Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Electrifying the production of sustainable aviation fuel: the risks, economics, and environmental benefits of emerging pathways including CO2†
R. Gary
Grim
 *a,
Dwarak
Ravikumar
a,
Eric C. D.
Tan
*a,
Dwarak
Ravikumar
a,
Eric C. D.
Tan
 a,
Zhe
Huang
a,
Jack R.
Ferrell
III
a,
Zhe
Huang
a,
Jack R.
Ferrell
III
 a,
Michael
Resch
a,
Michael
Resch
 a,
Zhenglong
Li
b,
Chirag
Mevawala
c,
Steven D.
Phillips
c,
Lesley
Snowden-Swan
c,
Ling
Tao
a,
Zhenglong
Li
b,
Chirag
Mevawala
c,
Steven D.
Phillips
c,
Lesley
Snowden-Swan
c,
Ling
Tao
 a and
Joshua A.
Schaidle
a and
Joshua A.
Schaidle
 *a
*a
aNational Renewable Energy Laboratory, 15013 Denver W Pkwy, Golden, CO 80401, USA. E-mail: gary.grim@nrel.gov; joshua.schaidle@nrel.gov
bOak Ridge National Laboratory, 1 Bethel Valley Rd, Oak Ridge, TN 37830, USA
cPacific Northwest National Laboratory, 902 Battelle Blvd, Richland, WA 99354, USA
First published on 7th October 2022
Abstract
Due to challenges related to weight and travel distance, the medium to long-haul aviation sector is expected to remain reliant on liquid hydrocarbon fuels into the foreseeable future, representing a persistent source of CO2 emissions within the anthropogenic carbon cycle. As the world grapples with the environmental fallout from rising CO2 emissions, a prevailing strategy to mitigate the impact of air travel is through the utilization of sustainable aviation fuels (SAF) produced from biogenic carbon sources such as fats, oils, greases, and biomass. However, with the demand for SAF expected to grow substantially in the coming decades, there is concern around the availability of these feedstocks at scale. Recent studies have proposed that this potential gap in supply could be closed by utilizing CO2 as a complementary source of carbon combined with renewable electricity to drive the chemical transformation. In this study, a cross-cutting comparison of an emerging CO2-to-SAF pathway with existing routes to SAF is performed, revealing the potential for CO2-derived SAF to be competitive both in terms of costs and carbon intensity, further diversifying future options for SAF and providing a complementary option for the conversion of CO2-to-SAF beyond the decades old methanol to olefins (MTO) and Fischer–Tropsch (FT) technologies. In addition, we discuss potential technical, market, and systems integration risks for the ultimate scale-up and commercialization of the pathway identified herein.
Broader contextAs governments world-wide continue to explore options for reducing emissions across the global economy, it is becoming clearer that there is unlikely to be a “one-size-fits-all” solution, but rather a diversified portfolio of technologies and sustainable feedstocks will be needed. This paradigm is on display in the ongoing efforts to produce sustainable aviation fuels where recent projections indicate current conversion strategies, focusing on (wet) organic feedstocks such as biomass, wastes, animal fats, and oils, may be unable to satisfy the growing global demand, requiring alternative approaches. In this contribution we explore an emerging route to SAF involving front-end “power-to-liquids” (P2L) technologies whereby renewable electricity and carbon dioxide are used to produce SAF precursors in combination with secondary downstream conversion steps. Using a consistent basis of assumptions, we perform cross-cutting technoeconomic and life-cycle analyses to compare P2L CO2-to-SAF technologies against existing SAF routes to highlight, on a like-for-like basis, the economic and environmental merits, R&D needs, and the risks associated with the scaling of these emerging technologies. |
Introduction
In the year 1903 humankind took flight for the first time, ushering in a new era for travel. Fast forward nearly 120 years and over 4.5 billion people are taking to the skies across 39 million flights each year.1 Along with the rise in air travel has come substantial economic growth and globalization in which goods, people, and services can now reach all corners of the globe with an ease never before seen. However, increased access to air travel has not come without cost. Recent data finds over 100 billion gallons of predominantly fossil-based jet fuel are consumed each year to power the global aviation sector, contributing to approximately 11% of all transportation-related CO2 emissions and 3% of total anthropogenic CO2 emissions.2,3 Further, in addition to CO2, combustion of fossil-derived jet fuel promotes SOx, NOx, particulate, and contrail formation in the upper atmosphere, which are believed to contribute to additional greenhouse warming of our planet.4 With demand for jet fuel expected to more than double by 20505 and triple by 2070,6 immediate efforts to decarbonize the aviation sector are needed to curtail rising emissions and avert the worst outcomes of climate change.In other areas of the transportation sector, decarbonization efforts have begun to achieve some measures of success through direct electrification via renewable electricity and battery storage. For example, as shown in Fig. 1, the International Energy Agency (IEA) now predicts we will see a peak in global passenger car emissions (i.e., gasoline consumption) around 2020, declining to zero by the year 2070. However, a comparable decrease in emissions is not forecasted for aviation. Aviation-related emissions are projected to continue rising during the 2020s and eventually overtake commercial trucking to become the largest source of transportation-related emissions by mid to late century. This resilience in medium to long-haul aviation emissions largely stems from the need for high density energy sources which, to date, cannot be met by battery systems and electricity directly, instead requiring the persistent use of energy-dense liquid fuels.
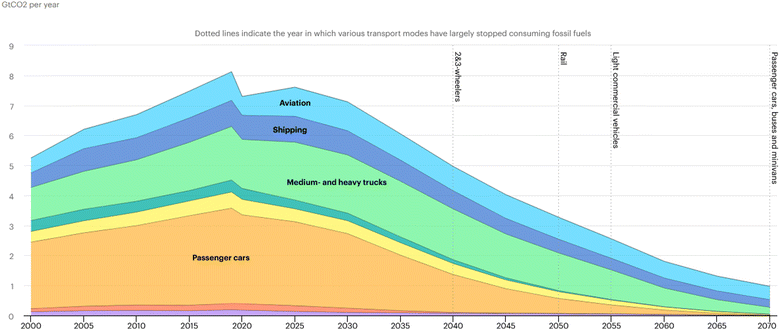 | ||
| Fig. 1 Estimated global CO2 emissions from the transportation sector in gigatonnes per year (2000–2070). Source: IEA, energy technology perspectives 2020, All rights reserved.6 | ||
To minimize the environmental impacts associated with the combustion of jet fuel, recent efforts have focused on the development and commercialization of sustainable aviation fuels (SAF) derived from non-fossil feedstocks. To date, ASTM International has certified seven pathways to make SAF predominantly involving biomass, municipal solid waste (MSW), fats, oils, and greases (FOG), sugars, and alcohol feedstocks paired with established conversion technologies such as Fischer–Tropsch (FT), hydro-processed esters and fatty acids (HEFA), and alcohol-to-jet (ATJ).5 SAF offers two main advantages relative to conventional jet fuel in that (1) the upstream utilization of sustainably sourced feedstocks can significantly lower the carbon intensity (CI) of the SAF product and provide the potential for a near carbon-neutral pathway to fuels, and (2) SAF is considered to be cleaner burning due to reduced formation of particulates and other harmful side products.4 However, the current focus on primarily organic feedstocks such as biomass and other plant and animal by-products, while a boon for sustainability, also presents a different set of challenges from the perspective of sourcing and supply logistics, scalability, cost, and land use. Whereas conventional jet fuel production is typically consolidated across a handful of oil-producing countries in large scale refineries at production rates of up to 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000s bbl day−1 to leverage benefits of economies of scale and reduced cost, the lower energy density and dispersed nature of current SAF feedstocks means SAF production is likely to be more distributed and smaller in scale, requiring an estimated future 5000–7000 individual bio-refineries worldwide co-located with feedstock supply.4 Combining these challenges with the anticipated future competition for sustainable sources of carbon, it is estimated that HEFA and other current bio-focused routes to SAF may only be able to meet approximately 50% of the global SAF demand by 2050.4
000s bbl day−1 to leverage benefits of economies of scale and reduced cost, the lower energy density and dispersed nature of current SAF feedstocks means SAF production is likely to be more distributed and smaller in scale, requiring an estimated future 5000–7000 individual bio-refineries worldwide co-located with feedstock supply.4 Combining these challenges with the anticipated future competition for sustainable sources of carbon, it is estimated that HEFA and other current bio-focused routes to SAF may only be able to meet approximately 50% of the global SAF demand by 2050.4
To expand the list of potential feedstocks beyond current options of biomass, FOG, MSW, etc., and meet the additional 50% of projected global SAF demand, researchers are now looking to e-fuels where, in a process known as “power-to-liquids” (P2L), renewable electricity and/or electrolytic H2 along with CO2 are converted into intermediate SAF precursors or, in some cases, directly into drop-in SAF products. While comparably lower in technical maturity relative to some existing SAF pathways, the utilization of CO2 offers a near limitless feedstock supply by taking advantage of carbon available in the anthropogenic carbon cycle, whilst simultaneously keeping virgin fossil carbon resources locked away beneath the earth. P2L technologies are also suggested to offer additional potential benefits relative to conventional production in terms of enhancing renewable energy deployment through increased storage capabilities, providing more flexibility in deployment location, enhanced electrical grid stability, and lower SAF CI depending on the source of electricity.7–10
However, to fully quantify the benefits of P2L CO2-to-SAF routes relative to other more established pathways, comparative techno-economic and life cycle analyses (TEA/LCA) are needed. While numerous TEA and LCA reports on SAF have been published previously, many focus on single conversion technologies and/or involve only biomass, FOG, or MSW feedstocks.11–17 With P2L CO2-derived SAF expected to comprise >50% of future global SAF supply, end-to-end analyses for P2L CO2-to-SAF conversion with renewable electricity are critically needed and are largely absent in the current literature. A notable exception is the work of Schmidt et al., in which P2L CO2 conversion through existing methanol-to-olefins (MTO) and FT pathways was studied.10 In this study, we fill this critical knowledge gap in three ways. First, expanding on the work from Schmidt, we look beyond the decades-old FT and MTO chemistries and identify, model, and analyze an emerging hybrid pathway for CO2-to-SAF including electro-, bio-, and thermo-chemistry. Second, using fully integrated Aspen Plus process models informed from the most current publicly available data and patents, we calculate the minimum jet fuel selling price (MJSP) and CI of CO2-derived SAF and provide a comparison with other notable SAF production routes. By using a consistent basis of assumptions across technologies, this cross-cutting analysis provides a like-for-like analysis of SAF production routes and clearly establishes the challenges and opportunities for CO2-to-SAF relative to existing routes. Lastly, as with any new technology, there will be risks to scale-up and commercialization. Herein, we identify potential technical, market, and systems integration risks relevant to the scale-up and commercialization of CO2-to-SAF pathways and propose key geographic and site-specific metrics relevant to early market adopters.
