Open Access Article
Open Access ArticleMetabolic regulation of (−)-epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid on the glucose uptake, lipid accumulation and insulin signalling in cardiac H9c2 cells
Esther
García-Díez
 a,
María Elvira
López-Oliva
b,
Jara
Pérez-Jiménez
a,
María Elvira
López-Oliva
b,
Jara
Pérez-Jiménez
 a,
María Angeles
Martín
a,
María Angeles
Martín
 ac and
Sonia
Ramos
ac and
Sonia
Ramos
 *a
*a
aDepartment of Metabolism and Nutrition, Institute of Food Science and Technology and Nutrition (ICTAN), Consejo Superior de Investigaciones Científicas (CSIC), José Antonio Novais 10, Ciudad Universitaria, 28040 Madrid, Spain. E-mail: s.ramos@ictan.csic.es; Fax: +34 91 549 36 27; Tel: +34 91 544 56 07
bSección Departamental de Fisiología. Facultad de Farmacia, Universidad Complutense de Madrid (UCM), Spain
cCentro de Investigación Biomédica en Red de Diabetes y Enfermedades Metabólicas Asociadas (CIBERDEM), ISCIII, Spain
First published on 26th April 2022
Abstract
Epicatechin (EC) and main colonic phenolic acids derived from flavonoid intake have been suggested to exert healthful effects, although their mechanism of action remains unknown. Heart damage is highly prevalent in metabolic diseases, and the failure of this organ is a major cause of death worldwide. In this study, the modulation of the energy metabolism and insulin signalling by the mentioned compounds in cardiac H9c2 cells was evaluated. Incubation of cells with EC (1–20 μM) and 2,3-dihydroxybenzoic acid (DHBA, 10 μM) reduced glucose uptake, and both compounds decreased lipid accumulation at concentrations higher than 0.5 μM. EC and DHBA also increased the tyrosine phosphorylated and total insulin receptor (IR) levels, and activated the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT) pathway in cardiac H9c2 cells. Interestingly, EC and DHBA did not modify glucose transporters (SGLT-1 and GLUT-1) levels, and increased GLUT-4 values. In addition, EC and DHBA decreased cluster of differentiation 36 (CD36) and fatty acid synthase (FAS) values, and enhanced carnitine palmitoyl transferase 1 (CPT1) and proliferator activated receptor α (PPARα) levels. By using specific inhibitors of AKT and 5′-AMP-activated protein kinase (AMPK), the participation of both proteins in EC- and DHBA-mediated regulation on glucose uptake and lipid accumulation was shown. Taken together, EC and DHBA modulate glucose uptake and lipid accumulation via AKT and AMPK, and reinforce the insulin signalling by activating key proteins of this pathway in H9c2 cells.
1. Introduction
Insulin signalling is very important for maintaining the energy metabolism balance and all physiological functions. Indeed, pathologies such as diabetes, obesity and cardiovascular disease, which are leading causes of morbidity and mortality worldwide,1,2 raise the risk of heart failure that has been associated to a generalized insulin resistance.3,4 Heart is one of the major energy consumers in the body, and this metabolic equilibrium constitutes a critical aspect in this organ. Under physiological conditions, cardiomyocyte energy derives from fatty acids (60–70%), glucose (30–40%) and lactate (10%).5At molecular level, in the heart, fatty acids are mainly transported into the cardiomyocyte by cluster of differentiation 36 (CD36), and glucose mainly enters into the cells via the glucose transporters-4 (GLUT-4) and -1 (GLUT-1).5–7 This transport of fatty acids and glucose is regulated by insulin and 5′-AMP-activated protein kinase (AMPK), which is a cellular energy major sensor.8 However, during a pathological situation, such as diabetes, there is an increased fatty acid uptake and oxidation with a concomitant reduced glucose metabolism.9 This accumulation of lipids leads to an attenuation of the insulin signalling pathway, decreased levels of GLUT-4 and GLUT-1, increased values of the sodium-glucose cotransporter 1 (SGLT-1), and diminished insulin-stimulated glucose uptake.7 Indeed, both lipid accumulation and insulin resistance contribute to alter the energy metabolism, which at long-term may lead to the heart failure.3 In addition, it has recently been reported that insulin resistance also affects the activation of contraction-stimulated glucose uptake, being AMPK a key protein for this metabolic process,8 and CD36 has been considered as a marker of this pathological situation.10 Consequently, strategies to stimulate the glucose uptake, reduce lipid accumulation, and/or reinforce the insulin route may be helpful to contribute to control the energy metabolism balance and protect against the loss of functionality in cardiac cells.
Natural compounds are considered good candidates for the prevention and/or treatment of chronic diseases because of their lack of toxicity and their ability to regulate multiple targets, which in turn is responsible for a broad range of potentially beneficial biological activities.11–14 Among these natural compounds, flavanols, such as (−)-epicatechin (EC) and derived proanthocyanidins, are abundant in cocoa, tea, grapes, carob, and other fruits and vegetables. However, it should be considered that more than 80% flavanols ingested in the diet are metabolized by the colonic microbiota producing different phenolic metabolites.15–17 In this regard, mono- and di-hydroxylated phenylpropionic and phenylacetic acids are the main colonic phenolic acids derived from the intake of flavanol-rich food, and have been detected in human serum at the micromolar range.15,17,18 Therefore, in order to evaluate the impact of polyphenols on health, the contribution of both food polyphenols and metabolites (including low molecular weight phenolic acids derived from the colonic intestinal microbiota) should be considered.12,15,17,19 In this line, to the best of our knowledge, whether EC and the microbial phenolic metabolites derived from flavanol intake have an effect on cardiac cells is unknown, and the mechanism for their potential preventive activities related to the glucose uptake and lipid accumulation, as well as insulin signalling in the heart has not been analysed.
In this context, the aim of the present study was to investigate the effects of EC and the microbial-derived flavanol metabolites 2,3-dihydroxybenzoic acid (DHBA), 3,4-dihydroxyphenylacetic acid (DHPAA), and 3-hydroxyphenylpropionic acid (HPPA) on key regulatory mechanisms related to the energy metabolism balance and insulin signalling in cardiac H9c2cells.
2. Materials and methods
2.1. Materials and chemicals
(−)-EC (>95% of purity), DHBA (≥99% of purity), DHPAA (>98% of purity), HPPA (>98% of purity), LY294002 (2-(4-morpholinyl)-8-phenyl-1(4H)-benzopyran-4-one hydrochloride), and compound C (6-[4-(2-piperidin-1-ylethoxy)phenyl]-3-pyridin-4-ylpyrazolo[1,5-a]pyrimidine) were purchased from Sigma Chemical (Madrid, Spain). The fluorescent probe D-glucose, 2-deoxy-2-((7-nitro-2,1,3-benzoxadiazol-4-yl)amino) (2-NBDG) was from Molecular Probes (Invitrogen, Madrid, Spain). Anti-protein kinase B (AKT) and anti-phospho-Ser473-AKT detecting levels of phospho- and total AKT, anti-glycogen synthase kinase-3 α/β (GSK3) and anti-phospho-GSK3 α/β recognizing phosphorylated Ser21/9 of GSK3, anti-glycogen synthase (GS) and anti-phospho-GS recognizing phosphorylated Ser641 of GS, as well as anti-AMPK and anti-phospho-Thr172-AMPK, anti-fatty acid synthase (FAS) and anti-β-actin were obtained from Cell Signalling Technology (Izasa, Madrid, Spain). Anti-phospho-insulin receptor-β (IRβ) recognizing levels of phosphorylated Tyr1150/1151 of IR, anti-IR β, anti-SGLT-1, anti-CPT1, and anti-PPARα were purchased from Santa Cruz (sc-81500, sc-711, sc-98974, sc-98834, and sc-9000, respectively, Qimigen, Madrid, Spain). Anti-GLUT-1 and anti-GLUT-4 were from Millipore (Madrid, Spain), and anti-CD36 was from Abcam (Cambridge, United Kingdom). Materials and chemicals for electrophoresis were from BioRad (BioRad Laboratories S.A., Madrid, Spain). Cell culture dishes, glutamine and cell culture medium were from Falcon (Cajal, Madrid, Spain) and Cultek (Madrid, Spain), respectively.2.2. Cell culture and treatments
Embrionic ventricular rat heart-derived H9c2 cardiomyoblasts (kindly provided by Prof. Dr Victoria Cachofeiro, Facultad de Medicina, UCM, Madrid, Spain) were grown in DMEM medium (24.5 mM D-glucose) supplemented with 10% fetal bovine serum (FBS) and antibiotics (penicillin and streptomycin [50 mg L−1]). After 48 h, cells were differentiated into adult cardiomyocytes for 6 days in DMEM supplemented with 1% FBS and 10 nM retinoic acid according to a previously described method.20,21 The medium was replaced every 48 h for 6 days. On day 7, the medium was changed to DMEM (5.5 mM D-glucose) containing 1% FBS and 2 mM glutamine, and the culture was continued. Next day, and always at the same daytime, the experimental treatment was carried out with different concentrations of EC, DHBA, DHPAA or HPPA (0.1–20 μM) diluted in DMEM containing 5.5 mM D-glucose, 2 mM glutamine and 1% FBS during 24 h. For the time-course experiments, cells were treated with 1 μM EC or 10 μM DHBA and then harvested at different times (1, 2, 4, 8, 18 and 24 h). In the experiments with the inhibitors, cells were preincubated with 10 μM LY294002 or 10 μM compound C for 1 h prior to 24 h of EC or DHBA treatment.2.3. Cell viability assay
Cell viability was determined by using the crystal violet assay.22 H9c2 cells were seeded (0.5 × 104 cells per well) in 24-well plates, and after the treatment were incubated with crystal violet (0.2% in ethanol) for 20 min. Plates were rinsed with distilled water, allowed to dry, and 1% sodium dodecylsulfate was added. The absorbance of each well was measured using a microplate reader at 570 nm.2.4. Glucose uptake
Cellular glucose uptake was quantified by the 2-NBDG assay using a microplate reader. The analysis takes advantage of the conversion of 2-NBDG into a non-fluorescent derivative (2-NBDG metabolite) after being taken by the cells. The assay has been described elsewhere.23,24 In brief, cells were plated in 24-well plates at a rate of 0.5 × 104 cells per well and after the treatments, 2-NBDG was added at 10 μM final concentration and incubated for 1 h at 37 °C. Then, cells were washed twice with PBS, serum-free medium was added and the fluorescence intensity was immediately measured in a microplate reader at an excitation wavelength of 485 nm and an emission wavelength of 530 nm.2.5. Oil red-O staining in cells
To measure cellular neutral lipid droplet accumulation, H9c2 cells were stained by the oil red-O method.25 H9c2 cells were seeded in 24-well plates (0.5 × 104 cells per well) and after the treatment were fixed with 3.7% formaldehyde for 20 min at room temperature. Later, cells were washed with PBS and stained with oil red-O solution (0.2 mg mL−1) for 2 h. Then, H9c2 cells were washed with PBS to remove unincorporated dye, isopropanol added and absorbance measured in a microplate reader at 500 nm.2.6. Preparation of cell lysates and western blot analysis
Cells were lysed in a buffer containing 25 mM HEPES (pH 7.5), 0.3 M NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.5 mM dithiothreitol, 0.1% Triton X-100, 200 mM β-glycerolphosphate, 0.1 mM Na3VO4, 2 μg mL−1 leupeptin and 1 mM phenylmethylsulfonyl fluoride at 4 °C. The supernatants were collected, assayed for protein concentration by using the Bio-Rad (Bio-Rad, Madrid, Spain) protein assay kit according to the manufacture's specifications, aliquoted and stored at −80° C until used for western blot analyses.Equal amounts of proteins were separated by SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride filters (Bio-Rad, Madrid, Spain). Membranes were probed with the corresponding primary antibody followed by incubation with peroxide-conjugated anti-rabbit (GE Healthcare, Madrid, Spain) or anti-mouse (Sigma, Madrid, Spain) immunoglobulin. All primary antibodies were used at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, except for IR, AKT, GS and AMPK that were employed at 1
1000, except for IR, AKT, GS and AMPK that were employed at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2000, β-actin was utilised at 1
2000, β-actin was utilised at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5000, and p-IR and CD36 at 1
5000, and p-IR and CD36 at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500. Blots were developed with the ECL system (GE Healthcare, Madrid, Spain). Normalization of western blot was ensured by β-actin and bands were quantified using a scanner and accompanying software.
500. Blots were developed with the ECL system (GE Healthcare, Madrid, Spain). Normalization of western blot was ensured by β-actin and bands were quantified using a scanner and accompanying software.
2.7. Statistics
Prior to statistical analysis, data were tested for homogeneity of variances by the test of Levene; for multiple comparisons, one-way ANOVA was followed by the Tukey test when variances were homogeneous or by the Tamhane test when variances were not homogeneous. P < 0.05 was considered significant. A SPSS version 27.0 program has been used.3. Results
3.1. Effects of EC and colonic phenolic metabolites on cell viability, glucose uptake and lipid accumulation
To discard the potential toxic effects of EC, DHBA, DHPAA and HPPA, and to analyse whether these phenolic compounds can modulate both glucose uptake and lipid accumulation, H9c2 cardiomyocytes were exposed to a range of concentrations (0.1–20 μM) for 24 h, which is recommended for in vitro studies, as similar values have been reported in biological fluids after the flavanol intake.15,26Treatment of H9c2 cells with EC or any of the mentioned microbial phenolic metabolites did not change the cell viability, indicating that the concentrations selected for the study did not damage cell integrity during the period of incubation (Table 1). Moreover, the glucose uptake was increased and lipid accumulation was decreased after treating the cells with EC and DHBA, but they did not change with DHPAA or HPPA (Table 1). Thus, the glucose uptake enhancement was detected at 1–20 μM EC and 10 μM DHBA, whilst the decrease in lipid accumulation was observed at 1–20 μM for both phenolic compounds. Thus, for subsequent experiments 1–10 μM EC and DHBA were selected, as these doses comprised the lowest and realistic range of concentrations22 for both compounds showing an effect on glucose uptake and lipid accumulation without causing any damaging effect in the cells, although a regulatory effect exerted by any other concentration tested on the cell signalling pathways evaluated could not be ruled out.
| Crystal violet staining (% of controls) | Glucose uptake (% of controls) | Oil red O staining (% of controls) | |
|---|---|---|---|
| C | 100.4 ± 4.7a | 100.0 ± 6.7a | 100.0 ± 6.3a |
| EC (μM) | |||
| 0.1 | 98.7 ± 4.2a | 101.2 ± 6.3a | 99.9 ± 6.1a |
| 0.25 | 100.3 ± 6.1a | 99.5 ± 5.5a | 100.2 ± 6.9a |
| 0.5 | 97.6 ± 5.0a | 101.9 ± 6.1a | 97.9 ± 6.2a |
| 1 | 97.2 ± 5.7a | 114.6 ± 6.0b | 81.5 ± 9.6b |
| 5 | 100.6 ± 8.8a | 115.9 ± 7.2b | 74.9 ± 8.3b |
| 10 | 98.9 ± 6.3a | 117.2 ± 5.2b | 84.4 ± 7.1b |
| 20 | 99.6 ± 6.8a | 110.5 ± 8.6ab | 85.7 ± 9.0b |
| DHBA (μM) | |||
| 0.1 | 101.4 ± 4.8a | 100.5 ± 6.7a | 101.3 ± 6.2a |
| 0.25 | 100.8 ± 5.3a | 100.4 ± 7.2a | 98.9 ± 7.4a |
| 0.5 | 102.5 ± 6.7a | 106.5 ± 8.5a | 97.4 ± 7.9a |
| 1 | 102.1 ± 4.2a | 105.2 ± 5.8a | 83.3 ± 3.5b |
| 5 | 102.7 ± 3.9a | 105.3 ± 5.3a | 78.3 ± 4.8b |
| 10 | 106.2 ± 6.3a | 118.1 ± 5.6b | 80.3 ± 7.5b |
| 20 | 99.4 ± 5.6a | 92.1 ± 9.9a | 85.3 ± 8.2b |
| DHPAA (μM) | |||
| 0.1 | 99.8 ± 6.1a | 102.2 ± 6.8a | 98.9 ± 9.9a |
| 0.25 | 100.4 ± 7.1a | 100.3 ± 5.5a | 103.5 ± 8.1a |
| 0.5 | 99.7 ± 5.7a | 106.4 ± 7.2a | 97.5 ± 7.5a |
| 1 | 97.9 ± 6.3a | 90.7 ± 9.0a | 96.9 ± 8.7a |
| 5 | 102.4 ± 5.7a | 102.7 ± 5.1a | 98.1 ± 4.0a |
| 10 | 101.0 ± 7.8a | 109.2 ± 9.1a | 97.7 ± 6.8a |
| 20 | 100.1 ± 5.0a | 101.7 ± 8.9a | 111.8 ± 8.1a |
| HPPA (μM) | |||
| 0.1 | 98.9 ± 7.3a | 101.2 ± 7.8a | 100.7 ± 4.8a |
| 0.25 | 100.1 ± 6.4a | 100.3 ± 6.1a | 99.8 ± 6.3a |
| 0.5 | 96.0 ± 8.5a | 106.8 ± 5.3a | 102.4 ± 7.0a |
| 1 | 96.3 ± 7.9a | 106.5 ± 3.4a | 93.1 ± 6.1a |
| 5 | 97.3 ± 7.9a | 106.0 ± 4.5a | 96.7 ± 2.5a |
| 10 | 97.5 ± 6.2a | 96.2 ± 8.0a | 101.2 ± 6.7a |
| 20 | 96.1 ± 9.5a | 103.8 ± 3.4a | 101.2 ± 7.4a |
3.2. Effects of EC and DHBA on tyrosine phosphorylation and protein levels of IR
Molecular mechanisms that regulate cardiac insulin signalling are not fully understood. Downregulation of the insulin-signalling pathway contributes to the insulin resistance in the heart, which is associated with altered energy metabolism and functionality.3,5 The modulation of IR constitutes the first step for recruiting and activating crucial downstream pathways for the uptake and use of glucose.3 To evaluate the potential modulatory effect of EC and DHBA on tyrosine phosphorylated and total levels of IR, H9c2 cells were incubated for 24 h with 1–10 μM of EC or DHBA.Treatment with all selected concentrations of EC (1–10 μM) and the highest dose of DHBA equally increased phosphorylated and total IR protein levels (Fig. 1). However, the lowest concentrations of DHBA (1 and 5 μM) did not modify the levels of the mentioned proteins, and they showed comparable values of phosphorylated and total IR to those of controls (untreated cells) (Fig. 1).
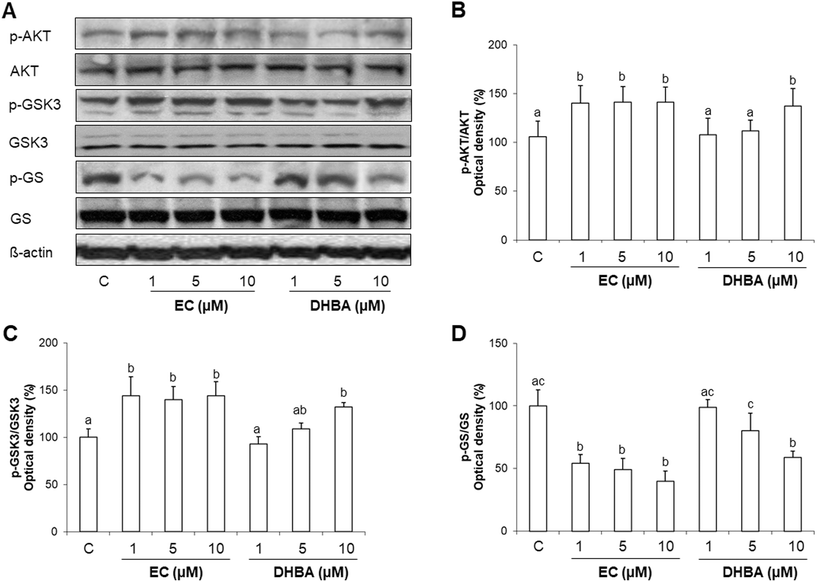
3.3. Effects of EC and DHBA on AKT, GSK3 and GS phosphorylation
IR activation leads to the stimulation of downstream proteins of the insulin route. Once AKT is activated, it promotes GSK3 phosphorylation that removes the repression of GS to facilitate the glycogen synthesis by its phosphorylation and inactivation.3,12 Therefore, phosphorylated and total levels of AKT, GSK3 and GS were assayed by western blot.As shown in Fig. 2A–C, EC (at all concentrations tested) and 10 μM DHBA enhanced the phosphorylated levels of AKT and GSK3. In agreement with these results, 1–10 μM EC and 10 μM DHBA diminished the phosphorylated levels of GS (Fig. 2A and D). There was no difference in the total levels of AKT, GSK3 and GS.
3.4. Effects of EC and DHBA on glucose transporter protein levels
Glucose uptake depends on the glucose transporters of the cells.7 Since the glucose uptake was regulated by EC and DHBA in H9c2 cells, the levels of the main glucose transporters in cardiomyocytes were evaluated after incubating the cells with EC or DHBA (1–10 μM) for 24 h.Treatment of H9c2 cells with the concentrations selected for EC or DHBA did not affect the expression levels of SGLT-1 and GLUT-1 (Fig. 3). However, EC or DHBA incubation increased GLUT-4 levels. DHBA (10 μM) induced the highest enhancement in the levels of GLUT-4 in comparison to the other concentrations of DHBA, and EC equally augmented the levels of GLUT-4 with all doses assayed (Fig. 3A and D).
3.5. Effect of EC and DHBA on AMPK levels
AMPK is a master sensor of the cellular energy balance in mammalian cells.8 Since EC and DHBA modulate glucose uptake and lipid accumulation in H9c2 cells, the expression levels of AMPK were analysed after a 24 h EC- or DHBA-treatment (1–10 μM).Fig. 4 shows that a 24 h treatment with EC or DHBA induced an increase in AMPK phosphorylated levels. EC induced a similar activation of AMPK with all concentrations tested, whereas DHBA (10 μM) displayed higher levels of p-AMPK than the lowest concentrations of DHBA, which presented similar values to those of control cells. The protein levels of total AMPK were not modified by EC or DHBA treatment.
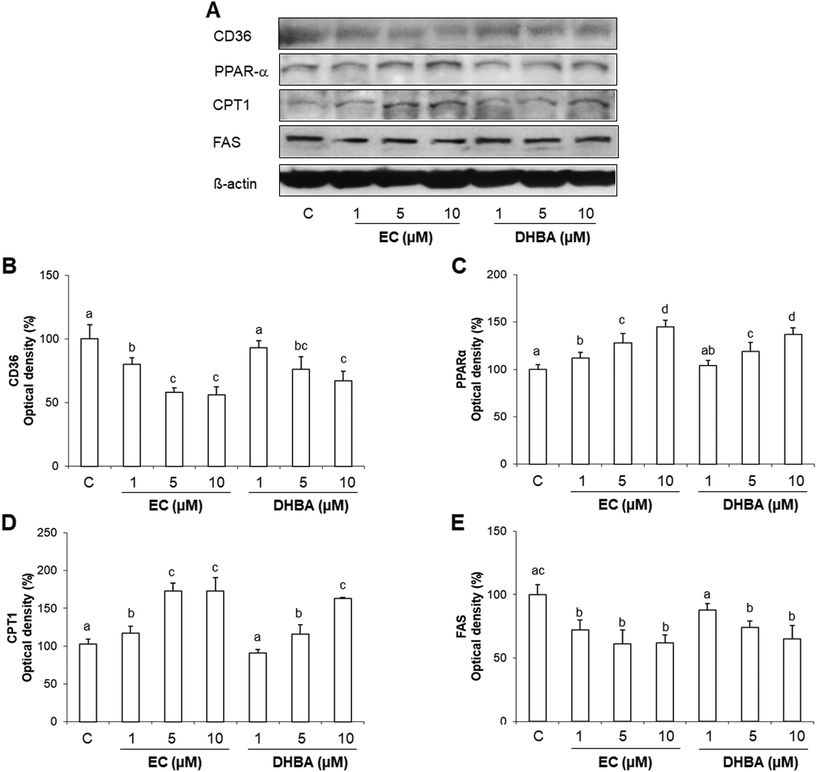
3.6. Effect of EC and DHBA on CD36, FAS, PPARα and CPT1 levels
CD36 promotes the lipid internalization. Then, short- and medium-chain fatty acids can enter the mitochondria directly, whilst long-chain acyl CoA requires carnitine palmitoyl transferase 1 (CPT1), the key regulatory enzyme that determines the rates of fatty acid oxidation.27,28 Moreover, 20% of fatty acids taken up by the heart are used for the synthesis of triacyglyerols, and these endogenous triacyglyerols contribute to the proliferator activated receptor α (PPARα) activation and to the basal fatty acid oxidation.27,28De novo lipid synthesis is regulated by fatty acid synthase (FAS).27,28 Then, to continue with the study of the cardiac metabolism, crucial proteins involved in the lipid transport, synthesis and degradation, such as CD36, CPT1, PPARα and FAS were evaluated by Western blot after a 24 h EC- or DHBA-treatment (1–10 μM).Levels of cardiac CD36 were dose-dependently diminished in EC-treated cells, whilst DHBA just decreased the values of this transporter at the highest concentrations tested (5 and 10 μM) (Fig. 5A and B). EC and DHBA induced a dose-dependent activation of PPARα (Fig. 5A and C), and CPT1 showed increased values after the 24 h treatment with all doses of EC tested, and the highest concentrations of DHBA (5 and 10 μM) (Fig. 5A and C). Similarly, all selected concentrations of EC, and 5 and 10 μM DHBA induced a comparable reduction in FAS levels, whereas the lowest concentration of DHBA did not modify the values of the mentioned protein (Fig. 5A and E).
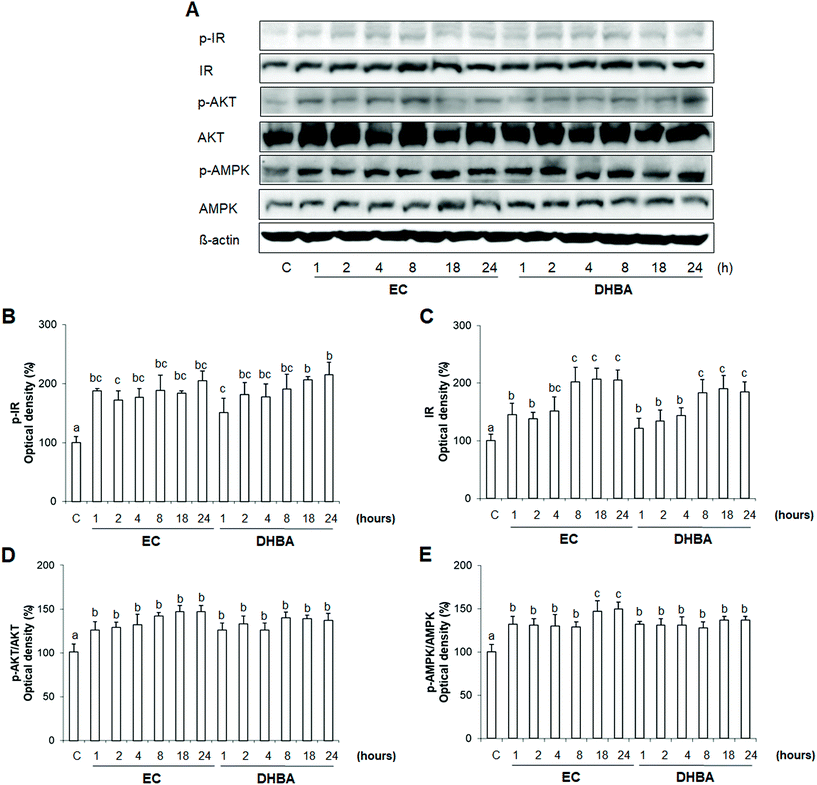
3.7. Time-course effect of EC and DHBA activation on IR, AKT and AMPK
To evaluate how EC and DHBA-induced activations of insulin signalling and AMPK were modulated through the time, H9c2 cells were treated with a fixed concentration of both phenolic compounds that clearly activated the mentioned proteins, i.e., 1 μM EC and 10 μM DHBA for different times (0–24 h). Then possible alterations either in total and/or phosphorylated levels of different proteins were analysed.EC and DHBA evoked an increase in total and phosphorylated levels of IR at all time-points analysed (Fig. 6A–C). IR values were augmented after 1 h-incubation with EC or DHBA, but after 8 h of treatment, levels were even higher, remaining these enhanced values up to 24 h.
As shown in Fig. 6A, D and E, total AKT and AMPK protein levels did not change during the incubation with EC or DHBA, whereas the increased phosphorylated values of both proteins were evident at the earliest time-point of treatment (1 h) and maintained up to 24 h.
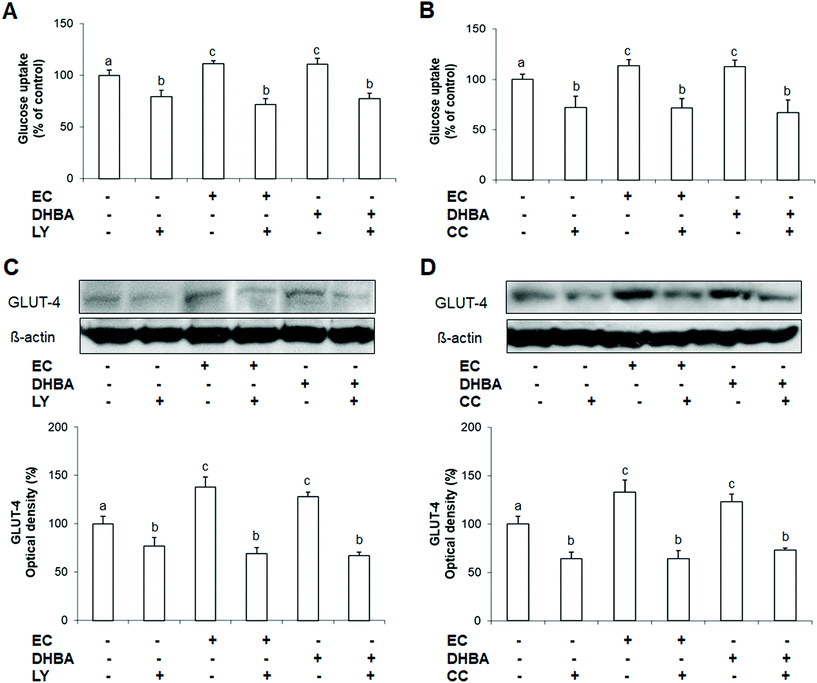
3.8. Effect of EC and DHBA activation of AKT and AMPK on glucose uptake
Glucose uptake and GLUT-4 levels constitute key aspects for the metabolic regulation in cardiomyocytes.3 In this regard, AKT seems to play an important role in this process, whilst the involvement of AMPK in the glucose uptake remains unclear.3 Since EC and DHBA increased the levels of p-AKT and p-AMPK, it was analysed whether any of these phenolic compounds were able to modulate the expression of GLUT-4, and the uptake of glucose via AKT and/or AMPK. To this end, H9c2 cells were exposed to a selective inhibitor of AKT (LY294002, LY) or AMPK (compound C, CC) for 1 h followed by a-24 h incubation with EC or DHBA, and then the glucose uptake and the levels of GLUT-4 were analysed. The concentrations selected for these analyses were the doses that clearly activated AKT, AMPK, and the glucose uptake, i.e. 1 μM for EC and 10 μM for DHBA.Treatment of H9c2 cells with EC or DHBA showed a comparable increase in the uptake of glucose, that is, decreased the percentage of 2-NBDG uptake in comparison to control cells (Fig. 7A and B). LY and CC alone diminished the glucose uptake, and these values similarly decreased in cells previously incubated with EC or DHBA (Fig. 7A and B). LY and CC treatments also reduced GLUT-4 levels in all cells incubated with these inhibitors, and the values of the glucose transporter were not recovered in the presence of EC or DHBA when compared to untreated cells (Fig. 7C and D).
3.9. Effect of EC and DHBA activation of AKT and AMPK on lipid accumulation
Lipids are the major energetic source of cardiomyocytes, and mainly are transported into cardiac cells by CD36, which is a marker of insulin resistance.10 In this regard, AMPK plays a crucial role in the lipid metabolism, and the insulin route also seems to be involved in the modulation of the accumulation and mobilization of lipids.5 To evaluate the involvement of AKT or AMPK on EC or DHBA-induced effects on lipid accumulation and CD36 levels, both kinases were inhibited by incubating the cells with LY or CC, respectively for 1 h prior to the treatment with EC or DHBA. As mentioned above, the concentrations selected for these analyses were the previously chosen (1 μM for EC and 10 μM for DHBA), since they showed a prominent effect on the phosphorylated levels of AKT and AMPK, as well as on the lipid accumulation.As illustrated in Fig. 8A and B, treatment with EC or DHBA alone equally decreased the lipid accumulation, whilst LY and CC increased the cellular lipid content in all cells treated with these inhibitors. Similar results were obtained for CD36 levels, as the values of this lipid transporter diminished after treating the cells with EC or DHBA alone, and enhanced by LY or CC in the presence or absence of EC or DHBA (Fig. 8C and D).
4. Discussion
The insulin signalling pathway is crucial for the maintenance of the cardiac energy metabolism and functionality.3 In the current study, it has been demonstrated, for the first time, that EC and the colonic DHBA metabolite diminished the lipid accumulation, increased the glucose uptake and levels of GLUT-4 in cardiomyocytes. In addition, EC and DHBA up-regulated the phosphorylated and total IR levels and activated the PI3K/AKT pathway and AMPK. EC and DHBA also diminished the levels of the key lipid transporter CD36, and FAS, increased PPARα and CPT1 values, and modulated the cardiac glucose uptake and lipid accumulation via AKT and AMPK.EC is present in fruits and beverages, such as grapes and tea, and more abundantly in cocoa.12,17,29 After the intake of flavanoid-rich foods, a small quantity of these compounds is absorbed in the small intestine, but in the colon most of them are transformed into low-molecular-weight metabolites (mono- and di-hydroxylated phenylpropionic, phenylacetic acids, and hydroxybenzoic acids) before being absorbed.15,17 Moreover, it is important to mention that pure compounds have been detected in plasma and urine, and accumulate in tissues as a consequence of a conjugation–deconjugation cycle.17,30 Taking together all these considerations, a pure compound and some of the most abundant metabolites found after the intake of flavanol-rich foods have been chosen to evaluate their effect on lipid accumulation, glucose uptake, and on the insulin pathway in cardiomyocytes at a range of realistic concentrations.15 Indeed, in biological fluids after the intake of flavanols circulating levels between 0.1–20 μM have been reported, and those concentrations are considered within the range recommended for in vitro studies.15,26
In the healthy heart, there is an energy metabolism balance related to the main substrates of this organ (lipid and glucose), which is crucial for maintaining the normal physiological cardiac function.5 In cardiomyocytes, GLUT-4 and -1 are the major glucose transporters, although it has been reported that SGLT-1 levels increase during certain diseases.7 In addition, during pathological situations, such as diabetes and obesity, there is a change in the substrate preference from glucose toward almost completely fatty acids (80–90%),31,32 leading to the lipid accumulation, the inhibition of the insulin signalling and, eventually to the failure of heart.3 In the present study, EC and DHBA modulated the uptake and accumulation of these substrates, as these natural compounds enhanced the glucose uptake and diminished the lipid content. These effects could be related to the regulation of GLUT-4 and CD36, as GLUT-1 and SGLT-1 remained unchanged. Similarly, an isoquinoline alkaloid extract increased the glucose uptake in cardiomyocytes and muscle cells, and that was accompanied by increased values of GLUT-4.33,34 Importantly, metformin, which is the first-line antidiabetic drug, alone and in combination with the antioxidant N-acetyl cysteine (NAC) avoided the diminished glucose uptake and GLUT-4 expression levels, as well as the enhanced lipid accumulation caused by a high concentration of glucose in H9c2 cells.35 Moreover, other natural compounds (berberine and aspalathin) have demonstrated to prevent the energy imbalance in cardiomyocytes incubated under high concentrations of palmitic acid or glucose by enhancing the glucose uptake, diminishing the lipid accumulation and avoiding the decrease of GLUT-4 values, contributing to the control of the glucose homeostasis and to reduce the glycaemia.20,33,34,36 Accordingly, the administration of a green tea polyphenolic extract increased GLUT-4 and diminished CD36 mRNA expression levels.37 In line with this, our results could suggest that EC and DHBA may contribute to increase the glucose uptake and reduce the lipid accumulation by regulating GLUT-4 and CD36 levels, respectively.
Insulin signalling also contributes to maintain the energy metabolism balance, and the inhibition of this route has also been associated to the loss of functionality in cardiac cells, as mentioned above.3,7,8 The activation of IR recruits the stimulation of the PI3K/AKT pathway, which is needed for the metabolic effects of insulin and it is responsible of the inhibition of GSK-3 and activation of GS.12 In this regard, EC and DHBA increased tyrosine phosphorylated and total levels of IR, as well as the phosphorylated values of AKT and GSK-3, and decreased p-GS levels in H9c2 cells. In concert, metformin alone or in combination with NAC prevented the increase of inhibitory p-(Ser)-IRS-1 values and the diminution of p-AKT levels in cardiomyocytes exposed to a high concentration of glucose.35 Correspondingly, berberine diminished the phosphorylated levels of p-(Ser)-IRS-1 and enhanced the phosphorylated levels of (Tyr)-IRS-1 without modifying the total levels of this protein, as well as increased the values of p-PI3K and p-AKT under physiological and pathological situations in muscle cells.34 In addition, in the heart of type 2 diabetic rats and in palmitate-treated H9c2 a down-regulation of AKT and GSK3 has been reported, whereas berberine restored the phosphorylated levels of the mentioned proteins in both experimental models.38 Similarly, impaired mRNA expression levels of IR, AKT and GSK3 were highly recovered in the heart of insulin-resistant rats by supplementing the diet with a green tea polyphenolic extract.37 These results were associated with an improved cardiac function and values of both glycaemia and insulinaemia in diabetic rats.38 Altogether, it could suggest that the phenolic compounds reinforce the insulin-signalling pathway in H9c2 cardiac cells.
Cardiac lipid accumulation is associated with cardiomyocyte dysfunction and with the development of cardiac diseases, hypertension, diabetes, obesity and metabolic syndrome.27 In the heart, CD36 plays a major role in the fatty acid uptake, and following the formation of long-chain acylcarnitine by CPT1, β-oxidation occurs. This metabolic process is importantly regulated by PPARα, which in turn, also modulates CPT1.32 Lipogenesis is mainly regulated by FAS.27 In this regard, EC and DHBA diminished CD36 and FAS values, and enhanced CPT1 and PPARα levels. In agreement with this, green tea polyphenols decreased CD36 levels, and key proteins related to the lipogenesis in the heart of insulin-resistant rats, which was associated to an improved cardiac situation.37 Indeed, an enhanced expression of CD36 has been associated to a reduced oxidative metabolism and cardiac hypertrophy.39 Moreover, the flavonoids apigenin, and quercetin increased the levels of myocardial PPARα and CPT1, which was connected to an enhanced fatty acid oxidation and led to the amelioration of the heart damage in hypertensive and metabolic syndrome-induced rats, respectively.40,41 In line with all this, EC and DHBA could contribute to modulate the lipid metabolism in the cardiac H9c2 cells.
AMPK is a metabolic sensor that plays a key role in multiple cellular processes.8,42 AMPK activation improves insulin sensitivity, inhibits lipolysis and lipogenesis, prevents hypertrophy, and has also been related to cardiomyocyte survival.8,43,44 In the present work, EC and DHBA increased p-AMPK levels in H9c2 cells. In agreement with our results, other phytochemicals, such as berberine and resveratrol, also enhanced p-AMPK levels in cultured cells.38,44,45 Similarly, baicalin and epigallocatechin-3-gallate have been reported to activate or prevent the inhibition of AMPK in different cultured cell lines and animal models under physiological and pathological conditions.24,43 In addition, AMPK activation stimulates fatty acid oxidation, which has been related to increased levels of CPT1 and PPARα.32,46 Thus, an AMPK activator, 5-aminoimidazole 1 carboxamide ribonucleoside (AICAR), restored PPARα levels, demonstrating a cardiac antihypertrophic function.47 In addition, AMPK modulates mitochondrial biogenesis by regulating proliferator-activated receptor γ coactivator 1α (PGC1α), which is also crucial for the fatty acid oxidation the interrelationship between PCG1α and PPARα.8,48 In line with this, the flavonoid naringenin activated PCG1α, leading to an improved mitochondrial function and mitochondrial biogenesis and the attenuation of cardiac damage following ischemia-reperfusion injury in vivo and in vitro; importantly, all these beneficial effects were abolished when AMPK was inhibited.49
AKT and AMPK have demonstrated to exert cardioprotective effects,43,44,50 and are known to modulate the glucose uptake and lipid metabolism in the heart.3,8 Activation of AKT and AMPK might be sufficient to increase GLUT-4 levels, and all these three proteins show decreased levels during type 2 diabetes, obesity and cardiovascular disease.3,8 In this line, other flavonoids have demonstrated early activations of the insulin pathway (IR-AKT) and AMPK, and the maintenance of those stimulations through the time, which was accompanied by an enhanced glucose uptake, in agreement with the present study.51–54 In addition, AMPK modulates the lipid metabolism, as mentioned above,8 and the PI3K/AKT pathway may also contribute to this regulation.3 Correspondingly, in this work it is shown that both kinases are involved in the modulation of GLUT-4 levels and glucose uptake, as well as in the regulation of CD36 values and lipid accumulation in H9c2 cells treated with EC and DHBA. Similarly, an alkaloid extract increased glucose uptake and GLUT-4 values, and that occurs together with enhanced levels of p-AKT and p-AMPK in insulin-sensitive and insulin-resistant muscle cells (physiological and pathological situations), suggesting the involvement of both kinases in the regulation of GLUT-4 and glucose uptake.34 Indeed, inhibition of AMPK diminished the glucose uptake in H9c2 berberine-treated cells, demonstrating at least in part that the activation of AMPK is linked to this metabolic process.45 Resveratrol and baicalin also stimulates the glucose uptake via AKT and AMPK in C2C12 myotube muscle cells, as this parameter decreased in the presence of pharmacological inhibitors for both kinases (LY294002 and compound C, respectively).24,54 Moreover, decreased p-AKT levels and glucose uptake together with enhanced CD36 values and impaired insulin signalling pathway have been reported in cardiac cells incubated with palmitate.55,56 Interestingly, empaglifozin, a SGLT2 inhibitor used for treating diabetic and cardiovascular patients, activates AMPK and reduces CD36 levels in the heart of Zucker diabetic fatty rats, which leads to a lower lipid accumulation, to an improved cardiac energy metabolism and, eventually to a protective effect against the development and progression of the diabetic cardiomyopathy.57 In this regard, green tea polyphenols alleviated the harmful effects detected in the heart of insulin-resistant rats by reducing mRNA CD36 values, and increasing AKT, GLUT-1 and -4 mRNA expression, as well as other key elements of the signalling route (enhanced IR and decreased GSK3 mRNA expression).37 All together points to the relevance of AKT and AMPK in the modulation of the glucose uptake and lipid accumulation in cardiac cells.
5. Conclusions
In summary, EC and DHBA regulate lipid accumulation, glucose uptake and strengthen the insulin-signalling pathway. Thus, EC and DHBA enhanced tyrosine phosphorylated and total levels of IR and activated the PI3K/AKT pathway and AMPK, together with an upregulation of GLUT-4, PPARα and CPT1 values and a downregulation of CD36 and FAS levels in cultured cardiomyocytes. Moreover, evidence is provided about a new mechanism by which EC and DHBA modulate the energetic metabolism via AKT and AMPK in cardiac H9c2 cells.Conflicts of interest
The authors have declared no conflict of interest.Acknowledgements
This work was supported by the grant RTI2018-095059-B-I00, funded by MCIN/AEI/10.13039/501100011033/ and by “ERDF A way of making Europe”. E. G.-D. was the recipient of a contract from Comunidad de Madrid (PEJ-2020-AI/BIO-18529). Sergio Castillo-González technical assistance is acknowledged (contracted by Comunidad de Madrid, PEJ-2018-TL/SAL-10236).Notes and references
- H. Schunkert, Obesity and target organ damage: the heart, Int. J. Obes., 2002, 26, S15–S20 CrossRef PubMed.
- M. J. Wilkinson, A. Zadourian and P. R. Taub, Heart failure and diabetes mellitus: Defining the problem and exploring the interrelationship, Am. J. Cardiol., 2019, 124, S3–S11 CrossRef CAS PubMed.
- C. Riehle and E. D. Abel, Insulin signaling and heart failure, Circ. Res., 2016, 118, 1151–1169 CrossRef CAS PubMed.
- M. Velez, S. Kohli and H. N. Sabbah, Animal models of insulin resistance and heart failure, Heart Failure Rev., 2014, 19, 1–13 CrossRef CAS PubMed.
- Y. Tan, Z. Zhang, C. Zheng, K. A. Wintergerst, B. B. Keller and L. Cai, Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: preclinical and clinical evidence, Nat. Rev. Cardiol., 2020, 17, 585–607 CrossRef PubMed.
- S. K. Banerjee, K. R. McGaffin, N. M. Pastor-Soler and F. Ahmad, SGLT1 is a novel cardiac glucose transporter that is perturbed in disease states, Cardiovasc. Res., 2009, 84, 111–118 CrossRef CAS PubMed.
- L. Szablewski, Glucose transporters in healthy heart and in cardiac disease, Int. J. Cardiol., 2017, 230, 70–75 CrossRef PubMed.
- S. M. Jeon, Regulation and function of AMPK in physiology and diseases, Exp. Mol. Med., 2016, 48, e245, DOI:10.1038/emm.2016.81.
- H. Bugger and E. D. Abel, Molecular mechanisms of diabetic cardiomyopathy, Diabetologia, 2014, 57, 660–671 CrossRef CAS PubMed.
- A. Handberg, K. Levin, K. Hojlund and H. Beck-Nielsen, Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: a novel marker of insulin resistance, Circulation, 2006, 114, 1169–1176 CrossRef CAS PubMed.
- D. Barreca, D. Trombetta, A. Smeriglio, G. Mandalari, O. Romeo, M. R. Felice, G. Gattuso and S. M. Nabavi, Food flavonols: nutraceuticals with complex health benefits and functionalities, Trends Food Sci. Technol., 2021, 117, 194–204 CrossRef CAS.
- M. A. Martín, L. Goya and S. Ramos, Antidiabetic actions of cocoa flavanols, Mol. Nutr. Food Res., 2016, 60, 1756–1769, DOI:10.1002/mnfr.201500961.
- A. Murakami and K. Ohnishi, Target molecules of food phytochemicals: Food science bound for the next dimension, Food Funct., 2012, 3, 462–476 RSC.
- E. Ramiro-Puig, G. Casadesus, H. G. Lee, X. Zhu, A. McShea, G. Perry, F. J. Perez-Cano, M. A. Smith and M. Castell, Neuroprotective effect of cocoa flavonoids on in vitro oxidative stress, Eur. J. Nutr., 2009, 48, 54–61 CrossRef CAS PubMed.
- M. Monagas, M. Urpi-Sarda, F. Sanchez-Patan, R. Llorach, I. Garrido, C. Gomez-Cordoves, C. Andres-Lacueva and B. Bartolome, Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites, Food Funct., 2010, 1, 233–253 RSC.
- J. I. Ottaviani, G. Borges, T. Y. Momma, J. P. E. Spencer, C. L. Keen, A. Crozier and H. Schroeter, The metabolome of [2-14C](−)-epicatechin in humans: Implications for the assessment of efficacy, safety, and mechanisms of action of polyphenolic bioactives, Sci. Rep., 2016, 6, 29034, DOI:10.1038/srep29034.
- G. Pereira-Caro, S. Gaillet, J. L. Ordóñez, P. Mena, L. Bresciani, K. A. Bindon, D. Del Rio, J.-M. Rouanet, J. M. Moreno-Rojas and A. Crozier, Bioavailability of red wine and grape seed proanthocyanidins in rat, Food Funct., 2020, 11, 3986, 10.1039/D0FO00350F.
- M. Urpi-Sarda, M. Monagas, N. Khan, R. Llorach, R. M. Lamuela-Raventos, O. Jauregui, R. Estruch, M. Izquierdo-Pulido and C. Andres-Lacueva, Targeted metabolic profiling of phenolics in urine and plasma after regular consumption of cocoa by liquid chromatography-tandem mass spectrometry, J. Chromatogr. A, 2009, 1216, 7258–7267 CrossRef CAS PubMed.
- V. M. Zamora-Gasga, E. Montalvo-González, G. Loarca-Piña, P. A. Vázquez-Landaverde, J. Tovar and S. G. Sáyago-Ayerdi, Microbial metabolites profile during in vitro human colonic fermentation of breakfast menus consumed by Mexican school children, Food Res. Int., 2017, 97, 7–14 CrossRef PubMed.
- R. Johnson, P. Dludla, E. Joubert, F. February, S. Mazibuko, S. Ghoor, C. Muller and J. Louw, Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis, Mol. Nutr. Food Res., 2016, 60, 922–934 CrossRef CAS PubMed.
- T. C. Karagiannis, A. J. E. Lin, K. Ververis, L. Chang, M. M. Tang, J. Okabe and A. El-Ost, Trichostatin A accentuates doxorubicin–induced hypertrophy in cardiac myocytes, Aging, 2010, 2, 659–668 CrossRef CAS PubMed.
- D. Álvarez-Cilleros, M. E. López-Oliva, M. A. Martín and S. Ramos, (−)-Epicatechin and the colonic metabolite 2,3-dihydroxybenzoic acid protect against high glucose and lipopolysaccharide-induced inflammation in renal proximal tubular cells through NOX-4/p38 signalling, Food Funct., 2020, 11, 8811, 10.1039/d0fo01805h.
- I. Cordero-Herrera, M. A. Martín, L. Goya and S. Ramos, Cocoa flavonoids protect hepatic cells against high glucose-induced oxidative stress. Relevance of MAPKs, Mol. Nutr. Food Res., 2015, 59, 597–609 CrossRef CAS PubMed.
- Y.-T. Kuo, C.-C. Lin, H.-T. Kuo, J.-H. Hung, C.-H. Liu, A. Jassey, M.-H. Yen, S.-J. Wu and L.-T. Li, Identification of baicalin from Bofutsushosan and Daisaikoto as a potent inducer of glucose uptake and modulator of insulin signaling-associated pathways, J. Food Drug Anal., 2019, 27, 240–248 CrossRef CAS PubMed.
- I. Cordero-Herrera, M. A. Martín, E. Fernández-Millán, C. Álvarez, L. Goya and S. Ramos, Cocoa and cocoa flavanol epicatechin improve hepatic lipid metabolism in in vivo and in vitro models. Role of PKCζ, J. Funct. Foods, 2015, 17, 761–773 CrossRef CAS.
- P. A. Kroon, M. N. Clifford, A. Crozier, A. J. Day, J. L. Donovan, C. Manach and G. Williamson, How should we assess the effects of exposure to dietary polyphenols in vitro?, Am. J. Clin. Nutr., 2004, 80, 15–21 CrossRef CAS PubMed.
- I. J. Goldberg, C. M. Trent and P. C. Schulze, Lipid metabolism and toxicity in the heart, Cell Metab., 2012, 15, 805–812 CrossRef CAS PubMed.
- T. D. Nguyen and P. C. Schulze, Lipid in the midst of metabolic remodeling-Therapeutic implications for the failing heart, Adv. Drug Delivery Rev., 2020, 159, 120–132 CrossRef CAS PubMed.
- J. A. Vinson, J. Proch, P. Bose, S. Muchler, P. Taffera, D. Shutta, N. Samman and G. A. C. Agbor, Chocolate is a powerful ex vivo and in vitro antioxidant, antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American diets, J. Agric. Food Chem., 2006, 54, 8071–8076 CrossRef CAS PubMed.
- F. Pérez-Vizcaíno, D. Bishop-Bailley, F. Lodi, J. Duarte, A. Cogolludo, L. Moreno, L. Boscá, J. Mitchell and T. Warner, The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells, Biochem. Biophys. Res. Commun., 2006, 346, 919–925 CrossRef PubMed.
- M. Bayeva, K. T. Sawicki and H. Ardehali, Taking diabetes to heart-deregulation of myocardial lipid metabolism in diabetic cardiomyopathy, J. Am. Heart Assoc., 2013, 2, e000433, DOI:10.1161/JAHA.113.000433.
- G. D. Lopaschuk, J. R. Ussher, C. L. Folmes, J. S. Jaswal and W. C. Stanley, Myocardial fatty acid metabolism in health and disease, Physiol. Rev., 2010, 90, 207–258 CrossRef CAS PubMed.
- W. Chang, L. Chen and G. M. Hatch, Berberine treatment attenuates the palmitate-mediated inhibition of glucose uptake and consumption through increased 1,2,3-triacyl-sn-glycerol synthesis and accumulation in H9c2 cardiomyocytes, Biochim. Biophys. Acta, 2016, 1861, 352–362 CrossRef CAS PubMed.
- L.-Z. Liu, S. C. K. Cheung, L.-L. Lan, S. K. S. Ho, H.-X. Xu, J. C. N. Chan and P. C. Y. Tong, Berberine modulates insulin signaling transduction in insulin-resistant cells, Mol. Cell. Endocrinol., 2010, 317, 148–153 CrossRef CAS PubMed.
- R. Johnson, N. F. Sangweni, S. E. Mabhida, P. V. Dludla, L. Mabasa, S. Riedel, C. Chapman, R. A. Mosa, A. P. Kappo, J. Louw and C. J. F. Muller, An in vitro study on the combination effect of metformin and N-acetyl cysteine against hyperglycaemia-induced cardiac damage, Nutrients, 2019, 11, 2850, DOI:10.3390/nu11122850.
- S. V. Penumathsa, M. Thirunavukkarasu, L. Zhan, G. Maulik, V. P. Menon, D. Bagchi and N. Maulik, Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium, J. Cell. Mol. Med., 2008, 12, 2350–2361 CrossRef CAS PubMed.
- B. Qin, M. M. Polansky, D. Harry and R. A. Anderson, Green tea polyphenols improve cardiac muscle mRNA and protein levels of signal pathways related to insulin and lipid metabolism and inflammation in insulin-resistant rats, Mol. Nutr. Food Res., 2010, 54, S14–S23 CrossRef CAS PubMed.
- W. Chang, M. Zhang, Z. Meng, Y. Yu, F. Yao, G. M. Hatch and L. Chen, Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5′-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells, Eur. J. Pharmacol., 2015, 769, 55–63 CrossRef CAS PubMed.
- D. P. Y. Koonen, M. Febbraio, S. Bonnet, J. Nagendran, M. E. Young, E. D. Michelakis and J. R. B. Dyck, CD36 expression contributes to age-induced cardiomyopathy in mice, Circulation, 2007, 116, 2139–2147 CrossRef CAS PubMed.
- Z.-Y. Zhu, T. Gao, Y. Huang, J. Xue and M.-L. Xie, Apigenin ameliorates hypertension-induced cardiac hypertrophy and down-regulates cardiac hypoxia inducible factor-lα in rats, Food Funct., 2016, 7, 1992–1998 RSC.
- S. K. Panchal, H. Poudyal and L. Brown, Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats, J. Nutr., 2012, 142, 1026–1032 CrossRef CAS PubMed.
- S. C. Bairwa, N. Parajuli and J. R. B. Dyck, The role of AMPK in cardiomyocyte health and survival, Biochim. Biophys. Acta, 2016, 1862, 2199–2210 CrossRef CAS PubMed.
- Y. Cai, L. Zhao, Y. Qin and X.-Q. Wu, EGCG blocked phenylephrin-induced hypertrophy in H9C2 cardiomyocytes, by activating AMPK-dependent pathway, Korean J. Physiol. Pharmacol., 2015, 19, 203–210 CrossRef CAS PubMed.
- J. T. Hwang, D. Y. Kwon, O. J. Park and M. S. Kim, Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells, Genes Nutr., 2008, 2, 323–326 CrossRef CAS PubMed.
- W. Chang, M. Zhang, J. Li, Z. Meng, S. Wei, H. Du, L. Chen and G. M. Hatch, Berberine improves insulin resistance in cardiomyocytes via activation of 5′-adenosine monophosphate-activated protein kinase, Metabolism, 2013, 62, 1159–1167 CrossRef CAS PubMed.
- T. T. Kim and J. R. B. Dyck, Is AMPK the savior of the failing heart?, Trends Endocrinol. Metab., 2015, 26, 40–48 CrossRef CAS PubMed.
- R. Meng, Z. Pei, A. Zhang, Y. Zhou, X. Cai, B. Chen, G. Liu, W. Mai, J. Wei and Y. Dong, AMPK activation enhances PPARa activity to inhibit cardiac hypertrophy via ERK1/2 MAPK signaling pathway, Arch. Biochem. Biophys., 2011, 511, 1–7 CrossRef CAS PubMed.
- C.-F. Cheng, H.-C. Ku and H. Lin, PGC-1α as a pivotal factor in lipid and metabolic regulation, Int. J. Mol. Sci., 2018, 19, 3447, DOI:10.3390/ijms19113447.
- L.-M. Yu, X. Dong, X.-D. Xue, J. Zhang, Z. Li, H.-J. Wu, Z.-L. Yang, Y. Yang and H.-S. Wang, Naringenin improves mitochondrial function and reduces cardiac damage following ischemia-reperfusion injury: the role of the AMPK-SIRT3 signaling pathway, Food Funct., 2019, 10, 2752–2765 RSC.
- A. Folino, A. E. Sprio, F. Di Scipio, G. N. Berta and R. Rastaldo, Alpha-linolenic acid protects against cardiac injury and remodelling induced by beta-adrenergic overstimulation, Food Funct., 2015, 6, 2231, 10.1039/c5fo00034c.
- D. Alvarez-Cilleros, M. A. Martín and S. Ramos, (-)-Epicatechin and the colonic 2,3-dihydroxybenzoic acid metabolite regulate glucose uptake, glucose production, and improve insulin signaling in renal NRK-52E cells, Mol. Nutr. Food Res., 2018, 1700470, DOI:10.1002/mnfr.201700470.
- I. Cordero-Herrera, M. A. Martín, L. Bravo, L. Goya and S. Ramos, Cocoa flavonoids improve insulin signalling and modulate glucose production via AKT and AMPK in HepG2 cells, Mol. Nutr. Food Res., 2013, 57, 974–985 CrossRef CAS PubMed.
- G. Montagut, S. Onnockx, M. Vaqué, C. Bladé, M. Blay, J. M. Fernández-Larrea, G. Pujadas, M. J. Salvadó, L. Arola, I. Pirson, A. Ardévol and M. Pinent, Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin, J. Nutr. Biochem., 2010, 21, 476–481 CrossRef CAS PubMed.
- C. E. Park, M.-J. Kim, J. H. Lee, B.-I. Min, H. Bae, W. Choe, S.-S. Kim and J. Ha, Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase, Exp. Mol. Med., 2007, 39, 222–229 CrossRef CAS PubMed.
- H. L. Nizami, P. Katare, P. Prabhakar, Y. Kumar, S. K. Arava, P. Chakraborty, S. K. Maulik and S. K. Banerjee, Vitamin D deficiency in rats causes cardiac dysfunction by inducing myocardial insulin resistance, Mol. Nutr. Food Res., 2019, 63, e1900109, DOI:10.1002/mnfr.201900109.
- M. A. H. Talukder, M. Preda, L. Ryzhova, I. Prudovsky and I. M. Pinz, Heterozygous caveolin-3 mice show increased susceptibility to palmitate-induced insulin resistance, Physiol. Rep., 2016, 4, e12736, DOI:10.14814/phy2.12736.
- A. Aragón-Herrera, S. Feijóo-Bandín, M. Otero-Santiago, L. Barral, M. Campos-Toimil, J. Gil-Longo, T. M. Costa-Pereira, T. García-Caballero, S. Rodríguez-Segade, J. Rodríguez, E. Tarazón, E. Roselló-Lletí, M. Portolés, O. Gualillo, J. R. González-Juanatey and F. Lago, Empagliflozin reduces the levels of CD36 and cardiotoxic lipids while improving autophagy in the hearts of Zucker diabetic fatty rats, Biochem. Pharmacol., 2019, 170, 113677, DOI:10.1016/j.bcp.2019.113677.
| This journal is © The Royal Society of Chemistry 2022 |