Open Access Article
Open Access ArticleLeafy vegetables fortification enhanced the nutritional profile and reduced the glycemic index of yellow cassava pasta
Oluranti M.
Lawal
 ab,
Vincenzo
Fogliano
ab,
Vincenzo
Fogliano
 a,
Imke
Rotte
a,
Tayo N.
Fagbemi
b,
Matthijs
Dekker
a and
Anita R.
Linnemann
*a
a,
Imke
Rotte
a,
Tayo N.
Fagbemi
b,
Matthijs
Dekker
a and
Anita R.
Linnemann
*a
aFood Quality and Design, Wageningen University and Research, P.O. Box 17, 6700 AA Wageningen, The Netherlands. E-mail: anita.linnemann@wur.nl; Tel: +31 317 482520
bFood Science and Technology Department, Federal University of Technology, Akure, Nigeria
First published on 11th May 2022
Abstract
Food-to-food fortification of yellow cassava flour with leafy vegetable powders (Amaranthus and Telfairia occidentalis) was employed in this study to develop cassava-vegetable spaghetti-like pasta products (YP, YPA5, YPA10, YPU5, YPU10, YPA5O). The nutritional profile, micronutrient retention, bioaccessibility, starch digestibility and in vitro glycemic index were assessed. The incorporation of leafy vegetable powder enhanced the nutritional quality of the yellow cassava pasta (YCP) products. The fortification increased (up to 3-fold) the protein in fortified YCP, increased the fibre (11%), doubled the ash and increased the beta-carotene (about 7-fold), iron (72%) and zinc contents by 10%. The phenolic content of fluted pumpkin leaf-fortified pasta with 10% leaf powder inclusion (YPU10) was 1100 μg GAE g−1, almost four times higher than that of the unfortified YCP. Leaf powders in the cassava pasta also favoured the retention of micronutrients during cooking and slowed down the starch digestibility. The retention during cooking was up to 91% in YPU10 for beta-carotene with no loss in iron, while the bioaccessibility of beta-carotene was impeded, the zinc retention was high and became significantly more bioaccessible with leaf addition and cooking. The estimated glycemic index of YCP was reduced by 19% and 15% in YPU10 and YPA10, respectively. The inclusion of the vegetables also reduced the glycemic index of the fortified YCP. Thus, adding leafy vegetable powder up to 10% into YCP is a promising approach to both valorise yellow provitamin A biofortified cassava and enhance the nutritional value.
1. Introduction
Cassava (Manihot esculenta Crantz) is an important African staple crop. However, the conventional, white-fleshed variety is deficient in protein and several micronutrients, of which vitamin A, iron, and zinc are especially lacking in cassava-based diets.1 Thus, yellow-fleshed cassava varieties, biofortified with provitamin A carotenoids and low in cyanide, were developed in large-scale breeding programmes.2 These varieties provide nutritional benefits over the white-fleshed cultivars.1,3 Thus, since 2011, provitamin A biofortified cassava varieties are being promoted and have received appreciable consumer acceptance in several sub-Saharan African (SSA) countries.4 Yellow cassava shows excellent potential to alleviate vitamin A deficiency but is still poor in iron and zinc. Moreover, studies revealed post-processing losses of beta-carotene up to 70% in yellow cassava products.5,6 In SSA, leafy vegetables are abundant, affordable and good sources of essential amino acids, fibre, vitamins and minerals (especially iron and zinc).7 Leafy vegetables are commonly prepared by boiling, stewing, frying and blanching, and eaten as a sauce or so-called soup with starchy staples such as cassava. Despite the health benefits, the current consumption of vegetables is insufficient to meet the daily requirements of people living on cassava-based diets.7 Raaijmakers et al.8 and Bakker et al.,9 also reported the low vegetable intake in Nigeria and Rwanda. Thus, fortifying starchy staples with leafy vegetable powder provides an effective and convenient means of ensuring vegetable consumption among the consumers. Food-to-food fortification is thus an emerging approach, mostly implemented in the developing world, to complement other strategies in combating micronutrient deficiencies.10,11 To date, this approach has not been used with biofortified yellow cassava. Based on compositional data, a food product combining leafy vegetables and yellow cassava into a convenient and popular food, such as pasta, seems a way to address the still prevailing nutritional deficiencies. Leafy vegetables can be dried and made into leaf powder, simplifying the possibilities for food-to-food fortification of staple foods with leafy vegetables12 such as fluted pumpkin (Telfairia occidentalis) and amaranth (Amaranthus cruentus), the two most preferred leafy vegetables in Nigeria.3Previous studies examined the rich nutritional profile of these leafy vegetables. Lawal et al.13 reported that Amaranthus contain 12.6 g per 100 g protein, 3.3 g per 100 g crude fibre, 6.4 mg per 100 g iron, and 1.7 mg per 100 g zinc on dry weight basis while Akwaowo et al.,14 noted that Telfairia occidentalis fresh leaves contain 22.4 g per 100 g protein, 14.2 g per 100 g crude fibre, 3.6 mg per 100 g iron, and 4.2 mg per 100 g zinc. The superior nutritional profile and the blood-glucose-lowering effect of these two vegetables have been reported by various authors15,16 thus their hypoglycemic activities could be utilised to produce functional food product.
This food design strategy meets the demand for functional foods with added health benefits, which is increasing worldwide due to growing consumer awareness of the role of these foods in preventing chronic diseases.17 Inclusion of dried leafy vegetables could also promote the development of low-glycemic-index (below 50) foods resulting in a slower rise in blood glucose and insulin level.18 In this respect, wheat-based products have been widely investigated in the last 50 years and research is still ongoing about the impact of the quality of gluten network on the glycemic index (GI). The research was boosted by the rising popularity of gluten-free products, not only for celiac people but for all consumers wishing to reduce gluten in their diet. This is an interesting opportunity for cassava-based products particularly gluten-free pasta whose market share is steadily growing.19
Pasta products are increasingly used as a carrier of functional ingredients in food fortification to enhance nutritional quality, improve health and reduce the risk of diseases.20 As a result of increasing urbanisation and changes in food habits, the pasta market is growing in the low- and middle-income countries of Africa. Several pasta products (e.g., macaroni, spaghetti, noodles) are becoming more popularly consumed in Africa within the last decade due to their delicious taste, extended shelf life, convenience and affordability21–23 Nigeria, for instance, has become the 12th largest (https://instantnoodles.org/en/noodles/market.html) instant noodle market in the world, with 1.76 billion servings of noodles annually24 while pasta has become a ‘street food in several African countries’.25,26
Several authors evaluated the fortification of wheat-based pasta with leaf powder and reported the improvement of the nutritional profile of the functional pasta. Borneo & Aguirre,27 reported that fortification of wheat-based pasta with amaranth leaf powder improved the nutritional composition of the functional pasta while Cárdenas-Hernández et al.,28 noted that the inclusion of amaranth flour and leaf powder enhanced the fibre and mineral profile of the fortified pasta. Simonato et al.,29 also described how moringa leaf powder enhanced the antioxidant activity, phenolics and mineral content of wheat pasta.
