Association between maternal vitamin D levels and risk of adverse pregnancy outcomes: a systematic review and dose–response meta-analysis†
Rui
Zhao
a,
Leilei
Zhou
a,
Shanshan
Wang
a,
Guoping
Xiong
b and
Liping
Hao
 *a
*a
aDepartment of Nutrition and Food Hygiene, Hubei Key Laboratory of Food Nutrition and Safety and the Ministry of Education (MOE) Key Laboratory of Environment and Health, School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China. E-mail: haolp@mails.tjmu.edu.cn; Fax: +0086-27-83693307; Tel: +86-27-83650523
bThe Central Hospital of Wuhan, Wuhan, Hubei, China
First published on 3rd December 2021
Abstract
Epidemiological studies have investigated the associations between vitamin D and the risk of adverse pregnancy outcomes; however, the results are conflicting and dose–response relationships remain to be confirmed. This study aimed to summarize previous studies on the associations of vitamin D levels with the risk of gestational diabetes mellitus (GDM), pre-eclampsia (PE), gestational hypertension (GH), and caesarean section (C-section), and to clarify the dose–response trends. PubMed, Embase, Scopus, and Web of Science were searched to identify eligible articles. A total of 69 prospective observational studies including cohort studies, case-cohort studies, or nested case-control studies were included in the current systematic review, of which 68 studies were available for meta-analysis. Compared with the lowest level, the highest level of 25(OH)D was significantly associated with a lower risk of GDM (RR: 0.76; 95% CI: 0.66–0.87), PE (RR: 0.74; 95% CI: 0.60–0.90;), and GH (RR: 0.87; 95% CI: 0.79–0.97); however, no significant relationship was found for C-section (RR: 1.00; 95% CI: 0.90–1.12). There was significant between-study heterogeneity for GDM (I2 = 69.2%; Pheterogeneity < 0.001), PE (I2 = 52.0%; Pheterogeneity = 0.001), and C-section (I2 = 59.1%; Pheterogeneity < 0.001), while no heterogeneity was found for GH (I2 = 0.0%; Pheterogeneity = 0.676). For each 25 nmol L−1 increase in 25(OH)D, the pooled RR was 0.92 (95% CI: 0.86–0.97) for GDM and 0.89 (95% CI: 0.84–0.94) for PE, respectively. Notably, the dose–response analysis showed a non-linear relationship between maternal 25(OH)D levels and the risk of PE (Pnon-linearity = 0.009). Our meta-analysis provides further scientific evidence of the inverse association between 25(OH)D levels and the risk of GDM, PE, and GH, which may be useful for the prevention of pregnancy complications. However, more evidence from prospective studies is needed regarding the dietary intake of vitamin D during pregnancy.
Introduction
Gestational diabetes mellitus (GDM), pre-eclampsia (PE), gestational hypertension (GH), and caesarean section (C-section) are serious adverse pregnancy outcomes that increase the risk of maternal and fetal/neonatal death and long-term health risks for the mother and offspring, such as diabetes mellitus, obesity, and cardiovascular disease.1–4 Vitamin D is an essential fat-soluble steroid hormone mainly produced through dietary intake and skin exposure to ultraviolet B rays from sunlight.5 However, increased air pollution, lifestyle changes, and the use of sunscreen products have further affected the synthesis of vitamin D, leading to a widespread prevalence of vitamin D deficiency, especially in pregnant women.6–8 In addition to the well-documented effect in regulating calcium and phosphorus balance and maintaining bone health, numerous studies have identified that vitamin D has anti-inflammatory and immunomodulatory functions,9,10 which take on pivotal roles in pregnancy.Observational studies have extensively investigated the associations of maternal vitamin D deficiency with the risk of adverse pregnancy outcomes, but the results are inconsistent.11–16 Some cohort studies have found that vitamin D deficiency is associated with a reduced risk of GDM16 and PE.11 However, the results of some other studies showed no significant association between vitamin D deficiency and the risk of GDM, PE, GH, or C-section.12–15 Since 2011, many meta-analyses of observational studies have been published, showing that maternal vitamin D status is inversely associated with the risk of GDM17,18 and PE,19 but not with C-section.20 However, previous studies had some limitations in their design and therefore no clear conclusions could be drawn. For example, some research included studies with cross-sectional designs or case-control studies, which may affect the reliability of the results. To the best of our knowledge, no meta-analysis has examined the relationship between vitamin D levels and the risk of GH, and only one study in 2013 assessed the relationship between vitamin D levels and the risk of C-section. In addition, most of the studies did not explore the dose–response relationship of vitamin D levels with the risk of adverse pregnancy outcomes.
Due to the lack of a comprehensive meta-analysis of prospective studies on pregnancy complications, we performed this meta-analysis to provide updated evidence on the association of maternal blood and dietary levels of vitamin D with the risk of adverse pregnancy outcomes.
Methods
Search strategy
This meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement (ESI Table S1†),21 and our protocol has been registered in PROSPERO (CRD42021244431). We conducted a systematic search of electronic databases including PubMed, Embase, Scopus, and Web of Science from the inception to January 19, 2021. In short, we searched for the following keywords: (“Vitamin D” OR “25-hydroxyvitamin D” OR “25(OH)D” OR “Cholecalciferol” OR “Ergocalciferol”) AND (“Gestational diabetes mellitus” OR “Pre-eclampsia” OR “Hypertension, pregnancy-induced” OR “Caesarean section” OR “Pregnancy outcome”). Details of the search strategy are provided in ESI Table S2.† Additionally, the bibliographies of relevant meta-analyses were manually searched to identify eligible literature.Study selection
We included studies that met the following criteria: (1) studies with prospective design (cohort, case-cohort, or nested case-control studies); (2) reported the intake of vitamin D or 25(OH)D level as exposure; (3) reported the incidence of pregnancy outcomes such as GDM, GH, PE, and C-section as the outcome variables; (4) reported risk estimates and 95% confidence intervals (CIs), or provided sufficient data to calculate these values; and (5) for dose–response analysis, studies should report at least three exposure categories and provide the number of cases and participants in each category. We excluded letters, commentaries, reviews, meta-analyses, conference abstracts, studies without original data, and non-English articles.Data extraction and quality assessment
Two investigators (RZ and LZ) independently extracted the following information from each eligible study using a standardized data collection form: first author's name, year of publication, country, study design, mean age or age range of participants, sample size, vitamin D assessment methods, type of outcomes, and adjustment factors. The study quality of selected studies was assessed using the Newcastle Ottawa Quality Assessment Scale (NOS).22 Studies scoring more than six stars are regarded as high in quality.Statistical methods
RRs and corresponding 95% CIs were used as the risk estimates for studies, and HRs and ORs were considered approximately equal to the RRs. A random-effects model was used to pool RRs and 95% CIs for the comparison of the highest versus lowest category of exposure.23 We used risk estimates from the multivariate models. Heterogeneity between studies was evaluated by Cochran's Q test (P < 0.10) and the I2 statistic test.24I2 values of 25%, 50%, and 75% were considered as low, moderate, and high heterogeneity, respectively.25 We conducted the subgroup analyses to identify sources of heterogeneity by potential influencing factors such as study type, geographic location, study quality, sample size, 25(OH)D assay methods, blood sample type, trimester of sample collection, and whether adjusting for important factors. Meta-regression was performed to explore the heterogeneity between subgroups.26 Funnel plots and Egger's regression test27 were used to assess the publication bias. We also performed sensitivity analyses with a random-effects model to assess the effect of excluding each study on the overall estimates.For the dose–response meta-analysis, 25(OH)D concentrations of ng mL−1 were converted to nmol L−1 by multiplying the values by 2.5. In studies that provided at least three categories of vitamin D levels, we extracted the mean or median vitamin D level in each category. When studies reported range values, we calculated the midpoint between the lower and upper limits of the category. If the highest category was open-ended, the width of the adjacent interval was used to calculate the upper bound. For studies that did not use the lowest category as the reference, we recalculated risk estimates using the method described by Hamling et al.28
A linear dose–response analysis of a random-effects model was performed using the generalized least squares regression to estimate the RRs for every 25 nmol L−1 increments in 25(OH)D levels.29 In addition, we examined the possible non-linear dose–response relationships by modeling the 25(OH)D levels through restricted cubic splines with 3 knots at the 10th, 50th, and 90th percentiles of the distribution.30,31 The non-linear P value (Pnon-linearity) was calculated by a likelihood ratio test.30,32 We used STATA version 15.1 (StataCorp, College Station, TX) for all analyses. Statistical tests were performed using a two-tailed method with a significance level of P < 0.05.
Results
Literature search and study characteristics
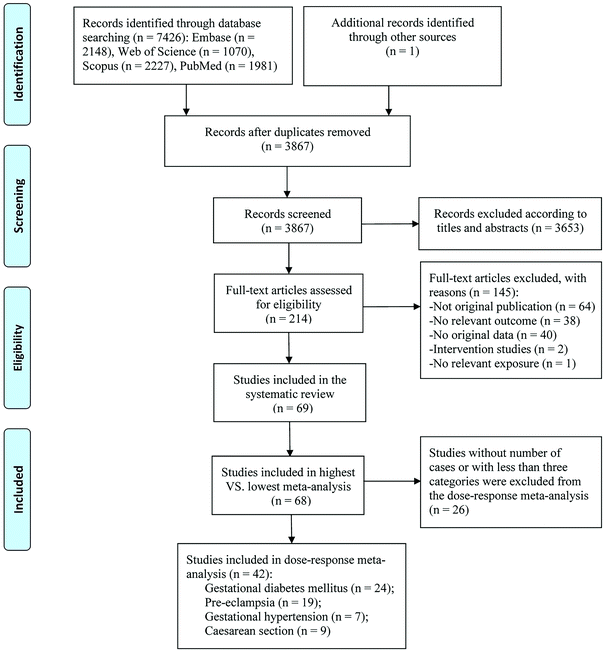
