Emerging investigator series: contributions of reactive nitrogen species to transformations of organic compounds in water: a critical review
Abstract
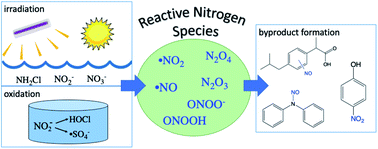
Reactive nitrogen species (RNS) pose a potential risk to drinking water quality because they react with organic compounds to form toxic byproducts. Since the discovery of RNS formation in sunlit surface waters, these reactive intermediates have been detected in numerous sunlit natural waters and engineered water treatment systems. This critical review summarizes what is known regarding RNS, including their formation, contributions to contaminant transformation, and products resulting from RNS reactions. Reaction mechanisms and rate constants have been described for nitrogen dioxide (˙NO2) reacting with phenolic compounds. However, significant knowledge gaps remain regarding reactions of RNS with other types of organic compounds. Promising methods to quantify RNS concentrations and reaction rates include the use of selective quenchers and probe compounds as well as electron paramagnetic resonance spectroscopy. Additionally, high resolution mass spectrometry methods have enabled the identification of nitr(os)ated byproducts that form via RNS reactions in sunlit surface waters, UV-based treatment systems, treatment systems that employ chemical oxidants such as chlorine and ozone, and certain types of biological treatment processes. Recommendations are provided for future research to increase understanding of RNS reactions and products, and the implications for drinking water toxicity.
- This article is part of the themed collections: Contaminant remediation and fate, Emerging Investigator Series, Environmental Science: Processes & Impacts: Recent Review Articles, Best Papers 2022 – Environmental Science: Processes & Impacts and Best Papers of 2022 from RSC’s Environmental Science journals


 Please wait while we load your content...
Please wait while we load your content...