Open Access Article
Open Access ArticleTemporal changes in salivary composition induced by oral exposure to different wine matrices and the relationship with the behaviour of aroma compounds in the mouth
Celia
Criado
,
Carolina
Muñoz-González
,
Blanca
Hernández-Ledesma
and
María Ángeles
Pozo-Bayón
 *
*
Instituto de Investigación en Ciencias de la Alimentación (CIAL), CSIC-UAM, C/Nicolás Cabrera, 9, 28049, Madrid, Spain. E-mail: m.delpozo@csic.es
First published on 14th March 2022
Abstract
The dynamic changes in saliva flow and composition (pH, total protein capacity (TPC), total polyphenol index (TPI) and saliva antioxidant activity (SAOX)) after the exposure of the oral cavity to aromatized wine matrices with different chemical compositions (dealcoholized, alcoholized, and synthetic wines) have been investigated. For this, stimulated saliva from ten volunteers were collected five days per week (from Monday to Friday) during three non-consecutive weeks, before (basal saliva) and after the oral intervention with the wines (5 and 15 minutes later) (n = 450). In order to know the relationship between the changes induced in salivary composition and the amount of aroma retained in the oral cavity, the expectorated wines were also collected (n = 150). Results showed differences in saliva composition (pH, TPI and SAOX) depending on the wine matrix that were only significant in the first five minutes after the oral exposure to the wines. The wines with ethanol produced significantly lower in-mouth aroma retention, while salivary TPI and, to a minor extent, SAOX, were positively related to the aroma retained. These results prove that not only wine aroma composition, but also the physiological changes in saliva induced by the non-volatile chemical composition of the wine play an important role in wine odorant compounds, and likely, in aroma perception.
1. Introduction
Saliva is a complex dilute aqueous solution. It contains numerous inorganic salts (sodium, calcium, potassium, chloride, phosphate, and bicarbonate),1 organic components such as enzymes (amylase, lipases, proteases, etc.),2–4 and proteins (mucins, proline rich proteins, histidine rich proteins, etc.).5 All of these components can be responsible for the different physiological effects that saliva exerts in the body such as oral lubrication, digestion, immunity, and the overall maintenance of homeostasis.6Additionally, in recent years, there has been an increase in evidence of the relationship between saliva and oral sensory perception.7 Among the more extensively investigated direct roles of saliva in some oral textural, trigeminal and gustative sensations, saliva could also affect the retronasal perception of odorant molecules in the mouth during food and beverage intake. Although this is an incipient field of research, saliva has been related to a myriad of physicochemical and biological effects on odorant molecules, such as dilution, retention by salivary proteins, the salting-out effect or enzymatic conversion, among others.8 In the case of wine, different studies have revealed a correlation between salivary parameters and oral aroma release or perception.9 Using temporal sensory methodologies, Criado and co-workers9,10 have shown a correlation between some salivary parameters (such as salivary flow and total protein content, TPC) and the dynamics of wine aroma perception upon wine tasting.9,10 These results can be explained, at least in part, by the effect of saliva on the dynamics of aroma release in the oral cavity.
Intrinsic factors, such as gender, age, ethnicity, etc., have been associated with interindividual variations in saliva composition11 that might differently affect oral aroma release.7,12 However, other exogenous factors, such as diet, can also affect saliva composition. For instance, DiSilvestro and co-workers13 showed that the oral exposure to pomegranate flavonoids for four weeks reduced salivary TPC and the enzymatic activity of aspartate, aminotransferase and α-glucosidase, and increased the radical scavenging capacity and the activity of the antioxidant enzyme ceruloplasmin. Additionally, green tea polyphenol epigallocatechin gallate (EGCG) can alter the properties of specific mucin proteins with lubricant properties likely involved in astringency perception.14 More recently, the interventions of a regular diet plus chocolate milk (with high polyphenol content) compared to a low polyphenol diet also induced changes in the salivary proteome.15
Many of these studies have shown an impact on saliva composition after the chronic exposure to food or specific food ingredients. In addition, changes in salivary composition, such as in the salivary proteome, could be also produced immediately after the stimulation with tastant/chemesthetic compounds.16 In this case, an immediate effect on salivary composition that in turn might affect the sensory perception of the food or beverage upon ingestion could be expected.
In the case of wine, which is highly appreciated for its aroma, studies involving the role of different wine components on saliva composition and properties are scarce. Despite this, pioneer works have already shown an effect of some wine components such as ethanol, tartaric acid or tannin on salivary flow.17,18
An increase in saliva flow with an increase in the concentration of tannic acid and their effect on gustatory (mostly sour) and trigeminal (irritation and tactile) stimulation have also been proven.19 Nonetheless, whether wine or wine components might affect salivary parameters related to the release of aroma compounds in the mouth affecting retronasal aroma is, to date, unknown.
Recent works have also suggested that some wine components (such as polyphenols) might inhibit salivary esterase enzymes, which are involved in the hydrolysis of wine carboxylic esters (e.g. ethyl hexanoate).20–23 Moreover, it has also been suggested that phenolic compounds might affect the saliva antioxidant capacity (SAOX),24 which could also be involved in the metabolism of certain salivary enzymes (e.g. NAD(P)H dependent enzymes) which catalyse the reduction of some types of aroma compounds (e.g. aldehydes to alcohols).25 At any rate, understanding the temporal changes in saliva composition during and after wine intake seems of utmost importance in order to better explain the effect of saliva on aroma release and perception during wine tasting, and likely to better understand consumer preferences of different types of wines.
In view of this, the objective of this study was to examine the dynamic changes in saliva flow, composition (pH, TPC, total polyphenol and TPI), and properties (SAOX) after the exposure of the oral cavity to aromatised wine matrices with different chemical compositions (dealcoholised, alcoholised, and synthetic wines). Saliva samples were collected from 10 volunteers five days per week (from Monday to Friday) during three non-consecutive weeks. In each week, a different type of wine was tested and saliva was collected before the oral exposure to the wine (basal saliva), and five and fifteen minutes after the oral exposure to the wine. Additionally, in order to determine the relationship between the changes in salivary composition induced by the different types of wine matrices and the amount of aroma retained in the oral cavity, the expectorated wines were also collected, and the aroma compounds were analysed by GC-MS.
2. Materials and methods
2.1. Wine matrices
A dealcoholised commercial rosé wine (“Matarromera” winery, 2017; Valbuena de Duero, Spain) made from Tempranillo and Verdejo grape varieties was used for this study (DW). The chemical composition was determined as previously described26 and it was: pH (3.00 ± 0.02), TPI (247.06 ± 11.62 mg gallic acid per L), procyanidins (97.28 ± 1.87 mg catechin per L), neutral polysaccharides (1204.90 ± 62.10 mg mannose per L), and free amino acids (654.04 ± 74.50 mg Leu per L).From the dealcoholised wine (DW), a second wine with ethanol (12%, v/v) was prepared (AW) by using food grade ethanol (Alcoholes Montplet S.A., Barcelona, Spain). In addition, a synthetic wine (SW) consisting of a 12% (v/v) hydroalcoholic solution, using food grade ethanol and adjusting the pH with tartaric acid (3.5) (Manuel Riesgo, Madrid, Spain) was also prepared.