Our search retrieved 7427 records, of which 7212 were excluded by the initial screening according to titles and abstracts. After the full-text screening, 145 articles were further excluded (ESI Table S3†), and we finally included 69 articles published from 2007 to 2021 (Fig. 1). Only one study reported the effect of vitamin D intake,33 the other 68 were studies on 25(OH)D levels, of which 36 studies were included in the meta-analysis for GDM,14–16,34–66 26 for PE,11,14,37,40,41,46,47,53,58,63,66–81 11 for GH,14,37,42,46,53,63,74,77,78,82,83 and 24 for C-section.12,16,37,38,41,42,46,47,53,56,66,84–96 For 25(OH)D levels, 17 articles detected 25(OH)D by LC-MS,12,36,37,39,40,44,49,50,52,59,61,69,70,74,75,80,89 10 by CLIA,11,14,41–43,55,64,79,81,91 eight by ECLIA,34,35,46,51,54,63,66,67 eight by ELISA,38,47,72,84,87,90,92,96 seven by RIA,15,45,57,60,71,77,82 six by HPLC,56,68,76,86,88,93 and three by EIA.65,78,95 Vitamin D intake was assessed using the food frequency questionnaire.33 Among all included articles, there were 46 cohort studies,12,14–16,33,34,37,38,40,42,44–51,53,54,56,59,62–64,66,67,74,76–79,81,83–94,96 21 nested case-control studies,11,35,39,41,43,52,55,57,58,60,61,65,68,70–73,75,80,82,95 and two case-cohort studies.36,69 Twenty articles were conducted in Asia,15,16,34,38,44,49,51,54,59,60,62–64,66,81,85,87,89,95,96 23 in North America,11,12,36,39,43,47,50,53,55,61,65,68–70,72,75–77,79,80,82,93,94 19 in Europe,33,37,41,45,46,52,56,57,67,71,73,74,78,83,84,88,90–92 five in Australia and New Zealand,14,40,42,48,58 and two in Africa.35,86 Forty-two studies were of high quality (ESI Tables S4 and S5†). The characteristics of included studies are shown in Table 1.| Author, year | Country, study type | Study period | Sample size | Age range or mean age (years) | Mean ± SD or median (IQR) vitamin D concentration | Exposure assessment method | Gestational week for vitamin D measurement | Categories of vitamin D level | Outcomes | NOS score | Adjusted variables |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbreviations: CI, confidence interval; CLIA, chemiluminescent Immunoassay; C-section, caesarean section; ECLIA, electrochemical luminescence immunoassay; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; FFQ, food frequency questionnaire; GDM, gestational diabetes mellitus; GH, gestational hypertension; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography-mass spectrometry; NOS, Newcastle Ottawa scale; NR, not reported; PE, pre-eclampsia; RIA, radioimmunoassay; RR, relative risk; T1, first trimester; T2, second trimester. Adjusted variables: (1) maternal age, (2) pre-pregnancy BMI/weight, (3) parity, (4) education, (5) race/ethnicity, (6) smoking, (7) alcohol consumption, (8) gestational age of blood sampling, (9) sampling season, (10) family history, (11) physical activity, (12) maternal height, (13)study site, (14) socioeconomic status,(15) gestational weeks at admission, (16) abnormal pregnancy history, (17) supplementation, (18) infant sex, (19) cholesterol, (20) high density lipoprotein, (21) triglyceride, (22) fasting plasma glucose, (23) CRP, (24) anaemia status, (25) CD4 cell count, (26) HIV RNA level, (27) ARV regimen, (28) skin color, (29) gestational weight gain, (30) sun exposure, (31) HbA1c, (32) menarche age, (33) menstrual cycle, (34) birth weight, (35) marital status, (36)religion, (37) blood pressure, (38) parathyroid hormone status, (39)gravidity, (40) homocysteine, (41) folate. | |||||||||||
| Chen et al. 202016 | China, Retrospective cohort | 2017–2018 | 2814 | 30.5 ± 4.98 | 53.1 ± 9.9 nmol L−1 | Colloidal gold immunochromatography | 16.3 ± 2.3 | <50 nmol L−1 | GDM, C-section | 8 | (1), (2), (3), (9) |
| ≥50 nmol L−1 | |||||||||||
| Xu et al. 201862 | China, Prospective cohort | 2015–2016 | 827 | Case: 29 (26–34), Control: 25 (22–28) | 15.3 (10.4–21.7) ng mL−1 | NR | At the first prenatal visit | <10.4 ng mL−1 | GDM | 7 | (1), (2), (5), (6), (8), (9), (10), (11), (14), (15), (16), (17), (19), (20), (21), (22), (23) |
| 10.4–15.3 ng mL−1 | |||||||||||
| 15.4–21.7 ng mL−1 | |||||||||||
| >21.7 ng mL−1 | |||||||||||
| Zhu et al. 201915 | China, Prospective cohort | 2013–2014 | 3110 | 26 7 ± 3 7 | 18.2 ± 8.4 ng mL−1 | RIA | <14 | <20 ng mL−1 | GDM | 9 | (1), (2), (3), (4), (6), (7), (9), (10), (14) |
| 20–30 ng mL−1 | |||||||||||
| >30 ng mL−1 | |||||||||||
| Yang et al. 201863 | China, Prospective cohort | 2013–2017 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
32 ± 4.2 | NR | ECLIA | 16 | <30 nmol L−1 | GDM, PE, GH | 5 | None |
| 30–50 nmol L−1 | |||||||||||
| >50 nmol L−1 | |||||||||||
| Al-Ajlan et al. 201834 | Saudi Arabia, Prospective cohort | NR | 419 | 28.7 ± 6.1 | 19.1 ± 15.1 nmol L−1 | ECLIA | 11.2 ± 3.4 | <50 nmol L−1 | GDM | 7 | (1), (2), (3), (9), (10), (11), (21), (29), (30), (31) |
| ≥50 nmol L−1 | |||||||||||
| Chen et al. 202085 | China, Retrospective cohort | 2015–2017 | 261 | 30.1 ± 4.0 | 22.2 ± 9.0 ng mL−1 | NR | 24–28 | <20 ng mL−1 | C-section | 4 | None |
| ≥20 ng mL−1 | |||||||||||
| Gernand et al. 201512 | U.S., Prospective cohort | 1959–1966 | 2798 | NR | 50.3 ± 27.8 nmol L−1 | LC-MS | ≤26 | <30 nmol L−1 | C-section | 7 | (2), (5), (13) |
| 30–49 nmol L−1 | |||||||||||
| 50–74 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Hemmingway et al. 201874 | Ireland, Prospective cohort | 2008–2011 | 1754 | 30.5 ± 4.5 | 22.7 ± 10.3 ng mL−1 | LC-MS | 15 | <30 nmol L−1 | GH, PE | 7 | GH: (2), (6), (11). PE: (2), (4), (17). SGA: (4), (11), (14) |
| 30–<75 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Yuan et al. 201796 | China, Prospective cohort | 2012–2015 | 1924 | Case: 30.2 ± 3.8, Control: 28.9 ± 3.0 | 43.4 (35.2–56.9) nmol L−1 | ELISA | T2 | <25 nmol L−1 | C-section | 8 | (1), (2), (3), (9), (10), (13), (16), (32), (33) |
| 25–37.4 nmol L−1 | |||||||||||
| 37.5–49.9 nmol L−1 | |||||||||||
| 50–74.9 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Al-Shafei et al. 202035 | Sudan, Nested case-control | 2017.1–2017.11 | Case: 60 Control: 60 | Case: 29.2 ± 6.0, Control: 28.0 ± 5.7 | Case: 7.3 (5.7–8.8) ng mL−1, Control: 8.4 (6.6–11.9) ng mL−1 | ECLIA | ≤14 | <6 ng mL−1 | GDM | 6 | None |
| ≥6 ng mL−1 | |||||||||||
| Yue et al. 202064 | China, Retrospective cohort | 2018–2020 | 8468 | NR | NR | CLIA | ≤20 | <20 ng mL−1 | GDM | 8 | (1), (2), (3), (19), (21) |
| 20–30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Abd Aziz et al. 202038 | Malaysia, Prospective cohort | NR | 60 | 34.8 ± 3.9 | 34.5 ± 14.1 nmol L−1 | ELISA | 12–14 | ≤50 nmol L−1 | GDM, C-section | 6 | None |
| >50 nmol L−1 | |||||||||||
| Dwarkanath et al. 201944 | India, Prospective cohort | 2008–2014 | 392 | 23.9 ± 3.8 | 34.4 (23.8–45.8) nmol L−1 | LC-MS | 12 | <30 nmol L−1 | GDM | 9 | (1), (2), (3), (4), (9), (11) |
| <50 nmol L−1 | |||||||||||
| <75 nmol L−1 | |||||||||||
| Li et al. 202051 | China, Retrospective cohort | 2014–2017 | 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 417 |
30.6 ± 3.5 | 42.9 (32.9–51.9) nmol L−1 | ECLIA | 16 | <50 nmol L−1 | GDM | 7 | (1) |
| ≥50 nmol L−1 | |||||||||||
| Xia et al. 201861 | U.S., Nested case-control | 2009–2013 | Cases: 107, Control: 214 | Case:30.5 ± 5.7, Control:30.4 ± 5.4 | NR | LC-MS | 10–14 | <50 nmol L−1 | GDM | 8 | (1), (2), (3), (5), (8), (9), (10), (11), (13) |
| 15–26 | ≥50 nmol L−1 | ||||||||||
| Thiele et al. 201994 | U.S., Retrospective cohort | 2009–2013 | 357 | 30.6 ± 4.5 | 29.9 ± 10.9 ng mL−1 | NR | <36 | ≤20.9 ng mL−1 | C-section | 5 | None |
| 21.0–29.9 ng mL−1 | |||||||||||
| >30 ng mL−1 | |||||||||||
| Shao et al. 202059 | China, Prospective cohort | 2011–2018 | 2789 | 28.7 ± 3.8 | 18.6 ± 8.6 ng mL−1 | LC-MS | 8–14 | <20 ng mL−1 | GDM | 9 | (1), (2), (3), (4), (8), (9), (11), (14), (29) |
| ≥20 ng mL−1 | |||||||||||
| Salakos et al. 202157 | French and Belgium, Nested case-control | 2012–2014 | Case: 250, Control: 941 | Case: 32.8 ± 5.3, Control: 32.3 ± 5.0 | Case: 21.1 ± 10 ng mL−1, Control: 22.7 ± 10 ng mL−1 | RIA | 10–14 | <10 ng mL−1 | GDM | 6 | None |
| 10–30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Öcal et al. 201990 | Turkey, Prospective cohort | 2012–2014 | 600 | Case: 18.4 ± 1.3, Control: 28.7 ± 5.4 | Case: 15.4 ± 7.9 ng mL−1, Control: 14.9 ± 4.7 ng mL−1 | ELISA | During pregnancy | <10.9 ng mL−1 | C-section | 4 | None |
| ≥10.9 ng mL−1 | |||||||||||
| Kısa et al. 202088 | Turkey, Prospective cohort | 2017 | 86 | 18–40 | 13.6 ± 6.6 ng mL−1 | HPLC | 11–13 | ≤10 ng mL−1 | C-section | 6 | None |
| >10 ng mL−1 | |||||||||||
| Iqbal et al. 202049 | India, Prospective cohort | 2019 | 290 | 24.9 ± 2.7 | Case: 33.5 ± 16.3 nmol L−1, Control: 38.2 ± 18.5 nmol L−1 | LC-MS | T1 | <30 nmol L−1 | GDM | 8 | (1), (2), (3), (4), (9), (11) |
| 30–50 nmol L−1 | |||||||||||
| 50–75 nmol L−1 | |||||||||||
| 50–75 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Bomba-Opon et al. 201483 | Poland, Prospective cohort | NR | 280 | NR | NR | NR | 11–13 | <20 ng mL−1 | GH | 5 | None |
| ≥20 ng mL−1 | |||||||||||
| Bozdag et al. 202041 | Turkey, Nested case-control | NR | 283 | NR | 9.5 ng mL−1 | CLIA | T1 | <10 ng mL−1 | GDM, PE, C-section | 5 | None |
| ≥10 ng mL−1 | |||||||||||
| Hajianfar et al. 201987 | Iran, Prospective cohort | NR | 812 | NR | NR | ELISA | 8–16 | <10 ng mL−1 | C-section | 6 | None |
| 32–34 | 10–29 ng mL−1 | ||||||||||
| >30 ng mL−1 | |||||||||||
| Griew et al. 201948 | Australia, Prospective cohort | 2011–2013 | 742 | 29.1 ± 4.9 | 43.5 ± 21.9 nmol L−1 | NR | 6–18 | <12.5 nmol L−1 | GDM | 6 | None |
| 12.5–29.9 nmol L−1 | |||||||||||
| 30–49.9 nmol L−1 | |||||||||||
| ≥50 nmol L−1 | |||||||||||
| Benachi et al. 201971 | French and Belgium, Nested case-control | 2012–2014 | Case: 83, Control: 319 | Case: 32.2 ± 5.9, Control: 31.7 ± 5.0 | Case: 20.1 ± 9.3 ng mL−1, Control: 22.3 ± 11.1 ng mL−1 | RIA | 10–14 | <10 ng mL−1 | PE | 5 | None |
| 10–30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Wilson et al. 201814 | Australia and New Zealand, Prospective cohort | 2004–2008 | 2800 | 28 ± 6 | 68.1 ±27.1 nmol L−1 | CLIA | 14–16 | <44 nmol L−1 | PE, GH, GDM | 9 | (1), (2), (5), (6), (7), (11), (13) |
| 44–63 nmol L−1 | |||||||||||
| 63–81 nmol L−1 | |||||||||||
| >81 nmol L−1 | |||||||||||
| Eggemoen et al. 201845 | Norway, Prospective cohort | 2008–2010 | 745 | 29.8 (29.5–30.2) | 50.2 (48.3–52.1) nmol L−1 | RIA | 15 | <50 nmol L−1 | GDM | 8 | (1), (3), (4), (5), (9), (13) |
| ≥50 nmol L−1 | |||||||||||
| Wen et al. 201795 | China, Nested case-control | 2012–2015 | 4718 | NR | 43.7 (35.5–57.9) nmol L−1 | EIA | Mid-late pregnancy | <25.0 nmol L−1 | C-section | 8 | (1), (2), (3), (8), (9), (10), (13), (16), (32), (33) |
| 25.0–37.4 nmol L−1 | |||||||||||
| 37.5–49.9 nmol L−1 | |||||||||||
| 50.0–74.9 nmol L−1 | |||||||||||
| >75.0 nmol L−1 | |||||||||||
| Gbadegesin et al. 201786 | Nigeria, Prospective cohort | 2012–2013 | 461 | 31.26 | NR | HPLC | 10–28 | 0–20 ng mL−1 | C-section | 5 | None |
| 21–30 ng mL−1 | |||||||||||
| >30 ng mL−1 | |||||||||||
| Van Weert et al. 201678 | The Netherlands, Prospective cohort | 2003–2004 | 2074 | 30.2 ± 4.6 | 60.0 ±29.8 nmol L−1 | EIA | <17 | <20 nmol L−1 | PE, GH | 8 | (1), (2), (4), (5), (6) |
| 20–29.9 nmol L−1 | |||||||||||
| 30–49.9 nmol L−1 | |||||||||||
| ≥50 nmol L−1 | |||||||||||
| Dodds et al. 201643 | Canada, Nested case-control | 2002–2010 | Case: 395, Control: 1925 | NR | Case: 45.5 (35.9–56.7) nmol L−1, Control: 51.9 (40.6–62.4) nmol L−1 | CLIA | <20 | <30 nmol L−1 | GDM | 8 | (1), (2), (8), (9), (13) |
| 30–<50 nmol L−1 | |||||||||||
| ≥50 nmol L−1 | |||||||||||
| Boyle et al. 201640 | New Zealand, Prospective cohort | 2005–2008 | 1710 | 30.3 ± 4.7 | 72.9 ± 27.0 nmol L−1 | LC-MS | 15 | <25 nmol L−1 | PE, GDM | 8 | (2), (5) |
| 25–49.9 nmol L−1 | |||||||||||
| 50–74.9 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Baca et al. 201669 | U.S., Case-cohort | 1999–2010 | Cases: 650 Sub-cohort: 2327 | NR | Case: 57.8 (57.3–58.3) nmol L−1, Sub-cohort: 64.6 (64.4–64.8) nmol L−1 | LC-MS | <20 | <25 nmol L−1 | PE | 8 | (1), (2), (3), (4), (5), (6), (8), (9), (14), (35) |
| 25–50 nmol L−1 | |||||||||||
| 50–75 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Ates et al. 201637 | Turkey, Prospective cohort | 2012–2014 | 229 | 29.5 ± 4.9 | 13 ± 9.4 ng mL−1 | LC-MS | 11–14 | <10 ng mL−1 | GDM, GH, PE, C-section | 7 | None |
| ≥10 ng mL−1 | |||||||||||
| Rodriguez et al. 201556 | Spain, Prospective cohort | 2003–2008 | 2382 | 32.0 ± 4.2 | 29.4 (21.8–37.2) ng mL−1 | HPLC | 13.5 ± 2 | <20 ng mL−1 | GDM, C-section | 9 | (1), (2), (3), (4), (6), (7), (13), (14), (18) |
| 20–29.99 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Nobles et al. 201553 | U.S., Prospective cohort | 2007–2012 | 237 | NR | 30.4 ± 12.0 ng mL−1 | NR | 15.2 ± 4.7 | <30 ng mL−1 | GDM, GH, PE, C-section | 9 | (1), (2), (5), (8), (9), (29) |
| ≥30 ng mL−1 | |||||||||||
| Loy et al. 201589 | Singapore, Prospective cohort | 2009–2010 | 940 | 30.5 ± 5.1 | 81.0 ± 27.2 nmol L−1 | LC-MS | 26–28 | ≤75 nmol L−1 | C-section | 8 | (1), (2), (3), (4), (5), (6), (11), (16), (18) |
| >75 nmol L−1 | |||||||||||
| Jain et al. 201560 | India, Nested case-control | NR | Case: 32, Control: 178 | <45 | Case:11.9 ± 3.4 nmol L−1, Control: 22.3 ± 15.3 nmol L−1 | RIA | <20 | <20 ng mL−1 | GDM | 6 | None |
| 20–29 ng mL−1 | |||||||||||
| >30 ng mL−1 | |||||||||||
| Gidlöf et al. 201573 | Sweden, Nested case-control | 1994–1995 | Case: 39, Control: 120 | Case: 29.2 ± 5.4, Control: 29.2 ± 4.6 | Case: 52.2 ± 20.5 nmol L−1, Control: 48.6 ± 20.5 nmol L−1 | NR | 12 | <50 nmol L−1 | PE | 6 | None |
| ≥50 nmol L−1 | |||||||||||
| Flood-Nichols et al. 201547 | U.S., Retrospective cohort | 2014 | 235 | 24.3 ± 4.4 | 27.6 (13–71.6) ng mL−1 | ELISA | 8–12 | <50 nmol L−1 | GDM, PE, C-section | 7 | (2), (5), (6), (9) |
| 50–75 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Davies-Tuck et al. 201542 | Australia, Prospective cohort | 2009–2010 | 1550 | 30.0 ± 5.4 | 47.0 (12–178) nmol L−1 | CLIA | 13.7 ± 3.3 | <50 nmol L−1 | GDM, GH, C-section | 7 | (1), (2), (3), (13) |
| 50–74 nmol L−1 | |||||||||||
| >74 nmol L−1 | |||||||||||
| Aydogmus et al. 201584 | Turkey, Prospective cohort | 2013–2014 | 148 | Groups I: 23.9 ± 4.6, Groups II: 24.9 ± 5.9 | Groups I: 10.8 ± 3.8 ng mL−1, Groups II: 23.8 ± 13.3 ng mL−1 | ELISA | >28 | <15 ng mL−1 | C-section | 5 | None |
| ≥15 ng mL−1 | |||||||||||
| Arnold et al. 201536 | U.S., Case-cohort | 1996–2008 | Case: 135, Control: 517 | Case: 33.5 ± 4.6, Control: 32.6 ± 4.4 | Case: 27.3 ± 8.7 ng mL−1, Control: 29.3 ± 8.3 ng mL−1 | LC-MS | 16 | <20 ng mL−1 | GDM | 8 | (1), (2), (5), (9), (10) |
| 20–29 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Anderson et al. 201582 | U.S., Nested case-control | NR | Case: 37, Control: 11 | Case: 25.3 ± 0.7, Control: 24.2 ± 0.6 | NR | RIA | T1 | <20 ng mL−1 | GH | 6 | None |
| 21–29 ng mL−1 | |||||||||||
| >30 ng mL−1 | |||||||||||
| Alvarez-Fernandez et al. 201567 | Spain, Retrospective cohort | 2010–2013 | 257 | NR | Case: 35.8 (27.6–46.0) nmol L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) , Control: 33.9 (23.8–44.9) nmol L−1 , Control: 33.9 (23.8–44.9) nmol L−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
ECLIA | 9–12 | <50 nmol L−1 | PE | 7 | None |
| ≥50 nmol L−1 | |||||||||||
| Achkar et al. 201511 | Canada, Nested case-control | 2002–2010 | Case: 169, Control: 1975 | NR | Case: 47.2 ±17.7 nmol L−1, Control: 52.3 ±17.2 nmol L−1 | CLIA | <20 | <30 nmol L−1 | PE | 8 | (1), (2), (3), (6), (8), (9), (13) |
| 30–<50 nmol L−1 | |||||||||||
| ≥50 nmol L−1 | |||||||||||
| Zhou et al. 201466 | China, Prospective cohort | 2010–2012 | 1953 | Group A: 29.2 ± 3.5, Group B: 29.5 ± 3.6, Group C: 30.3 ± 3.9 | 27.03 ± 7.92 ng mL−1 | ECLIA | 16–20 | ≤20 ng mL−1 | GDM, PE, C-section | 6 | None |
| 21–29 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Wetta et al. 201480 | U.S., Nested case-control | 2007–2008 | Case: 89, Control: 177 | Case: 26.1 ± 5.5, Control: 25.2 ± 6 | Case: 27.4 ± 14.4 ng mL−1, Control: 28.6 ± 12.6 ng mL−1 | LC-MS | 15–21 | <15 ng mL−1 | PE | 9 | (1), (2), (3), (5), (6), (8), (9), (16) |
| <30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Park et al. 201454 | Korea, Prospective cohort | 2011–2012 | 523 | Case: 34.8 ± 3.6, Control: 33.6 ± 3.7 | Case: 35.3 ± 16.5 nmol L−1, Control: 32 ± 14.5 nmol L−1 | ECLIA | 12–14 | <25.0 nmol L−1 | GDM | 8 | (1), (2), (8), (9), (16), (17) |
| 25.0–49.9 nmol L−1 | |||||||||||
| ≥50.0 nmol L−1 | |||||||||||
| Schneuer et al. 201458 | Australia, Nested case-control | 2006–2007 | 5109 | NR | 56.4 (43.3–69.8) nmol L−1 | NR | 10–14 | <37.5 nmol L−1 | PE, GDM | 8 | (1), (2), (3), (6), (9), (16), (13), (14) |
| 37.5–49.9 nmol L−1 | |||||||||||
| 50–75 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Reichetzeder et al. 201492 | Germany, Prospective cohort | 2007–2008 | 547 | 30.9 ± 6.1 | 18 ± 19 nmol L−1 | ELISA | Prior to delivery | <1 nmol L−1 | C-section | 5 | None |
| 1–25 nmol L−1 | |||||||||||
| ≥25 nmol L−1 | |||||||||||
| Lacroix et al. 201450 | Canada, Prospective cohort | NR | 655 | 28.4 ± 4.5 | 63.0 ± 18.8 nmol L−1 | LC-MS | 6–13 | <50 nmol L−1 | GDM | 6 | None |
| 50–74.9 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Scholl et al. 201376 | U.S., Prospective cohort | 2001–2007 | 1141 | 22.8 ± 5.4 | NR | HPLC | 13.7 ± 5.7 | <12 ng mL−1 | PE | 8 | (1), (2), (3), (5), (6), (15) |
| 12–15.9 ng mL−1 | |||||||||||
| 16.0–19.9 ng mL−1 | |||||||||||
| ≥20.0 ng mL−1 | |||||||||||
| Wei et al. 201279 | Canada, Prospective cohort | 2004–2006 | 697 | Case: 30.9 ± 5.3, Control: 30.3 ± 4.8 | Case: 51.1 ± 14.8 nmol L−1, Control: 56.0 ± 19.1 nmol L−1 | CLIA | 12–18 | <50 nmol L−1 | PE | 7 | (1), (2), (6), (9) |
| ≥50 nmol L−1 | |||||||||||
| Scholl et al. 201293 | U.S., Prospective cohort | 2001–2007 | 1153 | NR | NR | HPLC | 13.7 ± 5.6 | <30 nmol L−1 | C-section | 8 | (1), (2), (3), (5), (6), (9), (15) |
| 30–49.9 nmol L−1 | |||||||||||
| 50–125.0 nmol L−1 | |||||||||||
| >125 nmol L−1 | |||||||||||
| Perez-Ferre et al. 201291 | Spain, Prospective cohort | 2010 | 266 | 33 (29–36) | 18.9 (11.5–24.7) ng mL−1 | CLIA | 24–28 | <20 ng mL−1 | C-section | 6 | (1), (2), (5), (6), (16) |
| ≥20 ng mL−1 | |||||||||||
| Parlea et al. 201255 | Canada, Nested case-control | 2008–2009 | Case: 116, Control: 219 | Case: 34.3 ± 4.3, Control: 34.3 ± 4.1 | Case: 56.3± 19.4 nmol L−1, Control: 62.0 ± 21.6 nmol L−1 | CLIA | 15–18 | <46.9 nmol L−1 | GDM | 5 | (2), (8) |
| 46.9–60.4 nmol L−1 | |||||||||||
| 60.4–73.5 nmol L−1 | |||||||||||
| ≥73.5 nmol L−1 | |||||||||||
| Fernandez-Alonso et al. 201246 | Spain, Prospective cohort | 2009–2010 | 466 | NR | 27.6 ±9.9 ng mL−1 | ECLIA | 11–14 | <20 ng mL−1 | GDM, PE, GH, C-section | 7 | None |
| 20–29.99 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Baker et al. 201239 | U.S., Nested case-control | 2004–2009 | Case: 60, Control: 120 | Case: 35 (31–37), Control: 33 (30–36) | 89 (73–106) nmol L−1 | LC-MS | 11–14 | <50 nmol L−1 | GDM | 8 | (1), (2), (8), (9), (14) |
| 50–74.9 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Makgoba et al. 201152 | UK, Nested case-control | NR | Case: 90, Control: 158 | Case: 34.2 ± 4.9, Control: 33.1±4.7 | Case: 18.9 ± 10.7 ng mL−1, Control: 19.0 ± 10.7 ng mL−1 | LC-MS | T1 | <25 nmol L−1 | GDM | 6 | None |
| 25–50 nmol L−1 | |||||||||||
| ≥50 nmol L−1 | |||||||||||
| Azar et al. 201168 | U.S., Nested case-control | NR | Case: 23, Control: 24 | Case: 28.5 ± 5.6, Control: 29.9 ± 3.8 | NR | HPLC | 12.2 ± 1.9 | <15 ng mL−1 | PE | 5 | None |
| 15–20 ng mL−1 | |||||||||||
| 20–30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Shand et al. 201077 | Canada, Prospective cohort | 2004–2008 | 221 | NR | 47.7 (34.2–67.9) nmol L−1 | RIA | 10–20 | <37.5 nmol L−1 | PE, GH | 6 | None |
| 37.5–49.9 nmol L−1 | |||||||||||
| 50–75 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Powe et al. 201075 | U.S., Nested case-control | 1998–2006 | Case: 39, Control: 131 | Case: 28.9 ± 6.4, Control: 30.4 ± 6 | Case: 27.4 ± 1.9 ng mL−1, Control: 28.8 ± 0.8 ng mL−1 | LC-MS | T1 | Q1–Q4 | PE | 7 | (2), (5), (9), (28) |
| Baker et al. 201070 | U.S., Nested case-control | 2004–2008 | Cases:43 Controls: 198 | Case: 30 (25–34), Control: 28 (23–32) | Case: 75 (47–107) nmol L−1, Control: 98 (68–113) nmol L−1 | LC-MS | 15–20 | <50 nmol L−1 | PE | 8 | (1), (2), (3), (8), (9) |
| 50–74.9 nmol L−1 | |||||||||||
| ≥75 nmol L−1 | |||||||||||
| Haugen et al. 200933 | Norway, Prospective cohort | 2007 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 423 423 |
NR | Case: 7.7 (1.5-30.0) μg d−1, Control: 8.4 (1.7-31.4) μg d−1 | FFQ | During the first 4–5 months of pregnancy | <5.0 μg d−1 | PE | 7 | (1), (2), (4), (6), (9), (12) |
| 5.0–9.9 μg d−1 | |||||||||||
| 10.0–14.9 μg d−1 | |||||||||||
| 15.0–20.0 μg d−1 | |||||||||||
| >20.0 μg d−1 | |||||||||||
| Zhang et al. 200865 | U.S., Nested case-control | 2002–2004 | Case: 57 Control: 114 | Case: 34.3 ± 4.8, Control: 33.1 ± 3.9 | Case: 24.2 ± 8.5 ng mL−1, Control: 30.1 ± 9.7 ng mL−1 | EIA | 16 | <20 ng mL−1 | GDM | 8 | (1), (2), (5), (10) |
| 20–29 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
| Bodnar et al. 200772 | U.S., Nested case-control | 1997–2001 | Case: 49 Control: 216 | NR | Case: 45.4 (38.6-53.4) nmol L−1, Control: 53.1 (47.1–59.9) nmol L−1 | ELISA | <22 | <37.5 nmol L−1 | PE | 6 | (2), (4), (5), (8), (9), (17) |
| 37.5–75 nmol L−1 | |||||||||||
| >75 nmol L−1 | |||||||||||
| Yue et al. 202181 | China, Retrospective cohort | 2017–2019 | 7976 | NR | NR | CLIA | <20 | <10 ng mL−1 | PE | 8 | (1), (2), (3), (19), (21), (37), (40), (41) |
| 10–20 ng mL−1 | |||||||||||
| 20–30 ng mL−1 | |||||||||||
| ≥30 ng mL−1 | |||||||||||
Maternal vitamin D levels and the risk of gestational diabetes mellitus
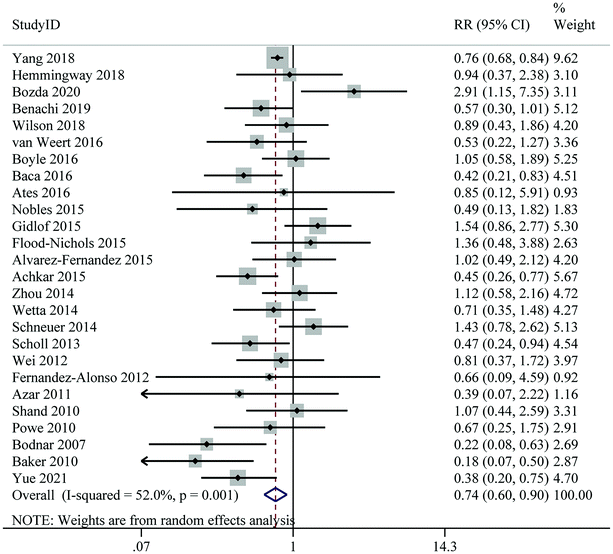
Thirty-six studies, including 101![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 116 individuals and 11
116 individuals and 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 127 cases, indicated that the highest level of 25(OH)D was significantly associated with a 24% reduction in the risk of GDM compared to the lowest level (RR: 0.76; 95% CI: 0.66–0.87); however, significant heterogeneity was found between studies (I2 = 69.2%, Pheterogeneity < 0.001) (Fig. 2 and Table 2). In most subgroups, a significant negative association between 25(OH)D levels and risk of GDM was still observed, particularly in nested case-control studies, participants from North America, and studies controlling for maternal age, BMI, and season in their analysis (Table 3).
127 cases, indicated that the highest level of 25(OH)D was significantly associated with a 24% reduction in the risk of GDM compared to the lowest level (RR: 0.76; 95% CI: 0.66–0.87); however, significant heterogeneity was found between studies (I2 = 69.2%, Pheterogeneity < 0.001) (Fig. 2 and Table 2). In most subgroups, a significant negative association between 25(OH)D levels and risk of GDM was still observed, particularly in nested case-control studies, participants from North America, and studies controlling for maternal age, BMI, and season in their analysis (Table 3).
 | ||
| Fig. 2 Maternal 25(Oh)D levels and risk of gestational diabetes mellitus, the highest versus lowest category. | ||
| Outcomes | Highest vs. lowest meta-analyses | Dose–response meta-analyses | ||||||
|---|---|---|---|---|---|---|---|---|
| N | RR (95% CI) | I 2 (%) | P heterogenity | N | RR (95% CI) | I 2 (%) | P heterogenity | |
| Abbreviations: CI, confidence interval; C-section, caesarean section; GDM, gestational diabetes mellitus; GH, gestational hypertension; PE, pre-eclampsia; RR, relative risk. | ||||||||
| GDM | 36 | 0.76 (0.66, 0.87) | 69.2 | <0.001 | 24 | 0.92 (0.86, 0.97) | 73.6 | <0.001 |
| PE | 26 | 0.74 (0.60, 0.90) | 52.0 | 0.001 | 19 | 0.89 (0.84, 0.94) | 49.4 | 0.008 |
| GH | 11 | 0.87 (0.79, 0.97) | 0.0 | 0.676 | 7 | 0.98 (0.92, 1.04) | 26.6 | 0.226 |
| C-section | 24 | 1.00 (0.90, 1.12) | 59.1 | <0.001 | 9 | 1.03 (0.99, 1.08) | 26.5 | 0.209 |
| GDM | PE | GH | C-section | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subgroups | N | RR (95% CI) | I 2 (%) | P h 1 | P h 2 | N | RR (95% CI) | I 2 (%) | P h 1 | P h 2 | N | RR (95% CI) | I 2 (%) | P h 1 | P h 2 | N | RR (95% CI) | I 2 (%) | P h 1 | P h 2 | |
| Abbreviations: CI, confidence interval; CLIA, chemiluminescent Immunoassay; C-section, caesarean section; ECLIA, electrochemical luminescence immunoassay; EIA, enzyme immunoassay; ELISA, enzyme-linked immunosorbent assay; GDM, gestational diabetes mellitus; GH, gestational hypertension; HPLC, high-performance liquid chromatography; LC-MS, liquid chromatography-mass spectrometry; NC, not calculable; NOS, Newcastle Ottawa scale; PE, pre-eclampsia; RIA, radioimmunoassay; RR, relative risk; T1, first trimester; T2, second trimester. Ph1 = P for heterogeneity within each subgroup. Ph2 = P for heterogeneity between subgroups with meta-regression. | |||||||||||||||||||||
| All studies | 36 | 0.76 (0.66, 0.87) | 69.2 | <0.001 | 26 | 0.74 (0.60, 0.90) | 52.0 | 0.001 | 11 | 0.87 (0.79, 0.97) | 0.0 | 0.676 | 24 | 1.00 (0.90, 1.12) | 59.1 | <0.001 | |||||
| Study type | |||||||||||||||||||||
| Cohort | 25 | 0.81 (0.70, 0.94) | 73.1 | <0.001 | 0.15 | 16 | 0.75 (0.67, 0.85) | 2.4 | 0.426 | 0.81 | 10 | 0.88 (0.79, 0.97) | 0.0 | 0.662 | 0.38 | 22 | 0.99 (0.88, 1.12) | 58.6 | <0.001 | 0.63 | |
| Nested case-control | 11 | 0.61 (0.46, 0.81) | 38.0 | 0.096 | 10 | 0.68 (0.42, 1.12) | 75.5 | <0.001 | 1 | 0.51 (0.15, 1.73) | — | — | 2 | 1.09 (0.75, 1.57) | 81.9 | 0.019 | |||||
| Geographic location | |||||||||||||||||||||
| Europe | 5 | 0.91 (0.72, 1.16) | 0.0 | 0.847 | 0.54 | 6 | 0.87 (0.59, 1.28) | 28.8 | 0.219 | 0.27 | 4 | 1.08 (0.69, 1.69) | 11.4 | 0.336 | 0.27 | 4 | 0.76 (0.48, 1.19) | 68.3 | 0.024 | 0.01 | |
| North America | 9 | 0.62 (0.46, 0.83) | 18.7 | 0.276 | 12 | 0.53 (0.39, 0.71) | 31.5 | 0.139 | 3 | 0.67 (0.34, 1.32) | 0.0 | 0.867 | 5 | 0.76 (0.60, 0.96) | 0.0 | 0.498 | |||||
| Asia | 16 | 0.79 (0.65, 0.95) | 81.6 | <0.001 | 5 | 0.89 (0.53, 1.52) | 70.6 | 0.009 | 2 | 0.85 (0.76, 0.95) | 0.0 | 0.759 | 13 | 1.05 (0.94, 1.17) | 52.5 | 0.014 | |||||
| Others | 6 | 0.64 (0.41, 1.01) | 50.3 | 0.074 | 3 | 1.13 (0.78, 1.63) | 0.0 | 0.592 | 2 | 1.01 (0.54, 1.91) | 0.0 | 0.549 | 2 | 1.40 (0.89, 2.21) | 65.2 | 0.090 | |||||
| Study quality | |||||||||||||||||||||
| <7 | 11 | 0.73 (0.53, 1.00) | 71.4 | <0.001 | 0.99 | 8 | 0.88 (0.59, 1.32) | 68.6 | 0.002 | 0.25 | 4 | 0.84 (0.76, 0.94) | 0.0 | 0.536 | 0.11 | 12 | 1.08 (0.92, 1.26) | 57.3 | 0.007 | 0.10 | |
| ≥7 | 25 | 0.75 (0.64, 0.88) | 67.7 | <0.001 | 18 | 0.67 (0.52, 0.85) | 38.7 | 0.048 | 7 | 1.12 (0.84, 1.50) | 0.0 | 0.906 | 12 | 0.91 (0.82, 1.01) | 24.5 | 0.203 | |||||
| Sample size | |||||||||||||||||||||
| <2000 | 26 | 0.64 (0.49, 0.84) | 68.2 | <0.001 | 0.14 | 19 | 0.79 (0.59, 1.04) | 49.8 | 0.007 | 0.39 | 8 | 1.02 (0.75, 1.38) | 0.0 | 0.562 | 0.64 | 20 | 1.03 (0.91, 1.17) | 54.0 | 0.002 | 0.26 | |
| ≥2000 | 10 | 0.86 (0.76, 0.98) | 68.8 | 0.001 | 7 | 0.64 (0.47, 0.89) | 60.9 | 0.018 | 3 | 0.86 (0.77, 0.95) | 0.0 | 0.723 | 4 | 0.87 (0.80, 0.95) | 0.0 | 0.742 | |||||
| Blood sample type | |||||||||||||||||||||
| Serum | 26 | 0.83 (0.72, 0.95) | 63.6 | <0.001 | 0.08 | 24 | 0.72 (0.58, 0.89) | 54.8 | 0.001 | 0.46 | 11 | 0.87 (0.79, 0.97) | 0.0 | 0.676 | NC | 20 | 1.03 (0.92, 1.16) | 61.4 | <0.001 | 0.16 | |
| Plasma | 9 | 0.54 (0.34, 0.86) | 79.5 | <0.001 | 2 | 0.97 (0.52, 1.80) | 0.0 | 0.434 | 0 | — | — | — | 4 | 0.84 (0.63, 1.12) | 40.3 | 0.170 | |||||
| 25(OH)D assay methods | |||||||||||||||||||||
| LC-MS/HPLC | 11 | 0.76 (0.60, 0.98) | 42.7 | 0.065 | 9 | 0.58 (0.40, 0.83) | 34.7 | 0.140 | 0.05 | 2 | 1.28 (0.88, 1.87) | 0.0 | 0.680 | 0.09 | 7 | 1.04 (0.75, 1.44) | 71.8 | 0.002 | 0.62 | ||
| ELISA/EIA | 3 | 0.63 (0.23, 1.68) | 19.8 | 0.287 | 0.64 | 3 | 0.54 (0.20, 1.43) | 66.3 | 0.051 | 1 | 0.93 (0.37, 2.35) | — | — | 8 | 0.98 (0.87, 1.11) | 9.3 | 0.358 | ||||
| RIA | 4 | 0.90 (0.57, 1.42) | 61.0 | 0.053 | 2 | 0.72 (0.40, 1.30) | 24.3 | 0.251 | 2 | 0.63 (0.27, 1.45) | 0.0 | 0.641 | 0 | — | — | — | |||||
| ECLIA | 7 | 0.88 (0.66, 1.17) | 84.5 | <0.001 | 4 | 0.77 (0.70, 0.86) | 0.0 | 0.594 | 2 | 0.85 (0.76, 0.95) | 0.0 | 0.757 | 2 | 1.10 (0.99, 1.23) | 0.0 | 0.794 | |||||
| CLIA | 6 | 0.61 (0.45, 0.84) | 40.2 | 0.138 | 5 | 0.77 (0.41, 1.43) | 73.9 | 0.004 | 2 | 1.01 (0.54, 1.91) | 0.0 | 0.549 | 3 | 0.84 (0.44, 1.62) | 84.0 | 0.002 | |||||
| Others | 5 | 0.71 (0.45, 1.12) | 79.4 | 0.001 | 4 | 1.08 (0.74, 1.56) | 49.0 | 0.117 | 2 | 0.58 (0.21, 1.58) | 0.0 | 0.327 | 4 | 0.87 (0.79, 0.96) | 0.0 | 0.519 | |||||
| Trimester of sample collection | |||||||||||||||||||||
| T1 | 17 | 0.72 (0.55, 0.94) | 66.9 | <0.001 | 0.60 | 9 | 1.29 (0.96, 1.73) | 0.3 | 0.431 | 0.01 | 4 | 0.63 (0.31, 1.27) | 0.0 | 0.643 | 0.74 | 7 | 1.22 (1.04, 1.44) | 0.0 | 0.452 | 0.49 | |
| T2 | 11 | 0.83 (0.69, 1.00) | 75.1 | <0.001 | 8 | 0.78 (0.60, 1.01) | 37.2 | 0.132 | 3 | 1.02 (0.73, 1.43) | 58.5 | 0.090 | 6 | 0.91 (0.74, 1.10) | 74.7 | 0.001 | |||||
| T3 | — | — | — | — | — | — | — | — | — | — | — | — | 4 | 0.92 (0.82, 1.04) | 0.0 | 0.428 | |||||
| During pregnancy | 9 | 0.65 (0.45, 0.95) | 61.1 | 0.008 | 9 | 0.47 (0.37, 0.61) | 0.0 | 0.604 | 4 | 0.82 (0.48, 1.39) | 0.0 | 0.990 | 8 | 0.99 (0.77, 1.28) | 61.5 | 0.011 | |||||
| Number of adjusted factors | |||||||||||||||||||||
| <6 | 22 | 0.79 (0.67, 0.93) | 65.5 | <0.001 | 0.78 | 17 | 0.87 (0.69, 1.10) | 44.0 | 0.027 | 0.06 | 9 | 0.87 (0.78, 0.96) | 0.0 | 0.545 | 0.76 | 18 | 1.05 (0.92, 1.21) | 64.8 | <0.001 | 0.16 | |
| ≥6 | 14 | 0.71 (0.53, 0.94) | 74.5 | <0.001 | 9 | 0.55 (0.39, 0.79) | 53.5 | 0.028 | 2 | 1.02 (0.53, 1.95) | 0.0 | 0.544 | 6 | 0.89 (0.80, 1.00) | 0.0 | 0.539 | |||||
| Adjusted for confounding factors | |||||||||||||||||||||
| Age | Yes | 21 | 0.74 (0.62, 0.88) | 72.5 | <0.001 | 0.75 | 11 | 0.56 (0.41, 0.77) | 50.1 | 0.029 | 0.03 | 4 | 0.94 (0.59, 1.52) | 0.0 | 0.909 | 0.91 | 9 | 0.86 (0.76, 0.98) | 37.2 | 0.121 | 0.001 |
| No | 15 | 0.77 (0.59, 1.00) | 61.7 | 0.001 | 15 | 0.91 (0.71, 1.17) | 44.0 | 0.035 | 7 | 0.90 (0.73, 1.11) | 12.6 | 0.334 | 15 | 1.13 (1.01, 1.28) | 33.1 | 0.103 | |||||
| BMI | Yes | 20 | 0.70 (0.56, 0.87) | 68.6 | <0.001 | 0.52 | 15 | 0.57 (0.44, 0.74) | 37.7 | 0.070 | 0.01 | 5 | 1.15 (0.85, 1.56) | 0.0 | 0.796 | 0.10 | 11 | 0.85 (0.76, 0.96) | 26.0 | 0.197 | 0.001 |
| No | 16 | 0.82 (0.68, 0.98) | 67.4 | <0.001 | 11 | 1.02 (0.76, 1.37) | 51.0 | 0.026 | 6 | 0.84 (0.76, 0.94) | 0.0 | 0.795 | 13 | 1.16 (1.03, 1.30) | 31.4 | 0.132 | |||||
| Season | Yes | 15 | 0.70 (0.55, 0.90) | 72.1 | <0.001 | 0.65 | 9 | 0.58 (0.37, 0.90) | 64.6 | 0.004 | 0.16 | 0 | — | — | — | NC | 5 | 0.86 (0.78, 0.94) | 9.0 | 0.355 | 0.03 |
| No | 21 | 0.80 (0.68, 0.95) | 65.6 | <0.001 | 17 | 0.82 (0.66, 1.02) | 38.8 | 0.052 | 11 | 0.87 (0.79, 0.97) | 0.0 | 0.676 | 19 | 1.08 (0.96, 1.21) | 45.1 | 0.018 | |||||
Twenty-four publications on the association between 25(OH)D levels and the risk of GDM were included in the dose–response analysis. No evidence of a non-linear association between 25(OH)D levels and GDM risk was found (Pnon-linearity = 0.695) (ESI Fig. S1†). For linear dose–response meta-analysis, we found a significant 8% reduction in the risk of GDM for each 25 nmol L−1 increase in 25(OH)D levels (RR: 0.92; 95% CI: 0.86–0.97), with high heterogeneity (I2 = 73.6%, Pheterogeneity < 0.001) (Fig. 3 and Table 2).
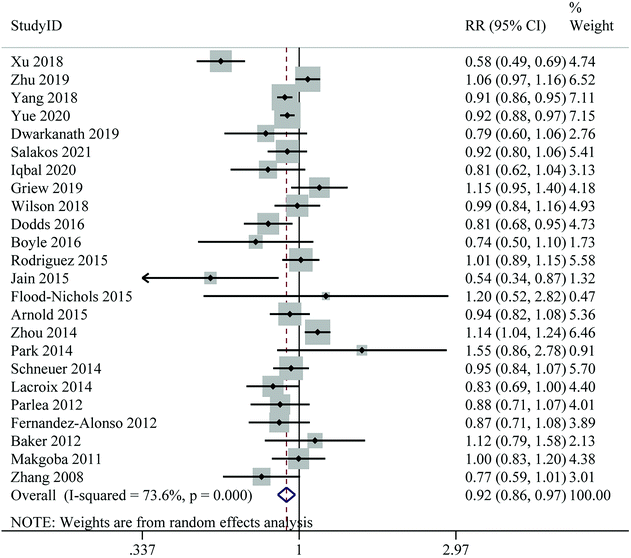 | ||
| Fig. 3 Linear dose–response meta-analysis of maternal 25(Oh)D levels (per 25 nmol L−1 increase) and risk of gestational diabetes mellitus. | ||
Maternal vitamin D levels and risk of pre-eclampsia
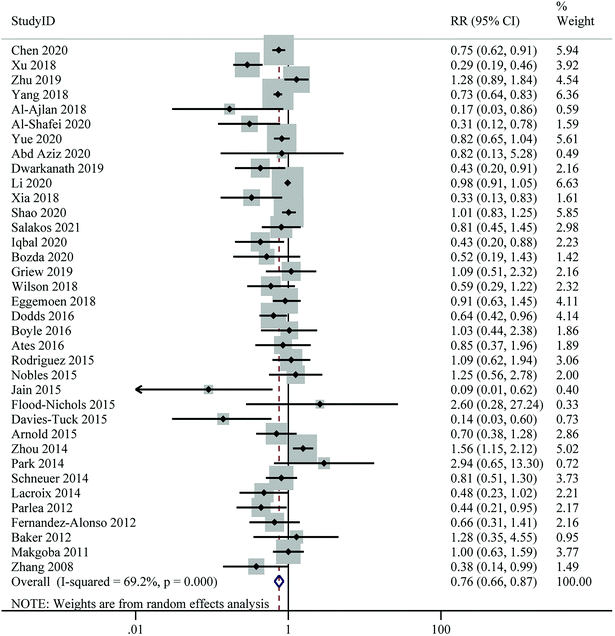
Twenty-six studies with a total of 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 203 participants and 4518 cases were included in this analysis. The pooled RR for the risk of PE, comparing the highest with the lowest level of 25(OH)D, was 0.74 (95% CI: 0.60–0.90), indicating a significant inverse association. However, there was evidence of moderate heterogeneity between studies (I2 = 52.0%, Pheterogeneity = 0.001) (Fig. 4 and Table 2). Subgroup analyses showed that the associations between 25(OH)D levels and risk of PE was significant in cohort studies, studies conducted in North America, studies of higher quality, studies using LC-MS/HPLC or ECLIA for 25(OH)D assays, and studies in which samples were collected throughout pregnancy. At the same time, the between-study heterogeneity was significantly reduced in these subgroups (Table 3).
203 participants and 4518 cases were included in this analysis. The pooled RR for the risk of PE, comparing the highest with the lowest level of 25(OH)D, was 0.74 (95% CI: 0.60–0.90), indicating a significant inverse association. However, there was evidence of moderate heterogeneity between studies (I2 = 52.0%, Pheterogeneity = 0.001) (Fig. 4 and Table 2). Subgroup analyses showed that the associations between 25(OH)D levels and risk of PE was significant in cohort studies, studies conducted in North America, studies of higher quality, studies using LC-MS/HPLC or ECLIA for 25(OH)D assays, and studies in which samples were collected throughout pregnancy. At the same time, the between-study heterogeneity was significantly reduced in these subgroups (Table 3).
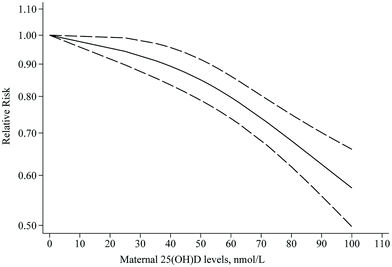
Nineteen articles with sufficient data were identified for inclusion in the dose–response meta-analysis of PE. We found that each 25 nmol L−1 increase in 25(OH)D levels was associated with an 11% lower risk of PE (RR: 0.89; 95% CI: 0.84–0.94), with moderate heterogeneity (I2 = 49.4%, Pheterogeneity = 0.008) (ESI Fig. S2† and Table 2). Results of the dose–response meta-analysis showed a non-linear trend between 25(OH)D levels and PE risk (Pnon-linearity = 0.009), where the RRs continued to decrease as 25(OH)D levels increased from zero to higher; however, the risk declined more significantly from 40 nmol L−1 onwards (Fig. 5).
We only retrieved one study from the Norwegian Mother and Child Cohort Study that examined the association between vitamin D intake and risk of PE. The result showed that the intake of vitamin D from supplements was associated with a reduced risk of PE.33 However, due to the small number of articles, we did not conduct a further meta-analysis.
Maternal vitamin D levels and the risk of gestational hypertension
A total of 11 studies with 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 657 participants and 2572 cases provided data on the relationship between the highest versus the lowest level of 25(OH)D and the risk of GH. Highest level of 25(OH)D in comparison with lowest level decreased risk of GH by 13% (RR: 0.87; 95% CI: 0.79–0.97), with no significant between-study heterogeneity (I2 = 0%, Pheterogeneity = 0.676) (Fig. 6 and Table 2). Subgroup analyses revealed that the associations between 25(OH)D levels and risk of GH was significant in cohort studies, studies from Asia, studies of lower quality, studies with sample sizes ≥2000, studies using ECLIA for 25(OH)D assays, studies with fewer than six adjusting factors, and studies without adjustment for BMI (Table 3).
657 participants and 2572 cases provided data on the relationship between the highest versus the lowest level of 25(OH)D and the risk of GH. Highest level of 25(OH)D in comparison with lowest level decreased risk of GH by 13% (RR: 0.87; 95% CI: 0.79–0.97), with no significant between-study heterogeneity (I2 = 0%, Pheterogeneity = 0.676) (Fig. 6 and Table 2). Subgroup analyses revealed that the associations between 25(OH)D levels and risk of GH was significant in cohort studies, studies from Asia, studies of lower quality, studies with sample sizes ≥2000, studies using ECLIA for 25(OH)D assays, studies with fewer than six adjusting factors, and studies without adjustment for BMI (Table 3).
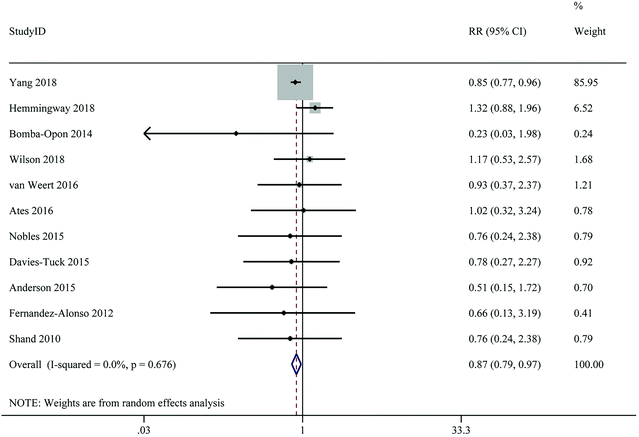 | ||
| Fig. 6 Maternal 25(Oh)D levels and risk of gestational hypertension, the highest versus lowest category. | ||
Seven publications were incorporated into the dose–response meta-analysis for GH. There was no evidence of a non-linear relationship between maternal 25(OH)D levels and the risk of GH (Pnon-linearity = 0.209) (ESI Fig. S3†). Moreover, the linear dose–response relationship between each 25 nmol L−1 increase in 25(OH)D levels and GH risk was not significant (RR: 0.98; 95% CI: 0.92–1.04; I2 = 26.6%, Pheterogeneity = 0.226) (ESI Fig. S4† and Table 2).
Maternal vitamin D levels and the risk of caesarean section
Twenty-four studies were included in the highest versus lowest meta-analysis on the relationship between maternal 25(OH)D levels and the risk of C-section, with a total of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 107 participants and 7670 cases. We found no significant association between maternal 25(OH)D levels and risk of C-section (RR: 1.00; 95% CI: 0.90–1.12; I2 = 59.1%, Pheterogeneity < 0.001) (ESI Fig. S5† and Table 2). Subgroup analyses revealed that the inverse association between maternal 25(OH)D levels and risk of C-section was significant in studies conducted in North America, studies with sample sizes ≥2000, and studies adjusted for maternal age, BMI, or season (Table 3).
107 participants and 7670 cases. We found no significant association between maternal 25(OH)D levels and risk of C-section (RR: 1.00; 95% CI: 0.90–1.12; I2 = 59.1%, Pheterogeneity < 0.001) (ESI Fig. S5† and Table 2). Subgroup analyses revealed that the inverse association between maternal 25(OH)D levels and risk of C-section was significant in studies conducted in North America, studies with sample sizes ≥2000, and studies adjusted for maternal age, BMI, or season (Table 3).
Out of 24 articles, nine studies were included in the dose–response analysis. In the non-linear dose–response analysis, the maternal 25(OH)D levels were not associated with the risk of C-section (Pnon-linearity = 0.773) (ESI Fig. S6†). Estimation of a linear dose–response trend demonstrated that an increase of 25 nmol L−1 in 25(OH)D was not associated with a higher risk of C-section (RR: 1.03; 95% CI: 0.99–1.08; I2 = 26.5%, Pheterogeneity = 0.209) (ESI Fig. S7† and Table 2).
Sensitivity analysis and publication bias
Due to the significant heterogeneity among studies between maternal 25(OH)D levels and the risk of GDM, we performed sensitivity analyses using a random effect model to test whether the pooled RR was significantly influenced by a specific study in the highest versus lowest meta-analysis (ESI Table S6†). The results suggested that the overall estimates were not substantially altered by excluding one study at a time, with the pooled RRs ranging from 0.73 to 0.80. None of the excluded studies explained the large degree of heterogeneity in the findings. Similarly, sensitivity analyses demonstrated that none of the individual studies significantly affected the overall results for PE, GH, and C-section. Based on the funnel plot and Egger's regression test, no evidence of publication bias was observed for PE (P = 0.698), GH (P = 0.858), or C-section (P = 0.983). For GDM, the funnel plot was asymmetrical and the Egger's test (P = 0.016) showed a significant publication bias (ESI Fig. S8–S11†).Discussion
Main findings
This meta-analysis of sixty-eight prospective studies systematically assessed the associations of vitamin D levels with the risk of GDM, PE, GH, and C-section by comparing the highest and lowest levels and performing dose–response analyses. The findings showed that the highest level of 25(OH)D was significantly correlated with reduced risk of GDM, PE, and GH compared to the lowest level. Further, the dose–response analysis suggested that each 25 nmol L−1 increase in 25(OH)D was associated with an 8% and 11% reduction in the risk of GDM and PE, respectively. There was evidence of a non-linear trend in the risk of PE, with a more dramatic decline in 25(OH)D from 40 nmol L−1. Moreover, we found no association between vitamin D levels and the risk of C-section.Strengths and limitations
Our study has several strengths. Firstly, this is the first comprehensive systematic review and dose–response meta-analysis to investigate the linear and non-linear relationships between maternal vitamin D levels and the risk of adverse pregnancy outcomes, including GDM, PE, GH, and C-section. Secondly, we incorporated studies that were truly prospective designed, excluding studies in which the gestational week of vitamin D measurement co-occurred as the endpoint or after the endpoint, to ensure a more plausible inference of causality. Thirdly, the large number of participants and cases provided sufficient statistical power to quantitatively assess the association of 25(OH)D levels with the risk of adverse pregnancy outcomes.Besides these strengths, this study also has some limitations that should be acknowledged. Firstly, there was significant heterogeneity among studies for GDM, PE, and C-section risk. We performed extensive subgroup analyses and sensitivity analyses to explore the potential source of heterogeneity. The results identified factors including geographic location, 25(OH)D assay methods, trimester of sample collection, and whether adjustment for confounding factors may be a significant source of heterogeneity. Secondly, although the RRs were derived from the multivariate models, our results could not completely rule out the unmeasured confounders. Thirdly, the gestational week vitamin D measured was not explicitly described in some of the included studies, limiting our estimate of the effect of vitamin D levels on outcomes at different trimesters. Moreover, there are few studies on the association between the dietary intake of vitamin D and pregnancy outcomes, so insufficient data are available to perform a meta-analysis. More studies which combine vitamin D intake with blood biomarkers are necessary for the future. Finally, there was a publication bias in this meta-analysis, which can be partly explained by the fact that some studies reporting negative results for the association of vitamin D levels with GDM risk were not published. In the future, more large sample population studies are needed to verify our results further.
Interpretation
In agreement with our findings, recent studies have found a significant protective effect of higher vitamin D levels on the risk of GDM.17,18,97 However, controversy still exists regarding the dose–response relationship between vitamin D and the risk of GDM. In a recent systematic review and meta-analysis by Sadeghian et al.,97 each 10 nmol L−1 increase in circulating 25(OH)D was associated with a 2% reduction in the risk of GDM. However, findings from another meta-analysis by Milajerdi et al.18 indicated a U-shaped non-linear association between serum vitamin D levels and risk of GDM. Several methodological limitations may restrict the validity of the estimated effect values from these studies. The authors included studies in which 25(OH)D was measured on the same day as or after screening for GDM and could not ensure the prospective property of the studies. Also, they only included studies with serum samples, which resulted in fewer GDM cases being included in the study. In contrast, our study included both serum and plasma samples and further explored differences in subgroup analyses. Overall, it is necessary to conduct well-designed studies to elucidate the dose–response relationship between vitamin D levels and GDM risk.We found a significant non-linear dose–response association between vitamin D levels and the risk of PE, with the risk decreasing more rapidly when vitamin D levels exceeded 40 nmol L−1. Several meta-analyses on the same topic were previously published, but they did not examine the potential non-linear and linear associations.19,20,98–100 Similar to our findings, some of these studies found that vitamin D deficiency or insufficiency was related to a higher risk of PE.19,20,100 However, two other studies showed that PE risk was not influenced by vitamin D levels during pregnancy.98,99 The discrepancies can be primarily attributed to the different inclusion criteria, with some studies including both cohort and cross-sectional studies, whereas the current meta-analysis included only prospective studies.
In this meta-analysis, maternal vitamin D levels in the highest category were protectively associated with the risk of GH compared with the lowest category. However, we did not find a significant dose–response relationship. To the best of our knowledge, this is the first meta-analysis to quantitatively summarize the association between vitamin D levels during pregnancy and GH. Nevertheless, the above results are based on a small number of studies, and further research is needed to shed light on this issue.
In line with the current study, the results of a systematic review and meta-analysis in 2013 showed no significant association between vitamin D levels and risk of C-section.20 Although vitamin D was found to reduce the common causes of the occurrence of C-section such as GDM and PE, and most studies did not distinguish whether the outcome was a primary C-section or whether it was an active elective cesarean delivery, which may somewhat influence the results. Future studies on this association need to consider and collect these essential factors associated with outcomes to improve the existing evidence.
The specific mechanisms behind the effects of vitamin D on adverse pregnancy outcomes are not well understood; however, the extra-skeletal effects of vitamin D may play a crucial role. For instance, vitamin D has an integral part in maintaining glucose and insulin homeostasis;101 therefore, higher vitamin D levels may reduce the risk of GDM. In addition, active vitamin D can inhibit the renin–angiotensin system (RAS),102 which is an essential pathway in the regulation of PE and GH.103 Furthermore, vitamin D is considered to have anti-inflammatory properties that may reduce the maternal inflammatory response.104 Consequently, these mechanisms may explain why higher vitamin D levels may reduce the risk of adverse pregnancy outcomes. However, more animal studies or clinical trials are needed to demonstrate the specific mechanisms.
In addition, our systematic review found that the mean 25(OH)D concentrations in pregnant women were highly varied in different regions, from 18 nmol L−1–98 nmol L−1.12,70,92 There are several possible influential factors that may contribute to discrepancies in vitamin D status between populations, such as sun exposure, diet, nutritional status, and renal function.105 Also, differences in the assay methods for blood 25(OH)D concentrations may have contributed to the discrepancy.105 Furthermore, the bioavailability of vitamin D intake varies among individuals, which may be explained in part by genetic variability in the vitamin D receptor (VDR).106,107
Conclusions
In conclusion, our comprehensive meta-analysis provides further evidence that higher 25(OH)D levels during pregnancy are associated with a lower risk of GDM and PE in a dose–response manner. However, the inverse association between maternal 25(OH)D levels and GH was significant in the highest versus lowest meta-analysis, but no dose–response relationship was found. Moreover, we found no association between vitamin D levels and the risk of C-section. More randomized controlled trials and animal experiments are needed to further evaluate the associations of vitamin D levels with adverse pregnancy outcomes to prove our findings.Author contributions
Rui Zhao: conceptualization, methodology, software, formal analysis, and writing – original draft. Leilei Zhou: methodology, validation, data curation, and writing – original draft. Shanshan Wang: methodology, resources, and validation. Guoping Xiong: conceptualization, supervision, writing – review and editing, and funding acquisition. Liping Hao: conceptualization, supervision, writing – review and editing, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declared no conflict of interest.Acknowledgements
We would like to thank all members of our research team for their engagement and the original authors of the included studies for their excellent work. Funding statement: This work was supported by the National Natural Science Foundation of China [No. 81773426 and No.82173513, to L. H.] and the Foundation of the Health Commission of Hubei Province [WJ2021F003, to G. X.].References
- P. Wu, R. Haththotuwa, C. S. Kwok, A. Babu, R. A. Kotronias, C. Rushton, A. Zaman, A. A. Fryer, U. Kadam, C. A. Chew-Graham and M. A. Mamas, Preeclampsia and Future Cardiovascular Health: A Systematic Review and Meta-Analysis, Circ.: Cardiovasc. Qual. Outcomes, 2017, 10, e003497 Search PubMed
.
- A. Hauspurg, W. Ying, C. A. Hubel, E. D. Michos and P. Ouyang, Adverse pregnancy outcomes and future maternal cardiovascular disease, Clin. Cardiol., 2018, 41, 239–246 CrossRef PubMed
.
- E. C. Johns, F. C. Denison, J. E. Norman and R. M. Reynolds, Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications, Trends Endocrinol. Metab., 2018, 29, 743–754 CrossRef CAS PubMed
.
- A. Słabuszewska-Jóźwiak, J. K. Szymański, M. Ciebiera, B. Sarecka-Hujar and G. Jakiel, Pediatrics Consequences of Caesarean Section-A Systematic Review and Meta-Analysis, Int. J. Environ. Res. Public Health, 2020, 17, 8031 CrossRef
.
- M. G. Kimlin, Geographic location and vitamin D synthesis, Mol. Aspects Med., 2008, 29, 453–461 CrossRef CAS
.
- S. Karras, S. A. Paschou, E. Kandaraki, P. Anagnostis, C. Annweiler, B. C. Tarlatzis, B. W. Hollis, W. B. Grant and D. G. Goulis, Hypovitaminosis D in pregnancy in the Mediterranean region: a systematic review, Eur. J. Clin. Nutr., 2016, 70, 979–986 CrossRef CAS PubMed
.
- M. F. Holick, The vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention, Rev. Endocr. Metab. Disord., 2017, 18, 153–165 CrossRef CAS PubMed
.
- R. M. Mogire, A. Mutua, W. Kimita, A. Kamau, P. Bejon, J. M. Pettifor, A. Adeyemo, T. N. Williams and S. H. Atkinson, Prevalence of vitamin D deficiency in Africa: a systematic review and meta-analysis, Lancet Global Health, 2020, 8, e134–e142 CrossRef
.
- N. Q. Liu, A. T. Kaplan, V. Lagishetty, Y. B. Ouyang, Y. Ouyang, C. F. Simmons, O. Equils and M. Hewison, Vitamin D and the regulation of placental inflammation, J. Immunol., 2011, 186, 5968–5974 CrossRef CAS
.
- B. Schröder-Heurich, C. J. P. Springer and F. von Versen-Höynck, Vitamin D Effects on the Immune System from Periconception through Pregnancy, Nutrients, 2020, 12, 1432 CrossRef
.
- M. Achkar, L. Dodds, Y. Giguère, J. C. Forest, B. A. Armson, C. Woolcott, S. Agellon, A. Spencer and H. A. Weiler, Vitamin D status in early pregnancy and risk of preeclampsia, Am. J. Obstet. Gynecol., 2015, 212, 511e511–511e517 CrossRef
.
- A. D. Gernand, M. A. Klebanoff, H. N. Simhan and L. M. Bodnar, Maternal vitamin D status, prolonged labor, cesarean delivery and instrumental delivery in an era with a low cesarean rate, J. Perinatol., 2015, 35, 23–28 CrossRef CAS
.
- A. D. Gernand, H. N. Simhan, K. M. Baca, S. Caritis and L. M. Bodnar, Vitamin D, pre-eclampsia, and preterm birth among pregnancies at high risk for pre-eclampsia: an analysis of data from a low-dose aspirin trial, BJOG, 2017, 124, 1874–1882 CrossRef CAS PubMed
.
- R. L. Wilson, A. J. Leviton, S. Y. Leemaqz, P. H. Anderson, J. A. Grieger, L. E. Grzeskowiak, P. E. Verburg, L. McCowan, G. A. Dekker, T. Bianco-Miotto and C. T. Roberts, Vitamin D levels in an Australian and New Zealand cohort and the association with pregnancy outcome, BMC Pregnancy Childbirth, 2018, 18, 251 CrossRef
.
- B. Zhu, K. Huang, S. Yan, J. Hao, P. Zhu, Y. Chen, A. Ye and F. Tao, VDR variants rather than early pregnancy Vitamin D concentrations
are associated with the risk of gestational diabetes: The Ma'anshan Birth Cohort (MABC) Study, J. Diabetes Res., 2019, 2019, 8313901 Search PubMed
.
- G. D. Chen, T. T. Pang, P. S. Li, Z. X. Zhou, D. X. Lin, D. Z. Fan, X. L. Guo and Z. P. Liu, Early pregnancy vitamin D and the risk of adverse maternal and infant outcomes: A retrospective cohort study, BMC Pregnancy Childbirth, 2020, 20, 465 CrossRef PubMed
.
- L. Wang, C. Zhang, Y. Song and Z. Zhang, Serum vitamin D deficiency and risk of gestational diabetes mellitus: a meta-analysis, Arch. Med. Sci., 2020, 16, 742–751 CrossRef CAS PubMed
.
- A. Milajerdi, F. Abbasi, S. M. Mousavi and A. Esmaillzadeh, Maternal vitamin D status and risk of gestational diabetes mellitus: A systematic review and meta-analysis of prospective cohort studies, Clin. Nutr., 2021, 40, 2576–2586 CrossRef CAS
.
- S. Akbari, B. Khodadadi, S. A. Y. Ahmadi, S. Abbaszadeh and F. Shahsavar, Association of vitamin D level and vitamin D deficiency with risk of preeclampsia: A systematic review and updated meta-analysis, Taiwan. J. Obstet. Gynecol., 2018, 57, 241–247 CrossRef
.
- F. Aghajafari, T. Nagulesapillai, P. E. Ronksley, S. C. Tough, M. O'Beirne and D. M. Rabi, Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies, Br. Med. J., 2013, 346, f1169 CrossRef
.
- D. Moher, A. Liberati, J. Tetzlaff, D. G. Altman and P. Group, Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement, PLoS Med., 2009, 6, e1000097 CrossRef
.
- A. Stang, Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses, Eur. J. Epidemiol., 2010, 25, 603–605 CrossRef
.
- R. DerSimonian and N. Laird, Meta-analysis in clinical trials revisited, Contemp. Clin. Trials, 2015, 45, 139–145 CrossRef
.
- J. P. Higgins and S. G. Thompson, Quantifying heterogeneity in a meta-analysis, Stat. Med., 2002, 21, 1539–1558 CrossRef
.
- J. P. Higgins, S. G. Thompson, J. J. Deeks and D. G. Altman, Measuring inconsistency in meta-analyses, Br. Med. J., 2003, 327, 557–560 CrossRef PubMed
.
- S. G. Thompson and J. P. Higgins, How should meta-regression analyses be undertaken and interpreted?, Stat. Med., 2002, 21, 1559–1573 CrossRef
.
- M. Egger, G. Davey Smith, M. Schneider and C. Minder, Bias in meta-analysis detected by a simple, graphical test, Br. Med. J., 1997, 315, 629–634 CrossRef CAS
.
- J. Hamling, P. Lee, R. Weitkunat and M. Ambühl, Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category, Stat. Med., 2008, 27, 954–970 CrossRef PubMed
.
- S. Greenland and M. P. Longnecker, Methods for trend estimation from summarized dose-response data, with applications to meta-analysis, Am. J. Epidemiol., 1992, 135, 1301–1309 CrossRef CAS
.
- L. Desquilbet and F. Mariotti, Dose-response analyses using restricted cubic spline functions in public health research, Stat. Med., 2010, 29, 1037–1057 Search PubMed
.
- N. Orsini, R. Li, A. Wolk, P. Khudyakov and D. Spiegelman, Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software, Am. J. Epidemiol., 2012, 175, 66–73 CrossRef PubMed
.
- V. Bagnardi, A. Zambon, P. Quatto and G. Corrao, Flexible meta-regression functions for modeling aggregate dose-response data, with an application to alcohol and mortality, Am. J. Epidemiol., 2004, 159, 1077–1086 CrossRef
.
- M. Haugen, A. L. Brantsaeter, L. Trogstad, J. Alexander, C. Roth, P. Magnus and H. M. Meltzer, Vitamin D supplementation and reduced risk of preeclampsia in nulliparous women, Epidemiology, 2009, 20, 720–726 CrossRef PubMed
.
- A. Al-Ajlan, S. Al-Musharaf, M. A. Fouda, S. Krishnaswamy, K. Wani, N. J. Aljohani, A. Al-Serehi, E. Sheshah, N. M. Alshingetti, I. Z. Turkistani, A. Afrah Alharbi, B. A. Alraqebah, A. M. Ali, G. Al-Saeed and N. M. Al-Daghri, Lower vitamin D levels in Saudi pregnant women are associated with higher risk of developing GDM, BMC Pregnancy Childbirth, 2018, 18, 86 CrossRef PubMed
.
- A. I. Al-Shafei, D. A. Rayis, A. H. Mohieldein, O. A. El-Gendy and I. Adam, Maternal early pregnancy serum level of 25-Hydroxyvitamin D and risk of gestational diabetes mellitus, Int. J. Gynecol. Obstet., 2020, 152, 382–385 CrossRef PubMed
.
- D. L. Arnold, D. A. Enquobahrie, C. Qiu, J. Huang, N. Grote, A. VanderStoep and M. A. Williams, Early Pregnancy Maternal Vitamin D Concentrations and Risk of Gestational Diabetes Mellitus, Paediatr. Perinat. Epidemiol., 2015, 29, 196–199 CrossRef PubMed
.
- S. Ates, O. Sevket, P. Ozcan, F. Ozkal, M. O. Kaya and B. Dane, Vitamin D status in the first-trimester: Effects of vitamin D deficiency on pregnancy outcomes, Afr. Health Sci., 2016, 16, 36–43 CrossRef
.
- N. H. A. Aziz, N. A. Yazid, R. A. Rahman, N. A. Rashid, S. K. Wong, N. V. Mohamad, P. S. Lim and K. Y. Chin, Is first trimester maternal 25-hydroxyvitamin d level related to adverse maternal and neonatal pregnancy outcomes? A prospective cohort study among Malaysian women, Int. J. Environ. Res. Public Health, 2020, 17, 3291 CrossRef CAS PubMed
.
- A. M. Baker, S. Haeri, C. A. Camargo, A. M. Stuebe and K. A. Boggess, First-trimester maternal vitamin D status and risk for gestational diabetes (GDM) a nested case-control study, Diabetes/Metab. Res. Rev., 2012, 28, 164–168 CrossRef CAS PubMed
.
- V. T. Boyle, E. B. Thorstensen, D. Mourath, M. B. Jones, L. M. E. McCowan, L. C. Kenny and P. N. Baker, The relationship between 25-hydroxyvitamin D concentration in early pregnancy and pregnancy outcomes in a large, prospective cohort, Br. J. Nutr., 2016, 116, 1409–1415 CrossRef CAS
.
- H. Bozdag and E. Akdeniz, Does severe vitamin D deficiency impact obstetric outcomes in pregnant women with thyroid autoimmunity?, J. Matern. – Fetal Neonat. Med., 2020, 33, 1359–1369 CAS
.
- M. Davies-Tuck, C. Yim, M. Knight, R. Hodges, J. C. G. Doery and E. Wallace, Vitamin D testing in pregnancy: Does one size fit all?, Aust. N. Z. J. Obstet. Gynaecol., 2015, 55, 149–155 CrossRef PubMed
.
- L. Dodds, C. G. Woolcott, H. Weiler, A. Spencer, J. C. Forest, B. A. Armson and Y. Giguère, Vitamin D Status and Gestational Diabetes: Effect of Smoking Status during Pregnancy, Paediatr. Perinat. Epidemiol., 2016, 30, 229–237 CrossRef
.
- P. Dwarkanath, P. Vinotha, T. Thomas, S. Joseph, A. Thomas, G. Shirley, C. N. Sheela, S. Mehta and A. V. Kurpad, Relationship of Early Vitamin D Concentrations and Gestational Diabetes Mellitus in Indian Pregnant Women, Front. Nutr., 2019, 6, 116 CrossRef
.
- Å. Eggemoen, C. W. Waage, L. Sletner, H. L. Gulseth, K. I. Birkeland and A. K. Jenum, Vitamin D, Gestational Diabetes, and Measures of Glucose Metabolism in a Population-Based Multiethnic Cohort, J. Diabetes Res., 2018, 2018, 8939235 Search PubMed
.
- A. M. Fernandez-Alonso, E. C. Dionis-Sanchez, P. Chedraui, M. D. Gonzalez-Salmeron and F. R. Perez-Lopez, First-trimester maternal serum 25-hydroxyvitamin D-3 status and pregnancy outcome, Int. J. Gynecol. Obstet., 2012, 116, 6–9 CrossRef CAS PubMed
.
- S. K. Flood-Nichols, D. Tinnemore, R. R. Huang, P. G. Napolitano and D. L. Ippolito, Vitamin D deficiency in early
pregnancy, PLoS One, 2015, 10, e0123763 CrossRef PubMed
.
- K. Griew, R. Nunn, G. Fairbrother and S. Tewari, Early pregnancy vitamin D deficiency and gestational diabetes: Exploring the link, Aust. J. Gen. Pract., 2019, 48, 797–802 CrossRef
.
- S. Iqbal, M. Malik and G. Bano, Serum Vitamin D levels and gestational diabetes mellitus: analysis of early pregnancy cohort from a teaching hospital of Kashmir Valley, J. Fam. Med. Prim. Care, 2020, 9, 4323–4328 CrossRef PubMed
.
- M. Lacroix, M. C. Battista, M. Doyon, G. Houde, J. Ménard, J. L. Ardilouze, M. F. Hivert and P. Perron, Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus, Acta Diabetol., 2014, 51, 609–616 CrossRef CAS PubMed
.
- H. Li, J. Ma, R. Huang, Y. Wen, G. Liu, M. Xuan, L. Yang, J. Yang and L. Song, Prevalence of vitamin D deficiency in the pregnant women: an observational study in Shanghai, China, Arch. Public Health, 2020, 78, 31 CrossRef PubMed
.
- M. Makgoba, S. M. Nelson, M. Savvidou, C. M. Messow, K. Nicolaides and N. Sattar, First-trimester circulating 25-hydroxyvitamin D levels and development of gestational diabetes mellitus, Diabetes Care, 2011, 34, 1091–1093 CrossRef CAS PubMed
.
- C. J. Nobles, G. Markenson and L. Chasan-Taber, Early pregnancy Vitamin D status and risk for adverse maternal and infant outcomes in a bi-ethnic cohort: The Behaviors Affecting Baby and You (B.A.B.Y.) Study, Br. J. Nutr., 2015, 114, 2116–2128 CrossRef CAS PubMed
.
- S. Park, H. K. Yoon, H. M. Ryu, Y. J. Han, S. W. Lee, B. K. Park, S. Y. Park, C. H. Yim and S. H. Kim, Maternal vitamin D deficiency in early pregnancy is not associated with gestational diabetes mellitus development or pregnancy outcomes in Korean pregnant women in a prospective study, J. Nutr. Sci. Vitaminol., 2014, 60, 269–275 CrossRef CAS
.
- L. Parlea, I. L. Bromberg, D. S. Feig, R. Vieth, E. Merman and L. L. Lipscombe, Association between serum 25-hydroxyvitaminD in early pregnancy and risk of gestational diabetes mellitus, Diabetic Med., 2012, 29, e25–e32 CrossRef CAS PubMed
.
- A. Rodriguez, R. García-Esteban, M. Basterretxea, A. Lertxundi, C. Rodríguez-Bernal, C. Iñiguez, C. Rodriguez-Dehli, A. Tardõn, M. Espada, J. Sunyer and E. Morales, Associations of maternal circulating 25-hydroxyvitamin D3 concentration with pregnancy and birth outcomes, BJOG, 2015, 122, 1695–1704 CrossRef CAS PubMed
.
- E. Salakos, T. Rabeony, M. Courbebaisse, J. Taieb, V. Tsatsaris, J. Guibourdenche, M. V. Senat, H. Haidar, J. C. Jani, D. Barglazan, E. Maisonneuve, M. C. Haguet, N. Winer, D. Masson, C. Elie, J. C. Souberbielle and A. Benachi, Relationship between vitamin D status in the first trimester of pregnancy and gestational diabetes mellitus - A nested case-control study, Clin. Nutr., 2021, 40, 79–86 CrossRef CAS PubMed
.
- F. J. Schneuer, C. L. Roberts, C. Guilbert, J. M. Simpson, C. S. Algert, A. Z. Khambalia, V. Tasevski, A. W. Ashton, J. M. Morris and N. Nassar, Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population, Am. J. Clin. Nutr., 2014, 99, 287–295 CrossRef CAS PubMed
.
- B. Shao, M. Mo, X. Xin, W. Jiang, J. Wu, M. Huang, S. Wang, X. Muyiduli, S. Si, Y. Shen, Z. Chen and Y. Yu, The interaction between prepregnancy BMI and gestational vitamin D deficiency on the risk of gestational diabetes mellitus subtypes with elevated
fasting blood glucose, Clin. Nutr., 2020, 39, 2265–2273 CrossRef CAS PubMed
.
- M. Jain, S. Kapry, S. Jain, S. K. Singh and T. B. Singh, Maternal Vitamin D Deficiency: A Risk Factor for Gestational Diabetes Mellitus in North India, Gynecol. Obstet., 2015, 5, 1000264 Search PubMed
.
- J. Xia, Y. Song, S. Rawal, J. Wu, S. Hinkle, M. Y. Tsai and C. Zhang, Vitamin d status during pregnancy and the risk of gestational diabetes mellitus-a longitudinal study in a multiracial cohort, Diabetes, 2018, 67, A385 CrossRef PubMed
.
- C. Xu, H. H. Ma and Y. Wang, Maternal Early Pregnancy Plasma Concentration of 25-Hydroxyvitamin D and Risk of Gestational Diabetes Mellitus, Calcif. Tissue Int., 2018, 102, 280–286 CrossRef CAS PubMed
.
- L. Yang, L. Song, X. Xu, Y. Liu, H. Li and L. Tang, Prevalence of Vitamin D Deficiency during Second Trimester of Pregnancy in Shanghai China, Risk Factors and Effects on Pregnancy Outcomes, Iran. J. Public Health, 2018, 47, 1145–1150 Search PubMed
.
- C. Y. Yue and C. M. Ying, Sufficience serum vitamin D before 20 weeks of pregnancy reduces the risk of gestational diabetes mellitus, Nutr. Metab., 2020, 17, 89 CrossRef CAS PubMed
.
- C. Zhang, C. Qiu, F. B. Hu, R. M. David, R. M. van Dam, A. Bralley and M. A. Williams, Maternal plasma 25-hydroxyvitamin D concentrations and the risk for gestational diabetes mellitus, PLoS One, 2008, 3, e3753 CrossRef PubMed
.
- J. Zhou, L. Su, M. Liu, Y. Liu, X. Cao, Z. Wang and H. Xiao, Associations between 25-hydroxyvitamin D levels and pregnancy outcomes: A prospective observational study in southern China, Eur. J. Clin. Nutr., 2014, 68, 925–930 CrossRef CAS PubMed
.
- I. Alvarez-Fernandez, B. Prieto, V. Rodriguez, Y. Ruano, A. I. Escudero and F. V. Alvarez, Role of vitamin D and sFlt-1/PlGF ratio in the development of early-and late-onset preeclampsia, Clin. Chem. Lab. Med., 2015, 53, 1033–1040 CAS
.
- M. Azar, A. Basu, A. J. Jenkins, A. J. Nankervis, K. F. Hanssen, H. Scholz, T. Henriksen, S. K. Garg, S. M. Hammad, J. A. Scardo, C. E. Aston and T. J. Lyons, Serum carotenoids and fat-soluble vitamins in women with type 1 diabetes and preeclampsia: A longitudinal study, Diabetes Care, 2011, 34, 1258–1264 CrossRef CAS PubMed
.
- K. M. Baca, H. N. Simhan, R. W. Platt and L. M. Bodnar, Low maternal 25-hydroxyvitamin D concentration increases the risk of severe and mild preeclampsia, Ann. Epidemiol., 2016, 26, 853–857.e1 CrossRef PubMed
.
- A. M. Baker, S. Haeri, C. A. Camargo Jr., J. A. Espinola and A. M. Stuebe, A nested case-control study of midgestation vitamin D deficiency and risk of severe preeclampsia, J. Clin. Endocrinol. Metab., 2010, 95, 5105–5109 CrossRef CAS PubMed
.
- A. Benachi, A. Baptiste, J. Taieb, V. Tsatsaris, J. Guibourdenche, M. V. Senat, H. Haidar, J. Jani, M. Guizani, J. M. Jouannic, M. C. Haguet, N. Winer, D. Masson, M. Courbebaisse, C. Elie and J. C. Souberbielle, Relationship between vitamin D status in pregnancy and the risk for preeclampsia: A nested case-control study, Clin. Nutr., 2019, 39, 440–446 CrossRef PubMed
.
- L. M. Bodnar, J. M. Catov, H. N. Simhan, M. F. Holick, R. W. Powers and J. M. Roberts, Maternal vitamin D deficiency increases the risk of preeclampsia, J. Clin. Endocrinol. Metab., 2007, 92, 3517–3522 CrossRef CAS PubMed
.
- S. Gidlöf, A. T. Silva, S. Gustafsson and P. G. Lindqvist, Vitamin D and the risk of preeclampsia-A nested case-control study, Acta Obstet. Gynecol. Scand., 2015, 94, 904–908 CrossRef PubMed
.
- A. Hemmingway, L. C. Kenny, L. Malvisi and M. E. Kiely, Exploring the concept of functional Vitamin D deficiency in pregnancy: Impact of the interaction between 25-hydroxyVitamin D and parathyroid hormone on perinatal outcomes, Am. J. Clin. Nutr., 2018, 108, 821–829 CrossRef PubMed
.
- C. E. Powe, E. W. Seely, S. Rana, I. Bhan, J. Ecker, S. A. Karumanchi and R. Thadhani, First trimester vitamin D, vitamin D binding protein, and subsequent preeclampsia, Hypertension, 2010, 56, 758–763 CrossRef CAS PubMed
.
- T. O. Scholl, X. Chen and T. P. Stein, Vitamin D, secondary hyperparathyroidism, and preeclampsia, Am. J. Clin. Nutr., 2013, 98, 787–793 CrossRef PubMed
.
- A. W. Shand, N. Nassar, P. Von Dadelszen, S. M. Innis and T. J. Green, Maternal vitamin D status in pregnancy and adverse pregnancy outcomes in a group at high risk for pre-eclampsia, BJOG, 2010, 117, 1593–1598 CrossRef CAS PubMed
.
- B. van Weert, D. van den Berg, E. J. Hrudey, A. J. Oostvogels, E. de Miranda and T. G. Vrijkotte, Is first trimester vitamin D status in nulliparous women associated with pregnancy related hypertensive disorders?, Midwifery, 2016, 34, 117–122 CrossRef PubMed
.
- S. Q. Wei, F. Audibert, N. Hidiroglou, K. Sarafin, P. Julien, Y. Wu, Z. C. Luo and W. D. Fraser, Longitudinal vitamin D status in pregnancy and the risk of pre-eclampsia, BJOG, 2012, 119, 832–839 CrossRef CAS PubMed
.
- L. A. Wetta, J. R. Biggio, S. Cliver, A. Abramovici, S. Barnes and A. T. N. Tita, Is midtrimester vitamin D status associated with spontaneous preterm birth and preeclampsia?, Am. J. Perinatol., 2014, 31, 541–546 Search PubMed
.
- C. Y. Yue, J. P. Gao, C. Y. Zhang and C. M. Ying, Is serum vitamin D deficiency before gestational 20 weeks a risk factor for preeclampsia?, Clin. Nutr., 2021, 40, 4430–4435 CrossRef CAS PubMed
.
- C. M. Anderson, J. L. Ralph, L. Johnson, A. Scheett, M. L. Wright, J. Y. Taylor, J. E. Ohm and E. Uthus, First trimester vitamin D status and placental epigenomics in preeclampsia among Northern Plains primiparas, Life Sci., 2015, 129, 10–15 CrossRef CAS PubMed
.
- D. A. Bomba-Opon, R. Brawura-Biskupski-Samaha, S. Kozlowski, P. Kosinski, Z. Bartoszewicz, T. Bednarczuk and M. Wielgos, First trimester maternal serum vitamin D and markers of preeclampsia, J. Matern.-Fetal Neonat. Med., 2014, 27, 1078–1079 CrossRef CAS PubMed
.
- S. Aydogmus, S. Kelekci, H. Aydogmus, S. Eriş, R. Desdicioʇlu, B. Yilmaz and G. Saʇlam, High prevalence of vitamin D deficiency among pregnant women in a Turkish population and impact on perinatal outcomes, J. Matern.-Fetal Neonat. Med., 2015, 28, 1828–1832 CrossRef PubMed
.
- W. Chen, Y. Li, B. Gao, J. Li, M. Zheng and X. Chen, Serum 25-hydroxyvitamin D levels in relation to lipids and clinical outcomes in pregnant women with gestational diabetes mellitus: An observational cohort study, BMJ Open, 2020, 10, e039905 CrossRef PubMed
.
- A. Gbadegesin, A. Sobande, O. Adedeji, E. Disu, O. Korede, A. Dosunmu and A. Shakunle, Maternal serum vitamin D levels and pregnancy outcomes: from Lagos, Nigeria, J. Obstet. Gynaecol., 2017, 37, 25–28 CrossRef CAS PubMed
.
- H. Hajianfar, A. Esmailzadeh, A. Feizi, Z. Shahshahan and L. Azadbakht, Association of Maternal Serum Vitamin D Level with Risk of Pregnancy-Related Complications and Neonatal Anthropometric Measures: A Prospective Observational Study, Int. J. Prev. Med., 2019, 10, 208 CrossRef PubMed
.
- B. Kısa, H. Kansu-Celik, T. Candar, E. M. Erol Koc, U. Y. Sert and O. Uzunlar, Severe 25-OH vitamin D deficiency as a reason for adverse pregnancy outcomes, J. Matern.-Fetal Neonat. Med., 2020, 33, 2422–2426 CrossRef PubMed
.
- S. L. Loy, N. Lek, F. Yap, S. E. Soh, N. Padmapriya, K. H. Tan, A. Biswas, G. S. H. Yeo, K. Kwek, P. D. Gluckman, K. M. Godfrey, S. M. Saw, F. Müller-Riemenschneider, Y. S. Chong, M. F. F. Chong, J. K. Y. Chan, A. Sheppard, A. Chinnadurai, A. E. N. Goh, A. Rifkin-Graboi, A. Qiu, B. W. Lee, B. F. P. Broekman, B. L. Quah, B. Shuter, C. K. Chng, C. Ngo, S. C. Y. Hsu, C. L. Bong, C. J. Henry, C. Y. I. Chee, D. Fok, H. Inskip, H. Chen, H. P. S. Van Bever, I. Magiati, I. B. Y. Wong, I. Y. M. Lau, J. Kapur, J. L. Richmond, J. D. Holbrook, J. J. Gooley, K. Niduvaje, L. Singh, L. L. Su, M. Daniel, L. P. C. Shek, M. V. Fortier, M. Hanson, M. Rauff, M. C. Chua, M. Meaney, M. T. Tint, O. H. Teoh, P. C. Wong, P. Agarwal, R. M. Van Dam, S. A. Rebello, S. C. Chong, S. Cai, S. B. Lim, V. S. Rajadurai, W. Stunkel, W. M. Han, W. W. Pang, Y. T. D. Goh, Y. B. Cheung, Y. H. Chan and Y. S. Lee, Association of maternal Vitamin D status with glucose tolerance and caesarean section in a multi-ethnic Asian cohort: The growing up in Singapore towards healthy outcomes study, PLoS One, 2015, 10, e0142239 CrossRef PubMed
.
- D. F. Öcal, Z. Aycan, G. Dağdeviren, N. Kanbur, T. Küçüközkan and O. Derman, Vitamin D deficiency in adolescent pregnancy and obstetric outcomes, Taiwan. J. Obstet. Gynecol., 2019, 58, 778–783 CrossRef PubMed
.
- N. Perez-Ferre, M. J. Torrejon, M. Fuentes, M. D. Fernandez, A. Ramos, E. Bordiu, L. Del Valle, M. A. Rubio, A. R. Bedia, C. Montañez and A. L. Calle-Pascual, Association of low serum 25-hydroxyvitamin D levels in pregnancy with glucose homeostasis and obstetric and newborn outcomes, Endocrinol. Pract., 2012, 18, 676–684 CrossRef PubMed
.
- C. Reichetzeder, H. Chen, M. Föller, T. Slowinski, J. Li, Y. P. Chen, F. Lang and B. Hocher, Maternal vitamin D deficiency and fetal programming - Lessons learned from humans and mice, Kidney Blood Pressure Res., 2014, 39, 315–329 CrossRef CAS PubMed
.
- T. O. Scholl, X. Chen and P. Stein, Maternal vitamin D status and delivery by cesarean, Nutrients, 2012, 4, 319–330 CrossRef CAS PubMed
.
- D. K. Thiele, E. N. Erickson and J. M. Snowden, High Prevalence of Maternal Serum 25-Hydroxyvitamin D Deficiency Is Not Associated With Poor Birth Outcomes Among Healthy White Women in the Pacific Northwest, J. Obstet. Gynecol. Neonatal Nurs., 2019, 48, 163–175 CrossRef PubMed
.
- J. Wen, Q. Hong, L. Zhu, P. Xu, Z. Fu, X. Cui, L. You, X. Wang, T. Wu, H. Ding, Y. Dai, C. Ji and X. Guo, Association of maternal serum 25-hydroxyvitamin D concentrations in second and third trimester with risk of gestational diabetes and other pregnancy outcomes, Int. J. Obes., 2017, 41, 489–496 CrossRef CAS PubMed
.
- Y. Yuan, H. Liu, C. Ji, X. Guo, L. Hu, J. Wen and M. Cai, Association of Maternal Serum 25-hydroxyvitamin D Concentrations in Second Trimester with Delivery Mode in A Chinese Population, Int. J. Med. Sci., 2017, 14, 1008–1014 CrossRef CAS PubMed
.
- M. Sadeghian, M. Asadi, S. Rahmani, M. Akhavan Zanjani, O. Sadeghi, S. A. Hosseini and A. Zare Javid, Circulating vitamin D and the risk of gestational diabetes: a systematic review and dose-response meta-analysis, Endocrine, 2020, 70, 36–47 CrossRef CAS PubMed
.
- M. J. Aguilar-Cordero, A. Lasserrot-Cuadrado, N. Mur-Villar, X. A. León-Ríos, T. Rivero-Blanco and I. M. Pérez-Castillo, Vitamin D, preeclampsia and prematurity: A systematic review and meta-analysis of observational and interventional studies, Midwifery, 2020, 87, 102707 CrossRef CAS PubMed
.
- S. J. Martínez-Domínguez, M. Tajada, P. Chedraui and F. R. Pérez-López, Systematic review and meta-analysis of Spanish studies regarding the association between maternal 25-hydroxyvitamin D levels and perinatal outcomes, Gynecol. Endocrinol., 2018, 34, 987–994 CrossRef PubMed
.
- M. Tabesh, A. Salehi-Abargouei, M. Tabesh and A. Esmaillzadeh, Maternal vitamin D status and risk of pre-eclampsia: a systematic review and meta-analysis, J. Clin. Endocrinol. Metab., 2013, 98, 3165–3173 CrossRef CAS PubMed
.
- S. G. Garbossa and F. Folli, Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose metabolism, Rev. Endocr. Metab. Disord., 2017, 18, 243–258 CrossRef CAS PubMed
.
- Y. C. Li, J. Kong, M. Wei, Z.-F. Chen, S. Q. Liu and L.-P. Cao, 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system, J. Clin. Invest., 2002, 110, 229–238 CrossRef CAS
.
- H. D. Mistry, M. V. H. Ogalde, F. Broughton Pipkin, G. Escher and L. O. Kurlak, Maternal, Fetal, and Placental Selectins in Women With Pre-eclampsia; Association With the Renin-Angiotensin-System, Front. Med., 2020, 7, 270 CrossRef PubMed
.
- C. C. Akoh, E. K. Pressman, E. Cooper, R. A. Queenan, J. Pillittere and K. O. O'Brien, Low Vitamin D is Associated With Infections and Proinflammatory Cytokines During Pregnancy, Reprod. Sci., 2018, 25, 414–423 CrossRef CAS PubMed
.
- K. D. Cashman, K. G. Dowling, Z. Škrabáková, M. Gonzalez-Gross, J. Valtueña, S. De Henauw, L. Moreno, C. T. Damsgaard, K. F. Michaelsen, C. Mølgaard, R. Jorde, G. Grimnes, G. Moschonis, C. Mavrogianni, Y. Manios, M. Thamm, G. B. Mensink, M. Rabenberg, M. A. Busch, L. Cox, S. Meadows, G. Goldberg, A. Prentice, J. M. Dekker, G. Nijpels, S. Pilz, K. M. Swart, N. M. van Schoor, P. Lips, G. Eiriksdottir, V. Gudnason, M. F. Cotch, S. Koskinen, C. Lamberg-Allardt, R. A. Durazo-Arvizu, C. T. Sempos and M. Kiely, Vitamin D deficiency in Europe: pandemic?, Am. J. Clin. Nutr., 2016, 103, 1033–1044 CrossRef CAS PubMed
.
- E. A. Torres Dominguez, A. Meza Peñafiel, A. Gómez Pedraza and E. E. Martínez Leo, Molecular mechanisms from insulin-mimetic effect of vitamin D: treatment alternative in Type 2 diabetes mellitus, Food Funct., 2021, 12, 6682–6690 RSC
.
- S. Shab-Bidar, T. R. Neyestani and A. Djazayery, The interactive effect of improvement of vitamin D status and VDR FokI variants on oxidative stress in type 2 diabetic subjects: a randomized controlled trial, Eur. J. Clin. Nutr., 2015, 69, 216–222 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo03033g |
| This journal is © The Royal Society of Chemistry 2022 |