The unique combination between increasing consumption of cassava pasta in countries where this crop is a staple food and the interest in gluten-free alternatives in Western countries prompted us to study the techno-functional characteristics of YCP fortified with leafy vegetables powders.13,21 Yellow cassava, having no gluten network to entrap the starch granules, has a high GI.30,31 However, we hypothesized that the insoluble dietary fibre of the leafy vegetables can also create a network to delay starch hydrolysis while the polyphenols in the vegetables can also reduce amylase activity. In principle, the ideal vegetable-fortified pasta product should have a low GI in combination with a high bioaccessibility of the micronutrients from the food matrix during digestion.
Consequently, this study aimed at (1) evaluating the effects of the addition of leaf powder (amaranth and fluted pumpkin leaves) on the retention and bioaccessibility of beta-carotene, iron and zinc in vegetable-fortified YCP, and (2) assessing the impact of the addition of leaf powder on the starch digestibility and in vitro GI of the leafy vegetable-fortified YCP.
2. Materials & methods
2.1. Materials
Yellow cassava flour made from low-cyanide sweet cassava variety (TMS 07/0593) was supplied by the International Institute for Tropical Agriculture (IITA) in Ibadan, Nigeria. The leafy vegetables, amaranth (Amaranthus cruentus) and fluted pumpkin (Telfairia occidentalis) leaves were cultivated at the Unifarm of Wageningen University and Research, The Netherlands. The leaves were harvested in the year 2020, ten weeks after planting, and washed, freeze-dried at −65 °C and milled to ∼5 μm particle sizes (6875D Freezer/Mill®, SPEX SamplePrep, UK). All the materials were packaged in amber bottles and stored at −20 °C until they were required for analysis. The chemicals used in the analyses were of analytical grade.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to form a dough which was manually kneaded and allowed to rest for 20 min. Long pasta strands (spaghetti-like) were produced with a small-scale manual pasta extruder (CuisinU RVS Compact Pasta machine, Roelofsarendsveen, the Netherlands) and laid out on an aluminium foil. The pasta was then dried in an incubator at 60 °C for 5–6 h. The pasta samples were stored in plastic amber bottles at −20 °C until needed for analysis. The pasta samples were weighed and cooked until the white core disappears using the method as described by Lawal et al.21 After cooking, the pasta was strained, cooled and weighed while 5 ml of sunflower oil was added to the water for cooking sample YPA5 O (YCP with 5% amaranth leaf powder plus 5 ml oil) to assess the impact of oil addition on the measured parameters.
1 to form a dough which was manually kneaded and allowed to rest for 20 min. Long pasta strands (spaghetti-like) were produced with a small-scale manual pasta extruder (CuisinU RVS Compact Pasta machine, Roelofsarendsveen, the Netherlands) and laid out on an aluminium foil. The pasta was then dried in an incubator at 60 °C for 5–6 h. The pasta samples were stored in plastic amber bottles at −20 °C until needed for analysis. The pasta samples were weighed and cooked until the white core disappears using the method as described by Lawal et al.21 After cooking, the pasta was strained, cooled and weighed while 5 ml of sunflower oil was added to the water for cooking sample YPA5 O (YCP with 5% amaranth leaf powder plus 5 ml oil) to assess the impact of oil addition on the measured parameters.
| Sample code | Composition |
|---|---|
| YP (YCP), YPA5 (YCP 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta), YPA5oil (YCP with 5 g amaranth leaf powder per 100 g of pasta with the addition of one teaspoon sunflower oil during cooking). | |
| YP | 100% yellow cassava flour, 0% leafy vegetables |
| YPA5 | 95% yellow cassava flour, 5% amaranth vegetable |
| YPA10 | 90% yellow cassava flour, 10% amaranth vegetable |
| YPU5 | 95% yellow cassava flour, 5% fluted pumpkin leaf vegetable |
| YPU10 | 90% yellow cassava flour, 10% fluted pumpkin leaf vegetable |
| YPA5 O | 95% yellow cassava flour, 5% amaranth vegetable with oil |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 while the total energy value was calculated per 100 grams of the sample using the Atwater conversion values. The sugar content was measured by HPLC based on AOAC 977.20 with minor modifications.34 The Apparent amylose content (AAC) of the pasta was determined following a modified method based on an iodine colourimetry method described by Man et al.36 The iron and zinc contents of the pasta and leaf powders were determined using inductively coupled plasma mass spectrometry ICP MS NEN-ISO 17053 according to AOAC 999.10
32 while the total energy value was calculated per 100 grams of the sample using the Atwater conversion values. The sugar content was measured by HPLC based on AOAC 977.20 with minor modifications.34 The Apparent amylose content (AAC) of the pasta was determined following a modified method based on an iodine colourimetry method described by Man et al.36 The iron and zinc contents of the pasta and leaf powders were determined using inductively coupled plasma mass spectrometry ICP MS NEN-ISO 17053 according to AOAC 999.10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35 Prior to the analysis, samples were dried at 70 °C overnight and ground to 0.425 mm Then, 300 mg of the ground sample was digested with concentrated nitric–hydrochloric acid mixture, and hydrogen–peroxide in a microwave digestion system (MarsXpress; CEM Corporation, Matthews, USA). After settling of the undissolved silica particles, the supernatant was analysed on the ICP-AES. mm and measurements were based on mg per kg dry weight.
35 Prior to the analysis, samples were dried at 70 °C overnight and ground to 0.425 mm Then, 300 mg of the ground sample was digested with concentrated nitric–hydrochloric acid mixture, and hydrogen–peroxide in a microwave digestion system (MarsXpress; CEM Corporation, Matthews, USA). After settling of the undissolved silica particles, the supernatant was analysed on the ICP-AES. mm and measurements were based on mg per kg dry weight.
The colour of the pasta samples was measured using a Hunter Lab flex colourimeter (Elscolab, USA).
The parameters were recorded as L* (lightness: L* = 0 black and L* = 100 white), a* (redness–greenness: −a* = greenness and +a* = redness) and b* (yellowness–blueness: −b* = blueness and +b* = yellowness) values. The colourimeter was standardised with a white plate supplied with the equipment, and three readings were done for each sample.
| % Inhibition of DPPH = (Abs control − Abs sample/Abs control) × 100 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) methanol
methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethyl acetate in 60
ethyl acetate in 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 ratio with 0.01% triethylamine (TEA).