2.2. Wine aromatisation
The three wine types (DW, AW and SW) were aromatised with a mixture of ten food grade aroma compounds (Sigma-Aldrich, Steinheim, Germany): isoamyl acetate, ethyl hexanoate, ethyl butanoate, ethyl pentanoate, furfural, E-2-hexenal, E-2-octenal, hexanal, octanal, and γ-nonalactone. They were all selected for being representative of the wine volatile profile and for having different physicochemical characteristics (Table 1).| Compounds | CAS number | MW (g mol−1) | BP (°C) | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P P |
Descriptor |
|---|---|---|---|---|---|
Descriptor for aroma compounds: https://www.flavornet.org; BP: boiling point; log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P: log of the octanol/water partition coefficient estimated from the molecular modelling software EPI Suit (U.S. EPA 2000–2007); MW: molecular weight. P: log of the octanol/water partition coefficient estimated from the molecular modelling software EPI Suit (U.S. EPA 2000–2007); MW: molecular weight. |
|||||
| Isoamyl acetate | 123-92-2 | 130.0 | 134.0 | 2.3 | Banana |
| Ethyl hexanoate | 123-66-0 | 144.0 | 167.0 | 2.8 | Apple, peel, fruit |
| Ethyl butanoate | 105-54-4 | 116.0 | 121.5 | 1.9 | Pineapple |
| Ethyl pentanoate | 539-82-2 | 130.0 | 145.0 | 1.7 | Fruity |
| Furfural | 98-01-1 | 96.0 | 162.0 | 0.8 | Bread, almond, sweet |
| E-2-Hexenal | 6728-26-3 | 98.0 | 47.0 | 1.6 | Green, leaf |
| E-2-Octenal | 2548-87-0 | 126.0 | 86.0 | 2.8 | Green, walnut |
| Hexanal | 66-25-1 | 100.0 | 130.0 | 1.8 | Grass, tallow, fat |
| Octanal | 124-13-0 | 128.0 | 171.0 | 3.5 | Fat, lemon, green |
| γ-Nonalactone | 104-61-0 | 156.0 | 121.0 | 2.1 | Coconut, peach |
For wine aromatisation, a stock solution of aroma compounds (2000 mg L−1) in food grade ethanol was firstly prepared, and from this, a second one (200 mg L−1) was then prepared. This solution was used to aromatise the wines just before starting the assay. To do so, 150 μL of the second stock solution was added to 15 mL of the wine already poured in the wine glass. The final concentration of each compound in the wine was 2 mg L−1.
2.3. Procedure for sample collection
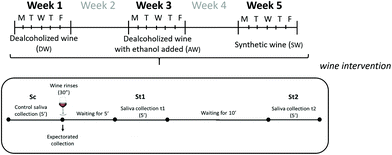
Ten healthy volunteers (8 females and 2 males) between 22 and 28 years old participated in the sessions. The sessions involved the collection of saliva before and after oral rinsing with the wine samples, and the expectorated wines. All trials were conducted every day in the mornings (from Monday to Friday), during three non-consecutive weeks, leaving a week between each type of wine tested.The experiment lasted for five weeks, and in each non-consecutive week, one of the wine matrices (DW, AW, and SW) was evaluated. Fig. 1 shows the procedure followed for saliva and expectorated wine collection.
Volunteers were informed of the nature of this study and gave their written consent to participate. This work was also approved by the Bioethical Committee from the Spanish National Research Council (CSIC).
A stimulated saliva sample was collected from the volunteers before testing the wines (basal or control saliva: Sc). Saliva collection was performed at the same time of the day (11 a.m.), in an attempt to reduce the compositional variability associated with circadian rhythms.27–29 Saliva was directly collected in a sterile tube (previously weighed). The subjects were told to avoid swallowing during the saliva collection process. For this, subjects chewed a piece of Parafilm™ and spat their saliva into the tube as many times as they wanted for 5 min. Saliva flow was calculated from the weight of saliva, and was expressed as mg mL−1, assuming 1 g being equal to 1 mL, as is commonly done in studies involving salivary flow measurements. After saliva collection, individuals were asked to perform a gentle mouth rinsing for 30 seconds with 15 mL of each of the wine matrices studied. The volunteers then expectorated the wine into a pre-weighed sterile tube. The expectorated wines were spiked with CaCl2 to inactivate salivary enzymes,22 and stored at −80 °C until their processing for aroma analysis as described in section 2.6.
Five and 15 minutes after the wine had been expectorated, two saliva samples, St1 and St2, respectively, were taken. Thus, for each day of the week, three saliva samples from each individual: one control saliva (Sc) before mouth rinsing, and two saliva samples: five minutes after wine rinsing (St1) and 15 minutes later (St2) (15 days × 3 sampling points × 10 individuals = 450 saliva samples), and 150 expectorated wines (15 days × 10 individuals) were collected for this study.
2.4. Saliva chemical and biochemical characterisation
Immediately after collection, the saliva was clarified by centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g for 15 min at 4 °C, which allowed the removal of bacteria and cellular debris. Subsequently, 200 μL of the supernatants were taken in order to measure the saliva pH using a pH meter (CP-505, ELMETRON, Poland). No more than 10 min elapsed between saliva collection and centrifugation, and during this time, saliva samples were kept in ice. To avoid any possible deterioration of the samples before their analysis, the centrifuged saliva was divided into 1.5 mL aliquots and stored at −80 °C. The saliva samples were kept in these conditions for less than two months before the analysis was performed. All the analytical determinations were performed in centrifuged saliva. TPC was measured using the commercial kit, Pierce™ BCA Protein Assay Kit (Pierce Thermo Scientific, Rockford, IL, USA) with bovine serum albumin as the calibration standard and the results were expressed in mg L−1. The analysis of the TPI was carried out using the Folin–Ciocalteu assay and the results were expressed as mg gallic acid per L.
000g for 15 min at 4 °C, which allowed the removal of bacteria and cellular debris. Subsequently, 200 μL of the supernatants were taken in order to measure the saliva pH using a pH meter (CP-505, ELMETRON, Poland). No more than 10 min elapsed between saliva collection and centrifugation, and during this time, saliva samples were kept in ice. To avoid any possible deterioration of the samples before their analysis, the centrifuged saliva was divided into 1.5 mL aliquots and stored at −80 °C. The saliva samples were kept in these conditions for less than two months before the analysis was performed. All the analytical determinations were performed in centrifuged saliva. TPC was measured using the commercial kit, Pierce™ BCA Protein Assay Kit (Pierce Thermo Scientific, Rockford, IL, USA) with bovine serum albumin as the calibration standard and the results were expressed in mg L−1. The analysis of the TPI was carried out using the Folin–Ciocalteu assay and the results were expressed as mg gallic acid per L.
2.5. Saliva antioxidant capacity (SAOX)
To determine the SAOX, a previously existing method30 based on the measurement of the Oxygen Radical Assay Capacity (ORAC) was adapted in this work for the saliva samples. For the blank wells, 20 μL of 75 mM PBS (pH 7.4) and 120 μL of fluorescein solution (Merck KGaA, Darmstadt, Germany) were added to 96-well black polystyrene plates (Corning Costar Corp., Corning, NY, USA). In the case of the standard, 20 μL of 75 mM PBS was substituted with 20 μL of 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox, Merck KGaA) at the range of 0.0002–0.0016 μmol per well. For the sample wells, 20 μL of the sample and 120 μL of fluorescein solution (final concentration: 70 nM) were added to the wells. The sample volume corresponded to PBS dilutions of the saliva samples collected during the assay; thus, the actual volume of the samples varied between 714 and 25 μL saliva per well. The plate was then incubated for 10 minutes at 37 °C. Afterwards, 60 μL of 2,2′-azobis(2-methylpropionamide)-dihydrochloride (AAPH) were added to the well (final concentration of 12 mM), and the plates were incubated for 95 minutes at 37 °C and were analyzed using a FLUOstar OPTIMA plate reader (BMG Labtech, Offenburg, Germany). During this time, fluorescence was measured every minute at the excitation and emission wavelengths of 485 nm and 520 nm, respectively. FluoStar Galaxy (version 4.11-0) software was used for data processing. Each sample was analysed in duplicate. Fluorescence measurements were normalized with the corresponding target curve (without antioxidants). From the normalised curves, the area under the curve (AUC) and the net AUC were calculated. The net curve AUC vs. saliva volume was represented, and the ORAC value was calculated dividing the slope of the curve corresponding to the sample by the slope of the curve corresponding to Trolox. This value was expressed as μmol Trolox equivalents (ET) per mL of saliva.2.6. Wine aroma analysis
Headspace stir bar sorptive extraction (HS-SBSE) methodology, which has been already described,26 was used to determine the amount of target aroma in the three aromatised wines (DW, AW and SW), and in the corresponding expectorated wines. To do so, 20 mm long glass-encapsulated magnetic stir bars (Twister®) with 0.5 mm of polydimethylsiloxane coating film thickness (Gesterl GmbH & Co. KG, Mülheim a/d Ruhr, Germany) were used. Briefly, 6 mL of the wines or the expectorated wines were placed in a 20 mL headspace vial (Gerstel) with 40 μL of the internal standard methyl nonanoate (2.5 mg L−1) (Sigma-Aldrich, CAS number 1713-84-6). Additionally, in the case of the wine samples, 1 mL of the saliva mixture (enzymatically inactivated with CaCl2) was also added to the vial, in order to calculate the percentage of aroma recovered after the oral processing of wine.31 This approach takes into consideration the potential effect of saliva proteins on wine aroma retention32 minimizing this effect when comparing the amount of aroma in the wine and the expectorated sample. The twister was exposed to the headspace of the hermetically closed vial. All the vials were heated at 36.5 °C for 60 min. After sorption, the stir bar was dried with a lint-free tissue and desorbed. Twisters were desorbed in a 6890N gas chromatograph (GC) coupled to an Agilent 5973N mass spectrometer (MS) (J&W Scientific, Folsom, CA, USA). Volatile compounds were separated on a DB-WAX polar capillary column (30 m × 0.25 mm i.d. × 0.50 μm film thickness) from Agilent. Helium was the carrier gas at a flow rate of 1 mL min−1. The GC was equipped with a thermal desorption unit (TDU) in combination with a CIS-4 injector (“cool injection system”) (Gerstel GmbH & Co. KG). This system allows the cryofocusing of aromatic compounds before their transfer to the analytical column. The thermal desorption in splitless mode was programmed between 40 °C and 240 °C (held for 5 minutes) at 60 °C min−1. After desorption, the analytes were cryofocused in a programmed cool injection system (CIS) at −100 °C using liquid nitrogen at −100 °C using a purge time of 2 min. Transfer from the trap to the column was performed with a 12 °C s−1 ramp from −100 °C to 240 °C and held for 5 minutes.The GC oven temperature was initially held at 40 °C for 2 min, then increased at 8 °C min−1 to 240 °C, and held for 15 min. For the MS system (Agilent 5973N), the temperatures of the transfer line, quadrupole and ion source were 270, 150 and 230 °C, respectively. Electron impact mass spectra were recorded at 70 eV ionization voltage, and the ionization current was 10 μA. The acquisitions were performed in scan (from 35 to 350 amu) and SIM modes. The identification of the 10 target aroma compounds added to the wines was based on the comparison of retention times and mass spectra. The mass spectra were compared with those from the NIST 2.0 database and with those from the reference compounds analysed under the same conditions. Relative peak areas (chromatographic peak area of a specific aroma compound/chromatographic peak area of the compound used as internal standard) were calculated for each tested aroma compound and used for quantitative comparison.
2.7. Aroma recovered after the oral processing of the wine matrices (AOP)
The percentage of aroma recovered after the oral processing (AOP) of the three wine matrices was calculated by comparing the initial amount of each compound in the wine matrix before and after the oral exposure as previously shown31 by applying the following expression:where a0 is the amount of aroma (relative area) determined in the aromatised wine and aexp is the amount of aroma (relative area) in the corresponding expectorated wine. The % AOP was calculated for each wine type (DW, AW and SW) by comparing them with their respective expectorated wines.