Methodology
Description of CO2-to-SAF pathway
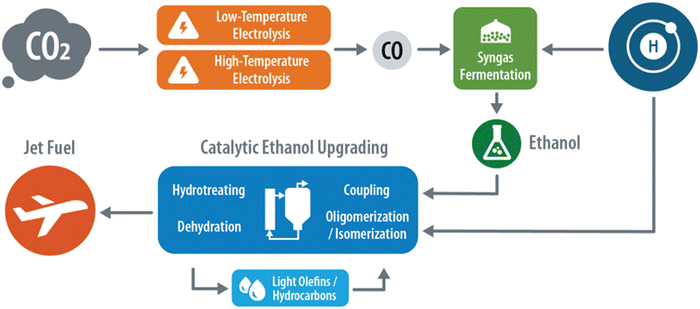
In the studied CO2-to-SAF pathway, it is assumed that SAF is produced via a three-step process, shown in Fig. 2, involving an electrolysis front-end to produce carbon monoxide (CO), a biological syngas fermentation stage to produce an ethanol intermediate, and a thermocatalytic ethanol upgrading step to produce the final SAF product. In this study we examine two cases for electrolysis, a low-temperature anion-exchange membrane (AEM) electrolyzer and high-temperature solid oxide electrolyzer cell (SOEC). We acknowledge that there are many potential pathways to produce SAF from CO2, such as MTO and FT noted earlier, as well as via the direct conversion of C4+ alcohols.18,19 We elected to model the three aforementioned steps as they (1) represent an emerging pathway of moderate technical maturity with a clear path towards commercialization, (2) utilize bioethanol which represents one of the largest sources of sustainable carbon in the United States, and (3) are compatible with P2L. The assumptions for each conversion step are discussed in detail below. It should be noted that the input values and assumptions for each conversion step were selected from publicly available data which were individually vetted by subject matter experts and are intended to provide a snapshot of the current state-of-technology for each respective process. The results presented in this report are specific only to the cases modeled herein and may not fully characterize and/or reflect the potential of other CO2-to-SAF pathways.| CO2 + H2O + 2e− → CO + 2OH− | (1) |
| Parameter | Value | Ref. |
|---|---|---|
| Low-temperature electrolysis | ||
| Whole cell voltage (V) | 3.0 | 23 |
| FE (%, CO) | 98 | 23 |
| Current density (mA cm−2) | 200 | 23 |
| Stability (h) | >3800 | 23 |
| Single-pass CO2 conversion (%) | 43 | 23 and 28 |
| Temperature (°C) | 50 | 23 |
| Total system cost ($ m−2) | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 739 |
29 |
| Stack fraction (−) | 0.74 | 29 |
| BOP fraction (−) | 0.26 | 29 |
| Replacement interval (years) | 3 | 29 |
| Replacement cost ($) | 15% stack | 29 |
| High-temperature electrolysis | ||
| Whole cell voltage (V) | 1.44 | 26 |
| FE (%, CO) | 99.5 | 24 |
| Current density (mA cm−2) | 772 | 24 |
| Stability (h) | >7000 | 24 and 26 |
| Single-pass CO2 conversion (%) | 50 | 30 |
| Temperature (°C) | 750 | 24 and 26 |
| Total system cost ($ m−2) | 7829 | 29 |
| Stack fraction (−) | 0.30 | 29 |
| BOP fraction (−) | 0.70 | 29 |
| Replacement interval (years) | 4 | 29 |
| Replacement cost ($) | 30% stack | 29 |
| Syn-gas fermentation | ||
| Ethanol productivity (g EtOH L−1 d−1) | 195 | 31 |
| Ethanol selectivity (%) | 95 | 32 |
| CO Single-pass conversion (%) | 95 | 32 |
| Product titer (g EtOH L−1) | 60 | 33 |
| Bioreactor temperature (°C) | 37 | 31 |
| Bioreactor temperature (bara) | 1–6 | 31 |
| Media recycle (%) | 80 | 32 |
| Co-product selectivity | ||
| Biomass/solids (%) | <2% | 32 |
| 2,3-Butanediol (%) | <2% | 32 |
| Acetic acid (%) | <2% | 32 |
| Thermocatalytic ethanol to jet | ||
| EtOH to light olefins | ||
| Ethanol conversion | 97 | 34 |
| Carbon selectivity to olefins | 81 | 34 |
| Carbon selectivity to oxygenates | 10 | 34 |
| Carbon selectivity to paraffins | 34 | |
| Catalyst | Ag–ZrO2/silica | 34 |
| Temperature (°C) | 325 | 34 |
| Pressure (bara) | 10 | 34 |
| WHSV (h−1) | 1.5 | 34 |
| Oligomerization | ||
| Single pass conversion (%) | 65 | 35 |
| Selectivity to C9+ olefins, single pass (%) | 66 | 35 |
| Catalyst | HZSM-5 | 35 |
| Temperature (°C) | 225 | 35 |
| Pressure (bara) | 23 | 35 |
| WHSV (h−1) | 0.46 | 35 |
| Hydrogenation | ||
| Catalyst | Pd on alumina | — |
| Temperature (°C) | 300 | — |
| Pressure (bara) | 21 | — |
| WHSV (h−1) | 5 | — |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 H2
1 H2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO
CO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CO2. Because H2O electrolysis is of high TRL and is being explored commercially,36 this step is not modelled explicitly, but rather treated as an operating expense. Further, with the goal of producing fuels with minimal CI, we assume the H2 used within the study is from renewable sources only and does not rely on fossil feedstocks (e.g., steam methane reforming or grid electricity mix). The mixture of gases is fed to a fermenter to produce ethanol following the reaction shown in eqn (2).37 The microbial productivity, selectivity, and conversion parameters are based on a proprietary anaerobic bacterium developed by LanzaTech, which has demonstrated compatibility across a range of H2, CO, and CO2 ratios.31 The LanzaTech process was selected for this conversion stage as it has commercially demonstrated the syngas to ethanol process at a large scale from steel mill waste gas, representing a high TRL option to produce ethanol.38
CO2. Because H2O electrolysis is of high TRL and is being explored commercially,36 this step is not modelled explicitly, but rather treated as an operating expense. Further, with the goal of producing fuels with minimal CI, we assume the H2 used within the study is from renewable sources only and does not rely on fossil feedstocks (e.g., steam methane reforming or grid electricity mix). The mixture of gases is fed to a fermenter to produce ethanol following the reaction shown in eqn (2).37 The microbial productivity, selectivity, and conversion parameters are based on a proprietary anaerobic bacterium developed by LanzaTech, which has demonstrated compatibility across a range of H2, CO, and CO2 ratios.31 The LanzaTech process was selected for this conversion stage as it has commercially demonstrated the syngas to ethanol process at a large scale from steel mill waste gas, representing a high TRL option to produce ethanol.38| 5H2 + CO + CO2 → C2H5OH + 2H2O | (2) |
Techno-economic analysis methodology
To model the end-to-end CO2 to SAF process, a fully integrated Aspen Plus model was developed based on the CO2-to-SAF scheme shown in Fig. 2. The design and technical parameters used within each conversion step (e.g., LTE, HTE, fermentation, thermocatalysis) were chosen to represent the current state of technology for each of the respective processes based on demonstrated process stability, TRL, and input from subject matter experts. All values and assumptions were pulled from publicly available literature.Process scale was based on an incoming flowrate of ∼52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 kg h−1 of pure CO2, equivalent to the off-gas of a ∼136 MMgal EtOH per year biorefinery. Mass and energy flowrates calculated from Aspen Plus were used to size and cost all major capital equipment. Unit costs for capital equipment were based on supplier quotes where available. In the absence of supplier data, Aspen Capital Cost Estimator (ACCE) was used. Equipment unit costs were typically scaled using an exponent term of 0.6, except in the case of the electrolyzers, which were scaled using a term of 1.0 based on similar PEM H2O electrolyzer systems.29,39 The material and energy flows along with capital cost information were input into a discounted cash flow rate of return economic model to calculate a minimum jet fuel selling price (MJSP) which corresponds to the minimum price that the SAF must sell for to generate a net present value of zero for a 10% internal rate of return. All costs are adjusted to 2016 United States dollars (2016$USD).
