Open Access Article
Open Access Articleβ(2→1) chicory and β(2→1)-β(2→6) agave fructans protect the human intestinal barrier function in vitro in a stressor-dependent fashion
Cynthia
Fernández-Lainez
 *abc,
Madelon J.
Logtenberg
d,
Xin
Tang
a,
Henk A.
Schols
d,
Gabriel
López-Velázquez
e and
Paul
de Vos
a
*abc,
Madelon J.
Logtenberg
d,
Xin
Tang
a,
Henk A.
Schols
d,
Gabriel
López-Velázquez
e and
Paul
de Vos
a
aImmunoendocrinology, Division of Medical Biology, Department of Pathology and Medical Biology, University Medical Centre Groningen, Hanzeplein 1, 9713 GZ, Groningen, The Netherlands. E-mail: c.fernandez.lainez@umcg.nl; lainezcynthia@hotmail.com; x.tang@umcg.nl; p.de.vos@umcg.nl; Fax: +3150-3611911; Tel: +3150-3618043
bLaboratorio de Errores innatos del Metabolismo y Tamiz, Instituto Nacional de Pediatría, Av. Iman 1, 04530, Ciudad de México, Mexico
cPosgrado en Ciencias Biológicas, Universidad Nacional Autónoma de México. Edificio D, 1° Piso. Circuito de Posgrados, Ciudad Universitaria, 04510, Ciudad de México, Mexico
dLaboratory of Food Chemistry, Wageningen University, Bornse Weilanden 9, 6708 WG, Wageningen, The Netherlands. E-mail: madelon.logtenberg@wur.nl; henk.schols@wur.nl
eLaboratorio de Biomoléculas y Salud Infantil, Instituto Nacional de Pediatría, Av. Iman 1, 04530, Cuidad de México, Mexico. E-mail: glv_1999@ciencias.unam.mx
First published on 20th May 2022
Abstract
Dietary fibers such as fructans can protect the intestinal epithelial barrier integrity, but the mechanisms underlying this protection are not completely understood. We aimed to study the protective effect of β(2→1)-β(2→6) branched graminan-type fructans (GTFs) on gut epithelial barrier function that was disrupted by three different agents which impact the barrier function via different cellular mechanisms. The effects of GTFs were compared with those of linear β(2→1) inulin-type fructans (ITFs). T84 intestinal epithelial monolayers were incubated with GTFs and ITFs. Afterwards, the monolayers were challenged with the barrier disruptors calcium ionophore A23187, 12-myristate 13-acetate (PMA) and deoxynivalenol (DON). Transepithelial resistance was measured with an electric cell–substrate impedance sensing system. All fructans studied prevented the barrier disruption induced by A23187. ITF II protected from the disruptive effects of PMA. However, none of the studied fructans influenced the disruption induced by DON. As a measure of disruption-induced inflammation, interleukin-8 (IL-8) production by the intestinal epithelium was determined by ELISA. The production of IL-8 induced by A23187 was decreased by all fructans, whereas IL-8 production induced by DON decreased only upon pre-treatment with ITF II. None of the studied fructans prevented PMA induced IL-8 production. GTFs just like ITFs can influence the barrier function and inflammatory processes in gut epithelial cells in a structure-dependent fashion. These distinct protective effects are dependent on the different signaling pathways that lead to gut barrier disruption.
1. Introduction
The gastrointestinal epithelium is considered as the gatekeeper of the human body that separates and protects the host from the harsh conditions in the gut lumen.1 To function as a barrier, the epithelial cells are linked by multiprotein complexes named tight junctions (TJs)2 that avoid the entry of larger, possibly immunologically active macromolecules, or other agents such as pathogenic organisms that are harmful to the host.3 These TJs allow the entry of molecules with radii between 3.5 and 6 Å4 but can actively regulate their permeability in response to physiological stress.5 Under normal barrier conditions, the transport of ions, water, and other molecules into the underlying lamina propria6 is actively regulated by the epithelial intestinal cells to provide the host with the desired luminal molecules.7Impairment of the intestinal barrier function has been proposed to be involved in the ever-growing list of Western diseases such as autoimmune diseases, allergies, some types of cancers, colitis, and inflammatory disorders.8 The disruption has been attributed to not only the more frequent use of barrier disrupting medications such as nonsteroidal anti-inflammatory drugs (NSAIDs)9 and proton pump inhibitors (PPIs)10,11 but also the consumption of westernized diets rich in barrier disrupting food additives, high fat, or the absence or lower consumption of dietary fibers.12,13 Although this emphasizes the importance of disrupted gut barrier function in disease development, it also opens venues to treat or prevent disease as many food molecules have been shown to stimulate gut barrier function.14,15
One such family of food molecules that have been shown to regulate the barrier function of gut epithelial cells are dietary fiber fructans.16 Fructans are mostly plant-derived polysaccharides that can be categorized by the Fn type, where F indicates fructose units, and the GFn type, where G indicates a glucose molecule linked to n number of fructose units.17 Fructans can be linked by β(2→1) bonds rendering them a linear structure.18 These fructans are of the inulin type19 and often extracted from chicory. These inulin-type fructans (ITFs) are often used in infant formulas20 and have been shown to protect the gut barrier function in a chain-length dependent fashion.16 Another group of fructans that is used as a food additive in infant formulas is fructans linked by both β(2→1) and β(2→6) bonds.21,22 These types of fructans are branched and known as graminans.23–25 These graminan-type fructans (GTFs) are extracted from plants of the Asparagaceae family such as Agave tequilana and are one of the most complex fructans described to date.26 It is unknown whether these branched fructans have any effect on gut barrier function.
Barrier disruption can occur via different cellular pathways in gut epithelial cells, but it is unknown by which pathways fructans protect epithelial cells from disruption. In vitro, barrier disruption can be induced by different agents and signaling mechanisms. Calcium ionophores, such as A23187, cause an increase of intracellular calcium leading to the disruption of the gut epithelial barrier.27 Another agent, 12-myristate 13-acetate (PMA), is a tumor-promoting phorbol ester which has been shown to disrupt the gut barrier function via the activation of protein kinase C (PKC).28 Another family of molecules that induce barrier disruption are trichothecenes such as the fungal toxin deoxynivalenol (DON) that act via MAPK signaling.29,30 This toxin is produced by the members of the fungal genus Fusarium, which represents the most common source of mycotoxin contamination of crops worldwide.31 As these agents disrupt the epithelial barrier via different mechanisms, they can be used to investigate the mechanisms underlying the protection against barrier disruption.
We aimed to determine whether and to what extent graminans of different molecular weights from agave could protect against gut barrier disruption. In addition, we compared the effects of GTFs with the barrier protective effect of ITFs from chicory. As it is unknown by which mechanisms fructans can protect against barrier disruption, we compared the effects induced by the calcium ionophore A23187, PMA and the fungal toxin DON which disrupt the epithelial integrity via different signaling pathways. Finally, interleukin-8 (IL-8) production by the intestinal epithelial cells was quantified as a measure of inflammatory stress induced by the above-mentioned disruptors and it was determined if fructans are able to protect from this inflammatory stress.
2. Materials & methods
2.1. Fructans
To study whether fructans with different structures and molecular sizes can protect against the disruption of the intestinal barrier of epithelial T84 cells, two formulations of β(2→1)-β(2→6)-linked graminan-type fructans from the Agave tequilana Weber blue variety (agave) and two formulations of β(2→1)-linked inulin-type fructans from Cichorium intybus (chicory) were included. Agave fructans GTF I (Metlos™) and GTF II (Metlin™) were provided by Nekutli™ (Guadalajara, México). Chicory fructans ITF I (Frutafit™ CLR) and ITF II (Frutafit™ TEX!) were provided by Sensus™ (B.V. Roosendaal, The Netherlands). These fructans have also been studied and analyzed in previous studies from our group.32,332.2. Cell lines
For determination of the potential protective effect of chicory and agave fructans on the barrier function of intestinal epithelial cells, we used colonic epithelial T84 cells (Sigma-Aldrich, Zwijndrecht, The Netherlands) between passages 28 and 34. Epithelial T84 cells were cultured in Dulbecco's modified Eagle's medium/nutrient mixture F-12 Ham with 15 mM HEPES and sodium bicarbonate (Sigma, Dorset, UK), supplemented with 10% heat-deactivated fetal bovine serum (Sigma-Aldrich, Dorset, UK) and gentamicin 50 mg ml−1 (Sigma-Aldrich, Dorset, UK) (complete medium). The epithelial T84 cells were cultured at 37 °C with 5% CO2, until 80% confluence. The medium was refreshed twice a week. For maintenance, the cells were passaged after treatment with trypsin (Sigma-Aldrich, Dorset, UK).2.3. Disruptors
The epithelial T84 monolayers were challenged using chemical compounds that disrupt the barrier function via different intracellular routes.27–29 The barrier disruptors used herein were the calcium ionophore A23187, also known as calcimycin, the food-contaminant mycotoxin DON, and PMA. The concentrations used herein were previously demonstrated to induce epithelial barrier disruption.14,162.4. Transepithelial electrical resistance measurements
The T84 cell-intestinal epithelial barrier function was monitored in real time with an electric cell–substrate impedance sensing system (ECIS, Applied BioPhysics™ model Zθ). First, epithelial T84 cells were seeded at a density of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per well in a final volume of 300 μl, in a 96-well PET plate with gold electrodes (96W20idf PET, Applied Biophysics). To increase the electrical stability of measurements, the plates were pre-incubated at room temperature (R.T.) for 30 min with a PBS solution of L-cysteine (2 mg ml−1) before seeding. Afterwards, the plates were washed with Dulbecco's modified Eagle's medium (DMEM) with 4.5 g L−1 glucose, 3.9 mM L-glutamine and 1 mM sodium pyruvate (Catalog number BE12-604F, Lonza, USA) and coated overnight at R.T. with a solution of 0.1% bovine serum albumin and 1% purified soluble collagen in DMEM. After washing the plates with complete medium, epithelial T84 cells were seeded and cultured at 37 °C with 5% CO2 for 21 days to allow the formation of a monolayer with stable transepithelial electrical resistance (TEER). The culture medium was refreshed every other day. For TEER measurements, the plates were placed in the ECIS equipment and the resistance was monitored for 5 hours at 400 Hz to ensure a stable measurement. Afterwards, the experiment was started. Epithelial T84 cells were pre-incubated for 24 hours with 10 mg ml−1 of the four studied fructans ITF I, ITF II, GTF I, and GTF II. This was followed by the addition of the disruptor calcium ionophore A23187 (4 μM, Sigma-Aldrich, UK), DON (8.4 μM, Sigma-Aldrich, UK), or PMA (1000 nM, Sigma-Aldrich, UK). After adding the disruptor, the TEER was monitored in the presence of the calcium ionophore A23187 for 3 hours, and with DON and PMA for 24 hours. The cells incubated only with the culture medium were used as untreated controls. The cells treated only with disruptors were included as positive controls. At least five independent experiments were performed with three technical replicates. To quantify the changes in the TEER after the different treatments, the area under the curve (AUC) was calculated. Untreated controls were set as 100% and the different conditions were related to the untreated controls.
000 cells per well in a final volume of 300 μl, in a 96-well PET plate with gold electrodes (96W20idf PET, Applied Biophysics). To increase the electrical stability of measurements, the plates were pre-incubated at room temperature (R.T.) for 30 min with a PBS solution of L-cysteine (2 mg ml−1) before seeding. Afterwards, the plates were washed with Dulbecco's modified Eagle's medium (DMEM) with 4.5 g L−1 glucose, 3.9 mM L-glutamine and 1 mM sodium pyruvate (Catalog number BE12-604F, Lonza, USA) and coated overnight at R.T. with a solution of 0.1% bovine serum albumin and 1% purified soluble collagen in DMEM. After washing the plates with complete medium, epithelial T84 cells were seeded and cultured at 37 °C with 5% CO2 for 21 days to allow the formation of a monolayer with stable transepithelial electrical resistance (TEER). The culture medium was refreshed every other day. For TEER measurements, the plates were placed in the ECIS equipment and the resistance was monitored for 5 hours at 400 Hz to ensure a stable measurement. Afterwards, the experiment was started. Epithelial T84 cells were pre-incubated for 24 hours with 10 mg ml−1 of the four studied fructans ITF I, ITF II, GTF I, and GTF II. This was followed by the addition of the disruptor calcium ionophore A23187 (4 μM, Sigma-Aldrich, UK), DON (8.4 μM, Sigma-Aldrich, UK), or PMA (1000 nM, Sigma-Aldrich, UK). After adding the disruptor, the TEER was monitored in the presence of the calcium ionophore A23187 for 3 hours, and with DON and PMA for 24 hours. The cells incubated only with the culture medium were used as untreated controls. The cells treated only with disruptors were included as positive controls. At least five independent experiments were performed with three technical replicates. To quantify the changes in the TEER after the different treatments, the area under the curve (AUC) was calculated. Untreated controls were set as 100% and the different conditions were related to the untreated controls.
2.5. Measurement of IL-8 production of epithelial T84 cells
To investigate whether disrupting molecules induce inflammation in T84 cells, the production of the pro-inflammatory cytokine IL-8 was measured. To that end, supernatants from the T84 cells were collected once the TEER experiments were finished. The supernatants were stored at −20 °C until further use. IL-8 was quantified with an ELISA kit (R&D Systems, Abingdon, UK) according to the manufacturer's protocol.2.6. Statistical analyses
GraphPad software version 9.2 was used for statistical analyses. The Shapiro–Wilk test was performed to test for the normality distribution of the data. Since AUC and IL-8 data are normally distributed, such data were expressed as mean ± SD. The significance was assessed with one-way ANOVA with Dunnet's multiple comparison test. A p-value < 0.05 was considered as statistically significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001.3. Results
To study whether the differences in their chemical characteristics are important for their effects on the modulation of the induced-disruption of the epithelial barrier, four fructan formulations were used. The graminan-type fructans studied are oligosaccharides with β(2→1) and β(2→6) linkages, which confer them a branched structure. GTF I is a mixture of fructans enriched with DP 3–4, although oligosaccharides with DP 7–45 are also present in lower amounts. GTF II is a mixture of mainly longer fructans whose DP ranges between 3 and 60 with most components around DP 17, although some higher molecular weight molecules are present. Both GTFs are composed of fructan structures of the Fn and GFn series of fructan structures (Table 1).33 In addition, in these two mixtures, there are molecules that represent the neo-levan type, which are characteristic of fructans extracted from agave plants.23 The inulin-type fructans included are oligosaccharides with only β(2→1) linkages, which confer them a linear structure. ITF I is a mixture of fructans with a DP range of 3–10, but it also possesses chains of DP up to 25. ITF II is a mixture of longer chain fructans with a DP of 10–60. ITF I contains both Fn and GFn types of oligosaccharides, whereas ITF II is constituted only of GFn units (Table 1).323.1. Agave and chicory fructans exert a protective effect on calcium ionophore-induced disruption of gut epithelial barrier function
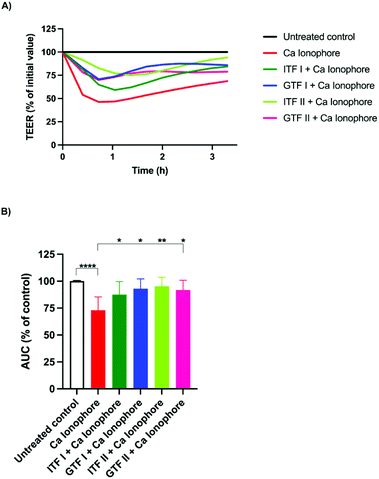
To test the potential protective effect of branched graminan-type fructans on the intestinal barrier function, epithelial T84 cell monolayers were preincubated with GTFs before adding the barrier disruptor calcium ionophore A23187 after which the TEER was measured for three hours. Linear inulin-type fructans were also tested to compare the effects between branched and linear fructan structures. After pre-incubation with fructans for 24 hours, A23187 was added to the cells followed by TEER measurements for three more hours. Fig. 1A shows a representative example of TEER measurements. These TEER values were used for calculating AUC. As shown in Fig. 1B, the incubation of T84 cells with A23187 decreased their AUC to 27% ± 5.5 (p < 0.001) compared with that of the untreated control. This effect of A23187 was strongly attenuated when the cells were preincubated with GTFs or ITFs since the AUC of these cells was similar to that of the untreated control. ITF II was the fructan with the strongest protective capacity against the barrier function disruption as the AUC decreased only by 4.7% ± 3.7, followed by GTF I (7% ± 4), GTF II (8.2% ± 4) and ITF I (12.5% ± 5.4).3.2. Linear long chain unbranched fructans exert protective effects against PMA-induced disruption of gut epithelial barrier function
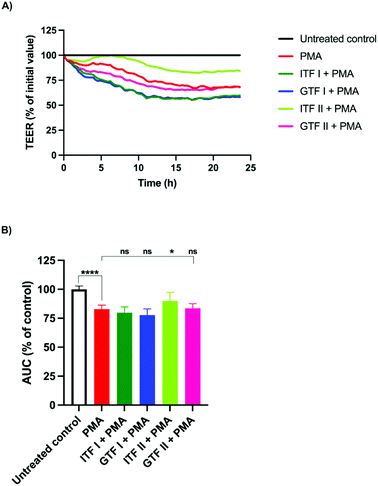
PMA is a well-known barrier disruptor which acts via the activation of PKC.28 We aimed to challenge the intestinal monolayers with this disruptor in order to study whether the protective effect exerted by chicory and agave fructans occurred via PKC. To that end, T84 monolayers were pre-incubated with fructans for 24 hours, followed by the addition of PMA and incubation for another 24 hours. A representative example of the TEER experiment is shown in Fig. 2A. These data were used for the calculation and plotting of the AUC percentage (Fig. 2B). Compared with the untreated control, PMA induced a reduction of 17% ± 1.5 (p < 0.01) in the AUC. This was lower in cells pre-incubated with ITF II where the AUC reduction was 10% ± 3.3 (p < 0.05) (Fig. 2B). The fructans ITF I, GTF I and GTF II did not influence the AUC reduction induced by PMA, since the AUC values obtained were 83.5% ± 1.8 for GTF II, 80% ± 2 for ITF I and 78% ± 2 for GTF I (Fig. 2B).3.3. No protective effect of fructans on DON induced disruption of gut epithelial barrier function
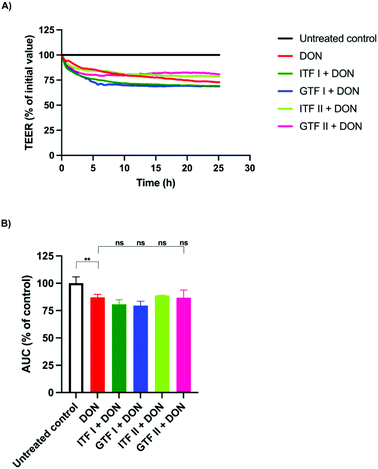
To investigate the ability of fructans to protect from epithelial barrier disruption exerted via MAPK signaling, epithelial T84 cells were also challenged with the fungal toxin DON. Monolayers of T84 cells were pre-incubated for 24 hours with the different fructans studied. Afterwards, DON was added followed by a subsequent incubation of another 24 hours. A representative example of the TEER measurements under different experimental conditions is shown in Fig. 3A. DON decreased the AUC significantly to 87.1% ± 1.3 (p < 0.01) compared to the control (Fig. 3B). This disruption could not be prevented with the fructans studied, since the AUC was 80.8% ± 2 for ITF I, 79.5% ± 1.9 for GTF I, 88.8% ± 0.24 for ITF II, and 86.7% ± 3.5 for GTF II (Fig. 3B).3.4. Fructans protect T84 intestinal epithelial cells from the inflammatory effect induced by the calcium ionophore and DON but not by PMA
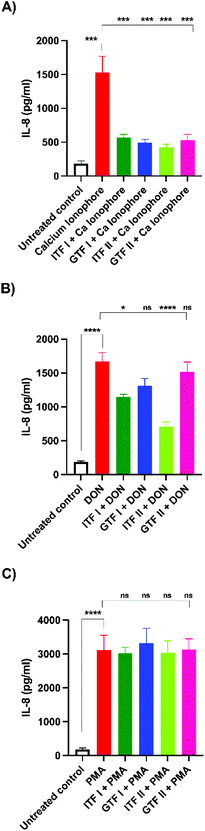
We aimed to investigate whether fructans could protect from the inflammation exerted by the different studied epithelial disruptors.To that end, IL-8 secretion from T84 cells was measured at the end of TEER experiments. The calcium ionophore strongly increased the production of IL-8 by T84 cells from 182 ± 18 pg ml−1 to 1531 ± 96 pg ml−1 (p < 0.001). Interestingly, pre-incubation with all the tested fructans significantly decreased the production of IL-8. Compared with the effect of the positive control (set as 100%), ITF II caused a reduction in IL-8 production of 72% ± 3 (p < 0.001), GTF I attenuated it to 68% ± 3 (p < 0.001), followed by GTF II which decreased it to 66% ± 6 (p < 0.001), while ITF I reduced IL-8 production by T84 cells to 63% ± 3 (p < 0.001) (Fig. 4A).
Incubation with DON increased the IL-8 production from 182 ± 18 pg ml−1 to 1671 ± 131 pg ml−1 (p < 0.0001). Pre-incubation with linear fructans ITF I and ITF II significantly decreased the IL-8 production of T84 cells. ITF I reduced IL-8 production to 31% ± 2 (p < 0.05), and ITF II reduced it to 57.5% ± 4 (p < 0.0001). In contrast, branched chain fructans did not influence the inflammation induced by DON, since there were no statistical differences between the IL-8 production of the positive control and cells pre-treated with these agave fructans (Fig. 4B).
PMA significantly stimulated IL-8 production in T84 cells. Under these conditions, IL-8 increased 17-fold the untreated control (from 182 ± 18 pg ml−1 to 3111 ± 118 pg ml−1). However, none of the studied fructans attenuated the inflammatory effect exerted by PMA, since the production of IL-8 was similar to that of the positive control. In cells pre-incubated with ITF I, the IL-8 value was 3020 ± 62 pg ml−1, 3321 ± 117 pg ml−1 for GTF I, 3034 ± 95 pg ml−1 for ITF II and 3136 ± 84 pg ml−1 for GTF II (Fig. 4C).
4. Discussion
Previous reports from our group and others have demonstrated the beneficial effects of inulin-type fructans on gut epithelial barrier function.16,34 These studies have shown that for ITF, this protection is dependent on the chain length of the fructans. One of the new findings in this study is that the ITF induced protection was dependent on the applied disrupter and specific biochemical pathways. This is not known yet for ITFs. In addition, we studied for the first time the effects of GTFs which are different from those of ITF. The ITFs are composed of fructose units with only β-2→1 linkages, which confer them a linear structure. The GTFs are extracted from agave plants and are more complex than ITFs. Fructans from agave are a mixture of oligosaccharides with β-2→1 and β-2→6 linkages, also known as graminans or agavins. This gives them a branched structure. Moreover, fructans from the neo-series have also been found in the mixtures extracted from agave.26 Their barrier-protective effects have never been studied. Since fructans from agave represent one of the most complex fructan types described to date, we compared their effects on gut epithelial barrier function with those of fructans with a linear structure and by which pathways they protect against barrier disruption. To the best of our knowledge, this is the first study where the protective effects of branched fructans from agave on gut epithelial barrier function are studied. The application of these fructans would be useful especially in regions where the agave plant is endemic, such as in the American continent, from the Canadian/United States border to the Northern region of South America.35To the best of our knowledge, this is the first study where it is investigated whether the protection of the intestinal barrier integrity exerted by fructans is dependent on the intracellular route by which the disruption is induced. To that end, three different disruptors which act via PKC or MAPK signaling were used. We found that the disruption induced via the PKC pathway was attenuated by ITF II, while the disruption induced by MAPK signaling was unaffected. Moreover, the protective effect of GTFs was not similar to that observed for ITFs, since ITF II was the only fructan that protected against the disruptive action of PMA, whereas the branched fructans did not influence the epithelial barrier impairment provoked by this molecule. This again illustrates that dietary fibers can attenuate barrier disruption depending on their chemical structure.
All fructans were effective in preventing barrier disruption induced by the calcium ionophore A23187. This effect was strongest with ITF II, followed by those with GTF I, GTF II, and ITF I. We hypothesize that the observed efficacy of fructans to attenuate A23187 induced barrier dysfunction is through a competition for the cell-binding site of the stressor on the cell membrane. Fructans possess a neutral charge. This neutral charge allows fructans to interact with the lipidic bilayer of cell membranes by the establishment of non-polar interactions.36–38 By this, fructans could occupy the binding sites of A23187, thus preventing, or reducing the binding of this disruptor. The binding mechanism of the calcium ionophore A23187 has been previously described. At physiological pH, the protonated form of A23187 prevails, and can strongly bind to the phospholipidic cellular membrane.39 To induce barrier disruption, A23187 binds and transports divalent calcium ions across the cell membrane, with a concomitant increase of intracellular calcium.40 Such an increase of intracellular calcium has been associated with changes in tight junctions and decreased resistance of T84 monolayers, a process ultimately mediated by PKC.14,27,41Fig. 5 schematically presents our proposed mechanism of protection of epithelial barrier function exerted by fructans.
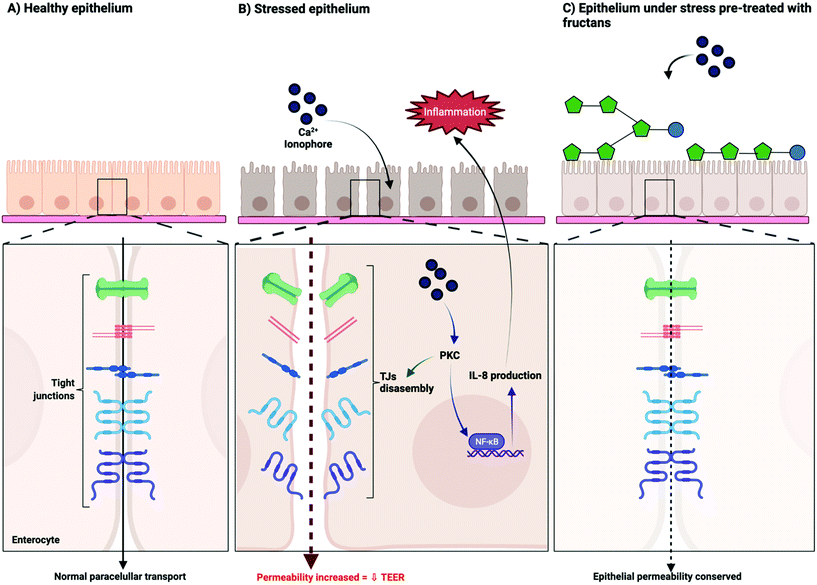 | ||
| Fig. 5 Proposed mechanism of action of the protective effect of linear β(2→1) and branched β(2→1)-β(2→6) fructans on the intestinal epithelial barrier function. | ||
The results were different with PMA induced barrier disruption. Only ITF II had a statistically significant effect on barrier disruption induced by PMA. Our findings indicate that protection against disruption by PMA is exclusive to linear structures of fructans and is chain-length dependent. The exclusive protection of ITF II from the disruptive action of PMA compared to those of other fructans could be explained by the long and linear structure of ITF II. Vereyken et al. studied the requirements for the establishment of interactions between inulins of different DP with mono- and bilayer lipidic systems. They found a strong dependency of the inulin chain length on the capacity to interact with lipid layers in favor of longer inulin that established the strongest interactions. In this study it was also proposed that the linearity and flexibility of furanose rings are the main characteristics responsible for the strong interaction between the long chain inulins and lipids.38 This would be different for GTFs since their branched structure would provoke a more spatially disordered arrangement of their chains, which would decrease their physical contact with the cell membrane, leading to more free spaces for PMA for establishing interactions.
We found that the long chain fructan ITF II was the only fructan that exerted protection from the disruptive action of PMA. This finding seems to contradict the findings of Vogt et al.16 where only non-statistical differences were found with long-chain inulins. Notably, however, differences in ITF dosing might be responsible for that, as Vogt et al. used a hundred-fold lower dose of fructans (0.1 mg ml−1) for pre-treating T84 cells compared to the present study.
The disruptive effects of both PMA and the calcium ionophore A23187 are PKC-dependent42 but the ways in which they lead to PKC-induced disruption are different. The intestinal barrier function depends on the assembly or disassembly of the proteins of the TJs. These TJs are regulated by the phosphorylation of the serine and threonine residues of their proteins. This regulation is achieved by protein kinases such as PKC. PKC is involved in TJ opening. At the cell membrane, PKC can phosphorylate TJ proteins such as occludin. This phenomenon has been observed when TJs are disassembled, and the barrier function is disrupted.42 In the present study, A23187 was used as it facilitates the transport of calcium ions into the cytoplasm. This sudden calcium influx activates the calcium-dependent PKC, leading to the disassembly of the TJs. PMA works differently. PMA is an analogue of diacylglycerol, one of the natural activators of PKC. In contrast to A23187, PMA binds directly to a specific region of PKC which leads to activation.43 This activation induces the translocation of PKC to the cell membrane and the disassembly of TJs.
At a structural level, A23187 and PMA also are different. A23187 is an antibiotic extracted from Streptomyces chartreusensis44 composed of a carboxylic acid with a pyrrole ketone moiety, a substituted benzoxazole group and a spiroketal ring.45 Two molecules of this compound chemically coordinate with a Ca2+ ion, which serves as a cation carrier into the cell.46 However, PMA possesses a phorbol group that contains a long acyl chain, which confers it a highly hydrophobic nature (PubChem database, https://pubchem.ncbi.nlm.nih.gov, consulted on November 9th, 2021). Due to this property, PMA possesses a very high affinity for cellular lipid membranes27 and probably a higher affinity than that of most fructans as fructans can only establish non-polar interactions with the cell membrane. This difference in affinity between A23187 and PMA for the cellular lipid membranes would likely be the reason for the differences in the effectiveness of fructans in protection from the barrier disruption induced by these agents.
We found that the long chain linear fructan ITF II had the strongest protective effect on epithelial barrier integrity when cells were challenged with A23187 and PMA. This confirms the findings of Wu et al. who showed that pre-incubation of Caco-2 Bbe1 monolayers or intestinal organoids exposed to short-chain and long-chain inulins protects the intestinal epithelial barrier from injury caused by enterohemorrhagic Escherichia coli. In addition, they also demonstrated that barrier function was reinforced through a PKC-dependent mechanism by a set of techniques that included the host-kinome with a 282-peptide immune array, gene enrichment analysis, and measurement of PKC isoform activities in the presence and absence of specific-isoform inhibitors.41
The epithelial barrier disruption provoked by the fungal toxin DON was not attenuated by the studied fructans. This toxin is known to decrease the transepithelial resistance via MAPK signaling.30 Our findings, therefore, suggest that fructans cannot prevent the MAPK pathway induced epithelial barrier disruption. This is in accordance with a previous report where the microbiota-independent effects of galacto-oligosaccharides (GOS) on intestinal epithelial integrity and the release of IL-8 were studied and compared with those of FOS and inulin, in combination with GOS or alone. To that end, the different oligosaccharides were added to Caco-2 monolayers, and after 24 hours of incubation the fungal toxin DON was added to the cultures. Afterwards, the TEER and lucifer yellow permeability were quantified to determine the integrity of the intestinal barrier. Only GOS and not the fructans had protective effects on the impairment induced by DON.34
In addition to protecting against barrier disruption, the studied fructans have also been shown to have other health benefits. The prebiotic roles of inulin-type fructans and fructans from agave have been studied by our group and others.22,47,48 These dietary fibers can exert an indirect beneficial effect on intestinal homeostasis by modifying the microbiota composition and supporting the production of its metabolic products such as short chain fatty acids (SCFAs).49 The beneficial effect of SCFAs was demonstrated in vitro by Commane et al. These researchers found that the epithelial barrier of Caco-2 cells was strengthened by the fermentation products of Bifidobacterium Bb 12, which was exposed to ITFs of different molecular sizes.50 This finding was later confirmed by Van den Abbeele et al. who demonstrated that the inulin-fermentation products from adult microbiota strengthened the gut barrier by increasing the TEER in Caco-2 cell monolayers via SCFA production.51 Moreover, our group demonstrated that inulin-type fructans contribute to the maturation of the glycocalyx, which is an important component of the gut epithelium composed of glycans and proteins. The glycocalyx serves as a scaffold for the binding of intestinal commensal microbiota and it strengthens the gut barrier. By this, it avoids the adhesion of pathogen microorganisms. Kong et al. demonstrated that the incubation of Caco-2 cells with inulin-type fructans increased the glycocalyx development by enhancing its thickness and area of coverage of molecules that constitute the glycocalyx such as albumin, heparan sulphate and hyaluronic acid.52
The protection by the studied fructans from the disrupting action of A23187 was also reflected in the attenuation of the inflammatory state of T84 cells, since along with the protection from the epithelial barrier disruption, the concentration of the pro-inflammatory cytokine IL-8 decreased significantly. IL-8, also known as the chemotactic factor CXCL8, is considered an essential pro-inflammatory cytokine that is constitutively expressed by epithelial cells. This chemokine functions as an early warning signal to cells in the underlying lamina propria. When a potential danger is detected, it is released which subsequently leads to inflammatory responses by recruiting immune cells such as neutrophils to the site of injury.53,54 Yu et al. previously demonstrated that A23187 stimulates IL-8 production in T84 cells following the release of intracellular calcium.55 The exacerbated expression of this cytokine is also strongly associated with intestinal inflammatory disorders such as ulcerative colitis.56
The observed regulatory effect of the studied fructans on IL-8 production by T84 cells exposed to A23187 indicates that fructans not only protect from barrier disruption but also contribute to the lowering of inflammatory events as reported before.16,57
In the present study, a 17-fold increase of IL-8 production was observed when stimulating T84 monolayers with PMA. It is known that this epithelial disruptor activates PKC signaling which leads to the up-regulation of IL-8 gene expression in T84 cells.55 The inflammatory effect observed was not influenced by the studied fructans. IL-8 production was also not attenuated by ITF II that protected from PMA induced barrier disruption, indicating that barrier disruption and regulation of inflammatory processes in epithelial cells might involve different pathways. A similar separate effect on barrier function inflammatory processes of fructans was observed for epithelial cells treated with DON. The production of IL-8 in T84 cells challenged with DON was significantly reduced by both studied ITFs in a chain-length dependent manner while barrier disruption was unaffected. These results suggest an MAPK-dependent regulatory role of ITFs on inflammatory processes but the absence of such an effect on the barrier function of gut epithelial cells.
5. Conclusions
To the best of our knowledge, this is the first study where the direct protective effects of GTFs on intestinal epithelial barrier function are addressed. We demonstrated that GTFs, just like ITFs, can prevent gut barrier disruption in a structure and size-dependent fashion. Their effect is most pronounced in calcium ionophore A23187-induced gut epithelial cell barrier disruption. This is different for ITFs that showed efficacy in not only A23187 but also PMA-induced gut barrier disruption. Specifically, the longer chain ITF II had such an effect. Linear ITFs in contrast to branched GTFs were able to reduce A23187 and DON-induced enhanced secretion of IL-8 by gut epithelial cells. These fructans were ineffective in reducing DON induced barrier dysfunction but efficacious in lowering inflammation, which suggest that different pathways are involved in how fructans can influence barrier function and inflammatory processes in gut epithelial cells.Our results may lead to better and broader uses of agave fructans in areas where agave is endemic and chicory inulin is not available such as in Latin America.35 There are several lines of applications of these fructans. They can be applied as a substitute for human milk oligosaccharides, which have health benefits in human milk. We show here that specific fructans might be used to protect against barrier disruption. Tailoring infant formulas supplemented with GTFs would be an option for babies with specific gastrointestinal issues such as premature babies.58 In addition, in adults our findings may lead to new applications. Gastrointestinal discomfort and also barrier disruption are very common issues in adults.59,60 Food with specific fructans may lead to lower frequencies of barrier disruption and lower discomfort. In the pharmaceutical sector, due to their non-digestible characteristics, ITFs have been used as drug stabilizers and carriers, improving drug dissolution and facilitation of controlled release of drugs to specific target sites such as the colon.61 Overall, the present study contributes to a better understanding of the health promoting effects of fructans and their applications in specific chemistries to support specific health benefits.
Author contributions
C. F. L. and P. D. V. designed the study. C. F. L. performed cell-based experiments. X. T. assisted with the cell-based experiments. C. F. L., M. J. L., H. A. S., G. L. V., and P. D. V. wrote the manuscript. P. D. V. supervised and administered the project. All authors have revised, improved, and approved the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was partially financed by the “Programa de Recursos Fiscales para Investigación” of the Instituto Nacional de Pediatría (Grant number 2019/062). C. F. L. was financially supported by the Abel Tasman Talent Program Sandwich PhD from the University of Groningen-University Medical Center Groningen, UG/UMCG in collaboration with Universidad Nacional Autónoma de México, UNAM and CONACyT (#260625).References
- D. Ulluwishewa, R. C. Anderson, W. C. McNabb, P. J. Moughan, J. M. Wells and N. C. Roy, Regulation of tight junction permeability by intestinal bacteria and dietary components, J. Nutr., 2011, 141, 769–776 CrossRef CAS PubMed.
- C. Zihni, C. Mills, K. Matter and M. S. Balda, Tight junctions: from simple barriers to multifunctional molecular gates, Nat. Rev. Mol. Cell Biol., 2016, 17, 564 CrossRef CAS PubMed.
- M. Camilleri, K. Madsen, R. Spiller, B. Van Meerveld and G. Verne, Intestinal barrier function in health and gastrointestinal disease, Neurogastroenterol. Motil., 2012, 24, 503–512 CrossRef CAS PubMed.
- C. M. Van Itallie, J. Holmes, A. Bridges, J. L. Gookin, M. R. Coccaro, W. Proctor, O. R. Colegio and J. M. Anderson, The density of small tight junction pores varies among cell types and is increased by expression of claudin-2, J. Cell Sci., 2008, 121, 298–305 CrossRef CAS PubMed.
- T. Suzuki, Regulation of intestinal epithelial permeability by tight junctions, Cell. Mol. Life Sci., 2013, 70, 631–659 CrossRef CAS PubMed.
- S. Aijaz, M. S. Balda and K. Matter, Tight junctions: molecular architecture and function, Int. Rev. Cytol., 2006, 248, 261–298 CrossRef CAS PubMed.
- L. Shen, C. R. Weber, D. R. Raleigh, D. Yu and J. R. Turner, Tight junction pore and leak pathways: a dynamic duo, Annu. Rev. Physiol., 2011, 73, 283–309 CrossRef CAS PubMed.
- M. F. McCarty and A. Lerner, Perspective: Prospects for nutraceutical support of intestinal barrier function, Adv. Nutr., 2021, 12, 316–324 CrossRef PubMed.
- F. Halter, Mechanisms of gastrointestinal toxicity of NSAIDS, Scand. J. Rheumatol., 1988, 17, 16–21 CrossRef PubMed.
- J. L. Wallace, S. Syer, E. Denou, G. de Palma, L. Vong, W. McKnight, J. Jury, M. Bolla, P. Bercik and S. M. Collins, Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis, Gastroenterology, 2011, 141, 1314–1322 CrossRef CAS PubMed.
- M. Vancamelbeke and S. Vermeire, The intestinal barrier: a fundamental role in health and disease, Expert Rev. Gastroenterol. Hepatol., 2017, 11, 821–834 CrossRef CAS PubMed.
- A. Lerner and T. Matthias, Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease, Autoimmun. Rev., 2015, 14, 479–489 CrossRef CAS PubMed.
- M. Martinez-Medina, J. Denizot, N. Dreux, F. Robin, E. Billard, R. Bonnet, A. Darfeuille-Michaud and N. Barnich, Western diet induces dysbiosis with increased E coli in CEABAC10 mice, alters host barrier function favouring AIEC colonisation, Gut, 2014, 63, 116–124 CrossRef PubMed.
- M. B. Kiewiet, M. I. G. Rodríguez, R. Dekkers, M. Gros, L. H. Ulfman, A. Groeneveld, P. de Vos and M. M. Faas, The epithelial barrier-protecting properties of a soy hydrolysate, Food Funct., 2018, 9, 4164–4172 RSC.
- L. Cheng, R. Akkerman, C. Kong, M. T. Walvoort and P. de Vos, More than sugar in the milk: human milk oligosaccharides as essential bioactive molecules in breast milk and current insight in beneficial effects, Crit. Rev. Food Sci. Nutr., 2020, 1–17 Search PubMed.
- L. Vogt, D. Meyer, G. Pullens, M. M. Faas, K. Venema, U. Ramasamy, H. A. Schols and P. de Vos, Toll-Like Receptor 2 Activation by b2→ 1-Fructans Protects Barrier Function of T84 Human Intestinal Epithelial Cells in a Chain Length–Dependent Manner, J. Nutr., 2014, 144, 1002–1008 CrossRef CAS PubMed.
- M. B. Roberfroid, Introducing inulin-type fructans, Br. J. Nutr., 2005, 93, S13–S25 CrossRef CAS PubMed.
- G. Kelly, Inulin-type prebiotics–a review: part 1, Altern. Med. Rev., 2008, 13(4), 315–329 Search PubMed.
- G. T. C. Delgado, W. M. Tamashiro and G. M. Pastore, Immunomodulatory effects of fructans, Food Res. Int., 2010, 43, 1231–1236 CrossRef CAS.
- M. Wiciński, E. Sawicka, J. Gębalski, K. Kubiak and B. Malinowski, Human milk oligosaccharides: health benefits, potential applications in infant formulas, and pharmacology, Nutrients, 2020, 12, 266 CrossRef PubMed.
- G. López-Velázquez, L. Díaz-García, A. Anzo, M. Parra-Ortiz, B. Llamosas-Gallardo, A. A. Ortiz-Hernández, J. Mancilla-Ramírez, J. M. Cruz-Rubio and P. Gutiérrez-Castrellón, Safety of a dual potential prebiotic system from Mexican agave” Metlin® and Metlos®”, incorporated to an infant formula for term newborn babies: A randomized controlled trial, Rev. Invest. Clin., 2013, 65, 483–490 Search PubMed.
- G. López-Velázquez, M. Parra-Ortiz, I. de la Mora-de la Mora, I. García-Torres, S. Enríquez-Flores, M. A. Alcántara-Ortigoza, A. González-del Angel, J. Velázquez-Aragón, R. Ortiz-Hernández and J. M. Cruz-Rubio, Effects of fructans from Mexican agave in newborns fed with infant formula: a randomized controlled trial, Nutrients, 2015, 7, 8939–8951 CrossRef PubMed.
- M. G. Lopez, N. A. Mancilla-Margalli and G. Mendoza-Diaz, Molecular structures of fructans from Agave tequilana Weber var. azul, J. Agric. Food Chem., 2003, 51, 7835–7840 CrossRef CAS PubMed.
- N. A. Mancilla-Margalli and M. G. López, Water-soluble carbohydrates and fructan structure patterns from Agave and Dasylirion species, J. Agric. Food Chem., 2006, 54, 7832–7839 CrossRef CAS PubMed.
- W. Praznik, R. Löppert, J. M. C. Rubio, K. Zangger and A. Huber, Structure of fructo-oligosaccharides from leaves and stem of Agave tequilana Weber, var. azul, Carbohydr. Res., 2013, 381, 64–73 CrossRef CAS PubMed.
- A. V. Pérez-López, J. Simpson, M. R. Clench, A. D. Gomez-Vargas and J. J. Ordaz-Ortiz, Localization and Composition of Fructans in Stem and Rhizome of Agave tequilana Weber var. azul, Front. Plant Sci., 2021, 11, 2309 Search PubMed.
- Y. H. Tai, J. Flick, S. A. Levine, J. L. Madara, G. W. Sharp and M. Donowitz, Regulation of tight junction resistance in T84 monolayers by elevation in intracellular Ca2+: a protein kinase C effect, J. Membr. Biol., 1996, 149, 71–79 CrossRef CAS PubMed.
- A. Y. Andreeva, J. Piontek, I. E. Blasig and D. I. Utepbergenov, Assembly of tight junction is regulated by the antagonism of conventional and novel protein kinase C isoforms, Int. J. Biochem. Cell Biol., 2006, 38, 222–233 CAS.
- J. Lucioli, P. Pinton, P. Callu, J. Laffitte, F. Grosjean, M. Kolf-Clauw, I. P. Oswald and A. P. F. Bracarense, The food contaminant deoxynivalenol activates the mitogen activated protein kinases in the intestine: interest of ex vivo models as an alternative to in vivo experiments, Toxicon, 2013, 66, 31–36 CrossRef CAS PubMed.
- T. Sergent, M. Parys, S. Garsou, L. Pussemier, Y.-J. Schneider and Y. Larondelle, Deoxynivalenol transport across human intestinal Caco-2 cells and its effects on cellular metabolism at realistic intestinal concentrations, Toxicol. Lett., 2006, 164, 167–176 CrossRef CAS PubMed.
- J. Van De Walle, B. Romier, Y. Larondelle and Y.-J. Schneider, Influence of deoxynivalenol on NF-κB activation and IL-8 secretion in human intestinal Caco-2 cells, Toxicol. Lett., 2008, 177, 205–214 CrossRef CAS PubMed.
- L. Vogt, U. Ramasamy, D. Meyer, G. Pullens, K. Venema, M. M. Faas, H. A. Schols and P. de Vos, Immune modulation by different types of β2→ 1-fructans is toll-like receptor dependent, PLoS One, 2013, 8, e68367 CrossRef CAS PubMed.
- C. Fernández-Lainez, R. Akkerman, M. Oerlemans, M. Logtenberg, H. Schols, L. Silva-Lagos, G. López-Velázquez and P. de Vos, β (2→ 6)-Type fructans attenuate proinflammatory responses in a structure dependent fashion via Toll-like receptors, Carbohydr. Polym., 2022, 277, 118893 CrossRef PubMed.
- P. Akbari, J. Fink-Gremmels, R. H. Willems, E. Difilippo, H. A. Schols, M. H. Schoterman, J. Garssen and S. Braber, Characterizing microbiota-independent effects of oligosaccharides on intestinal epithelial cells: Insight into the role of structure and size, Eur. J. Nutr., 2017, 56, 1919–1930 CrossRef CAS PubMed.
- A. V. Pérez-López and J. Simpson, The Sweet Taste of Adapting to the Desert: Fructan Metabolism in Agave Species, Front. Plant Sci., 2020, 11, 324 CrossRef PubMed.
- R. Demel, E. Dorrepaal, M. Ebskamp, J. Smeekens and B. De Kruijff, Fructans interact strongly with model membranes, Biochim. Biophys. Acta, Biomembr., 1998, 1375, 36–42 CrossRef CAS.
- I. J. Vereyken, V. Chupin, R. A. Demel, S. C. Smeekens and B. De Kruijff, Fructans insert between the headgroups of phospholipids, Biochim. Biophys. Acta, Biomembr., 2001, 1510, 307–320 CrossRef CAS.
- I. J. Vereyken, J. A. Van Kuik, T. H. Evers, P. J. Rijken and B. de Kruijff, Structural requirements of the fructan-lipid interaction, Biophys. J., 2003, 84, 3147–3154 CrossRef CAS.
- M. Kolber and D. Haynes, Fluorescence study of the divalent cation-transport mechanism of ionophore A23187 in phospholipid membranes, Biophys. J., 1981, 36, 369–391 CrossRef CAS PubMed.
- P. W. Reed and H. A. Lardy, A23187: a divalent cation ionophore, J. Biol. Chem., 1972, 247, 6970–6977 CrossRef CAS PubMed.
- R. Y. Wu, M. Abdullah, P. Määttänen, A. V. C. Pilar, E. Scruten, K. C. Johnson-Henry, S. Napper, C. O'Brien, N. L. Jones and P. M. Sherman, Protein kinase C δ signaling is required for dietary prebiotic-induced strengthening of intestinal epithelial barrier function, Sci. Rep., 2017, 7, 1–10 CrossRef CAS PubMed.
- L. González-Mariscal, R. Tapia and D. Chamorro, Crosstalk of tight junction components with signaling pathways, Biochim. Biophys. Acta, Biomembr., 2008, 1778, 729–756 CrossRef PubMed.
- K. Kolczynska, A. Loza-Valdes, I. Hawro and G. Sumara, Diacylglycerol-evoked activation of PKC and PKD isoforms in regulation of glucose and lipid metabolism: a review, Lipids Health Dis., 2020, 19, 1–15 CrossRef PubMed.
- Q. Wu, J. Liang, S. Lin, X. Zhou, L. Bai, Z. Deng and Z. Wang, Characterization of the biosynthesis gene cluster for the pyrrole polyether antibiotic calcimycin (A23187) in Streptomyces chartreusis NRRL 3882, Antimicrob. Agents Chemother., 2011, 55, 974–982 CrossRef CAS PubMed.
- M. O. Chaney, P. V. Demarco, N. D. Jones and J. L. Occolowitz, Structure of A23187, a divalent cation ionophore, J. Am. Chem. Soc., 1974, 96, 1932–1933 CrossRef CAS PubMed.
- G. Smith and W. Duax, Crystal and molecular structure of the calcium ion complex of A23187, J. Am. Chem. Soc., 1976, 98, 1578–1580 CrossRef CAS.
- M. J. Logtenberg, R. Akkerman, R. An, G. D. Hermes, B. J. de Haan, M. M. Faas, E. G. Zoetendal, H. A. Schols and P. de Vos, Fermentation of Chicory Fructo–Oligosaccharides and Native Inulin by Infant Fecal Microbiota Attenuates Pro–Inflammatory Responses in Immature Dendritic Cells in an Infant–Age–Dependent and Fructan–Specific Way, Mol. Nutr. Food Res., 2020, 64, 13 CrossRef PubMed.
- R. Akkerman, M. J. Logtenberg, M. Beukema, B. J. De Haan, M. M. Faas, E. G. Zoetendal, H. A. Schols and P. De Vos, Chicory inulin enhances fermentation of 2′-fucosyllactose by infant fecal microbiota and differentially influences immature dendritic cell and T-cell cytokine responses under normal and Th2-polarizing conditions, Food Funct., 2021, 12, 9018–9029 RSC.
- N. Gasaly, P. De Vos and M. A. Hermoso, Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation, Front. Immunol., 2021, 1807 Search PubMed.
- D. M. Commane, C. T. Shortt, S. Silvi, A. Cresci, R. M. Hughes and I. R. Rowland, Effects of fermentation products of pro-and prebiotics on trans-epithelial electrical resistance in an in vitro model of the colon, Nutr. Cancer, 2005, 51, 102–109 CrossRef CAS PubMed.
- P. Van den Abbeele, B. Taminiau, I. Pinheiro, C. Duysburgh, H. Jacobs, L. Pijls and M. Marzorati, Arabinoxylo-Oligosaccharides and Inulin Impact Inter-Individual Variation on Microbial Metabolism and Composition, Which Immunomodulates Human Cells, J. Agric. Food Chem., 2018, 66, 1121–1130 CrossRef CAS PubMed.
- C. Kong, M. Elderman, L. Cheng, B. J. de Haan, A. Nauta and P. de Vos, Modulation of intestinal epithelial glycocalyx development by human milk oligosaccharides and non–digestible carbohydrates, Mol. Nutr. Food Res., 2019, 63, 1900303 CrossRef PubMed.
- Y. Yu, H. Zeng, S. Lyons, A. Carlson, D. Merlin, A. S. Neish and A. T. Gewirtz, TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism, Am. J. Physiol.: Gastrointest. Liver Physiol., 2003, 285, G282–G290 CrossRef CAS PubMed.
- L. Eckmann, H. C. Jung, C. Schürer-Maly, A. Panja, E. Morzycka-Wroblewska and M. F. Kagnoff, Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8, Gastroenterology, 1993, 105, 1689–1697 CrossRef CAS.
- Y. Yu, C. De Waele and K. Chadee, Calcium-dependent interleukin-8 gene expression in T84 human colonic epithelial cells, Inflammation Res., 2001, 50, 220–226 CrossRef CAS PubMed.
- Y. Zhu, S. Yang, N. Zhao, C. Liu, F. Zhang, Y. Guo and H. Liu, CXCL8 chemokine in ulcerative colitis, Biomed. Pharmacother., 2021, 138, 111427 CrossRef PubMed.
- X. Wang, Y. Li, X. Yang and J. Yao, Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells, Int. J. Biol. Macromol., 2013, 61, 347–352 CrossRef CAS PubMed.
- R. Akkerman, M. M. Faas and P. de Vos, Non-digestible carbohydrates in infant formula as substitution for human milk oligosaccharide functions: Effects on microbiota and gut maturation, Crit. Rev. Food Sci. Nutr., 2019, 59, 1486–1497 CrossRef CAS PubMed.
- M. B. Kiewiet, M. E. Elderman, S. El Aidy, J. G. Burgerhof, H. Visser, E. E. Vaughan, M. M. Faas and P. de Vos, Flexibility of Gut Microbiota in Ageing Individuals during Dietary Fiber Long–Chain Inulin Intake, Mol. Nutr. Food Res., 2021, 65, 2000390 CrossRef CAS PubMed.
- R. An, E. Wilms, M. J. Logtenberg, M. P. van Trijp, H. A. Schols, A. A. Masclee, H. Smidt, D. M. Jonkers and E. G. Zoetendal, In vitro metabolic capacity of carbohydrate degradation by intestinal microbiota of adults and pre-frail elderly, ISME Commun., 2021, 1, 1–12 CrossRef.
- X. Wan, H. Guo, Y. Liang, C. Zhou, Z. Liu, K. Li, F. Niu, X. Zhai and L. Wang, The physiological functions and pharmaceutical applications of inulin: A review, Carbohydr. Polym., 2020, 246, 116589 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |