Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Perilla seed oil in combination with nobiletin-rich ponkan powder enhances cognitive function in healthy elderly Japanese individuals: a possible supplement for brain health in the elderly†
Michio
Hashimoto
 *a,
Kentaro
Matsuzaki
*a,
Kentaro
Matsuzaki
 a,
Koji
Maruyama
b,
Shahdat
Hossain
a,
Koji
Maruyama
b,
Shahdat
Hossain
 c,
Eri
Sumiyoshi
c,
Eri
Sumiyoshi
 a,
Harumi
Wakatsuki
a,
Setsushi
Kato
d,
Miho
Ohno
d,
Yoko
Tanabe
a,
Yoko
Kuroda
e,
Shuhei
Yamaguchi
f,
Koji
Kajima
b,
Yasushi
Ohizumi
g and
Osamu
Shido
a
a,
Harumi
Wakatsuki
a,
Setsushi
Kato
d,
Miho
Ohno
d,
Yoko
Tanabe
a,
Yoko
Kuroda
e,
Shuhei
Yamaguchi
f,
Koji
Kajima
b,
Yasushi
Ohizumi
g and
Osamu
Shido
a
aDepartment of Environmental Physiology, Faculty of Medicine, Shimane University, Izumo, Shimane, Japan. E-mail: michio1@med.shimane-u.ac.jp; Fax: +81-853-20-2730; Tel: +81 -853-20-2730
bSankyo Holdings Co., Ltd, Fuji, Shizuoka, Japan
cDepartment of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka, Bangladesh
dKato Hospital, Jinjukai Healthcare Corporation, Kawamoto, Shimane, Japan
eDepartment of Internal Medicine III, Faculty of Medicine, Shimane University, Izumo, Shimane, Japan
fShimane Prefecture Hospital Bureau, Izumo, Shimane, Japan
gKansei Fukushi Research Institute, Tohoku Fukushi University, Sendai, Miyagi, Japan
First published on 9th February 2022
Abstract
Perilla (Perilla frutescens) seed oil (PO), rich in α-linolenic acid (ALA), can improve cognitive function in healthy elderly Japanese people. Here, supplements containing either PO alone or PO with nobiletin-rich air-dried immature ponkan powder were examined for their effects on cognitive function in 49 healthy elderly Japanese individuals. Patients were enrolled in a 12-month randomized, double-blind, parallel-armed study. Randomized participants in the PO group received soft gelatin capsules containing 1.47 mL (0.88 g of ALA) of PO daily, and those in the PO + ponkan powder (POPP) group received soft gelatin capsules containing both 1.47 mL of PO and 1.12 g ponkan powder (2.91 mg of nobiletin) daily. At the end of intervention, the POPP group showed significantly higher cognitive index scores than the PO group. The pro-cognitive effects of POPP treatment were accompanied by increases in ALA and docosahexaenoic acid levels in red blood cell plasma membranes, serum brain-derived neurotropic factor (BDNF) levels, and biological antioxidant potential. We demonstrate that 12-month intervention with POPP enhances serum BDNF and antioxidant potential, and may improve age-related cognitive impairment in healthy elderly people by increasing red blood cell ω-3 fatty acid levels. Clinical Trial Registry, UMIN000040863.
1. Introduction
Perilla (Perilla frutescens) seed oil (PO) is mainly composed of α-linolenic acid (ALA; C18:3 ω-3; 54–64%), which is an essential ω-3 polyunsaturated fatty acid (PUFA).1 ALA has been reported to be an antithrombotic,2 antiarrhythmic,3 anti-inflammatory,4 and neuroprotective5 fatty acid. PO also exhibits antidepressant properties, and PO consumption ameliorates cognitive function by generating new hippocampal neural membrane structures. It has also been shown to induce the expression of specific proteins involved in numerous functions, including regulation of energy metabolism, cytoskeleton, transport, apoptosis, and neurogenesis in rats.6 An epidemiological study reported that serum ALA level was negatively associated with the risk of disabling dementia.7 Recently, we have shown the potential benefits of consistent PO intake on mental traits, such as depression and apathy, in healthy Japanese adults8 and on mental status and cognitive function in healthy Japanese elderly.9ALA is converted to other ω-3 PUFAs, including docosahexaenoic acid (DHA, C22:6) and eicosapentaenoic acid (EPA, C20:5), in animals. Omega-3 PUFAs, especially DHA, are known to have beneficial effects on brain function.10,11 Our previous randomized, double-blind, placebo-controlled studies reported that foods rich in DHA prevented age-related cognitive impairment in elderly Japanese.12,13 However, in humans, the metabolic conversion of ALA to other ω-3 PUFAs, particularly DHA, is very limited.14 Accordingly, it is important to study whether ALA consumption can provide EPA and DHA to critical organs. Interestingly, rodent and epidemiological studies indicate that the conversion of ALA to EPA and DHA is increased via the simultaneous nutritive consumption of flavonoids.15,16
Citrus peel is rich in poly-methoxylated flavones and has been widely used as a crude drug in traditional herbal medicine. Nobiletin is a flavonoid that is abundant in the peel of citrus fruits such as Citrus poonensis (ponkan). Nobiletin was found to improve cognitive and motor dysfunction in various animal models of neurodegenerative disorders by exerting beneficial effects against pathological features, including neuroinflammation, oxidative stress, amyloid-β pathology, tau hyperphosphorylation, cholinergic neurodegeneration, dysfunctional synaptic plasticity, and related signalling cascades.17–19 However, few intervention studies have been conducted in healthy elderly individuals to verify the effects of nobiletin supplementation on cognitive function.
ALA and nobiletin are characteristic compounds in plant-based neuroprotective diets. Therefore, taking ALA and nobiletin together may have a greater beneficial effect on cognitive function than taking either alone. However, to our knowledge, there have been no intervention studies assessing the combined effects of ALA and nobiletin on cognitive function in the elderly. In this study, we prepared two types of soft gelatine capsules (SGCs) containing either PO alone or PO combined with nobiletin-rich immature ponkan powder, and conducted a 12-month randomized, double-blind, intervention study in healthy elderly Japanese individuals to compare the effects of the two supplements on brain function. Our study presents a beneficial and easy-to-consume dietary supplement for maintaining cognitive function in the elderly.
2. Materials and methods
2.1. Participant selection and study design
Healthy elderly individuals aged 60 to 85 were recruited from Shimane Prefecture, Japan, and a 12-month, randomized, double-blinded, parallel-armed study was carried out. All volunteers underwent a medical examination, including assessments of anthropometry, blood biochemical levels, haematological parameters, cognitive function, and mental health. Volunteers were excluded if there was any evidence of a medical disorder, including respiratory, hepatic, renal, or cardiac disease; diabetes mellitus; endocrine, metabolic, or haematological diseases; allergy or hypersensitivity; or use of any psychotropic drugs/supplements that might significantly affect the results of the study. Volunteers with a total Mini-Mental State Examination (MMSE; see section 2.4) score of 23 or less were excluded.This study was approved by the Shimane University Ethics Committee (Study No.: 3194, 3497) and was performed according to the principles of the Declaration of Helsinki and Good Clinical Practice. All volunteers provided written informed consent prior to their participation. This intervention trial was performed between 2018 and 2020. Before deciding on the final participants, volunteers who wanted to participate were asked to respond to a self-reported general lifestyle questionnaire, which included questions related to their medical/medication history.
Two types of SGCs were produced by Sankyo Holdings Co., Ltd, Fuji, Japan. Taking into account the ease of swallowing one capsule, the maximum amount of PO in the capsule was set to 0.098 mL. Therefore, one SGC contained 0.098 mL of PO (58.8 mg ALA) alone, and the other contained 0.098 mL of PO and 0.075 g of nobiletin-rich air dried immature ponkan powder (nobiletin-rich ADPP; 0.19 mg nobiletin). The nutrient compositions of PO and ADPP are shown in Tables 1 and 2, respectively. ADPP is high in flavonoids, such as nobiletin, hesperidin, and narirutin.
| Proximate analysis | N = 5 | Fatty acids | N = 5 | ||
|---|---|---|---|---|---|
| Values are means ± SE. Nutritional values per 100 g of perilla seed oil. Data on proximate analysis was obtained from the Shimane Institute for Industrial Technology (Matsue, Japan) and data on fatty acids were obtained from Japan Food Research laboratories (JFRL, Tokyo, Japan). N, number of samples analysed. | |||||
| Energy | (kcal) | 931 ± 22 | Palmitic acid (C16:0) | (g) | 5.8 ± 0.1 |
| Protein | (g) | 0 | Stearic acid (C18:0) | (g) | 2.1 ± 0.1 |
| Fat | (g) | 101 ± 2 | Oleic acid (C16:1 ω-9) | (g) | 13.4 ± 0.6 |
| Carbohydrate | (g) | 0 | Linoleic acid (C18:2 ω-6) | (g) | 13.2 ± 0.4 |
| Fiber | (g) | 0 | α-linolenic acid (C18:3 ω-3) | (g) | 62.9 ± 1.5 |
| Moisture | (g) | 0 | |||
| Ash | (g) | 0 | |||
| Proximate analysis | Mineral and materials | ||||
|---|---|---|---|---|---|
| Nutritional values per 100 g of dry powder. Data was obtained from Hiyoshi Co., Shiga, Japan (ESI 1,† Table 2). The value of “other polyphenols” is the value excluding hesperidin, nobiletin and narirutin. | |||||
| Energy | (kcal) | 257 | Sodium | (mg) | 3 |
| Protein | (g) | 9.0 | |||
| Fat | (g) | 2.1 | Hesperidin | (g) | 16 |
| Carbohydrate | (g) | 22.5 | Nobiletin | (mg) | 260 |
| Fiber | (g) | 56.1 | Narirutin | (g) | 1.2 |
| Moisture | (g) | 6.6 | Other Polyphenols | (g) | 4.1 |
| Ash | (g) | 3.7 | |||
Forty-nine subjects (24 men and 25 women) were distributed in two groups, and each took 15 SGCs in three even doses each day for 12 months, between or immediately after meals. The PO group (n = 24) received 1.47 mL daily of PO alone, and the PO plus ADPP (POPP) group (n = 25) received both 1.47 mL of PO and 1.12 g of nobiletin-rich ADPP daily. Group allocation was performed by stratified random assignment according to total MMSE score, sex, and age, as described previously.8,20–22 Randomized code lists were generated by the medical statistics advisor, and the investigators, participants, and sponsor were blinded to these codes. Neither the participants nor the researchers knew which capsules were being consumed. Prior to the intervention trial, a blind sensory test was performed to confirm that there was no difference in the appearance or taste of the two SGCs (data not shown).
2.2. Anthropometry, body composition, and analysis of dietary intake
The body weight, height, and abdominal circumference of all participants were measured. Body composition was analysed using a bioelectrical impedance analyser WB-150 (TANITA Co., Tokyo, Japan).To demonstrate the effects of PO and POPP treatment on health status and lifestyle in participants, all participants were asked to record their daily SGC intake and health/mental status during the trials. Dietary intake before and after 12 months of intervention trial was approximated using a validated, brief, self-administered diet history questionnaire (BDHQ) for the Japanese elderly.23
2.3. Blood sampling
Blood was sampled in the morning or afternoon at the beginning of the trial (baseline) and after 12 months, after confirming that the participants had not eaten breakfast or lunch, respectively. After preparations to measure haematological parameters, blood was separated into serum and red blood cells (RBCs). Fresh serum samples were used to measure blood biochemical items, brain-derived neurotrophic factor (BDNF), and biological antioxidant potential (BAP). RBC samples were used to measure the fatty acid profiles of RBC plasma membranes (RBC-PM), and the remaining serum was stored at −80 °C within 4 h of collection until use.2.4. Evaluation of cognitive function and mental health in participants
Cognitive abilities were assessed using the Hasegawa's Dementia Scale-Revised (HDS-R),24 the MMSE,25 and the Japanese version of the Montreal Cognitive Assessment (MoCA-J).26 The MMSE test is commonly used to assess the cognitive impairments seen in association with dementia status, especially in Alzheimer's disease patients and elderly people with mild cognitive impairment (MCI).25 The HDS-R contains nine simple questions and has been widely accepted in Asian populations for clinical use and for the epidemiological screening of cognitive dysfunction.24 The MoCA-J is a reliable and valid cognitive screening test designed to assist Health Professionals in the detection of MCI.26Apathy and depression were assessed using the Japanese version of the apathy scale27 and the Zung Self-Rating Depression Scale (SDS),28 respectively. The apathy scale was developed as a tool for measuring apathy resulting from brain-related pathology.27 This test is commonly used in clinical trials and epidemiological surveys aimed at evaluating apathy. SDS is an established norm-referenced screening measure used worldwide to identify the presence of depressive symptoms in adults.28
Tests were performed before treatment (baseline) and again after the 12-month intervention period.
2.5. Blood biochemical analysis, fatty acid profile, and apolipoprotein E genotyping in participants
Blood biochemical analyses included evaluation of the levels of gamma-glutamyl transpeptidase, alanine aminotransferase, aspartate aminotransferase, albumin, total cholesterol, blood urea nitrogen, triglycerides (TG), creatinine, blood sugar, and high-and low-density lipoprotein cholesterol levels using an automatic analyser, the BiOLis 24i (Tokyo Boeki Medical System, Tokyo, Japan). In addition, haemoglobin A1c levels were determined using a commercially available kit (TFB Inc., Tokyo, Japan).Haematological analyses included evaluation of the number of RBCs, white blood cells, and platelets, and the levels of haemoglobin, mean corpuscular volume, mean corpuscular haemoglobin, and mean corpuscular haemoglobin concentration using an automated haematology analyser (XS-1000i, Sysmex Corporation, Kobe, Japan).
Serum BDNF levels were quantified using an enzyme-linked immunosorbent assay kit (Mature BDNF ELISA Kit Wako, FUJIFILM Wako Pure Chemical, Osaka, Japan) according to the manufacturer protocol.
Serum BAP levels were measured using a corresponding measurement kit (BAP test; Wismerll Co. Ltd, Tokyo, Japan) as previously described.9 This test is based on the ability of the serum to reduce ferric (Fe3+) to ferrous (Fe2+) ions, according to the principle of the ferric reducing antioxidant power assay.29 RBC-PM fatty acid profile,13,30 and apolipoprotein E (APOE) gene statuses12 were determined as previously described.
2.6. Statistical analysis
Results are indicated as the mean ± standard error (SE). Data distribution was assessed using the Shapiro–Wilk test. The comparison between baseline and endpoint (12 months) values for each group was assessed by paired t-tests or Wilcoxon signed-rank tests. Comparisons between the two groups were performed using an independent t-test or Mann–Whitney U-test. Allelic distribution of the APOE gene was analysed by the Pearson Chi-square test. Correlations were evaluated using Pearson's correlation coefficients. Analysis of covariance was used to compare the differences between groups regarding cognitive outcomes, serum BDNF, and BAP levels. Multiple linear regression analyses were conducted using cognitive outcomes as the dependent variable, and serum levels of BDNF and BAP as independent variables. For the analysis, baseline value of outcomes, blood biochemical levels, and participant characteristics (e.g., age, sex, BMI) were included in the models as covariates. All analyses were performed using PASW Statistics software (version 23.0, SPSS Inc., Chicago, IL, USA). All statistical tests were two-tailed, and significance was set at p < 0.05.3. Results
3.1. Clinical characteristics and demographics of participants and evaluation of dietary intake
The 12-month study was completed by 21 PO and 23 POPP group participants (Fig. 1). The five participants who left the study did not drop out due to participation-related illness or side effects, but withdrew for personal reasons, including the inconvenience of taking 15 SGCs daily. We used pre-protocol analysis instead of intention-to treat analysis in this study.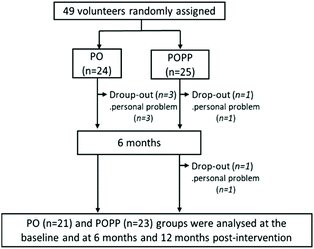 | ||
| Fig. 1 Flow diagram of participant selection. PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group. | ||
The final participants (n = 44) showed high adherence to the study protocol for 12 months (93.9 ± 3.2% for the PO group and 96.1 ± 2.0% for the POPP group). Data obtained from the general questionnaire on medical/medication history and lifestyle habits at baseline and after 12 months of intervention indicated no remarkable differences between time points. During the intervention trial, participants in both groups experienced no significant harmful effects (e.g., stomach irritation, allergic reactions, palpitations, etc.) that influenced their daily lives. Participant characteristics at baseline and at 12 months are shown in Table 3. There were no significant differences in baseline anthropometry, blood pressure, blood biochemical parameters, or haematological parameters between the two groups.
| PO (n = 21) | POPP (n = 23) | Change (12 months-baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POPP | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. Significant differences from the PO group, ##p < 0.05. BUN, Blood urea nitrogen; GOT, glutamate oxaloacetate transaminase; GPT, glutamic pyruvic transaminase; γ-GTP, γ-glutamyl transpeptidase; HbA1c, haemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MCH, mean corpuscular haemoglobin; MCHC, mean corpuscular haemoglobin concentration; MCV, mean corpuscular volume; PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group; RBC, red blood cell; WBC, white blood cell. | ||||||
