Open Access Article
Open Access ArticleBiological properties and therapeutic applications of garlic and its components
Lucía
Melguizo-Rodríguez
ab,
Enrique
García-Recio
bc,
Concepción
Ruiz
 abd,
Elvira
De Luna-Bertos
ab,
Rebeca
Illescas-Montes
abd,
Elvira
De Luna-Bertos
ab,
Rebeca
Illescas-Montes
 *ab and
Víctor J.
Costela-Ruiz
be
*ab and
Víctor J.
Costela-Ruiz
be
aBiomedical Group (BIO277), Department of Nursing, Faculty of Health Sciences, University of Granada, Avda. Ilustración 60, 18016, Granada, Spain
bInstitute of Biosanitary Research, Ibs.Granada, C/Doctor Azpitarte 4, 4a Planta, 18012, Granada, Spain
cBiomedical Group (BIO277), Department of Nursing, Faculty of Health Sciences of Melilla, University of Granada, C/Santander, 1, 52005, Melilla, Spain
dInstitute of Neuroscience Federico Olóriz, University of Granada, Centro de Investigación Biomédica (CIBM), Parque de Tecnológico de La Salud (PTS), Granada, Spain
eBiomedical Group (BIO277), Department of Nursing, Faculty of Health Sciences of Ceuta, University of Granada, C/Cortadura del Valle, Sn, 51001 Ceuta, Spain
First published on 15th February 2022
Abstract
Garlic is one of the most widely employed condiments in cooking. It has also been used since ancient times in traditional plant-based medicine, largely based on its organosulfur compounds. The objective of this study was to provide updated information on the biological and therapeutic garlic properties. Garlic has been found to possess important biological properties with high therapeutic potential, which is influenced by the mode of its utilization, preparation, and extraction. It has been attributed with antioxidant, anti-inflammatory, and immunomodulatory capacities. Garlic, in particular its organosulfur compounds, can maintain immune system homeostasis through positive effects on immune cells, especially by regulating cytokine proliferation and expression. This may underlie their usefulness in the treatment of infectious and tumor processes. These compounds can also offer vascular benefits by regulating lipid metabolism or by exerting antihypertensive and antiaggregant effects. However, further clinical trials are warranted to confirm the therapeutic potential of garlic and its derivatives.
Introduction
Garlic (Allium sativum) has been widely employed in cooking and medicine since ancient times.1 It continues to serve as a therapeutic resource in traditional healing, which uses plants with medicinal properties to treat diseases.2–4 Garlic contains phenolic compounds, saponins, polysaccharides, and organosulfur compounds, among other components.5 Its organosulfur compounds are responsible for its most important biological and pharmacological properties and are extracted and isolated for therapeutic purposes.6,7 Compounds derived from garlic have been attributed with anti-inflammatory, antioxidant, and antimicrobial activities with beneficial effects against cardiovascular diseases, cancers, and immune system disorders.2,4,7Garlic can serve as a poultice in solid powder form but is more frequently prepared as a liquid in water, oil, or alcohol solvents. The mode of preparation influences its composition and its biological effects.4,8 However, the separation and isolation of the organosulfur components of garlic is challenging due to their physicochemical properties, and variability in their proportions among garlic preparations hampers reproduction and validation of the effects observed in different studies.6 Also, despite the fact that garlic supplementation has been classified as a safe product, some non-serious gastrointestinal side effects have been reported.9,10
The aim of this study was to provide an update of scientific evidence on the biological properties of garlic and its chemical components and their relevance for the management of health and disease.
Characteristics and composition of garlic
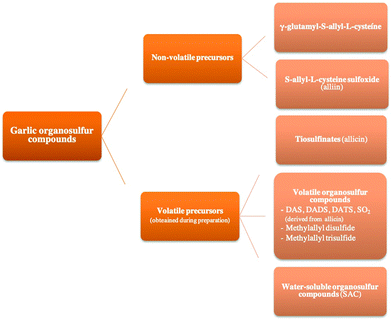
Garlic contains more than 200 chemical compounds with multiple properties. It is 65% water, 28% carbohydrates, 2.3% organosulfur compounds, 2% proteins, 1.2% free amino-acids, and 1.5% fiber. It also contains fat-soluble vitamins (vitamin A, vitamin K, and vitamin E), water-soluble vitamins (vitamin C, B-complex vitamins: B1, B2, B3, B6, and B8), and minerals (Ca, Fe, Mg, P, K, Na, and Zn). Organosulfur compounds give garlic its characteristic taste and odor as well as its pharmacological properties.11Moreover, garlic has both non-volatile and volatile organosulfur compounds. Non-volatile precursor compounds of sulfur in garlic include γ-glutamyl-S-allyl-L-cysteine and its sulfoxide, known as alliin. Alliin is only present in intact garlic, being transformed into other compounds when the bulb is macerated or crushed. In contrast, volatile organosulfur compounds are generated during the processing of garlic products, being divided into three subgroups according to their chemical nature (Fig. 1): (a) thiosulfinates, which result from the conversion of sulfoxides by enzymatic reaction when raw garlic is processed. (b) Volatile organosulfurs such as allicin (diallyl thiosulfinate), one of the most biologically active compounds, which is absent in intact garlic but produced by the reaction of alliin with the enzyme alliinase when the bulb is ground, crushed, or damaged. Allicin is highly unstable and instantly decomposes into diallyl sulfide (DAS), diallyl disulfide (DADS), diallyl trisulfide (DATS), and/or sulfur dioxide (SO2), among other compounds. This subgroup also includes methyl allyl disulfide and methyl allyl trisulfide. (c) Water-soluble organosulfur compounds, generated during the aqueous or alcoholic extraction of garlic through the decomposition of γ-glutamyl-S-allyl-L-cysteine into S-allyl-L-cysteine (SAC).
In summary, intact bulbs contain SAC and γ-glutamyl-S-allyl-L-cysteine sulfoxide, vapor-distilled oils contain volatile sulfide compounds (DADS, DATS, DAS), dry garlic powder is rich in alliin and DADS, and garlic macerates are rich in organosulfur compounds, ditiins, and E,Z-ajoenes. Finally, mashed and ethanol-soaked aged garlic extract (AGE) contains SAC and S-allyl-mercaptocysteine (SAMC) and has shown more biological properties in comparison to the aforementioned preparations.12–15 Multiple factors influence the bioactivity of garlic components, such as extraction, preparation, temperature or storage. In this context, it is reported that longer cooking time and increased temperature may reduce the proportion of phenolic compounds, such as hesperetin and 3/4-hydroxybenzoic acid, and flavonoid.16,17 It should be noted that absorption, metabolism and excretion are the main indices of bioavailability of an ingested compound. Allicin and allicin-derived compounds are rapidly metabolized to allyl methyl sulfide, which is the main metabolite of allicin and an active metabolite (Lawson and Wang, 2001).18 However, information on the bioavailibity of organosulfur compounds is limited. Thus, Miękus et al.19 suggested the need to conduct a series of clinical trials in a significant number of volunteers to optimize doses and understand the factors that determine bioavailability.
Biological activity of garlic
The different biological mechanisms of garlic are summarized in Table 1.| Biological acivity | Mechanism of action | References |
|---|---|---|
| SOD: superoxide dismutase; GSH: glutathione; Nrf2: nuclear factor erythroid 2-related factor 2; GCL: glutamate-cysteine ligase; AGE: aged garlic extract; HO-1: heme oxygenase-1; IL: interleukin; MCP-1: monocyte chemotactic protein; TNF-α: tumor necrosis factor α; NF-κB: nuclear kappa B transcription factor; NA: neuraminidase; DATS; diallyl trisulfide; HIV-1: human immunodeficiency virus type 1. | ||
| Antioxidant activity | Garlic phytochemicals could promote the expression of antioxidant enzymes, such as SOD or catalase, and increase cellular GSH levels. | Liu et al. 1992 |
| Borlinghaus et al. 2014 | ||
| Supplementation with 80–4000 mg day−1 of garlic for 2–24 weeks increases serum SOD levels, increasing total antioxidant capacity through a reduction of malondialdehyde. | Askari et al. 2021 | |
| Ajoene exerts its antioxidant function through the activation of Nrf2, which regulates GCL expression and increases GSH levels, among other mechanisms of action. Likewise, AGEs could reduce the oxidative stress of endothelial cells by activating the Nrf2 pathway, through an increase in the expression of GCL and HO-1. | Kay et al. 2010 | |
| Allicin attenuates oxidative stress responses caused by different factors through the Nrf2 pathway and regulation of apoptosis. | Hiramatsu et al. 2016 | |
| Zhang et al. 2017 | ||
| Liu et al. 2015 | ||
| AGEs play an antioxidant role against damage to DNA structures caused by ultraviolet radiation, performing antimutagenic, anticarcinogenic and reparative activities on damaged structures. | Corzo-Martínez et al. 2007 | |
| Khanum et al. 2004 | ||
| Anti-inflammatory activity | A reduction in adipocyte IL-6 and MCP-1 secretion, as well as up-regulation of the expression of genes involved in inflammatory response has been documented after alliin treatment. | Quintero-Fabián et al. 2013 |
| Treatment with garlic extracts on mononuclear cells showed inhibition of IL-17 secretion. | Moutia et al. 2016 | |
| IL-14 secretion was not affected after treatment with garlic extract on mononuclear cells. | ||
| AGE reduces the production of IL-12, TNF-α and IL-6. | Hodge, Hodge, and Han 2002 | |
| Garlic and its components may modulate the inflammatory response induced by T helper cells. | Larypoor et al. 2013 | |
| Liu et al. 2009 | ||
| Garlic components have the potential to inhibit NF-κB, thereby reducing the inflammatory response mediated by TNF-α, IL-1, IL-6, MCP-1 and IL-12, among others. | Rodrigues & Percival 2019 | |
| Antimicrobial activity | Garlic extracts have been shown to have antibiofilm and antibacterial effects against Bacillus cereus, Staphylococcus aureus, Micrococcus spp., Escherichia coli, Klebsiella spp., Proteus spp., Aspergillus versicolor, Penicillum citrinum, and Penicillium expansum. | Marefati et al. 2020 |
| Fratianni et al. 2016 | ||
| Nakamoto et al. 2020 | ||
| Anti-fungal activity of garlic against Penicillium funiculosum and Candida albicans has been demonstrated. | Li et al. 2014 | |
| Li et al. 2016 | ||
| Garlic has been shown to have antiviral activity through inhibition of retrotranscription and viral DNA synthesis capacity. | Rouf et al. 2020 | |
| Garlic and its organosulphur and flavonoid components exert antiviral action against SARS-CoV-2 infection via inhibition of the 6LU7 protein of the active region of the pathogen. | Pandey et al. 2019 | |
| Sen et al. 2020 | ||
| Thuy et al. 2020 | ||
| Garlic has therapeutic action in COVID-19 disease by strengthening immune system cells and decreasing the secretion of proinflammatory cytokine and leptin. | Donma & Donma 2020 | |
| Garlic extract inhibits the penetration and proliferation of the influenza A (H1N1) virus. | Mehrbod et al. 2009 | |
| Ajoene obtained from garlic inhibits the NA protein of the influenza A (H1N1) virus, which is responsible for the onset of viral infection. | Sahoo et al. 2016 | |
| Garlic-derived DATS treatment applied to human lung epithelial cells reduced viral load, increased viral gene expression, and decreased proinflammatory cytokine expression. | Ming et al. 2021 | |
| Garlic compounds such as glutamyl cysteine, DATS, allin or allicin possess HIV-1 inhibitory activity. | Gökalp et al. 2018 | |
| Immuno-modulatory activity | The consumption of garlic and its derivatives has been shown to exert immunomodulatory activity depending on the dose administered. | Kuttan 2000 |
| Charron et al. 2015 Arreola et al. 2015 | ||
| Colić et al. 2002 | ||
| Garlic's protein fraction and AGE have been shown to stimulate lymphocytes, natural killer cells and macrophages. | Ebrahimi et al. 2013 | |
| Moutia, Habti, & Badou 2018 | ||
| Rodrigues & Percival 2019 | ||
| Percival 2016 | ||
Antioxidant activity
The oxidation of DNA, proteins, and lipids by reactive oxygen species (ROS) plays a key role in aging and in the development of cancers and cardiovascular, inflammatory, neurodegenerative, and age-related diseases.20,21The antioxidant potential of garlic largely derives from its saponins, flavonoids, organosulfur compounds, and phenolic compounds. This potential is higher in aqueous extracts (e.g., AGE) than in fresh garlic or other preparations, likely due to the presence of water-soluble organosulfur compounds with antioxidant capacity (e.g., SAC and SAMC). Furthermore, a synergetic antioxidant effect has been observed between these compounds and flavonoids, saponins, and certain micro- and macro-nutrients also present in aqueous garlic extracts.11,14 Phytochemicals in these extracts can exert a double action by favoring the expression of antioxidant enzymes (e.g., superoxide dismutase [SOD] and catalase) and increasing cell glutathione (GSH) levels.22,23 In a recent meta-analysis of randomized controlled trials, serum SOD levels and total antioxidant capacity were found to be increased by supplementation with 80–4000 mg day−1 of garlic for 2–24 weeks, with a reduction in malondialdehyde production.24 An in vitro study found that ajoene, a chemically stable by product of garlic, can activate nuclear factor 2 (Nrf2), regulating the expression of glutamate-cysteine ligase (GCL), raising levels of GSH, a potent antioxidant, and increasing the expression of other genes that encode cysteine-metabolizing enzymes.25 Nrf2 controls the expression of numerous genes involved in the defense against xenobiotic agents and oxidative stress and is considered a key regulator of oxidative stress and the inflammatory response. For instance, it induces the gene expression of heme oxygenase-1 (HO-1), an enzyme that catalyzes degradation of the hemo group, generating carbon monoxide (CO), biliverdin, and iron. This enzyme is activated by oxidative stress or by the presence of cytokines or other mediators during inflammatory processes.25 In a study of human endothelial cells, AGE was found to reduce oxidative stress in a dose-dependent manner by activating the Nrf2 pathway through an increase in the expression of GCL and HO-1.26 Oral administration of AGE (100–200 mg kg−1) to male albino rats with indomethacin-induced gastric inflammation had a gastroprotective effect that was attributed to its anti-inflammatory and antioxidant properties.27 In this regard, it was reported in an in vitro study that allicin attenuates oxidative stress responses induced by lipopolysaccharides (LPS) of Gram-negative bacteria via the Nrf2 pathway.28 It was also observed that allicin has a protective effect in vitro against traumatic or ischemic neuronal damage by regulating apoptosis and oxidative stress pathways.29
The antioxidant properties of garlic also act by reducing free radicals. Thus, Corzo-Martínez et al. reported that AGE can counter ultraviolet radiation-induced DNA damage.30 Antimutagenic activity has been shown by certain sulfur components (e.g., DAS) through their action on DNA repair mechanisms, avoiding the onset of carcinogenesis.31
Locatelli et al.6 highlighted the antioxidant capacity of allicin, ajoenes, and vinyldithiins (2-VD) in garlic, reporting that allicin can eliminate 1,1-diphenyl-2-picrylhydrazyl radical (DPPH˙) and 2,20-azino-bis-(3-ethylbenzo thiazoline-6-sulfonic acid) diammonium salt (ABTS) and reduce iron levels.
