Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Mg3(Bi,Sb)2-based thermoelectric modules for efficient and reliable waste-heat utilization up to 750 K†
Yuntian
Fu‡
 a,
Qihao
Zhang‡
a,
Qihao
Zhang‡
 *b,
Zhongliang
Hu
a,
Meng
Jiang
*b,
Zhongliang
Hu
a,
Meng
Jiang
 a,
Aibin
Huang
a,
Aibin
Huang
 c,
Xin
Ai
b,
Shun
Wan
d,
Heiko
Reith
b,
Lianjun
Wang
c,
Xin
Ai
b,
Shun
Wan
d,
Heiko
Reith
b,
Lianjun
Wang
 *a,
Kornelius
Nielsch
*be and
Wan
Jiang
af
*a,
Kornelius
Nielsch
*be and
Wan
Jiang
af
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials & College of Materials Science and Engineering, Donghua University, Shanghai, 201620, China. E-mail: wanglj@dhu.edu.cn
bInstitute for Metallic Materials, Leibniz Institute for Solid State and Materials Research Dresden (IFW Dresden), Dresden, 01069, Germany. E-mail: k.nielsch@ifw-dresden.de; q.zhang@ifw-dresden.de
cState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai, 200050, China
dCenter for High Pressure Science and Technology Advanced Research (HPSTAR), Shanghai, 201203, China
eInstitute of Materials Science, Dresden University of Technology, Dresden, 01062, Germany
fInstitute of Functional Materials, Donghua University, Shanghai, 201620, China
First published on 14th June 2022
Abstract
Thermoelectric modules can directly convert the waste heat released by plants or vehicles into useful electricity, providing a clean and sustainable way to use fossil energy more efficiently. However, their commercial application in power generation, especially at temperatures above 500 K, has proceeded very slowly due to the low module efficiency, scarce or toxic constituent elements, and lack of stable metallization at high temperatures. Here, we develop a highly efficient, environmentally friendly and cost-effective thermoelectric module by using n-type Se-doped Mg3(Bi,Sb)2 and p-type CoSb3-based skutterudite. The module can operate robustly up to 750 K and achieves a conversion efficiency of over 9% under a temperature difference of 450 K, superior to that of state-of-the-art single-stage thermoelectric modules. These achievements result from a comprehensive study involving optimization of the material composition and microstructure, screening for effective diffusion barrier layers, and rational design of the module structure. Our work demonstrates the feasibility and scalability of efficient and reliable thermoelectric modules based on sustainable elements for broad applications in mid-temperature waste heat recovery.
Introduction
The worldwide energy crisis caused by the increasing use of fossil fuels due to enhanced energy consumption and thereby the production of very large amounts of greenhouse gases such as CO2 is one of the most severe problems faced by human beings. Excess heat, as a byproduct of combustion and industrial processes, is a ubiquitous unutilized energy resource that is still primarily considered waste. Effective harnessing of this heat energy would enable significant improvements in the energy efficiency of fossil fuel utilization and thereby mitigation of CO2 emissions. There have been various innovative attempts to harness waste heat to convert it into usable forms of energy, such as electricity. Among them, thermoelectric (TE) modules based on the Seebeck effect stand out owing to their advantages of solid-state operation without moving parts, virtually maintenance-free nature, and compactness for integration into existing systems.1,2 A TE module is commonly made up of a number of n- and p-type TE legs that are connected electrically in series and thermally in parallel. The theoretical maximum efficiency (ηmax) of a TE module depends on the average zT (zTave) of TE materials over a wide temperature range as well as the temperature difference between the hot (Th) and cold (Tc) sides of the module as follows: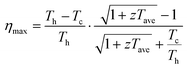 | (1) |
Over the past decades, research into TE materials has substantially grown. A variety of approaches, including electronic band engineering,3,4 design of multiscale hierarchical architectures,5 and entropy engineering,6 have succeeded in pushing zTave above 1.0. These remarkable advances are widely considered sufficient to enable next-generation TE modules with efficiencies exceeding the previous values by more than a factor of two.1 However, the development of corresponding TE modules has proceeded quite slowly. Currently, commercially available TE modules for power generation are mainly Bi2Te3-based modules.7,8 The optimal operating temperature of these modules is still below 500 K due to the unfavorable bipolar effect induced by the narrow bandgap of Bi2Te3,8 and they are limited to niche applications due to the low conversion efficiency (approximately 5–6%)9,10 and scarcity of elemental Te.9
In practice, recovery of waste heat up to the mid-temperature range (500–800 K),11 which matches the majority of industrial waste heat sources suitable for energy harvesting,12 offers greater application potential and is thus the area for which TE materials have been most intensively developed, such as PbTe,3,5 GeTe,11,13 SnSe,4,14 skutterudite (SKD),15 Cu2Se,16 and half-Heusler (HH).17 Among them, TE modules based on PbTe-related alloys are the most developed and have demonstrated successful use as radioisotope TE generators in several space missions.1,18 However, their large-scale industrial application is impeded by the toxicity of Pb and the scarcity of Te. The development of TE modules using other materials is also plagued by different challenges, such as the high cost of GeTe,19 the extreme oxygen sensitivity in the synthesis of SnSe,14 and the instability of Cu2Se due to electromigration of Cu ions.16 Therefore, strong desire for next-generation mid-temperature TE modules warrants the development of high-efficiency, cost-effective and stable alternatives.
In recent years, Mg3(Bi,Sb)2 alloys have shown exceptional TE properties from 300 K to 750 K and are regarded as one of the most promising materials for large-scale applications in the future because of their nontoxic nature, abundant constituent elements, and excellent mechanical robustness.20–24 Several groups have thereby embarked on module development and have recently demonstrated remarkable outcomes.8–10 Nevertheless, most of these research activities target modules that operate below 600 K, aiming to replace traditional Bi2Te3 alloys. Despite the advances, these studies still fall far from achieving the optimum application potential of Mg3(Bi,Sb)2 alloys. The goal of using Mg3(Bi,Sb)2-based TE modules for power generation above 600 K has not yet been realized, as issues related to material design, contact optimization, module assembly and service reliability are more challenging at higher temperatures.
Herein, through full exploitation from material modification to module engineering, we demonstrate for the first time high-efficiency and cost-effective Mg3(Bi,Sb)2-based TE modules for waste heat utilization up to 750 K (Fig. 1). To achieve this, we first tailored the band structure and microstructure of the Mg3Bi2–Mg3Sb2 compounds, and thus realized a zTave >1 from 300 to 700 K in Mg3.2Bi0.996SbSe0.004. Then, by developing a novel high-throughput screening method, we identified Nb as a suitable barrier material that enables a low interfacial contact resistivity (ρc of 9.7 μΩ cm2) and exceptional interfacial stability (ρc of 26 μΩ cm2 after accelerated thermal ageing at 773 K for 360 h). In addition, we investigated the compatibility of currently available p-type TE materials that feature high performance and environmentally friendliness with the Mg3Bi2–Mg3Sb2 compounds and employed finite element simulations to design the geometrical configuration of the TE modules. All these efforts resulted in a module consisting of n-type Mg3.2Bi0.996SbSe0.004 and p-type Ce0.9Fe3CoSb12 with a high conversion efficiency of 9.1% at a heat source temperature (Theater) of 748 K, surpassing that of state-of-the-art single-stage TE modules under the same temperature gradient (Fig. 1a). Moreover, our module does not contain toxic elements and offers a higher power/cost ratio than existing modules (Fig. 1b, and Supplementary note, ESI,†), e.g., two orders of magnitude more cost effective than GeTe-based modules, which spurs prospects for large-scale commercial applications.
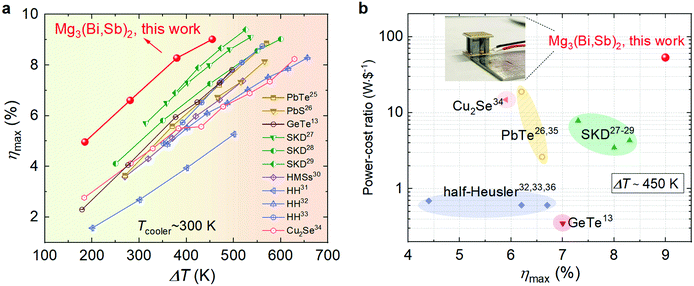 | ||
| Fig. 1 Performance of our Mg3(Bi,Sb)2-based TE module in comparison to other mid-temperature counterparts. (a) Maximum conversion efficiency (ηmax) as a function of temperature difference (ΔT) for the Mg3.2Bi0.996SbSe0.004/Ce0.9Fe3CoSb12 module. Literature results of the state-of-the-art TE modules are included for comparison.13,25–34 (b) Comparisons of the cost effectiveness and ηmax of different TE modules under a ΔT of 450 K (PbTe,26,35 GeTe,13 SKD,27–29 HH,32,33,36 Cu2Se34). The cost of TE materials is used for the comparison, which is calculated by using the element prices from IYPT 2019.37 The inset shows a photograph of our module. | ||
Results and discussion
TE properties of Se-doped Mg3.2Bi2−xSbx
Mg3Bi2 is a semimetal, and Mg3Sb2 is a semiconductor;22 and it has been reported that alloying Mg3Bi2 with Mg3Sb2 can enhance the bandgap and suppress the bipolar contribution of Bi-rich compositions, pushing the maximum zT (zTmax) to higher temperatures.22 However, to obtain satisfactory zTave towards mid-temperature power generation applications, further optimization on the carrier concentration and modulation of electron–phonon interaction are necessary. Herein, we synthesized a series of samples with the nominal composition of Mg3.2Bi2−x−ySbxSey (x = 0.3, 0.5, 1, 1.5, 1.7, y = 0.004, 0.008, 0.012). Given the scarcity of Te, Se is used as the anionic dopant to optimize the carrier concentration with the aim of achieving cost-effective TE materials for large-scale applications. In addition, recent reports suggest that Se can substitute Te as an effective n-type dopant in Mg3Sb2-based materials,38–41 which underlines their possible application for next-generation mid-temperature TE modules with earth-abundant and inexpensive component elements.Small samples in a typical dimension – a disk with a diameter of 10 mm and a thickness of 2 mm – were first prepared for structural and performance analysis. Powder X-ray diffraction (XRD) patterns (Fig. S1, ESI†) and energy dispersive spectroscopy (EDS) analyses (Fig. S2, ESI†) verify the single phase of all samples. Temperature-dependent electronic and thermal transport properties of small-sized Mg3.2Bi1.996−xSbxSe0.004 samples are shown in Fig. S3 (ESI†), along with a detailed discussion. As a result, zTmax of approximately 1.3 is obtained for both Mg3.2Bi0.996SbSe0.004 and Mg3.2Bi0.496Sb1.5Se0.004 samples (Fig. 2a). The zTave values from 300 to 700 K are calculated, and the maximum zTave exceeds 1.0 for the Mg3.2Bi0.996SbSe0.004 sample, which is superior to that of most Se-doped Mg3Bi2–Mg3Sb2 alloys and is comparable to the high value recently reported by Mo et al.41 (Fig. 2b). The high zTave of our Mg3.2Bi0.996SbSe0.004 sample is mainly attributed to the high electrical conductivity due to the large grain size (Fig. S4, ESI†) achieved by using a high sintering temperature (Table S2, ESI†) without compromising the low thermal conductivity. This agrees with earlier reports. For example, Kanno et al. considerably enhanced the room temperature zT values of coarse-grained Mg3.2Sb1.5Bi0.49Te0.01 samples by increasing the sintering temperature from 873 K to 1123 K.42 Larger grains lead to the reduction of detrimental grain boundary scattering,42,43 resulting in higher carrier mobility. Furthermore, Pan et al. also found that the lattice thermal conductivity of n-type Mg3(Bi,Sb)2 polycrystalline samples is nearly independent of grain size, whereas electron mobility exhibits a significant increase with larger grain size.24
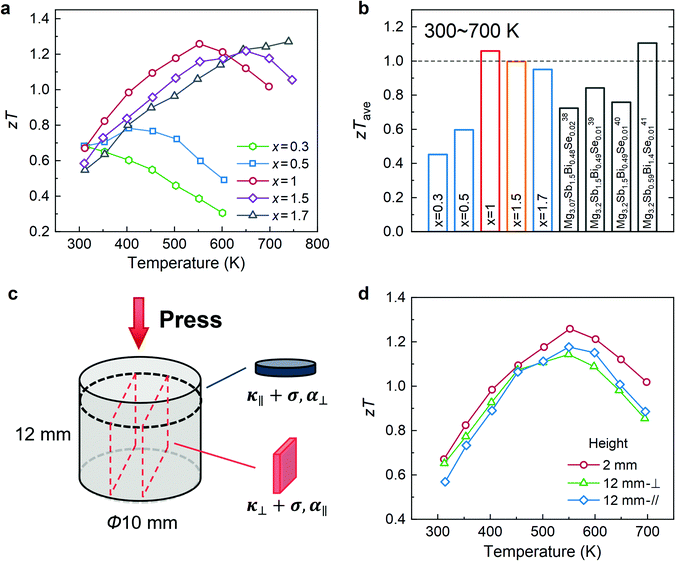 | ||
| Fig. 2 TE transport properties of n-type Mg3.2Bi1.996−xSbxSe0.004. (a) Temperature dependence of zT. (b) zTave of Mg3.2Bi1.996−xSbxSe0.004 in this work compared with those of reported Se-doped Mg3(Bi,Sb)2 alloys.38–41 (c) Schematic slicing diagram of the large-sized cylinders, showing the directions that TE transport properties were measured. (d) Comparison of the TE properties of large- and small-sized samples. | ||
In addition, we also investigated the effect of different Se doping (y = 0.004, 0.008, 0.012) on the TE properties of x = 1.0 samples and found that the simultaneous increases in the power factor and thermal conductivity result in almost unchanged zTave values (Fig. S8, ESI†). Similar results can be also observed in the recent work of Mo et al.41 This implies that slight fluctuations in the elemental content of Se do not seriously deteriorate the TE properties of Mg3.2Bi0.996SbSe0.004, which ensures excellent reproducibility of the material during batch preparation and thus facilitates the fabrication of TE modules. More critically, considering that TE modules usually utilize the TE properties of sintered samples along the pressing direction, we further prepared large-sized cylinders to confirm the consistency of their properties (Fig. 2c). As a result, the electronic and thermal transport properties of the large- and small-sized samples are comparable (Fig. 2d and Fig. S9, ESI†), and each property is substantially isotropic. zTave of 1.0 is maintained for the large-sized samples (Fig. S9e, ESI†), enabling the development of high-performance TE modules.
