Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Emerging investigator series: perspectives on toxicokinetics of nanoscale plastic debris in organisms
Fazel
Abdolahpur Monikh
 *ab,
Martina G.
Vijver
*ab,
Martina G.
Vijver
 c,
Raine
Kortet
c,
Raine
Kortet
 a,
Iseult
Lynch
a,
Iseult
Lynch
 d and
Willie J. G. M.
Peijnenburg
d and
Willie J. G. M.
Peijnenburg
 ce
ce
aDepartment of Environmental & Biological Sciences, University of Eastern Finland, P.O. Box 111, FI-80101 Joensuu, Finland. E-mail: fazel.monikh@uef.fi; f.a.monikh@gmail.com
bDepartment of Experimental Limnology, Leibniz Institute for Freshwater Ecology and Inland Fisheries, Berlin, Germany
cInstitute of Environmental Sciences (CML), Leiden University, P.O. Box 9518, 2300 RA Leiden, Netherlands
dSchool of Geography, Earth and Environmental Sciences, University of Birmingham, Birmingham, B15 2TT, UK
eNational Institute of Public Health and the Environment (RIVM), Center for Safety of Substances and Products, Bilthoven, Netherlands
First published on 30th March 2022
Abstract
Fragmentation of plastic waste in the environment can lead to the formation of nanoscale plastic debris (NPD) of size < 1 μm. Although it is reported that NPD can be taken up by organisms, the current lack of knowledge regarding its toxicokinetics is a problem. It is currently unknown whether/how NPD passes through physiological barriers, and subsequently is biodistributed, biotransformed and/or excreted from organisms. New methods and techniques are being developed at a rapid pace that facilitates gaining insights into the uptake and toxicokinetics of NPD even in complex biotic samples. However, the required knowledge is generated slowly, which hinders environmental risk assessment. In this perspective, we outline the current understanding of the toxicokinetics of NPD in organisms by transferring the acquired knowledge on the toxicokinetics of engineered polymeric NMs to NPD. We briefly discuss the absorption, distribution, metabolism (e.g., biotransformation), and excretion (ADME) of NPD and highlight the knowledge gaps and research required to address them. Building on this, a perspective on toxicokinetics modeling of NPD using physiologically based pharmacokinetic (PBPK) models is presented, discussing the factors that might influence the modeling data and providing recommendations on the factors that need to be considered for developing PBPK models for NPD.
Environmental significancePlastics in the environment can be degraded to nanoscale plastic debris (NPD) with a size smaller than 1 μm. But it is unknown how the physicochemical properties of NPD influence their interaction with organisms and their toxicokinetics in the organisms' body. This study uses the knowledge gained from investigating engineered polymeric nanomaterials for medical purposes to shed some light on the toxicokinetics of aged NPD. The study describes the ADME and behavior of NPD in organisms. This perspective helps further studies to direct their focus toward the existing knowledge gaps. It discusses the limitations of applying toxicokinetic models developed for soluble chemicals for modeling the toxicokinetics of NPD and provides recommendations for adaptions to the current approaches where necessary. |
Introduction
Nanoscale plastic debris (NPD) is small and irregularly shaped pieces of weathered or degraded plastic with a wide range of synthetic polymeric compositions. It is heterogeneous in nature, composed of different polymer types and occurring in different sizes and forms such as particles, films, fibers, and fragments. Currently, there is a debate on defining and classifying NPD, particularly for environmental risk assessment. In this perspective, we define NPD as weathered plastic pieces with a size smaller than 1 μm. Although it is assumed that the majority of plastic particles found in the environment are generated as a result of fragmentation (degradation of the polymeric backbone) and weathering of larger plastics in the environment, polymeric nanomaterials (P-NMs) which are purposefully engineered and functionalized at the nanoscale for different applications e.g., for medical research and for application in paints,1 could also be present in the environment. Most NPD is thus unintentionally present in the environment, and the constituent plastics were never designed to be ingested or internalized by organisms. For two decades researchers have wondered whether the plastic particles present in food, water and the air are able to pass through the physiological barriers of organisms, assuming that they are too large. New results are emerging however, showing that vegetables such as carrots and lettuce are able to take up small sized (0.2 μm) P-NMs.2 It is also expected that NPD is potentially able to penetrate physiological barriers such as the gut barrier3 and enter the organisms' cells and tissues.4 It is, therefore, critical to comprehensively understand the disposition and toxicokinetics of NPD in organisms for understanding the impact of these particles on human health and effects on other organisms.Nevertheless, investigating the toxicokinetics of NPD is a challenging task, mostly due to the limitations in the current analytical techniques in terms of detecting and characterizing relevant concentrations of NPD in organisms' bodies which are complex biological samples.5 Great effort has been made to understand the toxicity of NPD by using P-NMs as models. Despite the analytical limitations, a considerable number of studies have investigated the uptake and toxicokinetics of P-NMs (for example by using fluorescent labels or more recently chemically doped P-NMs), which significantly advanced our understanding of their absorption, distribution, metabolism, and excretion (ADME) within organisms' bodies and revealed many challenges,6 although release of fluorescent labels from particles can confound results.7 Most of these studies are, however, medically oriented, where the particles are directly administered into, e.g., a vein, to have 100% bioavailability. Moreover, such medical P-NMs are designed to have a longer circulation time in the blood and to be biodegradable. In Table 1 we compared some of the expected factors that could influence the toxicokinetics of particles including NPD and P-NMs. A key question concerns the extent to which knowledge of these clinical and spherical P-NMs can be transferred to heterogeneous NPD, which is persistent against biodegradation.
| Factors | Polymeric engineered nanomaterials | Nanoscale plastic debris |
|---|---|---|
| Size | Designed with known size | Highly polydisperse |
| Shape | Designed with known shape | Occurs in various shapes |
| Surface functional groups | Functionalized intentionally for medical applications | Surface functionalization in the environment e.g., natural organic matter or functional groups like hydroxyl |
| Chemical compositions | One type of polymer, e.g., polyethylene glycol, polylactide | Various types of polymers may be co-exposed e.g., PS, PVC |
| Durability | Biodegradable | Non-biodegradable |
| Hydrophobicity | Low hydrophobicity | Various hydrophobicities |
| Additives | No additives | Different metallic and chemical additives |
| Surface charge | Negative surface charge to avoid interactions with biogenic substances in blood circulation | The surface charge depends on the environment in which the particle resides |
| Molar mass | Defined molar masses | Various molar masses |
| Solubility | Soluble in water or undergoes swelling of the polymer | Non-soluble in water and swelling of the polymer unlikely to occur in water |
There are some considerable differences between NPD and P-NMs, particularly regarding their chemistry and shape, which may challenge the direct transfer of knowledge between the two particle types. For example, some polymers like polyvinyl chloride (PVC) undergo photo-aging when exposed to UV irradiation which can lead to generation of hydroxyl radicals.8 Such functional groups like hydroxyl can significantly influence the interaction of the NPD particles with organisms, whereas P-NMs injected directly into the body do not undergo any weathering-induced transformation. Moreover, aged NPD typically contains an increased oxygen content, an altered carbonyl index, and an increased oxygen-to-carbon ratio as a result of more O-containing functional groups and some free volume between molecules, as observed also during weathering of microplastics.9,10 Despite these differences, we hypothesize that some of the knowledge generated on P-NMs and other NMs (e.g., metal-bearing NMs) could be transferred towards understanding and predicting the impacts of NPD and can provide insights into the likely mechanisms of uptake and the biological fate of NPD in organisms in the absence of the required data. We also expect that models that have been applied to date to understand the ADME of small molecules and extrapolated to P-NMs might also be a useful tool for NPD hazard assessment.
This perspective is organized into two sections. In the first section we provide a brief discussion on the potential biological fate and ADME of NPD in vivo by transferring knowledge generated on the biological fate of P-NMs and provide a perspective on research needed to understand the toxicokinetics of heterogeneous NPD. The second section addresses the toxicokinetic modeling of NPD and provides a perspective on the factors to be considered for model development and application for NPD. This perspective excludes polymeric materials that are designed to be biodegradable, such as polyvinyl alcohol, polyethylene glycol, polyvinylpyrrolidone, polyphenylene sulfide, etc.,11 and focuses mainly on the most common polymer types (Table 2) found in the environment.
| Type of polymer | Chemical properties and changes in plastics in the environment | Possible biodegradation processes |
|---|---|---|
| Low density polyethylene (LD-PE) and high-density polyethylene (HD-PE) | - LD-PE: rubbery polymer with a density (0.94 g cm−3) lower than water (freshwater = 0.99 g cm−3 and seawater = 1.02 g cm−3) is exposed to photooxidation in water | - After aging, LD-PE is susceptible to biodegradation by bacteria such as Pseudomonas spp23 and fungi such as Aspergillus and Cladosporium18 |
| - Swelling is unlikely to happen to pristine LD-PE. LD-PE, however, is an oxo-degradable polymer (contains a photosensitizer compound) and can be oxidized using oxidizing agents and some solvents such as acid, which results in softening or swelling of the polymer18 | - Low pH in organism intestines and the presence of hydrochloric acid might facilitate hydrolyzation of LD-PE | |
| - Exhibit a large amount of free volume between molecules, flexible for the diffusion of oxidative agents into the bulk of the polymer | - Worms or larval stages of moths, such as Galleria mellonella and Plodia interpunctella, might biodegrade LDPE and metabolize them to ethylene glycol24,25 | |
| - HD-PE, density (0.97 g cm−3) lower than water and exposed to photooxidation | ||
| - Absorption of sunlight causes cleavage of polymer chains by chain scission | ||
| - In soil, e.g., as mulches, mechanical degradation can create cracks.21 On the surface of soil, it is exposed to photo-thermal oxidation | ||
| - LD-PE has branched chains and a weaker structure compared to HD-PE. HD-PE has a more a compact structure, lower permeability, and fewer accessible active sites.22 Thus, the influence of weathering on LD-PE is expected to be higher than that on HD-PE | ||
| Polyethylene terephthalate (PET) | - Hydrophobic; polymer swelling is unlikely to occur to pristine NPD in water | - It is likely that lipases and esterases can attack specifically carboxylic linkages and biodegrade PET17 |
| - Density (1.38 g cm−3) slightly higher than water and is not exposed to photooxidation for long times | - The bacteria Aspergillus Niger increased the roughness and swelling ability of PET containing 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mmol% of nitroterephthalic acid and decreased its average molecular weight29 mmol% of nitroterephthalic acid and decreased its average molecular weight29 |
|
| - It is a linear (saturated) polyester and a semi crystalline polymer26 | - Activity of the fungi Trichoderma enhanced the enzymatic hydrolysis of PET30 | |
| - Possibility of formation of free radicals due to the presence of carboxylic end groups within PET27 | ||
| - When exposed to UV light, hydroxyl and carboxylic end-groups are produced | ||
| - Thermal degradation results in a decrease in molecular weight and an increase in carboxyl end-groups28 | ||
| Polypropylene | - Highly hydrophobic and no polymer swelling occurs when in water | - Lipase and esterase enzymes may attack the carboxylic linkages of the polymers17 |
| - Rubbery polymer with a density (0.92 g cm−3) lower than water and thus exposed to photooxidation at the water surface | - PP can be biodegraded in the gut of mealworms via gut microbiomes33 | |
| - Exhibits a large amount of free volume between molecules and presents great mobility and flexibility for the diffusion of oxidative agents into the bulk of the polymer | ||
| - It is an oxo-degradable polymer and the photodegradation of PP films can be activated using oxidative agents e.g. metal oxides31 | ||
| - Photo-oxidation can initiate formation of alkyl radicals | ||
| - The reaction of radicals with oxygen leads to formation of hydroperoxide groups | ||
| - Hydroperoxide groups initiate chain scission and chain-branching, and then formation of carbonyl groups on the surface | ||
| - Micro-cracks form on the surface because of weathering32 | ||
| Polystyrene (PS) | - Glassy polymer with a density (1.05 g cm−3) slightly higher than freshwater and lower than seawater and is exposed to photooxidation only in seawater | - Enzymatic degradation and hydrolysis are possible after photo-oxidation due to the presence of oxygenated functional groups |
| - Foamed PS has a density of 0.01 to 0.19 g cm−3 which is lower than water. Thus, foam PS is exposed to photooxidation | - PS with ether linkage is susceptible to monooxygenase attack35 | |
| - Highly hydrophobic and no polymer swelling occurs when in water | - Low pH in intestine and the presence of hydrochloric acid might facilitate degradation of PS27 | |
| - Dense structure with little void space minimizing the diffusion of oxidative agents into the bulk of the polymer | - The bacteria Enterobacter sp., and Alcaligenes sp. can degrade PS36 | |
| - Photo-oxidation can break the chains of PS, containing hydrophilic oxygenated groups34 | ||
| Polyvinyl chloride (PVC) | - Glassy polymer with density (1.38 g cm−3) higher than water and not exposed to photooxidation | - PVC can be biodegraded by the bacterium Pseudomonas putida37 |
| - Highly hydrophobic and no polymer swelling occurs when in water | - Enzymatic degradation is possible due to the presence of hydroxyl functional groups | |
| - Dense structure with little void space and presents higher cohesive forces, minimizing the diffusion of oxidative agents into the bulk of the polymer | - Low pH in the intestine of some organisms can facilitate degradation of PVC with hydroxyl functional groups | |
| - Photo-oxidation of PVC can generate hydroxyl radicals7 | ||
| - Plasticizers make up to 40% of its mass. The presence of plasticizers and stabilizers in PVC increases the possibility of biodegradation | ||
| - Degradation of stabilizers can increase the susceptibility of PVC to oxidation26 | ||
| Polyurethane (PUR) | - Hydrophobic; polymer swelling is unlikely to occur in water | - Papain, proteases and esterase and urease degraded polyester PUR39 – after degradation papain can diffuse into PUR and hydrolyzes the urethane and urea linkages producing free amine and hydroxyl groups, causing breaks in the structural integrity |
| - Available in a wide range of densities (12–30 g cm−3) all much denser than water | - Bacteria such as Pseudomonas sp., and Arthrobacter globiformis degrade PUR | |
| - The chain is not composed exclusively of carbon atoms but rather of heteroatoms, oxygen, carbon and nitrogen which make it susceptible to degradation e.g., to hydrolysis38 | - Fungi such as Stemphylium and Cladosporium degrade PU | |
| - Numerous sites (ester, urethane, urea, and other groups) where a hydrolytic reaction, either chemical or enzymic, can take place | - It is likely that the bond cleaved by endopeptidases is an amide group17 |
Absorption, distribution, metabolism, excretion (ADME) of NPD
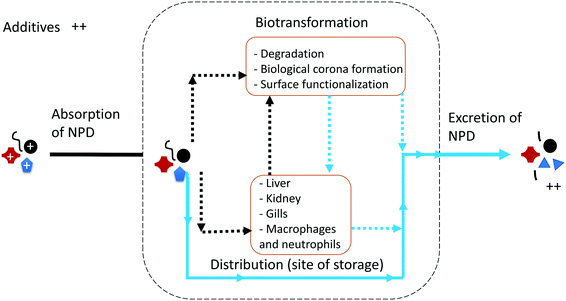
An overview of the expected ADME for NPD is illustrated in Fig. 1, which was generated using data reported for the biological fate of NMs (P-NMs and metal-bearing NMs). Each step is briefly discussed in the next section to highlight the challenges and research needed to fill the knowledge gaps for NPDs.Absorption of NPD
Herein, we consider absorption as the process by which NPD proceeds from the external exposure site into organisms by different routes of exposure. In ecosystems, absorption of NPD might occur via inhalation (e.g., gills), ingestion, and through skin absorption. The absorption is influenced by the physicochemical properties of NPD such as size, shape, surface chemistry and the chemical composition of the particles. Eyes in mammals, and probably in fish, could be a potential pathway for diffusive entry of NPD into the optic nerve directly as reported for certain other substances.12 Ingestion and inhalation are believed to be the most common exposure pathways for NPD, and the respiratory and gastrointestinal tract, which has a large surface area, represents the primary exposure site for NPD.13 Previous studies on nanoparticles have documented the ability of the lung to clear particles either through the immune system or absorption into the blood.14,15 There is no evidence about pulmonary clearance of NPD. Because of their small size, NPD is not ingested intentionally by organisms, as observed for its large microplastic counterparts which are typically mistaken for food by the organisms,16 but they could be ingested unintentionally through dietary uptake of food contaminated with NPD and via drinking contaminated water, or as a result of non-specific recognition resulting from the growth of a biofilm on the plastic surface.The main research question at present is “In what state does NPD reach organisms?”. Before entering organisms, NPD undergoes various abiotic and biotic transformations and degradation reactions, which might bring NPD into a state that is more susceptible to biodegradation in the organisms' bodies and can dramatically change its bioavailability to organisms. In Table 2, we summarize the possible degradation processes and reactions that may happen to NPD before its entry into the organisms' bodies. Aging of NPD is governed by polymer characteristics such as its mobility, crystallinity, the presence and type of additives, molecular weight, degree of cross-linking (bonds that link polymer chains to one another), the type of functional group, etc.17 Polymer chains with high N and O atom contents, such as functional groups containing e.g., ester, amide and other groups, are more susceptible to oxidation and enzymatic degradation than those with mainly C and H. Moreover, polymers with large crystalline domains are less susceptible to degradation because their well-organized molecular frameworks prevent the diffusion of O2, H2O and enzymes into the bulk of NPD.18 As a result of aging, NPD might undergo a significant change in chemical structure under environmental conditions, which leads to the loss of some properties that may vary from polymer to polymer and significantly influence the absorption of NPD by organisms. We recommend that future studies perform aging on NPD before exposure to organisms as this is the main feature differentiating NPD from other P-NMs. Aging can be performed by considering the type of polymer and the possible weathering process that might occur for that polymer (see Table 2). For example, polyethylene (PE), which has a lower density than water, might be more susceptible to photooxidation than PVC, which has a higher density than water and thus might sink to the bottom of the water column (Table 2).
Before entering an organism, NPD encounters the physiological barriers of organisms such as the mucosal barriers of gills, lungs and gut epithelia. Mucus in many vertebrates is similar, consisting of water (∼97%), a complex network of highly branched glycoproteins (such as mucin fibers), lipids, cellular and serum macromolecules, electrolytes, cells, and other cellular debris with highly conserved sialic acid, carboxylic acid, and sulphated residues.19 This complex hydrogel biopolymeric network is the first barrier through which NPD must diffuse before physical contact with the epithelial cell membrane. The interactions of NPD with the mucosal barrier and its effect on the NPD penetration into an organism are highly relevant but remain to be elucidated. The charge distribution within mucus provides a charge selective diffusion barrier, where negatively charged particles can penetrate deeply into this layer19 and positively charged particles are trapped at a shallow depth. Future studies which focus on NPD absorption in organisms should perform comprehensive particle characterization to understand the charge of the particles, for example, by measuring the zeta potential using laser Doppler electrophoresis.
Distribution and biotransformation of NPD
The next step after exposure is the transfer of NPD across the physiological barrier, which consists of endocytosis through the apical membrane into the epithelial cell and then transport through the cell and exocytosis to the blood system, a process called transcytosis. As reported for P-NMs, cellular uptake of NPD is likely to occur through multiple pathways, e.g., clathrin-independent uptake and active energy-dependent processes.40 NPD can bind with different biomolecules in the gut and be absorbed across the epithelium as reported also for ultrafine particles.41 Lessons from NM studies showed that cellular uptake, in general, depends on the physicochemical properties of the pristine particles, like shape, size, and chemical composition, and on their surface chemistry. This knowledge can be extrapolated to NPD with a size smaller than 100 nm analogous to NMs. In addition, it is likely that polymer-dependent mechanical properties of NPD, such as the degree of cross-linking, crystallinity (crystalline or amorphous), glass transition temperature (which determines the glassy or rubbery feature of polymers) and the amounts and types of additives in the plastics may play significant roles in its uptake. For example, polymers with a higher glass transition temperature are much stiffer under physiological conditions and show a higher cellular uptake compared to those with lower glass temperatures which are less stiff.42 It is necessary to perform systematic studies to understand how the physicochemical properties of NPD modulate its cellular uptake into organisms. Eventually, the internalised particles pass into the bloodstream from where they are transported to different organs. Despite numerous studies on the distribution of P-NMs into tissues and organs following direct administration, no investigation is available yet that focuses on biodistribution of NPD. The target organs for NPD could be the liver, kidney and spleen as reported for other P-NMs.43 It is also possible that uptake via phagocytosis, which is mediated by macrophages, leads to NPD accumulation in the reticuloendothelial or in the mononuclear phagocyte system.44 The distribution of NPD might be dependent on the physicochemical properties of the particles and their interaction with the living system including the acquisition and evolution of an eco-corona.45Lessons learned from P-NMs revealed that when particles enter the physiological medium of an organism, they undergo different biotransformations. In a traditional viewpoint, such transformations can be considered as metabolism, which refers to the degradation or enzymatic transformation of a chemical to its metabolites. In the case of metallic NMs, for example, it was reported that silver, cerium oxide and zinc oxide NMs are metabolized in physiological media. This leads to dissolution of the particles which can occur before, during and after uptake and transfer across biological barriers.46 NPD, however, is non-biodegradable in physiological media. It is possible that upon entering the acidic lysosomal environment, additives are released from the particles which can, in turn, weaken the structure of NPD. Moreover, the surface characteristics of NPD can change due to interaction with biomolecules such as proteins and metabolites.47 Particles rapidly adsorb various (bio)molecules on their surface to form a so-called biomolecule ‘corona’, which seems to affect the particle in situ identity and its toxicokinetics.48 The adsorbed corona defines the particle surface and mediates further interactions between the particles and the surrounding microenvironment.49 The biomolecule corona can influence the biodistribution, degradation, biopersistence (the duration that an NPD remains in an organism without being metabolized or excreted from the organism), and clearance of NPD in organisms. For example, the biomolecule corona can cause the indirect transformation of the core particles by altering their colloidal stability e.g., either by inducing steric stabilization, which leads to the stability of the particles, or by protein-mediated bridging, which can lead to agglomeration of the particles. As expected, NPD-biomolecule corona within tissues may reside in the extracellular space, attach to the surface of macrophages or cells of the tissue, or enter the cells. Within the tissues, partitioning of NPD could occur between the body fluid and the cells as a function of the physicochemical properties of NPD and their biomolecule corona. After entering cells, partitioning also may take place between different parts of the cells, e.g., membrane, organelles, lipid, and fluid cytosol.
Excretion of NPD
Like other P-NMs, the physicochemical properties of NPD could influence its excretion (i.e., removal from the body of the organism, with or without degradation). It is reported that P-NMs are mostly excreted from the liver and the kidneys,50 but the mechanism of excretion and the responsible organs for excretion of NPD is yet to be explored.It is known that in general enzymes add polar groups to the hydrophobic molecules and consequently increase the water solubility of the molecules and thus their elimination kinetics from the organisms. Nevertheless, NPD are hydrophobic particles and behave differently to conventional molecules. It means that the excretion of NPD might be different despite having a similar hydrophobic nature to its molecular counterparts. It has been reported that P-NMs can use the hepatic excretion route, which relies on exocytosis and ∼200 nm vesicular trafficking, to undergo clearance. The renal excretion route has also reported for NMs with a hydrodynamic diameter < 100 nm.51 Future studies should investigate whether these approaches could also be used by < 200 nm NPD and whether NPD can pass through the glomerular filter of vertebrate kidneys with a molecular weight cut off of ∼60 kDa. In the case of the gills, where extensive vesicular trafficking is unlikely to happen and whereby fish use branchial excretion by reversing the diffusion gradients of toxicants to the outward direction,14 the excretion of NPD with an enlarged size seems unlikely. We recommend future studies to consider also the biotransformation (e.g., biomolecule corona) of NPD in investigating NPD excretion.
Perspective on toxicokinetic modelling of NPD
Toxicokinetic models can predict the time course of the internal concentration of a pollutant as a function of uptake, distribution, metabolism, and excretion,52 mostly with the assumption of being a highly dissolvable compound. Several toxicokinetic models have been developed for aquatic and terrestrial organisms.53 Nevertheless, application of these equilibrium models to NMs is challenging in general,6,54,55 which we discuss in the next section. In the absence of analytical techniques for understanding the toxicokinetics of NPD, modeling the experimental data could assist in filling the existing knowledge gaps. For example, physiologically based pharmacokinetic (PBPK) models may help to understand the concentration of NPD in tissues and identify factors that influence the NPD distribution in organs and, thus, its potential toxicity. PBPK models have been applied for NMs, for example, cerium oxide56 and silver57 NMs and also for polymeric NMs,6 with relative success. PBPK models may thus assist in ADME and toxicity studies of NPD. These models have been previously described in detail58 and their application for NMs has been evaluated by Yuan et al.6 The strength of PBPK models is that they allow interspecies extrapolation which can help to extrapolate animal data to humans.59Recommendations for toxicokinetics modelling of NPD
Toxicokinetic models are based on steady-state assumptions which consider the equilibrium between accumulation of a compound and its excretion from organisms.60 Lessons from NM modeling studies clearly show that equilibrium assumptions do not hold for NMs, i.e., both the exposure conditions and the biodistribution of NMs, which influence the uptake and excretion of the particles, cannot be considered as constant.61 NPD of the same type may also take different uptake and excretion pathways, rejecting the steady-state assumption. Moreover, there is great diversity in the physicochemical properties of NPD such as particle size, shape, chemistry, surface charge and surface composition. These properties can deviate the modelled data from the experimental data if not fully accounted for. For example, small variations in particle size or charge can significantly alter the systemic disposition of typically heterogeneous NPD. Here we describe some of the factors that might influence the simulation data for NPD and recommend factors that could be considered for PBPK modelling of NPD. Note that in this section we do not propose a way forward in modeling NPD, but rather highlight the parameters that should be considered while developing toxicokinetics models for NPD or extending existing models to cover NPD. We describe some of the assumptions in Table 3 and evaluate the limitations of transferring these assumptions from PBPK of persistent organic pollutants (POPs) and NMs to PBPK of NPD.| Assumption | Limitation of application | Possible modification of the assumption or interim solution |
|---|---|---|
| Concentration of chemicals in the exposure medium and organisms is easily measured or known | No technique or methods are available yet to track and measure NPD in complex exposure media or in the body5 | Development of new methods to measure NPD in exposure medium and the organisms' bodies is required |
| Assumptions based on macrophage loading and endocytosis rates can be applied to estimate the internalized dose | ||
| Mass is applied as the dose metric and the concentration of chemicals is homogeneous in the exposure matrix | Particle number and volume specific surface area can be used as dose metrics for NPD and the concentration of NPD is heterogeneous in the exposure matrix66 | Mass can be used as a dose metric for NPD. By using sonication, a relatively homogeneous dispersion of NPD can be provided for lab-based uptake and biodistribution studies |
| Note that commercial engineered polymeric nanoparticles are not a realistic proxy for NPD particles which are more heterogeneous in shape, size and weathering/transformation, but can provide at least baseline data under idealized conditions | ||
| Unchanged chemicals are taken up | NPD particles have a dynamic behavior in exposure systems, and they change due to environmental interactions including undergoing aggregation67 | Consider NPD of the same size and shape and type in the exposure matrices and stabilize the particles against aggregation and account for any biotransformation by undertaking characterisation of NPD under the exposure conditions over the exposure duration |
