Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Wax based oleogels and their application in sponge cakes†
Till
Wettlaufer
 * and
Eckhard
Flöter
* and
Eckhard
Flöter
Technische Universität Berlin, Faculty III Process Sciences, Department of Food Technology and Food Chemistry, Chair of Food Process Engineering, Germany. E-mail: till.wettlaufer@tu-berlin.de
First published on 16th August 2022
Abstract
The use of high amounts of saturated fatty acids mainly obtained from tropical fats and oils gains increasing rejection from consumers. Use of liquid plant based oils, however, does not deliver necessary functionalities. In this contribution, sunflower-(SFW), bees-wax (BW), ricebran wax (RBW) and a BW–wax mixture (BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh) were investigated as a potential alternative fat phase in low-density bakery products. Since the food product matrix is composed of complex ingredients, key-functionalities (foam-stabilization, viscoelastic properties, and oil-binding) were first investigated for pure oleogels as oleofoams. It could be demonstrated that all waxes investigated were able to form oleofoams. The location of wax crystal aggregates, at the oil–air interface or in the bulk, was shown to be a significant factor regarding oil-binding and viscoelastic properties. However, it was not possible to transfer all findings made for the oleofoams to the ones made for the oleogel based sponge cakes. There, all oleogels showed improvement compared to the canola oil variant regarding oil-leaping and visual appearance (volume). Sensory evaluation attested satisfactory results for all wax-based oleogel applications. This contribution aims to deliver novel findings for wax-based oleogels as oleofoams as well as an alternative fat phase in low-density bakery products. The gathered results aim to enable a target-oriented characterization of oleogel applications and hence facilitate future use to deliver beneficial products to the market.
SFWh) were investigated as a potential alternative fat phase in low-density bakery products. Since the food product matrix is composed of complex ingredients, key-functionalities (foam-stabilization, viscoelastic properties, and oil-binding) were first investigated for pure oleogels as oleofoams. It could be demonstrated that all waxes investigated were able to form oleofoams. The location of wax crystal aggregates, at the oil–air interface or in the bulk, was shown to be a significant factor regarding oil-binding and viscoelastic properties. However, it was not possible to transfer all findings made for the oleofoams to the ones made for the oleogel based sponge cakes. There, all oleogels showed improvement compared to the canola oil variant regarding oil-leaping and visual appearance (volume). Sensory evaluation attested satisfactory results for all wax-based oleogel applications. This contribution aims to deliver novel findings for wax-based oleogels as oleofoams as well as an alternative fat phase in low-density bakery products. The gathered results aim to enable a target-oriented characterization of oleogel applications and hence facilitate future use to deliver beneficial products to the market.
Introduction
In many food applications fat phases are relevant to achieve consumer relevant characteristics. The functionalities of the fat phase are manifold; some of them are: liquid oil prevents dehydration of water-rich products, and as a bulk fat or gel it is responsible for hardness and particle integration.1 However, edible fats also interact with other ingredients and stabilize also gases and water. Many desirable functionalities are basically due to high concentrations of high melting triglycerides, which are saturated to an extended degree. Due to these high melting properties, stable crystals can be formed which entrap the lower melting continuous phase. Unfortunately, the properties that are essential for the functionality of the fat phase, saturated and trans fatty acids, are also those that are of nutritional concern. In addition, these are mostly obtained from fractionated and modified palm oil, which is increasingly being rejected by consumers. For this reason, many food producers are striving to remove palm fat from the ingredient list and replace it with alternative and regional fat phases to meet customer demands. Oleogels, which are defined by non-triglyceride structuring of liquid oils, are an attractive alternative and have gained increased attention in the past years. Many structuring agents like ethylcellulose,2–4 waxes,5 sterol–sterolester combination6 and many others were identified. Since availability, costs and a low effective concentration are essential economical properties of structuring agents, waxes are considered as the most promising candidates.Waxes are multicomponent materials naturally preventing plants and insects from water loss. Depending on the source, waxes exhibit varying amounts of wax esters (WE), fatty acids (FA), fatty alcohols (FaOH), hydrocarbons (HC) and minor components. The composition of waxes plays an important role regarding the effectiveness of structuring liquid oils. Wax esters are said to be the major components of waxes being responsible for efficient structure development, but every other component mentioned above is able to form gels likewise. Since wax esters are composed of esterified long chain aliphatic fatty acids and alcohols, they also exhibit a strong saturated character. Nevertheless, studies showed that high amounts (<50%) of waxes are excreted undigested in mammals,7 which underlines their beneficial usage in food products. Additionally, waxes appear to be able to structure liquid oils at significantly lower levels than saturated triacylglycerols (TAGs). Similar to TAGs, waxes form solid bodies through interconnected solid particles. Through the absence of digestion and the lower concentrations necessary to deliver functionality, nutritionally beneficial fat phases can be created. Although right now only approved as glazing and coating agents,8–10 waxes like rice bran wax (RBW), sunflower wax (SFW) and bees wax (BW) might be attractive oil structurants for future food formulations.
Although the tailoring of wax gel properties was vastly investigated in terms of wax type and concentration, solvent quality, cooling rate and shear, it is quite difficult to predict their functional performance in product applications. Nevertheless, some propulsive work has already been conducted. Doan et al. (2016)11 used bees wax (BW) as a partial palm oil replacer in hazelnut fillings and found equivalent product characteristics at a roughly 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 BW
5 BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) palm oil ratio (17% BW oleogel). Oleogels in cream cheese products with rice bran wax,12 bouillon cubes with sunflower wax (SFW)13 and BW as pork fat replacement in fermented sausages14 were successfully demonstrated. In general, it is only reasonable to use oleogels where pure oil cannot be used due to a lack of functionality. In many bakery products such as pastry and cakes, the portion of the fat phase exceeds 25% w/w and is responsible for the texture, volume, taste and water loss prevention. Commonly, margarine or shortenings are used. Hwang et al. (2014)15 and Doan et al. (2018)16 produced margarines and shortenings with SFW and obtained promising results. Regarding the applications of wax based oleogels, some work has already been presented. Table 1 gives a brief overview of the reported applications of wax based oleogels in bakery products. Exemplarily, Mert et al. (2016)17 produced short doughs with carnauba and candelilla wax oleogels and increased product quality compared to a pure oil application. Kim et al. (2017)18 also investigated carnauba wax oleogels and increased the total fat phase stepwise to 50% of aerated batters. They found similar gas stabilization but denser products when oleogels were used.
palm oil ratio (17% BW oleogel). Oleogels in cream cheese products with rice bran wax,12 bouillon cubes with sunflower wax (SFW)13 and BW as pork fat replacement in fermented sausages14 were successfully demonstrated. In general, it is only reasonable to use oleogels where pure oil cannot be used due to a lack of functionality. In many bakery products such as pastry and cakes, the portion of the fat phase exceeds 25% w/w and is responsible for the texture, volume, taste and water loss prevention. Commonly, margarine or shortenings are used. Hwang et al. (2014)15 and Doan et al. (2018)16 produced margarines and shortenings with SFW and obtained promising results. Regarding the applications of wax based oleogels, some work has already been presented. Table 1 gives a brief overview of the reported applications of wax based oleogels in bakery products. Exemplarily, Mert et al. (2016)17 produced short doughs with carnauba and candelilla wax oleogels and increased product quality compared to a pure oil application. Kim et al. (2017)18 also investigated carnauba wax oleogels and increased the total fat phase stepwise to 50% of aerated batters. They found similar gas stabilization but denser products when oleogels were used.
| Structurants | Inclusion level (% w/w)a | Oil type | Model system | Findings | Authors |
|---|---|---|---|---|---|
| a Related to the oleogel phase. | |||||
| CDW | 3, 6 | Canola oil | Cookies | • Oleogel cookies had soft characteristics and higher spreadablility after baking | Jang et al. 2015![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
| CDW + shortening | 3, 6 | Canola oil | Cookies | • Oleogels performed better compared with pure oil, but underperformed regarding shortening | Mert and Demirkesen 2016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17 17 |
| • Curable by blending oleogel and shortening | |||||
| CRW, CDW | 2.5, 5 | Sunflower oil | Short-dough cookies | • Improvement compared to pure oil | Mert and Demirkesen 2016![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
| • Insufficient characteristics compared to shortening cookies | |||||
| SFW | 1, 2, 3, 4 | Expeller-pressed corn germ oil, high oleic soybean oil | Cookies | • Increasing SFW concentration lead to firmer gels for the soy bean formulations; the ones with corn germ oil contrarily hardened | M. Zhao et al. 2020![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 21 |
| • The variant with corn germ oil further was better processable | |||||
| CRW | 0.1 | Canola oil | Aerated cakes | • Replacement of PO-shortening successful | Kim et al. 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
| • Up to 25% equal characteristics | |||||
| RBW, BW, CLW | 0.1 | Sunflower oil | Cake | • Low-viscosity batters with oleogels | Oh et al. 2017![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 22 |
| • BW soft product in contrast to RBW and CLW, which were hard and chewy | |||||
| • BW overall comparable with the conventional shortening product | |||||
| CRW | 0.05 | High oleic sunflower oil | Cake | • No adverse effects of product characteristics, also indicated by sensory tests | Pehlivanoglu et al. 2018![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
| BW | 10, (8, 6, 4.5, 3, 1.5) | Sunflower oil | Gluten-free cake | • Full-substitution, 10%, leads to undesired product characteristics | Demirkesen and Mert 2019![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 24 24 |
| • Partial substitution leads to acceptable properties | |||||
For aerated batters it is well known that liquid oils deliver reasonable product characteristics, although the volume is slightly reduced. Unfortunately, an increased oil migration from the product and consequently undesired staining of the packaging are associated with it. Many contributions unfortunately omitted the use of pure oil as a reference. As stated earlier, this appears to be relevant to assess the benefits of the oleogel application. Furthermore, the assessment is often limited to the illustration of the feasibility of product production. Specific functionality assessment of the oleogel versus the reference product is rarely documented as correlations of oleogel properties to product properties and performance.
Since bakery products are sensitive to the properties of the fat phase and aerated batters in particular require a crystalline fat phase to gain softness,25 we investigated sponge cakes as model systems of low-density bakery products, sponge cakes. Since food products are always composed of different qualities and quantities of different edible materials (proteins, lipids, carbohydrates, etc.), the functionalities of the oleogels were first investigated as pure, ‘clean’ systems. The key-functionalities of the fat phase in low-density bakery products were determined to be sufficient foam-stabilization, texture contribution and prevention of oil migration. Similar to hardstock fats (TAGs), waxes might be able to deliver these functionalities. The utilized waxes in this study can be regarded as attractive waxes since they are either approved by legislative organs or are structure efficient. This study investigates the feasibility of these materials as fat-replacers in sponge cakes. The methods used to characterize ‘clean’ oleogel systems, solely the oleogel without other cake ingredients, as oleofoams were also used for the final products to allow systematic comparison of the obtained data.
In this contribution we aim to deliver the full set of investigations of the wax-based oleogels’ ability to fulfill their techno-functional role as well as correlating this behavior with the final properties in more complex applications. Utilization of conventional fat phases as references allowed a more detailed interpretation of the results obtained. The functionalities which need to be delivered by the fat phase, here the oleogels, are air stabilization, prevention of oil-leakage, texture and of course sensory approval.
Materials
Refined canola oil was obtained from Gustav Heess GmbH (Leonberg, Germany) and sponge cakes from Raps Ölsaatenverarbeitung GmbH (Kiel, Germany). Palm fat type Vamoline 36 KH was obtained from Vandermoortele (Gent, Belgium). Sunflower wax (SFW, “6607L”, Lot.nr.: F2136022), ricebran wax (RBW, “2811”, Lot.nr.: F2135009), beeswax (BW, “8108LM”, Lot.nr.: F2111028) and sunflower wax hydrolysate (SFWh, “6607H”, Lot.nr.: F2134017) were contributed by KahlWax GmbH (Trittau, Germany). The non-fat bakery ingredients (flour, whole egg, and sugar) were obtained from Kuchenmeister GmbH (Soest, Germany) and they exhibited basic industrial quality.Methods
Preparation of oleofoams
Oleofoams were produced with oleogels stabilized for 48 h at room temperature. The gels were produced by heating canola oil up to 90 °C on a heating plate with a coupled temperature sensor to prevent overshoot. The solutions were continuously stirred with a magnetic stirrer. After reaching the target temperature, waxes were added in quantities of 1, 5 and 10% w/w to obtain 400 g oleogels. After 30 minutes the hot solutions were poured into glass beakers and stored at room temperature (20 ± 1 °C) for 48 h. The oleogels were transferred into a 5000 ml mixing vessel of a conventional kitchen mixer (1000 watt) and were mixed with a whisk up to 30 minutes at the highest stage, which is a representative time for whipping procedures.25,26 At an interval of 5 minutes, the samples were taken for microscopy examinations.Preparation of sponge cakes
The recipe was basically the classically used ‘pound-on-pound’ procedure, where 25% of egg, wheat flour, sugar and the fat phase, respectively, are used. Additionally, a rising agent and emulsifier were added to meet the industrial standard and reproduce the manufacturing conditions. The composition of the 25% fat phase was varied. Palm fat served as a positive reference fat while pure canola was a second reference. Alternative fat phases were pure canola oil, oleogels composed of 95% w/w canola oil and 5% w/w SFW, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively. Prior to usage, the oleogels were stabilized for 48 h at room temperature.
1, respectively. Prior to usage, the oleogels were stabilized for 48 h at room temperature.
For the production of sponge cakes, all fat phases were used as a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 substitute. The ingredients were added to the mixing bowl and stirred for 30 seconds at low speed with a whipping tool to ensure homogeneous netting and mixing. The suspension was then mixed for an additional 3 minutes at high speed. The produced batter was weighed in pre-oil-coated sponge cake molds with a mass of 400 ± 1 g, respectively. The cakes were baked in a plate stove (Friedrich Solingen, Solingen, Germany) at 180 °C top and bottom heat for 55 minutes.
1 substitute. The ingredients were added to the mixing bowl and stirred for 30 seconds at low speed with a whipping tool to ensure homogeneous netting and mixing. The suspension was then mixed for an additional 3 minutes at high speed. The produced batter was weighed in pre-oil-coated sponge cake molds with a mass of 400 ± 1 g, respectively. The cakes were baked in a plate stove (Friedrich Solingen, Solingen, Germany) at 180 °C top and bottom heat for 55 minutes.
Oil leaping capacity
The oil binding capacity was measured according to a method presented by Conty et al. (2021)27 and Flöter et al. (2021).28 The authors introduced a novel method to measure oil binding of oleogels by inserting filter paper strips and examined the vertical oil leap by capillary forces over time. In this work oleofoams were carefully filled in cylindrical glass sample containers of 22.3 ml in triplicate. The containers were filled in slight excess and skimmed at the open end of the container. Subsequently, pre-cut Whatman 595 filter paper strips of 1 cm × 10 cm (maximum achievable filter-length through circular filters) were placed at a depth of 1 cm into the oelofoam-samples and were fixed at the top to prevent further immersion into the foams. The oil leap was recorded with fully soaked filter paper strips in a temperature and humidity-controlled environment (HP 110, Memmert, Schwabach, Germany).Additionally, cylindrical glass sample containers filled with oleofoam samples, as described before, were stored at room temperature (20 °C ± 1 °C) to investigate oil accumulation upon storage. The accumulating oil was detected from the height of the precipitating oil phase, also in triplicate.
For sponge cakes, cubic samples of 4 × 4 cm were cut out of the center of the cakes, ensuring that the bottom of the cake was also the bottom of the cubes. The aforementioned filter paper strips (1 cm × 10 cm) were inserted 1 cm into the cakes at the center of the upper horizontal surface after precutting the cakes with a scalpel. The oil leap was recorded for 85 h at room temperature (20 °C1 °C) for each of the duplicates.
The described setups are depicted in Fig. 1 for clarity. The measurements were conducted until the filter paper strip was fully soaked or the increase of the oil level was stagnant.
Images were taken at defined intervals and the distance of oil leap, or height of oil accumulation, was determined with an image analysis software (ImageJ Version 1.52a). A pre-placed 10 mm mark on the filter paper strips, used also for the accumulation-tests on the glass containers, was used for the calibration of the distance-measurement-tool.
Density determination
Samples were carefully poured into glass containers of known volume. The mass of the samples was measured gravimetrically and the density was calculated. The overrun as % of stabilized air was calculated as follows: | (1) |
Viscoelastic properties
Rheological examinations were carried out with an MCR 302 rheometer (Anton Paar, Graz, Austria). The samples were placed on a 51 mm diameter Peltier plate and a sand blasted geometry PP50S was driven onto the sample. After trimming with a silicon separator tool, amplitude and frequency sweeps were carried out at a fixed gap of 1 mm. Amplitude sweeps were executed, with an angular velocity of 10 rad s−1, from 0.01 to 200% strain. Frequency sweeps were carried out at a strain within the linear viscoelastic region of the sample ranging from 1 to 100 rad s−1.Firmness measurements
Firmness determination was conducted with a universal testing machine zwickiLine (Zwick Roell, Ulm, Germany) equipped with a 1 kN load cell (trigger force: 0.02 N). The penetration tests were carried out as two cycles of penetration. According to AACC Method 74-09, a 25 mm thick sample was penetrated for 40% (10 mm) of the detected sample height with a velocity of 100 mm min−1. The probe head had a 25 mm diameter with flattened edges. Parameters of the texture profile analysis29 were computed with Origin Pro 9 (OriginLab, Northampton, MA, USA) software.Microstructural analysis
Samples (<1 ml) of the oleofoams were extracted from the mixing process at 5-minute sampling intervals. The foams were placed on glass slides equipped with an 8 mm × 10 mm × 1 mm O-ring as a spacer to prevent excessive shear stress of the samples. A glass slide was carefully administered on the top of the O-ring. Micrographs were gathered with an Axio Scope. An A1KMAT microscope (Carl Zeiss Microscopy GmbH, Oberkochen, Germany) and the associated Zen 1.3 (blue edition) software were used. Pictures were analyzed with ImageJ 1.52 software in terms of bubble size. Therefore, scaling was carried out at the scale bar and up to 200 bubbles were manually measured per micrograph. At least 3 pictures of oleofoams per mixing interval were gathered.Truncated sponge cakes were carefully deposited on a conventional document scanner besides a prearranged scale. Images were obtained with a resolution of 600 dpi and analyzed again with ImageJ software. The binary images were adjusted by automatic threshold setting and were further processed with the ‘dilate’, ‘erode’ and ‘watershed’ functions to delimit pores. The pore and bubble size distribution was obtained with Origin Pro 9 (OriginLab, Northampton, MA, USA).
Sensory evaluation
The produced sponge cakes were stored for one week at 20 °C (±1 °C) prior to sensory evaluation. A ranking test according to DIN ISO 8587:2006 was carried out with a panel consisting of eleven untrained persons.30 Consistently 0.5 cm thick slices of the respective sponge cakes were collected and handed to the testers. The coding was always the same. The following categories were queried: visual appearance, odor, taste, density, crumbliness, off-flavor and overall impression. Testers were instructed to rank the samples in the respective categories from best (1) to worst (5). To test for significance the Friedman-test was performed.30Results and discussion
Since the properties of the oleogels are not included in the following sections, Table 2 gives a brief overview over the most important characteristics, including the critical gelling concentration (CGC), solubilities, degree of homogeneity (DoH), wax ester (WE) content, structure efficiency and waxes’ morphologies. It is observed that SFW shows a paramount structure efficiency, which is based on a high wax ester content with narrowly distributed wax esters, as indicated by the DoH. This results in low solubilities and a small CGC which is also due to the large platelet-like morphology. Although RBW exhibits also a high WE content, the resulting properties are significantly different. The lower DoH indicates a poorer distribution of the WE, resulting in less efficient scaffolding due to the formation of dendritic aggregates. BW and the mixture with the SFW–hydrolysate (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) exhibit lower WE contents and a higher solubility. The wax mixture, BW
1) exhibit lower WE contents and a higher solubility. The wax mixture, BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh, however indicates non-WE based structure formation and shows increased structure formation, which might be due to interactions of FA and FaOH as previously introduced.31
SFWh, however indicates non-WE based structure formation and shows increased structure formation, which might be due to interactions of FA and FaOH as previously introduced.31
| SFW | RBW | BW | BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh SFWh |
|
|---|---|---|---|---|
| CGC (% w/w) | 0.5[A], 0.14[D] | 1[B], 5[C] | 6[A], 2.18[D] | 0.58[D] |
| Solubility (% w/w) | 0.8[E] | 1.4[F], 1.5[F] | 3.1[E] | 1.9[E] |
| DoH (−) | 14.27[F] | 11.07[F] | 8.89[F] | 4.88[E] |
| WE (%) | 96[G] | 93[G] | 58[G] | 39[G,calc] |
| Structure efficiency (N/% solids) | 4.3[F], 2.9[F] | 0.7[F], 0.3[F] | 0.01[F], 0.3[F] | 1.39[E,D,calc] |
| Morphology | Platelet | Dendritic | Small-needle-like | Small-grain-like |
Density of oleofoams
The density of the produced oleofoams was measured according to the method described above. The results are shown in Fig. 2. The determined density of canola oil, 0.9144 g ml−1 (dotted horizontal line, 2ndy-axis), was in accordance with the reported literature values of 0.914–0.917 g ml−1 from Eskin et al. (2003)39 and was used as a benchmark for aeration success.The aim of the mixing process was primarily to reduce the density, hence whipping air into the oleogels. Since the presented data were gathered from oleofoams after 30 minutes of mixing, it can be stated without ambiguity that a density reduction could be achieved. The evolution of densities as a function of the wax inclusion level is illustrated as diamonds in Fig. 2. Increasing the wax concentration in SFW oleogels shows a steep increase in aeration from 1% w/w to 5% w/w by 26.08% but stays fairly constant at the highest inclusion level, 10% w/w as observable by investigating the overrun and lower density. At 5% w/w, counterintuitively RBW and BW show density values of oleofoams comparable to pure canola oil, indicating limited bubble stabilization-power.
At a medium inclusion level, 5% w/w, SFW stabilized the whipped air most effectively compared to RBW and BW. The 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of BW and SFWh, BW
1 mixture of BW and SFWh, BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, surprisingly showed better results compared to oleofoams produced with RBW and BW. The wax mixture was chosen based on earlier findings regarding synergistic firmness effects in oleogels.31 The data presented suggest that the long chain fatty acids present in the hydrolyzed wax (SFWh) are surface active and thus stabilize phase boundaries more effectively than BW solely. As mentioned before, increasing the concentration did not result in significant changes for SFW oleofoams. Although RBW consists of a similar composition to SFW, air stabilization cannot be observed to the same extent. Even the highest concentrations of 10% w/w RBW does not deliver significant gas phase stabilization. In fact, the RBW particles evoke only 6.6% density reduction. The other waxes, BW and BW
1, surprisingly showed better results compared to oleofoams produced with RBW and BW. The wax mixture was chosen based on earlier findings regarding synergistic firmness effects in oleogels.31 The data presented suggest that the long chain fatty acids present in the hydrolyzed wax (SFWh) are surface active and thus stabilize phase boundaries more effectively than BW solely. As mentioned before, increasing the concentration did not result in significant changes for SFW oleofoams. Although RBW consists of a similar composition to SFW, air stabilization cannot be observed to the same extent. Even the highest concentrations of 10% w/w RBW does not deliver significant gas phase stabilization. In fact, the RBW particles evoke only 6.6% density reduction. The other waxes, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, showed a greater impact with 11.9 and 12.9%, respectively.
1, showed a greater impact with 11.9 and 12.9%, respectively.
An important factor in the assessment of oleogels and their properties is the amount of dissolved material in the continuous phase. Many authors utilize the critical gelling concentration (CGC) to ensure comparable conditions for different structuring systems. Indeed, the CGC is a useful value to express the amount of structurant necessary to form a weak gel. However, network formation by aggregation, the crystallization conditions and the geometry of the container play an important role in CGC determination. To avoid these factors introducing ambiguity, we extrapolated the minimal amount of material necessary to form crystals in previous contribution.36 Regarding the ability to form oleofoams, the CGC might be a characteristic value since it incorporates the effectiveness of scaffolding and also the solubility. Stabilization of the incorporated bubbles may hence be a function of the microstructure and solid quantity present. The solubility concentration of the structurant in the liquid oil needs to be increased in the mixture prior to considering any solid formation. The solubility of SFW in canola oil is low, 0.77% w/w; see Table 2. RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 have similar solubilities of 1.5 and 1.9% w/w, respectively. BW, an ingredient composed of more components, has a significantly higher solubility, 3.1% w/w. In this context, it is not surprising that BW administered at 5% w/w shows a poor whipping performance. Nevertheless, RBW, even though having a low solubility, appears to be much less potent in bubble stabilization than SFW. As already mentioned, the waxes differ in chemical composition. The high WE-waxes SFW and RBW show very different behavior as oleogels and oleofoams. The differences found might be explained by the different DoH, hence WE chain length distributions. While SFW forms large platelet-like structures due to narrow WE, RBW shows dendritic structures which might be due to less effectively crystallizing WEs. This implies that larger and platelet shaped aggregates are more effective in air stabilization. However, regarding the chemically more inhomogeneous waxes, BW and BW
1 have similar solubilities of 1.5 and 1.9% w/w, respectively. BW, an ingredient composed of more components, has a significantly higher solubility, 3.1% w/w. In this context, it is not surprising that BW administered at 5% w/w shows a poor whipping performance. Nevertheless, RBW, even though having a low solubility, appears to be much less potent in bubble stabilization than SFW. As already mentioned, the waxes differ in chemical composition. The high WE-waxes SFW and RBW show very different behavior as oleogels and oleofoams. The differences found might be explained by the different DoH, hence WE chain length distributions. While SFW forms large platelet-like structures due to narrow WE, RBW shows dendritic structures which might be due to less effectively crystallizing WEs. This implies that larger and platelet shaped aggregates are more effective in air stabilization. However, regarding the chemically more inhomogeneous waxes, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh, one can observe in contrast to RBW an increase in bubble-stabilization at 10% w/w. This might be due to higher portions of amphiphilic molecules, as for instance free fatty acids. This hypothesis might be supported by the higher overrun of the hydrolysate mixture (BW
SFWh, one can observe in contrast to RBW an increase in bubble-stabilization at 10% w/w. This might be due to higher portions of amphiphilic molecules, as for instance free fatty acids. This hypothesis might be supported by the higher overrun of the hydrolysate mixture (BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh).
SFWh).
Microstructure of oleofoams
Micrographs of oleofoams are depicted in Fig. 3 and 4. The results of the image analysis are illustrated in Fig. 5. The influence of the inclusion level on oleofoam evolution as a function of mixing time with the example of SFW is shown in Fig. 3 and presents at first sight darker gas bubbles for SFW 1% w/w in a range from 5 to 25 minutes of mixing time.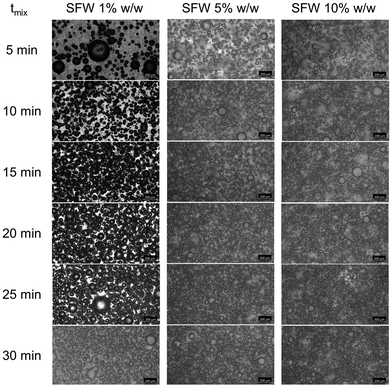 | ||
| Fig. 3 Micrographs of oleofoams based on different inclusion levels (1, 5 and 10% w/w) of SFW over a mixing period of 30 minutes. | ||
 | ||
Fig. 4 Micrographs of oleofoams based on different inclusion levels (5 and 10% w/w) of RBW, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1 SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 over a mixing period of 30 minutes. 1 over a mixing period of 30 minutes. | ||
 | ||
| Fig. 5 Bubble size (length) distribution (BSD) of oleofoams as a function of mixing time (minutes). Curves were computed with lognormal distribution type; bars were binned to a total of 30. | ||
In general, the amount of bubbles seems to increase with increasing mixing duration (top to bottom rows). This visual impression is confirmed by the presented bubble size distribution (BSD) in Fig. 5. Using 1% w/w SFW resulted in the broadest of monomodal BSD at every mixing interval ranging from 130.4 μm at 5 minutes to 97.6 μm at 30 minutes (total span width, not shown in Fig. 5). Other wax inclusion levels remained nearly constant over the mixing time and exhibited values around 60 μm (5% w/w = 59.5 ± 4.1 μm; 10% w/w = 60.6 ± 6.3 μm). Since the median value showed little differences compared to the mean bubble size, only the mean value will be further discussed and presented here (Fig. 5, horizontal black line). Although the span width showed only differences for 1% w/w wax, increasing the SFW inclusion level resulted in smaller bubble sizes for a 10% w/w compared to 5% w/w wax dosage. Although the evolution of mean BSD showed only small deviations as a function of the mixing time, the final mean bubble size after 30 minutes was 30.5 ± 1.3, 28.8 ± 1.3, and 25.0 ± 0.4 for 1, 5 and 10% w/w respectively. Doubling the wax inclusion from 5 to 10% w/w did not reveal significant changes in BSD. It is hypothesized that the platelet-like aggregates already covered the bubbles to an extended degree and prevented further fragmentation of the bubbles. The residual, non-bubble-covering aggregates are thought to remain in the continuous oil phase. Why these aggregates do not further stabilize incorporated air during increasing mixing time, but rather show a decrease in overrun (as shown in Fig. 2), remains undisclosed.
Micrographs of oleofoams produced with RBW, BW, and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh at concentrations of 5 and 10% w/w are shown in Fig. 4. First, RBW 5% w/w and BW 5% w/w show somewhat darker and smooth air bubbles with particles in the bulk phase. However, doubling the wax concentration leads to a higher number of smaller and overlapping gas bubbles for all systems. The visual impression suggests a reduction of the bubble size, which is also confirmed by the BSDs (Fig. 5).
SFWh at concentrations of 5 and 10% w/w are shown in Fig. 4. First, RBW 5% w/w and BW 5% w/w show somewhat darker and smooth air bubbles with particles in the bulk phase. However, doubling the wax concentration leads to a higher number of smaller and overlapping gas bubbles for all systems. The visual impression suggests a reduction of the bubble size, which is also confirmed by the BSDs (Fig. 5).
As the BSDs indicate, the distribution width of bubble sizes is reduced from 5 to 10% w/w dosage for BW or RBW. The BW-mixture, BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh, in contrast shows a minute shift to larger bubble sizes when a higher wax concentration is used. Nevertheless, BW 5% w/w showed the broadest BSD distribution width of all wax foams, which in fact enlarged after 20 minutes of whipping. However, the mean bubble size of this system decreased from 58.9 ± 6.4 μm after 5 minutes to 22.6 ± 2.3 μm after 30 minutes of whipping. RBW and BW
SFWh, in contrast shows a minute shift to larger bubble sizes when a higher wax concentration is used. Nevertheless, BW 5% w/w showed the broadest BSD distribution width of all wax foams, which in fact enlarged after 20 minutes of whipping. However, the mean bubble size of this system decreased from 58.9 ± 6.4 μm after 5 minutes to 22.6 ± 2.3 μm after 30 minutes of whipping. RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh achieved mean bubble sizes of 16.1 ± 0.4 μm and 11.5 ± 0.6 μm after 30 minutes. Higher wax concentration leads to smaller mean bubble sizes for BW, RBW and BW
SFWh achieved mean bubble sizes of 16.1 ± 0.4 μm and 11.5 ± 0.6 μm after 30 minutes. Higher wax concentration leads to smaller mean bubble sizes for BW, RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh. In fact, the sizes obtained were smaller than those of SFW after 30 minutes which ranged from 31 to 25 μm. BW, RBW and BW
SFWh. In fact, the sizes obtained were smaller than those of SFW after 30 minutes which ranged from 31 to 25 μm. BW, RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh exhibited mean bubble sizes of 21.7 ± 1.2 μm, 11.5 ± 0.8 μm and 10.4 ± 0.9 μm, respectively.
SFWh exhibited mean bubble sizes of 21.7 ± 1.2 μm, 11.5 ± 0.8 μm and 10.4 ± 0.9 μm, respectively.
Obviously, the quantity of solids, and vice versa the solubility, is a significant factor in the stabilization of air bubbles. In particular, for RBW and BW, a decrease in BSD could be observed with higher dosage of waxes, which might be explained by the increased viscosity of the continous phase. In contrast to that, SFW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh did not show the same behavior, but instead showed relatively steady and small BSD also as a function of mixing time. As previously formulated, platelet-like aggregates of SFW might effectively shield air bubbles through pickering-stabilization and prevent further fragmentation. For BW
SFWh did not show the same behavior, but instead showed relatively steady and small BSD also as a function of mixing time. As previously formulated, platelet-like aggregates of SFW might effectively shield air bubbles through pickering-stabilization and prevent further fragmentation. For BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh however it is hypothesized that the stabilization might be due to interfacial stabilization by amphiphilic species. As the BSD shows, this stabilization is effective at as low as 5%. Further addition of wax does not lead to significant smaller bubbles with increased mixing time. M. Callau et al. (2020)40 identified different combinations of FA and FaOH being able to effectively stabilize oleofoams as solid particles. Since we utilized more complex structurant mixtures, a conclusive causal relationship cannot be established at this point.
SFWh however it is hypothesized that the stabilization might be due to interfacial stabilization by amphiphilic species. As the BSD shows, this stabilization is effective at as low as 5%. Further addition of wax does not lead to significant smaller bubbles with increased mixing time. M. Callau et al. (2020)40 identified different combinations of FA and FaOH being able to effectively stabilize oleofoams as solid particles. Since we utilized more complex structurant mixtures, a conclusive causal relationship cannot be established at this point.
Vertical oil leap
The ability to retain liquid oil in the particle network can be referred to as the oil binding capacity, OBC. In particular, for the characterization of networks and for product applications, the OBC is an important parameter.27 It is known that some products, as for example sponge cakes, can be produced by replacing saturated hardstock fats with solely liquid oil. Unfortunately, the products might exhibit undesired oil migration.The question to be answered here is if oleogels, by fulfilling their functionality (here as air bubble stabilizers), also improve the oil binding capacity. This is particularly of interest, because the relationship of oil leakage and wax crystals is unclear. The relatively small amount of wax crystals might be completely concentrated at the interface of the air bubbles. This would leave limited crystalline material for the immobilization of the liquid and hence leave the oil in a similar state to that in cakes made from straight oil. On the other hand, the surface of Pickering-stabilized air bubbles might have a much better wettability by oil and hence suppress drainage.
The data obtained by this method were fitted with an adapted Michaelis Menten type equation:41
 | (2) |
Fig. 6 shows the vertical oil leaps of the produced oleofoams after 30 minutes of whipping. Canola oil was used as a benchmark for a wax free system (red line). All waxes utilized exhibited different gel forming properties as reported earlier, and are compared in Table 2. Different morphologies, solid material compositions (undissolved components), particle surface polarity and sintering may play decisive roles in oleofoam formation and stabilization. SFW is an effective oil structurant, which is attributed to its homogeneous composition of 96% of wax esters.38 RBW exhibits nearly the same amount of wax esters (93%), but shows poor structuring properties which is thought to be explained by different wax ester qualities and wider variance of fatty acid and fatty alcohol moieties in the wax esters.37 BW shows medium structuring properties, which can be improved by a substitution of 50% of hydrolyzed SFW and SFWh.
Regarding the oil leaping results illustrated in Fig. 6, no univocal statement can be formulated for all waxes at first sight. In general, it can be observed that higher inclusion levels of wax do not necessitate a lower oil binding capacity, hence lowering the amount of leaped oil. The effect of wax concentration can easily be demonstrated with SFW (black lines). Counterintuitively, 5% w/w SFW showed a higher maximum oil leap compared to 10% w/w and canola oil. Utilization of 1% w/w presented the fastest oil leap. Although the other oleofoams were produced with only 5% w/w and 10% w/w inclusion levels, similar results could be obtained. Even though the amount of wax was doubled, RBW showed slightly higher oil leakage compared to 5% w/w wax (grey lines). Regarding BW (light blue lines) and its combination with SFW hydrolysate (BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (blue lines), the effect of wax concentration was manifested by a decrease in the oil rise. For BW
1) (blue lines), the effect of wax concentration was manifested by a decrease in the oil rise. For BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh, a lower concentration showed higher values compared to pure canola oil.
SFWh, a lower concentration showed higher values compared to pure canola oil.
Combining the observations made for the microstructure of the oleofoams from Fig. 4 it seems that the position of the crystal aggregates plays a crucial role in oil leakage. Investigation of the microstructure of the oleofoams with polarized light (PL) revealed that compared to other waxes, only SFW showed birefringement at the bubbles’ surfaces. Fig. 7 shows PL micrographs of 5% w/w SFW and BW oleofoams. The figure illustrates that BW and also RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (not shown) present crystal agglomeration in the bulk phase and smooth air bubbles. Schröder et al. (2018)42 reported that birefringement at the oil–gas interface can be attributed to the presence of crystals as Pickering-stabilizers. However, the obtained micrographs also revealed that birefringement was reduced for all samples with increasing mixing time. Consequently, the loss of birefringement might be considered as destabilization of air bubbles. It hence might be justified that the crystals help increase bulk viscosity rather than directly stabilizing the gas bubbles at the interface. Since Saha et al. (2020)43 used tripalmitin in their studies, it is quite possible that stabilization by wax crystal aggregates results in less intense birefringement caused by the platelet shaped wax esters’ multiple diffraction. Metilli et al. (2021)44 likely found a buckled interface when a mixture of paraffin and a synthetic wax were used to create oleofoams and found interface stabilizing properties.
1 (not shown) present crystal agglomeration in the bulk phase and smooth air bubbles. Schröder et al. (2018)42 reported that birefringement at the oil–gas interface can be attributed to the presence of crystals as Pickering-stabilizers. However, the obtained micrographs also revealed that birefringement was reduced for all samples with increasing mixing time. Consequently, the loss of birefringement might be considered as destabilization of air bubbles. It hence might be justified that the crystals help increase bulk viscosity rather than directly stabilizing the gas bubbles at the interface. Since Saha et al. (2020)43 used tripalmitin in their studies, it is quite possible that stabilization by wax crystal aggregates results in less intense birefringement caused by the platelet shaped wax esters’ multiple diffraction. Metilli et al. (2021)44 likely found a buckled interface when a mixture of paraffin and a synthetic wax were used to create oleofoams and found interface stabilizing properties.
 | ||
| Fig. 7 Polarized light micrographs of 5% w/w SFW and BW oleofoams after 5 minutes of whipping at 200-fold magnification. | ||
BW and RBW, which exhibited crystals in the bulk phase and smooth surfaces of air bubbles, presented reduced oil leaping. This could be explained by a denser oleogel/viscous fluid since the available solid matter is more frequently present in the continuous phase. Systems with Pickering-stabilized interfaces are consequently not able to prevent oil leakage due to lower concentration of solids in the bulk.
Comparing BW and RBW, it is obvious that an increase in wax concentration is more effective for BW. This might be rooted in a better spatial obstruction by platelet shaped BW aggregates.31 Nevertheless, some systems exhibit higher oil leaps than pure canola oil. It may be reasonable to assume an increased driving force in these cases. To be rather speculative, this behavior might be explained by recrystallization processes causing densification of the aggregates and hence expelling excess oil from the large-meshed pores similar to oiling-out of margarines. Metilli et al. (2021)44 reported air bubble dissolution in sunflower oil with wax Pickering-stabilized oleofoams. Although this is reportedly more pronounced at higher temperatures, gas dissolution might result in a shift in phase equilibrium at room temperature and cause recrystallization. This hypothesis, speculative though, might additionally explain the increased oil leaping values, especially for SFW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
To also investigate the stability of the oleofoams, the accumulation of the continuous phase at the bottom was investigated. Fig. 8 shows the oil accumulation of oleofoams as a function of storage time. The oil accumulation was normalized to the calculated oil content of the respective oleofoams to obtain meaningful data.
 | ||
Fig. 8 Oil accumulation of oleofoams (n = 3). O = SFW, □ = RBW, ◊ = BW, Δ = BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh. Open symbols = 10% w/w, filled symbols = 5% w/w. Errors omitted for clarity. SFWh. Open symbols = 10% w/w, filled symbols = 5% w/w. Errors omitted for clarity. | ||
Obviously, the 5% w/w wax samples (filled symbols) show greater oil accumulation compared to 10% w/w wax samples (open symbols). For RBW and BW 5% w/w no phase separation, but a dull suspension, could be observed. Hence, only values for 5% w/w SFW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 are shown for this inclusion level. This makes the evaluation of the difference in oil holding capacity difficult. Considering the 10% w/w wax oleofoams, RBW and BW accumulated more oil compared to SFW and BW
1 are shown for this inclusion level. This makes the evaluation of the difference in oil holding capacity difficult. Considering the 10% w/w wax oleofoams, RBW and BW accumulated more oil compared to SFW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh. The differences between the different 10%-samples remain small though. Furthermore, the data suggest that the drainage process comes to an end after relatively short storage times, 2 to 5 days. Since RBW and BW show rather increased bulk viscosity than stabilization of air bubbles, similar oil accumulation to other structurants is somewhat surprising.
SFWh. The differences between the different 10%-samples remain small though. Furthermore, the data suggest that the drainage process comes to an end after relatively short storage times, 2 to 5 days. Since RBW and BW show rather increased bulk viscosity than stabilization of air bubbles, similar oil accumulation to other structurants is somewhat surprising.
Viscoelastic properties of oleofoams
To gather information about the nature and characteristics of oleofoams, amplitude strain sweeps were carried out. The results are illustrated in Fig. 9. First of all, it could be determined that most of the oleofoams exhibited a gel character since the elastic modulus G′ exceeded the loss modulus’ G′′ at low amplitudes. This is by no means obvious; despite the wax concentration in excess of the respective CGC (Table 2), Pickering stabilization binds wax crystals at the bubble interface and possibly depletes the bulk from wax crystals. However, Fameau et al. (2020)45 reported contributions from the crystals at the bubble surface and the bulk oleogel to the rheological characteristics of oleofoams. Hence, the rheological signal is complex due to superimposing contributions of the dispersed gas phase and the wax crystals.Amplitude sweeps (Fig. 9) illustrated features of the oleofoams that deviate strongly from those of oleogels. For SFW it could be observed that although the elastic moduli increase with increasing SFW inclusion levels there is a qualitative difference emerging with increasing wax concentration. The oleofoams stabilized with 1% w/w and 5% w/w (G′ < G′′) show in contrast to the 10% wax foam no gel character (G′ > G′′). Only 10% w/w SFW is characterized as a semi-solid with a limited linear viscoelastic region (LVR), γL = 0.037 ± 0.003%. Also the flow point is found at low values γf = 0.23 ± 0.119% (349 ± 18.2 Pa). RBW showed a comparable behavior with 10% w/w again resulting in a gel like system. This gel presented a short LVR, γL = 0.0410.009%, and a minute shift towards higher yield strain values (γf = 0.54 ± 0.126% (68.47 3 Pa)). In contrast to that, gel characteristics were observed for both concentrations (5 and 10%) for BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh systems. Although BW showed higher initial G′ values for 5% w/w, BW
SFWh systems. Although BW showed higher initial G′ values for 5% w/w, BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 exhibited the highest elastic values of 10% w/w oleofoams with 7515 Pa. The increase of the wax concentration leads to shortening of the LVR for BW to an extent of 88% (γL,5% BW = 0.331 ± 0.043%; γL,10% BW = 0.039 ± 0.051%). For the mixed structurant BW
1 exhibited the highest elastic values of 10% w/w oleofoams with 7515 Pa. The increase of the wax concentration leads to shortening of the LVR for BW to an extent of 88% (γL,5% BW = 0.331 ± 0.043%; γL,10% BW = 0.039 ± 0.051%). For the mixed structurant BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh the linear range, in contrast, extended by 282% (γL,5% BW:SFWh = 0.03 ± 0.0025%; γL,10% BW:SFWh = 0.085 ± 0.0471%). Regarding the crossover points a similar evolution is found.
SFWh the linear range, in contrast, extended by 282% (γL,5% BW:SFWh = 0.03 ± 0.0025%; γL,10% BW:SFWh = 0.085 ± 0.0471%). Regarding the crossover points a similar evolution is found.
Frequency sweeps (not shown) confirm the findings obtained by the amplitude sweeps. Systems with a gel character maintained this property independently from the applied angular velocity. The observed increase of moduli at increasing frequency is of cross-linked networks and gels. Nevertheless, the ratio of elastic and viscous moduli should exceed 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.46 In the cases presented, the maximum achieved ratio is 5
1.46 In the cases presented, the maximum achieved ratio is 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for BW
1 for BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh. This implies that oleofoams can at best be considered as ‘weak’ gels.
SFWh. This implies that oleofoams can at best be considered as ‘weak’ gels.
However, the slight increase in elastic and viscous values for SFW-based oleofoams at higher amplitudes indicates an increased resistance to shearing. Since this effect is only observable for SFW-oleofoams which delivered the highest air incorporation (Fig. 3), this effect might be linked to mechanically induced (shear) collisions of big and small air bubbles with adhered solid wax aggregates (Fig. 7, left). The obtained signal at high deformations might hence be a result of compacting air bubbles pressing against each other resulting in an increase in pressure. Gunes et al. (2017) reported also higher signals in Pickering stabilized oleofoams and attributed this to the jamming of solids upon measurment.25
In summary, the rheological studies on the oleofoams show that the foaming behavior of different oleogels cannot be straightforwardly predicted for the properties of the oleogels.31,37 This is due to several factors. The oleofoams studied vary significantly in the volume of the dispersed gas phase (Fig. 2), the bubble size distributions and the amount and type of crystalline material. In the interpretation of the observations it is consequently necessary to consider numerous effects next to the basic parameters mentioned above. These are in particular the amount of crystals adhering to the gas–oil interface and the remaining crystal concentration in the bulk oil phase. SFW clearly performs the best as a structurant for oleofoams.
Sponge cakes
Viscoelastic properties of sponge cake batters
To evaluate the functionality of oleofoams in sponge cakes, rheological measurements of cake batters were carried out in line with those applied to the oleofoams. After the whipping process, the densities of the batters were 73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 g l−1, 7755 g l−1, 7455 g l−1, 815 ± 5 g l−1, and 780 ± 10 g l−1 for palm fat, canola oil, SFW, BW and BW
010 g l−1, 7755 g l−1, 7455 g l−1, 815 ± 5 g l−1, and 780 ± 10 g l−1 for palm fat, canola oil, SFW, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 5% w/w based batters, respectively. These values clearly indicate that the density of whipped fat phases (Fig. 2) does not simply propagate into batter densities. Fig. 10 depicts the data from amplitude sweeps of the cake batters produced with reference fat phases (A) and oleogels (B). First, it needs to be mentioned that the y-axes are differently scaled.
SFWh 5% w/w based batters, respectively. These values clearly indicate that the density of whipped fat phases (Fig. 2) does not simply propagate into batter densities. Fig. 10 depicts the data from amplitude sweeps of the cake batters produced with reference fat phases (A) and oleogels (B). First, it needs to be mentioned that the y-axes are differently scaled.
Although the batters are known to be viscous suspensions, all investigated systems exhibited gel-like properties. The two reference fat phases, palm fat and canola oil (A), result in significantly different viscoelastic behavior of the cake batters. The initial elastic modulus in amplitude sweeps of palm fat based batters exceeded that of canola oil by 45-fold ( = 17
= 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 966 Pa,
966 Pa,  = 399.7 Pa). The use of oleogels produced batters with elastic moduli in a range from 677 to 1126 Pa. Counterintuitively, SFW caused least elastic batters of all applied oleogels. BW and BW
= 399.7 Pa). The use of oleogels produced batters with elastic moduli in a range from 677 to 1126 Pa. Counterintuitively, SFW caused least elastic batters of all applied oleogels. BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh exhibited almost identical values and courses. These findings could again be confirmed by frequency sweeps (data not shown).
SFWh exhibited almost identical values and courses. These findings could again be confirmed by frequency sweeps (data not shown).
The significant differences between the two references, canola oil and palm fat, impressively illustrate that the rheological properties of the fat phase contribute substantially to the rheological characteristics of the batter. In this light, it is astonishing that SFW, as the most efficient oil structurant, creates the softest batters among the oleogelators. Nevertheless, this is in line with the results obtained for oleofoams. At 5% w/w wax dosage, SFW was the only wax not resulting in an oleofoam with gel properties. At 5% w/w dosage, BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and pure BW oleofoams showed quite similar rheological behavior (Fig. 9). Also, their respective batters are rheologically almost indistinguishable. The densities of the complex matrices obtained after mixing the batter ingredients (fat, egg, sugar and flour) does not correspond to the oleofoam densities. The viscoelastic properties of the batters appear to correlate with those of the oleofoams; compare Fig. 9 and 10.
1 and pure BW oleofoams showed quite similar rheological behavior (Fig. 9). Also, their respective batters are rheologically almost indistinguishable. The densities of the complex matrices obtained after mixing the batter ingredients (fat, egg, sugar and flour) does not correspond to the oleofoam densities. The viscoelastic properties of the batters appear to correlate with those of the oleofoams; compare Fig. 9 and 10.
Properties of oleogel sponge cakes
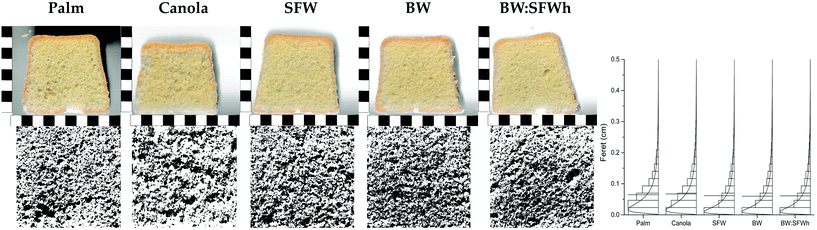
Already during the baking process, a higher leavening of the oleogel-based products was observed. However, in the final products (Fig. 11) only minute changes were observed. This could be caused by the box molds used. The products showed only minute visually observable differences. The colors of the crust and crumb were rated as identical.The processed images of the truncated sponge cake slices highlight the respective pore structure (Fig. 11, bottom row). The pore size distribution (PSD) is illustrated on the right in Fig. 11. The mean feret diameter remains practically invariant over all cakes, 0.063 ± 0.003 cm. Also the pore coverage as %area is found to be invariant. It is however found that the spatial arrangement of pores is less homogeneous for both references (palm fat and canola oil). Detailed investigation of the truncated cakes with a density analysis, however, might give better results.
The textural properties of the sponge cakes were studied by texture profile analysis (TPA). All calculated parameters are shown for clarity. It can be observed in general that significant differences are found between palm oil and canola oil and wax-oleogel applications. Only the resilence and springiness index showed deviating behavior.
Fig. 12 shows the force measured during double penetration. It was found that the reference cake with palm fat was clearly the hardest in the first and second penetration.
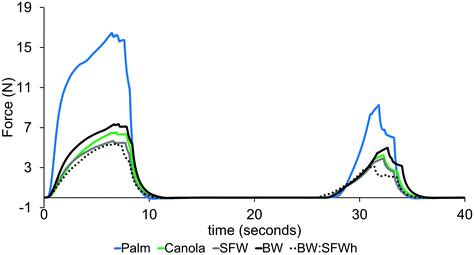 | ||
| Fig. 12 Force–time diagrams utilized for the TPA of sponge cakes with different fat phase compositions. | ||
All samples showed qualitatively the same force–distance evolution. These relative differences between the samples correspond to the rheological characterization of the cake batters. Taking a more quantitative look at the cake structure Table 3 reveals that the palm oil based cake is most distinct from the other cakes. It is only with respect to the springiness indistinguishable from the canola oil based cake. Among the cakes based on either straight canola oil or oleogels, significant differences are found in springiness and resilience, see superscripts. Furthermore, no significant differences were found between canola oil or oleogel based cakes.
| Properties | Palm fat | Canola oil | SFW 5% | BW 5% | BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 5% SFWh 5% |
|---|---|---|---|---|---|
| Hardness | 16.72 ± 0.80a | 6.59 ± 0.01b | 5.69 ± 0.20b | 7.47 ± 0.15b | 5.45 ± 0.22b |
| Cohesiveness | 0.32 ± 0.01a | 0.45 ± 0.00b | 0.43 ± 0.00b | 0.46 ± 0.01b | 0.48 ± 0.03b |
| Resilience | 0.41 ± 0.02a | 0.55 ± 0.01b | 0.47 ± 0.00ac | 0.46 ± 0.02ac | 0.50 ± 0.00bc |
| Adhesiveness | −0.22 ± 0.02a | −0.03 ± 0.01b | −0.03 ± 0.00b | −0.03 ± 0.00b | −0.06 ± 0.01b |
| Springiness index | 0.97 ± 0.01a | 0.98 ± 0.02a | 0.84 ± 0.02b | 0.78 ± 0.03b | 0.76 ± 0.02b |
| Gumminess | 5.29 ± 0.47a | 2.94 ± 0.02b | 2.48 ± 0.10b | 3.40 ± 0.11b | 2.63 ± 0.28b |
| Chewiness | 5.16 ± 0.51a | 2.88 ± 0.07b | 2.08 ± 0.05b | 2.65 ± 0.18b | 1.98 ± 0.17b |
Another important consumer relevant criterion in sponge cakes is oil migration. The presence of liquid oil or fat staining is unwanted on the products’ surface or packaging. To quantify the risk of these phenomena, the sponge cakes were also subjected to the oil leap test. Preliminary tests revealed that this method developed for oleogels was also applicable for sponge cakes. The results obtained are presented in Fig. 13.
 | ||
Fig. 13 Oil leap of 4 × 4 cm sponge cake cubes over a period of up to 90 h (n = 2). □ = reference (black = palm; grey = canola), ◊ = BW, Δ = BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh, o = SFW, all 5% w/w on fat. SFWh, o = SFW, all 5% w/w on fat. | ||
Cubes of the sponge cakes (4 × 4 cm) were studied for up to 90 h. Cakes made with pure canola oil exhibited the fastest oil leaping rate among all cakes. After 23 h, the 8 cm filter paper strip was fully soaked in oil. In contrast to that, palm fat showed only limited oil migration and reached 2.8 cm after 85 h. This difference is in line with expectation due to the fact that palm oil is at least semi-solid at room temperature.
The use of waxes as oil structurants, 5% w/w in the fat phase, reduced the oil loss significantly. Among the waxes BW was most efficient in reducing oil loss. The rate of oil loss found is approximately half way between those of palm oil and pure canola oil. Compared to canola oil the other waxes also showed superior performance. The overall assessment, e.g., the fact that SFW was the least effective oil binder in sponge cakes, is in line with the data on the oil binding of oleofoams, Fig. 6. It should be noted here that during the baking process the waxes are completely liquid, so that it cannot necessarily be assumed that the crystallization of the wax on cooling down of the cakes is related to oleogelation or rather heterogeneous crystallization at the surface of the other ingredients. With this in mind, it is surprising to find that some characteristics derived for oleogels are found in cakes. However, any attempt to formulate a hypothesis on a mechanism of action is doomed to be too speculative.
A sensory test evaluating the visual appearance, odor, taste, density, crumbliness and off-flavor of the different sponge cakes was performed. For the categories, no significant differences among all sponge cakes could be established. Despite this lack of discrimination, significant differences in the overall impression were found. The ranking sum, and hence cumulative disliking increased from the oleogel samples (SFW (24), BW (30), and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh (29)) to the two reference fat phases (canola oil (32) and palm fat (50)). The oleogel samples were thus the preferred product. The results obtained also indicate that the untrained testers found it difficult to identify and describe differences. This assessment indicates that the wax-based oleogels perform well in sponge cakes.
SFWh (29)) to the two reference fat phases (canola oil (32) and palm fat (50)). The oleogel samples were thus the preferred product. The results obtained also indicate that the untrained testers found it difficult to identify and describe differences. This assessment indicates that the wax-based oleogels perform well in sponge cakes.
Conclusion
In this contribution we report on the investigation of the key functionalities of fat phases in low density bakery products with emphasis on the application of wax based oleogels in sponge cakes. For this instance, four different waxes (SFW, BW, RBW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were used to produce 5 and 10% w/w (1% w/w additionally for SFW) oleogels. The oleogels were studied to possibly establish relationships between the functionality in the low-density bakery products and oleogel properties. To this end ‘clean’ oleogels (solely wax and oil) were investigated in terms of air stabilization, microstructure, viscoelastic properties and the oil binding capacity as oleofoams. This was done to test for the product specific functionalities in a minimally complex environment possible. It was found that all oleogels were able to stabilize air bubbles. SFW and the wax mixture BW
1) were used to produce 5 and 10% w/w (1% w/w additionally for SFW) oleogels. The oleogels were studied to possibly establish relationships between the functionality in the low-density bakery products and oleogel properties. To this end ‘clean’ oleogels (solely wax and oil) were investigated in terms of air stabilization, microstructure, viscoelastic properties and the oil binding capacity as oleofoams. This was done to test for the product specific functionalities in a minimally complex environment possible. It was found that all oleogels were able to stabilize air bubbles. SFW and the wax mixture BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 generated the highest overruns. Although the oil accumulation of the oleofoams was shown to be dependent on the wax concentration, vertical oil leaping did not reveal the same trend. Only 5 and 10% w/w BW and RBW as well as 10% w/w BW
1 generated the highest overruns. Although the oil accumulation of the oleofoams was shown to be dependent on the wax concentration, vertical oil leaping did not reveal the same trend. Only 5 and 10% w/w BW and RBW as well as 10% w/w BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh showed lower oil leaping compared to the pure canola oil reference. SFW appeared to even promote oil-migration. Micrographs revealed that the different positions of the wax crystal aggregates (bubble surface versus bulk) most likely play a role in oil immobilization. The effect of crystal aggregation at the bubble surface also seems to influence the viscoelastic properties. To test for the projection and scalability of the functionalities gathered in the whipping trials (oleofoams), the performance of the wax-based oleogels (here only SFW, BW and BW
SFWh showed lower oil leaping compared to the pure canola oil reference. SFW appeared to even promote oil-migration. Micrographs revealed that the different positions of the wax crystal aggregates (bubble surface versus bulk) most likely play a role in oil immobilization. The effect of crystal aggregation at the bubble surface also seems to influence the viscoelastic properties. To test for the projection and scalability of the functionalities gathered in the whipping trials (oleofoams), the performance of the wax-based oleogels (here only SFW, BW and BW![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) SFWh 1
SFWh 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was investigated as alternative fat phases in sponge cakes. Canola oil and palm fat were used as references. It was found that the densities of the batters do not correspond to those of the oleofoams. This illustrates the complex interactions between the recipe constituents. The viscoelastic properties of the cake batters differed significantly. This behaviour corresponded to the rheological characteristics of the respective oleofoams. The evaluation of the sponge cakes showed that there are technically only minor differences depending on the different fat phases used. Only with respect to the oil binding capacity palm oil proved to be most efficient while canola oil showed the worst performance. BW-based oleogels were most efficient in oil immobilization, delivering migration rates halfway between those of palm oil and canola oil. Sensory tests illustrated that the different sponge cakes do not differ much. Except for the overall liking, no significant differences could be established. In the overall liking, however, the oleogel based sponge cakes actually scored better than those based on canola oil or palm oil.
1) was investigated as alternative fat phases in sponge cakes. Canola oil and palm fat were used as references. It was found that the densities of the batters do not correspond to those of the oleofoams. This illustrates the complex interactions between the recipe constituents. The viscoelastic properties of the cake batters differed significantly. This behaviour corresponded to the rheological characteristics of the respective oleofoams. The evaluation of the sponge cakes showed that there are technically only minor differences depending on the different fat phases used. Only with respect to the oil binding capacity palm oil proved to be most efficient while canola oil showed the worst performance. BW-based oleogels were most efficient in oil immobilization, delivering migration rates halfway between those of palm oil and canola oil. Sensory tests illustrated that the different sponge cakes do not differ much. Except for the overall liking, no significant differences could be established. In the overall liking, however, the oleogel based sponge cakes actually scored better than those based on canola oil or palm oil.
In summary, the study illustrated that low density bakery products can succesfully be produced almost irrespective of the oil phase characteristics, from liquid oil via wax-based oleogels to palm oil. It was found that the main functional differences in the sponge cakes studied are found in the resistence to oil leakage. Since the discrimination between the different sponge cakes is almost impossible, it is not surprising that the properties of the sponge cakes do not correspond to differences established between oleofoams and batters. The good technical performance of the oleogels against the reference is a necessary but not sufficient condition to claim feasibility. However, the sensory tests performed also indicate at least equal quality of the oleogel based sponge cakes.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This IGF project AiF 20285N of the FEI is supported via AiF within the programme for promoting the Industrial Collective Research (IGF) of the Federal Ministry of Economic Affairs and Climate Action (BMWK), based on a resolution of the German Parliament. Special thanks go to Matthias Weber and Rüdiger Jank (Kuchenmeister GmbH, Soest) who supported us with their in-depth expertise regarding baked goods and enabled the application in sponge cakes in their facility. Furthermore, we would like to thank Luiza Fritsche for her patient support with the image analysis.References
- Crystallization of lipids, ed. K. Sato, John Wiley & Sons Ltd, West Sussex, 1st edn, 2018 Search PubMed.
- A. K. Zetzl, PhD Thesis, Univeristy of Guelph, 2013.
- M. Davidovich-Pinhas, S. Barbut and A. G. Marangoni, The gelation of oil using ethyl cellulose, Carbohydr. Polym., 2015, 117, 869–878 CrossRef CAS PubMed.
- M. Davidovich-Pinhas, A. J. Gravelle, S. Barbut and A. G. Marangoni, Temperature effects on the gelation of ethylcellulose oleogels, Food Hydrocolloids, 2015, 46, 76–83 CrossRef CAS.
- C. D. Doan, I. Tavernier, P. K. Okuro and K. Dewettinck, Internal and external factors affecting the crystallization, gelation and applicability of wax-based oleogels in food industry, Innovative Food Sci. Emerging Technol., 2018, 45, 42–52 CrossRef CAS.
- A. Bot, Y. S. J. Veldhuizen, R. den Adel and E. C. Roijers, Non-TAG structuring of edible oils and emulsions, Food Hydrocolloids, 2009, 23, 1184–1189 CrossRef CAS.
- A. R. Place, Comparative aspects of lipid digestion and absorption: physiological correlates of wax ester digestion, Am. J. Physiol., 1992, 263, R464–R471 CAS.
- FDA, GRAS Notice (GRN) No. 720, Rice bran wax, 2017.
- F. Aguilar, R. Crebelli, B. Dusemund, P. Galtier, D. Gott, U. Gundert-Remy, J. König, C. Lambré, J.-C. Leblanc, A. Mortensen, P. Mosesso, D. Parent-Massin, I. Stankovic, P. Tobback, I. Waalkens-Berendsen, R. Antonius Woutersen and M. Wright, Scientific Opinion on the re-evaluation of carnauba wax (E 903) as a food additive, EFS2, 2012, 10, 1 Search PubMed.
- F. Aguilar, R. Crebelli, B. Dusemund, P. Galtier, D. Gott, U. Gundert-Remy, J. König, C. Lambré, J.-C. Leblanc, A. Mortensen, P. Mosesso, D. Parent-Massin, I. Stankovic, P. Tobback, I. Waalkens-Berendsen, R. Antonius Woutersen and M. Wright, Scientific Opinion on the re-evaluation of candelilla wax (E 902) as a food additive, EFSA J., 2012, 10, 2946 CrossRef.
- C. D. Doan, A. R. Patel, I. Tavernier, N. de Clercq, K. van Raemdonck, D. v. d. Walle, C. Delbaere and K. Dewettinck, The feasibility of wax-based oleogel as a potential co-structurant with palm oil in low-saturated fat confectionery fillings, Eur. J. Lipid Sci. Technol., 2016, 118, 1903–1914 CrossRef CAS.
- H. L. Bemer, M. Limbaugh, E. D. Cramer, W. J. Harper and F. Maleky, Vegetable organogels incorporation in cream cheese products, Food Res. Int., 2016, 85, 67–75 CrossRef CAS PubMed.
- V. Conty, S. Theierl and E. Flöter, Improving the nutritional profile of culinary products: oleogel-based bouillon cubes, Food Funct., 2021, 12, 7185–7197 RSC.
- D. Franco, A. J. Martins, M. López-Pedrouso, M. A. Cerqueira, L. Purriños, L. M. Pastrana, A. Vicente, C. Zapata and J. M. Lorenzo, Evaluation of linseed oil oleogels to partially replace pork backfat in fermented sausages, J. Sci. Food Agric., 2020, 100, 218–224 CrossRef CAS PubMed.
- H. S. Hwang, M. Singh, J. K. Winkler-Moser, E. L. Bakota and S. X. Liu, Preparation of margarines from organogels of sunflower wax and vegetable oils, J. Food Sci., 2014, 79, C1926–C1932 CrossRef CAS PubMed.
- C. D. Doan, I. Tavernier, S. Danthine, T. Rimaux and K. Dewettinck, Physical compatibility between wax esters and triglycerides in hybrid shortenings and margarines prepared in rice bran oil, J. Sci. Food Agric., 2018, 98, 1042–1051 CrossRef CAS PubMed.
- I. Mert and I. Demirkesen, Reducing saturated fat with oleogel/shortening blends in a baked product, Food Chem., 2016, 199, 809–816 CrossRef PubMed.
- J. Y. Kim, J. Lim, J. H. Lee, H.-S. Hwang and S. Lee, Utilization of Oleogels as a Replacement for Solid Fat in Aerated Baked Goods: Physicochemical, Rheological, and Tomographic Characterization, J. Food Sci., 2017, 82, 445–452 CrossRef CAS PubMed.
- A. Jang, W. Bae, H.-S. Hwang, H. G. Lee and S. Lee, Evaluation of canola oil oleogels with candelilla wax as an alternative to shortening in baked goods, Food Chem., 2015, 187, 525–529 CrossRef CAS PubMed.
- B. Mert and I. Demirkesen, Evaluation of highly unsaturated oleogels as shortening replacer in a short dough product, LWT – Food Sci. Technol., 2016, 68, 477–484 CrossRef CAS.
- M. Zhao, M. Xu, E. Monono, J. Rao and B. Chen, Unlocking the potential of minimally processed corn germ oil and high oleic soybean oil to prepare oleogels for bakery application, Food Funct., 2020, 11, 10329–10340 RSC.
- K. Oh, C. Amoah, J. Lim, S. Jeong and S. Lee, Assessing the effectiveness of wax-based sunflower oil oleogels in cakes as a shortening replacer, LWT – Food Sci. Technol., 2017, 86, 430–437 CrossRef.
- H. Pehlivanoglu, G. Ozulku, R. M. Yildirim, M. Demirci, O. S. Toker and O. Sagdic, Investigating the usage of unsaturated fatty acid-rich and low-calorie oleogels as a shortening mimetics in cake, J. Food Process. Preserv., 2018, 42, e13621 CrossRef.
- I. Demirkesen and B. Mert, Utilization of Beeswax Oleogel–Shortening Mixtures in Gluten–Free Bakery Products, J. Am. Oil Chem. Soc., 2019, 96, 545–554 CrossRef CAS.
- D. Z. Gunes, M. Murith, J. Godefroid, C. Pelloux, H. Deyber, O. Schafer and O. Breton, Oleofoams: Properties of Crystal-Coated Bubbles from Whipped Oleogels-Evidence for Pickering Stabilization, Langmuir, 2017, 33, 1563–1575 CrossRef CAS PubMed.
- B. P. Binks and I. Marinopoulos, Ultra-stable self-foaming oils, Food Res. Int., 2017, 95, 28–37 CrossRef CAS PubMed.
- V. Conty and E. Flöter, Oil binding capacity, 2021 Search PubMed.
- E. Flöter, T. Wettlaufer, V. Conty and M. Scharfe, Oleogels—Their Applicability and Methods of Characterization, Molecules, 2021, 26, 1673 CrossRef PubMed.
- M. C. Bourne, J. F. Kenny and J. Barnard, Computer–Assisted Readout of Data from Texture Profile Analysis Curves1, 1978, 481–494 Search PubMed.
- DIN, DIN ISO 8587:2006, ICS 67.240, ICS 67.240.
- T. Wettlaufer, B. Hetzer and E. Flöter, Characterization of Oleogels Based on Waxes and Their Hydrolyzates, Eur. J. Lipid Sci. Technol., 2021, 123, 2000345 CrossRef CAS.
- A. R. Patel, M. Babaahmadi, A. Lesaffer and K. Dewettinck, Rheological profiling of organogels prepared at critical gelling concentrations of natural waxes in a triacylglycerol solvent, J. Agric. Food Chem., 2015, 63, 4862–4869 CrossRef CAS PubMed.
- L. S. K. Dassanayake, D. R. Kodali, S. Ueno and K. Sato, Physical Properties of Rice Bran Wax in Bulk and Organogels, J. Am. Oil Chem. Soc., 2009, 86, 1163–1173 CrossRef CAS.
- C. D. Doan, D. van de Walle, K. Dewettinck and A. R. Patel, Evaluating the Oil-Gelling Properties of Natural Waxes in Rice Bran Oil: Rheological, Thermal, and Microstructural Study, J. Am. Oil Chem. Soc., 2015, 92, 801–811 CrossRef CAS.
- T. Wettlaufer and E. Flöter, On the Effect of Storage Time on Wax-Wax-Hydrolyzate Canola oil Oleogels, Eur. J. Lipid Sci. Technol., 2022 Search PubMed , in review.
- T. Wettlaufer and E. Flöter, Effect of Cooling Rate on the Properties of Wax-Wax-Hydrolyzate based Oleogels, Food Biophys., 2022 Search PubMed , https://link.springer.com/article/10.1007/s11483-022-09725-y.
- T. Wettlaufer, H. Brykczynski and E. Flöter, Wax based Oleogels – Properties in Medium Chain Triglycerides (MCT) and Canola Oil, Eur. J. Lipid Sci. Technol., 2022, 124, 2100114 CrossRef CAS.
- C. D. Doan, C. M. To, M. d. Vrieze, F. Lynen, S. Danthine, A. Brown, K. Dewettinck and A. R. Patel, Chemical profiling of the major components in natural waxes to elucidate their role in liquid oil structuring, Food Chem., 2017, 214, 717–725 CrossRef CAS PubMed.
- N. A. M. Eskin and R. Przybylski, Rape Seed Oil/Canola, 2003, pp. 4911–4916 Search PubMed.
- M. Callau, K. Sow-Kébé, N. Jenkins and A.-L. Fameau, Effect of the ratio between fatty alcohol and fatty acid on foaming properties of whipped oleogels, Food Chem., 2020, 333, 127403 CrossRef CAS PubMed.
- U. Deichmann, S. Schuster, J.-P. Mazat and A. Cornish-Bowden, Commemorating the 1913 Michaelis-Menten paper Die Kinetik der Invertinwirkung: three perspectives, FEBS J., 2014, 281, 435–463 CrossRef CAS PubMed.
- A. Schröder, J. Sprakel, K. Schroën, J. N. Spaen and C. Berton-Carabin, Coalescence stability of Pickering emulsions produced with lipid particles: A microfluidic study, J. Food Eng., 2018, 234, 63–72 CrossRef.
- S. Saha, B. Saint-Michel, V. Leynes, B. P. Binks and V. Garbin, Stability of bubbles in wax-based oleofoams: decoupling the effects of bulk oleogel rheology and interfacial rheology, Rheol. Acta, 2020, 59, 255–266 CrossRef CAS.
- L. Metilli, A. Lazidis, M. Francis, S. Marty-Terrade, J. Ray and E. Simone, The Effect of Crystallization Conditions on the Structural Properties of Oleofoams Made of Cocoa Butter Crystals and High Oleic Sunflower Oil, Cryst. Growth Des., 2021, 21, 1562–1575 CrossRef CAS.
- A.-L. Fameau and A. Saint-Jalmes, Recent Advances in Understanding and Use of Oleofoams, Front. Sustain. Food Syst., 2020, 4, 2621 Search PubMed.
- T. G. Mezger, Das Rheologie Handbuch, Vincentz, 5th edn, 2016 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00563h |
| This journal is © The Royal Society of Chemistry 2022 |