| Anthropometry | ||||||
| Sex (male/female) | 21 (11/10) | — | 23 (12/11) | — | ||
| Age (years) | 70.2 ± 1.4 | 71.3 ± 1.3 | 68.7 ± 1.2 | 69.7 ± 1.2 | ||
| Height (cm) | 157.1 ± 1.5 | 157.3 ± 1.4 | 159.8 ± 2.0 | 158.6 ± 1.8 | 0.2 ± 0.5 | −1.1 ± 0.3 |
| Body weight (kg) | 54.1 ± 2.0 | 53.9 ± 2.0 | 59.7 ± 2.6 | 60.0 ± 2.6 | −0.3 ± 0.3 | 0.3 ± 0.4 |
| Body mass Index (kg m−2) | 21.8 ± 0.6 | 21.8 ± 0.6 | 23.2 ± 0.6 | 23.3 ± 0.6 | −0.1 ± 0.1 | 0.1 ± 0.1 |
| Waist circumference (cm) | 81.5 ± 2.2 | 80.7 ± 2.1 | 84.4 ± 2.2 | 84.0 ± 2.2 | −0.8 ± 0.4 | −0.4 ± 0.4 |
| Body fat (%) | 24.82 ± 1.29 | 25.11 ± 1.30 | 27.62 ± 1.33 | 28.40 ± 1.39** | 0.29 ± 0.3 | 0.80 ± 0.3 |
| Blood pressure (BP) | ||||||
| Systolic BP (mmHg) | 141 ± 5 | 140 ± 4 | 144 ± 4 | 143 ± 4 | −1 ± 3 | −1 ± 3 |
| Diastolic BP (mmHg) | 85 ± 4 | 84 ± 3 | 81 ± 2 | 80 ± 3 | −1 ± 2 | −1 ± 2 |
| Blood biochemistry | ||||||
| GOT (U L−1) | 26.7 ± 2.7 | 27.9 ± 3.5 | 23.7 ± 1.0 | 22.7 ± 0.8 | 1.2 ± 2.4 | −0.9 ± 1.0 |
| GPT (U L−1) | 19.8 ± 1.5 | 20.3 ± 1.8 | 21.6 ± 2.0 | 21.6 ± 1.3 | 0.5 ± 1.6 | 0.0 ± 1.8 |
| γ−GTP (IU L−1) | 30.7 ± 8.0 | 32.7 ± 8.9 | 35.3 ± 5.6 | 38.0 ± 5.5 | 2.0 ± 1.4 | 2.6 ± 2.9 |
| Albumin (g dL−1) | 4.26 ± 0.05 | 4.44 ± 0.05** | 4.35 ± 0.05 | 4.33 ± 0.03 | 0.18 ± 0.05 | −0.02 ± 0.02## |
| Total cholesterol (mg dL−1) | 211.8 ± 8.1 | 219.2 ± 7.6 | 213.6 ± 7.1 | 210.7 ± 5.7 | 7.5 ± 4.9 | −3.0 ± 6.4 |
| Triglyceride (mg dL−1) | 120.94 ± 13.8 | 98.44 ± 11.75** | 97.79 ± 10.0 | 98.57 ± 10.2 | −23.11 ± 10.2 | 0.78 ± 10.7 |
| BUN (mg dL−1) | 15.8 ± 0.7 | 17.9 ± 0.7** | 17.7 ± 0.7 | 18.9 ± 0.8 | 2.0 ± 0.5 | 1.2 ± 0.6 |
| Creatinine (mg dL−1) | 0.8 ± 0.04 | 0.8 ± 0.03 | 0.8 ± 0.03 | 0.7 ± 0.04 | 0.01 ± 0.01 | −0.02 ± 0.02 |
| Blood sugar (mg dL−1) | 104.6 ± 3.8 | 95.3 ± 2.3** | 109.6 ± 4.8 | 108.3 ± 3.1 | −9.3 ± 3.6 | −1.3 ± 5.1 |
| HDL-C (mg dL−1) | 67.3 ± 3.9 | 67.6 ± 3.5 | 64.0 ± 3.3 | 63.2 ± 3.1 | 0.2 ± 1.8 | −0.8 ± 1.6 |
| LDL-C (mg dL−1) | 125.0 ± 6.2 | 132.1 ± 6.3 | 123.7 ± 6.6 | 125.3 ± 6.0 | 7.1 ± 4.0 | 1.7 ± 5.7 |
| HbA1c (NGSP) (%) | 5.7 ± 0.09 | 5.7 ± 0.1 | 5.8 ± 0.08 | 5.8 ± 0.1 | −0.03 ± 0.03 | −0.04 ± 0.05 |
| Hematological parameters | ||||||
| WBC (×103 μL−1) | 5.5 ± 0.4 | 5.5 ± 0.4 | 5.7 ± 0.3 | 5.8 ± 0.3 | −0.05 ± 0.3 | 0.08 ± 0.2 |
| RBC (×104 μL−1) | 434.8 ± 8.6 | 445.7 ± 8.2** | 441.5 ± 8.7 | 444.3 ± 8.4 | 10.9 ± 4.2 | 2.8 ± 3.7 |
| Hemoglobin (g dL−1) | 13.65 ± 0.16 | 13.94 ± 0.17** | 13.92 ± 0.31 | 14.00 ± 0.30 | 0.29 ± 0.1 | 0.08 ± 0.1 |
| Hematocrit (%) | 40.66 ± 0.65 | 41.40 ± 0.56* | 40.74 ± 0.8 | 40.76 ± 0.7 | 0.74 ± 0.4 | 0.02± 0.4 |
| Platelet (×104 μL−1) | 20.5 ± 1.3 | 20.8 ± 1.2 | 22.0 ± 1.0 | 22.7 ± 1.1 | 0.3 ± 0.6 | 0.7 ± 0.5 |
| MCV (fL) | 93.67 ± 0.85 | 93.10 ± 0.86** | 92.32 ± 0.56 | 91.82 ± 0.67 | −0.57 ± 0.3 | −0.50 ± 0.3 |
| MCH (pg) | 31.5 ± 0.4 | 31.3 ± 0.4 | 31.5 ± 0.3 | 31.5 ± 0.3 | −0.1 ± 0.1 | 0.0 ± 0.1 |
| MCHC (g dL−1) | 33.6 ± 0.2 | 33.7 ± 0.2 | 34.1 ± 0.2 | 34.3 ± 0.2 | 0.1 ± 0.1 | 0.2 ± 0.1 |
APOE-ε4 alleles are known to be the strongest and most prevalent genetic risk factor for sporadic late-onset Alzheimer's disease, and individuals carrying alleles with the apolipoprotein E-ε4 have an increased risk of developing this condition.31–33 The frequency of different APOE alleles was surveyed in the PO or POPP group. The frequencies of the APOE2/2, APOE2/3, and APOE3/3 genotypes were 18 (85.7%) in the PO group and 15 (65.2%) in the POPP group. The frequencies of the APOE2/4 and APOE3/4 genotypes were 3 (14.3%) in the PO group and 8 (34.8%) in the POPP group. The frequency of the APOE4/4 genotype was 0 (0%) in both groups. These results indicate that there were no significant differences in the distribution of APOE alleles between the two groups (p > 0.05).
There were no significant differences in mean nutritional intake based on BDHQ reports between baseline and 12-month responses in either the PO or POPP group (Table 4), nor in the overall change for either group, indicating that treatment did not influence nutritional intake.
| PO (n = 21) | POPP (n = 23) | Change (12 months-baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POPP | |
| Values are means ± SE. ALA, α-linolenic acid; BDHQ, brief-type self-administered diet history questionnaire; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group. The intake of perilla seed oil in SGCs was not reflected in dietary intake after 12 months of intervention. | ||||||
| Energy (KJ d−1) | 7945 ± 461 | 8280 ± 615 | 7886 ± 518 | 7178 ± 254 | 334 ± 450 | −709 ± 430 |
| Protein (g d−1) | 75.2 ± 5.9 | 79.4 ± 7.4 | 77.1 ± 6.4 | 68.8 ± 3.8 | 4.2 ± 6.8 | −8.4 ± 6.3 |
| Fat (g d−1) | 53.5 ± 4.5 | 56.6 ± 5.1 | 52.9 ± 4.3 | 49.1 ± 3.1 | 3.1 ± 5.3 | −3.8 ± 4.1 |
| Carbohydrate (g d−1) | 254.2 ± 16.1 | 263.6 ± 23.5 | 234.4 ± 16.9 | 218.6 ± 10.1 | 9.4 ± 14.8 | −15.8 ± 15.6 |
| Total dietary fibre (g d−1) | 12.9 ± 1.2 | 13.8 ± 1.6 | 13.1 ± 1.2 | 12.8 ± 1.1 | 0.9 ± 1.3 | −0.3 ± 1.0 |
| Saturated fat (g d−1) | 14.1 ± 1.3 | 14.9 ± 1.4 | 13.9 ± 1.3 | 13.1 ± 1.0 | 0.8 ± 1.5 | −0.7 ± 1.2 |
| Monounsaturated fat (g d−1) | 18.7 ± 1.6 | 19.6 ± 1.9 | 18.3 ± 1.4 | 17.0 ± 1.1 | 0.9 ± 2.0 | −1.3 ± 1.4 |
| Polyunsaturated fat (g d−1) | 13.2 ± 1.0 | 14.3 ± 1.2 | 13.3 ± 1.1 | 12.4 ± 0.8 | 1.2 ± 1.2 | −0.9 ± 1.0 |
| ω6 polyunsaturated fat (g d−1) | 10.2 ± 0.7 | 11.1 ± 0.8 | 10.2 ± 0.8 | 9.8 ± 0.6 | 0.9 ± 0.8 | −0.5 ± 0.7 |
| ω-3 polyunsaturated fat (g d−1) | 3.0 ± 0.3 | 3.2 ± 0.4 | 3.0 ± 0.3 | 2.6 ± 0.2 | 0.2 ± 0.4 | −0.4 ± 0.3 |
| ALA (C18:3 ω-3) (g d−1) | 1.55 ± 0.11 | 1.77 ± 0.15 | 1.56 ± 0.14 | 1.52 ± 0.11 | 0.21 ± 0.14 | −0.04 ± 0.11 |
| EPA (C20:5 ω-3) (g d−1) | 0.42 ± 0.07 | 0.43 ± 0.10 | 0.45 ± 0.08 | 0.34 ± 0.04 | 0.01 ± 0.09 | −0.11 ± 0.10 |
| DHA (C22:6 ω-3) (g d−1) | 0.71 ± 0.11 | 0.70 ± 0.15 | 0.74 ± 0.12 | 0.57 ± 0.06 | −0.01 ± 0.14 | −0.17 ± 0.14 |
Subject characteristics after 12 months of treatment are shown in Table 3. Comparing baseline and 12-month characteristics, serum albumin, blood urea nitrogen, RBC, and haemoglobin levels were significantly elevated, and serum TG, blood sugar, and mean corpuscular volume levels were significantly decreased in the PO group. Similarly, body fat levels were significantly increased in the POPP group at 12 months compared to baseline levels. The remaining parameters were unchanged in both the PO and POPP groups (Table 3). The mean change in serum albumin levels was higher in the POPP group than in the PO group. The mean change in the remaining parameters of anthropometry, blood pressure, and blood biochemical parameters, excluding serum albumin after 12 months of intervention, showed no significant differences between the two groups.
In both groups, some parameters, such as serum albumin and blood sugar, at 12 months showed significant fluctuations compared to before the study, but all of them were within standard values, and it was considered that there were no clinical problems. Taken together, these results indicate that no adverse events were reported in this study, and that long-term intake of either PO or POPP capsules did not alter hepatic function, renal function, lipid metabolism, or haematopoiesis, suggesting the potential safety of consuming PO or POPP capsules for 12 months.