10 ratio with 0.01% triethylamine (TEA).
 | (2) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 U mL−1), 5 μL of 0.3 M CaCl2, then HCl (1 M) was added to lower the pH to 3.0 and water up to 20 mL. The reaction vessel was placed into a shaking water bath at 37 °C for 2 h. During the intestinal phase, the gastric mixture was mixed with 11 mL of simulated intestinal fluid (SIF) electrolyte stock solution, 5.0 mL of pancreatin solution (800 U mL−1), 2.5 mL of bile salt solution (160 mM) and 40 mL of CaCl2 (0.3 M). NaOH (1 M) was then added to neutralise the mixture to pH 7.0 and Milli Q water up to 40 mL. Additionally, we also cooked YPA5 samples with 2 mL of sunflower oil to determine the impact of oil on beta-carotene, iron and zinc bioaccessibility. The bioaccessibility was calculated as the percentage of micronutrients present in the supernatant after in vitro digestion and centrifugation (IVD), based on the concentration and volumes in the cooked samples (adapted from Oomen et al.43).
000 U mL−1), 5 μL of 0.3 M CaCl2, then HCl (1 M) was added to lower the pH to 3.0 and water up to 20 mL. The reaction vessel was placed into a shaking water bath at 37 °C for 2 h. During the intestinal phase, the gastric mixture was mixed with 11 mL of simulated intestinal fluid (SIF) electrolyte stock solution, 5.0 mL of pancreatin solution (800 U mL−1), 2.5 mL of bile salt solution (160 mM) and 40 mL of CaCl2 (0.3 M). NaOH (1 M) was then added to neutralise the mixture to pH 7.0 and Milli Q water up to 40 mL. Additionally, we also cooked YPA5 samples with 2 mL of sunflower oil to determine the impact of oil on beta-carotene, iron and zinc bioaccessibility. The bioaccessibility was calculated as the percentage of micronutrients present in the supernatant after in vitro digestion and centrifugation (IVD), based on the concentration and volumes in the cooked samples (adapted from Oomen et al.43). | (3) |
| Rapidly digestible starch (RDS) = (G20 × F × 0.9 × 100)/W | (4) |
| Slowly digestible starch (SDS) = ((G120 − G20) × F × 0.9 × 100)/W | (5) |
| Total starch (TS) = (GTS × F × 0.9 × 100)/W | (6) |
| Resistant starch (RS) = TS − (RDS + SDS) | (7) |
2.2.6.1. Estimation of the glycemic index of cassava pasta samples. A first-order non-linear equation model described by Goñi et al.,47 was applied to describe the kinetics of starch hydrolysis and the estimate glycemic index (GI) of the cooked pasta samples where C = C∞ (1 − e−kt) where C represented the percentage of starch hydrolysed at time t (min), C∞ is the maximum hydrolysis extent, and k is the kinetic constant.48 The parameters, C and k, were estimated for each cassava pasta sample based on the in vitro starch digestion data. The hydrolysis index (HI) was then calculated by dividing the Area Under Curve (AUC) of each starch hydrolysis by the AUC of the reference food (white bread). The HI is expressed as a percentage representing the rate of starch digestion. The estimated GI indicated the digestibility of the pasta starch with the digestibility of starch in the reference material white bread. The estimated glycemic index of YCP was calculated from eqn (8):
| Estimated GI = 39.71 + 0.54HI. | (8) |
3. Results and discussion
3.1. Proximate composition, elemental analysis, colour measurements and apparent amylose content of YCP
| Parameter | YP | YPA5 | YPA10 | YPU5 | YPU10 | YPA5oil | ALP | ULP |
|---|---|---|---|---|---|---|---|---|
| The data expressed as mean values ± standard deviation was of three independent experiments on a dry weight basis (n = 3). Different superscript letters on a row indicate significant differences among samples (p ≤ 0.05), nd – not detected, Nd* – not determined. YP (YCP), YPA5 (YCP 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta), YPA5oil (YCP with 5 g amaranth leaf powder per 100 g of pasta with oil added during cooking), ALP (amaranth dry leaf powder), ULP (fluted pumpkin leaf powder), (moisture content, protein content, fat content, ash content, total dietary fibre) in g per 100 g on a dry weight basis, CHO-carbohydrate content calculated by difference, EO-energy value in kcal per 100 g, mineral composition (iron content and zinc content) in mg kg−1 on a dry weight basis, sugar content (fructose, glucose, sucrose) in mg g−1, apparent amylose content expressed in percentages. | ||||||||
| Moisture content | 11.71 ± 0.70e | 11.91 ± 0.98d | 11.21 ± 0.08d | 11.02 ± 0.04d | 11.10 ± 2.32c | 11.61 ± 0.32b | 11.97 ± 0.09ab | 15.67 ± 0.11a |
| Protein content | 0.99 ± 0.02e | 2.48 ± 0.21cd | 2.65 ± 0.06cd | 2.88 ± 0.03c | 2.95 ± 0.01c | 2.43 ± 0.15cd | 31.49 ± 1.44b | 34.70 ± 0.02a |
| Fat content | 0.20 ± 0.02b | 0.32 ± 0.06b | 0.21 ± 0.04b | 0.31 ± 0.06b | 0.54 ± 0.11b | 1.43 ± 0.11b | 2.53 ± 0.18ab | 6.33 ± 0.11a |
| Ash content | 1.55 ± 0.04b | 2.26 ± 0.00b | 4.70 ± 0.30b | 5.10 ± 0.01b | 4.10 ± 0.11b | 4.77 ± 0.13b | 15.41 ± 0.03a | 15.78 ± 0.16a |
| Carbohydrate content | 94.60 | 92.40 | 92.40 | 91.10 | 90.40 | 91.33 | 36.60 | 27.31 |
| Energy value | 384.10 | 382.30 | 373.00 | 369.00 | 369.00 | 377.00 | 73.10 | 82.08 |
| Dietary fibre | 9.00 ± 0.03bc | 9.10 ± 0.02bc | 9.15 ± 0.11bc | 9.30 ± 0.04bc | 10.00 ± 0.13b | 9.10 ± 0.15bc | 11.20 ± 0.11ab | 12.15 ± 0.22a |
| Iron content | 25.00 | 32.00 | 35.00 | 34.00 | 43.00 | 33.00 | 97.00 | 110.50 |
| Zinc content | 9.10 | 9.80 | 10.00 | 8.90 | 7.30 | 8.50 | 49.00 | 6.70 |
| Fructose | 0.49 ± 0.01ab | 0.90 ± 0.12a | 1.03 ± 0.04a | 0.92 ± 0.05a | 1.18 ± 0.01a | 1.11 ± 0.02a | Nd | 0.11 ± 0.05b |
| Glucose | 0.54 ± 0.09a | 0.70 ± 0.03a | 0.55 ± 0.01a | 0.86 ± 0.05a | 0.96 ± 0.02a | 0.58 ± 0.07a | Nd* | 0.21 ± 0.01a |
| Sucrose | nd | 0.12 ± 0.01c | nd | 1.53 ± 0.18a | 1.13 ± 0.04ab | 0.11 ± 0.01c | Nd* | 0.09 ± 0.04c |
| pH | 6.15 ± 0.05a | 6.17 ± 0.01a | 6.42 ± 0.02a | 6.16 ± 0.10a | 6.14 ± 0.04a | 6.11 ± 0.01a | nd | nd |
| AAC | 21.90 ± 0.04e | 25.01 ± 0.05de | 27.91 ± 0.11c | 29.37 ± 0.04b | 30.85 ± 0.08a | 25.43 ± 0.09d | nd | nd |
The food-in-food strategy however moderately improved the dietary fibre content of the YCP samples from 9.0 to 10.0 g per 100 g, mainly due to the yellow cassava flour's high fibre and resistant starch content rather than the high fibre in the leafy vegetables. The dietary fibre content of fortified YCP samples were thus slightly improved with leaf powder fortification. Sato et al.,57 however reported higher protein levels when dried leaves of Pereskia aculeata Miller were utilised to fortify wheat-based pasta (1.9 to 10.2%). The intake of dietary fibre rich products is desirable to meet nutritional recommendations as it helps in the proper control and management of diabetes and obesity. The structure provided by the dietary fibre network can control the kinetic of glucose release in the blood.58 The ash content of fortified pasta ranged from 2.3 g per 100 g–5.1 g per 100 g (dry weight), which was significantly different (P > 0.05) from the unfortified yellow pasta YP (1.6 g per 100 g), a consequence of the high ash content of cassava. This ash content was higher than those obtained by Sato et al.,57 when 10% Pereskia aculeata Miller leaf powder was incorporated into wheat pasta (2.2 g per 100 g). Generally, ash, protein and dietary fibre contents were lower in the control unfortified YCP than the fortified pasta samples while a higher level of fortification with leaf powder also enhanced the nutritional value of the pasta.