| Chemical is eliminated via passive diffusion | The elimination pathway of NPD is unknown. The elimination of NPD depends on the type, size and shape of NPD, pathways | For simplicity this assumption might be transferable to NPD |
| Lab-based depuration studies should be carried out on the exposed organisms. The above note that spherical commercial particles might not translate directly to more irregularly shaped particles and should be considered here also | ||
| Elimination pathways such as macrophage-uptake and shedding of exoskeleton or other species-specific routes may apply and should be explored | ||
| Metabolism of the chemical always follows the same pathway e.g., catalyzed by P450 | NPD particles are covered by a dynamic biomolecule corona and the biotransformation of each type of NPD may differ compared to any other type of NPD68 | Formation of the biomolecule corona can be considered as the main assumption for biotransformation of NPD |
| Enzymatic interactions may also result in degradation of NPD, as is increasingly emerging for carbon family materials such as carbon nanotubes and graphene oxide, wherein internalization by neutrophils leads to degradation through production of extracellular trapping networks containing proteases such as myeloperoxidase. However, the role of the acquired corona also needs to be considered as it may affect enzyme access to the NPD surface69 | ||
| Organic chemicals can be eliminated by receiving functional groups to increase their hydrophilicity | NPD particles are covered by the biological corona and surface modification of NPD might not be possible by enzymes | Formation of the biological corona can increase the hydrophilicity of NPD particles and increase their clearance |
A critical issue is the principle of mass as a dose metric for assessing ADME which is applicable for chemicals and molecules, as the mass and number of molecules are fully proportional. However, in the case of NPD, there is typically a (wide) distribution of particle sizes which breaks the relationship between the mass and the number of particles. Additionally, if degradation occurs, individual particle mass may decrease while the particle number remains roughly constant or increases slightly, although agglomeration may lead to apparent decreases in particle number. The appropriate dose-metric for PBPK modeling of NMs has also been a hotly debated topic. This has consequences for NPD modelling if not considered, because the current PBPK models, in general, are fed with experimental data based on mass as the input. In our opinion, like other particles (e.g., NMs, ultrafine particles, and fine particles), particle number rather than mass is a more appropriate dose metric for assessing the ADME of NPD and could be considered as an input in the models.
Another issue is related to the surface composition of the particles. Although NPD is basically a hydrophobic material and may favor lipid rich organs in the organisms' bodies, weathering processes considerably influence the NPD chemistry. For PBPK modeling, we recommend that NPD is treated as a hydrophilic material rather than hydrophobic because it is covered by proteins in the organisms' bodies and is highly likely to be a water dispersible material after aging and biotransformation. The presence of biomolecules on the surface of particles influences their interactions with the cell membrane, their uptake pathways, and their biodistribution in the organisms' bodies.45,62 Thus, the biomolecule corona is a critical parameter in developing PBPK models for NPD.
The dynamic behavior of NPD in organisms could be a considerable challenge to tackle in model development. Some NPD particles can undergo substantial changes in size when they are present in physiological medium, whether through weight loss and surface topography changes, which might result from the degradation of the polymer and through loss of various additives as a result of polymer swelling.18 This means that they can swell from the original nano-size to even micron size, thus possessing different sizes in the wet and dry states.18,63 Although swelling is not the case for very hydrophobic NPD, it might happen to some types of NPD after aging such as LD-PE and PET (see Table 2). It is also unknown whether the swelling occurs in hydrophobic NPD particles when they reside in lipid-rich organs or hydrophobic microenvironments such as between the phospholipid bilayers of cell membranes.
Enzymes have relatively high molecular weights at several kDa, which make them unable to penetrate the polymer matrix, particularly for polymers with a high degree of crystallinity. Thus, enzymatic hydrolysis occurs typically via surface erosion.64 Some NPD particles have free volume (up to a few nanometers) between their polymer chains, whether due to application purposes (e.g. LD-PE) of the plastic, or due to weathering in the environment, and some also have internal nanosized pores (e.g., glassy polymers such as PVC and PS). For example, weathering due to ultraviolet radiation, which mostly occurs in marine plastic debris, causes bond cleavages in the polymeric matrix and thus the formation of cracks.65 The porosity may increase the diffusion of enzymes and metabolites as well as oxidative agents into the NPD matrix and may accelerate the degradation of NPD. This is expected to take place in physiological media where a high concentration of diffusing biomolecules is present.
The distribution of NPD after uptake and partitioning into different organs must be known for PBPK modelling. Thus, whether accumulation in secondary organs such as the liver and kidneys, which are mainly responsible for the metabolism of chemicals, occurs in the case of NPD must be explored. The accumulation and biotransformation of NPD therefore need to be considered to facilitate the excretion kinetics, which is important in the context of understanding the elimination and biopersistence of NPD. The biodistribution of NPD could be size dependent, as reported for NMs, which highlights the importance of particle size in the modeling. The point is not to develop a model working solely for NPD with a size smaller than 1 μm but to develop models or extend the existing models to cover the proposed size ranges of heterogeneous NPD. Note that this size range is arbitrarily proposed by the scientific community and the model does not need to be limited to this range of sizes, but it must cover this size. Even more precisely, the developed models should cover the biologically relevant size of NPD, i.e., particle sizes that can be taken up by organisms and biodistributed in the organisms' bodies to reach different target organs.
General conclusions
An increasing number of reports show that NPD is potentially able to penetrate biological barriers, leaving no doubt that NPD is an emerging environmental pollutant of high concern. Nevertheless, the existing knowledge gaps regarding uptake pathways, biodistribution, biotransformation, storage and clearance are hindering proper environmental risk assessment and adaptation of suitable mitigation strategies. Complete risk assessment might not be possible until a fit-for-purpose methodology is available to allow tracking, quantification, and characterization of NPD in the environment and in biota. It is necessary to develop suitable methodologies to facilitate understanding and prediction of the toxicokinetics of NPD. Once suitable methods are available, it will be possible to explore how the physicochemical properties of NPD and its aging in the environment influence its toxicokinetics. This would be a fruitful avenue to ultimately better understand the magnitude of the ecological and evolutionary threats that NPD may pose to ecosystems. In summary, NPD undergoes different aging processes which make it susceptible to further degradation and biodegradation whether in the environment or in biota. We recommend that future studies utilise aged NPD for understanding the toxicology and toxicokinetics of NPD rather than pristine P-NMs which are not representative of the particles actually present in the environment and those that interact with physiological barriers.Previously, researchers faced the same problems in performing risk assessment of non-soluble chemical substances of emerging concern. Although such persistent particles violate the basic assumption of being soluble, toxicokinetic models appeared highly advantageous in terms of allowing formulation of testable hypotheses and providing mechanistic understanding of the chemical mode of action and predictions regarding the chemical's toxicokinetics. However, application of toxicokinetic models might be a challenge for NPD until a novel methodology is developed to quantify NPD in organisms. That said, considerable progress has been made in PBPK modelling of NMs, much of which we believe can be used also for NPD with some caveats. Care must be taken when transferring PBPK models developed for engineered NMs to NPD, as the NM models are themselves still being evolved and refined and are not yet approved for regulatory purposes. For instance, because some metallic NMs are quickly dissolved, they might fit the existing models for metals, whereas NPD does not dissolve in physiological media or might undergo very slow degradation. There are also models that fit the first target organs but use assumptions to describe how particles reach the secondary targets. Such predictions are typically verified experimentally by performing an in vitro experiment and not assessing the in vivo distribution across the whole organism. An additional important advantage of PBPK modelling is that it provides interspecies extrapolation even to humans, which enhances the risk assessment and mitigation strategy without the requirement to perform experimental tests for each type, size and shape of NPD.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by the University of Eastern Finland water program funded by Saastamoinen foundation, Wihuri foundation and Olvi foundation. This study was also partially funded by the European Union's Horizon 2020 research and innovation program, via the project PLASTICSFATE (project number 965367). IL acknowledges funding from the Leverhulme Trust grant “PlasticRivers” and the European Union's Horizon 2020 project NanoSolveIT (Grant Agreement No 814572).References
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella, D. Rejeski and M. S. Hull, Nanotechnology in the Real World: Redeveloping the Nanomaterial Consumer Products Inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- L. Li, Y. Luo, R. Li, Q. Zhou, W. J. G. M. Peijnenburg, N. Yin, J. Yang, C. Tu and Y. Zhang, Effective Uptake of Submicrometre Plastics by Crop Plants via a Crack-Entry Mode, Nat. Sustain., 2020, 3, 929–937 CrossRef.
- M. Forte, G. Iachetta, M. Tussellino, R. Carotenuto, M. Prisco, M. De Falco, V. Laforgia and S. Valiante, Polystyrene Nanoparticles Internalization in Human Gastric Adenocarcinoma Cells, Toxicol. In Vitro, 2016, 31, 126–136 CrossRef CAS PubMed.
- J. E. Ward and D. J. Kach, Marine Aggregates Facilitate Ingestion of Nanoparticles by Suspension-Feeding Bivalves, Mar. Environ. Res., 2009, 68, 137–142 CrossRef CAS PubMed.
- F. Abdolahpur Monikh, M. G. Vijver, D. M. Mitrano, H. A. Leslie, Z. Guo, P. Zhang, I. Lynch, E. Valsami-Jones and W. J. G. M. Peijnenburg, The Analytical Quest for Sub-Micron Plastics in Biological Matrices, Nano Today, 2021, 41, 101296 CrossRef CAS.
- D. Yuan, H. He, Y. Wu, J. Fan and Y. Cao, Physiologically Based Pharmacokinetic Modeling of Nanoparticles, J. Pharm. Sci., 2019, 108, 58–72 CrossRef CAS PubMed.
- C. Schür, S. Rist, A. Baun, P. Mayer, N. B. Hartmann and M. Wagner, When Fluorescence Is Not a Particle: The Tissue Translocation of Microplastics in Daphnia Magna Seems an Artifact, Environ. Toxicol. Chem., 2019, 38, 1495–1503 CrossRef PubMed.
- C. Wang, Z. Xian, X. Jin, S. Liang, Z. Chen, B. Pan, B. Wu, Y. S. Ok and C. Gu, Photo-Aging of Polyvinyl Chloride Microplastic in the Presence of Natural Organic Acids, Water Res., 2020, 183, 116082 CrossRef CAS PubMed.
- J. Jacquin, J. Cheng, C. Odobel, C. Pandin, P. Conan, M. Pujo-Pay, V. Barbe, A.-L. Meistertzheim and J.-F. Ghiglione, Microbial Ecotoxicology of Marine Plastic Debris: A Review on Colonization and Biodegradation by the “Plastisphere”, Front. Microbiol., 2019, 10, 885 CrossRef PubMed.
- N. B. Hartmann, T. Hüffer, R. C. Thompson, M. Hassellöv, A. Verschoor, A. E. Daugaard, S. Rist, T. Karlsson, N. Brennholt, M. Cole, M. P. Herrling, M. C. Hess, N. P. Ivleva, A. L. Lusher and M. Wagner, Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris, Environ. Sci. Technol., 2019, 53, 1039–1047 CrossRef CAS PubMed.
- S. Doppalapudi, A. Jain, W. Khan and A. J. Domb, Biodegradable Polymers-an Overview, Polym. Adv. Technol., 2014, 25, 427–435 CrossRef CAS.
- R. D. Handy, T. B. Henry, T. M. Scown, B. D. Johnston and C. R. Tyler, Manufactured Nanoparticles: Their Uptake and Effects on Fish—a Mechanistic Analysis, Ecotoxicology, 2008, 17, 396–409 CrossRef CAS PubMed.
- A. D. Vethaak and J. Legler, Microplastics and Human Health, Science, 2021, 371, 672–674 CrossRef CAS PubMed.
- A. Buckley, J. Warren, A. Hodgson, T. Marczylo, K. Ignatyev, C. Guo and R. Smith, Slow Lung Clearance and Limited Translocation of Four Sizes of Inhaled Iridium Nanoparticles, Part. Fibre Toxicol., 2017, 14, 5 CrossRef PubMed.
- S. G. Han, J. S. Lee, K. Ahn, Y. S. Kim, J. K. Kim, J. H. Lee, J. H. Shin, K. S. Jeon, W. S. Cho, N. W. Song, M. Gulumian, B. S. Shin and I. J. Yu, Size-Dependent Clearance of Gold Nanoparticles from Lungs of Sprague–Dawley Rats after Short-Term Inhalation Exposure, Arch. Toxicol., 2015, 89, 1083–1094 CrossRef CAS PubMed.
- M. Cole, P. Lindeque, E. Fileman, C. Halsband, R. Goodhead, J. Moger and T. S. Galloway, Microplastic Ingestion by Zooplankton, Environ. Sci. Technol., 2013, 47, 6646–6655 CrossRef CAS PubMed.
- W. Cooper and G. Vaughan, Recent Developments in the Polymerization of Conjugated Dienes, Prog. Polym. Sci., 1967, 1, 91–160 CrossRef CAS.
- N. Lucas, C. Bienaime, C. Belloy, M. Queneudec, F. Silvestre and J.-E. Nava-Saucedo, Polymer Biodegradation: Mechanisms and Estimation Techniques – A Review, Chemosphere, 2008, 73, 429–442 CrossRef CAS PubMed.
- J. S. Crater and R. L. Carrier, Barrier Properties of Gastrointestinal Mucus to Nanoparticle Transport, Macromol. Biosci., 2010, 10, 1473–1483 CrossRef CAS PubMed.
- T. P. Haider, C. Völker, J. Kramm, K. Landfester and F. R. Wurm, Plastics of the Future? The Impact of Biodegradable Polymers on the Environment and on Society, Angew. Chem., Int. Ed., 2019, 58, 50–62 CrossRef CAS PubMed.
- D. Briassoulis, Mechanical Behaviour of Biodegradable Agricultural Films under Real Field Conditions, Polym. Degrad. Stab., 2006, 91, 1256–1272 CrossRef CAS.
- S.-J. Royer, S. Ferrón, S. T. Wilson and D. M. Karl, Production of Methane and Ethylene from Plastic in the Environment, PLoS One, 2018, 13, e0200574 CrossRef PubMed.
- B. M. Kyaw, R. Champakalakshmi, M. K. Sakharkar, C. S. Lim and K. R. Sakharkar, Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species, Indian J. Microbiol., 2012, 52, 411–419 CrossRef CAS PubMed.
- P. Bombelli, C. J. Howe and F. Bertocchini, Polyethylene Bio-Degradation by Caterpillars of the Wax Moth Galleria Mellonella, Curr. Biol., 2017, 27, R292–R293 CrossRef CAS PubMed.
- E. Huerta Lwanga, B. Thapa, X. Yang, H. Gertsen, T. Salánki, V. Geissen and P. Garbeva, Decay of Low-Density Polyethylene by Bacteria Extracted from Earthworm's Guts: A Potential for Soil Restoration, Sci. Total Environ., 2018, 624, 753–757 CrossRef CAS PubMed.
- J. Szostak-Kotowa, Biodeterioration of Textiles, Int. Biodeterior. Biodegrad., 2004, 53, 165–170 CrossRef CAS.
- D. N. Bikiaris and G. P. Karayannidis, Effect of Carboxylic End Groups on Thermooxidative Stability of PET and PBT, Polym. Degrad. Stab., 1999, 63, 213–218 CrossRef CAS.
- H. Zimmerman and N. T. Kim, Investigations on Thermal and Hydrolytic Degradation of Poly(Ethylene Terephthalate), Polym. Eng. Sci., 1980, 20, 680–683 CrossRef CAS.
- M. S. Marqués-Calvo, M. Cerdà-Cuéllar, D. P. R. Kint, J. J. Bou and S. Muñoz-Guerra, Enzymatic and Microbial Biodegradability of Poly(Ethylene Terephthalate) Copolymers Containing Nitrated Units, Polym. Degrad. Stab., 2006, 91, 663–671 CrossRef.
- L. Espino-Rammer, D. Ribitsch, A. Przylucka, A. Marold, K. J. Greimel, E. H. Acero, G. M. Guebitz, C. P. Kubicek and I. S. Druzhinina, Two Novel Class Ii Hydrophobins from Trichoderma Spp. Stimulate Enzymatic Hydrolysis of Poly(Ethylene Terephthalate) When Expressed as Fusion Proteins, Appl. Environ. Microbiol., 2013, 79, 4230–4238 CrossRef CAS PubMed.
- S. Shawaphun, T. Manangan and S. Wacharawichanant, Thermo- and Photo-Degradation of LDPE and PP Films Using Metal Oxides as Catalysts, Adv. Mater. Res., 2010, 93–94, 505–508 CAS.
- A. M. Resmeriţă, A. Coroaba, R. Darie, F. Doroftei, I. Spiridon, B. C. Simionescu and P. Navard, Erosion as a Possible Mechanism for the Decrease of Size of Plastic Pieces Floating in Oceans, Mar. Pollut. Bull., 2018, 127, 387–395 CrossRef PubMed.
- S. S. Yang, M. Q. Ding, L. He, C. H. Zhang, Q. X. Li, D. F. Xing, G. L. Cao, L. Zhao, J. Ding, N. Q. Ren and W. M. Wu, Biodegradation of Polypropylene by Yellow Mealworms (Tenebrio Molitor) and Superworms (Zophobas Atratus) via Gut-Microbe-Dependent Depolymerization, Sci. Total Environ., 2020, 756, 144087 CrossRef PubMed.
- J. Shang, M. Chai and Y. Zhu, Photocatalytic Degradation of Polystyrene Plastic under Fluorescent Light, Environ. Sci. Technol., 2003, 37, 4494–4499 CrossRef CAS PubMed.
- B. Schink, P. H. Janssen and J. Frings, Microbial Degradation of Natural and of New Synthetic Polymers, FEMS Microbiol. Lett., 1992, 103, 311–316 CrossRef CAS PubMed.
- V. C. Sekhar, K. M. Nampoothiri, A. J. Mohan, N. R. Nair, T. Bhaskar and A. Pandey, Microbial Degradation of High Impact Polystyrene (HIPS), an e-Plastic with Decabromodiphenyl Oxide and Antimony Trioxide, J. Hazard. Mater., 2016, 318, 347–354 CrossRef CAS PubMed.
- A. S. Danko, M. Luo, C. E. Bagwell, R. L. Brigmon and D. L. Freedman, Involvement of Linear Plasmids in Aerobic Biodegradation of Vinyl Chloride, Appl. Environ. Microbiol., 2004, 70, 6092–6097 CrossRef CAS PubMed.
- J. P. Santerre, R. S. Labow, D. G. Duguay, D. Erfle and G. A. Adams, Biodegradation Evaluation of Polyether and Polyester-urethanes with Oxidative and Hydrolytic Enzymes, J. Biomed. Mater. Res., 1994, 28, 1187–1199 CrossRef CAS PubMed.
- G. T. Howard, Biodegradation of Polyurethane: A Review, Int. Biodeterior. Biodegrad., 2002, 49, 245–252 CrossRef CAS.
- N. R. Yacobi, L. DeMaio, J. Xie, S. F. Hamm-Alvarez, Z. Borok, K. J. Kim and E. D. Crandall, Polystyrene Nanoparticle Trafficking across Alveolar Epithelium, Nanomed.: Nanotechnol., Biol. Med., 2008, 4, 139–145 CrossRef CAS PubMed.
- M. C. E. Lomer, R. P. H. Thompson and J. J. Powell, Fine and Ultrafine Particles of the Diet: Influence on the Mucosal Immune Response and Association with Crohn's Disease, Proc. Nutr. Soc., 2002, 61, 123–130 CrossRef PubMed.
- B. Eshaghi, N. Alsharif, X. An, H. Akiyama, K. A. Brown, S. Gummuluru and B. M. Reinhard, Stiffness of HIV-1 Mimicking Polymer Nanoparticles Modulates Ganglioside-Mediated Cellular Uptake and Trafficking, Adv. Sci., 2020, 7, 2000649 CrossRef CAS PubMed.
- T. Wu and M. Tang, Review of the Effects of Manufactured Nanoparticles on Mammalian Target Organs, J. Appl. Toxicol., 2018, 38, 25–40 CrossRef CAS PubMed.
- O. Lunov, T. Syrovets, C. Loos, J. Beil, M. Delacher, K. Tron, G. U. Nienhaus, A. Musyanovych, V. Mailänder, K. Landfester and T. Simmet, Differential Uptake of Functionalized Polystyrene Nanoparticles by Human Macrophages and a Monocytic Cell Line, ACS Nano, 2011, 5, 1657–1669 CrossRef CAS PubMed.
- L. Xu, M. Xu, R. Wang, Y. Yin, I. Lynch and S. Liu, The Crucial Role of Environmental Coronas in Determining the Biological Effects of Engineered Nanomaterials, Small, 2020, 16, 2003691 CrossRef CAS PubMed.
- Z. Guo, P. Zhang, S. Chakraborty, A. J. Chetwynd, F. Abdolahpur Monikh, C. Stark, H. Ali-Boucetta, S. Wilson, I. Lynch and E. Valsami-Jones, Biotransformation modulates the penetration of metallic nanomaterials across an artificial blood–brain barrier model, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, 1–10 Search PubMed.
- M. Lundqvist, J. Stigler, G. Elia, I. Lynch, T. Cedervall and K. A. Dawson, The Crucial Role of Environmental Coronas in Determining the Biological Effects of Engineered Nanomaterials, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14265–14270 CrossRef CAS PubMed.
- J. Bozich, S. Lohse and M. Torelli, Surface Chemistry, Charge and Ligand Type Impact the Toxicity of Gold Nanoparticles to Daphnia Magna, Environ. Sci.: Nano, 2014, 1, 260–270 RSC.
- J. Klein, Probing the Interactions of Proteins and Nanoparticles, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2029–2030 CrossRef CAS PubMed.
- R. Landsiedel, E. Fabian, L. Ma-Hock, W. Wohlleben, K. Wiench, F. Oesch and B. van Ravenzwaay, Toxico-/Biokinetics of Nanomaterials, Arch. Toxicol., 2012, 86, 1021–1060 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Rapid translocation of nanoparticles from the lung airspaces to the body, Nat. Biotechnol., 2009, 25, 1165–1170 CrossRef PubMed.
- A. Kretschmann, R. Ashauer, T. G. Preuss, P. Spaak, B. I. Escher and J. Hollender, Toxicokinetic Model Describing Bioconcentration and Biotransformation of Diazinon in Daphnia Magna, Environ. Sci. Technol., 2011, 45, 4995–5002 CrossRef CAS PubMed.
- T. Jager, C. Albert, T. G. Preuss and R. Ashauer, General Unified Threshold Model of Survival - a Toxicokinetic-Toxicodynamic Framework for Ecotoxicology, Environ. Sci. Technol., 2011, 45, 2529–2540 CrossRef CAS PubMed.
- W. Utembe, H. J. Clewell, N. Sanabria, P. Doganis and M. Gulumian, Current approaches and techniques in physiologically based pharmacokinetic (PBPK) modelling of nanomaterials, Nanomaterials, 2020, 10, 1–32 CrossRef PubMed.
- A. Henrique Silva, E. Lima, M. Vasquez Mansilla, R. D. Zysler, M. L. Mojica Pisciotti, C. Locatelli, R. K. R. Rajoli, A. Owen, T. B. Creczynski-Pasa and M. Siccardi, A physiologically based pharmacokinetic model to predict the superparamagnetic iron oxide nanoparticles (SPIONs) accumulation in vivo. European Journal of, Nanomedicine, 2017, 9, 79–90 CAS.
- D. Li, M. Morishita, J. G. Wagner, M. Fatouraie, M. Wooldridge, W. E. Eagle, J. Barres, U. Carlander, C. Emond and O. Jolliet, In vivo biodistribution and physiologically based pharmacokinetic modeling of inhaled fresh and aged cerium oxide nanoparticles in rats, Part. Fibre Toxicol., 2015, 13, 45 CrossRef PubMed.
- F. C. Klaessig, PBPK Modeling of Slightly Soluble Silver Nanomaterials and Regulatory Acceptance, Small, 2020, 16, 1907667 CrossRef CAS PubMed.
- I. Nestorov, Whole Body Pharmacokinetic Models, Clin. Pharmacokinet., 2003, 42, 883–908 CrossRef CAS PubMed.
- R. M. J. Ings, Interspecies Scaling and Comparisons in Drug Development and Toxicokinetics, Xenobiotica, 1990, 20, 1201–1231 CrossRef CAS PubMed.
- A. Grech, C. Brochot, J.-L. Dorne, N. Quignot, F. Y. Bois and R. Beaudouin, Toxicokinetic Models and Related Tools in Environmental Risk Assessment of Chemicals, Sci. Total Environ., 2017, 578, 1–15 CrossRef CAS PubMed.
- M. G. Vijver, Y. Zhai, Z. Wang and W. J. G. M. Peijnenburg, Emerging Investigator Series: The Dynamics of Particle Size Distributions Need to Be Accounted for in Bioavailability Modelling of Nanoparticles, Environ. Sci.: Nano, 2018, 5, 2473–2481 RSC.
- K. E. Wheeler, A. J. Chetwynd, K. M. Fahy, B. S. Hong, J. A. Tochihuitl, L. A. Foster and I. Lynch, Environmental Dimensions of the Protein Corona, Nat. Nanotechnol., 2021, 16, 617–629 CrossRef CAS PubMed.
- O. Hollóczki and S. Gehrke, Can Nanoplastics Alter Cell Membranes?, ChemPhysChem, 2019, 21, 9–12 CrossRef PubMed.
- B. Laycock, M. Nikolić, J. M. Colwell, E. Gauthier, P. Halley, S. Bottle and G. George, Lifetime Prediction of Biodegradable Polymers, Prog. Polym. Sci., 2017, 71, 144–189 CrossRef CAS.
- N. B. Hartmann, S. Rist, J. Bodin, L. H. S. Jensen, S. N. Schmidt, P. Mayer, A. Meibom and A. Baun, Microplastics as Vectors for Environmental Contaminants: Exploring Sorption, Desorption, and Transfer to Biota, Integr. Environ. Assess. Manage., 2017, 13, 488–493 CrossRef PubMed.
- F. Abdolahpur Monikh, L. Chupani, M. G. Vijver and W. J. G. M. Peijnenburg, Parental and trophic transfer of nanoscale plastic debris in an assembled aquatic food chain as a function of particle size, Environ. Pollut., 2021, 269, 116066 CrossRef CAS PubMed.
- Z. Peng, X. Liu, W. Zhang, Z. Zeng, Z. Liu, C. Zhang, Y. Liu, B. Shao, Q. Liang, W. Tang and X. Yuan, Advances in the Application, Toxicity and Degradation of Carbon Nanomaterials in Environment: A Review, Environ. Int., 2020, 134, 105298 CrossRef CAS PubMed.
- P. V. Silva, C. A. M. van Gestel, R. A. Verweij, A. G. Papadiamantis, S. F. Gonçalves, I. Lynch and S. Loureiro, Toxicokinetics of Pristine and Aged Silver Nanoparticles in Physa Acuta, Environ. Sci.: Nano, 2020, 7, 3849–3868 RSC.
- A. K. Singh, Nanoparticle Pharmacokinetics and Toxicokinetics, in Engineered Nanoparticles, Elsevier, 2016, vol. 201, pp. 229–293 Search PubMed.
| This journal is © The Royal Society of Chemistry 2022 |