Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insights into gut microbiota metabolism of dietary lipids: the case of linoleic acid
Zongyao
Huyan
 a,
Nicoletta
Pellegrini
a,
Nicoletta
Pellegrini
 ab,
Wilma
Steegenga
c and
Edoardo
Capuano
ab,
Wilma
Steegenga
c and
Edoardo
Capuano
 *a
*a
aFood Quality and Design Group, Wageningen University, Wageningen, The Netherlands. E-mail: edoardo.capuano@wur.nl; Tel: +31 317485690
bDepartment of Agricultural, Food, Environmental and Animal Sciences, University of Udine, Udine, Italy
cDivision of Human Nutrition and Health, Wageningen University & Research, Wageningen, The Netherlands
First published on 29th March 2022
Abstract
It has been recognized that, next to dietary fibre and proteins, gut microbiota can metabolize lipids producing bioactive metabolites. However, the metabolism of dietary lipids by human gut microbiota has been poorly explored so far. This study aimed to examine the change in lipids, particularly linoleic acid (LA), induced by the chemical form of lipids and the presence of the plant matrix. Short-chain fatty acid (SCFA) production was monitored to get an insight into microbial activity. Free LA, glyceryl trilinoleate and soybean oil as well as digested intact (DS) and broken (BS) soybean cells were subjected to in vitro fermentation using human faecal inoculums. Confocal microscopy was used to visualize the soybean cell integrity. Three LA metabolites, including two conjugated fatty acids (CLAs, 9z,11e and 9e,11e) and 12hydroxy, 9z C18:1, were identified and monitored. Free LA addition improved the LA metabolite production but reduced SCFA concentrations compared to trilinoleate and soybean oil. Breaking cell integrity had impacts on CLA, hydroxy C18:1 and SCFA production and free fatty acid release within the first 24 h of fermentation, but this effect vanished with time. In contrast, soybean oil only increased free LA release and hydroxy C18:1 production. The content of several FAs decreased during fermentation suggesting a substantial conversion in microbial metabolites. Besides, LA metabolites were also identified in the fermentation pellets suggesting the incorporation of microbial FA metabolites into bacterial cells. This study expands our understanding of microbial metabolism of dietary lipids with a special emphasis on the role of food- and diet-related factors.
1 Introduction
The human gut hosts trillions of microorganisms, known collectively as the gut microbiota.1 Gut microbiota makes a vitally important contribution to host well-being, including the production of metabolites, whose health-related effects are widely described.2,3 Whereas the microbial metabolism of carbohydrates and proteins in the gut is well studied, the interaction between human gut microbiota and dietary fats requires more investigation.The microbial metabolism of dietary lipids is considered a detoxifying mechanism,4,5 through which several bacterial genera, e.g. lactobacilli, Roseburia and bifidobacteria, can transform growth-inhibiting polyunsaturated fatty acids (PUFAs) into less toxic fatty acids (FAs), from many of which humans obtain benefits.6–8 In particular, during this process linoleic acid (LA) is converted into conjugated linoleic acids (CLAs) which were reported to contribute to gut health, e.g. ameliorating chemical-induced acute colitis in animal models and inhibiting the growth of human colon cancer cells in vitro.9–12 Other biologically-active LA metabolites, e.g. 10-hydroxy-cis-12-octadecenoic acid and 10-oxo-trans-11-octadecenoic acid, are also produced as intermediates showing different physiological effects.7,13–16Although the metabolic pathway through which bacteria metabolize LA is known, few studies have explored how the dietary lipids are provided to the gut microbiota as well as how the presence of lipids changes microbial activity.
Dietary lipids are present in food ingredients and meals as free oils or emulsions, mostly (95% of total lipids) in the form of triglycerides. Most of the dietary triglycerides are hydrolyzed by gastric and pancreatic lipase and the resulting FA absorbed in the small intestine. The lipid fraction that escapes digestion in the small intestine (5% of the total dietary lipids) and enters the large intestine presumably presents itself in the form of a mixture of triglycerides, mono- and diglycerides and free fatty acids (FFA). We can hypothesize that microbial utilization of those FAs may differ depending on the form provided, because the utilization of FAs may be simpler when they are provided in a free form compared to as esterified glycerol in triglycerides. Whereas animal fat is easily accessed by digestive fluids, in intact plant tissues, dietary lipids are located within intact cells which have a strong impact on the gastrointestinal utilization of food components.17–19 The presence of a cell wall in plant foods can impede access to digestive enzymes,20–22 hence, a variable fraction of food components will escape digestion in the small intestine and may be metabolized by the gut microbiota. This fraction varies mostly depending on the level of cell integrity at the point of swallowing. Apart from this, it was also shown that cell integrity affects the accessibility of food compounds to the gut microbiota in the colon. Microbial catabolism of tryptophan by the human microbiota is different in isolated soybean cells and particles compared to a free tryptophan supplement.20 Higher amounts of short-chain fatty acid (SCFA) are produced in vitro when cell integrity is lost or permeability of the cell wall is increased,23,24 which suggests that the accessibility of nutrients to microbes also depends on cell integrity. However, the effects of cell integrity on the microbial utilization of lipids, next to that of proteins and carbohydrates, has been largely overlooked. Furthermore, it was reported that microbial CLA production would depend on the FA composition that the lipids display, e.g. the presence of other PUFAs.25–28 Therefore, we hypothesize that the form in which dietary lipids are provided to gut microbiota would affect microbial LA metabolism and thereby influence the formation of LA metabolites in the human gut.