| L* | a* | b* | |
|---|---|---|---|
| Mean ± SD, n = 3, columns with different superscripts are significantly different (p < 0.05) L* scale: 0–50 (dark); 51–100 (light); a* scale: +ve value (red); −ve value (green); b* scale: +ve value (yellow); −ve value (blue). YP (YCP), YPA5 (YCP with 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta), YPA5oil (YCP with 5 g amaranth leaf powder per 100 g of pasta with the addition of one teaspoon sunflower oil during cooking). | |||
| YP | 70.0 ± 0.7d | 1.2e ± 0.1 | 15.1b ± 0.3 |
| YPA5 | 79.7e ± 0.4 | −7.3b ± 0.1 | 11.2a ± 0.1 |
| YPA10 | 66.2c ± 0.0 | −7.9a ± 0.2 | 16.6c ± 0.2 |
| YPU5 | 58.5b ± 0.1 | −6.4d ± 0.1 | 26.2d ± 0.1 |
| YPU10 | 52.0a ± 0.0 | −6.7c ± 0.0 | 27.1e ± 0.1 |
3.2. Total phenolics, flavonoids and antioxidant activity of YCP and leaf powders
The phenolic contents of the YCP samples varied according to the presented food matrix and ranged from 226.6 ± 2.22 μg GAE g−1 in YCP to 1098.3 ± 5.26 μg GAE g−1 in YPU10 (Table 4), showing that the leaf powder addition significantly (p ≤ 0.05) increased the total phenolics content of fortified YCP. Thus, the highest phenolic content was found in the fluted pumpkin leaf-fortified pasta YPU10 and was almost four times higher than found in the unfortified YCP. Phenolic compounds have excellent antioxidant properties due to their ability to bind metal ions, reduce peroxides, and promote the potency of anti-oxidative enzymes.65 They may also inhibit digestive enzymes (α-amylase and amyloglucosidase) through chemical interactions that lead to precipitation of the enzymes, thus limiting their activity on the digestion of carbohydrate foods. Moreover, starch-phenolics complexes have been reported to significantly slow down starch digestion.66 Fluted pumpkin and amaranth leaves are particularly rich in flavonoids, the largest and most abundant group of secondary metabolites with marked antioxidant properties in leafy vegetables.67 In this study, fluted pumpkin leaves were found to contain higher total phenolics (931.1 GAE μg per g DW) than the amaranth leaves (862 GAE per μg DW) but fewer flavonoids (101.5 μg RE per g DW) than the amaranth leaves (111.1 μg RE per g DW). This is higher than values reported by other authors for amaranth68 (total phenolics 14.8 GAE per μgg FW, total flavonoids 62.5 RE per μgg DW) while Okonwu et al.,69 reported 7.7–13.4 g per 100 g flavonoids in fluted pumpkin leaves. The highest flavonoid content was found in the fluted pumpkin fortified pasta YPU10 (80% higher than in the unfortified sample). In comparison, the highest antioxidant activity as measured by the DPPH free radical scavenging activity among the pasta samples was observed for the amaranth-fortified pasta, YPA10 (0.98 mmol kg−1) which is 122% higher than in unfortified YCP. The DPPH profile of the YCP reported in this study suggests significant antioxidant activities thus a comparison method such as ABTS (2,20-azinobis-(3-ethylbenzthiazoline-6-sulfonic acid)) with Butylhydroxytoluene (BHT) used as standard antioxidant in the experiments could be used in future research to validate the results and the IC50 values should also be determined.| Parameters | DPPH (mmol kg−1) DW | Total phenolics (GAE μg g−1) DW | Total flavonoids (RE μg g−1) DW | Beta-carotene (μg g−1) DW |
|---|---|---|---|---|
| Values are presented as means or range; different superscript letters (each column) represent significant differences (p < 0.05). YP (yellow cassava pasta), YPA5 (YCP 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta), YPA5oil (YCP with 5 g amaranth leaf powder per 100 g of pasta with oil added during cooking) *separately analysed ALP (amaranth leaf powder), FLP (fluted pumpkin leaf powder). | ||||
| YP | 0.44 ± 0.04b | 226.6 ± 2.22c | 34.1 ± 0.15c | 0.48 ± 0.01bc |
| YPA5 | 0.88 ± 0.02a | 445.17 ± 3.22b | 58.0 ± 0.09ab | 2.11 ± 0.09b |
| YPA10 | 0.98 ± 0.12a | 449.97 ± 2.18b | 57.8 ± 0.07b | 3.81 ± 0.08ab |
| YPU5 | 0.41 ± 0.04bc | 893.05 ± 3.77ab | 57.7 ± 1.05b | 2.91 ± 0.01ab |
| YPU10 | 0.73 ± 0.16b | 1098.32 ± 5.26a | 61.6 ± 0.05a | 7.82 ± 0.23a |
| YPA5 O | 0.87 ± 0.06ab | 432.08 ± 1.21b | 55.3 ± 0.32bc | 1.50 ± 0.14b |
| *ALP | 1.75 ± 0.03 | 862.02 ± 4.15 | 111.5 ± 0.10 | 9.40 ± 0.03 |
| *FLP | 2.83 ± 0.01 | 931.10 ± 3.12 | 101.5 ± 0.09 | 13.60 ± 0.04 |
3.3. Beta-carotene content of leaf powders and YCP (fortified and unfortified)
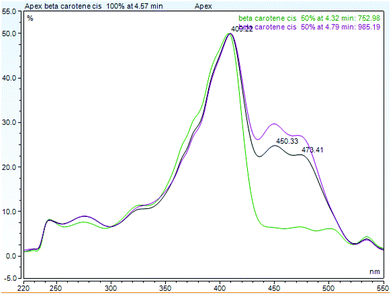