3.2. Cognitive function and mental health assessment
The mean HDS-R, MMSE, and MoCA-J total scores of all subjects (n = 44) at baseline were 28.5 ± 0.3/30, 28.5 ± 0.3/30, and 26.4 ± 0.5/30, respectively. Comparing the cognition index before and at 12 months after the intervention trial, MMSE total scores were significantly increased in the POPP group (p = 0.044), but not in the PO group (Table 5). The total HDS-R and MoCA-J scores showed no significant changes across the 12 months in both the PO and POPP groups.| PO (n = 21) | POPP (n = 23) | Change (12 months – baseline) | ||||
|---|---|---|---|---|---|---|
| Score | Baseline | 12 months | Baseline | 12 months | PO | POPP |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. Significant differences from the PO, ##p < 0.05, 0.05 < #p < 0.1. ACWM, attention, concentration and working memory; HDS-R, Hasegawa's dementia scale-revised; MMSE, Mini-Mental State Examination; MoCA-J, Japanese version of Montreal Cognitive Assessment; PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group. SDS, Self-rating depression scale; SMRT, short-term memory recall task. | ||||||
| Cognitive index | ||||||
| MMSE | ||||||
| Total | 28.7 ± 0.3 | 29.1 ± 0.3 | 28.3 ± 0.4 | 29.2 ± 0.2** | 0.4 ± 0.4 | 0.8 ± 0.4 |
| Subitem “orientation” | 10.0 ± 0.00 | 9.81 ± 0.11 | 9.96 ± 0.04 | 10.0 ± 0.00 | −0.19 ± 0.11 | 0.04 ± 0.04## |
| Subitem “attention and calculation” | 4.43 ± 0.16 | 4.81 ± 0.11* | 4.17 ± 0.20 | 4.65 ± 0.14** | 0.38 ± 0.19 | 0.48 ± 0.18 |
| Subitem “language” | 8.76 ± 0.12 | 8.86 ± 0.08 | 8.78 ± 0.09 | 9.00 ± 0.00** | 0.10 ± 0.12 | 0.22 ± 0.09 |
| HDS-R | ||||||
| Total | 28.5 ± 0.3 | 28.6 ± 0.3 | 28.4 ± 0.4 | 28.8 ± 0.3 | 0.0 ± 0.4 | 0.4 ± 0.4 |
| Subitem “orientation in times” | 4.00 ± 0.00 | 3.86 ± 0.08 | 4.00 ± 0.00 | 4.00 ± 0.00 | −0.14 ± 0.08 | 0.00 ± 0.00# |
| Subitem “serial subtractions” | 1.81 ± 0.09 | 1.91 ± 0.07 | 1.74 ± 0.09 | 1.91 ± 0.06** | 0.10 ± 0.10 | 0.17 ± 0.08 |
| MoCA-J | ||||||
| Total | 26.4 ± 0.6 | 27.0 ± 0.8 | 26.4 ± 0.6 | 26.9 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 0.5 |
| Subscale “SMRT” | 2.8 ± 0.4 | 3.5 ± 0.4* | 3.0 ± 0.4 | 3.1 ± 0.3 | 0.7 ± 0.4 | 0.2 ± 0.3 |
| Subscale “ACWM” | 5.57 ± 0.19 | 5.52 ± 0.18 | 5.39 ± 0.15 | 5.70 ± 0.13** | −0.05 ± 0.20 | 0.30 ± 0.12# |
| Subscale “orientation” | 6.00 ± 0.00 | 5.86 ± 0.08 | 5.96 ± 0.04 | 6.00 ± 0.00 | −0.14 ± 0.08 | 0.04 ± 0.04## |
| Emotional index | ||||||
| SDS | 34.8 ± 1.6 | 32.9 ± 1.2 | 34.7 ± 1.3 | 34.3 ± 1.5 | −1.9 ± 1.1 | −0.3 ± 1.1 |
| Apathy | 10.5 ± 1.2 | 10.0 ± 1.0 | 10.1 ± 1.2 | 8.9 ± 1.1 | −0.5 ± 0.8 | −1.2 ± 0.8 |
When analysing the scores for the MMSE and HDS-R subitems and the MoCA-J subscales, MMSE subitem “attention and calculation” (p = 0.013) and “language” (p = 0.022) scores, the HDS-R subitem “serial subtractions” score (p = 0.043), and the MoCA-J subscale “attention, concentration and working memory” (p = 0.016) score in the POPP group were significantly increased over the 12 months (Table 5). Similarly, the MMSE subitem “attention and calculation” score (p = 0.057) and the MoCA-J subscale “short-term memory recall task” score (p = 0.070) in the PO group tended to increase over the 12 months (Table 5).
The mean changes in the MMSE subitem “orientation” score (p = 0.042) and the MoCA-J subscale “orientation” score (p = 0.049) were significantly greater in the POPP group than in the PO group (Table 5). The mean changes in the HDS-R subitem “orientation in times” score (p = 0.079) and the MoCA-J subscale “attention, concentration, and working memory” score (p = 0.069) tended to be higher in the POPP group than in the PO group. The remaining subitems or subscales for MMSE, HDS-R, and MoCA-J were not significantly different before and after 12 months of intervention trial in both groups. Therefore, the mean change scores of the remaining subitems or subscales of the MMSE, HDS-R, and MoCA-J showed no notable differences between the two groups (Table 5).
There were no noteworthy differences in apathy and SDS scores between baseline and 12 months in either group (Table 5).
3.3 Fatty acid profiles of RBC-PM
Table 6 shows the RBC-PM fatty acid profiles at baseline and after 12 months of intervention. Comparing the fatty acid levels of RBC-PM in the PO group at 0 and 12 months, the ALA, arachidonic acid, EPA, and docosapentaenoic acid levels significantly increased, whereas palmitic acid, lignoceric acid, and nervonic acid levels significantly decreased, but the DHA levels did not change. Similarly, in the POPP group, the ALA, arachidonic acid, docosapentaenoic acid, and DHA levels significantly increased, and the palmitic acid, lignoceric acid, nervonic acid, and ω-6/ω-3 levels significantly decreased. The significant increase in DHA levels in the POPP group, but not in the PO group, may suggest flavonoid-induced DHA synthesis.| PO (n = 21) | POPP (n = 23) | Change (12 months – baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POPP | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05, 0.05 < *p < 0.1. PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group. AA: arachidonic acid; ALA: α-linolenic acid; C24:0: lignoceric acid; C24:1: nervonic acid; DHA: docosahexaenoic acid; DPA: docosapentaenoic acid; EPA: eicosapentaenoic acid; LLA: linoleic acid; OLA: oleic acid; PLA: palmitic acid; STA: stearic acid. | ||||||
| PLA (C16:0) | 24.4 ± 0.88 | 22.8 ± 0.32** | 24.8 ± 0.86 | 22.7 ± 0.24** | −1.6 ± 0.72 | −2.1 ± 0.74 |
| STA (C18:0) | 17.2 ± 0.22 | 16.8 ± 0.26 | 17.6 ± 0.41 | 16.9 ± 0.25 | −0.4 ± 0.35 | −0.7 ± 0.38 |
| OLA (C18:1 ω-9) | 15.1 ± 1.16 | 15.8 ± 0.37 | 17.0 ± 0.52 | 16.2 ± 0.35 | 0.7 ± 1.04 | −0.8 ± 0.39 |
| LLA (C18:2 ω-6) | 13.0 ± 0.56 | 12.3 ± 0.38* | 11.9 ± 0.36 | 12.0 ± 0.31 | −0.8 ± 0.40 | 0.1 ± 0.30 |
| ALA (C18:3 ω-3) | 0.25 ± 0.02 | 0.32 ± 0.01** | 0.23 ± 0.02 | 0.31 ± 0.02** | 0.07 ± 0.02 | 0.08 ± 0.02 |
| AA (C20:4 ω-6) | 11.1 ± 0.89 | 12.8 ± 0.39** | 10.2 ± 0.80 | 12.6 ± 0.23** | 1.7 ± 0.76 | 2.5 ± 0.73 |
| EPA (C20:5 ω-3) | 1.7 ± 0.13 | 2.1 ± 0.14** | 1.7 ± 0.17 | 2.0 ± 0.15* | 0.3 ± 0.15 | 0.1 ± 0.17 |
| DPA (C22:5 ω-3) | 1.5 ± 0.11 | 1.8 ± 0.06** | 1.3 ± 0.13 | 1.7 ± 0.07** | 0.3 ± 0.12 | 0.4 ± 0.12 |
| C24:0 | 4.7 ± 0.11 | 4.2 ± 0.09** | 4.7 ± 0.14 | 4.3 ± 0.09** | −0.5 ± 0.10 | −0.5 ± 0.1 |
| DHA (C22:6 ω-3) | 6.7 ± 0.57 | 7.4 ± 0.25 | 6.1 ± 0.59 | 7.4 ± 0.33** | 0.7 ± 0.54 | 1.3 ± 0.44 |
| C24:1 | 3.8 ± 0.09 | 3.3 ± 0.07** | 3.9 ± 0.10 | 3.4 ± 0.06** | −0.4 ± 0.09 | −0.4 ± 0.10 |
| ω-6/ω-3 | 2.6 ± 0.21 | 2.2 ± 0.10* | 2.8 ± 0.24 | 2.3 ± 0.14** | −0.4 ± 0.21 | −0.5 ± 0.21 |
The mean changes in the RBC-PM fatty acid profiles indicated no significant variations between the two groups (Table 6).
3.4. Serum levels of BDNF and BAP
Table 7 shows serum BDNF and BAP levels in participants. At baseline, there were no noticeable differences in serum levels of BDNF and BAP between both groups. Comparing data at 0 and 12 months, the BDNF levels significantly increased during the intervention trial in the POPP group, but not in the PO group, and the serum BAP levels significantly increased in both groups. The mean changes in the levels of serum BDNF and BAP were not significantly different between the two groups.| PO (n = 21) | POPP (n = 23) | Change (12 months – baseline) | ||||
|---|---|---|---|---|---|---|
| Baseline | 12 months | Baseline | 12 months | PO | POPP | |
| Values are means ± SE. Significant differences from the baseline values, **p < 0.05. BAP, biological antioxidant potential; BDNF, brain-derived neurotrophic factor; PO, perilla seed oil group; POPP, PO and nobiletin-rich air-dried immature ponkan powder group. | ||||||
| BDNF (pg mL−1) | 3652 ± 356 | 3803 ± 453 | 3636 ± 298 | 4304 ± 393** | 151 ± 306 | 668 ± 269 |
| BAP (mmol L−1) | 2443 ± 104 | 2608 ± 118** | 2375 ± 82 | 2584 ± 108** | 164 ± 41 | 209 ± 48 |
3.5. Correlation between cognitive test scores, RBC-PM fatty acid profiles, and serum levels of BDNF and BAP
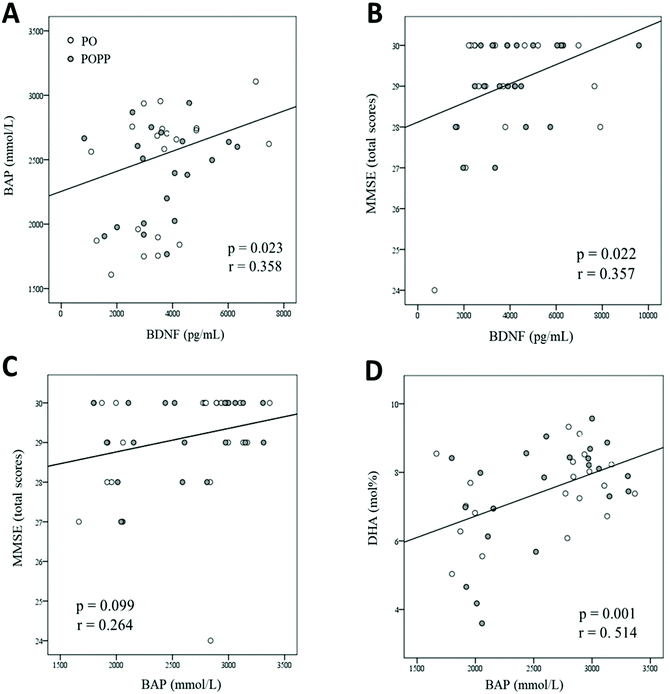
Participants showed a positive (r = 0.358, p = 0.023) baseline correlation between serum BDNF and BAP levels (Fig. 2A).At 12 months, the MMSE total score was positively (r = 0.357, p = 0.022) correlated with serum BDNF levels (Fig. 2B), and tended to be slightly positively (r = 0.264, p = 0.099) correlated with serum BAP levels (Fig. 2C). Furthermore, the MMSE subitem “language” score tended to be positively (r = 0.301, p = 0.056) correlated with serum BDNF levels (data not shown). At 12 months after the intervention trial, serum BAP levels were positively (r = 0.514, p = 0.001) correlated with RBC-PM DHA levels (Fig. 2D).
