Atomistic insights into the hydrodefluorination of PFAS using silylium catalysts†
Abstract
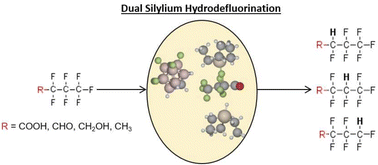
Fluorochemicals are a persistent environmental contaminant that require specialized techniques for degradation and capture. In particular, recent attention on per- and poly-fluoroalkyl substances (PFAS) has led to numerous explorations of different techniques for degrading the super-strong C–F bonds found in these fluorochemicals. In this study, we investigated the hydrodefluorination mechanism using silylium-carborane salts for the degradation of PFAS at the density functional theory (DFT) level. We find that the degradation process involves both a cationic silylium (Et3Si+) and a hydridic silylium (Et3SiH) to facilitate the defluorination and hydride-addition events. Additionally, the role of carborane ([HCB11H5F6]−) is to force unoccupied anti-bonding orbitals to be partially occupied, weakening the C–F bond. We also show that changing the substituents on carborane from fluorine to other halogens weakens the C–F bond even further, with iodic carborane ([HCB11H5I6]−) having the greatest weakening effect. Moreover, our calculations reveal why the C–F bonds are resistant to degradation, and how the silylium-carborane chemistry is able to chemically transform these bonds into C–H bonds. We believe that our results are further applicable to other halocarbons, and can be used to treat either our existing stocks of these chemicals or to treat concentrated solutions following filtration and capture.
- This article is part of the themed collections: Environmental Science: Processes & Impacts Recent HOT Articles and Contaminant remediation and fate


 Please wait while we load your content...
Please wait while we load your content...