Hence, in the present study, we aimed at unravelling the effects on the microbial utilization of dietary fat by human microbiota, in particular, the microbial metabolism of linoleic acid. Soybean oil (SO), glyceryl trilinoleate (TLA) and soybean were selected as sources of LA. The soybean was cooked and samples with different levels of cell integrity were prepared. The materials underwent in vitro colonic fermentation using human microbiota obtained from faecal samples from three healthy volunteers. After fermentation, the effect of lipid supplementation on microbial activity was studied and analyzed by the amount of SCFA produced.
2 Methods and materials
2.1 Materials
Soybean seeds, harvested in June 2020 in China, were purchased from a local supermarket (Wageningen, the Netherlands) and stored at room temperature in a dry place. SO was extracted from soybean seeds using Soxhlet extraction and stored at 4 °C before use. All the samples were used within five weeks from their purchase. LA, TLA and other chemicals were of analytical grade and purchased from Sigma Aldrich (St Louis, MO, USA). Standard samples of CLAs, cis-9, trans-11-CLA (9z,11e CLA), trans-9, trans-11-CLA (9e,11e CLA), and hydroxy FAs, 12-hydroxy-9(Z)-octadecenoic acid and 12-hydroxy-9(E)-octadecenoic acid were purchased from Larodan (Solna, Sweden). Long-chain FAs, i.e. palmitic acid (C16:0), n-heptadecanoic acid (C17:0), stearic acid (C18:0), oleic acid (C18:1), LA and linolenic acid (C18:3), and SCFAs, i.e. acetate, propionate, butyrate, valerate, isobutyrate and isovalerate were purchased from Sigma-Aldrich (St Louis MO, USA).2.2 Preparation of intact and broken digested soybean seeds
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, w/v). The cotyledons were gently mashed and sieved. Particles of size ranging from 125 to 250 μm were used based on the results from Zahir et al.22 who reported that such a size range would only contain intact cells. Confocal laser scanning microscopy (CLSM) was used to confirm the intactness of the produced cells. The obtained material was stored at 5 °C and in vitro pre-digested within 48 h.
3, w/v). The cotyledons were gently mashed and sieved. Particles of size ranging from 125 to 250 μm were used based on the results from Zahir et al.22 who reported that such a size range would only contain intact cells. Confocal laser scanning microscopy (CLSM) was used to confirm the intactness of the produced cells. The obtained material was stored at 5 °C and in vitro pre-digested within 48 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4, w/w) and the mixture (5 g) was subjected to the oral phase by mixing with SSF for 2 min to obtain a final ratio of 50
4, w/w) and the mixture (5 g) was subjected to the oral phase by mixing with SSF for 2 min to obtain a final ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v). Following oral digestion, the resulting mixture (10 mL) was mixed with SGF to obtain a final ratio of 50
50 (v/v). Following oral digestion, the resulting mixture (10 mL) was mixed with SGF to obtain a final ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) to stimulate the gastric phase of digestion. The pH was adjusted to 3.0 with 1 M HCl and CaCl2 was added to achieve 0.075 mM in the mixture. Porcine pepsin was added to achieve an activity of 2000 U mL−1 in the digest. The digest was incubated at 37 °C and shaken for 2 h. Finally, the pH of the gastric digest was raised to 7.0 with 1 M NaOH and mixed with pre-warmed SIF. Fresh bile salts and CaCl2 were added to achieve 10 mM and 0.3 mM, respectively, in the final digest. Pancreatin was added to achieve a lipase activity of 2000 U mL−1. The mixture was shaken at 37 °C for 2 h to stimulate the intestinal phase. After the intestinal phase, the entire system was centrifuged at 4500g for 20 min to obtain pellets, which represent DS. DS was collected and divided into two equal parts. One part of DS pellets was weighed and stored at −20 °C, and the other part was frozen and ground to break all cell walls. This latter sample will be indicated as the broken soybean cells (BS) from now on and stored at −20 °C. The intactness of the cell wall of DS and BS was checked by CLSM (see section 2.5). Both DS and BS pellets underwent in vitro colonic fermentation within 72 h.
50 (v/v) to stimulate the gastric phase of digestion. The pH was adjusted to 3.0 with 1 M HCl and CaCl2 was added to achieve 0.075 mM in the mixture. Porcine pepsin was added to achieve an activity of 2000 U mL−1 in the digest. The digest was incubated at 37 °C and shaken for 2 h. Finally, the pH of the gastric digest was raised to 7.0 with 1 M NaOH and mixed with pre-warmed SIF. Fresh bile salts and CaCl2 were added to achieve 10 mM and 0.3 mM, respectively, in the final digest. Pancreatin was added to achieve a lipase activity of 2000 U mL−1. The mixture was shaken at 37 °C for 2 h to stimulate the intestinal phase. After the intestinal phase, the entire system was centrifuged at 4500g for 20 min to obtain pellets, which represent DS. DS was collected and divided into two equal parts. One part of DS pellets was weighed and stored at −20 °C, and the other part was frozen and ground to break all cell walls. This latter sample will be indicated as the broken soybean cells (BS) from now on and stored at −20 °C. The intactness of the cell wall of DS and BS was checked by CLSM (see section 2.5). Both DS and BS pellets underwent in vitro colonic fermentation within 72 h.