Diffusion barrier material screening
In a TE module, p- and n-type TE materials are usually connected by electrodes to realize electric connection in series. During practical operation, the hot-side temperature usually reaches hundreds of degrees. As a result, commonly used electrode materials, such as Ni and Cu, can undergo severe chemical reactions and/or atomic diffusion with TE materials,47 leading to degradation and instability of the module. Therefore, searching for a suitable diffusion barrier material that is almost chemically inert to TE materials, but offers low electrical contact resistivity (ρc) and good mechanical bonding, is an urgent task for any kind of TE material for mid-temperature applications.Currently, Fe, Ni, 304 stainless steel and Mg2Cu have been investigated as diffusion barrier materials for Mg3Bi2–Mg3Sb2 alloys.8–10,44,45 Despite some advances, studies on interfacial stability are scarce and have mainly focused on temperatures below 600 K. The Mg3.2Sb1.5Bi0.49Te0.01/304 stainless steel junction exhibited a quite low initial ρc of 5.6 μΩ cm2.44,46 However, it significantly increased to 25 μΩ cm2 after thermal ageing under 673 K for 200 h.46 The stability of the interface is unsatisfactory. Furthermore, previous attempts to identify barrier materials are based on a trial-and-error approach,48 which is both time-consuming and less than ideal for finding the optimal combination. Recently, Xing et al. developed a high-throughput strategy to identify viable diffusion barrier layers for GeTe.13 This approach is productive but still suffers from shortcomings, such as deactivation of candidate powders due to the inter-reaction or diffusion between themselves, and random dispersion of candidate powders making tracking the specific interfaces after thermal ageing difficult.
Herein, we develop a modified process for rapid and efficient screening of the diffusion barrier layer (Fig. 3a). First, the powders of different barrier layer candidates were separately mixed with TE powders at the same ratio. All mixtures were then loaded layer by layer into a graphite die and consolidated into a bulk sample by one-step sintering. Finally, segmented samples were obtained by cutting the sintered bulk along the direction of the sintering pressure and then used for characterization and thermal ageing. As a case study, we selected eight kinds of elemental powders (W, Nb, Ni, Mo, Fe, Ti, Cr, Cu) and mixed them with Mg3.2Bi0.996SbSe0.004 powders at 1 vol%. Sintering was conducted by spark plasma sintering (SPS) technique following the sintering parameters for Mg3.2Bi0.996SbSe0.004. Accelerated thermal ageing tests were carried out at 773 K for 360 h to identify the elemental reaction or diffusion in a shorter time. More details can be found in the Methods section.
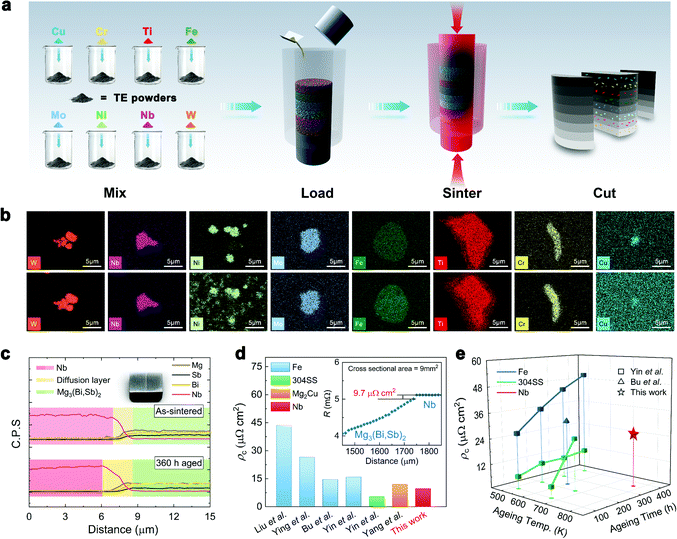 | ||
| Fig. 3 Barrier layer design and interfacial stability. (a) Schematic diagram for high-throughput screening of the diffusion barrier layer for Mg3.2Bi0.996SbSe0.004. (b) EDS mapping results showing the elemental distribution of different barrier layer candidates within the Mg3.2Bi0.996SbSe0.004 matrix before and after thermal ageing at 773 K for 360 h in vacuum. (c) EDS line scanning results of the Mg3.2Bi0.996SbSe0.004/Nb junction before and after ageing. The inset is a photo of the tested sample. (d) Measured contact resistivity (ρc) of the as-sintered Mg3.2Bi0.996SbSe0.004/Nb junction in comparison with literature results.8–10,44,45 The inset shows a typical resistance scanning result. (e) ρc of the Mg3.2Bi0.996SbSe0.004/Nb junction as a function of thermal ageing temperature and time. Data from the literature are included for comparison.8,44,46 | ||
Benefiting from the new approach, we can accurately track the changes in specific elements at specific locations after thermal ageing (Fig. 3b, Fig. S10 and S11, ESI†). As Fig. 3b shows, the microstructures of typical interfaces between Mg3.2Bi0.996SbSe0.004 and different metals can be divided into three categories. The first category includes W. There is no obvious reaction or diffusion between the W particles and the Mg3.2Bi0.996SbSe0.004 matrix before and after thermal ageing, indicating that W fails to form effective bonds with the matrix. The second category includes Ni, Cu, and Ti. All of them can form intimate contact with the surrounding Mg3.2Bi0.996SbSe0.004 matrix by forming a reaction layer during the sintering process. However, the reaction is so violent that the metal particles are severely eroded away after thermal ageing, and therefore, these materials cannot satisfy the requirement of long-term operation at high temperatures. The third category includes Fe, Mo, Cr, and Nb. They all bond well to the matrix after sintering but do not generate visible reaction layers. After thermal ageing, reaction layers can be found in the vicinity of Fe particles (Fig. 3b and Fig. S11a, ESI†), but this is not the case with the other three particles. However, Mo particles shrink (Fig. 3b and Fig. S11b, ESI†) while Cr particles swell (Fig. 3b and Fig. S11c, ESI†) due to thermal ageing. Only Nb particles barely have any change (Fig. 3b and Fig. S11d, ESI†), suggesting that Nb is more appropriate as the diffusion barrier material for Mg3Bi2–Mg3Sb2 alloys in mid-temperature applications.
After confirming the chemical inertness of the barrier layer candidates through rapid screening, we further selected Nb to investigate the interfacial contact resistance. Nb foil was sintered with Mg3.2Bi0.996SbSe0.004 to form sandwich-structured legs (inset of Fig. 3c), which were then used for accelerated thermal ageing experiments and characterization of the interfaces. There is a thin interfacial reaction layer of less than 2 μm at the as-sintered Mg3.2Bi0.996SbSe0.004/Nb interface (Fig. 3c), resulting in a low contact resistivity (ρc) of ∼9.7 μΩ cm2 (Fig. 3d). The actual ρc value should be lower because the minimum probe step is 12.5 μm in this work due to the limitations of the measurement instrument, which is much larger than the thickness of the interfacial reaction layer shown in Fig. 3c. Despite this, this value is still lower than most of the reported data (Fig. 3d). More critically, even after accelerated thermal ageing at 773 K for 360 h, the interfacial reaction layer remains thin at less than 3 μm (Fig. 3c), yielding a ρc as low as 26 μΩ cm2 (Fig. 3e).
This is the first study of the long-term service performance of a Mg3(Bi,Sb)2-based TE junction at temperatures as high as 773 K (Fig. 3e), and the junction shows exceptional interfacial stability. This superiority may be attributed to the combination of a low interfacial bonding energy and a large activation energy barrier for elemental migration between Nb and Mg3.2Bi0.996SbSe0.004,29 which facilitates the application of Mg3(Bi,Sb)2-based TE modules at elevated temperatures. Moreover, despite the difference in the coefficient of thermal expansion (CTE) between the Nb and Mg3Bi2–Mg3Sb2 alloys, no cracks are observed near the interface areas (Fig. S12, ESI†) due to the use of thin Nb foil (thickness of 30 μm, see Methods).
P-type material selection and module geometry design
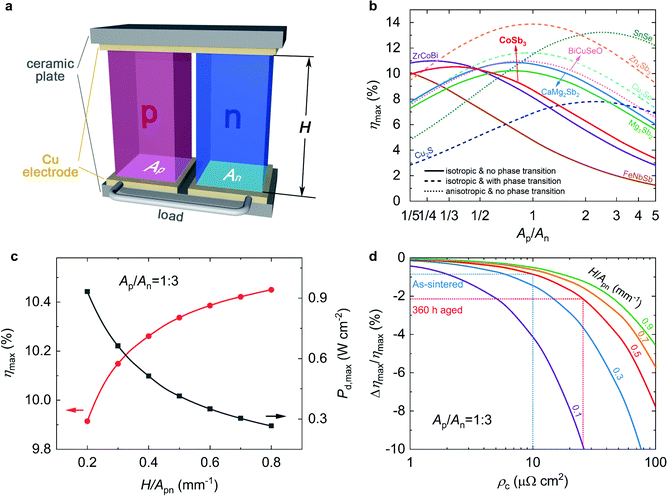
To fabricate mid-temperature TE modules for large-scale applications, p-type high-performance TE materials consisting of abundant and nontoxic elements are also necessary. In this regard, we selected ten state-of-the-art p-type Pb&Te-free TE materials from previous reports14,27,36,49–55 and predicted the power generation performance of the modules constructed using them along with n-type Mg3.2Bi0.996SbSe0.004 (Fig. 4a and b). Finite element simulations were employed to obtain the optimal geometrical configuration, especially the cross-sectional area ratio of the p- and n-legs (Ap/An), for the maximum conversion efficiency (ηmax). We can see that at temperatures of 700 K and 300 K for the hot-side (Th) and cold-side (Tc) ceramic plates, respectively, an ηmax of over 10% can be achieved by combining a variety of p-type materials with Mg3.2Bi0.996SbSe0.004 (Fig. 4b), such as ZrCoBi, CoSb3, Zn4Sb3, Cu2Se, SnSe, BiCuSeO, and CaMg2Bi2. This is motivating, as we have a wider choice of materials. However, in terms of developing practical modules, there are some challenging issues, such as the anisotropic TE properties of SnSe and BiCuSeO, phase transition and elemental migration in Zn4Sb3 and Cu2Se, that block full development. In this regard, Ce0.9Fe3CoSb12 is preferred because it offers good stability up to 750 K,29 isotropic characteristics and, more importantly, well-established production.27,56After determining an appropriate p-type TE material, we further optimized the ratio of the height and cross-sectional area (H/Apn) for optimal power generation performance. Ap/An is set to 1/3 based on the above results. Clearly, ηmax and the maximum power density (Pd,max) show opposite trends as a function of H/Apn (Fig. 4c). This is because the effective temperature difference across the TE legs is enlarged by increasing the leg height, which leads to an increase in ηmax.56 However, the module resistance is also enlarged, resulting in a decrease in Pd,max. We can also notice that ηmax increases more and more slowly and tend to stabilize with the increase of H/Apn, while Pd,max significantly decreases with increasing H/Apn (Fig. 4c). For example, when H/Apn is increased from 0.2 to 0.8 mm−1, the ηmax increases by ∼5%, while the corresponding Pd,max decreases by ∼70%. Therefore, H/Apn should be determined by balancing the ηmax and Pd,max requirements for practical applications.