The mean changes in the HDS-R subitem “orientation in time” scores tended to be positively (r = 0.278, p = 0.078) correlated with serum BDNF levels (data not shown).
Serum BDNF and BAP levels were identified as the significant factors associated positively with each cognitive score, and serum BDNF was a more effective predictor than BAP (Table 8).
| Dependent variable | Independent variable | Standardized coefficient, β | t | p | R 2 |
|---|---|---|---|---|---|
| Adjustment factors were baseline value of cognitive outcomes, biochemical and age, sex, body mass index. BAP, biological antioxidant potential; BDNF, brain-derived neurotrophic factor; HDS-R, Hasegawa's dementia scale-revised; MMSE, Mini-Mental State Examination. | |||||
| MMSE (total) | BDNF | 0.319 | 2.836 | 0.008 | 0.399 |
| BAP | 0.181 | 1.495 | 0.112 | ||
| MMSE (language) | BDNF | 0.322 | 2.577 | 0.021 | 0.316 |
| BAP | −0.06 | −0.622 | 0.583 | ||
| HDS-R (total) | BDNF | 0.392 | 2.586 | 0.014 | 0.369 |
| BAP | 0.19 | 1.456 | 0.151 | ||
| HDS-R (serial subtractions) | BDNF | 0.362 | 2.881 | 0.022 | 0.435 |
| BAP | 0.319 | 2.174 | 0.036 | ||
4. Discussion
In this study, we reported that a 12-month intervention with a combination of PO and ADPP supplementation significantly improved age-related decline in cognitive function in healthy elderly Japanese participants compared to PO supplementation alone. The observed improvement was related to increases in RBC-PM ALA and DHA levels and increases in serum BDNF and BAP levels. Furthermore, our findings indicated that consistent intake of PO or POPP supplementation daily for 12 months at the indicated doses was not associated with any significant harmful effects. Participant compliance was also excellent in regard to consuming 15 SGCs daily for 12 months. To the best of our knowledge, this is the first long-term, double-blind, randomized, controlled trial to evaluate the combined efficacy of ALA-rich PO and nobiletin-rich ADPP on cognitive function in elderly Japanese individuals with age-related cognitive decline.The results of increased levels of ALA in the RBC-PMs in both the PO and POPP groups indicate an effective incorporation of ω-3 PUFAs into the RBC-PMs through ingestion of PO. The results are consistent with our recent investigation, in which we reported that dietary intake of PO (7 mL day−1) for 12 months was associated with an increase in RBC-PM ALA levels, with a concomitant alleviation of age-related cognitive decline in healthy elderly Japanese individuals.9 Similarly, a community-based prospective study in Japan reported that serum ALA levels, but not those of EPA or DHA, were negatively correlated with the risk of dementia.7 ALA exhibits neuroprotective and plasticity-enhancing properties in the brain.34 In this study, the intake of PO-only significantly increased the levels of ALA in the RBC-PMs (Table 6), but did not affect cognitive function in healthy elderly Japanese individuals (Table 5). Consistent with the current findings, Kamalashiran et al. (2019)35 also reported that the intake of PO, along with standard clinical therapy, in patients with mild to moderate dementia did not affect cognitive outcomes. The exact mechanism(s) by which the PO only group in this study, with the same 12 months of intervention as the groups in our previous study, did not show a change in cognitive ability remains to be clarified. However, the discrepancy may relate to the differences in dosage of PO between the two intervention.9 The amount of PO ingested in this study was 1.47 mL daily; the amount of dietary PO intake in our previous two interventional trials was 7 mL daily. The reduced dose in this study was due to two factors. First, the dose of ponkan powder was set based on the amount of nobiletin confirmed as safe and effective.36,37 Second, dosage was limited due to the logistical reasons described in section 2.1. Thus, a lower dose of PO was used in this study to demonstrate the effect of PO alone or with ADPP on cognitive function in the elderly. We believe that the differences in the PO intake caused a difference in the beneficial effects on the brain cognition-related outcomes in the elderly.
ALA can act as a precursor molecule for ω-3 PUFA, EPA, and DHA, which is thought to prevent age-related decline in memory cognition.38 However, conversion ability in humans is low, with only 0.1–21% of ALA converted to EPA and 0.1–0.9% to DHA.14 More importantly, the enzymes (e.g., desaturase and elongase) used to biosynthesize long chain DHA are most highly expressed in the liver as compared to brain.39–41 This corresponds to more than 30-fold higher rates of DHA synthesis in the liver than those in the brain.42 Consequently, DHA synthesis does not contribute significantly to brain DHA homeostasis.43 In this study, both PO and POPP supplementation for 12 months significantly increased levels of RBC-PM ALA and EPA, but not those of DHA (Table 6). These findings agree with those of Hamazaki et al., (2006)44 who showed that ALA-rich PO consumption did not affect DHA levels in human plasma or phospholipid fraction. The increases in ALA and EPA, but not DHA, following PO intervention observed in this study are also consistent with the results of our two recent PO intervention studies8,9 and other ALA intervention studies.45 Barceló-Coblijn et al. (2008)46 also reported that 2.4–3.6 g d−1 ALA intake for 10 weeks increased ALA and EPA, but not DHA, content in RBCs. In contrast, Bemelmans et al. (2002)47 suggested that long-term ALA supplementation for two years increased blood DHA levels, indicating that fatty acid composition of erythrocytes is affected in a dose- and time-dependent manner. Therefore, the lack of increase in DHA in the PO group observed in the present study was likely due to an inadequate ALA dose. In this study, POPP supplementation for 12 months, however, significantly increased DHA levels in the RBC-PM, although there was no significant difference in the mean change of RBC-PM DHA levels between the PO and POPP groups (Table 6). This led us to infer that the polyphenols and flavonoids contained in the ADPP contributed to the enhancement of DHA levels in the POPP group. The speculation is supported by Toufektsian et al., (2011)16 who reported that chronic consumption of the plant-derived flavonoid anthocyanin increased plasma EPA and DHA levels in rats. This was further corroborated by Wu et al. (2017),48 who reported that dietary consumption of ALA with curcumin, a flavonoid polyphenol, enhanced the synthesis of DHA from ALA and related enzymes. Contrarily, Burak et al. (2017)45 showed that the chronic consumption of the flavonoid quercetin did not increase EPA or DHA in serum phospholipids and erythrocytes, suggesting that quercetin did does affect the conversion of ALA to EPA and DHA.
In this intervention trial, the mean changes in the MMSE subitem “orientation” score and the MoCA-J subscale “orientation” score from baseline measurements to month 12 were notably greater in the POPP group than in the PO group (Table 5). This indicates a greater beneficial effect of POPP supplementation over that of PO only on age-related cognitive decline. Apart from anthocyanin, curcumin, and quercetin, nobiletin, an O-methylated flavonoid (methoxy flavonoid), isolated from citrus peels, has also been reported in a multicentre, randomized, double-blind, placebo-controlled study to have beneficial effects for improving memory dysfunction in healthy elderly subjects.49 One clinical study found that nobiletin-rich citrus peel extract reduced cognitive impairment in Alzheimer's disease patients on donepezil therapy.36 In numerous animal models of dementia, nobiletin has also been shown to ameliorate cognitive impairment by exhibiting neuroprotective effects against neuroinflammation and oxidative stress.18 Interestingly, nobiletin has been shown to augment hippocampal aminomethylphosphonic acid receptor-mediated synaptic transmission, improving in memory cognition.50 Moreover, nobiletin and its bioactive metabolites can easily penetrate the blood–brain barrier,51 and are thought to be a promising phytomedicine against neurodegenerative and age-related cognitive decline.17,18 Cognitive dysfunction is crucially correlated with oxidative damage, and reinforcement of antioxidant ability could defend cognitive functions in the elderly.52 Moreover, nobiletin regulates gene expression associated with oxidative and endoplasmic reticulum stress, as well as protein activities related to synaptic plasticity and memory formation. In this study, serum BAP levels, which represent total antioxidant capacity53 increased by a greater extent in the POPP group than in the PO group (P < 0.05; Table 7).
Antioxidant capacity is highly correlated with the tissue DHA level.54,55 In this study, we observed that POPP, but not PO supplementation alone, for 12 months significantly increased RBC-PM DHA levels (Table 6), and that the serum BAP levels were positively correlated with the RBC-PM DHA levels (Fig. 2D), reinforcing the association between RBC-PM DHA and serum BAP levels in the elderly. Given this evidence, it is plausible that the cognitive amelioration seen in the POPP group may be due, at least in part, to the effects of nobiletin. Although the significant ingredient of ADPP is unclear, nobiletin might is a likely candidate for the improved cognitive improvement of the POPP subjects. In addition to nobiletin, flavonoid diglycosides, such as hesperidin and narirutin, contained in the ADPP (Table 2) may contribute to the increased cognitive ability of the POPP group. Flavanones can cross the blood–brain barrier, localize in the brain,56 and exert anti-inflammatory and antioxidative activities57 in the hippocampus, striatum, and frontal cortex.58 Further research is needed to clarify the mechanisms underlying the potential effects of POPP on the conversion of ALA to DHA, and on cognitive improvement.
Regardless of mechanism for increased DHA levels (either from ALA or indirectly by flavonoid induction), elevated DHA levels contribute to cognitive abilities. DHA is generally decreased in the brain and RBC-PM of the elderly and in patients with Alzheimer's disease.59 Epidemiological and animal studies have reported that DHA may be useful for preventing dementia.10,11 Our previous intervention trials have reported that DHA-enriched food12,28 and beverage intake60 prevents age-related decline in cognitive function in healthy elderly Japanese people,12 and these observations were associated with increases in RBC-PM DHA levels. Therefore, an increase in DHA levels and/or DHA synthesis by the intake of POPP supplementation may have contributed to the improvement of cognitive function in the POPP group.
Epidemiological studies have reported that high serum TG levels are associated with decline in memory function in a middle-aged population,61 and with MCI in an older population.62 Animal studies have also shown a similar effect of hypertriglyceridemia on cognitive impairment.63,64 In this study, serum TG levels significantly decreased in the PO group, but not in the POPP group after 12 months of treatment (Table 3). These findings correspond with a previous report by Egert et al., (2009)65 who found that fasting serum TG concentrations in normolipidemic individuals significantly decreased in ALA intervention trials. These results suggest that the lowering effects on serum TG levels by PO intervention may prevent cognitive impairment in elderly people with MCI.
Nobiletin-rich ADPP intake did not affect the lipid profiles or haematological parameters after intervention in the POPP group compared to those in the PO group (Table 3). It remains unclear why TG levels decreased only in the PO group. Although the effects of PO66–68 and/or polyphenols on TG levels in animals69 are well known, their effects on human TG levels are yet to be clarified. In humans, polyphenols have been paradoxically reported both to reduce and have no effects on TG levels.70 Similarly, vegetable oils containing ω3-PUFAs have been reported to reduce TG levels in hyper-triglyceridemic individuals, and to either reduce or have no effects on TG levels in normo-triglyceridemic subjects71 This indicates that the effects of ω3-PUFAs on TG metabolism may be inconsistent. We speculate that the hypo-triglyceridemic effect observed in this study was largely influenced by baseline TG levels, which were 23% higher in the PO group than in the POPP group. In order to reduce plasma TG levels, the unsaturated fatty acids in the PO and/or the polyphenols in the ADPP must have the ability to inhibit TG absorption, hepatic synthesis, and secretion. This could occur through the VLDL to LDL dilapidation cascade, increasing energy expenditure from TG, and leading to its excretion through biliary paths. In the present study, it remains unknown what stages of TG metabolism were affected by the ω3-PUFAs in PO to reduce serum TG levels in the PO group. Similarly, it is not known whether this process was interrupted by ADPP polyphenols in the POPP group. Additionally, the reason why the POPP group did not exhibit haematological changes remains to be explained in future investigations.
We evaluated the effects of PO and POPP supplementation on the serum levels of BDNF, a key molecule involved in plastic changes related to learning, memory, and cognition. Although several studies have reported a positive correlation between serum BDNF levels and cognitive abilities,72,73 other did not find an association.74,75 This discrepancy led us to examine the correlation between BDNF and MMSE scores, which were significantly correlated after 12 months of intervention. Total MMSE score was positively correlated with serum BDNF levels (Fig. 2), and the score of MMSE subitem “language” tended to be positively correlated with BDNF levels (p = 0.056). Moreover, a significant correlation of serum BDNF as the independent variable persisted in a multiple regression analysis with cognitive outcomes as the dependent variable (Table 8). These results suggest an association between serum BDNF levels and cognitive function in the Japanese elderly. While other confounding factors may act on this correlation, it is important to note that this relationship was observed only in the POPP group and not in the PO group. Therefore, we believe that DHA levels contribute to the relationship between BDNF and cognition. Oral administration of ω-3 PUFAs, including DHA and EPA, increased serum BDNF levels concurrently with serum DHA levels in an amyloid-β-treated Alzheimer's disease rat model.76 These results suggest that the relationship between mean change in DHA levels (ΔDHA) and serum BDNF after 12 months of intervention trials contributed to the cognitive improvements observed. BDNF-induced increases in neural spine density, synaptogenesis, and thereby synaptic function,77,78 and could potentially account for the improvement of cognitive scores after prolonged intake of ADPP. However, why PO alone had no impact on serum BDNF levels in remains to be clarified.
We have recently reported that 7 mL of daily dietary PO intake for 12 months has the potential to improve mental conditions, such as depression and apathy in Japanese adults8 and the healthy elderly.9 However, contrary to these results, we observed in this study that both the apathy and SDS scores in both groups remained unchanged during the 12-month intervention trial (Table 5). It is possible that the difference in PO dosage reduced the beneficial effects on brain function in the elderly. However, further studies are required to clarify these differences.
This study had several limitations. First, the participants were healthy elderly people without cognitive impairments due to physical or psychological illness, so observed impairments in cognition and mental health were mostly age-related. Therefore, there was no significant difference in total test scores for cognitive function and mental health between the PO and POPP groups. Future research is needed to study the impact of consuming SGCs containing either PO or PO with nobiletin-rich ADPP on participants with cognitive dysfunction and mental illness, including neurodegenerative disorders. Second, the conversion efficiency of ALA to EPA and DHA in humans is much lower than that in rodents, and thus, to accurately elucidate the mechanism of ALA action, it is necessary to determine whether it is an action by ALA alone or an action via EPA and DHA. This distinction cannot be clarified in human intervention studies. Third, the sample size in this study was relatively small, with subjects from a localized population, which limits the generalizability of our present results. Fourth, we had no information of the subjects other than APOE status, and did not pursue follow-up after the end of the trial. Fifth, we could not add two control groups, a placebo group and a group taking nobiletin-rich ADPP alone, in this intervention trial, since participants in this study were volunteers who did not want to take daily 15 SGCs that had no and/or unknown effects. As a result, the power of this intervention trial was weaker than that in a case with all four experimental groups. Further intervention trials on these four groups are needed in the future.
5. Conclusions
Taking the PO combined with nobiletin-rich ADPP supplement was clinically safe, and improved cognitive function in healthy elderly Japanese individuals, presumably due to the increased BDNF associated with increased ω-3 PUFAs in the brain. Although further research is needed to clarify the mechanisms underlying the beneficial effects of PO and PO combined with nobiletin-rich ADPP on cognitive function, these effective and easy-to-consume dietary supplements may be a feasible and accessible option for maintaining brain health in the elderly.Author contributions
Michio Hashimoto: Conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, supervision, visualization, writing-original draft, writing-review & editing. Kentaro Matsuzaki: Data curation, investigation, software, formal analysis, visualization, writing-original draft, writing-review & editing. Koji Maruyama: Conceptualization, project administration, funding acquisition, validation. Shahdat Hossain: Validation, writing-original draft, visualization, writing-review & editing. Eri Sumiyoshi: Validation, visualization, writing-review & editing. Harumi Wakatsuki, Data curation, formal analysis, investigation, methodology. Setsushi Kato: methodology, resources, supervision, validation. Miho Ohno: investigation, methodology, resources. Yoko Tanabe: Investigation. Yoko Kuroda: Investigation, methodology. Shuhei Yamaguchi: Supervision. Koji Kajima: Conceptualization, funding acquisition, supervision. Yasushi Ohizumi: Conceptualization, project administration, funding acquisition, writing-review & editing, supervision. Osamu Shido: Supervision, validation.Conflicts of interest
Koji Maruyama and Koji Kajima, employees of Sankyo Holdings Co., Ltd, contributed to the experiments. Other authors have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was partly supported by the grant from Sankyo Holdings Co., Ltd (Shimane University Project code: E1D28018). We sincerely thank the participants of the study. We would also like to thank Editage (http://www.editage.com) for English language editing.References
- H. M. Ahmed, Ethnomedicinal, Phytochemical and Pharmacological Investigations of Perilla frutescens (L.) Britt, Molecules, 2018, 24, 102 CrossRef PubMed.
- H. Campos, A. Baylin and W. C. Willett, Alpha-linolenic acid and risk of nonfatal acute myocardial infarction, Circulation, 2008, 118, 339–345 CrossRef CAS PubMed.
- C. M. Albert, K. Oh, W. Whang, J. E. Manson, C. U. Chae, M. J. Stampfer, W. C. Willett and F. B. Hu, Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease, Circulation, 2005, 112, 3232–3238 CrossRef CAS PubMed.
- G. Zhao, T. D. Etherton, K. R. Martin, P. J. Gillies, S. G. West and P. M. Kris-Etherton, Dietary alpha-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects, Am. J. Clin. Nutr., 2007, 85, 385–391 CrossRef CAS PubMed.
- C. Nguemeni, B. Delplanque, C. Rovère, N. Simon-Rousseau, C. Gandin, G. Agnani, J. L. Nahon, C. Heurteaux and N. Blondeau, Dietary supplementation of alpha-linolenic acid in an enriched rapeseed oil diet protects from stroke, Pharmacol. Res., 2010, 61, 226–233 CrossRef CAS PubMed.
- J. Lee, S. Park, J. Y. Lee, Y. K. Yeo, J. S. Kim and J. Lim, Improved spatial learning and memory by perilla diet is correlated with immunoreactivities to neurofilament and α-synuclein in hilus of dentate gyrus, Proteome Sci., 2012, 10, 72 CrossRef CAS PubMed.
- K. Yamagishi, A. Ikeda, C. L. Chei, H. Noda, M. Umesawa, R. Cui, I. Muraki, T. Ohira, H. Imano, T. Sankai, T. Okada, T. Tanigawa, A. Kitamura, M. Kiyama and H. Iso, Serum α-linolenic and other ω-3 fatty acids, and risk of disabling dementia: Community-based nested case-control study, Clin. Nutr., 2017, 36, 793–797 CrossRef CAS PubMed.
- M. Hashimoto, K. Matsuzaki, S. Kato, S. Hossain, M. Ohno and O. Shido, Twelve-month Studies on Perilla Oil Intake in Japanese Adults-Possible Supplement for Mental Health, Foods, 2020, 9, 530 CrossRef CAS PubMed.
- M. Hashimoto, K. Matsuzaki, S. Hossain, T. Ito, H. Wakatsuki, Y. Tanabe, M. Ohno, S. Kato, K. Yamashita and O. Shido, Perilla Seed Oil Enhances Cognitive Function and Mental Health in Healthy Elderly Japanese Individuals by Enhancing the Biological Antioxidant Potential, Foods, 2021, 10, 1130 CrossRef CAS PubMed.
- M. Hashimoto, S. Hossain, A. Al Mamun, K. Matsuzaki and H. Arai, Docosahexaenoic acid: one molecule diverse functions, Crit. Rev. Biotechnol., 2017, 37, 579–597 CrossRef CAS PubMed.
- G. Y. Sun, A. Simonyi, K. L. Fritsche, D. Y. Chuang, M. Hannink, Z. Gu, C. M. Greenlief, J. K. Yao, J. C. Lee and D. Q. Beversdorf, Docosahexaenoic acid (DHA): An essential nutrient and a nutraceutical for brain health and diseases, Prostaglandins, Leukotrienes Essent. Fatty Acids, 2018, 136, 3–13 CrossRef CAS PubMed.
- M. Hashimoto, S. Kato, Y. Tanabe, M. Katakura, A. A. Mamun, M. Ohno, S. Hossain, K. Onoda, S. Yamaguchi and O. Shido, Beneficial effects of dietary docosahexaenoic acid intervention on cognitive function and mental health of the oldest elderly in Japanese care facilities and nursing homes, Geriatr. Gerontol. Int., 2017, 17, 330–337 CrossRef PubMed.
- M. Hashimoto, K. Yamashita, S. Kato, T. Tamai, Y. Tanabe, M. Mitarai, I. Matsumoto and M. Ohno, Beneficial effects of daily dietary omega-3 polyunsaturated fatty acid supplementation on age-related cognitive decline in elderly Japanese with very mild dementia: A 2-year randomized, double-blind, placebo-controlled trial, J. Aging Res. Clin. Pract., 2012, 1, 193–201 Search PubMed.
- G. Burdge, Alpha-linolenic acid metabolism in men and women: nutritional and biological implications, Curr. Opin. Clin. Nutr. Metab. Care, 2004, 7, 137–144 CrossRef CAS PubMed.
- M. de Lorgeril, P. Salen, J. L. Martin, F. Boucher and J. de Leiris, Interactions of wine drinking with omega-3 fatty acids in patients with coronary heart disease: a fish-like effect of moderate wine drinking, Am. Heart J., 2008, 155, 175–181 CrossRef CAS PubMed.
- M. C. Toufektsian, P. Salen, F. Laporte, C. Tonelli and M. de Lorgeril, Dietary flavonoids increase plasma very long-chain (n-3) fatty acids in rats, J. Nutr., 2011, 141, 37–41 CrossRef CAS PubMed.
- Y. Ohizumi, A new strategy for preventive and functional therapeutic methods for dementia–approach using natural products, Yakugaku Zasshi, 2015, 135, 449–464 CrossRef CAS PubMed.
- A. Nakajima and Y. Ohizumi, Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer's Disease and Parkinson's Disease, Int. J. Mol. Sci., 2019, 20, 3380 CrossRef CAS PubMed.
- K. Matsuzaki and Y. Ohizumi, Beneficial Effects of Citrus-Derived Polymethoxylated Flavones for Central Nervous System Disorders, Nutrients, 2021, 13, 145 CrossRef CAS PubMed.
- E. Sumiyoshi, K. Matsuzaki, N. Sugimoto, Y. Tanabe, T. Hara, M. Katakura, M. Miyamoto, S. Mishima and O. Shido, Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial, Nutrients, 2019, 11, 2800 CrossRef CAS PubMed.
- M. Hashimoto, Y. Tanabe, S. Hossain, K. Matsuzaki, M. Ohno, S. Kato, M. Katakura and O. Shido, Intake of Alpha-Linolenic Acid-Rich Perilla frutescens Leaf Powder Decreases Home Blood Pressure and Serum Oxidized Low-Density Lipoprotein in Japanese Adults, Molecules, 2020, 25, 2099 CrossRef CAS PubMed.
- T. Ichinose, K. Matsuzaki, M. Kato, Y. Tanabe, N. Tachibana, M. Morikawa, S. Kato, S. Ohata, M. Ohno, H. Wakatsuki, S. Hossain, O. Shido and M. Hashimoto, Intake of Docosahexaenoic Acid-Enriched Milk Beverage Prevents Age-Related Cognitive Decline and Decreases Serum Bone Resorption Marker Levels, J. Oleo Sci., 2021, 70, 1829–1838 CrossRef CAS PubMed.
- S. Kobayashi, K. Murakami, S. Sasaki, H. Okubo, N. Hirota, A. Notsu, M. Fukui and C. Date, Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults, Public Health Nutr., 2011, 14, 1200–1211 CrossRef PubMed.
- Y. Imai and K. Hasegawa, The revised Hasegawa's dementia scale (HDS-R)---Evaluation of its usefulness as a screening test for dementia, J. Hong Kong Coll. Psychiatr., 1994, 4, 20–24 Search PubMed.
- M. F. Folstein, S. E. Folstein and P. R. McHugh, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res., 1975, 12, 189–198 CrossRef CAS PubMed.
- Y. Fujiwara, H. Suzuki, M. Yasunaga, M. Sugiyama, M. Ijuin, N. Sakuma, H. Inagaki, H. Iwasa, C. Ura, N. Yatomi, K. Ishii, A. M. Tokumaru, A. Homma, Z. Nasreddine and S. Shinkai, Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment, Geriatr. Gerontol. Int., 2010, 10, 225–232 CrossRef PubMed.
- K. Okada, S. Kobayashi, K. Aoki, N. Suyama and S. Yamagata, Assessment of moti-vational loss in poststroke patients using the Japanese version of Starkstein's Apathy Scale (in Japanese), Jpn. J. Stroke, 1998, 20, 318–327 CrossRef.
- W. W. Zung, A self-rating depression scale, Arch. Gen. Psychiatry, 1965, 12, 63–70 CrossRef CAS PubMed.
- I. F. Benzie and J. J. Strain, The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay, Anal. Biochem., 1996, 239, 70–76 CrossRef CAS PubMed.
- M. Hashimoto, K. Shinozuka, S. Gamoh, Y. Tanabe, M. S. Hossain, Y. M. Kwon, N. Hata, Y. Misawa, M. Kunitomo and S. Masumura, The hypotensive effect of docosahexaenoic acid is associated with the enhanced release of ATP from the caudal artery of aged rats, J. Nutr., 1999, 129, 70–76 CrossRef CAS PubMed.
- W. J. Strittmatter, A. M. Saunders, D. Schmechel, M. Pericak-Vance, J. Enghild, G. S. Salvesen and A. D. Roses, Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease, Proc. Natl. Acad. Sci. U. S. A., 1993, 90, 1977–1981 CrossRef CAS PubMed.
- A. Kurz, K. Altland, N. Lautenschlager, R. Zimmer, R. Busch, I. Gerundt, H. Lauter and U. Müller, Apolipoprotein E type 4 allele and Alzheimer's disease: effect on age at onset and relative risk in different age groups, J. Neurol., 1996, 243, 452–456 CrossRef CAS PubMed.
- C. C. Liu, T. Kanekiyo, H. Xu and G. Bu, Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy, Nat. Rev. Neurol., 2013, 9, 106–118 CrossRef CAS PubMed.
- N. Blondeau, C. Nguemeni, D. N. Debruyne, M. Piens, X. Wu, H. Pan, X. Hu, C. Gandin, R. H. Lipsky, J. C. Plumier, A. M. Marini and C. Heurteaux, Subchronic alpha-linolenic acid treatment enhances brain plasticity and exerts an antidepressant effect: a versatile potential therapy for stroke, Neuropsychopharmacology, 2009, 34, 2548–2559 CrossRef CAS PubMed.
- C. Kamalashiran, K. Sriyakul, J. Pattaraarchachai and S. Muengtaweepongsa, Outcomes of Perilla Seed Oil as an Additional Neuroprotective Therapy in Patients with Mild to Moderate Dementia: A Randomized Control Trial, Curr. Alzheimer Res., 2019, 16, 146–155 CrossRef CAS PubMed.
- T. Seki, T. Kamiya, K. Furukawa, M. Azumi, S. Ishizuka, S. Takayama, S. Nagase, H. Arai, T. Yamakuni and N. Yaegashi, Nobiletin-rich Citrus reticulata peels, a kampo medicine for Alzheimer's disease: a case series, Geriatr. Gerontol. Int., 2013, 13, 236–238 CrossRef PubMed.
- H. Endo, Development of fundamental technology for analysis and evaluation of functional agricultural products and functional foods. 18: Research on medical safety and usefulness of nobiletin for dementia, Res. Rep. Ministry Agric., Forestry Fisheries, 2015, 529, 337–379 Search PubMed . (in Japanese).
- M. Kouba and J. Mourot, A review of nutritional effects on fat composition of animal products with special emphasis on n-3 polyunsaturated fatty acids, Biochimie, 2011, 93, 13–17 CrossRef CAS PubMed.
- H. P. Cho, M. Nakamura and S. D. Clarke, Cloning, expression, and fatty acid regulation of the human delta-5 desaturase, J. Biol. Chem., 1999, 274, 37335–37339 CrossRef CAS PubMed.
- H. P. Cho, M. T. Nakamura and S. D. Clarke, Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase, J. Biol. Chem., 1999, 274, 471–477 CrossRef CAS PubMed.
- A. E. Leonard, B. Kelder, E. G. Bobik, L. T. Chuang, C. J. Lewis, J. J. Kopchick, P. Mukerji and Y. S. Huang, Identification and expression of mammalian long-chain PUFA elongation enzymes, Lipids, 2002, 37, 733–740 CrossRef CAS PubMed.
- M. Igarashi, K. Ma, L. Chang, J. M. Bell and S. I. Rapoport, Dietary n-3 PUFA deprivation for 15 weeks upregulates elongase and desaturase expression in rat liver but not brain, J. Lipid Res., 2007, 48, 2463–2470 CrossRef CAS PubMed.
- M. Igarashi, J. C. DeMar, Jr., K. Ma, L. Chang, J. M. Bell and S. I. Rapoport, Docosahexaenoic acid synthesis from alpha-linolenic acid by rat brain is unaffected by dietary n-3 PUFA deprivation, J. Lipid Res., 2007, 48, 1150–1158 CrossRef PubMed.
- K. Hamazaki, M. Itomura, T. Hamazaki and S. Sawazaki, Effects of cooking plant oils on recurrent aphthous stomatitis: a randomized, placebo-controlled, double-blind trial, Nutrition, 2006, 22, 534–538 CrossRef PubMed.
- C. Burak, S. Wolffram, B. Zur, P. Langguth, R. Fimmers, B. Alteheld, P. Stehle and S. Egert, Effects of the flavonol quercetin and α-linolenic acid on n-3 PUFA status in metabolically healthy men and women: a randomised, double-blinded, placebo-controlled, crossover trial, Br. J. Nutr., 2017, 117, 698–711 CrossRef CAS PubMed.
