxiety or increase in job satisfaction associated with HK-PS23 use only occurred in subgroups exhibiting higher initial anxiety or stress levels, respectively. The result of increase in job satisfaction among those that perceived higher stress at baseline may agree with past literature. Benton et al. described that although no significant changes in depression or anxiety were found among healthy volunteers consuming probiotics, there was an increase in those reporting themselves as ‘happy’ rather than ‘depressed’ in the participants whose mood condition ranked the bottom third at baseline.49 As for the anxiolytic effects found only in people with initial higher anxiety, Rao et al. also reported patients with chronic fatigue syndrome who had possible subclinical anxiety symptoms but no anxiety disorder comorbidity had decreased anxiety levels after consuming 8 weeks of L. casei.50
Our results might indicate HK-PS23's potential to ameliorate anxiety symptoms or subjective perception of stress is the greatest in those with subclinical or clinical anxiety and/or mental health issues. It has been proposed that L. paracasei might help prevent a stress-induced decrease in positive mood.51 Previous studies on mice which were administered PS23 showed that the decrease in anxiety-like behaviors was correlated with high levels of blood BDNF and brain monoamines.13,23 Similar to past literature that described the possible mechanisms of probiotics on depression may be associated with the antidepressant activity of brain neurotransmitters,13,19,52,53 researchers have suggested the anxiolytic effects were possibly mediated by dopamine, norepinephrine, and serotonin neurotransmitter pathways in the brain.21,23 These neurotransmitters may help regulate the HPA axis and expression of stress-related glucocorticoid and mineralocorticoid receptors in the hippocampus, striatum, and frontal cortex regions.13,21 It is possible that probiotics may modulate the microbiome and reduce inflammation in the gut, then connect with the central nervous system through the enteric nervous system and the vagal sensory nerve fibers, further influencing neurotransmissions in the brain.19,21,50,54 However, such explanation remains an assumption without much available data supporting the detailed causal pathways. Future investigations on possible biological mechanisms underlying the anxiolytic effects of HK-PS23 associated with anti-inflammatory factors, connections between the gut and the brain neurotransmitters, and influences on the HPA axis are still warranted.55
Although no significant between-group differences were found from our Fitbit data in the main analysis, upon looking at subgroup data, differences were found between the treatment and placebo cohorts. Among participants with higher levels of perceived stress, mean-from-baseline total sleep time and REM stage percentage was decreased in those taking HK-PS23 compared with placebo. Those with higher anxiety levels saw shorter average WASO and light sleep stage durations if taking HK-PS23. In line with previous studies that described improvements in sleep after probiotics use,20,56–58 reductions in the percentage of REM sleep, average time of WASO, and light sleep in our HK-PS23 group may be signs of increased quality of sleep. However, a lower total sleep time in our HK-PS23 group compared to the placebo does not necessarily mean that the participants did not have sufficient sleep. The subjective perception of sleep restoration varied from person to person.59 Since participants in both groups did not show significant differences in mean changes from baseline on the ISI, it is possible that these changes detected from the Fitbit were not sufficient to cause changes in the subjective perception of sleep conditions.
In our results, the time of completing question #1 in the Trails Marking Test (TMT) Part B was significantly different between endpoint and the baseline in both groups, but no group-by-time interaction was noted. Our original purpose to include the TMT was intended to measure whether possible alleviations in stress or anxiety associated with psychobiotic use in this study population may help improve their executive function at work. The TMT Part B was therefore used to measure ability associated with working memory and inhibition control. Our finding that there was a time effect in the test performance indicated that the ability was improved after the 8-week trial. However, the result of lacking group-by-time interaction implied that, as with our other psychological measures in the main analysis, the improvements over time were similar between the HK-PS23 and the placebo groups. It may be that HK-PS23 is no better than placebo in improving executive function. However, it is also possible that although our participants perceived that they were under significant stress, their mental health conditions were still within an acceptable range because they had not yet reached the severity of clinical depression or anxiety. Such condition implied that they maintained fair performance at their job or executive function before and after the interventions, the efficacy of improvements in concentration or working memory in probiotics may not reach significant level as compared to that of the placebo. Perhaps in the future, it may be feasible to investigate whether psychobiotics might have clinical efficacy in improvements of attention, memory, or executive function using other neuropsychological assessments in the patient population with clinical diagnoses of anxiety or depression.
Strengths and limitations
The main strengths of this study are its prospective, double-blind, randomized placebo control design and comprehensive measures for comparing mood, stress, sleep, and biological changes before and after the trial. The first limitation is the relatively small sample size of this study with almost all of the participants being female and not all being shift workers. A larger sample is needed to obtain more conclusive results, particularly in terms of analyses of a highly stressed or anxious population. However, the current study may be regarded as a pilot and explorative randomized controlled trial, which demonstrated feasibility and tolerability profiles. Second, although significant results were found within a particular subgroup of nurses in this study, these findings may more adequately apply to female nurses. Generalizations of our results to other nurses, healthcare workers, or employees in different industries may still be restricted. A third limitation is that, despite the multiple stress- or anxiety-related biological markers tested, there were still potential confounders, such as variations in individual workload, life stressors, or dietary habits, that we were not able to control. Although we gave each participant a fitness tracker to record stages of their sleep, we only require them to wear the Fitbit while they sleep, and not 24 hours a day. Therefore, not all physical activities were recorded. In future research, dietary or records of physical activities and assessments of other neuropsychological or biological examinations (e.g., gut permeability or other inflammatory markers) may help expand the understanding of possible biological mechanisms.Conclusions
This study showed good tolerability and safety profiles of the psychobiotic HK-PS23 in female nurses, who work in a high stress condition. HK-PS23 demonstrated potential benefits in reducing the level of cortisol compared to placebo and might improve perceived anxiety states in nurses under particularly higher levels of anxiety. The mechanism of action in these improvements has yet to be determined. We suggest further studies involving microbiota profiles and brain imaging to clarify the complex relationships among stress, anxiety, inflammation, immunomodulation, and neurology are still needed.Author contributions
SIW: conceptualization, investigation, formal analysis, data curation, writing – original Draft. CCW: conceptualization, methodology, writing – review & editing. LHC: formal analysis, data curation, writing – review & editing. SWN: writing – review & editing. CJL: writing – review & editing. YHL: writing – review & editing. CJL: writing – review & editing. CCH: methodology, funding acquisition. WLC: project administration, investigation, formal analysis, data curation. PJT: conceptualization, methodology. PHK: writing – review & editing. YCT: methodology, writing – review & editing, visualization.Conflicts of interest
CCW, PJT, LHC, SWN, and CCH are employees of Bened Biomedical Co., Ltd. YCT owns stock in Bened Biomedical Co., Ltd. The views of this article reflect those of the authors and not necessarily those of the funder. Other authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
The authors thank Editage for their suggestions on English grammar and writing. The authors appreciate Ms. Fang-Ju Sun, the statistician from the Department of Medical Research, Mackay Memorial Hospital, Taipei, Taiwan, for assistance with the statistical analyses.References
- H. Dong, Q. Zhang, Z. Sun, F. Sang and Y. Xu, Sleep problems among Chinese clinical nurses working in general hospitals, Occup. Med., 2017, 67, 534–539 CrossRef CAS PubMed.
- M. Arimura, M. Imai, M. Okawa, T. Fujimura and N. Yamada, Sleep, mental health status, and medical errors among hospital nurses in Japan, Ind. Health, 2010, 48, 811–817 CrossRef PubMed.
- Q. Hao, D. Wang, M. Xie, Y. Tang, Y. Dou, L. Zhu, Y. Wu, M. Dai, H. Wu and Q. Wang, Prevalence and Risk Factors of Mental Health Problems Among Healthcare Workers During the COVID-19 Pandemic: A Systematic Review and Meta-Analysis, Front. Psychiatry, 2021, 12, 567381 CrossRef PubMed.
- H. S. Jung and B. Lee, Factors associated with the occurrence of functional dyspepsia and insomnia in shift-working nurses, Work, 2016, 54, 93–101 Search PubMed.
- J. Purdy, Chronic physical illness: a psychophysiological approach for chronic physical illness, Yale J. Biol. Med., 2013, 86, 15–28 Search PubMed.
- W. Chin, Y. L. Guo, Y. J. Hung, C. Y. Yang and J. S. Shiao, Short sleep duration is dose-dependently related to job strain and burnout in nurses: a cross sectional survey, Int. J. Nurs. Stud., 2015, 52, 297–306 CrossRef PubMed.
- G. Jones, M. Hocine, J. Salomon, W. Dab and L. Temime, Demographic and occupational predictors of stress and fatigue in French intensive-care registered nurses and nurses’ aides: a cross-sectional study, Int. J. Nurs. Stud., 2015, 52, 250–259 CrossRef PubMed.
- A. A. Al Balushi, M. Alameddine, M. F. Chan, M. Al Saadoon, K. Bou-Karroum and S. Al-Adawi, Factors associated with self-reported medical errors among healthcare workers: a cross-sectional study from Oman, Int. J. Qual. Health Care, 2021, 33, mzab102 CrossRef PubMed.
- E. Kakemam, Z. Chegini, A. Rouhi, F. Ahmadi and S. Majidi, Burnout and its relationship to self-reported quality of patient care and adverse events during COVID-19: A cross-sectional online survey among nurses, J. Nurs. Manage., 2021, 29, 1974–1982 CrossRef PubMed.
- S. Khani and J. A. Tayek, Cortisol increases gluconeogenesis in humans: its role in the metabolic syndrome, Clin. Sci., 2001, 101, 739–747 CrossRef CAS.
- K. Šik Novak, N. Bogataj Jontez, S. Kenig, M. Hladnik, A. Baruca Arbeiter, D. Bandelj, M. Černelič Bizjak, A. Petelin, N. Mohorko and Z. Jenko Pražnikar, The effect of COVID-19 lockdown on mental health, gut microbiota composition and serum cortisol levels, Stress, 2022, 25, 246–257 CrossRef PubMed.
- R. S. Thompson, M. Gaffney, S. Hopkins, T. Kelley, A. Gonzalez, S. J. Bowers, M. H. Vitaterna, F. W. Turek, C. L. Foxx, C. A. Lowry, F. Vargas, P. C. Dorrestein, K. P. Wright, R. Knight and M. Fleshner, Ruminiclostridium 5, Parabacteroides distasonis, and bile acid profile are modulated by prebiotic diet and associate with facilitated sleep/clock realignment after chronic disruption of rhythms, Brain, Behav., Immun., 2021, 97, 150–166 CrossRef CAS PubMed.
- C. L. Wei, S. Wang, J. T. Yen, Y. F. Cheng, C. L. Liao, C. C. Hsu, C. C. Wu and Y. C. Tsai, Antidepressant-like activities of live and heat-killed Lactobacillus paracasei PS23 in chronic corticosterone-treated mice and possible mechanisms, Brain Res., 2019, 1711, 202–213 CrossRef CAS PubMed.
- G. De Palma, P. Blennerhassett, J. Lu, Y. Deng, A. Park, W. Green, E. Denou, M. Silva, A. Santacruz and Y. Sanz, Microbiota and host determinants of behavioural phenotype in maternally separated mice, Nat. Commun., 2015, 6, 7735 CrossRef CAS PubMed.
- R. D. Heijtz, S. Wang, F. Anuar, Y. Qian, B. Björkholm, A. Samuelsson, M. L. Hibberd, H. Forssberg and S. Pettersson, Normal gut microbiota modulates brain development and behavior, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 3047–3052 CrossRef CAS PubMed.
- A. Sarkar, S. M. Lehto, S. Harty, T. G. Dinan, J. F. Cryan and P. W. J. Burnet, Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals, Trends Neurosci., 2016, 39, 763–781 CrossRef CAS PubMed.
- L. H. Cheng, Y. W. Liu, C. C. Wu, S. Wang and Y. C. Tsai, Psychobiotics in mental health, neurodegenerative and neurodevelopmental disorders, J. Food Drug Anal., 2019, 27, 632–648 CrossRef CAS PubMed.