000 kg h−1 of pure CO2, equivalent to the off-gas of a ∼136 MMgal EtOH per year biorefinery. Mass and energy flowrates calculated from Aspen Plus were used to size and cost all major capital equipment. Unit costs for capital equipment were based on supplier quotes where available. In the absence of supplier data, Aspen Capital Cost Estimator (ACCE) was used. Equipment unit costs were typically scaled using an exponent term of 0.6, except in the case of the electrolyzers, which were scaled using a term of 1.0 based on similar PEM H2O electrolyzer systems.29,39 The material and energy flows along with capital cost information were input into a discounted cash flow rate of return economic model to calculate a minimum jet fuel selling price (MJSP) which corresponds to the minimum price that the SAF must sell for to generate a net present value of zero for a 10% internal rate of return. All costs are adjusted to 2016 United States dollars (2016$USD).
The economic and market assumptions applied in this study fall under one of three scenarios: conservative, optimistic, and aggressive. Our conservative economic assumptions represent values currently obtainable if a project were to be deployed today; however, acknowledging the likelihood for a more favorable future environment, we also consider an optimistic and an aggressive case as shown in Table 2. The optimistic market scenario reflects near-term future opportunities provided by falling renewable energy costs while keeping the current technology-specific assumptions from Table 1. The aggressive market case reflects very optimistic economic assumptions for future deployment coupled with moderate technological improvements across the comparably lower TRL electrolysis conversion steps, specifically related to metrics such as cell voltage, current density, and capital cost. Several of the most aggressive assumptions are aligned with strategic areas of interest, such as the U.S. Department of Energy's 2031 “Earthshot” goal of $1.00 per kg H2.40 All other TEA assumptions can be found in Table S1 (ESI†).
| Economic parameters | Assumed basis |
|---|---|
| a Assumes electrolytic H2 production. | |
| Conservative market case | |
| H2 pricea ($ kg−1) | 4.50 |
| CO2 price ($ tonne−1) | 40 |
| Electricity price ($ kW h−1) | 0.068 |
| Optimistic market case | |
| H2 pricea ($ kg−1) | 2.00 |
| CO2 price ($ tonne−1) | 25 |
| Electricity price ($ kW h−1) | 0.02 |
| Aggressive market case (+technological improvements) | |
| H2 pricea ($ kg−1) | 1.00 |
| CO2 price ($ tonne−1) | 0 |
| Electricity price ($ kW h−1) | 0.01 |
| LTE voltage (V) | 2.5 |
| LTE current density (mA cm−2) | 1000 |
| LTE replacement interval (years) | 7 |
| LTE capital cost ($ m−2) | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| HTE voltage (V) | 1.0 |
| HTE current density (mA cm−2) | 2000 |
| HTE replacement interval (years) | 7 |
| HTE capital cost ($ m−2) | 5000 |
| HTE replacement cost ($) | 15% stack |
Life cycle analysis methodology
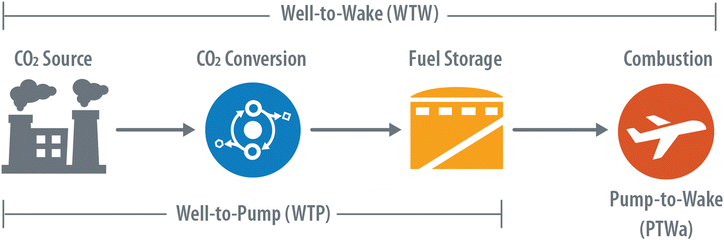
In this study we define the CO2-to-SAF system boundary for LCA to include four stages highlighted in Fig. 3: (1) the capture and purification of the CO2 feedstock, (2) the conversion of CO2-to-SAF, (3) the transportation and storage of SAF, and (4) the final combustion of SAF. The first three steps, commonly referred to as “well to pump” (WTP) emissions, are explicitly calculated in this work, whereas the final “pump to wake” (PTWa) step is considered constant and independent of the upstream technology and a value of 72.88 gCO2e per MJ is applied directly from the Argonne National Laboratory GREET model.41For the capture and purification of CO2, a variety of ad- and absorption technologies are available across the many possible sources of CO2. Due to the wide range in purity, concentration, and capture methods, the first key LCA assumption is around the CI of acquiring the CO2 feedstock. In this study, we consider six possible sources of CO2: (1) a bioethanol plant, (2) a cement plant, (3) a natural gas combined cycle (NGCC) power plant, and (4–6) three direct air capture (DAC) strategies including an industry average approach, vacuum temperature-swing adsorption (VTS), or hydroxide-based sorbents. Herein the classification of the CO2 (e.g., biogenic vs. fossil) was not explicitly accounted for and it was assumed that all captured CO2 would have otherwise either been emitted to the atmosphere or, in the case of DAC, remained in the atmosphere.
To calculate the CI associated with the capture step from each point source, our base case assumption was to apply the average estimated energy intensity and associated energy emissions factors as reported by von der Assen et al.42 and other literature sources for DAC.43,44 These data assume the use of primarily grid electricity and/or fossil energy which is representative of how such processes are conducted today. Acknowledging the evolving energy landscape, we also add two comparison cases of CO2 capture with DAC paired with renewable electricity (denoted below as RE) to show the potential for future emissions reductions through increased penetration of renewables and technology advancements. Across all cases, the calculated CI for sourcing CO2 ranged from 0–0.53 kg CO2e emitted per kg CO2 captured, as shown in Table 3 depending on the source of CO2, carbon footprint of the energy source, and compression. For more information on assumptions see Tables S2–S5 (ESI†).
| CO2 source | Emissions factor (kg CO2e per kg CO2 captured) | Emissions factor @ 10 MPa (kg CO2e per kg CO2 captured) |
|---|---|---|
| a Average energy intensity for DAC systems using fossil/grid energy.42 b Hydroxide-based DAC technology with renewable energy.43 c Vacuum temperature-swing technology with renewable energy.44 d Assumes grid electricity/fossil energy usage.42 | ||
| DAC (Avg + FE Blend)a | 0.48 | 0.53 |
| DAC (Hydroxide + RE)b | 0.08 | 0.14 |
| DAC (VTS + RE)c | 0.06 | 0.12 |
| Cementd | 0.29 | 0.35 |
| NGCCd | 0.16 | 0.21 |
| Bioethanold | 0.0 | 0.05 |
To calculate the greenhouse gas emissions associated with the CO2-to-SAF conversion stage, we tabulate the major process inputs and outputs derived from the Aspen Plus model in a life cycle inventory table. Multiplying these major mass and energy flows by their associated emissions factor yields the total CO2e emission rates for the process. Note herein we consider two primary data sources for emissions factors, the GREET database and the commercial LCA platform SimaPro which uses the EcoInvent database, when available. A major difference between the two platforms as it relates to P2L LCA is in the handling of renewable electricity emissions.
GREET assumes an emissions factor of 0 for renewable electricity (and renewable H2) by default, whereas SimaPro and other literature sources45,46 assigns a small but non-negligible value to renewable electricity generation to account for the upstream manufacturing, maintenance, and operation. Due to the high energy demands of P2L conversion, we only consider the use of renewable electricity for the conversion stage(s) and consequently, this small difference in carbon accounting for renewable electricity generation can have a significant impact on the final CI number. We report both data sets for full transparency with and without compression to 10 MPa to capture both bolt-on and centralized utilization requiring feedstock transport. The life cycle inventory, complete list of emissions factors, and carbon intensity calculations are provided in Tables S6–S8 (ESI†).
Results and discussion
Life cycle analysis
Multiplying the major mass and energy flows of the CO2-to-SAF model by their associated emissions factors yields the total hourly CO2 equivalent (CO2e) flow (provided in Table S8, ESI†). The total CO2e flow normalized by the energy output in the SAF product yields CI in terms of gCO2e per MJ. In Table 4, we show that depending on the source of CO2 and the assumptions in emissions factors for renewable energy (i.e., GREET vs. SimaPro), the CI for the default modeled CO2-to-SAF process (LTE case) can range from as low as ∼1 gCO2e per MJ to as high as 61 gCO2e per MJ.| CO2 source | CI-GREET (gCO2e per MJ) | CI-SimaPro (gCO2e per MJ) |
|---|---|---|
| a Vacuum temperature-swing technology with renewable energy.44 b Hydroxide-based DAC technology with renewable energy.43 c Average energy intensity for DAC systems using fossil/grid energy.42 | ||
| Bioethanol | 1 | 21 |
| Bioethanol (10 MPa) | 5 | 25 |
| Cement | 24 | 44 |
| Cement (10 MPa) | 28 | 48 |
| NGCC | 13 | 33 |
| NGCC (10 MPa) | 17 | 37 |
| DAC (VTS + RE)a | 6 | 26 |
| DAC (VTS + RE, 10 MPa) | 10 | 30 |
| DAC (Hydroxide + RE)b | 7 | 27 |
| DAC (Hydroxide + RE, 10 MPa) | 12 | 31 |
| DAC (Avg + FE)c | 37 | 57 |
| DAC (Avg + FE, 10 MPa) | 42 | 61 |
These results highlight that the impact of selecting a CO2 source extends beyond a simple difference in the raw input feedstock cost; rather, the concentration and purity of the source, and thus, by extension the assumptions around the energy intensity of the capture and purification step can dramatically impact the CI of the final SAF product. As shown in Tables 3 and 4, in some cases such as the most dilute CO2 sources, over 50% of the total CO2 consumed for use as a feedstock may be remitted during the capture step (on a net basis) due to high energy intensity and/or the associated emissions of sourcing the feedstock, increasing the final CI for SAF. Consequently, in an environment where making fuels with the lowest CI is becoming increasingly valued, both socially and from the perspective of qualifying for policy incentives, these data indicate the highest purity (i.e., lowest energy intensive) CO2 sources are likely to be highly coveted for early adopters as they can offer a greater reduction in CI relative to more dilute sources (i.e., more energy intensive) shown in Fig. 4.
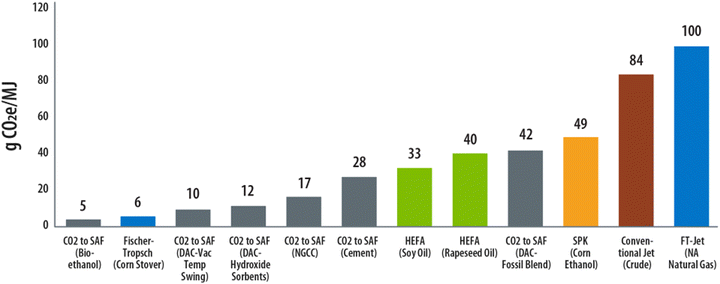 | ||
| Fig. 4 Comparison of WTWa CI values across SAF pathways using the GREET methodology assuming compression to 10 MPa. Blue = Fischer–Tropsch, Grey = CO2 utilization technologies, Green = HEFA, Orange = SPK, Red = conventional. All non-CO2 routes to SAF sourced from GREET.41,48 DAC VTS and DAC Hydroxide cases assume the use of renewable energy. | ||
To minimize this disparity, research and development into conversion strategies that are compatible with dilute CO2 streams and/or involve combined reactive capture + conversion47 is warranted to reduce the energy burden on the upstream CO2 collection stage(s). Further, reducing the CI of the energy used for CO2 capture by implementing a higher fraction of renewables will also serve to significantly lower the overall CI, as shown by the VTS and hydroxide DAC cases which assumed the use of renewable energy only.
With electricity being the major energy input for P2L processes, the results of the LCA also highlight another key finding with respect to how renewable electricity emissions are modeled and the implications for cross-comparing technologies on a like-for-like basis. Specifically, in the GREET model the emissions associated with renewable electricity and H2 produced from green electricity sources are assumed as zero by default. Conversely, the EcoInvent database in the commercial LCA platform SimaPro as well as other literature sources45,46 attribute a small amount of emissions to the manufacturing and operation of renewable energy infrastructure. Our data in Table 4 shows that for an identical set of process data these differences in accounting of renewable electricity emissions, in this case related to wind-derived electricity, contributes to a difference in ∼20 gCO2e per MJ to the SAF CI (LTE case) between the two methodologies, while keeping all other assumptions constant. Considering both methodologies are widely used in scientific literature, harmonizing these assumptions, maintaining transparency, and using consistent reporting methods will be critical to progressing the field further as the prevalence of reporting CI becomes more commonplace in scientific literature.
In Fig. 4 we provide a comparison of P2L CO2-to-SAF CI values against four ASTM-International certified SAF pathways and two pathways to conventional jet fuel evaluated using GREET methodology and electricity assumptions.48 These data show that across all CO2 sources considered, the LTE CO2-to-SAF pathway can offer an approximately 50–94% reduction in CI relative to conventional jet fuel production (84 gCO2e per MJ) depending on the source of CO2.41 Further, the LTE CO2-to-SAF CI is in general in line with and/or lower than the shown ASTM-certified SAF pathways.
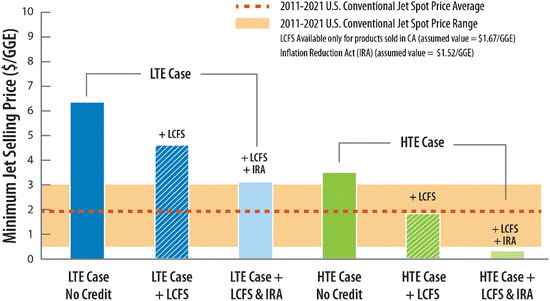
While all SAF pathways included herein represent an improvement in CI relative to the established fossil pathways, the difference in calculated CI becomes significant when considering incentives and credits where the monetary value is typically linked to the CI and GHG reduction potential. For instance, using California's low-carbon fuel standard (LCFS) as an example, SAF with a CI value of 42 gCO2e per MJ (average DAC CO2 source) would qualify for a $0.94 per gallon gasoline equivalent (GGE) incentive based on the price of the underlying credit (assumed here as $171 per ton), whereas at a CI of 5 gCO2e per MJ (bioethanol CO2 source), the same incentive increases in value by 78% to approximately $1.67 per GGE.49 Additionally, recently passed legislation in the United States further widens the gap in economic incentives for low CI fuels. In the 2022 “Inflation Reduction Act” (IRA),50 an additional $1.25 per gal incentive is provided for qualifying SAF pathways showing >50% reduction in CI relative to conventional jet fuel. This incentive is increased an additional $0.01 per gal for each percent reduction in CI above 50%, further benefiting the top performing pathways with the lowest CI. Between just these two credits, the LCFS and IRA, we show that the difference in the monetary value of these credits for our modelled cases (e.g., CI of 42 and CI of 5) could be as wide as $1.17 per GGE in total, which stands to strongly incentivize those pathways with the lowest CI (e.g., high-purity CO2 sources) and encourage integration with renewable electricity, especially for energy intensive capture and purification of dilute CO2 sources. For more details and examples of incentive calculations, see ESI.†
Techno-economic analysis and minimum jet selling price
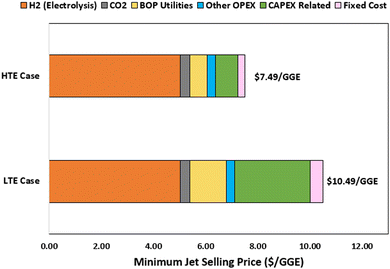
In Table 5, the calculated MJSP is presented for both the LTE and HTE P2L CO2-to-SAF cases. We show that before the application of any credits or incentives, the MJSP under the conservative economic assumptions falls in the range of $7.49–10.49 per GGE, several multiples higher than that of the trailing 10 year U.S. jet fuel range. However, as more favorable market and technological assumptions are applied in the “optimistic market” and “aggressive market + tech” scenarios defined in Table 2, we find that the MJSPs are reduced substantially to the range of $3.97–6.45 per GGE under optimistic economic assumptions, and $2.25–2.53 per GGE, under the most aggressive economic assumptions with moderate technological improvements.| MJSP ($ per GGE) | |
|---|---|
| a Data reflects current cost estimates across major SAF pathways based on ref. 51–58. b EIA U.S. Gulf Coast Kerosene-Type Jet Fuel Spot Price FOB.59 | |
| LTE case (conservative) | 10.49 |
| LTE case (optimistic) | 6.45 |
| LTE case (aggressive + tech) | 2.53 |
| HTE case (conservative) | 7.49 |
| HTE case (optimistic) | 3.97 |
| HTE case (aggressive + tech) | 2.25 |
| Estimated SAF production costa | 1.26–13.30 |
| 2011–2021 U.S. Jet spot price rangeb | 0.55–2.97 |
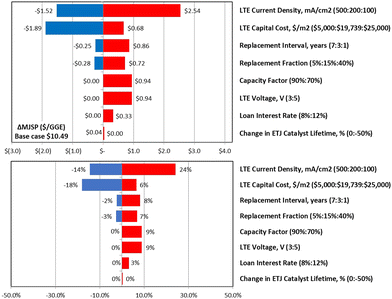
The major contributors to MJSP across the two studied cases under the conservative economic assumptions are provided in Fig. 5, showing that independent of the specific pathway, the number one cost driver impacting process economics is input electricity cost, as accounted for both directly in the CO2 electrolysis step (BOP utilities) and indirectly for the H2 generation step (H2 electrolysis).
These data indicate, as discussed above, the economic viability of CO2-to-SAF processes will be inextricably linked to available electricity price and maintaining a low-cost, high-capacity factor source of electricity will be crucial to the commercial success of P2L CO2-to-SAF. Second to electricity price, we find the next largest contributor to MJSP is the process CAPEX, primarily due to the high capital intensity of the low and high temperature electrolyzers. In comparing the two pathways within the conservative market scenario, we show that the CAPEX contribution is significantly larger in the LTE case adding $2.90 per GGE to the MJSP compared to only $0.86 per GGE in the alternative HTE case. This difference stems from the higher assumed unit cost for low temperature electrolyzers of $19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 739 per m2 (versus $7830 per m2 for HTE) combined with the lower operating current densities, which necessitates more surface area and consequently larger systems. Additionally, due to the lack of longer duration stability testing of electrolyzers in a commercial setting, both electrolysis systems are modeled with relatively short replacement frequencies (3–4 years), which also contribute to the overall production cost as well as charges related to the depreciation of the high overall cost of capital. It is anticipated that as supply chains grow and economies of scale are leveraged, these capital costs will begin to fall; however, at this time the electrolyzer capital costs are major burdens on total plant costs. These results highlight over the near term and until R&D on the LTE systems lowers unit cost and raises operating current density, economic gains may be seen by using HTE systems for converting CO2 to CO on the upstream portion of the process. However, high temperature electrolysis systems will require significant heat input which could be a detriment to deployment depending upon fuel source, location constraints, and availability at low cost.
739 per m2 (versus $7830 per m2 for HTE) combined with the lower operating current densities, which necessitates more surface area and consequently larger systems. Additionally, due to the lack of longer duration stability testing of electrolyzers in a commercial setting, both electrolysis systems are modeled with relatively short replacement frequencies (3–4 years), which also contribute to the overall production cost as well as charges related to the depreciation of the high overall cost of capital. It is anticipated that as supply chains grow and economies of scale are leveraged, these capital costs will begin to fall; however, at this time the electrolyzer capital costs are major burdens on total plant costs. These results highlight over the near term and until R&D on the LTE systems lowers unit cost and raises operating current density, economic gains may be seen by using HTE systems for converting CO2 to CO on the upstream portion of the process. However, high temperature electrolysis systems will require significant heat input which could be a detriment to deployment depending upon fuel source, location constraints, and availability at low cost.
Relative to the moving 10-year average U.S. jet fuel spot price of approximately $1.89 per GGE,59 these data show that in the absence of policy-driven incentives, the two modeled CO2-to-SAF cases show an average calculated price approximately 4.75× higher than that of fossil jet fuel under the conservative economic scenario. When compared to other pathways to produce SAF which are estimated to range from $1.26–13.30 per GGE depending on feedstock and conversion technology, we show in Fig. 6 that under conservative assumptions P2L CO2-to-SAF currently falls on the upper end of this range and on the mid- to low-end of the range when optimistic and aggressive assumptions are applied. The economic competitiveness of CO2-to-SAF could be further enhanced if policy incentives are considered, as discussed above, the potential for a lower CI final product can contribute to larger subsidies and credits to help offset any higher costs.
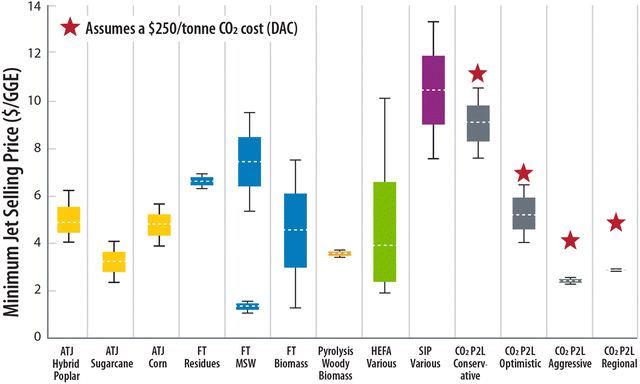 | ||
| Fig. 6 Comparison of minimum jet selling price across SAF pathways (FOG = fats, oils, and grease; UCO = used cooking oil). Red stars reflect cost of CO2-derived SAF if CO2 price were fixed at $250 per tonne. Data from ref. 51–58. | ||
Energy efficiency for CO2-to-SAF
The energy inputs and outputs for the as-modeled CO2-to-SAF process fall across five categories: (1) CO2 capture, (2) CO2 electrolysis, (3) H2 generation, (4) balance of plant work and heat, and (5) energy out in the form of SAF. Tabulating these mass and energy flows and multiplying by their respective intensity factors (see Table S9, ESI†) allows for the calculation of total process energy efficiency (EE). Shown in Table 6, we calculate that depending on the CO2 source and associated energy intensity for purification, the end-to-end energy efficiencies for the as-modeled process ranges from approximately 28–37%. When compared to the energy efficiency reported for other more mature ASTM-certified routes (HEFA, FT, ATJ) we find that all our CO2-to-SAF cases fell on the lower end for EE. While our best case of using CO2 sourced from a bioethanol refinery performed similarly to the baseline ATJ and FT cases, all CO2 cases significantly underperformed HEFA, which showed top end EE values approaching 74%. The top three largest energy sinks in our CO2-to-SAF processes were H2 generation for fermentation and hydrotreating at an average of 59% of total energy input, electric demand for CO2 electrolysis at an average of 21% total energy input, and CO2 capture + process separations contributing to an average of 20% of the total energy input required. Based on these data, possible avenues for improving the energy efficiency for CO2-to-SAF moving forward may include minimizing H2 usage (e.g., through other fermentation stoichiometries), performing electrolysis reactions more efficiently (e.g., lower cell voltages), and minimizing energy demands for CO2 capture and purification (e.g., one-step reactive capture + conversion).| CO2 source | Energy efficiency (%) | Energy intensity (MJ per kg HC fuels) |
|---|---|---|
| a Ref. 60. b Ref. 61. c Ref. 62. | ||
| CO2 (DAC) | 31.5 | 138.3 |
| CO2 (DAC, hydroxide) | 31.1 | 140.1 |
| CO2 (DAC, VTSA) | 28.4 | 153.24 |
| CO2 cement | 33.5 | 129.9 |
| CO2 NGCC | 35.2 | 123.5 |
| CO2 ethanol | 36.8 | 118.3 |
| HEFA FOGa | 74.0% | 59.3 |
| Fischer tropschb | 43.6% | 101.0 |
| Alcohol-to-jetc | 39.3% | 111.0 |
Region-specific analysis
At the onset of an analysis, it can be unclear precisely when, where, and how a technology would be deployed. Consequently, in the absence of data on commissioning year, country/state of operation, status of the technology, etc., a common first step is the use of averaged global or country-specific economic and technical assumptions, which while useful, can be overly conservative and not necessarily representative of localized opportunities present for the early adopters at a smaller scale. Early adopters will typically seek strategic deployment of assets in these targeted areas with the most favorable economic conditions to raise profit margins and ease the higher cost of pioneer plant deployment. Below we explore possible deployment locations within the United States for early adopters of the CO2-to-SAF process and highlight notable economic opportunities to produce CO2-derived SAF at a lower price point.From the MJSP cost breakdown data presented in Fig. 5, access to a reliable (i.e., high-capacity factor) and cheap source of electricity is expected to be the most significant contributor to the final SAF cost. With most electric grids worldwide still fueled to some degree by fossil resources for the foreseeable future, direct connection to the electrical grid is not considered herein nor recommended currently due to the associated high carbon footprint and impact on SAF CI (see Fig. S2, ESI†). Consequently, the first major challenge for P2L CO2-to-SAF commercialization will be sourcing low-cost renewable electricity. Secondary to the cost of electricity, early adopters of CO2-to-SAF will also consider the logistics of securing access to a reliable, pure, and cheap source of CO2. With utility prices subject to supply and demand fluctuations and varying over time, we evaluate current region-specific opportunities based on published United States power purchase agreement (PPA) data for solar and wind derived electricity for the years 2016–2021.63,64 A PPA represents a contract between an electricity supplier and a consumer where the producer finances the upfront capital, construction, and operating charges, and in return sets a fixed electricity price which the consumer is locked in for a negotiated term of typically 5–20 years. In Table 7, we provide the average negotiated PPA rate for solar and wind energy from 2016–2021, along with the installed capacity for the PPA agreements across the 11 unique operating regions of the U.S. grid shown in Fig. 7 (Hawaii not shown).
| Grid ISO region | Average solar PPA price ($ per kW h) | Installed PPA solar capacity (MW-AC) | Average wind PPA price ($ per kW h) | Installed PPA wind capacity (MW-AC) |
|---|---|---|---|---|
| CAISO | 0.031 | 1704 | 0.042 | 451 |
| ERCOT | 0.030 | 464 | 0.022 | 200 |
| FRCC | — | — | — | — |
| Hawaii | 0.095 | 110 | — | — |
| ISO-NE | 0.080 | 287 | 0.039 | 101 |
| MISO | 0.038 | 270 | 0.023 | 2888 |
| NYISO | 0.098 | 621 | — | — |
| PJM | 0.049 | 610 | 0.034 | 649 |
| SERC | 0.034 | 1332 | 0.018 | 479 |
| SPP | 0.051 | 10 | 0.016 | 3918 |
| WECC | 0.026 | 4754 | 0.025 | 3218 |
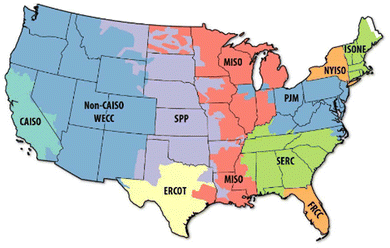 | ||
| Fig. 7 United States electrical grid operating regions. Reproduced from ref. 65. | ||
Across the regions of the electric grid, we show that on average the lowest cost renewable electricity is provided via wind energy in the SPP region at an average price of $0.016 per kW h. Further, SPP also contains the largest PPA-supply of installed wind energy resources at 3918 MW-AC during the period of 2016–2021. While these data only provide an averaged snapshot across the various regions and one-off opportunities for lower electricity pricing may exist in other regions (e.g., SERC-wind, WECC-solar), the combined low price and high supply of wind energy available in the SPP region make for an attractive location for a first-of-a-kind deployment of P2L CO2-to-SAF.
Within the SPP region most of the installed wind capacity, both PPA and non PPA, is primarily concentrated into two states, Texas and Iowa (see Fig. S3, ESI†), suggesting from the perspective of cost and installed production either of these two states could represent a promising opportunity for P2L CO2-to-SAF deployment.
For the purposes of this case study we selected Texas as it is also home to some of the largest solar energy projects in the U.S., providing an attractive option to supplement the low-cost wind energy and increase overall capacity factor.63 Further, Texas offers a diversified selection of CO2 point sources as well as access to dedicated CO2 pipeline infrastructure (Fig. S4, ESI†).
From this set of regional economic data we calculate MJSP for the aforementioned LTE and HTE cases, highlighting the opportunity for an early adopter of P2L CO2-to-SAF specific to the West Texas region. Shown in Fig. 8, under these regional assumptions (full list provided in Table S10, ESI†) we find that the LTE case deployed in the West Texas region could achieve a MJSP of approximately $6.27 per GGE, over $4 cheaper than our default conservative economic scenario which assumes U.S. domestic averaged values. The major driver for the difference in cost is in the assumptions around electricity price which, in the regional economic scenario, is much lower at $0.016 per kW h compared to $0.068 per kW h assumed in the other conservative scenario(s). The regional LTE MJSP is further decreased when considering the application of policy incentives. Applying the low-carbon fuel standard (LCFS) noted above, which provides a financial incentive for fuels sold for use in California, we find that based on the calculated CI for our SAF product of 5 gCO2e per MJ, the LCFS incentive (based on a credit value assumed here as $171 per ton), is approximately $1.67 per GGE, lowering the MJSP further to $4.60 per GGE (see Fig. S5 for more information, ESI†). Applying the second Inflation Reduction Act (IRA) incentive, we find that, assuming a SAF CI of 5 gCO2e per MJ, it would offer an additional discount of $1.69 per gal ($1.52 per GGE), bringing the MJSP down to only $3.08 per GGE. Applying the same input cost and incentive assumptions to the HTE case, we show the MJSPs range from $3.51 per GGE down to as low as ∼$0.32 per GGE with both incentives. These data suggest that with incentives, pockets of opportunity exist throughout the U.S. and likely abroad in which P2L CO2-to-SAF processes could be cost-competitive not only with other routes to SAF, but also conventional jet fuel production.
Risks for CO2-to-SAF commercialization
The development, scale-up, and commercialization of new technologies is often rife with risk. Identifying and mitigating potential risks as early as possible in the commercialization process is critical to increasing the chances of success and long-term viability. With the complete end-to-end CO2-to-SAF process discussed herein having never been demonstrated previously, we acknowledge the heightened probability for a variety of technical, market, and systems integration risks. To characterize and identify these specific risks, we engaged with a team of subject matter experts with experience across each of the proposed conversion steps as well as technology providers with expertise in process scale up and commercialization. Working with this team, we developed a risk register to characterize risks based on probability and impact to inform and guide future R&D and investments in P2L CO2-to-SAF. Below we summarize the key takeaways as related to technical, market, and systems integration risks. The full register of risks can be found in Tables S11–S14 (ESI†).| Source of risk | TEA variable | Variable range |
|---|---|---|
| Unknown durability profile | Electrolyzer replacement interval (years) | 1–7 |
| Electrolyzer replacement fraction (% cost) | 5–40 | |
| Performance degradation & impact of impurities | LTE voltage (V) | 3.0–5.0 |
| LTE current density (mA cm−2) | 100–500 | |
| ETJ catalyst price increase (%) | 0–100 | |
| ETO conversion rate (%) | 70–98 | |
| Difficulty in finding investors | Loan interest rate (%) | 8.0–12.0 |
| Handling process turndown and intermittency | Capacity factor (%) | 70–90 |
| Availability of critical components | LTE capital cost ($ per m2) | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–22 000–22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Although much of the technological foundation has been laid for P2L CO2-to-SAF, major research and development is still needed, particularly on the electrolysis stage(s) and general process intensification. Scaling the production of electrolyzers to drive down unit costs (and size) while running concurrent long term testing to evaluate and identify failure mechanisms are two top R&D needs to design and build systems capable of competing commercially. Further, with renewable energy utilization being a cornerstone of P2L, more research is needed to understand the impacts of intermittency and specifically on how to buffer individual processes, each with their own unique technology elements (e.g., electro-, bio-, and thermo-chemistry), during system turndowns. Finally, and more broadly, there is a need for systems analysis and systems of systems analysis which considers not only the standalone process, but evaluates how such a P2L process would play a part within a network of larger energy systems across the grid as the penetration of renewable electricity continues to expand around the globe.
Conclusion
In this study, the techno-economic and life cycle merits of an emerging P2L CO2-to-SAF pathway were evaluated and compared against several ASTM-approved SAF production routes. Our analysis shows P2L CO2-to-SAF can be an attractive future option for SAF production being competitive both in terms of costs and CI, providing a diversified and complementary option for the conversion of CO2 to SAF beyond the decades old MTO and FT technologies. When compared to petroleum jet fuel, the models and analysis presented herein indicate that currently CO2-to-SAF is more costly and that the gap can be reduced, but not fully closed, by pursuing high-efficiency, high-yield technologies in locations with access to low-cost renewable electricity. Under the most aggressive economic scenario considered, we find that with advancements to the underlying technologies combined with substantial reductions in the price of electricity down to $0.01 per kW h, SAF costs as low as $2.25–2.53 per GGE may be obtainable in the future without incentives. Specific to the United States, our analysis suggests that near-term deployment of P2L CO2-to-SAF in the West Texas region could see production costs as low as $3.51 per GGE using currently available technology, with the potential for MJSPs as low as $0.32 per GGE driven by $3.19 per GGE total applied incentives ($1.67 from LCFS and $1.52 from the Inflation Reduction Act). Producing SAF from CO2 is expected to lower the CI of the fuel by 50–94% relative to fossil jet fuel, similar to other SAF production methods. Further minimizing SAF CI will rely on sourcing high purity CO2 feedstocks, the utilization of low energy intensity conversion pathways, and by ensuring that front-end CO2 capture and purification stages are fully integrated with renewable energy sources.Abbreviations
| ACCE | Aspen capital cost estimator |
| AEM | Anion-exchange membrane |
| ASTM | American society for testing and materials |
| ATJ | Alcohol-to-jet |
| BBL | Barrel |
| CAPEX | Capital expense |
| CI | Carbon intensity |
| CO | Carbon monoxide |
| CO2e | CO2 equivalent |
| DAC | Direct air capture |
| ETO | Ethanol-to-olefins |
| EtOH | Ethanol |
| FE | Fossil energy |
| FOG | Fats, oils, and greases |
| FT | Fischer-tropsch |
| GGE | Gallon of gasoline equivalent |
| GREET | Greenhouse gases, regulated emissions, and energy use in technologies model |
| HEFA | Hydroprocessed esters and fatty acids |
| THE | High-temperature electrolysis |
| IEA | International energy agency |
| LCA | Life cycle analysis |
| LCFS | Low-carbon fuel standard |
| LTE | Low-temperature electrolysis |
| MJSP | Minimum jet fuel selling price |
| MSW | Municipal solid waste |
| MTO | Methanol to olefins |
| NGCC | Natural gas combined cycle |
| P2L | Power-to-liquids |
| PNNL | Pacific northwest national laboratory |
| PPA | Power purchase agreement |
| PTW | Pump-to-wake |
| SAF | Sustainable aviation fuel |
| SOEC | Solid-oxide electrochemical cell |
| SPK | Synthetic paraffinic kerosene |
| SSA | Sustainable skies act |
| TEA | Techno-economic analysis |
| VTS | Vacuum temperature swing |
| WTP | Well-to-pump |
| WTWa | Well-to-wake |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was authored by the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC, for the U.S. Department of Energy (DOE) under Contract No. DE-AC36-08GO28308. Funding provided by the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office. The views expressed in the article do not necessarily represent the views of the DOE or the U.S. Government. The U.S. Government retains and the publisher, by accepting the article for publication, acknowledges that the U.S. Government retains a nonexclusive, paid-up, irrevocable, worldwide license to publish or reproduce the published form of this work, or allow others to do so, for U.S. Government purposes.References
- The International Civil Aviation Organization, The World of Air Transport in 2019, https://www.icao.int/annual-report-2019/Pages/the-world-of-air-transport-in-2019.aspx, (accessed 12/14, 2021).
- IEA, Transport sector CO2 emissions by mode in the Sustainable Development Scenario, 2000-2030, https://www.iea.org/data-and-statistics/charts/transport-sector-co2-emissions-by-mode-in-the-sustainable-development-scenario-2000-2030, (accessed 12/14, 2021).
- IEA, Tracking Aviation 2020, https://www.iea.org/reports/tracking-aviation-2020, (accessed 12/14, 2021).
- ICF, Fueling Net Zero: How the aviation industry can deploy sufficient sustainable aviation fuel to meet climate demands, https://www.icf.com/insights/transportation/deploying-sustainable-aviation-fuel-to-meet-climate-ambition, (accessed 12/14, 2021).
- U.S. Department of Energy, Sustainable Aviation Fuel: Review of Technical Pathways, https://www.energy.gov/sites/prod/files/2020/09/f78/beto-sust-aviation-fuel-sep-2020.pdf, (accessed 12/14, 2021).
- IEA, Energy Technology Perspectives 2020, https://www.iea.org/reports/energy-technology-perspectives-2020, (accessed 12/14, 2021).
- M. Bailera, P. Lisbona, L. M. Romeo and S. Espatolero, Renewable Sustainable Energy Rev., 2017, 69, 292–312 CrossRef CAS.
- A. Buttler and H. Spliethoff, Renewable Sustainable Energy Rev., 2018, 82, 2440–2454 CrossRef CAS.
- R. G. Grim, Z. Huang, M. T. Guarnieri, J. R. Ferrell, L. Tao and J. A. Schaidle, Energy Environ. Sci., 2020, 13, 472–494 RSC.
- P. Schmidt, V. Batteiger, A. Roth, W. Weindorf and T. Raksha, Chem. Ing. Tech., 2018, 90, 127–140 CrossRef CAS.
- A. Alam, M. F. H. Masum and P. Dwivedi, GCB Bioenergy, 2021, 13, 1800–1813 CrossRef CAS.
- A. Diniz, R. Sargeant and G. J. Millar, Biotechnol. Biofuels, 2018, 11, 161 CrossRef PubMed.
- P. Laveille, J. Uratani, J. G. G. Barron, M. Brodeur-Campbell, N. R. Chandak, A. George, S. Morin, A. R. Galvan and M. Berthod, Biofuels, Bioprod. Biorefin., 2021, 16(1), 27–42 CrossRef.
- R. Shen, L. Tao and B. Yang, BioFPR, 2019, 13, 486–501 CAS.
- A. H. Tanzil, K. Brandt, M. Wolcott, X. Zhang and M. Garcia-Perez, Biomass Bioenergy, 2021, 145, 105942 CrossRef CAS.
- A. H. Tanzil, X. Zhang, M. Wolcott, K. Brandt, C. Stöckle, G. Murthy and M. Garcia-Perez, Biomass Bioenergy, 2021, 146, 105937 CrossRef CAS.
- W.-C. Wang and L. Tao, Renewable Sustainable Energy Rev., 2016, 53, 801–822 CrossRef CAS.
- S. Geleynse, K. Brandt, M. Garcia-Perez, M. Wolcott and X. Zhang, ChemSusChem, 2018, 11, 3728–3741 CrossRef CAS PubMed.
- D. Silva Braz and A. Pinto Mariano, Bioresour. Technol., 2018, 268, 9–19 CrossRef CAS PubMed.
- Y. Chen, C. W. Li and M. W. Kanan, J. Am. Chem. Soc., 2012, 134, 19969–19972 CrossRef CAS PubMed.
- B. Kim, S. Ma, H.-R. Molly Jhong and P. J. A. Kenis, Electrochim. Acta, 2015, 166, 271–276 CrossRef CAS.
- K. P. Kuhl, E. R. Cave, D. N. Abram and T. F. Jaramillo, Energy Environ. Sci., 2012, 5, 7050–7059 RSC.
- Z. Liu, H. Yang, R. Kutz and R. I. Masel, J. Electrochem. Soc., 2018, 165, J3371–J3377 CrossRef CAS.
- R. Kungas, P. Blennow, T. Heiredal-Clausen, T. H. Norby, J. Rass-Hansen, J. B. Hansen and P. G. Moses, ECS Trans., 2019, 91, 215–223 CrossRef.
- C. Mittal, C. Hadsbjerg and P. Blennow, Chem. Eng. World, 2017, 44–46 Search PubMed.
- R. Küngas, J. Electrochem. Soc., 2020, 167(4), 044508 CrossRef.
- C. Duan, R. Kee, H. Zhu, N. Sullivan, L. Zhu, L. Bian, D. Jennings and R. O’Hayre, Nat. Energy, 2019, 4, 230–240 CrossRef CAS.
- E. Jeng and F. Jiao, Reaction Chem. Eng., 2020, 5, 1768–1775 RSC.
- NREL, H2A: Hydrogen Analysis Production Models, https://www.nrel.gov/hydrogen/h2a-production-models.html, (accessed 03/19, 2021).
- K. Xie, Y. Zhang, G. Meng and J. T. S. Irvine, J. Mater. Chem., 2011, 21, 195–198 RSC.
- F. Liew, M. E. Martin, R. C. Tappel, B. D. Heijstra, C. Mihalcea and M. Kopke, Front. Microbiol., 2016, 7, 694 Search PubMed.
- LanzaTech, CCU-Now: fuels and chemicals from waste, (https://ec.europa.eu/energy/sites/ener/files/documents/25_sean_simpson-lanzatech.pdf).
- R. M. Handler, D. R. Shonnard, E. M. Griffing, A. Lai and I. Palou-Rivera, Ind. Eng. Chem. Res., 2015, 55, 3253–3261 CrossRef.
- V. L. Dagle, A. D. Winkelman, N. R. Jaegers, J. Saavedra-Lopez, J. Hu, M. H. Engelhard, S. E. Habas, S. A. Akhade, L. Kovarik, V.-A. Glezakou, R. Rousseau, Y. Wang and R. A. Dagle, ACS Catal., 2020, 10, 10602–10613 CrossRef CAS.
- M. A. Lilga, R. T. Hallen, K. O. Albrecht, A. R. Cooper, J. G. Frye and K. K. Ramasamy, US Pat., 9771533B2, 2017.
- U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen Production: Electrolysis, https://www.energy.gov/eere/fuelcells/hydrogen-production-electrolysis, (accessed 12/17, 2021).
- Z. Huang, G. Grim, J. Schaidle and L. Tao, Appl. Energy, 2020, 280, 115964 CrossRef CAS.
- LanzaTech, World's First Commercial Waste Gas to Ethanol Plant Starts Up, https://www.lanzatech.com/2018/06/08/worlds-first-commercial-waste-gas-ethanol-plant-starts/, (accessed 1/3, 2022).
- NREL, Current Central Hydrogen Production from Polymer Electrolyte Membrane (PEM) Electrolysis (2019) version 3.2018, https://www.nrel.gov/hydrogen/assets/docs/current-central-pem-electrolysis-v3-2018.xlsm, (accessed 09/30, 2022).
- U.S. Department of Energy, Office of Energy Efficiency and Renewable Energy, Hydrogen Shot, https://www.energy.gov/eere/fuelcells/hydrogen-shot, (accessed 1/3, 2022).
- Argonne National Laboratory, GREET WTW Calculator, https://greet.es.anl.gov/tools, (accessed 1/3, 2022).
- N. von der Assen, L. J. Muller, A. Steingrube, P. Voll and A. Bardow, Environ. Sci. Technol., 2016, 50, 1093–1101 CrossRef CAS PubMed.
- M. M. J. de Jonge, J. Daemen, J. M. Loriaux, Z. J. N. Steinmann and M. A. J. Huijbregts, Int. J. Greenhouse Gas Control, 2019, 80, 25–31 CrossRef CAS.
- S. Deutz and A. Bardow, Nat. Energy, 2021, 6, 203–213 CrossRef CAS.
- J. Burkhardt, A. Patyk, P. Tanguy and C. Retzke, Appl. Energy, 2016, 181, 54–64 CrossRef.
- V. Fthenakis and E. Leccisi, Prog. Photovoltaics Res. Appl., 2021, 29, 1068–1077 CrossRef CAS.
- T. G. Deutsch, S. Baker, P. Agbo, D. R. Kauffman, J. Vickers and J. A. Schaidle, Summary Report of the Reactive CO2 Capture: Process Integration for the New Carbon Economy Workshop, https://www.nrel.gov/docs/fy21osti/78466.pdf, (accessed 1/3, 2022).
- Argonne National Laboratory, GREET.Net: A fresh design for GREET life cycle analysis tool, https://greet.es.anl.gov/index.php?content=greetdotnet, (accessed 1/03, 2022).
- California Air Resource Board, Weekly LCFS Credit Transfer Activity Reports, https://www.arb.ca.gov/fuels/lcfs/credit/lrtweeklycreditreports.htm, (accessed 12/15, 2021).
- J. A. Yarmuth 117th Congress, H.R.5376 - Inflation Reduction Act of 2022, https://www.congress.gov/bill/117th-congress/house-bill/5376, (accessed 09/30, 2022).
- J. T. Crawford, C. W. Shan, E. Budsberg, H. Morgan, R. Bura and R. Gustafson, Biotechnol. Biofuels, 2016, 9, 141 CrossRef PubMed.
- S. de Jong, R. Hoefnagels, A. Faaij, R. Slade, R. Mawhood and M. Junginger, Biofuels, Bioprod. Biorefin., 2015, 9, 778–800 CrossRef CAS.
- B. C. Klein, M. F. Chagas, T. L. Junqueira, M. C. A. F. Rezende, T. D. F. Cardoso, O. Cavalett and A. Bonomi, Appl. Energy, 2018, 209, 290–305 CrossRef CAS.
- U. Neuling and M. Kaltschmitt, Fuel Process. Technol., 2018, 171, 54–69 CrossRef CAS.
- M. F. Shahriar and A. Khanal, Fuel, 2022, 325, 124905 CrossRef CAS.
- R. Shen, L. Tao and B. Yang, Biofuels, Bioprod. Biorefin., 2018, 13, 486–501 CrossRef.
- O. Winjobi, D. R. Shonnard and W. Zhou, ACS Sustainable Chem. Eng., 2017, 5, 4529–4540 CrossRef CAS.
- Z. Yang, K. Qian, X. Zhang, H. Lei, C. Xin, Y. Zhang, M. Qian and E. Villota, Energy, 2018, 154, 289–297 CrossRef CAS.
- EIA, U.S. Gulf Coast Kerosene-Type Jet Fuel Spot Price FOB, https://www.eia.gov/dnav/pet/hist/eer_epjk_pf4_rgc_dpgD.htm, (accessed 3/15/2022, 2022).
- L. Tao, A. Milbrandt, Y. Zhang and W. C. Wang, Biotechnol. Biofuels, 2017, 10, 261 CrossRef PubMed.
- Y. Zhang, A. H. Sahir, E. C. D. Tan, M. S. Talmadge, R. Davis, M. J. Biddy and L. Tao, Green Chem., 2018, 20, 5358–5373 RSC.
- L. Tao, J. N. Markham, Z. Haq and M. J. Biddy, Green Chem., 2017, 19, 1082–1101 RSC.
- M. Bolinger, J. Seel, C. Warner and D. Robson, Utility-Scale Solar, 2021 Edition, https://emp.lbl.gov/utility-scale-solar, (accessed 1/5, 2022).
- R. Wiser, M. Bolinger, B. Hoen, D. Millstein, J. Rand, G. Barbose, N. Darghouth, W. Gorman, S. Jeong, A. Mills and B. Paulos, Land-Based Wind Market Report: 2021 Edition, https://emp.lbl.gov/wind-technologies-market-report/, (accessed 1/5, 2022).
- P. Denholm, Y. Sun and T. Mai, An Introduction to Grid Services: Concepts, Technical Requirements, and Provision from Wind, https://www.nrel.gov/docs/fy19osti/72578.pdf, (accessed 1/5, 2022).
- LanzaTech, Carbon Engineering and LanzaTech partner to advance jet fuel made from air, https://www.lanzatech.com/2021/07/26/carbon-engineering-and-lanzatech-partner-to-advance-jet-fuel-made-from-air/, (accessed 1/5, 2022).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/D2EE02439J |
| This journal is © The Royal Society of Chemistry 2022 |