Anti-inflammatory activity
The anti-inflammatory activity of garlic extracts is well documented. Quintero-Fabián et al. reported that treatment of LPS-stimulated 3 T3-L1 adipocytes with alliin reduces the gene expression and protein synthesis of interleukin-6 (IL-6) and monocyte chemotactic protein (MCP-1) and upregulates genes related to the immune response.32 Other authors found that treatment with garlic extract inhibited the expression of IL-17 by peripheral blood mononuclear cells without affecting their expression of IL-4.33In another in vitro study, Hodge et al. reported that AGE reduces the production of IL-12 and other proinflammatory cytokines and increases IL-10, which inhibits the production of tumor necrosis factor α (TNF-α) and IL-6.34 Inhibition of Th1 proinflammatory cytokines by allicin was observed by Larypoor et al.35 However, a study in rats found that garlic oil had dual effects on the balance of T helper cells (Th1–Th2) according to the dose, with low doses obtaining a Th1 response whereas high doses a Th2 response.36
Nuclear kappa B transcription factor (NF-κB) is a key regulator of the expression of genes involved in inflammation, including the enzymes nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). NF-κB overactivation is associated with various chronic inflammatory diseases, and suppression of the NF-κB pathway by anti-inflammatory drugs favors their prevention and treatment.26 The NF-κB transcription factor can be inhibited by caffeic acid, SAC, and DATS, among other compounds derived from garlic, thereby inhibiting multiple genes that encode cytokines related to the proinflammatory response (e.g., TNF-α, IL-1, IL-6, MCP-1, and IL-12).14 In another investigation, a garlic protein of 14 kDa isolated from AGE significantly inhibited the production of NO, prostaglandin E (PGE), TNF-α, and IL-1β by LPS-activated macrophages in a dose-dependent manner with no toxic effects. As observed in other studies, the anti-inflammatory effect was largely mediated by the inhibition of iNOS and COX-2 expressions through inactivation of the NF-κB pathway.37
Antimicrobial activity
Ample evidence has also been published on the antimicrobial and antifungal properties of garlic and some of its components.38 Fresh garlic extract was found to inhibit the growth of Bacillus cereus, Staphylococcus aureus, Micrococcus spp., Escherichia coli, Klebsiella spp., and Proteus spp., using 1% chloramphenicol as reference antibiotic.39 In addition, Fratianni et al. observed that “Rosato” and “Caposele” garlic varieties exert antimicrobial action against Aspergillus versicolor, Penicillum citrinum, and Penicillium expansum.40 The sulfurous compounds of garlic can impair biofilm formation by acting as quorum-sensing inhibitors, hampering early bacterial adhesion and the secretion of extracellular polymeric substances and virulence factors.36 There is evidence that garlic has antifungal activity against Penicillium funiculosum and Candida albicans.41,42 It has also been demonstrated antiviral activity against multiple pathogens. It acts by blocking the entry of viruses into cells, inhibiting viral polymerase-RNA, reverse transcriptase, DNA synthesis, and the transcription of immediate-early gene 1 (IEG1) and downregulating the extracellular signal-regulated kinase (ERK)/mitogen activated protein kinase (MAPK) signaling pathway.43 Various researchers have explored the therapeutic potential of garlic against SARS-CoV-2 infection disease. Some authors have observed that garlic and its bioactive compounds may act against this disease by inhibiting target protein 6LU7 of SARS-CoV-2.44–46 It has also been proposed that garlic may mitigate the effects of COVID-19 on the immune system by strengthening immune system cells and inhibiting the secretion of proinflammatory cytokines and leptin.47 Moreover, some authors point out that garlic and its components may also have antiviral activity against influenza A (H1N1) and avian influenza (H9N2) viruses.48–50 Similarly, garlic compounds such as glutamyl cysteine, DATS, allin and allicin have been shown to inhibit the activity of human immunodeficiency virus type 1 (HIV-1).51 However, numerous studies indicate that supplementation with garlic or its derivatives could exert an undesirable interaction with prescribed antiretroviral drugs against HIV disease.52,53Immunomodulatory activity
Garlic can contribute to maintaining immune system homeostasis through its immunomodulatory action on different cell populations, alongside its antiapoptotic, antiparasitic, and anticarcinogenic effects.13Animal experiments showed that garlic consumption can increase the number of immune cells and the total number of bone marrow cells.54 A study in humans found that expression of the gene encoding the NFAM1 protein is increased by the addition of 5 g of raw mashed garlic to one meal every day. NFAM1 is a type I membrane receptor that activates cytokine gene promoters (e.g., IL-13 and TNF-α) and is involved in B cell development and signaling.55
Various garlic derivatives have been found to have a dual effect, exerting either a stimulatory or inhibitory effect on monocytes and lymphocytes according to the dose.13,56,57 For instance, concanavalin A-stimulated lymphocyte proliferation is inhibited by high doses of garlic extract but increased by low doses.48 The same dual effect has been observed with DATS.57
The protein fraction of garlic has demonstrated stimulatory effects on lymphocytes, natural killer (NK) cells, and macrophages. Specifically, it was found to increase the mitogenic activity of human peripheral blood lymphocytes and CD8+ T cells, producing an increase in the delayed hypersensitivity reaction and promoting an effective cellular response.58
AGE has also exhibited this immunomodulatory property, activating and increasing the population of NK cells. Supplementation with AGE in mice increases the activation of NK and cytotoxic T cells in mice and improves the immune response in humans by augmenting the proliferation of NK and Tγδ cells.11,13,14,59
Therapeutic activity against diseases
Given its antioxidant, immunomodulatory, and anti-inflammatory properties, researchers have explored the usefulness of garlic in the treatment of various diseases (Table 2).| Therapeutic applications | Mechanism of action | Ref. |
|---|---|---|
| DADS: diallyl disulfide; DATS: diallyl trisulfide; SAC: S-allyl-L-cysteine; SAMC: S-allyl-mercaptocysteine; AGE: aged garlic extract; LDL: low-density lipoprotein; HDL: high-density lipoprotein. | ||
| Infectious processes | The administration of allicin and garlic oil has been shown to have beneficial effects against respiratory tract infections. | Yarnell (2018)60 |
| Ko et al. (2018)61 | ||
| Oral allicin supplementation treatment may prevent common cold virus infection. | Josling (2001)63 | |
| Daily administration of 300 mg extended-release garlic capsules for 20 weeks reduced the morbidity of acute respiratory infections in children aged 10–12 years. | Andrianova et al. (2003)62 | |
| Treatment with garlic extract in a sepsis mouse model showed favourable results against the infectious process. | Lee et al. (2015)64 | |
| Tumoural processes | Anti-tumour properties have been found in garlic, mainly in some compounds such as DADS, DATS, SAC, and SAMC. | Miraghajani et al. (2018)72 |
| Liu et al. (2019)67 | ||
| Zhang et al. (2020)70 | ||
| Zhou et al. (2020)68 | ||
| Treatments with garlic extract and garlic protein fractions reduced the tumour activity of rat sarcoma cells and breast tumour cells. | Hu et al. (2002)73 | |
| Ebrahimi et al. (2013)58 | ||
| The protein fraction of garlic and AGE was shown to modulate the activity of natural killer cells in both healthy populations and individuals with advanced cancer pathologies. | Moutia, Habti and Badou (2018)11 | |
| Rodrigues and Percival (2019)14 | ||
| Ishikawa et al. (2006)74 Fallah-Rostami et al. (2013)75 | ||
| Garlic and its components showed a beneficial effect in the treatment of tumours by modulating cytokine novels, such as TNF-α, IFN-γ, IL-2, as well as the activity exerted by T-cells. | Suddek (2014)76 | |
| Hodge et al. (2008)77 | ||
| Larypoor et al. (2013)35 | ||
| Vitamin supplementation in combination with garlic for 7.3 years showed to reduce the prevalence of precancerous gastric lesions. | You et al. (2006)78 | |
| Oral vitamin and garlic supplementation treatment administered for 7.3 years was found to reduce the risk of mortality of gastric cancer after a follow-up of more than 22 years. | Li et al. (2019)79 | |
| Daily supplementation treatment with AGE (500 mg) for 6 months in patients with advanced cancer of the digestive system (pancreatic, colon and liver cancer) showed an improvement of NK cell activity. | Ishikawa et al. (2006)80 | |
| A 12-month supplemental treatment with high-dose AGE (2.4 mL day−1) suppressed the number and size of colorectal adenomas. | Tanaka et al. (2006)81 | |
| Activity at vascular level | Garlic and its constituents may reduce disorders related to the cardiovascular system by reducing triglyceride and cholesterol levels. | Gupta and Porter (2001)84 |
| Silagy and Neil (1994)83 | ||
| Ried (2016)98 | ||
| The components of garlic may regulate lipid metabolism in adipocytes. | Baek et al. (2019)86 | |
| Oral garlic supplementation (400 mg per day) for 12 weeks was shown to significantly reduce blood concentrations of transaminases and cholesterol in patients with non-alcoholic fatty liver disease. | Sangouni et al. (2020)87 | |
| Daily supplementation of 800 mg of garlic in patients with polycystic ovary syndrome reduces plasma levels of total cholesterol, LDL, but not HDL. | Zadhoush et al. (2021)88 | |
