Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Increase in the active ingredients of traditional Chinese medicine Isatis indigotica through iron nanoparticles supplementation versus carbon nanotubes: a comparative study†
Pingfan
Zhou‡
a,
Binbin
Long‡
a,
Ruisi
Wang‡
a,
Yaqi
Jiang
a,
Weichen
Zhao
a,
Yuanbo
Li
a,
Mingshu
Li
a,
Zhiling
Guo
 b,
Peng
Zhang
b,
Peng
Zhang
 *bc,
Yukui
Rui
*bc,
Yukui
Rui
 *a and
Iseult
Lynch
*a and
Iseult
Lynch
 b
b
aBeijing Key Laboratory of Farmland Soil Pollution Prevention and Remediation, College of Resources and Environmental Sciences, China Agricultural University, Beijing 100193, China. E-mail: ruiyukui@163.com
bSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Edgbaston, Birmingham, B15 2TT, UK. E-mail: p.zhang.1@bham.ac.uk
cDepartment of Environmental Science and Engineering, University of Science and Technology of China, Hefei, 230026, China
First published on 4th July 2022
Abstract
The low yield and active ingredient content of medicinal plants is always a challenge. However, nanotechnology offers new tools for enhancing the biomass and metabolism of medicinal plants. In the present study, the effects of two nanomaterials (NMs) on the growth, photosynthesis, antioxidant system, mineral homeostasis, C/N accumulation, and content of active ingredients of Isatis indigotica (BLG) were investigated. BLG was grown in soil supplemented with 10–250 mg kg−1 of multi-walled carbon nanotubes (CNTs) or iron oxide nanoparticles (Fe2O3 NPs) for 60 days in a greenhouse. The results showed that a low concentration (10 mg kg−1) of Fe2O3 NPs promoted the growth of BLG, while the opposite trend was observed in the CNT treatment. Fe2O3 NPs at 10 mg kg−1 effectively increased the shoot length and biomass by 21% and 14%, respectively. Notably, the Fe2O3 NPs could also effectively enhance the officinal ingredient content of BLG by regulating the antioxidant system, mineral element homeostasis, and carbon and nitrogen synthesis. Fe2O3 NPs at 10 mg kg−1 increased the flavonoids, soluble sugars, and total protein content of BLG roots by 55%, 124%, and 59%, respectively. In conclusion, our results suggest that the Fe2O3 NPs can be used as a bio-fortifier to enhance the yield and officinal quality of BLG.
Environmental significanceThe low yield and active ingredient content of medicinal plants is a widespread and unresolved problem. For the first time, this study reports the effects of CNTs and Fe2O3 NPs on the plant growth and medicinal active ingredients of Isatis indigotica (BLG), and reveals the mechanism of nanomaterials as biofortification agents to promote the medicinal active ingredients. Furthermore, this study also demonstrated that Fe2O3 NPs at 10 mg kg−1 are considered to be a more effective biological enhancer than CNTs to promote the plant yield and medicinal active contents. |
1 Introduction
Isatis indigotica Fort. (Cruciferae family, abbreviated as BLG), a biennial herbaceous plant, is widely distributed and cultivated in Southeast Asian countries.1 The dried roots and leaves, named “Banlangen (BLG)” and “Daqingye (DQY)” in Chinese,2 respectively, have been used in treating diseases for hundreds of years in China, especially for the treatment of influenza, colds, fever, and infection.3 Various compounds isolated from BLG have been identified as effective components, mainly as polysaccharides, phenols, amino acids, alkaloids, and flavonoids,4–6 which have high contents and low side effects. Moreover, the compound preparation of BLG and DQY or its monomer extract has attracted extensive research and has application value, for Coronavirus disease 2019, COVID-19.7 Cheng et al. (2021) reported that the Chinese patented medicine Fufang Banlangen Keli (CBLGG, containing BLG and DQY) is effective in the treatment of symptoms caused by COVID-19.8 Despite the importance of BLG as a medicine, the contents of the active ingredients in the plant (and yield) are usually low; therefore, methods that can increase their active ingredient levels also can increase their medical values and effectiveness.Nanomaterials (NMs) have recently shown the potential to improve the nutritional quality, growth, and yield of plants.9 Using NMs as inducers for increasing plant metabolite production is becoming an emerging approach.10 The application of NMs may lead to a direct increase in plant production or an indirect increase in the synthesis and accumulation of secondary metabolites by stimulating the stress system of the plant.11,12 Among the various NMs studied, multi-walled carbon nanotubes (CNTs) and iron oxide nanoparticles (Fe2O3 NPs) are considered as potential candidates for enhancing plant growth, defense systems, and metabolic acceleration with less toxicity.13–16 For example, foliar applications of 3 mg L−1 Fe2O3 NPs enhanced the antioxidant enzyme activity as well as anthocyanins and flavonoids levels in Hibiscus sabdariffa plants.14 In addition, an approximately 4-fold increase in rosmarinic acid (RA) in the leaves of Salvia verticillata plants sprayed with 50 mg L−1 CNTs was observed compared to the control, which may have been due to the enhanced RA synthase activity and gene expression.17 However, high concentrations of treatment might cause toxicity to plants.10
So far, the effects of CNTs and Fe2O3 NPs on the growth and the content of medicinal ingredients of BLG have not been studied. Since the biological effects of NMs on plants are highly related to the physicochemical properties of the NMs,18 the effects of CNTs and Fe2O3 NPs on BLG growth might be different due to their distinct compositions, i.e., Fe2O3 is metal based while CNTs are carbon based. Also, CNTs are more stable in the soil environment, while Fe2O3 NPs are susceptible to dissolution and release Fe ions.19,20
In this study, we investigated the effects of different concentrations of CNTs and Fe2O3 NPs on the growth of BLG by measuring phenotypic and physiological parameters, including biomass, plant length, photosynthetic pigments, and organic and inorganic nutrient contents. The stress status and plant tolerance to stress were assessed by measuring the level of antioxidant enzyme activity in the plants. Finally, the contents of flavonoids, total amino acids, soluble sugars, and total phenols in BLG were also determined and correlated with the observed biological effects.
2 Materials and methods
2.1 Characterization of nanomaterials
The powders of α-Fe2O3 NPs (99.99% purity) and CNTs (99.99% purity) were purchased from Shanghai P.T Nano Powder Co. Ltd. The morphology of the NMs was characterized by transmission electron microscopy (TEM)21,22 (ESI† Fig. S1). The ζ potential and hydrodynamic sizes of the NMs in deionized water were analyzed with a Zetasizer Nano ZS90 system (Malvern, UK) (Table S1†). TEM analysis showed some agglomeration of the MWCNTs with cross-sectional diameters of about 25.1 ± 3.7 nm; while the Fe2O3 NPs were spherical with an average size of 29.4 ± 2.6 nm. The zeta potentials of CNTs and Fe2O3 NPs in deionized water were 10.4 ± 2.6 mV and 4.3 ± 0.5 mV, respectively.2.2 Pot experiment design
The plant exposure experiments were conducted at the greenhouse in China Agricultural University (Beijing, China) under controlled environmental conditions. Surface soil was collected from the Shang Zhuang Research Center of China Agricultural University, Beijing (Detailed location: N40.137312, E116.185430). Detailed properties of the soil are presented in Table S3.† After air-drying and sieving with a 2 mm mesh, the soil was mixed with CNTs or Fe2O3 NPs to achieve final NM concentrations of 10, 50, and 250 mg kg−1. Each pot was filled with 2.0 kg of soil. A pot without the addition of NMs was used as the control. Seeds of Isatis indigotica (hereinafter referred to as BLG) were purchased from the Chinese Academy of Agricultural Sciences, Beijing. The seeds were sterilized with 5% H2O2 for 30 min and rinsed with deionized water thoroughly before germination. One filter paper was placed in a 15 cm × 10 cm tray and immersed in 15 mL deionized water. The BLG seeds were arrayed in the tray, with another filter paper covering on top to reduce direct sun exposure during the germination. The tray was then sealed tightly with parafilm to avoid water loss and placed in a climate incubator allowing germination for 7 days (22/18 °C, 16/8 h light and dark cycle). Seedlings with equal sizes were planted into the pot soil and allowed to grow for 60 days before harvest. All the seedlings were rinsed thoroughly with tap water and then with deionized water to remove the surface adsorbed soil and NMs. The plant height was measured with a ruler. Fresh seedlings were dried in an oven at 65 °C. The dry weight of seedlings was recorded with an electronic balance. Fresh root and shoots from another set of samples were frozen immediately by liquid nitrogen and stored at −80 °C for further biochemical analysis.2.3 Biochemical analysis and chlorophyll content
The malondialdehyde (MDA) content and the activities of the antioxidant enzymes, including catalase (CAT), superoxide dismutase (SOD), and peroxidase (POD), were determined using commercial assay kits (Nanjing Jiancheng Biotechnology Co., Ltd.). Briefly, 0.2 g of fresh shoot and root tissues were ground into powder by ball milling in liquid nitrogen and the samples were then suspended in 0.8 ml PBS (pH 7.4, 50 mM). The suspensions were centrifuged at 5000 rpm and 4 °C for 10 min and the supernatants were collected for measurement following the manufacturer's instructions. To ensure accuracy and linearity, standard SOD, PDO, CAT, and MDA solutions of known concentrations (6 concentrations) were prepared and analyzed according to the same procedure described in the sample analysis kit. Six duplicate samples were tested at each data point and the experiment was repeated four times.To measure the chlorophyll content, fresh shoots with large veins removed (0.5 g) were placed in 10 ml 95% (V/V) ethanol solution and extracted in the dark for 1 week. The contents of chlorophyll a (Cha), chlorophyll b (Chb), carotenoid (Cc), and total chlorophyll (Ct) were determined by UV spectrophotometry (TU-1900, China) at 665 nm and 649 nm. The contents of Ca, Cb, and Ct were calculated according to a previously described method as per the following formulas:23
| Ca = 13.95A665 − 6.88A649 |
| Cb = 24.96A649 − 7.32A665 |
| Ctotal = Ca + Cb |
| Cc = (1000A470 − 2.05Ca − 114.8Cb) |
2.4 Determination of carbon, nitrogen, and mineral elements
Following the method of Shakoor et al. (2022),22 samples were dried at 105 °C for 30 min and further dried at 65 °C for 24 h. The total C and total N in the dry powders of the roots and shoots were determined using an organic elemental analyzer (Vario EL, Elementar, Germany). The dry samples (0.25 g) were transferred to digest tubes containing 10 mL nitric acid overnight and then digested by a microwave digestion system (MARS 6, CEM, UK). The microwave digestion program was set to 120 °C for 5 min, 160 °C for 10 min, and 180 °C for 10 min. The solutions were evaporated to 1 mL using an electric plate (VB20, LabTech, China) at 210 °C. Then the residual solutions were filtered through a 0.25 μm PTFE membrane and diluted to 25 mL with deionized water. Finally, the concentrations of the elements in the digestion solutions were measured by ICP-MS (Elan DRC-e, Perkin Elmer, USA). Spiking recovery experiments and the analysis of certified reference materials (GBW 07603) were performed for analytical method validation. The QA/QC data for the assay are described in Table S4.†2.5 Determination of the medicinal active components
The contents of amino acids, total phenols, soluble sugars, and flavonoids in the BLG roots and shoots were determined using Nanjing Jiancheng Bioreagent assay kits. First, 0.1 g of fresh shoot and root tissues were ground into powder by ball milling in liquid nitrogen. The powder samples were then suspended in 0.8 ml of PBS (pH 7.4, 50 mM). The suspensions were centrifuged at 3000 rpm and 4 °C for 10 min and the supernatants were collected for measurement following the manufacturer's instructions. Briefly, the mechanism is that the copper ions can complex with amino acids to produce blue complexes, which show absorbance at 650 nm. The OD values were converted to obtain the total amino acid contents. Phenols can be reduced by tungsten–molybdic acid to produce blue compounds, which are then calculated after measuring the absorbance at 760 nm. Concentrated sulfuric acid dehydrates sugars and condenses them with anthrone to produce a blue compound, which shows absorbance at 620 nm. In alkaline nitrite solutions, flavonoids can form red complexes with aluminum ions, which were calculated after measuring the absorbance value at 502 nm.2.6 Data analysis
Statistical analyses were performed with SPSS 25.0. Significances of the difference between groups were analyzed using one-way ANOVA. The mean values for each treatment were compared using the Duncan test at a p < 0.05 confidence level. Data are expressed as the mean ± SD (n = 4). The different lowercase letters indicate significant differences at p < 0.05.3 Results and discussion
3.1 Impacts of the nanomaterials on the phenotypes
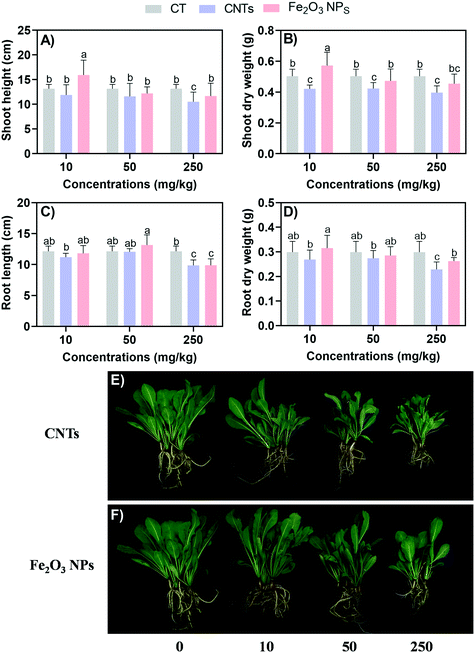
After 60 days of exposure, all the BLG plants were harvested (Fig. 1). Overall, the CNTs exhibited stronger negative effects on BLG than Fe2O3 NPs. In the Fe2O3 NPs treatment, only 250 mg kg−1 could significantly reduce the root length of BLG by 18% (Fig. 1C). The CNT treatment did not promote the growth of BLG, while Fe2O3 NPs at the concentration of 10 mg kg−1 significantly enhanced the shoot height and dry weight by 21% and 14%, respectively (Fig. 1A and B). With the high concentration (250 mg kg−1) of the CNT treatment, the plant height, shoot dry weight, root length, and root dry weight were reduced by 20%, 21%, 19%, and 23%, respectively, as compared to the control.Compared with other plants (e.g., rice, wheat, and other food crops) reported in previous studies, medicinal plants seem more sensitive to NMs in terms of both the positive and negative effects24–26 (Table S2†). The reason for this might be due to that crop having stronger stress resistance after long-term cultivation than medicinal plants.27,28 In addition, the crop seeds used in some previous research work were transgenic varieties. For example, a previous study reported that CuO NPs were more toxic to non-transgenic cotton than to transgenic cotton.29
The different effects between CNTs and Fe2O3 are highly related to their physicochemical distinctions. Fe2O3 is made of Fe, which can provide nutrient value to support plant growth. CNTs had a higher zeta potential than Fe2O3 NPs in this study (Table S1†), and thus are more likely to be adsorbed on the negatively charged root surface.30 The larger size of CNTs and their agglomerations mean they may adhere to the root surface and block water channels, thus preventing the normal uptake of nutrients and water by BLG.31
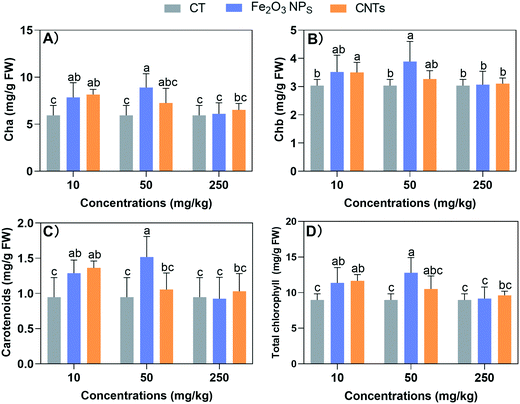
3.2 Impacts of the nanomaterials on the photosynthetic system
Since the biomasses were affected by the NM treatments, we further examined the photosynthesis system, which produces carbohydrates for biomass accumulation. All the NM treatments showed no significant negative effects on the content of photosynthesis pigments (Fig. 2). At the low concentration (10 mg kg−1), both Fe2O3 NPs and CNTs could effectively enhance the photosynthetic pigments. Specifically, Fe2O3 NPs and CNTs at 10 mg kg−1 significantly increased the photosynthetic pigments by 16–36% and 15–43%, respectively. At 50 mg kg−1, the CNT treatment showed no significant effects; while Fe2O3 NPs at 50 mg kg−1 enhanced the levels of Cha, Chb, carotenoids, and total photosynthetic pigments by 49%, 28%, 60%, and 43%, respectively. These results suggest that the enhanced photosynthesis contributed to the increase in BLG biomass and length. The increase in photosynthetic pigments indirectly led to an increase in photosynthetic rate, thus enabling BLG to accumulate more organic matter.Previous studies demonstrated that the foliar application of 20 mg L−1 Fe2O3 NPs increased chlorophyll a, b, and carotenoid concentrations by 70%, 139%, and 119% in wheat leaves, respectively.32 The beneficial effects of Fe-based nanomaterials can be attributed to the fact that Fe is an essential metal element involved in the electron transfer and various enzymatic reactions in the photosynthesis process. For carbon-based materials, previous studies showed that carbon dots could significantly enhance photosynthesis in maize by boosting photosynthetic system activity.33 However, although 10 mg kg−1 CNTs enhanced the photosynthetic pigments levels, they did not increase the biomass accumulation of BLG. Therefore, we next investigated the mineral homeostasis and antioxidant systems in BLG to further explore the reasons for these results.
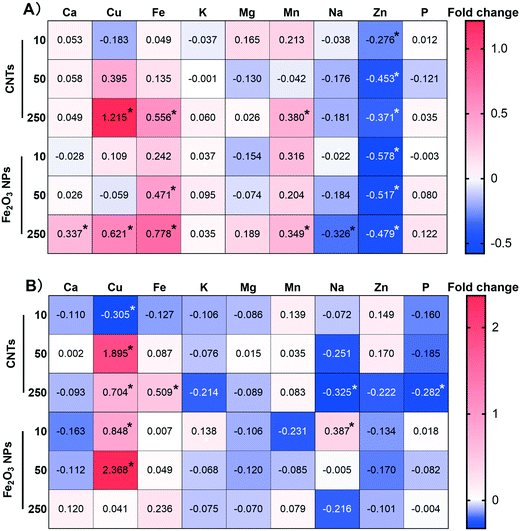
3.3 Impacts of the nanomaterials on mineral nutrient homeostasis
The highest concentration (250 mg kg−1) of NMs induced significant changes in the mineral homeostasis of BLG (Fig. 3). Fe2O3 NPs induced more significant changes in six elements (Ca, Cu, Fe, Mn, Na, Zn) in the root system compared to CNTs (Cu, Fe, Mn, Zn). However, the effects of Fe2O3 NPs were not significant in the shoots. In contrast, four mineral elements (Cu, Fe, Na, P) in the shoots were significantly altered by the treatment with 250 mg kg−1 CNTs, which may explain the fact that CNTs are more toxic than Fe2O3 NPs.At concentration of 10 mg kg−1, the Fe2O3 NPs treatment increased the Cu content in the shoots by 0.848-fold, whereas the CNT treatment significantly downregulated it by 0.305-fold (Fig. 3B). Similarly, 10 mg kg−1 CNT treatment resulted in a 0.106–0.127-fold downregulation of Fe, K, and P in the shoots, but this difference was not statistically significant. High concentrations of Fe2O3 NPs resulted in a significant 0.778-fold increase in Fe content in the root system due to Fe supplementation. Interestingly, high concentrations of CNTs also resulted in a significant increase of 0.556- and 0.509-fold in the Fe content in the roots and shoots, respectively. Similarly, Zhang et al. (2020) observed that carbon-based NMs induced Fe overload in rice, resulting in plant growth inhibition.24 The mechanism, however, is still unclear and needs further studies.