Beta-carotene accounts for more than 90% of the total carotenoids in vegetables,70 thus the fortification of YCP with beta-carotene rich leafy vegetables (amaranth-9.4 μg g−1 and fluted pumpkin leaf −13.6 μg g−1) enhanced the beta-carotene contents of the YCP (Table 4). The beta-carotene content of YCP was thus improved with increasing leaf powder fortification, as beta-carotene was six-fold higher (2.5–4.3 μg g−1) in the fortified than in the unfortified YCP. However, beta-carotene found in the YCP were mainly in trans-form while the cis-isomers were mostly below detection limits due to the analytical methods employed. Processing such as boiling, steaming, stir-frying, blanching or par-boiling has been reported to result in the formation of cis-isomers which possess different biological properties such as decreased provitamin A activity and altered bioavailability and antioxidant capacity.41 A limitation of this analysis is thus the poor detection of the cis-isomers and the interference/non-separation of the chlorophyll content of the leafy vegetables (Appendix 1) as well as the lack of evaluation of their influence on the shelf life of YCP. Future studies into the effect of chlorophyll on the storability of the YCP and a more in-depth study of the cis-isomerization or degradation is thus recommended.713.4. Retention and bioaccessibility of beta-carotene, iron and zinc in cooked yellow cassava pasta
The effect of cooking on the retention of beta-carotene, iron and zinc in the cooked YCP are shown in Table 5. As previously reported, processing (boiling) led to losses in beta-carotene content of yellow cassava food products.6,72 The fortified YCP however showed higher retention of beta-carotene (60–91.6%) than the unfortified YCP (11.1%), an indication of the carotenoid stability conferred on the product by the addition of leaf powder. The values are higher than beta-carotene retention values reported by Taleon et al.73 for other processed yellow cassava products such as fufu-fermented porridge (21.6–35.7%), chickwangue-stiff dough (1.5–5.6%). As reported by Lawal et al.6gari and pupuru (traditional products of cassava in Nigeria), similarly had low beta-carotene retention (24.4–35.2% and 34.7–39.9%, respectively), while Eyinla et al.5 reported an even lower β-carotene retention in yellow cassava chips (13.7%), flour (11.7%) and dough (5.5%) while Vimala et al.,72 showed that yellow-fleshed cassava retained 51.3–81% and 44.1–83.9% of beta-carotene after boiling and stir-frying. Iron retention in the cooked vegetable-fortified YCP was from 62.5 to 100% in line with Pereira et al.74 who also reported high retention of iron (>94%) in solar dried amaranth leaves. The zinc content of the fortified YPU10 was also substantially improved on cooking, as similarly reported by other authors, probably due to the leaching of the cooking pots into the cooked samples.59| Samples | Uncooked samples | % True retention after cooking | % Bioaccessibility after in vitro digestion |
|---|---|---|---|
| YP (YCP), YPA5 (YCP 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta), YPA5oil (YCP with 5 g of amaranth leaf powder per 100 g of pasta with a teaspoon of sunflower oil added). | |||
| Beta-carotene (μg g −1 ) | |||
| YP | 0.48 ± 0.01 | 11.1 | 62.5 |
| YPA5 | 2.11 ± 0.09 | 66.6 | 3.4 |
| YPA10 | 3.81 ± 0.08 | 87.8 | 3.9 |
| YPU5 | 2.91 ± 0.01 | 70.1 | 14.8 |
| YPU10 | 7.82 ± 0.23 | 91.6 | 17.5 |
| YPA5 O | 1.50 ± 0.14 | 60.0 | 2.8 |
| Iron (mg kg −1 ) | |||
| YP | 25.00 | 80.00 | 33.0 |
| YPA5 | 32.00 | 100.00 | 21.6 |
| YPA10 | 35.00 | 94.30 | 36.4 |
| YPU5 | 34.00 | 81.80 | 26.7 |
| YPU10 | 43.00 | 81.40 | 20.9 |
| YPA5 O | 33.00 | 62.50 | 32.0 |
| Zinc (mg kg −1 ) | |||
| YP | 9.10 | 53.8 | 326.5 |
| YPA5 | 9.80 | 78.6 | 194.8 |
| YPA10 | 10.00 | 93.0 | 129.0 |
| YPU5 | 8.90 | 71.9 | 218.8 |
| YPU10 | 7.30 | 113.7 | 168.7 |
| YPA5 O | 8.50 | 58.2 | 193.0 |
The bioaccessibility of beta carotene was however impeded by the addition of leaf powder mostly due to the presence of the bioactive compounds. Similar low bioaccessibility of beta-carotene had been reported (<0.15%) in leafy vegetables and (0.6% to 3.0%) in orange-fleshed sweet potatoes.75,76 In contrast, higher bioaccessibility of beta-carotene ranging from 8% to 40% was reported by Berni et al.77 for orange-fleshed sweet potato. Iron bioaccessibility was also found to be low in this study (20.9–36.4%) while zinc was significantly more bioaccessible. Icard-Vernière et al.60 in their study on bioaccessibility of iron and zinc in leafy vegetables, reported up to 17% bioaccessibility of iron in amaranth vegetable sauces while the study by Gautam et al.78 showed up to 193% bioaccessibility for zinc in cereal food products. Kruger et al.,10 also reported increased bioaccessible iron by 519% and zinc by 295% with the addition of Vigna unguiculata leaves to whole grain maize porridge. The addition of oil to YCP did not show any significant effect on bioaccessibility of the micronutrients, as the % bioaccessibility values of beta carotene, iron and zinc in YPA5 and YPA5 O were similar. Since the beneficial effects of bioactive compounds of food depend not only on their content and the amount consumed but also on their bioavailability/bioaccessibility, the nutritional content of food should be ultimately bioavailable/bioaccessible.
3.5. Starch digestibility and in vitro glycemic index of YCP
The rapidly available glucose content (RAG) varied significantly among the pasta samples from 21.9–27.6 with the highest in unfortified YCP (Table 6). Conversely, the slowly digestible starch (SDS) was found to be the lowest in the unfortified yellow pasta. The RS for the fortified cassava pasta samples was in the range of 1.78–2.45% (Table 6), higher than Eyinla et al.30 reported for traditional unfortified cassava products. RAG and RS content of starchy foods are significant determinants of their glycemic response.44 Furthermore, several studies had reported the beneficial impact of RS in starch digestion and its consequent lower glycemic responses. The fractions of RDS varied among the YCP samples (40.9–47.11), with YCP fortified with the highest amount of fluted pumpkin (YPU10), having the lowest value. In this case, leaf addition is significant as the phenolic compounds in the leafy vegetables inhibit starch hydrolysis, slowing down starch digestion, thus lowering the glycemic response. The inclusion of fluted pumpkin leaves was also observed to have a higher impact than the amaranth leaves on the SDS in the pasta samples (107% higher). Foster-Powell et al.79 and Arvidsson-Lenner et al.80 estimated the glycemic index of pasta products in the range between 40 and 78 depending on the processing method and plant material used. In addition, the glycemic index (GI) of a food is influenced by the relative presence of rapidly digested starch and slowly digested starch.45 Foods with high SDS (42.8–47.1%), such as the vegetable fortified cassava pasta, are ideal for diabetic patients. Its consumption could help manage diabetes due to its lower RDS and lower glycemic index (58.1–61.4) compared to unfortified YCP (71.7) or bread (100).| RAG | RDS | SDS | RS | Estimated GI | |
|---|---|---|---|---|---|
| Mean ± SD, n = 3, columns with different superscripts are significantly different (p < 0.05). RAG (rapidly available glucose), RDS (rapidly digestible starch), SDS (slowly digestible starch), RS (resistant starch). YP (YCP), YPA5 (YCP 5 g of amaranth leaf powder per 100 g of pasta), YPA10 (YCP with 10 g of amaranth leaf powder per 100 g of pasta), YPU5 (YCP with 5 g of fluted pumpkin leaf powder per 100 g of pasta), YPU10 (YCP with 10 g of fluted pumpkin leaf powder per 100 g of pasta). | |||||
| YP | 27.60 ± 1.16f | 42.23 ± 0.17f | 40.90 ± 0.15c | 1.12 ± 0.03d | 71.72 ± 1.12d |
| YPA5 | 25.48 ± 0.35e | 38.98 ± 1.02e | 42.78 ± 1.08b | 1.78 ± 0.01c | 61.39 ± 0.07c |
| YPA10 | 22.80 ± 0.25ab | 34.81 ± 0.15b | 43.99 ± 0.91ab | 2.05 ± 0.08c | 61.11 ± 0.14b |
| YPU5 | 24.11 ± 0.23c | 36.88 ± 0.18d | 45.06 ± 0.75a | 2.31 ± 0.11b | 59.68 ± 0.33ab |
| YPU10 | 21.88 ± 1.30a | 33.47 ± 0.03a | 47.11 ± 0.61a | 2.45 ± 0.07a | 58.14 ± 0.15a |
| YPA5oil | 23.38 ± 0.58d | 35.77 ± 0.19c | 43.01 ± 1.10b | 2.23 ± 0.05c | 60.19 ± 0.03cd |
4. Conclusions
The fortification of YCP with amaranth and fluted pumpkin leaf powders enhanced the pasta's nutritional profile and can be utilised to combat micronutrient deficiencies (vitamin A, iron and zinc). The inclusion of amaranth and fluted pumpkin leaf powders also boosted the iron, zinc, phenolics and flavonoids contents of the formulated pasta. Furthermore, the addition of amaranth and fluted pumpkin leaf powders delayed the kinetic of glucose digestion as shown by the value of RDS, SDS, RS, TS and RAG. Consequently, it reduced the estimated glycemic index of YCP thus proving useful as a low glycemic index product. However, the low beta-carotene bioaccessibility of YCP require further investigation to ascertain degree of isomerization due to cooking. Also, the impact of chlorophyll on the shelf life of the fortified YCP products should be ascertained. The results obtained from this study showed that incorporating leaf powder in YCP is a feasible way to obtain pasta products with unique micronutrient profiles and improved glycemic response. The YCP as a functional food product is nutritious, convenient and affordable and can help in tackling nutritional deficiencies, especially among low-income consumers in cassava consuming countries.Compliance with ethical standards
The authors complied with all the ethical standards stipulated.Author contributions
All authors provided feedback on the manuscript and approved the submitted version.Conflicts of interest
The authors declare no conflict of interest.Appendix 1
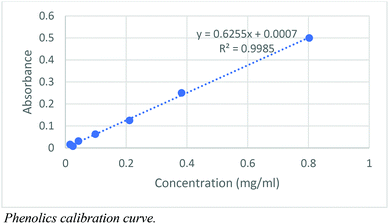
Phenolics calibration curve.
Acknowledgements
The authors are grateful to the TETFund, Nigeria, for the scholarship to conduct the research. We also thank Unifarm, Wageningen University and Research, the Netherlands, for their excellent care of the vegetables, Peter Iluebbey and Clara Alajo of IITA, Ibadan, Nigeria, for the provision of the yellow cassava flour. Finally, the Food Quality & Design technical team is much appreciated.References
- O. Ayetigbo, S. Latif, A. Abass and J. Müller, Sustainability, 2018, 10, 3089 CrossRef CAS.
- P. Ilona, H. E. Bouis, M. Palenberg, M. Moursi and A. Oparinde, Afr. J. Food, Agric., Nutr. Dev., 2017, 17(2), 12000–12025 CAS.
- O. M. Lawal, E. F. Talsma, E. Bakker, V. Fogliano and A. R. Linnemann, J. Sci. Food Agric., 2021a, 101(14), 6027–6035 Search PubMed.
- A. Bechoff, K. Tomlins, G. Fliedel, L. A. Becerra Lopez-lavalle, A. Westby, C. Hershey and D. Dufour, Crit. Rev. Food Sci. Nutr., 2018, 58, 547–567 CrossRef CAS PubMed.
- T. E. Eyinla, B. Maziya-Dixon, O. E. Alamu and R. A. Sanusi, Foods, 2019, 8(5), 177 CrossRef CAS PubMed.
- O. M. Lawal, A. B. Adebanjo and T. N. Fagbemi, J. Food Technol. Res., 2020, 7, 154–162 CrossRef.
- N. P. Uusiku, A. Oelofse, K. G. Duodu, M. J. Bester and M. Faber, J. Food Compos. Anal., 2010, 23, 499–509 CrossRef CAS.
- I. Raaijmakers, H. Snoek, B. Maziya-Dixon and T. Achterbosch, Sustainability, 2018, 10, 4771 CrossRef.
- S. Bakker, D. Mc Mahon and V. Uwase, Patterns and determinants of fruit and vegetable consumption in urban Rwanda: results of an urban consumer study in Kigali and North-western Rwanda, Wageningen Centre for Development Innovation, Wageningen, 2020 Search PubMed.
- J. Kruger, J. R. Taylor, M. G. Ferruzzi and H. Debelo, Compr. Rev. Food Sci. Food Saf., 2020, 19(6), 3618–3658 CrossRef PubMed.
- M. Affonfere, F. J. Chadare, F. T. K. Fassinou, A. R. Linnemann and K. G. Duodu, Food Rev. Int., 2021, 1–29 CrossRef.
- M. Getachew and H. Admassu, Cogent Food Agric., 2020, 6, 1 Search PubMed.
- O. M. Lawal, L. Stuijvenberg, N. Boon, O. Awolu, V. Fogliano and A. R. Linnemann, J. Food Sci., 2021b, 8, 1750–3841 Search PubMed.
- E. U. Akwaowo, B. A. Ndon and E. U. Etuk, Food Chem., 2000, 70, 235–240 CrossRef CAS.
- T. M. Salman, I. A. Alagbonsi, S. A. Biliaminu, O. A. Ayandele, O. K. Oladejo and O. A. Adeosun, Biokemistri, 2013, 25(3), 133–139 Search PubMed.
- P. E. Aba and I. R. Udechukwu, J. Basic Clin. Physiol. Pharmacol., 2017, 29(4), 313–320 Search PubMed.
- D. Sun-Waterhouse, Int. J. Food Sci. Technol., 2011, 46, 899–920 CrossRef CAS.
- G. Livesey, R. Taylor, H. F. Livesey, A. E. Buyken, D. J. A. Jenkins, L. S. A. Augustin, J. L. Sievenpiper, A. W. Barclay, S. Liu, T. M. S. Wolever, W. C. Willett, F. Brighenti, J. Salas Salvadó, I. Björck, S. W. Rizkalla, G. Riccardi, C. L. Vecchia, A. Ceriello, A. Trichopoulou, A. Poli, J. C. Brand-Miller, A. Astrup, W. Cyril, C. Kendall, M.-A. Ha and S. Baer-Sinnott, Nutrients, 2019, 11(6), 1280 CrossRef CAS PubMed.
- Y. Gao, M. E. Janes, B. Chaiya, M. A. Brennan, C. S. Brennan and W. Prinyawiwatkul, Int. J. Food Sci. Technol., 2018, 53(1), 19–32 CrossRef CAS.
- T. Oliviero and V. Fogliano, Trends Food Sci. Technol., 2016, 51, 58–64 CrossRef CAS.
- O. M. Lawal, O. Sanni, M. O. Oluwamukomi, V. Fogliano and A. R. Linnemann, Food Struct., 2021c, 30, 100241 Search PubMed.
- N. D. Qumbisa and N. Ngobese, Afr. J. Food, Agric., Nutr. Dev., 2020, 20, 16099–16111 CAS.
- T. Deb and E. Giokos, https://edition.cnn.com/2019/01/25/africa/indomie-giant-in-nigeria-intl/index.html (Accessed February 12, 2022).
- International Pasta Organisation, 2020.
- J. N. Ihedioha, E. E. Okali, R. N. Ekere and C. C. Ezeofor, Iran. J. Toxicol., 2019, 13, 1 Search PubMed.
- S. Marras and M. AgBendech, Street Food in Urban Ghana, F. A. O. U, 2016, 1 Search PubMed.
- R. Borneo and A. Aguirre, LWT–Food Sci. Technol., 2008, 41, 1748–1751 CrossRef CAS.
- A. Cárdenas-Hernández, T. Beta, G. Loarca-Piña, E. Castaño-Tostado, J. O. Nieto-Barrera and S. Mendoza, J. Cereal Sci., 2016, 72, 84–90 CrossRef.
- B. Simonato, T. Roberta, R. Giada, R. Corrado, S. Davide Sega, R. Gabriele, L. L. Lucini and G. Gianluca, J. Sci. Food Agric., 2021, 101, 1920–1925 CrossRef CAS PubMed.
- T. E. Eyinla, R. A. Sanusi and B. Maziya-Dixon, Food Chem., 2021, 356, 129664 CrossRef CAS PubMed.
- M. Palermo, N. Pellegrini and V. Fogliano, J. Sci. Food Agric., 2014, 94(6), 1057–1070 CrossRef CAS PubMed.
- AOAC, Official methods of analysis, Association of the official analytical chemist, Washington D.C., USA, 19th edn, 2012 Search PubMed.
- AOAC, Association of Official Analytical Chemists, Official Methods of Analysis, AOAC International, Washington D.C., USA, 18th edn, 2005 Search PubMed.
- AOAC, Official Methods of Analysis of AOAC International, Arlington, VA, 20th edn, 2006 Search PubMed.
- AOAC, Determination of Heavy Metals in Food by Inductively Coupled Plasma–Mass Spectrometry: First Action 2015.01, J. AOAC Int., 2015, 98, 4 Search PubMed.
- J. Man, Y. Yang, C. Zhang, X. Zhou, Y. Dong, F. Zhang, Q. Liu and C. Wei, J. Agric. Food Chem., 2012, 60(36), 9332–9341 CrossRef CAS PubMed.
- Y. Li, D. Ma, D. Sun, C. Wang, J. Zhang, Y. Xie and T. Guo, Crop J., 2015, 3(4), 328–334 CrossRef.
- D. Plank, W. Szpylka, J. Sapirstein, H. D. Woollard, C. M. Zapf, V. Lee, C.-Y. O. Chen, R. H. Liu, R. Tsao, A. Düsterloh, S. Baugh, … and M. Stringer, J. AOAC Int., 2012, 95(6), 1562–1569 CrossRef CAS PubMed.
- A. Serpen, V. Gökmen, N. Pellegrini and V. Fogliano, J. Cereal Sci., 2008, 48(3), 816–820 CrossRef CAS.
- G. Sadler, J. Davis and D. Dezman, J. Food Sci., 1990, 55(5), 1460–1461 CrossRef CAS.
- S. Lee, Y. Choi, H. S. Jeong, J. Lee and J. Sung, Effect of different cooking methods on the content of vitamins and true retention in selected vegetables, Food Sci. Biotechnnol., 2018, 27, 333–342 CAS.
- M. Minekus, M. Alminger, P. Alvito, S. Ballance, T. Bohn, C. Bourlieu, F. Carrière, R. Boutrou, M. Corredig, D. Dupont, C. Dufour, L. Egger, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. Mackie, … and A. Brodkorb, Food Funct., 2014, 5(6), 1113–1124 RSC.
- A. G. Oomen, A. Hack, M. Minekus, E. Zeijdner, C. Cornelis, G. Schoeters, W. Verstraete, T. Van de Wiele, J. Wragg, C. J. M. Rompelberg, A. J. Sips and J. H. Van Wijnen, Environ. Sci. Technol., 2002, 36(15), 3326–3334 CrossRef CAS PubMed.
- K. N. Englyst, S. Vinoy, H. N. Englyst and V. Lang, Br. J. Nutr., 2003, 89(3), 329–339 CrossRef CAS PubMed.
- K. N. Englyst, H. N. Englyst, G. J. Hudson, T. J. Cole and H. Cummings, Am. J. Clin. Nutr., 1999, 69(3), 448–454 CrossRef CAS PubMed.
- A. Hawkins and S. K. Johnson, Int. J. Food Sci. Nutr., 2005, 56(3), 147–155 CrossRef CAS PubMed.
- I. Goñi, A. Garcia-Alonso and F. Saura-Calixto, Nutr. Res., 1997, 17(3), 427–437 CrossRef.
- V. Gallo, A. Romano and P. Masi, Food Struct., 2020, 24, 100139 CrossRef.
- G. Lorenzo, M. Sosa and A. Califano, in Alternative and Replacement Foods, 2018, pp. 433–458 Search PubMed.
- T. Ogawa and S. Adachi, Drying Technol., 2017, 35(16), 1919–1949 CrossRef CAS.
- D. Nyadanu and S. T. Lowor, Genet. Resour. Crop Evol., 2015, 62(1), 131–140 CrossRef.
- H. C. Schönfeldt and B. Pretorius, J. Food Compos. Anal., 2011, 24(8), 1141–1146 CrossRef.
- F. A. Oguntoyinbo, V. Fusco, G. S. Cho, J. Kabisch, H. Neve, W. Bockelmann, M. Huch, L. Frommherz, B. Trierweiler, B. Becker, N. Benomar, N. Gálvez, A. H. Abriouel, W. H. Holzapfel and M. A. P. Franz, Front. Microbiol., 2016, 7, 1 Search PubMed.
- O. C. Aworh, Food Res. Int., 2015, 76, 986–991 CrossRef.
- Z. Islam, S. M. R. Islam, F. Hossen, K. Mahtab-ul-Islam, Md. R. Hasan and R. Karim, Int. J. Food Sci., 2021, 2021, 1–11 CrossRef PubMed.
- R. van der Merwe, J. Kruger, M. G. Ferruzzi, K. G. Duodu and J. R. N. Taylor, J. Food Sci. Technol., 2019, 56, 2244–2256 CrossRef CAS PubMed.
- R. Sato, L. P. de L. Cilli, B. E. de Oliveira, V. B. V. Maciel, A. C. Venturini and C. M. P. Yoshida, Food Sci. Technol., 2019, 39, 28–34 CrossRef.
- D. Patel, S. Prasad, R. Kumar and S. Hemalatha, Asian Pac. J. Trop. Biomed., 2012, 2(4), 320–330 CrossRef CAS PubMed.
- N. Perez-Moral, S. Saha, M. Philo, D. J. Hart, M. S. Winterbone, W. J. Hollands, M. Spurr, J. Bows, V. van der Velpen, P. A. Kroon and P. J. Curtis, J. Funct. Foods, 2018, 48, 410–419 CrossRef CAS PubMed.
- C. Icard-Vernière, C. Picq, L. Courbis and C. Mouquet-Rivier, Food Funct., 2016, 7(2), 1103–1110 RSC.
- J. Singh, A. Dartois and L. Kaur, Trends Food Sci. Technol., 2010, 21(4), 168–180 CrossRef CAS.
- M. Frei, P. Siddhuraju and K. Becker, Food Chem., 2003, 83(3), 395–402 CrossRef CAS.
- B. Biernacka, D. Dziki, A. Miś, S. Rudy, A. Krzykowski, R. Polak and R. Różyło, Int. Agrophys., 2013, 33(3), 323–330 CrossRef.
- E. Carini, E. Curti, F. Cassotta, N. E. O. Najm and E. Vittadini, Food Chem., 2014, 144, 74–79 CrossRef CAS PubMed.
- L. Gong, W. Cao, H. Chi, J. Wang, H. Zhang, J. Liu and B. Sun, Food Res. Int., 2018, 103, 84–102 CrossRef CAS PubMed.
- L. Kan, T. Oliviero, R. Verkerk, V. Fogliano and E. Capuano, J. Funct. Foods, 2020, 68, 103924 CrossRef CAS.
- E. Corradini, P. Foglia, P. Giansanti, R. Gubbiotti, R. Samperi and A. Lagana, Nat. Prod. Res., 2011, 25, 469–495 CrossRef CAS PubMed.
- U. Sarker, Md. M. Hossain and S. Oba, Sci. Rep., 2020, 10, 1336 CrossRef CAS PubMed.
- K. Okonwu, L. A. Akonye and S. I. Mensah, J. Appl. Sci. Environ. Manage., 2018, 22, 259 CAS.
- H. S. Black, F. Boehm, R. Edge and T. G. Truscott, Antioxidants, 2020, 9, 264 CrossRef CAS PubMed.
- A. Schieber and R. Carle, Trends Food Sci. Technol., 2005, 16, 416–422 CrossRef CAS.
- B. Vimala, R. Thushara, B. Nambisan and J. Sreekumar, Int. J. Food Sci. Technol., 2011, 46, 166–169 CrossRef CAS.
- V. Taleon, D. Sumbu, T. Muzhingi and S. Bidiaka, J. Sci. Food Agric., 2019, 99(3), 1434–1441 CrossRef CAS PubMed.
- E. J. Pereira, L. M. J. Carvalho, G. M. Dellamora-Ortiz, F. S. N. Cardoso, J. L. V. Carvalho, D. S. Viana, S. C. Freitas and M. M. Rocha, Food Nutr. Res., 2014, 58(1), 20694 CrossRef PubMed.
- B. de la Fuente, G. López-García, V. Mañez, A. Alegría, R. Barberá and A. Cilla, Foods, 2019, 8(7), 250 CrossRef CAS PubMed.
- D. Mbogo, T. Muzhingi and S. Janaswamy, J. Food Sci., 2021, 86(3), 901–906 CrossRef CAS PubMed.
- P. Berni, C. Chitchumroonchokchai, S. G. Canniatti-Brazaca, F. F. De Moura and M. L. Failla, Plant Foods Hum. Nutr., 2015, 70(1), 1–8 CrossRef CAS PubMed.
- S. Gautam, K. Platel and K. Srinivasan, Food Chem., 2010, 122, 668–672 CrossRef CAS.
- K. Foster-Powell, S. H. Holt and J. C. Brand-Miller, Am. J. Clin. Nutr., 2002, 76(1), 5–56 CrossRef CAS PubMed.
- R. Arvidsson-Lenner, N.-G. Asp, M. Axelsen, S. Bryngelsson, E. Haapa, A. Järvi, B. Karlström, A. Raben, A. Sohlström, I. Thorsdottir and B. Vessby, Scand. J. Nutr., 2004, 48(2), 84 CrossRef.
| This journal is © The Royal Society of Chemistry 2022 |