| Supplementation daily with 900 mg of AGE in men with moderate hypercholesterolemia for 12 weeks significantly reduced serum triglyceride and LDL levels, as well as lowered systolic and diastolic blood pressure. | Adler and Holub (1997)89 | |
| Supplementation treatment of 9.6 mg allicin for 12 weeks in patients with hypercholesterolemia reduced total, LDL and HDL cholesterol levels. | Kannar et al. (2001)90 | |
| Garlic supplementation (2 g day−1) in patients with hyperlipidemia significantly reduced plasma levels of total and LDL cholesterol, and increased HDL after 60 days. Similarly, this supplementation therapy reduced systolic blood pressure values, but not dyastolic blood pressure. | Zeb et al. (2018)91 | |
| Oral black AGE supplementation treatment in patients with mild hypercholesterolemia (6 g day−1) for 12 weeks did not show a reduction in serum LDL and triglyceride levels, although it did significantly increase HDL levels. | Jung et al. (2014)92 | |
| Thiosulfinates act as potent inhibitors of platelet aggregation. | Briggs et al. (2000)93 | |
| Components of garlic have been shown to have antihypertensive properties, with similar results to drugs commonly used to treat hypertension. | Dubey et al. (2017)95 | |
| Chaupis-Meza et al. (2014)96 | ||
| Gao et al. (2020)97 | ||
| Allicin has a protective effect on blood vessels by increasing endothelial cell proliferation, and decreasing lipopolysaccharide-induced apoptosis and inflammation. | Zhang et al. (2017)28 | |
| Prebiotic agent | Fructane present in garlic may stimulate the proliferation of Bifidobacteria. | Zhang et al. (2013)101 |
| Supplementation with AGE for 3 months showed a significant increase in intestinal microbiota populations such as Lactobacillus and Clostridia. | Ried (2020)102 | |
| Therapeutic endocrine and metabolic effects | Treatments with garlic extracts have demonstrated an improvement in glucose, fructosamine and glycosylated hemoglobin levels, and a weight loss in diabetic rats. | Askari et al. (2021)24 |
| Kaur et al. (2016)103 | ||
| Thomson et al. (2016)104 | ||
| Wang et al. (2017)105 | ||
| In humans, garlic has been demonstrated to improve components of the metabolic syndrome such as increasing high-density cholesterol and reducing waist circumference, weight, arterial blood pressure and insulin resistance. | Sangouni et al. (2021)106 | |
| Yang et al. (2016)107 | ||
Infectious processes
Various authors have studied the action of garlic and its components against infectious processes. Thus, the severity and duration of respiratory tract infections and their symptoms were reduced after the administration of allicin versus placebo.60 In a mouse study, garlic oil and DADS were found to have protective effects against respiratory tract inflammation caused by cigarette smoke, reducing the inflammatory cell count and the production of IL-1β, IL-6, and TNF-α.61 Similarly, experimental studies in adults and children showed that daily supplementation with allicin or long-releasing garlic capsules for 12 or 20 weeks respectively, significantly reduced common cold virus infections in adults and acute respiratory infections in children, with no side effects as a result of the administration.62,63In a mouse model of induced polymicrobial sepsis, the administration of methyl sucrose 3-formyl-4-methylpentanoate, isolated from garlic, reduced peritoneal fluid colony-forming units (CFUs) produced by the stimulation of ROS generation, diminished lymphocyte apoptosis in the spleen, and inhibited the production of proinflammatory cytokines (e.g., TNF-α, IL-1β, and IL-6).64
Lee et al. evaluated the growth and immune response of laying chickens fed with a mixture of phytogenic extracts (garlic and cilantro) and probiotics.65 They reported an increase in the proliferation of lamina propria leukocytes and in their cytotoxic activity against tumor cells, also observing an improvement in the egg production but not growth of the animals. The inhibition of LPS-induced TNF-α and IFN-γ secretion and the reduction in fecal E. coli count were similar between chickens fed with this mixture and those treated with antibiotics.
Tumoural processes
Garlic has demonstrated anticarcinogenic properties against colorectal, breast, liver, and gastric cancers, among others.66–69 In 2020, Zhang et al. reported that garlic had therapeutic potential not only to prevent but also to treat cancer, proposing possible mechanisms of action.70 The aforementioned beneficial effects of garlic on the immune system would also contribute to controlling tumor processes.11In vitro and in vivo studies have demonstrated the capacity of garlic to inhibit cell division, block carcinogen activation, reduce microbiological growth, scavenge ROS, induce superoxide dismutase, increase proinflammatory cytokine release, and reduce cell proliferation.71 These effects have been associated with specific compounds of garlic, especially DADS, DATS, SAC, and SAMC.72 Treatment with garlic extract attenuated cell migration and induced death in rat sarcoma cells, and pre-exposure to the extract completely inhibited the growth of implanted tumor cells.73 Likewise, treatment with protein fractions extracted from garlic reduced the tumor size in mice with implanted breast tumor cells, increasing T cell activation and the intra-tumor infiltration of lymphocytes at all studied doses.56The protein fraction of garlic has been found to modulate NK cell activity in mice, while AGE can increase the number and activity of NK cells in healthy individuals and in patients with advanced cancers.11,14,74,75
Cytokines also play an important role in antitumor immunity. Supplementation with allicins of Ehrlich ascites carcinoma (EAC) cells treated with tamoxifen (antiestrogenic drug for breast cancer) increased the expression of cytotoxic damage markers and TNF-α levels.76 In another study, AGE significantly increased the apoptosis of acute lymphoblastic leukemia cells without altering the activation of T cells or the production of IFN-γ, IL-2, and TNF-α, whereas T cell proliferation and cytokine production were reduced by treatment with cytostatic agents.77 In a mouse study, treatment with AGE produced a cytokine release pattern that favored Th1 cell action and antitumor immunity.35
Other human randomized clinical trials in have evaluated the effectiveness of garlic against disorders resulting from infection with the bacterium Helicobacter pylori. It was found that combined vitamin and garlic supplementation treatment (extract and oil) for 7.3 years reduced the prevalence of precancerous gastric lesions and reduced the risk of mortality from gastric cancer after a follow-up of 22 years.78,79 Indeed, this is in accordance with the findings of Ishikawa et al., who observed an improvement in NK cell activity after 6 months of AGE supplementation in patients with advanced pancreatic, liver and colon cancer.80 Similarly, other authors demonstrated that daily supplementation with high-dose AGE (2.4 mL) could inhibit the progression of colorectal adenoma.81 None of these studies found significant adverse effects as a consequence of supplementary therapy.
Activity at vascular level
Garlic has been attributed with beneficial effects on the cardiovascular system by improving lipid profile, endothelial function, and blood pressure.7,82A meta-analysis published by Silagy and Neil in 1994 confirmed that garlic and its derivatives can reduce levels of triglycerides and cholesterol.83 Allicin can also suppress cholesterol biosynthesis by inhibiting the key enzymes squalene-monooxygenase and acetyl-CoA synthetase.84,85 In addition, (1R,3S)-1-methyl-1,2,3,4-tetrahydro-β-carboline-3-carboxylic acid obtained from garlic was found to prevent adiposity by regulating the expression of genes involved in the lipid metabolism of adipocytes, downregulating lipogenic genes and upregulating lipolytic genes.86 Likewise, experimental studies are in agreement with these findings, observing garlic supplementation to be beneficial in reducing plasma levels of total cholesterol and LDL in patients with nonalcoholic fatty liver disease,87 polycystic ovary syndrome,88 and hyperlipidemia.89–91 In contrast, Jung et al. observed that daily supplementation of 6 g of black AGE in patients with hypercholesterolemia did not decrease LDL, although they did report an increase in HDL.92 No adverse effects were reported in any of the patients.
In relation to cardiovascular disorders, thiosulfinates such as allicin have been reported to act as potent inhibitors of platelet aggregation, thereby contributing to the prevention of cardiac and/or cerebral ischemia.93 Allicin derived from garlic has also demonstrated antihypertensive properties,94,95 attributed to the action of red blood cells on these polysulfides, which leads to the release of H2S.94 H2S is a powerful signaling molecule that relaxes smooth muscle cells surrounding blood vessels and allows their expansion, thereby reducing blood pressures. Chaupis-Meza et al. found that macerated garlic extract had a dose-dependent hypotensive effect in an in vivo rat model, observing a dose-dependent hypotensive effect comparable to that of captopril, widely prescribed for this purpose.96 Dubey et al. were not able to identify the underlying mechanism.95 However, Gao et al., who verified the anti-hypertensive effects of garlic and its hydrolysates, proposed that they may act by inhibiting the activity of angiotensin-converting enzyme (ACE), reducing the formation of ACE-II, and protecting bradykinin activity.97 A meta-analysis of human studies confirmed that garlic contributes to reducing the blood pressure of hypertensive individuals and improving their cholesterol levels.98 However, its effect on normotensive individuals has not been established.
Garlic also has a protective effect at endothelial level. Thus, allicin increases the proliferation in vitro of human umbilical vein endothelial cells.28 In this regard, the proliferative capacity of this cell population is diminished in response to LPS and the cells release lactate-dehydrogenase, a marker of cytotoxicity. The presence of allicin has been found to decrease LPS-induced apoptosis, suppress ROS overproduction, reduce lipid peroxidation, and decrease endogenous antioxidant enzymatic activities in endothelial cells.99 These protective effects have been attributed to the inhibition of mitochondrial dysfunction through a reduction in potential mitochondrial membrane collapse, cytochrome c synthesis, and mitochondrial ATP release.28 It has been reported that allicin not only inhibits the LPS-induced inflammatory response but also dose-dependently increases the expression of liver X receptors α (LXRα), and the nuclear receptors LXR α and β play a crucial role in regulating carbohydrate and lipid metabolism.28 It was also been observed that allicin can activate Nrf2 transcription factor, which regulates defenses against oxidative stress and inflammation (see above).28,100 In conclusion, allicin is able to attenuate the LPS-induced inflammatory response at blood vessel level.
Other effects
Besides the aforementioned benefits, various studies have demonstrated the therapeutic potential of garlic as a prebiotic agent capable of acting on and increasing the diversity of intestinal microbiota. Thus, garlic fructane was found to stimulate the proliferation of Bifidobacteria,101 and a meta-analysis by Ried et al. concluded that supplementation with AGE for three months significantly augments populations of Lactobacillus and Clostridia.102 Garlic has also shown therapeutic effects at endocrinal and metabolic level. For instance, treatment of type I and II diabetic rats with garlic and certain garlic extracts (e.g., AGE) improved their weight and their levels of glucose, fructosamine, and glycosylated hemoglobin, thereby reducing the damage to pancreatic cells.24,103–105 Garlic has also been reported to improve components of the metabolic syndrome in humans by increasing high-density cholesterol and reducing waist circumference, weight, arterial pressure, and insulin resistance.106,107Conclusions
Garlic and its multiple derivatives possess biological activities with high therapeutic potential against infectious, immunological, vascular, and tumor processes. They can be prepared in raw, macerated, or powder form or as an aqueous, oily, or alcoholic extract. Little clinical advantage has been taken of this natural resource, and further research is warranted in humans to support its therapeutic application.Author contributions
Formulation of research question: L. M. R. and C. R.; conception and design of study: L. M. R., R. I. M., C. R. and. V. J. C. R.; literature search and data collection: L. M. R., E. G. R. and C. R.; data analysis: L. M. R., E. G. R., C. R., E. L. B., R. I. M. and V. J. C. R.; writing—original draft preparation, L. M. R., C. R., R. I. M. and V. J. C. R.; writing—review and editing: L. M. R., E. G. R., C. R., E. L. B., R. I. M. and V. J. C. R.; supervision: R. I. M. and C. R. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by research group BIO277 (Junta de Andalucía) and Department of Nursing (University of Granada).Notes and references
- E. Ayaz and H. C. Alpsoy, Garlic (Allium sativum) and traditional medicine, Turkiye Parazitol. Derg., 2007, 31, 145–149 Search PubMed.
- M. Foroutan-Rad, K. H. Tappeh and S. Khademvatan, Antileishmanial and Immunomodulatory Activity of Allium sativum (Garlic): A Review, J. Evidence-Based Complementary Altern. Med., 2017, 22, 141–155 CrossRef CAS PubMed.
- K. Sharma, N. Mahato, S. H. Nile, E. T. Lee and Y. R. Lee, Economical and environmentally-friendly approaches for usage of onion (Allium cepa L.) waste, Food Funct., 2016, 7, 3354–3369 RSC.
- X. Lu, N. Li, X. Qiao, Z. Qiu and P. Liu, Composition analysis and antioxidant properties of black garlic extract, J. Food Drug Anal., 2017, 25, 340–349 CrossRef CAS PubMed.
- K. A. Szychowski, K. Rybczyńska-Tkaczyk, K. Gaweł-Bęben, M. Świeca, M. Karaś, A. Jakubczyk, M. Matysiak, U. E. Binduga and J. Gmiński, Characterization of Active Compounds of Different Garlic (Allium sativum L.) Cultivars, Pol. J. Food Nutr. Sci., 2018, 68, 73–81 CrossRef CAS.
- D. A. Locatelli, M. A. Nazareno, C. M. Fusari and A. B. Camargo, Cooked garlic and antioxidant activity: Correlation with organosulfur compound composition, Food Chem., 2017, 220, 219–224 CrossRef CAS PubMed.
- G. El-Saber Batiha, A. Magdy Beshbishy, L. G. Wasef, Y. H. A. Elewa, A. A. Al-Sagan, M. E. Abd El-Hack, A. E. Taha, Y. M. Abd-Elhakim and H. Prasad Devkota, Chemical Constituents and Pharmacological Activities of Garlic (Allium sativum L.): A Review, Nutrients, 2020, 12, 872 CrossRef PubMed.
- N. Martins, S. Petropoulos and I. C. F. R. Ferreira, Chemical composition and bioactive compounds of garlic (Allium sativum L.) as affected by pre- and post-harvest conditions: A review, Food Chem., 2016, 211, 41–50 CrossRef CAS PubMed.
- D. Gyawali, R. Vohra, D. Orme-Johnson, S. Ramaratnam and R. H. Schneider, A Systematic Review and Meta-Analysis of Ayurvedic Herbal Preparations for Hypercholesterolemia, Medicina, 2021, 57, 546 CrossRef PubMed.
- M. K. Ang-Lee, J. Moss and C. S. Yuan, Herbal medicines and perioperative care, J. Am. Med. Assoc., 2001, 286, 208–216 CrossRef CAS PubMed.
- M. Moutia, N. Habti and A. Badou, In Vitro and In Vivo Immunomodulator Activities of Allium sativum L, J. Evidence-Based Complementary Altern. Med., 2018, 2018, 4984659 Search PubMed.
- H. Amagase, Clarifying the real bioactive constituents of garlic, J. Nutr., 2006, 136, 716S–725S CrossRef CAS PubMed.
- R. Arreola, S. Quintero-Fabián, R. I. López-Roa, E. O. Flores-Gutiérrez, J. P. Reyes-Grajeda, L. Carrera-Quintanar and D. Ortuño-Sahagún, Immunomodulation and anti-inflammatory effects of garlic compounds, J. Immunol. Res., 2015, 2015, 401630 Search PubMed.
- C. Rodrigues and S. S. Percival, Immunomodulatory Effects of Glutathione, Garlic Derivatives, and Hydrogen Sulfide, Nutrients, 2019, 11, 295 CrossRef CAS PubMed.
- A. Shang, S.-Y. Cao, X.-Y. Xu, R.-Y. Gan, G.-Y. Tang, H. Corke, V. Mavumengwana and H.-B. Li, Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.), Foods, 2019, 8, 246 CrossRef CAS PubMed.
- T. Alide, P. Wangila and A. Kiprop, Effect of cooking temperature and time on total phenolic content, total flavonoid content and total in vitro antioxidant activity of garlic, BMC Res. Notes, 2020, 13, 564 CrossRef CAS PubMed.
- J. F. Rinaldi de Alvarenga, P. Quifer-Rada, S. Hurtado-Barroso, M. Illan, X. Torrado-Prat and R. M. Lamuela-Raventós, Cuisinomics: MS-based untargeted approach reveals chemical modulation by a recipe during home cooking, Food Res. Int., 2020, 138, 109787 CrossRef CAS PubMed.
- L. D. Lawson and Z. J. Wang, Low allicin release from garlic supplements: a major problem due to the sensitivities of alliinase activity, J. Agric. Food Chem., 2001, 49, 2592–2599 CrossRef CAS PubMed .15.
- N. Miękus, K. Marszałek, M. Podlacha, A. Iqbal, C. Puchalski and A. H. Świergiel, Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds, Molecules, 2020, 25, 3804 CrossRef PubMed.
- C. Borek, Dietary antioxidants and human cancer, Integr. Cancer Ther., 2004, 3, 333–341 CrossRef CAS PubMed.
- K. A. Awan, M. S. Butt, F. Ashfaq, H. Munir and H. A. R. Suleria, Prophylactic Potential of Conventional and Supercritical Garlic Extracts to Alleviate Diet Related Malfunctions, Recent Pat. Food, Nutr. Agric., 2019, 10, 34–47 CrossRef CAS PubMed.
- J. Liu, M. W. Albers, T. J. Wandless, S. Luan, D. G. Alberg, P. J. Belshaw, P. Cohen, C. MacKintosh, C. B. Klee and S. L. Schreiber, Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity, Biochemistry, 1992, 31, 3896–3901 CrossRef CAS PubMed.
- J. Borlinghaus, F. Albrecht, M. C. H. Gruhlke, I. D. Nwachukwu and A. J. Slusarenko, Allicin: chemistry and biological properties, Molecules, 2014, 19, 12591–12618 CrossRef PubMed.
- M. Askari, H. Mozaffari, M. Darooghegi Mofrad, A. Jafari, P. J. Surkan, M. R. Amini and L. Azadbakht, Effects of garlic supplementation on oxidative stress and antioxidative capacity biomarkers: A systematic review and meta-analysis of randomized controlled trials, Phytother. Res., 2021, 35, 3032–3045 CrossRef CAS PubMed.
- H. Y. Kay, J. Won Yang, T. H. Kim, D. Y. Lee, B. Kang, J.-H. Ryu, R. Jeon and S. G. Kim, Ajoene, a stable garlic by-product, has an antioxidant effect through Nrf2-mediated glutamate-cysteine ligase induction in HepG2 cells and primary hepatocytes, J. Nutr., 2010, 140, 1211–1219 CrossRef CAS PubMed.
- K. Hiramatsu, T. Tsuneyoshi, T. Ogawa and N. Morihara, Aged garlic extract enhances heme oxygenase-1 and glutamate-cysteine ligase modifier subunit expression via the nuclear factor erythroid 2-related factor 2-antioxidant response element signaling pathway in human endothelial cells, Nutr. Res., 2016, 36, 143–149 CrossRef CAS PubMed.
- E. N. Lee, Y. W. Choi, H. K. Kim, J. K. Park, H. J. Kim, M. J. Kim, H. W. Lee, K.-H. Kim, S. S. Bae, B. S. Kim and S. Yoon, Chloroform extract of aged black garlic attenuates TNF-α-induced ROS generation, VCAM-1 expression, NF-κB activation and adhesiveness for monocytes in human umbilical vein endothelial cells, Phytother. Res., 2011, 25, 92–100 CrossRef CAS PubMed.
- M. Zhang, H. Pan, Y. Xu, X. Wang, Z. Qiu and L. Jiang, Allicin Decreases Lipopolysaccharide-Induced Oxidative Stress and Inflammation in Human Umbilical Vein Endothelial Cells through Suppression of Mitochondrial Dysfunction and Activation of Nrf2, Cell. Physiol. Biochem., 2017, 41, 2255–2267 CrossRef CAS PubMed.
- S.-G. Liu, P.-Y. Ren, G.-Y. Wang, S.-X. Yao and X.-J. He, Allicin protects spinal cord neurons from glutamate-induced oxidative stress through regulating the heat shock protein 70/inducible nitric oxide synthase pathway, Food Funct., 2015, 6, 321–330 Search PubMed.
- M. Corzo-Martínez, N. Corzo and M. Villamiel, Biological properties of onions and garlic, Trends Food Sci. Technol., 2007, 18, 609–625 CrossRef.
- F. Khanum, K. R. Anilakumar and K. R. Viswanathan, Anticarcinogenic properties of garlic: a review, Crit. Rev. Food Sci. Nutr., 2004, 44, 479–488 CrossRef CAS PubMed.
- S. Quintero-Fabián, D. Ortuño-Sahagún, M. Vázquez-Carrera and R. I. López-Roa, Alliin, a garlic (Allium sativum) compound, prevents LPS-induced inflammation in 3 T3-L1 adipocytes, Mediators Inflammation, 2013, 2013, 381815 Search PubMed.
- M. Moutia, F. Seghrouchni, O. Abouelazz, A. Elouaddari, A. Al Jahid, A. Elhou, S. Nadifi, J. Jamal Eddine, N. Habti and A. Badou, Allium sativum L. regulates in vitro IL-17 gene expression in human peripheral blood mononuclear cells, BMC Complementary Altern. Med., 2016, 16, 377 CrossRef PubMed.
- G. Hodge, S. Hodge and P. Han, Allium sativum (garlic) suppresses leukocyte inflammatory cytokine production in vitro: potential therapeutic use in the treatment of inflammatory bowel disease, Cytometry, 2002, 48, 209–215 CrossRef CAS PubMed.
- M. Larypoor, M. Bayat, M. H. Zuhair, A. Akhavan Sepahy and M. Amanlou, Evaluation of The Number of CD4(+) CD25(+) FoxP3(+) Treg Cells in Normal Mice Exposed to AFB1 and Treated with Aged Garlic Extract, Cell J., 2013, 15, 37–44 Search PubMed.
- C.-T. Liu, H.-M. Su, C.-K. Lii and L.-Y. Sheen, Effect of supplementation with garlic oil on activity of Th1 and Th2 lymphocytes from rats, Planta Med., 2009, 75, 205–210 CrossRef CAS PubMed.
- S. Z. T. Rabe, T. Ghazanfari, Z. Siadat, M. Rastin, S. Z. T. Rabe and M. Mahmoudi, Anti-inflammatory effect of garlic 14 kDa protein on LPS-stimulated-J774A.1 macrophages, Immunopharmacol. Immunotoxicol., 2015, 37, 158–164 CrossRef PubMed.
- M. Nakamoto, K. Kunimura, J.-I. Suzuki and Y. Kodera, Antimicrobial properties of hydrophobic compounds in garlic: Allicin, vinyldithiin, ajoene and diallyl polysulfides (Review), Exp. Ther. Med., 2020, 19, 1550–1553 CAS.
- N. Marefati, V. Ghorani, F. Shakeri, M. Boskabady, F. Kianian, R. Rezaee and M. H. Boskabady, A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents, Pharm. Biol., 2016, 59, 287–302 CrossRef PubMed.
- F. Fratianni, R. Riccardi, P. Spigno, M. N. Ombra, A. Cozzolino, P. Tremonte, R. Coppola and F. Nazzaro, Biochemical Characterization and Antimicrobial and Antifungal Activity of Two Endemic Varieties of Garlic (Allium sativum L.) of the Campania Region, Southern Italy, J. Med. Food, 2016, 19, 686–691 CrossRef CAS PubMed.
- W.-R. Li, Q.-S. Shi, H.-Q. Dai, Q. Liang, X. Xie, X.-M. Huang, G. Zhao and L.-X. Zhang, Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans, Sci. Rep., 2016, 6, 22805 CrossRef CAS PubMed.
- W.-R. Li, Q.-S. Shi, Q. Liang, X.-M. Huang and Y.-B. Chen, Antifungal effect and mechanism of garlic oil on Penicillium funiculosum, Appl. Microbiol. Biotechnol., 2014, 98, 8337–8346 CrossRef CAS PubMed.
- R. Rouf, S. J. Uddin, D. K. Sarker, M. T. Islam, E. S. Ali, J. A. Shilpi, L. Nahar, E. Tiralongo and S. D. Sarker, Antiviral potential of garlic (Allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data, Trends Food Sci. Technol., 2020, 104, 219–234 CrossRef CAS PubMed.
- P. Pandey, F. Khan, A. Kumar, A. Srivastava and N. K. Jha, Screening of potent inhibitors against 2019 novel coronavirus (Covid-19) from alliumsativum and allium cepa: An in silico approach, Biointerface Res. Appl. Chem., 2021, 11, 7981–7993 CAS.
- D. Sen, P. Debnath, B. Debnath, S. Bhaumik and S. Debnath, J, Identification of potential inhibitors of SARS-CoV-2 main protease and spike receptor from 10 important spices through structure-based virtual screening and molecular dynamic study, Biomol. Struct. Dyn., 2020, 1–22 CAS.
- B. T. Thuy, T. T. A. My, N. T. T. Hai, L. T. Hieu, T. T. Hoa, H. T. P. Loan, N. T. Triet, T. T. V. Anh, P. T. Quy, P. V. Tat, N. V. Hue, D. T. Quang, N. T. Trung, V. T. Tung, L. K. Huynh and N. T. A. Nhung, Investigation into SARS-CoV-2 Resistance of Compounds in Garlic Essential Oil, ACS Omega, 2020, 5, 8312–8320 CrossRef CAS PubMed.
- M. M. Donma and O. Donma, The effects of allium sativum on immunity within the scope of COVID-19 infection, Med. Hypotheses, 2020, 144, 109934 CrossRef CAS PubMed.
- P. Mehrbod, E. Amini and M. Tavassoti-Kheiri, Antiviral activity of garlic extract on Influenza virus, Iran. J. Virol., 2009, 3, 19–23 CrossRef.
- L. Ming, Z. Li, X. Li, L. Tang and G. He, Antiviral activity of diallyl trisulfide against H9N2 avian influenza virus infection in vitro and in vivo, Virol. J., 2021, 18, 171 CrossRef CAS PubMed.
- M. Sahoo, L. Jena, S. N. Rath and S. Kumar, Identification of Suitable Natural Inhibitor against Influenza A (H1N1) Neuraminidase Protein by Molecular Docking, Genomics Inform., 2016, 14, 96–103 CrossRef PubMed.
- F. Gökalp, The inhibition effect of garlic-derived compounds on human immunodeficiency virus type 1 and saquinavir, J. Biochem. Mol. Toxicol., 2018, 32, e22215 CrossRef PubMed.
- M. A. Jalloh, P. J. Gregory, D. Hein, Z. R. Cochrane and A. Rodriguez, Dietary supplement interactions with antiretrovirals: a systematic review, Int. J. STD AIDS, 2017, 28, 4–15 CrossRef CAS PubMed.
- C. Bordes, G. Leguelinel-Blache, J.-P. Lavigne, J.-M. Mauboussin, D. Laureillard, H. Faure, I. Rouanet, A. Sotto and P. Loubet, Interactions between antiretroviral therapy and complementary and alternative medicine: a narrative review, Clin. Microbiol. Infect., 2020, 26, 1161–1170 CrossRef CAS PubMed.
- G. Kuttan, Immunomodulatory effect of some naturally occuring sulphur-containing compounds, J. Ethnopharmacol., 2000, 72, 93–99 CrossRef CAS PubMed.
- C. S. Charron, H. D. Dawson, G. P. Albaugh, P. M. Solverson, B. T. Vinyard, G. I. Solano-Aguilar, A. Molokin and J. A. Novotny, A Single Meal Containing Raw, Crushed Garlic Influences Expression of Immunity- and Cancer-Related Genes in Whole Blood of Humans, J. Nutr., 2015, 145, 2448–2455 CrossRef CAS PubMed.
- Z. H. Feng, G. M. Zhang, T. L. Hao, B. Zhou, H. Zhang and Z. Y. Jiang, Effect of diallyl trisulfide on the activation of T cell and macrophage-mediated cytotoxicity, J. Tongji Med. Univ., 1994, 14, 142–147 CrossRef CAS PubMed.
- M. Colić, D. Vucević, V. Kilibarda, N. Radicević and M. Savić, Modulatory effects of garlic extracts on proliferation of T-lymphocytes in vitro stimulated with concanavalin A, Phytomedicine, 2002, 9, 117–124 CrossRef PubMed.
- M. Ebrahimi, Z. Mohammad Hassan, A. Mostafaie, N. Zare Mehrjardi and T. Ghazanfari, Purified Protein Fraction of Garlic Extract Modulates Cellular Immune Response against Breast Transplanted Tumors in BALB/c Mice Model, Cell J., 2013, 15, 65–75 Search PubMed.
- S. S. Percival, Aged Garlic Extract Modifies Human Immunity, J. Nutr., 2016, 146, 433S–436S CrossRef CAS PubMed.
- E. Yarnell, Herbs for Viral Respiratory Infections, Altern. Complement. Ther., 2018, 24, 35–43 CrossRef.
- J.-W. Ko, S.-H. Jeong, H.-J. Kwon, N.-R. Shin, Y.-S. Seo, J.-C. Kim, I.-S. Shin and J.-S. Kim, Preventive Effect of Garlic Oil and Its Organosulfur Component Diallyl-Disulfide on Cigarette Smoke-Induced Airway Inflammation in Mice, Nutrients, 2018, 10, 1659 CrossRef PubMed.
- I. V. Andrianova, I. A. Sobenin, E. V. Sereda, L. I. Borodina and M. I. Studenikin, Effect of long-acting garlic tablets ‘allicor’ on the incidence of acute respiratory viral infections in children, Ter. Arkh., 2003, 75, 53–56 CAS.
- P. Josling, Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey, Adv. Ther., 2001, 18, 189–193 CrossRef CAS PubMed.
- S. K. Lee, Y. J. Park, M. J. Ko, Z. Wang, H. Y. Lee, Y. W. Choi and Y.-S. Bae, A novel natural compound from garlic (Allium sativum L.) with therapeutic effects against experimental polymicrobial sepsis, Biochem. Biophys. Res. Commun., 2015, 464, 774–779 CrossRef CAS PubMed.
- J. S. Lee, M. J. Kim, S. H. Park, S. B. Lee, T. Wang, U. S. Jung, J. Im, E. J. Kim, K. W. Lee and H. G. Lee, Effects of dietary mixture of garlic (Allium sativum), coriander (Coriandrum sativum) and probiotics on immune responses and caecal counts in young laying hens, J. Anim. Physiol. Anim. Nutr., 2017, 101, e122–e132 CrossRef CAS PubMed.
- Z. Li, X. Ying, F. Shan and J. Ji, The association of garlic with Helicobacter pylori infection and gastric cancer risk: A systematic review and meta-analysis, Helicobacter, 2018, 23, e12532 CrossRef PubMed.
- X. Liu, A. Baecker, M. Wu, J.-Y. Zhou, J. Yang, R.-Q. Han, P.-H. Wang, A.-M. Liu, X. Gu, X.-F. Zhang, X.-S. Wang, M. Su, X. Hu, Z. Sun, G. Li, Z.-Y. Jin, S. Y. Jung, L. Mu, N. He, Q.-Y. Lu, L. Li, J.-K. Zhao and Z.-F. Zhang, Raw Garlic Consumption and Risk of Liver Cancer: A Population-Based Case-Control Study in Eastern China, Nutrients, 2019, 11, 2038 CrossRef CAS PubMed.
- X. Zhou, H. Qian, D. Zhang and L. Zeng, Garlic intake and the risk of colorectal cancer: A meta-analysis, Medicine, 2020, 99, e18575 CrossRef CAS PubMed.
- A. Muhammad, M. A. Ibrahim, O. L. Erukainure, I. Malami and A. Adamu, Spices with Breast Cancer Chemopreventive and Therapeutic Potentials: A Functional Foods Based-Review, Anticancer Agents Med. Chem., 2018, 18, 182–194 CrossRef CAS PubMed.
- Y. Zhang, X. Liu, J. Ruan, X. Zhuang, X. Zhang and Z. Li, Phytochemicals of garlic: Promising candidates for cancer therapy, Biomed. Pharmacother., 2020, 123, 109730 CrossRef PubMed.
- A. Pandrangi, Cancer Chemoprevention by Garlic – A Review, Hereditary Genet., 2015, 4, 147 Search PubMed.
- M. Miraghajani, N. Rafie, H. Hajianfar, B. Larijani and L. Azadbakht, Aged Garlic and Cancer: A Systematic Review, Int. J. Prev. Med., 2018, 9, 84 CrossRef PubMed.
- X. Hu, B. N. Cao, G. Hu, J. He, D. Q. Yang and Y. S. Wan, Attenuation of cell migration and induction of cell death by aged garlic extract in rat sarcoma cells, Int. J. Mol. Med., 2002, 9, 641–643 CAS.
- H. Ishikawa, T. Saeki, T. Otani, T. Suzuki, K. Shimozuma, H. Nishino, S. Fukuda and K. Morimoto, Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer, J. Nutr., 2006, 136, 816S–820S CrossRef CAS PubMed.
- F. Fallah-Rostami, M. A. Tabari, B. Esfandiari, H. Aghajanzadeh and M. Y. Behzadi, Immunomodulatory activity of aged garlic extract against implanted fibrosarcoma tumor in mice, N. Am. J. Med Sci., 2013, 5, 207–212 CrossRef PubMed.
- G. M. Suddek, Allicin enhances chemotherapeutic response and ameliorates tamoxifen-induced liver injury in experimental animals, Pharm. Biol., 2014, 52, 1009–1014 CrossRef CAS PubMed.
- G. Hodge, S. Davis, M. Rice, H. Tapp, B. Saxon and T. Revesz, Garlic compounds selectively kill childhood pre-B acute lymphoblastic leukemia cells in vitro without reducing T-cell function: Potential therapeutic use in the treatment of ALL, Biologics, 2008, 2, 143–149 CAS.
- W. You, L. M. Brown, L. Zhang, J. Li, M. Jin, Y. Chang, J. Ma, K. Pan, W. Liu, Y. Hu, S. Crystal-Mansour, D. Pee, W. J. Blot, J. F. Fraumeni, G. Xu and M. H. Gail, Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions, J. Natl. Cancer Inst., 2006, 98, 974–983 CrossRef PubMed.
- W.-Q. Li, J.-Y. Zhang, J.-L. Ma, Z.-X. Li, L. Zhang, Y. Zhang, Y. Guo, T. Zhou, J.-Y. Li, L. Shen, W.-D. Liu, Z.-X. Han, W. J. Blot, M. H. Gail, K.-F. Pan and W.-C. You, Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial, Br. Med. J., 2019, 366, l5016 CrossRef PubMed.
- H. Ishikawa, T. Saeki, T. Otani, T. Suzuki, K. Shimozuma, H. Nishino, S. Fukuda and K. Morimoto, Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer, J. Nutr., 2006, 136, 816S–820S CrossRef CAS PubMed.
- S. Tanaka, K. Haruma, M. Yoshihara, G. Kajiyama, K. Kira, H. Amagase and K. Chayama, Aged garlic extract has potential suppressive effect on colorectal adenomas in humans, J. Nutr., 2006, 136, 821S–826S CrossRef CAS PubMed.
- H. R. Ramírez-Concepción, L. N. Castro-Velasco and E. Martínez-Santiago, Efectos Terapéuticos del Ajo (Allium Sativum), Salud Adm, 2016, 3, 39–47 Search PubMed.
- C. Silagy and A. Neil, Garlic as a lipid lowering agent–a meta-analysis, J. R. Coll. Physicians Lond., 1994, 28, 39–45 CAS.
- N. Gupta and T. D. Porter, Garlic and garlic-derived compounds inhibit human squalene monooxygenase, J. Nutr., 2001, 131, 1662–1667 CrossRef CAS PubMed.
- M. Focke, A. Feld and K. Lichtenthaler, Allicin, a naturally occurring antibiotic from garlic, specifically inhibits acetyl-CoA synthetase, FEBS Lett., 1990, 261, 106–108 CrossRef CAS PubMed.
- S. C. Baek, K. H. Nam, S. A. Yi, M. S. Jo, K. H. Lee, Y. H. Lee, J. Lee and K. H. Kim, Anti-adipogenic Effect of β-Carboline Alkaloids from Garlic (Allium sativum), Foods, 2019, 8, 673 CrossRef CAS PubMed.
- A. A. Sangouni, M. R. Mohammad Hosseini Azar and M. Alizadeh, Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: a double-blind randomised controlled clinical trial, Br. J. Nutr., 2020, 124, 450–456 CrossRef CAS PubMed.
- R. Zadhoush, A. Alavi-Naeini, A. Feizi, E. Naghshineh and M. R. Ghazvini, The effect of garlic (Allium sativum) supplementation on the lipid parameters and blood pressure levels in women with polycystic ovary syndrome: A randomized controlled trial, Phytother. Res., 2021, 35, 6335–6342 CrossRef CAS PubMed.
- A. J. Adler and B. J. Holub, Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men, Am. J. Clin. Nutr., 1997, 65, 445–450 CrossRef CAS PubMed.
- D. Kannar, N. Wattanapenpaiboon, G. S. Savige and M. L. Wahlqvist, Hypocholesterolemic effect of an enteric-coated garlic supplement, J. Am. Coll. Nutr., 2001, 20, 225–231 CrossRef CAS PubMed.
- F. Zeb, M. Safdar, S. Fatima, S. Khan, S. Alam, M. Muhammad, A. Syed, F. Habib and H. Shakoor, Supplementation of garlic and coriander seed powder: Impact on body mass index, lipid profile and blood pressure of hyperlipidemic patients, Pak. J. Pharm. Sci., 2018, 31, 1935–1941 CAS.
- E.-S. Jung, S.-H. Park, E.-K. Choi, B.-H. Ryu, B.-H. Park, D.-S. Kim, Y.-G. Kim and S.-W. Chae, Reduction of blood lipid parameters by a 12-wk supplementation of aged black garlic: a randomized controlled trial, Nutrition, 2014, 30, 1034–1039 CrossRef CAS PubMed.
- W. H. Briggs, H. Xiao, K. L. Parkin, C. Shen and I. L. Goldman, Differential inhibition of human platelet aggregation by selected Allium thiosulfinates, J. Agric. Food Chem., 2000, 48, 5731–5735 CrossRef CAS PubMed.
- G. A. Benavides, G. L. Squadrito, R. W. Mills, H. D. Patel, T. S. Isbell, R. P. Patel, V. M. Darley-Usmar, J. E. Doeller and D. W. Kraus, Hydrogen sulfide mediates the vasoactivity of garlic, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 17977–17982 CrossRef CAS PubMed.
- H. Dubey, A. Singh, A. M. Patole and C. R. Tenpe, Antihypertensive effect of allicin in dexamethasone-induced hypertensive rats, Integr. Med. Res., 2017, 6, 60–65 CrossRef PubMed.
- D. Chaupis-Meza, J. Rojas, M. Gasco and G. F. Gonzales, Hypotensive effect of extract of macerated garlic (Allium sativum) for 18 weeks in an in vivo experimental model, Rev. Peru Med. Exp. Salud Publica, 2014, 31, 461–466 Search PubMed.
- X. Gao, Z. Xue, Q. Ma, Q. Guo, L. Xing, R. K. Santhanam, M. Zhang and H. Chen, Antioxidant and antihypertensive effects of garlic protein and its hydrolysates and the related mechanism, J. Food Biochem., 2020, 44, e13126 Search PubMed.
- K. Ried, Garlic Lowers Blood Pressure in Hypertensive Individuals, Regulates Serum Cholesterol, and Stimulates Immunity: An Updated Meta-analysis and Review, J. Nutr., 2016, 146, 389S–396S CrossRef CAS PubMed.
- X. Chen, S. Pang, J. Lin, J. Xia and Y. Wang, Allicin prevents oxidized low-density lipoprotein-induced endothelial cell injury by inhibiting apoptosis and oxidative stress pathway, BMC Complementary Altern. Med., 2016, 16, 133 CrossRef PubMed.
- C.-L. Li, X.-H. Liu, Y. Qiao, L.-N. Ning, W.-J. Li, Y.-S. Sun, D.-S. Liu, W. Gao and C.-M. Ma, Allicin alleviates inflammation of diabetic macroangiopathy via the Nrf2 and NF-kB pathway, Eur. J. Pharmacol., 2020, 876, 173052 CrossRef CAS PubMed.
- N. Zhang, X. Huang, Y. Zeng, X. Wu and X. Peng, Study on prebiotic effectiveness of neutral garlic fructan in vitro, Food Sci. Hum., 2013, 2, 119–123 Search PubMed.
- K. Ried, Garlic lowers blood pressure in hypertensive subjects, improves arterial stiffness and gut microbiota: A review and meta-analysis, Exp. Ther. Med., 2020, 19, 1472–1478 CAS.
- G. Kaur, R. Padiya, R. Adela, U. K. Putcha, G. S. Reddy, B. R. Reddy, K. P. Kumar, S. Chakravarty and S. K. Banerjee, Garlic and Resveratrol Attenuate Diabetic Complications, Loss of β-Cells, Pancreatic and Hepatic Oxidative Stress in Streptozotocin-Induced Diabetic Rats, Front. Pharmacol., 2016, 7, 360 CAS.
- M. Thomson, K. K. Al-Qattan, D. Js and M. Ali, Anti-diabetic and anti-oxidant potential of aged garlic extract (AGE) in streptozotocin-induced diabetic rats, BMC Complementary Altern. Med., 2016, 16, 17 CrossRef PubMed.
- J. Wang, X. Zhang, H. Lan and W. Wang, Effect of garlic supplement in the management of type 2 diabetes mellitus (T2DM): a meta-analysis of randomized controlled trials, Food Nutr. Res., 2017, 61, 1377571 CrossRef PubMed.
- A. A. Sangouni, M. Alizadeh, A. Jamalzehi and K. Parastouei, Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: A randomized clinical trial, Phytother. Res., 2021, 35, 4433–4441 CrossRef CAS PubMed.
- D. J. Yang, S. H. Moh, D. H. Son, S. You, A. W. Kinyua, C. M. Ko, M. Song, J. Yeo, Y.-H. Choi and K. W. Kim, Gallic Acid Promotes Wound Healing in Normal and Hyperglucidic Conditions, Molecules, 2016, 21, 899 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2022 |