- G. Barceló-Coblijn, E. J. Murphy, R. Othman, M. H. Moghadasian, T. Kashour and J. K. Friel, Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid, Am. J. Clin. Nutr., 2008, 88, 801–809 CrossRef PubMed.
- W. J. Bemelmans, J. Broer, E. J. Feskens, A. J. Smit, F. A. Muskiet, J. D. Lefrandt, V. J. Bom, J. F. May and B. Meyboom-de Jong, Effect of an increased intake of alpha-linolenic acid and group nutritional education on cardiovascular risk factors: the Mediterranean Alpha-linolenic Enriched Groningen Dietary Intervention (MARGARIN) study, Am. J. Clin. Nutr., 2002, 75, 221–227 CrossRef CAS PubMed.
- A. Wu, E. E. Noble, E. Tyagi, Z. Ying, Y. Zhuang and F. Gomez-Pinilla, Curcumin boosts DHA in the brain: Implications for the prevention of anxiety disorders, Biochim. Biophys. Acta, 2015, 1852, 951–961 CrossRef CAS PubMed.
- S. Yamada, M. Shirai, K. Ono, T. Teruya, A. Yamano and J. T. Woo, Beneficial effects of a nobiletin-rich formulated supplement of Sikwasa (C. depressa) peel on cognitive function in elderly Japanese subjects; A multicenter, randomized, double-blind, placebo-controlled study, Food Sci. Nutr., 2021, 9, 6844–6853 CrossRef PubMed.
- K. Matsuzaki, K. Miyazaki, S. Sakai, H. Yawo, N. Nakata, S. Moriguchi, K. Fukunaga, A. Yokosuka, Y. Sashida, Y. Mimaki, T. Yamakuni and Y. Ohizumi, Nobiletin, a citrus flavonoid with neurotrophic action, augments protein kinase A-mediated phosphorylation of the AMPA receptor subunit, GluR1, and the postsynaptic receptor response to glutamate in murine hippocampus, Eur. J. Pharmacol., 2008, 578, 194–200 CrossRef CAS PubMed.
- D. Saigusa, M. Shibuya, D. Jinno, H. Yamakoshi, Y. Iwabuchi, A. Yokosuka, Y. Mimaki, A. Naganuma, Y. Ohizumi, Y. Tomioka and T. Yamakuni, High-performance liquid chromatography with photodiode array detection for determination of nobiletin content in the brain and serum of mice administrated the natural compound, Anal. Bioanal. Chem., 2011, 400, 3635–3641 CrossRef CAS PubMed.
- N. T. Akbaraly, H. Faure, V. Gourlet, A. Favier and C. Berr, Plasma carotenoid levels and cognitive performance in an elderly population: results of the EVA Study, J. Gerontol., Ser. A, 2007, 62, 308–316 CrossRef PubMed.
- E. H. Jansen and T. Ruskovska, Comparative Analysis of Serum (Anti)oxidative Status Parameters in Healthy Persons, Int. J. Mol. Sci., 2013, 14, 6106–6115 CrossRef CAS PubMed.
- M. S. Hossain, M. Hashimoto, S. Gamoh and S. Masumura, Antioxidative effects of docosahexaenoic acid in the cerebrum versus cerebellum and brainstem of aged hypercholesterolemic rats, J. Neurochem., 1999, 72, 1133–1138 CrossRef CAS PubMed.
- M. Hashimoto, S. Hossain, T. Shimada, K. Sugioka, H. Yamasaki, Y. Fujii, Y. Ishibashi, J. Oka and O. Shido, Docosahexaenoic acid provides protection from impairment of learning ability in Alzheimer's disease model rats, J. Neurochem., 2002, 81, 1084–1091 CrossRef CAS PubMed.
- K. A. Youdim, M. S. Dobbie, G. Kuhnle, A. R. Proteggente, N. J. Abbott and C. Rice-Evans, Interaction between flavonoids and the blood-brain barrier: in vitro studies, J. Neurochem., 2003, 85, 180–192 CrossRef CAS PubMed.
- P. Kumar and A. Kumar, Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington's like symptoms in rats: possible role of nitric oxide, Behav. Brain Res., 2010, 206, 38–46 CrossRef CAS PubMed.
- E. T. Menze, M. G. Tadros, A. M. Abdel-Tawab and A. E. Khalifa, Potential neuroprotective effects of hesperidin on 3-nitropropionic acid-induced neurotoxicity in rats, Neurotoxicology, 2012, 33, 1265–1275 CrossRef CAS PubMed.
- M. Söderberg, C. Edlund, K. Kristensson and G. Dallner, Fatty acid composition of brain phospholipids in aging and in Alzheimer's disease, Lipids, 1991, 26, 421–425 CrossRef PubMed.
- T. Ichinose, M. Kato, K. Matsuzaki, Y. Tanabe, N. Tachibana, M. Morikawa, S. Kato, S. Ohata, M. Ohno, H. Wakatsuki, S. Hossain, O. Shido and M. Hashimoto, Beneficial effects of docosahexaenoic acid-enriched milk beverage intake on cognitive function in healthy elderly Japanese: A 12-month randomized, double-blind, placebo-controlled trial, J. Funct. Foods, 2020, 74, 8 Search PubMed.
- E. C. Leritz, R. E. McGlinchey, D. H. Salat and W. P. Milberg, Elevated levels of serum cholesterol are associated with better performance on tasks of episodic memory, Metab. Brain Dis., 2016, 31, 465–473 CrossRef CAS PubMed.
- Q. Yao, G. X. Jiang, Z. M. Zhou, J. M. Chen and Q. Cheng, Metabolic Syndrome and Mild Cognitive Impairment: A Case-Control Study among Elderly in a Shanghai Suburb, J. Alzheimers Dis., 2016, 51, 1175–1182 CAS.
- S. Sharma, R. Taliyan and S. Ramagiri, Histone deacetylase inhibitor, trichostatin A, improves learning and memory in high-fat diet-induced cognitive deficits in mice, J. Mol. Neurosci., 2015, 56, 1–11 CrossRef CAS PubMed.
- S. Sharma and R. Taliyan, Epigenetic modifications by inhibiting histone deacetylases reverse memory impairment in insulin resistance induced cognitive deficit in mice, Neuropharmacology, 2016, 105, 285–297 CrossRef CAS PubMed.
- S. Egert, F. Kannenberg, V. Somoza, H. F. Erbersdobler and U. Wahrburg, Dietary alpha-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans, J. Nutr., 2009, 139, 861–868 CrossRef CAS PubMed.
- F. Wang, H. Zhu, M. Hu, J. Wang, H. Xia, X. Yang, L. Yang and G. Sun, Perilla Oil Supplementation Improves Hypertriglyceridemia and Gut Dysbiosis in Diabetic KKAy Mice, Mol. Nutr. Food Res., 2018, 62, e1800299 CrossRef PubMed.
- H. H. Chang, C. S. Chen and J. Y. Lin, Dietary perilla oil lowers serum lipids and ovalbumin-specific IgG1, but increases total IgE levels in ovalbumin-challenged mice, Food Chem. Toxicol., 2009, 47, 848–854 CrossRef CAS PubMed.
- H. K. Kim, S. Choi and H. Choi, Suppression of hepatic fatty acid synthase by feeding alpha-linolenic acid rich perilla oil lowers plasma triacylglycerol level in rats, J. Nutr. Biochem., 2004, 15, 485–492 CrossRef CAS PubMed.
- S. Mineo, A. Noguchi, Y. Nagakura, K. Kobori, T. Ohta, E. Sakaguchi and T. Ichiyanagi, Boysenberry Polyphenols Suppressed Elevation of Plasma Triglyceride Levels in Rats, J. Nutr. Sci. Vitaminol., 2015, 61, 306–312 CrossRef CAS PubMed.
- C. Bladé, L. Arola and M. J. Salvadó, Hypolipidemic effects of proanthocyanidins and their underlying biochemical and molecular mechanisms, Mol. Nutr. Food Res., 2010, 54, 37–59 CrossRef PubMed.
- S. H. Goodnight, Jr., W. S. Harris, W. E. Connor and D. R. Illingworth, Polyunsaturated fatty acids, hyperlipidemia, and thrombosis, Arteriosclerosis, 1982, 2, 87–113 Search PubMed.
- F. Angelucci, G. Spalletta, F. di Iulio, A. Ciaramella, F. Salani, L. Colantoni, A. E. Varsi, W. Gianni, G. Sancesario, C. Caltagirone and P. Bossù, Alzheimer's disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels, Curr. Alzheimer Res., 2010, 7, 15–20 CrossRef CAS PubMed.
- J. Nettiksimmons, E. M. Simonsick, T. Harris, S. Satterfield, C. Rosano and K. Yaffe, The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: a longitudinal study, PLoS One, 2014, 9, e91339 CrossRef PubMed.
- P. A. Lapchak, D. M. Araujo, K. D. Beck, C. E. Finch, S. A. Johnson and F. Hefti, BDNF and trkB mRNA expression in the hippocampal formation of aging rats, Neurobiol. Aging, 1993, 14, 121–126 CrossRef CAS PubMed.
- I. Driscoll, B. Martin, Y. An, S. Maudsley, L. Ferrucci, M. P. Mattson and S. M. Resnick, Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults, PLoS One, 2012, 7, e35217 CrossRef CAS PubMed.
- M. Hashimoto, R. Tozawa, M. Katakura, H. Shahdat, A. M. Haque, Y. Tanabe, S. Gamoh and O. Shido, Protective effects of prescription n-3 fatty acids against impairment of spatial cognitive learning ability in amyloid β-infused rats, Food Funct., 2011, 2, 386–394 RSC.
- R. J. Tolwani, P. S. Buckmaster, S. Varma, J. M. Cosgaya, Y. Wu, C. Suri and E. M. Shooter, BDNF overexpression increases dendrite complexity in hippocampal dentate gyrus, Neuroscience, 2002, 114, 795–805 CrossRef CAS PubMed.
- T. Numakawa and H. Odaka, Brain-Derived Neurotrophic Factor Signaling in the Pathophysiology of Alzheimer's Disease: Beneficial Effects of Flavonoids for Neuroprotection, Int. J. Mol. Sci., 2021, 22, 5719 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo03508h |
| This journal is © The Royal Society of Chemistry 2022 |