Open Access Article
Open Access ArticlePomegranate juice alters the microbiota in breast milk and infant stool: a pilot study†
Susanne M.
Henning
 *,
Jieping
Yang
*,
Jieping
Yang
 ,
Ru-Po
Lee
,
Jianjun
Huang
,
Gail
Thames
,
Michelle
Korn
,
Dina
Ben-Nissan
,
David
Heber
and
Zhaoping
Li
,
Ru-Po
Lee
,
Jianjun
Huang
,
Gail
Thames
,
Michelle
Korn
,
Dina
Ben-Nissan
,
David
Heber
and
Zhaoping
Li
Center for Human Nutrition, David Geffen School of Medicine, Department of Medicine, Los Angeles, CA 90095, USA. E-mail: shenning@mednet.ucla.edu; Tel: +1 310-825-9345
First published on 5th May 2022
Abstract
Pomegranate juice (PomJ) contains ellagitannins (ETs) that are metabolized to ellagic acid (EA). Intestinal bacteria convert EA further to urolithins that are absorbed into the circulation and may provide health benefits. PomJ consumption by pregnant women was reported to be neuroprotective for their infants. In order to determine whether EA and metabolites are transferred from breast milk of mothers consuming PomJ to nursing infants, we performed an interventional pilot study and enrolled ten healthy women with full-term, exclusively breast-fed infants, consuming 8 oz. of PomJ daily for two weeks. Breast milk, plasma, urine and stool samples were collected from the mothers and the urine and stool samples from the infants before and after two weeks of PomJ consumption. Samples were analyzed using liquid chromatography-mass spectrometry to identify EA metabolites and 16S rRNA sequencing to determine changes in the microbiota. EA metabolite conjugates (dimethyl EA-glucuronide DMEAG and urolithin A-glucuronide UAG) were found in breast milk, plasma and urine from mothers and in urine of infants after 14 days of PomJ consumption. In addition, urolithin B-glucuronide (UBG) was found in breast milk, plasma and urine from two participants and urine from their infants. PomJ consumption was associated with a significant decrease in breast milk of Lactococcus, Subdoligranulum, and Acinetobacter, while the abundance of Firmicutes/Faecalibacterium increased significantly. In breast milk Escherichia/Shigella was inversely correlated to breast milk UAG. In infant stools, the abundance of Lachnoclostridium and Staphylococcus was increased. Infant stool Blautia was positively correlated to breast milk and mother plasma UBG. This pilot study demonstrates that EA and its metabolites are absorbed by the nursing infant from breast milk, excreted in urine and impact the infant gut microbiome. The concentration of EA metabolites in breast milk increased over time. Phenolic compounds in breast milk could be a way to promote neuroprotective, antioxidant and anti-inflammatory health benefits in infants.
1. Introduction
Pomegranate fruits have been used for centuries in ancient cultures for their medicinal purposes. The pomegranate antioxidant and anti-inflammatory effects led to studies of dietary supplementation with pomegranate juice (PomJ). These studies have shown protective effects of PomJ on cardiovascular disease, inflammatory bowel disease and prostate cancer.1–3Most health benefits of PomJ have been attributed to the presence of ellagitannins (ETs), mainly punicalagins and ellagic acid (EA).4,5 ETs are hydrolyzed in the intestine and absorbed as EA over six hours after administration of PomJ.6–8 After PJ consumption, the remaining ETs and EA are retained unabsorbed in the gut lumen where they interact with the complex intestinal bacterial microflora to form a family of urolithins in the gut.9,10 There are inter-individual variations of ET metabolism producing either urolithin A (UA), urolithin B (UB), isourolithin or no urolithins (i.e. non-producers).11–13 The term “metabotypes” is used to describe the differences among individuals in ET metabolism and these differences have also been related to biomarkers of risk for cardiovascular disease and other chronic diseases of aging.14,15 These differences in metabolism have been associated with differences in the population of intestinal microflora.11 There is growing interest in the potential of dietary supplements for the protection of placental oxidative stress and neuroprotection in the brain. In a study by Chen et al., PomJ supplementation before delivery has been found to reduce oxidative stress and stimulus-induced apoptosis in the placenta.16 In addition, supplementation of pomegranate in the drinking water of pregnant and nursing rodent dams was found to significantly decrease focal brain ischemia and periventricular white matter injury in either inflammatory or hypoxic-ischemic models.17–22
In a small pilot study, we found that EA and UA are present in breast milk and a study by Cortes-Martin et al. found urolithins in breast milk formed from mothers consuming walnuts.23,24 As yet, whether pomegranate metabolites are absorbed by nursing infants and whether the metabotype of the mother is transferred to the infant has not been studied. The present pilot study has been designed to determine whether consumption of PomJ by the nursing mother will increase ET metabolites in the infants’ urine and whether changes in the mothers’ milk and stool microbiota are related to changes in the infants’ stool microbiota.
2. Materials and methods
2.1. Study design
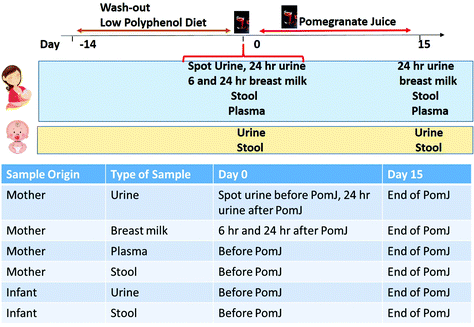
This was an interventional, open label pilot study. Twelve women and infants were recruited at the UCLA Center for Human Nutrition (Fig. 1). The clinical protocol was approved by the Institutional Review Board. The study was registered at clinicaltrials.gov (NCT04341961). All subjects gave written informed consent prior to the start of the study. The mothers were asked to consume 8 oz of PomJ daily for 2 weeks. A dose of 8 oz of PomJ was selected based on previous studies demonstrating health beneficial effects such as antioxidant, cholesterol lowering, anti-inflammatory and delaying prostate cancer recurrence resulting from consumption of 8 oz of PomJ or supplement with equivalent polyphenol content.3,25 Dietary guidelines recommend consuming no more than 1 cup of juice daily.At baseline and after 14 days of PomJ consumption, a blood sample, breast milk sample, 6 and 24 hours urine sample after PomJ consumption and stool sample were obtained from the mother and a spot urine and stool samples were collected from the infants (Fig. 1). Urolithins and EA levels were determined in the maternal plasma, breast milk and urine, and in the infant urine. The stool microbiome was analyzed in breast milk and in the maternal and infant stool samples. Two weeks prior to the baseline visit, participants were asked to consume a low polyphenol diet. The low polyphenol diet includes no more than 3 servings of polyphenol rich fruits/vegetables and no more than 10 g of fiber. A list of foods containing high fiber and phenolic compounds was provided.
Following the pre-study washout phase, mothers consumed 8 oz of PomJ (PomWonderful Co, Los Angeles, CA) daily for two weeks. Participants were asked to return the unused study product to assess compliance. On the first day of PomJ consumption, mothers also collected their breast milk at 6 and 24 hours after consuming the first dose of PomJ. On the last day of PomJ consumption breast milk samples were collected at the same times. Stool samples were obtained from the mother and infants prior to PJ consumption and on the last day of PomJ consumption.
2.2. Participants
Healthy women who were postpartum less than 6 months with a full term baby born vaginally and who were exclusively breast feeding were enrolled in the study. Exclusion criteria included: (a) antibiotics or laxatives use during the 2 months before the study; (b) consuming pre- or probiotics or anti-inflammatory medication; (c) screening laboratory values outside of the normal range that were considered clinically significant for study participation by the investigator; and (d) allergy to pomegranate. There was one screen failure and one drop out for unknown reasons, and ten women completed the study.2.3. Urine collection for infants
In female infants, the area around the urethra was thoroughly washed with soap or cleansing wipes. A plastic bag with an adhesive strip on one end was placed around the genital area. For male infants, the bag was placed over the entire penis and the adhesive was attached to the skin. For females, the bag was placed over the labia on both sides. A diaper was placed over the urine collection bag to help prevent dislocation of the urine bag if the infant is active. Once the infant voided, the bagged specimen was transferred into a collection container for sample evaluation.2.4. Stool collection
An aliquot of a daily stool specimen was collected by the mothers using a standard procedure and stool from the infant diapers was collected. Stool samples were refrigerated and delivered to the UCLA Center for Human Nutrition in a cooler with ice pack (∼4 °C) no later than 12 hours after collection. At the laboratory, stool was aliquoted into smaller vials and frozen immediately and stored at −80 °C. Approximately 1 g of stool was weighed and dried in a vacuum drying oven (15 in Hg) at 80 °C for 48 hours, then weighed again to establish the moisture content so that all counts could be corrected to dry weight.2.5. Reagents and instruments
All solvents were HPLC grade and purchased from Fisher Scientific (Tustin, CA, USA).2.6. Pomegranate metabolites in breast milk, plasma and urine
1 mL urine samples were processed as previously reported.6 5 mL of breast milk was mixed with 20 mL acetonitrile and mixed by vortex. The mixture was centrifuged at 4000 rpm for 10 min. The acetonitrile phase was dried using vacuum (speedvac) and reconstituted in 1 mL water, and further purified using solid-phase extraction (SPE) on C18 cartridges (Waters WAT 036945) and eluted with 2 mL methanol. The elutant was placed in the SpeedVac to dry, reconstituted in 200 μl methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water 1
water 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v), and analyzed for ETs and ET metabolites, dimethylellagic acid glucuronide (DMEAG) and urolithin A-glucuronide (UAG) and urolithin B-glucuronide (UBG) by LC-MS/MS system to determine the presence and levels of as previously reported.6 The concentrations were calculated based on UAG and UBG standards (Toronto Research Chemicals, Canada).
v), and analyzed for ETs and ET metabolites, dimethylellagic acid glucuronide (DMEAG) and urolithin A-glucuronide (UAG) and urolithin B-glucuronide (UBG) by LC-MS/MS system to determine the presence and levels of as previously reported.6 The concentrations were calculated based on UAG and UBG standards (Toronto Research Chemicals, Canada).
2.7. Fecal 16S rRNA gene sequencing and taxonomic analysis
DNA extraction and sequencing of the 16S rRNA gene were performed as previously described by UCLA Microbiome Core.26 Briefly, DNA from stool was extracted using the DNeasy power soil DNA isolation kit with bead beating (Qiagen, Valencia, CA) and from breast milk using the Qiagen DNeasy PowerFood Microbial Kit (Qiagen, Valencia, CA). For stool and breast milk samples, the 16S rRNA gene V4 variable region was amplified and barcoded using F515/R806 primers followed by 250 × 2 bp sequencing on an Illumina HiSeq 2500.26Amplicon sequence variants (ASVs) were identified using DADA2 and annotated them against the SILVA v138 database. Alpha diversity metrics (Chao1 and Shannon index) were calculated after rarefication to a depth of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 sequences per sample and tested for significance using the Wilcoxon signed ranked test at p < 0.05. Beta-diversity was calculated using Bray–Curtis dissimilarity. The relationships of samples across groups were determined by Permutational Multivariate Analysis of Variance (PERMANOVA) using the Adonis command provided by Vegan in R and were displayed via Principal Coordinate Analysis (PCoA) ordination.27 DESeq2 was used to identify abundance changes at the genus level and differences that occurred between day 15 and day 29 samples following the PomJ intervention. P-Values were adjusted for multiple testing using Benjamini Hochberg false discovery rate correction in DESeq2. As Bonferroni correction is often considered overly conservative, we listed genera with p-value < 0.05 and marked those with adjusted p < 0.05 using *. Spearman rank correlations were calculated and plotted as a heatmap between the Pom metabolites (breast milk and blood) and bacteria (breast milk and infant stool) at day 15 using R (version 3.5.2).28 *** p < 0.001, ** p < 0.01, * p < 0.05.
000 sequences per sample and tested for significance using the Wilcoxon signed ranked test at p < 0.05. Beta-diversity was calculated using Bray–Curtis dissimilarity. The relationships of samples across groups were determined by Permutational Multivariate Analysis of Variance (PERMANOVA) using the Adonis command provided by Vegan in R and were displayed via Principal Coordinate Analysis (PCoA) ordination.27 DESeq2 was used to identify abundance changes at the genus level and differences that occurred between day 15 and day 29 samples following the PomJ intervention. P-Values were adjusted for multiple testing using Benjamini Hochberg false discovery rate correction in DESeq2. As Bonferroni correction is often considered overly conservative, we listed genera with p-value < 0.05 and marked those with adjusted p < 0.05 using *. Spearman rank correlations were calculated and plotted as a heatmap between the Pom metabolites (breast milk and blood) and bacteria (breast milk and infant stool) at day 15 using R (version 3.5.2).28 *** p < 0.001, ** p < 0.01, * p < 0.05.
2.8. Statistical analysis
This is the first study to examine the bioavailability of pomegranate metabolites in breast milk and potential impact on the baby. For sample size justification, we considered the formation of metabolites. Our published pilot study in 2 individuals demonstrated that breast milk concentration of dimethyl ellagic acid glucuronide (DMEAG) increased from 0.2 ± 0.3 to 0.7 ± 0.3 (nmol L−1) and urolithin A glucuronide (UAG) increased from 3.0 ± 4.2 to 28.9 ± 10.4 (nmol L−1) after two weeks of 8 oz. of PomJ consumption daily.23 Assuming a correlation of 0.5 between baseline and 2 weeks PomJ consumption, a sample of sensitivity analysis demonstrated that N = 12 participants would provide >80% power to detect the change in DMEAG and UAG.Summary statistics (mean, SD, IQR and frequency distribution) and graphs were generated for baseline demographics and PomJ metabolites in mother urine, plasma, breast milk and infant urine using Graph Pad Prism 6 (San Diego, CA).
3. Results
3.1. Demographic data
The demographic data are shown in Table 1. The maternal age ranged from 30–38 years. The infants’ ages at the time of screening ranged from 1 to 6 months. All mothers were in excellent health without any chronic medical conditions. All infants were delivered vaginally at an academic medical center without any complications. Compliance with PomJ consumption was 99%. Ten mothers with infants completed the study.| Baseline demographics | |
|---|---|
| Data are means ± SD; N = 12. | |
| Mother age (year) | 34 ± 2.8 |
| Infant age at start of PJ (month) | 3.7 ± 1.4 |
| Race/ethnicity of mother | |
| Asian | 17% |
| Black/AA | 0 |
| White | 83% |
| Hispanic | 17% |
| Non-hispanic | 83% |
3.2. Pomegranate metabolite concentration
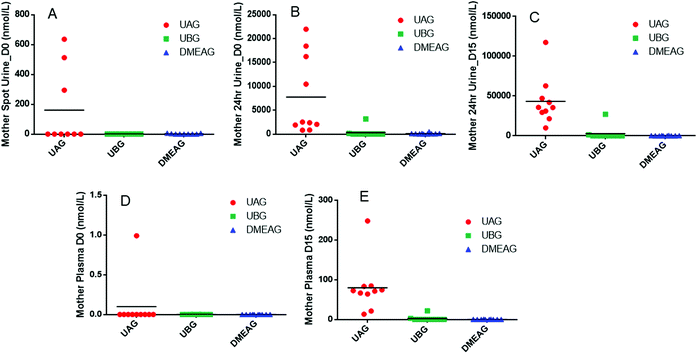
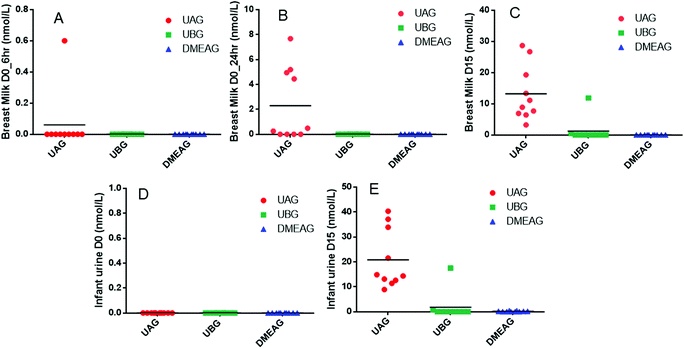
Following the low polyphenol-diet washout phase and prior to the first 8 oz of PomJ consumption, small amounts of Pom metabolites UAG and DMEAG were found in urine from 3 mothers (Fig. 2A). At baseline, 24 hours after the first 8 oz of PomJ consumption, urine samples from all mothers contained UAG (IQR: 12.8 μmol L−1 [0.8–22.0 μmol L−1]) and DMEAG μmol L−1 (IQR: 0.07 μmol L−1 [0.02–0.48 μmol L−1]) (Fig. 2B) and 24 h urine samples from two mothers contained UBG (Fig. 2B). After 14 days of consumption all Pom metabolite concentrations were increased compared to the baseline-24 h urine samples for UAG (IQR: 15.9 μmol L−1 [10–117 μmol L−1]) and DMEAG (IQR: 0.24 μmol L−1 [0.03–0.71 μmol L−1]). Urine samples from two mothers contained higher concentration of UBG.At baseline breast milk from one mother contained a small amount of UAG 6 hours after PomJ consumption (Fig. 3) and breast milk from 6 mothers contained UAG 24 hours after PomJ consumption. At the end of the study, all breast milk samples contained UAG (IQR: 10.7 nmol L−1 [3.2–28.7 nmol L−1]). The same two participants with UBG in urine, also showed UBG (0.5 and 11.9 nmol L−1) in breast milk (Fig. 3). Infant urine samples at baseline did not contain any EA metabolites (Fig. 3), while at end of the study all infant urine samples contained UAG (IQR: 18.1 nmol L−1 [8.9–40.3 nmol L−1]). Infant urine from the same two mothers whose urine and breast milk samples contained UBG also contained UBG (1.8 ± 5.5 nmol L−1 [0–17.5 nmol L−1]). Urine from five other infants contained DMEAG (0.8 and 17.5 nmol L−1) (Fig. 3).
3.3. Breast milk and stool microbiota
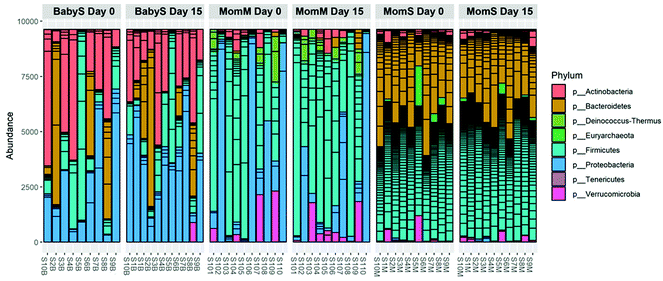
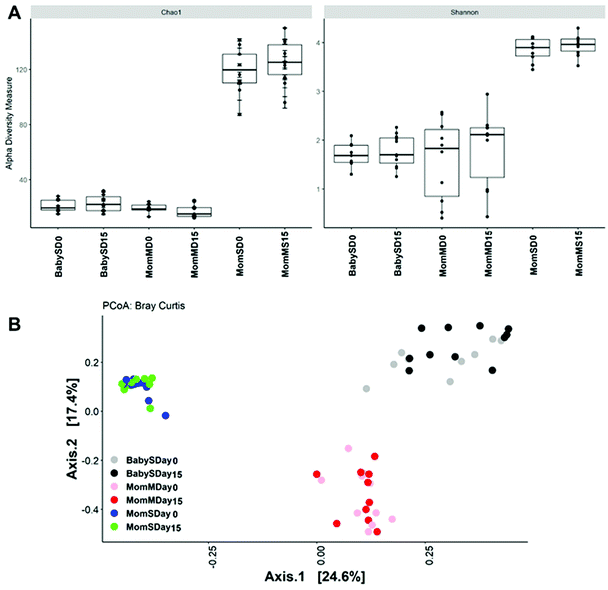
At the phylum level, breast milk microbiota contained Firmicutes > Proteobacteria > Verrucomicrobia > Deinococcus-Thermus > Actinobacteria (Fig. 4). Infant stool samples mainly contained Actinobacteria ≥ Bacteroidetes ≥ Firmicutes ≥ Proteobacteria with large interindividual differences (Fig. 4). The microbiota in the mothers’ stool samples contained mostly Firmicutes, Bacteroidetes and some Verrucomicrobia and Actinobacteria and minor amounts of Proteobacteria and Euryarchaeota (Fig. 4).Alpha diversity was higher in mother stool samples compared to breast milk and infant stool. No change in alpha diversity was observed between baseline and 14 days of PomJ consumption (Fig. 5A and Fig. S2†).
Using Bray Curtis beta diversity analysis a distinct separation between breast milk, infant stool and mother stool clusters was observed (Fig. 5B). But no change in beta diversity was observed between samples collected at baseline and after 14 days of PomJ consumption (Fig. 5, ESI Table S1†).
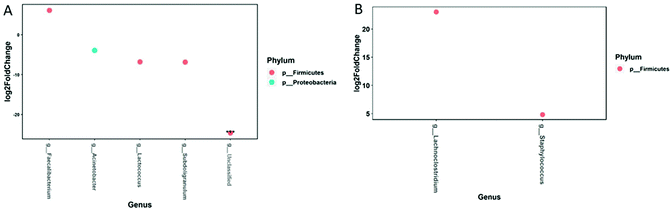
In breast milk, changes in abundance of bacteria comparing before and after the PomJ intake showed a significant decrease in three bacteria from the phylum Firmicutes, genera Lactococcus (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 −6.8), Subdoligranulum (log
2 −6.8), Subdoligranulum (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2: −6.9), unknown (log
2: −6.9), unknown (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 −24) and one phylum Proteobacteria genus Acinetobacter (log
2 −24) and one phylum Proteobacteria genus Acinetobacter (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2: −3.9) (Fig. 6A), while the relative abundance of Firmicutes/Faecalibacterium increased significantly (log
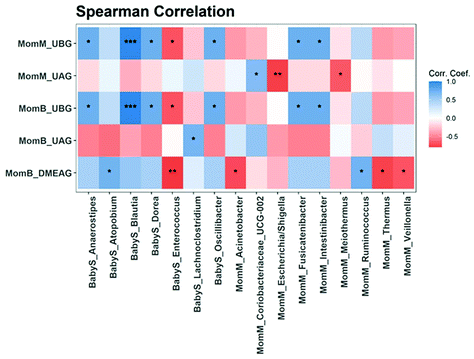
2: −3.9) (Fig. 6A), while the relative abundance of Firmicutes/Faecalibacterium increased significantly (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2: 6.1). A difference in response to PomJ consumption was determined when analyzing the changes in abundance in breast milk and infant stool during the PomJ intake separately for the two metabotypes (Fig. S3 and S4†). We performed a Spearman correlation analysis testing the correlation between the microbiota in breast milk (MomM) and infant stool (BabyS) and Pom metabolites in breast milk and mother blood (MomB) after 14 days of PomJ consumption (Fig. 7). Breast milk genus Escherichia/Shigella was negatively correlated with breast milk UAG (Fig. 7).
2: 6.1). A difference in response to PomJ consumption was determined when analyzing the changes in abundance in breast milk and infant stool during the PomJ intake separately for the two metabotypes (Fig. S3 and S4†). We performed a Spearman correlation analysis testing the correlation between the microbiota in breast milk (MomM) and infant stool (BabyS) and Pom metabolites in breast milk and mother blood (MomB) after 14 days of PomJ consumption (Fig. 7). Breast milk genus Escherichia/Shigella was negatively correlated with breast milk UAG (Fig. 7).
 | ||
| Fig. 6 Microbial genera identified to be significantly different in abundance between day 0 and day 15 after PomJ consumption in (A) breast milk and (B) infant stool. N = 10. | ||
In the infant stool samples, changes in abundance of bacteria occurring during the mothers’ PomJ consumption increased significantly for two bacteria from the phylum Firmicutes, genus Lachnoclostridium (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 23.1) and Staphylococcus (log
2 23.1) and Staphylococcus (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 4.8) (Fig. 6B). Infants with mothers from the metabotype B showed significant differences after PomJ consumption for in 4 bacteria, with 2 bacteria from the phylum Firmicutes (Veillonella log
2 4.8) (Fig. 6B). Infants with mothers from the metabotype B showed significant differences after PomJ consumption for in 4 bacteria, with 2 bacteria from the phylum Firmicutes (Veillonella log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 23.9), 2 Bacteroidetes (Bacteroides log
2 23.9), 2 Bacteroidetes (Bacteroides log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 25.4, Parabacteroides og
2 25.4, Parabacteroides og![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 23.4) and 1 Actinobacter (Bifidobacterium log
2 23.4) and 1 Actinobacter (Bifidobacterium log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 25.0) (Fig. S4†). Spearman correlation analysis demonstrated that infant stool Blautia was positively corelated to breast milk and plasma UBG (Fig. 7). In addition infant stool Enterococcus was inversely related to breast milk UBG, plasma UBG and DMEAG.
2 25.0) (Fig. S4†). Spearman correlation analysis demonstrated that infant stool Blautia was positively corelated to breast milk and plasma UBG (Fig. 7). In addition infant stool Enterococcus was inversely related to breast milk UBG, plasma UBG and DMEAG.
In the maternal stool samples, changes were observed in the bacteria abundance occurring during the PomJ consumption of one bacterium from the phylum Firmicutes (Anaerostipes log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 1.2) that was slightly increased. In the metabotype B group one bacterium from the phylum Firmicutes (Sutterella log
2 1.2) that was slightly increased. In the metabotype B group one bacterium from the phylum Firmicutes (Sutterella log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 8.9) was increased significantly (data not presented).
2 8.9) was increased significantly (data not presented).
4. Discussion
The present study confirms findings from our previous pilot study demonstrating the presence of pomegranate metabolites in breast milk of mothers consuming PomJ. The novel finding in the present study is that Pom metabolites such as UAG, UBG and DMEAG were absorbed by the infants from breast milk and excreted in the infants’ urine. All mothers were UA producers, and two mothers had the intestinal microbiota to form both UA and UB with UBG present in their plasma, urine, breast milk and their children's urine.Pomegranate ellagitannins affect bacteria in the adult gut with acute and chronic exposure11 and could also indirectly affect the microbiome of newborn infants. We propose that EA and urolithins ingested from breast milk are further metabolized by the infant's intestinal bacteria, leading to alteration in the infant's gut microbiota. In addition, there is evidence that gut associated anaerobes are transferred from the mother's intestine to neonates’ intestines via breastfeeding.29–31 In the present study, at the end of the washout phase, the relative abundance of bacteria were similar in breast milk and infant stool samples with abundance of Actinobacteria, Proteobacteria, and Firmicutes, while maternal stool samples were populated mainly with Firmicutes and Bacteroidetes. A published study by Pannaraj et al. showed similar results with main phyla in breast milk being Proteobacteria and Firmicutes and in infant stool being Proteobacteria and Actinobacteria.32
In the present study, changes in microbiota during the PomJ consumption in the breast milk including all participants demonstrated an increase in Faecalibacteria and a decrease in Subdoligranulum and Acinetobacter. In infant stool samples the relative abundance of Lachnoclostridium (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 23.1) and Staphylococcus (log
2 23.1) and Staphylococcus (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 4.8) were increased after 14 days of PomJ consumption (Fig. 6B). In addition, Blautia in infant stool, was positively correlated to breast milk and plasma UBG. Lachnoclostridium and Faecalibacterium are common members of the mammary gland specific microbiome, which may colonize the mammary gland via physiological translocation before lactation.33 They are able to colonize the gut of breastfeeding infants due to growth promotion by milk oligosaccharides.33
2 4.8) were increased after 14 days of PomJ consumption (Fig. 6B). In addition, Blautia in infant stool, was positively correlated to breast milk and plasma UBG. Lachnoclostridium and Faecalibacterium are common members of the mammary gland specific microbiome, which may colonize the mammary gland via physiological translocation before lactation.33 They are able to colonize the gut of breastfeeding infants due to growth promotion by milk oligosaccharides.33
The anaerobic Faecalibacterium, Lachnoclostridium, Blautia and Acinetobacter have been shown to transfer from the maternal to infant gut during lactation.33Faecalibacteria, Subdoligranulum and Lachnoclostridium belong to the class Clostridia and are anaerobic butyrate-producers.29 These bacteria have been found in maternal fecal and breast milk samples.29 Butyrate is a short-chain fatty acid (SCFA) which has beneficial effects on host physiology, including influencing the stability and physiology of the intestinal environment and serving as an energy source for colonocytes. Among several other roles, butyrate helps to maintain the integrity of the mucosa, protecting against cellular inflammation and promoting the removal of dysfunctional cells.34 Formation of butyrate in breast milk might enhance butyrate in the infants’ intestine and provide benefits to the infants as well.
A publication by Jost et al. showed that in breast milk and infant stool samples, the facultative anaerobic genus Staphylococcus, that increased in breast milk, was among the predominant members of the Firmicutes phylum.29,35Lactococcus, which decreased in breast milk belongs to the genus (https://en.wikipedia.org/wiki/Genus) of lactic acid bacteria (https://en.wikipedia.org/wiki/Lactic_acid_bacteria) and is commonly found in breast milk.35 The Sperman analysis showed an inverse correlation between breast milk UAG and breast milk Escherichia/Shigella. The abundance of Escherichia/Shigella in infants stool, has been associated with diarrhea in infants.36 Our findings might demonstrate another benefit of PomJ consumption by nursing mothers.
Interindividual variation in urolithin formation has been observed in association with differences in microbiota populations. In a previous study from our laboratory, based on urinary and fecal content of the POM metabolite urolithin A (UA), we observed that 30% of individuals consuming Pom extract for 3 weeks did not form UA, while 40% formed UA and 30% experienced induction of UA formation during the 3 weeks of Pom extract consumption.11 Selma et al. demonstrated that among healthy individuals 12–30% did not form urolithins and among the urolithin producers 57% formed urolithin A (UA), 31% urolithin B (UB).9,11 In the present study, all participants formed urolithins at 24 h after PomJ consumption. This observation might be unique to breast feeding mothers, who also eat a healthy diet during pregnancy and breast feeding enabling their intestinal microflora to form urolithins. Only small changes were observed in relative bacterial abundance in the mother stool samples from day 0 to 15 demonstrating the stability of the microbiota in healthy individuals.
Two mothers were metabotype B (UB producers), which was associated with a difference in the effects of PomJ consumption on microbiota composition in breast milk and infant stool samples (ESI Fig. S3 and S4†). Future studies with larger numbers of participants are necessary to analyze the difference in microbiota changes between metabotype A and B.
Limitations of this intervention study include the lack of dietary assessment of food intake of the mothers. Knowing the dietary pattern of the participants would assist in interpreting the microbiota data. In addition, the small number of participants limited the number of participants with metabotype B, which reduced the chance of finding differences in the microbiota between the metabotypes.
In the present study the concentrations of urolithins in breast milk and mothers’ urine samples match results from our previous pilot study.23 EA and urolithins are bioavailable, which allows the absorption from breast milk by the infants. However, we found that infant urinary concentration of UAG was ∼2000-fold lower compared to the mother urine.
More and more evidence has indicated that EA as well as its microbial metabolites urolithins are involved in many biological activities.10 The antioxidant and anti-inflammation effects of EA have been studied.10,37 The developing fetal brain is particularly vulnerable to the harmful effects of oxidative stress, with the result often similar to that of neurological injury caused by a hypoxic-ischemic event around the time of birth.38 Neuroprotective potential in infants whose mothers consumed pomegranate juice demonstrated lower risk for brain injury, including any white or cortical grey matter injury compared to placebo.38,39 Demonstrating health benefits of pomegranate consumption on infant health could provide a greater incentive for women to breast feed.
5. Conclusions
In this study, we showed for the first time that ellagic acid metabolites (DMEAG and UAG) can be absorbed by the infant from breast milk and are excreted in the infant urine. However, the Pom metabolite concentration in the infants’ urine is much lower compared to the mothers’ urine. The presence of PomJ metabolites in the infant's intestine was associated with an increase in the relative proportion of Lachnoclostridium (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 23.1) and Staphylococcus (log
2 23.1) and Staphylococcus (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 4.8), that could contribute to the health benefits of maternal Pom J consumption. This study provides important information demonstrating the need for future studies to investigate the impact of phenolic compounds from PomJ on neonatal health and development.
2 4.8), that could contribute to the health benefits of maternal Pom J consumption. This study provides important information demonstrating the need for future studies to investigate the impact of phenolic compounds from PomJ on neonatal health and development.
Author contributions
SMH: Investigation, original draft; DH, ZL and MK: conceptualization, review & editing; JY formal analysis, review & editing; RPL, JH and DBN: methodology; GT: project administration.Conflicts of interest
There are no conflicts of interest to declare.References
- W. Marx, J. Kelly, S. Marshall, S. Nakos, K. Campbell and C. Itsiopoulos, The Effect of Polyphenol-Rich Interventions on Cardiovascular Risk Factors in Haemodialysis: A Systematic Review and Meta-Analysis, Nutrients, 2017, 9, 1345 CrossRef PubMed.
- F. Danesi and L. R. Ferguson, Could Pomegranate Juice Help in the Control of Inflammatory Diseases?, Nutrients, 2017, 9, 958 CrossRef PubMed.
- A. J. Pantuck, C. A. Pettaway, R. Dreicer, J. Corman, A. Katz, A. Ho, W. Aronson, W. Clark, G. Simmons and D. Heber, A randomized, double-blind, placebo-controlled study of the effects of pomegranate extract on rising PSA levels in men following primary therapy for prostate cancer, Prostate Cancer Prostatic Dis., 2015, 18, 242–248 CrossRef CAS PubMed.
- M. I. Gil, F. A. Tomas-Barberan, B. Hess-Pierce, D. M. Holcroft and A. A. Kader, Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing, J. Agric. Food Chem., 2000, 48, 4581–4589 CrossRef CAS PubMed.
- N. P. Seeram, L. S. Adams, S. M. Henning, Y. Niu, Y. Zhang, M. G. Nair and D. Heber, In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice, J. Nutr. Biochem., 2005, 16, 360–367 CrossRef CAS PubMed.
- N. P. Seeram, S. M. Henning, Y. Zhang, M. Suchard, Z. Li and D. Heber, Pomegranate juice ellagitannin metabolites are present in human plasma and some persist in urine for up to 48 hours, J. Nutr., 2006, 136, 2481–2485 CrossRef CAS PubMed.
- S. U. Mertens-Talcott, P. Jilma-Stohlawetz, J. Rios, L. Hingorani and H. Derendorf, Absorption, metabolism, and antioxidant effects of pomegranate (Punica granatum l.) polyphenols after ingestion of a standardized extract in healthy human volunteers, J. Agric. Food Chem., 2006, 54, 8956–8961 CrossRef CAS PubMed.
- M. Larrosa, A. Gonzalez-Sarrias, M. T. Garcia-Conesa, F. A. Tomas-Barberan and J. C. Espin, Urolithins, ellagic acid-derived metabolites produced by human colonic microflora, exhibit estrogenic and antiestrogenic activities, J. Agric. Food Chem., 2006, 54, 1611–1620 CrossRef CAS.
- M. V. Selma, A. Gonzalez-Sarrias, J. Salas-Salvado, C. Andres-Lacueva, C. Alasalvar, A. Orem, F. A. Tomas-Barberan and J. C. Espin, The gut microbiota metabolism of pomegranate or walnut ellagitannins yields two urolithin-metabotypes that correlate with cardiometabolic risk biomarkers: Comparison between normoweight, overweight-obesity and metabolic syndrome, Clin. Nutr., 2018, 37, 897–905 CrossRef CAS PubMed.
- J. C. Espin, M. Larrosa, M. T. Garcia-Conesa and F. Tomas-Barberan, Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: the evidence so far, Evidence-Based Complementary Altern. Med., 2013, 2013, 270418 Search PubMed.
- Z. Li, S. M. Henning, R. P. Lee, Q. Y. Lu, P. H. Summanen, G. Thames, K. Corbett, J. Downes, C. H. Tseng, S. M. Finegold and D. Heber, Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers, Food Funct., 2015, 6, 2487–2495 RSC.
- X. Mora-Cubillos, S. Tulipani, M. Garcia-Aloy, M. Bullo, F. J. Tinahones and C. Andres-Lacueva, Plasma metabolomic biomarkers of mixed nuts exposure inversely correlate with severity of metabolic syndrome, Mol. Nutr. Food Res., 2015, 59, 2480–2490 CrossRef CAS PubMed.
- R. Gonzalez-Barrio, P. Truchado, H. Ito, J. C. Espin and F. A. Tomas-Barberan, UV and MS identification of Urolithins and Nasutins, the bioavailable metabolites of ellagitannins and ellagic acid in different mammals, J. Agric. Food Chem., 2011, 59, 1152–1162 CrossRef CAS.
- F. A. Tomas-Barberan, A. Gonzalez-Sarrias, R. Garcia-Villalba, M. A. Nunez-Sanchez, M. V. Selma, M. T. Garcia-Conesa and J. C. Espin, Urolithins, the rescue of “old” metabolites to understand a “new” concept: Metabotypes as a nexus among phenolic metabolism, microbiota dysbiosis, and host health status, Mol. Nutr. Food Res., 2017, 61(1) DOI:10.1002/mnfr.201500901.
- A. Gonzalez-Sarrias, R. Garcia-Villalba, M. Romo-Vaquero, C. Alasalvar, A. Orem, P. Zafrilla, F. A. Tomas-Barberan, M. V. Selma and J. C. Espin, Clustering according to urolithin metabotype explains the interindividual variability in the improvement of cardiovascular risk biomarkers in overweight-obese individuals consuming pomegranate: A randomized clinical trial, Mol. Nutr. Food Res., 2017, 61(5) DOI:10.1002/mnfr.201600830.
- B. Chen, M. G. Tuuli, M. S. Longtine, J. S. Shin, R. Lawrence, T. Inder and D. Michael Nelson, Pomegranate juice and punicalagin attenuate oxidative stress and apoptosis in human placenta and in human placental trophoblasts, Am. J. Physiol.: Endocrinol. Metab., 2012, 302, E1142–E1152 CrossRef CAS PubMed.
- Y. E. Kim, C. J. Hwang, H. P. Lee, C. S. Kim, D. J. Son, Y. W. Ham, M. Hellstrom, S. B. Han, H. S. Kim, E. K. Park and J. T. Hong, Inhibitory effect of punicalagin on lipopolysaccharide-induced neuroinflammation, oxidative stress and memory impairment via inhibition of nuclear factor-kappaB, Neuropharmacology, 2017, 117, 21–32 CrossRef CAS.
- T. West, M. Atzeva and D. M. Holtzman, Pomegranate polyphenols and resveratrol protect the neonatal brain against hypoxic-ischemic injury, Dev. Neurosci., 2007, 29, 363–372 CrossRef CAS PubMed.
- V. Tapias, J. R. Cannon and J. T. Greenamyre, Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease, Neurobiol. Aging, 2014, 35, 1162–1176 CrossRef PubMed.
- M. A. Ahmed, E. M. El Morsy and A. A. Ahmed, Pomegranate extract protects against cerebral ischemia/reperfusion injury and preserves brain DNA integrity in rats, Life Sci., 2014, 110, 61–69 CrossRef CAS PubMed.
- Z. Amri, A. Ghorbel, M. Turki, F. M. Akrout, F. Ayadi, A. Elfeki and M. Hammami, Effect of pomegranate extracts on brain antioxidant markers and cholinesterase activity in high fat-high fructose diet induced obesity in rat model, BMC Complementary Altern. Med., 2017, 17, 339 CrossRef.
- D. J. Loren, N. P. Seeram, R. N. Schulman and D. M. Holtzman, Maternal dietary supplementation with pomegranate juice is neuroprotective in an animal model of neonatal hypoxic-ischemic brain injury, Pediatr. Res., 2005, 57, 858–864 CrossRef CAS PubMed.
- S. M. Henning, M. B. Wallenstein, N. Weigel, C. Johnson, J. Yang, R.-P. Lee, M. Korn, M. Scala, D. Stevenson, D. Ben-Nissan, D. Heber and Z. Li, Appearance of Ellagic Acid Metabolites from Pomegranate Juice in Breast Milk: A Case Report, Ann. Clin. Case Rep., 2019, 4, 1738 Search PubMed.
- A. Cortes-Martin, R. Garcia-Villalba, I. Garcia-Mantrana, A. Rodriguez-Varela, M. Romo-Vaquero, M. C. Collado, F. A. Tomas-Barberan, J. C. Espin and M. V. Selma, Urolithins in Human Breast Milk after Walnut Intake and Kinetics of Gordonibacter Colonization in Newly Born: The Role of Mothers’ Urolithin Metabotypes, J. Agric. Food Chem., 2020, 68, 12606–12616 CrossRef CAS PubMed.
- M. Rosenblat, N. Volkova, J. Attias, R. Mahamid and M. Aviram, Consumption of polyphenolic-rich beverages (mostly pomegranate and black currant juices) by healthy subjects for a short term increased serum antioxidant status, and the serum's ability to attenuate macrophage cholesterol accumulation, Food Funct., 2010, 1, 99–109 RSC.
- J. P. Jacobs, L. Lin, M. Goudarzi, P. Ruegger, D. P. McGovern, A. J. Fornace Jr., J. Borneman, L. Xia and J. Braun, Microbial, metabolomic, and immunologic dynamics in a relapsing genetic mouse model of colitis induced by T-synthase deficiency, Gut Microbes, 2017, 8, 1–16 CrossRef CAS PubMed.
- J. Yang, Y. Guo, R. Lee, S. M. Henning, J. Wang, Y. Pan, T. Qing, M. Hsu, A. Nguyen, S. Prabha, R. Ojha, G. W. Small, D. Heber and Z. Li, Pomegranate Metabolites Impact Tryptophan Metabolism in Humans and Mice, Curr. Dev. Nutr., 2020, 4, nzaa165 CrossRef CAS PubMed.
- R. Core Team, R: A language and environment for statistical computing, 2018, https://www.R-project.org/.
- T. Jost, C. Lacroix, C. P. Braegger, F. Rochat and C. Chassard, Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding, Environ. Microbiol., 2014, 16, 2891–2904 CrossRef CAS PubMed.
- L. Ruiz, R. Bacigalupe, C. Garcia-Carral, A. Boix-Amoros, H. Arguello, C. B. Silva, M. de Los Angeles Checa, A. Mira and J. M. Rodriguez, Microbiota of human precolostrum and its potential role as a source of bacteria to the infant mouth, Sci. Rep., 2019, 9, 8435 CrossRef PubMed.
- J. M. Rodriguez, The origin of human milk bacteria: is there a bacterial entero-mammary pathway during late pregnancy and lactation?, Adv. Nutr., 2014, 5, 779–784 CrossRef PubMed.
- P. S. Pannaraj, F. Li, C. Cerini, J. M. Bender, S. Yang, A. Rollie, H. Adisetiyo, S. Zabih, P. J. Lincez, K. Bittinger, A. Bailey, F. D. Bushman, J. W. Sleasman and G. M. Aldrovandi, Association Between Breast Milk Bacterial Communities and Establishment and Development of the Infant Gut Microbiome, JAMA Pediatr., 2017, 171, 647–654 CrossRef PubMed.
- C. Qi, J. Zhou, H. Tu, R. Tu, H. Chang, J. Chen, D. Li, J. Sun and R. Yu, Lactation-dependent vertical transmission of natural probiotics from the mother to the infant gut through breast milk, Food Funct., 2022, 13, 304–315 RSC.
- N. Gutierrez and D. Garrido, Species Deletions from Microbiome Consortia Reveal Key Metabolic Interactions between Gut Microbes, mSystems, 2019, 4, e00185-19 CrossRef PubMed.
- L. Fernandez, P. S. Pannaraj, S. Rautava and J. M. Rodriguez, The Microbiota of the Human Mammary Ecosystem, Front. Cell. Infect. Microbiol., 2020, 10, 586667 CrossRef CAS PubMed.
- H. Niu, X. Zhou, X. Zhang, T. Liu, Y. Wu, L. Lyu, C. Liang, S. Chen, P. Gong, J. Zhang, X. Han, S. Jiang and L. Zhang, Breast milk contains probiotics with anti-infantile diarrhoea effects that may protect infants as they change to solid foods, Environ. Microbiol., 2021, 23, 1750–1764 CrossRef CAS PubMed.
- N. P. Seeram, M. Aviram, Y. Zhang, S. M. Henning, L. Feng, M. Dreher and D. Heber, Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States, J. Agric. Food Chem., 2008, 56, 1415–1422 CrossRef CAS PubMed.
- M. M. Ross, S. Cherkerzian, N. D. Mikulis, D. Turner, J. Robinson, T. E. Inder and L. G. Matthews, A randomized controlled trial investigating the impact of maternal dietary supplementation with pomegranate juice on brain injury in infants with IUGR, Sci. Rep., 2021, 11, 3569 CrossRef CAS PubMed.
- L. G. Matthews, C. D. Smyser, S. Cherkerzian, D. Alexopoulos, J. Kenley, M. G. Tuuli, D. M. Nelson and T. E. Inder, Maternal pomegranate juice intake and brain structure and function in infants with intrauterine growth restriction: A randomized controlled pilot study, PLoS One, 2019, 14, e0219596 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00280a |
| This journal is © The Royal Society of Chemistry 2022 |