Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Metabolic alterations in alga Chlamydomonas reinhardtii exposed to nTiO2 materials†
Wei
Liu
 a,
Mengting
Li
a,
Weiwei
Li
b,
Arturo A.
Keller
a,
Mengting
Li
a,
Weiwei
Li
b,
Arturo A.
Keller
 b and
Vera I.
Slaveykova
b and
Vera I.
Slaveykova
 *a
*a
aUniversity of Geneva, Faculty of Sciences, Earth and Environment Sciences, Department F.-A. Forel for Environmental and Aquatic Sciences, Environmental Biogeochemistry and Ecotoxicology, Uni Carl Vogt, 66 Blvd Carl-Vogt, CH 1211 Geneva, Switzerland. E-mail: vera.slaveykova@unige.ch
bBren School of Environmental Science & Management, University of California, Santa Barbara, California 93106-5131, USA
First published on 1st July 2022
Abstract
Nano-sized titanium dioxide (nTiO2) is one of the most commonly used materials, however the knowledge about the molecular basis for metabolic and physiological changes in phytoplankton is yet to be explored. In the present study we use a combination of targeted metabolomics, transcriptomics and physiological response studies to decipher the metabolic perturbation in green alga Chlamydomonas reinhardtii exposed for 72 h to increasing concentrations (2, 20, 100 and 200 mg L−1) of nTiO2 with primary sizes of 5, 15 and 20 nm. Results show that the exposure to all three nTiO2 materials induced perturbation of the metabolism of amino acids, nucleotides, fatty acids, tricarboxylic acids, antioxidants but not in the photosynthesis. The alterations of the most responsive metabolites were concentration and primary size-dependent despite the significant formation of micrometer-size aggregates and their sedimentation. The metabolic perturbations corroborate the observed physiological responses and transcriptomic results and confirmed the importance of oxidative stress as a major toxicity mechanism for nTiO2. Transcriptomics revealed also an important influence of nTiO2 treatments on the transport, adenosine triphosphate binding cassette transporters, and metal transporters, suggesting a perturbation in a global nutrition of the microalgal cell, which was most pronounced for exposure to 5 nm nTiO2. The present study provides for the first-time evidence for the main metabolic perturbations in green alga C. reinhardtii exposed to nTiO2 and helps to improve biological understanding of the molecular basis of these perturbations.
Environmental significanceA significant knowledge gap exists regarding the metabolic perturbations induced by engineered nanomaterials on phytoplankton species, in spite of their essential role in global biogeochemical cycles and food-web dynamics in aquatic environments. We have combined targeted metabolomics, transcriptomics and physiological response studies to explore quantitatively for the first time the metabolic alterations in green algae exposed to increasing concentrations of nano-sized titanium dioxide (nTiO2) materials with three different primary sizes. We selected nTiO2 as one of the most commonly used nanomaterials. Results revealed that exposure to nTiO2 at concentrations higher than those usually found in freshwater environments induces significant alteration of the metabolism of amino acids, nucleotides, fatty acids, tricarboxylic acids and antioxidants, as well as disturbance of the global nutrient status of the algae. For most of the responsive metabolites the changes in the abundance upon nTiO2 treatments were exposure concentration and primary size dependent. |
Introduction
Nano-sized titanium dioxide (nTiO2) is one of the top five most commonly used materials.1,2 Recent measurements revealed that the concentrations of Ti-containing nanoparticles range between 104 and 107 nanoparticles per mL (corresponding to mass concentrations <10 μg L−1) in global surface waters.3 However in some hotspots, such as the runoff from the façade of a new building, much higher concentrations were detected.4 Once released into the environment, nTiO2 are subject of different physical, chemical and biological transformations, depending on the nanoparticle characteristics, environmental and biological factors.5–7 nTiO2 are considered as moderately stable in freshwater (global stability index of 0.46 for artificial freshwater).8 Accompanying the well-established use and increasing environmental concentrations of nTiO2, a significant amount of research has been conducted to assess their potential toxicity to different biota, including phytoplankton species.9–16 nTiO2 have been classified as “harmful” for the environment.17 Evidence is accumulating that nTiO2 can induce the enhanced production of reactive oxygen species (ROS) and oxidative stress,7,9,18–21 the disruption of the cell membrane and interruption of energy transductions,22 DNA and genotoxic damage23,24 and the reduction of light available to algal cells due to the trapping of the algal cells in the nTiO2 aggregates.25 For a given organism, the toxicity of nTiO2 depends on the size, crystalline structure and surface chemistry of nanoparticles in water.26–29 The advances of ‘omic’ technologies allow a deeper insight into the metabolic perturbation in different phytoplankton species and the modes-of-action of environmental contaminants, including metal-containing nanoparticles.30–35 For instance, nCeO2 with different surface coatings affected the transcripts encoding flagella, indicating an effect on algal mobility.36 Exposure to MoS2 nanosheets altered significantly the nitrogen metabolism in cyanobacterium Nostoc.37 Similarly, exposure of golden-brown alga Poterioochromonas malhamensis to nAg and released silver ions induced perturbation of the metabolism of amino acids, nucleotides, fatty acids, tricarboxylic acids, photosynthesis and photorespiration.38 Dissolvable nAg decreased the abundance of D-galactose, sucrose, and D-fructose, carbohydrates involved in the synthesis and repair of cell walls, in a concentration dependent manner in Scenedesmus obliquus.39 nAg affected the phosphorus assimilation metabolism in green alga Chlorella vulgaris40 as well as altered the arginine and proline metabolism, indole alkaloid biosynthesis, and phospholipid metabolism in cyanobacterium Microcystis aeruginosa.41 nAg exposure increased the levels of transcripts encoding components of the cell wall and the flagella C. reinhardtii.42 Despite these advancements, little published research has focused on the cellular and molecular mechanisms involved in the stress tolerance of microalgae exposed to nTiO2 with different primary sizes. The only “omic” study revealed that nTiO2 raised the levels of transcripts encoding subunits of the proteasome, suggesting proteasome inhibition in C. reinhardtii.42The main objective of the present study is to obtain a novel comprehensive understanding about the metabolic perturbations induced in microalgae exposed to nTiO2. To this end, green alga C. reinhardtii, a representative aquatic primary producer, was exposed to increasing concentrations (2 to 200 mg L−1) of nTiO2 with primary sizes of 5, 15 and 20 nm for 72 h. We hypothesized that the observed metabolic perturbations will be dependent on the primary size and exposure concentration of nTiO2. To verify this hypothesis, we have performed a targeted metabolomics study. The metabolomics results were further corroborated with the biological responses at physiological (growth inhibition, oxidative stress, membrane damage and changes in photosynthetic activity) and transcriptomic levels. In addition, the behaviour of nTiO2 in the exposure medium was characterized in terms of aggregation and sedimentation. The findings of this exploratory study contribute to the improvement of the current knowledge regarding nTiO2 toxicity pathways in this species, and possibly other species of microalgae.
Experimental
nTiO2 materials
Powdered nano-sized TiO2 particles with different primary sizes (anatase 5 nm (A5), anatase 15 nm (A15) and anatase/rutile 20 nm (AR20)) were purchased from Nanostructured & Amorphous Materials Inc, USA. The primary characteristics of the materials as provided by the manufacturer are given in Table S1, ESI.† Stock suspensions of 2.0 g L−1 nTiO2 were prepared by dispersing nanoparticles in ultrapure water (Millipore, Darmstadt, Germany) using sonication for 10 min (50 W L−1 at 40 kHz). The stock suspensions were used to prepare suspensions of nTiO2 in the exposure medium containing 2, 20, 100 and 200 mg L−1 of A5, A15 or AR20. The exposure medium consisted of 8.2 × 10−4 M CaCl2·2H2O, 3.6 × 10−4 M MgSO4·7H2O, 2.8 × 10−4 M NaHCO3, 1.0 × 10−4 M KH2PO4 and 5.0 × 10−6 M NH4NO3 with a pH of 7.0 ± 0.2 and an ionic strength of 2.75 mM, as in our previous studies.21,43 The selected test concentrations of nTiO2 are much higher than those found in freshwater environments,3 but cover a range of ecotoxicologically relevant concentrations.44Characterization of nTiO2 in algal exposure medium
The Z-average hydrodynamic diameter and zeta potential of the three types of nTiO2 materials suspended in the exposure medium were measured at 72 h using a Malvern Zetasizer Nano-ZS (Malvern Instruments, Worcestershire, UK) at 20 °C. Results are the means of 3 sample measurements, 5 runs for each. The suspended fraction of the nTiO2 suspensions was determined by using a UV-vis spectrophotometer (PerkinElmer UV/visible spectrophotometer Lambda 365, wave range of 200–800 nm) at the beginning of the experiment and at 72 h. The suspended fraction was determined as the ratio between the final (A) and initial absorbance (A0) at the maximum absorption peak wavelength of 243 nm (A/A0) as detailed previously.43Bioassays with Chlamydomonas reinhardtii
Wild-type C. reinhardtii (CPCC11, Canadian Phycological Culture Centre, Waterloo, Canada) was grown axenically at 20.2 ± 0.5 °C, 115 rpm and 110 μmol phot m−2 s−1. Algal cells were cultured in 4× diluted tris-acetate-phosphate (4× TAP) medium45 until mid-exponential growth (62 hours post-inoculation), centrifuged (10 minutes, 1300g), rinsed and re-suspended (∼106 cells per mL) in the exposure medium enriched with 2, 20, 100, and 200 mg L−1 A5, A15 or AR20 for 72 h. Negative controls (NC) in the absence of nTiO2 were also performed. All the experiments were performed in 3 independent biological replicates. Changes in algal growth and physiology (excessive ROS generation, membrane damage and alteration of photosynthetic activity), alteration of key metabolites induced by nTiO2 exposure and transcriptomic response were determined as presented below.Evaluation of the nTiO2 effect on algal growth and physiology
The effect of nTiO2 on the algal growth was assessed following the changes of the algal cell densities by flow cytometry (FCM). The measurements were performed with a BD Accuri C6 flow cytometer equipped with a CSampler (BD Biosciences, San Jose, CA). The 488 nm argon excitation laser and fluorescence detection channels with band pass emission filters at 530 ± 15 nm (FL1), 585 ± 20 nm (FL2) and a long pass emission filter for >670 nm (FL3) were used. Data acquisition and analysis were performed with the BD Accuri C6 Software 264.15. The primary threshold was set to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events on FSC-H. Information on algal cell densities and chlorophyll fluorescence (in FL3) was obtained in a single run after 72 h incubation. Algal cells were discriminated from nTiO2 aggregates with similar size by applying a gating strategy, as described in our earlier study.21 The ROS generation and membrane damage were examined using the fluorescent probes CellROX® green (Life Technologies Europe B.V., Zug, Switzerland) and propidium iodide (PI) (Sigma-Aldrich, Buchs, Switzerland) and the number of affected cells was followed by FCM. The detailed staining procedures and gating strategies are presented in our previous studies.21,46 The photosynthetic activity of C. reinhardtii was determined prior to and after nTiO2 treatment using a Multiple Excitation Wavelength Chlorophyll Fluorescence Analyzer (Multi-Color-PAM, Walz, Germany). The maximal fluorescence yield of photosystem II, Fm, and maximal variable fluorescence, Fv (Fv/Fm) and non-photochemical quenching (NPQ) were measured after 72 h exposure following a 20 min dark acclimation. These parameters are well-known indicators for alteration of photosynthetic activity by different biotic and abiotic stressors.38
000 events on FSC-H. Information on algal cell densities and chlorophyll fluorescence (in FL3) was obtained in a single run after 72 h incubation. Algal cells were discriminated from nTiO2 aggregates with similar size by applying a gating strategy, as described in our earlier study.21 The ROS generation and membrane damage were examined using the fluorescent probes CellROX® green (Life Technologies Europe B.V., Zug, Switzerland) and propidium iodide (PI) (Sigma-Aldrich, Buchs, Switzerland) and the number of affected cells was followed by FCM. The detailed staining procedures and gating strategies are presented in our previous studies.21,46 The photosynthetic activity of C. reinhardtii was determined prior to and after nTiO2 treatment using a Multiple Excitation Wavelength Chlorophyll Fluorescence Analyzer (Multi-Color-PAM, Walz, Germany). The maximal fluorescence yield of photosystem II, Fm, and maximal variable fluorescence, Fv (Fv/Fm) and non-photochemical quenching (NPQ) were measured after 72 h exposure following a 20 min dark acclimation. These parameters are well-known indicators for alteration of photosynthetic activity by different biotic and abiotic stressors.38
Assessment of the metabolic response by liquid chromatography-mass spectrometry targeted metabolomics
Changes in concentrations of key primary metabolites in C. reinhardtii exposed to the three nTiO2 materials of different primary sizes at four concentrations (2, 20, 100, and 200 mg L−1) and unexposed controls were determined by liquid chromatography-mass spectrometry (LC-MS) targeted metabolomics. At the end of the exposure for 72 h, the cells were placed in liquid nitrogen to stop metabolic activity, then frozen at −80 °C for 24 h and freeze-dried. Eighty metabolites, including antioxidants, amino acids, organic acids/phenolics, nucleobase/side/tide, sugar/sugar alcohols and fatty acids, were extracted in 80% methanol containing 2% formic acid, following a previously developed methodology.38,47 Targeted analyses of these metabolites were performed using an Agilent 6470 liquid chromatography triple quadrupole mass spectrometer according to previously established methods.47–50 The absolute concentrations of the metabolites in each sample were quantified using 7 point calibration standards, with isotopically-labeled metabolites as internal standards.Statistical analyses of the metabolomics data were performed for exposed algae and control using MetaboAnalyst 5.0.51 One-way analysis of variance (ANOVA) followed by Fisher's LSD post hoc analysis with p < 0.05 was completed to screen for metabolites differing between nTiO2 treatments and controls, as well as differing between different nTiO2 concentrations or primary sizes. Unsupervised principal component analysis (PCA) and supervised partial least squares-discriminant analysis (PLS-DA) were performed to get a global overview of the metabolic changes obtained in treatments with nTiO2 of different primary sizes and different concentrations. Metabolites with a variable importance in the projection (VIP) greater than 1 were regarded as significant and responsible for group separation.52 Metabolites significantly dysregulated by exposure to nTiO2, as identified via ANOVA and PLS-DA, were further considered as responsive metabolites. Pathway analyses were performed with MetaboAnalyst 5.0 with respect to the KEGG pathway built-in metabolic library of green alga Chlorella variables.53 Pathways with threshold >0.1 were considered as significantly dysregulated.54,55
Assessment of transcriptomic response using nCounter
To corroborate the results of targeted metabolomics, 117 transcripts were selected for analysis, including those involved in the response and toxicity pathway of C. reinhardtii to different pollutants56 available in the Gene Expression Omnibus database (GSE65109). 94 of the transcripts have an annotation in MapMan ontology,57 representing amino acid metabolism (16 transcripts), carbohydrate metabolism (17), stress (16), transport (30), metal binding (5) and photosynthesis (9). All selected transcripts were regulated by more than 2-fold in at least three conditions, and all of them had annotations to genes with known functions. Probes were designed and synthesized by NanoString nCounter Technologies (Table S2†). This technology offers a medium-throughput quantitative approach to study differential transcript expression. Total RNA was extracted from the C. reinhardtii exposed to the three types of nTiO2 materials at two concentrations (2 and 20 mg L−1) for 72 h. These concentrations were chosen based on the preliminary tests at exposure concentrations of 2, 20, 100 and 200 mg L−1 of nTiO2 and considering the quality of extracted RNA. Untreated cells were used as control. For each replicate, approximately 100 mg fresh weight of algal cells from the control and experimental groups was harvested by centrifugation at 4 °C at 3200 × g for 5 min. Total RNA was extracted following the previously established protocol for C. reinhardtii.58,59 The concentration of RNA was determined using the Qubit RNA Broad Range Assay Kit and a Qubit 2.0 fluorometer (Thermo Fisher Scientific, USA) following the manufacturer's instructions. The purity of RNA samples was estimated by assaying 2 μL of total RNA extract on a NanoDrop 2000c spectrophotometer (Thermo Scientific, USA). The quality of the RNA was confirmed (A 260/A 280: 1.8–2.0; A 260/A 230: 1.8–2.2).100 ng of total RNA was hybridized with multiplexed Nanostring probes and samples were processed according to a published procedure.60 Background correction was made by subtracting from the raw counts the mean ± 2 standard deviation of counts obtained with negative controls. Values <1 were fixed to 1 to avoid negative values after log transformation. Then, counts for target transcripts were normalized with the geometric mean of six housekeeping genes (Cre06_g260950_t1_2.1, Cre06_g272950_t1_1.1, Cre08_g370550_t1_1.1, Cre09_g411100_t1_2.1, Cre12_g519180_t1_1, Cre06_g260950_t1_2.1) selected as the most stable using the geNorm algorithm.61 Normalized data were log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 transformed for further analyses.
2 transformed for further analyses.
Statistical analysis of nCounter data was carried out with R, a free software environment available at https://www.r-project.org. After importing the normalized data, the significance of differential transcript expression between the groups was determined by computing the moderated t-statistics and false discovery rate (FDR) with the BioConductor package limma, available at https://www.bioconductor.org. p-Values were corrected for multiple testing by the use of the FDR method.62 A significance threshold p < 0.05 associated with a fold change value of 2 or more was applied. Graphical representations were computed in GraphPad Prism 9.0 (Prism, GraphPad Software, San Diego).
Results and discussion
Characterization of nTiO2 suspensions in the exposure medium
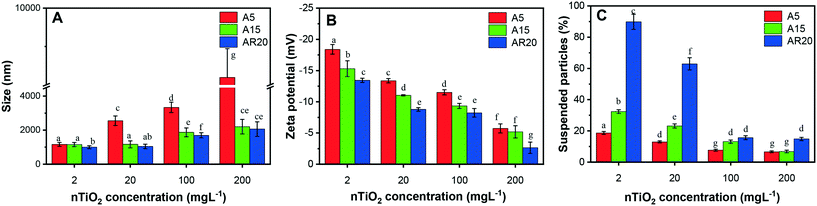
Since the stability of nTiO2 dispersions in exposure medium plays an important role in the induced biological responses, we characterized nTiO2 suspensions in the exposure medium in terms of their aggregation and sedimentation (Fig. 1). All three types of nTiO2 aggregated substantially in the algal exposure medium at 72 h even at 2 mg L−1, forming aggregates with sizes above 1000 nm (Fig. 1A). An increase of nTiO2 concentration resulted in a rise in aggregate size, which was particularly pronounced for the A5 material with a primary size of 5 nm. The changes in average size with increasing concentration were less pronounced for A15 and AR20. A5 formed much bigger aggregates than A15 and AR20 at higher concentrations. For example, the z-average hydrodynamic diameter of A5 was about 3.9 times higher than those of A15 and AR20 in suspensions containing 200 mg L−1. No significant difference was found between the aggregate size of A15 and AR20 at the four tested concentrations. All nTiO2 suspensions in exposure medium exhibited a negative zeta potential value (Fig. 1B): −18.1 mV for A5, −15.3 mV for A15 and −13.4 mV for AR20 at a concentration of 2 mg L−1. Increasing nTiO2 concentration resulted in a significant decrease in the absolute values of the zeta potential for the three tested nTiO2 materials, which was consistent with the formation of bigger nTiO2 aggregates. As shown in our preceding studies, the three nTiO2 materials form rapidly micrometer aggregates in the exposure medium (2 h exposure,21) and the size of the aggregates stayed stable over the time interval 24–96 h.43The percentage of suspended TiO2 decreased with increasing concentration (Fig. 1C), reflecting the larger aggregates formed at higher concentrations and their sedimentation rates. For example, only about 19%, 32% and 88% of particles for A5, A15 and AR20, respectively, were still suspended after 72 h at a concentration of 2 mg L−1. Our previous kinetic study revealed that the micrometer aggregates of nTiO2 settled down rapidly within the first 24 h and the percentage of the suspended particles tended to stay constant from 24 to 96 h.43 The above observations are consistent with the fact that the nTiO2 suspensions at higher concentrations are more susceptible to aggregation due to the increased collision probability between the particles.63 It is currently accepted that the aggregation state of nTiO2 suspensions is a result of the van der Waals attraction forces,64 Coulomb repulsion caused by the surface charge of the particles or the action of the electrostatic double layer, and steric hindrance.65
Effect of nTiO2 treatments on algal physiology
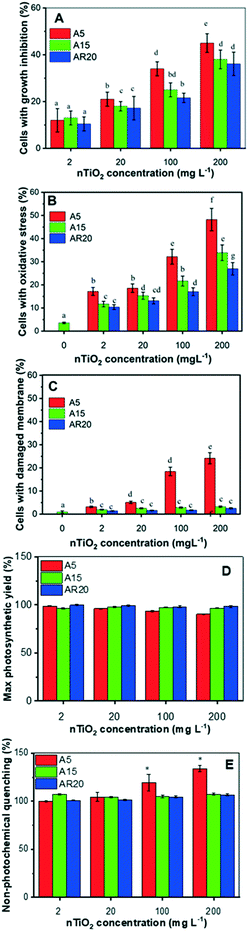
All three tested nTiO2 materials induced growth inhibition in less than 50% of the exposed alga and the effect was concentration dependent (Fig. 2A). Exposure to A5 resulted in the most important growth inhibition, compared with the A15 and AR20 treatments, showing that the nTiO2 with lower size inhibited the growth of C. reinhardtii more significantly (p < 0.05). These observations agree with the finding that the toxicity outcome of exposure to nTiO2 depends on the primary size.20,27,29 These findings would also suggest that C. reinhardtii is more tolerant to nTiO2 in comparison with other green algae and diatoms.20,28,29,44 However, direct comparison is not possible given different exposure medium compositions and different nTiO2 materials used. The generation of excessive cellular ROS concentrations is considered the most likely toxicity mechanism of nTiO2.20 Here all three types of nTiO2 induced oxidative stress in C. reinhardtii even at the lowest tested concentration of 2 mg L−1 (Fig. 2B). The oxidative stress became more pronounced with increasing nTiO2 concentration for all tested materials. A5 induced stronger oxidative stress than A15 and AR20, as already observed at short term exposure.21Changes in membrane permeability induced by 72 h-exposure to A15 and AR20 were negligible. The percentage of affected cells was comparable to unexposed controls. However, exposure of C. reinhardtii to A5 resulted in a significant percentage of cells with membrane damage at a nTiO2 concentration of 200 mg L−1 (Fig. 2C). This result is also consistent with the observed trapping of the algal cells in aggregates of A5 at 96 h exposure.43
nTiO2 treatments had no measurable effect on algal photosynthesis. No significant difference (p > 0.05) of the Fv/Fm and NPQ values was found in all treatments except for A5 at a concentration of 200 mg L−1 in comparison with the control after 72 h (Fig. 2D and E). The decrease in Fv/Fm reveals an inhibition in algal photosynthetic activity related to light energy utilization caused by 200 mg L−1 A5. In agreement with Fv/Fm, no significant differences in NPQ were observed after exposure to the three nTiO2 materials. A significant effect (p < 0.05) on NPQ was found only upon exposure to 200 mg L−1 A5, indicating that the absorbed light energy exceeded the capacity of its utilization in photosynthetic processes, considered as a primary defense mechanism employed to dissipate the stress.
Overview of metabolic profiles in C. reinhardtii under different nTiO2 treatments
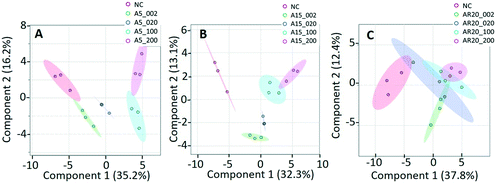
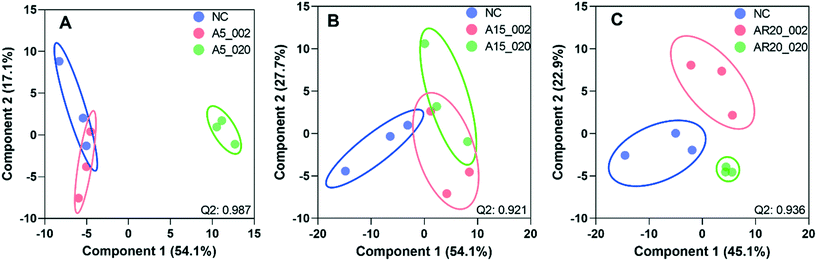
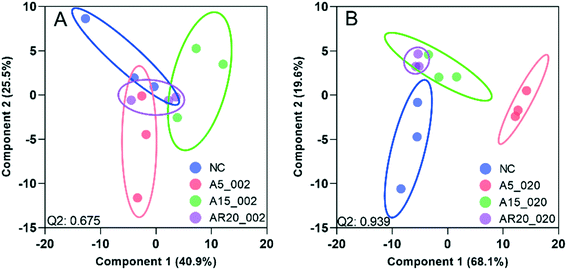
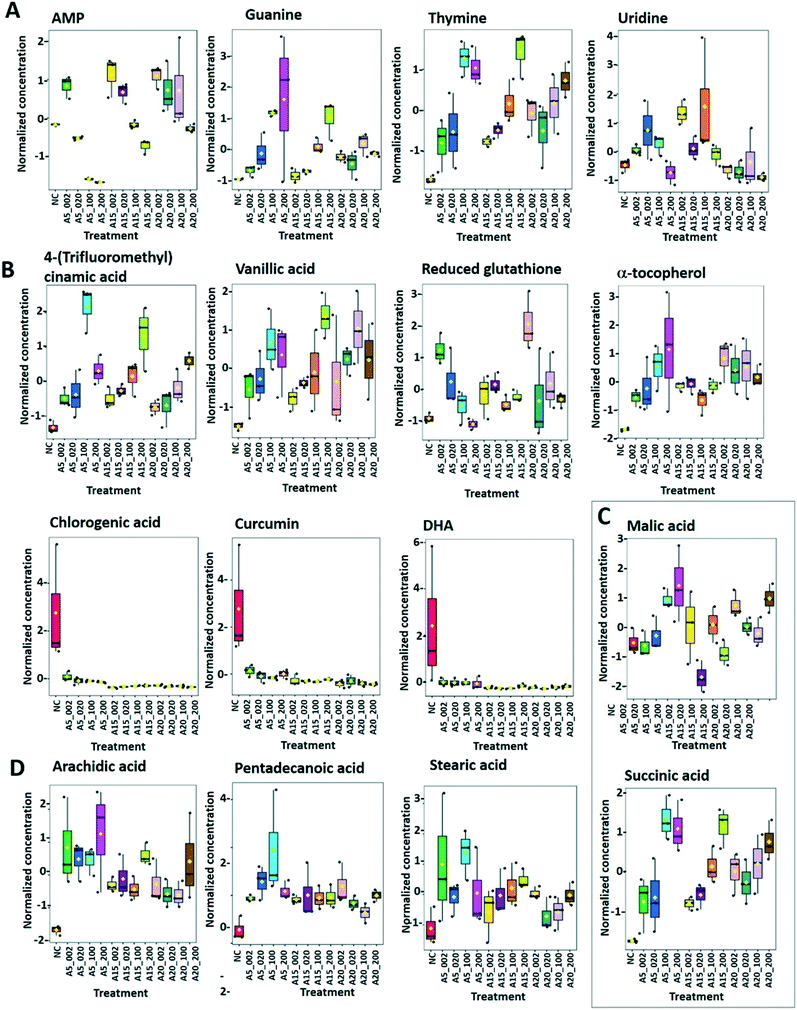
Of a total of 80 metabolites analyzed, 50 were found above their limits of detection and determined in the controls and treatments with nTiO2 of different primary sizes and concentrations. A general overview of the different treatment groups was obtained by unsupervised PCA (Fig. S1†) and supervised PLS-DA for concentration treatments (Fig. 3) and different size treatments (Fig. S2† and 4). The PLS-DA score plot (Fig. 3A and B) showed excellent separation of A5 and A15 treatments and unexposed controls, as well as treatments with different nTiO2 concentrations, highlighting the importance of tracking the alteration of the metabolic response with exposure concentrations. For the AR20 exposure, the separation of the nTiO2 treatments from controls and among the treatments was not very good (Fig. 3C). Based on a VIP score >1 (Fig. S3†) and ANOVA (Tables S3–S5†), a total of 26 responsive metabolites were identified to be significant in the A5-treatments in comparison with the untreated control; 25 for A15 and 26 for AR20.The results of the supervised PLS-DA for treatments with a given concentration of nTiO2 of different primary sizes (Fig. 4) showed very good separation between the control and different primary size treatments for all concentrations, demonstrating the importance of considering the primary size of nTiO2 in metabolic perturbations. Based on a VIP score >1 (Fig. S4†) and ANOVA (Tables S6–S9†), a total of 28 responsive metabolites were found for 2 mg L−1, 21 for 20 mg L−1, 34 for 100 mg L−1 and 29 for 200 mg L−1 nTiO2, for different size treatments. With a small exception, the abundance of the same metabolites was affected in different treatments with a rather clear size dependence for the same initial nTiO2 concentration. It is necessary to keep in mind that in addition to the differences in size there is a small difference in the crystalline structure: A5 and A15 are composed of anatase, whereas AR20 contains 80–90% anatase and 10–20% rutile. nTiO2 in anatase crystal structure was shown to be more toxic for algae than rutile.28
Overall, most of the responsive metabolites were common for different nTiO2 concentration- and size-treatments. They included mainly amino acids, antioxidants, some fatty and carboxylic acids and nucleobases/sides/tides. However, the intensity of the responses and size- or concentration-dependences differed, as discussed further.
The responsive metabolites were also grouped by heatmap clustering. In different concentration treatments (Fig. S5–S7†), three large groups were identified corresponding to (i) metabolites with higher abundance in algae treated with nTiO2 than in the control (12 for A5, 12 for A15 and 15 for AR20). In the group, the metabolites with increasing abundance with the nTiO2 concentrations were 9 for A5, 5 for A15, and 5 for AR20; (ii) metabolites with lower abundance in nTiO2 treatments than in the untreated control (11 for A5, 11 for A15 and 12 for AR20). Within this group, the number of metabolites with decreasing abundance with nTiO2 concentrations was 9 for A5, 11 for A15, and 10 for AR20; and (iii) metabolites with no clear concentration dependence.
Clustering of the treatments as a function of the primary size of nTiO2 revealed five clusters including (Fig. S8–S11†): (i) metabolites with higher abundance than the control; increase of metabolite concentrations with primary size (metabolites with the highest abundance in AR20 treatments: 11 at 2 mg L−1, 8 at 20 mg L−1, 3 at 100 mg L−1, 1 at 200 mg L−1); (ii) metabolites with higher abundance than the control, decrease of metabolite concentrations with primary size (metabolites with the highest abundance in A5 treatments: 2 at 2 mg L−1, 2 at 20 mg L−1, 11 at 100 mg L−1, 8 at 200 mg L−1); (iii) metabolites with lower abundance than the control; decrease of the metabolite concentrations with the primary size (metabolites with the lowest abundance in AR20 treatments: 7 at 2 mg L−1, 7 at 20 mg L−1, 7 at 100 mg L−1, 6 at 200 mg L−1); (iv) metabolites with lower abundance than the control; increase of the metabolite concentration with primary size (metabolites with the lowest abundance in A5 treatments – 6 at 2 mg L−1, 3 at 20 mg L−1, 6 at 100 mg L−1, 5 at 200 mg L−1). The number of metabolites with the highest or lowest abundance at 2 and 20 mg L−1 for AR20 treatments could be related to the much higher percentage of AR20 dispersed in the suspensions of 2 and 20 mg L−1 nTiO2 than 100 and 200 mg L−1 nTiO2 treatments; (v) metabolites with no clear concentration dependence.
Furthermore, the responsive metabolites identified by PLS-DA and ANOVA corresponded to 11impacted pathways (Fig. S12 and S13†). Interestingly, the number of the significantly impacted pathways increased with the decrease of the primary size (Fig. S12†) and the increase of nTiO2 exposure concentrations (Fig. S13†). The glutathione metabolism, alanine, aspartate and glutamate metabolism, cysteine and methionine metabolism, phenylalanine metabolism and arginine biosynthesis were commonly affected in different treatments. Exposure to A5 resulted in the strongest impact on the glutathione metabolism.
Overview of transcriptomic profiles in C. reinhardtii under different nTiO2 treatments
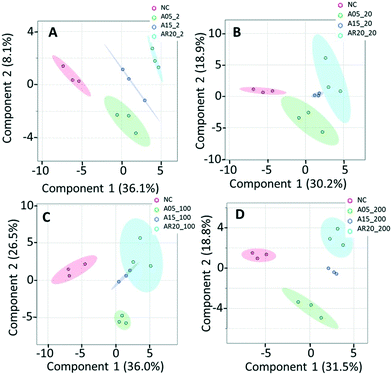
PCA and PLS-DA of the differences in transcript expression between the unexposed controls, treatments with nTiO2 of different concentrations (Fig. S14† and 5) and different primary sizes (Fig. S15† and 6) were conducted. A good separation of A5 and AR20 treatments from the control as well as treatments with different concentrations of nTiO2 was observed (Fig. 5A and C). Poor separation between A15 treatments and the control was found (Fig. 5B).At 2 mg L−1 no separation between the treatments with different sizes and controls was observed (Fig. 6A). However, at 20 mg L−1, good separation of the treatments with all three nTiO2 materials and controls was found. In addition, the distribution of principal components of A5 was clearly separate from A15 and AR20 (Fig. 6B). These results highlighted that the three nTiO2 altered significantly the transcriptomic expression in C. reinhardtii in a primary size dependent way at concentrations of 20 mg L−1.
Of the 117 transcripts tested with nCounter, only a few transcripts allowed discrimination between the treatments. For A5 treatment, the abundance of 3 and 49 transcripts was significantly altered upon exposure to A5 at 2 (Fig. S16A†) and 20 mg L−1 (Fig. S16B†), respectively. However, the expression of few transcripts was significantly altered in A15 and AR20 treatments: 9 for A15 at 2 and 20 mg L−1 (Fig. S16C and D†), 3 and 8 for AR20 at 2 and 20 mg L−1 (Fig. S16E and F†), respectively. This analysis confirmed that for exposure to 20 mg L−1, nTiO2 of smaller primary size (A5) has a significantly larger effect on transcriptome expression of C. reinhardtii when compared with the A15 and AR20 treatments. The number of upregulated transcripts for both treatments was larger than the downregulated transcripts. Despite the observed increase in the number of transcripts with dysregulated expression observed for A5, it is not possible to make conclusions about the concentration dependence of the responses, given the impossibility to extract RNA of good quality in the treatments with 100 and 200 mg L−1 nTiO2. Moreover, 36.7% of dysregulated genes had a fold change >4 or <−4 in 20 mg L−1 A5 treatment, suggesting that the exposure to the smaller size at the higher concentration of 20 mg L−1 nTiO2 resulted in a stronger dysregulation than other treatments.
Significantly dysregulated transcripts corresponded to biological pathways that include regulation of cell processes, energy metabolism (photosynthesis, carbohydrate metabolism), amino acid metabolism, stress and transport (Fig. S17, Table S10†), thus providing an indication about the cellular targets in C. reinhardtii. The number of dysregulated genes depended on the primary size of the tested nTiO2 and increased with concentration from 2 to 20 mg L−1. The exposure to two lower concentrations of nTiO2 resulted in significant changes in the adaptation of the nutrition pathways and metabolic production of energy as well as the induction of oxidative stress, and consequently, the activation of cellular protective response.
Metabolic perturbation in Chlamydomonas reinhardtii exposed to nTiO2
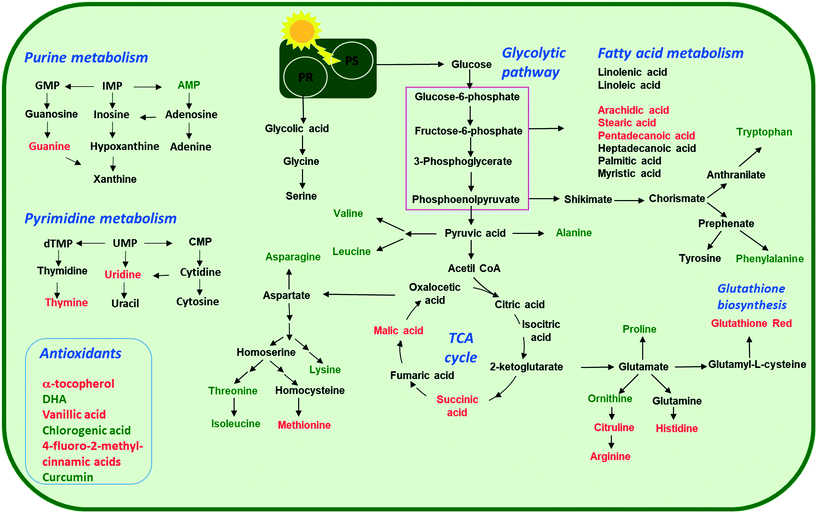
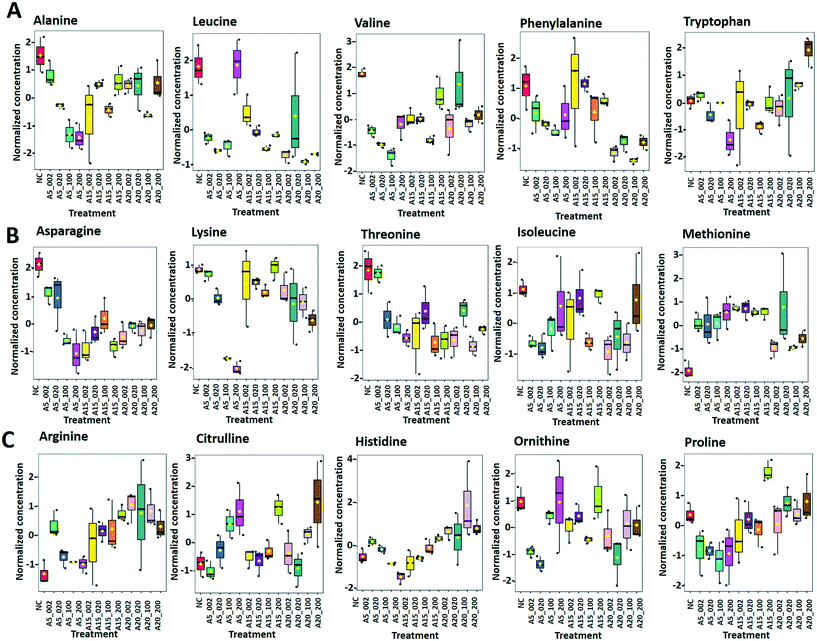
Arginine, citrulline and histidine (Fig. 8C) biosynthesized from the TCA metabolite α-ketoglutarate71 increased in cells exposed to the three nTiO2. The abundance of citrulline increased with higher nTiO2 exposure concentrations, as well as the abundance of arginine in A15 and AR20 treatments.
Ornithine was depleted in nTiO2 treatments with respect to the control and the depletion was more pronounced for A5. Histidine and proline exhibited a more complex pattern with an increase in abundance with exposure concentration for A15, a decrease for A5 and lack of concentration dependence for AR20 treatments. As it is an amino acid needed for growth and development of algal cells,72 the accumulation reveals an impact on algal growth and cell development. In addition, histidine and arginine take part in deamination,73 showing that this process can be also altered. Proline plays an important role in osmo- and redox-regulation, metal chelation, and scavenging of free radicals induced by different metals in plants,74,75 therefore a decrease in abundance suggests a defensive response to oxidative stress.
Levels of aromatic amino acids, phenylalanine and tryptophan, derived from phosphoenolpyruvate were significantly reduced in a concentration dependent manner in A5 treatments only. Concentrations of two other amino acids, glycine and serine, measured in nTiO2 treatments and controls were comparable. As these amino acids are synthesized by the photorespiratory glycolate cycle in algae,66 the fact that no changes were observed in their concentration suggests no effect of nTiO2 on algal photorespiratory activity. These findings are in line with no observed changes in maximum photosynthetic yield and NPQ in most nTiO2 treatments (Fig. 2D and E), as well as with the lack of changes in the abundance of glucose which is a primary product of photosynthesis.
The metabolomics results are consistent with the strong dysregulation of the following transcripts involved in amino-acid metabolism. Four transcripts involved in amino acid metabolism were significantly affected by exposure to A5 at 20 mg L−1, but not to A15 and AR20 (Table S10†). The transcripts in genes coding for “phosphoserine aminotransferase” involved in the phosphorylated pathway of serine biosynthesis76 (Cre07.g331550.t1.2, FC −2.81, FDR 7.63 × 10−9) and “dihydrodipicolinate reductase” involved in L-lysine biosynthesis77 (Cre16.g656300.t1.3, FC −2.07, FDR 6.77 × 10−7), as well as peroxisomal 3-ketoacyl-CoA thiolase 3 in the isoleucine degradation pathway78 (Cre17.g723650.t1.2, FC −2.08, FDR 7.63 × 10−9), were down-regulated. The transcripts coding for fumarylacetoacetate hydrolase, which are involved in the final step of the tyrosine degradation pathway79 (Cre12.g549450.t1.2, FC 4.50, FDR 3.45 × 10−10), were up-regulated.
Dehydroascorbic acid (DHA, Fig. 9B), an oxidized form of ascorbic acid, was significantly depleted in the treatments with all three nTiO2 as compared with the control. However, no clear dependence on the concentration as a function of nTiO2 primary size or exposure concentration was found. Such a decrease of DHA probably corresponds to its consumption, via quenching of the ROS induced by nTiO2 exposure, to prevent the damage to important cellular components.
DHA depletion could suggest depletion of ascorbic acid and/or an acceleration of the recycling and regeneration of ascorbic acid used to eliminate ROS damage and enhance stress tolerance.85 Indeed, ascorbic acid participates in diverse cellular processes associated with photosynthetic functions and stress tolerance.86 For example, nAg and Ag+ induced an accumulation of ascorbic acid in P. melhamensis.38
α-Tocopherol accumulated in the all nTiO2 treatments in comparison to the control (Fig. 9B). The α-tocopherol abundance increased with the A5 and A15 exposure concentrations. However, no concentration dependence was found in AR20 treatments. α-Tocopherol is a major plastid prenyllipid antioxidant able to quench and scavenge singlet oxygen (1O2), as well as scavenge superoxide (O2˙−) and lipid radicals.87 The above observation is consistent with the literature showing an increase in the content of α- and γ-tocopherol in C. reinhardtii upon acclimation to chronic exposure to Cr2O72−, Cd2+ and Cu2+.88 However, α-tocopherol concentration decreased significantly in C. reinhardtii exposed to pyrazolate.89
The abundance of polyphenols and phenolic acids, known as antioxidants, was also altered upon nTiO2 exposure. The concentrations of vanillic and 4-fluoro-2-methylcinnamic acids (Fig. 9B) significantly increased in all three nTiO2 material treatments in an exposure concentration dependent manner. By contrast, chlorogenic acid (CGA) and curcumin were significantly depleted in the treatments with the three nTiO2. Similar depletion of these antioxidants was found in cyanobacterium Nostoc sphaeroides exposed to nAg and Ag+.90 CGA, the ester of caffeic acid and quinic acid, is known to play the role of an intermediate in lignin biosynthesis. The results indicate that the antioxidant defense system of C. reinhardtii was activated in nTiO2 exposure, which resulted in the alteration of the abundance of the ROS-scavenging metabolites to cope with the enhanced generation of ROS. No dependence in the changes of chlorogenic acid and curcumin levels with exposure concentration or primary size of nTiO2 was observed. Taken together, the above finding indicates that even at lower tested concentration of nTiO2 (i.e. 2 mg L−1) the antioxidant defense system of C. reinhardtii was affected, which resulted in an accumulation of the ROS-scavenging metabolites to cope with the enhanced generation of ROS (Fig. 2B). Nevertheless, the antioxidant system was overwhelmed in particularly for exposure to 100 and 200 mg L−1 nTiO2 as can be seen by the important percentage of cells experiencing oxidative stress.
The metabolomics results were also consistent with the transcriptomic profiling. A5 at 20 mg L−1 down-regulated the expression of transcripts of genes involved in reduction–oxidation reaction hemostasis, such as thioredoxin (Cre16.g656600.t1.2, TY2, FC −3.37, FDR 5.89 × 10−10) and glutathione peroxidase (Cre10.g458450.t1.3, GPX5, FC −5.07, and FDR 1.16 × 10−8). Indeed, thioredoxin is a key molecule in the response of C. reinhardtii to oxidative stress induced by dissolved metals and nanoparticles.91,92 In addition, the transcripts of genes associated with the stress response, e.g. “stress.abiotic.heat” such as chaperone DnaJ-domain superfamily protein were strongly up-regulated (Cre12.g488500.t1,2, FC 6.42, and FDR 1.82 × 10−8). The transcripts of genes related to oxidative stress tolerance coding for the cysteine-rich secretory protein family93 (Cre11.g467672.t1.1, FC 3.33, and FDR 4.96 × 10−8, Cre06.g278160.t1.1, FC 2.83, and FDR 1.24 × 10−9) and glycosyl hydrolase family protein94 (Cre13.g570700.t1.3, FC 2.36, and FDR 2.63 × 10−10) were also up-regulated. Moreover, genes involved in calcium-transporting ATPase 3, endoplasmic reticulum-type (ECA3), were also up-regulated indicating an increase of intracellular ROS production. These observations indicate an increased production of ROS induced by 20 mg L−1 A5 and subsequent modification of RedOx homeostasis balance in C. reinhardtii.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), stearic (octadecanoic acid, 18
0), stearic (octadecanoic acid, 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0), arachidic (icosanoic acid, 20
0), arachidic (icosanoic acid, 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0)) accumulated in cells exposed to nTiO2 (Fig. 7 and 9D). However, no clear dependences on nTiO2 primary size or exposure concentrations were observed. These results suggest that algae exposed to nTiO2 modify the membrane fluidity to make it more tolerant to oxidation, thus preserving membrane integrity under oxidative stress conditions.96 Indeed, saturated acids and in particular palmitic acid are known to be less prone to oxidation than other fatty acids.96 Interestingly, changes in the abundance of unsaturated linoleic and linolenic acids were not significantly different from the unexposed control (all p > 0.05) with the exception of treatments with 2 mg L−1 A5, A15 and AR20. These findings suggest no significant unsaturation of the lipid membranes and alteration of integrity of lipid membranes. Indeed, this finding agrees with the relatively low percentage of cells with the affected membrane permeability found by PI in nTiO2 treatments (Fig. 2C).
0)) accumulated in cells exposed to nTiO2 (Fig. 7 and 9D). However, no clear dependences on nTiO2 primary size or exposure concentrations were observed. These results suggest that algae exposed to nTiO2 modify the membrane fluidity to make it more tolerant to oxidation, thus preserving membrane integrity under oxidative stress conditions.96 Indeed, saturated acids and in particular palmitic acid are known to be less prone to oxidation than other fatty acids.96 Interestingly, changes in the abundance of unsaturated linoleic and linolenic acids were not significantly different from the unexposed control (all p > 0.05) with the exception of treatments with 2 mg L−1 A5, A15 and AR20. These findings suggest no significant unsaturation of the lipid membranes and alteration of integrity of lipid membranes. Indeed, this finding agrees with the relatively low percentage of cells with the affected membrane permeability found by PI in nTiO2 treatments (Fig. 2C).
Similar accumulation of fatty acids has been observed in algae under toxic metal stress.97 However, the concentrations of arachidic and stearic acids decreased in P. malhamensis treated with nAg.38 nAg and Ag+ also induced a reduction in the abundance of monounsaturated and polyunsaturated fatty acids of Chlorella vulgaris.98 Plants were also shown to regulate the composition of fatty acids in the membrane to rebuild membrane integrity, as was shown for cucumber leaves exposed to nAg and Ag+.99
Influence of nTiO2 treatments on nutrient transport
Exposure to 20 mg L−1 A5 up-regulated the expression of most transcripts involved in transport, adenosine triphosphate (ATP) binding cassette (ABC) transporters, and metal transporters. Among the dysregulated ABC transporters, the multidrug resistance-associated protein 12 (Cre10.g458450.t1.3, MRP12, FC 7.38, FDR 6.40 × 10−10) and P-loop containing nucleoside triphosphate hydrolase superfamily protein (Cre10.g444700.t1.1, FC 7.96, FDR 6.71 × 10−11) were strongly up-regulated suggesting the involvement of the cellular mechanism in metal detoxification in algal.100,101 Several zinc (Zn)-regulated transporters, iron (Fe)-regulated transporter-like proteins (ZIP), transporters of Fe and Zn, and Zn transporters were also up-regulated. These regulations suggest the impact of A5 on the global nutrition of the microalgal.102 A15 and AR20 significantly dysregulated similar numbers and categories of genes (Table S10†), mostly involved in the ABC transporters and metal transporters at both concentrations.Overall the present results revealed that 72 h exposure of green alga to nTiO2 with different primary sizes altered the metabolism of amino acids, nucleotides, fatty acids, tricarboxylic acids and antioxidants, and resulted in a disturbance in a global nutrition in a concentration- and primary size-dependent way, despite the formation of micrometer-size aggregates and their sedimentation. These disturbances were consistent with the observed oxidative stress and growth inhibition observations. However, it is worth noting that the bioassays were performed at concentrations of nTiO2 much higher than those typically found in the freshwater environment. As the metabolic perturbations and modes of toxic action may not be the same at a low or high exposure concentration, an extrapolation of the present observations to natural environment conditions is limited. In addition, the exposure of 72 h corresponding to chronic toxicity tests for algae was selected to gain a mechanistic understanding of the adverse outcome in an advanced time frame relative to when the physiological responses are typically assessed. However, the prolonged exposure could trigger other modes of action over time and different defense/detoxification mechanisms.35 Despite these limitations, the study provides a unique and novel biological insight into the perturbation of the algal metabolism by nTiO2 and their aggregates and potentially directs further mechanistic studies with other phytoplankton species and nanomaterials.
Conclusion
The present exploratory study provides, for the first-time, evidence of the metabolic perturbations in green alga C. reinhardtii exposed to increasing concentration of nTiO2 with different primary sizes. The results revealed that despite the important aggregation and sedimentation, the exposure to increasing concentrations of nTiO2 with primary sizes of 5, 15 and 20 nm altered the abundance of metabolites involved in various pathways corresponding to amino acid, nucleotides, fatty acid, TCA and antioxidant metabolism. Most of the responsive metabolites were common for all the treatments, however the intensity of the response and the existence or not of concentration- and nanoparticle primary size-dependences differed among the treatments. The metabolomics results correlate well with the transcriptomics and physiological results and confirmed that oxidative stress is a major toxicity mechanism for nTiO2. The findings contribute to an improvement of knowledge concerning the molecular basis of these perturbations and thus to the understanding of environmental implications of one of the most used engineered nanomaterials.Author contributions
V. I. S., W. L. and M. T. conceived and designed the study. M. T. performed the nTiO2 characterization, bioassays for physiological response assessment, exposure assays for metabolomics, analyzed the physiological response data and provided interpretations, and wrote this part of the manuscript; W. L. performed exposure for transcriptomics, RNA extraction, and data processing, and wrote the transcriptomics part of the manuscript; W. W. L. performed the LC-MS measurements and data processing; V. I. S. performed analysis and interpretation of metabolomics results, wrote the metabolomics part of the manuscript, and overviewed the overall study. A. A. K. took part in the data interpretation, manuscript writing, and overviewed the overall study. All the authors critically commented and revised the manuscript. All the authors have approved the paper submission.Conflicts of interest
The authors declare no competing interests.Acknowledgements
V. I. S. and A. A. K. acknowledge the partial financial support from the Swiss National Science Foundation (grant number IZSEZ0_180186). M. T. L. acknowledges the financial support of Fondation Ernst et Lucie Schmidheiny. Dr M. Docquier and team (Genomics Platform, University of Geneva) are acknowledged for Nanostring RNA profiling and bioinformatics. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the funding agencies.References
- E. Rollerova, J. Tulinska, A. Liskova, M. Kuricova, J. Kovriznych, A. Mlynarcikova, A. Kiss and S. Scsukova, Titanium dioxide nanoparticles: some aspects of toxicity/focus on the development, Endocr. Regul., 2015, 49, 97–112 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Predicted releases of engineered nanomaterials: From global to regional to local, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- A. Azimzada, I. Jreije, M. Hadioui, P. Shaw, J. M. Farner and K. J. Wilkinson, Quantification and characterization of Ti-, Ce-, and Ag-nanoparticles in global surface waters and precipitation, Environ. Sci. Technol., 2021, 55, 9836–9844 CrossRef CAS PubMed.
- R. Kaegi, A. Ulrich, B. Sinnet, R. Vonbank, A. Wichser, S. Zuleeg, H. Simmler, S. Brunner, H. Vonmont, M. Burkhardt and M. Boller, Synthetic TiO2 nanoparticle emission from exterior facades into the aquatic environment, Environ. Pollut., 2008, 156, 233–239 CrossRef CAS PubMed.
- K. Li, D. F. Xu, H. Liao, Y. Xue, M. Y. Sun, H. Su, X. J. Xiu and T. Y. Zhao, A review on the generation, discharge, distribution, environmental behavior, and toxicity (especially to microbial aggregates) of nano-TiO2 in sewage and surface-water and related research prospects, Sci. Total Environ., 2022, 824, 153866 CrossRef CAS PubMed.
- G. V. Lowry, K. B. Gregory, S. C. Apte and J. R. Lead, Transformations of nanomaterials in the environment, Environ. Sci. Technol., 2012, 46, 6893–6899 CrossRef CAS PubMed.
- K. Kansara, S. Bolan, D. Radhakrishnan, T. Palanisami, A. A. H. Al-Muhtaseb, N. Bolan, A. Vinu, A. Kumar and A. Karakoti, A critical review on the role of abiotic factors on the transformation, environmental identity and toxicity of engineered nanomaterials in aquatic environment, Environ. Pollut., 2022, 296, 118726 CrossRef CAS PubMed.
- A. Brunelli, A. Foscari, G. Basei, G. Lusvardi, C. Bettiol, E. Semenzin, A. Marcomini and E. Badetti, Colloidal stability classification of TiO2 nanoparticles in artificial and in natural waters by cluster analysis and a global stability index: Influence of standard and natural colloidal particles, Sci. Total Environ., 2022, 829, 154658 CrossRef CAS PubMed.
- F. Li, Z. Liang, X. Zheng, W. Zhao, M. Wu and Z. Wang, Toxicity of nano-TiO2 on algae and the site of reactive oxygen species production, Aquat. Toxicol., 2015, 158, 1–13 CrossRef CAS PubMed.
- V. K. Sharma, Aggregation and toxicity of titanium dioxide nanoparticles in aquatic environment—a review, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2009, 44, 1485–1495 CrossRef CAS PubMed.
- L. Chen, L. Zhou, Y. Liu, S. Deng, H. Wu and G. Wang, Toxicological effects of nanometer titanium dioxide (nano-TiO2) on Chlamydomonas reinhardtii, Ecotoxicol. Environ. Saf., 2012, 84, 155–162 CrossRef CAS PubMed.
- Q. Abbas, B. Yousaf, H. Ullah, M. U. Ali, Y. S. Ok and J. Rinklebe, Environmental transformation and nano-toxicity of engineered nano-particles (ENPs) in aquatic and terrestrial organisms, Crit. Rev. Environ. Sci. Technol., 2020, 50, 2523–2581 CrossRef CAS.
- H. M. R. Abdel-Latif, M. A. O. Dawood, S. Menanteau-Ledouble and M. El-Matbouli, Environmental transformation of n-TiO2 in the aquatic systems and their ecotoxicity in bivalve mollusks: A systematic review, Ecotoxicol. Environ. Saf., 2020, 200, 110776 CrossRef CAS PubMed.
- M. F. Gutierrez, A. Ale, V. Andrade, C. Bacchetta, A. Rossi and J. Cazenave, Metallic, metal oxide, and metalloid nanoparticles toxic effects on freshwater microcrustaceans: An update and basis for the use of new test species, Water Environ. Res., 2021, 93, 2505–2526 CrossRef CAS PubMed.
- Z. Luo, Z. Q. Li, Z. Xie, I. M. Sokolova, L. Song, W. Peijnenburg, M. H. Hu and Y. J. Wang, Rethinking nano-TiO2 safety: Overview of toxic effects in humans and aquatic animals, Small, 2020, 16, 2002019 CrossRef CAS PubMed.
- J. Zhao, M. Q. Lin, Z. Y. Wang, X. S. Cao and B. S. Xing, Engineered nanomaterials in the environment: Are they safe?, Crit. Rev. Environ. Sci. Technol., 2021, 51, 1443–1478 CrossRef.
- A. Kahru and H.-C. Dubourguier, From ecotoxicology to nanoecotoxicology, Toxicology, 2010, 269, 105–119 CrossRef CAS PubMed.
- D. Xiong, T. Fang, L. Yu, X. Sima and W. Zhu, Effects of nano-scale TiO2, ZnO and their bulk counterparts on zebrafish: acute toxicity, oxidative stress and oxidative damage, Sci. Total Environ., 2011, 409, 1444–1452 CrossRef CAS PubMed.
- H. An, C. Ling, M. Xu, M. Hu, H. Wang, J. Liu, G. Song and J. Liu, Oxidative damage induced by nano-titanium dioxide in rats and mice: a systematic review and meta-analysis, Biol. Trace Elem. Res., 2020, 194, 184–202 CrossRef CAS PubMed.
- J. Hou, L. Wang, C. Wang, S. Zhang, H. Liu, S. Li and X. Wang, Toxicity and mechanisms of action of titanium dioxide nanoparticles in living organisms, J. Environ. Sci., 2019, 75, 40–53 CrossRef PubMed.
- M. Li, W. Liu and V. I. Slaveykova, NanoTiO2 materials mitigate mercury uptake and effects on green alga Chlamydomonas reinhardtii in mixture exposure, Aquat. Toxicol., 2020, 224, 105502 CrossRef CAS PubMed.
- I. M. Sadiq, S. Dalai, N. Chandrasekaran and A. Mukherjee, Ecotoxicity study of titania (TiO2) NPs on two microalgae species: Scenedesmus sp. and Chlorella sp, Ecotoxicol. Environ. Saf., 2011, 74, 1180–1187 CrossRef CAS PubMed.
- M. Sendra, D. Sánchez-Quiles, J. Blasco, I. Moreno-Garrido, L. M. Lubián, S. Pérez-García and A. Tovar-Sánchez, Effects of TiO2 nanoparticles and sunscreens on coastal marine microalgae: Ultraviolet radiation is key variable for toxicity assessment, Environ. Int., 2017, 98, 62–68 CrossRef CAS PubMed.
- D. Minetto, G. Libralato and A. Volpi Ghirardini, Ecotoxicity of engineered TiO2 nanoparticles to saltwater organisms: An overview, Environ. Int., 2014, 66, 18–27 CrossRef CAS PubMed.
- V. Aruoja, H.-C. Dubourguier, K. Kasemets and A. Kahru, Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata, Sci. Total Environ., 2009, 407, 1461–1468 CrossRef CAS PubMed.
- T. Brzicová, J. Sikorová, A. Milcová, K. Vrbová, J. Kléma, P. Pikal, Z. Lubovská, V. Philimonenko, F. Franco and J. Topinka, Nano-TiO2 stability in medium and size as important factors of toxicity in macrophage-like cells, Toxicol. In Vitro, 2019, 54, 178–188 CrossRef PubMed.
- X. He, C. Xie, Y. Ma, L. Wang, X. He, W. Shi, X. Liu, Y. Liu and Z. Zhang, Size-dependent toxicity of ThO2 nanoparticles to green algae Chlorella pyrenoidosa, Aquat. Toxicol., 2019, 209, 113–120 CrossRef CAS PubMed.
- L. Clément, C. Hurel and N. Marmier, Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants – Effects of size and crystalline structure, Chemosphere, 2013, 90, 1083–1090 CrossRef PubMed.
- A. Menard, D. Drobne and A. Jemec, Ecotoxicity of nanosized TiO2. Review of in vivo data, Environ. Pollut., 2011, 159, 677–684 CrossRef CAS PubMed.
- M. Kulasza and L. Skuza, Changes of gene expression patterns from aquatic organisms exposed to metal nanoparticles, Int. J. Environ. Res. Public Health, 2021, 18, 8361 CrossRef CAS PubMed.
- M. Revel, A. Chatel and C. Mouneyrac, Omics tools: New challenges in aquatic nanotoxicology?, Aquat. Toxicol., 2017, 193, 72–85 CrossRef CAS PubMed.
- M. Mortimer, Y. Wang and P. A. Holden, Molecular mechanisms of nanomaterial-bacterial interactions revealed by omics-The role of nanomaterial effect level, Front. Bioeng. Biotechnol., 2021, 9, 683520 CrossRef PubMed.
- S. Majumdar and A. A. Keller, Omics to address the opportunities and challenges of nanotechnology in agriculture, Crit. Rev. Environ. Sci. Technol., 2021, 51, 2595–2636 CrossRef CAS.
- H. M. Kim and J. S. Kang, Metabolomic studies for the evaluation of toxicity induced by environmental toxicants on model organisms, Metabolites, 2021, 11, 485 CrossRef CAS PubMed.
- G. T. Ankley, R. S. Bennett, R. J. Erickson, D. J. Hoff, M. W. Hornung, R. D. Johnson, D. R. Mount, J. W. Nichols, C. L. Russom, P. K. Schmieder, J. A. Serrrano, J. E. Tietge and D. L. Villeneuve, Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment, Environ. Toxicol. Chem., 2010, 29, 730–741 CrossRef CAS PubMed.
- E. Morel, I. Jreije, V. Tetreault, C. Hauser, W. Zerges and K. J. Wilkinson, Biological impacts of Ce nanoparticles with different surface coatings as revealed by RNA-Seq in Chlamydomonas reinhardtii, Nanoimpact, 2020, 19, 100228 CrossRef.
- S. Chen, N. Shi, M. Huang, X. Tan, X. Yan, A. Wang, Y. Huang, R. Ji, D. Zhou, Y.-G. Zhu, A. A. Keller, J. L. Gardea-Torresdey, J. C. White and L. Zhao, MoS2 nanosheets–cyanobacteria interaction: Reprogrammed carbon and nitrogen metabolism, ACS Nano, 2021, 15, 16344–16356 CrossRef CAS PubMed.
- W. Liu, S. Majumdar, W. Li, A. A. Keller and V. I. Slaveykova, Metabolomics for early detection of stress in freshwater alga Poterioochromonas malhamensis exposed to silver nanoparticles, Sci. Rep., 2020, 10, 1–13 CrossRef PubMed.
- P. Wang, B. Zhang, H. Zhang, Y. L. He, C. N. Ong and J. Yang, Metabolites change of Scenedesmus obliquus exerted by AgNPs, J. Environ. Sci., 2019, 76, 310–318 CrossRef PubMed.
- R. Qu, Q. Xie, J. Tian, M. Zhou and F. Ge, Metabolomics reveals the inhibition on phosphorus assimilation in Chlorella vulgaris F1068 exposed to AgNPs, Sci. Total Environ., 2021, 770, 145362 CrossRef CAS PubMed.
- J. L. Zhang, Z. P. Zhou, Y. Pei, Q. Q. Xiang, X. X. Chang, J. Ling, D. Shea and L. Q. Chen, Metabolic profiling of silver nanoparticle toxicity in Microcystis aeruginosa, Environ. Sci.: Nano, 2018, 5, 2519–2530 RSC.
- D. F. Simon, R. F. Domingos, C. Hauser, C. M. Hutchins, W. Zerges and K. J. Wilkinson, Transcriptome sequencing (RNA-seq) analysis of the effects of metal nanoparticle exposure on the transcriptome of Chlamydomonas reinhardtii, Appl. Environ. Microbiol., 2013, 79, 4774–4785 CrossRef CAS PubMed.
- M. Li and V. I. Slaveykova, Kinetic aspects of the interactions between TiO2 nanoparticles, mercury and the green alga Chlamydomonas reinhardtii, Environments, 2022, 9, 44 CrossRef.
- A. Kahru and A. Ivask, Mapping the Ddawn of nanoecotoxicological research, Acc. Chem. Res., 2013, 46, 823–833 CrossRef CAS PubMed.
- E. H. Harris, The Chlamydomonas sourcebook: a comprehensive guide to biology and laboratory use, Elsevier, 2013 Search PubMed.
- G. Cheloni, C. Cosio and V. I. Slaveykova, Antagonistic and synergistic effects of light irradiation on the effects of copper on Chlamydomonas reinhardtii, Aquat. Toxicol., 2014, 155, 275–282 CrossRef CAS PubMed.
- V. I. Slaveykova, S. Majumdar, N. Regier, W. Li and A. A. Keller, Metabolomic responses of green alga Chlamydomonas reinhardtii exposed to sublethal concentrations of inorganic and methylmercury, Environ. Sci. Technol., 2021, 55, 3876–3887 CrossRef CAS PubMed.
- Y. Huang, W. Li, A. S. Minakova, T. Anumol and A. A. Keller, Quantitative analysis of changes in amino acids levels for cucumber (Cucumis sativus) exposed to nano copper, NanoImpact, 2018, 12, 9–17 CrossRef.
- Y. Huang, A. S. Adeleye, L. Zhao, A. S. Minakova, T. Anumol and A. A. Keller, Antioxidant response of cucumber (Cucumis sativus) exposed to nano copper pesticide: Quantitative determination via LC-MS/MS, Food Chem., 2019, 270, 47–52 CrossRef CAS PubMed.
- S. Majumdar, L. Pagano, J. A. Wohlschlegel, M. Villani, A. Zappettini, J. C. White and A. A. Keller, Proteomic, gene and metabolite characterization reveal the uptake and toxicity mechanisms of cadmium sulfide quantum dots in soybean plants, Environ. Sci.: Nano, 2019, 6, 3010–3026 RSC.
- Z. Pang, J. Chong, G. Zhou, D. A. de Lima Morais, L. Chang, M. Barrette, C. Gauthier, P.-É. Jacques, S. Li and J. Xia, MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights, Nucleic Acids Res., 2021, 49, W388–W396 CrossRef CAS PubMed.
- Y. Jung, Y. G. Ahn, H. K. Kim, B. C. Moon, A. Y. Lee, D. H. Ryu and G. S. Hwang, Characterization of dandelion species using 1H NMR- and GC-MS-based metabolite profiling, Analyst, 2011, 136, 4222–4231 RSC.
- J. Chong, D. S. Wishart and J. Xia, Using MetaboAnalyst 4.0 for Comprehensive and Integrative Metabolomics Data Analysis, Curr. Protoc. Bioinf., 2019, 68, e86 Search PubMed.
- J. Xia and D. S. Wishart, Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst, Nat. Protoc., 2011, 6, 743 CrossRef CAS PubMed.
- J. Xia and D. S. Wishart, MSEA: a web-based tool to identify biologically meaningful patterns in quantitative metabolomic data, Nucleic Acids Res., 2010, 38, W71–W77 CrossRef CAS PubMed.
- R. Beauvais-Flück, V. I. Slaveykova and C. Cosio, Cellular toxicity pathways of inorganic and methyl mercury in the green microalga Chlamydomonas reinhardtii, Sci. Rep., 2017, 7, 1–12 CrossRef PubMed.
- O. Thimm, O. Bläsing, Y. Gibon, A. Nagel, S. Meyer, P. Krüger, J. Selbig, L. A. Müller, S. Y. Rhee and M. Stitt, MAPMAN: a user-driven tool to display genomics data sets onto diagrams of metabolic pathways and other biological processes, Plant J., 2004, 37, 914–939 CrossRef CAS PubMed.
- R. Beauvais-Flück, V. I. Slaveykova, S. Ulf and C. Cosio, Towards early-warning gene signature of Chlamydomonas reinhardtii exposed to Hg-containing complex media, Aquat. Toxicol., 2019, 214, 105259 CrossRef PubMed.
- P. Dranguet, C. Cosio, S. Le Faucheur, R. Beauvais-Flück, A. Freiburghaus, I. A. Worms, B. Petit, N. Civic, M. Docquier and V. I. Slaveykova, Transcriptomic approach for assessment of the impact on microalga and macrophyte of in-situ exposure in river sites contaminated by chlor-alkali plant effluents, Water Res., 2017, 121, 86–94 CrossRef CAS PubMed.
- G. K. Geiss, R. E. Bumgarner, B. Birditt, T. Dahl, N. Dowidar, D. L. Dunaway, H. P. Fell, S. Ferree, R. D. George and T. Grogan, Direct multiplexed measurement of gene expression with color-coded probe pairs, Nat. Biotechnol., 2008, 26, 317–325 CrossRef CAS PubMed.
- J. Vandesompele, K. De Preter, F. Pattyn, B. Poppe, N. Van Roy, A. De Paepe and F. Speleman, Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes, Genome Biol., 2002, 3, 1–12 CrossRef PubMed.
- Y. Hochberg and Y. Benjamini, More powerful procedures for multiple significance testing, Stat. Med., 1990, 9, 811–818 CrossRef CAS PubMed.
- A. A. Keller, H. Wang, D. Zhou, H. S. Lenihan, G. Cherr, B. J. Cardinale, R. Miller and Z. Ji, Stability and aggregation of metal oxide nanoparticles in natural aqueous matrices, Environ. Sci. Technol., 2010, 44, 1962–1967 CrossRef CAS PubMed.
- J. Wang, Y. Sun, M. Yu, X. Lu, S. Komarneni and C. Yang, Emulsions stabilized by highly hydrophilic TiO2 nanoparticles via van der Waals attraction, J. Colloid Interface Sci., 2021, 589, 378–387 CrossRef CAS PubMed.
- I. M. Nadeem, L. Hargreaves, G. T. Harrison, H. Idriss, A. L. Shluger and G. Thornton, Carboxylate adsorption on rutile TiO2 (100): Role of Coulomb repulsion, relaxation, and steric hindrance, J. Phys. Chem. C, 2021, 25, 13770–13779 CrossRef PubMed.
- O. Vallon and M. H. Spalding, in The Chlamydomonas Sourcebook, ed. E. H. Harris, D. B. Stern and G. B. Witman, Academic Press, London, 2nd edn, 2009, pp. 115–158, DOI:10.1016/B978-0-12-370873-1.00012-5.
- N. L. Taylor, J. L. Heazlewood, D. A. Day and A. H. Millar, Lipoic acid-dependent oxidative catabolism of alpha-keto acids in mitochondria provides evidence for branched-chain amino acid catabolism in Arabidopsis, Plant Physiol., 2004, 134, 838–848 CrossRef CAS PubMed.
- B. G. Forde and P. J. Lea, Glutamate in plants: metabolism, regulation, and signalling, J. Exp. Bot., 2007, 58, 2339–2358 CrossRef CAS PubMed.
- M. Wirtz and M. Droux, Synthesis of the sulfur amino acids: Cysteine and methionine, Photosynth. Res., 2006, 86, 345–362 CrossRef PubMed.
- K. Lameka, M. D. Farwell and M. Ichise, in Handbook of Clinical Neurology, ed. J. C. Masdeu and R. G. González, Elsevier, 2016, vol. 135, pp. 209–227 Search PubMed.
- H. Less and G. Galili, Principal transcriptional programs regulating plant amino acid metabolism in response to abiotic stresses, Plant Physiol., 2008, 147, 316–330 CrossRef CAS PubMed.
- M. A. Lieberman and R. Ricer, Brs Biochemistry, Molecular Biology, And Genetics, Wolters Kluwer, Lippincott Williams and Wilkins, 6th edn, 2013 Search PubMed.
- T. M. Hildebrandt, A. Nunes Nesi, W. L. Araújo and H.-P. Braun, Amino acid catabolism in plants, Mol. Plant, 2015, 8, 1563–1579 CrossRef CAS PubMed.
- J. Matysik, Alia, B. Bhalu and P. Mohanty, Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants, Curr. Sci., 2002, 82, 525–532 CAS.
- S. S. Sharma and K.-J. Dietz, The significance of amino acids and amino acid-derived molecules in plant responses and adaptation to heavy metal stress, J. Exp. Bot., 2006, 57, 711–726 CrossRef CAS PubMed.
- B. Sekula, M. Ruszkowski and Z. Dauter, Structural analysis of phosphoserine aminotransferase (Isoform 1) from Arabidopsis thaliana–the enzyme involved in the phosphorylated pathway of serine biosynthesis, Front. Plant Sci., 2018, 9, 876 CrossRef PubMed.
- H. Andre'O, C. Bless, P. Macedo, S. P. Chatterjee, B. K. Singh, C. Gilvarg and T. Leustek, Biosynthesis of lysine in plants: evidence for a variant of the known bacterial pathways, Biochim. Biophys. Acta, Gen. Subj., 2005, 1721, 27–36 CrossRef PubMed.
- C. Carrie, M. W. Murcha, A. H. Millar, S. M. Smith and J. Whelan, Nine 3-ketoacyl-CoA thiolases (KATs) and acetoacetyl-CoA thiolases (ACATs) encoded by five genes in Arabidopsis thaliana are targeted either to peroxisomes or cytosol but not to mitochondria, Plant Mol. Biol., 2007, 63, 97–108 CrossRef CAS PubMed.
- C. Han, C. Ren, T. Zhi, Z. Zhou, Y. Liu, F. Chen, W. Peng and D. Xie, Disruption of fumarylacetoacetate hydrolase causes spontaneous cell death under short-day conditions in Arabidopsis, Plant Physiol., 2013, 162, 1956–1964 CrossRef CAS PubMed.
- N. V. Bhagavan and C.-E. Ha, Nucleotide Metabolism, in Essentials of Medical Biochemistry, ed. N. V. Bhagavan and C.-E. Ha, Imprint: Academic Press, 2015, p. 752, vol. eBook ISBN: 9780124166974 Search PubMed.
- C. H. Foyer and G. Noctor, Ascorbate and glutathione: The heart of the redox hub, Plant Physiol., 2011, 155, 2–18 CrossRef CAS PubMed.
- A. Jamers, R. Blust, W. De Coen, J. L. Griffin and O. A. H. Jones, An omics based assessment of cadmium toxicity in the green alga Chlamydomonas reinhardtii, Aquat. Toxicol., 2013, 126, 355–364 CrossRef CAS PubMed.
- T. L. Stoiber, M. M. Shafer and D. E. Armstrong, Differential effects of copper and cadmium exposure on toxicity endpoints and gene expression in Chlamydomonas reinhardtii, Environ. Toxicol. Chem., 2010, 29, 191–200 CrossRef CAS PubMed.
- A. Jamers, R. Blust, W. De Coen, J. L. Griffin and O. A. Jones, Copper toxicity in the microalga Chlamydomonas reinhardtii: an integrated approach, BioMetals, 2013, 26, 731–740 CrossRef CAS PubMed.
- M. Xiao, Z. Li, L. Zhu, J. Wang, B. Zhang, F. Zheng, B. Zhao, H. Zhang, Y. Wang and Z. Zhang, The multipleroles of ascorbate in the abiotic stress response of plants: Antioxidant, cofactor, and regulator, Front. Plant Sci., 2021, 12, 598173 CrossRef PubMed.
- N. Gest, H. Gautier and R. Stevens, Ascorbate as seen through plant evolution: the rise of a successful molecule?, J. Exp. Bot., 2012, 64, 33–53 CrossRef PubMed.
- B. Nowicka and J. Kruk, Plastoquinol is more active than α-tocopherol in singlet oxygen scavenging during high light stress of Chlamydomonas reinhardtii, Biochim. Biophys. Acta, Bioenerg., 2012, 1817, 389–394 CrossRef CAS PubMed.
- B. Nowicka, B. Pluciński, P. Kuczyńska and J. Kruk, Physiological characterization of Chlamydomonas reinhardtii acclimated to chronic stress induced by Ag, Cd, Cr, Cu and Hg ions, Ecotoxicol. Environ. Saf., 2016, 130, 133–145 CrossRef CAS PubMed.
- B. Nowicka, T. Fesenko, J. Walczak and J. Kruk, The inhibitor-evoked shortage of tocopherol and plastoquinol is compensated by other antioxidant mechanisms in Chlamydomonas reinhardtii exposed to toxic concentrations of cadmium and chromium ions, Ecotoxicol. Environ. Saf., 2020, 191, 110241 CrossRef CAS PubMed.
- M. Huang, A. A. Keller, X. Wang, L. Tian, B. Wu, R. Ji and L. Zhao, Low concentrations of silver nanoparticles and silver ions perturb the antioxidant defense system and nitrogen metabolism in N2-fixing cyanobacteria, Environ. Sci. Technol., 2020, 54, 15996–16005 CrossRef CAS PubMed.
- S. D. Lemaire, B. Guillon, P. Le Maréchal, E. Keryer, M. Miginiac-Maslow and P. Decottignies, New thioredoxin targets in the unicellular photosynthetic eukaryote Chlamydomonas reinhardtii, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 7475–7480 CrossRef CAS PubMed.
- F. Montrichard, F. Alkhalfioui, H. Yano, W. H. Vensel, W. J. Hurkman and B. B. Buchanan, Thioredoxin targets in plants: the first 30 years, J. Proteomics, 2009, 72, 452–474 CrossRef CAS PubMed.
- X. Liu, H. Zhang, H. Jiao, L. Li, X. Qiao, M. R. Fabrice, J. Wu and S. Zhang, Expansion and evolutionary patterns of cysteine-rich peptides in plants, BMC Genomics, 2017, 18, 1–14 CrossRef PubMed.
- W. Chen, X. Jiang and Q. Yang, Glycoside hydrolase family 18 chitinases: The known and the unknown, Biotechnol. Adv., 2020, 43, 107553 CrossRef CAS PubMed.
- S. Gonçalves, M. Kahlert, S. F. P. Almeida and E. Figueira, Assessing Cu impacts on freshwater diatoms: biochemical and metabolomic responses of Tabellaria flocculosa (Roth) Kützing, Sci. Total Environ., 2018, 625, 1234–1246 CrossRef PubMed.
- R. G. Upchurch, Fatty acid unsaturation, mobilization, and regulation in the response of plants to stress, Biotechnol. Lett., 2008, 30, 967–977 CrossRef CAS PubMed.
- E. Pinto, T. C. S. Sigaud-kutner, M. A. S. Leitão, O. K. Okamoto, D. Morse and P. Colepicolo, Heavy metal-induced ooxidative stress in algae, J. Phycol., 2003, 39, 1008–1018 CrossRef CAS.
- M. Behzadi Tayemeh, M. Esmailbeigi, I. Shirdel, H. S. Joo, S. A. Johari, A. Banan, H. Nourani, H. Mashhadi, M. J. Jami and M. Tabarrok, Perturbation of fatty acid composition, pigments, and growth indices of Chlorella vulgaris in response to silver ions and nanoparticles: A new holistic understanding of hidden ecotoxicological aspect of pollutants, Chemosphere, 2020, 238, 124576 CrossRef CAS PubMed.
- H. Zhang, W. Du, J. R. Peralta-Videa, J. L. Gardea-Torresdey, J. C. White, A. Keller, H. Guo, R. Ji and L. Zhao, Metabolomics reveals how cucumber (Cucumis sativus) reprograms metabolites to cope with silver ions and silver nanoparticle-induced oxidative stress, Environ. Sci. Technol., 2018, 52, 8016–8026 CrossRef CAS PubMed.
- A. Georgantzopoulou, S. Cambier, T. Serchi, M. Kruszewski, Y. L. Balachandran, P. Grysan, J.-N. Audinot, J. Ziebel, C. Guignard and A. C. Gutleb, Inhibition of multixenobiotic resistance transporters (MXR) by silver nanoparticles and ions in vitro and in Daphnia magna, Sci. Total Environ., 2016, 569, 681–689 CrossRef PubMed.
- J. Park, W. Y. Song, D. Ko, Y. Eom, T. H. Hansen, M. Schiller, T. G. Lee, E. Martinoia and Y. Lee, The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury, Plant J., 2012, 69, 278–288 CrossRef CAS PubMed.
- M. L. Guerinot, The ZIP family of metal transporters, Biochim. Biophys. Acta, Biomembr., 2000, 1465, 190–198 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00260d |
| This journal is © The Royal Society of Chemistry 2022 |