Emerging investigator series: long-term exposure of amorphous silica nanoparticles disrupts the lysosomal and cholesterol homeostasis in macrophages†
Ronglin
Ma‡
a,
Xiaoming
Cai‡
b,
Ye
Zhou
c,
Xi
Liu
a,
Di
Wu
a,
Huizhen
Zheng
a,
Yanxia
Pan
a,
Jun
Jiang
a,
Shujuan
Xu
a,
Qianqian
Xie
a,
Jie
Jiang
a,
Weili
Wang
 a,
Nikolai
Tarasenko
d,
Fangjun
Wang
a,
Nikolai
Tarasenko
d,
Fangjun
Wang
 *c and
Ruibin
Li
*c and
Ruibin
Li
 *a
*a
aState Key Laboratory of Radiation Medicine and Protection, School for Radiological and Interdisciplinary Sciences (RAD-X), Collaborative Innovation Center of Radiological Medicine of Jiangsu Higher Education Institutions, Soochow University, Suzhou 215123, Jiangsu, China. E-mail: liruibin@suda.edu.cn; Tel: +86 512 65880062
bCenter for Genetic Epidemiology and Genomics, School of Public Health, Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Soochow University, Suzhou 215123, Jiangsu, China
cCAS Key Laboratory of Separation Sciences for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences (CAS), Dalian 116023, Liaoning, P. R. China. E-mail: wangfj@dicp.ac.cn; Tel: +86 411 82464150
dB. I. Stepanov Institute of Physics, National Academy of Sciences of Belarus, 68 Nezalezhnasti Ave., 220072 Minsk, Belarus
First published on 19th November 2021
Abstract
Amorphous silica nanoparticles (ASiNPs) are generally considered to be biocompatible with limited acute toxicity. These nanoparticles were therefore exploited in diverse nanoproducts (e.g. foods and cosmetics) and may be released into the environment in daily life. This has led to substantial concerns about their long-term exposure effects. Herein, RAW 264.7 cells were subjected to long-term exposure to ASiNPs for assessment of their potential cytotoxicity. Two ASiNPs with diameters of 50–100 nm (ASiNP1) and 450–500 nm (ASiNP2) were synthesized and comprehensively characterized. The acquired cells were denoted as RAW 264.7-NP1 (ASiNP1 treatment) and RAW 264.7-NP2 (ASiNP2 treatment) after long-term exposure. Both the small and large ASiNPs showed similar cellular distributions in lysosomes. We therefore examined the impacts of ASiNPs on the lysosomal density, size and function by microscopy imaging and proteomics analysis. As a result, RAW 264.7-NP1 cells displayed 2.3-fold higher lysosomal density and a 149% increase of average lysosomal area compared to parent cells. Proteomics analysis of lysosomes revealed 25 significantly changed proteins closely related to 5 cellular effects, including autophagy and lipid metabolism. Confocal imaging and immunoblotting of LC3 proteins indicated an accumulation of autophagosomes in RAW 264.7-NP1 cells. Elevation of cholesterol was also demonstrated in RAW 264.7-NP1 cells by visualization and quantification of cellular cholesterols. Notably, the cholesterol elevation in RAW 264.7-NP1 cells could be ameliorated by acceleration of autophagic flux (rapamycin treatment) but aggregated by blockage of autolysosome formation (chloroquine treatment), indicating the critical role of autophagic flux in lipid metabolism. Overall, the discovery of these hazard effects raised warnings for the long-term use of ASiNP-based nanoproducts and may facilitate the decision making for environmental exposure thresholds of ASiNPs.
Environmental significanceWhile amorphous silica nanomaterials (ASiNPs) are generally considered to be biocompatible in acute toxicity studies, these nanoparticles were exploited in diverse nanoproducts (e.g. foods and cosmetics) and may be released into environments in daily life. The environmental release of these fine particulates from some nanoproducts provides the possibility for them to enter a mammal's digestive system through inhalation and swallowing. Therefore, biosafety assessments of ASiNPs in long-term exposure are relevant to possible environmental exposure in real life. Since ASiNPs could be taken by macrophages through endocytosis and mainly accumulated in lysosomes, we therefore examined the impacts of long-term ASiNP exposure on lysosomes in RAW 264.7 cells. We found that long-term exposure to ASiNPs, especially small-sized ones led to a significant increase of lysosome densities and sizes in the cells. Second, the autophagic flux and lipid metabolism were altered, resulting in the accumulation of autophagosomes and elevation of cholesterol in cells. Based on the discovery of these hazard effects, we believe long-term exposure to ASiNPs could disrupt the lysosome and cholesterol homeostasis in macrophage-like cells, which may elicit inflammatory pathways and increase the formation of macrophage foam cells. |
1. Introduction
The large-scale production and wide application of silica nanoparticles (SiNPs) in various areas, such as agriculture,1 dietary,2 consumer products3 and biomedicine,4–6 have raised increasing public awareness on the exposure risks of SiNPs to mammals. During the past few decades, crystalline silica particles have been demonstrated to elicit oxidative stresses,7 genotoxic effects,8,9 membranolysis,10 mitochondria dysfunction11 and pro-inflammatory cytokine release,12,13 in macrophages, red blood cells, embryonic cells and lung epithelial cells. In the occupational scenarios of inhalation exposure, these hazard signals may eventually cumulate into severe respiratory diseases, such as silicosis,14,15 chronic obstructive pulmonary disease16 and lung cancer.17,18 Therefore, crystalline silica has been classified as a group 1 carcinogenic compound by the International Agency for Research on Cancer (IARC).19Since amorphous silica nanoparticles (ASiNPs) were often applied in cancer therapy or diagnosis with a single or a few repetitive doses, most of their biosafety studies focused on the effects of short-term exposure to mammalian cells.20,21 In contrast to crystalline silica, ASiNPs are generally thought to be biocompatible in cells and animals. ASiNPs were found to display a three-stage degradation behaviour in simulated biological fluids and is favourable for cargo release.22 Meng et al. found that ASiNPs could be internalized into lysosomes in 6–36 h exposure and had limited effects in the metabolic activity of macrophage-like (THP-1), KB-31, HeLa and A549 cells.23–25 Moreover, ASiNPs had shown low haemolytic activity,26,27 and cytotoxicity in bronchial epithelial cells, macrophages and pancreatic cancer cells after 24 h exposure.28,29 Consistently, we found that unlike rare earth nanoparticles, ASiNPs neither disrupted autophagic flux nor induced inflammatory responses in THP-1 cells after 24 h exposure.30 Due to their large surface area and good biocompatibility, ASiNPs have been explored extensively for imaging and therapeutic delivery of agents into various tumor cells.26 Recently, Cornell dots consisting of ultra-small inorganic hybrid silica nanoparticles (<10 nm) were approved by the US FDA in stage I human clinical trial for real-time image-guided intraoperative mapping of nodal metastases.31 Besides drug delivery, ASiNPs were explored for applications in implants,32 antibacterial materials,33 food packaging,34 cosmetics,35 water purification membranes,36 industrial catalysis,37etc. The wide application and production of amorphous silica may result in persistent release of fine particulates into the environment. For example, Boccuni et al. characterized the workplace exposed SiNPs and found that the concentration of SiNPs (with an aerodynamic diameter <250 nm) was up to 2.78 μg m−3 in air.38 Besides, SiNPs may be released into water bodies, soil and landfill.39 It has been predicted that 258–2899 metric tons of SiNPs could be released into water bodies in Asia per year. After environmental release, these particulates may enter the human body by inhalation, swallowing or skin contact,40 and may be captured by the reticuloendothelial system for a persistent interaction with mammalian cells. However, the long-term impacts of amorphous silica on mammalian cells were rarely explored.
Considering that macrophages are the major cell types responsible for the removal of exogenous fine particulates,40,41 we deliberately selected a macrophage-like cell line (RAW 264.7) to examine the impacts of long-term exposure (50 passages in 150 days) to ASiNPs. The acquired cells were denoted as RAW 264.7-NP1/NP2. Given the endocytic internalization of ASiNPs in cells, we collected the lysosomes from RAW 264.7-NP1 cells for proteomics analysis. The protein profile allowed us to identify the potential cytotoxicity of ASiNPs by bioinformatics. The discovered hazard effects were further validated by imaging of lysosomes and autophagosomes as well as cholesterol quantification.
2. Materials and methods
2.1 Reagents and materials
Hoechst 33342 was purchased from InvivoGen (San Diego, USA). Fluorescein isothiocyanate labeled albumin bovine serum (FITC-BSA) was obtained from Solarbio (Beijing, China). LysoTracker DND-99 was obtained from Yeasen (Shanghai, China). The MTS cell proliferation colorimetric assay kit was purchased from Promega (Madison, USA). The cholesterol/cholesteryl ester detection kit and anti-LC3 antibody were from Abcam (Massachusetts, USA). The lysosome enrichment kit was purchased from Thermo Scientific (Rockford, USA). Styrene monomer was from Kanto Chemical (Tokyo, Japan). Octane, lysine, hexadecyltrimethylammonium bromide (CTAB), tetraethylorthosilicate (TEOS), 2,20-azobis (2-methylpropionamide) dihydrochloride (AIBA) and N-(3-dimethylaminopropyl)-N′-ethyl-carbodiimide hydrochloride (EDC) were from Sigma-Aldrich (St. Louis, USA).2.2 In-house synthesis of ASiNPs
The ASiNPs were synthesized by an organic template method as described in a previous study.42 Briefly, 0.1 g CTAB was dissolved in 30 mL of aqueous solution at 60 °C in a three-necked flask reactor. For the synthesis of small-sized ASiNP1, 15 mL of octane, 15 mg of styrene monomer, 22 mg of lysine, 1.0 g of TEOS and 25 mg of AIBA were added into CTAD solution, and the mixture was heated at 60 °C for 3 h under a N2 atmosphere. For the synthesis of large-sized ASiNP2, 15 mL of octane, 15 mg of styrene monomer, 50 mg of hydrazine, 5.0 g of TEOS and 25 mg of AIBA were used, and the reaction time was increased to 8 h. The reaction solution was cooled to room temperature and decanted for 12 h. Silica nanoparticles were collected by centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm, 5 min) and the templates were removed by heat treatment at 500 °C, followed by sufficient acid and ethanol washing.
000 rpm, 5 min) and the templates were removed by heat treatment at 500 °C, followed by sufficient acid and ethanol washing.
2.3 Physicochemical characterization of ASiNPs
The morphology of ASiNPs was observed by transmission electron microscopy (TEM, Tecnai G20, FEI, USA) at a voltage of 120 kV. To prepare the TEM samples, a drop of ASiNP suspension (50 μg mL−1 in DI water) was placed on a grid and air-dried at room temperature. The hydrodynamic diameters and surface charges of ASiNPs in water or cell culture medium were measured by dynamic light scattering and analysis of zeta potentials on a Zetasizer Nano ZS90 (Malvern, UK) instrument as described previously.302.4 Fluorescent labeling of ASiNPs
Fluorescein isothiocyanate conjugated with bovine serum albumin (FITC-BSA) was used to label ASiNPs by an amidation reaction. In detail, 10 mg of EDC and 20 mg of NHS were added into 4 mL of ASiNPs (100 μg mL−1) in DI H2O and stirred for 2 h at 25 °C. Then ASiNP pellets were obtained by centrifugation at 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and then reacted with 1 mL of FITC-BSA solution (0.1 mg mL−1) under magnetic stirring for 2 h. FITC-BSA labeled ASiNPs were washed three times with DI H2O to remove non-covalent bound FITC-BSA and suspended in 400 μL DI H2O.
000 rpm for 10 min and then reacted with 1 mL of FITC-BSA solution (0.1 mg mL−1) under magnetic stirring for 2 h. FITC-BSA labeled ASiNPs were washed three times with DI H2O to remove non-covalent bound FITC-BSA and suspended in 400 μL DI H2O.
2.5 Cytotoxicity test
RAW 264.7 cells obtained from ATCC were cultured in high-glucose DMEM (supplemented with 10% fetal bovine serum) at 37 °C, 5% CO2. The cells were seeded in 96 well plates at 3 × 104 cells per well for overnight incubation, and then exposed to ASiNPs at 0–200 μg mL−1 for 24 h. After removal of the supernatants, an aliquot of 120 μL diluted MTS solution was added to the cell pellets and incubated at 37 °C for 3 h. Then centrifugation (3000 rpm, 5 min) was performed to transfer the supernatants (100 μL per well) into new plates for absorbance detection at OD 490 nm using a microplate reader (Synergy NEO HTS, Biotek, USA). The cell viability was determined by the following equation: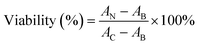 , where AN, AC, and AB represent the absorbances of ASiNP-treated cells, untreated cells and blank.
, where AN, AC, and AB represent the absorbances of ASiNP-treated cells, untreated cells and blank.
2.6 Long-term exposure of macrophage cells to ASiNPs
RAW 264.7 cells were seeded at a density of 1 × 106 cells per well in 6-well plates and exposed to 100 μg mL−1 ASiNP1 or ASiNP2 solutions that were freshly prepared in DMEM media and fully dispersed by probe sonication at 32 W for 10 s. The cells were cultured at 37 °C with 5% CO2 and passaged every 48–72 hours. After exposure to ASiNP solutions for 50 generations, the cells were then subcultured in ASiNP-free medium for additional 3 passages. The acquired cells by ASiNP1 and ASiNP2 treatment were denoted as RAW 264.7-NP1 and RAW 264.7-NP2, respectively.2.7 Confocal microscopy imaging
An UltraView VoX confocal microscope (PerkinElmer, USA) was used to image labeled ASiNPs, lysosomes and autophagosomes in RAW 264.7 cells. High-magnification images were taken at the 600× objective. To visualize the ASiNPs in cells, RAW 264.7 cells were incubated with FITC-BSA labeled ASiNPs (50 μg mL−1) for 24 h. For detection of the LC3 complex, the untreated, chloroquine (CQ, 62.5 μM, 12 h) treated RAW 264.7 cells and RAW 264.7-NP1/NP2 cells were fixed and stained with DyLight 488 labeled anti-LC3 antibodies. To visualize lysosomes, membranes, autophagosomes and mitochondria, the cells were stained with Hoechst 33342 (100 ng mL−1) and LysoTracker DND-99, WGA-594, an LC3II labeling kit or mito-tracker dye.2.8 ICP-OES quantification of the intracellular silica content
RAW 264.7 cells were seeded at a density of 1 × 106 cells per well in 6-well plates and exposed to 100 μg mL−1 ASiNP1 or ASiNP2 solutions for 24 h. After that, the cells were washed thrice with PBS and subjected to further incubation in ASiNP-free culture media for 0, 24 and 48 h. After washing with PBS, the treated cells were collected in 1 mL PBS to count the number of cells, followed by centrifugation at 1000g for 5 min. The cell pellets were digested in 1 mL of nitric acid (HNO3, 65–70%) supplemented with 0.5 mL of hydrogen peroxide and heated at 120 °C for 1 h. The resulting solutions were mixed with 33 μL potassium hydroxide (KOH, 1.0 M) and stirred overnight at room temperature. The pH of the final digestion solutions was adjusted to 2 with sulphuric acid (H2SO4, 2.25 M), and the solution was diluted in 5 mL DI H2O before ICP-OES analysis (Thermo Fisher, ICAP 7200).2.9 TEM detection of lysosomes in RAW 264.7 cells
Cells were washed twice with PBS and fixed with 2% glutaraldehyde in PBS for 30 min. After treatment with propylene oxide, cells were embedded in Epon and cut into 50–70 nm thick sections using a Reichert-Jung Ultracut E ultramicrotome (Leica Microsystems, Shanghai, China). Cell sections were stained with uranyl acetate and Reynolds lead citrate on copper grids before visualization by TEM. In TEM images, lysosomes displayed a lower electron density than other organelles. The lysosome numbers were counted manually, and the sizes of the lysosomes were measured using ImageJ.2.10 Isotope-labelled proteomic quantification of lysosomes
Lysosomes were collected according to the manufacturer's protocol of the lysosome enrichment kit. Briefly, RAW 264.7 cell (1 × 106) pellets were harvested by centrifugation and resuspended in 800 μL lysosome enrichment reagent A (1× extraction buffer) with a protease inhibitor. After incubation on ice for exactly 2 min, the cells were lysed by sonication and mixed with 800 μL reagent B (1× gradient dilution buffer), followed by centrifugation at 500 × g for 10 min at 4 °C. The supernatant (1500 μL) was then added to an ultracentrifuge tube and mixed with 500 μL of Optiprep cell separation media, which was overlaid onto a discontinuous Optiprep density gradient (30%, 27%, 23%, 20%, and 17%) and ultracentrifuged at 145![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 2 h at 4 °C. The lysosome band, which was at the top of the gradient, was carefully removed and washed with PBS, and then kept in PBS with a protease inhibitor at −20 °C until downstream processing. For protein extraction, the lysosomes were lysed with buffer containing 8 M urea, 100 mM TEAB (pH 8), and 1% protease cocktail (v/v). The protein concentration was determined by Bradford assay.
000 × g for 2 h at 4 °C. The lysosome band, which was at the top of the gradient, was carefully removed and washed with PBS, and then kept in PBS with a protease inhibitor at −20 °C until downstream processing. For protein extraction, the lysosomes were lysed with buffer containing 8 M urea, 100 mM TEAB (pH 8), and 1% protease cocktail (v/v). The protein concentration was determined by Bradford assay.
The extracted protein samples were reduced with 10 mM DTT at 60 °C for 1 h and alkylated with 20 mM IAA in the dark at room temperature for 30 min. After that, the urea concentration was diluted to 1 M with 100 mM TEAB buffer (pH 8), and digested with trypsin (enzyme/substrate, 1/25 (w/w)) overnight at 37 °C. Mass-differentiated isotopic labelling was performed by adding 5 mM CH2O, 5 mM NaBH3CN for light labeling and 5 mM CD2O and 5 mM NaBH3CN for heavy labeling as described in a previous study.43 The lysosome proteins from ASiNP treated and control cells were labelled with heavy and light isotope labelling reagents, respectively. After stopping the labeling reaction with 50 mM NH4HCO3, the heavy and light isotope labelled samples were mixed, and desalted using an OASIS HLB column (Waters, Milford, MA) and subjected to LC-MS/MS analysis with an LTQ-Orbitrap Velos MS. The samples were separated on a reversed phase C18 capillary column (15 cm × 75 μm i.d.). The mobile phase A was 0.1% formic acid and mobile phase B was acetonitrile with 0.1% formic acid. The gradient separation was set as 10–35% B over 78 min at a flow rate of 300 nL min−1. The full MS scans were collected from m/z 400 to 2000 with a resolution of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (m/z 400). The MS/MS scans were performed in a data dependent analysis mode by selecting the 20 most intense ions in the full MS scan for collision-induced dissociation. The normalized collision energy for MS/MS analysis was 35%. The dynamic exclusion duration was set to 120 s, the repeat count was 1, and the repeat duration was 60 s.
000 (m/z 400). The MS/MS scans were performed in a data dependent analysis mode by selecting the 20 most intense ions in the full MS scan for collision-induced dissociation. The normalized collision energy for MS/MS analysis was 35%. The dynamic exclusion duration was set to 120 s, the repeat count was 1, and the repeat duration was 60 s.
The obtained raw files were searched with MaxQuant by using a mouse protein database. Cysteine carboxyamidomethylation was set as a static modification, methionine oxidation and acetylation of protein N-term were set as variable modifications. The maximum number of missed trypsin cleavages was set to 2. Precursor ion mass tolerances were 10 ppm, and the fragment ion mass tolerance was 0.8 Da. The false discovery rate (FDR) for peptide, and protein and protein phosphorylation sites were all set as <1% and a minimum length of six amino acids was used for peptide identification. A peptide with a Mascot score ≥25 was selected for protein identification. Proteins were identified with at least one unique peptide. Doublets were selected as the quantification mode with the dimethyl Lys 0 and N-term 0 as light labels and dimethyl Lys 4 and N-term 4 as heavy labels. All other parameters are the default setting in MaxQuant. For protein quantification, the average of all quantified peptides was calculated.
2.11 Quantification of cholesterol
Cholesterol and cholesterol esters in parent and acquired RAW 264.7 cells (1 × 106) were extracted with 200 μL mixture of chloroform, isopropanol and alkyl phenyl polyoxyethylene ether (NP-40) (CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA
IPA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NP-40, 7
NP-40, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1). After centrifugation at 15
0.1). After centrifugation at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g for 5 min, the supernatant was transferred into a new tube and dried at 50 °C under vacuum. To detect total cholesterol, the samples were resuspended in 44 μL of cholesterol assay buffer to hydrolyze cholesterol ester with 2 μL of cholesterol enzyme, followed by incubation at 37 °C for 30 min. The absorbance of the samples was measured at 450 nm using a microplate reader and cholesterol was quantified by plotting against the standard curve.
000 × g for 5 min, the supernatant was transferred into a new tube and dried at 50 °C under vacuum. To detect total cholesterol, the samples were resuspended in 44 μL of cholesterol assay buffer to hydrolyze cholesterol ester with 2 μL of cholesterol enzyme, followed by incubation at 37 °C for 30 min. The absorbance of the samples was measured at 450 nm using a microplate reader and cholesterol was quantified by plotting against the standard curve.
2.12 Statistical analysis
All of the results are shown as mean ± SD. The statistical significance was evaluated using one-way ANOVA or two-tailed Student's T test. Unless otherwise noted, at least three independent experiments were performed, and statistical significances were set at p < 0.05.3. Results
3.1 Physicochemical characterization of ASiNPs
Two ASiNPs (named as ASiNP1 and ASiNP2) were selected to study their effects after long-term exposure on macrophage cells. These two nanoparticles displayed uniform spherical morphologies with mesoporous holes at 5–10 nm (Fig. 1). Their primary sizes, hydrodynamic diameters and zeta potentials were fully characterized. As shown in Table 1, the primary sizes of ASiNP1 and ASiNP2 were 50–100 nm and 400–450 nm, respectively. These two particles could be well dispersed in DI water and cell culture media, evidenced by only 15–32% increase of hydrodynamic sizes. The zeta potentials of ASiNP1 and ASiNP2 in DI H2O were all negative, with values of −25 to −22 mV, but increased to −10 to −8 mV in cell culture media due to the adsorption of proteins on the particle surface.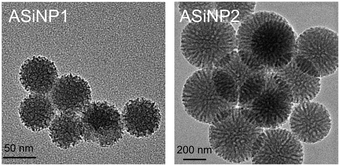 | ||
| Fig. 1 Morphological images of detected ASiNPs using TEM. A drop of ASiNP suspension (50 μg mL−1) was placed on a grid, and then dried at room temperature for TEM observation. | ||
| ASiNPs | Hydrodynamic diameter (nm) | Zeta-potential (mV) | ||
|---|---|---|---|---|
| Water medium | DMEM medium | Water | DMEM | |
| ASiNP1 | 86.5 ± 3.8 | 114.7 ± 1.0 | −24.9 ± 0.5 | −9.6 ± 0.3 |
| ASiNP2 | 413.9 ± 22.9 | 475.0 ± 15.2 | −21.6 ± 0.1 | −8.1 ± 0.5 |
3.2 Cellular internalization of ASiNPs
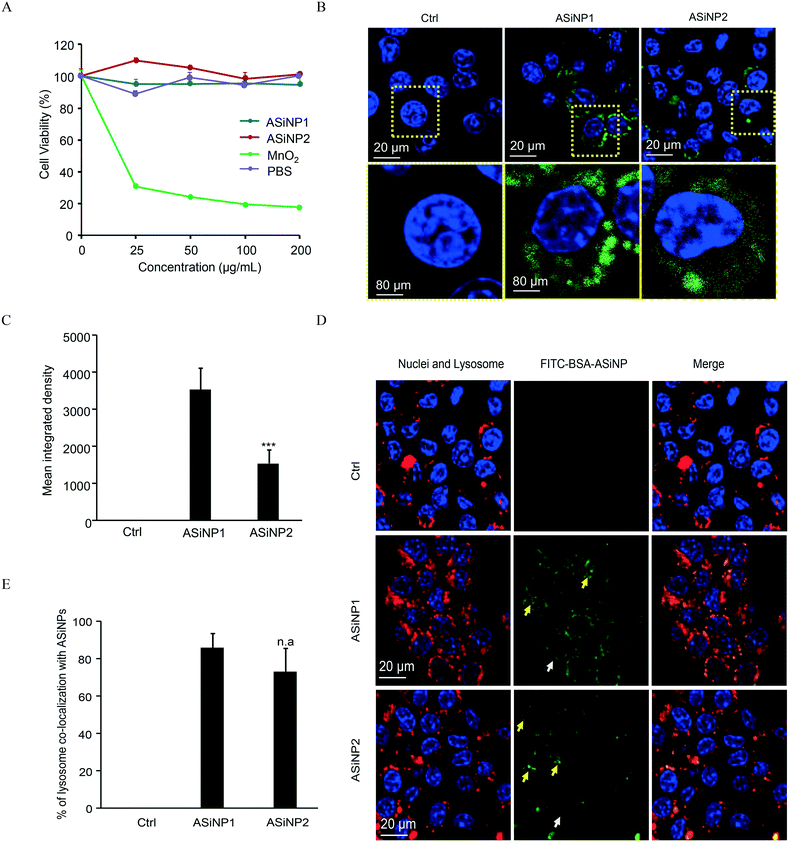
The lungs and gastrointestinal tract are two major target organs for environmentally released nanoparticles.40,44,45 Substantial reports showed that fine particulates may induce inflammations in these organs due to the recruitment of immune cells.46–49 Among them, macrophage is the major cell line responsible for the clearance of these external nanosized stimuli.30,48,50,51 Therefore, macrophages were selected for long-term exposure. To select a suitable dosage for long-term exposure, we assessed the acute cytotoxicity of the two ASiNPs in RAW 264.7 cells. Both nanoparticles had limited cytotoxic effects in a wide dose range of 0–200 μg mL−1 (Fig. 2A). MnO2 has been found to display high toxicity in previous studies and was used as the positive control.49,50 Considering the exposure doses of ASiNPs in real scenarios, RAW 264.7 cells were then treated with 100 μg mL−1 nanoparticles for long-term exposure. We first compared the cellular uptake of ASiNP1 and ASiNP2. As shown in Fig. 2B and C, both nanoparticles labeled with FITC-BSA were present in cells as green dots using confocal microscopy. RAW 264.7 cells had better appetites for smaller sized ASiNP1, displaying ca. 2.3-folder higher fluorescence intensity than ASiNP2 treatment. The higher cellular uptake of ASiNP1 was confirmed by quantification of intracellular silica contents using ICP-OES (Fig. S1†). While the intracellular ASiNP1 was 45.89 pg per cell after 24 h incubation, the amount of ASiNP2 was 21.41 pg per cell. Then we determined the cellular distribution of ASiNPs by extensive examination of their co-localization with subcellular organelles, including membranes, lysosomes, mitochondria, autophagosomes, etc. (Fig. S2†). Notably, both ASiNPs had nice co-localizations with lysosomes indexed with red dots, evidenced by >70% overlap by ImageJ analysis (Fig. 2D and E). These results indicated that ASiNPs displayed size-dependent cellular internalizations but had little difference in their distribution patterns.3.3 Impacts of ASiNPs on lysosomes after long-term exposure
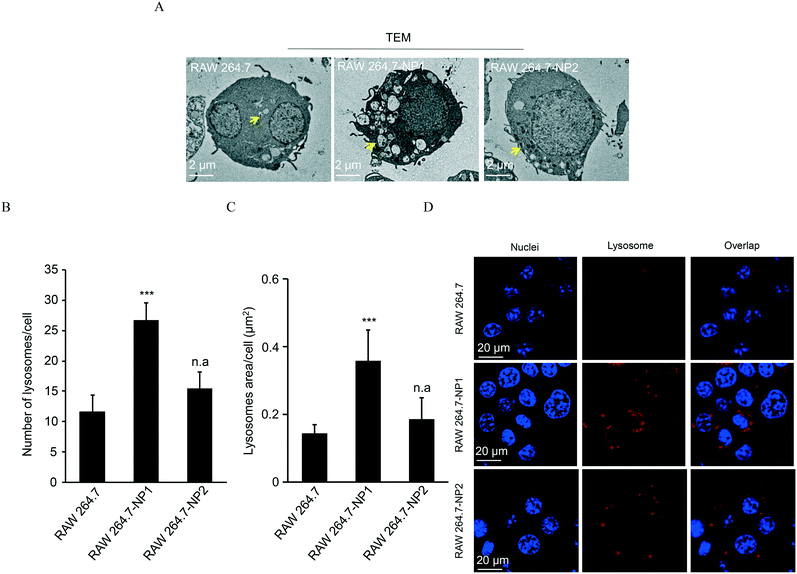
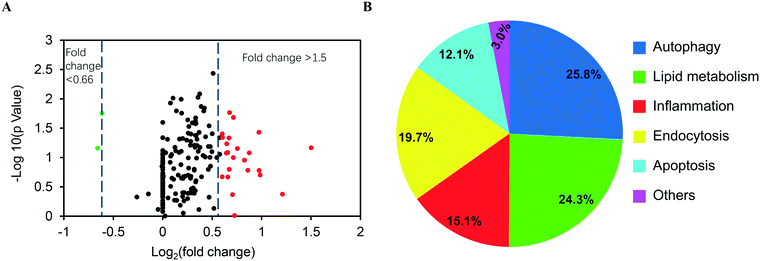
Since ASiNPs are mainly distributed in lysosomes, we were interested to see if long-term ASiNP exposure elicited changes in lysosomes. For this purpose, RAW 264.7 cells were exposed repeatedly to 100 μg mL−1 ASiNPs for 50 passages to acquire RAW 264.7-NP1 and RAW 264.7-NP2 cells. After three additional passages in ASiNP-free media, we examined the density and morphological changes of lysosomes in the acquired cells by TEM. Since lysosomes are full of acidic and enzymatic juices to digest aged biomolecules and/or organelles, these compartments displayed a lower electron-density than other organelles. As shown in Fig. 3A, TEM detected a significant lysosomal density increase in RAW 264.7-NP1 cells when compared to parent cells. An average of 27 lysosomes per RAW 264.7-NP1 cell was counted by statistical analysis of the lysosome numbers in the TEM images, 2.3-fold higher than RAW 264.7 cells (Fig. 3B). Besides density changes, lysosomes in RAW 264.7-NP1 cells had a larger area and were more heterogeneous than those in parent cells. We measured the mean area of lysosomes by TEM as described in previous studies.52,53 As a result, RAW 264.7-NP1, RAW 264.7-NP2 and parent cells showed average lysosomal sizes at 0.36 μm2, 0.18 μm2 and 0.14 μm2, respectively (Fig. 3C). To further validate this, the lysosomes in the acquired cells were stained with LysoTracker DND-99 for confocal imaging. Consistently, dramatic increments of lysosome numbers were detected in RAW 264.7-NP1 cells (Fig. 3D).Given the morphology and density changes in RAW 264.7-NP1 cells, we speculated that long-term exposure to ASiNPs may affect the biological function of lysosomes. To confirm this, the lysosomes in RAW 264.7-NP1 cells were isolated by ultracentrifugation and subjected to proteomics analysis since lysosomal proteins often serve as executors of signalling pathways related to various cellular processes.54 Proteins extracted from lysosomes were labelled with stable isotopes as described in the Materials and methods section. The isotope-labeled protein samples were analysed by nano-LC-MS/MS and 225 lysosomal proteins were quantified. To discover the proteins associated with the adverse outcomes of long-term ASiNP1 exposure, we calculated the fold changes of identified proteins between RAW 264.7-NP1 and RAW 264.7 cells. Consequently, 25 differential proteins with fold changes >1.5 or <0.66, were identified. As shown in Fig. 4A, long-term ASiNP1 exposure induced the up-regulation of 23 proteins and down-regulation of 2 proteins. Then, we investigated the molecular functions and related cellular processes of the 25 proteins by literature research and database search in KEGG, UniprotKB, etc. These proteins were involved mainly in five biological processes, including autophagy, lipid metabolism, inflammation, endocytosis, apoptosis, etc. (Fig. 4B and Table S1†). Among the 25 changed proteins, 17 of them were associated with autophagy pathways, and 16 were involved in lipid metabolism (Table S1†). Altogether, these results indicated that long-term ASiNP1 exposure could significantly increase the average size and density of lysosomes and likely affected autophagy and lipid metabolism processes.
3.4 Examination of autophagic flux and cholesterol degradation in RAW 264.7-NP1 cells
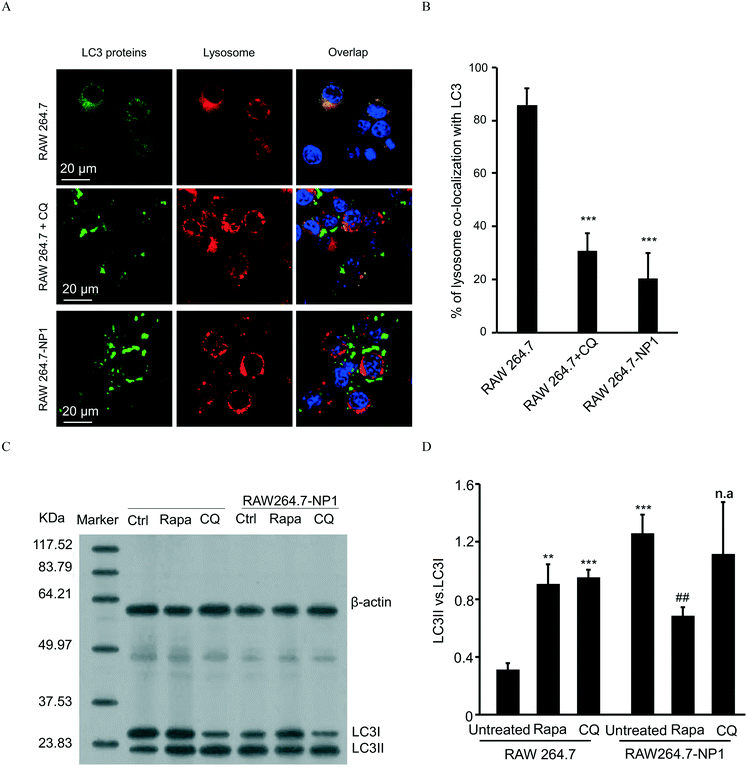
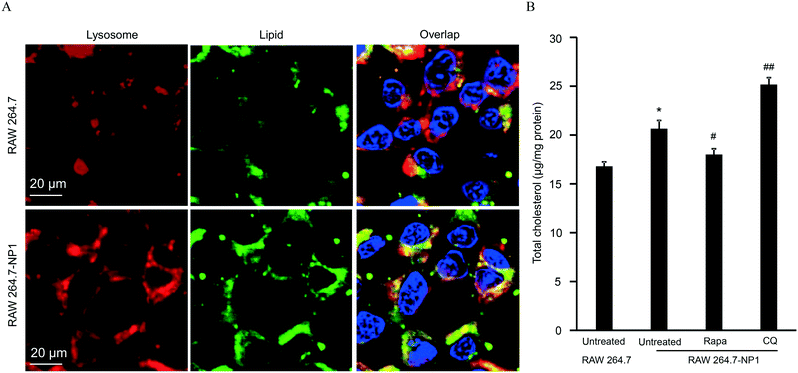
Autophagy is well-known as a self-degradative process, during which subcellular protein and organelles are wrapped in a double membrane organelle known as the autophagosome.55 The formation of autophagosomes involves ubiquitination of protein complexes and lipidation of LC3 protein complexes in the cytosol.56 After packing biomolecules and organelles, autophagosomes are then fused with lysosomes, leading to the degradation of cytosolic components.57 Disruption of the autophagic flux can result in assembly of autophagosomes and lipidated LC3II complexes. To assess this effect, LC3 complexes were stained with DyLight 488 (green)-labeled anti-LC3 antibodies and visualized by confocal microscopy. As shown in Fig. 5A, autophagosomes were significantly increased in RAW 264.7-NP1 cells compared to the control. Moreover, the co-localization between LC3 complexes and lysosomes was obviously lower in RAW 264.7-NP1 cells than that in the control, indicating a blockage of the fusion process between autophagosomes and lysosomes (Fig. 5B). Immunoblotting was used to further assess the level of lipidated LC3II complexes in RAW 264.7-NP1 cells. As shown in Fig. 5C and D, western blotting confirmed that the LC3II level was upregulated in the RAW 264.7-NP1 cells, which is similar to cells treated with the well-known autophagy inhibitor chloroquine (CQ). These results suggested that long-term exposure to ASiNP1 may affect the fusion of autophagosomes and lysosomes, resulting in a blockage of autophagic flux.As lysosomes play a key role in regulating the metabolism of low-density lipoprotein cholesterol (LDL-C),58 we speculated that long-term exposure to ASiNPs may also affect the degradation of LDL-C. To examine this, LDL-C and lysosomes were labeled with BODIPY (blue) and LysoTracker DND-99 (red), respectively, for confocal imaging. As shown in Fig. 6A, the increased level of LDL-C could be observed in RAW 264.7-NP1 cells by confocal microscopy. The majority of total LDL-C co-localized with lysosomes in the RAW 264.7-NP1 cells. Meanwhile, cellular cholesterol was measured using a commercial assay kit. The results showed that the level of total cholesterol was significantly higher in RAW 264.7-NP1 cells than that in control cells (Fig. 6B). Interestingly, 12 of the changed proteins identified in proteomic analysis were found to be associated with both autophagy and lipid metabolism (Table S1†). We therefore speculated that the blockage of autophagic flux may be closely related to the alteration of lipid metabolism. To examine this, we measured the cholesterol levels in RAW 264.7-NP1 cells pre-treated with rapamycin and CQ for acceleration and blockage of autophagic flux, respectively. As shown in Fig. 6B, the increase of cellular cholesterol in RAW 264.7-NP1 cells could be reversed by introduction of rapamycin. In contrast, treatment with autophagy inhibitor CQ could further increase the level of cellular cholesterol. Thus, we concluded that the blockage of autophagic flux may inhibit lipid metabolism in lysosomes and result in the accumulation of total cholesterol in cells.
4. Discussion
ASiNPs can adsorb and/or entrap large amounts of molecules due to their large surface area and tuneable pores, and thus are ideal additive materials in industries, especially in foods and cosmetics. The wide use of ASiNPs in food production and storage increased their exposure risks in the gastrointestinal (GI) tract.59 It has been reported that the daily intake of silica NPs through oral administration is up to 1.8 mg kg−1,60,61 and all these particles can reach the small intestine.62 Assuming that ASiNPs are homogeneously distributed in the intestine and cell culture media and the surface area of the human small intestine is 2 × 106 cm2, the corresponding in vitro dose (96-well plates, 100 μL per well, 0.32 cm2 area per well) for a 50 kg person exposed to silica NPs daily over a 5-year time period could be calculated according to an established method.40,63 Based on this calculation approach, in vitro doses at 0–262.8 μg mL−1 are comparable to the real exposure levels of ASiNPs. Therefore, the in vitro (100 μg mL−1) dose used in this study is relevant to possible oral exposure in real life.Despite generally being recognized as “biocompatible and safe”, the adverse effects of ASiNPs were also reported in a few reports. Studies have shown acute exposure to ASiNPs could induce a few routine hazard signals, such as reactive oxygen species (ROS) generation,61 pro-inflammatory cytokine release,64etc. These conflicted results could be attributed to the residual template molecules in ASiNP synthesis, exposure doses, cell models, and surface functionalities. Compared to these toxicity studies, our study focused on the impacts of long-term exposure on lysosomes in macrophage-like cells. We found ASiNP treatment disrupted the lysosome homeostasis and autophagic flux, which may lead to the accumulation of cholesterol in cells. It is well known that extracellular lipoproteins containing cholesterol are internalized via endocytosis and delivered to lysosomes.43 The lipoproteins are then degraded by acid lipases in lysosomes and the lipid moieties including free cholesterol are released into cytoplasm for utilization or stored as cholesterol esters in lipid droplets.43 The lipid droplets can be removed by the autophagic pathway via a process recently termed as “lipophagy”.65 This process involves the engulfing of small lipid droplets or sequestering parts of large lipid droplets by LC3II positive membranes to form autophagosomes and subsequently delivering the lipid cargo to lysosomes for degradation and cholesterol efflux.58 Therefore, inhibition of autophagy could block the lipophagy and cholesterol metabolism in cells. The hydrolysis of lipid droplets and cholesterol efflux in macrophages is important for reverse cholesterol transport in atherosclerotic plaques. Blockage of lipid metabolism and accumulation of cholesterol may elicit inflammatory pathways and increase the formation of macrophage foam cells. Macrophage cells become foamy with the increase of cytoplasmic lipid droplets where the accumulated cholesterol ester was stored. Foam cells may secrete various substances such as cytokines, growth factors, pro-oxidants, and matrix-degrading metallo-proteases that are thought to be involved in plaque growth.66 On the other hand, excessive free cholesterol in the cells may lead to the formation of cholesterol crystals, which are hard to dissolve and remove.66–68 The deposited crystalline cholesterol in atheromatous plaque can further promote the increase of inflammatory response and expansion of the necrotic core.69,70 Therefore, foam cells are recognized as a key component in the initiation and progression of atherosclerosis in mammals.71
5. Conclusion
In summary, the effects of long-term ASiNP exposure on lysosomes were assessed in RAW 264.7 cells. ASiNP treatment induced lysosomal density and size increments as well as 25 protein changes. These proteins were involved in processes, especially for autophagy and lipid metabolism. These two hazard effects were validated in cells by imaging autophagosomes and quantification of cholesterol levels. Notably, long-term exposure elicited significant autophagosome and cholesterol accumulation in cells. Blockage of autophagic flux further aggravated the cholesterol elevation. These findings aroused a rethinking of cytotoxicity for the long-term use of amorphous silica nanoparticles.Author contributions
R. M., X. C., F. W. and R. L. conceived and designed the study. R. M. and X. C. performed most of the experiments and participated in the writing of the manuscript. Y. Z. completed the proteomic analysis. D. W. and H. Z. participated in the studies on confocal imaging. Y. P. and J. J. performed the TEM characterization. S. X. and Q. X. participated in the studies on lysosome enrichment. J. J., W. W. and N. T. participated in the studies on the synthesis of ASiNPs. The writing of this paper was led by R. L. with the participation of R. M. and X. C.Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
This work was supported by the grants from the National Key R&D Program of China (2018YFE0120400, 2020YFA0710700), the National Natural Science Foundation of China (No. 21804096, 21976126), the Key Project of Social Development of Jiangsu Province (BE2018653), and the Natural Science Foundation of Jiangsu Province (No. BK20180840).References
- Y. H. Gao, Y. H. Zhang, S. He, Y. N. Xiao, X. Y. Qin, Y. Zhang, D. L. Li, H. J. Ma, H. You and J. H. Li, Fabrication of a hollow mesoporous silica hybrid to improve the targeting of a pesticide, Chem. Eng. J., 2019, 364, 361–369 CrossRef CAS.
- M. Mesa, Chitosan and silica as dietary carriers: Potential application for beta-galactosidase, silicon and calcium supplementation, Food Hydrocolloids, 2020, 108, 106067 CrossRef CAS.
- C. Contado, J. Mejia, O. L. Garcia, J. P. Piret, E. Dumortier, O. Toussaint and S. Lucas, Physicochemical and toxicological evaluation of silica nanoparticles suitable for food and consumer products collected by following the EC recommendation, Anal. Bioanal. Chem., 2016, 408, 271–286 CrossRef CAS PubMed.
- J. G. Croissant, Y. Fatieiev, A. Almalik and N. M. Khashab, Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications, Adv. Healthcare Mater., 2018, 7, 1700831 CrossRef PubMed.
- Z. Li, Y. T. Zhang and N. P. Feng, Mesoporous silica nanoparticles: synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery, Expert Opin. Drug Delivery, 2019, 16, 219–237 CrossRef CAS PubMed.
- F. Q. Tang, L. L. Li and D. Chen, Mesoporous Silica Nanoparticles: Synthesis, Biocompatibility and Drug Delivery, Adv. Mater., 2012, 24, 1504–1534 CrossRef CAS PubMed.
- Z. Zhang, H. M. Shen, Q. F. Zhang and C. N. Ong, Involvement of oxidative stress in crystalline silica-induced cytotoxicity and genotoxicity in rat alveolar macrophages, Environ. Res., 2000, 82, 245–252 CrossRef CAS PubMed.
- Z. Q. Chu, Y. J. Huang, L. L. Li, Q. Tao and Q. Li, Physiological pathway of human cell damage induced by genotoxic crystalline silica nanoparticles, Biomaterials, 2012, 33, 7540–7546 CrossRef CAS PubMed.
- P. J. A. Borm, P. Fowler and D. Kirkland, An updated review of the genotoxicity of respirable crystalline silica, Part. Fibre Toxicol., 2018, 15, 23 CrossRef PubMed.
- C. Pavan, R. Santalucia, R. Leinardi, M. Fabbiani, Y. Yakoub, F. Uwambayinema, P. Ugliengo, M. Tomatis, G. Martra, F. Turci, D. Lison and B. Fubini, Nearly free surface silanols are the critical molecular moieties that initiate the toxicity of silica particles, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 27836–27846 CrossRef CAS PubMed.
- R. R. Wu, J. Hogberg, M. Adner, P. Ramos-Ramirez, U. Stenius and H. Y. Zheng, Crystalline silica particles cause rapid NLRP3-dependent mitochondrial depolarization and DNA damage in airway epithelial cells, Part. Fibre Toxicol., 2020, 17, 39 CrossRef CAS PubMed.
- T. Skuland, M. Lag, A. C. Gutleb, B. C. Brinchmann, T. Serchi, J. Ovrevik, J. A. Holme and M. Refsnes, Pro-inflammatory effects of crystalline- and nano-sized non-crystalline silica particles in a 3D alveolar model, Part. Fibre Toxicol., 2020, 17, 13 CrossRef CAS PubMed.
- V. Hornung, F. Bauernfeind, A. Halle, E. O. Samstad, H. Kono, K. L. Rock, K. A. Fitzgerald and E. Latz, Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization, Nat. Immunol., 2008, 9, 847–856 CrossRef CAS PubMed.
- C. C. Leung, I. T. S. Yu and W. H. Chen, Silicosis, Lancet, 2012, 379, 2008–2018 CrossRef CAS.
- S. L. Cassel, S. C. Eisenbarth, S. S. Iyer, J. J. Sadler, O. R. Colegio, L. A. Tephly, A. B. Carter, P. B. Rothman, R. A. Flavell and F. S. Sutterwala, The Nalp3 inflammasome is essential for the development of silicosis, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9035–9040 CrossRef CAS PubMed.
- E. Hnizdo and V. Vallyathan, Chronic obstructive pulmonary disease due to occupational exposure to silica dust: a review of epidemiological and pathological evidence, Occup. Environ. Med., 2003, 60, 237–243 CrossRef CAS PubMed.
- L. Kachuri, P. J. Villeneuve, M. E. Parent, K. C. Johnson, S. A. Harris and C. C. Registries, Occupational exposure to crystalline silica and the risk of lung cancer in Canadian men, Int. J. Cancer, 2014, 135, 138–148 CrossRef CAS PubMed.
- O. Wong, The epidemiology of silica, silicosis and lung cancer: Some recent findings and future challenges, Ann. Epidemiol., 2002, 12, 285–287 CrossRef PubMed.
- P. J. A. Borm, L. Tran and K. Donaldson, The carcinogenic action of crystalline silica: A review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism, Crit. Rev. Toxicol., 2011, 41, 756–770 CrossRef CAS PubMed.
- X. M. Zang, J. M. Li, Y. Wang, Y. Y. Liu, Z. B. Wei, L. Y. Yang and A. J. Miao, A facile method to study the bioaccumulation kinetics of amorphous silica nanoparticles by quantum dot embedding, Environ. Sci.: Nano, 2018, 5, 2830–2841 RSC.
- B. Grotz, M. Geppert, R. Mills-Goodlet, S. Hofer, N. Hofstatter, C. Asam, A. Feinle, K. Kocsis, T. Berger, O. Diwald, N. Husing, M. Wallner, F. Ferreira, A. Duschl and M. Himly, Biologic effects of nanoparticle-allergen conjugates: time-resolved uptake using an in vitro lung epithelial co-culture model of A549 and THP-1 cells, Environ. Sci.: Nano, 2018, 5, 2184–2197 RSC.
- Q. J. He, J. L. Shi, F. Chen, M. Zhu and L. X. Zhang, An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles, Biomaterials, 2010, 31, 3335–3346 CrossRef CAS PubMed.
- H. A. Meng, M. Liong, T. A. Xia, Z. X. Li, Z. X. Ji, J. I. Zink and A. E. Nel, Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line, ACS Nano, 2010, 4, 4539–4550 CrossRef CAS PubMed.
- H. A. Meng, M. Xue, T. A. Xia, Y. L. Zhao, F. Tamanoi, J. F. Stoddart, J. I. Zink and A. E. Nel, Autonomous in Vitro Anticancer Drug Release from Mesoporous Silica Nanoparticles by pH-Sensitive Nanovalves, J. Am. Chem. Soc., 2010, 132, 12690–12697 CrossRef CAS PubMed.
- H. Meng, S. Yang, Z. X. Li, T. Xia, J. Chen, Z. X. Ji, H. Y. Zhang, X. Wang, S. J. Lin, C. Huang, Z. H. Zhou, J. I. Zink and A. E. Nel, Aspect Ratio Determines the Quantity of Mesoporous Silica Nanoparticle Uptake by a Small GTPase-Dependent Macropinocytosis Mechanism, ACS Nano, 2011, 5, 4434–4447 CrossRef CAS PubMed.
- J. G. Croissant, K. S. Butler, J. I. Zink and C. J. Brinker, Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications, Nat. Rev. Mater., 2020, 5, 886–909 CrossRef CAS.
- Y. S. Lin and C. L. Haynes, Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS PubMed.
- H. Y. Zhang, D. R. Dunphy, X. M. Jiang, H. Meng, B. B. Sun, D. Tarn, M. Xue, X. Wang, S. J. Lin, Z. X. Ji, R. B. Li, F. L. Garcia, J. Yang, M. L. Kirk, T. Xia, J. I. Zink, A. Nel and C. J. Brinker, Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic, J. Am. Chem. Soc., 2012, 134, 15790–15804 CrossRef CAS PubMed.
- M. Liong, J. Lu, M. Kovochich, T. Xia, S. G. Ruehm, A. E. Nel, F. Tamanoi and J. I. Zink, Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery, ACS Nano, 2008, 2, 889–896 CrossRef CAS PubMed.
- R. B. Li, Z. X. Ji, H. Q. Qin, X. D. Kang, B. B. Sun, M. Y. Wang, C. H. Chang, X. Wang, H. Y. Zhang, H. F. Zou, A. E. Nel and T. Xia, Interference in Autophagosome Fusion by Rare Earth Nanoparticles Disrupts Autophagic Flux and Regulation of an Interleukin-1 beta Producing Inflammasome, ACS Nano, 2014, 8, 10280–10292 CrossRef CAS PubMed.
- The U. S. Food and drug administration, https://clinicaltrials.gov/ct2/show/NCT02106598?term=cornell+dot&draw=2&rank=1, (accessed 11/20/2020, 2020).
- W. S. Wang, X. T. Sun, H. L. Zhang, C. Yang, Y. Liu, W. L. Yang, C. F. Guo and C. S. Wang, Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia-reperfusion injury, Int. J. Nanomed., 2016, 11, 3255–3263 CrossRef CAS PubMed.
- Y. Zhang, Y. He, C. X. Shi, M. D. Sun, C. Yang, H. J. Li, F. M. Chen, Z. M. Chang, X. Zheng, Z. Wang, W. F. Dong, J. J. She and D. Shao, Tannic Acid-Assisted Synthesis of Biodegradable and Antibacterial Mesoporous Organosilica Nanoparticles Decorated with Nanosilver, ACS Sustainable Chem. Eng., 2020, 8, 1695–1702 CrossRef.
- C. Wu, Y. Zhu, T. Wu, L. Wang, Y. Yuan, J. Chen, Y. Hu and J. Pang, Enhanced functional properties of biopolymer film incorporated with curcurmin-loaded mesoporous silica nanoparticles for food packaging, Food Chem., 2019, 288, 139–145 CrossRef CAS PubMed.
- Z. Lu, T. Zhang, J. Yang, J. Wang, J. Shen, X. Wang, Z. Xiao, Y. Niu, G. Liu and X. Zhang, Effect of mesoporous silica nanoparticles-based nano-fragrance on the central nervous system, Eng. Life Sci., 2020, 20, 535–540 CrossRef CAS PubMed.
- Y. X. Bao, X. M. Yan, W. Du, X. N. Xie, Z. Q. Pan, J. L. Zhou and L. S. Li, Application of amine-functionalized MCM-41 modified ultrafiltration membrane to remove chromium (VI) and copper (II), Chem. Eng. J., 2015, 281, 460–467 CrossRef CAS.
- X. Wang, Y. He, Y. Ma, J. Liu, Y. Liu, Z.-A. Qiao and Q. Huo, Architecture of yolk-shell structured mesoporous silica nanospheres for catalytic applications, Dalton Trans., 2018, 47, 9072–9078 RSC.
- F. Boccuni, R. Ferrante, F. Tombolini, C. Natale, A. Gordiani, S. Sabella and S. Iavicoli, Occupational exposure to graphene and silica nanoparticles. Part I: workplace measurements and samplings, Nanotoxicology, 2020, 14, 1280–1300 CrossRef CAS PubMed.
- A. A. Keller and A. Lazareva, Predicted Releases of Engineered Nanomaterials: From Global to Regional to Local, Environ. Sci. Technol. Lett., 2014, 1, 65–70 CrossRef CAS.
- X. M. Cai, X. Liu, J. Jiang, M. Gao, W. L. Wang, H. Z. Zheng, S. J. Xu and R. B. Li, Molecular Mechanisms, Characterization Methods, and Utilities of Nanoparticle Biotransformation in Nanosafety Assessments, Small, 2020, 16, 1907663 CrossRef CAS PubMed.
- R. E. Yanes, D. Tarn, A. A. Hwang, D. P. Ferris, S. P. Sherman, C. R. Thomas, J. Lu, A. D. Pyle, J. I. Zink and F. Tamanoi, Involvement of Lysosomal Exocytosis in the Excretion of Mesoporous Silica Nanoparticles and Enhancement of the Drug Delivery Effect by Exocytosis Inhibition, Small, 2013, 9, 697–704 CrossRef CAS PubMed.
- A. B. D. Nandiyanto, S.-G. Kim, F. Iskandar and K. Okuyama, Synthesis of spherical mesoporous silica nanoparticles with nanometer-size controllable pores and outer diameters, Microporous Mesoporous Mater., 2009, 120, 447–453 CrossRef CAS.
- Y. Meng, S. Heybrock, D. Neculai and P. Saftig, Cholesterol Handling in Lysosomes and Beyond, Trends Cell Biol., 2020, 30, 452–466 CrossRef CAS PubMed.
- Z. Ju, G. F. Ren, M. L. Zhou, J. Jing, J. Xiang, X. D. Liu, R. X. Huang and P. K. Zhou, Exposure to a combination of silica nanoparticles and low-dose radiation aggravates lung fibrosis in mice via gut microbiota modulation, Environ. Sci.: Nano, 2020, 7, 3979–3998 RSC.
- X. M. Cai, J. Dong, J. Liu, H. Z. Zheng, C. Kaweeteerawat, F. J. Wang, Z. X. Ji and R. B. Li, Multi-hierarchical profiling the structure-activity relationships of engineered nanomaterials at nano-bio interfaces, Nat. Commun., 2018, 9, 1–12 CrossRef PubMed.
- C. M. Nogueira, W. M. de Azevedo, M. L. Z. Dagli, S. H. Toma, A. Z. D. Leite, M. L. Lordello, I. Nishitokukado, C. L. Ortiz-Agostinho, M. I. S. Duarte, M. A. Ferreira and A. M. Sipahi, Titanium dioxide induced inflammation in the small intestine, World J. Gastroenterol., 2012, 18, 4729–4735 CrossRef CAS PubMed.
- B. B. Sun, S. Pokhrel, D. R. Dunphy, H. Y. Zhang, Z. X. Ji, X. Wang, M. Y. Wang, Y. P. Liao, C. H. Chang, J. Y. Dong, R. B. Li, L. Madler, C. J. Brinker, A. E. Nel and T. Xia, Reduction of Acute Inflammatory Effects of Fumed Silica Nanoparticles in the Lung by Adjusting Silanol Display through Calcination and Metal Doping, ACS Nano, 2015, 9, 9357–9372 CrossRef CAS PubMed.
- Y. Huang, X. H. Li, S. J. Xu, H. Z. Zheng, L. L. Zhang, J. W. Chen, H. X. Hong, R. Kusko and R. B. Li, Quantitative structure–activity relationship models for predicting inflammatory potential of metal oxide nanoparticles, Environ. Health Perspect., 2020, 128, 067010 CrossRef CAS PubMed.
- H. Y. Zhang, Z. X. Ji, T. Xia, H. Meng, C. Low-Kam, R. Liu, S. Pokhrel, S. J. Lin, X. Wang, Y. P. Liao, M. Y. Wang, L. J. Li, R. Rallo, R. Damoiseaux, D. Telesca, L. Madler, Y. Cohen, J. I. Zink and A. E. Nel, Use of Metal Oxide Nanoparticle Band Gap To Develop a Predictive Paradigm for Oxidative Stress and Acute Pulmonary Inflammation, ACS Nano, 2012, 6, 4349–4368 CrossRef CAS PubMed.
- X. M. Cai, A. Lee, Z. X. Ji, C. Huang, C. H. Chang, X. Wang, Y. P. Liao, T. Xia and R. B. Li, Reduction of pulmonary toxicity of metal oxide nanoparticles by phosphonate-based surface passivation, Part. Fibre Toxicol., 2017, 14, 13 CrossRef PubMed.
- A. Ramsperger, V. K. B. Narayana, W. Gross, J. Mohanraj, M. Thelakkat, A. Greiner, H. Schmalz, H. Kress and C. Laforsch, Environmental exposure enhances the internalization of microplastic particles into cells, Sci. Adv., 2020, 6, eabd1211 CrossRef CAS PubMed.
- G. Huang, F. Zhang, Q. Ye and H. Wang, The circadian clock regulates autophagy directly through the nuclear hormone receptor Nr1d1/Rev-erb and indirectly via Cebpb/(C/ebp) in zebrafish, Autophagy, 2016, 12, 1292–1309 CrossRef CAS PubMed.
- J. Okarmus, H. Bogetofte, S. I. Schmidt, M. Ryding, S. Garcia-Lopez, B. J. Ryan, A. Martinez-Serrano, P. Hyttel and M. Meyer, Lysosomal perturbations in human dopaminergic neurons derived from induced pluripotent stem cells with PARK2 mutation, Sci. Rep., 2020, 10, 10278 CrossRef CAS PubMed.
- A. Ballabio and J. S. Bonifacino, Lysosomes as dynamic regulators of cell and organismal homeostasis, Nat. Rev. Mol. Cell Biol., 2020, 21, 101–118 CrossRef CAS PubMed.
- L. Yu, Y. Chen and S. A. Tooze, Autophagy pathway: Cellular and molecular mechanisms, Autophagy, 2018, 14, 207–215 CrossRef CAS PubMed.
- N. Mizushima and T. Yoshimori, How to interpret LC3 immunoblotting, Autophagy, 2007, 3, 542–545 CrossRef CAS PubMed.
- N. Mizushima, B. Levine, A. M. Cuervo and D. J. Klionsky, Autophagy fights disease through cellular self-digestion, Nature, 2008, 451, 1069–1075 CrossRef CAS PubMed.
- M. Ouimet, V. Franklin, E. Mak, X. H. Liao, I. Tabas and Y. L. Marcel, Autophagy Regulates Cholesterol Efflux from Macrophage Foam Cells via Lysosomal Acid Lipase, Cell Metab., 2011, 13, 655–667 CrossRef CAS PubMed.
- R. Li, K. Navab, G. Hough, N. Daher, M. Zhang, D. Mittelstein, K. Lee, P. Pakbin, A. Saffari, M. Bhetraratana, D. Sulaiman, T. Beebe, L. Wu, N. Jen, E. Wine, C.-H. Tseng, J. A. Araujo, A. Fogelman, C. Sioutas, M. Navab and T. K. Hsiai, Effect of Exposure to Atmospheric Ultrafine Particles on Production of Free Fatty Acids and Lipid Metabolites in the Mouse Small Intestine, Environ. Health Perspect., 2015, 123, 34–41 CrossRef PubMed.
- S. Dekkers, P. Krystek, R. J. B. Peters, D. P. K. Lankveld, B. G. H. Bokkers, P. H. van Hoeven-Arentzen, H. Bouwmeester and A. G. Oomen, Presence and risks of nanosilica in food products, Nanotoxicology, 2011, 5, 393–405 CrossRef CAS PubMed.
- Y. D. Deng, X. D. Zhang, X. S. Yang, Z. L. Huang, X. Wei, X. F. Yang and W. Z. Liao, Subacute toxicity of mesoporous silica nanoparticles to the intestinal tract and the underlying mechanism, J. Hazard. Mater., 2021, 409, 124520 CrossRef PubMed.
- R. Peters, E. Kramer, A. G. Oomen, Z. E. H. Rivera, G. Oegema, P. C. Tromp, R. Fokkink, A. Rietveld, H. J. P. Marvin, S. Weigel, A. A. C. M. Peijnenburg and H. Bouwmeester, Presence of Nano-Sized Silica during In Vitro Digestion of Foods Containing Silica as a Food Additive, ACS Nano, 2012, 6, 2441–2451 CrossRef CAS PubMed.
- W. Wang, Y. Kong, J. Jiang, Q. Xie, Y. Huang, G. Li, D. Wu, H. Zheng, M. Gao, S. Xu, Y. Pan, W. Li, R. Ma, M. X. Wu, X. Li, H. Zuilhof, X. Cai and R. Li, Engineering the Protein Corona Structure on Gold Nanoclusters Enables Red-Shifted Emissions in the Second Near-infrared Window for Gastrointestinal Imaging, Angew. Chem., Int. Ed., 2020, 59, 22431–22435 CrossRef CAS PubMed.
- A. M. Mahmoud, E. M. Desouky, W. G. Hozayen, M. Bin-Jumah, E.-S. El-Nahass, H. A. Soliman and A. A. Farghali, Mesoporous Silica Nanoparticles Trigger Liver and Kidney Injury and Fibrosis Via Altering TLR4/NF-kappa B, JAK2/STAT3 and Nrf2/HO-1 Signaling in Rats, Biomolecules, 2019, 9, 528 CrossRef CAS PubMed.
- C. Ward, N. Martinez-Lopez, E. G. Otten, B. Carroll, D. Maetzel, R. Singh, S. Sarkar and V. I. Korolchuk, Autophagy, lipophagy and lysosomal lipid storage disorders, Biochim. Biophys. Acta, Mol. Cell Biol. Lipids, 2016, 1861, 269–284 CrossRef CAS PubMed.
- E. Trogan, R. P. Choudhury, H. M. Dansky, J. X. Rong, J. L. Breslow and E. A. Fisher, Laser capture microdissection analysis of gene expression in macrophages from atherosclerotic lesions of apolipoprotein E-deficient mice, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 2234–2239 CrossRef CAS PubMed.
- N. Varsano, F. Beghi, N. Elad, E. Pereiro, T. Dadosh, I. Pinkas, A. J. Perez-Berna, X. Jin, H. S. Kruth, L. Leiserowitz and L. Addadi, Two polymorphic cholesterol monohydrate crystal structures form in macrophage culture models of atherosclerosis, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 7662–7669 CrossRef CAS PubMed.
- R. S. Lim, J. L. Suhalim, S. Miyazaki-Anzai, M. Miyazaki, M. Levi, E. O. Potma and J. T. Bruce, Identification of cholesterol crystals in plaques of atherosclerotic mice using hyperspectral CARS imaging, J. Lipid Res., 2011, 52, 2177–2186 CrossRef CAS PubMed.
- P. Duewell, H. Kono, K. J. Rayner, C. M. Sirois, G. Vladimer, F. G. Bauernfeind, G. S. Abela, L. Franchi, G. Nuñez, M. Schnurr, T. Espevik, E. Lien, K. A. Fitzgerald, K. L. Rock, K. J. Moore, S. D. Wright, V. Hornung and E. Latz, NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals, Nature, 2010, 464, 1357–1361 CrossRef CAS PubMed.
- K. J. Moore, F. J. Sheedy and E. A. Fisher, Macrophages in atherosclerosis: a dynamic balance, Nat. Rev. Immunol., 2013, 13, 709–721 CrossRef CAS PubMed.
- A. Lnsis, Atherosclenrosis, Nature, 2000, 407, 233–241 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1en00696g |
| ‡ Equal contributions to this paper. |
| This journal is © The Royal Society of Chemistry 2022 |