In addition, the determination of H/Apn is closely related to the interface contact resistance. This is because a higher ρc leads to higher losses in the conversion efficiency, and the losses increase when H/Apn decreases (Fig. 4d). As discussed above, the ρc for the Mg3.2Bi0.996SbSe0.004/Nb interface in this work is 9.7 and 26 μΩ cm2 before and after thermal ageing, respectively. Therefore, taking these conflicting factors into account, we chose H/Apn = 0.5 mm−1 to fabricate the TE modules considering that the corresponding efficiency loss is below 3% (Fig. 4d).
Module fabrication and performance evaluation
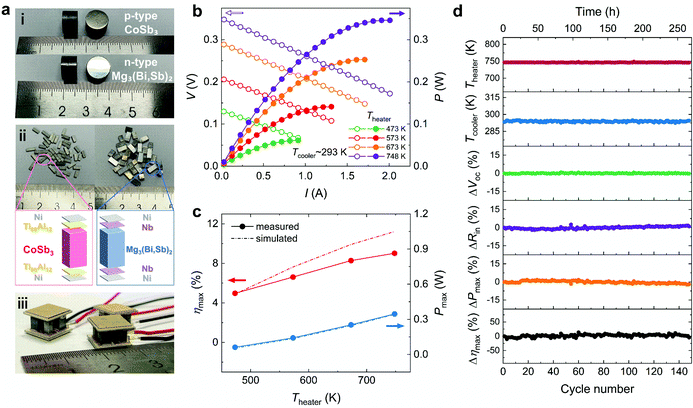
Based on the above achievements in optimization of TE materials and module engineering, we fabricated TE modules by using n-type Mg3.2Bi0.996SbSe0.004 and p-type Ce0.9Fe3CoSb12 (Fig. 5a, Fig. S13, ESI,† and Methods). The current (I), output voltage (V), output power (P), and TE conversion efficiency were measured under different ΔT with a Theater of up to 748 K (see Methods). Circulating water with a fixed temperature of 288 K was used to cool the cold side. ΔT = Theater − Tcooler in this work, where Tcooler is the temperature of the cold-side Cu block (Fig. S14, ESI†). Note that thermal resistances between the TE module and external heat/cold sources are inevitable due to imperfect contacts, leading to an additional temperature drop, and therefore, the effective temperature difference across the TE module is lower than ΔT. According to our previous calibration experiments,56 when Theater reaches 748 K, Th is probably ∼20 K lower than Theater, and Tc may be ∼10 K higher than Tcooler.Tests reveal that each V–I curve exhibits a good linear relationship (Fig. 5b), where the slope indicates internal resistance (Rin) of the module at different ΔT, and the y-intercept represents the corresponding open-circuit voltage (Voc). The maximum power output (Pmax) is reached when the external load resistance is equal to Rin. The conversion efficiency at each current is calculated by using the output power and cold-side heat flow through eqn (2). As shown in Fig. 5c, the measured ηmax increases with increasing Theater, exceeding 9% at a Theater of 748 K with a corresponding ΔT of 455 K. We noticed that an efficiency of 9% was recently reported by Yin et al.,44 which is quite impressive and inspiring, but it was achieved on a single n-type Mg3.2Sb1.5Bi0.49Te0.01 leg. The single-leg efficiency is very useful for assessing the potential of a single TE material, but is still far from real applications. Towards industrial applications, modules consisting of both n-type and p-type TE materials need to be developed. To the best of our knowledge, this is the first Mg3(Bi,Sb)2-based TE module that can operate at temperatures up to 750 K (Fig. S15, ESI†). More critically, the ηmax of our module is higher than that of the state-of-the-art single-stage TE modules under the same temperature gradient (Fig. 1a and Fig. S16, ESI†). In addition, higher ηmax is predicted, which could be achieved by optimizing the filling factor of our module and reducing the heat leakage from the gaps between TE legs.9,26
Furthermore, we conducted thermal cycling experiments to examine the long-term reliability of the module. During the measurements, Tcooler was maintained at ∼293 K while Theater was set to cycle between 473 K and 750 K (Fig. 5d). One cycle lasted approximately 110 min, and the module power generation performance was evaluated whenever Theater was stabilized at 750 K. Measurements were carried out in a chamber filled with argon at an initial pressure of ∼200 Pa. After 150 cycles (∼275 h), Voc remains stable (Fig. 5d). We did not observe an abrupt increment in Rin as reported in other mid-temperature TE modules,13 indicating a much better module reliability. Both Pmax and ηmax are comparable to those before thermal cycling, showing a slight decrease of <5%. This demonstrates the great feasibility of Mg3(Bi,Sb)2-based TE modules for mid-temperature applications owing to their record-high conversion efficiency and excellent service stability.
Conclusions
TE modules can harvest waste heat and directly convert it to electricity, thereby improving fuel efficiency and mitigating CO2 emissions. The tremendous advances in high-performance TE materials achieved over the past decades have raised expectations regarding the use of TE generators in various energy saving and energy management applications. However, the commercialization of TE generators, particularly those that can operate above 500 K, proceeds slowly due to the low conversion efficiency, high costs, toxicity of the constituent elements, and limited long-term service stability. We have therefore developed a novel high-performance TE module in this work by using the environmentally friendly and low-cost TE materials of n-type Se-doped Mg3(Bi,Sb)2 and p-type CoSb3-based SKD. The module exhibits exceptional characteristics, including a high operating temperature of up to ∼750 K, a record-high conversion efficiency of 9.1% under a temperature difference of ∼450 K, a higher efficiency/price ratio than that of state-of-the-art TE modules, excellent interfacial stability, and good module reliability during thermal cycling. These achievements result from the full exploration involving tailoring of the material composition and microstructure, high-throughput screening of effective diffusion barrier layers, and optimization of the module structure. Our study has opened the door to the development of Mg3(Bi,Sb)2-based TE modules for mid-temperature waste heat recovery. We believe that higher zTave values could be achieved by further optimization, such as by further doping at the cation site of Mg3(Bi,Sb)2, which will soon lead to module efficiency exceeding 10%. In this regard, our findings here bridge the gap between material discovery and module development, providing a viable route to accelerate subsequent research on fabricating efficient and stable TE modules.Experimental
Material synthesis
High-purity elements of Bi (99.999%, Sinopharm Chemical Reagent Ltd), Sb (99.99%, Sinopharm Chemical Reagent Co.), Se (99%, Sinopharm Chemical Reagent Ltd), and Mg (99.9%, Sinopharm Chemical Reagent Ltd) were weighed according to the composition of Mg3.2Bi2−x−ySbxSey (x = 0.3, 0.5, 1, 1.5, 1.7, y = 0.004, 0.008, 0.012). An excess Mg of 0.2 was chose based on previous studies (Table S1, ESI†). All the elements in total of 8 g were weighed at once and then loaded into a stainless-steel ball milling jar in a glove box under an argon atmosphere with oxygen and water levels below 0.1 ppm. The mixture was ball milled for 15 h at 550 rpm using a planetary mill machine (RETSCH PM100). Next, the ball-milled powders were loaded into a graphite die with an inner diameter of 10 mm. SPS (Dr Sinter: 2000) was then carried out to obtain dense bulk samples under a uniaxial pressure of 50 MPa at different temperatures (973–1073 K, Table S2, ESI†) for 3 min. The p-type Ce0.9Fe3CoSb12 samples were synthesized following our previous work,27 and the corresponding TE properties are shown in Fig. S17 (ESI†).In the diffusion barrier screening experiments, high purity powders of W (99.95%, Adamas), Nb (99.99%, Alfa Aesar, 325 mesh), Ni (99.99% Macklin, 200 mesh), Mo (99.9%, Alfa Aesar, 100 mesh), Fe (99.99%, Adamas), Ti (99.99%, Macklin, 300 mesh), Cr (99.94%, Alfa Aesar, 200 mesh), and Cu (99.5%, Alfa Aesar, 150 mesh) were separately mixed with Mg3.2Bi0.996SbSe0.004 powder at 1 vol%. The eight mixed powders were then sequentially added to a graphite die and sintered into a bulk by using the SPS technique following the above sintering parameters for Mg3.2Bi0.996SbSe0.004.