- A. A. Mohammadi, S. Jazayeri, K. Khosravi-Darani, Z. Solati, N. Mohammadpour, Z. Asemi, Z. Adab, M. Djalali, M. Tehrani-Doost, M. Hosseini and S. Eghtesadi, The effects of probiotics on mental health and hypothalamic-pituitary-adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers, Nutr. Neurosci., 2016, 19, 387–395 CrossRef CAS PubMed.
- G. Akkasheh, Z. Kashani-Poor, M. Tajabadi-Ebrahimi, P. Jafari, H. Akbari, M. Taghizadeh, M. R. Memarzadeh, Z. Asemi and A. Esmaillzadeh, Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial, Nutrition, 2016, 32, 315–320 CrossRef CAS PubMed.
- S. I. Wu, C. C. Wu, P. J. Tsai, L. H. Cheng, C. C. Hsu, I. K. Shan, P. Y. Chan, T. W. Lin, C. J. Ko, W. L. Chen and Y. C. Tsai, Psychobiotic Supplementation of PS128TM Improves Stress, Anxiety, and Insomnia in Highly Stressed Information Technology Specialists: A Pilot Study, Front. Nutr., 2021, 8, 614105 CrossRef PubMed.
- J. F. Liao, C. C. Hsu, G. T. Chou, J. S. Hsu, M. T. Liong and Y. C. Tsai, Lactobacillus paracasei PS23 reduced early-life stress abnormalities in maternal separation mouse model, Benefic. Microbes, 2019, 10, 425–436 CrossRef CAS PubMed.
- Y. W. Liu, W. H. Liu, C. C. Wu, Y. C. Juan, Y. C. Wu, H. P. Tsai, S. Wang and Y. C. Tsai, Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naive adult mice, Brain Res., 2016, 1631, 1–12 CrossRef CAS PubMed.
- S. Y. Huang, L. H. Chen, M. F. Wang, C. C. Hsu, C. H. Chan, J. X. Li and H. Y. Huang, Lactobacillus paracasei PS23 Delays Progression of Age-Related Cognitive Decline in Senescence Accelerated Mouse Prone 8 (SAMP8) Mice, Nutrients, 2018, 10, 894 CrossRef PubMed.
- P. L. Liao, C. C. Wu, T. Y. Chen, Y. C. Tsai, W. S. Peng, D. J. Yang and J. J. Kang, Toxicity Studies of Lactobacillus plantarum PS128TM Isolated from Spontaneously Fermented Mustard Greens, Foods, 2019, 8, 668 CrossRef CAS PubMed.
- E. Panel, O. B. Hazards, A. Ricci, A. Allende, D. Bolton, M. Chemaly, R. Davies, R. Girones, K. Koutsoumanis, R. Lindqvist, B. Nørrung, L. Robertson, G. Ru, P. S. Fernández Escámez, M. Sanaa, M. Simmons, P. Skandamis, E. Snary, N. Speybroeck, B. Ter Kuile, J. Threlfall, H. Wahlström, P. S. Cocconcelli, L. Peixe, M. P. Maradona, A. Querol, J. E. Suarez, I. Sundh, J. Vlak, F. Barizzone, S. Correia and L. Herman, Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 7: suitability of taxonomic units notified to EFSA until September 2017, EFSA J., 2018, 16, e05131 Search PubMed.
- S. Cohen, T. Kamarck and R. Mermelstein, A global measure of perceived stress, J. Health Soc. Behav., 1983, 24, 385–396 CrossRef CAS PubMed.
- L. C. Chu and H. S. R. Kao, The Moderation of Meditation Experience and Emotional Intelligence on the Relationship between Perceived Stress and Negative Mental Health, Chin. J. Psychol., 2005, 47, 157–179 Search PubMed.
- H. Alharbi and A. Alshehry, Perceived stress and coping strategies among ICU nurses in government tertiary hospitals in Saudi Arabia: a cross-sectional study, Ann. Saudi Med., 2019, 39, 48–55 CrossRef PubMed.
- T. R. Jordan, J. Khubchandani and M. Wiblishauser, The Impact of Perceived Stress and Coping Adequacy on the Health of Nurses: A Pilot Investigation, Nurs. Res. Pract., 2016, 2016, 5843256 Search PubMed.
- A. Ezzati, J. Jiang, M. J. Katz, M. J. Sliwinski, M. E. Zimmerman and R. B. Lipton, Validation of the Perceived Stress Scale in a community sample of older adults, Int. J. Geriatr. Psychiatry, 2014, 29, 645–652 CrossRef PubMed.
- Y. H. Chiu, F. J. Lu, J. H. Lin, C. L. Nien, Y. W. Hsu and H. Y. Liu, Psychometric properties of the Perceived Stress Scale (PSS): measurement invariance between athletes and non-athletes and construct validity, PeerJ, 2016, 4, e2790 CrossRef PubMed.
- N. Çelmeçe and M. Menekay, The Effect of Stress, Anxiety and Burnout Levels of Healthcare Professionals Caring for COVID-19 Patients on Their Quality of Life, Front. Psychol., 2020, 11, 597624 CrossRef PubMed.
- Y. Hacimusalar, A. C. Kahve, A. B. Yasar and M. S. Aydin, Anxiety and hopelessness levels in COVID-19 pandemic: A comparative study of healthcare professionals and other community sample in Turkey, J. Psychiatr. Res., 2020, 129, 181–188 CrossRef PubMed.
- D. T. Shek, The Chinese version of the State-Trait Anxiety Inventory: its relationship to different measures of psychological well-being, J. Clin. Psychol., 1993, 49, 349–358 CrossRef CAS PubMed.
- P. J. Chang and Y. C. Yeh, The Relationships between Gender, Birth Order, Family Structure, Emotion, Creative Personalities and Technological Creativity of Fifth Graders, National Chung-Shan University, 2003.
- S. I. Liu, Z. T. Yeh, H. C. Huang, F. J. Sun, J. J. Tjung, L. C. Hwang, Y. H. Shih and A. W. Yeh, Validation of Patient Health Questionnaire for depression screening among primary care patients in Taiwan, Compr. Psychiatry, 2011, 52, 96–101 CrossRef PubMed.
- C. Gagnon, L. Belanger, H. Ivers and C. M. Morin, Validation of the Insomnia Severity Index in primary care, J. Am. Board Fam. Med., 2013, 26, 701–710 CrossRef PubMed.
- J. Endicott, J. Nee, W. Harrison and R. Blumenthal, Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure, Psychopharmacol. Bull., 1993, 29, 321–326 CAS.
- L. Diop, S. Guillou and H. Durand, Probiotic food supplement reduces stress-induced gastrointestinal symptoms in volunteers: a double-blind, placebo-controlled, randomized trial, Nutr. Res., 2008, 28, 1–5 CrossRef CAS PubMed.
- F. X. Lesage and S. Berjot, Validity of occupational stress assessment using a visual analogue scale, Occup. Med., 2011, 61, 434–436 CrossRef CAS PubMed.
- J. Llinàs-Reglà, J. Vilalta-Franch, S. López-Pousa, L. Calvó-Perxas, D. Torrents Rodas and J. Garre-Olmo, The Trail Making Test, Assessment, 2017, 24, 183–196 CrossRef PubMed.
- H. E. Montgomery-Downs, S. P. Insana and J. A. Bond, Movement toward a novel activity monitoring device, Sleep Breath., 2012, 16, 913–917 CrossRef PubMed.
- J. D. Cook, M. L. Prairie and D. T. Plante, Utility of the Fitbit Flex to evaluate sleep in major depressive disorder: A comparison against polysomnography and wrist-worn actigraphy, J. Affective Disord., 2017, 217, 299–305 CrossRef PubMed.
- S. Otsuji, H. Shibata and M. Umeda, Turbidimetric immunoassay of serum C-reactive protein, Clin. Chem., 1982, 28, 2121–2124 CrossRef CAS.
- L. Ibanez, N. Potau, M. V. Marcos and F. de Zegher, Corticotropin-releasing hormone as adrenal androgen secretagogue, Pediatr. Res., 1999, 46, 351–353 CrossRef CAS PubMed.
- S. P. Kane, Sample Size Calculator. ClinCalc: https://clincalc.com/stats/samplesize.aspx, (accessed August 27, 2021, https://clincalc.com/stats/samplesize.aspx).
- A. Kazemi, S. Soltani, S. Ghorabi, A. Keshtkar, E. Daneshzad, F. Nasri and S. M. Mazloomi, Effect of probiotic and synbiotic supplementation on inflammatory markers in health and disease status: A systematic review and meta-analysis of clinical trials, Clin. Nutr., 2020, 39, 789–819 CrossRef CAS PubMed.
- A. Keskitalo, A. K. Aatsinki, S. Kortesluoma, J. Pelto, L. Korhonen, L. Lahti, M. Lukkarinen, E. Munukka, H. Karlsson and L. Karlsson, Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months, Stress, 2021, 24, 551–560 CrossRef CAS PubMed.
- D. Benton, C. Williams and A. Brown, Impact of consuming a milk drink containing a probiotic on mood and cognition, Eur. J. Clin. Nutr., 2007, 61, 355–361 CrossRef CAS PubMed.
- A. V. Rao, A. C. Bested, T. M. Beaulne, M. A. Katzman, C. Iorio, J. M. Berardi and A. C. Logan, A randomized, double-blind, placebo-controlled pilot study of a probiotic in emotional symptoms of chronic fatigue syndrome, Gut Pathog., 2009, 1, 6 CrossRef PubMed.
- M. Murata, J. Kondo, N. Iwabuchi, S. Takahashi, K. Yamauchi, F. Abe and K. Miura, Effects of paraprobiotic Lactobacillus paracasei MCC1849 supplementation on symptoms of the common cold and mood states in healthy adults, Benefic. Microbes, 2018, 9, 855–864 CrossRef CAS PubMed.
- H. M. Chen, P. S. Kuo, C. Y. Hsu, Y. H. Chiu, Y. W. Liu, M. L. Lu and C. H. Chen, Psychophysiological Effects of Lactobacillus plantarum PS128 in Patients with Major Depressive Disorder: A Preliminary 8-Week Open Trial, Nutrients, 2021, 13, 3731 CrossRef CAS PubMed.
- N. Zhang, Y. Zhang, M. Li, W. Wang, Z. Liu, C. Xi, X. Huang, J. Liu, J. Huang, D. Tian, J. Mu, X. Liao and S. Zhai, Efficacy of probiotics on stress in healthy volunteers: A systematic review and meta-analysis based on randomized controlled trials, Brain Behav., 2020, 10, e01699 Search PubMed.
- F. Huang and X. Wu, Brain Neurotransmitter Modulation by Gut Microbiota in Anxiety and Depression, Front. Cell Dev. Biol., 2021, 9, 649103 CrossRef PubMed.
- T. G. Dinan, C. Stanton and J. F. Cryan, Psychobiotics: a novel class of psychotropic, Biol. Psychiatry, 2013, 74, 720–726 CrossRef CAS PubMed.
- A. Lin, C. T. Shih, C. L. Huang, C. C. Wu, C. T. Lin and Y. C. Tsai, Hypnotic Effects of Lactobacillus fermentum PS150TM on Pentobarbital-Induced Sleep in Mice, Nutrients, 2019, 11, 2409 CrossRef CAS PubMed.
- K. Nishida, D. Sawada, T. Kawai, Y. Kuwano, S. Fujiwara and K. Rokutan, Para-psychobiotic Lactobacillus gasseri CP2305 ameliorates stress-related symptoms and sleep quality, J. Appl. Microbiol., 2017, 123, 1561–1570 CrossRef CAS PubMed.
- M. Takada, K. Nishida, A. Kataoka-Kato, Y. Gondo, H. Ishikawa, K. Suda, M. Kawai, R. Hoshi, O. Watanabe, T. Igarashi, Y. Kuwano, K. Miyazaki and K. Rokutan, Probiotic Lactobacillus casei strain Shirota relieves stress-associated symptoms by modulating the gut-brain interaction in human and animal models, Neurogastroenterol. Motil., 2016, 28, 1027–1036 CrossRef CAS PubMed.
- D. Shrivastava, S. Jung, M. Saadat, R. Sirohi and K. Crewson, How to interpret the results of a sleep study, J. Community Hosp. Intern. Med. Perspect., 2014, 4, 24983–24983 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |