Open Access Article
Open Access ArticleDaily consumption of cranberry improves endothelial function in healthy adults: a double blind randomized controlled trial†
Christian
Heiss
 abc,
Geoffrey
Istas
ad,
Rodrigo P.
Feliciano
a,
Timon
Weber
a,
Brian
Wang
d,
Claudia
Favari
abc,
Geoffrey
Istas
ad,
Rodrigo P.
Feliciano
a,
Timon
Weber
a,
Brian
Wang
d,
Claudia
Favari
 e,
Pedro
Mena
e,
Pedro
Mena
 ef,
Daniele
Del Rio
ef,
Daniele
Del Rio
 efg and
Ana
Rodriguez-Mateos
efg and
Ana
Rodriguez-Mateos
 *ad
*ad
aDivision of Cardiology, Pulmonology, and Vascular Medicine, Medical Faculty, University Düsseldorf, Düsseldorf, Germany
bDepartment of Clinical and Experimental Medicine, University of Surrey, Guildford, UK
cSurrey and Sussex Healthcare NHS Trust, East Surrey Hospital, Redhill, UK
dDepartment of Nutritional Sciences, School of Life Course and Population Health Sciences, Faculty of Life Sciences and Medicine, King's College London, Franklin-Wilkins Building, 150 Stamford Street, London, SE1 9NH, UK. E-mail: ana.rodriguez-mateos@kcl.ac.uk; Tel: +44(0) 207 848 4349
eHuman Nutrition Unit, Department of Food & Drug, University of Parma, Parma, Italy
fMicrobiome Research Hub, University of Parma, Parma, Italy
gSchool of Advanced Studies on Food and Nutrition, University of Parma, Parma, Italy
First published on 22nd March 2022
Abstract
Background: Previous studies indicate cardiovascular health benefits of cranberry juice consumption. However, whether daily consumption of whole cranberries will have sustained vascular benefits in healthy individuals is currently unknown. Objective: To investigate the vascular effects of acute and daily consumption of freeze dried whole cranberry in healthy men and how effects relate to circulating cranberry (poly)phenol metabolites. Methods: A double-blind, parallel-group, randomized controlled trial was conducted in 45 healthy male adults randomly allocated to 1 month daily consumption of either cranberry (9 g powder solubilized in water equivalent to 100 g of fresh cranberries, 525 mg total (poly)phenols) or control (9 g powder, no (poly)phenols). Flow-mediated dilation (FMD, primary outcome), pulse wave velocity (PWV), aortic augmentation index (AIx), blood pressure, heart rate, blood lipids, and blood glucose were assessed at baseline and at 2 h on day 1 and after 1 month. Plasma and 24 h-urine were analyzed before and after treatment using targeted quantitative LC-MS methods including 137 (poly)phenol metabolites. Results: Cranberry consumption significantly increased FMD at 2 h and 1-month (1.1% (95% CI: 1.1%, 1.8%); ptreatment ≤ 0.001; ptreatment![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) time = 0.606) but not PWV, AIx, blood pressure, heart rate, blood lipids, and glucose. Of the 56 and 74 (poly)phenol metabolites quantified in plasma and urine, 13 plasma and 13 urinary metabolites significantly increased 2 h post-consumption and on day 1, respectively, while 4 plasma and 13 urinary metabolites were significantly higher after 1-month of cranberry consumption, in comparison with control. A multi-variable stepwise linear regression analysis showed that plasma cinnamic acid-4′-glucuronide, 4-hydroxybenzoic acid-3-sulfate, 2,5-dihydroxybenzoic acid, 3′-hydroxycinnamic acid, and 5-O-caffeoylquinic acid were significant independent predictors of 2 h FMD effects (R2 = 0.71), while 3′-hydroxycinnamic acid, 4-methoxycinnamic acid-3′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid 3′-sulfate, and 3-(4′-methoxyphenyl)propanoic acid 3′-glucuronide predicted the 1-month FMD effects (R2 = 0.52). Conclusions: Acute and daily consumption of whole cranberry powder for 1 month improves vascular function in healthy men and this is linked with specific metabolite profiles in plasma. The National Institutes of Health (NIH)-randomized trial records held on the NIH ClinicalTrials.gov website (NCT02764749). https://clinicaltrials.gov/ct2/show/NCT02764749
time = 0.606) but not PWV, AIx, blood pressure, heart rate, blood lipids, and glucose. Of the 56 and 74 (poly)phenol metabolites quantified in plasma and urine, 13 plasma and 13 urinary metabolites significantly increased 2 h post-consumption and on day 1, respectively, while 4 plasma and 13 urinary metabolites were significantly higher after 1-month of cranberry consumption, in comparison with control. A multi-variable stepwise linear regression analysis showed that plasma cinnamic acid-4′-glucuronide, 4-hydroxybenzoic acid-3-sulfate, 2,5-dihydroxybenzoic acid, 3′-hydroxycinnamic acid, and 5-O-caffeoylquinic acid were significant independent predictors of 2 h FMD effects (R2 = 0.71), while 3′-hydroxycinnamic acid, 4-methoxycinnamic acid-3′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid 3′-sulfate, and 3-(4′-methoxyphenyl)propanoic acid 3′-glucuronide predicted the 1-month FMD effects (R2 = 0.52). Conclusions: Acute and daily consumption of whole cranberry powder for 1 month improves vascular function in healthy men and this is linked with specific metabolite profiles in plasma. The National Institutes of Health (NIH)-randomized trial records held on the NIH ClinicalTrials.gov website (NCT02764749). https://clinicaltrials.gov/ct2/show/NCT02764749
Introduction
Cranberries are fruits rich in (poly)phenols that have been widely investigated because of their potential health benefits.1 They are particularly rich in A-type proanthocyanidins (PACs), but also contain significant amounts of anthocyanins, flavonols, and phenolic acids.2,3 The A-type PACs are unique to cranberries and a few other foods,4 while B-type PACs are abundant in many commonly consumed fruits such as grapes, apples and blueberries, and cocoa products. This may be important as some reports exist on different bioactivities between A and B type PACs.5–7 Few randomized control trials have investigated the effects of cranberry juice on biomarkers of cardiovascular (CVD) risk, mainly in individuals at high risk or with CVD, with mixed results.8–13 More recent studies in healthy populations conducted by our group have demonstrated acute improvements in vascular function after single consumption of cranberry juice.13–15 This effect was linked to the presence of specific metabolites in plasma that we had newly identified. We demonstrated a dose–response relationship between the amount of cranberry (poly)phenols consumed and circulating (poly)phenol metabolites.14–16 As opposed to the few studies performed with cranberry juice, no study has investigated the cardiovascular effects of whole cranberry freeze-dried powder in humans, which has a significant different phytochemical profile than the juice and is richer in anthocyanins and flavonols and has the additional advantage of having less sugar than commercial cranberry juices.17,18 It is, therefore, not known whether consumption of whole cranberry powder in relevant amounts can improve vascular function when given over longer and more relevant time periods.The aim of this study was to investigate the acute (2 h) and chronic effects (1 month) of whole cranberry powder on vascular function, as determined by flow-mediated vasodilation (FMD; primary endpoint), arterial stiffness (pulse wave velocity [PWV], aortic augmentation index [AIx]) blood pressure, blood lipids, and glucose in healthy individuals. Furthermore, plasma and urinary concentrations of cranberry (poly)phenols were quantified to explore a link between circulating metabolites and vascular outcomes to guide further mechanistic work in the future.
Materials and methods
Study subjects
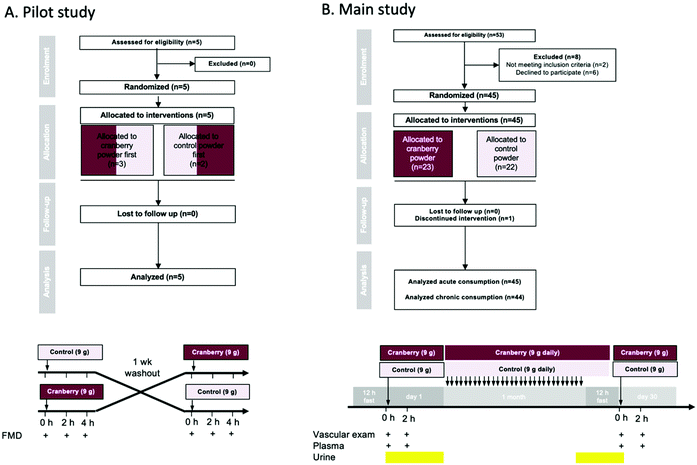
Fifty healthy male participants aged 18 to 45 years were recruited for the pilot (n = 5) and main study (n = 45). These subjects were well-characterized healthy participants. Health was ascertained by a routine clinical physical examination and specific cardiovascular history performed by a cardiovascular specialist (cardiovascular medicine consultant). Manifest cardiovascular disease including coronary artery disease, cerebrovascular disease and peripheral artery disease, diabetes mellitus, acute inflammation, terminal renal failure, malignancies, and heart rhythm other than sinus were exclusion criteria.Cranberry and control powders
The whole cranberry and placebo powder were supplied by the Cranberry Institute (Carver, MA, USA). The 9 g of cranberry powder ingested daily contained 525 mg of total (poly)phenols, of which 374 mg were PACs analyzed using the 4-(dimethylamino)cinnamaldehyde (DMAC) for soluble PACs and the butanol–hydrochloric acid method (BuOH–HCl) method for insoluble PACs, using a standardized cranberry reference standard (c-PAC)19,20 (Table 1). Individual (poly)phenols and phenolic acids were analyzed using HPLC-MS/MS as previously described.21 The amount of cranberry powder used in the study was chosen as the amounts of (poly)phenols contained in the powder is equivalent to 100 g fresh cranberries and is close to the amount (‘effective dose’) to exert half maximal effects to improved FMD (ED50 = 442 mg) based on our previous study18 (see ESI Fig. S1†). The powder was produced from a blend of cranberry varieties that approximate the market profile at the time of production (56% Stevens, and 11% each of Ben Lear, Grygleski, Pilgrim, and HyRed). The berries were individually frozen after harvest, freeze-dried, and ground into powder form (20 mesh). The control powder had the same colour and similar taste than the cranberry powder, and was produced from a blend of water, maltodextrin (CPC Maltrin M-180), citric acid, artificial cranberry flavor (Lorann oils), fructose, red color (Lorann oils), and grape shade (Esco Foods), and was then freeze-dried (processed in the U.S. Department of Agriculture, Agricultural Research Service, Western Regional Research Center, Healthy Processed Foods Pilot Plant, Albany, CA).| Sum of (poly)phenols, mg | 525 |
| Total proanthocyanidins (PACs), mg | 374.2 |
| Soluble PACs (c-PAC, DMAC) | 280.8 |
| Insoluble PACs (BuOH–HCl) | 93.4 |
| Epicatechin (LC-MS) | 0.493 |
| Catechin (LC-MS) | 0.019 |
| Total flavonols, mg | 81 |
| Quercetin-3-rhamnoside eq., mg (HPLC) | 81 |
| Quercetin, μg (LC-MS) | 0.153 |
| Kaempferol, μg (LC-MS) | 0.001 |
| Total anthocyanins (cyanidin-3-galact eq.), mg | 54 |
| Phenolic acids, mg | 17 |
| 3′,4′-Dihydroxycinnamic acid eq., (caffeic acid), mg (HPLC) | 16 |
| 5-O-Caffeoylquinic acid (chlorogenic acid), μg (LC-MS) | 0.720 |
| 3,4-Dihydroxybenzoic acid (protocatechuic acid), μg (LC-MS) | 0.051 |
| 4′-Hydroxycinnamic acid (p-coumaric acid), μg (LC-MS) | 0.034 |
| 4′-Hydroxy-3′,5′-dimethoxycinnamic acid (sinapic acid), μg (LC-MS) | 0.010 |
| 4′-Hydroxy-3′-methoxycinnamic acid (ferulic acid), μg (LC-MS) | 0.007 |
| 3-Hydroxybenzoic acid, μg (LC-MS) | 0.006 |
| 3,4-Dihydroxybenzaldehyde, μg (LC-MS) | 0.004 |
| 2,5-Dihydroxybenzoic acid, μg (LC-MS) | 0.003 |
| 2-Hydroxybenzoic acid, μg (LC-MS) | 0.003 |
| Dihydrocaffeic acid, μg (LC-MS) | 0.001 |
| 4-Hydroxybenzoic acid, μg (LC-MS) | 0.001 |
| 2′-Hydroxycinnamic acid (o-coumaric acid), μg (LC-MS) | 0.000 |
| 3′-Hydroxy-4′-methoxycinnamic acid (isoferulic acid), μg (LC-MS) | 0.000 |
| 4-Hydroxybenzaldehyde, μg (LC-MS) | 0.000 |
Study design
The primary endpoint was an improvement of endothelial vasodilator function as measured by FMD using high-resolution ultrasound. No other parameters were tested.
The primary endpoint was changes in endothelial vasodilator function as measured by FMD after 1 month of daily consumption. Secondary endpoints were changes in key determinants of vascular function and include acute changes in FMD and acute and chronic changes in PWV, AIx, and office blood pressure as determined automatically by applanation tonometry and a blood pressure monitoring system.
Tertiary endpoints included changes in blood lipids, plasma glucose and plasma and urinary cranberry-derived (poly)phenol metabolites.
A qualified researcher enrolled participants on the study. Participants and researchers administering interventions and assessing study outcomes were blinded to the interventions. An independent researcher generated the random allocation to treatment sequence (using a Williams design) and implemented the allocation sequence. All studies were conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Duesseldorf Research Ethics Committee (Ref.: 5360R). Informed consents were obtained from all participants of this study. The study was also registered with the National Institutes of Health randomized trial records held on the ClinicalTrials.gov website (NCT02764749). This study was conducted with 45 volunteers of which 44 completed the study from June until October 2016 at the University of Duesseldorf outpatient clinic.
Assessment of total dietary and (poly)phenol intake
Dietary data were obtained from FFQs collected at baseline and were based on version 6 of the EPIC FFQ. Input values from the FFQs of participants were analyzed using the FETA-software.22 The Phenol Explorer database was used to estimate the (poly)phenol content of foods. See ESI Table S1† for total energy, macronutrients, and micronutrients and ESI Table S2† for (poly)phenol intake.Vascular measurements
FMD was measured as previously described.23 Briefly, the diameter and flow velocity of the brachial artery (BA) was measured using a 12 MHz transducer (Vivid I, GE) and automatic edge-detection software (Brachial Analyzer, Medical Imaging Applications, Iowa City, IA, USA) yielding standard deviations of mean differences between repeated measurements of less than 1%. BA diameter was measured 2 cm proximal to the elbow. Reactive hyperaemia was induced by 5 min of lower arm occlusion with a sphygmomanometer cuff inflated to 250 mmHg. After cuff deflation, 20, 40, 60, and 80 s the diameter was assessed and FMD calculated as maximal relative diameter gain relative to baseline. The FMD was expressed as [(diametermax − diameterbaseline)/(diameterbaseline × 100)].Office blood pressure was measured in the supine position three times after 10 min of rest using an automated clinical digital sphygmomanometer (Dynamap, Tampa, FL, USA) with appropriately sized cuff placed around the upper arm at heart level.
Central blood pressure and pulse wave analysis for AIx and PWV determination were measured by applanation tonometry using the SphygmoCor® (SMART medical, Gloucestershire, UK) system. Via a transfer function, the pressure waveform of the ascending aorta was synthesized. PWV was determined from measurements taken at the carotid and femoral artery as previously described.24
Clinical biochemical analyses
All clinical chemistry parameters including total, LDL and HDL-cholesterol, triglycerides (enzymatic photometric assay; RocheDiagnostics), HbA1c, glucose (hexokinase assay) and whole blood count (flow cytometry; Sysmex) were measured using standard techniques by the Institute for Clinical Chemistry, University Hospital Duesseldorf, Germany.Analysis of plasma and urine (poly)phenol metabolites
Analysis of 137 (poly)phenol metabolites (ESI Table S1†) in plasma and urine was performed using microelution solid phase extraction coupled with ultra-high-performance liquid chromatography quadrupole time of flight coupled to mass spectrometry (UPLC-Q-TOF MS) and authentic standards for quantification using a validated method as previously described.25 Phenyl-γ-valerolactones (PVLs) and phenylvaleric acids (PVAs), for a total of 67 potential metabolites, were analysed by ultra-high-performance liquid chromatography coupled with triple quadrupole mass spectrometry (UHPLC-ESI-QqQ-MS/MS) following a validated method.26 For PVLs and PVAs, plasma samples were prepared by solid phase extraction as previously indicated, while urine samples followed another procedure.27 Metabolite identification was carried out by comparison of the retention time with in-house synthesized standards and/or MS/MS fragmentation patterns. When available, quantification was performed with calibration curves of standards, when not available, with the most structurally similar compound.16Power calculation and statistical analysis
Power calculations were performed for the primary endpoint, change in FMD response. Power was based on the inter-individual variability of the operator that performed the FMD analysis (SD = 1%). At 0.8 power and at 0.05 significance level, the number of subjects required to detect a difference of 0.9% in the response of matched pairs in a parallel study is 40. The characteristics of the study population are expressed as mean values ± SDs.In the pilot study, the primary test for an effect was a repeated measurements ANOVA (2 within subject factors: intervention and time) followed by post hoc pairwise comparisons comparing the responses due to the cranberry and the control powder at 2 h and 4 h. Responses to treatments were calculated as changes in FMD: 2 and 4 h values minus baseline values at 0 h.
The primary test for an effect in the main study was a univariate analysis of covariance (ANCOVA) followed by post hoc pairwise comparisons comparing the responses due to the cranberry and the control powder (fixed factors) at 1 month (dependent) with baseline values as covariates to account for baseline differences. Responses to treatments were calculated as changes in respective parameters (e.g., FMD): 1 month values minus baseline values on day 1 adjusted to average baseline value. Mean values of parameters are presented as means ± SEMs, and differences between responses are presented as means with Bonferroni-adjusted 95% CIs. We also analysed the difference between responses at 2 h after acute consumption of the 2 interventions on day 1 and at 1 month as compared with the 0 h baseline on day 1 by the use of repeated-measurements ANCOVA, with baseline values as covariates to account for variations in baseline value. Cranberry related effects were estimated as change after cranberry corrected for changes after control. Multivariate stepwise linear regression analyses were performed to identify which changes in cranberry (poly)phenol metabolites were significant (independent variables) predictors of FMD changes (dependent variable). Analyses were computed with SPSS 26 (IBM Corp.).
Results
Baseline characteristics of study participants
A total of 5 and 53 volunteers were screened for recruitment in the pilot study and the main study, respectively. In the pilot study, 5 volunteers were recruited and completed the study. In the main study, 45 were included and randomly allocated a treatment (23 received cranberry powder and 22 the control powder) (Fig. 1). One volunteer discontinued the treatment after 15 days due to multiple episodes of diarrhea. The baseline characteristics of the groups of healthy young males were all within normal limits (Table 2). No significant differences between the two groups regarding the baseline daily dietary intake of macronutrients, micronutrients (ESI Table S1†) and (poly)phenols (ESI Table S2†) were found. The test powders were well tolerated by all subjects, except for the one drop-out mentioned above.| Cranberry (n = 22) | Control (n = 22) | |
|---|---|---|
| a Values are mean ± SD; AIx, aortic augmentation index; BMI, body mass index; CRP, C-reactive protein; FMD, flow-mediated dilation; DBP, diastolic blood pressure; GGT, γ-glutamyltransferase; GPT, glutamyl pyruvate transferase; GOT, glutamate oxaloacetate transaminase; HbA1c, glycated hemoglobin; HDL, high density lipoprotein; LDL, low density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure. | ||
| Age, years | 25 ± 3 | 25 ± 3 |
| Weight, kg | 77 ± 13 | 80 ± 10 |
| Height, cm | 182 ± 8 | 181 ± 6 |
| BMI, kg m−2 | 23 ± 3 | 24 ± 3 |
| FMD, % | 6.3 ± 0.8 | 7.3 ± 0.6 |
| PWV, m s−1 | 5.3 ± 1.1 | 5.2 ± 1.1 |
| SBP, mmHg | 125 ± 7 | 126 ± 9 |
| DBP, mmHg | 69 ± 9 | 69 ± 7 |
| AIx, % | −3.8 ± 14.8 | −8.4 ± 11.8 |
| Total cholesterol, mg dL−1 | 161 ± 23 | 163 ± 39 |
| Triglycerides, mg dL−1 | 90 ± 75 | 73 ± 35 |
| LDL cholesterol, mg dL−1 | 96 ± 20 | 94 ± 36 |
| HDL cholesterol, mg dL−1 | 56 ± 11 | 54 ± 11 |
| HbA1c, % | 4.8 ± 0.2 | 4.8 ± 0.3 |
| Fasting plasma glucose, mg dL−1 | 85 ± 6 | 86 ± 7 |
| Total bilirubin, mg dL−1 | 1.0 ± 0.9 | 0.7 ± 0.4 |
| CRP, mg dL−1 | 0.1 ± 0.1 | 0.1 ± 0.1 |
| GOT, U L−1 | 24 ± 5 | 27 ± 6 |
| GPT, U L−1 | 22 ± 4 | 25 ± 11 |
| GGT, U L−1 | 18 ± 8 | 24 ± 19 |
| Creatinine, mg dL−1 | 0.9 ± 0.1 | 1.0 ± 0.1 |
Whole cranberry powder acutely improves endothelial function (pilot study)
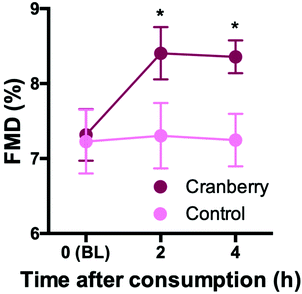
In the pilot study (n = 5), cranberry powder increased FMD at 2 h by 1.0% (95% CI: 0.3%, 1.7%) and at 4 h by 1.0% (95% CI: 0.3%, 1.7%) as compared to control (pintervention = 0.018) (Fig. 2). The response did not differ between 2 and 4 h (ptime = 0.743). The effect size of the cranberry powder (containing 525 mg total (poly)phenols) reflected what would be expected based on our previously published dose–response experiments with cranberry juice (see ESI Fig. S1†).Acute and daily consumption of whole cranberry powder improves endothelial function (main study)
During this 1 month randomized controlled trial, the consumption of whole cranberry powder led to a significant improvement in FMD as compared with the control (Table 3) by 1.1% (95% CI: 0.6%, 1.6%). Of note this estimate effect is adjusted for baseline FMD. Fig. 3 shows that similar significant improvements (pintervention < 0.001) were observed at 2 h after the first cranberry intervention on day 1 but that there were no further improvements at 1 month and 2 h after consumption of the last cranberry powder. There was no significant time × intervention interaction (p = 0.606), suggesting that the effect of cranberry did not differ between the time points and was independent of baseline FMD (p = 0.269). One month FMD values were significantly increased compared to day 1 baseline only in the cranberry group (6.3 ± 0.2% to 7.5 ± 0.2%, ptime![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intervention < 0.001) but not the control group (7.3 ± 0.2% vs. 7.2 ± 0.2%). When comparing the acute changes exerted by cranberry at day 1 with effects at 1 month (each relative to the fasting morning values on same day) the results showed that cranberry acutely increased FMD on both days (day 1: 1.6 ± 0.1%, 1 month: 0.4 ± 0.2%, ptime
intervention < 0.001) but not the control group (7.3 ± 0.2% vs. 7.2 ± 0.2%). When comparing the acute changes exerted by cranberry at day 1 with effects at 1 month (each relative to the fasting morning values on same day) the results showed that cranberry acutely increased FMD on both days (day 1: 1.6 ± 0.1%, 1 month: 0.4 ± 0.2%, ptime![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intervention < 0.001) but that the effect was significantly lower at 1 month (ptime
intervention < 0.001) but that the effect was significantly lower at 1 month (ptime![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ×
×![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) intervention < 0.001). Baseline diameter of the brachial artery remained unaffected by cranberry or placebo (data not shown).
intervention < 0.001). Baseline diameter of the brachial artery remained unaffected by cranberry or placebo (data not shown).
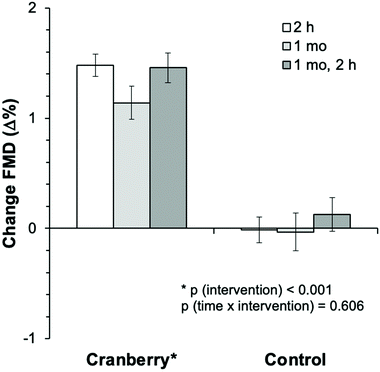 | ||
| Fig. 3 Changes in flow-mediated dilation as compared to baseline (day 1) after 2 hours, 1 month and 1 month, 2 hours of cranberry or control (n = 44). Bar graphs are mean, error bars are SEM. | ||
| Cranberry | Control | Estimated effect Cranberryb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| (Change from day 1 baseline) | (Change from day 1 baseline) | (Difference Cranberry − control) | p Treatmentc | p Treatment × time | p Baseline | |||||
| 2 h | 1 mo | 1 mo, 2 h | 2 h | 1 mo | 1 mo, 2 h | |||||
| a Values are mean ± SEM or x (Bonferroni-corrected 95% CIs). Significant values are in bold. AIx, aortic augmentation index; DBP, diastolic blood pressure; FMD, flow-mediated dilation; LDL, low-density lipoprotein; HBA1C, hemoglobin A1C; HDL, high-density lipoprotein; PWV, pulse wave velocity; SBP, systolic blood pressure. b Overall estimated marginal means adjusted for baseline values (Bonferroni). c Repeated measurement (FMD, PWV, SBP, DBP, AIx) or univariate ANCOVA comparing changes at each timepoint to baseline at day 0 h with baseline values as a covariate. Bonferroni corrected for multiple comparisons. | ||||||||||
| Primary end point | ||||||||||
| FMD, % | 1.5 ± 0.1 | 1.1 ± 0.1 | 1.5 ± 0.1 | 0.0 ± 0.1 | 0.0 ± 0.2 | −0.1 ± 0.2 | 1.4 (1.1, 1.8) | <0.001 | 0.606 | 0.269 |
| Secondary end points | ||||||||||
| PWV, m s−1 | −0.15 ± 0.18 | −0.15 ± 0.24 | −0.01 ± 0.20 | −0.11 ± 0.20 | 0.17 ± 0.24 | −0.06 ± 0.23 | −0.10 (−0.59, 0.39) | 0.673 | 0.788 | 0.004 |
| SBP, mmHg | −4.7 ± 1.3 | −1.3 ± 2.1 | −5.4 ± 1.4 | −3.4 ± 1.3 | −0.8 ± 2.0 | −3.0 ± 1.4 | −1.4 (−5.1, 2.3) | 0.445 | 0.556 | 0.060 |
| DBP, mmHg | −2.3 ± 1.2 | −2.2 ± 1.1 | −4.8 ± 1.0 | −1.5 ± 1.2 | −3.1 ± 1.1 | −1.9 ± 1.0 | −0.9 (−3.4, 1.5) | 0.445 | 0.250 | 0.003 |
| AIx, % | −3.1 ± 2.6 | −0.3 ± 2.8 | −4.1 ± 2.5 | −5.4 ± 2.6 | 0.0 ± 2.8 | −6.3 ± 2.5 | 1.4 (−4.4, 7.2) | 0.629 | 0.982 | <0.001 |
| Total cholesterol, mg dL−1 | −4.9 ± 4.2 | 0.5 ± 13.9 | −5.3 (−31.4, 20.8) | 0.682 | 0.282 | |||||
| Triglycerides, mg dL−1 | 11.9 ± 8.7 | −6.5 ± 8.6 | 20.0 (−5.9, 45.9) | 0.125 | 0.424 | |||||
| HDL cholesterol, mg dL−1 | −4.9 ± 1.5 | 2.6 ± 4.4 | −4.2 (−11.1, 2.6) | 0.213 | <0.001 | |||||
| LDL cholesterol, mg dL−1 | −2.0 ± 4.6 | 1.5 ± 12.6 | −4.4 (−28.4, 19.5) | 0.708 | 0.091 | |||||
| HbA1C, % | −0.08 ± 0.11 | 0.09 ± 0.09 | −0.16 (−0.44, 0.12) | 0.250 | 0.015 | |||||
| Glucose, mg dL−1 | 0.1 ± 1.9 | 1.5 ± 2.3 | −0.4 (−5.1, 4.3) | 0.863 | <0.001 | |||||
The remaining secondary outcomes (BP, PWV, AIx, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, HbA1c, glucose) did not show any significant differences between responses to cranberry and control interventions (Table 3).
Characterization of cranberry (poly)phenol metabolites in plasma
Of the targeted 137 (poly)phenol metabolites in plasma, we detected 64 and quantified 56 (see ESI Tables S3 and S4†), including 13 PVLs and PVAs deriving from the gut microbial metabolism of cranberry catechins and PACs. Results showed that the concentrations of PVLs and PVAs in circulation were higher in the subjects treated with cranberry powder than in those treated with the placebo. Mean plasma concentrations were very low though (0.1–10 nmol L−1) for the majority of the metabolites detected (5-phenyl-γ-valerolactone-4′-glucuronide, 5-phenyl-γ-valerolactone-3′-sulfate, 5-(4′-hydroxyphenyl)-γ-valerolactone-3′-glucuronide, and 5-phenylvaleric acid-sulfate-glucuronide), except for 5-(hydroxyphenyl)-γ-valerolactone-sulfate, for which higher plasma concentrations were found (10–30 nmol L−1). This compound is the sum of two isomers, 5-(3′-hydroxyphenyl)-γ-valerolactone-4′-sulfate and 5-(4′-hydroxyphenyl)-γ-valerolactone-3′-sulfate and is the main PVL found in circulation upon consumption of flavan-3-ols.27 The low concentrations of PVLs and PVAs in plasma observed in this study were expected, as these compounds typically have maximum concentrations in plasma at 4 to 8 hours post-consumption16 and fasting blood was collected approximately 24 h after the last cranberry powder was consumed.The most abundant compounds found in plasma after cranberry powder consumption included hippuric acids, benzoic acids, and cinnamic acids derivatives. Cranberry powder had a significant main effect on the changes (2 h, 1 month or 1 month/2 h values minus baseline) of 28 metabolites (ANCOVA with baseline values as covariate). While cranberry significantly decreased 4 metabolites in plasma, it increased 24 (Fig. 4), suggesting that they represent circulating cranberry derived (poly)phenols metabolites: α-hydroxyhippuric acid, 4′-hydroxyhippuric acid, 2′-hydroxyhippuric acid, 2-hydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, 3-methoxybenzoic acid-4-sulfate, 4-hydroxybenzoic acid-3-glucuronide, 3-hydroxybenzoic acid-4-sulfate, 4-hydroxybenzoic acid-3-sulfate, 3′-hydroxycinnamic acid, 2′-hydroxycinnamic acid, 3′-methoxycinnamic acid-4′-sulfate, 3-(3′-methoxyphenyl)propanoic acid-4′-sulfate, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, 5-O-caffeoylquinic acid, 3′-methoxycinnamic acid-4′-glucuronide, 4′-methoxycinnamic acid-3′-glucuronide, 3-(3′-methoxyphenyl)propanoic acid-4′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide, cinnamic acid-4′-glucuronide, 3′-hydroxycinnamic acid-4′-sulfate, 4′-hydroxy-3′-methoxyphenylacetic acid, and 5-(3′-hydroxyphenyl)-γ-valerolactone-4′-glucuronide.
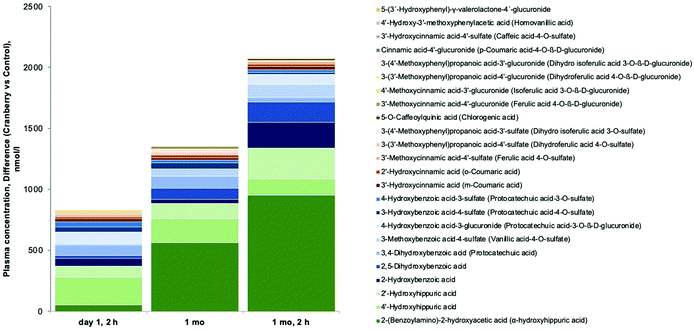 | ||
| Fig. 4 Overview of changes in plasma concentrations of (poly)phenol metabolites. Stacked columns of (poly)phenol metabolites changes on day 1 at 2 h, 1 month and 1 month, 2 hours (relative to baseline) that were significantly increased by cranberry as compared to control that were significant and positive (see ESI Table S4†). | ||
With regard to dominant (poly)phenols at different timepoints, cranberry led to a significantly greater increase over baseline as compared to control of 13 (poly)phenols at 2 h (2′-hydroxyhippuric acid (226 μmol L−1 [95% CI: 94 μM, 358 μM]), cinnamic acid-4′-glucuronide, 2,5-dihydroxybenzoic acid, 3-(3′-methoxyphenyl)propanoic acid-4′-glucuronide, 3-methoxybenzoic acid-4-sulfate, 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide, 3′-hydroxycinnamic acid, 2′-hydroxycinnamic acid, 3′-methoxycinnamic acid-4′-glucuronide, 4-hydroxybenzoic acid-3-glucuronide, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, 5-O-caffeoylquinic acid, and 3′-hydroxycinnamic acid-4′-sulfate) and 13 at 1 month (a-hydroxyhippuric acid (564 μM [95% CI: 61 μM, 1057 μM]), 2′-hydroxyhippuric acid, 2,5-dihydroxybenzoic acid, 4′-hydroxyhippuric acid, 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide, 3-(3′-methoxyphenyl)propanoic acid-4′-sulfate, 3-methoxybenzoic acid-4-sulfate, 4′-methoxycinnamic acid-3′-glucuronide, 3′-hydroxycinnamic acid, 3′-methoxycinnamic acid-4′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, 3,4-dihydroxybenzoic acid, 4-hydroxybenzoic acid-3-glucuronide).
Plasma (poly)phenol metabolites as independent predictors of changes in FMD
A multi-variable stepwise linear regression analysis showed that plasma cinnamic acid-4′-glucuronide, 4-hydroxybenzoic acid-3-sulfate, 2,5-dihydroxybenzoic acid, 3′-hydroxycinnamic acid, and 5-O-caffeoylquinic acid were significant independent predictors of 2 h FMD effects that together explained 71% of variability in FMD changes (R2 = 0.71). One month chronic effects were predicted by 3′-hydroxycinnamic acid, 4′-methoxycinnamic acid-3′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, and 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide together explaining 52% of FMD variability (R2 = 0.52).Change of metabolite excretion profile over time
Of the 137 targeted metabolites, we detected 79 and quantified 74 cranberry (poly)phenol metabolites in 24 h urine. The urinary excretion of PVLs and PVAs reached mean values ranging from 0.1 to 10 excreted μmol (see Fig. 5 and ESI Table S5†). Quantitatively, the most excreted metabolites were 5-(4′-hydroxyphenyl)-γ-valerolactone-3′-glucuronide, 5-(3′-hydroxyphenyl)-γ-valerolactone-4′-glucuronide, 5-(phenyl)-γ-valerolactone-sulfate-glucuronide and 5-(hydroxyphenyl)-γ-valerolactone-sulfate (sum of two 3′,4′ isomers). Other compounds quantified in urine samples for most of the subjects were 5-phenyl-γ-valerolactone-4′-glucuronide, 5-phenyl-γ-valerolactone-3′-glucuronide, 5-phenyl-γ-valerolactone-3′-sulfate, and 5-phenylvaleric acid-sulfate-glucuronide. As in plasma, the most abundant urinary metabolites belonged to the families of phenylacetic acids, hippuric acids, benzoic acids, and cinnamic acids.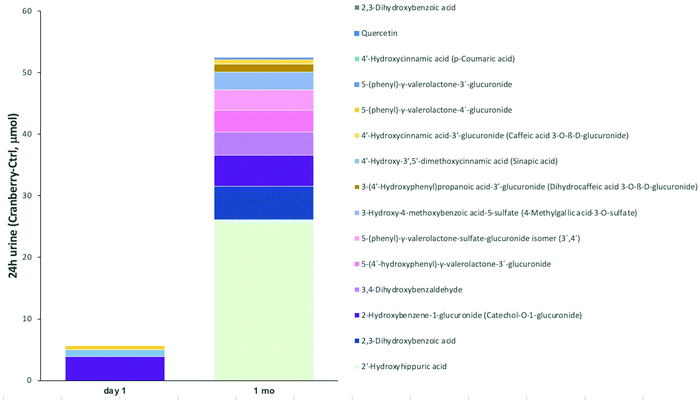 | ||
| Fig. 5 Overview of changes in 24-hour urinary excretion of (poly)phenol metabolites. Stacked columns show differences of excreted (poly)phenol metabolite amounts between cranberry and control at day 1 and 1 month that were significantly increased by cranberry as compared to control and positive (see ESI Table S5†). | ||
Cranberry powder consumption led to significantly higher excretion of 4 and 13 (poly)phenol metabolites in 24 h urine as compared to control at day 1 and 1 month, respectively. On day 1, the 4 compounds that were significantly higher in the cranberry group were phenylacetic acid (51 μmol [95% CI: 6 μmol, 95 μmol]), 2-hydroxybenzene-1-glucuronide, 4′-hydroxy-3′,5′-dimethoxycinnamic acid, and 5-phenyl-γ-valerolactone-4′-glucuronide. At 1 month postfd1consumption, the major changes were found for 2′-hydroxyhippuric acid (26 μmol [95% CI: 7 μmol, 45 μmol]), 2,5-dihydroxybenzoic acid, 2-hydroxybenzene-1-glucuronide, 3,4-dihydroxybenzaldehyde, 5-(4′-hydroxyphenyl)-γ-valerolactone-3′-glucuronide, 5-(phenyl)-γ-valerolactone-sulfate-glucuronide, 3-hydroxy-4-methoxybenzoic acid-5-sulfate, 3-(4′-hydroxyphenyl)propanoic acid-3′-glucuronide, 4′-hydroxycinnamic acid-3′-glucuronide, 5-phenyl-γ-valerolactone-3′-glucuronide, 4′-hydroxycinnamic acid, quercetin, and 2,3-dihydroxybenzoic acid. Interestingly, at day 1 cranberry led to significantly decreased excretion of 7 (poly)phenol metabolites (3-(3′-methoxyphenyl)propanoic acid-4′-glucuronide (−21 μmol [95% CI: −41 μmol, −2 μmol]), 2,6-dihydroxybenzene-1-sulfate, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, 3-(4′-hydroxyphenyl)propanoic acid-3′-sulfate, 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid, 3-(4′-hydroxyphenyl)propanoic acid-3′-glucuronide, 3-(3′-methoxyphenyl)propanoic acid-4′-sulfate).
In 16 metabolites, there was a significant difference between cranberry associated differences at day 1 and 1 month. In 15 metabolites there was a greater cranberry associated increase in excreted metabolites (4-hydroxybenzoic acid-sulfate (2546 μmol [95% CI: 329 μmol, 4763 μmol]), 3-hydroxyphenylacetic acid, 3′-methoxycinnamic acid-4′-sulfate, 3-(3′-methoxyphenyl)propanoic acid-4′-glucuronide, 2-hydroxyhippuric acid, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, 3-(4′-hydroxyphenyl)propanoic acid-3′-sulfate, 3,4-dihydroxybenzaldehyde, 3-(4′-hydroxy-3′-methoxyphenyl)propanoic acid, 2,5-dihydroxybenzoic acid, 3-(4′-hydroxyphenyl)propanoic acid-3′-glucuronide, quercetin, quercetin-7-glucuronide, 4-hydroxybenzoic acid-3-glucuronide, and 2,3-dihydroxybenzoic acid) and phenylacetic acid excretion decreased (−78 μmol [95% CI: −134 μmol, −21 μmol]).
Discussion
In this study, we found significant improvements in our primary outcome, changes in endothelial function measured as FMD at 2 hours after first consumption and chronic effects determined fasted after 1 month of daily consumption of cranberry powder as compared to control treatment in a group of healthy men. Importantly, different patterns of metabolites predicted a large proportion of the effect.To our knowledge, this is the first study to investigate improvements in vascular function after daily whole cranberry powder intake in healthy humans. Only one study has tested the chronic effect of cranberry juice on FMD and PWV in subjects with coronary artery disease.10 In an acute pilot study of this paper, they observed significant increases in (upper arm occlusion) FMD at 4 h after cranberry juice containing 834 mg polyphenols similar to our previous study in healthy volunteers14 and the present study. However, in a consecutive 4 weeks randomized, controlled double-blind study also performed in CAD patients showed no significant effects on FMD, blood pressure, blood lipids and glucose but a significant decrease in PWV was detected.10 This discrepancy of results could be explained by many factors including different methodology, populations, medication, or differences in bioavailability or compliance. Unfortunately, plasma or urinary (poly)phenol concentrations were not reported in the study. Notably, these patients were obese, on regular medication including lipid lowering medication, ACEI/ARBs and platelet inhibitors and there was a large proportion of people with diabetes, arterial hypertension and smokers. The methodology of measuring FMD with upper arm occlusion significantly differs from the lower arm occlusion method used in the present study and comparisons need to made with caution.28
The acute FMD increase (1.5 ± 0.4%) in the current study with cranberry powder containing 525 mg total polyphenols is comparable to the effect that would be expected based on our previous dose–response study with cranberry juice achieving a maximal response of about 1.7 ± 0.2%, with a dose to achieve half-maximal effects at 442 mg14 (Fig. S1†). While the effect size appears comparable, the composition of cranberry products differed considerably in some regards. For instance, juice with the same amount of total (poly)phenols has a higher proportion of PACs, but lower amounts of anthocyanins and flavonols.17,18 Bearing in mind that PACs are barely, if even at all, absorbed in the upper gastrointestinal tract after acute consumption, the role of the other flavonoids (anthocyanins, phenolic acids, and flavonols) in the intervention products might be more relevant in mediating early FMD responses. Only a few studies have linked the presence of plasma (poly)phenol metabolites with improvements in biomarkers for cardiovascular health.14,29–31
Despite the fact that PAC oligomers and polymers are rather not absorbed acutely (2 h), they are metabolised to some extent by the gut microbiota after chronic consumption.32 Numerous in vivo and in vitro studies indicate that proanthocyanin dimers and flavanol monomers can be degraded into phenylvaleric acids (PVAs) and phenyl-γ-valerolactone (PVLs) derivatives, phenylacetic acids, propionic acids, hydroxybenzoic acids, hydroxycinnamic acids and hippuric acids by the gut microbiota.33–40 Studies on the metabolic fate of anthocyanins, which were the second highest abundant (poly)phenols in the powder, indicates that they undergo colonic degradation to form a plethora of phenolic acids including hydroxycinnamic acids, benzaldehydes, propionic acids, phenylacetic acids, and benzoic acids.41,42 Interestingly, we observed that after 1 month cranberry consumption the pattern of metabolites that increased at 2 h changed as compared to acute metabolites on day 1 of the study. This may be explained by changes in the expression of metabolising enzymes or could be interpreted as indirect evidence that chronic consumption of cranberry may modulate the gut microbiota, which plays a major role in polyphenol metabolism. However, whether cranberry consumption affects the gut microbiota needs to be confirmed in future studies.
To investigate the relationship between the circulating cranberry related (poly)phenol metabolites and vascular function improvements, we performed a targeted analysis of 138 and quantified 56 of the 64 detectable cranberry-derived (poly)phenol metabolites in plasma upon cranberry powder and placebo consumption (ESI Table S1†). In addition, bioavailability data previously published by our group was used to compare the plasma (poly)phenol concentration with that after acute intake of cranberry juices.15 The total plasma (poly)phenols at 2 h did not differ between juice and powder. However, the abundance of individual (poly)phenol metabolites differed between juice and powder. The highest plasma concentration changes at 2 h after consumption of the powder were phenylacetic acids (phenylacetic acid, 4-hydroxyphenylacetic acid, and 3-hydroxyphenylacetic acid) accounting for 80% of detected metabolites. Upon intake of juice, hippuric acids (mainly α-hydroxyhippuric acid and 4′-hydroxyhippuric acid) together with benzene diols (2-hydroxybenzene-1-sulfate and 2-hydroxy-4-methylbenzene-1-sulfate) increased the most, accounting for 79% of all (poly)phenols quantified in plasma. All of these metabolites have been reported as breakdown products of anthocyanins. However, without adequate isotope- or radiolabelled standards it is difficult to establish if these compounds, which are also abundant endogenous metabolites and could come from microbial metabolism of other (poly)phenols present in the diet or other metabolic processes, are indeed anthocyanin metabolites.42 Nevertheless, only a distinct set of metabolites correlated with and statistically predicted FMD changes after consumption of the powder in comparison with the juice. In the current study, cinnamic acid-4′-glucuronide, 4-hydroxybenzoic acid-3-sulfate, 2,5-dihydroxybenzoic acid, 3′-hydroxycinnamic acid, and 5-O-caffeoylquinic acid were significant independent predictors of 2 h FMD effects (R2 = 0.71) and 3′-hydroxycinnamic acid, 4′-methoxycinnamic acid-3′-glucuronide, 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate, and 3-(4′-methoxyphenyl)propanoic acid-3′-glucuronide of 1 month chronic effects (R2 = 0.52). From the metabolites found 2 h after intake of the cranberry powder that correlated with FMD changes in the current study (9 compounds), only 3-(4′-methoxyphenyl)propanoic acid-3′-sulfate was found to also correlate with FMD changes at 2 h after intake of the juice (8 compounds).14 The non-straightforward complexity of associations points toward potential differences in mechanisms of action among cranberry products despite apparent similar efficacy to improve vascular function. We cannot discard though important confounding factors such as the effects of the background diet of volunteers participating in both studies, and the effect of (poly)phenols found in different matrices (solid vs. liquid, powder vs. juice) with potentially different bioaccessibility and different kinetics in biological fluids.
The association between specific metabolites and improvements in FMD may help to gain insight into potential mechanisms of action of cranberry (poly)phenols. While association is not causation, our data can form the basis of hypotheses to be tested in future studies. For instance, we cautiously hypothesise that the metabolites that correlated with 2 h FMD improvements are causally related to these improvements. Due to the timeframe of responses and close temporal association, the mechanisms are likely not related to changes on a gene transcription or epigenetic level, but rather relate to modulation of signalling pathways directly or indirectly affecting FMD. These could be receptor mediated, posttranscriptional enzyme modulatory (eNOS activity) or even related to physicochemical effects (e.g. redox balance) and they could involve flow sensing systems or signal transduction (AKT/PI3K) down to eNOS in endothelial cells, NO bioavailability or smooth muscle responses to NO (sGC, PD). The correlations with the effects at 1 month may include effects on the level of expression genes directly (e.g. eNOS or AKT143,44) or indirectly (e.g. GLUT445) related to vascular function, and potentially involving transcription factors.46,47 These effects are harder to interpret as the presence of one metabolite at a certain timepoint is compared with an effect that was likely triggered a long time ago and potentially by different metabolites. The fact that chronic effects are not further increasing by acute consumption at 1 month as seen with other food bioactives,48 may mean that acute and chronic effects are linked or saturated. Taken together, our current results deliver a set of promising metabolite candidates to be tested as isolated compounds in physiologically relevant model systems (e.g. mouse FMD43 and concentrations.
A few potential limitations not previously mentioned are worth discussing. Firstly, the study was conducted in a healthy cohort of young male volunteers and is, therefore, limited in its generalisability towards the general population and people at increased risk. Similar to most other studies in the field, the short, 1-month duration of our study limits conclusions with regards to potential clinically relevant health benefits that would require that the short-term vascular protective effects are maintained over long periods of time. Along these lines, some potential health benefits that may take longer to manifest may be not detected. For instance, one would expect that sustained improvement in endothelial function over longer time periods in the order of years could protect blood vessels and thereby slow age-related vascular stiffening which would be detected as a slower rate of PWV increase over time.49 Of note, the lack of cranberry effect on PWV in the current study does not reflect arterial stiffness as a marker vascular ageing but rather functional arterial stiffness which can be affected by bioactives such as cocoa flavanols within hours.50–52 Another limitation is that, although volunteers followed a restricted (poly)phenol diet during the 24 h urinary collection period, we cannot discard that they may not have been fully compliant with the low (poly)phenol diet given to them. Finally, we tried to link the vascular function improvements with plasma (poly)phenol metabolite concentrations quantified at the same time of the measurements providing the most comprehensive metabolomic analysis to date. While the results of the regression analysis are limited by the small sample size they provide important correlative and hypothesis generating insight. It is tempting to assume that the identified metabolites may be bioactive compounds mediating improvement in FMD, but they may also only be a marker of some other underlying process. The significant correlations described herein should rather be taken into account when planning future mechanistic studies to try and establish cause-and-effect relationships using isolated compounds.
In conclusion, our current study shows that acute (2 h) and chronic (1 month) consumption of a freeze-dried whole cranberry powder equivalent to 100 g fresh cranberries improved endothelial function in healthy young males. The findings were paralleled by significant increases in total plasma (poly)phenols and patterns of (poly)phenol metabolites statistically explained a large proportion of changes in FMD. Moreover, the amounts of cranberry used in this work could realistically be achieved daily, which further underlines the relevance of this study in the context of primary prevention of CVD in the general population.
Data share statement
Data described in the manuscript, code book, and analytic code will be made available upon request to the authors.Abbreviations
| AIx | Aortic augmentation index |
| CVD | Cardiovascular disease |
| CAD | Coronary artery disease |
| CHD | Coronary heart disease |
| FMD | Flow-mediated dilation |
| PWV | Pulse wave velocity |
| RCT | Randomized controlled trial |
| TP | Total (poly)phenols |
| ACN | Anthocyanins |
| PAC | Proanthocyanidins |
| PVLs | Phenyl-γ-valerolactones |
| PVAs | Phenylvaleric acids |
Author contributions
Conceptualization: Ana Rodriguez-Mateos, Christian Heiss; methodology: Geoffrey Istas, Rodrigo R. P. Feliciano and Brian Wang; investigation: Ana Rodriguez-Mateos, Geoffrey Istas, Timon Weber, Rodrigo R. P. Feliciano, Brian Wang, Claudia Favari, Pedro Mena; data Curation: Ana Rodriguez-Mateos and Christian Heiss; statistical analysis: Geoffrey Istas, Christian Heiss; writing-original draft preparation: Christian Heiss, and Rodriguez-Mateos, Geoffrey Istas, Brian Wang; writing-review & editing: Ana Rodriguez-Mateos, Christian Heiss, Claudia Favari, Pedro Mena, Daniele Del Rio; funding acquisition: Ana Rodriguez-Mateos, Christian Heiss, Pedro Mena and Daniele Del Rio.Conflicts of interest
The authors declare no conflict of interest other than the acknowledged funding sources.Acknowledgements
This study was funded by the Cranberry Institute and by the Research Committee of the Medical Faculty of Heinrich-Heine University Dusseldorf (grant number 9772574). The authors also acknowledge a Susanne Bunnenberg Heart Foundation grant to Dusseldorf Heart Centre.References
- A. Rodriguez-Mateos, C. Heiss, G. Borges and A. Crozier, Berry (poly)phenols and cardiovascular health, J. Agric. Food Chem., 2014, 62(18), 3842–3851 Search PubMed.
- G. Cunningham David, A. Vannozzi Sarah, R. Turk, R. Roderick, E. O'Shea and K. Brilliant, Cranberry Phytochemicals and Their Health Benefits, American Chemical Society, 2003, pp. 35–51 Search PubMed.
- E. Pappas and K. M. Schaich, Phytochemicals of Cranberries and Cranberry Products: Characterization, Potential Health Effects, and Processing Stability, Crit. Rev. Food Sci. Nutr., 2009, 49(9), 741–781 Search PubMed.
- L. Gu, M. A. Kelm, J. F. Hammerstone, G. Beecher, J. Holden, D. Haytowitz and R. L. Prior, Screening of Foods Containing Proanthocyanidins and Their Structural Characterization Using LC-MS/MS and Thiolytic Degradation, J. Agric. Food Chem., 2003, 51(25), 7513–7521 Search PubMed.
- R. P. Feliciano, J. J. Meudt, D. Shanmuganayagam, C. G. Krueger and J. D. Reed, Ratio of “A-type” to “B-type” proanthocyanidin interflavan bonds affects extra-intestinal pathogenic Escherichia coli invasion of gut epithelial cells, J. Agric. Food Chem., 2014, 62(18), 3919–3925 Search PubMed.
- J. B. Blumberg, T. A. Camesano, A. Cassidy, P. Kris-Etherton, A. Howell, C. Manach, L. M. Ostertag, H. Sies, A. Skulas-Ray and J. A. Vita, Cranberries and Their Bioactive Constituents in Human Health, Adv. Nutr., 2013, 4(6), 618–632 Search PubMed.
- A. B. Howell, J. D. Reed, C. G. Krueger, R. Winterbottom, D. G. Cunningham and M. Leahy, A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity, Phytochemistry, 2005, 66(18), 2281–2291 Search PubMed.
- G. Ruel, A. Lapointe, S. Pomerleau, P. Couture, S. Lemieux, B. Lamarche and C. Couillard, Evidence that cranberry juice may improve augmentation index in overweight men, Nutr. Res., 2013, 33(1), 41–49 Search PubMed.
- I. T. Lee, Y. C. Chan, C. W. Lin, W. J. Lee and W. H. Sheu, Effect of cranberry extracts on lipid profiles in subjects with Type 2 diabetes, Diabetic Med., 2008, 25(12), 1473–1477 Search PubMed.
- M. M. Dohadwala, M. Holbrook, N. M. Hamburg, S. M. Shenouda, W. B. Chung, M. Titas, M. A. Kluge, N. Wang, J. Palmisano and P. E. Milbury, et al., Effects of cranberry juice consumption on vascular function in patients with coronary artery disease, Am. J. Clin. Nutr., 2011, 93(5), 934–940 Search PubMed.
- D. S. Hsia, D. J. Zhang, R. S. Beyl, F. L. Greenway and C. Khoo, Effect of daily consumption of cranberry beverage on insulin sensitivity and modification of cardiovascular risk factors in adults with obesity: a pilot, randomised, placebo-controlled study, Br. J. Nutr., 2020, 124(6), 577–585 Search PubMed.
- B. Chew, B. Mathison, L. Kimble, D. McKay, K. Kaspar, C. Khoo, C. O. Chen and J. Blumberg, Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: a randomized controlled trial, Eur. J. Nutr., 2019, 58(3), 1223–1235 Search PubMed.
- J. A. Novotny, D. J. Baer, C. Khoo, S. K. Gebauer and C. S. Charron, Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults, J. Nutr., 2015, 145(6), 1185–1193 Search PubMed.
- A. Rodriguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 1–11 Search PubMed.
- R. P. Feliciano, C. E. Mills, G. Istas, C. Heiss and A. Rodriguez-Mateos, Absorption, Metabolism and Excretion of Cranberry (Poly)phenols in Humans: A Dose Response Study and Assessment of Inter-Individual Variability, Nutrients, 2017, 9(3), 268 Search PubMed.
- C. Favari, P. Mena, C. Curti, G. Istas, C. Heiss, D. Del Rio and A. Rodriguez-Mateos, Kinetic profile and urinary excretion of phenyl-gamma-valerolactones upon consumption of cranberry: a dose-response relationship, Food Funct., 2020, 11(5), 3975–3985 Search PubMed.
- J. B. Blumberg, A. Basu, C. G. Krueger, M. A. Lila, C. C. Neto, J. A. Novotny, J. D. Reed, A. Rodriguez-Mateos and C. D. Toner, Impact of Cranberries on Gut Microbiota and Cardiometabolic Health: Proceedings of the Cranberry Health Research Conference 2015, Adv. Nutr., 2016, 7(4), 759S–770S Search PubMed.
- A. Rodriguez-Mateos, R. P. Feliciano, A. Boeres, T. Weber, C. N. dos Santos, M. R. Ventura and C. Heiss, Cranberry (poly)phenol metabolites correlate with improvements in vascular function: A double-blind, randomized, controlled, dose-response, crossover study, Mol. Nutr. Food Res., 2016, 60(10), 2130–2140 Search PubMed.
- C. G. Krueger, J. D. Reed, R. P. Feliciano and A. B. Howell, Quantifying and characterizing proanthocyanidins in cranberries in relation to urinary tract health, Anal. Bioanal. Chem., 2013, 405(13), 4385–4395 Search PubMed.
- E. R. Gullickson, C. G. Krueger, A. Birmingham, M. Maranan and J. D. Reed, Development of a Cranberry Standard for Quantification of Insoluble Cranberry (Vaccinium macrocarpon Ait.) Proanthocyanidins, J. Agric. Food Chem., 2020, 68(10), 2900–2905 Search PubMed.
- M. A. Martín, S. Ramos, R. Mateos, J. P. J. Marais, L. Bravo-Clemente, C. Khoo and L. Goya, Chemical characterization and chemo-protective activity of cranberry phenolic powders in a model cell culture. Response of the antioxidant defenses and regulation of signaling pathways, Food Res. Int., 2015, 71, 68–82 Search PubMed.
- A. A. Mulligan, R. N. Luben, A. Bhaniani, D. J. Parry-Smith, L. O'Connor, A. P. Khawaja, N. G. Forouhi and K. T. Khaw, A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability, BMJ Open, 2014, 4(3), e004503 Search PubMed.
- A. Rodriguez-Mateos, M. Hezel, H. Aydin, M. Kelm, J. O. Lundberg, E. Weitzberg, J. P. E. Spencer and C. Heiss, Interactions between cocoa flavanols and inorganic nitrate: Additive effects on endothelial function at achievable dietary amounts, Free Radicals Biol. Med., 2014, 80, 121–128 Search PubMed.
- A. Rodriguez-Mateos, T. Cifuentes-Gomez, I. Gonzalez-Salvador, J. I. Ottaviani, H. Schroeter, M. Kelm, C. Heiss and J. P. Spencer, Influence of age on the absorption, metabolism, and excretion of cocoa flavanols in healthy subjects, Mol. Nutr. Food Res., 2015, 59(8), 1504–1512 Search PubMed.
- R. P. Feliciano, E. Mecha, M. R. Bronze and A. Rodriguez-Mateos, Development and validation of a high-throughput micro solid-phase extraction method coupled with ultra-high-performance liquid chromatography-quadrupole time-of-flight mass spectrometry for rapid identification and quantification of phenolic metabolites i, J. Chromatogr. A, 2016, 1464, 21–31 Search PubMed.
- N. Brindani, P. Mena, L. Calani, I. Benzie, S. W. Choi, F. Brighenti, F. Zanardi, C. Curti and D. Del Rio, Synthetic and analytical strategies for the quantification of phenyl-gamma-valerolactone conjugated metabolites in human urine, Mol. Nutr. Food Res., 2017, 61(9), 1700077 Search PubMed.
- F. Castello, G. Costabile, L. Bresciani, M. Tassotti, D. Naviglio, D. Luongo, P. Ciciola, M. Vitale, C. Vetrani and G. Galaverna, et al., Bioavailability and pharmacokinetic profile of grape pomace phenolic compounds in humans, Arch. Biochem. Biophys., 2018, 646, 1–9 Search PubMed.
- K. L. Berry, R. A. Skyrme-Jones and I. T. Meredith, Occlusion cuff position is an important determinant of the time course and magnitude of human brachial artery flow-mediated dilation, Clin. Sci., 2000, 99(4), 261–267 Search PubMed.
- C. Valls-Pedret, R. M. Lamuela-Raventós, A. Medina-Remón, M. Quintana, D. Corella, X. Pintó, M.Á Martínez-González, R. Estruch and E. Ros, Polyphenol-rich foods in the Mediterranean diet are associated with better cognitive function in elderly subjects at high cardiovascular risk, J. Alzheimer's Dis., 2012, 29(4), 773–782 Search PubMed.
- G. Istas, R. P. Feliciano, T. Weber, R. Garcia-Villalba, F. Tomas-Barberan, C. Heiss and A. Rodriguez-Mateos, Plasma urolithin metabolites correlate with improvements in endothelial function after red raspberry consumption: A double-blind randomized controlled trial, Arch. Biochem. Biophys., 2018, 651, 43–51 Search PubMed.
- A. Rodriguez-Mateos, C. Rendeiro, T. Bergillos-Meca, S. Tabatabaee, T. W. George, C. Heiss and J. P. Spencer, Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: a randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity, Am. J. Clin. Nutr., 2013, 98(5), 1179–1191 Search PubMed.
- J. I. Ottaviani, C. Kwik-Uribe, C. L. Keen and H. Schroeter, Intake of dietary procyanidins does not contribute to the pool of circulating flavanols in humans, Am. J. Clin. Nutr., 2012, 95(4), 851–858 Search PubMed.
- M. M. Appeldoorn, J. P. Vincken, A. M. Aura, P. C. Hollman and H. Gruppen, Procyanidin dimers are metabolized by human microbiota with 2-(3,4-dihydroxyphenyl)acetic acid and 5-(3,4-dihydroxyphenyl)-gamma-valerolactone as the major metabolites, J. Agric. Food Chem., 2009, 57(3), 1084–1092 Search PubMed.
- X. Meng, S. Sang, N. Zhu, H. Lu, S. Sheng, M. J. Lee, C. T. Ho and C. S. Yang, Identification and characterization of methylated and ring-fission metabolites of tea catechins formed in humans, mice, and rats, Chem. Res. Toxicol., 2002, 15(8), 1042–1050 Search PubMed.
- K. Ou, P. Sarnoski, K. R. Schneider, K. Song, C. Khoo and L. Gu, Microbial catabolism of procyanidins by human gut microbiota, Mol. Nutr. Food Res., 2014, 58(11), 2196–2205 Search PubMed.
- M. Margalef, Z. Pons, F. I. Bravo, B. Muguerza and A. Arola-Arnal, Plasma kinetics and microbial biotransformation of grape seed flavanols in rats, J. Funct. Foods, 2015, 12, 478–488 Search PubMed.
- A. Engemann, F. Hubner, S. Rzeppa and H. U. Humpf, Intestinal metabolism of two A-type procyanidins using the pig cecum model: detailed structure elucidation of unknown catabolites with Fourier transform mass spectrometry (FTMS), J. Agric. Food Chem., 2012, 60(3), 749–757 Search PubMed.
- L. Y. Rios, M. P. Gonthier, C. Remesy, I. Mila, C. Lapierre, S. A. Lazarus, G. Williamson and A. Scalbert, Chocolate intake increases urinary excretion of polyphenol-derived phenolic acids in healthy human subjects, Am. J. Clin. Nutr., 2003, 77(4), 912–918 Search PubMed.
- R. P. Feliciano, A. Boeres, L. Massacessi, G. Istas, M. R. Ventura, C. Audia, N. D. Santos, C. Heiss and A. Rodriguez-Mateos, Identification and quantification of novel cranberry-derived plasma and urinary (poly)phenols, Arch. Biochem. Biophys., 2016, 599, 31–41 Search PubMed.
- P. Mena, L. Bresciani, N. Brindani, I. A. Ludwig, G. Pereira-Caro, D. Angelino, R. Llorach, L. Calani, F. Brighenti and M. N. Clifford, et al., Phenyl-gamma-valerolactones and phenylvaleric acids, the main colonic metabolites of flavan-3-ols: synthesis, analysis, bioavailability, and bioactivity, Nat. Prod. Rep., 2019, 36(5), 714–752 Search PubMed.
- R. M. de Ferrars, C. Czank, Q. Zhang, N. P. Botting, P. A. Kroon, A. Cassidy and C. D. Kay, The pharmacokinetics of anthocyanins and their metabolites in humans, Br. J. Pharmacol., 2014, 171(13), 3268–3282 Search PubMed.
- R. Gonzalez-Barrio, C. A. Edwards and A. Crozier, Colonic catabolism of ellagitannins, ellagic acid, and raspberry anthocyanins: in vivo and in vitro studies, Drug Metab. Dispos., 2011, 39(9), 1680–1688 Search PubMed.
- D. Schuler, R. Sansone, T. Freudenberger, A. Rodriguez-Mateos, G. Weber, T. Y. Momma, C. Goy, J. Altschmied, J. Haendeler and J. W. Fischer, et al., Measurement of endothelium-dependent vasodilation in mice–brief report, Arterioscler., Thromb., Vasc. Biol., 2014, 34(12), 2651–2657 Search PubMed.
- P. Tsakiroglou, J. Weber, S. Ashworth, C. Del Bo and D. Klimis-Zacas, Angiogenesis is Differentially Modulated by Anthocyanin and Phenolic Acid Extracts from Wild Blueberry (V. angustifolium) Through PI3K Pathway, J. Med. Food, 2021, 24(3), 226–235 Search PubMed.
- T. Wallerath, H. Li, U. Godtel-Ambrust, P. M. Schwarz and U. Forstermann, A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase, Nitric Oxide, 2005, 12(2), 97–104 Search PubMed.
- K. D. Croft, Y. Yamashita, H. O'Donoghue, D. Shirasaya, N. C. Ward and H. Ashida, Screening plant derived dietary phenolic compounds for bioactivity related to cardiovascular disease, Fitoterapia, 2018, 126, 22–28 Search PubMed.
- D. Milenkovic, A. Rodriguez-Mateos, M. Lucosz, G. Istas, K. Declerck, R. Sansone, R. Deenen, K. Kohrer, K. F. Corral-Jara and J. Altschmied, et al., Flavanol Consumption in Healthy Men Preserves Integrity of Immunological-Endothelial Barrier Cell Functions: Nutri(epi)genomic Analysis, Mol. Nutr. Food Res., 2022, e2100991 Search PubMed.
- R. Sansone, A. Rodriguez-Mateos, J. Heuel, D. Falk, D. Schuler, R. Wagstaff, G. G. Kuhnle, J. P. Spencer, H. Schroeter and M. W. Merx, et al., Cocoa flavanol intake improves endothelial function and Framingham Risk Score in healthy men and women: a randomised, controlled, double-masked trial: the Flaviola Health Study, Br. J. Nutr., 2015, 114(8), 1246–1255 Search PubMed.
- A. Diaz, M. Tringler, S. Wray, A. J. Ramirez and E. I. Cabrera Fischer, The effects of age on pulse wave velocity in untreated hypertension, J. Clin. Hypertens., 2018, 20(2), 258–265 Search PubMed.
- C. Heiss, R. Sansone, H. Karimi, M. Krabbe, D. Schuler, A. Rodriguez-Mateos, T. Kraemer, M. M. Cortese-Krott, G. G. Kuhnle and J. P. Spencer, et al., Impact of cocoa flavanol intake on age-dependent vascular stiffness in healthy men: a randomized, controlled, double-masked trial, Age, 2015, 37(3), 9794 Search PubMed.
- R. Sansone, J. I. Ottaviani, A. Rodriguez-Mateos, Y. Heinen, D. Noske, J. P. Spencer, A. Crozier, M. W. Merx, M. Kelm and H. Schroeter, et al., Methylxanthines enhance the effects of cocoa flavanols on cardiovascular function: randomized, double-masked controlled studies, Am. J. Clin. Nutr., 2017, 105(2), 352–360 Search PubMed.
- A. Rodriguez-Mateos, T. Weber, S. S. Skene, J. I. Ottaviani, A. Crozier, M. Kelm, H. Schroeter and C. Heiss, Assessing the respective contributions of dietary flavanol monomers and procyanidins in mediating cardiovascular effects in humans: randomized, controlled, double-masked intervention trial, Am. J. Clin. Nutr., 2018, 108(6), 1229–1237 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2fo00080f |
| This journal is © The Royal Society of Chemistry 2022 |