Open Access Article
Open Access ArticleUnraveling the impact of viscosity and starch type on the in vitro starch digestibility of different gels
Maria
Santamaria
a,
Leticia
Montes
b,
Raquel
Garzon
a,
Ramón
Moreira
b and
Cristina M.
Rosell
 *ac
*ac
aInstitute of Agrochemistry and Food Technology (IATA-CSIC), C/Agustin Escardino, 7, 46980 Paterna, Valencia, Spain. E-mail: crosell@iata.csic
bDepartment of Chemical Engineering, Universidade de Santiago de Compostela, rúa Lope Gómez de Marzoa, Santiago de Compostela, E-15782, Spain
cDepartment of Food and Human Nutritional Sciences, University of Manitoba, Winnipeg, Canada
First published on 7th June 2022
Abstract
Starch is one of the most important carbohydrates that is present in many foods. Gelatinization is an important property of starch, associated with physical changes that promote an increase in viscosity. The objective of this research was to understand how the viscosity of starch gels affects their hydrolysis and whether that effect was dependent on the type of starch. Different gels (corn, wheat, and rice) with variable or constant viscosity were analyzed using diverse methodologies to determine the changes in the pasting behavior. A rapid force analyzer, a vibration viscometer and a rheometer were used to differentiate the gels based on the starch source and concentration. At a fixed starch concentration, corn gel displayed the highest viscosity, slowing the enzymatic starch hydrolysis. The higher viscosity of those gels prepared with a fixed starch concentration significantly enhanced the slowly digestible starch (SDS) and reduced the kinetic constant (k). Nevertheless, gels with constant viscosity (550 mPa s) showed comparable hydrolysis kinetics, obtaining similar SDS, total hydrolyzed starch and k. The correlation matrix confirmed the relationship between k and gel viscosity (r = −0.82), gelatinization rate (α-slope) (r = −0.87), breakdown (r = −0.84) and elastic modulus (G′ 37 °C) (r = −0.73). Therefore, these parameters could be used as predictors of the hydrolysis performance of starch gels as well as in reverse engineering for the design of healthy foods.
Introduction
Starch is a polysaccharide extensively used as a functional ingredient in many foods due to its applications as a thickener, stabilizer, gelling agent, and water retention agent.1 Because of that, besides intrinsic properties like amylose content, granule size, length of amylopectin branches and crystallinity, the pasting properties or viscosity performance (peak viscosity, final viscosity, breakdown and setback viscosity) of the slurries during heating and cooling are always reported as key properties for starch characterization.2Consumers’ health concerns have prompted the evaluation of food-related properties that could contribute to human well-being and prevent diseases. In that scenario, starch hydrolysis plays a fundamental role pertaining to postprandial glucose levels and in consequence the glycemic index of the foods.3 Starch digestion by the action of enzymes in the small intestine and the subsequent rate of absorption of the released glucose have been used to categorize starch into rapidly digestible starch (RDS), slowly digestible starch (SDS) and resistant starch (RS).4 These facts have pointed out the importance of starch hydrolysis kinetics. Thus, besides the intrinsic features of starch previously mentioned, the digestive performance of different starches is usually included in the studies of starch characterization.5 Different strategies have been developed to modulate carbohydrate digestion, which include reducing the amount of available carbohydrates, reducing the rate of digestion or reducing the glucose absorption rate.6 In response to that, starches with low digestibility have been developed, like those rich in resistant starch either present in the native starch or obtained after chemical modification or processing.7
Nevertheless, the digestion of starch is not only affected by its features but also by the physical properties of the media which can modulate the rate of enzyme diffusion to starch substrates.7 Literature studies have confirmed the role of bulk viscosity in gastric emptying and the reduction of glycemic index, thus opening the opportunity to modulate digestion with compounds that affect viscosity. This has been explored with diverse starches and hydrocolloids, which might restrict enzyme accessibility to starch by interacting with the surface of starch granules or creating a hydrated network surrounding that encapsulates the granule, or increasing the bulk viscosity.8,9 In fact, results with different polysaccharides (guar gum and chitosan) indicated a negative correlation between the peak viscosity (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 814–14
814–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 535 mPa s) and the SDS fraction of potato starches, suggesting that the effect might be more related to physical properties than chemical interactions.10 Nevertheless, very limited studies have correlated the viscosity of starch gels with the digestion parameters. For instance, a higher peak viscosity (480–5076 mPa s) and viscosity breakdown, defined as the difference between the peak viscosity and the lowest viscosity of potato starches during the holding stage at 95 °C (24–3540 mPa s), were correlated with lower hydrolysis rates of native starches but that correlation was not observed with gelatinized starches.11 Bajaj et al. (2018)2 reported a reverse relationship between gel hardness and gelatinization temperatures with the RS amount, but no relationship with the peak viscosity in the range of 2183 to 8387 mPa s. Velásquez-Barreto et al. (2021)12 have recently reported the positive relationship of SDS, obtained in in vitro digestibility studies, with the Rapid Visco Analyzer (RVA) peak viscosity of gels (290–370 mPa s) and the viscosity upon cooling the starch gels isolated from unconventional Peruvian tubers up to 60 °C (92–180 mPa s). Furthermore, other researchers used rheometric techniques to relate starch rheological behavior with its hydrolysis.13 Yield stress (σ0) or the minimum force required to initiate the flow of starch paste was positively correlated with the peak viscosity (4647–8303 mPa s) in pearl millet starches and negatively correlated with the RS amount.13 Overall, although previous research has characterized the rheological properties of different starch gels and their hydrolysis, the results do not allow the identification of the potential role of viscosity in explaining the encountered divergences.
535 mPa s) and the SDS fraction of potato starches, suggesting that the effect might be more related to physical properties than chemical interactions.10 Nevertheless, very limited studies have correlated the viscosity of starch gels with the digestion parameters. For instance, a higher peak viscosity (480–5076 mPa s) and viscosity breakdown, defined as the difference between the peak viscosity and the lowest viscosity of potato starches during the holding stage at 95 °C (24–3540 mPa s), were correlated with lower hydrolysis rates of native starches but that correlation was not observed with gelatinized starches.11 Bajaj et al. (2018)2 reported a reverse relationship between gel hardness and gelatinization temperatures with the RS amount, but no relationship with the peak viscosity in the range of 2183 to 8387 mPa s. Velásquez-Barreto et al. (2021)12 have recently reported the positive relationship of SDS, obtained in in vitro digestibility studies, with the Rapid Visco Analyzer (RVA) peak viscosity of gels (290–370 mPa s) and the viscosity upon cooling the starch gels isolated from unconventional Peruvian tubers up to 60 °C (92–180 mPa s). Furthermore, other researchers used rheometric techniques to relate starch rheological behavior with its hydrolysis.13 Yield stress (σ0) or the minimum force required to initiate the flow of starch paste was positively correlated with the peak viscosity (4647–8303 mPa s) in pearl millet starches and negatively correlated with the RS amount.13 Overall, although previous research has characterized the rheological properties of different starch gels and their hydrolysis, the results do not allow the identification of the potential role of viscosity in explaining the encountered divergences.
Recently, the authors studied the impact of the viscosity of corn starch gels, obtained by varying the starch concentration, on in vitro hydrolysis and observed that the hydrolysis kinetics constant is inversely dependent on gel viscosity due to enzyme diffusion limitation.14 Specifically, a positive significant relationship was defined between gel viscosity and the starch fraction SDS (R2 = 0.95) and RS (R2 = 0.96). In the case of RDS, the results suggested that a viscosity threshold is required to affect enzyme accessibility. Nevertheless, that impact of viscosity was only tested with corn starch gels, and thus what happens with other cereal starches remains to be investigated.
The possible correlation between starch gel characteristics and starch digestion might contribute to reverse engineering in the design of starch-based systems. In this way, foods could be designed based on the knowledge of the targeted final food characteristics. For this reason, the present study aims to validate the relationship of gel characteristics with the in vitro hydrolysis of starch gels obtained from different cereals. Starch gels from corn, wheat, and rice with variable viscosity (VV) or constant viscosity (CV) were rheologically characterized and their properties were correlated with the in vitro hydrolysis parameters.
Materials and methods
Materials
Commercial food grade starches, having similar amylose content, from corn (20.15% amylose content and 12.43% moisture content) and wheat (23.98% amylose content and 12.72% moisture content) were supplied by EPSA (Valencia, Spain) and rice starch (20.71% amylose content and 10.30% moisture content) was purchased from Sigma Aldrich (Sigma Chemical, St Louis, USA). The enzymes used were type VI-B α-amylase from porcine pancreas (EC 3.2.1.1) from Sigma Aldrich (Sigma Chemical, St Louis, USA) and amyloglucosidase (EC 3.2.1.3) from Novozymes (Bagsvaerd, Denmark). A D-Glucose Assay Kit (GOPOD) was provided by Megazyme (Megazyme International Ireland Ltd., Bray, Ireland). Other chemicals were of analytical grade.Preparation of starch gels with constant amounts of starch (variable viscosity) or constant viscosity
Two sets of gels were prepared: the first one using a fixed amount of starch; those gels were referred to as variable viscosity (VV), and the second one by varying the amount of starch to obtain constant viscosity (CV). For gels under VV notation, 5 g of starch (based on 14% moisture content) was suspended in 20 g of water. Starches (corn, wheat, and rice) were manually dispersed in deionized water and the slurries were heated in a boiling water bath for 20 minutes and manual stirring was applied every five minutes. The resulting gels were cooled down to 37 °C for further analysis.The viscosity of the rice gel, prepared as previously described, was measured at 37 °C using a vibration viscometer VL7-100B-d15 (Hydramotion Ltd, Malton, United Kingdom). Although the viscosity is measured at high shears, when reaching the Newtonian plateau, the complexity associated with shear-thinning materials is removed. Preliminary assays were conducted with corn and wheat starches to identify the amount of starch required to obtain a viscosity similar to the one obtained with the rice gel. Afterwards, the second set of gels was prepared with starch: water, setting up the ratio for rice, corn, and wheat at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, 1
4, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 and 1
5.5 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.2, respectively, to obtain gels with similar viscosities, referred to as constant viscosity (CV).
5.2, respectively, to obtain gels with similar viscosities, referred to as constant viscosity (CV).
The amount of total starch (TS) in the gels was quantified using a commercial assay kit (K.TSTA) (Megazyme International Ireland Ltd., Bray, Ireland) following the determination of the total starch content of the samples containing resistant starch (RTS-NaOH procedure is recommended).
Rapid force analyzer
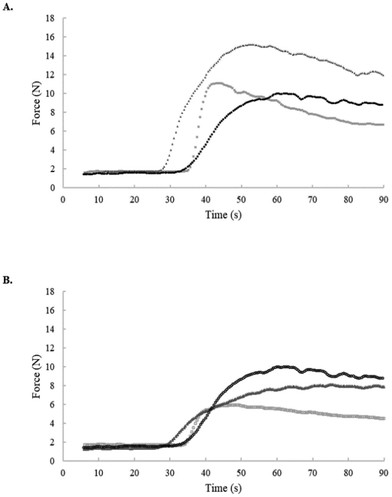
The force changes during starch gelatinization were studied using a rapid force analyzer (RFA, Amylab® Chopin Technologies, Villeneuve-la-Garenne, Cedex, France), as previously described by Garzon and Rosell et al. (2021).15 Briefly, the starch slurry was placed into the precision test tubes of the device and manually shaken for 30 s. After immersing the stirring rod into the slurry, the tube was capped with a plunger and placed into the holder of the device. The rapid test consisted of heating the sample at 100 °C for 90 s and subjecting it to continuous shearing. The plots recorded the force, expressed in Newtons, of the slurry/gel under continuous heating/shearing. The parameters defined include the onset time indicating the start of gelatinization, the initial (F0) and maximum force (F1), the α-slope among F0 and F1, the final force at 90 s (F2) and the force difference between F1 and F2 related to starch breakdown.Gels viscoelastic behavior
The viscoelastic characterization was made using a stress-controlled rheometer (MCR 301; Anton Paar, Graz, Austria) using a starch pasting cell (ST24-2D/2V/2V-30, gap 2.460 mm, bob radius 12 mm) with a solvent trap kit to minimize water evaporation during the tests. Different starches (corn, wheat, and rice) were dispersed in water (total weight 20 g) with constant and variable gel viscosity and poured into the rheometer cuvette at 95 °C. First, a pre-shear of 100 s−1 was made for 1 min to homogenize the sample at 95 °C. Secondly, a time sweep was carried out at 30 Pa, 1 Hz and 95 °C for 19 min (previous assays were performed to ensure that frequency sweeps were carried out inside the linear viscoelastic region of tested gels). Then, a cooling profile was made from 95 °C to 37 °C at 3 °C min−1 with a constant stress of 30 Pa and a constant frequency of 1 Hz. The frequency sweep was carried out from 0.1 to 10 Hz at 1% strain and 37 °C. Afterwards, a time sweep was carried out at 30 Pa, 1 Hz and at 37 °C for 30 min to observe the maturation of the gel. A second frequency sweep was made under the same conditions as the first one.In vitro digestibility
The digestibility of the starch gels was determined following the method described by Santamaria et al. (2021),14 with a few modifications. A fresh gel (200 mg) was mixed with 4 mL of 0.1 M sodium maleate buffer (pH 6.9) containing porcine pancreatic α-amylase (0.9 U mL−1) by using an Ultra Turrax T18 basic homogenizer (IKA-Werke GmbH and Co. KG, Staufen, Germany). The slurry was incubated in a shaker incubator (SKI 4; ARGO Lab, Carpi, Italy) at 37 °C for 3 h under constant stirring (200 rpm). Aliquots were taken to quantify glucose release. The remnant starch after the 24 h hydrolysis was solubilized with 2 mL of 1.7 M NaOH using an Ultra-Turrax T18 homogenizer (IKA-Werke GmbH and Co. KG, Staufen, Germany) for 5 min at 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm in an ice bath and hydrolyzed with amyloglucosidase (143 U mL−1) at 50 °C for 30 min in a shaking water bath for its complete hydrolysis. Glucose determination was performed using a glucose oxidase-peroxidase (GOPOD) kit. The absorbance was measured using a SPECTROstar Nano microplate reader (BMG LABTECH, Ortenberg, Germany) at 510 nm. Starch was calculated as glucose (mg) × 0.9.
000 rpm in an ice bath and hydrolyzed with amyloglucosidase (143 U mL−1) at 50 °C for 30 min in a shaking water bath for its complete hydrolysis. Glucose determination was performed using a glucose oxidase-peroxidase (GOPOD) kit. The absorbance was measured using a SPECTROstar Nano microplate reader (BMG LABTECH, Ortenberg, Germany) at 510 nm. Starch was calculated as glucose (mg) × 0.9.
From the hydrolysis results, rapidly digestible starch (RDS) or the percentage of total starch hydrolyzed within 20 min of incubation, slowly digestible starch (SDS) or the starch fraction hydrolyzed within 20 and 120 min, digestible starch or total starch hydrolyzed after 24 h (DS), and resistant starch (RS) that remained after 24 h of incubation were calculated.
The in vitro hydrolysis data were fit to a first-order equation (eqn (1)) to describe the kinetic parameters of starch hydrolysis as reported by Goñi et al. (1997).16
| C = C∞ (1 − e−kt) | (1) |
Statistical analysis
All experiments were carried out in triplicate and the experimental data were statistically analyzed by the Statgraphics Centurion XVII software (Statistical Graphics Corporation, Rockville, MD, USA). Data were subjected to multivariate analysis of variance (MANOVA) and the values were expressed as a mean ± standard deviation. Fisher's least significant differences test (LSD) was used to estimate the significant differences among experimental mean values with a significance level of p ≤ 0.05. Furthermore, Pearson correlation analysis was used to identify the possible relationship between the rheological and hydrolysis parameters.Results and discussion
Two different types of gels were prepared using corn, wheat or rice starches to identify the role of viscosity in the pasting properties, viscoelastic properties, and digestibility performance. The first set of gels was prepared with the same amount of starch and thus variable viscosity (VV). The initial amount of starch selected for those gels was based on a previous study,14 where the concentration (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 starch
4 starch![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water) for corn starch gels was the most limiting one regarding the relationship among the closed gel structure, the higher viscosity, and the slowest and more limited starch hydrolysis. In contrast, the second set was prepared with varying amounts of starch for obtaining gels with the same viscosity (CV). The amount of total starch in samples with variable gel viscosity was 17.20 ± 0.20 g per 100 g. On the other hand, the constant viscosity was 12.63 ± 0.08 g per 100 g, 12.60 ± 0.18 g per 100 g and 16.93 ± 0.15 g per 100 g of starch for corn, wheat, and rice gels, respectively.
water) for corn starch gels was the most limiting one regarding the relationship among the closed gel structure, the higher viscosity, and the slowest and more limited starch hydrolysis. In contrast, the second set was prepared with varying amounts of starch for obtaining gels with the same viscosity (CV). The amount of total starch in samples with variable gel viscosity was 17.20 ± 0.20 g per 100 g. On the other hand, the constant viscosity was 12.63 ± 0.08 g per 100 g, 12.60 ± 0.18 g per 100 g and 16.93 ± 0.15 g per 100 g of starch for corn, wheat, and rice gels, respectively.
The viscosity of the gels prepared at VV was significantly (p < 0.05) influenced by the starch source (Table 1). The corn gel presented the highest viscosity (1170 mPa s) at 37 °C, followed by the wheat gel (834 mPa s), and finally the rice gel (525 mPa s). The viscosity of the rice starch was selected as the target to obtain CV gels.
| Variable gel viscosity (VV) | Constant gel viscosity (CV) | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|
| Corn VV | Wheat VV | Rice VV, Rice CV | Corn CV | Wheat CV | Source | Viscosity | ||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5 5.5 |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.2 5.2 |
||||
Values followed by different letters within the same row denote significant differences p < 0.05. Parameters: η (viscosity), onset (starch gelatinization initial time), F0 (initial force), α-slope (between F0 and F1), F1 (maximum force), F2 (final force), breakdown (difference between F1 and F2), G′ (storage modulus) G′′ (loss modulus), and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (damping factor). δ (damping factor). |
||||||||
| η adjustment | Vibration viscosimeter | |||||||
| η (mPa s) | 1170 ± 293a | 834 ± 81b | 525 ± 15c | 542 ± 88c | 553 ± 55c | 0.0297 | 0.0044 | |
| Gel development | RFA parameters | |||||||
| Onset (s) | 36 ± 1a | 28 ± 0b | 34 ± 2a | 34 ± 1a | 28 ± 3b | 0.0005 | 0.7310 | |
| F 0 (N) | 2.10 ± 0.28 | 1.98 ± 0.49 | 1.90 ± 0.76 | 1.72 ± 0.12 | 1.51 ± 0.62 | 0.8749 | 0.3515 | |
| α-Slope | 1.23 ± 0.00a | 0.99 ± 0.01b | 0.57 ± 0.02c | 0.52 ± 0.04c | 0.39 ± 0.02d | 0.1314 | 0.0043 | |
| F1 (N) | 11.39 ± 0.30b | 15.29 ± 0.55a | 9.93 ± 0.86b | 6.11 ± 0.26d | 8.08 ± 0.68c | 0.1626 | 0.0060 | |
| F2 (N) | 6.74 ± 0.25c | 11.99 ± 1.14a | 8.78 ± 1.03b | 4.54 ± 0.02d | 7.92 ± 0.62bc | 0.0030 | 0.0189 | |
| Breakdown (N) | 4.65 ± 0.05a | 3.19 ± 0.44b | 1.16 ± 0.17c | 1.57 ± 0.28c | 0.15 ± 0.06d | 0.0394 | 0.0046 | |
| Gel behavior | Rheometric parameters | |||||||
| Cooling profile (initial and end values, at 1 Hz) | ||||||||
| G′ 95 °C (Pa) | 301 ± 2c | 575 ± 7a | 340 ± 8b | 171 ± 6d | 293 ± 16c | 0.0134 | 0.0102 | |
| G′′ 95 °C (Pa) | 108 ± 39b | 233 ± 42a | 81 ± 21b | 73 ± 19b | 79 ± 0b | 0.1073 | 0.0488 | |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ 95 °C δ 95 °C |
0.359 ± 0.125ab | 0.405 ± 0.069ab | 0.237 ± 0.057b | 0.428 ± 0.095a | 0.269 ± 0.016ab | 0.0824 | 0.6637 | |
| G′ 37 °C (Pa) | 3025 ± 49b | 3580 ± 141a | 872 ± 4e | 1380 ± 85d | 1580 ± 99c | 0.0049 | 0.0045 | |
| G′′ 37 °C (Pa) | 155 ± 31b | 344 ± 4a | 99 ± 12c | 92 ± 9c | 173 ± 5b | 0.0022 | 0.0175 | |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ 37 °C δ 37 °C |
0.051 ± 0.011b | 0.096 ± 0.003a | 0.113 ± 0.013a | 0.067 ± 0.011b | 0.109 ± 0.004a | 0.0001 | 0.1211 | |
| Mechanical spectra | ||||||||
| Slope linear G’ (0.1–10 Hz) | 0.020 ± 0.001 | 0.022 ± 0.002 | 0.026 ± 0.008 | 0.019 ± 0.003 | 0.023 ± 0.002 | 0.6419 | 0.1769 | |
| Slope linear G′′ (0.1–10 Hz) | 0.213 ± 0.035 | 0.195 ± 0.074 | 0.235 ± 0.042 | 0.247 ± 0.019 | 0.246 ± 0.002 | 0.1919 | 0.9474 | |
| G’ (0.1 Hz) | 4620 ± 71b | 5775 ± 7a | 1075 ± 35e | 2675 ± 148d | 3955 ± 92c | 0.0000 | 0.0042 | |
| G′′ (0.1 Hz) | 154 ± 61ab | 255 ± 87a | 97 ± 24b | 68 ± 6b | 109 ± 14b | 0.1148 | 0.0387 | |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (0.1 Hz) δ (0.1 Hz) |
0.033 ± 0.013b | 0.044 ± 0.015b | 0.090 ± 0.020a | 0.025 ± 0.001b | 0.028 ± 0.004b | 0.0003 | 0.3128 | |
Starch performance during gelatinization and the viscoelastic properties of gels
After setting up the conditions to obtain the two types of gels, their textural performance during gelatinization was recorded using a rapid force analyzer (RFA).15 It uses a rapid (90 s) thermal method under continuous shearing. The force required to stir the slurries during gelatinization was different for each starch gel (Fig. 1). A very low force was detected at the beginning of the test, which was high enough till heating to promote the onset of starch swelling with a simultaneous increase in the stirring force. The pasting performance of the gels was dependent on the source of starch and, obviously, on the amount of starch. However, the observed changes in the plots revealed not only the starch dilution but also the changes in the force pattern of the gels. The parameters defined to analyze the gel performance in the RFA are shown in Table 1. Upon adapting viscosity (CV), to have constant gel viscosity, differences within the RFA plots were reduced, particularly during gelatinization. Regarding specific parameters, the starch source significantly (p < 0.05) affected the onset of gelatinization, force at 90 s (F2) and breakdown, whereas the gel viscosity (CV or VV gels) factor affected significantly (p < 0.05) the α-slope, maximum (F1) and final force (F2), and breakdown. Wheat gels showed the lowest onset indicating that gelatinization began at lower temperatures.15 Among the VV gels made with the same amount of starch, the corn gel showed a higher α-slope, indicating faster gelatinization, and the wheat gel displayed the highest maximum force (F1). Garzon and Rosell et al. (2021)15 observed the same trend and correlated higher force with more porous gels, revealing thicker walls and big holes. The corn gel presented a higher breakdown, indicating lower resistance to physical rupture during starch granule swelling. A similar result was reported using the RVA when comparing corn and rice starches and it was related to the higher swelling of granules.17 When adapting gels to obtain CV, corn and wheat gels showed lower forces with respect to rice gel along gelatinization. The starches showed significant differences with regard to F1 but the onset, α-slope and breakdown of the rice and corn starches were similar, confirming the proximity of the physical behavior of the starch gels when adapting viscosity.All starch gels, after fully developing a stable network structure, showed a solid like behavior (G′ > G′′) (Table 1). During the cooling profile from 95 to 37 °C, both moduli increased, but greater differences were observed on G′ than G′′. In VV gels, ΔG′ and ΔG′′ were higher for corn and wheat starches than for rice starch. At 37 °C, the rice starch led to the weakest gel with the lowest elastic modulus (872 Pa), Table 1. Meanwhile, the strongest gel (high G′ value) was obtained with wheat starch (in respective sets of CV and VV gels). This property is relevant to measuring the easiness of the gel to be fragmented into small pieces under shear rates. The rheological tests confirmed that CV gels had closer values of viscous modulus. At 37 °C, the gels were subjected to two frequency sweeps (time 0 and 30 min) and the viscoelastic behavior with angular frequency was almost constant, meaning that gel maturation took place mainly during cooling and when the gel achieved the lowest temperature, the maturation was practically complete (data not shown). Strong and weak gels can be classified as such based on their mechanical spectra. In all the cases, G′ > G′′ from 0.1 to 10 s−1, with G′ being relatively independent of frequency (slope <0.03) and G′′ increasing with increasing frequency (Fig. 2). In fact, the slope of G′′ with frequency varied in a narrow range (from 0.20 up to 0.25) and no significant difference (p > 0.05) was found between the tested starch gels, Table 1. This type of spectrum is usually associated with a weak gel.18 Upon small deformations, weak gels resemble strong gels, but as the deformations increase, the three-dimensional networks undergo a progressive (and reversible) breakdown.19 The tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′) values at 0.1 Hz for VV gels were 0.033, 0.044 and 0.090 for corn, wheat, and rice gels, respectively, indicating that the viscous character is low, but more relevant in rice gels. No significant difference (p > 0.05) between the tan
δ (G′′/G′) values at 0.1 Hz for VV gels were 0.033, 0.044 and 0.090 for corn, wheat, and rice gels, respectively, indicating that the viscous character is low, but more relevant in rice gels. No significant difference (p > 0.05) between the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ values of CV gels and VV gels from the same starch was observed. Therefore, some differences in the viscoelastic behavior of the tested starch gels were found in relation to the formation of firmer (higher G′) or more stable (low damping factor) structures.
δ values of CV gels and VV gels from the same starch was observed. Therefore, some differences in the viscoelastic behavior of the tested starch gels were found in relation to the formation of firmer (higher G′) or more stable (low damping factor) structures.
In vitro hydrolysis of starch gels
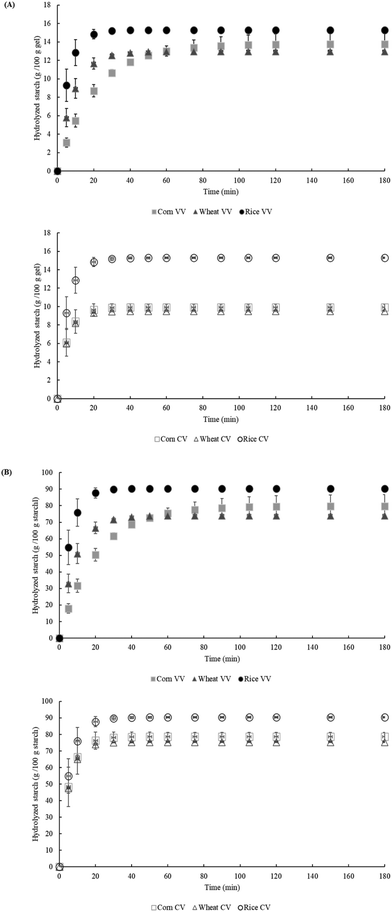
Starch gels were subjected to enzymatic hydrolysis with digestive enzymes (Fig. 3). Intrinsic properties like amylose size and chain size distribution of amylopectin have been related to the in vitro digestion of native starches, but in the gel state that molecular order and their contribution might no longer be crucial and be more related to the new molecular organization in which the initial amorphous structure is more susceptible to enzyme hydrolysis.20 Therefore, if only structural features were responsible for the starch hydrolysis kinetics, no differences would be detected due to viscosity changes.To assess the impact of the amount of starch, the results are expressed in grams of hydrolyzed starch per 100 g of gel (Fig. 3A) and grams of hydrolyzed starch per 100 g of starch (Fig. 3B). Regarding VV gel hydrolysis, the rice gel showed faster and higher hydrolysis (Fig. 3A VV), which could be related to its lower viscosity at 37 °C (Table 1), compared to the wheat and corn gels. In highly viscous systems, like wheat and corn gels, enzyme diffusion encounters the external resistance (viscosity) of the gels that affects the hydrolysis. A similar behavior has been observed when modulating the viscosity by incorporating hydrocolloids in starch gels and it has been attributed to the limitations of the enzyme accessibility to starch.21,22 However, when comparing gels having the same viscosity (CV) different enzymatic hydrolyses were observed (Fig. 3A CV). The CV gels of wheat and corn displayed a similar hydrolysis behavior but the CV gel of rice showed more extensive hydrolysis. Although that trend could be initially attributed to its higher starch content, the hydrolysis plots normalized to the amount of starch revealed the same trend (Fig. 3B). Therefore, the results confirmed that gel hydrolysis was not only affected by starch content, and considering they had similar viscosity, gel physical properties like viscoelasticity might also influence the hydrolysis of gels. This behavior might be related either to the lower G′ of the rice gel (Table 1), which suggested a weaker gel structure, or to more porous gels, as previously mentioned high force gels (F1 in Table 1) were related to porosity as reported by Garzon and Rosell et al. (2021).15 Both effects would favor enzyme accessibility to the gel, explaining the more extensive hydrolysis of CV rice gels.
Starch fractions (RDS, SDS, DS and RS), according to the rate of glucose release, presented statistically significant differences (p < 0.05) (Table 2). The starch source significantly (p < 0.05) affected the RDS, whereas gel viscosity significantly (p < 0.05) impacted the amounts of SDS and RS. VV gels made of corn starch had the lowest amount of RDS, which agrees with the findings of Zhang et al. (2006)23 by studying different raw cereal starches. Corn VV gel had the highest viscosity and thus the variability in the starch gel characteristics mainly affect the RDS. In addition, the corn VV gel had the highest amount of SDS (Table 2). Nevertheless, gels made at constant viscosity did not present statistically significant differences in SDS, and rice gel gave the highest RDS and RS.
| Variable gel viscosity | Constant gel viscosity | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Corn VV | Wheat VV | Rice VV, Rice CV | Corn CV | Wheat CV | Source | Viscosity | |
| Means within the same row followed by different letters indicate significant differences p < 0.05. C∞ and k were determined by the equation, C = C∞ (1 − e−kt).a Rapidly digestible starch (RDS), slowly digestible starch (SDS), digestible starch (DS), resistant starch (RS), kinetic constant (k), equilibrium concentration (C∞), area under the hydrolysis curve after 180 min (AUC), total starch content (TS) and hydrolysis percentage (C∞/TS). | |||||||
| RDS (%) | 8.70 ± 0.66c | 11.66 ± 0.60b | 14.84 ± 0.51a | 9.64 ± 0.65c | 9.32 ± 0.05c | 0.0001 | 0.4246 |
| SDS (%) | 5.02 ± 1.79a | 1.30 ± 0.73b | 0.45 ± 0.43b | 0.30 ± 0.31b | 0.18 ± 0.00b | 0.1190 | 0.0461 |
| DS (%) | 14.26 ± 2.76a | 11.51 ± 1.91ab | 13.26 ± 0.26ab | 11.83 ± 0.45ab | 10.26 ± 0.81b | 0.0756 | 0.1604 |
| RS (%) | 20.15 ± 1.71a | 17.85 ± 1.94a | 17.24 ± 2.79a | 7.76 ± 3.57b | 10.62 ± 1.03b | 0.4312 | 0.0169 |
| k (min−1) | 0.05 ± 0.01b | 0.12 ± 0.03ab | 0.19 ± 0.06a | 0.20 ± 0.07a | 0.20 ± 0.00a | 0.2488 | 0.0383 |
| C ∞ (%) | 13.77 ± 1.20b | 12.96 ± 0.13b | 15.29 ± 0.08a | 9.93 ± 0.34c | 9.50 ± 0.05c | 0.0022 | 0.0063 |
| AUC | 2194 ± 114b | 2215 ± 4b | 2661 ± 39a | 1729 ± 78c | 1656 ± 8c | 0.0003 | 0.0058 |
| C ∞ /TS (%) | 79.77 ± 7.10b | 74.08 ± 3.36b | 90.36 ± 0.31a | 78.64 ± 3.21b | 75.86 ± 0.46b | 0.0003 | 0.9064 |
In addition, the kinetic parameters derived from in vitro hydrolysis plots (Fig. 3A) are shown in Table 2. The kinetic constant (k) or the hydrolysis rate was significantly (p < 0.05) affected by gel viscosity, being faster when decreasing the viscosity, but a similar k (p > 0.05) was obtained with the gels obtained at CV. Therefore, the loss of the gel crystalline structure did not determine the k,24 but the physical properties are significantly affecting hydrolysis. With regard to variable viscosity, the corn gel showed the slowest kinetic constant. A decrease in the k was accompanied by a simultaneous increase in the SDS content. For this reason, gel viscosity could be a modulating factor as it can limit the enzyme diffusion rate and slow down the enzymatic hydrolysis. Regarding the equilibrium concentration of the hydrolyzed starch (C∞) and the area under the hydrolysis curve (AUC), they were significantly (p < 0.05) affected by both factors: starch source and gel viscosity. The maximum hydrolysis (C∞) indicates the extent of the hydrolysis when the curve reaches a plateau and the area under the curve is related to the glucose release in 180 minutes of hydrolysis. As previously mentioned, the rice gel presented the largest hydrolysis (Fig. 3A), even when comparing the starch gels made at constant viscosity. In samples with constant viscosity, these parameters decreased due to the lower starch content of the gels.
The relationship between the equilibrium concentration of hydrolyzed starch and the total starch content of each gel was significantly affected by the type of starch. The rice gel had a higher hydrolysis percentage (90.36%), while the corn and wheat gels displayed similar results. Consequently, gel viscosity is a factor with a great impact on the reaction rate (k) and on the starch fractions, particularly the SDS. This result agrees with the findings of Velásquez-Barreto et al. (2021)12 who studied tuber starches and observed positive correlations between gel viscosities and SDS amounts.
Correlation matrix
A correlation matrix was established to find any significant relationship between the parameters recorded from the pasting behaviour, the viscoelastic characterization, and the in vitro hydrolysis of tested gels (Table 3). The viscosity at 37 °C showed a strong positive correlation with SDS (r = 0.83) and moderate correlations with DS (r = 0.65) and RS (r = 0.63). Therefore, the results confirmed that the viscosity of the gels affects the hydrolysis behaviour. Likely, the viscosity of the system retards the binding of α-amylase-starch or modifies the starch structure thus affecting the α-amylase activity.25 In fact, a significant negative correlation (r = −0.82) was observed between the viscosity at 37 °C and the kinetic constant (k), thus confirming that viscosity limits the mass transfer and affects the hydrolysis reaction rate. These results support that higher viscosity in a food matrix increases SDS content, which has been associated with a lower glycemic index, greater satiety and slower enzymatic hydrolysis.22,26 A positive correlation was observed between the α-slope of RFA with SDS (r = 0.84) and RS (r = 0.74). Interestingly, a strong negative correlation (r = −0.87) was observed between the α-slope and kinetic constant (k), indicating that faster gelatinization led to gels with reduced kinetic constant. This fact is also related to gel firmness (G′) and negative correlation (r = −0.73), because gels with a higher gelatinization rate give firmer gels that undergo slower hydrolysis.15 A positive moderate correlation was observed between maximum force (F1) and RS (r = 0.74). Garzon and Rosell et al. (2021)15 related the force with gel structure, suggesting that higher force was required for obtaining gels with a more porous structure. Breakdown was positively correlated with SDS (r = 0.83) and RS (r = 0.65) and negatively correlated with kinetic constant (r = −0.84), which agree with previous results.8 It has been reported that the loss of crystalline structure in gelatinized starch is not a determining factor for starch digestion.24 Nevertheless, it seems that higher breakdown, and consequently lower stability during heating, allowed higher structural disorganization of the gels, which could be recrystallized during cooling giving more structured gels that offer more resistance to hydrolysis as indicated by the higher SDS and lower k. This assumption was also supported by the significant negative correlation observed between the SDS and tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′) values of the gels after cooling (r = −0.72), relating starch hydrolysis with the level of the gel structure. Regarding the rheometric properties, those that showed the most significant correlations (p < 0.01) were in mechanical spectra. A significant negative correlation (r = −0.78) was observed between G′ (0.1 Hz) and the hydrolysis percentage (C∞/TS). This could mean that a characteristic such as elasticity can influence the percentage of hydrolysis. In native starches, the chain length distribution has been correlated with the starch digestibility,20 but that fundamental property does not seem to explain the hydrolysis behaviour of the gels. The digestibility of the gel depends on the ability of the enzyme to penetrate into the gel; consequently, strong structures (high firmness) of gels seem to delay the hydrolysis. In addition, there was a high correlation between the tan
δ (G′′/G′) values of the gels after cooling (r = −0.72), relating starch hydrolysis with the level of the gel structure. Regarding the rheometric properties, those that showed the most significant correlations (p < 0.01) were in mechanical spectra. A significant negative correlation (r = −0.78) was observed between G′ (0.1 Hz) and the hydrolysis percentage (C∞/TS). This could mean that a characteristic such as elasticity can influence the percentage of hydrolysis. In native starches, the chain length distribution has been correlated with the starch digestibility,20 but that fundamental property does not seem to explain the hydrolysis behaviour of the gels. The digestibility of the gel depends on the ability of the enzyme to penetrate into the gel; consequently, strong structures (high firmness) of gels seem to delay the hydrolysis. In addition, there was a high correlation between the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ (G′′/G′) values at 0.1 Hz with RDS (r = 0.89), C∞ (r = 0.71), AUC (r = 0.82), and C∞/TS (r = 0.69), which suggested that less structured gels (high damping factor) favoured the initial hydrolysis of starch, for the first 20 minutes, and also the extent of the gels hydrolysis.
δ (G′′/G′) values at 0.1 Hz with RDS (r = 0.89), C∞ (r = 0.71), AUC (r = 0.82), and C∞/TS (r = 0.69), which suggested that less structured gels (high damping factor) favoured the initial hydrolysis of starch, for the first 20 minutes, and also the extent of the gels hydrolysis.
| RDS (%) | SDS (%) | DS (%) | RS (%) | k | C ∞ (%) | AUC | C ∞ /TS (%) | |
|---|---|---|---|---|---|---|---|---|
| Bold values indicate significant correlations. ** Indicates p < 0.01. * Indicates p < 0.05. | ||||||||
| η (mPa s) | −0.41 | 0.83** | 0.65* | 0.63* | −0.82** | 0.30 | 0.14 | −0.23 |
| Onset (s) | −0.05 | 0.42 | 0.68* | 0.12 | −0.25 | 0.31 | 0.24 | 0.49 |
| F0 (N) | 0.08 | 0.42 | 0.25 | 0.38 | −0.31 | 0.44 | 0.38 | 0.20 |
| α-Slope | −0.21 | 0.84** | 0.50 | 0.74** | −0.87** | 0.52 | 0.36 | −0.16 |
| F1 (N) | 0.25 | 0.37 | 0.15 | 0.74* | −0.54 | 0.57 | 0.53 | −0.17 |
| F2 (N) | 0.46 | −0.06 | −0.12 | 0.51 | −0.14 | 0.41 | 0.46 | −0.10 |
| Breakdown (N) | −0.25 | 0.83** | 0.50 | 0.65* | −0.84** | 0.46 | 0.31 | −0.16 |
| G′ 95 °C | 0.37 | 0.06 | −0.08 | 0.57 | −0.30 | 0.42 | 0.44 | −0.24 |
| G′′ 95 °C | 0.15 | 0.06 | −0.07 | 0.43 | −0.34 | 0.19 | 0.20 | −0.54 |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ 95 °C δ 95 °C |
−0.33 | 0.01 | 0.09 | −0.07 | −0.23 | −0.32 | −0.34 | −0.68* |
| G′ 37 °C | −0.34 | 0.58 | 0.07 | 0.53 | −0.73* | 0.16 | 0.03 | −0.58 |
| G′′ 37 °C | 0.00 | 0.05 | −0.20 | 0.35 | −0.30 | 0.04 | 0.03 | −0.62 |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ 37 °C δ 37 °C |
0.66* | −0.72* | −0.30 | −0.13 | 0.65* | 0.04 | 0.21 | 0.18 |
| Slope lin G′ (0.1–10 Hz) | −0.02 | −0.26 | −0.47 | −0.49 | 0.40 | −0.24 | −0.21 | 0.18 |
| Slope lin G′′ (0.1–10 Hz) | 0.52 | −0.27 | −0.12 | 0.20 | 0.16 | 0.29 | 0.37 | 0.35 |
| G′ 0.1 Hz | −0.54 | 0.41 | −0.17 | 0.28 | −0.56 | −0.19 | −0.30 | −0.78** |
| G′′ 0.1 Hz | 0.03 | 0.24 | 0.24 | 0.57 | −0.48 | 0.23 | 0.20 | −0.43 |
tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ 0.1Hz δ 0.1Hz |
0.89** | −0.21 | 0.42 | 0.39 | 0.20 | 0.71* | 0.82** | 0.69* |
Conclusions
The rheological performance of starch gels, besides their in vitro hydrolysis, allows the assessment of global starch functionality, namely the technological behaviour for industrial applications and the prediction of their comportment during digestion. Viscosity plays a fundamental role in starch gel functionality, being an important parameter that modulates those functionalities. Starch gels from different cereals have significantly different viscosities when produced at constant starch concentrations, and as a consequence, different viscoelastic properties and in vitro hydrolysis kinetics. Particularly, wheat and corn gels displayed higher forces and solid like behaviour. Conversely, rice gel showed a lower gelatinization rate and weak behaviour. Nevertheless, force along gelatinization and the viscoelastic properties of cereal starch gels were closer when comparing gels of similar viscosity, showing alike hydrolysis rates. The results allowed the correlation of the rheological properties with the hydrolysis parameters, thus confirming the importance of gel viscosity, which was positively correlated with the SDS fraction (r = 0.83) and RS (r = 0.63), and negatively correlated with the kinetic constant (r = −0.82). Therefore, a higher viscosity in the range of 550–1170 mPa s will slow down enzymatic hydrolysis. Therefore, apart from the already well-known factors (amylose/amylopectin ratio, chain length, gel structure, and so on) that affect starch digestion, gel viscosity could be a rapid indicator for estimating starch kinetic hydrolysis. Overall, the gel viscosity of cereal starches greatly affects the hydrolysis kinetics, which opens the opportunity to apply reverse engineering in the design of starch-based systems to reduce postprandial glucose levels. Further in vivo studies will be undertaken to confirm the results obtained from the model systems.Author contributions
Credit roles: MS: Conceptualization; data curation; formal analysis; investigation; methodology; and roles/writing – original draft; LM: Investigation and methodology; RG: Methodology; supervision; and data curation; RM: Formal analysis; writing – review & editing; and funding acquisition; CMR: Conceptualization; funding acquisition; investigation; supervision; and writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial support of Grant RTI2018-095919-B-C2 funded by MCIN/AEI/10.13039/501100011033, “ERDF A way of making Europe” by the “European Union”, the Generalitat Valenciana (Project Prometeo 2017/189) and the Xunta de Galicia (Consolidation Project ED431B 2019/01).References
- Y. Ai and J.-L. Jane, Gelatinization and rheological properties of starch, Starch – Stärke, 2015, 67, 213–224 CrossRef CAS.
- R. Bajaj, N. Singh, A. Kaur and N. Inouchi, Structural, morphological, functional and digestibility properties of starches from cereals, tubers and legumes: a comparative study, J. Food Sci. Technol., 2018, 55, 3799–3808 CrossRef CAS PubMed.
- J. Singh, A. Dartois and L. Kaur, Starch digestibility in food matrix: a review, Trends Food Sci. Technol., 2010, 21, 168–180 CrossRef CAS.
- H. N. Englyst, S. M. Kingman and J. H. Cummings, Classification and measurement of nutritionally important starch fractions, Eur. J. Clin. Nutr., 1992, 46(Suppl. 2), S33–S50 Search PubMed.
- H. Kaur, B. S. Gill and B. L. Karwasra, In vitro digestibility, pasting, and structural properties of starches from different cereals, Int. J. Food Prop., 2018, 21, 70–85 CrossRef CAS.
- M. S. M. Wee and C. J. Henry, Reducing the glycemic impact of carbohydrates on foods and meals: Strategies for the food industry and consumers with special focus on Asia, Compr. Rev. Food Sci. Food Saf., 2020, 19, 670–702 CrossRef CAS PubMed.
- L. A. Bello-Perez, P. C. Flores-Silva, E. Agama-Acevedo and J. Tovar, Starch digestibility: past, present, and future, J. Sci. Food Agric., 2020, 100, 5009–5016 CrossRef CAS PubMed.
- M. A. Gularte and C. M. Rosell, Physicochemical properties and enzymatic hydrolysis of different starches in the presence of hydrocolloids, Carbohydr. Polym., 2011, 85, 237–244 CrossRef CAS.
- N. Qadir, I. A. Wani and F. A. Masoodi, Physicochemical, functional properties, and in vitro digestibility studies of starch from rice cultivars grown in indian temperate region, Starch – Stärke, 2021, 73, 2000188 CrossRef CAS.
- T. Sasaki, Influence of anionic, neutral, and cationic polysaccharides on the in vitro digestibility of raw and gelatinized potato starch, J. Sci. Food Agric., 2020, 100, 2435–2442 CrossRef CAS PubMed.
- T. Noda, S. Takigawa, C. Matsuura-Endo, T. Suzuki, N. Hashimoto, N. S. Kottearachchi, H. Yamauchi and I. S. M. Zaidul, Factors affecting the digestibility of raw and gelatinized potato starches, Food Chem., 2008, 110, 465–470 CrossRef CAS PubMed.
- F. F. Velásquez-Barreto, L. A. Bello Pérez, C. Nuñez Santiago, H. Yee Madeira and C. E. Velezmoro Sánchez, Relationships among molecular, physicochemical and digestibility characteristics of Andean tuber starches, Int. J. Biol. Macromol., 2021, 182, 472–481 CrossRef PubMed.
- K. S. Sandhu and A. K. Siroha, Relationships between physicochemical, thermal, rheological and in vitro digestibility properties of starches from pearl millet cultivars, LWT – Food Sci. Technol., 2017, 83, 213–224 CrossRef CAS.
- M. Santamaria, R. Garzon, R. Moreira and C. M. Rosell, Estimation of viscosity and hydrolysis kinetics of corn starch gels based on microstructural features using a simplified model, Carbohydr. Polym., 2021, 273, 118549 CrossRef CAS PubMed.
- R. Garzon and C. M. Rosell, Rapid assessment of starch pasting using a rapid force analyzer, Cereal Chem., 2021, 98, 305–314 CrossRef CAS.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, A starch hydrolysis procedure to estimate glycemic index, Nutr. Res., 1997, 17, 427–437 CrossRef.
- M. Gupta, A. S. Bawa and A. D. Semwal, Morphological, thermal, pasting, and rheological properties of barley starch and their blends, Int. J. Food Prop., 2009, 12, 587–604 CrossRef.
- Y. Y. Feng, T. H. Mu, M. Zhang and M. M. Ma, Effects of different polysaccharides and proteins on dough rheological properties, texture, structure and in vitro starch digestibility of wet sweet potato vermicelli, Int. J. Biol. Macromol., 2020, 148, 1–10 CrossRef CAS PubMed.
- I. Rosalina and M. Bhattacharya, Dynamic rheological measurements and analysis of starch gels, Carbohydr. Polym., 2002, 48, 191–202 CrossRef CAS.
- M. M. Martinez, C. Li, M. Okoniewska, I. Mukherjee, D. Vellucci and B. Hamaker, Slowly digestible starch in fully gelatinized material is structurally driven by molecular size and A and B1 chain lengths, Carbohydr. Polym., 2018, 197, 531–539 CrossRef CAS PubMed.
- S. Tomoko and K. Kaoru, Effect of non-starch polysaccharides on the in vitro digestibility and rheological properties of rice starch gel, Food Chem., 2011, 127, 541–546 CrossRef PubMed.
- Y. S. Ma, Y. Pan, Q. T. Xie, X. M. Li, B. Zhang and H. Q. Chen, Evaluation studies on effects of pectin with different concentrations on the pasting, rheological and digestibility properties of corn starch, Food Chem., 2019, 274, 319–323 CrossRef CAS PubMed.
- G. Zhang, Z. Ao and B. R. Hamaker, Slow digestion property of native cereal starches, Biomacromolecules, 2006, 7, 3252–3258 CrossRef CAS PubMed.
- P. Guo, J. Yu, L. Copeland, S. Wang and S. Wang, Mechanisms of starch gelatinization during heating of wheat flour and its effect on in vitro starch digestibility, Food Hydrocolloids, 2018, 82, 370–378 CrossRef CAS.
- S. Dhital, F. J. Warren, P. J. Butterworth, P. R. Ellis and M. J. Gidley, Mechanisms of starch digestion by α-amylase—Structural basis for kinetic properties, Crit. Rev. Food Sci. Nutr., 2017, 57, 875–892 CrossRef CAS PubMed.
- Y. Zhu, W. H. Hsu and J. H. Hollis, The impact of food viscosity on eating rate, subjective appetite, glycemic response and gastric emptying rate, PLoS One, 2013, 8, e67482 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |