Open Access Article
Open Access ArticleHelichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench infusions in reversing the traits of metabolic syndrome: a double-blind randomized comparative trial
Saša
Kenig
 a,
Katja
Kramberger
a,
Karin
Šik Novak
a,
Katja
Kramberger
a,
Karin
Šik Novak
 a,
Igor
Karnjuš
a,
Dunja
Bandelj
b,
Ana
Petelin
a and
Zala
Jenko Pražnikar
a,
Igor
Karnjuš
a,
Dunja
Bandelj
b,
Ana
Petelin
a and
Zala
Jenko Pražnikar
 *a
*a
aFaculty of Health Sciences, University of Primorska, Polje 42, Izola, Slovenia. E-mail: zala.praznikar@upr.si; Fax: +386-5-662-6480; Tel: +386-5-662-6469
bFaculty of Mathematics, Natural Sciences and Information Technologies, University of Primorska, 6000 Koper, Slovenia
First published on 24th June 2022
Abstract
Health impairments characteristic for metabolic syndrome such as increased body mass, a dysregulated lipid or glucose profile and elevated blood pressure can be reversed by appropriate lifestyle modifications. Supplementing the normal diet with herbal infusions is a promising strategy. We conducted a randomised double-blind comparative study in which participants with at least two traits of metabolic syndrome consumed an infusion of either Helichrysum italicum subsp. italicum (HI, n = 14) or Helichrysum arenarium (HA, n = 13) daily for 28 days. Anthropometric and biochemical parameters were measured at baseline, at the end of the intervention and after a 2-week washout period. HI infusion consumption had a beneficial effect on anthropometric traits; significant reductions in body weight, body mass index, and visceral and total body fat were observed. In the HA group, there was a greater reduction in serum glucose levels and an improvement in the lipid profile. In both groups, high LDL levels were measured at baseline, but two weeks after the intervention, in 84% of participants in the HA group and 71% in the HI group, the levels were within the reference range. Both interventions caused a decrease in HDL but also improved serum antioxidant properties. Consuming either infusion could thus be recommended as a simple, profitable habit for individuals with traits of metabolic syndrome.
1. Introduction
Metabolic syndrome is defined as a complex combination of several co-occurring conditions. These include upper-body obesity, atherogenic dyslipidaemia, insulin resistance and hypertension.1,2 Systemic low-grade inflammation is also tightly linked to its initiation and progression.3 The parameters are interrelated and have more than a merely additive effect on the development of a number of non-communicable diseases, such as coronary heart disease and other atherosclerotic cardiovascular diseases, as well as type 2 diabetes,4 all of which are among the leading causes of death worldwide.5 The prevalence of metabolic syndrome is somewhat difficult to determine due to discrepancies in the published criteria, but is estimated to be around a quarter of the world's population.6 The condition can be reversed with changes in lifestyle; dietary changes, such as caloric restriction,7 adherence to the Mediterranean or DASH diet7,8 or the induction of nutritional ketosis9 have been proposed in this context.10 Favourable outcomes have also been confirmed from the consumption of functional foods, such as pro- and prebiotics,11 fruits, vegetables and herbal teas.12Herbal medicines and nutraceuticals are traditionally used as part of primary health care and their use is increasingly accepted in both developing and developed countries.13 Several of them have already been studied in the context of metabolic syndrome and been proved at least moderately effective, either to target dyslipidemia14 or to manage insulin resistance,15 whereas the evidence for the use of herbal preparations for efficient weight loss is less convincing.16
Products from Helichrysum italicum (Roth) G. Don (also called immortelle or everlasting, abbreviated as HI) and Helichrysum arenarium (L.) Moench (sandy everlasting, abbreviated as HA), two members of the Asteraceae family, are widely used in ethnomedicine,17 mostly in the form of infusions/decoctions. Only HA is an approved medicinal plant and is as such available on the market. Its efficacy has been confirmed in increasing bile secretion and treating dyspeptic disorders.18 Importantly, no adverse effects have been reported.18 In an in vitro model, the anti-atherosclerotic and anti-inflammatory activities of flavonoids isolated from HA were recently reported.19 Moreover, HA extracts could scavenge free radicals20 and diminish lipid peroxidation.21 In a clinical trial, the consumption of naringenin, the main flavonoid in HA, reduced total and LDL cholesterol and triglycerides, and increased HDL levels in overweight/obese adults.22
HI has not yet been evaluated by the European Medicines Agency (EMA). However, we have recently shown in in vitro experiments in cell lines that HI exerts a higher antioxidative potential than HA.23 In addition, a study examining the acute effect of a single ingestion found increased fat oxidation and increased resting energy expenditure in healthy male participants.24 In a review of clinical trials, we found only six studies on the internal use of HI.25 Even though it can be confirmed from those studies that the use of HI is safe, since no negative side effects were reported, not much can be concluded about the efficacy, as in most studies HI was used in combination with other herbs.
In vitro data and the data investigating the separate compounds present in the HI and HA infusions suggest that they may have beneficial effects for patients with metabolic syndrome. Clinical trials are sparse and the need for them has been widely expressed.17 Both infusions have been thoroughly chemically characterized using HPLC-MS26 and differ in the composition of polyphenols. The HI infusion has a higher abundance of hydroxycinnamic acids and pyrones, whereas HA contains more flavonoides, in particular flavonones. HI is also rich in cyclic polyols, isobenzofuranones, and neolignans. Kaempferol and quercetin derivatives were detected only in HI, whereas there were no flavanones. The relative abundances of caffeoylquinic acid, total hydroxybenzoic acids, and cyclic polyols in HA were only somewhat lower than those in HI. Some compounds detected in HI were absent in HA; these were flavonols and flavones, pyrones, and coumarins. The sum of the identified phenolic compounds was approximately three times higher in HI.23,26 Based on this data, regular consumption could lead to beneficial but differential outcomes. In the present randomized clinical trial, we therefore compared the effects of the daily consumption of HI or HA infusion on anthropometric parameters, blood pressure, lipid profile, glucose levels and antioxidative status in adults exhibiting at least two metabolic syndrome traits. Sensory evaluation was performed concomitantly.
2. Methods
2.1. Study participants
Thirty-five asymptomatic overweight/obese adults were recruited into the study. The inclusion criteria were BMI > 25 kg m−2 and at least two parameters of metabolic syndrome (increased waist circumference, high blood pressure, increased serum glucose, increased serum triacyclglycerols and decreased serum HDL cholesterol), no more than a 3% change in body mass within the last three months, and age between 40 and 65 years. The exclusion criteria were cardiovascular, gastrointestinal, or liver disease, type 2 diabetes mellitus, taking medications for lipid disorders, anti-inflammatory drugs, antibiotics or nutritional supplements during the last 3 months, and being pregnant or lactating. The Caucasian male and female volunteers were recruited through advertisements posted on internet forums, sent via e-mail lists and published in local newspapers. Five participants did not meet the inclusion criteria; therefore, the final analytical sample was thirty volunteers. The sample size was determined using G*Power 3.1.9.7 (Heinrich Heine University, Duesseldorf, Germany), and was based on the effectiveness of chamomile tea in lowering cholesterol levels by approximately 10% in patients with type 2 diabetes.27 Therefore, we assumed a value of 0.6 mmol L−1 for the mean of the differences between the pairs for total cholesterol, whereas the standard deviation of the differences was set to 0.8 mmol L−1. Based on the predicted values, we calculated a priori that a sample size of 14 subjects in each group was required to achieve a statistical power of 80% and a significance level of 5% (two-sided). The number of participants was increased to 15 per group to account for the expected dropout rate. Eligible participants were included after we received their written informed consent.2.2. Study protocol
This study was conducted in accordance with the Declaration of Helsinki and all procedures were approved by the Slovenian National Medical Ethics Committee (no. 0120-557/2017/4). The protocol was registered on Clinicaltrials.gov (NCT04866628). The study is a double-blind randomized, comparative trial and was conducted at the University of Primorska, Faculty of Health Sciences, between May and July 2021. Thirty participants were randomly assigned into two groups – the HA group or the HI group. Randomization with stratification was performed using free open-source desktop application, MinimPy (https://sourceforge.net/projects/minimpy).28 The stratified variables were gender, age and BMI; we used two randomly assigned separate sequences for males and females, three randomly assigned separate sequences for age groups (<47 years; 48–55 years; >56 years), and three randomly assigned separate sequences for BMI groups (<30 kg m−2; 30–35 kg m−2; >35 kg m−2); therefore, both groups (HA and HI) were equal with regard to sex, age and BMI. The study was double blind; dried plant material was packaged and coded by a research assistant who did not know the aim of the study. Also, both infusions were similar in terms of colour, smell, and flavour and thus could not be distinguished by the participants.The study consisted of a 4 week intervention period, during which subjects ingested an infusion (2 dl day−1) every evening, and a 2 week follow-up period without ingestion of any tea beverage. Subjects were instructed to ingest the infusion every evening 2 hours after dinner and not to consume it simultaneously with their meals in order to prevent confounding effects of the macronutrients. The subjects were advised to maintain their physical activity level and their diet. They recorded their food intake for 3 days and these dietary data were analysed using the Open Platform for Clinical Nutrition (OPEN), accessible through the website https://opkp.si/. Anthropometric and biochemical measurements were performed at baseline, after 4 weeks of consumption and after a 2-week follow-up period. Compliance with the supplementation protocol was supervised by a researcher who contacted the subjects once a week.
2.3. Plant material and infusion preparation
The plant material used in the present study was obtained from two Helichrysum species: dried plant material of commercially available Helichrysum arenarium (L.) Moench tea, purchased from Flora Ltd, Rogatec, Slovenia, and Helichrysum italicum (Roth) G. Don subspecies italicum plants grown in a commercial plantation in Dragonja, Slovenian Istria (45°27′05′′ N 13°41′31′′ E). The plant material was the same as that in Kramberger et al.23 and was morphologically and genetically characterized by an expert biologist, Dr Dunja Bandelj. The morphological evaluation of HI subsp. italicum included the most indicative qualitative and quantitative characteristics, such as the presence of axillary leaf fascicles, the caulinar leaf length, leaf margin undulation, and the number of capitula per synflorescence, while genetic characterization employed eight newly developed microsatellite markers.23,29 The herbarium specimen of HI is deposited at the University of Primorska, Faculty of Mathematics, Natural Sciences and Information Technologies, Slovenia, under accession number HIa_UP20. Chemical composition analysis was performed using high-performance liquid chromatography–mass spectrometry (HPLC-MS) as discussed in our previous reports.23,26After baseline measurements, the participants received blinded HA or HI tea filter bags, containing 1 g of milled dried plant material. They were instructed to prepare hot water infusions by immersing the herbs enclosed in the tea filter paper in hot water (200 mL, 100 °C) for 10 minutes just before consumption.
2.4. Sensory evaluation of infusions
During the first and third weeks of the intervention the participants were instructed to smell and drink the infusion and perform sensory evaluation. The participants were provided with a prescribed questionnaire adopted from Adnan et al. (2013) with adjustments.30 It contained five items: overall impression; the likeability of its taste; bitterness; smell; and pleasantness. The sensory evaluation score was from 1 (dislike very much) to 6 (like very much) at either end of an unstructured 15 cm line scale.2.5. Appetite
The appetite items were assessed using a validated Simplified Nutritional Appetite Questionnaire (SNAQ) during the first and third weeks of the intervention and in the follow-up period.31 The SNAQ is a self-report appetite assessment tool, with short single-domain questions. Participants subjectively assessed their appetite in the preceding week on a scale from 1 (very poor) to 5 (very good) and the taste of food on the scale from 1 (very bad) to 5 (very good). They reported the frequency of feeling full after a meal on a scale from 1 (feeling full after a few bites) to 5 (hardly ever feel full) and the average number of meals they consumed in one day on a scale from 1 (less than one meal per day) to 5 (five or more meals per day). Their appetite for sweet foods and snacks was assessed using VAS, an unstructured 10 cm line scale from 1 (very poor) to 10 (very good).32 Subjects were requested to make a vertical mark on the line that best matched how they had been feeling in the last few days. Each score was determined by measuring the distance from the left side of the line to the mark. The average subjective appetite value was calculated as the average of the scales for all visual scales.2.6. Anthropometric measurements
All anthropometric measurements were performed between 7 a.m. and 9 a.m. in standardized conditions by the same examiner. First, systolic blood pressure (SBP), diastolic blood pressure (DBP) and heart rate (HR) were measured on the right upper arm, in a seated position, after resting for five minutes, with an automatic device (automatic blood pressure monitor Model SEM-1, Omron Healthcare Company, Singapore). Then, body weight was measured in light clothing without shoes to the nearest 0.1 kg and height to the nearest 0.1 cm, using a Leicester Height Measure (Invicta Plastics Limited, Oadby, UK). Body weight, height, body fat, fat free mass (FFM), visceral fat and total body water (TBW) were measured while subjects were in a standing position with bioelectrical impedance analysis using a Tanita MC-980MA (Maeno-cho, Japan) and corresponding software (GMON Pro-Tanita). The BMI was calculated as weight (kg) divided by height (m) squared. Each set of measurements lasted about 15–20 min.2.7. Blood parameters
Blood samples were collected from the median cubital vein immediately after anthropometric measurements. Serum samples were prepared after clot formation 15 min after withdrawal by centrifugation of the full blood at 2000g for 15 min. Serum aliquots were stored at −80 °C. Serum concentrations of glucose, triacylglycerols (TAG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), aminotransferase aspartate (AST), and aminotransferase alanine (ALT), C-reactive protein (CRP), bilirubin and uric acid (UA) were measured using Cobass reagents and performed on a Cobass c111 analyzer (Roche, Basel, Switzerland).2.8. Serum radical scavenging activity
Serum radical scavenging activity (RSA) was determined using 1,1-diphenyl-2-picrylhydrazyl (DPPH) reagent (Sigma-Aldrich, St Louis, MO). Samples were deproteinized with an equal volume of acetonitrile and centrifuged to remove the precipitate.33 Ascorbic acid served as a positive control. Samples were diluted 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 with phosphate buffered saline (PBS), mixed with 0.2 mM DPPH methanol solution and incubated at room temperature in the dark for 1 h. Absorbance was measured at 515 nm on an Infinite F200 spectrophotometer (Tecan Group Ltd, Zürich, Switzerland) and the percentage of DPPH discoloration was calculated as a measure of radical scavenging activity using the equation:
3 with phosphate buffered saline (PBS), mixed with 0.2 mM DPPH methanol solution and incubated at room temperature in the dark for 1 h. Absorbance was measured at 515 nm on an Infinite F200 spectrophotometer (Tecan Group Ltd, Zürich, Switzerland) and the percentage of DPPH discoloration was calculated as a measure of radical scavenging activity using the equation:| (A (DPPH with PBS) − A (PBS)) − (A (sample with DPPH) − A (sample))/(A (DPPH with PBS) − A (PBS)) × 100 |
2.9. RNA extraction, quantitative real-time PCR and data analysis
Peripheral blood mononuclear cells were isolated with Histopaque-1077 reagent (Sigma-Aldrich, St Louis, MO) from 2 mL of fresh blood samples collected into EDTA vacutainers (BD, Franklin Lakes, NJ). RNA was isolated using TRIzol reagent (Thermo-Fisher Scientific, Waltham, MA) and 2 μg were transcribed to cDNA with a cDNA Archive kit (Applied Biosystems, Waltham, MA). For the quantitative RT-PCR reactions, a QuantStudio® 5 Real-Time PCR System and a QuantiNova SYBR Green PCR kit (Qiagen, Hilden, Germany) were used. Primers with the following IDs were selected from the PrimerBank:34 48762945c1 for superoxide dismutase 1 (SD), 260436906c3 for catalase, 305410788c1 for glutathione reductase (GR), 196049379c2 for 3-hydroxy-3-methylglutaryl–CoA reductase (HMG–CoA-R), and 148298676c2 for 3-hydroxy-3-methylglutaryl–CoA synthase 1 (HMG–CoA-S). 18S rRNA was used as an internal control. The PCR conditions were 50 °C for 2 min, 95 °C for 10 min and 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The data were analyzed using the ΔΔCt algorithm. Melting curves were inspected to ensure primer specificity. One sample t-test was calculated to determine if the fold-change was greater than 1. An independent t-test was used to compare fold changes between groups.2.10. Statistics
Statistical analysis was performed using SPSS version 23.0 (IBM Corp., Armonk, NY). The normality of the variables was tested using the Shapiro–Wilk test. Data are presented as the mean value with the standard deviation, the median value with the minimum and maximum or a percentage. The effects of the interventions within each group were analyzed using Student's paired samples t-test or the Wilcoxon signed-rank test, whereas the comparison of mean changes between the 2 groups was done using an independent t-test or Mann–Whitney U test. P-Values <0.05 were considered statistically significant.3. Results
3.1. Baseline characteristics of study participants
In this study, 30 subjects started the experiments; three subjects dropped out due to scheduling problems and the samples of these three subjects were not included due to incomplete profiles at week 4 (one subject) or after the follow-up period (two subjects). The final sample size was therefore 27 subjects (19 women, 8 men). At baseline, groups were not significantly different with respect to age, sex, smoking habits, blood pressure, and anthropometry. In addition, the energy intake and macronutrient composition of the diets did not differ between the two groups (Tables 1 and 2). No adverse effects were reported during the clinical trial. According to the interviews with the participants at the end of the intervention, compliance was very good and we did not receive any unused tea filter bags at the end of the intervention period.| Helichrysum arenarium group (N = 13) | Helichrysum italicum group (N = 14) | P-Value | |
|---|---|---|---|
| Abbreviations: BMI, body mass index; F, female; M, male; MUFA, monounsaturated fatty acids; PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids. Values are expressed as means ± SD. P-Value denotes the difference between groups using an independent samples t-test or Mann–Whitney U test. | |||
| Gender (% F, M) | 69% F, 31% M | 71% F, 29% M | 0.905 |
| Age (years) | 54.1 ± 7.7 | 52.4 ± 8.0 | 0.573 |
| BMI (kg m−2) | 29.2 ± 3.7 | 32.0 ± 7.2 | 0.224 |
| Not smoking (%) | 92% | 86% | 0.784 |
| Energy intake (kcal) | 1939 ± 602 | 1888 ± 414 | 0.809 |
| Proteins (g) | 86 ± 35 | 81 ± 24 | 0.669 |
| Carbohydrates (g) | 207 ± 59 | 209 ± 66 | 0.937 |
| Fats (g) | 80 ± 34 | 79 ± 28 | 0.908 |
| SFA (g) | 28 ± 10 | 25 ± 7 | 0.381 |
| MUFA (g) | 24 ± 14 | 26 ± 21 | 0.814 |
| PUFA (g) | 10 ± 5 | 10 ± 4 | 0.810 |
| Cholesterol (mg) | 283 ± 179 | 283 ± 141 | 0.991 |
| Helichrysum arenarium group (N = 13) | Helichrysum italicum group (N = 14) | |||||
|---|---|---|---|---|---|---|
| Baseline | Week 4 | Follow-up | Baseline | Week 4 | Follow-up | |
| Abbreviations: ALT, alanine aminotransferase; AST, aspartate transaminase; BIL, bilirubin; BMI, body mass index; BP, blood pressure; DBP, diastolic blood pressure; FFM, free fat mass; GLU, glucose; HDL, high-density lipoprotein; HR, heart rate; LDL, low-density lipoprotein; RSA, radical scavenging activity; SBP, systolic blood pressure; TAG, triacylglycerols; TBW, total body water. TC, total cholesterol; UA, uric acid. Values are expressed as means ± SD or median ± SD (for CRP). aP-Value denotes significant (P < 0.05) difference in the change from baseline between Helichrysum italicum and Helichrysum arenarium groups, determined by independent t-test. bP-Value denotes significant (P < 0.05) difference from the initial value within the group determined by Student's paired samples t-test or the Wilcoxon signed-rank test. cP-Value denotes significant (P < 0.05) difference between the two groups (Helichrysum italicum vs. Helichrysum arenarium) at a given time point. | ||||||
| Body weight (kg) | 86.7 ± 12.6 | 86.6 ± 13.3 | 86.2 ± 13.7 | 91.3 ± 18.8 | 89.6 ± 18.8a,b | 88.4 ± 18.7a,b |
| BMI (kg m−2) | 29.2 ± 3.7 | 29.2 ± 3.9 | 29.0 ± 4.1 | 32.0 ± 7.2 | 31.4 ± 7.1a,b | 31.3 ± 7.2a,b |
| Body fat (%) | 34.9 ± 6.9 | 33.8 ± 7.0b | 33.6 ± 7.3b | 35.7 ± 7.4 | 35.0 ± 7.1b | 34.6 ± 7.1b |
| FFM (kg) | 56.5 ± 10.2 | 57.3 ± 10.3b | 57.1 ± 10.4b | 58.4 ± 11.7 | 58.0 ± 11.8a | 57.4 ± 10.7 |
| Visceral fat | 10.2 ± 2.3 | 9.9 ± 3.1 | 9.9 ± 3.1 | 10.9 ± 3.3 | 10.4 ± 3.2b | 10.1 ± 3.4b |
| TBW (%) | 45.9 ± 4.4 | 46.7 ± 4.6b | 46.9 ± 4.7b | 45.4 ± 4.8 | 45.8 ± 4.5b | 46.1 ± 4.6b |
| SBP (mm Hg) | 140 ± 12 | 135 ± 11 | 139 ± 13 | 142 ± 18 | 142 ± 19 | 137 ± 20 |
| DBP (mm Hg) | 88 ± 11 | 84 ± 10 | 90 ± 9 | 85 ± 8 | 89 ± 8a,b | 89 ± 11b |
| BP > 130/85 | 6/13 | 5/13 | 7/13 | 7/14 | 7/14 | 7/14 |
| HR (beats per min) | 76 ± 13 | 73 ± 9 | 71 ± 11 | 73 ± 11 | 72 ± 11 | 74 ± 12 |
| GLU (mmol L−1) | 5.27 ± 0.62 | 5.33 ± 0.97 | 4.90 ± 0.87b | 5.42 ± 1.07 | 5.19 ± 0.62 | 5.23 ± 0.77 |
| TC (mmol L−1) | 5.32 ± 1.06 | 5.18 ± 0.94 | 4.05 ± 0.71b | 4.98 ± 0.84 | 4.49 ± 0.83 | 4.29 ± 0.99b |
| LDL (mmol L−1) | 3.92 ± 0.82 | 3.77 ± 0.82 | 2.96 ± 0.49b | 3.80 ± 0.78 | 3.32 ± 0.64b | 3.15 ± 0.95b |
| HDL (mmol L−1) | 1.53 ± 0.53 | 1.49 ± 0.42 | 1.12 ± 0.37b | 1.29 ± 0.38 | 1.18 ± 0.36b | 1.08 ± 0.26b |
| LDL/HDL | 2.76 ± 0.86 | 2.69 ± 0.86 | 2.83 ± 0.81 | 3.20 ± 1.09 | 3.02 ± 0.94 | 3.06 ± 1.04 |
| TAG (mmol L−1) | 1.31 ± 0.55 | 1.32 ± 0.48 | 1.02 ± 0.36b | 1.29 ± 0.40 | 1.25 ± 0.41 | 1.18 ± 0.40 |
| AST (Ucat L−1) | 0.36 ± 0.12 | 0.35 ± 0.09 | 0.23 ± 0.06b | 0.45 ± 0.42 | 0.31 ± 0.11 | 0.24 ± 0.07 |
| ALT (Ucat L−1) | 0.36 ± 0.18 | 0.35 ± 0.14 | 0.25 ± 0.11b | 0.52 ± 0.55 | 0.37 ± 0.19 | 0.32 ± 0.16 |
| UA (μmol L−1) | 298 ± 87 | 314 ± 63 | 259 ± 48 | 259 ± 53 | 264 ± 55c | 254 ± 63 |
| BIL (μmol L−1) | 7.82 ± 4.56 | 8.14 ± 3.69 | 4.63 ± 2.79b | 7.02 ± 4.72 | 5.88 ± 3.01 | 4.99 ± 3.65b |
| CRP (μg L−1) | 1.82 ± 1.43 | 1.33 ± 1.86 | 0.89 ± 1.55 | 1.15 ± 2.40 | 2.91 ± 2.53 | 1.56 ± 3.15 |
| RSA (%) | 29.8 ± 4.3 | 32.6 ± 7.6 | 35.5 ± 5.1b | 28.2 ± 6.1 | 30.5 ± 9.1 | 35.8 ± 7.6b |
| High LDL | 11/13 | 9/13 | 2/13 | 11/14 | 7/14 | 4/14 |
| 3 or more MST | 4/13 | 2/13 | 2/13 | 6/14 | 4/14 | 4/14 |
| 2 or less MST | 9/13 | 11/13 | 11/13 | 8/14 | 10/14 | 10/14 |
3.2. Effects of Helichrysum infusions on anthropometric parameters
The paired t-tests revealed significant decreases in body weight, BMI, percentage of body fat, and visceral fat in the HI group, while the percentage of TBW and DBP significantly increased in the same group after week 4 and during the follow-up (Table 2). In the HA group, a significant decrease in the percentage of body fat and a significant increase in FFM and the percentage of TBW were found after week 4 and during follow-up. In addition, significant differences in the changes from baseline between HI and HA were observed for body weight and BMI at week 4 and after follow-up, and for FFM and DBP at week 4. To clarify, HI supplementation had a slightly bigger beneficial impact on some physiological changes (body weight and BMI) and these changes were also noted during follow-up. On the other hand, the less desirable change in DBP was also detected only in the HI group. Neither of the interventions had any impact on SBP or HR (Table 2). Considering blood pressure values over 130/85, which is defined as the threshold for metabolic syndrome, the percentage of the participants above this value was constant throughout the study.3.3. Effects of Helichrysum infusion consumption on metabolic profile
The baseline concentration of glucose, the lipid profile, liver enzymes, endogenic antioxidants (UA and bilirubin) and CRP did not differ significantly between the two groups (Table 2). In the HA group, glucose, total cholesterol, LDL cholesterol, HDL cholesterol and TAG were significantly lower during follow-up compared with the baseline values. Although the decrease in TC and LDL levels can already be observed at week 4, the difference was not significant. Additionally, during follow-up, the HA group showed a significant decrease in the AST and ALT levels and bilirubin levels compared with the baseline values. Similarly, in the HI group, there was a significant decrease in TC, HDL cholesterol and bilirubin during follow-up. LDL cholesterol was significantly decreased as early as after week 4 and remained so during follow-up. Neither of the interventions caused any improvement in the LDL/HDL ratio and the same was observed for CRP levels. However, radical scavenging activity of both groups improved in the follow-up period. At baseline, most participants (84% in HA and 79% in HI) had LDL levels above reference value; at the end of the HA and HI interventions, 30% (9/13) and 50% (7/14) of participants, respectively, had LDL values within the reference range. In the follow-up period further improvements were observed, as normal values of LDL were measured in 85% of the HA group and 72% of the HI group (Table 2). Due to the similar trend in the dynamics of biochemical parameters through the time period, no significant differences in the changes from baseline values between the two groups were observed.3.4. Effects of Helichrysum infusion consumption on appetite
Participants were instructed to maintain their normal diet. During the first and the third weeks of the intervention and in the follow-up period, changes in appetite, number of meals per day and cravings were followed anyway due to their possible influence on anthropometric parameters (Table 3). In the HA group the feeling of fullness was significantly decreased during the third week of consumption. On the other hand, in the group that consumed HI, cravings for sweet food were significantly reduced in the follow-up period compared with the first week and cravings for snacks were at the same time point significantly lower than during week 3. Appetite and number of meals per day remained constant throughout the study in both groups.| Helichrysum arenarium group (N = 13) | Helichrysum italicum group (N = 14) | |||||
|---|---|---|---|---|---|---|
| Week 1 | Week 3 | Follow-up | Week 1 | Week 3 | Follow-up | |
| Values are expressed as means ± SD. bP-Value denotes significant (P < 0.05) difference from the initial value within the group determined by the Wilcoxon signed-rank test. dP-Value denotes significant (P < 0.05) difference between follow-up and week 3 within the group using the Wilcoxon signed-rank test. | ||||||
| Appetite | 4.08 ± 0.64 | 4.00 ± 0.82 | 3.92 ± 0.64 | 3.79 ± 0.89 | 3.57 ± 0.65 | 3.64 ± 0.75 |
| Number of meals per day | 4.54 ± 0.88 | 4.46 ± 1.13 | 4.69 ± 0.75 | 4.29 ± 0.61 | 4.36 ± 0.93 | 4.21 ± 1.05 |
| Feeling of fullness | 4.15 ± 0.56 | 3.54 ± 0.88b | 4.00 ± 0.58 | 3.71 ± 0.61 | 3.71 ± 0.73 | 3.57 ± 0.85 |
| Craving for sweet foods | 6.08 ± 2.57 | 5.77 ± 2.24 | 5.31 ± 2.14 | 6.50 ± 2.71 | 5.50 ± 1.74 | 5.00 ± 2.04b |
| Craving for snacks | 4.62 ± 2.93 | 5.15 ± 2.34 | 4.85 ± 2.51 | 5.07 ± 2.46 | 5.14 ± 1.41 | 4.00 ± 1.71d |
3.5. Changes in gene expression
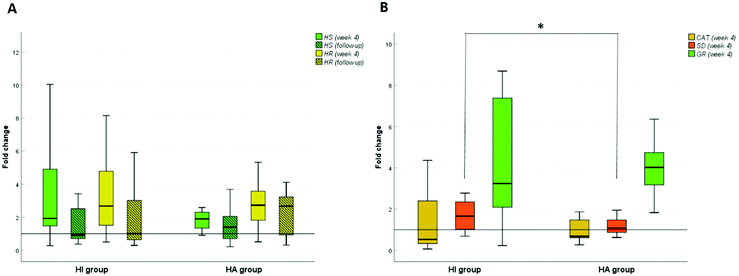
Due to the observed effects of HA and HI on serum cholesterol levels, we additionally examined two genes for the cholesterol metabolic pathway. We analyzed the expression levels of HMG-CoA-R and HMG-CoA-S in peripheral lymphocytes in both groups at baseline, after 4 weeks of supplementation and during follow-up (Fig. 1A). In week 4, HMG-CoA-S was 1.89 ± 3.05-fold (P < 0.05) upregulated in HA and 1.76 ± 2.68-fold (P < 0.05) in HI. In the follow-up period, the expression in HA was only slightly and not significantly above baseline (1.40 ± 1.83) and even below baseline (0.91 ± 1.83) in HI. The up-regulation of HMG-CoA-R was even more marked in week 4 (2.73 ± 2.94, P < 0.05 in HA and 2.64 ± 3.55, P < 0.05 in HI). In the follow-up period, it remained up-regulated (2.67 ± 1.36-fold, P < 0.05 compared with baseline) in HA, whereas in the HI group it returned to baseline (1.00 ± 2.28).3.6. Changes in radical scavenging activity
To explain the observed changes in radical scavenging activity (Table 2) and due to the previously reported antioxidant activity of HI and HA on cell lines,23 we were also interested in evaluating the expression of genes related to oxidative stress. We analyzed three genes for oxidative stress at baseline and after 4 weeks of the nutritional intervention. Catalase was downregulated to 0.68 ± 0.55 and 0.53 ± 1.48 (P < 0.05) in the HA and HI groups, respectively. Superoxide dismutase expression remained unchanged in HA, but was 1.66 ± 1.03-fold (P < 0.05) up-regulated after the intervention with HI; the difference between the groups was significant (P = 0.029). There was no significant difference in the expression of the GR gene between the groups; this gene was markedly up-regulated in both groups (4.02 ± 3.58, P < 0.05 in HA and 3.23 ± 10.13, P < 0.05 in HI). Looking at separate participants, the GR gene was upregulated in 84.6% and 85.7% of subjects after 4 weeks of HA or HI ingestion, respectively.3.7. Overall impression and sensory evaluation of Helichrysum infusions
Helichrysum infusions are beverages with a bitter taste; therefore, we were interested in overall likeability of these products over time. In general, the overall impression and overall likeability scores were higher in the HA group than in the HI group, but the significant difference was only in the pleasantness of ingestion of the infusion at week 1 (4.62 ± 1.33 for HA vs. 3.43 ± 1.34 for HI). Moreover, the pleasantness of HA significantly decreased over time (4.62 ± 1.33 at week 1 vs. 3.77 ± 1.42 at week 3). On the other hand, the results for the overall impression, the likeability of the taste, bitterness, and smell were not significantly different at the two time points or between the two groups (Table 4). All scores were above 3, suggesting that both products are acceptable for consumers.| Scores | Helichrysum arenarium group (N = 13) | Helichrysum italicum group (N = 14) | ||
|---|---|---|---|---|
| Week 1 | Week 3 | Week 1 | Week 3 | |
| Values are expressed as means ± SD. bP-Value denotes significant (P < 0.05) difference from the initial value within the group using a Wilcoxon signed-rank test. cP-Value denotes significant (P < 0.05) difference between the two groups (Helichrysum italicum vs. Helichrysum arenarium) at a given time point. | ||||
| Overall impression | 4.15 ± 1.44 | 4.08 ± 1.26 | 3.29 ± 1.73 | 3.29 ± 1.67 |
| Likeability of the taste | 4.31 ± 1.60 | 3.69 ± 1.44 | 3.14 ± 1.89 | 2.93 ± 1.94 |
| Likeability of the bitterness | 4.08 ± 1.55 | 3.85 ± 1.63 | 3.29 ± 1.59 | 3.43 ± 1.74 |
| Likeability of the smell | 4.69 ± 1.70 | 4.08 ± 1.50 | 3.50 ± 1.51 | 3.71 ± 1.59 |
| Pleasantness of ingestion | 4.62 ± 1.33 | 3.77 ± 1.42b | 3.43 ± 1.34c | 3.50 ± 1.83 |
4. Discussion
A four-week intervention where participants supplemented their normal diet with a daily intake of HI or HA infusion had beneficial effects on the components of metabolic syndrome. As expected, based on the differential chemical composition of the two infusions,23 the effects were only partially overlapping. The HI infusion proved effective in reducing body weight, BMI, total body fat and visceral fat, which could all be attributed to its ability to increase fat oxidation, as was shown in vivo for acute ingestion and in vitro in hepatocytes.24 The result is also in line with data on the separate polyphenolic compounds predominating in HI; chlorogenic acid, for example, reduced body mass and body fat,35 and green coffee beans, with abundant chlorogenic acid, caused a decrease in abdominal fat area and visceral fat.36 In animal models, of the compounds detected in HI, myricetins reduced body weight through brown adipose tissue activation37 together with an improved lipid profile,38 and kaempferol supplementation had a similar effect on weight loss as it can increase energy expenditure.39 An 8-week intervention with an intake of quercetin, detected in HI but not in HA,23 caused a decrease in waist circumference but not in BMI.40 Caffeoylquinic acids are also present in HA in lower amounts,23 and indeed, a decrease in the percentage of body fat with a concomitant increase in FFM was also observed in this group, despite there being no change in total body mass. In obese subjects, reduced cardiometabolic risk factors, i.e. multi-organ insulin sensitivity, improved beta-cell function and reduced levels of glucose and triglycerides, were achieved with a 5% weight loss.41 Our participants consuming HI infusion achieved a 4% body mass reduction, supporting the physiological relevance of the observed changes.In the follow-up period, two weeks after both interventions, total and LDL cholesterol were significantly decreased. The reduction was greater in case of HA intake, whereas HI exhibited a milder effect that was already detectable at the end of the 4-week period. HDL was also decreased in both interventions and consequently the LDL/HDL ratio remained constant. Only in the HA group did levels of glucose and triacylglycerols also drop in the follow-up period. Previously, an HA methanol extract was shown to diminish the glucose rise after sucrose ingestion,42 while a reduction of triacylglycerols was only demonstrated in investigations of separate compounds, such as naringenin.22 Apigenin, present in HA but not in HI, was also efficient in reducing serum triglycerides and total cholesterol in mice.43
The influence of HA on the metabolism of cholesterol is well known. Thus far there has not been a clinical trial, but HA's cholagogue and choleretic activities are the main reasons for its wide use in traditional medicine.44 In fact, its ability to stimulate bile secretion18 is the most likely mechanism leading to reduced cholesterol levels. When the bile acid pool is reduced, de novo synthesis from serum cholesterol is stimulated, which finally leads to reduced levels of total or LDL cholesterol.45 Apart from increased secretion, reduced endogenous synthesis could also cause reduced cholesterol levels. However, the detected increased expressions of HMG–CoA-S and HMG–CoA-R during both interventions oppose this hypothesis. It should be noted that the expressions were measured in peripheral lymphocytes and not in the liver, where most of endogenous synthesis takes place. The transient increase in the expression of genes involved in endogenous synthesis could compensate for decreased intestinal absorption.46 In cultured lymphocytes a similar compensatory mechanism, where an abundance of LDL particles could inhibit HMG–CoA-R and -S expression, has been shown.47 Bioactive compounds detected in infusions were shown to reduce serum cholesterol also through other mechanisms. Neolignans can downregulate proprotein convertase subtilisin/kexin type 9 (PCSK9) and upregulate LDL receptors, and with this increase the intake of LDL from serum to the liver.48 Several alpha-pyrones inhibit pancreatic cholesterol esterase, which results in poorer cholesterol absorption,49 but the exact same pyrones as found in HI (helipyrone, italipyrone and micropyrone) have not yet been evaluated in vivo.
Cravings for sweet foods and snacks were reduced in the follow-up period after the HI intervention but not during or after the HA intervention. Even though this fact could partially contribute to the observed changes in anthropometric and metabolic parameters, the contribution is probably not instrumental, as reduced cravings were not reflected in a reduced number of meals per day. There was also no change in appetite in general or in the feeling of fullness. On the contrary, the feeling of fullness was decreased in the HA group in agreement with its well-established traditional use.50
A decrease in serum levels of ALT and AST points to a hepatoprotective effect of HA. This is in line with previous reports,44,51 where such an effect was suggested based on the polyphenolic composition; however, no clinical study has been performed before. Use in chronic liver inflammation is also described in the Assessment Report on HA by the EMA.18 Of the bioactive compounds, isosalipurposide has been shown to exert a cytoprotective effect against oxidative damage in hepatocytes.52 Naringenin successfully reduced the NAFLD grade in human together with a decrease in serum cholesterol but not ALT and AST,22 whereas in an animal model it prevented a nanoparticle-induced rise in ALT and AST.53 In our HI group, ALT and AST were not significantly changed but the decrease in bilirubin was similar in both groups, pointing to a positive influence of both infusions on liver health.
The total serum antioxidative potential increased in the follow-up period after both interventions. Both infusions contain several antioxidative compounds; arzanol is the most abundant in HI,54 and in HA apigenin, naringenin and other flavanones are the main antioxidative constituents.55 The fact that radical scavenging activity increased only after the end of the interventions suggests that rather than absorption of the bioactive compounds per se, there was a hormetic response of the body to the intake of infusions. A beneficial compensation can be seen as a marked up-regulation of GR in both groups and a modest up-regulation of SD in the HI group. The endogenous antioxidants UA and bilirubin were not affected in the same way.
It is known that chlorogenic acid can also exert antihypertensive effects.56 Surprisingly, while in our study there was no effect of HI or HA on systolic blood pressure and in both groups the number of participants with high BP was constant throughout the study, DBP increased in the HI group. This is in contrast to our previous report where DBP decreased transiently 30 minutes after its acute intake.24 On the other hand, increased blood pressure in patients with hypertension is mentioned as a possible side effect of HA ingestion.18
5. Conclusions
Taken together, we show here that the intake of HI or HA has some favourable effects on the traits of metabolic syndrome. Interestingly, although only HA is an officially recognized medicinal plant, HI has some advantages when the physiological effects of the two are compared. It is more effective in improving anthropometric features and reducing cravings, whereas HA improves other components of metabolic syndrome, namely glucose and the lipid profile. On the down-side, both interventions caused a decrease in HDL. The study was intentionally designed in such a way that it could be followed in an everyday life. Consuming the infusion could be a simple and readily available profitable habit for people with components of metabolic syndrome, as it is known that HI and HA are grown in home gardens in different parts of the world; HA is especially common in Poland, Russia, Ukraine and Lithuania44 and HI in the Mediterranean regions.57 To this end it is also important that the product is sensorially acceptable to the consumer. It remains to be elucidated whether the infusions act through the direct absorption of bioactive compounds, the influence on the intestinal wall integrity, or the composition of the microbiota. Also, the relatively small numbers in this study can be considered a limitation and further studies with higher numbers of participants could be recommended based on the promising results.Author contributions
Conceptualization: S. K., Z. J. P., A. P.; Data curation: A. P., Z. J. P.; Formal analysis: S. K., K. K., A. P., Z. J. P.; Funding acquisition: Z. J. P., D. B.; Investigation: K. Š. N., K. K., I. K., A. P., S. K., Z. J. P.; Methodology: S. K:, A. P., Z. J. P.; Project administration: Z. J. P.; Resources: D. B.; Software: A. P., Z. J. P.; Supervision: S. K., Z. J. P.; Validation: A. P., Z. J. P.; Writing – original draft: S. K:; Writing – review & editing; A. P., D. B., K. K., S. K., Z. J. P.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was financially supported by the Slovenian Research Agency (research program P1-0386), the European Agricultural Fund for Rural Development LAG Istra (project 33152-247/217/15), and the Republic of Slovenia Ministry of Education, Science and Sport and European Union from European Social Funds (project SMILJ).References
- P. L. Huang, A comprehensive definition for metabolic syndrome, Dis. Models Mech., 2009, 2, 231–237 CrossRef CAS PubMed.
- S. M. Grundy, Metabolic syndrome update, Trends Cardiovasc. Med., 2016, 26, 364–373 CrossRef PubMed.
- K. Kempf, B. Rose, C. Herder, U. Kleophas, S. Martin and H. Kolb, Inflammation in metabolic syndrome and type 2 diabetes: Impact of dietary glucose, Ann. N. Y. Acad. Sci., 2006, 1084, 30–48 CrossRef CAS PubMed.
- E. Kassi, P. Pervanidou, G. Kaltsas and G. Chrousos, Metabolic syndrome: Definitions and controversies, BMC Med., 2011, 9, 48 CrossRef PubMed.
- World Health Organization, Global health estimates: Leading causes of death; Cause-specific mortality, 2000–2019, 2022, https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death, (accessed March 2022) Search PubMed.
- M. G. Saklayen, The global epidemic of the metabolic syndrome, Curr. Hypertens. Rep., 2018, 20, 12 CrossRef PubMed.
- S. Castro-Barquero, A. M. Ruiz-León, M. Sierra-Pérez, R. Estruch and R. Casas, Dietary strategies for metabolic syndrome: A comprehensive review, Nutrients, 2020, 12, E2983 CrossRef PubMed.
- M. Mirabelli, et al., Mediterranean Diet nutrients to turn the tide against insulin resistance and related diseases, Nutrients, 2020, 12, E1066 CrossRef PubMed.
- V. M. Gershuni, S. L. Yan and V. Medici, Nutritional ketosis for weight management and reversal of metabolic syndrome, Curr. Nutr. Rep., 2018, 7, 97–106 CrossRef CAS PubMed.
- T. N. Akbaraly, et al., Overall diet history and reversibility of the metabolic syndrome over 5 years, Diabetes Care, 2010, 33, 2339–2341 CrossRef PubMed.
- M. Green, K. Arora and S. Prakash, Microbial medicine: Prebiotic and probiotic functional foods to target obesity and metabolic syndrome, Int. J. Mol. Sci., 2020, 21, E2890 CrossRef PubMed.
- S. Mohamed, Functional foods against metabolic syndrome (obesity, diabetes, hypertension and dyslipidemia) and cardiovasular disease, Trends Food Sci. Technol., 2014, 35(2), 114–128, DOI:10.1016/j.tifs.2013.11.001.
- M. Ekor, The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety, Front. Pharmacol., 2014, 4, 177 Search PubMed.
- M. S. Adel Mehraban, O. Tabatabaei-Malazy, R. Rahimi, M. Daniali, P. Khashayar and B. Larijani, Targeting dyslipidemia by herbal medicines: A systematic review of meta-analyses, J. Ethnopharmacol., 2021, 280, 114407 CrossRef CAS PubMed.
- N. Tran, B. Pham and L. Le, Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery, Biology, 2020, 9, 252 CrossRef CAS PubMed.
- R. Farrington, I. F. Musgrave and R. W. Byard, Evidence for the efficacy and safety of herbal weight loss preparations, J. Integr. Med., 2019, 17, 87–92 CrossRef PubMed.
- D. Antunes Viegas, A. Palmeira-de-Oliveira, L. Salgueiro, J. Martinez-de-Oliveira and R. Palmeira-de-Oliveira, Helichrysum italicum: From traditional use to scientific data, J. Ethnopharmacol., 2014, 151, 54–65 CrossRef PubMed.
- Committee on Herbal Medicinal Products, Assessment report on Helichrysum arenarium (L.) Moench, flos, 2016, pp. 1–24 Search PubMed.
- Z. Mao, et al., Anti-atherosclerotic activities of flavonoids from the flowers of Helichrysum arenarium L. Moench through the pathway of anti-inflammation, Bioorg. Med. Chem. Lett., 2017, 27, 2812–2817 CrossRef CAS PubMed.
- E. Czinner, K. Hagymási, A. Blázovics, Á. Kéry, É. Szőke and É. Lemberkovics, In vitro antioxidant properties of Helichrysum arenarium (L.) Moench, J. Ethnopharmacol., 2000, 73, 437–443 CrossRef CAS PubMed.
- E. Czinner, K. Hagymási, A. Blázovics, Á. Kéry, É. Szőke and É. Lemberkovics, The in vitro effect of Helichrysi flos on microsomal lipid peroxidation, J. Ethnopharmacol., 2001, 77, 31–35 CrossRef CAS PubMed.
- Z. Namkhah, F. Naeini, S. Mahdi Rezayat, M. Yaseri, S. Mansouri and M. Javad Hosseinzadeh-Attar, Does naringenin supplementation improve lipid profile, severity of hepatic steatosis and probability of liver fibrosis in overweight/obese patients with NAFLD? A randomised, double-blind, placebo-controlled, clinical trial, Int. J. Clin. Pract., 2021, 75, e14852 CAS.
- K. Kramberger, Z. Jenko Pražnikar, A. Baruca Arbeiter, A. Petelin, D. Bandelj and S. Kenig, A comparative study of the antioxidative effects of Helichrysum italicum and Helichrysum arenarium infusions, Antioxidants, 2021, 10, 380 CrossRef CAS PubMed.
- S. Kenig, et al., Helichrysum italicum ssp. italicum infusion promotes fat oxidation in hepatocytes and stimulates energy expenditure and fat oxidation after acute ingestion in humans: A pilot study, Plants, 2021, 10, 1516 CrossRef CAS PubMed.
- K. Kramberger, S. Kenig, Z. Jenko Pražnikar, N. Kočevar Glavač and D. Barlič Maganja, A review and evaluation of the data supporting internal use of Helichrysum italicum, Plants, 2021, 10, 2021 CrossRef PubMed.
- K. Kramberger, et al., HPLC-DAD-ESI-QTOF-MS determination of bioactive compounds and antioxidant activity comparison of the hydroalcoholic and water extracts from two Helichrysum italicum species, Metabolites, 2020, 10, E403 CrossRef PubMed.
- M. Rafraf, M. Zemestani and M. Asghari-Jafarabadi, Effectiveness of chamomile tea on glycemic control and serum lipid profile in patients with type 2 diabetes, J. Endocrinol. Invest., 2015, 38, 163–170 CrossRef CAS PubMed.
- M. Saghaei and S. Saghaei, Implementation of an open-source customizable minimization program for allocation of patients to parallel groups in clinical trials, J. Biomed. Sci. Eng., 2011, 4, 734–739 CrossRef.
- A. Baruca Arbeiter, M. Hladnik, J. Jakše and D. Bandelj, First set of microsatellite markers for immortelle (Helichrysum italicum (Roth) G. Don): A step towards the selection of the most promising genotypes for cultivation, Ind. Crops Prod., 2021, 162, 113298 CrossRef CAS.
- M. Adnan, A. Ahmad, A. Ahmed, N. Khalid, I. Hayat and I. Ahmed, Chemical composition and sensory evaluation of tea (Camellia sinensis) commercialized in Pakistan, Pak. J. Bot., 2013, 45, 901–907 Search PubMed.
- M. M. G. Wilson, et al., Appetite assessment: Simple appetite questionnaire predicts weight loss in community-dwelling adults and nursing home residents, Am. J. Clin. Nutr., 2005, 82, 1074–1081 CrossRef CAS PubMed.
- C. P. Sepple and N. W. Read, Gastrointestinal correlates of the development of hunger in man, Appetite, 1989, 13, 183–191 CrossRef CAS PubMed , 1989.
- J. Chrzczanowicz, et al., Simple method for determining human serum 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity – Possible application in clinical studies on dietary antioxidants, Clin. Chem. Lab. Med., 2008, 46, 342–349 CAS.
- A. Spandidos, X. Wang, H. Wang and B. Seed, PrimerBank: A resource of human and mouse PCR primer pairs for gene expression detection and quantification, Nucleic Acids Res., 2010, 38, D792–D799 CrossRef CAS PubMed.
- E. Thom, The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people, J. Int. Med. Res., 2007, 35, 900–908 CrossRef CAS PubMed.
- T. Watanabe, S. Kobayashi, T. Yamaguchi, M. Hibi, I. Fukuhara and N. Osaki, Coffee abundant in chlorogenic acids reduces abdominal fat in overweight adults: A randomized, double-blind, controlled trial, Nutrients, 2019, 11, E1617 CrossRef PubMed.
- T. Hu, X. Yuan, G. Wei, H. Luo, H. J. Lee and W. Jin, Myricetin-induced brown adipose tissue activation prevents obesity and insulin resistance in db/db mice, Eur. J. Nutr., 2018, 57, 391–403 CrossRef CAS PubMed.
- H. C. Chao, P. F. Tsai, S. C. Lee, Y. S. Lin and M. C. Wu, Effects of myricetin-containing ethanol solution on high-fat diet induced obese rats, J. Food Sci., 2017, 82, 1947–1952 CrossRef CAS PubMed.
- P. A. Romero-Juárez, et al., Dietary flavonoid kaempferol reduces obesity-associated hypothalamic microglia activation and promotes body weight loss in mice with obesity, Nutr. Neurosci., 2021, 1–15, DOI:10.1080/1028415X.2021.2012629.
- M. Pfeuffer, et al., Effect of quercetin on traits of the metabolic syndrome, endothelial function and inflammation in men with different APOE isoforms, Nutr., Metab. Cardiovasc. Dis., 2013, 23, 403–409 CrossRef CAS PubMed.
- F. Magkos, et al., Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity, Cell Metab., 2016, 23, 591–601 CrossRef CAS PubMed.
- T. Morikawa, et al., Dipeptidyl peptidase-IV inhibitory activity of dimeric dihydrochalcone glycosides from flowers of Helichrysum arenarium, J. Nat. Med., 2015, 69, 494–506 CrossRef CAS PubMed.
- K. Zhang, W. Song, D. Li and X. Jin, Apigenin in the regulation of cholesterol metabolism and protection of blood vessels, Exp. Ther. Med., 2017, 13, 1719–1724 CrossRef CAS PubMed.
- D. Pljevljakušić, D. Bigović, T. Janković, S. Jelačić and K. Šavikin, Sandy everlasting (Helichrysum arenarium (L.) Moench): Botanical, chemical and biological properties, Front. Plant Sci., 2018, 9, 2018 Search PubMed.
- A. Capurso, et al., Increased bile acid excretion and reduction of serum cholesterol after crenotherapy with salt-rich mineral water, Aging, 1999, 11, 273–276 CAS.
- S. D. Turley and J. M. Dietschy, The intestinal absorption of biliary and dietary cholesterol as a drug target for lowering the plasma cholesterol level, Prev. Cardiol., 2003, 6, 29–33 CrossRef CAS PubMed.
- J. A. Cuthbert and P. E. Lipsky, Differential regulation of the expression of 3-hydroxy-3-methylglutaryl coenzyme A reductase, synthase, and low density lipoprotein receptor genes, J. Lipid Res., 1992, 33, 1157–1163 CrossRef CAS.
- H. S. Chae, et al., Identification of neolignans with PCSK9 downregulatory and LDLR upregulatory activities from Penthorum chinense and the potential in cholesterol uptake by transcriptional regulation of LDLR via SREBP2, J. Ethnopharmacol., 2021, 278, 114265 CrossRef CAS PubMed.
- L. M. Deck, M. L. Baca, S. L. Salas, L. A. Hunsaker and D. L. Vander Jagt, 3-Alkyl-6-chloro-2-pyrones: Selective inhibitors of pancreatic cholesterol esterase, J. Med. Chem., 1999, 42, 4250–4256 CrossRef CAS PubMed.
- Committee on Herbal Medicinal Products, European Union herbal monograph on Helichrysum arenarium (L.) Moench, flos, 2015, p. 5 Search PubMed.
- A. D. Kshirsagar, R. Mohite, A. S. Aggrawal and U. R. Suralkar, Hepatoprotective medicinal plants of Ayurveda – A review, Asian J. Pharm. Clin., 2011, 4, 1–8 Search PubMed.
- J. Y. Han, et al., The chalcone compound isosalipurposide (ISPP) exerts a cytoprotective effect against oxidative injury via Nrf2 activation, Toxicol. Appl. Pharmacol., 2015, 287, 77–85 CrossRef CAS PubMed.
- D. I. El-Wafaey, O. E. Nafea and E. M. Faruk, Naringenin alleviates hepatic injury in zinc oxide nanoparticles exposed rats: Impact on oxido-inflammatory stress and apoptotic cell death, Toxicol. Mech. Methods, 2022, 32, 58–66 CrossRef CAS PubMed.
- J. Werner, et al., Pyrone derivatives from Helichrysum italicum, Fitoterapia, 2019, 133, 80–84 CrossRef CAS PubMed.
- A. C. Gradinaru, M. Silion, A. Trifan, A. Miron and A. C. Aprotosoaie, Helichrysum arenarium subsp. arenarium: Phenolic composition and antibacterial activity against lower respiratory tract pathogens, Nat. Prod. Res., 2014, 28, 2076–2080 CrossRef CAS PubMed.
- R. Revuelta-Iniesta and E. A. S. Al-Dujaili, Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: A pilot crossover study using green and black coffee, BioMed Res. Int., 2014, 2014, 2014 Search PubMed.
- T. Ninčević, M. Grdiša, Z. Šatović and M. Jug-Dujaković, Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity, Ind. Crops Prod., 2019, 138, 111487 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |