Open Access Article
Open Access ArticleCombining galacto-oligosaccharides and 2′-fucosyllactose alters their fermentation kinetics by infant fecal microbiota and influences AhR-receptor dependent cytokine responses in immature dendritic cells†
Renate
Akkerman‡
 *a,
Madelon J.
Logtenberg‡
b,
Martin
Beukema
*a,
Madelon J.
Logtenberg‡
b,
Martin
Beukema
 a,
Bart J.
de Haan
a,
Marijke M.
Faas
a,
Erwin G.
Zoetendal
c,
Henk A.
Schols
b and
Paul
de Vos
a
a,
Bart J.
de Haan
a,
Marijke M.
Faas
a,
Erwin G.
Zoetendal
c,
Henk A.
Schols
b and
Paul
de Vos
a
aImmunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University of Groningen and University Medical Centre Groningen, Groningen, The Netherlands. E-mail: r.akkerman@umcg.nl
bLaboratory of Food Chemistry, Wageningen University & Research, Wageningen, The Netherlands
cLaboratory of Microbiology, Wageningen University & Research, Wageningen, The Netherlands
First published on 20th May 2022
Abstract
Galacto-oligosaccharides (GOS) and 2′-fucosyllactose (2′-FL) are non-digestible carbohydrates (NDCs) that are often added to infant formula to replace the functionalities of human milk oligosaccharides (HMOs). It is not known if combining GOS and 2′-FL will affect their fermentation kinetics and subsequent immune-modulatory effects such as AhR-receptor stimulation. Here, we used an in vitro set-up for the fermentation of 2′-FL and GOS, either individually or combined, by fecal microbiota of 8-week-old infants. We found that GOS was fermented two times faster by the infant fecal microbiota when combined with 2′-FL, while the combination of GOS and 2′-FL did not result in a complete degradation of 2′-FL. Fermentation of both GOS and 2′-FL increased the relative abundance of Bifidobacterium, which coincided with the production of acetate and lactate. Digesta of the fermentations influenced dendritic cell cytokine secretion differently under normal conditions and in the presence of the AhR-receptor blocker CH223191. We show that, combining GOS and 2′-FL accelerates GOS fermentation by the infant fecal microbiota of 8-week-old infants. In addition, we show that the fermentation digesta of GOS and 2′-FL, either fermented individually or combined, can attenuate DC cytokine responses in a similar and in an AhR-receptor dependent way.
Introduction
Infant formulas are often supplemented with non-digestible carbohydrates (NDCs) or enzymatically produced human milk oligosaccharide (HMO) to mimic some HMO functions from human milk (HM).1 This includes stimulation of gut microbiota colonization and guidance and development of balanced gut immune responses.2 Galacto-oligosaccharides (GOS) and the HMO analog 2-fucosyllactose (2′-FL) are commonly applied in infant formula,3 either individually, or in case of GOS in combination with long-chain fructo-oligosaccharides (lcFOS) in a typical ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.4 To date, there are only a few other combinations of NDCs and/or HMOs known that are used in infant formulas, while the list of NDCs that are allowed for their application in infant formula is growing.
1.4 To date, there are only a few other combinations of NDCs and/or HMOs known that are used in infant formulas, while the list of NDCs that are allowed for their application in infant formula is growing.
GOS is very broadly applied in infant formula and comprised of a mixture of galactose chains with a terminal galactose or glucose unit.5 The degree of polymerization (DP) of GOS molecules usually ranges from 2–8, with a large variation in glycosidic linkages and level of branching between different structures. A recent study by Logtenberg et al. identified over 100 different structures in commercially available Vivinal GOS.6 In various studies, supplementation of GOS to infant formulas was shown to increase the abundance of Bifidobacterium in infants of different ages when compared to infants who received formula without GOS.7,8 The utilization of GOS by specific bacterial species might also influence gut immune responses, as GOS fermentation leads to the formation of short chain fatty acids (SCFAs), such as propionate, butyrate and acetate, which exert immuno-regulatory properties.9,10 However, there are also studies showing direct immune-regulatory effects of GOS. For example, it was shown that GOS could attenuate pro-inflammatory responses in dendritic cells induced by co-cultured epithelial cells.11
Since a few years, the HMO 2′-FL is also applied in infant formulas, as it can now be produced as a fermentation product of genetically engineered microorganisms such as E. coli12 or yeast.13 This makes it possible to produce 2′-FL in sufficient quantities and in a cost-effective way to allow its application in infant formula. In contrast to GOS, 2′-FL is applied as an individual molecule in infant formulas. 2′-FL is composed of a lactose molecule decorated with an α(1–2)-linked fucose unit. In HM, 2′-FL is the most prevalent HMO that makes up around 30% of all HMOs.14 Like GOS, 2′-FL has been shown to have immune regulating effects. Supplementation of infant formula with 2′-FL restored the level of inflammatory cytokines in healthy term formula-fed infants, to more similar levels as in breastfed children.15
Next to immune effects, 2′-FL is considered to play an important role in the colonization of bifidobacteria as infants fed with HM of non-secretor mothers who lack α1–2-fucosylated HMOs like 2′-FL, were found to have difficulties in acquiring bifidobacterial species.16 2′-FL indeed has been shown to stimulate Bifidobacterium adolescentis and butyrate-producing bacteria in a validated in vitro gut model using microbiota of 6-month-old formula-fed infants.17 However, in contrast to GOS, 2′-FL appears to be more selectively fermented by specific infant gut microbiota as illustrated by the differentiation of infant fecal inocula into ‘slow and fast 2′-FL fermenters’.18 The ‘slow 2′-FL fermenters’ might benefit from a combination of 2′-FL with other more widely fermented oligosaccharides such as GOS. In addition, the combination of NDCs and/or HMOs with their unique structure-specific immune effects19,20 could result in novel supplements for the application in tailored infant formula to meet the demands of specific groups of infants of different ages or health statuses.
The immune effects resulting from NDC fermentation are most often attributed to the SCFAs that are formed during the fermentation. However, nowadays more evidence becomes available that NDC fermentation might also influence tryptophan metabolism and thereby the formation of indole-derivatives.21 Microbial tryptophan metabolites resulting from proteolysis can influence host health by binding to aryl hydrocarbon receptors (AhR) and thereby exert anti-inflammatory effects.21 In addition, there are studies suggesting that indole-derivatives can improve the gut barrier function.22,23 Furthermore, indole-derivatives also serve as important signaling molecules within the microbial community in the gut.24 However, to date, minor knowledge is available on the effect of indole-formation and AhR activation on immune responses in early life and how this is influenced by the fermentation of NDCs and HMOs in infants.
In the present study we used an in vitro fermentation set-up with pooled fecal inoculum of 8-week-old infants to investigate whether combining the commonly used NDC GOS with the HMO 2′-FL influenced the fermentation kinetics of specific oligosaccharides. GOS and 2′-FL were fermented either individually or combined at a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, with 2′-FL representing the amount of DP3 molecules in GOS. The degradation of GOS and 2′-FL by the infant fecal microbiota was studied as well as the microbiota compositions and the production of SCFAs during fermentation. Fermentation digesta collected at different timepoints were incubated with immature dendritic cells under normal cell-culture conditions or in presence of the AhR-receptor blocker CH223191 to study the effect of the fermentation products on dendritic cell cytokine responses.
1, with 2′-FL representing the amount of DP3 molecules in GOS. The degradation of GOS and 2′-FL by the infant fecal microbiota was studied as well as the microbiota compositions and the production of SCFAs during fermentation. Fermentation digesta collected at different timepoints were incubated with immature dendritic cells under normal cell-culture conditions or in presence of the AhR-receptor blocker CH223191 to study the effect of the fermentation products on dendritic cell cytokine responses.
Materials and methods
Substrates
2′-FL and purified Vivinal GOS (<3% monomers and lactose (w/w dry matter)) were provided by FrieslandCampina Ingredients (Amersfoort, The Netherlands). The purified Vivinal GOS was obtained from Vivinal GOS through the removal of lactose and monomers by lactase treatment and nanofiltration.Fermentation of GOS and 2′-FL by infant fecal inoculum
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 (v/v) with a total volume of 54 ml. GOS and 2′-FL were fermented in combination in a ratio of 4
10 (v/v) with a total volume of 54 ml. GOS and 2′-FL were fermented in combination in a ratio of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) and individually. In all the fermentations, the final concentration of the substrates in the fermentation liquid was 10 mg ml−1. Samples were collected with a syringe in triplicate at the start and after 12, 18, 24 and 36 h of fermentation. To preserve the bacteria for further analysis, one sample was immediately frozen in liquid nitrogen and stored at −80 °C. Samples for other analysis were heated for 5 min in a boiling water bath to inactivate all enzymes. Subsequently, the samples were stored at −20 °C until further analysis. The following control fermentations were included: (1) GOS and 2′-FL without inoculum to monitor contamination, (2) inoculum without GOS and 2′-FL to monitor background fermentation.
1 (v/v) and individually. In all the fermentations, the final concentration of the substrates in the fermentation liquid was 10 mg ml−1. Samples were collected with a syringe in triplicate at the start and after 12, 18, 24 and 36 h of fermentation. To preserve the bacteria for further analysis, one sample was immediately frozen in liquid nitrogen and stored at −80 °C. Samples for other analysis were heated for 5 min in a boiling water bath to inactivate all enzymes. Subsequently, the samples were stored at −20 °C until further analysis. The following control fermentations were included: (1) GOS and 2′-FL without inoculum to monitor contamination, (2) inoculum without GOS and 2′-FL to monitor background fermentation.
Fate of GOS and 2′-FL during fermentation
Microbial composition analysis
To determine the impact of the fermentation of GOS and/or 2′-FL on the microbiota composition, 16S rRNA gene amplicon sequencing was performed as described previously.19 Subsequent to DNA extraction, the V5–V6 region of 16S ribosomal RNA (rRNA) genes were amplified by polymerase chain reactions (PCR) in triplicate with the unique barcoded primer pair BSF784 (RGGATTAGATACCC) and R1064 (CGACRRCCATGCANACCT). For samples collected at the start of the fermentation, 10 μl of DNA template was used in PCR reactions. The PCR reactions of samples collected at later time points were performed with 0.7 μl of DNA template. Two synthetic communities with known composition were included as positive controls.27The PCR products were purified and prepared for sequencing as reported elsewhere.19 In short, an amplicon pool with a volume of 40 μl was formed by combining 200 ng of each uniquely barcoded sample followed by a concentration step using the HighPrep PCR kit (MagBio Genomics, Alphen aan den Rijn, The Netherlands). The library was sent for adapter ligation and sequencing with Illumina Hiseq2500 (GATC-Biotech, Konstanz, Germany).
The 16S rRNA gene amplicon sequencing data were processed and analyzed using the NG-Tax 2.0 pipeline, with default settings and R version 3.5.0.28 SILVA database release 132![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29 was used for taxonomic classification.
29 was used for taxonomic classification.
Production of SCFAs and other acids during fermentation
The production of acetate, propionate and butyrate during fermentation of GOS and 2′-FL was monitored by gas chromatography (GC) as described in previous study.19 High performance liquid chromatography (HPLC) was applied for the monitoring of lactate and succinate production during the fermentation.30Stimulation of DCs with fermentation digesta
After 24 h, cells were attached to the plate and pre-incubated with normal DC-MM culture medium or medium containing 20 μM CH223191 for 1 h. Before replacing the medium, by medium containing fermentation digesta, bacteria were removed from the fermentation digesta by centrifugation (10 min, RT, 12000g). Subsequently, supernatants were filtered through a 0.2 μm filter and the digesta was diluted in DC-MM culture medium (MatTek Corporation, Ashland, MA, USA) containing Polymyxin-B (50 ng ml−1) (Invivogen) or in DC-MM culture medium containing Polymyxin-B (50 ng ml−1) and 20 μM CH223191 at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The pH was set at 7.4 by the addition of 2N NaOH. DCs were then incubated with 200 μl per well medium containing the fermentation samples for 48 h. After incubation supernatants were collected and stored at −20 °C until further analysis. Experiments were repeated 6 times.
10. The pH was set at 7.4 by the addition of 2N NaOH. DCs were then incubated with 200 μl per well medium containing the fermentation samples for 48 h. After incubation supernatants were collected and stored at −20 °C until further analysis. Experiments were repeated 6 times.
Results
Combination of GOS and 2′-FL alters the fermentation kinetics by infant fecal microbiota
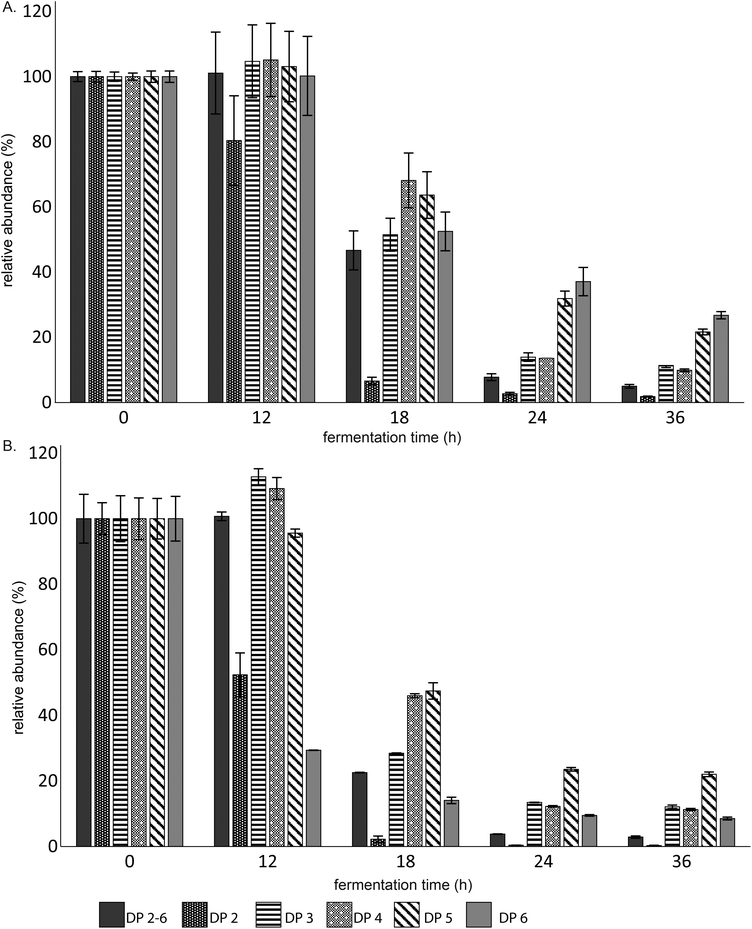
The fermentation digesta of both the individual and combined fermentations were analyzed by UHPLC-PGC-MS and HPAEC to determine if the combination of GOS and 2′-FL altered the fermentation behaviour of infant fecal microbiota. When GOS was fermented individually, the fecal microbiota of 8-week-old infants preferably fermented GOS oligomers with a lower degree of polymerization (DP) (Fig. 1A). After 36 h of fermentation, only 5 ± 0.6% of the total GOS preparation was remaining, while still more than 20% of the DP5 and 6 oligomers present in the GOS preparation were remaining.Interestingly, GOS was fermented two times faster by the infant fecal microbiota when combined with 2′-FL (Fig. 1B). In addition, the size-dependency as observed during the individual fermentation was absent. After 36 h of fermentation, DP6 oligomers (8.5 ± 0.4%) were less abundant than oligomers with DP 3–5 present in the GOS preparation, respectively 12.1 ± 0.5, 11.3 ± 0.3 and 22 ± 0.7%.
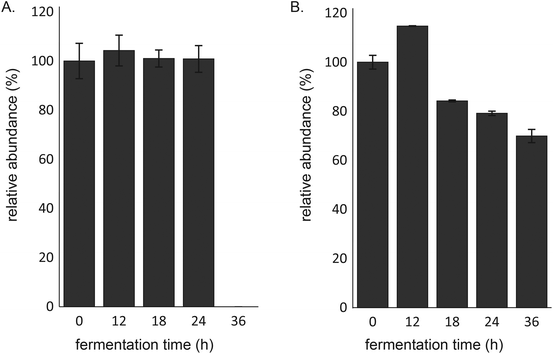
The fecal microbiota of 8-week-old infants degraded 2′-FL completely between 24 and 36 h of fermentation (Fig. 2A). However, whereas 2′-FL remained intact in the first 24 h of fermentation of 2′-FL alone, more than 20% of 2′-FL was degraded after 24 h of fermentation when fermented in combination with GOS (Fig. 2B). Nevertheless, the combination of 2′-FL and GOS did not result in the complete degradation of 2′-FL. After 36 h of fermentation, 70 ± 2.7% of 2′-FL was still remaining.
Increase in Bifidobacterium during fermentation of GOS and 2′-FL by infant fecal microbiota
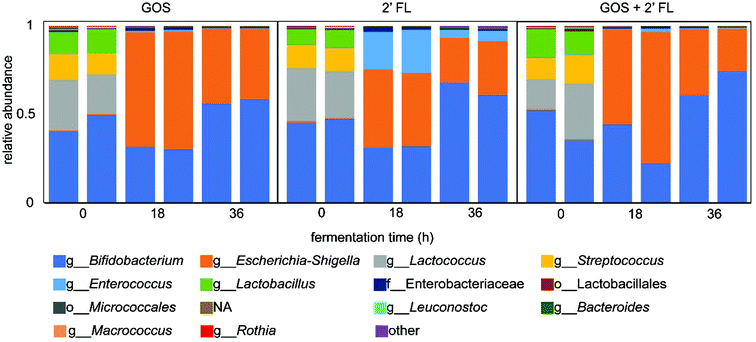
To investigate the changes in microbiota composition upon fermentation, the digesta collected during the fermentation of GOS and/or 2′-FL by fecal microbiota of 8-week-old infants were analyzed by 16S rRNA gene amplicon sequencing. A fermentation without added GOS and/or 2′-FL served as control and showed an enrichment in bacteria belonging to the genus Escherichia-Shigella and Enterococcus, which are suggested to be stimulated by the protein-rich SIEM (Fig. S1†).31The fermentation of GOS increased the relative abundance of Bifidobacterium, from 45% at the start of the fermentation to 57% after 36 h of fermentation (Fig. 3). The decrease in relative abundance in the first 18 h of fermentation can be attributed to the background fermentation on SIEM constituents by bacteria belonging to the genus Escherichia-Shigella. This background fermentation resulted in an increase in 16S rRNA gene copy numbers in the initial stage of the fermentation.20 In comparison to GOS, the fermentation of 2′-FL by infant fecal microbiota increased the relative abundance of Bifidobacterium to a larger degree (Fig. 3). The relative abundance of Bifidobacterium increased from 45% at the start of the fermentation to 64% after 36 h of fermentation. Next to the increase in Bifidobacterium, an increase in the abundance of Escherichia-Shigella and Enterococcus was observed which was likely a result of background fermentation of SIEM constituents.
The fermentation of the combination of GOS and 2′-FL by fecal microbiota of 8-week-old infants slightly enhanced the bifidogenic effect (Fig. 3). At the start of the fermentation, 44% of the bacteria belonged to the genus Bifidobacterium. This increased up to 68% after 36 h of fermentation which is 4% higher than with 2′-FL alone and 11% higher than with GOS. Similar to the individual fermentations, bacteria belonging to the genus Escherichia-Shigella were also enriched upon fermentation.
Organic acid production during fermentation of GOS and 2′-FL by infant fecal microbiota
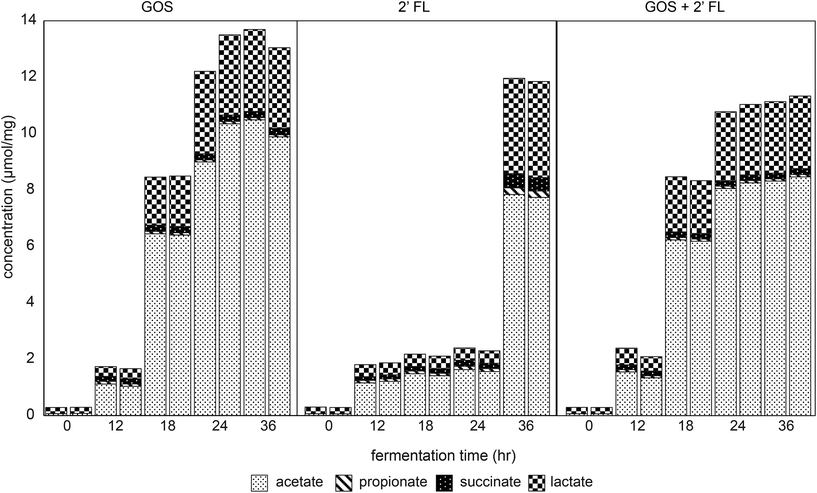
The fermentation digesta were analyzed by GC and HPLC to monitor the production of immune-active SCFAs, lactate and succinate32,33 during fermentation of GOS and/or 2′-FL by fecal microbiota of 8-week-old infants. A control fermentation without added GOS and/or 2′-FL resulted in minor production of organic acids with a total level of 2.3 ± 0.1 μmol mg−1 after 36 h of fermentation (Fig. S2†). Butyrate and branched-chain fatty acids were not detected in any of the fermentation digesta.The fermentation of GOS by fecal microbiota of 8-week-old infants resulted in a higher total production of organic acids acetate, lactate, succinate and propionate compared to the control fermentation (Fig. 4). The total organic acid concentration measured 8.5 μmol mg−1 after 18 h of fermentation, which further increased up to 13.4 ± 0.3 μmol mg−1 after 36 h of fermentation. Acetate, lactate, succinate and propionate were present in a ratio of 76![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21
21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In comparison to GOS, the organic acid concentration in fermentation digesta of 2′-FL increased only after 24 h of fermentation with a lower total organic acid level (Fig. 4). The total organic acid concentration measured 2.1 μmol mg−1 after 18 h of fermentation, which further increased up to 11.9 ± 0.1 μmol mg−1 after 36 h of fermentation. Acetate, lactate, succinate and propionate were present in a ratio of 65
1. In comparison to GOS, the organic acid concentration in fermentation digesta of 2′-FL increased only after 24 h of fermentation with a lower total organic acid level (Fig. 4). The total organic acid concentration measured 2.1 μmol mg−1 after 18 h of fermentation, which further increased up to 11.9 ± 0.1 μmol mg−1 after 36 h of fermentation. Acetate, lactate, succinate and propionate were present in a ratio of 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 29
29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.
2.
The fermentation of the combination of GOS and 2′-FL by infant fecal microbiota resulted in similar organic acid production as observed with the fermentation of GOS alone (Fig. 4). The total organic acid concentration measured 8.4 ± 0.1 μmol mg−1 after 18 h of fermentation, which further increased up to 11.2 ± 0.1 μmol mg−1 after 36 h of fermentation. Acetate, lactate, succinate and propionate were present in a ratio of 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
Cytokine responses induced by fermentation digesta are AhR-receptor dependent
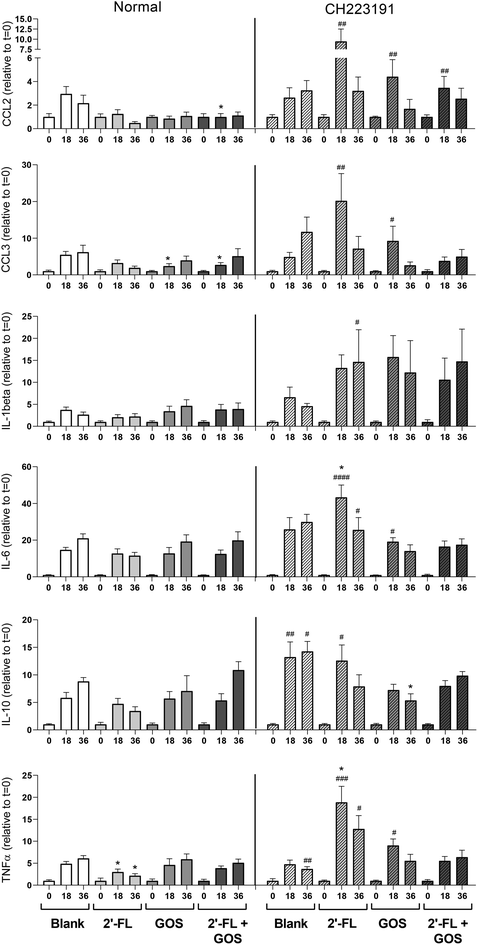
In vivo, DCs are abundantly present under the epithelial lining of the infant's gut and play an important role in the initiation of mucosal immune responses.34 To investigate the effect of the digesta of 2′-FL, GOS and the combination of 2′-FL/GOS fermented by fecal inoculum of 8-week-old infants on DC cytokine responses, DCs were incubated with digesta taken at different timepoints during the fermentation. The digesta of the blank fermentation increased cytokine production in DCs in a fermentation time dependent manner (Fig. 5).Incubation of DCs with both the t = 18 and t = 36 digesta from the 2′-FL fermentation resulted in a significant lower TNFα production when compared to the digesta of the blank fermentation. Incubation with the t = 18 digesta of the GOS fermentation resulted in significant lower secretion of MIP-1α/CCL3 and incubation of DCs with the t = 18 of the 2′-FL/GOS combination resulted in significant lower levels of MCP-1/CCL2 and MIP-1α/CCL3 when compared to the digesta of the blank control.
To investigate the influence of indole-derivatives present in the digesta on DC cytokine responses, DCs were also incubated with fermentation digesta in presence of the specific AhR-receptor antagonist CH223191.35 This also resulted in differences in cytokine secretion patterns between the digesta of the blank and the NDC fermentations. Incubation of the DCs with the t = 18 digesta of the 2′-FL fermentation resulted in a significant higher production of TNFα, whereas incubation with the t = 36 digesta of GOS resulted in a significant lower production of IL-10 when compared to the corresponding digesta of the blank fermentations. Incubation of DCs with CH223191 alone had no effect on cytokine responses when compared to DCs cultured in normal cell culture (Fig. S3†).
In addition, cytokine responses of DCs incubated with the fermentation digesta under normal circumstances or in presence of the specific AhR-receptor antagonist CH223191 were compared. Incubation of DCs with the t = 18 digesta of the blank fermentation resulted in significant higher secretion of IL-10 when incubated in presence of CH223191 compared to normal conditions. The t = 36 digesta of the blank induced higher secretion of both IL-10 and TNFα when incubated in presence of CH223191. Incubation of DCs with the t = 18 digesta of 2′-FL in presence of CH223191 resulted in a significant higher secretion of MCP-1/CCL2, MIP-1α/CCL3, IL-6, IL-10 and TNFα, while the incubation with the t = 36 digesta of the 2′-FL fermentation resulted in significant higher levels of IL-1β, IL-6 and TNFα when compared to DCs incubated with the digesta under normal conditions.
Incubation of DCs with the t = 18 digesta of the GOS fermentation resulted in significant higher levels of MCP-1/CCL2, MIP-1α/CCL3, IL-6 and TNFα, when compared to DCs incubated with the t = 18 GOS digesta under normal conditions. Incubation with the t = 18 digesta of the 2′-FL/GOS combination fermentation resulted in higher levels of MCP-1/CCL2 when compared to DCs incubated under normal conditions.
Discussion
Nutrition plays an important role in early life as it guides the colonization of the gut microbiota and it contributes to the maturation of the gut mucosal immune system.3 Nowadays, NDCs are added to infant nutrition to mimic some HMO functions, including their prebiotic properties and their immune regulating effects.2,36 GOS and the HMO analog 2′-FL are both added to infant formulas. However, it is unknown whether combining these NDCs impacts these beneficial health effects and whether fermentation of these NDCs has an effect on AhR activation and immune responses in early life. For this reason, we fermented GOS and 2′-FL, both individually and combined, using infant fecal inoculum and investigated the effect of the digesta on DC cytokine responses under normal conditions and in presence of the AhR antagonist CH223191.GOS was fermented in a size-specific fashion by the fecal microbiota of 8-week-old infants, which is in line with the observations in our previous study.25 Compared to GOS, the infant fecal microbiota required more time to adapt to the fermentation of 2′-FL as this substrate remained intact during the first 24 h of fermentation. After this adaptation period, 2′-FL was completely degraded between 24 and 36 h of fermentation. Fermentation of GOS in combination with 2′-FL expedited the fermentation of both GOS and 2′-FL, i.e. GOS was fermented two times faster and the fermentation of 2′-FL started earlier. The early onset of 2′-FL degradation could be explained by the immediate stimulation of GOS-degrading bacteria which are also capable of expressing enzymes needed to break down 2′-FL, like Bifidobacterium longum subsp. infantis expressing both β-galactosidases for the breakdown of GOS37 and 1,2-α-L-fucosidases for the breakdown of 2′-FL.38 Nevertheless, GOS degradation remained preferred over 2′-FL degradation as illustrated by an incomplete degradation of 2′-FL when combined with GOS. In addition, the expedited GOS degradation confirms the preference of GOS over 2′-FL by the infant fecal microbiota.
The fermentation of both GOS and 2′-FL by the fecal microbiota of 8-week-old infants increased the relative abundance of Bifidobacterium. This coincided with the production of both lactate and acetate, which are important end products of the bifid shunt, a specific metabolic pathway in bifidobacteria for the degradation of hexose carbohydrates.39 The bifidogenic effect of GOS in vitro is supported by many studies40,41 as well as by various in vivo studies as reviewed by Vandenplas et al.42 For 2′-FL there are also multiple studies showing bifidogenic effects in vitro43,44 and in vivo.16 The combination of GOS and 2′-FL did not result in significant synergistic bifidogenic effects on genus level, which might be explained by the capacity of several Bifidobacterium species to degrade both GOS and 2′-FL.37,38 In addition, background fermentation occurred in the protein-rich SIEM medium as illustrated by an increased relative abundance of the protein-degrading bacteria Escherichia-Shigella in each fermentation.31 The protein-degrading bacteria Enterococcus was only enhanced during the individual 2′-FL fermentation and the control fermentation without added substrate.
The digesta of the fermentations of GOS, 2′-FL and the GOS/2′-FL combination all induced cytokine responses in immature dendritic cells under normal conditions. We observed significant differences between the cytokine responses induced by the digesta of these fermentations when compared to the cytokine responses induced by the digesta of the blank fermentation. This might be related to the formation of different bacterial metabolites upon the fermentation of the different NDCs, like the SCFAs acetate and lactate or other molecules expressed and produced by bacterial species that were specifically increased in abundance i.e., Bifidobacterium, during the NDC fermentations. Lactate has been shown to support the development of immunosuppressive phenotypes in DCs through the attenuation of cell maturation.45 Acetate was also reported to have slight effects on chemokine secretion patterns in DCs before. In a study by Nastasi et al. incubation with acetate reduced the release of MIP-1α/CCL3.33 In addition, metabolites and surface molecules produced by specific bacterial strains might influence the cytokine secretion patterns as well. For example, Bifidobacterium, which increased in abundance in all NDC fermentation groups but not in the control fermentation, is known for the production of immune regulating exopolysaccharides.46 Also, other bacterial products formed upon fermentation, including ATP, lipoteichoic acid, polysaccharide A, nucleic acid, and peptidoglycan are likely present in the digesta and can interact with pattern recognition receptors (PRRs) like Toll-like receptors (TLRs) and C-type lectins expressed on the cell surface of DCs.47
Incubation of DCs with the digesta of the different fermentations in presence of the AhR-receptor blocker CH223191 resulted in significant different cytokine secretion patterns when compared to incubation under normal conditions. Especially incubation with the different t = 18 samples resulted in the secretion of significant higher cytokine levels. The effect was most pronounced for the NDC fermentation samples rather than for the blank fermentation samples. This might indicate that stimulation of the AhR-receptor by certain bacterial metabolites present in the digesta of the NDC fermentations has an immune attenuating effect in DCs. There are currently many different substances identified as agonist for the AhR-receptor, including synthetic compounds48 and naturally occurring derivatives of tryptophan including indole, indole-3-acetate and tryptamine.49
The gut microbiota metabolizes tryptophan, which is abundantly present in the protein-rich SIEM medium, into derivatives via the action of the enzyme tryptophanase (TnaA), which is expressed in many Gram-negative, as well as Gram-positive bacterial species including Escherichia coli, Clostridium spp. and Bacteroides spp.50 A recent study by Laursen et al.51 reported that Bifidobacterium species also have the ability to convert aromatic amino acids (tryptophan, phenylalanine and tyrosine) into aromatic lactic acids, respectively indolelactate, phenyllactate and 4-hydroxyphenyllactate.51 Considering the composition of the microbiota, with a high relative abundance of Bifidobacterium species in the fermentations of GOS, 2′-FL and GOS/2′-FL, it is likely that AhR ligands were formed during the NDC fermentations.
Stimulation of AhR-receptors is associated with immunoregulatory effects as, in DCs, AhR-receptors control both the differentiation and function of the cells.52 A study by Kado et al. showed that AhR-receptor stimulation also influences TLR responses in DCs. They found that TLR stimulated DCs express higher levels of AhR and are more sensitive to AhR ligands and that AhR stimulation in these DCs decreased the expression of surface markers and TLR induced cytokine expression.53 In addition, there are studies showing that decreased stimulation of the AhR-receptor is associated with pro-inflammatory responses as Lamas et al. showed for intestinal diseases like celiac disease.54 Our results also show that AhR-receptor stimulation by metabolites present in the fermentation digesta of GOS and/or 2′-FL contribute partially to the attenuation of DC cytokine responses by NDC fermentation digesta.
Conclusions
Our study provides insight in the effect of combining the NDCs GOS and 2′-FL that are currently applied separately in infant formulas. Our data shows that combining GOS and 2′-FL accelerates GOS fermentation by the infant fecal microbiota of 8-week-old infants. In addition, we show that the fermentation digesta of GOS and 2′-FL, either fermented individually or combined, can attenuate DC cytokine responses in an AhR-receptor dependent way.Author contributions
R. A., M. J. L, H. A. S. and P. D. V. designed the study. M. J. L performed the fermentation experiments. R. A. and M. B. performed the cell-based experiments. M. J. L performed the microbiota analysis, supported by E. Z., M. M. F. and B. J. D. H. assisted with the cell-based experiments. R. A., M. J. L., H. A. S. and P. D. V. wrote the manuscript. All authors have revised and improved the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Research of Renate Akkerman and Madelon J. Logtenberg was performed in the public-private partnership ‘CarboKinetics’ coordinated by the Carbohydrate Competence Center (CCC, https://www.cccresearch.nl). CarboKinetics is financed by participating industrial partners Agrifirm Innovation Center B.V., Cooperatie Avebe U.A., DSM Food Specialties B.V., FrieslandCampina Nederland B.V., Nutrition Sciences N.V., VanDrie Holding N.V. and Sensus B.V., and allowances of the Dutch Research Council (NWO).The authors thank Ineke Heikamp-de Jong for her support during the sequencing preparations.
References
- R. Akkerman, M. M. Faas and P. de Vos, Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation, Crit. Rev. Food Sci. Nutr., 2019, 59(9), 1486–1497 CrossRef CAS PubMed.
- L. Bode, Human milk oligosaccharides: every baby needs a sugar mama, Glycobiology, 2012, 22(9), 1147–1162 CrossRef CAS PubMed.
- C. Kong, M. M. Faas, P. de Vos and R. Akkerman, Impact of dietary fibers in infant formulas on gut microbiota and the intestinal immune barrier, Food Funct., 2020, 11, 9445–9467 RSC.
- J. Knol, P. Scholtens, C. Kafka, J. Steenbakkers, S. Gro, K. Helm, M. Klarczyk, H. Schöpfer, H. M. Böckler and J. Wells, Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants, J. Pediatr. Gastroenterol. Nutr., 2005, 40, 36–42 CrossRef CAS PubMed.
- D. P. M. Torres, M. P. F. Gonçalves, J. A. Teixeira and L. R. Rodrigues, Galacto-oligosaccharides: Production, properties, applications, and significance as prebiotics, Compr. Rev. Food Sci. Food Saf., 2010, 9(5), 438–454 CrossRef CAS PubMed.
- M. J. Logtenberg, K. M. H. Donners, J. C. M. Vink, S. S. van Leeuwen, P. de Waard, P. de Vos and H. A. Schols, Touching the high complexity of prebiotic Vivinal galacto-oligosaccharides using UHPLC-PGC-MS, J. Agric. Food Chem., 2020, 68(29), 7800–7808 CrossRef CAS PubMed.
- T. Matsuki, S. Tajima, T. Hara, K. Yahagi, E. Ogawa and H. Kodama, Infant formula with galacto-oligosaccharides (OM55N) stimulates the growth of indigenous bifidobacteria in healthy term infants, Benefic. Microbes, 2016, 7(4), 453–461 CrossRef CAS PubMed.
- C. Sierra, M. J. Bernal, J. Blasco, R. Martínez, J. Dalmau, I. Ortuño, B. Espín, M. I. Vasallo, D. Gil, M. L. Vidal, D. Infante, R. Leis, J. Maldonado, J. M. Moreno and E. Román, Prebiotic effect during the first year of life in healthy infants fed formula containing GOS as the only prebiotic: a multicentre, randomised, double-blind and placebo-controlled trial, Eur. J. Nutr., 2015, 54, 89–99 CrossRef CAS PubMed.
- W. N. D'Souza, J. Douangpanya, S. Mu, P. Jaeckel, M. Zhang, J. R. Maxwell, J. B. Rottman, K. Labitzke, A. Willee, H. Beckmann, Y. Wang, Y. Li, R. Schwandner, J. A. Johnston, J. E. Towne and H. Hsu, Differing roles for short chain fatty acids and GPR43 agonism in the regulation of intestinal barrier function and immune responses, PLoS One, 2017, 12(7), e0180190 CrossRef PubMed.
- C. Alexander, K. S. Swanson, G. C. Fahey Jr. and K. A. Garleb, Perspective: Physiologic importance of short-chain fatty acids from nondigestible carbohydrate fermentation, Adv. Nutr., 2019, 10(4), 576–589 CrossRef PubMed.
- M. Bermudez-Brito, N. M. Sahasrabudhe, C. Rosch, H. A. Schols, M. M. Faas and P. de Vos, The impact of dietary fibers on dendritic cell responses in vitro is dependent on the differential effects of the fibers on intestinal epithelial cells, Mol. Nutr. Food Res., 2015, 59(4), 698–710 CrossRef CAS PubMed.
- F. Baumgärtner, L. Seitz, G. A. Sprenger and C. Albermann, Construction of Escherichia coli strains with chromosomally integrated expression cassettes for the synthesis of 2′-fucosyllactose, Microb. Cell Fact., 2013, 12, 40 CrossRef PubMed.
- G. A. Sprenger, F. Baumgärtner and C. Albermann, Production of human milk oligosaccharides by enzymatic and whole-cell microbial biotransformations, J. Biotechnol., 2017, 258, 79–91 CrossRef CAS PubMed.
- Y. Vandenplas, B. Berger, V. P. Carnielli, J. Ksiazyk, H. Lagström, M. Sanchez Luna, N. Migacheva, J. Mosselmans, J. Picaud, M. Possner, A. Singhal and M. Wabitsch, Human milk oligosaccharides: 2′-fucosyllactose (2′-FL) and lacto-N-neotetraose (LNnT) in infant formula, Nutrients, 2018, 10(9), 1161 CrossRef PubMed.
- K. C. Goehring, B. J. Marriage, J. S. Oliver, J. A. Wilder, E. G. Barrett and R. H. Buck, Similar to those who are breastfed, infants fed a formula containing 2′-fucosyllactose have lower inflammatory cytokines in a randomized controlled trial, J. Nutr., 2016, 146(12), 2559–2566 CrossRef CAS PubMed.
- Z. T. Lewis, S. M. Totten, J. T. Smilowitz, M. Popovic, E. Parker, D. G. Lemay, M. L. Van Tassell, M. J. Miller, Y. Jin, J. B. German, C. B. Lebrilla and D. A. Mills, Maternal fucosyltransferase 2 status affects the gut bifidobacterial communities of breastfed infants, Microbiome, 2015, 3(1), 1–21 CrossRef PubMed.
- P. Van den Abbeele, C. Duysburgh, E. Vazquez, J. Chow, R. Buck and M. Marzorati, 2′-Fucosyllactose alters the composition and activity of gut microbiota from formula-fed infants receiving complementary feeding in a validated intestinal model, J. Funct. Foods, 2019, 61, 103484 CrossRef CAS.
- K. Salli, H. Anglenius, J. Hirvonen, A. A. Hibberd, I. Ahonen, M. T. Saarinen, K. Tiihonen, J. Maukonen and A. C. Ouwehand, The effect of 2′-fucosyllactose on simulated infant gut microbiome and metabolites, a pilot study in comparison to GOS and lactose, Sci. Rep., 2019, 9(1), 13232 CrossRef PubMed.
- R. Akkerman, M. J. Logtenberg, R. An, M. A. Van Den Berg, B. J. de Haan, M. M. Faas, E. G. Zoetendal, P. de Vos and H. A. Schols, Endo-1,3(4)-β-glucanase-treatment of oat β-glucan enhances fermentability by infant fecal microbiota, stimulates Dectin-1 activation and attenuates inflammatory responses in immature dendritic cells, Nutrients, 2020, 12(6), 1660 CrossRef CAS PubMed.
- M. J. Logtenberg, R. Akkerman, R. An, G. D. A. Hermes, B. J. de Haan, M. M. Faas, E. G. Zoetendal, H. A. Schols and P. de Vos, Fermentation of chicory fructo-oligosaccharides and native inulin by infant fecal microbiota attenuates pro-inflammatory responses in immature dendritic cells in an infant-age-dependent and fructan-specific way, Mol. Nutr. Food Res., 2020, 64(13), 2000068 CrossRef CAS PubMed.
- Z. M. Kundi, J. C. Lee, J. Pihlajamäki, C. B. Chan, K. S. Leung, S. S. Y. So, E. Nordlund, M. Kolehmainen and H. El-Nezami, Dietary fiber from oat and rye brans ameliorate western diet–induced body weight gain and hepatic inflammation by the modulation of short–chain fatty acids, bile acids, and tryptophan metabolism, Mol. Nutr. Food Res., 2020, 65, 1900580 CrossRef PubMed.
- H. M. Roager and T. R. Licht, Microbial tryptophan catabolites in health and disease, Nat. Commun., 2018, 9(1), 3294 CrossRef PubMed.
- M. Jennis, C. R. Cavanaugh, G. C. Leo, J. R. Mabus, J. Lenhard and J. Hornby, Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo, Neurogastroenterol. Motil., 2018, 30(2), e13178 CrossRef PubMed.
- J. H. Lee and J. Lee, Indole as an intercellular signal in microbial communities, FEMS Microbiol. Rev., 2010, 34(4), 426–444 CrossRef CAS PubMed.
- M. J. Logtenberg, R. Akkerman, R. G. Hobé, K. M. H. Donners, S. S. van Leeuwen, G. D. A. Hermes, B. J. de Haan, M. M. Faas, P. L. Buwalda, E. G. Zoetendal, P. de Vos and H. A. Schols, Structure-specific fermentation of galacto-oligosaccharides, isomalto-oligosaccharides and isomalto/malto-polysaccharides by infant fecal microbiota and impact on dendritic cell cytokine responses, Mol. Nutr. Food Res., 2021, 65(16), 2001077 CrossRef CAS PubMed.
- M. J. Logtenberg, J. C. M. Vink, R. M. Serierse, R. An, G. D. A. Hermes, H. Smidt and H. A. Schols, Pooled faecal inoculum can predict infant fiber fermentability despite high inter-individual variability of microbiota composition, Bioact. Carbohydr. Diet. Fibre, 2020, 24, 100235 CrossRef CAS.
- J. Ramiro-Garcia, G. D. A. Hermes, C. Giatsis, D. Sipkema, E. G. Zoetendal, P. J. Schaap and H. Smidt, NG-Tax, a highly accurate and validated pipeline for analysis of 16S rRNA amplicons from complex biomes, F1000Research, 2016, 5, 1791–1791 Search PubMed.
- W. Poncheewin, G. D. A. Hermes, J. C. J. van Dam, J. J. Koehorst, H. Smidt and P. J. Schaap, NG-Tax 2.0: A semantic framework for high-throughput amplicon analysis, Front. Genet., 2020, 10(1366), 1664–802 Search PubMed.
- C. Quast, E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies and F. O. Glöckner, The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acids Res., 2012, 41(D1), D590–D596 CrossRef PubMed.
- S. E. Ladirat, F. H. J. Schuren, M. H. C. Schoterman, A. Nauta, H. Gruppen and H. A. Schols, Impact of galacto-oligosaccharides on the gut microbiota composition and metabolic activity upon antibiotic treatment during in vitro fermentation, FEMS Microbiol. Ecol., 2014, 87(1), 41–51 CrossRef CAS PubMed.
- A. J. Richardson, N. McKain and R. J. Wallace, Ammonia production by human faecal bacteria, and the enumeration, isolation and characterization of bacteria capable of growth on peptides and amino acids, BMC Microbiol., 2013, 13, 6–6 CrossRef CAS PubMed.
- P. M. Smith, M. R. Howitt, N. Panikov, M. Michaud, C. A. Gallini, M. Bohlooly-Y, J. N. Glickman and W. S. Garrett, The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis, Science, 2013, 341(6145), 569–573 CrossRef CAS PubMed.
- C. Nastasi, M. Candela, C. M. Bonefeld, C. Geisler, M. Hansen, T. Krejsgaard, E. Biagi, M. H. Andersen, P. Brigidi, N. Odum, T. Litman and A. Woetmann, The effect of short-chain fatty acids on human monocyte-derived dendritic cells, Sci. Rep., 2015, 5, 16148 CrossRef CAS PubMed.
- H. Tezuka and T. Ohteki, Regulation of IgA production by intestinal dendritic cells and related cells, Front. Immunol., 2019, 10, 1891 CrossRef CAS PubMed.
- B. Zhao, D. E. Degroot, A. Hayashi, G. He and M. S. Denison, CH223191 is a ligand-selective antagonist of the Ah (Dioxin) receptor, Toxicol. Sci., 2010, 117(2), 393–403 CrossRef CAS PubMed.
- L. Cheng, R. Akkerman, C. Kong, M. T. C. Walvoort and P. de Vos, More than sugar in the milk: human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects, Crit. Rev. Food Sci. Nutr., 2020, 61(3), 1–17 Search PubMed.
- V. Ambrogi, F. Bottachi, J. O'Sullivan, M. O'Connell Motherway, C. Linqiu, B. Schoemaker, M. Schoterman and D. van Sinderen, Characterization of GH2 and GH42 β-galactosidases derived from bifidobacterial infant isolates, AMB Express, 2019, 9, 9 CrossRef PubMed.
- K. Salli, J. Hirvonen, J. Siitonen, I. Ahonen, H. Anglenius and J. Maukonen, Selective utilization of the human milk oligosaccharides 2′-fucosyllactose, 3-fucosyllactose, and difucosyllactose by various probiotic and pathogenic bacteria, J. Agric. Food Chem., 2021, 69(1), 170–182 CrossRef CAS PubMed.
- R. J. Palframan, G. R. Gibson and R. A. Rastall, Carbohydrate preferences of Bifidobacterium species isolated from the human gut, Curr. Issues Intest. Microbiol., 2003, 4, 71–75 CAS.
- L. A. van den Broek, S. W. A. Hinz, G. Beldman, J. P. Vincken and A. G. J. Voragen, Bifidobacterium carbohydrases: their role in breakdown and synthesis of (potential) prebiotics, Mol. Nutr. Food Res., 2008, 52, 146–163 CrossRef CAS PubMed.
- B. Rodriguez-Colinas, S. Kolida, M. Baran, A. O. Ballesteros, R. A. Rastall and F. J. Plou, Analysis of fermentation selectivity of purified galacto-oligosaccharides by in vitro human faecal fermentation, Appl. Microbiol. Biotechnol., 2013, 97, 5743–5752 CrossRef CAS PubMed.
- Y. Vandenplas, I. Zakharova and Y. Dmitrieva, Oligosaccharides in infant formula: more evidence to validate the role of prebiotics, Br. J. Nutr., 2015, 113, 1339–1344 CrossRef CAS PubMed.
- H. Ashida, A. Miyake, M. Kiyohara, J. Wada, E. Yoshida, H. Kumagai, T. Katayama and K. Yamamoto, Two distinct α-L-fucosidases from Bifidobacterium bifidum are essential for the utilization of fucosylated milk oligosaccharides and glycoconjugates, Glycobiology, 2009, 19(9), 1010–1017 CrossRef CAS PubMed.
- T. Thongaram, J. L. Hoeflinger, J. Chow and M. J. Miller, Human milk oligosaccharide consumption by probiotic and human-associated bifidobacterial and lactobacilli, J. Dairy Sci., 2017, 100(10), 7825–7833 CrossRef CAS PubMed.
- R. Sangsuwan, B. Thuamsang, N. Pacifici, R. Allen, H. Han, S. Miakicheva and J. S. Lewis, Lactate exposure promotes immunosuppressive phenotypes in innate immune cells, Cell. Mol. Bioeng., 2020, 13, 541–557 CrossRef CAS PubMed.
- M. M. P. Oerlemans, R. Akkerman, M. Ferrari, M. T. C. Walvoort and P. de Vos, Benefits of bacteria-derived exopolysaccharides on gastrointestinal microbiota, immunity and health, J. Funct. Foods, 2021, 76, 104289 CrossRef CAS.
- B. Ruiter and W. G. Shreffler, The role of dendritic cells in food allergy, J. Allergy Clin. Immunol., 2012, 129(4), 921–928 CrossRef CAS PubMed.
- D. E. DeGroot, G. He, D. Fraccalvieri, L. Bonati, A. Pandini and M. S. Denison, AhR ligands: promiscuity in binding and diversity in response, in The AH receptor in biology and toxicology, ed. R. Pohjanvirta, Wiley, Hoboken, NJ., 2011, pp. 63–79 Search PubMed.
- U. Jin, S. Lee, G. Sridharan, K. Lee, L. A. Davidson, A. Jayaraman, R. S. Chapkin, R. Alaniz and S. Safe, Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities, Mol. Pharmacol., 2014, 85(5), 777–788 CrossRef PubMed.
- J. H. Lee and J. Lee, Indole as an intercellular signal in microbial communities, FEMS Microbiol. Rev., 2010, 34, 426–444 CrossRef CAS PubMed.
- M. F. Laursen, M. Sakanaka, N. von Burg, D. Andersen, J. M. Moll, C. T. Pekmez, A. Rivollier, K. F. Michaelsen, C. Molgaard, M. V. Lind, L. O. Dragsted, T. Katayama, H. L. Frandsen, A. M. Vinggaard, M. I. Bahl, S. Brix, W. Agace, T. R. Licht and H. M. Roager, Bifidobacterium species associated with breastfeeding produce aromatic lactic acids in the infant gut, Nat. Microbiol., 2021, 6, 1367–1382 CrossRef CAS PubMed.
- F. J. Quintana, A. Yeste and I. D. Mascanfroni, Role and therapeutic value of dendritic cells in central nervous system autoimmunity, Cell Death Differ., 2015, 22, 215–224 CrossRef CAS PubMed.
- S. Kado, W. L. W. Chang, A. N. Chi, M. Wolny, D. M. Shepherd and C. F. A. Vogel, Aryl hydrocarbon receptor signaling modifies Toll-like receptor-regulated responses in human dendritic cells, Arch. Toxicol., 2016, 91, 2209–2221 CrossRef PubMed.
- B. Lamas, L. Hernandez-Galan, H. J. Galipeau, M. Constante, A. Clarizio, J. Jury, N. M. Breyer, A. Caminero, G. Rueda, C. L. Hayes, J. L. McCarville, M. Bermudez-Brito, J. Planchais, N. Rolhion, J. A. Murray, P. Langella, L. M. P. Loonen, J. M. Wells, P. Bercik, H. Sokol and E. F. Verdu, Aryl hydrocarbon receptor ligand production by the gut microbiota is decreased in celiac disease leading to intestinal inflammation, Sci. Transl. Med., 2020, 12(566), eaba0624 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00550f |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2022 |