2.3 In vitro colonic fermentation
In vitro colonic fermentation was followed as described elsewhere30 with modification. Briefly, three healthy adults (25–27 years old), declaring no smoking and no antibiotic consumption for 6 months, donated fresh faecal samples on six different days. Healthy volunteers gave written consent for a single fecal donation and their anonymity was maintained at all times. According to the guidelines of the Medical Ethical Advisory Committee of Wageningen University (METC-WU), this research did not need an ethics approval. The faecal sample was prepared with anaerobic sterilized phosphate buffer (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, w/v), which consisted of 8.8 g L−1 K2HPO4, 6.8 g L−1 KH2PO4 and 0.1 g of sodium thioglycolate in demi-water as well as the addition of 15 mg L−1 sodium dithionite before use, using a Stomacher 400 circulator (Seward, UK). Two sets of experiments were carried out. In the first, in vitro colonic fermentation of free linoleic acid, TLA and SO containing the same content of linoleic acid (0.06 g) was conducted to study the oil matrix effects. In the second set of experiments, the fermentation of BS and DS was carried out. Each treatment was prepared by filling sterilized penicillin bottles with an amount of food containing 0.12 g of oil and 10% faecal inoculum. Before the addition of faecal inoculum, vessels were flushed with N2/CO2 (80/20, v/v) gases to create an anaerobic condition. A basal medium containing 5.22 g L−1 K2HPO4, 16.32 g L−1 KH2PO4, 2 g L−1 NaHCO3, 2 g L−1 yeast extract, 2 g L−1 peptone, 1 g L−1 mucin, and 0.5 g L−1L-cysteine HCl was used. The final volume was 70 mL and each fermentation was carried out in triplicate. In the second set of fermentations, next to BS and DS samples, one extra sample containing SO (0.12 g, i.e. the same oil amount as in BS and DS samples), was also fermented as a positive control. A negative control (blank) was also included containing only the standard media with no extra lipid added. According to the results from a preliminary experiment, DS and BS samples were taken after 8, 24, and 48 h, whereas SO, LA and TLA samples were sampled at 24 h and 48 h. At each time point, the content of the whole bottle was collected and the pressure within the bottles was monitored to ensure that no gas exchange would occur with the external environment. After centrifugation at 4500g for 20 min, the supernatants and pellets were separated and stored at −20 °C for further analysis.
20, w/v), which consisted of 8.8 g L−1 K2HPO4, 6.8 g L−1 KH2PO4 and 0.1 g of sodium thioglycolate in demi-water as well as the addition of 15 mg L−1 sodium dithionite before use, using a Stomacher 400 circulator (Seward, UK). Two sets of experiments were carried out. In the first, in vitro colonic fermentation of free linoleic acid, TLA and SO containing the same content of linoleic acid (0.06 g) was conducted to study the oil matrix effects. In the second set of experiments, the fermentation of BS and DS was carried out. Each treatment was prepared by filling sterilized penicillin bottles with an amount of food containing 0.12 g of oil and 10% faecal inoculum. Before the addition of faecal inoculum, vessels were flushed with N2/CO2 (80/20, v/v) gases to create an anaerobic condition. A basal medium containing 5.22 g L−1 K2HPO4, 16.32 g L−1 KH2PO4, 2 g L−1 NaHCO3, 2 g L−1 yeast extract, 2 g L−1 peptone, 1 g L−1 mucin, and 0.5 g L−1L-cysteine HCl was used. The final volume was 70 mL and each fermentation was carried out in triplicate. In the second set of fermentations, next to BS and DS samples, one extra sample containing SO (0.12 g, i.e. the same oil amount as in BS and DS samples), was also fermented as a positive control. A negative control (blank) was also included containing only the standard media with no extra lipid added. According to the results from a preliminary experiment, DS and BS samples were taken after 8, 24, and 48 h, whereas SO, LA and TLA samples were sampled at 24 h and 48 h. At each time point, the content of the whole bottle was collected and the pressure within the bottles was monitored to ensure that no gas exchange would occur with the external environment. After centrifugation at 4500g for 20 min, the supernatants and pellets were separated and stored at −20 °C for further analysis.
2.4 Analyses
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/v) of chloroform/methanol/1.5 KCl in H2O (2
2, v/v) of chloroform/methanol/1.5 KCl in H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), according to the procedure of Bligh–Dyer.31 The Bligh–Dyer solution was added to those bottles in the first experiment which studied lipid form effects (i.e. FLA, TLA and SO samples) without centrifugation.
1, v/v), according to the procedure of Bligh–Dyer.31 The Bligh–Dyer solution was added to those bottles in the first experiment which studied lipid form effects (i.e. FLA, TLA and SO samples) without centrifugation.
The extracted lipids were concentrated and separated into two parts, one for FFA determination and the other for derivatization to prepare FA methyl esters (FAMEs), which were prepared by mixing the extracted lipids with NaOH/MeOH as previously described.32
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 5 min, 4 °C) and amounts of 2 mL were filtered (15 mm ∅, 0.2 μm regenerated cellulose filter, Phenomenex, Torrance, USA). An internal standard of 2-ethylbutyric acid in 0.3 M HCl and 0.9 M oxalic acid was also added for better SCFA separation and quantification before injection into a gas chromatography system equipped with a flame ionization detector (GC-FID, GC-2014, Shimadzu, Hertogenbosch, Netherlands). Nitrogen was used as a carrier gas. The temperature of GC-FID started at 100 °C, then increased to 180 °C for 2 min at a rate of 10.8 °C min−1. Then, it increased at 50 °C min−1 to 240 °C and was maintained at 240 °C for 2 min. Standard calibration curves of acetic, propionic, butyric, valeric, iso-butyric and iso-valeric acids were prepared in the range of 0–50 mM.
000g, 5 min, 4 °C) and amounts of 2 mL were filtered (15 mm ∅, 0.2 μm regenerated cellulose filter, Phenomenex, Torrance, USA). An internal standard of 2-ethylbutyric acid in 0.3 M HCl and 0.9 M oxalic acid was also added for better SCFA separation and quantification before injection into a gas chromatography system equipped with a flame ionization detector (GC-FID, GC-2014, Shimadzu, Hertogenbosch, Netherlands). Nitrogen was used as a carrier gas. The temperature of GC-FID started at 100 °C, then increased to 180 °C for 2 min at a rate of 10.8 °C min−1. Then, it increased at 50 °C min−1 to 240 °C and was maintained at 240 °C for 2 min. Standard calibration curves of acetic, propionic, butyric, valeric, iso-butyric and iso-valeric acids were prepared in the range of 0–50 mM.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v), and the homogenized sample (30 μL) was placed in a glass slide. A CLSM type 510 (Zeiss, Oberkochen, Germany) using a 405 nm blue/violet diode laser for calcofluor white, a 543 nm HeNe laser for rhodamine B and a 488 nm argon laser for BODIPY were used for visualization. All images were acquired using a 10/20 EC Plan-Neofluar/0.5 A lens and analyzed with ZEN blue edition (Carl Zeiss Microscopy).
1, v/v), and the homogenized sample (30 μL) was placed in a glass slide. A CLSM type 510 (Zeiss, Oberkochen, Germany) using a 405 nm blue/violet diode laser for calcofluor white, a 543 nm HeNe laser for rhodamine B and a 488 nm argon laser for BODIPY were used for visualization. All images were acquired using a 10/20 EC Plan-Neofluar/0.5 A lens and analyzed with ZEN blue edition (Carl Zeiss Microscopy).
2.5 Statistical analysis
The mean values ± standard error of the mean (SEM, N = 3) were used to express the results unless stated otherwise. Statistical analysis was performed by GraphPad Prism 9.1.0 (GraphPad Software, La Jolla, CA). Differences were evaluated using a one-way repeated measures analysis of variance (ANOVA) followed by Tukey's post-hoc test unless stated otherwise. A value of p < 0.05 was considered statistically significant.3 Results
3.1 FA composition and the amount of lipid digested by in vitro digestion of cooked soybean
The results presented in Table 1 show the total lipid content, as well as the FA composition of cooked soybean, DS, BS and SO. After in vitro INFOGEST digestion, around half of the lipids initially present in soybean cells were digested. FA analysis shows that the lipid content and composition of DS and BS were not significantly different, as expected (since BS were produced from DS just by breaking cells). Compared to the FA composition of cooked soybean and SO, the FA composition of both BS and DS was slightly enriched in C18:0 and depleted in C18:2. Besides, no CLA or hydroxy FAs were found in the starting materials (data not shown).| Sample | FA composition (mg g−1 of food) | Lipid contenta (mg g−1 of dry matter) | ||||
|---|---|---|---|---|---|---|
| C16:0 | C18:0 | C18:1 | C18:2 | C18:3 | ||
| Note: results are expressed as mean ± standard deviation.a The lipid content of each sample was the sum value of the detected FAs (C16:0, C18:0, C18:1, C18:2, and C18:3). Values followed by the same letter in a column are not significantly different (p < 0.05, one-way ANOVA followed by a Tukey post-hoc test). Abbreviations: FA, fatty acid; BS, cell broken digested soybean; DS, digested soybean; SO, soybean oil. | ||||||
| Cooked soybean | 21.73 ± 0.72a | 8.82 ± 0.44a | 49.73 ± 0.71a | 109.1 ± 0.71a | 16.8 ± 1.74a | 206.18 ± 4.32b |
| DS | 11.94 ± 0.45a | 6.63 ± 0.93b | 24.24 ± 1.28a | 55.02 ± 0.44b | 8.88 ± 0.23a | 106.71 ± 3.33c |
| BS | 12.73 ± 0.21b | 6.69 ± 0.47b | 26.36 ± 0.28a | 56.04 ± 0.28b | 8.58 ± 0.21a | 110.40 ± 1.45c |
| SO | 116.5 ± 4.18b | 30.00 ± 3.23b | 232.9 ± 2.11a | 511.10 ± 1.13b | 77.00 ± 2.13a | 967.5 ± 12.78a |
3.2 Microstructure of soybean cotyledons after different treatments
CLSM was used to characterize the microstructure of cooked soybean, DS, BS and fermented DS and BS (Fig. 1). Soybeans preserved their cell intactness after cooking, and proteins and lipids are distributed uniformly within the cell, with big clusters of lipid droplets mainly at the periphery of the cells and surrounding the protein bodies. The observation of DS showed that the majority of cells retained their integrity though some cells showed gaps between the cell wall and proteins because of digestion of the lipid droplets, as previously reported.22 The lipid arrangement in DS was highly modified compared to cooked soybean, with lipids coalescing and forming bigger clusters inside the cells which moved to the periphery of the cells (Fig. 1B). In comparison, cells in BS had broken cell walls which enabled protein and lipids to be released (Fig. 1(C)). The lipids in BS sample formed big clusters, proteins were found in various sizes and shapes, and no intact cell walls were observed, which indicated cells were heavily damaged.After fermentation, the pellets of DS and BS fermentation were also collected and observed, as shown in Fig. 1(D and E). The fermented DS sample still had intact cell walls but they appear lighter compared to those of unfermented DS, possibly because of the fermentation of stainable material by the gut microbiota. Furthermore, the protein areas in most of the cells had further shrunk and larger clusters of lipids were observed at the periphery of the cells compared to the DS sample. In the fermented BS sample, fewer oil droplets were observed.
3.3 Effects of different LA forms on microbial metabolism of linoleic acid
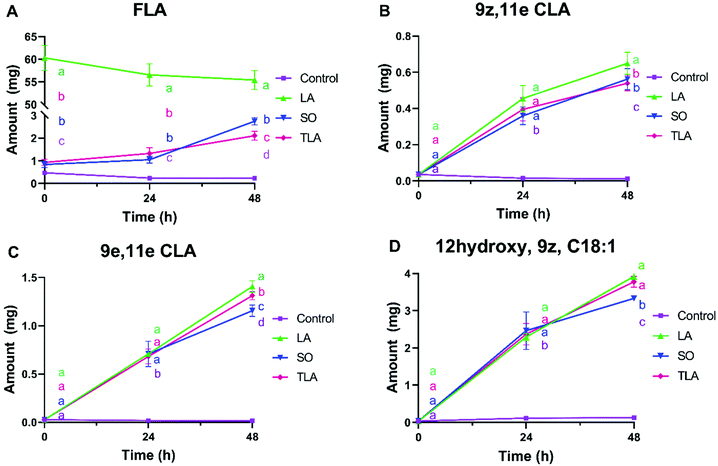
The changes in the amounts of free linoleic acid (FLA) and LA metabolites in the supernatants during the fermentation of LA, TLA and SO is reported in Fig. 2. The amount of FLA was much higher in the LA sample than in the SO and TLA samples, as shown in Fig. 2(A). Although the three samples contained the same amount of LA, this was differently accessible to microorganisms. The LA sample provided direct accessible FLA to the microbiota, while in SO and TLA samples the release of LA from triglycerides required microbial lipolytic activity. In addition, FLA amount in the LA sample slightly decreased during fermentation, compared to that in SO and TLA, in which FLA amounts increased with time. Although LA in both SO and TLA samples was present in the form of triglycerides, the amount of FLA was higher in the SO sample after 48 h, in which around 4% of FLA was released from triglycerides vs. 3.5% of that in TLA.Fig. 2(B–E) shows the level of 9z,11e CLA, 9e,11e CLA and 12hydroxy, 9z, C18:1 FA, (important intermediates in the microbial conversion of LA to CLA) in the supernatants.7 No significant difference was observed in the concentration of the selected FAs among LA, TLA and SO at 24 h. However, after 48 h of fermentation, the concentration of all LA metabolites was significantly higher in the LA sample. Furthermore, the concentration of 9e,11e CLA and 12hydroxy, 9z, C18:1 was higher in the TLA sample than in SO. Since 12hydroxy, 9z, C18:1 is an intermediate in the metabolic conversion of LA into CLAs, this suggests that a more active LA metabolism activity occurs in TLA than in SO.
In addition to microbial metabolism of lipids, we have also investigated if the presence of the lipids would affect the microbial activity of gut microbiota by measuring the amount of six SCFAs (Fig. 3). Acetate and propionate were the two major FAs produced during fermentation, followed by butyrate, isovalerate, valerate, and isobutyrate. Acetate, propionate, butyrate and total SCFA were significantly lower in LA than in the other samples including the control treatment (no lipids added) starting from 24 h. This suggests that LA added in the free form at the beginning of fermentation inhibited the microbial activity unlike triglycerides or free oil.
3.4 The effects of cell wall encapsulation on microbial metabolism of linoleic acid
Pre-digested soybean particles (i.e. BS and DS) were subjected to in vitro colonic fermentation to explore the effects of the soybean matrix on microbial metabolism of linoleic acid. SO was also included as an additional sample wherein the same amount of oil was free from the matrix. Again, an amount of DS, BS and SO was used that contained the same level of LA. Apart from LA metabolites, FLA and SCFA, the content of FFAs in the supernatants and total lipid amount in the whole fermentation were quantified.Despite the different amounts of FLA in SO, BS and DS over time, the amount of 9z,11e CLA was not significantly different among the three treatments, with a conversion rate of linoleic acid into this CLA around 5–6% (5.28%, 5.72% and 5.79%, respectively). Conversely, the amount, as well as the conversion rate of 9e,11e CLA was higher in BS and SO than in DS at 24 h but was higher in BS (5.91%) and DS (5.51%) than in SO (3.66%) after 48 h. The amount of 12hydroxy, 9z, C18:1 was not significantly different between BS and DS until 24 h but higher in DS compared to BS after 48 h. Furthermore, the amount of 12hydroxy, 9z, C18:1 was significantly higher in the SO sample (with a conversion rate at 9.30%) than in the soybean samples (BS, 4.40%; DS, 6.79%). Additionally, the amount of 9z,11e CLA was slightly higher than that of 9e,11e CLA in BS and DS compared to SO in which that of 9e,11e CLA was higher.
As shown in Fig. 5, besides being present in the supernatants of the fermentation (i.e. the liquid fraction available for absorption in the large intestine), LA, two CLAs and 12 hydroxy, 9z C18:1 were also found in the pellets, which contained unfermented soybean material and/or bacterial cells. Because the pellets of the SO sample only contained bacterial cells, the presence of those FAs in the pellets suggests that LA metabolites are incorporated into bacterial membranes.
Interestingly, the FLA amount in pellets was higher at any time in the BS than in the DS sample. However, unlike in the supernatants where the amount of LA metabolites kept increasing, the amounts of LA metabolites detected in the pellets increased until 24 h and then remained constant or slightly decreased. It was observed that the content of the selected FAs was higher in the BS sample than in other treatments, whereas no difference was observed for 12hydroxy, 9z, C18:1 amount in DS at 48 h. Nevertheless, unlike supernatants where a large amount of 12hydroxy, 9z, C18:1 was detected, a limited amount of that was observed in the pellets.
When the results from both supernatants and pellets are combined, we can conclude that soybean cell–matrix limits the level of accessible FLA to microbiota but it enables the production of higher levels of CLAs compared to that of free oil. Loss of cellular integrity can result in higher levels of CLAs but the effect vanished with time, as already reported with intracellular starch fermentation.24 Additionally, free oil can produce higher levels of 12hydroxy, 9z, C18:1 than soybean samples.
The level of FFAs was the highest in SO samples until 24 h followed by BS and then DS, suggesting that free oil and damaged cells allowed oil to have higher accessibility to lipase/hydrolysis and resulted in a higher FFA content similar to the FLA data in Fig. 2A and 4A. However, no significant difference was observed at 48 h between BS and DS (p > 0.05). This indicates that the effects of cell integrity on the release of FFA is temporary and vanishes with time. Besides, it was found that 56.3% and 76.6% of total FAs in supernatants of BS and DS fermentations, respectively, were FFAs.
The FA composition of the whole system was also determined and reported in Fig. 7. In each treatment, the content in C18:1, C18:2 and C18:3 dramatically decreased with time, indicating a large consumption of those FAs. Since values of C18:0 did not significantly change, these FA were converted into other unsaturated FA, such as FAs with functional groups, including CLA isomers and hydroxy FA, which content increased during fermentation. Among three treatments, BS and DS contained higher values of each CLA than SO after 48 h fermentation, while no significant difference was observed between BS and DS samples. However, hydroxy C18:1 was found higher in SO, followed by DS and BS. This suggests that the LA metabolism outcomes are related to how LA is supplied. In brief, we detected a trend for a decrease in the sum of the selected FA in time, most likely due to the conversion in other FA that were not monitored.
4 Discussion
It has been long recognized that, next to dietary fibre and proteins, gut microbiota can metabolize lipids producing a range of bioactive metabolites. Metabolism of unsaturated FAs as LA and linolenic acid is of particular interest. The metabolic pathways through which gut microbiota metabolizes LA have been widely investigated as it has potential beneficial effects on intestinal health of certain LA metabolites.7,34,35 An increasing body of evidence shows that structural and compositional aspects of foods and meals could modulate the production of e.g. SCFA and tryptophan metabolites,20,23 but the effect of those factors in the modulation of the production of LA metabolites is unknown. Hence, this study, we believe for the first time, explored the effects of dietary factors and food-related factors on the microbial production of LA metabolites by human microbiota. This was done by supplying LA in different forms (free, in TAGs, in oil, in oil within intact or broken pre-digested soybean cells) and monitoring LA metabolism by tracking the amounts of three LA metabolites.Our results showed that the microbial metabolism of LA is different depending on the form in which LA is provided. In the first place, utilization of LA would depend on whether LA is in a free form or bound to glycerol in TAGs. When LA is provided in a free form, more LA metabolites are produced. This may be because a much higher level of the precursor LA is available to microbes from the early-stage (Fig. 2). Our results on the production of SCFA (Fig. 3) suggests also that higher amounts of FLA may inhibit microbial growth and accelerate the transformation of FLA into less toxic FAs, i.e. LA metabolites.5,36 In contrast, when lipids are supplied in the form of triglycerides bacterial lipases are required to release FLA37 and only part of the FA is released. This results in lower levels of FLA at each time point. Besides, it was also observed that when LA was provided in the form of free oil (SO) higher amount of FLA and lower amount of LA metabolites were produced compared to LA provided as trilinoleate, although the amount of SCFA produced was not significantly different. This may be ascribed to the higher content in FA in SO, including FA other than LA, e.g. linolenic acid, which would also represent a substrate for microbial metabolism and thereby influences the metabolism of LA.27
Apart from the chemical structure of the lipid provided, it was also reported that the presence of an intact cell wall, such as in isolated intact cells and cells in the core of food particles, can affect the microbial metabolism of food compounds.20,23 Hence, we investigated whether plant cell integrity would affect microbial LA metabolism. Our data show that damaging cell integrity allowed to produce slightly higher levels of LA metabolites, at least at 24 h of fermentation, as well as slightly higher, albeit statistically significant, concentrations of butyrate. This is because of the release of more intracellular components, including LA and proteins from the broken cells which, at an early phase of the fermentation, might contribute to sustain the microbial growth and activity in the BS sample. This higher availability of intracellular proteins may explain the higher levels of butyrate after 24 h, since butyrate can be produced by bacterial metabolism of certain amino acids. However, the levels of all the remaining SCFA were not affected by cell integrity and, as expected, total SCFA was little affected by the presence of higher levels of easily available intracellular proteins and lipids because bacteria would preferentially use carbohydrates, i.e. cell wall components for their growth. In contrast, studies using easily fermentable starch-containing peas and beans reported that broken cells would produce higher levels of SCFA.23,38 Moreover, LA metabolites were found not only in supernatants but also in pellets of BS, DS and SO fermentations, which contained cells of soybean and/or bacteria. In the supernatants, higher CLA amounts were observed in BS and DS samples, despite the higher amount of FLA and 12hydroxy, 9z, C18:1 in the SO sample. In the pellets, a higher amount of LA metabolites was also found in BS and DS samples than in SO. This resulted in a higher amount of total LA metabolites in BS and DS than in the SO sample. Since LA metabolites were not found in the starting materials, i.e. BS, DS and SO, we speculate that the LA metabolites in pellets were those incorporated into bacterial cells, mostly in cell membranes. The physiological relevance of those FA metabolites on host's health and microbial ecology is not clear and deserves further investigation. The reason for the higher amount of LA metabolites in BS and DS than in SO might be the presence of extra nutrients in DS and BS, in the form of cell wall polysaccharides and intracellular proteins, which promoted LA metabolism, possibly through sustaining the general bacterial growth, as confirmed by the SCFA results. This also suggests that the higher CLA levels observed in BS possibly link to the population of CLA-producers. Besides, the finding of higher 12hydroxy, 9z, C18:1 level in SO also suggests that the outcomes of microbial metabolism of lipids may also be related lipid forms.
In the present study, we also characterized the change in FA composition during fermentation of lipid-rich matrices, something that is poorly reported. Here we observed an effect of the presence of an intact cell wall on the production of FFA in soybean isolated cells but this effect vanished with time. This is similar to what was reported on the effect of cell integrity on starch utilization in red kidney beans.24,39 Since the metabolism of LA involves a hydrogenation step, we expected a decrease of the unsaturation level and a simultaneous increase in stearic acid, i.e. C18:0 which is the final product of LA saturation.40,41 We found a consistent decrease in the content of C18:1, C18:2 and C18:3 which is possibly explained by the conversion in other unsaturated FA, some of which were monitored in the present study. However, the overall content in saturated FA, especially stearic acid also decreased or at least did not increase which suggests that microbial LA metabolism by human gut microbiota will produce CLAs without largely increasing the overall level of saturation in 48 h fermentation. Overall, when the total FA content was calculated combining supernatants and pellets we found a trend for a slight decrease in the total amount of FAs (p = 0.0634 for BS, p = 0.0810 for DS, and p = 0.0515 for SO, using two-way ANOVA followed by Tukey's post-hoc test) and this trend appeared in all the treatments.
5 Conclusion
Our results provided evidence that food-related and dietary factors such as the form in which LA is provided to gut microbes or the presence of additional substrates in the food matrix can affect microbial LA metabolism. Higher levels of LA metabolites may be produced in the large intestine if dietary lipids are present in ileal effluents as FFAs. When the lipids are encapsulated in a plant matrix, the level of LA metabolites would be the result of a balance between the higher accessibility of LA (as well as other FA) and the extra energy provided by cell wall polysaccharides and proteins which will sustain microbial growth and conversion of LA. Specifically, the presence of additional fermentable components promotes FLA conversion compared to free oil. In contrast to the intact cells, the damaged soybean cells provide both easily accessible energy and FLA at the start, thereby explaining the higher content of LA metabolites in the whole system. However, this effect on the CLA production in the supernatants of fermentation vanished with time. Taking the effect of lipids and cell components together, the microbial metabolism of LA depends on the nutrient level in the media: with sufficient nutrient supplies, the LA conversion is accelerated due to higher microbial activity and more CLA-producers, while a more intense detoxification of PUFA by CLA-producers to improve the living environment would occur with less available carbohydrate or protein nutrients requires. Hence, further research is needed to understand the role of the nutrients level and composition to be able to modulate microbial lipid metabolite profile in a desired way. Moreover, the effect of the microbial community structure on lipid metabolism was not addressed in the present study. However, further insight into the changes induced by diet on the microbial community and on microbial metabolism of dietary lipids is advised in future studies.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank Geert Meijer and Christos Fryganas for setting up the GC method. Zongyao Huyan received a PhD scholarship from the China Scholarship Council (CSC no. 201906300025).References
- I. Rowland, G. Gibson, A. Heinken, K. Scott, J. Swann, I. Thiele and K. Tuohy, Eur. J. Nutr., 2018, 57, 1–24 CrossRef CAS PubMed.
- Y. Heianza, W. Ma, J. A. E. Manson, K. M. Rexrode and L. Qi, J. Am. Heart Assoc., 2017, 6, 1–20 Search PubMed.
- K. A. Verbeke, A. R. Boobis, A. Chiodini, C. A. Edwards, A. Franck, M. Kleerebezem, A. Nauta, J. Raes, E. A. F. Van Tol and K. M. Tuohy, Nutr. Res. Rev., 2015, 28, 42–66 CrossRef CAS PubMed.
- L. Gorissen, F. Leroy, L. De Vuyst, S. De Smet and K. Raes, Crit. Rev. Food Sci. Nutr., 2015, 55, 1561–1574 CrossRef CAS PubMed.
- S. Kishino, M. Takeuchi, S.-B. Park, A. Hirata, N. Kitamura, J. Kunisawa, H. Kiyono, R. Iwamoto, Y. Isobe, M. Arita, H. Arai, K. Ueda, J. Shima, S. Takahashi, K. Yokozeki, S. Shimizu and J. Ogawa, Proc. Natl. Acad. Sci. U. S. A., 2013, 110, 17808–17813 CrossRef CAS PubMed.
- J. Ogawa, S. Kishino, A. Ando, S. Sugimoto, K. Mihara and S. Shimizu, J. Biosci. Bioeng., 2005, 100, 355–364 CrossRef CAS PubMed.
- A. S. Salsinha, L. L. Pimentel, A. L. Fontes, A. M. Gomes and L. M. Rodríguez-Alcalá, Microbiol. Mol. Biol. Rev., 2018, 82 Search PubMed.
- E. Devillard, F. M. McIntosh, S. H. Duncan and R. J. Wallace, J. Bacteriol., 2007, 189, 2566–2570 CrossRef CAS PubMed.
- R. Hontecillas, M. J. Wannemeulher, D. R. Zimmerman, D. L. Hutto, J. H. Wilson, D. U. Ahn and J. Bassaganya-Riera, J. Nutr., 2002, 132, 2019–2027 CrossRef CAS PubMed.
- S. Borniquel, C. Jädert and J. O. Lundberg, J. Nutr., 2012, 142, 2135–2140 CrossRef CAS PubMed.
- D. Brandão and L. Ribeiro, Int. J. Food Sci. Nutr., 2018, 69, 437–450 CrossRef PubMed.
- F. Beppu, M. Hosokawa, L. Tanaka, H. Kohno, T. Tanaka and K. Miyashita, J. Nutr. Biochem., 2006, 17, 830–836 CrossRef CAS PubMed.
- F. M. McIntosh, K. J. Shingfield, E. Devillard, W. R. Russell and R. J. Wallace, Microbiology, 2009, 155, 285–294 CrossRef CAS PubMed.
- E. Devillard, F. M. McIntosh, D. Paillard, N. A. Thomas, K. J. Shingfield and R. J. Wallace, Microbiology, 2009, 155, 513–520 CrossRef CAS PubMed.
- H. E. Yang, Y. Li, A. Nishimura, H. F. Jheng, A. Yuliana, R. Kitano-Ohue, W. Nomura, N. Takahashi, C. S. Kim, R. Yu, N. Kitamura, S. B. Park, S. Kishino, J. Ogawa, T. Kawada and T. Goto, Mol. Nutr. Food Res., 2017, 61, 1700064 CrossRef PubMed.
- T. Nanthirudjanar, H. Furumoto, J. Zheng, Y. Il Kim, T. Goto, N. Takahashi, T. Kawada, S. B. Park, A. Hirata, N. Kitamura, S. Kishino, J. Ogawa, T. Hirata and T. Sugawara, Lipids, 2015, 50, 1093–1102 CrossRef CAS PubMed.
- G. Somaratne, M. J. Ferrua, A. Ye, F. Nau, J. Floury, D. Dupont and J. Singh, Crit. Rev. Food Sci. Nutr., 2020, 60, 3753–3769 CrossRef CAS PubMed.
- G. Mandalari, N. M. Rigby, C. Bisignano, R. B. Lo Curto, F. Mulholland, M. Su, M. Venkatachalam, J. M. Robotham, L. A. N. Willison, K. Lapsley, K. H. Roux and S. K. Sathe, LWT–Food Sci. Technol., 2014, 59, 439–447 CrossRef CAS.
- H. Sengul, E. Surek and D. Nilufer-Erdil, Food Res. Int., 2014, 62, 1069–1079 CrossRef CAS.
- Z. Huang, T. Schoones, J. M. Wells, V. Fogliano and E. Capuano, Mol. Nutr. Food Res., 2021, 2100092 CrossRef CAS PubMed.
- L. Kan, T. Oliviero, R. Verkerk, V. Fogliano and E. Capuano, J. Funct. Foods, 2020, 68, 103924 CrossRef CAS.
- M. Zahir, V. Fogliano and E. Capuano, Food Funct., 2018, 9, 6326–6336 RSC.
- N. Guan, X. He, S. Wang, F. Liu, Q. Huang, X. Fu, T. Chen and B. Zhang, J. Agric. Food Chem., 2020, 68, 1091–1100 CrossRef CAS PubMed.
- A. M. Rovalino-Córdova, V. Fogliano and E. Capuano, J. Funct. Foods, 2020, 73, 104087 CrossRef.
- A. Ando, J. Ogawa, S. Kishino and S. Shimizu, J. Am. Oil Chem. Soc., 2003, 80, 889–894 CrossRef CAS.
- A. Roy, G. P. Mandal and A. K. Patra, Vet. World, 2017, 10, 11–16 CrossRef CAS PubMed.
- T. A. Wood, N. Mckain, X. Shen, C. Atasoglu and R. J. Wallace, Anim. Feed Sci. Technol., 2010, 161, 28–37 CrossRef CAS.
- M. Adamczak, U. T. Bornscheuer and W. Bednarski, Eur. J. Lipid Sci. Technol., 2008, 110, 491–504 CrossRef CAS.
- A. Brodkorb, L. Egger, M. Alminger, P. Alvito, R. Assunção, S. Ballance, T. Bohn, C. Bourlieu-Lacanal, R. Boutrou, F. Carrière, A. Clemente, M. Corredig, D. Dupont, C. Dufour, C. Edwards, M. Golding, S. Karakaya, B. Kirkhus, S. Le Feunteun, U. Lesmes, A. Macierzanka, A. R. Mackie, C. Martins, S. Marze, D. J. McClements, O. Ménard, M. Minekus, R. Portmann, C. N. Santos, I. Souchon, R. P. Singh, G. E. Vegarud, M. S. J. Wickham, W. Weitschies and I. Recio, Nat. Protoc., 2019, 14, 991–1014 CrossRef CAS PubMed.
- B. Guo, T. Oliviero, V. Fogliano, Y. Ma, F. Chen and E. Capuano, J. Agric. Food Chem., 2020, 68, 1844–1850 CrossRef CAS PubMed.
- E. G. Bligh and W. J. Dyer, Can. J. Biochem. Physiol., 1959, 37, 911–917 CrossRef CAS PubMed.
- K. Raes, S. De Smet and D. Demeyer, Anim. Sci., 2001, 73, 253–260 CrossRef CAS.
- M. Zahir, Effect of cell integrity on soybean protein digestion and fermentation: an in vitro study, Wageningen University & Research, 2021.
- B. Yang, H. Gao, C. Stanton, R. P. Ross, H. Zhang, Y. Q. Chen, H. Chen and W. Chen, Prog. Lipid Res., 2017, 68, 26–36 CrossRef CAS PubMed.
- S. Basak and A. K. Duttaroy, Nutrients, 2020, 12, 1–7 Search PubMed.
- C. E. Polan, J. J. McNeill and S. B. Tove, J. Bacteriol., 1964, 88, 1056–1064 CrossRef CAS PubMed.
- A. S. Martin-Rubio, P. Sopelana and M. D. Guillén, J. Sci. Food Agric., 2019, 99, 4793–4800 CrossRef CAS PubMed.
- R. R. Bhattarai, S. Dhital, B. A. Williams, H. J. Yang, D. Mikkelsen, B. M. Flanagan and M. J. Gidley, Food Hydrocolloids, 2021, 113, 106538 CrossRef CAS.
- A. M. Rovalino-Córdova, V. Fogliano and E. Capuano, Food Funct., 2021, 12, 4983–4994 RSC.
- C. R. Kepler, W. P. Tucker and S. B. Tove, J. Biol. Chem., 1970, 245, 3612–3620 CrossRef CAS.
- M. Coakley, R. P. Ross, M. Nordgren, G. Fitzgerald, R. Devery and C. Stanton, J. Appl. Microbiol., 2003, 94, 138–145 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |