Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Association between functional foods and cardiometabolic health in a real-life setting: a longitudinal observational study using objective diet records from an electronic purchase system†
Yoshiro
Shirai
 *a,
Masae
Sakuma
b,
Yuji
Nagasaka
c,
Naoki
Takeda
d,
Kunio
Matsui
c and
Mieko
Nakamura
*a,
Masae
Sakuma
b,
Yuji
Nagasaka
c,
Naoki
Takeda
d,
Kunio
Matsui
c and
Mieko
Nakamura
 e
e
aDepartment of Food and Nutritional Environment, Kinjo Gakuin University, Aichi, Japan. E-mail: y-shirai@kinjo-u.ac.jp
bDepartment of Food and Health Sciences, International College of Arts and Sciences, Fukuoka Women's University, Fukuoka, Japan. E-mail: m-sakuma@fwu.ac.jp
cAgriculture & Biotechnology Business Division, Toyota Motor Corporation, Japan. E-mail: yuji_nagasaka@mail.toyota.co.jp; kunio_matsui@mail.toyota.co.jp
dSafety & Health Promotion Division, Toyota Motor Corporation, Aichi, Japan. E-mail: nao-ki_takeda_aa@mail.toyota.co.jp
eDepartment of Community Health and Preventive Medicine, Hamamatsu University School of Medicine, Shizuoka, Japan. E-mail: miekons@hama-med.ac.jp
First published on 31st January 2022
Abstract
The effects of the regular consumption of soy, barley, and green tea in a real-life setting are unclear. This longitudinal observational study showed the associations of their intake with cardiometabolic health when employees freely selected these foods in the workplace cafeteria of an industrial company in Japan. The consumption was objectively assessed by an electronic purchase system using integrated circuit chip-equipped tableware and personal identification cards. The associations between the cumulative number of servings of each food during the 12 weeks prior to a health examination and changes in cardiometabolic measurements were examined among Japanese male workers (n = 890). Higher total intake of soy products was associated with significant lower levels in low-density lipoprotein cholesterol. Higher total intake of rice with barley was marginally associated with lower levels in systolic blood pressure and glycated hemoglobin. These associations were attenuated after adjustment for the baseline values of the dependent variables. Serving soy and barley products in the workplace cafeteria possibly promotes real-life benefits to employees’ cardiometabolic health.
Introduction
Metabolic syndrome (visceral obesity, dyslipidemia, hypertension, and hyperglycemia) is a common health disorder that results from dietary habits.1 Experimental and cohort studies have identified various foods that protect against metabolic syndrome. Many randomized controlled trials (RCTs) and meta-analyses have shown the positive effects of a high intake of soy,2–5 barley,6–8 and green tea9–11 on low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TGs), glucose metabolism, of which glycated hemoglobin (HbA1c) is a typical indicator, and systolic blood pressure (SBP). Notably, the beneficial effect of soy protein on plasma lipoprotein concentrations is so well-established that the US Food and Drug Administration has approved a coronary heart disease risk reduction claim.12 In Japan, Foods for Specified Health Uses (FOSHUs), which are scientifically accepted for their usefulness in maintaining and promoting health, and Foods with Function Claims (FFCs), which were introduced to make more products available that are clearly labeled with certain health benefits, have been approved by the government as a system that consumers can use to choose healthy diets.13 Several soy, barley, and green tea products are registered as FOSHUs and FFCs, and RCTs have validated their efficacy in experimental settings with high doses and for relatively short dosing periods. However, whether the actual consumption of those foods shows the functionalities has hardly been examined in real-life settings.Here, we present an uncontrolled real-life prospective observational study for Japanese male workers. Functional foods (barley, soybeans, and green tea) were assessed objectively and accurately using an electronic purchasing system with records of free will consumption by workers in their workplace cafeteria. The functional properties of these foods were displayed on tabletop pops. The workplace annual health examination records were used to assess the cardiometabolic health (body mass index [BMI], SBP, LDL-C, HDL-C, TG, and HbA1c) before and after the functional foods were offered in the cafeteria. The purpose of this study was to observe in a real-life setting whether the consumption of functional foods by workers of their own free will was associated with changes in cardiometabolic measurements.
Materials and methods
Subjects and study design
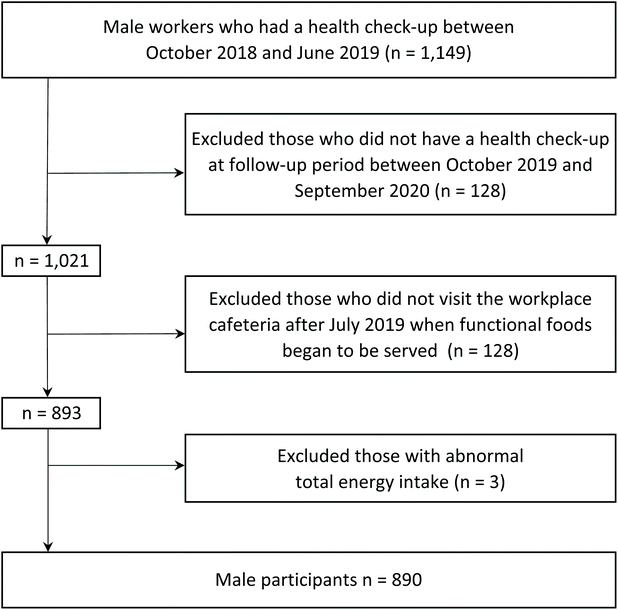
This longitudinal observational study was initiated in November 2019 to examine the association between the voluntary selection of functional foods, which are defined as foods possessing health-beneficial components, at lunchtime in the setting of a workplace cafeteria and cardiometabolic measurements in a Japanese automobile-manufacturing factory. A total of 1149 male workers who underwent a health examination between October 2018 and June 2019 were retrospectively selected for this study. The latest health examinations conducted during this period were determined to be the baseline survey, whereas the latest health examinations conducted between October 2019 and September 2020 were determined to be the follow-up survey. Changes in health examination results between the baseline and follow-up surveys were set as outcome (i.e., dependent) variables. Given that functional foods were introduced to the workplace cafeteria in July 2019, dietary data for the 12 weeks preceding the follow-up survey were set as exposure (i.e., independent) variables. Those who had no follow-up survey (n = 128), those who did not use the workplace cafeteria after July 2019 when functional foods began to be served (n = 128), and those with abnormal total energy intake (n = 3) (Fig. 1), the final study cohort included 890 participants.Institutional review board statement
The study conformed to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Boards of Toyota Motor Corporation (2019BJ0070: October 7, 2019), Hamamatsu University School of Medicine (19-255: November 19, 2019), and Sugiyama Jogakuen University (2019-12: November 19, 2019). All participants provided informed consent through an opt-out approach. To ensure ample opportunity to refuse study participation, we made the study information available to the participants at the workplace cafeteria from the time we introduced the serving of functional foods to the end of the study and at the health check-up site. Fortunately, no one refused.Functional foods
Functional foods served in the workplace cafeteria from July 2019 included soy protein in soy products, β-glucan in barley, and catechin in green tea, and their functional information was displayed on tabletop pops in the workplace cafeteria. Workers could freely select these functional foods and other dishes at their own discretion.Soy products, including tofu and natto (fermented soybeans), had occasionally been included in some menus as items and were provided before July 2019. One portion of tofu priced at ¥60 (tax-excluded Japanese yen) was defined as 75 g, including 5.8 g of soy protein, and that of natto (¥100) was defined as 40 g, including 6.2 g of soy protein. Therefore, choosing one dish of soy products provided approximately 6 g of soy protein.
Barley was served as boiled white rice mixed with barley (50% w/w; hereafter rice with barley). A product called “Mocchiri Mugi” was used from July 2019 to January 2020, and a product called “Mainichi Ichizen Mochimugi Blend” was used after January 2020. The barley cultivar of these products was CDC Fibar, which characteristically contains high levels of β-glucan.14 One bowl of rice with barley includes approximately 3.0 g of β-glucan. The cafeteria served a menu with a choice of boiled rice with barley (¥100) or boiled white rice (¥80).
The catechin-rich green tea (hereafter green tea) served in the cafeteria was a business-use product with added catechins (15.6 g of catechins per 100 g of powder; Mitsui Norin Co., Ltd). One cup of green tea (almost 200 mL of cold beverage with dissolved green tea powder) included approximately 218.4 mg of catechins. Self-service green tea was available for all cafeteria users and used a dedicated cup. Green tea was provided free of charge because many cafeterias serve free water and green tea in Japan. Even before the present study, regular green tea (not rich in catechins) was offered free of charge.
Food and nutrient intake using dietary records obtained from the electronic purchase system
Dietary data, including that on functional foods, were obtained using dietary records from the electronic purchase system at the workplace cafeteria. Accordingly, each tableware has an embedded integrated circuit chip linked to the menu information, and after choosing their meal, the users place their trays on the payment table, where the information is read from the chips, and pay with their employee identification cards. Therefore, individual dietary information is automatically and objectively collected on the basis of the tableware chosen. However, information about leftover food is not recorded by the company that operates the cafeteria (Nakagawa Kyushoku Co., Ltd). The workplace cafeteria is mainly open Monday through Friday, and 99.5% of visits from July 2019 to September 2020 were on weekdays. We retrospectively extracted the dietary records, including that for functional foods, from July 2019 (i.e., when functional foods were introduced) until September 2020. The number of purchases of functional foods and other menu items as well as cafeteria visits were summarized weekly. Food group (“eggs”, “fish, mollusks, and crustaceans”, “fruits”, “grain (cereal) foods”, “pulses”, “meats”, “milk and milk products”, “mushrooms”, “nuts and seeds”, “fats and oils”, “potatoes and starches”, “algae”, “sugars and sweeteners”, and “vegetables”) and nutrient intakes were calculated with the number of purchase records of menu items and its recipe data based on the Standard Tables of Food Composition in Japan – 2015 – (Seventh Revised Version).15 The statistical models used food and nutrient intake data for the 12 weeks prior to the week of the follow-up health examination for each subject.Data from annual workplace health examination
The company's workers were required to undergo a health examination in the same month every year (approximately 150–200 persons per month) at the on-site company health check-up site. Health examination data prior to July 2019 (i.e., when functional foods were initially offered in the cafeteria) were used as the baseline, whereas data from October 2019 to September 2020 were used as follow-up data. The health examination included anthropometric measurements (BMI, SBP, and diastolic blood pressure [DBP]) and blood tests (LDL-C, HDL-C, TGs, and HbA1c; once every 2 years for those aged <35 years old and annually for those aged ≥35 years old), a self-administered questionnaire (including exercise, drinking, and smoking habits), and a medical interview (including information on hospital visits and medications).Health examinations were performed by the company's doctors, nurses, and public health nurses who were blinded regarding the participants’ dietary records, and the laboratory analysis of the obtained blood samples was outsourced to a professional company (Good Life Design Inc.). LDL-C, HDL-C, and TGs were measured using an automated analyzer (JCA-BM6070; JEOL Ltd) by the selective solubilization method, selective inhibition method, and free glycerol elimination method, respectively. HbA1c was measured using an automated analyzer (JCA-BM9130; JEOL Ltd) by an enzymatic method. SBP and DBP were measured using an automated blood pressure monitor (HBP-9020; Omron Corp.).
Statistical analysis
Participants who had missing values at baseline or follow-up for LDL-C, HDL-C, and TGs (n = 216) and HbA1c (n = 247) were excluded from the analysis by pairwise deletion. The number of cafeteria visits per week (total number of cafeteria visits during the 12 weeks preceding the follow-up health examination divided by 12) and the average intake of functional foods per week (total number of portions consumed during the 12 weeks preceding follow-up health examination divided by 12) were calculated. Prespecified outcome variables (BMI, SBP, LDL-C, HDL-C, TG, and HbA1c) and potential confounders (age, medication, BMI, alcohol, smoking, exercise, total energy intake, intake of functional foods other than the explanatory variable of interest, and baseline value of outcome variables) were used in the analyses. The change in cardiometabolic measurements was calculated by subtracting the baseline value from the follow-up value. Those who answered that their exercise habit was once a week (n = 282), two to six times a week (n = 264), and seven times a week (n = 90) were combined into group considered as participants with habitual exercise. Those who answered that their drinking habit was less than three times a week (n = 360) and more than three times a week (n = 309) were combined into a group considered as drinkers (n = 669). Those who smoked <10 cigarettes a day (n = 53) and >10 cigarettes a day (n = 275) were combined into a group considered as smokers.Multivariate regression analysis was used to investigate the association between changes in health examination measurements and the intake of rice with barley, soy products, and green tea at the workplace cafeteria. The dependent variable was the change in cardiometabolic measurements, and the independent variables were the total amounts of functional foods consumed during the 12 weeks prior to the health examination. Adjustments were made for the following potential confounders: model 1: age; model 2: medication (hypertension for SBP; hyperlipidemia for LDL-C, HDL-C, and TGs; or diabetes for HbA1c), BMI (except when the dependent variable was BMI), alcohol, smoking, exercise, and total energy intake, with further adjustments for the intake of rice with barley, soy products, or green tea (e.g., for rice with barley, adjust soy products and green tea); and model 3: baseline values of each dependent variable.
Post hoc subgroup analysis by baseline cardiometabolic risk based on the classification of the 2020 edition of the Japan Society of Ningen Dock16 was performed. Each high-risk group was defined as follows: BMI >25 kg m−2, SBP >130 mmHg or DBP 85 >mmHg, LDL-C >120 mg dL−1, HDL-C <40 mg dL−1, TGs >150 mg dL−1, and HbA1c >5.6%.