2.8. Statistical analysis
One-way ANOVA was used to check the significant differences (95% significance) in the basal salivary composition during the three weeks of the study, and before and after the oral intervention within the same wine type. Additionally, it was also applied to check the effect of the wine matrix composition on the saliva composition collected at the same sampling point (St1). Tukey's test was applied for mean comparison. To explore the relationship between saliva composition after the oral exposure to wine and the AOP (%), Pearson's correlation analysis and principal component analysis (PCA) were applied. The XLSTAT program (v.19.01) (Addinsoft, Paris, France) was used for data processing.3. Results
3.1. Stability of the basal saliva composition of the cohort through the duration of the study
The differences in the basal saliva composition (before the oral intervention with the wines) over the three saliva collection weeks (corresponding to the first, third and fifth week over a total 5-week period) were assessed. For this, Sc samples collected each day (from Monday to Friday) from the 10 volunteers for three weeks were compared. For each week, fifty saliva samples (five days × ten volunteers) were analysed in triplicate. One-way ANOVA was applied in order to check for significant differences (p < 0.05) in the saliva composition over the three weeks (Table 2). As it can be seen, the ANOVA results showed that there were no significant differences (p < 0.05) in any of the salivary parameters. The average value of the stimulated salivary flow was 1.32 ± 0.31 mL min−1. In the same way, the pH values were quite consistent during the three weeks with an average value of 7.23 ± 0.14. TPC ranged between 904.21 ± 333.83 mg L−1 (determined at week 5) and 1041.58 ± 298.09 mg L−1 (determined at week 3). Nonetheless, these changes were not statistically significant. TPI was also determined to obtain a basal value of this parameter that might be modified after the oral exposure to the wine matrices (DW and AW). As it can be seen, neither TPI nor SAOX changed over the five weeks exhibiting average values of 36.64 ± 4.68 mg gallic acid per L and 1.72 ± 0.60 (μmol TE per mL saliva), respectively.| Week | SF | pH | TPC | TPI | SAOX | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Average | Max. | Min. | Average | Max. | Min. | Average | Max. | Min. | Average | Max. | Min. | Average | Max. | Min. | |
| SF: salivary flow (mL min−1); TPC: total protein content (mg L−1); TPI: total polyphenol index (mg ac gallic per L); SAOX: antioxidant activity (μmol Trolox equivalents (TE) per mL saliva). | |||||||||||||||
| 1 | 1.25a | 2.21 | 0.57 | 7.23a | 7.78 | 6.48 | 1041.58a | 1769.82 | 344.55 | 37.33a | 55.69 | 19.56 | 1.89a | 5.90 | 0.90 |
| 3 | 1.31a | 2.26 | 0.64 | 7.20a | 7.54 | 6.78 | 1050.65a | 1661.32 | 459.55 | 35.09a | 65.33 | 16.76 | 1.65a | 3.39 | 0.76 |
| 5 | 1.42a | 2.07 | 0.74 | 7.25a | 7.55 | 6.87 | 904.21a | 2239.25 | 364.95 | 37.51a | 68.71 | 20.38 | 1.61a | 3.00 | 0.59 |
| Pr > F | 0.52 | 0.79 | 0.28 | 0.55 | 0.54 | ||||||||||
3.2. Temporal changes in the saliva composition after the oral exposure to the wine matrices
The temporal changes in the salivary flow, composition (pH, TPC and TPI) and SAOX after the oral exposure to the three different wine types were explored by one-way ANOVA and Tukey's test (Table 3). The table also shows the average values of each salivary parameter in Sc. For this, Sc values from the three weeks were pooled once, proving the absence of significant changes in the basal salivary composition of the cohort over the five weeks of the study (Table 2).| DW | AW | SW | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Sc | St1 | St2 | Sc | St1 | St2 | Sc | St1 | St2 | |
| SF: salivary flow (mL min−1); TPC: total protein content (mg L−1); TPI: total polyphenol index (mg gallic acid per L); SAOX: antioxidant activity (μmoL Trolox equivalents (TE) per mL of saliva). | |||||||||
| SF | 1.32 ± 0.31 | 1.39 ± 0.35 | 1.31 ± 0.41 | 1.32 ± 0.31 | 1.44 ± 0.27 | 1.20 ± 0.28 | 1.32 ± 0.31 | 1.57 ± 0.29 | 1.32 ± 0.30 |
| pH | 7.23 ± 0.14 | 7.15 ± 0.28 | 7.27 ± 0.16 | 7.23 ± 0.14 | 7.16 ± 0.26 | 7.21 ± 0.12 | 7.23 ± 0.14b | 7.54 ± 0.15a | 7.28 ± 0.12b |
| TPC | 998.81 ± 126.74 | 945.03 ± 119.05 | 982.47 ± 135.16 | 998.81 ± 126.74 | 944.79 ± 206.82 | 982.15 ± 238.36 | 998.81 ± 126.74 | 877.59 ± 136.44 | 879.97 ± 234.14 |
| TPI | 36.64 ± 4.68b | 46.26 ± 5.73a | 37.65 ± 7.56b | 36.64 ± 4.68a | 40.05 ± 4.95ab | 33.12 ± 3.9b | 36.64 ± 4.68 | 34.99 ± 3.77 | 36.72 ± 4.75 |
| SAOX | 1.72 ± 0.60 | 2.01 ± 0.79 | 2.01 ± 0.79 | 1.72 ± 0.60 | 1.67 ± 0.60 | 1.67 ± 0.60 | 1.72 ± 0.60 | 1.53 ± 0.53 | 1.53 ± 0.53 |
As can be seen in the table, only pH and TPI showed significant differences (p < 0.05) between the saliva before (Sc) and after the oral intervention with wine (St1 and St2), albeit the magnitude of this effect was dependent on the wine type. For instance, the oral exposure to SW produced a slight but significant increase in salivary pH (from 7.23 in Sc to 7.54 in St1). However, values returned to basal 15 minutes after the intervention with this simple wine system (pH = 7.28 in St2). A similar trend was found in the salivary flow after the oral exposure to SW; although in this case, results were not statistically significant (Table 3). On the other hand, a significant increase in TPI was also observed in the saliva St1 collected five minutes after the oral exposure to DW and AW. This increase was more noticeable in the case of the intervention with DW (from 36.64 mg gallic acid per L to 46.26 mg gallic acid per L) compared to AW (from 36.64 mg gallic acid per L to 40.05 mg gallic acid per L). However, as shown in the table, TPI determined in St2 did not differ from that of Sc. This shows that the impact of the wine on TPI quickly reverted, and 15 minutes after the intervention with the wines, the salivary parameters were practically the same as those determined in the basal saliva. A slight increase in SAOX immediately after the intervention with DW that lasted 15 minutes after the oral intervention with the wine was also observed, although these results were not statistically significant. This trend was not observed, however, after the oral exposure to AW, and even, a reduction (statistically not significant) in SAOX was observed after the oral intervention with SW. As it can also be seen in the table, none of the wines produced significant changes in the salivary flow or TPC.
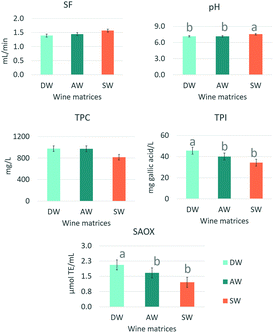
Results from Table 3 show that the largest variations in the saliva composition induced by the wines were only noticed in the first collection point (St1). To compare the extent of this effect at this time point depending on the type of wine matrix, a one-way ANOVA and Tukey's test for mean comparison were applied. These results are shown in Fig. 2.
As shown in Fig. 2, three salivary parameters, pH, TPI and AOX, exhibited significant differences depending on the wine type. Nonetheless, the type of wine affected neither salivary flow nor TPC. The oral exposure to SW produced the highest values of salivary pH (7.54), while the oral exposure to DW and AW showed similar (7.15 and 7.16, respectively) and lower pH values than SW. Nonetheless, the oral exposure to DW produced the highest salivary TPI (46.26 mg gallic acid per L) and SAOX (2.01 μmol TE per mL of saliva) values. Interestingly, the figure also shows that SAOX values in the three types of saliva (DW, AW and SW) seem to be in line with the TPI values determined in the same saliva samples. The higher the TPI in saliva (after the oral exposure to DW and AW wines), the higher the SAOX value. The significance of this correlation was further confirmed by Pearson's correlation analysis (Pearson's coefficient = 0.356; p < 0.0001) (data not shown).
3.3. Recovery of aroma compounds after the oral exposure to the wines and relationship with the changes induced in the saliva composition
To relate the changes in the saliva composition after the oral stimulation with the wines and their potential impact on wine aroma, the amount of aroma after oral processing (AOP) was firstly determined.Fig. 3 shows the average AOP (percentage) calculated after the oral exposure to the three different wine matrices (5 expectorates × 10 individuals × 3 wine types). The oral exposure to SW produced the highest AOP for all compounds (between 91.03% for γ-nonalactone and 96.53% for furfural). Additionally, the oral exposure to AW produced slight, but significant lower AOP values (between 82.49% for γ-nonalactone and 86.03% for octenal) compared to SW. Interestingly, the lowest aroma recovered was found after the oral exposure to DW (32.36% for γ-nonalactone and 61.60% for octanal). The figure also shows that for all the aroma compounds, except for γ-nonalactone, significant differences (p < 0.05) in AOP were found among the three wine matrices.
From the comparison of AOP values among wine types (Fig. 4), it was evident that differences were larger between DW and the ethanolic wine matrices (AW and SW), the latter being quite similar among them. In fact, the percentage of γ-nonalactone recovered after the oral exposure to both ethanolic wine matrices did not show significant differences (82.49% and 91.03% for AW and SW, respectively). It is also worth mentioning that while for the alcohol containing wines (AW and SW), AOP values were very similar for most odorant molecules, in the case of DW, there were more differences among compound types. In this sense, for the oral exposure to DW, the lowest AOP (35.60–36.90%) corresponded to the four esters (isoamyl acetate, ethyl butanoate, ethyl pentanoate and ethyl hexanoate), while the two linear aldehydes (hexanal and octanal) showed the highest AOP values (above 60%). However, the corresponding saturated aldehydes (hexenal and octenal) showed low AOP (39.09 and 45.60%, respectively) similar to furfural (41.18%). As previously shown, γ-nonalactone exhibited the lowest recovery (32.36%).
For further investigation of the relationship between AOP and the composition of saliva collected after the oral exposure to the wines (St1), Pearson's correlation analysis was performed (data not shown). From all tested salivary parameters, only pH, TPI and SAOX showed a significant (p < 0.05) correlation with AOP. Nonetheless, while pH exhibited a positive correlation (r = 0.4–0.5), TPI (r = −0.5 to 0.6) and SAOX (r = −0.4 to 0.5) showed a negative one, showing that a higher aroma recovered from the mouth was correlated to a higher salivary pH and lower salivary TPI and SAOX values.
Additionally, a PCA was also performed using the individual AOP values obtained from the intervention with the three wines. Data from the salivary composition of St1 were also included as supplementary variables. In this case, only the three salivary parameters that better correlated with AOP (pH, TPI and SAOX) were considered. The graphic representation is shown in Fig. 4. In the plot, each data point corresponded to the average value of five determinations per individual. As it can be seen, AOP data are well grouped according to the wine type. The first PCA (F1) explained most of the data variability (97.70%) since it was strongly and positively related to AOP. SW and AW were positively correlated to this component, exhibiting the highest AOP values, as already shown in Fig. 4. This shows the great impact of the ethanol content on the aroma recovered from the mouth. DW wines showed the opposite trend, being negatively correlated to F1 and then to AOP. Interestingly, the individual variability seemed to have much lower influence compared to the strong effect of wine type in AOP. From the salivary parameters, TPI also exhibited a negative correlation with F1, while pH and SAOX did not strongly contribute to the distinct effect of wine type on AOP. This shows that from all tested salivary parameters, TPI was the most related to AOP.
4. Discussion
In this study, the temporal change in salivary parameters (flow, pH, TPC, TPI and SAOX) as a physiological response to the oral exposure to different types of wine matrices was assessed.Firstly, the stability of the basal saliva composition of the cohort over the 5-week duration of the study was checked. The results in Table 2 show that when averaging the values of all individuals obtained in each saliva collection week (first, third and fifth), no significant differences were found in the salivary flow, nor in any of the selected compositional parameters (pH, TPC, TPI and SAOX). Interestingly, although large interindividual differences in the salivary composition and flow were previously determined,9–11 these differences are less pronounced when considering longer studies, following the same cohort over time. These results agree with those reported by Neyraud and co-workers4 who did not find significant changes in the salivary composition (salivary flow, lipolysis, proteolysis, and total antioxidant status) when monitoring the same individuals during an 8-month period. In any case, the average values determined for pH (7.23 ± 0.14), salivary flow (1.72 ± 0.60) and TPC (998.81 ± 126.74 mg L−1) for the participants in the present study were also within the normal values already determined in the centrifuged salivary samples.9–11 In the case of TPI, average values of 36.64 ± 4.68 mg gallic acid per L were found in basal saliva, which might reflect the residual polyphenols coming from the diet.24 Interestingly, despite the individuals not following any particular diet, saliva TPI was almost constant during the 5-week period of this study. Additionally, the average SAOX value (1.72 ± 0.60 μmol TE per mL) was similar to the values previously reported using a similar (ORAC) method for healthy elderly individuals (n = 15).33 The origin of this antioxidant activity could not only be related to the combined activity of different endogenous saliva antioxidants (albumin, enzymes, uric acid, and ascorbate), but also to the antioxidant activity provided by certain diet components such as polyphenols,24 which as previously indicated, were also found in the saliva.
Compared to the basal salivary composition (Sc), the oral exposure to the wines produced changes in the salivary pH and in the TPI, which depended on the type of the wine matrix (Table 2). These differences were only statistically significant with the basal saliva in the first collection point (St1), but not in the second one (St2), suggesting that the “wine matrix effect” is only noticed in the saliva composition during the first minutes after the intervention, but it did not persist over longer time (e.g. 15 min). The constant saliva replenishment in the oral cavity and the “washing off” of any residue from food or beverage intake from the oral cavity could explain this fact. From a sensory point of view, the changes in the saliva composition during these first minutes after wine intake could be of relevance in explaining retro-olfactory responsiveness, given the impact of saliva on flavor perception.9,10 Results from Table 2 show however that these changes depend on the wine composition. The increase in saliva pH (from 7.23 to 7.54) followed by oral exposure to SW but not after the oral intervention with DW and AW could be due to the slight but still higher pH of SW (3.5) compared to DW and AW (pH = 3.0). Additionally, the higher buffering capacity of saliva in the case of real wines (having different salts) compared to simple ethanolic solutions (SW) could have also influenced the lack of changes in the salivary pH after the intervention with real wine matrices. Previous works already reported a decrease in the salivary pH following the ingestion of acidic beverages (fruity juices). However, while one study showed how changes in pH quickly reverted over the first five minutes after ingestion,34 another one took thirty minutes.35
On the other hand, the intervention with the three wine types also induced differences in salivary TPI. A significant increase in the TPI was observed in the saliva (St1) collected after the oral exposure to the wines (DW and AW), compared to the saliva collected after the exposure to the synthetic wine (SW) (Table 2). This increase should be motivated by the presence of polyphenols in DW and AW, which were, however, absent in SW. Previous works also found an increase in the TPI after the consumption of polyphenol rich beverages, such as dark chocolate, expresso coffee or tea.36,37 It was also found that TPI returned to basal salivary levels fifteen minutes after the oral exposure to the wine, which contrasts with some previous results in which the presence of Folin-positive agents were found in saliva several hours after the consumption of polyphenol rich beverages.36
Surprisingly, none of the three wines produced an effect in the salivary flow, which contrasts with previous results.17,18 In these works, a sialogogic (saliva-flow-producing) effect of water solutions containing wine components (ethanol, sucrose and tannic acid) individually or in a combination was found. Also, it is also worth mentioning that this effect was higher with water solutions than with real wines (Chenin blanc and Pinot noir). In these works, the authors collected non-stimulated resting saliva to compare the effect of the tastant stimuli on salivary flow. The basal salivary flow values were, therefore, much lower than those reported in the present study, which correspond to mechanically stimulated saliva. Additionally, in the previous studies, only parotid saliva was monitored, in contrast with the whole mouth saliva collected in this study, which although to a greater extent would come from the parotid gland, the contribution of other minor glands (submandibular/sublingual) should not be underestimated. In any case, the flow and composition of stimulated saliva has been recently reported to correlate with sensory perception.9,10
In addition to salivary flow, results also showed that TPC was not affected by the oral exposure to the wines either. This result is not surprising considering that previous results on the effect of tastant stimuli in salivary proteins are somehow little conclusive and difficult to compare, given the high variability of foods and experimental conditions previously used. For instance, Neyraud and colleagues38 showed that the whole saliva proteome varied between tastants, where citric acid produced the greatest differences in protein patterns compared to glucose.38 Additionally, DiSilvestro13 showed a reduction in TPC in saliva after mouth rinsing with pomegranate juice (three times per day) for 4 weeks. In contrast, Enberg and colleagues39 showed the opposite results, with an increase in salivary TPC when studying the effect of acute alcohol consumption (0.7 g alcohol per kg of body weight for men and 0.6 g alcohol per kg of body weight for women). These results might reflect the stimulus-specificity of dietary taste compounds on the salivary proteome alteration, as previously indicated.16
Despite the lower salivary pH induced by SW, the most interesting fact regarding the impact of wine matrix composition was the significantly higher TPI and SAOX values found in the saliva after the stimulation with AW and DW wines (Fig. 3). As previously mentioned, the binding of polyphenols contained in the wines onto oral surfaces could be the reason behind this, which is also supported by the higher TPI in these saliva samples compared to those collected after stimulation with SW. It has also been suggested that these polyphenols may function as a slow release device capable of maintaining enhanced SAOX.36 Although a significant correlation between SAOX and salivary TPI was demonstrated (r = 0.356; p < 0.0001), the amount of polyphenols in saliva did not seem sufficient in explaining the increase in SAOX after the oral exposure to AW and DW. Other contributors could be some salivary proteins, (e.g. histatins), which can be induced by certain polyphenols and have antioxidant activity.40 In spite of this, the presumably low tannin concentration of the rosé wines used in this study does not seem to support this hypothesis. New studies using wines exhibiting a wide range and types of polyphenols should be carried out to better explain the relationship between SAOX and salivary TPI.
Another important objective of this study was to understand whether differences in the saliva composition induced by the three wines might affect the fate of aroma compounds in the mouth. For this, the AOP was calculated by using SOOM methodology.31,41,42 This method determines the amount of single odorants lost by certain “in-mouth” reactions mainly involving saliva (e.g. adsorption/resorption in the oral cavity, aroma interaction with salivary components, aroma metabolism, etc.).43 Regarding AOP values, it could be assumed that the higher the AOP, the higher the recovery of an odorant from the mouth after the oral intervention with wine will be, and therefore, a lower retention in the oral cavity and a lower involvement in different in-mouth reactions could be expected.
Interestingly, significant differences among AOP values for all target aroma compounds (except γ-nonalactone) (Fig. 3) were found depending on the type of the wine matrix. The higher AOP values were obtained for SW, followed by AW and DW.
These results seem to indicate the large impact of ethanol and polyphenols present in the wine, favouring the retention of aroma molecules in the oral cavity (therefore, exhibiting the lowest AOP values). Different studies have pointed out that wine polyphenols could form binary/ternary complexes with saliva proteins from the free circulating saliva or from the mucosa pellicle, being able to encapsulate aroma molecules.32,44,45 These complexes could form reservoirs of aroma molecules in the mouth, which might decrease the immediate aroma release,46,47 but can contribute to the long lasting aroma perception (aroma persistence), when they are progressively released.48,49 In fact, the adsorption of polyphenols to salivary proteins24 or to oral epithelial cells50 has been shown, proving that they can persist several hours after the intake of polyphenol-rich beverages (coffee and chocolate beverages).24
Nonetheless, in the presence of a well-known organic solvent, such as ethanol, polyphenol–salivary protein–aroma complexes could be washed off from the oral cavity, which might explain the higher AOP after the oral exposure to the alcoholic wine matrices. Interestingly, when considering the same ethanol concentration, as in the case of the AW and SW matrices, significantly lower AOP values were found for AW. This would support the higher oral retention of aroma compounds in the presence of polyphenols. This is in agreement with the higher saliva TPI after the exposure of AW, which, as explained, might favour the retention of aroma molecules in the oral cavity. As far as we know, this is the first work in the literature, in which the relationship between salivary TPI and the behaviour of aroma compounds in the oral cavity has been established. These results also agree with the correlation found between the TPI of commercial wines and the oral release of aroma compounds (esters).46
It is also worth mentioning the large differences in AOP among certain groups of compounds depending on the wine type (Fig. 3). For instance, in the case of DW, the high hydrophobicity of γ-nonalactone could determine its low recovery from the oral cavity. It has been suggested that the higher retention of certain odorants in the oral mucosa, such as β-ionone, was due to their high hydrophobicity allowing them to bind with mucins present in the oral mucosal pellicle.46 Nonetheless, the lower recovery of esters (above 34–36%) after the oral exposure to DW compared to other compounds, such as the lineal aldehydes hexanal or octanal (AOP values above 60%) cannot be exclusively explained by the differences in hydrophobicity. Both groups of compounds have similar log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values (Table 1). The log
P values (Table 1). The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of the four esters ranged between 1.70 and 2.83, while both aldehydes have log
P of the four esters ranged between 1.70 and 2.83, while both aldehydes have log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P values of 1.38 and 3.5 for hexanal and octanal, respectively (Table 1). This shows that mechanisms other than hydrophobicity might also influence the amount of these compounds retained in the mouth. Previously, the existence of salivary esterase able to hydrolyse esters has been shown using ex vivo saliva models.22,51 This could be the reason for the lower recovery of esters in the expectorated DW compared to compounds with similar hydrophobicity (aldehydes). Muñoz-González and co-workers,25 using ex vivo saliva models, also showed that some linear aldehydes (e.g. octanal) could be metabolised by salivary enzymes, giving rise to the corresponding linear alcohol (octanol), which could affect their recovery into the expectorated wines.
P values of 1.38 and 3.5 for hexanal and octanal, respectively (Table 1). This shows that mechanisms other than hydrophobicity might also influence the amount of these compounds retained in the mouth. Previously, the existence of salivary esterase able to hydrolyse esters has been shown using ex vivo saliva models.22,51 This could be the reason for the lower recovery of esters in the expectorated DW compared to compounds with similar hydrophobicity (aldehydes). Muñoz-González and co-workers,25 using ex vivo saliva models, also showed that some linear aldehydes (e.g. octanal) could be metabolised by salivary enzymes, giving rise to the corresponding linear alcohol (octanol), which could affect their recovery into the expectorated wines.
On the other hand, it has recently been proven that at the usual wine ethanol concentration (above 10–12%), the activity of salivary esterase enzymes is highly reduced.23 Although the effect of ethanol in NAD(P)H dependent enzymes, which could be involved in the metabolism of aldehydes, has not been described so far, the effect of ethanol on these enzymes should not be neglected. Then, the presence of ethanol could contribute to an explanation for the higher recovery of certain aroma molecules (esters and aldehydes) after the oral exposure to AW and SW wine types by inhibiting their oral metabolism. This is in agreement with the higher oral persistence of esters described in the presence of ethanol.12
5. Conclusions
Results from this work have shown an effect of the oral exposure to different wine types (mainly differing in ethanol and macromolecule contents) on the changes in the saliva composition. These changes are mostly produced within the first five minutes after the oral intervention with the wines, and they returned to the basal composition after fifteen minutes. The most affected salivary parameters were pH, TPI and SAOX. Nonetheless, these changes depended on the matrix composition. The intervention with a SW produced an increase in salivary pH, while the intervention with a DW and the same wine with ethanol added (AW) produced an increase in the TPI and SAOX. The increase in salivary TPI has been related to a higher retention of aroma molecules in the oral cavity, which was highly relevant in the wine without ethanol. Ethanol increased the recovery of aroma compounds in the expectorated wines, suggesting a limitation in their in-mouth availability. This could be explained by higher solubilisation of the aroma–saliva protein–polyphenol complexes, or, in the case of certain aroma compounds (esters), it could also be due to the inhibition of the enzymatic reactions involving esterase enzymes. The increase in SAOX after the oral intervention with the wine might also have a potential impact on some types of redox reactions involving aroma molecules (e.g. aldehydes). New studies focused on determining the impact of wines with different types and concentrations of polyphenols on salivary TPI and SAOX and their effect on odorant molecules prone to reduction reactions will be conducted in oncoming works in order to understand the relevance of these reactions on flavour perception.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
Authors greatly thank the volunteers for their participation in this study and Irene Vazquez and María Pérez for their technical assistance. This work was supported by the Spanish MICINN through the PID 2019-11734RB-100 Project. C. M.-G. thanks the AT program (Comunidad de Madrid, Spain) for her postdoctoral contract.References
- R. K. Drobitch and C. K. Svensson, Therapeutic Drug Monitoring in Saliva, Clin. Pharmacokinet., 1992, 23, 365–379 CrossRef CAS PubMed.
- A. Buettner, Influence of human salivary enzymes on odorant concentration changes occurring in vivo. 1. Esters and thiols, J. Agric. Food Chem., 2002, 50, 3283–3289 CrossRef CAS PubMed.
- A. Buettner, Influence of human saliva on odorant concentrations. 2. Aldehydes, alcohols, 3-alkyl-2-methoxypyrazines, methoxyphenols, and 3-hydroxy-4, 5-dimethyl-2(5H)-furanone, J. Agric. Food Chem., 2002, 50, 7105–7110 CrossRef CAS PubMed.
- E. Neyraud, O. Palicki, C. Schwartz, S. Nicklaus and G. Feron, Variability of human saliva composition: possible relationships with fat perception and liking, Arch. Oral Biol., 2012, 57, 556–566 CrossRef CAS.
- J. M. McRae and J. A. Kennedy, Wine and grape tannin interactions with salivary proteins and their impact on astringency: a review of current research, Molecules, 2011, 16, 2348–2364 CrossRef CAS PubMed.
- M. D. Contreras-Aguilar and F. Gómez-García, Salivary glands’ anatomy and physiology, Springer Nature, 2020 Search PubMed.
- C. Criado, M. Pérez-Jiménez, C. Muñoz-González and A. Pozo-Bayón, in Proceedings of the 16th Weurman Flavour Research Symposium, ed. J.-L. Le Quere, 2021 Search PubMed.
- C. Muñoz-González, G. Feron and F. Canon, Main effects of human saliva on flavour perception and the potential contribution to food consumption, Proc. Nutr. Soc., 2018, 77, 423–431 CrossRef PubMed.
- C. Criado, C. Muñoz-González and M. Á. Pozo-Bayón, Differences in salivary flow and composition between age groups are correlated to dynamic retronasal aroma perception during wine consumption, Food Qual. Prefer., 2021, 87, 104046 CrossRef.
- C. Criado, C. Chaya, V. Fernández-Ruíz, M. D. Álvarez, B. Herranz and M. Á. Pozo-Bayón, Effect of saliva composition and flow on inter-individual differences in the temporal perception of retronasal aroma during wine tasting, Food Res. Int., 2019, 126, 108677 CrossRef CAS.
- E. Neyraud, O. Palicki, C. Schwartz, S. Nicklaus and G. Feron, Variability of human saliva composition: Possible relationships with fat perception and liking, Arch. Oral Biol., 2012, 57, 556–566 CrossRef CAS.
- C. Muñoz-González, M. Pérez-Jiménez and M. Á. Pozo-Bayón, Oral persistence of esters is affected by wine matrix composition, Food Res. Int., 2020, 135, 109286 CrossRef PubMed.
- R. A. DiSilvestro, D. J. DiSilvestro and D. J. DiSilvestro, Pomegranate extract mouth rinsing effects on saliva measures relevant to gingivitis risk, Phytother. Res., 2009, 23, 1123–1127 CrossRef PubMed.
- H. S. Davies, P. D. A. Pudney, P. Georgiades, T. A. Waigh, N. W. Hodson, C. E. Ridley, E. W. Blanch and D. J. Thornton, Reorganisation of the salivary mucin network by dietary components: insights from green tea polyphenols, PLoS One, 2014, 9, e108372 CrossRef PubMed.
- C. R. Crawford and C. A. Running, Addition of chocolate milk to diet corresponds to protein concentration changes in human saliva, Physiol. Behav., 2020, 225, 113080 CrossRef CAS PubMed.
- M. Bader, T. Stolle, M. Jennerwein, J. Hauck, B. Sahin and T. Hofmann, Chemosensate-induced modulation of the salivary proteome and metabolome alters the sensory perception of salt taste and odor-active thiols, J. Agric. Food Chem., 2018, 66, 7740–7749 CrossRef CAS PubMed.
- S. Martin and R. M. Pangborn, Human parotid secretion in response to ethyl alcohol, J. Dent. Res., 1971, 50, 485–490 CrossRef CAS PubMed.
- R. J. Hyde and R. M. Pangborn, Parotid salivation in response to tasting wine, Am. J. Enol. Vitic., 1978, 29, 87–91 Search PubMed.
- J.-X. Guinard, C. Zoumas-Morse and C. Walchak, Relation between parotid saliva flow and composition and the perception of gustatory and trigeminal stimuli in foods, Physiol. Behav., 1997, 63, 109–118 CrossRef CAS.
- M. Pérez-Jiménez and M. Á. Pozo-Bayón, Development of an in-mouth headspace sorptive extraction method (HSSE) for oral aroma monitoring and application to wines of different chemical composition, Food Res. Int., 2019, 121, 97–107 CrossRef.
- M. Perez-Jiménez, A. Esteban-Fernández, C. Muñoz-González and M. A. Pozo-Bayón, Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility, Molecules, 2020, 25, 1701 CrossRef PubMed.
- M. Pérez-Jiménez, C. Muñoz-González and M. A. Pozo-Bayón, Understanding human salivary esterase activity and its variation under wine consumption conditions, RSC Adv., 2020, 10, 24352–24361 RSC.
- M. Pérez-Jiménez, C. Muñoz-González and M. Á. Pozo-Bayón, Front. Nutr., 2021, 8, 849 CrossRef PubMed.
- I. Ginsburg, E. Koren, M. Shalish, J. Kanner and R. Kohen, Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity, Arch. Oral Biol., 2012, 57, 1327–1334 CrossRef CAS PubMed.
- C. Muñoz-González, G. Feron, M. Brulé and F. Canon, Understanding the release and metabolism of aroma compounds using micro-volume saliva samples by ex vivo approaches, Food Chem., 2018, 240, 275–285 CrossRef.
- M. Pérez-Jiménez and M. Á. Pozo-Bayón, Development of an in-mouth headspace sorptive extraction method (HSSE) for oral aroma monitoring and application to wines of different chemical composition, Food Res. Int., 2019, 121, 97–107 CrossRef PubMed.
- C. Dawes, Circadian rhythms in human salivary flow rate and composition, J. Physiol., 1972, 220, 529–545 CrossRef CAS PubMed.
- C. Dawes, Circadian rhythms in the flow rate and composition of unstimulated and stimulated human submandibular saliva, J. Physiol., 1975, 244, 535–548 CrossRef CAS.
- E. Brandão, S. Soares, N. Mateus and V. de Freitas, Human saliva protein profile: Influence of food ingestion, Food Res. Int., 2014, 64, 508–513 CrossRef.
- B. Hernández-Ledesma, A. Dávalos, B. Bartolomé and L. Amigo, Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS, J. Agric. Food Chem., 2005, 53, 588–593 CrossRef.
- A. Esteban-Fernández, N. Rocha-Alcubilla, C. Muñoz-González, M. V. Moreno-Arribas and M. Á. Pozo-Bayón, Intra-oral adsorption and release of aroma compounds following in-mouth wine exposure, Food Chem., 2016, 205, 280–288 CrossRef.
- C. Muñoz-González, G. Feron, E. Guichard, J. J. Rodríguez-Bencomo, P. J. Martín-Álvarez, M. V. Moreno-Arribas and M. Á. Pozo-Bayón, Understanding the role of saliva in aroma release from wine by using static and dynamic headspace conditions, J. Agric. Food Chem., 2014, 62, 8274–8288 CrossRef PubMed.
- C. Muñoz-González, M. Brulé, G. Feron and F. Canon, Does interindividual variability of saliva affect the release and metabolization of aroma compounds ex vivo? The particular case of elderly suffering or not from hyposalivation, J. Texture Stud., 2019, 50, 36–44 CrossRef PubMed.
- A. Millward, L. Shaw, E. Harrington and A. J. Smith, Continuous monitoring of salivary flow rate and pH at the surface of the dentition following consumption of acidic beverages, Caries Res., 1997, 31, 44–49 CrossRef CAS PubMed.
- L. Kiran Banan and A. Hegde, Plaque and salivary pH changes after consumption of fresh fruit juices, J. Clin. Pediatr. Dent., 2006, 30, 9–13 CrossRef.
- I. Ginsburg, R. Kohen, M. Shalish, D. Varon, E. Shai and E. Koren, The oxidant-scavenging abilities in the oral cavity may be regulated by a collaboration among antioxidants in saliva, microorganisms, blood cells and polyphenols: a chemiluminescence-based study, PLoS One, 2013, 8, e63062 CrossRef CAS PubMed.
- M.-J. Lee, J. D. Lambert, S. Prabhu, X. Meng, H. Lu, P. Maliakal, C.-T. Ho and C. S. Yang, Delivery of tea polyphenols to the oral cavity by green tea leaves and black tea extract, Cancer Epidemiol., Biomarkers Prev., 2004, 13, 132–137 CrossRef CAS PubMed.
- E. Neyraud, T. Sayd, M. Morzel and E. Dransfield, Proteomic analysis of human whole and parotid salivas following stimulation by different tastes, J. Proteome Res., 2006, 5, 2474–2480 CrossRef CAS PubMed.
- N. Enberg, H. Alho, V. Loimaranta and M. Lenander-Lumikari, Saliva flow rate, amylase activity, and protein and electrolyte concentrations in saliva after acute alcohol consumption, Oral. Surg., Oral. Med., Oral. Pathol. Oral. Radiol., 2001, 92, 292–298 CrossRef CAS.
- M. Schwartz, F. Neiers, G. Feron and F. Canon, The Relationship Between Salivary Redox, Diet, and Food Flavor Perception, Front. Nutr., 2021, 7, 384 Search PubMed.
- A. Buettner and P. Schieberle, Exhaled odorant measurement (EXOM)—a new approach to quantify the degree of in-mouth release of food aroma compounds, LWT – Food Sci. Technol., 2000, 33, 553–559 CrossRef CAS.
- C. Muñoz-Gonzalez, M. Perez-Jimenez, C. Criado and M. A. Pozo-Bayon, Effects of Ethanol Concentration on Oral Aroma Release After Wine Consumption, Molecules, 2019, 24, 83253 Search PubMed.
- Flavor perception, ed. A. J. Taylor and D. D. Roberts, John Wiley & Sons, 2008 Search PubMed.
- A. Mitropoulou, E. Hatzidimitriou and A. Paraskevopoulou, Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva, Food Res. Int., 2011, 44, 1561–1570 CrossRef CAS.
- A. Genovese, P. Piombino, A. Gambuti and L. Moio, Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations, Food Chem., 2009, 114, 100–107 CrossRef CAS.
- A. Esteban-Fernández, C. Muñoz-González, A. Jiménez-Girón, M. Pérez-Jiménez and M. Á. Pozo-Bayón, Aroma release in the oral cavity after wine intake is influenced by wine matrix composition, Food Chem., 2018, 243, 125–133 CrossRef PubMed.
- M. Perez-Jiménez, C. Chaya and M. Á. Pozo-Bayón, Individual differences and effect of phenolic compounds in the immediate and prolonged in-mouth aroma release and retronasal aroma intensity during wine tasting, Food Chem., 2019, 285, 147–155 CrossRef PubMed.
- C. Muñoz-González, F. Canon, G. Feron, E. Guichard and M. A. Pozo-Bayón, Assessment wine aroma persistence by using an in vivo PTR-ToF-MS approach and its relationship with salivary parameters, Molecules, 2019, 24, 1277 CrossRef PubMed.
- C. Muñoz-González, M. Brule, C. Martin, G. Feron and F. Canon, Molecular mechanisms of aroma persistence: from noncovalent interactions between aroma compounds and oral mucosa to metabolization of aroma compounds by saliva and oral cells, Food Chem., 2022, 373, 131467 CrossRef PubMed.
- C. Payne, P. K. Bowyer, M. Herderich and S. E. P. Bastian, Interaction of astringent grape seed procyanidins with oral epithelial cells, Food Chem., 2009, 115, 551–557 CrossRef CAS.
- M. Pérez-Jiménez, N. Rocha-Alcubilla and M. Á. Pozo-Bayón, Effect of saliva esterase activity on ester solutions and possible consequences for the in-mouth ester release during wine intake, J. Texture Stud., 2019, 50, 62–70 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |