Open Access Article
Open Access ArticleHypertension- and glycaemia-lowering effects of a grape-pomace-derived seasoning in high-cardiovascular risk and healthy subjects. Interplay with the gut microbiome†
Diego
Taladrid
a,
Miguel
de Celis
b,
Ignacio
Belda
b,
Begoña
Bartolomé
a and
M. Victoria
Moreno-Arribas
 *a
*a
aInstitute of Food Science Research (CIAL), CSIC-UAM, C/Nicolás Cabrera 9, 28049 Madrid, Spain. E-mail: victoria.moreno@csic.es; Tel: +34 910017900
bDepartment of Genetics, Physiology and Microbiology, Complutense University of Madrid, 28040-Madrid, Spain
First published on 12th January 2022
Abstract
Purpose: Grape pomace (GP) is a winery by-product rich in polyphenols and dietary fibre. Some recent results suggest that GP-derived extracts could be promising additives in food, specially recommended for low-salt diets. The hypothesis tested in this paper is that the regular consumption of GP-derived seasonings could help in the control of hypertension and glycaemia. Methods: A randomized intervention study (6 weeks) was performed in high-risk cardiovascular subjects (n = 17) and in healthy subjects (n = 12) that were randomly allocated into intervention (2 g day−1 of GP seasoning) or control (no seasoning consumed) groups. Blood samples, faeces, urine and blood pressure (BP) were taken at the baseline and at the end of the intervention. Faecal samples were analysed for microbiota composition (16S rRNA gene sequencing) and microbial-derived metabolites (short chain fatty acids and phenolic metabolites). Results: Among the clinical parameters studied, BP and fasting blood glucose significantly decreased (p < 0.05) after the seasoning intervention, but not for the control group. Notably, application of a novel approach based on ASV (Amplicon Sequence Variant) co-occurrence networks allowed us to identify some bacterial communities whose relative abundances were related with metadata. Conclusion: Our primary findings suggest that GP-seasoning may help in the modulation of cardiometabolic risk factors, mainly in the early stages. Furthermore, it evidences modulation of gut microbiota and functional bacterial communities by grape pomace, which might mediate the cardiometabolic effects of this by-product.
1. Introduction
Prevention of cardiometabolic risk factors, such as hypertension and glycaemia, is a great challenge worldwide. Both high blood pressure and high fasting glucose level, together with other factors, cluster what is termed metabolic syndrome (MeS), a pathological condition that predisposes adults to develop diabetes, cardiovascular disease or both. Estimations from the Global Burden of Diseases indicate that in 2015, 19.4% and 8.2% of the world population showed high systolic blood pressure and high fasting plasma glucose, respectively, being responsible for 16 million global deaths.1 Issues such as age, gender, genetic background, physical activity, dietary patterns and microbiota composition have been associated with these MetS risk factors.2 Hypocaloric and low-salt diets are the first strategies to control both hypertension and glycaemia.3 Moreover, consumption of certain foods rich in fibre and polyphenols has also been found effective in the prevention of MetS risk factors (see ref. 4 for a review). These food components have demonstrated protective cardiometabolic effects through diverse intricate mechanisms.5,6 In addition, they may influence gut microbiota composition and/or functionality and hence, impact on microbiota-related diseases.7,8The gut microbiome exerts digestive, metabolic, immunomodulatory and protective functions that affect the general health status of the host.9,10 Several authors reported a decrease in microbial richness and diversity, and an impact on the relative abundance of specific genera and family in pre-hypertensive and hypertensive individuals11,12 and people suffering from glycaemic imbalances.13,14 Although it is known that diet can promote beneficial cardiometabolic effects associated with gut microbiota metabolism (i.e., production of short chain fatty acids from dietary fibre15), the causal relationships among cardiovascular diseases and MetS risk factors, diet and gut microbiota still remain to be completely clarified.16
Grape pomace (GP) is a winery by-product composed of grape seeds, skin and stems, and is rich in dietary fibre and polyphenols. Extracts from GP are suitable for different applications in the food industry, also representing a profitable and environment-friendly utilization way for this by-product that is generated in large quantities (25 kg per 100 kg of fresh grapes).17 Based on their potential biological activities, several intervention studies with GP supplements have been carried out in humans.5,18,19
Some recent results suggest that GP-derived ingredients could be promising additives in food,20 and specially recommended as sodium salt replacers. Moreover, sensory evaluation of several GP-derived seasonings incorporated into everyday dishes has confirmed their good sensory acceptability by consumers, which may enhance consumer liking and adherence to low-sodium diets.21 From this background, the aim of the present study was to address the effects of the regular consumption of GP-derived seasonings in subjects suffering from hypertension and/or glycaemia. For that, a complete biochemical study, a description of the gut microbiome composition and structure, and the analysis of faecal metabolites (i.e., short chain fatty acids and phenolic metabolites) were performed in samples from high-cardiovascular risk subjects (HCR subjects) and healthy subjects, before and after a nutritional intervention with a GP-derived seasoning (Fig. 1). The relationship between the clinical parameters and sequencing data was also investigated through the application of novel approaches based on bacterial co-occurrence networks defined by the presence of ASV (Amplicon Sequence Variant) in samples (Fig. 1). Overall, this paper brings new findings about the health effects of GP-derived seasonings and, in general, about the interplay between MetS risk factors, diet and gut microbiota.
 | ||
| Fig. 1 Flow chart of this study, including details about intervention, sample collection, analytical determinations, and data analysis. | ||
2. Materials and methods
2.1. Grape pomace extracts
A seasoning obtained from red grape pomace whose sensory acceptability by consumers was previously confirmed21 was used in this study. It was manufactured in the industrial facilities of Bodega Matarromera (Valbuena de Duero, Valladolid, Spain). Its total phenolic content was 47.96 ± 4.08 mg of gallic acid equivalents per g as determined by the Folin–Ciocalteau's method, being rich in phenolic acids (1843.03 μg−1), flavan-3-ols (276.31 μg−1) and flavonoids (72.76 μg−1).21 As indicated by the manufacturer, other components were: protein (77 mg g−1), complex polysaccharides (810 mg g−1), dietary fibre (19 mg g−1), fat (l < 10 mg g−1) and ash (39 mg g−1).2.2. Clinical trial
The intervention study (ISRTC 13100451) was conducted in accordance with the Clinical Ethics Committee at the Health Department of Comunidad de Madrid (Madrid, Spain) and the Spanish National Research Council's Bioethics Committee (Madrid, Spain) (reference RTC-2016-4556-1). All experiments were performed in compliance with the Declaration of Helsinki. A total of 32 volunteers (45.24 ± 15.41 years old), including people with cardiometabolic risk factors (HCR subjects) and healthy subjects, were recruited at the initial stage. The including criteria for the group with metabolic imbalance were to present raised BP (systolic BP (SPB) > 130 mmHg and/or diastolic BP (DBP) > 85 mmHg) and/or increased fasting plasma glucose (>100 mg dL−1). By contrast, healthy subjects had to present normal values of the mentioned parameters and no diagnosed disease. Exclusion criteria for both groups were: diagnosed with serious cardiovascular disease, antibiotic or nutraceutical intake up to 3 months before the study, endocrine and gastrointestinal disorders, addiction to drugs and restrictive diets (i.e. vegetarians). None of the HCR subjects was under medication. The following formula was employed for calculating the sample size:where Zα represents a p value lower than 0.05 (Zα = 1.96), Zβ a power of 80% (Zβ = 0.842), while d and S are based, respectively, on the mean reduction of 8 mmHg and the standard deviation of 7.5 mmHg in MBP, both reported in previous studies carried out under similar conditions.22–24
Before starting, 31 volunteers received full information about the study and a survey of dietary habits and of recipes low in sodium, and they signed an informed consent to participate. Then, they were allocated randomly into the intervention or the control groups by lottery employing sequentially numbered containers. Both intervention and control groups had to reduce the consumption of sodium salt during the 6 weeks of the clinical trial. Furthermore, the intervention group consumed 2 g day−1 of the GP-derived seasoning as a sodium salt replacer in their foods (Fig. 1). A total of 29 volunteers successfully completed the study: 17 from the intervention group (9 HCR subjects and 8 healthy subjects) and 12 from the control group (7 HCR subjects and 5 healthy subjects) (Table 1). Samples of urine, faeces and blood and the data of height, weight, waist circumference and BP were taken before and after the intervention period. Mean Blood Pressure (MBP) was calculated using the following equation:
| Control | Intervention | |||
|---|---|---|---|---|
| Healthy subjects | HCR subjects | Healthy subjects | HCR subjects | |
| n (total) | 5 | 7 | 8 | 9 |
| n (females) | 2 | 4 | 6 | 5 |
| n (males) | 3 | 3 | 2 | 4 |
| Age | 33.00 ± 11.28 | 57.50 ± 4.97 | 35.00 ± 14.48 | 52.00 ± 12.49 |
| Weight (kg) | 70.89 ± 19.37 | 79.33 ± 15.47 | 60.90 ± 11.00 | 81.96 ± 14.75 |
| BMI (kg m−2) | 25.31 ± 5.79 | 29.16 ± 3.96 | 22.65 ± 3.10 | 30.37 ± 3.98 |
| SBP (mmHg) | 118.3 ± 9.8 | 137.7 ± 9.3 | 108.9 ± 11.6 | 135.0 ± 5.2 |
| DBP (mmHg) | 75.00 ± 13.78 | 90.46 ± 7.27 | 68.89 ± 7.82 | 88.89 ± 11.67 |
| Glucose (mg dL−1) | 80.00 ± 10.00 | 98.50 ± 15.59 | 76.22 ± 3.19 | 89.63 ± 12.29 |
| Cholesterol (mg dL−1) | 189.5 ± 28.1 | 185.2 ± 46.6 | 167.7 ± 25.4 | 217.3 ± 23.1 |
| Triglycerides (mg dL−1) | 98.67 ± 38.51 | 118.3 ± 95.1 | 69.44 ± 17.09 | 140.8 ± 41.9 |
| HDL (mg dL−1) | 56.17 ± 17.19 | 53.60 ± 15.44 | 60.33 ± 12.64 | 58.25 ± 19.65 |
| LDL (mg dL−1) | 113.5 ± 30.9 | 98.20 ± 33.45 | 93.56 ± 19.68 | 130.8 ± 23.6 |
| MBP (mmHg) | 89.44 ± 12.37 | 106.2 ± 6.6 | 82.22 ± 8.66 | 98.33 ± 20.08 |
Before sequencing and metabolite analysis, faecal samples were thawed and 1 gram was diluted in 10 ml of PBS. After a vigorous homogenization, five aliquots of 1 mL were prepared and then centrifuged at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 minutes at 4 °C.
000 rpm for 10 minutes at 4 °C.
2.3. Plasma biochemical parameter measurement
Serum biochemical parameters were measured in plasma using an automated biochemical auto-analyser in an accredited laboratory. The tests included the measurement of glucose levels, lipids, transaminases, and ions and haematology (Table 2). Insulin was measured with the IMMULITE 2000 analyzer (DPC, LA, USA). All determinations were carried out at least in duplicate.| Control (n = 12) | Intervention (n = 17) | |||||
|---|---|---|---|---|---|---|
| Initial | Final | pval | Initial | Final | pval | |
| Anthropometric measurements | ||||||
| BMI (kg m−2) | 27.23 ± 5.14 | 26.75 ± 5.1 | 0.343 | 26.29 ± 5.24 | 26.09 ± 5.22 | 1.000 |
| Abdomen (cm) | 94.08 ± 14.87 | 94.86 ± 13.69 | 1.000 | 93.53 ± 14.07 | 93.38 ± 16.9 | 1.000 |
| Waist (cm) | 96.08 ± 18.8 | 100.23 ± 13.15 | 0.762 | 98.53 ± 16.95 | 96.75 ± 15.74 | 0.983 |
| SBP (mmHg) | 129.41 ± 13.54 | 129.46 ± 15.17 | 0.888 | 122.63 ± 15.93 | 104.44 ± 15.42 | 0.003** |
| DBP (mmHg) | 83.83 ± 12.83 | 82.85 ± 10.02 | 0.330 | 78.89 ± 14.1 | 67.78 ± 9.43 | 0.013* |
| MBP (mmHg) | 99.02 ± 12.51 | 91.36 ± 28.22 | 0.582 | 90.7 ± 17.41 | 77.71 ± 8.05 | 0.004** |
| Blood biochemistry | ||||||
| Leukocytes (103 μL−1) | 6.78 ± 2.55 | 7.12 ± 2.99 | 1.000 | 6.6 ± 1.03 | 6.57 ± 1.04 | 0.720 |
| Erythrocytes (103 μL−1) | 5.02 ± 0.51 | 4.96 ± 0.55 | 0.241 | 4.89 ± 0.46 | 4.79 ± 0.37 | 0.079 |
| Hemoglobin (g dL−1) | 14.61 ± 1.16 | 14.55 ± 1.32 | 0.735 | 14.29 ± 1.34 | 14.21 ± 1.07 | 0.294 |
| Hematocrit (%) | 44.80 ± 3.50 | 44.28 ± 3.99 | 0.075 | 43.05 ± 3.43 | 42.62 ± 2.73 | 0.222 |
| MCV (fL) | 89.5 ± 4.29 | 89.6 ± 3.56 | 0.770 | 88.26 ± 4.24 | 89.12 ± 4.01 | 0.083 |
| MCH (pg) | 29.15 ± 1.63 | 29.39 ± 1.79 | 0.160 | 29.27 ± 1.46 | 29.72 ± 1.73 | 0.201 |
| MCHC (g dL−1) | 32.64 ± 1.32 | 32.8 ± 1.29 | 0.084 | 33.17 ± 0.89 | 33.33 ± 0.9 | 0.842 |
| RDW (5) | 13.08 ± 0.64 | 13.3 ± 0.79 | 0.359 | 13.34 ± 1.18 | 13.33 ± 0.62 | 0.889 |
| Platelets (103 μL−1) | 273.25 ± 49.18 | 274.36 ± 53.26 | 0.414 | 284.88 ± 60.7 | 300.75 ± 67.07 | 0.730 |
| MPV (fl) | 9.11 ± 0.86 | 9.26 ± 0.67 | 1.000 | 8.89 ± 0.98 | 8.96 ± 1.36 | 0.909 |
| Lymphocytes (103 μL−1) | 2.15 ± 0.75 | 2.3 ± 0.6 | 0.193 | 2.14 ± 0.67 | 2.27 ± 0.67 | 0.074 |
| % Lymphocytes | 33.11 ± 9.21 | 35.35 ± 10.38 | 0.169 | 32.49 ± 9.00 | 34.61 ± 9.01 | 0.018* |
| Monocytes (103 μL−1) | 0.45 ± 0.15 | 0.51 ± 0.4 | 0.092 | 0.39 ± 0.10 | 0.36 ± 0.08 | 0.096 |
| % Monocytes | 6.94 ± 1.45 | 6.78 ± 2.07 | 0.123 | 5.96 ± 1.34 | 5.54 ± 0.92 | 0.027* |
| Neutrophils (103 μL−1) | 3.87 ± 1.9 | 4.02 ± 2.4 | 0.922 | 3.83 ± 0.83 | 3.66 ± 0.88 | 0.140 |
| % Neutrophils | 55.73 ± 10.16 | 53.83 ± 10.14 | 0.375 | 58.11 ± 8.83 | 55.65 ± 8.9 | 0.017* |
| Eosinophils (103 μL−1) | 0.19 ± 0.14 | 0.19 ± 0.12 | 0.834 | 0.20 ± 0.12 | 0.25 ± 0.17 | 0.011* |
| % Eosinophils | 2.56 ± 1.79 | 2.65 ± 1.59 | 0.235 | 2.96 ± 1.64 | 3.7 ± 2.31 | 0.012* |
| Basophils (103 μL−1) | 0.05 ± 0.04 | 0.02 ± 0.03 | 0.269 | 0.03 ± 0.01 | 0.03 ± 0.01 | 0.874 |
| % Basophils | 0.62 ± 0.34 | 0.49 ± 0.19 | 0.673 | 0.48 ± 0.19 | 0.5 ± 0.21 | 0.722 |
| Glucose (mg dL−1) | 89.25 ± 15.79 | 88.82 ± 15.56 | 0.959 | 82.53 ± 10.9 | 77.94 ± 12.29 | 0.028* |
| Creatinine (mg dL−1) | 0.73 ± 0.12 | 0.74 ± 0.11 | 0.944 | 0.74 ± 0.14 | 0.76 ± 0.15 | 0.438 |
| Bilirubin (mg dL−1) | 0.71 ± 0.38 | 0.62 ± 0.24 | 0.295 | 0.62 ± 0.25 | 0.61 ± 0.25 | 0.830 |
| Uric acid (mg mL−1) | 5.41 ± 1.41 | 5.01 ± 1.46 | 0.284 | 5.24 ± 2.05 | 4.91 ± 1.97 | 0.589 |
| Albumin (g dL−1) | 4.34 ± 0.55 | 4.3 ± 0.48 | 0.396 | 4.47 ± 0.22 | 4.4 ± 0.23 | 0.168 |
| Calcium (mg dL−1) | 9.51 ± 0.36 | 9.57 ± 0.3 | 0.833 | 9.30 ± 0.41 | 9.33 ± 0.34 | 0.955 |
| Phosphorus (mg dL−1) | 3.32 ± 0.25 | 3.14 ± 0.55 | 0.726 | 3.62 ± 0.58 | 3.51 ± 0.63 | 0.310 |
| GPT (UI L−1) | 25.17 ± 11.65 | 23.00 ± 14.80 | 0.213 | 26.53 ± 17.56 | 25 ± 14.71 | 0.278 |
| GGT (UI L−1) | 25.58 ± 20.3 | 21.36 ± 19.32 | 0.858 | 23 ± 18.47 | 21.94 ± 20.77 | 0.972 |
| Sodium (mEq L−1) | 139.36 ± 1.8 | 141 ± 1.73 | 0.159 | 140.53 ± 2.29 | 141.69 ± 1.54 | 0.138 |
| Potassium (mEq L−1) | 4.47 ± 0.31 | 4.16 ± 0.27 | 0.219 | 4.41 ± 0.65 | 4.33 ± 0.55 | 0.124 |
| Cholesterol (mg dL−1) | 187.33 ± 36.82 | 187.91 ± 52.11 | 0.414 | 191 ± 34.73 | 192.25 ± 29.26 | 1.000 |
| Triglycerides (mg dL−1) | 108.5 ± 69.92 | 107.18 ± 35.43 | 0.441 | 103 ± 47.54 | 94.81 ± 29.92 | 0.649 |
| HDL (mg dL−1) | 55 ± 15.65 | 55.91 ± 14.33 | 0.361 | 59.35 ± 15.81 | 60.81 ± 13.28 | 0.842 |
| LDL (mg dL−1) | 106.55 ± 31.46 | 110.55 ± 45.59 | 0.553 | 111.06 ± 28.34 | 112.44 ± 24.96 | 0.798 |
| Insulin (μUI mL−1) | 6.63 ± 3.11 | 6.53 ± 2.86 | 1.000 | 9.21 ± 5.79 | 8.42 ± 4.85 | 0.639 |
| Urine biochemistry | ||||||
| Proteins (mg dL−1) | 3.07 ± 1.71 | 5.19 ± 2.86 | 0.678 | 5.81 ± 4.51 | 5.93 ± 6.85 | 0.631 |
| Creatinine (mg dL−1) | 82.18 ± 34.62 | 107.27 ± 54.5 | 0.435 | 108.06 ± 62.59 | 73.31 ± 35.78 | 0.198 |
| Total calcium (mg dL−1) | 9.16 ± 5.76 | 11.74 ± 6.89 | 0.937 | 8.98 ± 5 | 8.77 ± 4.8 | 0.831 |
| Sodium (mEq L−1) | 91.82 ± 33.48 | 119.91 ± 60.81 | 0.204 | 91.82 ± 48.3 | 107.94 ± 60.01 | 0.291 |
| Potassium (mEq L−1) | 37.44 ± 18.31 | 39.78 ± 19.71 | 0.425 | 49.22 ± 22.29 | 43.83 ± 20.75 | 0.407 |
| Excreted sodium (mEq min−1) | 157.83 ± 67.08 | 162.25 ± 63.02 | 0.572 | 128.02 ± 86.05 | 162.58 ± 73.55 | 0.775 |
| Excreted potassium (mEq min−1) | 62.81 ± 29.08 | 55.02 ± 24.99 | 0.510 | 63.13 ± 25.4 | 66.17 ± 22.5 | 0.582 |
| Creatinine clearance (mL min−1) | 128.37 ± 45.65 | 130.74 ± 36 | 0.226 | 121.89 ± 34.83 | 102.51 ± 35.59 | 0.618 |
2.4. DNA extraction and sequencing
Faecal pellets were employed to bacterial DNA extraction using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany), following the manufacturer's recommended protocol. The two-step PCR Illumina® protocol was followed to prepare libraries, including PCR blockers in the first process for minimizing the amplification of mitochondrial and chloroplast DNA.25 The V3–V4 region of the ribosomal RNA gene was amplified using the forward primer CCTACGGGNBGCASCAG and the reverse primer GACTACNVGGGTATCTAATCC. Sequencing was subsequently carried out on Illumina® MiSeq instrument (Illumina®, San Diego, CA, USA) using 2 × 300 paired-end reads and then it was analysed by amplifying and sequencing the 16S rRNA v3–v4 gene. Raw files are available in the National Center for Biotechnology (NCBI) repository under the project code PRJNA717111.2.5. Sequence processing
The DADA2 algorithm was employed to denoise, filter, align pairs and filter out chimeras in the raw data.26 This algorithm also applies an error correction model that allows the differentiation of even a single nucleotide, leading to the formation of Amplicon Sequence Variants (ASVs). A total of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 886
886![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 843 good quality reads were obtained for faecal DNA. The taxonomic assignment was performed using the naïve Bayesian classifier implemented in DADA2 using the reference database Silva,27 with a bootstrap cut-off of 80%.
843 good quality reads were obtained for faecal DNA. The taxonomic assignment was performed using the naïve Bayesian classifier implemented in DADA2 using the reference database Silva,27 with a bootstrap cut-off of 80%.
2.6. Analysis of short chain fatty acids (SCFA)
Analysis of SCFA in faeces was carried out in duplicate following the SPME-GCMS method described previously.28 Two vials were obtained by sampling, each from a different aliquot. Quantitative data were obtained using calibration curves of each of their corresponding standards compared to the internal standard (2-methylvaleric acid).2.7. Analysis of phenolic metabolites
Analysis of phenolic metabolites in faeces was carried out following the UPLC–ESI–MS/MS methodology described previously.29 Three aliquots per sample of faecal supernatants were employed. Data were collected in the multiple reaction monitoring (MRM) mode. The MS/MS parameters (cone voltage, collision energy, and MRM transition) of the targeted phenolic compounds were reported previously.30 All metabolites were quantified using the calibration curves of their corresponding standards compared to the internal standard (4-hydroxybenzoic-2,3,5,6-d4 acid), except for the phenyl-4-hydroxyvaleric acid that was quantified as (−)-epicatechin. For this reason, this compound was excluded from the sum of phenolic compound contents (∑ phenolic compounds).2.8. Statistical analysis
All statistical analyses were carried out on R. The Wilcoxon signed-rank test (for paired samples) was employed to check the differences in the clinical parameters, bacterial taxa and metabolites between the samples collected before and after the intervention period. Microbial features (i.e., genera and species) most likely to explain the differences between the microbiota data from healthy subjects and HCR subjects were determined by LEfSe. Bacterial co-occurrence networks were constructed in Gephi® to observe the relationships between the microbiome, the metabolites and the clinical data. Keystone members of each community were identified by a mean of the grade, the betweenness centrality and the closeness centrality of each ASV (node) in the network,31 with all three being determined using the igraph package and standardized to a scale of 1 to 0 prior the mean.3. Results
From the 31 volunteers selected for the study, a total of 29 participants completed the study (two participants could not attend the final sample delivery) in two stages between November 2018 and November 2019 (Fig. 1).3.1. Changes in clinical parameters after intervention with the GP-derived seasoning
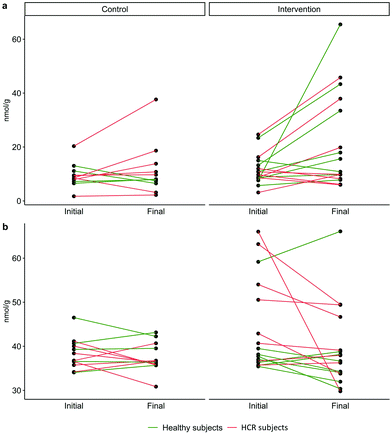
After the intervention period, the evolution of the clinical parameters in people who consumed 2 g day−1 of the GP-derived seasoning (intervention group, n = 17) and the controls (n = 12) was evaluated by the Wilcoxon test (Fig. 2). Blood pressure (i.e., SBP, DBP and MBP) and fasting blood glucose significantly decreased after 6-weeks of consumption of the GP-derived seasoning, whereas no significant change was observed in the rest of the clinical parameters (Table 2). MBP decreased in 16 out of the 17 volunteers in the intervention group with a mean reduction of 14.32%, while no general trend was observed for the control group (Fig. 2a). This outcome was accentuated when we separated the trends of HCR subjects (16.71% reduction after the intervention period, p value = 0.007) and healthy subjects (9.15% reduction after the intervention period, p value = 0.011). A similar outcome was observed in the glycemic profile (Fig. 2b), in which the majority of the volunteers conforming to the intervention group (14 out of 17) exhibited a mean reduction by 5.56% in glucose levels, being similar for the subgroups of healthy subjects and HCR subjects (5.86% and 5.23% reduction after the intervention period, respectively). Less relevant, some leukocyte populations (i.e., eosinophils) significantly increased after intervention with the GP-derived seasoning (Table 2).3.2. Description of global gut microbiota patterns
A total of 3942 ASVs were sequenced from the faecal samples analysed. No differential patterns in terms of alpha and beta diversity were observed between the healthy subjects and HCR subjects at the initial sampling (Fig. S1a and S2b†) or between the initial and final times for both intervention and control groups (Fig. S2a and S2b†). In general, a clear dominance of the phyla Firmicutes, covering around the 60% of microbiota, and Bacteroidetes, with half the abundance, was observed (Fig. S3†). Ruminicoccaceae (34.23%) and Lachnospiraceae (11.62%) were the most abundant families within the Firmicutes phylum, whereas Bacteroidaceae (18.65%) pertains to the phylum Bacteriodetes. A noticeable occurrence of Akkermansia, the only member of the Akkermansiaceae (7.54%) family (Verrucomicrobiota phylum) was also observed (Fig. S3†).Initially, there were several microbial features differentiating the groups of HCR and healthy volunteers (Fig. S4†). From the genus identified as Escherichia/Shigella to the phylum it belongs to (Proteobacteria), both taxa included, all the taxonomic categories were significantly higher in HCR subjects. Other genera with differential abundance in HCR subjects were Tyzerella 4, Gordonibacter and Fournierella. By contrast, healthy subjects were enriched with Subdoligranulum and Ruminococcaceae UCG-013, both members of the family Ruminicoccaceae, and Faecalitalea. This pattern was also observed for some of these microbial features, like Escherichia/Shigella (and its upper taxonomic categories), Subdoligranulum and Ruminicoccaceae UCG-013, when microbial features between the healthy subjects and HCR subjects were represented in a heat tree (Fig. S5†).
None of these genera was affected by the seasoning consumption, neither in healthy subjects nor in HCR subjects. However, some families such as Streptococcaceae experienced a significant increase (143%) (p < 0.05) in their relative abundance after the intervention period whereas others experienced a significant reduction (p < 0.05), as occurred for Eggerthellaceae (52% reduction) and Coribacteriales Incertae Sedis (81% reduction) (Fig. 3). Significant changes (p < 0.05) after the intervention were also noticed in the relative abundance of some genera, such as Peptoniphilus (86% reduction), Clostridiaceae 1 (394% increase), Clostridium sensu stricto 1 (363% increase), Ezakiella (74% reduction), Streptococcus (143% increase), Lachnospiraceae ND3007 group (53% reduction), Paraprevotella (17% increase) and Senegalimassilia (44% reduction) (Fig. 3).
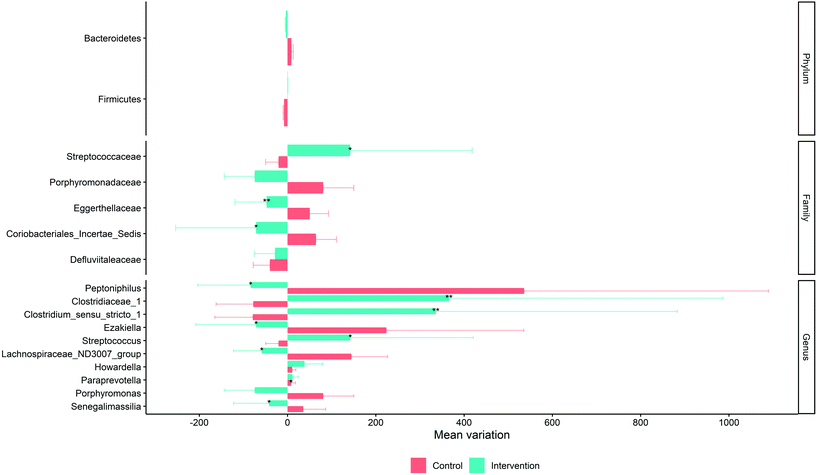 | ||
| Fig. 3 Magnitude of change at the phylum, family and genus levels with the intervention. The Wilcoxon signed-rank test was employed to check statistical differences. | ||
3.3. Changes in faecal metabolites
Acetic, propionic and butyric acids were found in a proportion of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in most of the volunteers (Table 3). Concerning phenolic metabolites, phenyl-4-hydroxyvaleric acid (quantified as (−)-epicatechin), 4-hydroxyphenylacetic acid and 3-(3-hydroxyphenyl) propionic acid were the most abundant. Significant changes (p < 0.05) after intervention with the GP-derived seasoning were found for propionic acid and protocatechuic acid, but not in the control group. After the intervention period, a mean reduction of 10.8% for propionic acid and a mean increase of 152.13% for protocatechuic acid were found for those volunteers who consumed the GP-derived seasoning. Fig. 4 details the individual evolution for both significant metabolites.
1 in most of the volunteers (Table 3). Concerning phenolic metabolites, phenyl-4-hydroxyvaleric acid (quantified as (−)-epicatechin), 4-hydroxyphenylacetic acid and 3-(3-hydroxyphenyl) propionic acid were the most abundant. Significant changes (p < 0.05) after intervention with the GP-derived seasoning were found for propionic acid and protocatechuic acid, but not in the control group. After the intervention period, a mean reduction of 10.8% for propionic acid and a mean increase of 152.13% for protocatechuic acid were found for those volunteers who consumed the GP-derived seasoning. Fig. 4 details the individual evolution for both significant metabolites.
| Concentration (nmol g−1) | Control | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Initial | Final | p | n | Initial | Final | p | |
| a Number of volunteers in which the metabolite was detected. | ||||||||
| Short chain fatty acids | ||||||||
| Acetic acid | 12 | 64.39 ± 20.57 | 66.38 ± 19.12 | 0.695 | 17 | 79.17 ± 29.03 | 72.2 ± 19.26 | 0.495 |
| Propionic acid | 12 | 38.29 ± 3.48 | 38.04 ± 3.68 | 0.322 | 17 | 41.93 ± 10.31 | 37.4 ± 5.65 | 0.030* |
| Butyric acid | 12 | 12.12 ± 4.86 | 15.44 ± 10.64 | 1.000 | 17 | 17.79 ± 15.16 | 16.14 ± 14.76 | 0.188 |
| Phenolic compounds | ||||||||
| Gallic acid | 7 | 6.55 ± 9.58 | 19.01 ± 29.21 | 0.183 | 10 | 12.97 ± 24.79 | 120.02 ± 426.57 | 0.293 |
| Protocatechuic acid | 11 | 9.81 ± 4.84 | 10.99 ± 9.5 | 0.577 | 16 | 11.7 ± 6.24 | 22.44 ± 18.27 | 0.018* |
| 3,4-Dihydroxyphenylacetic acid | 10 | 43.93 ± 85.98 | 44.27 ± 65.35 | 0.831 | 12 | 136.88 ± 380.65 | 112.31 ± 281.89 | 0.802 |
| 3-O-Methylgallic acid | 2 | 0.2 ± 0.5 | 0.47 ± 0.86 | 0.584 | 3 | 1.63 ± 4.07 | 1.17 ± 3.28 | 0.361 |
| 4-Hydroxybenzoic acid | 10 | 2.91 ± 3.94 | 4.88 ± 10.07 | 0.343 | 11 | 3.82 ± 5.35 | 6.99 ± 10.08 | 0.118 |
| Y-valerolactone | 4 | 6.72 ± 10.21 | 9.68 ± 20.02 | 0.590 | 6 | 8.21 ± 18.2 | 5.2 ± 7.33 | 0.185 |
| (+)-Catequin | 2 | 0.99 ± 3.57 | 1.02 ± 2.77 | 1.000 | 2 | 0 | 2.44 ± 6.48 | 0.371 |
| 4-Hydroxyphenylacetic acid | 12 | 413.95 ± 1397.45 | 80.95 ± 192.01 | 0.898 | 11 | 18.64 ± 15.58 | 22.41 ± 20.71 | 0.950 |
| 3-(3,4-Dihydroxyphenyl)propionic acid | 8 | 308.06 ± 1092.53 | 510.18 ± 1755.56 | 0.308 | 10 | 17.26 ± 41.01 | 118.07 ± 436.78 | 0.572 |
| 3-Hydroxybenzoic acid | 6 | 1.57 ± 3.51 | 3.78 ± 3.13 | 0.024* | 6 | 4.81 ± 6.79 | 5.62 ± 11.01 | 0.906 |
| Caffeic acid | 8 | 8.72 ± 21.84 | 7.24 ± 16.11 | 0.541 | 8 | 6.33 ± 6.24 | 31.72 ± 110.55 | 0.415 |
| 3-Hydroxyphenylacetic acid | 10 | 54.93 ± 104.11 | 65.25 ± 61.46 | 0.359 | 10 | 44.4 ± 52.12 | 55.72 ± 73.85 | 0.556 |
| Dihydroxyphenyl-4-hydroxyvaleric acid | 2 | 0.25 ± 0.92 | 4.05 ± 10.06 | 0.371 | 3 | 15.1 ± 38.61 | 4.52 ± 12.34 | 0.295 |
| Dihydroxyphenylvalerolactone | 0 | 0 | 0 | 2 | 3.2 ± 11.99 | 31.14 ± 120.28 | 0.789 | |
| 4-Hydroxy-3-methoxyphenylacetic acid | 6 | 6.9 ± 10.28 | 11.92 ± 14.71 | 0.529 | 8 | 13.38 ± 18.69 | 16.61 ± 19.46 | 0.126 |
| p-Cumaric acid | 8 | 1.41 ± 1.96 | 1.68 ± 2.46 | 0.107 | 12 | 1.74 ± 2.3 | 5.04 ± 11.53 | 0.222 |
| 3-(3-Hydroxyphenylpropionic) acid | 12 | 252.42 ± 810.9 | 313.18 ± 721.86 | 0.365 | 14 | 413.38 ± 1126.2 | 148.73 ± 261.61 | 0.597 |
| Phenylacetic acid | 10 | 434.95 ± 480.71 | 310.55 ± 408.48 | 0.147 | 11 | 352.2 ± 338.83 | 413.58 ± 209.47 | 0.625 |
| Phenylpropionic acid | 3 | 205.13 ± 322.61 | 108.75 ± 254.17 | 0.789 | 3 | 193.58 ± 385.27 | 141.2 ± 293.43 | 0.181 |
| Phenyl-4-hydroxyvaleric acid | 10 | 1505.51 ± 2324.81 | 1295.19 ± 1577.43 | 0.838 | 13 | 1413.22 ± 1598.94 | 1236.31 ± 1378.65 | 0.940 |
| Total phenolic compounds | 12 | 1759.38 ± 3052.12 | 1507.82 ± 2657.43 | 0.912 | 16 | 1259.23 ± 1337.24 | 1264.93 ± 1315.49 | 0.747 |
3.4. Bacterial co-occurrence networks and relationship with clinical parameters
To dive deeper into the relationships between the microbiome and the clinical data we constructed a network by representing microbial communities composed of strains (ASVs) that tend to co-occur and develop in a similar metabolic context. Moreover, essential ASVs of each community (keystone members) were identified. Specifically, four well-differentiated communities significantly correlated with different clinical parameters (Fig. 5). The complete taxonomic composition of each functional community is described in ESI Table 1.† Only two taxa, Bacteroides and Ruminococcaceae, were present in the four communities, with the last one also being the clear dominator in all of them (Table S1†). A first functional community was formed by ASVs whose relative abundance correlated positively with cholesterol and glucose [module (+)cholesterol (+)glucose] and characterised by several strains acting as the keystone members, highlighting those belonging to Prevotellaceae and Erysipelotrichaceae (Table S1†). A second functional community showed positive correlation with BP and negative correlation with butyric acid and total phenolic content [module (+)BP (−)butyric acid (−)phenolics], with few keystone members belonging mainly to Bacteroides and Christensenellaceae (Table S1†). Another community correlated negatively with weight, creatinine and leukocytes [module (−)weight (−)creatinine (−)leukocytes]; again, the presence of several keystone members belonging to Bacteroides, Marinifilaceae and Acidaminococcaceae was shown (Table S1†). Finally, a fourth community was negatively correlated with the total phenolic content [module −phenolics], being more frequent as the keystone species belonging to Muribaculaceae (Table S1†). Therefore, relationships between the gut microbiota composition and clinical parameters seemed to be more based on functional communities composed of bacteria belonging to different genus and even families, rather than on bacterial taxa (i.e., genera, species).4. Discussion
Nowadays, there is greater awareness of dietary strategies for the prevention and treatment of specific MetS risk factors and their comorbidities.32 In this context, this paper reports, for the first time, scientific evidence of hypertension- and glycaemia-lowering effects associated with the regular consumption of a GP-derived seasoning. Although the reduction rate observed after the nutritional intervention (2 g of seasoning per day; 6 weeks) was relatively moderate, it can be inferred that the dietary intervention with this kind of seasoning as a salt replacement could help in the control of cardiometabolic risk factors and MetS, especially at the initial stages.The hypertension- and glycaemia-lowering effects found in this study for GP-derived seasonings were in line with the prior studies with GP extracts33 reporting reductions by 25% and 10% in MBP and glucose, respectively, for HCR subjects after consumption of red wine polyphenols (around 700 mg GAE per day), but no changes for healthy volunteers. Even so, we also perceived lowering effects in healthy subjects, which may be related with the polyphenol dose of our intervention (96 mg GAE per day). It seems that lower doses of polyphenols from grape (around 100 mg GAE per day) may work better in the management of glycaemia and BP in healthy subjects,18 than high doses (around 700 mg GAE per day).33–35 Individuals with metabolic imbalance usually present a higher degree of oxidative status, so they respond better to high doses, with reductions in the glucose level of 3–25% and in the BP of 2–14.5% for doses from 8.4 mg GAE per day to 800 mg GAE per day.19,22,23,33,36,37 No changes in blood pressure nor glucose were found in T2DM patients after prolonged supplementation with grape extract (151 mg GAE per day, one year).38 Besides polyphenols, the dietary fibre contained in the GP-derived seasoning may also contribute to the antihypertensive and hypoglycaemic effects observed after intervention. It has been reported that the digestive fibre fraction presented in GP forms viscous solutions that may cause difficulty in the absorption of glucose and lipids39 and facilitate the assimilation of potassium,40 which acts indirectly on the maintenance of the blood pressure.41 Notwithstanding the above, it should be noted that one of the main limitations of our study was the small sample size of both cohorts of participants (healthy subjects and HCR subjects).
As expected from its richness in polyphenols and dietary fibre, the consumption of the GP-derived seasoning should promote the production of microbial metabolites such as SCFA and phenolic metabolites. In the faecal samples, SCFA (acetic, propionic and butyric acids) were found in physiological concentrations42 with a mean proportion of 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, in concordance with earlier studies.18 Other trials with GP extracts reported increases in acetic and propionic acids after dietary supplementation,18 whereas we only observed a significant (p < 0.05) decrease in propionic acid after the intervention. However, it is important to mention that faecal SCFA concentrations are greatly influenced by the type and amount of fermentable available substrates ingested in the diet.43 Regarding phenolic metabolites, the faecal profile and concentrations found in this study were similar to those reported in earlier studies.18,30 Significant increases after intervention with the GP-derived seasoning were only observed for one phenolic metabolite (protocatechuic acid). Again, we believe that diet may have blunted the expected effect of supplementation. In addition, the relatively low number of volunteers and the variability in the gut microbiota functionality among them may also influence the results, since differences in gut microbiota metabolism have been suggested as one of the factors responsible for the great interindividual variability observed in polyphenol absorption, distribution, metabolism and excretion (ADME) in humans.44,45
1, in concordance with earlier studies.18 Other trials with GP extracts reported increases in acetic and propionic acids after dietary supplementation,18 whereas we only observed a significant (p < 0.05) decrease in propionic acid after the intervention. However, it is important to mention that faecal SCFA concentrations are greatly influenced by the type and amount of fermentable available substrates ingested in the diet.43 Regarding phenolic metabolites, the faecal profile and concentrations found in this study were similar to those reported in earlier studies.18,30 Significant increases after intervention with the GP-derived seasoning were only observed for one phenolic metabolite (protocatechuic acid). Again, we believe that diet may have blunted the expected effect of supplementation. In addition, the relatively low number of volunteers and the variability in the gut microbiota functionality among them may also influence the results, since differences in gut microbiota metabolism have been suggested as one of the factors responsible for the great interindividual variability observed in polyphenol absorption, distribution, metabolism and excretion (ADME) in humans.44,45
From the initial characterization of the faecal microbiota, we found that the HCR group showed significantly (p < 0.05) higher relative abundance for Escherichia/Shigella, Tyzerella 4, Gordonibacter and Fournierella, whereas faecal microbiota in healthy subjects was relatively richer in Subdoligranulum, Ruminococcaceae UCG-013, and Faecalitalea. Some of these microbial features were also reported in previous studies characterizing faecal microbiota from hypertensive and/or T2DM patients. For example, Enterobacteraceae and its genera Enterobacter and Escherichia/Shigella were enriched in hypertensive subjects,12,46 as occurred with Tyzerella 4 in diabetics and people at cardiovascular risk.47,48 In addition, Ruminococcaceae was enriched in normotensive subjects,12 and in a cross-sectorial study, Calderón-Pérez and collaborators49 found that some genera of this family follow the same pattern. Therefore, coinciding results were found in the genus Subdoligranulum, a member of Ruminococcaceae, and were traditionally related to the normal values of BP since it is a butyrate producer.12,50
Among the parameters that regulate bacterial abundances and interrelations in the gut microbiome, diet and clinical conditions are the main contributors to the great variance observed between the subjects.51,52 Recent reports confirm that some dietary patterns such as following the Mediterranean diet could be useful to restore the potentially beneficial members of the gut microbiota associated with MetS risk factors.53,54 In our study, overall compositional aspects of the microbial community (alpha- and beta-diversity patterns) were not significantly different after the consumption of the GP-derived seasoning (2 g day−1, 6 weeks). This may be due to the buffer effect exerted by the gut microbiota environment on its composition, even after diet modifications.55 However, some significant changes in the relative abundance of specific families and genera after intervention were found. In particular, the Streptococcaceae family, and more specifically the Streptococcus genus, registered a notably increase (143%) in its relative abundance. As said above, this genus has been associated with a healthy gut status since it was found in a greater abundance in faeces from healthy volunteers than hypertensive12 and T2DM56 patients, and it was described as a butyrate producer.12 Through this ability, Streptococcus could stimulate the G protein-coupled receptors present in the smooth muscle cells of blood vessels, modulating vasodilatation and reducing blood pressure.57 In fact, some strains of S. thermophilus have been tested as probiotics, further leading to conspicuous reduction in BP and LDL in patients with metabolic imbalance.58 Moreover, it has been reported that the viability and growth rate of S. thermophilus used as a probiotic were enhanced when the formulation included GP polyphenols59 or apple polyphenols.60 A similar pattern was observed for the genera Clostridiaceae 1 and Clostridium sensu stricto 1, whose relative abundances increased after supplementation. Both genera were previously correlated with low values of blood pressure14,61 and it was also reported that Clostridiales, the order to which they belong, was found in lower magnitude in hypertensive patients.12 There is no evidence of the effect of polyphenol/fibre supplementation on the viability of both genera, whereas Gil-Sánchez et al.18 observed an increase in close species belonging to the genus Clostridium in a similar intervention study with GP in healthy women. By contrast, the family Eggerthellaceae and the genera Peptoniphilus and Lachnospiraceae ND3007 group experienced a decrease in relative abundance. This reduction gains consistency since the three cases are well-known non-desirable taxa. Eggerthellaceae is associated with gastrointestinal pathologies and endotoxemia,62 the Lachnospiraceae ND3007 group belongs to a family related with hypercaloric diets,63 obesity64 and hypertension,49 and Peptoniphilus is a pathogen-like bacterium involved in the formation of ulcers.65
Gut bacteria obtain nutrients from the food compounds released after the digestion. Different bacterial enzymatic pathways take place in this process, and enzyme expression depends on the intestinal environment. Moreover, there are several cross-feeding associations between the strains, leading to complex metabolic communities which vary along the gastrointestinal tract. These facts highlight the necessity of studying the complex functional relationships in the gut microbiome, a research approach that is gaining popularity in recent years.9 Previous studies have successfully employed bacterial networks to explore their interrelationships and to find out the key species that largely influence the functionality of the human microbiome.66 These networks are mainly based on species co-occurrence to lead to bacterial communities. In most of the cases, microbial communities are composed of strains close to the functional level, so that few functional differences exist among the people despite the taxonomic variations of their microbiota.67 Following this approach, we found four well-defined communities associated with some clinical parameters and metabolites.
Our network was constructed from a co-occurrence matrix composed of bacteria belonging to the core microbiome, defined as those ASVs identified in more than 10% and less than 90% of the participants. Moreover, ASVs which tend to co-occur in only one individual with a probability of 99.9% were excluded from the network. The four communities identified were related with key clinical parameters, such as BP, glucose or cholesterol. All communities presented a complex and varied composition, so we focused on the keystone members of each community, which were the central ASV of the network, on whose functionality relied the viability of the entire community. This idea minimizes the importance of bacterial taxa identification in favour of bacterial functionality, that is governed by induction/repression of enzymatic activities depending on the environment.68 We found many taxa related with both healthy and harmful responses in humans in the same community or repeated in several communities, which supported the strain-dependent effect modulated by the environment. One example of this was the presence of Bacteroides in all the communities but with a minor relevance in that related with (−)phenolics, which may make sense observing the beneficial role of polyphenols in the growth of this genus in previous studies.18,33,37 Moreover, Bacteroides is widely related to a good health status in previous bibliography,53,69,70 while here it plays a key role in communities related to the rising values of BP, glucose and cholesterol, suggesting that correlations between the clinical data and the microbiome were conditioned by particular strains and the dynamics of the trophic community in which they grow. To reinforce this idea, another example of controversy could be the members of Ruminococcaceae, mainly associated with a good health status49 but dominant among our four defined communities. Subdoligranulum, a member of the family, and previously reported as increased in healthy subjects, was present in three of the four communities, even in that related with (+)BP (−)butyric acid (−)phenolics, despite being a butyrate producer genus, while it is true that it has a lower importance in that community compared to the others. Overall, these results strengthen the hypothesis for future work that relationships among the clinical cardiometabolic parameters and the gut microbiome should be based not only on specific taxonomic data (i.e., genera, species) but also on bacteria communities defining specific metabolic functionalities.
5. Conclusions
This study found that the culinary use of a grape pomace-based seasoning (2 g day−1, 6 weeks) significantly reduced BP and fasting glucose in both healthy people and HCR subjects, which may suggest a promising dietary strategy to manage risk factors at the initial stage of cardiometabolic imbalance. Faecal metabolites remained similar to the basal conditions despite the GP intervention, revealing that diet is the most important regulator of the gut metabolome and microbiome beyond the intervention. Nevertheless, small differences between healthy and HCR subjects were found in terms of microbiota and we checked that GP could also modify the abundance of some bacterial taxa. However, we are aware of the limitations of this study, as it is necessary to extend the study to a higher number of volunteers and health status and to the analysis of the complete faecal metabolome, for a robust confirmation of the results obtained here.In line with previous studies, some of our bacteria correlated with metabolites and clinical markers indicating a clear interrelation between health and the gut microbiome. However, the construction of a network based on bacteria co-occurrence and the correlations mentioned before allow us to emphasize the considerable importance of identifying environment-dependent functional communities against a simple taxa identification. Presumably, there are taxa which tend to promote a good health status like Akkermansia muciniphila or some species of Bacteroides possessing a similar genome, but above the taxonomic identification there exists a strain-dependent effect and an environmental influence that may lead to different microbiome functionalities.
Abbreviations
| ASV | Amplicon sequence variant |
| BP | Blood pressure |
| DBP | Diastolic blood pressure |
| FPG | Fasting plasma glucose |
| GAE | Gallic acid equivalents |
| MBP | Mean blood pressure |
| MetS | Metabolic syndrome |
| SBP | Systolic blood pressure |
| T2DM | Type 2 diabetes mellitus |
Competing of interest
All the authors declare no conflict of interest. The seasoning used in this study, obtained from red grape pomace, was provided by Bodega Matarromera S.L. (in the framework of the Project RTC-2016-4556-1) that is both the manufacturer and seller of this product.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are deeply indebted to the individuals who volunteered to participate in this trial. This research was funded by the Spanish Ministry of Science and Innovation (Spain), grant number RTC-2016-4556-1. Additional funding came from grants AGL2015-64522-C2-1-R and PID2019-108851RB-C21 (Spanish Ministry of Science and Innovation), and ALIBIRD-CM 2020 P2018/BAA-4343 (Community of Madrid).References
- M. H. Forouzanfar, A. Afshin, L. T. Alexander, S. Biryukov, M. Brauer, K. Cercy, F. J. Charlson, A. J. Cohen and J. Zhu, Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015, Lancet, 2016, 388, 1659–1724 CrossRef.
- J. D. Hernández-Camacho and M. Hernández-Camacho, Clinical update on metabolic syndrome, Rev. Esp. Nutr. Hum. Diet., 2017, 21, 384–392 CrossRef.
- P. Almeda-Valdes, R. J. Herrera-Mercadillo, C. A. Aguilar-Salinas, M. Uribe and N. Méndez-Sánchez, The Role of Diet in Patients with Metabolic Syndrome, Curr. Med. Chem., 2019, 26, 3613–3619 CrossRef CAS PubMed.
- J. J. Van Den Driessche, J. Plat and R. P. Mensink, Effects of superfoods on risk factors of metabolic syndrome: A systematic review of human intervention trials, Food Funct., 2018, 9, 1944–1966 RSC.
- G. Costabile, M. Vitale, D. Luongo, D. Naviglio, C. Vetrani, P. Ciciola, A. Tura, F. Castello, P. Mena, D. Del Rio, B. Capaldo, A. A. Rivellese, G. Riccardi and R. Giacco, Grape pomace polyphenols improve insulin response to a standard meal in healthy individuals: A pilot study, Clin. Nutr., 2019, 38, 2727–2734 CrossRef CAS PubMed.
- H. Quesada, J. M. Del Bas, D. Pajuelo, S. Díaz, J. Fernandez-Larrea, M. Pinent, L. Arola, M. J. Salvadó and C. Bladé, Grape seed proanthocyanidins correct dyslipidemia associated with a high-fat diet in rats and repress genes controlling lipogenesis and VLDL assembling in liver, Int. J. Obes., 2009, 33, 1007–1012 CrossRef CAS PubMed.
- J. C. Espín, A. González-Sarrías and F. A. Tomás-Barberán, The gut microbiota: A key factor in the therapeutic effects of (poly)phenols, Biochem. Pharmacol., 2017, 139, 82–93 CrossRef PubMed.
- M. V. Moreno-Arribas, B. Bartolomé, J. L. Peñalvo, P. Pérez-Matute and M. J. Motilva, Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer's Disease, Nutrients, 2020, 12, 3082 CrossRef CAS PubMed.
- H. Antushevich, Fecal microbiota transplantation in disease therapy, Clin. Chim. Acta, 2020, 503, 90–98 CrossRef CAS PubMed.
- S. M. Jandhyala, R. Talukdar, C. Subramanyam, H. Vuyyuru, M. Sasikala and D. N. Reddy, Role of the normal gut microbiota, World J. Gastroenterol., 2015, 21, 8836–8847 CrossRef PubMed.
- J. Li, F. Zhao, Y. Wang, J. Chen, J. Tao, G. Tian, S. Wu, W. Liu, Q. Cui, B. Geng, W. Zhang, R. Weldon, K. Auguste, L. Yang, X. Liu, L. Chen, X. Yang, B. Zhu and J. Cai, Gut microbiota dysbiosis contributes to the development of hypertension, Microbiome, 2017, 5(1), 1–19 CrossRef PubMed.
- Y. Chang, Y. Chen, Q. Zhou, C. Wang, L. Chen, W. Di and Y. Zhang, Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia, Clin. Sci., 2020, 134, 289–302 CrossRef CAS PubMed.
- J. Wang, J. Qin, Y. Li, Z. Cai, S. Li, J. Zhu, F. Zhang, S. Liang, W. Zhang, Y. Guan, D. Shen, Y. Peng, D. Zhang, Z. Jie, W. Wu, Y. Qin, W. Xue, J. Li, L. Han, D. Lu, P. Wu, Y. Dai, X. Sun, Z. Li, A. Tang, S. Zhong, X. Li, W. Chen, R. Xu, M. Wang, Q. Feng, M. Gong, J. Yu, Y. Zhang, M. Zhang, T. Hansen, G. Sanchez, J. Raes, G. Falony, S. Okuda, M. Almeida, E. Lechatelier, P. Renault, N. Pons, J. M. Batto, Z. Zhang, H. Chen, R. Yang, W. Zheng, S. Li, H. Yang, S. D. Ehrlich, R. Nielsen, O. Pedersen, K. Kristiansen and J. Wang, A metagenome-wide association study of gut microbiota in type 2 diabetes, Nature, 2012, 490, 55–60 CrossRef PubMed.
- L. F. Gomez-Arango, H. L. Barrett, H. D. McIntyre, L. K. Callaway, M. Morrison and M. Dekker Nitert, Increased Systolic and Diastolic Blood Pressure is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy, Hypertension, 2016, 68, 974–981 CrossRef CAS PubMed.
- S. Moossavi and F. Bishehsari, Microbes: possible link between modern lifestyle transition and the rise of metabolic syndrome, Obes. Rev., 2019, 20, 407–419 CrossRef CAS PubMed.
- A. Cortés-Martín, C. E. Iglesias-Aguirre, A. Meoro, M. V. Selma and J. C. Espín, There is No Distinctive Gut Microbiota Signature in the Metabolic Syndrome: Contribution of Cardiovascular Disease Risk Factors and Associated Medication, Microorganisms, 2020, 8, 416 CrossRef PubMed.
- P. Rondeau, F. Gambier, F. Jolibert and N. Brosse, Compositions and chemical variability of grape pomaces from French vineyard, Ind. Crops Prod., 2013, 43, 251–254 CrossRef CAS.
- I. Gil-Sánchez, A. Esteban-Fernández, D. González de Llano, M. Sanz-Buenhombre, A. Guadarrana, N. Salazar, M. Gueimonde, C. G. de los Reyes-Gavilánc, L. Martín Gómez, M. L. García Bermejo, B. Bartolomé and M. V. Moreno-Arribas, Supplementation with grape pomace in healthy women: Changes in biochemical parameters, gut microbiota and related metabolic biomarkers, J. Funct. Foods, 2018, 45, 34–46 CrossRef.
- I. Urquiaga, S. D'Acuña, D. Pérez, S. Dicenta, G. Echeverría, A. Rigotti and F. Leighton, Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial, Biol. Res., 2015, 48, 1–10 CrossRef PubMed.
- D. Taladrid, L. Laguna, B. Bartolomé and M. V. Moreno-Arribas, Plant-derived seasonings as sodium salt replacers in food, Trends Food Sci. Technol., 2020, 99, 194–202 CrossRef CAS.
- D. Taladrid, L. Laguna, V. D. Vendrell, A. Guadarrana, M. V. Moreno-Arribas and B. Bartolomé, Sensory acceptability of winery by-products as seasonings for salt replacement, Eur. Food Res. Technol., 2020, 246(11), 2359–2369 CrossRef CAS.
- G. Belcaro, A. Ledda, S. Hu, M. R. Cesarone, B. Feragalli and M. Dugall, Grape Seed Procyanidins in Pre- and Mild Hypertension: A Registry Study, Evidence-Based Complementary Altern. Med., 2013, 2013, 313142 Search PubMed.
- R. T. Ras, P. L. Zock, Y. E. M. P. Zebregs, N. R. Johnston, D. J. Webb and R. Draijer, Effect of polyphenol-rich grape seed extract on ambulatory blood pressure in subjects with pre- and stage I hypertension, Br. J. Nutr., 2013, 110, 2234–2241 CrossRef CAS PubMed.
- I. Urquiaga, S. D'Acuña, D. Pérez, S. Dicenta, G. Echeverría, A. Rigotti and F. Leighton, Wine grape pomace flour improves blood pressure, fasting glucose and protein damage in humans: A randomized controlled trial, Biol. Res., 2015, 48, 49 CrossRef PubMed.
- D. S. Lundberg, S. Yourstone, P. Mieczkowski, C. D. Jones and J. L. Dangl, Practical innovations for high-throughput amplicon sequencing, Nat. Methods, 2013, 10, 999–1002 CrossRef CAS PubMed.
- B. J. Callahan, P. J. McMurdie, M. J. Rosen, A. W. Han, A. J. A. Johnson and S. P. Holmes, DADA2: High-resolution sample inference from Illumina amplicon data, Nat. Methods, 2016, 13, 581–583 CrossRef CAS PubMed.
- C. Quast, E. Pruesse, P. Yilmaz, J. Gerken, T. Schweer, P. Yarza, J. Peplies and F. O. Glöckner, The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools, Nucleic Acids Res., 2013, 41, D590 CrossRef CAS PubMed.
- C. Cueva, A. Jiménez-Girón, I. Muñoz-González, A. Esteban-Fernández, I. Gil-Sánchez, M. Dueñas, P. J. Martín-Álvarez, M. A. Pozo-Bayón, B. Bartolomé and M. V. Moreno-Arribas, Application of a new Dynamic Gastrointestinal Simulator (SIMGI) to study the impact of red wine in colonic metabolism, Food Res. Int., 2015, 72, 149–159 CrossRef CAS.
- A. Jiménez-Girón, M. I. Queipo-Ortuño, M. Boto-Ordóñez, I. Muñoz-González, F. Sánchez-Patán, M. Monagas, P. J. Martín-Álvarez, M. Murri, F. J. Tinahones, C. Andrés-Lacueva, B. Bartolomé and M. V. Moreno-Arribas, Comparative study of microbial-derived phenolic metabolites in human feces after intake of gin, red wine, and dealcoholized red wine, J. Agric. Food Chem., 2013, 61, 3909–3915 CrossRef PubMed.
- I. Muñoz-González, A. Jiménez-Girón, P. J. Martín-Álvarez, B. Bartolomé and M. V. Moreno-Arribas, Profiling of microbial-derived phenolic metabolites in human feces after moderate red wine intake, J. Agric. Food Chem., 2013, 61, 9470–9479 CrossRef PubMed.
- S. Banerjee, K. Schlaeppi and M. G. A. van der Heijden, Keystone taxa as drivers of microbiome structure and functioning, Nat. Rev. Microbiol., 2018, 16, 567–576 CrossRef CAS PubMed.
- S. Castro-Barquero, A. M. Ruiz-León, M. Sierra-Pérez, R. Estruch and R. Casas, Dietary Strategies for Metabolic Syndrome: A Comprehensive Review, Nutrients, 2020, 12, 2983 CrossRef CAS PubMed.
- I. Moreno-Indias, L. Sánchez-Alcoholado, P. Pérez-Martínez, C. Andrés-Lacueva, F. Cardona, F. Tinahones and M. I. Queipo-Ortuño, Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients, Food Funct., 2016, 7, 1775–1787 RSC.
- M. I. Queipo-Ortuño, M. Boto-Ordóñez, M. Murri, J. M. Gomez-Zumaquero, M. Clemente-Postigo, R. Estruch, F. Cardona Diaz, C. Andrés-Lacueva and F. J. Tinahones, Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers, Am. J. Clin. Nutr., 2012, 95, 1323–1334 CrossRef PubMed.
- D. Martínez-Maqueda, B. Zapatera, A. Gallego-Narbón, M. P. Vaquero, F. Saura-Calixto and J. Pérez-Jiménez, A 6-week supplementation with grape pomace to subjects at cardiometabolic risk ameliorates insulin sensitivity, without affecting other metabolic syndrome markers, Food Funct., 2018, 9, 6010–6019 RSC.
- I. Urquiaga, D. Troncoso, M. J. Mackenna, C. Urzúa, D. Pérez, S. Dicenta, P. M. de la Cerda, L. Amigo, J. C. Carreño, G. Echeverría and A. Rigotti, The consumption of beef burgers prepared with wine grape pomace flour improves fasting glucose, plasma antioxidant levels, and oxidative damage markers in humans: A controlled trial, Nutrients, 2018, 10(10), 1388 CrossRef PubMed.
- M. I. Queipo-Ortuño, M. Boto-Ordóñez, M. Murri, J. M. Gomez-Zumaquero, M. Clemente-Postigo, R. Estruch, F. Cardona Diaz, C. Andrés-Lacueva and F. J. Tinahones, Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers, Am. J. Clin. Nutr., 2012, 95, 1323–1334 CrossRef PubMed.
- J. Tomé-Carneiro, M. Larrosa, M. J. Yáñez-Gascón, A. Dávalos, J. Gil-Zamorano, M. Gonzálvez, F. J. García-Almagro, J. A. Ruiz Ros, F. A. Tomás-Barberán, J. C. Espín and M. T. García-Conesa, One-year supplementation with a grape extract containing resveratrol modulates inflammatory-related microRNAs and cytokines expression in peripheral blood mononuclear cells of type 2 diabetes and hypertensive patients with coronary artery disease, Pharmacol. Res., 2013, 72, 69–82 CrossRef PubMed.
- L. B. Bindels, R. R. Segura Munoz, J. C. Gomes-Neto, V. Mutemberezi, I. Martínez, N. Salazar, E. A. Cody, M. I. Quintero-Villegas, H. Kittana, C. G. de los Reyes-Gavilán, R. J. Schmaltz, G. G. Muccioli, J. Walter and A. E. Ramer-Tait, Resistant starch can improve insulin sensitivity independently of the gut microbiota, Microbiome, 2017, 5, 12 CrossRef PubMed.
- M. Streppel, M. Ocke, H. Boshuizen, F. Kok and D. Kromhout, Dietary fiber intake in relation to coronary heart disease and all-cause mortality over 40 y: the Zutphen Study, Am. J. Clin. Nutr., 2008, 88, 1119–1125 CrossRef CAS PubMed.
- A. Binia, J. Jaeger, Y. Hu, A. Singh and D. Zimmermann, Daily potassium intake and sodium-to-potassium ratio in the reduction of blood pressure: A meta-analysis of randomized controlled trials, J. Hypertens., 2015, 33, 1509–1520 CrossRef CAS PubMed.
- D. Rios-Covian, N. Salazar, M. Gueimonde and C. G. de los Reyes-Gavilan, Shaping the metabolism of intestinal Bacteroides population through diet to improve human health, Front. Microbiol., 2017, 8, 376 Search PubMed.
- K. A. Verbeke, A. R. Boobis, A. Chiodini, C. A. Edwards, A. Franck, M. Kleerebezem, A. Nauta, J. Raes, E. A. F. Van Tol and K. M. Tuohy, Towards microbial fermentation metabolites as markers for health benefits of prebiotics, Nutr. Res. Rev., 2015, 28, 42–66 CrossRef CAS PubMed.
- C. Morand and F. A. Tomás-Barberán, Interindividual Variability in Absorption, Distribution, Metabolism, and Excretion of Food Phytochemicals Should Be Reported, J. Agric. Food Chem., 2019, 67, 3843–3844 CrossRef CAS PubMed.
- C. Cueva, I. Gil-Sánchez, B. Ayuda-Durán, S. González-Manzano, A. M. González-Paramás, C. Santos-Buelga, B. Bartolomé and M. Victoria Moreno-Arribas, An integrated view of the effects of wine polyphenols and their relevant metabolites on gut and host health, Molecules, 2017, 22(1), 99 CrossRef PubMed.
- Q. Ma, Y. Li, J. Wang, P. Li, Y. Duan, H. Dai, Y. An, L. Cheng, T. Wang, C. Wang, T. Wang and B. Zhao, Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing, Biomed. Pharmacother., 2020, 124, 109873 CrossRef CAS PubMed.
- S. Ma, Y. You, L. Huang, S. Long, J. Zhang, C. Guo, N. Zhang, X. Wu, Y. Xiao and H. Tan, Alterations in Gut Microbiota of Gestational Diabetes Patients During the First Trimester of Pregnancy, Front. Cell. Infect. Microbiol., 2020, 10, 58 CrossRef CAS PubMed.
- T. N. Kelly, L. A. Bazzano, N. J. Ajami, H. He, J. Zhao, J. F. Petrosino, A. Correa and J. He, Gut Microbiome Associates with Lifetime Cardiovascular Disease Risk Profile among Bogalusa Heart Study Participants, Circ. Res., 2016, 119, 956–964 CrossRef CAS PubMed.
- L. Calderón-Pérez, M. J. Gosalbes, S. Yuste, R. M. Valls, A. Pedret, E. Llauradó, N. Jimenez-Hernandez, A. Artacho, L. Pla-Pagà, J. Companys, I. Ludwig, M. P. Romero, L. Rubió and R. Solà, Gut metagenomic and short chain fatty acids signature in hypertension: a cross-sectional study, Sci. Rep., 2020, 10, 1–16 CrossRef PubMed.
- K. Zuo, J. Li, Q. Xu, C. Hu, Y. Gao, M. Chen, R. Hu, Y. Liu, H. Chi, Q. Yin, Y. Cao, P. Wang, Y. Qin, X. Liu, J. Zhong, J. Cai, K. Li and X. Yang, Dysbiotic gut microbes may contribute to hypertension by limiting vitamin D production, Clin. Cardiol., 2019, 42, 710–719 CrossRef PubMed.
- G. Falony, M. Joossens, S. Vieira-Silva, J. Wang, Y. Darzi, K. Faust, A. Kurilshikov, M. J. Bonder, M. Valles-Colomer, D. Vandeputte, R. Y. Tito, S. Chaffron, L. Rymenans, C. Verspecht, L. De Sutter, G. Lima-Mendez, K. D′hoe, K. Jonckheere, D. Homola, R. Garcia, E. F. Tigchelaar, L. Eeckhaudt, J. Fu, L. Henckaerts, A. Zhernakova, C. Wijmenga and J. Raes, Population-level analysis of gut microbiome variation, Science, 2016, 352, 560–564 CrossRef CAS PubMed.
- A. Zhernakova, A. Kurilshikov, M. J. Bonder, E. F. Tigchelaar, M. Schirmer, T. Vatanen, Z. Mujagic, A. V. Vila, G. Falony, S. Vieira-Silva, J. Wang, F. Imhann, E. Brandsma, S. A. Jankipersadsing, M. Joossens, M. C. Cenit, P. Deelen, M. A. Swertz, R. K. Weersma, E. J. M. Feskens, M. G. Netea, D. Gevers, D. Jonkers, L. Franke, Y. S. Aulchenko, C. Huttenhower, J. Raes, M. H. Hofker, R. J. Xavier, C. Wijmenga and J. Fu, Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity, Science, 2016, 352, 565–569 CrossRef CAS PubMed.
- C. Haro, S. Garcia-Carpintero, J. F. Alcala-Diaz, F. Gomez-Delgado, J. Delgado-Lista, P. Perez-Martinez, O. A. Rangel Zuñiga, G. M. Quintana-Navarro, B. B. Landa, J. C. Clemente, J. Lopez-Miranda, A. Camargo and F. Perez-Jimenez, The gut microbial community in metabolic syndrome patients is modified by diet, J. Nutr. Biochem., 2016, 27, 27–31 CrossRef CAS PubMed.
- A. Camargo, C. Vals-Delgado, J. F. Alcala-Diaz, A. Villasanta-Gonzalez, F. Gomez-Delgado, C. Haro, A. Leon-Acuña, M. P. Cardelo, J. D. Torres-Peña, I. Guler, M. M. Malagon, J. M. Ordovas, P. Perez-Martinez, J. Delgado-Lista and J. Lopez-Miranda, A Diet-Dependent Microbiota Profile Associated with Incident Type 2 Diabetes: From the CORDIOPREV Study, Mol. Nutr. Food Res., 2020, 64, 2000730 CrossRef CAS PubMed.
- G. D. Wu, J. Chen, C. Hoffmann, K. Bittinger, Y. Y. Chen, S. A. Keilbaugh, M. Bewtra, D. Knights, W. A. Walters, R. Knight, R. Sinha, E. Gilroy, K. Gupta, R. Baldassano, L. Nessel, H. Li, F. D. Bushman and J. D. Lewis, Linking long-term dietary patterns with gut microbial enterotypes, Science, 2011, 334, 105–108 CrossRef CAS PubMed.
- P. F. de Groot, C. Belzer, Ö. Aydin, E. Levin, J. H. Levels, S. Aalvink, F. Boot, F. Holleman, D. H. van Raalte, T. P. Scheithauer, S. Simsek, F. G. Schaap, S. W. M. Olde Damink, B. O. Roep, J. B. Hoekstra, W. M. de Vos and M. Nieuwdorp, Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study, PLoS One, 2017, 12, e0188475 CrossRef PubMed.
- J. Miyamoto, M. Kasubuchi, A. Nakajima, J. Irie, H. Itoh and I. Kimura, The role of short-chain fatty acid on blood pressure regulation, Curr. Opin. Nephrol. Hypertens., 2016, 25, 379–383 CrossRef CAS PubMed.
- M. Ito, S. Kusuhara, W. Yokoi, T. Sato, H. Ishiki, S. Miida, A. Matsui, K. Nakamori, C. Nonaka and K. Miyazaki, Streptococcus thermophilus fermented milk reduces serum MDA-LDL and blood pressure in healthy and mildly hypercholesterolaemic adults, Benefic. Microbes, 2017, 8, 171–178 CrossRef CAS PubMed.
- P. O. de Souza de Azevedo, B. Aliakbarian, A. A. Casazza, J. G. LeBlanc, P. Perego and R. P. de Souza Oliveira, Production of fermented skim milk supplemented with different grape pomace extracts: Effect on viability and acidification performance of probiotic cultures, PharmaNutrition, 2018, 6, 64–68 CrossRef.
- D. Sun-Waterhouse, J. Zhou and S. S. Wadhwa, Effects of Adding Apple Polyphenols Before and After Fermentation on the Properties of Drinking Yoghurt, Food Bioprocess Technol., 2012, 5, 2674–2686 CrossRef CAS.
- H. Li, B. Liu, J. Song, Z. An, X. Zeng, J. Li, J. Jiang, L. Xie and W. Wu, Characteristics of Gut Microbiota in Patients with Hypertension and/or Hyperlipidemia: A Cross-Sectional Study on Rural Residents in Xinxiang County, Henan Province, Microorganisms, 2019, 7, 399 CrossRef CAS PubMed.
- S. K. P. Lau, P. C. Y. Woo, A. M. Y. Fung, K. M. Chan, G. K. S. Woo and K. Y. Yuen, Anaerobic, non-sporulating, Gram-positive bacilli bacteraemia characterized by 16S rRNA gene sequencing, J. Med. Microbiol., 2004, 53, 1247–1253 CrossRef CAS PubMed.
- K. Lippert, L. Kedenko, L. Antonielli, I. Kedenko, C. Gemeier, M. Leitner, A. Kautzky-Willer, B. Paulweber and E. Hackl, Gut microbiota dysbiosis associated with glucose metabolism disorders and the metabolic syndrome in older adults, Benefic. Microbes, 2017, 8, 545–556 CrossRef CAS PubMed.
- D. Hou, Q. Zhao, L. Yousaf, J. Khan, Y. Xue and Q. Shen, Consumption of mung bean (Vigna radiata L.) attenuates obesity, ameliorates lipid metabolic disorders and modifies the gut microbiota composition in mice fed a high-fat diet, J. Funct. Foods, 2020, 64, 103687 CrossRef.
- S. E. Dowd, Y. Sun, P. R. Secor, D. D. Rhoads, B. M. Wolcott, G. A. James and R. D. Wolcott, Survey of bacterial diversity in chronic wounds using Pyrosequencing, DGGE, and full ribosome shotgun sequencing, BMC Microbiol., 2008, 8, 1–15 CrossRef PubMed.
- M. Layeghifard, H. Li, P. W. Wang, S. L. Donaldson, B. Coburn, S. T. Clark, J. D. Caballero, Y. Zhang, D. E. Tullis, Y. C. W. Yau, V. Waters, D. M. Hwang and D. S. Guttman, Microbiome networks and change-point analysis reveal key community changes associated with cystic fibrosis pulmonary exacerbations, npj Biofilms Microbiomes, 2019, 5, 1–12 CrossRef PubMed.
- M. Loftus, S. A. D. Hassouneh and S. Yooseph, Bacterial associations in the healthy human gut microbiome across populations, Sci. Rep., 2021, 11, 2828 CrossRef CAS PubMed.
- Z. Liu, A. Ma, E. Mathé, M. Merling, Q. Ma and B. Liu, Network analyses in microbiome based on high-throughput multi-omics data, Briefings Bioinf., 2020, 2020, 1–17 Search PubMed.
- L. Crovesy, D. Masterson and E. L. Rosado, Profile of the gut microbiota of adults with obesity: a systematic review, Eur. J. Clin. Nutr., 2020, 74, 1251–1262 CrossRef PubMed.
- X. Zhang, D. Shen, Z. Fang, Z. Jie, X. Qiu, C. Zhang, Y. Chen and L. Ji, Human Gut Microbiota Changes Reveal the Progression of Glucose Intolerance, PLoS One, 2013, 8, e71108 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo03942c |
| This journal is © The Royal Society of Chemistry 2022 |