Post hoc sensitivity analyses were conducted using multivariate regression analysis with an additional adjustment of intake of fruits, vegetables, or fish, mollusks, and crustaceans for SBP, intake of nuts and seeds, pulses, or meats for LDL-C, intake of fish, mollusks, and crustaceans, nuts and seeds, or meats for TGs, and intake of grain (cereal) foods, pulses, or sugars and sweeteners for HbA1c, with reference to a meta-analysis estimating the effect of each food group on the markers of chronic disease;17 additional adjustment of those with positions within the company (e.g., general manager and section chief) and those without; the exclusion of participants who visited the cafeteria less than 3 days per week (n = 315); the exclusion of participants who changed their medication status after baseline (n = 13 for hypertension; n = 9 for dyslipidemia; n = 2 for diabetes); and exclusion of participants with baseline TGs ≥500 mg dL−1 (n = 11) because high concentrations of TGs may be associated with hypertriglyceridemic HDL particles18 and an increased proportion of small, dense LDL particles.19
Moreover, missing data were imputed using multiple imputation as sensitivity analyses. Missing data values were predicted using multivariate imputation by chained equations using all covariates included in the analysis and variables considered to be associated with the missingness mechanism. The convergence times of the fully conditional specification algorithm was set to twice the convergence times of the EM algorithm, while the predictive mean matching method was used to generate 100 imputed data sets. The rates of missing data from 1145 males, after excluding 4 participants with abnormal energy intake from the 1149 included in health examination, at the item level ranged from 10.7% (difference in BMI) to 34.8% (difference in HbA1c). Given that blood tests are conducted once every 2 years for those under 35 years of age and that some workers did not undergo health examinations during the relevant period, missing data were inevitable.
All analyses were performed using R 4.0.3, and the multivariate regression model was fitted by the lm function of the stats package.20 The mice package 3.13 was estimated using multivariate regression analyses separately applied to each imputed dataset.21 These estimates and their standard errors were combined using Rubin's rules. P values were two-tailed and a P value of <0.05 was considered statistically significant.
Results
Study population
Table 1 presents selected baseline characteristics of participants. The mean age (standard deviation [SD]) in the 890 male participants was 39.9 (11.2) years. The mean number of visits to the workplace cafeteria per week (SD) was 3.0 (1.8). A total of 115 participants (12.9%) never visited to the workplace cafeteria during the 12 weeks prior to the follow-up health examination, and 454 participants (51%) used the cafeteria more than four times per week on average. The mean (SD) portion of functional foods consumed per week per cafeteria user during the 12 weeks prior to the health examination was 0.2 (0.5) bowls for rice with barley, 1.2 (1.1) dishes for soy products, and 1.7 (2.1) cups for green tea, with the distribution as follows: rice with barley: not consumed, 70.3% (n = 545); <2 times per weeks, 27.4% (n = 212); 2–4 times per week 1.9% (n = 15); and ≥4 times per weeks 0.4% (n = 3). Soy products: not consumed, 9.7% (n = 75); <2 times per weeks, 72.6% (n = 563); 2–4 times per weeks 14.2% (n = 110); and ≥4 times per weeks, 3.5% (n = 27). Green tea: not consumed, 37.5% (n = 291); <2 times per weeks, 25.5% (n = 198); 2–4 times per weeks, 16.1% (n = 125); and ≥4 times per weeks, 20.8% (n = 161). Table S1† lists the characteristics of the cafeteria meals and the mean intake amount of food groups per visit of the users. The mean intake of fish, mollusks, and crustaceans; fruits; grain (cereal) foods; pulses; meats; nuts and seeds; and vegetables that were associated with cardiovascular measurements17 was 15.9, 3.9, 159.3, 23.1, 102.5, 0.3, and 112.4 g, respectively. Furthermore, Table S2† summarizes the correlation between the intake of functional foods and the intake amount of each food group per visit 12 weeks before the follow-up examinations. The intake of soy products showed a significantly weak correlation with the intake of mushrooms, nuts and seeds, and potatoes and starches, with correlation coefficients of 0.30, 0.27, and 0.32, respectively.| Health examination results at baseline | Results | |
|---|---|---|
| a Excluded 216 participants with missing data at baseline or follow-up health examination. b Excluded 247 participants with missing data at baseline or follow-up health examination. c Excluded 115 participants with no cafeteria visits HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol. | ||
| Age, year, mean (SD) | 39.9 | (11.2) |
| BMI, kg m−2, mean (SD) | 23.0 | (3.4) |
| Systolic blood pressure, mmHg, mean (SD) | 121.4 | (12.4) |
| Diastolic blood pressure, mmHg, mean (SD) | 72.7 | (11.0) |
| LDL-C, mg dL−1, mean (SD)a | 115.5 | (30.2) |
| HDL-C, mg dL−1, mean (SD)a | 60.0 | (14.9) |
| Triglycerides, mg dL−1, mean (SD)a | 117.1 | (122.7) |
| Hemoglobin A1c, %, mean (SD)b | 5.4 | (0.5) |
| Exercise, number (%) | ||
| <1 time per week | 254 | (31.7) |
| ≥1 time per week | 636 | (68.3) |
| Alcohol use, number (%) | ||
| No drinking | 221 | (24.8) |
| Drinking | 669 | (75.2) |
| Smoking, number (%) | ||
| No smoking | 562 | (63.1) |
| Smoking | 328 | (36.9) |
| Dietary behavior in the 12 weeks preceding the health examination at follow-up | ||
| Number of cafeteria visits per week, mean (SD) | 3.0 | (1.8) |
| Average intake of white rice with barley per week, bowl, mean (SD)c | 0.2 | (0.5) |
| Average intake of soy products per week, dish, mean (SD)c | 1.2 | (1.1) |
| Average intake of catechin-rich green tea per week, cup, mean (SD)c | 1.7 | (2.1) |
| Average intake of total energy/visit, kcal, mean (SD)c | 825.5 | (0.5) |
| Medication status | ||
| Hypertension medication, number (%) | ||
| Continued medication before baseline | 65 | (7.3) |
| Continued medication after baseline | 13 | (1.5) |
| No medication | 812 | (91.2) |
| Dyslipidemia medication, number (%) | ||
| Continued medication before baseline | 57 | (6.4) |
| Continued medication after baseline | 8 | (0.9) |
| Started medication after baseline and finished before follow-up | 1 | (0.1) |
| No medication | 824 | (92.6) |
| Diabetes medication, number (%) | ||
| Continued medication before baseline | 25 | (2.8) |
| Continued medication after baseline | 2 | (0.2) |
| No medication | 863 | (97.0) |
Association between total functional foods intake and cardiometabolic measurements
The mean changes from baseline to follow-up in cardiometabolic measurements (SD) were as follows: 0.2 (0.8) kg m−2 for BMI, 2.2 (12.1) mmHg for SBP, 1.5 (20.2) mg dL−1 for LDL-C, 1.0 (8.4) mg dL−1 for HDL-C, −5.1 (111.3) mg dL−1 TGs, and −0.1% (0.3%) for HbA1c. The associations between the intake of rice with barley, soy products, and green tea and changes in cardiometabolic measurements are shown in Table 2. Higher total intake of soy products was significantly associated with lower levels in LDL-C in model 2 (β: −0.161, P = 0.030), and the association was slightly attenuated by adjustment with the baseline value of LDL-C in model 3 (β: −0.122, P = 0.084). Higher total intake of rice with barley was marginally associated with lower levels in SBP (β: −0.111, P = 0.097) and HbA1c (β: −0.003, P = 0.082) in model 2; however, the associations were attenuated by adjustment with each baseline value in model 3. No association was found between green tea and changes in cardiometabolic measurements.| Cardiometabolic measurement | Model 1b | Model 2c | Model 3d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| a General linear model was used to estimate β, SE, and P values for changes of health examination measurements with intakes of white rice with barley, soy products, and catechin-rich green tea. b Model 1: age-adjusted. c Model 2: added medication (hypertension for SBP; hyperlipidemia for LDL-C, HDL-C, and TGs; or diabetes for HbA1c), BMI, alcohol, smoking, exercise, total energy intake, and intake of white rice with barley, soy products, and catechin-rich green tea (e.g., for rice with barley, adjust soy products and green tea). d Model 3: added baseline values of each dependent variable. BMI, body mass index; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipo-protein cholesterol; SBP, systolic blood pressure; SE, standard error; TG, triglyceride. | |||||||||
| White rice with barley | |||||||||
| Change of BMI, kg m−2 | −0.001 | 0.005 | 0.784 | −0.001 | 0.005 | 0.853 | −0.000 | 0.005 | 0.929 |
| Change of SBP, mmHg | −0.118 | 0.066 | 0.073 | −0.111 | 0.067 | 0.097 | −0.060 | 0.056 | 0.285 |
| Change of LDL-C, mg dL−1 | 0.129 | 0.116 | 0.269 | 0.185 | 0.119 | 0.119 | 0.184 | 0.112 | 0.103 |
| Change of HDL-C, mg dL−1 | −0.052 | 0.048 | 0.286 | −0.034 | 0.049 | 0.486 | 0.016 | 0.048 | 0.730 |
| Change of TGs, mg dL−1 | −0.813 | 0.642 | 0.206 | −1.056 | 0.657 | 0.108 | −0.570 | 0.442 | 0.197 |
| Change of HbA1c, % | −0.004 | 0.002 | 0.027 | −0.003 | 0.002 | 0.082 | −0.002 | 0.001 | 0.254 |
| Soy products | |||||||||
| Change of BMI, kg m−2 | −0.001 | 0.002 | 0.706 | −0.003 | 0.003 | 0.306 | −0.002 | 0.003 | 0.333 |
| Change of SBP, mmHg | −0.004 | 0.030 | 0.901 | 0.019 | 0.036 | 0.602 | 0.011 | 0.030 | 0.708 |
| Change of LDL-C, mg dL−1 | −0.157 | 0.061 | 0.011 | −0.161 | 0.074 | 0.030 | −0.122 | 0.070 | 0.084 |
| Change of HDL-C mg dL−1 | −0.012 | 0.026 | 0.640 | 0.012 | 0.031 | 0.691 | 0.003 | 0.029 | 0.925 |
| Change of TGs, mg dL−1 | 0.186 | 0.341 | 0.585 | 0.159 | 0.410 | 0.697 | 0.075 | 0.275 | 0.786 |
| Change of HbA1c, % | −0.001 | 0.001 | 0.213 | −0.001 | 0.001 | 0.724 | −0.001 | 0.001 | 0.365 |
| Catechin-rich green tea | |||||||||
| Change of BMI, kg m−2 | −0.001 | 0.001 | 0.594 | −0.002 | 0.001 | 0.172 | −0.002 | 0.001 | 0.208 |
| Change of SBP, mmHg | −0.013 | 0.017 | 0.432 | −0.002 | 0.019 | 0.900 | 0.001 | 0.016 | 0.955 |
| Change of LDL-C, mg dL−1 | 0.008 | 0.032 | 0.623 | 0.030 | 0.037 | 0.416 | 0.040 | 0.035 | 0.257 |
| Change of HDL-C, mg dL−1 | −0.006 | 0.013 | 0.640 | 0.007 | 0.015 | 0.640 | 0.003 | 0.015 | 0.829 |
| Change of TGs, mg dL−1 | −0.019 | 0.179 | 0.916 | −0.039 | 0.205 | 0.849 | 0.140 | 0.138 | 0.311 |
| Change of HbA1c, % | 0.000 | 0.000 | 0.791 | 0.001 | 0.001 | 0.130 | 0.001 | 0.000 | 0.123 |
Subgroup analysis by baseline cardiometabolic risk
In the subgroup analysis by baseline cardiometabolic risk (Table 3), significant inverse associations between total intake of soy products and change of LDL-C were observed only in participants with LDL-C ≥120 mg dL−1 in model 2 (β: −0.214, P = 0.031), and the association was slightly attenuated in model 3 (β: −0.183, P = 0.055). Regarding the intake of rice with barley, lower levels in SBP (model 2, β: −0.151, P = 0.052, and model 3, β: −0.128, P = 0.063) were marginally observed only in participants with SBP <130 mmHg and DBP <85 mmHg. Significant lower levels in HbA1c (β: −0.017, P = 0.005) and TGs (β: −9.151, P = 0.031) with rice with barley were observed only in participants with HbA1c ≥5.6% and TGs ≥150 mg dL−1 in model 2, respectively; however, the association was not observed in model 3, in which each baseline value was further adjusted. A significant higher level of SBP was observed with green tea intake in participants with SBP ≥130 mmHg or DBP ≥85 mmHg (model 3, β: 0.076, P = 0.020).| Cardiometabolic measurement | Model 1 | Model 2 | Model 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| β | SE | P value | β | SE | P value | β | SE | P value | |
| a Participants were divided into two groups: the high-risk group with abnormalities of a mild level or greater and the group with low risk, based on the classification of the 2020 edition of the Japan Society of Ningen Dock.16 A general linear model was used to estimate β, SE, and P values for change of health examination measurements with the intake of white rice with barley, soy products, and catechin-rich green tea DBP, diastolic blood pressure; HbA1c, glycated hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; SBP, systolic blood pressure; TG, triglyceride. | |||||||||
| White rice with barley | |||||||||
| Change of BMI, kg m−2 | |||||||||
| BMI ≥25 (n = 217) | −0.016 | 0.010 | 0.135 | −0.015 | 0.011 | 0.171 | −0.014 | 0.011 | 0.181 |
| BMI <25 (n = 673) | 0.004 | 0.005 | 0.373 | 0.005 | 0.005 | 0.341 | 0.005 | 0.005 | 0.325 |
| Change of SBP, mmHg | |||||||||
| SBP ≥130 or DBP ≥85 (n = 261) | 0.037 | 0.104 | 0.724 | −0.002 | 0.109 | 0.982 | 0.014 | 0.098 | 0.883 |
| SBP <130 and DBP <85 (n = 629) | −0.177 | 0.077 | 0.022 | −0.151 | 0.078 | 0.052 | −0.128 | 0.069 | 0.063 |
| Change of LDL-C, mg dL−1 | |||||||||
| LDL-C ≥120 (n = 328) | 0.195 | 0.163 | 0.233 | 0.133 | 0.162 | 0.414 | 0.158 | 0.157 | 0.313 |
| LDL-C <120 (n = 346) | 0.092 | 0.153 | 0.549 | 0.162 | 0.156 | 0.299 | 0.162 | 0.157 | 0.300 |
| Change of HDL-C, mg dL−1 | |||||||||
| HDL-C <40 mg dL−1 (n = 36) | −0.080 | 0.207 | 0.702 | −0.149 | 0.246 | 0.550 | −0.132 | 0.235 | 0.579 |
| HDL-C ≥40 mg dL−1 (n = 638) | 0.097 | 0.116 | 0.405 | 0.138 | 0.118 | 0.242 | 0.150 | 0.120 | 0.212 |
| Change of TGs, mg dL−1 | |||||||||
| TGs ≥150 (n = 136) | −8.341 | 3.905 | 0.035 | −9.151 | 4.196 | 0.031 | 0.583 | 2.573 | 0.821 |
| TGs <150 (n = 538) | −0.103 | 0.258 | 0.690 | −0.097 | 0.267 | 0.716 | −0.136 | 0.266 | 0.610 |
| Change of HbA1c, % | |||||||||
| HbA1c ≥5.6 (n = 201) | −0.020 | 0.006 | <0.001 | −0.017 | 0.006 | 0.005 | −0.001 | 0.005 | 0.817 |
| HbA1c <5.6 (n = 4 42) | −0.0003 | 0.001 | 0.820 | −0.0002 | 0.001 | 0.876 | −0.0001 | 0.00 | 0.916 |
| Soy products | |||||||||
| Change of BMI, kg m−2 | |||||||||
| BMI ≥25 (n = 217) | −0.003 | 0.005 | 0.474 | −0.004 | 0.006 | 0.512 | −0.004 | 0.006 | 0.456 |
| BMI <25 (n = 673) | −0.00004 | 0.002 | 0.987 | −0.002 | 0.003 | 0.483 | −0.002 | 0.003 | 0.520 |
| Change of SBP, mmHg | |||||||||
| SBP ≥130 or DBP ≥85 (n = 261) | 0.040 | 0.052 | 0.434 | 0.047 | 0.065 | 0.473 | 0.011 | 0.059 | 0.852 |
| SBP <130 and DBP <85 (n = 629) | −0.022 | 0.033 | 0.517 | 0.026 | 0.040 | 0.509 | 0.012 | 0.035 | 0.733 |
| Change of LDL-C, mg dL−1 | |||||||||
| LDL-C ≥120 (n = 328) | −0.149 | 0.085 | 0.080 | −0.214 | 0.099 | 0.031 | −0.183 | 0.095 | 0.055 |
| LDL-C <120 (n = 346) | −0.100 | 0.083 | 0.232 | 0.021 | 0.101 | 0.833 | 0.021 | 0.102 | 0.833 |
| Change of HDL-C, mg dL−1 | |||||||||
| HDL-C <40 mg dL−1 (n = 36) | −0.198 | 0.075 | 0.013 | −0.142 | 0.109 | 0.205 | −0.113 | 0.106 | 0.297 |
| HDL-C ≥40 mg dL−1 (n = 638) | −0.001 | 0.027 | 0.970 | 0.011 | 0.032 | 0.722 | 0.010 | 0.031 | 0.757 |
| Change of TGs, mg dL−1 | |||||||||
| TGs ≥150 (n = 136) | 1.341 | 1.382 | 0.334 | 0.893 | 1.780 | 0.617 | −0.291 | 1.060 | 0.784 |
| TGs <150 (n = 538) | −0.084 | 0.150 | 0.575 | −0.048 | 0.180 | 0.792 | −0.051 | 0.179 | 0.774 |
| Change of HbA1c, %c | |||||||||
| HbA1c ≥5.6 (n = 201) | −0.002 | 0.003 | 0.399 | 0.002 | 0.003 | 0.587 | −0.0004 | 0.002 | 0.880 |
| HbA1c <5.6 (n = 442) | −0.001 | 0.001 | 0.220 | −0.001 | 0.001 | 0.145 | −0.001 | 0.001 | 0.120 |
| Catechin-rich green tea | |||||||||
| Change of BMI, kg m−2 | |||||||||
| BMI ≥25 (n = 217) | −0.002 | 0.003 | 0.521 | −0.002 | 0.003 | 0.598 | −0.002 | 0.003 | 0.539 |
| BMI <25 (n = 673) | −0.001 | 0.001 | 0.518 | −0.002 | 0.001 | 0.205 | −0.002 | 0.001 | 0.237 |
| Change of SBP, mmHg | |||||||||
| SBP ≥130 or DBP ≥85 (n = 261) | 0.048 | 0.030 | 0.117 | 0.063 | 0.036 | 0.079 | 0.076 | 0.032 | 0.020 |
| SBP <130 and DBP <85 (n = 629) | −0.048 | 0.019 | 0.010 | −0.037 | 0.021 | 0.069 | −0.02 | 0.018 | 0.166 |
| Change of LDL-C, mg dL−1 | |||||||||
| LDL-C ≥120 (n = 328) | 0.073 | 0.049 | 0.138 | 0.063 | 0.056 | 0.260 | 0.060 | 0.054 | 0.270 |
| LDL-C <120 (n = 346) | −0.035 | 0.040 | 0.383 | 0.003 | 0.045 | 0.945 | 0.003 | 0.045 | 0.946 |
| Change of HDL-C, mg dL−1 | |||||||||
| HDL-C <40 mg dL−1 (n = 36) | −0.062 | 0.063 | 0.339 | −0.003 | 0.090 | 0.969 | 0.008 | 0.086 | 0.923 |
| HDL-C ≥40 mg dL−1 (n = 638) | −0.004 | 0.014 | 0.785 | 0.011 | 0.016 | 0.488 | 0.006 | 0.015 | 0.678 |
| Change of TGs, mg dL−1 | |||||||||
| TGs ≥150 (n = 136) | 0.263 | 0.831 | 0.994 | −0.084 | 1.027 | 0.935 | 0.874 | 0.613 | 0.156 |
| TGs <150 (n = 538) | 0.006 | 0.076 | 0.937 | 0.023 | 0.087 | 0.789 | 0.019 | 0.086 | 0.830 |
| Change of HbA1c, % | |||||||||
| HbA1c ≥5.6 (n = 201) | 0.0003 | 0.001 | 0.863 | 0.002 | 0.001 | 0.139 | 0.0001 | 0.001 | 0.910 |
| HbA1c <5.6 (n = 442) | 0.0003 | 0.0003 | 0.454 | 0.0003 | 0.0004 | 0.399 | 0.0004 | 0.0004 | 0.272 |
Sensitivity analyses
On the additional adjustment for nuts and seeds intake, model 3 showed the attenuated associations between the total intake of soy products and the low levels of LDL-C (β: −0.117, P = 0.101). The marginal inverse association between the total intake of soy products and change of LDL-C among participants with LDL-C ≥120 mg dL−1 in model 3 was apparent in the results that excluded the 225 participants who visited the workplace cafeteria less than 3 days per week (β: −0.234, P = 0.024). The marginal association between the high total intake of rice with barley and the low levels of HbA1c in model 2 was attenuated among those excluded 225 participants who visited the workplace cafeteria less than 3 days per week (β: −0.002, P = 0.164) but not among those participants with HbA1c ≥5.6% (β: −0.015, P = 0.025). The associations between the high total intake of soy products and the low levels of LDL-C remained by excluding nine participants who changed their medication status after baseline, but model 2 (β: −0.141, P = 0.062) and model 3 (β: −0.112, P = 0.119) showed a decrease in its significance. By excluding 13 participants who changed their medication status, the associations between the high total intake of rice with barley and low levels of SBP in model 2 were attenuated (β: −0.102, P = 0.130) but not the association with HbA1c levels in model 2 (β: −0.003, P = 0.082). In addition, the high total intake of rice with barley was marginally associated with low levels of TGs in model 2 (β: −1.245, P = 0.061) and model 3 (β: −0.802, P = 0.061). The association between a lower level of TGs with rice with barley among participants with TGs ≥150 mg dL−1 disappeared in the analysis that excluded 11 participants with baseline TGs ≥500 mg dL−1 (model 2, β: 1.017, P = 0.687). All other associations were not substantially changed by additional adjustment and exclusion.Integrated results for the analysis of the imputed data sets showed that the association between SBP and total intake of rice with barley in model 2 for attenuated (β: −0.102, p = 0.125), whereas that for HbA1c remained (β: −0.003, p = 0.050). The association between soy products and LDL-C was slightly attenuated (model 2, β: −0.139, P = 0.065, and model 3, β: −0.119, P = 0.099).
Discussion
Our uncontrolled, real-world observational study using an electronic purchase system in a workplace cafeteria as a dietary assessment method revealed that the high intake of soy products was significantly associated with lower levels of LDL-C and the high intake of rice with barley was marginally associated with lower levels of SBP and HbA1c among male workers in an automobile-manufacturing factory. To our knowledge, no studies have examined the associations of functional foods consumed under free will on cardiometabolic health in a real-life setting that objectively assessed the dietary data.The inverse association between soy product intake and changes in LDL-C is completely in line with previous meta-analyses of RCTs.2,3,5,22 In the later meta-analysis, the intervention of soy products with a median intake of 25 g d−1 of soy protein caused a significant net reduction of 3.27 mg dL−1 of LDL-C.5 In another meta-analysis, significant reductions of LDL-C were more apparent in patients with hypercholesterolemia than in healthy subjects.2 Likewise, lower levels of LDL-C with soy product intake were significant in participants with LDL-C ≥120 mg dL−1 in our study. Several mechanisms have been proposed for the cholesterol-lowering effect of soy products, including the inhibition of bile acid or cholesterol absorption, inhibition of cholesterol synthesis, and stimulation of LDL receptor transcription.23
In contrast to LDL-C, no significant association was observed between soy product and TGs or HDL-C in this study, despite several meta-analyses2,5,24 reporting the TG-lowering and HDL-C-increasing effect of soy products. It is possible that the amount of soy product intake in our study was insufficient to obtain such an association. In our study, the estimated change in LDL-C was slight, with only −0.122 mg dL−1 caused by the intake of one portion of soy protein. The amount consumed in our study was much smaller than that in an RCT setting.
The high intake of rice with barley was marginally associated with lower levels of HbA1c in our study. Whole-grain and cereal fiber intake have been inversely associated with insulin resistance, the prevalence of metabolic syndrome, and the risk of type 2 diabetes in previous cohort studies.24,25 The glycemic index (GI) is a ranking of carbohydrate-containing foods based on their effect on postprandial glycemic response,26 and a meta-analysis of 14 RCTs showed the amelioration of HbA1c through a low-GI diet.27 Barley has the lowest GI of all major grains.28 A study in healthy Japanese subjects revealed an improvement in their glucose metabolism as the result of consuming 50% barley compared with white rice as a staple food.6 Furthermore, dietary GI was independently associated with HbA1c in Japanese female subjects whose dietary GI was primarily determined based on the GI of white rice.29 Replacing white rice with 50% barley as a part of a meal may be useful for glycemic control.
It has been reported that barley may not only have a beneficial effect on the glycemic response after the meal in which it is consumed but also lower glycemic response, appetite, and energy intake after subsequent meals (the so-called second-meal effect).30,31 Therefore, in our study, the beneficial associations of barley consumed during lunch may have been maintained during dinner.
In our study, the high intake of barley was also marginally associated with lower levels of SBP. A systematic review and meta-analysis of RCTs of healthy individuals reported that the high consumption of dietary fiber, especially β-glucan, was associated with a lower SBP.7 Another study in healthy subjects reported that replacing about 20% of the energy from refined carbohydrates with whole-grain foods lowered blood pressure.32 The barley provided in our study was characterized by its high content of γ-aminobutyric acid as well as β-glucan and contained 20 mg per 100 g in the shape of rice grains (analysis of one sample by Nagakura Seibaku K. K.). γ-Aminobutyric acid has been approved as an FFC by the Japanese government to improve not only blood pressure but also stress relief and sleep.13 Thus, it is suggested that these effects may be synergistically involved in lowering blood pressure.
Overall, no association was found between green tea intake and changes in cardiometabolic measurements in our study. In a recent meta-analysis of RCTs, the association between green tea and cardiometabolic factors was inconclusive. Green tea consumption was reported to improve LDL-C9,33 and SBP,10 but no effect was reported in short-term (4–24 weeks) trials (the analysis also included black tea).34 Trials of the effect of green tea consumption on BMI for individuals who were overweight have reported both a beneficial35 and non-beneficial effect.36 Green tea consumption has shown no benefit on HDL, TGs,33 or HbA1c11 in recent studies. However, the intake of green, black, and oolong tea was reported as associated with cardiovascular health in a recent large-scale meta-analysis of 37 population-based studies.37 Therefore, the period between the introduction of green tea and the comparison with baseline cardiometabolic values or the observation period our study may have been too short to demonstrate the association with cardiometabolic health in real life.
Additionally, catechins reach their peak blood concentration 1–2 hours after intake and have a half-life of 2–5 hours,38 and potential effects of green tea depend on the amount consumed.37 The green tea intake at lunch observed in our study may be insufficient in terms of the frequency and amount of daily intake to obtain a beneficial association. In addition, the association with cardiometabolic factors may be affected by other dietary factors, such as diet quality, amount consumed, or snacking.
The chief strength of our study is the longitudinal analysis of real-world data, which includes the intake of functional foods consumed according to the participants’ free will in a workplace cafeteria and collected objectively using an electronic purchase system as a dietary assessment method. Therefore, the various biases associated with dietary surveys were minimized, and the measurement error was minimal and more realistically represented the potential for improving cardiometabolic health by serving functional foods at lunchtime in real-life workplace cafeterias. Furthermore, the health examiners were blinded regarding the participants’ dietary intake, including that of functional foods. However, participants with a higher risk for cardiometabolic health might be more likely to consume functional foods with health benefits, suggesting a potential bias. Therefore, we conducted the post hoc subgroup analysis according to baseline cardiometabolic risk.
This study has further some limitations. First, this is an observational study. Although we accounted for several potential confounding factors, residual unmeasured confounders may still remain. Second, this study cannot exclude the possibility of selection bias because the participants were male workers who used the cafeteria in a single factory. Further studies, including female workers and other factories, are necessary to enhance generalizability. In addition, there were considerable missing data (especially blood lipids and blood glucose measurements). Although sensitivity analysis, in which missing values were imputed via multiple imputation, showed considerable attenuation of the association between rice with barley and SBP, other associations remained unchanged. Third, only lunch, mostly on weekdays, was assessed as dietary data, and the influences of breakfast, dinner, and snacks could not be considered. Thus, the observed associations on cardiometabolic measurements may include those of dietary preferences at other times of the day. Regarding the effect of the entire lunch on the outcome, the participants consumed other foods associated with cardiovascular measurements, such as nuts and seeds and vegetables,17 in addition to the functional foods focused in the present study. Additionally, although no detailed data on nutrients are available in this study, those participants with a higher intake of soy products also had higher intakes of mushrooms, nuts and seeds, and potatoes and starches; thus, they may have had higher fiber intake and better fatty acid balance. Therefore, the overall quality of the meal in the cafeteria may also be related. Fourth, we were unable to evaluate the intake amount and the amount of leftover food. Because intake was assessed on the basis of tableware, the intake amount could have been less if there were leftover food. Fifth, because of the extremely strong correlation between the baseline value and the amount of change in the health examination results, it is possible that even the association of functional foods was over adjusted in model 3. Finally, although functional foods were not sufficiently consumed, especially rice with barley, we did not actively recommend their consumption because we examined the association between the intake of functional foods consumed at will with changes in cardiometabolic measurements. Further studies are necessary to examine how active encouragement can increase the intake of functional foods in a real-life setting and how much they can be positively linked to cardiometabolic measurements and affect long-term health.
Conclusions
In conclusion, this is the first real-life observational study to examine the associations of functional foods consumed by male employees at lunch of their own free will in a workplace cafeteria. An electronic purchasing system was used as the objective dietary assessment method. Our results more realistically suggested that soy products and rice with barley served in workplace cafeterias may positively impact cardiometabolic health by the voluntary consumption of these foods among Japanese male workers.Data availability statement
In the original data used in this study are unavailable to the public as per of the policy of Toyota Motor Corporation.Author contributions
The authors’ responsibilities were as follows: Conceptualization and methodology, Y. S., M. S., Y. N., K. M., and M. N.; investigation, Y. N., N. T., and K. M.; data curation, Y. S., Y. N., N. T., and K. M.; formal analysis, Y. S.; supervision, M. N.; writing – original draft, Y. S., M. S., and M. N.; writing – review & editing, all authors read and approved the final manuscript.Conflicts of interest
The white rice mixed with barley used in the cafeteria is a product called Mainichi Ichizen Mochimugi Blend, jointly developed by Toyota Motor Corporation and Nagakura Seibaku K. K., and Toyota Motor Corporation has applied for a patent on this product. Nagakura Seibaku K. K. sells the developed product, and Toyota Motor Corporation has received technology royalties from October 2020, but no products were offered for this study by Nagakura Seibaku K. K. Y. S., M. S., and M. N. received research funding from Toyota Motor Corporation. None of the authors had other conflicts of interest to declare.Acknowledgements
We express our sincere appreciation to the employees of Toyota Motor Corporation and its affiliated companies for their cooperation in conducting this study. The authors would like to thank Enago (http://www.enago.jp) for the English language review. This study was an observational study, and the functional foods served in the cafeteria were paid for and consumed by the workers. No financial support was received from companies or other organizations for the functional foods provided during this study. This research and the APC were funded by Toyota Motor Corporation. Y. S., M. S., and M. N. received research funding from Toyota Motor Corporation for this joint research (Y. S. 19SEI007, M. S.: 1106106000, and M. N.: 931950918).References
- K. G. Alberti, R. H. Eckel, S. M. Grundy, P. Z. Zimmet, J. I. Cleeman, K. A. Donato, J.-C. Fruchart, W. P. T. James, C. M. Loria and S. C. Smith Jr., International Diabetes Federation Task Force on Epidemiology and Prevention, National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society, International association for the study of obesity. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Circulation, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity, 2009, vol. 120, pp. 1640–1645.
- O. A. Tokede, T. A. Onabanjo, A. Yansane, J. M. Gaziano and L. Djoussé, Soya products and serum lipids: a meta-analysis of randomised controlled trials, Br. J. Nutr., 2015, 114, 831–843 CrossRef CAS PubMed.
- S. Blanco Mejia, M. Messina, S. S. Li, E. Viguiliouk, L. Chiavaroli, T. A. Khan, K. Srichaikul, A. Mirrahimi, J. L. Sievenpiper, P. Kris-Etherton and D. J. A. Jenkins, A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults, J. Nutr., 2019, 149, 968–981 CrossRef PubMed.
- J. W. Anderson, B. M. Johnstone and M. E. Cook-Newell, Meta-analysis of the effects of soy protein intake on serum lipids, N. Engl. J. Med., 1995, 333, 276–282 CrossRef CAS PubMed.
- M. Moradi, E. Daneshzad and L. Azadbakht, The effect of isolated soy protein, isolated soy isoflavones and soy protein containing isoflavones on serum lipids in postmenopausal women: a systematic review and meta-analysis, Crit. Rev. Food Sci. Nutr., 2020, 60, 3414–3428 CrossRef CAS PubMed.
- M. Higa, Y. Fuse, N. Miyashita, A. Fujitani, K. Yamashita, T. Ichijo, S. Aoe and T. Hirose, Effect of high ∝-glucan barley on postprandial blood glucose levels in subjects with normal glucose tolerance: assessment by meal tolerance test and continuous glucose monitoring system, Clin. Nutr. Res., 2019, 8, 55–63 CrossRef PubMed.
- C. E. Evans, D. C. Greenwood, D. E. Threapleton, C. L. Cleghorn, C. Nykjaer, C. E. Woodhead, C. P. Gale and V. J. Burley, Effects of dietary fibre type on blood pressure: a systematic review and meta-analysis of randomized controlled trials of healthy individuals, J. Hypertens., 2015, 33, 897–911 CrossRef CAS PubMed.
- H. V. T. Ho, J. L. Sievenpiper, A. Zurbau, S. Blanco Mejia, E. Jovanovski, F. Au-Yeung, A. L. Jenkins and V. Vuksan, A systematic review and meta-analysis of randomized controlled trials of the effect of barley ∝-glucan on LDL-C, non-HDL-C and apoB for cardiovascular disease risk reductioni−iv, Eur. J. Clin. Nutr., 2016, 70, 1239–1245 CrossRef CAS PubMed.
- W. Liu, C. Wan, Y. Huang and M. Li, Effects of tea consumption on metabolic syndrome: a systematic review and meta-analysis of randomized clinical trials, Phytother. Res., 2020, 34, 2857–2866 CrossRef CAS PubMed.
- R. Xu, K. Yang, J. Ding and G. Chen, Effect of green tea supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials, Medicine, 2020, 99, e19047 CrossRef PubMed.
- R. Xu, Y. Bai, K. Yang and G. Chen, Effects of green tea consumption on glycemic control: a systematic review and meta-analysis of randomized controlled trials, Nutr. Metab., 2020, 17, 56 CrossRef CAS PubMed.
- Food and Drug Administration, Department of Health and Human Services, Food labeling: health claims; soy protein and coronary heart disease. Final rule, Fed. Regist., 1999, 64, 57700–57733 Search PubMed.
- Consumer Affairs Agency, What are “foods with function claims” ?, 2015, https://www.caa.go.jp/en/policy/food_labeling/, accessed 2 Feb 2021.
- The official website of the Government of Canada. Canadian Food Inspection Agency. Plant varieties, Plant breeders’ rights, 2015, https://www.inspection.gc.ca/english/plaveg/pbrpov/cropreport/bar/app00005281e.shtml, accessed 2 March 2021.
- Ministry of Education, Culture, Sports, Science and Technology. Standard Tables of Food Composition in Japan, 2015 – (Seventh Revised Version), 2021, https://www.mext.go.jp/en/policy/science_technology/policy/title01/detail01/1374030.htm, accessed 10 March 2021.
- Japan Society of Ningen Dock, Criteria category 2020 (In Japanese), 2020, https://www.ningen-dock.jp/wp/wp-content/uploads/2013/09/69ab66f1e59f3c9e295b3e00e161ff30.pdf, accessed 19 January 2021.
- L. Schwingshackl, G. Hoffmann, K. Iqbal, C. Schwedhelm and H. Boeing, Food groups and intermediate disease markers: a systematic review and network meta-analysis of randomized trials, Am. J. Clin. Nutr., 2018, 108, 576–586 CrossRef PubMed.
- J. W. Skeggs and R. E. Morton, LDL and HDL enriched in triglyceride promote abnormal cholesterol transport, J. Lipid Res., 2002, 43, 1264–1274 CrossRef CAS.
- M. A. Austin, M. C. King, K. M. Vranizan and R. M. Krauss, Atherogenic lipoprotein phenotype. a proposed genetic marker for coronary heart disease risk, Circulation, 1990, 82, 495–506 CrossRef CAS PubMed.
- R Core Team, R: A language and environment for statistical computing, 2020, https://www.eea.europa.eu/data-and-maps/indicators/oxygen-consuming-substances-in-rivers/r-development-core-team-2006, accessed 20 January 2021. R Foundation for Statistical Computing, Vienna, Austria.
- S. van Buuren and K. Groothuis-Oudshoorn, Mice: multivariate Imputation by Chained Equations in R, J. Stat. Softw., 2011, 45, 1–67 Search PubMed.
- J. W. Anderson, B. M. Johnstone and M. E. Cook-Newell, Meta-analysis of the effects of soy protein intake on serum lipids, N. Engl. J. Med., 1995, 333, 276–282 CrossRef CAS PubMed.
- S.-J. Cho, M. A. Juillerat and C.-H. Lee, Cholesterol lowering mechanism of soybean protein hydrolysate, J. Agric. Food Chem., 2007, 55, 10599–10604 CrossRef CAS PubMed.
- N. M. McKeown, J. B. Meigs, S. Liu, E. Saltzman, P. W. F. Wilson and P. F. Jacques, Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort, Diabetes Care, 2004, 27, 538–546 CrossRef PubMed.
- J. Montonen, P. Knekt, R. Järvinen, A. Aromaa and A. Reunanen, Whole-grain and fiber intake and the incidence of type 2 diabetes, Am. J. Clin. Nutr., 2003, 77, 622–629 CrossRef CAS PubMed.
- D. J. Jenkins, T. M. Wolever, R. H. Taylor, H. Barker, H. Fielden, J. M. Baldwin, A. C. Bowling, H. C. Newman, A. L. Jenkins and D. V. Goff, Glycemic index of foods: a physiological basis for carbohydrate exchange, Am. J. Clin. Nutr., 1981, 34, 362–366 CrossRef CAS PubMed.
- J. Brand-Miller, S. Hayne, P. Petocz and S. Colagiuri, Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials, Diabetes Care, 2003, 26, 2261–2267 CrossRef PubMed.
- F. S. Atkinson, K. Foster-Powell and J. C. Brand-Miller, International tables of glycemic index and glycemic load values: 2008, Diabetes Care, 2008, 31, 2281–2283 CrossRef PubMed.
- K. Murakami, S. Sasaki, Y. Takahashi, H. Okubo, Y. Hosoi, H. Horiguchi, E. Oguma and F. Kayama, Dietary glycemic index and load in relation to metabolic risk factors in Japanese female farmers with traditional dietary habits, Am. J. Clin. Nutr., 2006, 83, 1161–1169 CrossRef CAS PubMed.
- T. Matsuoka, A. Tsuchida, A. Yamaji, C. Kurosawa, M. Shinohara, I. Takayama, H. Nakagomi, K. Izumi, Y. Ichikawa, N. Hariya, S. Yamashita and K. Mochizuki, Consumption of a meal containing refined barley flour bread is associated with a lower postprandial blood glucose concentration after a second meal compared with one containing refined wheat flour bread in healthy Japanese: a randomized control trial, Nutrition, 2020, 72, 110637 CrossRef CAS PubMed.
- S. Aoe, T. Ikenaga, H. Noguchi, C. Kohashi, K. Kakumoto and N. Kohda, Effect of cooked white rice with high ∝-glucan barley on appetite and energy intake in healthy Japanese subjects: a randomized controlled trial, Plant Foods Hum. Nutr., 2014, 69, 325–330 CrossRef CAS PubMed.
- K. M. Behall, D. J. Scholfield and J. Hallfrisch, Whole-grain diets reduce blood pressure in mildly hypercholesterolemic men and women, J. Am. Diet. Assoc., 2006, 106, 1445–1449 CrossRef PubMed.
- R. Xu, K. Yang, S. Li, M. Dai and G. Chen, Effect of green tea consumption on blood lipids: a systematic review and meta-analysis of randomized controlled trials, Nutr. J., 2020, 19, 48 CrossRef CAS PubMed.
- E. Igho-Osagie, K. Cara, D. Wang, Q. Yao, L. P. Penkert, A. Cassidy, M. Ferruzzi, P. F. Jacques, E. J. Johnson, M. Chung and T. Wallace, Short-term tea consumption is not associated with a reduction in blood lipids or pressure: a systematic review and meta-analysis of randomized controlled trials, J. Nutr., 2020, 150, 3269–3279 CrossRef PubMed.
- Y. Lin, D. Shi, B. Su, J. Wei, M.-A. Găman, M. M. Sedanur, I. J. Borges do Nascimento and N. S. Guimaraes, The effect of green tea supplementation on obesity: a systematic review and dose-response meta-analysis of randomized controlled trials, Phytother. Res., 2020, 34, 2459–2470 CrossRef CAS PubMed.
- T. M. Jurgens, A. M. Whelan, L. Killian, S. Doucette, S. Kirk and E. Foy, Green tea for weight loss and weight maintenance in overweight or obese adults, Cochrane Database Syst. Rev., 2012, 12, CD008650 Search PubMed.
- M. Chung, N. Zhao, D. Wang, M. Shams-White, M. Karlsen, A. Cassidy, M. Ferruzzi, P. F. Jacques, E. J. Johnson and T. C. Wallace, Dose-response relation between tea consumption and risk of cardiovascular disease and all-cause mortality: a systematic review and meta-analysis of population-based studies, Adv. Nutr., 2020, 11, 790–814 CrossRef PubMed.
- U. Ullmann, J. Haller, J. P. Decourt, N. Girault, J. Girault, A. S. Richard-Caudron, B. Pineau and P. Weber, A single ascending dose study of epigallocatechin gallate in healthy volunteers, J. Int. Med. Res., 2003, 31, 88–101 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1fo02434e |
| This journal is © The Royal Society of Chemistry 2022 |