Lithium deposition mechanism on Si and Cu substrates in the carbonate electrolyte†
Abstract
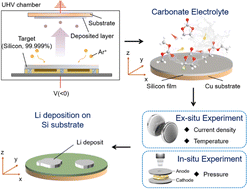
Electrochemical Li deposition occurs in fast-charging Li-ion batteries and Li metal batteries. The morphologies of the Li deposits govern the reversibility of the deposition/stripping reaction, and affect the tendency of internal-shorting, therefore determining the performance and safety of Li-ion and Li metal batteries. Many different morphologies, including hemi-sphere, granular, columnar, whisker, and dendrite, have been observed. However, how Li deposits grow into different morphologies largely remains elusive. In this work, we reveal the mechanism of Li growth on Si and Cu substrates in a commercial carbonate electrolyte by combing electron imaging, optical imaging, molecular dynamics simulation and electrochemical tests with theoretical analysis. Li growth dynamics is analyzed by comparing images of Li deposits at different currents, capacities, and temperatures. For Si substrates, a 3D-2D growth mechanism is found almost irrespective of the current density. For Cu substrates, the growth follows a 3D-2D mechanism under quasi-equilibrium conditions but transitions to a different mechanism under high current density, in which a 1D Li whisker grows out from Li islands. The tendency of whisker growth on both substrates is quantified and its dependence on current density is determined. It is revealed that all Li deposits on Cu become whiskers beyond a critical current density (Jw = 0.79 ± 0.10 mA cm−2) while Si substrates have a much larger Jw (>10 mA cm−2), leading to virtually no whisker formation. In addition, correlations between whisker growth, coulombic efficiency, and the tendency of internal shorting are determined. The nucleation and growth mechanism, as well as the transition between morphologies, are comprehensively discussed based on the results of this work and state-of-the-art findings on Li deposition.
- This article is part of the themed collection: 2022 Journal of Materials Chemistry Lectureship runners-up: Jessica Wade and Luisa Whittaker-Brooks


 Please wait while we load your content...
Please wait while we load your content...