Plant growth and development cannot be achieved without various mineral nutrient supply.34 A deficiency or excess of even one of these elements can lead to growth and developmental limitations.35 Our results showed that the effect of low-concentration NMs on altering mineral element homeostasis was not significant, except for Zn in the roots and Cu in the shoots. Compared with the CNT treatment, low-concentration Fe2O3 NPs led to higher contents of Cu, Fe, K, and P in BLG. K and P are essential macro elements for plant growth,36 and the difference between them can explain the ability of Fe2O3 NPs to effectively promote the growth of the plant. In addition, Fe is an essential component of iron redox proteins in plants and is involved in various processes, such as plant respiration, chlorophyll biosynthesis and photosynthesis, and biological nitrogen fixation.37 Cu also plays a key role in processes such as chlorophyll synthesis, photosynthesis and respiratory electron-transport chain, and is also an important component of antioxidant enzymes (e.g., SOD).38 Therefore, enhancement of the Fe and Cu contents induced by Fe2O3 NPs treatment could effectively promote photosynthesis. However, the Zn content was reduced in both BLG cases, which may be because the NMs may affect the Zn transporter protein as reported previously.39 Alteration of inorganic mineral homeostasis by NPs treatment has been reported in many previous studies;18,22 however, the exact mechanism remains unclear. Part of the reason may be that plants absorb metal ions as chemical equivalents and there is competitive adsorption between ions.40,41 Therefore, the variation of one mineral nutrient element may affect the uptake of other elements.
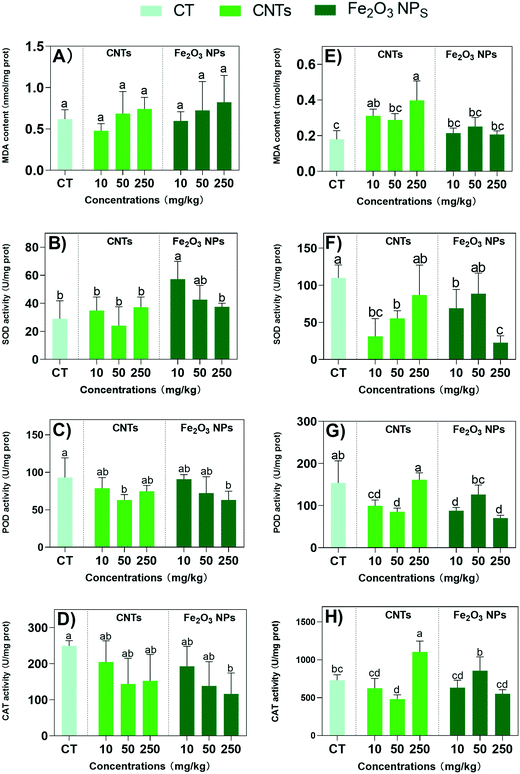
3.4 Impacts of the nanomaterials on the antioxidant system
The content of secondary metabolites in plants is closely related to environmental stresses, as secondary metabolites are involved in plant defense against environmental stresses.42 Previous reports suggested that NMs triggered oxidative responses and regulated the expression of secondary metabolites synthesis proteins,10 which may effectively enhance the medicinal active ingredients of BLG. Therefore, we further examined the antioxidant system of BLG in response to the degree of plant stress (Fig. 4). Excessive reactive oxygen species (ROS) affect the structure and function of proteins and lipid membranes, causing damage to cells.43 The oxidative damage was assessed here by examining the level of MDA, an end product of lipid peroxidation caused by ROS. Both CNTs and Fe2O3 NPs did not induce MDA overaccumulation in the shoots (Fig. 4A). However, CNTs induced significant MDA accumulation in the roots at all exposure concentrations, suggesting oxidative stress (Fig. 4E). Again, Fe2O3 NPs also did not affect MDA levels in the roots. This suggest that overall Fe2O3 NPs did not cause significant oxidative damage in the BLG plants.The oxidative responses were further examined by measuring the antioxidant enzyme activities. CNT treatments did not cause any change in SOD, PDO, and CAT activities in the shoots (Fig. 4B–D), except for the POD activity being reduced by 25% at 50 mg kg−1 (Fig. 4C). However, in the roots, all the enzyme activities were reduced by 17–25% at 10 and 50 mg kg−1 concentrations of CNTs (Fig. 4F–H). This suggest an overall reduced antioxidative capacity of the plants after CNT treatments. In contrast, Fe2O3 NPs at 10 mg kg−1 markedly increased SOD activity by 97% in the shoots (Fig. 4B). However, the SOD activities of the roots were reduced by 37% and 79% upon 10 and 250 mg kg−1 Fe2O3 treatments (Fig. 4F). Overall, the POD and CAT activities were not affected or reduced by the Fe2O3 NPs treatments in the roots and shoots, with the most significant reduction observed for the highest concentrations.
The detailed mechanism of the oxidative responses in plants are complicated. The same enzyme might be upregulated or downregulated as a response to stress or a growth enhancer, which needs to be interpreted together with other data. For example, the enhanced SOD activity in the shoots by the addition of 10 mg kg−1 Fe2O3 NPs suggested an enhanced antioxidative capacity because the other data showed the overall promotive effects at this concentration; whereas the reduced SOD activities by a high concentration of Fe2O3 NPs suggested oxidative stress considering the overall negative impacts under this treatment.
3.5 Impacts of the nanomaterials on N and C accumulation
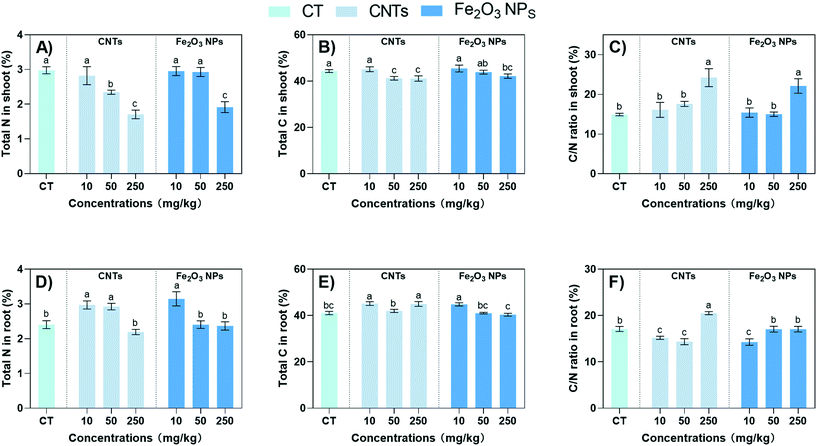
The carbon-based secondary metabolites in plants (such as phenols, terpenoids, and other compounds with only C, H, and O as their main structures) are positively correlated with the C/N (carbon/nutrient) ratio in plants.44 Therefore, the addition of NMs may also affect the carbon and nitrogen accumulation patterns of plants and influence the synthesis of secondary metabolites.45 For example, CNTs and Fe2O3 NPs at 250 mg kg−1 significantly decreased the total N content by 43% and 36%, respectively (Fig. 5A). However, the effect of 10 mg kg−1 NMs on the total N content in the shoots was not significant. The opposite trend was observed in the roots, where the total N content was significantly increased by 24% and 31% after exposure to 10 mg kg−1 CNTs and Fe2O3 NPs, respectively, indicating increased N assimilation in the roots (Fig. 5D). The accumulation pattern of total C was similar to total N. The total C content of the roots was significantly increased by 10% and 9% after exposure to 10 mg kg−1 CNTs and Fe2O3 NPs, respectively (Fig. 5E). Since secondary metabolites are mainly composed of C, the increase in total C content may be related to the accumulation of secondary metabolites.46The C/N ratio was affected by the change in the accumulation mode of total C and N. The C/N ratio of plants is often regarded as a convenient indicator of growth and quality, as well as a reliable indicator of C allocation to defense-related metabolites.47 Here, the C/N ratio in the plant shoots increased significantly after treatment with 250 mg kg−1 of NMs (Fig. 5C), indicating that BLG regulated the C/N ratio in response to the high level of the NM stress. In the roots part, the CNTs and Fe2O3 NPs at 10 mg kg−1 significantly decreased the C/N ratio by 10% and 17% (Fig. 5F), indicating an overall stimulation of C/N metabolism in the roots. In agreement with our results, Hu et al. (2021) also found that 100 mg kg−1 of CNTs significantly promoted N accumulation in maize roots and downregulated the C/N ratio.15 Similarly, Deng et al. (2017) also demonstrated that CNTs could improve the N-utilization efficiency of collard greens.48 These results suggest that 10 mg kg−1 CNTs and Fe2O3 NPs could improve the C and N assimilation by BLG roots to synthesize more secondary metabolites.
3.6 Impacts of the nanomaterials on the medicinal active components
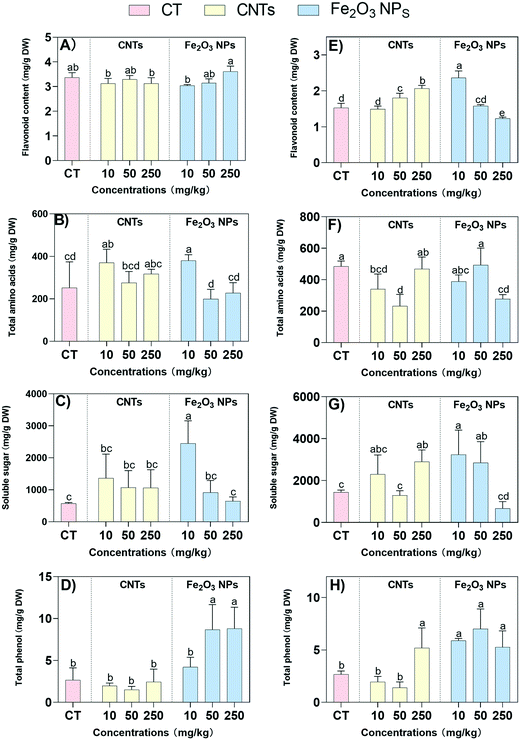
The primary and secondary metabolites associated with the medicinal active ingredients, including flavonoids, amino acids, soluble sugar, and total phenol, were determined to further confirm our hypothesis, Modern pharmacological studies have demonstrated that BLG has various pharmacological effects, such as antibacterial, antiviral, antitumor, and the scavenging of oxygen free radicals.7 Among them, flavonoids have been shown to have potent antiviral and antibacterial effects.49 Here, the flavonoid content in the shoots was not significantly changed under the action of the two NMs (Fig. 6A). As for the roots, the content in the Fe2O3 NPs treatment showed a decreasing trend with the increase in NM concentration, while the opposite was observed for CNT treatment (Fig. 6E). Fe2O3 NPs at 10 mg kg−1 caused the highest increase in flavonoids content in the roots with a 55% increase, indicating that a low concentration of Fe2O3 NPs could effectively stimulate the production of flavonoids in the roots. Amino acids are essential primary metabolites and are closely related to the synthesis of secondary metabolites, such as antibodies, enzymes, and hormones.50 The changes in amino acid content in the shoots and roots showed significant differences, with low concentrations (10 mg kg−1) of CNTs and Fe2O3 NPs elevating the total amino acid content in the shoots by 47% and 51%, respectively (Fig. 6B). However, the roots showed 30% and 52% decreases in the total amino acid content with 10 and 50 mg kg−1 CNT treatments, respectively (Fig. 6F), which was consistent with the results for the antioxidant enzyme activity (SOD, POD, and CAT) in the roots.Soluble sugars are the main product of photosynthesis and are associated with plant stress tolerance.51 Here, the soluble sugar content was significantly elevated with 10 mg kg−1 of Fe2O3 NPs treatment compared to the control. Specifically, at 10 mg kg−1 Fe2O3 NPs concentration, the soluble sugars in the shoots and roots were significantly increased by 328% and 124%, respectively (Fig. 6C and G). For the CNT treatment, the soluble sugars in the roots were significantly increased by 101% at 250 mg kg−1 treatment only, indicating that a low concentration of Fe2O3 NPs could enhance plant stress tolerance more effectively. Similarly, the total phenolics have strong antioxidant effects, as well as antiviral, antibacterial, and therapeutic effects on cardiovascular diseases. A significant increase in total phenols in the shoots was observed only for the 50 and 250 mg kg−1 Fe2O3 NPs treatments, with 227% and 232% increases, respectively (Fig. 6D). It was noteworthy that all concentrations of Fe2O3 NPs significantly elevated the total phenols in the roots, compared to only 250 mg kg−1 of CNTs which significantly elevated total phenols in the roots by 93% (Fig. 6H). Once again, the results demonstrated that low concentrations of Fe2O3 NPs could enhance plant stress tolerance more effectively than the CNT treatment.
The total amino acids is another important indicator for evaluating the plant nutritional quality. The total amino acids in the shoots were significantly upregulated by both NMs at low concentrations, while low and medium concentrations of CNTs resulted in a significant downregulation of the total amino acids in the roots. These results were consistent with the phenotypic trends of the roots, which were more significantly inhibited by CNTs compared to Fe2O3 NPs treatment. The contents of soluble sugar and total phenolics were closely related to the plant stress tolerance. The higher the soluble sugar content, the less likely the plant can lose water from plant cells and thus the more likely it is to survive under stress.52 Similarly, total phenols can effectively scavenge ROS and thus enhance the resistance to stress.53 Fe2O3 NPs at 10 mg kg−1 significantly increased the soluble sugar content and total phenolic, which was consistent with the physiological phenotypic results. The soluble sugar content showed a decreasing trend with increasing NM concentration, which was consistent with the results for chlorophyll. Plant photosynthesis may be impaired by the stress of high NM concentrations, and thus the amount of organic matter produced is reduced as a result. In addition, an increase in total phenol content implies that more ROS are scavenged, i.e., there will be less damage to the cell membrane structure.
The results showed that a small amount of Fe2O3 NPs could effectively increase the content of flavonoids, soluble sugars, and total phenol in the roots of BLG. In contrast, only high concentrations of CNTs could increase the contents of these compounds in the roots, which may be related to the difference in the nature of the NMs. CNTs are more likely to adhere to the root or cell surface and affect water and nutrient element uptake due to their higher zeta potential and larger agglomeration size than Fe2O3 NPs.30 Compared with CNT treatment, BLG shoots treated with a low concentration of Fe2O3 NPs had higher contents of Cu, Fe, K, and P. As a result, there were differences in the availability of the two massive elements of P and K, resulting in differences in plant growth. Furthermore, various physiological and biochemical reactions that depend on the availability of mineral elements are likely to be affected, including the synthesis of photosynthetic pigments, the intensity of photosynthesis, and the enzymatic activity of the antioxidant system.54,55 The synthesis of organic matter, such as sugars, is promoted by the enhanced photosynthetic system and leads to an increase in carbon and nitrogen accumulation in plants. Finally, the enhancement of the antioxidant system and the C/N ratio contribute to an increase in the synthesis of secondary metabolites.44,45
It is worth noting that the magnitude of stress is likely one of the key factors affecting the content of secondary metabolites.46 Some previous reports claimed that the synthesis of plant secondary metabolites was reduced when environmental stress severely threatened plant survival.56,57 In the present study, treatment with 250 mg kg−1 of Fe2O3 NPs resulted in a decrease in the activities of antioxidant enzymes in the shoots, including SOD, POD, and CAT, which caused a decrease in the content of the corresponding medicinal active substances (flavonoid content, amino acids, soluble sugar, and total phenol). In addition, the response of the medicinal active ingredients to NMs was more sensitive in the roots than in the shoots. The reason was that the root system was the direct site of NM exposure; even 10 mg kg−1 of CNTs resulted in a significant increase in MDA content in the roots, which could inhibit the levels of the medicinal active components. The synthesis of secondary metabolites is a dynamic and complex process that is regulated by a combination of factors.42 In addition to some of the mechanisms explored in this study, a previous report suggested that Fe2O3 NPs could also indirectly promote phenolic synthesis by enhancing the PAL enzyme activity.58 Further studies are required to explore the complex underlying mechanisms.
In summary, this study for the first time reported the effects of CNTs and Fe2O3 NPs on plant growth, the antioxidant system, mineral element homeostasis, C/N accumulation, and levels of medicinal active ingredients in BLG (Isatis indigotica). Overall, a small amount of Fe2O3 NPs or high dose of CNTs could increase the flavonoid content in the roots and improve the medicinal efficacy of BLG. Notably, the impacts of CNTs and Fe2O3 NPs on medical plants can be made complex by the soil conditions and different agricultural management. Future studies in different conditions are thus needed. Considering the dose used and the total active ingredient content in BLG, Fe2O3 NPs at 10 mg kg−1 are considered to be a more effective biological enhancer than CNTs to promote the plant yield and medicinal active contents.
Author contributions
Pingfan Zhou, Binbin Long, Ruisi Wang: investigation and writing – original draft, visualization, conceptualization. Yaqi Jiang, Weichen Zhao, Yuanbo Li, Mingshu Li, Zhiling Guo, Iseult Lynch: writing – review & editing. Peng Zhang, Yukui Rui: writing – review & editing, conceptualization, supervision.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The project was supported by the National Key R&D Program of China (2017YFD0801300, 2017YFD0801103). Additional support from EU H2020 project NanoSolveIT (Grant Agreement 814572), NanoCommons (Grant Agreement 731032) and The Engineering and Physical Sciences Research Council Impact Acceleration Accounts Developing Leaders (Grant No. 1001634) was acknowledged.References
- X. Yuan, P. Guo, X. Qi, N. Ning, H. Wang, H. Wang, X. Wang and Y. Yang, Safety of herbicide Sigma Broad on Radix Isatidis (Isatis indigotica Fort.) seedlings and their photosynthetic physiological responses, Pestic. Biochem. Physiol., 2013, 106(1), 45–50 CrossRef CAS.
- J. G. ZHU, Profile of the Chinese Pharmacopoeia (1990), Pharmacopeial forum, 1990, 16(6), 1276–1277 Search PubMed.
- B. Wu, L. Wang, L. Jiang, L. Dong, F. Xu, Y. Lu, J. Jin, Z. Wang, G. Liang and X. Shan, n-butanol extract from Folium isatidis inhibits the lipopolysaccharide-induced downregulation of CXCR1 and CXCR2 on human neutrophils, Mol. Med. Rep., 2018, 17(1), 179–185 CAS.
- M. Chen, S. Lin, L. Li, C. Zhu, X. Wang, Y. Wang, B. Jiang, S. Wang, Y. Li, J. Jiang and J. Shi, Enantiomers of an Indole Alkaloid Containing Unusual Dihydrothiopyran and 1,2,4-Thiadiazole Rings from the Root of Isatis indigotica, Org. Lett., 2012, 14(22), 5668–5671 CrossRef CAS PubMed.
- X. Wu, G. Qin, K. K. Cheung and K. F. Cheng, New alkaloids from Isatis indigotica, Tetrahedron, 1997, 53(39), 13323–13328 CrossRef CAS.
- X. Deng, G. Gao, S. Zheng and F. Li, Qualitative and quantitative analysis of flavonoids in the leaves of Isatis indigatica Fort. by ultra-performance liquid chromatography with PDA and electrospray ionization tandem mass spectrometry detection, J. Pharm. Biomed. Anal., 2008, 48(3), 562–567 CrossRef CAS PubMed.
- J. Chen, Z. Zhu, T. Gao, Y. Chen, Q. Yang, C. Fu, Y. Zhu, F. Wang and W. Liao, Isatidis Radix and Isatidis Folium: A systematic review on ethnopharmacology, phytochemistry and pharmacology, J. Ethnopharmacol., 2022, 283, 114648 CrossRef CAS PubMed.
- L. Cheng, F. Wang, S. B. Zhang and Q. Y. You, Network Pharmacology Integrated Molecular Docking Reveals the Anti-COVID-19 and SARS Mechanism of Fufang Banlangen Keli, Nat. Prod. Commun., 2021, 16(1), 1–11 CrossRef.
- P. Zhou, M. Adeel, N. Shakoor, M. Guo, Y. Hao, I. Azeem, M. Li, M. Liu and Y. Rui, Application of Nanoparticles Alleviates Heavy Metals Stress and Promotes Plant Growth: An Overview, Nanomaterials, 2021, 11(1), 26 CrossRef CAS PubMed.
- K. Kralova and J. Jampilek, Responses of Medicinal and Aromatic Plants to Engineered Nanoparticles, Appl. Sci., 2021, 11(4), 1813 CrossRef CAS.
- R. Sharifi-Rad, S. Esmaeilzadeh Bahabadi, A. Samzadeh-Kermani and M. Gholami, The Effect of Non-biological Elicitors on Physiological and Biochemical Properties of Medicinal Plant Momordica charantia L, Iran. J. Sci. Technol., 2020, 44(5), 1315–1326 CrossRef.
- S. H. Moazzami Farida, R. Karamian and B. R. Albrectsen, Silver nanoparticle pollutants activate oxidative stress responses and rosmarinic acid accumulation in sage, Physiol. Plant., 2020, 170(3), 415–432 CrossRef CAS PubMed.
- A. Ali, T. Shah, R. Ullah, P. Zhou, M. Guo, M. Ovais, Z. Tan and Y. Rui, Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications, Front. Chem., 2021, 9, 629054 CrossRef CAS PubMed.
- H. Kiapour, P. Moaveni, B. Sani, F. Rajabzadeh and H. Mozafari, Investigating the effect of magnesium and iron oxide nanoparticles on the levels of enzymatic and non-enzymatic antioxidants in roselle, J. Medicinal Plants By-products, 2020, 9(1), 19–31 Search PubMed.
- Y. Hu, P. Zhang, X. Zhang, Y. Liu, S. Feng, D. Guo, T. Nadezhda, Z. Song and X. Dang, Multi-Wall Carbon Nanotubes Promote the Growth of Maize (Zea mays) by Regulating Carbon and Nitrogen Metabolism in Leaves, J. Agric. Food Chem., 2021, 69(17), 4981–4991 CrossRef CAS PubMed.
- S. Cai, P. Zhang, Z. Guo, F. Jin, J. Wang, Z. Song, T. Nadezhda, I. Lynch and X. Dang, Multi-walled carbon nanotubes improve nitrogen use efficiency and nutritional quality in Brassica campestris, Environ. Sci.: Nano, 2022, 9(4), 1315–1329 RSC.
- N. Rahmani, T. Radjabian and B. M. Soltani, Impacts of foliar exposure to multi-walled carbon nanotubes on physiological and molecular traits of Salvia verticillata L., as a medicinal plant, Plant Physiol. Biochem., 2020, 150, 27–38 CrossRef CAS PubMed.
- P. Zhang, X. Wu, Z. Guo, X. Yang, X. Hu and I. Lynch, Stress Response and Nutrient Homeostasis in Lettuce (Lactuca sativa) Exposed to Graphene Quantum Dots Are Modulated by Particle Surface Functionalization, Adv. Biol., 2021, 5(4), 2000778 CrossRef CAS PubMed.
- P. Cervantes-Aviles, X. Huang and A. A. Keller, Dissolution and Aggregation of Metal Oxide Nanoparticles in Root Exudates and Soil Leachate: Implications for Nanoagrochemical Application, Environ. Sci. Technol., 2021, 55(20), 13443–13451 CrossRef CAS PubMed.
- W. Zhang, Z. Zeng, Z. Liu, J. Huang, R. Xiao, B. Shao, Y. Liu, Y. Liu, W. Tang, G. Zeng, J. Gong and Q. He, Effects of carbon nanotubes on biodegradation of pollutants: Positive or negative?, Ecotoxicol. Environ. Saf., 2020, 189, 109914 CrossRef CAS PubMed.
- M. Adeel, T. Farooq, J. White, Y. Hao, Z. He and Y. Rui, Carbon-based nanomaterials suppress Tobacco Mosaic Virus (TMV) infection and induce resistance in Nicotiana benthamian, J. Hazard. Mater., 2020, 404, 124167 CrossRef PubMed.
- N. Shakoor, M. Adeel, M. Zain, P. Zhang, M. A. Ahmad, T. Farooq, P. Zhou, I. Azeem, M. Rizwan, K. Guo, G. Jilani, S. Ahmar, S. Maqbool, X. Ming and Y. Rui, Exposure of cherry radish (Raphanus sativus L. var. Radculus Pers) to iron-based nanoparticles enhances its nutritional quality by trigging the essential elements, NanoImpact, 2022, 25, 100388 CrossRef CAS PubMed.
- H. K. Lichtenthaler and A. R. Wellburn, Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents, Biochem. Soc. Trans., 1983, 11(5), 591–592 CrossRef CAS.
- P. Zhang, Z. Guo, W. Luo, F. A. Monikh, C. Xie, E. Valsami-Jones, I. Lynch and Z. Zhang, Graphene Oxide-Induced pH Alteration, Iron Overload, and Subsequent Oxidative Damage in Rice (Oryza sativa L.): A New Mechanism of Nanomaterial Phytotoxicity, Environ. Sci. Technol., 2020, 54(6), 3181–3190 CrossRef CAS PubMed.
- Y. Hao, C. Ma, Z. Zhang, Y. Song, W. Cao, J. Guo, G. Zhou, Y. Rui, L. Liu and B. Xing, Carbon nanomaterials alter plant physiology and soil bacterial community composition in a rice-soil-bacterial ecosystem, Environ. Pollut., 2018, 232, 123–136 CrossRef CAS PubMed.
- M. Li, P. Zhang, M. Adeel, Z. Guo, A. J. Chetwynd, C. Ma, T. Bai, Y. Hao and Y. Rui, Physiological impacts of zero valent iron, Fe3O4 and Fe2O3 nanoparticles in rice plants and their potential as Fe fertilizers, Environ. Pollut., 2021, 269, 116134 CrossRef CAS PubMed.
- S. L. Chen, H. Yu, H. M. Luo, Q. Wu, C. F. Li and A. Steinmetz, Conservation and sustainable use of medicinal plants: problems, progress, and prospects, Chin. Med., 2016, 11, 1–10 CrossRef CAS PubMed.
- D. Q. Fuller, T. Denham, M. Arroyo-Kalin, L. Lucas, C. J. Stevens, L. Qin, R. G. Allaby and M. D. Purugganan, Convergent evolution and parallelism in plant domestication revealed by an expanding archaeological record, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(17), 6147–6152 CrossRef CAS PubMed.
- N. Le Van, C. Ma, J. Shang, Y. Rui, S. Liu and B. Xing, Effects of CuO nanoparticles on insecticidal activity and phytotoxicity in conventional and transgenic cotton, Chemosphere, 2016, 144, 661–670 CrossRef PubMed.
- Z. Li, Y. Liu, Y. Zheng and R. Xu, Zeta potential at the root surfaces of rice characterized by streaming potential measurements, Plant Soil, 2015, 386(1), 237–250 CrossRef CAS.
- J. E. Cañas, M. Long, S. Nations, R. Vadan, L. Dai, M. Luo, R. Ambikapathi, E. H. Lee and D. Olszyk, Effects of functionalized and nonfunctionalized single-walled carbon nanotubes on root elongation of select crop species, Environ. Toxicol. Chem., 2008, 27(9), 1922–1931 CrossRef PubMed.
- M. Adrees, Z. S. Khan, S. Ali, M. Hafeez, S. Khalid, M. Z. Ur Rehman, A. Hussain, K. Hussain, S. A. Shahid Chatha and M. Rizwan, Simultaneous mitigation of cadmium and drought stress in wheat by soil application of iron nanoparticles, Chemosphere, 2020, 238, 124681 CrossRef CAS PubMed.
- H. Yang, C. Wang, F. Chen, L. Yue, X. Cao, J. Li, X. Zhao, F. Wu, Z. Wang and B. Xing, Foliar carbon dot amendment modulates carbohydrate metabolism, rhizospheric properties and drought tolerance in maize seedling, Sci. Total Environ., 2022, 809, 151105 CrossRef CAS PubMed.
- P. Zhou, P. Zhang, M. Guo, M. Li, L. Wang, M. Adeel, N. Shakoor and Y. Rui, Effects of age on mineral elements, amino acids and fatty acids in Chinese chestnut fruits, Eur. Food Res. Technol., 2021, 247(8), 2079–2086 CrossRef CAS.
- P. Zhou, M. Adeel, M. Guo, L. Ge, N. Shakoor, M. Li, Y. Li, G. Wang and Y. Rui, Characterisation of biochar produced from two types of chestnut shells for use in remediation of cadmium- and lead-contaminated soil. Crop and Pasture, Science, 2022, 21297 Search PubMed.
- X. Lv, J. Han, Y. Liao and Y. Liu, Effect of phosphorus and potassium foliage application post-anthesis on grain filling and hormonal changes of wheat, Field Crops Res., 2017, 214, 83–93 CrossRef.
- T. Kobayashi, T. Nozoye and N. K. Nishizawa, Iron transport and its regulation in plants, Free Radical Biol. Med., 2019, 133, 11–20 CrossRef CAS PubMed.
- M. Rehman, L. J. Liu, Q. Wang, M. H. Saleem, S. Bashir, S. Ullah and D. X. Peng, Copper environmental toxicology, recent advances, and future outlook: a review, Environ. Sci. Pollut. Res., 2019, 26(18), 18003–18016 CrossRef CAS PubMed.
- I. Josko, M. Kusiak, B. Xing and P. Oleszczuk, Combined effect of nano-CuO and nano-ZnO in plant-related system: From bioavailability in soil to transcriptional regulation of metal homeostasis in barley, J. Hazard. Mater., 2021, 416, 126230 CrossRef CAS PubMed.
- S. Qin, H. Liu, Z. Nie, Z. Rengel, W. Gao, C. Li and P. Zhao, Toxicity of cadmium and its competition with mineral nutrients for uptake by plants: A review, Pedosphere, 2020, 30(2), 168–180 CrossRef.
- Y. Cui, X. Zhang and Y. Zhu, Does copper reduce cadmium uptake by different rice genotypes?, J. Environ. Sci., 2008, 20(3), 332–338 CrossRef CAS.
- S. Khare, N. B. Singh, A. Singh, I. Hussain, K. Niharika, V. Yadav, C. Bano, R. K. Yadav and N. Amist, Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints, J. Plant Biol., 2020, 63(3), 203–216 CrossRef CAS.
- Y. Hao, C. Ma, J. C. White, M. Adeel, R. Jiang, Z. Zhao, Y. Rao, G. Chen, Y. Rui and B. Xing, Carbon-based nanomaterials alter the composition of the fungal endophyte community in rice (Oryza sativa L.), Environ. Sci.: Nano, 2020, 7(7), 2047–2060 RSC.
- J. P. Bryant, F. S. Chapin and D. R. Klein, Carbon/Nutrient Balance of Boreal Plants in Relation to Vertebrate Herbivory, Oikos, 1983, 40(3), 357–368 CrossRef CAS.
- P. Pant, S. Pandey and S. Dall'Acqua, The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review, Chem. Biodiversity, 2021, 18(11), 2100345 CrossRef PubMed.
- L. Gobbo-Neto and N. P. Lopes, Medicinal plants: Factors of influence on the content of secondary metabolites, Quim. Nova, 2007, 30(2), 374–381 CrossRef CAS.
- M. Royer, R. Larbat, J. Le Bot, S. Adamowicz and C. Robin, Is the C:N ratio a reliable indicator of C allocation to primary and defence-related metabolisms in tomato?, Phytochemistry, 2013, 88, 25–33 CrossRef CAS PubMed.
- Y. Deng, B. Eitzer, J. C. White and B. Xing, Impact of multiwall carbon nanotubes on the accumulation and distribution of carbamazepine in collard greens (Brassica oleracea), Environ. Sci.: Nano, 2017, 4(1), 149–159 RSC.
- W. Y. Ren, Z. H. Qiao, H. W. Wang, L. Zhu and L. Zhang, Flavonoids: Promising anticancer agents, Med. Res. Rev., 2003, 23(4), 519–534 CrossRef CAS PubMed.
- Z. Q. Liu, W. Long, D. A. Fryburg and E. J. Barrett, The regulation of body and skeletal muscle protein metabolism by hormones and amino acids, J. Nutr., 2006, 136(1), 212S–217S CrossRef CAS PubMed.
- Y. Y. Ma, Y. L. Zhang, J. Lu and H. B. Shao, Roles of plant soluble sugars and their responses to plant cold stress, Afr. J. Biotechnol., 2009, 8(10), 2004–2010 CAS.
- G. Feng, F. S. Zhang, X. L. Li, C. Y. Tian, C. Tang and Z. Rengel, Improved tolerance of maize plants to salt stress by arbuscular mycorrhiza is related to higher accumulation of soluble sugars in roots, Mycorrhiza, 2002, 12(4), 185–190 CrossRef CAS PubMed.
- M. Y. Ashraf, A. R. Azmi, A. H. Khan and S. A. Ala, Effect of Water-Stress On Total Phenols, Peroxidase-Activity and Chlorophyll Content in Wheat (Triticum-Aestivum L), Acta Physiol. Plant., 1994, 16(3), 185–191 CAS.
- P. Fincheira, G. Tortella, A. B. Seabra, A. Quiroz, M. C. Diez and O. Rubilar, Nanotechnology advances for sustainable agriculture: current knowledge and prospects in plant growth modulation and nutrition, Planta, 2021, 254(4), 66 CrossRef CAS PubMed.
- M. Hasanuzzaman, M. Bhuyan, K. Nahar, M. Hossain, J. Mahmud, M. Hossen, A. Masud, A. Moumita and M. Fujita, Potassium: A Vital Regulator of Plant Responses and Tolerance to Abiotic Stresses, Agronomy, 2018, 8(3), 31 CrossRef.
- D. A. Herms and W. J. Mattson, The dilemma of plants: to grow or defend, Q. Rev. Biol., 1992, 67(3), 283–335 CrossRef.
- F. A. Bazzaz, N. R. Chiariello, P. D. Coley and L. F. Pitelka, Allocating Resources to Reproduction and Defense: New assessments of the costs and benefits of allocation patterns in plants are relating ecological roles to resource use, BioScience, 1987, 37(1), 58–67 CrossRef.
- M. Taghizadeh, F. Nasibi, K. M. Kalantari and F. Ghanati, Evaluation of secondary metabolites and antioxidant activity in Dracocephalum polychaetum Bornm. cell suspension culture under magnetite nanoparticles and static magnetic field elicitation, Plant Cell, Tissue Organ Cult., 2019, 136(3), 489–498 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00488g |
| ‡ The three authors contributed equally to the manuscript. |
| This journal is © The Royal Society of Chemistry 2022 |