Open Access Article
Open Access ArticleA pilot study on extractable organofluorine and per- and polyfluoroalkyl substances (PFAS) in water from drinking water treatment plants around Taihu Lake, China: what is missed by target PFAS analysis?†
Enmiao
Jiao
 a,
Zhiliang
Zhu
a,
Zhiliang
Zhu
 a,
Daqiang
Yin
a,
Yanling
Qiu
*a,
Anna
Kärrman
a,
Daqiang
Yin
a,
Yanling
Qiu
*a,
Anna
Kärrman
 b and
Leo W. Y.
Yeung
b and
Leo W. Y.
Yeung
 *b
*b
aKey Laboratory of Yangtze River Water Environment, College of Environmental Science and Engineering, Tongji University, China. E-mail: ylqiu@tongji.edu.cn
bMan-Technology-Environment Research Centre (MTM), School of Science and Technology, Örebro University, Sweden. E-mail: Leo.Yeung@oru.se
First published on 10th June 2022
Abstract
Per- and polyfluoroalkyl substances (PFAS) have raised concerns due to their worldwide occurrence and adverse effects on both the environment and humans as well as posing challenges for monitoring. Further collection of information is required for a better understanding of their occurrence and the unknown fractions of the extractable organofluorine (EOF) not explained by commonly monitored target PFAS. In this study, eight pairs of raw and treated water were collected from drinking water treatment plants (DWTPs) around Taihu Lake in China and analyzed for EOF and 34 target PFAS. Mass balance analysis of organofluorine revealed that at least 68% of EOF could not be explained by target PFAS. Relatively higher total target concentrations were observed in 4 DWTPs (D1 to D4) when compared to other samples with the highest sum concentration up to 189 ng L−1. PFOA, PFOS and PFHxS were the abundant compounds. Suspect screening analysis identified 10 emerging PFAS (e.g., H-PFAAs, H-PFESAs and OBS) in addition to target PFAS in raw or treated water. The ratios PFBA/PFOA and PFBS/PFOS between previous and current studies showed significant replacements of short-chain to long-chain PFAS. The ratios of the measured PFAS concentrations to the guideline values showed that some of the treated drinking water exceeds guideline values, appealing for efforts on drinking water safety guarantee.
Environmental significancePer- and polyfluoroalkyl substances (PFAS) are man-made substances which have been manufactured and used extensively as additives in consumer products since the 1950s. Releases of PFAS have resulted in their detections in various environmental media, especially in drinking water which was identified as one of the major exposure pathways to humans. However, current monitoring of PFAS is far short of what is representative of the entire class of the thousands of compounds. Organofluorine mass balance analysis, therefore, was performed to investigate the extractable organofluorine (EOF) levels and to figure out the levels of unidentified components. This study describes a pilot study of EOF analysis in drinking water, and the results demonstrate a large percentage of unidentified EOF, indicating underestimation of the organofluorine pollution levels. This work also provides important information for drinking water safety assurance and the necessity to identify the unknown compounds. |
Introduction
Per- and polyfluoroalkyl substances (PFAS) are a group of fluorinated substances that contain at least one fully fluorinated methyl or methylene carbon atom (without any H/Cl/Br/I atom attached to it)1 according to the 2021 definition by the Organization for Economic Co-operation and Development (OECD) and have been manufactured and used around the globe since the 1950s.2 Their unique properties including high thermal stability and oil/water repellency3 have made them extensively used as additives in consumer products4 and have resulted in their prevalent presence in abiotic and biotic media.5–10 Some have raised environmental concerns, and among them, perfluorooctanesulfonic acid (PFOS) and perfluorooctanoic acid (PFOA) were listed in the Stockholm Convention, while perfluorohexane sulfonic acid (PFHxS) is now under review by the committee.11 The regulations have led to the phase-out of these substances, and manufacturers have shifted to the compensatory production and usage of replacements such as some novel PFAS (mainly the substitutes of PFOA and PFOS) which already showed ubiquitous occurrence.12,13 Besides, ultra-short PFAS (C2 and C3) also attract increasing attention due to their mobile properties, and among them, trifluoroacetate (TFA) has already been widely reported in surface water, rainwater, the atmosphere, and sediments.14–17According to OECD, there are nearly 5000 individual PFAS.18 Monitoring of all these registered PFAS, however, is challenging since they include a large number of structurally different chemicals.19 Occurrence of unknown PFAS, including transformation products, may result in increased environmental and human exposure.20 The extractable organofluorine (EOF) has emerged to complement the current PFAS analysis21 and plays an important role in providing insight into the amount of unidentified organofluorines through an organofluorine mass balance approach.22 Suspect screening analysis performed by high-resolution mass spectrometry (HRMS) techniques (e.g., quadrupole time-of-flight mass spectrometry (QTOF) and Orbitrap) allows better understanding of other emerging PFAS that are not regularly monitored. Recent studies have identified EOF and different classes of emerging PFAS in various environmental media.23–29 These techniques, however, were mostly applied to wastewater or biota samples. Studies focusing on drinking water are limited.
Humans are exposed to PFAS through contact with contaminated media; among them, drinking water consumption can be a major source of PFAS exposure.3 The US Environmental Protection Agency (EPA) and some other agencies or organizations30–32 have recommended guideline values for different PFAS in drinking water considering the human health. In China, the limit values of PFOA and PFOS are 80 and 40 ng L−1, respectively. Other PFAS are not the candidate chemicals for regulatory monitoring, making it essential to understand the levels of PFAS in drinking water and to provide data support for further related standards.
Therefore, the present study was conducted to analyze PFAS in water samples collected from eight drinking water treatment plants (DWTPs) around the Taihu Lake Basin, which serves as a major drinking water source in one of the most populous and economically developed regions of China.33 The objectives of this study were: (i) to conduct extractable organofluorine analysis in drinking water around Taihu Lake; (ii) to investigate the removal efficiency under current DWTP processes in China; (iii) to identify any emerging PFAS in water samples using suspect screening analysis; and (iv) to evaluate the PFAS levels with reference to the guideline values.
Methods and materials
Chemicals and reagents
Extractable organofluorine (EOF) and 34 target PFAS were studied (Table S1†). Native standards of PFCAs (C4–C14, C16, and C18), PFSAs (C2, C4–C10, and C12), fluorotelomer sulfonic acid (FTSAs), novel compounds (hexafluoropropylene oxide dimer acid (HFPO-DA), 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 chlorinated polyfluorinated ether sulfonate (6
2 chlorinated polyfluorinated ether sulfonate (6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cl-PFESA), 8
2 Cl-PFESA), 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 chlorinated polyfluorinated ether sulfonate (8
2 chlorinated polyfluorinated ether sulfonate (8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cl-PFESA), 3H-perfluoro-3-[(3-methoxy-propoxy) propanoic acid] (ADONA), and perfluoro-4-ethylcyclohexanesulfonate (PFECHS)), isotope-labeled PFCAs (C4–C12, C14, and C16), PFSAs (C4, C6, and C8) and isotope-labeled HFPO-DA were all purchased from Wellington Laboratories (Guelph, ON, Canada). TFA and perfluoropropanoic acid (PFPrA) were purchased from Sigma Aldrich (Darmstadt, Germany), and perfluoroethane sulfonic acid (PFEtS) was purchased from Kanto Chemical Co. Inc (Tokyo, Japan). All standard solutions were prepared in HPLC grade methanol. For EOF analysis, a PFOS standard from Sigma-Aldrich was used.
2 Cl-PFESA), 3H-perfluoro-3-[(3-methoxy-propoxy) propanoic acid] (ADONA), and perfluoro-4-ethylcyclohexanesulfonate (PFECHS)), isotope-labeled PFCAs (C4–C12, C14, and C16), PFSAs (C4, C6, and C8) and isotope-labeled HFPO-DA were all purchased from Wellington Laboratories (Guelph, ON, Canada). TFA and perfluoropropanoic acid (PFPrA) were purchased from Sigma Aldrich (Darmstadt, Germany), and perfluoroethane sulfonic acid (PFEtS) was purchased from Kanto Chemical Co. Inc (Tokyo, Japan). All standard solutions were prepared in HPLC grade methanol. For EOF analysis, a PFOS standard from Sigma-Aldrich was used.
Sample collection and pretreatment
The investigated DWTPs are located in 5 cities around the Taihu Lake Basin, China (Fig. 1). River and river network water were used as water sources in D5 and D8, while the water sources of the other 6 DWTPs were lake water. The overall treatment process includes pre-ozonation, coagulation, sedimentation, sand filtration, post-ozonation, bio-activated carbon and disinfection, although there are some differences between DWTPs. Both raw and treated water samples were collected in August 2019. In each sampling site, two water samples including raw and the corresponding treated water (each approximately 2 L) were collected. All samples were collected in pre-cleaned polypropylene bottles, shipped to the laboratory, and kept in the cooling room (4 °C) until analysis.The water sample (500 mL) was first filtered by using a 0.47 μm membrane. Then the membrane was sonicated for 10 min in MeOH, and the MeOH was poured into the water sample. After this, 2 ng internal standard was spiked into the water samples. The solid phase extraction (SPE) method with Oasis WAX cartridges (Waters 150 mg, 6 mL, and 30 mm) was used for extraction. In brief, the cartridges were pre-conditioned with 4 mL 0.1% NH4OH/methanol, 4 mL methanol and 4 mL Milli-Q water. After loading the samples, the cartridges were washed with 4 mL of MilliQ-water, 4 mL of an ammonium acetate buffer solution (pH = 4) and then dried under vacuum for 30 min. Eluted with 4 mL 0.1% NH4OH/methanol, the anionic fraction was then evaporated under nitrogen gas to a final volume of 200 μL. Then, aliquots of the samples were taken and mixed with different volumes of the aqueous mobile phase to obtain different compositions of the organic solvent of 80% (ultrashort PFAS analysis) and 40% (remaining PFAS analysis) in the vial with the addition of 2 ng recovery standard for PFAS analysis.
Sample pretreatment of the extractable organofluorine
The pretreatment of EOF was similar to the method mentioned above, except that both the internal standard and recovery standard were not added. In addition, a more extensive wash was used after loading the samples; cartridges were washed with 20 mL 0.01% NH4OH/water (to remove inorganic fluoride), 10 mL MilliQ three times, followed by 4 mL ammonium acetate buffer and 4 mL 20% methanol/water. Aliquots of the samples were subjected to PFAS analysis as described above, to enable calculation of the EOF mass balance, and 100 μL of the aliquot was subjected to EOF analysis.Instrumental analysis
Separation of the ultra-short analytes was performed using an Acquity Ultra Performance Convergence Chromatography (UPC2) system coupled with a tandem mass spectrometer (Waters Corporation, Milford, USA) that was operated in the electrospray negative ionization mode with the source temperature at 150 °C. The extracts were injected into a Torous™ DIOL column (3 mm × 150 mm, 1.7 μm; Waters Corporation, Milford, USA) with supercritical CO2 (A) and 0.1% ammonium hydroxide in methanol (B) as the mobile phase. Details of the method are provided elsewhere.34The remaining target compounds were analyzed by using the Acquity UPLC system coupled with the Xevo TQ-S tandem mass spectrometer (Waters Corporation, Milford, USA) that was operated in the electrospray negative ionization mode. The chromatographic separation was accomplished by using an Acquity BEH C18 column (2.1 mm × 100 mm, 1.7 μm) (Waters Corporation Milford, USA). A gradient mobile phase of (A) 2 mM ammonium acetate (in 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, methanol: MilliQ) with 5 mM 1-methyl piperidine and (B) 2 mM ammonium acetate (in MeOH) with 5 mM 1-methyl piperidine at a flow rate of 0.30 mL min−1 was used. Details of the LC and MS conditions are provided elsewhere.35
70, methanol: MilliQ) with 5 mM 1-methyl piperidine and (B) 2 mM ammonium acetate (in MeOH) with 5 mM 1-methyl piperidine at a flow rate of 0.30 mL min−1 was used. Details of the LC and MS conditions are provided elsewhere.35
The EOF were analyzed by combustion ion chromatography (CIC) (Metrohm, Switzerland), consisting of a combustion module, a 920 absorbent module and a 930 compact IC flex. In brief, the sample was placed in a quartz boat, and all fluorine was converted to hydrogen fluoride and absorbed into the water after combustion at 1000–1050 °C. The dissolved fluoride was then analyzed by using the ion chromatograph. Separation of anions was completed by using an ion exchange column (Metrosep A Supp5, 4 mm × 150 mm) with 64 mM sodium carbonate and 20 mM sodium bicarbonate in water as the eluent solution. Details of the method are provided elsewhere.36
Suspect screening analysis was performed by using an Acquity UPLC system coupled with a quadrupole time-of-flight mass spectrometer (QTOF) (G2-XS, Waters Corporation, Milford, USA) in the electrospray negative ionization mode.37 The separation of the compounds was performed by using an Acquity BEH C18 column (2.1 mm × 100 mm; 1.7 μm; Waters Corporation, Milford, USA) with the mobile phase (A) 2 mM ammonium acetate (in 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70, methanol: MilliQ) and (B) 2 mM ammonium acetate (in MeOH). A data independent acquisition mode (MSE) was used to obtain the precursor and fragment ions. Details about the parameters are provided in Table S2† and elsewhere.37
70, methanol: MilliQ) and (B) 2 mM ammonium acetate (in MeOH). A data independent acquisition mode (MSE) was used to obtain the precursor and fragment ions. Details about the parameters are provided in Table S2† and elsewhere.37
Quality control and quality assurance
To avoid interference of background contamination, poly tetrafluoroethylene (PTFE) and fluoropolymer materials from the instruments and other possible sources were removed.For UPLC-MS/MS and UPC2-MS/MS analyses, recovery samples for each batch of sample analysis were prepared using Milli-Q water to monitor contamination or loss during the whole extraction. Native and internal standards (2 ng) were added to the recovery samples in the same way as for the samples. For blanks, internal standards (2 ng) were spiked into Milli-Q water which was prepared in the lab. For blank spikes, native standards (2 ng) were spiked into the bottle containing Milli-Q water in addition to internal standards. All blanks and recovery samples were extracted and stored in the same way as for the real samples. The recoveries of internal standards for blanks all ranged from 71% to 111%. The native compounds in the recovery samples ranged from 73% to 102% (Table S2 and S3†). Matrix spike recovery tests were also carried out using tap water. Two ng each of all of the target analytes was spiked into 500 mL of tap water. Except for long-chain PFAS (PFUnDA and PFDoDA), all other PFASs showed acceptable recoveries, ranging from 75% to 103%. Although each target compound was analyzed using the isotope-labelled standards as internal standards, establishing exact matching internal standards for some compounds was not possible due to the lack of the isotope-labelled standards. Therefore, the internal standards with the closest retention time under the same analytical method were selected. For example, TFA and PFPrA were corrected using 13C-PFBA. TFMS, PFEtS and PFPrS were corrected using 13C-PFBS. PFTrDA was corrected using 13C-PFDoDA. PFOcDA was corrected using 13C-PFHxDA. PFPeS was corrected using 13C-PFHxS. PFHpS, PFDS, PFNS and PFDoDS were corrected using 13C-PFOS. F-53B was corrected using 13C-PFOS. 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA and 8
2 FTSA and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA were corrected using 6
2 FTSA were corrected using 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA. Except for these compounds, all the other compounds used their corresponding isotope-labelled standards as internal standards.
2 FTSA. Except for these compounds, all the other compounds used their corresponding isotope-labelled standards as internal standards.
Quantification of PFAS was based on an internal calibration method using corresponding isotope-labelled internal standards. The method detection limit (MDL) of PFAS was determined as average concentrations in procedural blanks plus three times the standard deviation. The method quantification limit (MQL) was determined as average concentrations in procedure blanks plus ten times the standard deviation. The lowest point of the calibration curve was used if the analyte was not found in the blanks (Table S5†).
Quantification of EOF was based on external calibration using PFOS as the standard that was combusted in the same way as the samples. Since the background fluoride signal was detected when an empty quartz boat was combusted, real sample analysis was conducted when variation of the level of the combustion blank (combustion of the empty quartz boat) was below 10%. Quantification of EOF in the blanks and samples was done after subtracting the combustion blanks injected before and after the sample. For EOF analysis, the MDL was 50 ng L−1 F−1. The sample concentrations were corrected for the blank level and were reported when their levels were at least two times higher than the MDL.
For suspect screening analysis, extraction blanks were also analyzed together with the samples. The identified emerging PFAS (target PFAS excluded) were not found in blanks. Considering the lack of standards for the PFAS identified through suspect screening, semi-quantification was performed using surrogate native standards24,26 (Table S8†). Mass errors for all of the classes were set to 5 ppm.
Extractable organofluorine analysis
The amount of EOF in the samples was assessed by CIC (ng L−1 F−1). To evaluate the extent of unidentified organofluorine, the target PFAS concentrations were first converted to fluoride through eqn (1). | (1) |
Results and discussion
Extractable organofluorine analysis in raw and treated water
Relatively high EOF concentrations were observed for four DWTPs (D3, D4, D5 and D8) as shown in Fig. 2. The EOF concentrations in raw water from D3, D4, D5 and D8 were 400, 378, 187 and 81.2 ng of F/L, respectively, while in treated water, the concentrations were 409, 340, 125 and 130 ng of F/L, respectively. The higher concentrations observed in D3 and D4 might be related to the industries (e.g., the textile industry and chemical industry) around these areas. In both raw and treated water samples from D3 and D4, the largest known contributors to EOF were PFCAs (8% and 13%, respectively), and PFSAs (11% and 13%, respectively). The novel PFAS and ultrashort PFAS only accounted for around 1% of EOF. In the samples from D5 and D8, the largest contributors to EOF were PFCAs (ranging from 4% to 25% of EOF). The PFSAs and novel PFAS made up less than 4% and 2% of EOF, respectively, with ultra-short PFAS accounting for less than 2%. The percentage of target PFAS to EOF ranged from 8% to 32%, indicating a large portion of unidentified origin. | ||
| Fig. 2 EOF concentrations (ng L−1 F−1) (left) and composition of EOF by converting the target PFAS concentration to fluorine equivalents (right) in four DWTPs (D3, D4, D5 and D8). | ||
Observable differences (>20%) in EOF after treatment were noted in D8 (increase), and the opposite phenomenon was observed in D5 (Table S6†); no observable changes were noted in D3 (2%) and D4 (−9.8%). It was hypothesized that any increase or decrease in EOF after treatment might be reflected in changes in target PFAS concentrations after treatment. However, no clear trends were concluded from the target PFAS results. As shown in D5, the decrease in EOF (33%) and the inconsistent increase or decrease for different classes of PFAS was observed after treatment, and a similar situation was observed in D8. It is not possible to speculate what types of unknown PFAS were removed or produced during the treatment processes. As discussed before, the overall treatment processes were quite similar between DWTPs. However, an additional biofilter technology was included in D5 which might help explain the decrease of EOF. Further investigation for the use of the biofilter technology is needed.
Likewise, some other researchers have also discovered that unidentified organofluorine in water matrices accounted for a large proportion of EOF.22,29,38,39 Precursor compounds might make a significant contribution to the unknown portion of EOF due to their incomplete transformation. Instead of fully converting to common perfluoroalkyl acids (PFAAs), they may also form some unknown intermediates under the treatment process which might also result in better binding abilities of the transformation products to WAX cartridges, explaining the increase of EOF levels in D8. Besides, fluorine-containing drugs could also contribute to EOF.40 Many fluorinated pharmaceuticals are included in the updated definition of PFASs. More efforts, therefore, are needed to understand what the unknowns are and how to improve the quality of water sources.
Suspect screening analysis
A total of 10 emerging PFAS of five classes were identified in the raw and treated water samples at different confidence levels based on the scale proposed by another study,41 which included hydro substituted PFCAs (H-PFCAs), hydro substituted PFSAs (H-PFSAs), chlorine substituted perfluoroalkyl ether sulfonates (Cl-PFESAs), hydro substituted perfluoroalkyl ether sulfonates (H-PFESAs) and p-perfluorous nonenoxybenzenesulfonate (OBS) (Table S8†). By comparing the HRMS spectral information with the database from other literature studies,24,27 six PFAS were identified at a confidence level of 2, while others were at a confidence level of 3. The semi-quantified concentrations and composition together with the detailed information of these emerging PFAS are presented in Table S8 and Fig. S1.†For H-PFCAs, C5, C8 and C9 were identified in this class. H-PFPeA showed the highest detection frequency of 50%, followed by H-PFOA (13%). H-PFNA was only identified in one sample (treated water from D3). The total concentrations of H-PFCAs ranged from 0.100 to 0.703 ng L−1, which further explained the EOF increase from 0.02% to 0.10%. No observable differences in concentrations for H-PFCAs were noted between raw and treated water (<20%). For H-PFSAs, H-PFBS and H-PFOS were identified, and increases were observed after the treatment processes (>20%). For Cl-PFESAs, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cl-PFESA was identified. For H-PFESAs, 2
2 Cl-PFESA was identified. For H-PFESAs, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H-PFESA, 4
2 H-PFESA, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H-PFESA and 6
2 H-PFESA and 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H-PFESA were identified with a detection frequency higher than 63%. 6
2 H-PFESA were identified with a detection frequency higher than 63%. 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H-PFESA was the most predominant compound among H-PFESAs, which was not unexpected as it has been found to be the transformation product of 6
2 H-PFESA was the most predominant compound among H-PFESAs, which was not unexpected as it has been found to be the transformation product of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cl-PFESA.42 The semi-quantified concentrations of 6
2 Cl-PFESA.42 The semi-quantified concentrations of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 H-PFESA (0.008–0.439 ng L−1) were lower than those of 6
2 H-PFESA (0.008–0.439 ng L−1) were lower than those of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Cl-PFESA, which was found to be consistent with the results from another study on surface water.42 OBS was identified in 11 out of the 16 samples; this compound was widely used in fire-fighting foam and oil production.43 The identified 10 emerging PFAS contributed to a further 0.01% to 0.28% of EOF. These identified emerging PFAS, therefore, raised concerns about their high presence in drinking water and potential health risk for humans.
2 Cl-PFESA, which was found to be consistent with the results from another study on surface water.42 OBS was identified in 11 out of the 16 samples; this compound was widely used in fire-fighting foam and oil production.43 The identified 10 emerging PFAS contributed to a further 0.01% to 0.28% of EOF. These identified emerging PFAS, therefore, raised concerns about their high presence in drinking water and potential health risk for humans.
Concentrations and profiles of target PFAS in raw and treated water
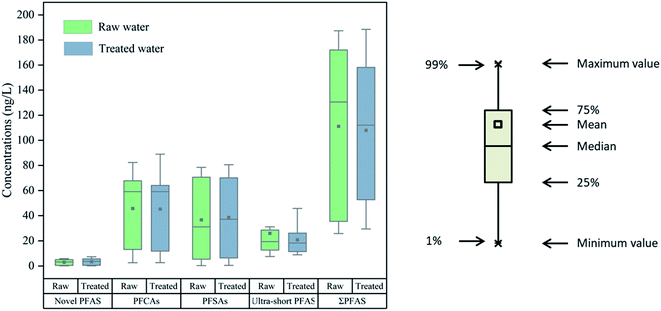
Out of the 34 target compounds, 22 PFAS were detected at levels above the MQLs in the raw (n = 8) and treated water samples (n = 8) collected from the 8 DWTPs. The results are presented in Fig. 3, 4 and Table S7.† PFCAs (C4–C6 and C8–C10) and PFSAs (C4, C6 and C8) were the most frequently detected compounds with a detection frequency of 100%, followed by F-53B (94% of total samples), PFHpA (88%) and PFPeS (88%). Ultra-short PFAS were detected at frequencies of 56% or higher. Lower frequencies of detection were observed for some long-chain PFAS. | ||
| Fig. 4 Profiles of the major PFAS classes (A), and the individual PFAS composition (B) in raw and treated water from 8 DWTPs. | ||
Total concentrations (∑PFAS) ranged from 25.8 to 187 ng L−1 in the raw water and 29.4 to 188 ng L−1 in the treated water. The concentrations of PFAS showed little differences between raw and treated water, indicating limited removal efficiency although activated carbon was already in use in these DWTPs.44 Among all target PFAS, 5 ultra-short PFAS (TFA, PFPrA, TFMS, PFEtS and PFPrS) were detected with the sum concentrations ranging from 7.53 to 78.7 ng L−1 contributing to 6% to 89% of ∑PFAS, which highlights the importance of these compounds. PFPrA and TFMS displayed generally high levels, accounting for 55% of ∑ultra-short PFAS or higher. F-53B, a substitute of PFOS produced in China, was also observed in 8 DWTPs (median: 2.70 ng L−1). Although 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 FTSA and HFPO-DA were also widely detected, the sum concentrations of these two compounds were at lower levels (less than 1.6 ng L−1), only accounting for 2% or lower of the total PFAS. For the sum of PFCAs and PFSAs, the concentrations ranged from 2.95 to 157 ng L−1 in the raw water and 3.21 to 170 ng L−1 in the treated water. Among the PFCAs and PFSAs, PFBA was at higher levels compared to the PFSAs with the same carbon chain length (PFBS), and PFOA (range: 1.36–49.0 ng L−1) was also abundant with its concentration generally higher than that of PFOS (range: 0.193–15.4 ng L−1), which might be due to the higher production and usage of PFOA, PFBA and their precursors.45 PFHxS was also one of the abundant compounds, ranging from 0.03 to 61.4 ng L−1. The ratios of PFBA to PFOA and PFBS to PFOS were calculated to reflect the extent to which PFOA and PFOS were replaced. The ratios of PFBS to PFOS showed an observable increase, ranging from 0.31 in tap water collected from Shanghai in 20083 to 1.63 in the treated water in this study. PFBA/PFOA also increased from 0.05 in tap water collected from Shanghai in 20083 to 0.42 in this study. Another study also found a high ratio of PFBA to PFOA in tap water collected from Shanghai in 201746 with a value up to 11.4, indicating the increasing usage of shorter chain alternatives.
2 FTSA and HFPO-DA were also widely detected, the sum concentrations of these two compounds were at lower levels (less than 1.6 ng L−1), only accounting for 2% or lower of the total PFAS. For the sum of PFCAs and PFSAs, the concentrations ranged from 2.95 to 157 ng L−1 in the raw water and 3.21 to 170 ng L−1 in the treated water. Among the PFCAs and PFSAs, PFBA was at higher levels compared to the PFSAs with the same carbon chain length (PFBS), and PFOA (range: 1.36–49.0 ng L−1) was also abundant with its concentration generally higher than that of PFOS (range: 0.193–15.4 ng L−1), which might be due to the higher production and usage of PFOA, PFBA and their precursors.45 PFHxS was also one of the abundant compounds, ranging from 0.03 to 61.4 ng L−1. The ratios of PFBA to PFOA and PFBS to PFOS were calculated to reflect the extent to which PFOA and PFOS were replaced. The ratios of PFBS to PFOS showed an observable increase, ranging from 0.31 in tap water collected from Shanghai in 20083 to 1.63 in the treated water in this study. PFBA/PFOA also increased from 0.05 in tap water collected from Shanghai in 20083 to 0.42 in this study. Another study also found a high ratio of PFBA to PFOA in tap water collected from Shanghai in 201746 with a value up to 11.4, indicating the increasing usage of shorter chain alternatives.
In general, the profiles in 7 DWTPs (except D7) were all dominated by PFCAs and PFSAs. PFBA, PFOA, PFHxS and PFOS were the main compounds, and the sum of the four substances accounted for 31% or higher of the total PFAS. It was notable that the concentration of PFHxS was at higher levels in this study, probably due to the increasing production and use of PFHxS in recent years, which was similar to another study that also found that PFHxS was predominant in Taihu Lake (45.9-351 ng L−1).47 In D7, however, ultra-short PFAS accounted for a larger proportion, reaching up to 89%, and PFPrA was the dominant substance (17.9 and 14.3 ng L−1 for raw and treated water, respectively). TFA and PFPrA can be the breakdown products of hydrofluorocarbons (HFCs) which are used mainly as refrigerants.48 The different profile in D7, therefore, might be explained in that there is a fluorine industry producing fluorine-containing refrigerants in its nearby cities. Besides, F-53B was prevalent, which was not surprising due to its extensive application in China. Compared to the raw water, the concentrations of PFAS in treated water showed a insignificant decrease and may even increase due to the transformation of some precursor compounds.49
The comparison of EOF levels between the present study and other studies
Recent publications have revealed the ubiquitous presence of unknown organofluorine substances by analyzing EOF. However, the knowledge of EOF in drinking water is scarce. With regard to EOF in water, the highest EOF concentration in the present study was around 400 ng L−1 F−1 which was comparable to the EOF concentration in surface water collected at two sites in Sweden (320 and 408 ng L−1 F−1, respectively), but much lower than that from other two sampling sites in Sweden (1110 to 3930 ng L−1 F−1, respectively).36 A high level of EOF was also found in surface water from Norway (up to 4030 ng L−1 F−1).29 Another study investigated the mass balance in wastewater in Nordic countries and found EOF ranging from 183 to 1460 ng L−1 F−1.50 However, target compounds can only explain at most 32% of EOF in this study. In other studies related to surface water and wastewater, they also discovered that a large percentage of EOF couldn't be explained by the monitored PFAS (>45%).For the target PFAS, an early study has reported PFOA and PFOS in 21 major cities from China; Shenzhen was found to have the highest concentration of PFOA (45.9 ng L−1) and PFOS (14.8 ng L−1) in tap water,51 which was similar to the highest PFOA (49.0 ng L−1) and PFOS (15.4 ng L−1) levels in the present study. PFHxS (max: 61.4 ng L−1) was also one of the predominant substances in this study. This compound was also found at high levels in nearby surface water (Taihu Lake) with the maximum concentration up to 292 ng L−1.12 Subsequent research studies were later conducted to investigate the PFAS contamination (mainly PFCAs and PFSAs) in different Chinese cities, showing the total concentration reaching up to 130 and 175 ng L−1, respectively.3,52 Similar total levels were detected in the present study, with the maximum sum concentrations of 188 ng L−1. Besides, the changes of the levels in DWTPs were also studied, and the limited removal efficiency of PFAS in each treatment process except for the activated carbon step was observed.53 Compared to other foreign studies, one study was conducted in several European countries, and predominant contaminants in the investigation of tap water in Amsterdam were PFBS (18.8 ng L−1) and PFOA (8.6 ng L−1), while PFOS (8.8 ng L−1) was the substance with the highest concentration in Sweden.54
In the present study, ultra-short PFAS were also detected. However, there is limited published data about their levels in drinking water. TFA was found to display an increase from not detected (2002) to 155 ng L−1 (2012) in tap water from Beijing,55 and PFPrA was also present in addition to the commonly investigated PFAS (max: 0.011 μg L−1) in tap water in Germany.56 They were even detected in bottled water with the maximum concentrations of 6.52 and 0.18 ng L−1 for PFPrA and PFPrS, respectively.57
However, it should be noted that many adverse effects of the novel substitutes (e.g., F-53B) have already been observed.58,59 Therefore, in view of the phase-out of some PFAS in China together with the known or unknown toxicity of some replacements, the substitutes (e.g., novel PFAS and ultra-short PFAS) have already become a new concern.44
PFAS levels with reference to some guideline values
Exposure through drinking water has become a concern that needs to be emphasized since it was reported that low levels of PFAS in drinking water could increase their levels in serum, indicating health effects for humans.60In China, the limit values of PFOA and PFOS are 80 and 40 ng L−1, respectively. The concentrations of these two substances in the treated water did not exceed the limits of 80 and 40 ng L−1. Therefore, a ratio between the measured concentrations in the water and the values proposed by other different government agencies were calculated (Fig. 5); the values proposed by different agencies are presented in Table 1.
 | ||
| Fig. 5 Ratios of measured PFAS concentrations in treated water and the standard and guideline values issued by agencies. | ||
| No. | Location and agencies | Finalized into law | Values (ng L−1) | References | |
|---|---|---|---|---|---|
| 1 | U.S. | United States environmental protection agency (EPA) | — | PFOA and PFOS (individual or combination) (70) | 30 |
| 2 | The state water resources control board (SWRCB), California (CA) | Yes | PFOA (10), PFOS (40), and PFBS (5000) | 61 and 62 | |
| 3 | Department of environmental protection (DEP), Massachusetts (MA) | Yes | Individual or sum of 6 PFAS (PFOA, PFOS, PFNA, PFHxS, PFHpA, and PFDA) (20) | 63 | |
| 4 | DEP, New Jersey (NJ) | Yes | PFOA (14), PFOS (13), and PFNA (13) | 64 | |
| 5 | Department of health (DOH), Vermont (VT) | Yes | PFOA + PFOS + PFHxS + PFHpA + PFNA (20) | 65 | |
| 6 | Department of environmental services (DES), New Hampshire (NH) | Yes | PFOA (12), PFOS (15), PFNA (11), and PFHxS (18) | 31 | |
| 7 | DOH, New York (NY) | Yes | PFOA (10) and PFOS (10) | 66 | |
| 8 | DOH, Washington (WA) | Yes | PFOA (10), PFOS (15), PFNA (9), PFBS (345), and PFHxS (65) | 67 | |
| 9 | EPA, Denmark | — | PFOA + PFOS + PFNA + PFHxS (2) | 68 | |
| 10 | DOH, Australia | — | PFOA (560) and PFOS + PFHxS (individual or combination) (70) | 69 | |
| 11 | National food agency (NFA), Sweden | — | Σ11PFAS (90) | 70 | |
The calculated ratios of all treated water for PFBS were far below 1 and are not presented in Fig. 5. For other individual PFAS, PFNA showed acceptable levels with the ratios of all the treated water below 1. However, when evaluating individual PFAS using the limit values from the U.S., it could be seen that ratios of PFAS (PFOA and PFHxS) in a majority of treated water samples were higher than 1 (up to 4.9 and 3.4, respectively), showing safety concerns. Treated water from 3 out of the 8 DWTPs showed excessive concentrations of PFOS when compared to the maximum contaminant level in New York. The ratios became much higher when considering the sum of PFAS. For the values issued by other agencies such as the Department of Health in Vermont, the Swedish action guideline and Danish EPA, the calculated ratios of the sum of several PFAS were much higher than 1, reaching up to 62.5 when compared with the health-based values in Denmark. Mixtures, therefore, might need more attention since they may interact synergistically,3 and the health effects are difficult to investigate. Besides, the unknown risk of ultra-short PFAS and other novel PFAS remains a concern. Measures, therefore, should be implemented to protect drinking water safety.
Conclusions
The occurrence of PFAS in water samples from China was investigated; PFOA, PFOS and PFHxS were the abundant compounds among the target PFAS. Higher total concentrations, ranging from 132 to 189 ng L−1, were observed in the samples from D1 to D4. No observable differences in PFAS concentrations were noted between raw water and its corresponding treated water, which indicated insignificant removal efficiency under the current treatment process. Apart from commonly monitored PFAS, EOF was also uncovered, and the contributions of target compounds to EOF ranged from 10% to 33%. The results from suspect screening analysis identified 10 emerging PFAS in raw and treated water. H-PFPeA, H-PFESAs and OBS showed higher presence, which contributed to a further 0.01% to 0.26% of the EOF. The ratios between the measured PFAS concentrations and the values proposed by different agencies showed that some of the treated drinking water exceeds guideline values, indicating potential health concerns.Data availability
All data used in this study are included in the main text and the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The project was financially supported by the Major Science and Technology Program for Water Pollution Control and Treatment (2017ZX07201005) and the National Key Research and Development Project of China (2021YFC3200801). The authors from ORU acknowledge the funding from the Knowledge Foundation (KKS) within the Enforce Research Profile (20160019), Sweden, and Swedish Research Council FORMAS (2020-02032). Authors also acknowledged scholarship (Ref: ORU 2019/03505) from Örebro University for promoting internationalization.References
- OECD, Reconciling Terminology of the Universe of Per- and Polyfluoroalkyl Substances:Recommendations and Practical Guidance, OECD Series on Risk Management, No. 61, OECD Publishing, Paris, 2021 Search PubMed.
- K. Prevedouros, I. T. Cousins, R. C. Buck and S. H. Korzeniowski, Sources, Fate and Transport of Perfluorocarboxylates, Environ. Sci. Technol., 2006, 40, 32–44 CrossRef CAS PubMed.
- Y. L. Mak, S. Taniyasu, L. W. Y. Yeung, G. Lu, L. Jin, Y. Yang, P. K. S. Lam, K. Kannan and N. Yamashita, Perfluorinated Compounds in Tap Water from China and Several Other Countries, Environ. Sci. Technol., 2009, 43, 4824–4829 CrossRef CAS PubMed.
- Occurrence and use of highly fluorinated substances and alternatives, Swedish Chemicals Agency, 2015 Search PubMed.
- A. de la Torre, I. Navarro, P. Sanz and M. d. l. Á. Mártinez, Occurrence and human exposure assessment of perfluorinated substances in house dust from three European countries, Sci. Total Environ., 2019, 685, 308–314 CrossRef CAS PubMed.
- X. Zhang, R. Lohmann, C. Dassuncao, X. C. Hu, A. K. Weber, C. D. Vecitis and E. M. Sunderland, Source Attribution of Poly- and Perfluoroalkyl Substances (PFASs) in Surface Waters from Rhode Island and the New York Metropolitan Area, Environ. Sci. Technol. Lett., 2016, 3, 316–321 CrossRef CAS PubMed.
- W. A. Gebbink, L. van Asseldonk and S. P. J. van Leeuwen, Presence of Emerging Per- and Polyfluoroalkyl Substances (PFASs) in River and Drinking Water near a Fluorochemical Production Plant in the Netherlands, Environ. Sci. Technol., 2017, 51, 11057–11065 CrossRef CAS PubMed.
- H. Fromme, A. Dreyer, S. Dietrich, L. Fembacher, T. Lahrz and W. Völkel, Neutral polyfluorinated compounds in indoor air in Germany – The LUPE 4 study, Chemosphere, 2015, 139, 572–578 CrossRef CAS PubMed.
- C. Bjerregaard-Olesen, C. C. Bach, M. Long, M. Ghisari, R. Bossi, B. H. Bech, E. A. Nohr, T. B. Henriksen, J. Olsen and E. C. Bonefeld-Jørgensen, Time trends of perfluorinated alkyl acids in serum from Danish pregnant women 2008–2013, Environ. Int., 2016, 91, 14–21 CrossRef CAS PubMed.
- H. Jin, L. Mao, J. Xie, M. Zhao, X. Bai, J. Wen, T. Shen and P. Wu, Poly- and perfluoroalkyl substance concentrations in human breast milk and their associations with postnatal infant growth, Sci. Total Environ., 2020, 713, 136417 CrossRef CAS PubMed.
- All POPs listed in the Stockholm Convention, https://chm.pops.int/TheConvention/ThePOPs/ListingofPOPs/tabid/2509/Default.aspx Search PubMed.
- Y. Pan, H. Zhang, Q. Cui, N. Sheng, L. W. Y. Yeung, Y. Sun, Y. Guo and J. Dai, Worldwide Distribution of Novel Perfluoroether Carboxylic and Sulfonic Acids in Surface Water, Environ. Sci. Technol., 2018, 52, 7621–7629 CrossRef CAS PubMed.
- J. Wang, Y. Pan, X. Wei and J. Dai, Temporal Trends in Prenatal Exposure (1998–2018) to Emerging and Legacy Per- and Polyfluoroalkyl Substances (PFASs) in Cord Plasma from the Beijing Cord Blood Bank, China, Environ. Sci. Technol., 2020, 54, 12850–12859 CrossRef CAS PubMed.
- F. Li, C. Zhang, Y. Qu, J. Chen, L. Chen, Y. Liu and Q. Zhou, Quantitative characterization of short- and long-chain perfluorinated acids in solid matrices in Shanghai, China, Sci. Total Environ., 2010, 408, 617–623 CrossRef CAS PubMed.
- Q. Wang, X. Wang and X. Ding, Rainwater trifluoroacetic acid (TFA) in Guangzhou, South China: Levels, wet deposition fluxes and source implication, Sci. Total Environ., 2014, 468–469, 272–279 CrossRef CAS PubMed.
- M. Scheurer, K. Nödler, F. Freeling, J. Janda, O. Happel, M. Riegel, U. Müller, F. R. Storck, M. Fleig, F. T. Lange, A. Brunsch and H.-J. Brauch, Small, mobile, persistent: trifluoroacetate in the water cycle – Overlooked sources, pathways, and consequences for drinking water supply, Water Res., 2017, 126, 460–471 CrossRef CAS PubMed.
- Y. Tian, Y. Yao, S. Chang, Z. Zhao, Y. Zhao, X. Yuan, F. Wu and H. Sun, Occurrence and Phase Distribution of Neutral and Ionizable Per- and Polyfluoroalkyl Substances (PFASs) in the Atmosphere and Plant Leaves around Landfills: A Case Study in Tianjin, China, Environ. Sci. Technol., 2018, 52, 1301–1310 CrossRef CAS PubMed.
- Oecd, Toward A New Comprehensive Global Database Of Per- And Polyfluoroalkyl Substances (Pfass), Summary Report On Updating The Oecd 2007 List Of Per- And Polyfluoroalkyl Substances (Pfass), OECD Series on Risk Management, No. 39, OECD Publishing, Paris, 2018 Search PubMed.
- A. Akhdhar, M. Schneider, A. Orme, L. Schultes, A. Raab, E. M. Krupp, J. P. Benskin, B. Welz and J. Feldmann, The use of high resolution graphite furnace molecular absorption spectrometry (HR-MAS) for total fluorine determination in extractable organofluorines (EOF), Talanta, 2020, 209, 120466 CrossRef CAS PubMed.
- L. Ahrens and M. Bundschuh, Fate and effects of poly- and perfluoroalkyl substances in the aquatic environment: a review, Environ. Toxicol. Chem., 2014, 33, 1921–1929 CrossRef CAS PubMed.
- L. W. Y. Yeung, A. O. De Silva, E. I. H. Loi, C. H. Marvin, S. Taniyasu, N. Yamashita, S. A. Mabury, D. C. G. Muir and P. K. S. Lam, Perfluoroalkyl substances and extractable organic fluorine in surface sediments and cores from Lake Ontario, Environ. Int., 2013, 59, 389–397 CrossRef CAS PubMed.
- Y. Miyake, N. Yamashita, P. Rostkowski, M. K. So, S. Taniyasu, P. K. S. Lam and K. Kannan, Determination of trace levels of total fluorine in water using combustion ion chromatography for fluorine: a mass balance approach to determine individual perfluorinated chemicals in water, J. Chromatogr. A, 2007, 1143, 98–104 CrossRef CAS PubMed.
- R. Aro, U. Eriksson, A. Kärrman and L. W. Y. Yeung, Organofluorine Mass Balance Analysis of Whole Blood Samples in Relation to Gender and Age, Environ. Sci. Technol., 2021, 55, 13142–13151 CAS.
- X. Wang, N. Yu, Y. Qian, W. Shi, X. Zhang, J. Geng, H. Yu and S. Wei, Non-target and suspect screening of per- and polyfluoroalkyl substances in Chinese municipal wastewater treatment plants, Water Res., 2020, 183, 115989 CrossRef CAS PubMed.
- Y. Wang, N. Yu, X. Zhu, H. Guo, J. Jiang, X. Wang, W. Shi, J. Wu, H. Yu and S. Wei, Suspect and Nontarget Screening of Per- and Polyfluoroalkyl Substances in Wastewater from a Fluorochemical Manufacturing Park, Environ. Sci. Technol., 2018, 52, 11007–11016 CrossRef CAS PubMed.
- Y. Liu, M. Qian, X. Ma, L. Zhu and J. W. Martin, Nontarget Mass Spectrometry Reveals New Perfluoroalkyl Substances in Fish from the Yangtze River and Tangxun Lake, China, Environ. Sci. Technol., 2018, 52, 5830–5840 CrossRef CAS PubMed.
- Q. Wang, Y. Ruan, L. Jin, X. Zhang, J. Li, Y. He, S. Wei, J. C. W. Lam and P. K. S. Lam, Target, Nontarget, and Suspect Screening and Temporal Trends of Per- and Polyfluoroalkyl Substances in Marine Mammals from the South China Sea, Environ. Sci. Technol., 2021, 55, 1045–1056 CrossRef CAS PubMed.
- R. Aro, U. Eriksson, A. Kärrman, K. Jakobsson and L. W. Y. Yeung, Extractable organofluorine analysis: A way to screen for elevated per- and polyfluoroalkyl substance contamination in humans?, Environ. Int., 2022, 159, 107035 CrossRef CAS PubMed.
- R. Aro, P. Carlsson, C. Vogelsang, A. Kärrman and L. W. Y. Yeung, Fluorine mass balance analysis of selected environmental samples from Norway, Chemosphere, 2021, 283, 131200 CrossRef CAS PubMed.
- United States Environmental Protection Agency, Drinking Water Health Advisories for PFOA and PFOS, 2016, https://www.epa.gov/ground-water-and-drinking-water/drinking-water-health-advisories-pfoa-and-pfos Search PubMed.
- New Hampshire Department of Environmental Services, Update on New Hampshire PFAS Drinking Water Standards (MCLs), 2020, https://www4.des.state.nh.us/nh-pfas-investigation/?p=1185 Search PubMed.
- Minnesota Department of Health, Perfluoroalkyl Substances (PFAS), https://www.health.state.mn.us/communities/environment/hazardous/topics/pfcs.html#safelevels Search PubMed.
- X. Hu, W. Shi, N. Yu, X. Jiang, S. Wang, J. P. Giesy, X. Zhang, S. Wei and H. Yu, Bioassay-directed identification of organic toxicants in water and sediment of Tai Lake, China, Water Res., 2015, 73, 231–241 CrossRef CAS PubMed.
- M. K. Björnsdotter, L. W. Y. Yeung, A. Kärrman and I. E. Jogsten, Ultra-Short-Chain Perfluoroalkyl Acids Including Trifluoromethane Sulfonic Acid in Water Connected to Known and Suspected Point Sources in Sweden, Environ. Sci. Technol., 2019, 53, 11093–11101 CrossRef PubMed.
- A.-M. Kaiser, M. Forsthuber, R. Aro, A. Kärrman, C. Gundacker, H. Zeisler, P. Foessleitner, H. Salzer, C. Hartmann, M. Uhl and L. W. Y. Yeung, Extractable Organofluorine Analysis in Pooled Human Serum and Placental Tissue Samples from an Austrian Subpopulation—A Mass Balance Analysis Approach, Environ. Sci. Technol., 2021, 55, 9033–9042 CrossRef CAS PubMed.
- A. Koch, A. Kärrman, L. W. Y. Yeung, M. Jonsson, L. Ahrens and T. Wang, Point source characterization of per- and polyfluoroalkyl substances (PFASs) and extractable organofluorine (EOF) in freshwater and aquatic invertebrates, Environ. Sci.: Processes Impacts, 2019, 21, 1887–1898 RSC.
- A. Koch, S. Yukioka, S. Tanaka, L. W. Y. Yeung, A. Kärrman and T. Wang, Characterization of an AFFF impacted freshwater environment using total fluorine, extractable organofluorine and suspect per- and polyfluoroalkyl substance screening analysis, Chemosphere, 2021, 276, 130179 CrossRef CAS PubMed.
- A. Kärrman, L. W. Y. Yeung, K. M. Spaan, F. T. Lange, M. A. Nguyen, M. Plassmann, C. A. de Wit, M. Scheurer, R. Awad and J. P. Benskin, Can determination of extractable organofluorine (EOF) be standardized? First interlaboratory comparisons of EOF and fluorine mass balance in sludge and water matrices, Environ. Sci.: Processes Impacts, 2021, 23, 1458–1465 RSC.
- A. Kärrman, T. Wang, R. Kallenborn, A. M. Langseter, S. M. Grønhovd, E. M. Ræder, J. L. Lyche, L. Yeung, F. Chen, U. Eriksson, R. Aro and F. Fredrikssion, PFASs in the Nordic Environment : Screening of Poly- and Perfluoroalkyl Substances (PFASs) and Extractable Organic Fluorine (EOF) in the Nordic Environment, Nordisk Ministerråd, Copenhagen, 2019 Search PubMed.
- J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011), Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed.
- E. L. Schymanski, J. Jeon, R. Gulde, K. Fenner, M. Ruff, H. P. Singer and J. Hollender, Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence, Environ. Sci. Technol., 2014, 48, 2097–2098 CrossRef CAS PubMed.
- Y. Lin, T. Ruan, A. Liu and G. Jiang, Identification of Novel Hydrogen-Substituted Polyfluoroalkyl Ether Sulfonates in Environmental Matrices near Metal-Plating Facilities, Environ. Sci. Technol., 2017, 51, 11588–11596 CrossRef CAS PubMed.
- Y. Bao, Y. Qu, J. Huang, G. Cagnetta, G. Yu and R. Weber, First assessment on degradability of sodium p-perfluorous nonenoxybenzene sulfonate (OBS), a high volume alternative to perfluorooctane sulfonate in fire-fighting foams and oil production agents in China, RSC Adv., 2017, 7, 46948–46957 RSC.
- Y. Qu, X. Jiang, G. Cagnetta, L. Liu, Y. Bao, W. Li, Q. Wang, C. Liang, J. Huang, H. Yang and G. Yu, Poly- and perfluoroalkyl substances in a drinking water treatment plant in the Yangtze River Delta of China: Temporal trend, removal and human health risk, Sci. Total Environ., 2019, 696, 133949 CrossRef CAS.
- Z. Wang, I. T. Cousins, M. Scheringer, R. C. Buck and K. Hungerbuhler, Global emission inventories for C4–C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources, Environ. Int., 2014, 70, 62–75 CrossRef CAS PubMed.
- Y. Li, J. Li, L. Zhang, Z. Huang, Y. Liu, N. Wu, J. He, Z. Zhang, Y. Zhang and Z. Niu, Perfluoroalkyl acids in drinking water of China in 2017: Distribution characteristics, influencing factors and potential risks, Environ. Int., 2019, 123, 87–95 CrossRef CAS PubMed.
- X. Ma, G. Shan, M. Chen, J. Zhao and L. Zhu, Riverine inputs and source tracing of perfluoroalkyl substances (PFASs) in Taihu Lake, China, Sci. Total Environ., 2018, 612, 18–25 CrossRef CAS PubMed.
- K. R. Solomon, G. J. Velders, S. R. Wilson, S. Madronich, J. Longstreth, P. J. Aucamp and J. F. Bornman, Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the Montreal and Kyoto Protocols, J. Toxicol. Environ. Health, Part B, 2016, 19, 289–304 CAS.
- S. Takagi, F. Adachi, K. Miyano, Y. Koizumi, H. Tanaka, M. Mimura, I. Watanabe, S. Tanabe and K. Kannan, Perfluorooctanesulfonate and perfluorooctanoate in raw and treated tap water from Osaka, Japan, Chemosphere, 2008, 72, 1409–1412 CrossRef CAS PubMed.
- R. Aro, U. Eriksson, A. Kärrman, F. Chen, T. Wang and L. W. Y. Yeung, Fluorine Mass Balance Analysis of Effluent and Sludge from Nordic Countries, ACS ES&T Water, 2021, 1, 2087–2096 Search PubMed.
- Y. H. Jin, W. Liu, I. Sato, S. F. Nakayama, K. Sasaki, N. Saito and S. Tsuda, PFOS and PFOA in environmental and tap water in China, Chemosphere, 2009, 77, 605–611 CrossRef CAS PubMed.
- Y. Li, J. Li, L. Zhang, Z. Huang, Y. Liu, N. Wu, J. He, Z. Zhang, Y. Zhang and Z. Niu, Perfluoroalkyl acids in drinking water of China in 2017: Distribution characteristics, influencing factors and potential risks, Environ. Int., 2019, 123, 87–95 CrossRef CAS PubMed.
- C. G. Pan, Y. S. Liu and G. G. Ying, Perfluoroalkyl substances (PFASs) in wastewater treatment plants and drinking water treatment plants: Removal efficiency and exposure risk, Water Res., 2016, 106, 562–570 CrossRef CAS PubMed.
- S. Ullah, T. Alsberg and U. Berger, Simultaneous determination of perfluoroalkyl phosphonates, carboxylates, and sulfonates in drinking water, J. Chromatogr. A, 2011, 1218, 6388–6395 CrossRef CAS PubMed.
- Z. Zhai, J. Wu, X. Hu, L. Li, J. Guo, B. Zhang, J. Hu and J. Zhang, A 17-fold increase of trifluoroacetic acid in landscape waters of Beijing, China during the last decade, Chemosphere, 2015, 129, 110–117 CrossRef CAS PubMed.
- K. N. ö Janda, H.-J. Brauch, C. Zwiener and F. T. Lange, Robust trace analysis of polar (C2–C8) perfluorinated carboxylic acids by liquid chromatography-tandem mass spectrometry: method development and application to surface water, groundwater and drinking water, Environ. Sci. Pollut. Res., 2019, 26, 7326–7336 CrossRef PubMed.
- S. J. Chow, N. Ojeda, J. G. Jacangelo and K. J. Schwab, Detection of ultrashort-chain and other per- and polyfluoroalkyl substances (PFAS) in U.S. bottled water, Water Res., 2021, 201, 117292 CrossRef CAS PubMed.
- G. Shi, Q. Cui, Y. Pan, N. Sheng, S. Sun, Y. Guo and J. Dai, 6
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 Chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos, Aquat. Toxicol., 2017, 185, 67–75 CrossRef CAS PubMed.
2 Chlorinated polyfluorinated ether sulfonate, a PFOS alternative, induces embryotoxicity and disrupts cardiac development in zebrafish embryos, Aquat. Toxicol., 2017, 185, 67–75 CrossRef CAS PubMed. - C.-H. Li, X.-M. Ren and L.-H. Guo, Adipogenic Activity of Oligomeric Hexafluoropropylene Oxide (Perfluorooctanoic Acid Alternative) through Peroxisome Proliferator-Activated Receptor γ Pathway, Environ. Sci. Technol., 2019, 53, 3287–3295 CrossRef CAS PubMed.
- G. B. Post, J. A. Gleason and K. R. Cooper, Key scientific issues in developing drinking water guidelines for perfluoroalkyl acids: contaminants of emerging concern, PLoS Biol., 2017, 15, e2002855 CrossRef PubMed.
- California State Water Resources Control Board, Response Levels Lowered for Water Systems Statewide as PFAS Investigation Continues, 2020, https://www.waterboards.ca.gov/press_room/press_releases/2020/pr02062020_pfoa_pfos_response_levels.pdf, (accessed 9th, February, 2022) Search PubMed.
- California State Water Resources Control Board, Drinking Water Notification Levels, 2021, https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/NotificationLevels.html, (accessed 9th, February, 2022) Search PubMed.
- Massachusetts Department of Environmental Protection, Massachusetts Drinking Water Standard and Health Information, 2020, https://www.mass.gov/info-details/per-and-polyfluoroalkyl-substances-pfas#massachusetts-drinking-water-standard-and-health-information-, (accessed 9th, February, 2022) Search PubMed.
- New Jersey Department of Environmental Protection and Drinking Water Quality, What are the MCLs for PFOA, PFOS, and PFNA and what do they mean?, 2020, https://www.nj.gov/dep/watersupply/pfas/ Search PubMed.
- Vermont Department of Health, What is the health advisory level for PFAS in drinking water?, 2020, https://www.healthvermont.gov/environment/drinking-water/perfluoroalkyl-and-polyfluoroalkyl-substances-pfas-drinking-water Search PubMed.
- New York Department of Health, Public Water Systems and NYS Drinking Water Standards for PFOA, PFOS and 1,4-Dioxane, 2020, https://www.health.ny.gov/environmental/water/drinking/docs/water_supplier_fact_sheet_new_mcls.pdf Search PubMed.
- Washington Department of Health, Rulemaking Activities—Office of Drinking Water, 2020, https://www.doh.wa.gov/CommunityandEnvironment/DrinkingWater/RegulationandCompliance/RuleMaking Search PubMed.
- Danish Environmental Protection Agency, Stricter requirements for per- and polyfluoroalkyl substances (PFAS), 2020, https://mst.dk/service/nyheder/nyhedsarkiv/2021/jul/skaerpede-krav-til-per-og-polyfluoralkyl-stoffer-pfas/ Search PubMed.
- Astralia Department of Health, Health Based Guidance Values for PFAS, 2017, https://www1.health.gov.au/internet/main/publishing.nsf/Content/2200FE086D480353CA2580C900817CDC/$File/HBGV-Factsheet-20190911.pdf Search PubMed.
- Swedish National Food Agency website, Recommendation – the risk management of PFAS in drinking water, 2014, https://www.livsmedelsverket.se/en/business-legislation-and-control/legislation-food-business/drinking-water-production-and-control/t#Recommendation_-_the_risk_management_of_PFAS_in_drinking_water Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See https://doi.org/10.1039/d2em00073c |
| This journal is © The Royal Society of Chemistry 2022 |