TE module fabrication
n-Type TE joints of Ni/Nb/Mg3.2Bi0.996SbSe0.004/Nb/Ni were fabricated by sequentially loading a Ni foil (thickness of 0.1 mm, Hebei Zhanmo Metal Materials Ltd, 99.9%), a Nb foil (thickness of 0.03 mm, Hebei Zhanmo Metal Materials Ltd, 99.9%), Mg3.2Bi0.996SbSe0.004 powders, another Nb foil, and another Ni foil into a graphite die with an inner diameter of 10 mm and then densifying them through a one-step SPS technique. Nb foils work as the diffusion barrier layer, and Ni foils work as the metallization layer. The sintering parameters were the same as those used to sinter the Mg3.2Bi0.996SbSe0.004 powder, except that the holding time was increased by three minutes, considering the increased thickness of the sample. p-Type TE joints of Ni/Ti88Al12/Ce0.9Fe3CoSb12/Ti88Al12/Ni were also fabricated by the one-step SPS technique, where a mixture of Ti and Al powders (thickness of 0.1 mm) was used as the barrier layer. The sintering temperature was 873 K, the pressure was 60 MPa, and the holding time was 8 min. After grinding and polishing, the joints were then cut by wire cutting (STX-202A) into dices with dimensions of 3 × 3 × 6 mm3 for the n-type and 1.7 × 1.7 × 6 mm3 for the p-type. Both the hot and cold sides of the TE joints were then soldered onto a double-sided direct bonded copper alumina plate using high-temperature Ag paste (CT2700R7S). The overall size of the module consisting of two couples was 10 × 10 × 9 mm3 (Fig. S13, ESI†). Glass fibers (GXZ aluminosilicate fiber paper) were then filled between TE legs to reduce heat losses due to convection and radiation. Copper wires were soldered to the cold-side Cu electrodes to measure the current and voltage. Prior to high temperature testing, the module resistance at room temperature was measured to ensure good interface bonding and module integrity. Note that BN coatings were sprayed onto the surfaces at hot side of the TE legs to restrain the surface oxidation and sublimation of Mg before the test. In addition, because Mg can react with water, resulting in degradation of the performance, we avoided their contact with water throughout the material processing and device integration process.Material and module characterization
The phase composition and crystal structure were characterized by powder XRD (Rigaku D/Max-2550 PC) using Cu-Kα radiation (λ = 1.541 Å) at 40 kV and 30 mA. The microstructure and chemical compositions were analyzed by field emission SEM (FE-SEM, TESCAN/MAIA3, Czech) equipped with an energy dispersive spectrometer. The thermal conductivity (κ) was calculated by κ = D·Cp·ρ, where D, Cp and ρ are the thermal diffusivity, specific heat and density, respectively. D was measured by a laser flash method using an LFA 457 (Netzsch Instruments, Germany), Cp was obtained from the equation in ref. 57, and ρ was measured by the Archimedes method. σ and α were simultaneously measured using a ZEM-3 (ULVAC-RIKO, Japan). The measurement uncertainties for α, σ, and D were ∼5%. The Hall coefficient (RH) was determined using a Hall measurement system (Lakeshore 8400 Series HMS, USA). The carrier concentration (nH) and mobility (μH) were calculated from nH = 1/eRH and μH = σRH, where e is the charge of the electron.The contact resistivity was measured using a homemade four-probe measurement system as detailed in ref. 56. Consolidated sandwiches, e.g. Mg3.2Bi0.996SbSe0.004/Nb/Mg3.2Bi0.996SbSe0.004, obtained by the same sintering process as described above were cut into rectangular bars with dimensions of 3 × 3 × 4 mm3 and used for the measurements. At least three different positions on each sample were measured to obtain an average value.
The power generation performance of the TE module was characterized using a commercial measurement system (Fig. S13, ESI†) developed by Shanghai Fuyue Vacuum Technology Ltd. The measurement principle is based on the steady-state method similar to that previously reported.27 Briefly, a TE module is sandwiched between a resistive heater and a circulating water-cooled Cu block. The cross-sectional area of the heater and Cu block is 10 × 10 mm2, which is the same as that of the TE module, thus avoiding heat leakage. The efficiency (η) of a TE module is defined as the ratio between the electric power output (P) and the sum of P and Qout,
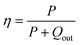 | (2) |
| Qout = ACuκCuΔT/L | (3) |
Finite element simulation
The simulation of TE modules was implemented using 3D finite element analyses through ANSYS-Workbench. A full-parameter model coupling the TE effects (conduction, Joule effect, Thomson effect and Peltier effect) and comprehensively considering temperature-dependent material properties was used for accurate simulation. Details can be found in our previous work.56Author contributions
Y. F. and L. W. conceived the ideas and designed the work. Y. F. carried out the experiments including material preparation and characterization, module fabrication and measurements. Z. H. and M. J. contributed to material preparation and interface design. Y. F., Q. Z., and A. H. performed the simulations. S. W. contributed to analysis of the properties of TE materials. Y. F. and Q. Z. wrote the draft. X. A., H. R., L. W., K. N., and W. J. contributed to the discussion and editing.Conflicts of interest
Y. F., L. W. and W. J. have filed a provisional patent application on the work described here. The status of the patent application is pending.Acknowledgements
This work was funded by Natural Science Foundation of China (No. 51871053, 52174343), the Innovation Program of Shanghai Municipal Education Commission (202101070003E00110), Shanghai Committee of Science and Technology (No. 20JC1415200). Q. H. Z. acknowledges the financial support from Alexander von Humboldt Foundation (No. CHN 1210297 HFST-P). The authors thank Dr Binbin Jiang from Southern University of Science and Technology for his support on the Mini-PEM measurements.References
- Q. Yan and M. G. Kanatzidis, Nat. Mater., 2022, 21, 503–513 CrossRef CAS PubMed.
- X. Shi and L. Chen, Nat. Mater., 2016, 15, 691–692 CrossRef CAS PubMed.
- Y. Pei, X. Shi, A. LaLonde, H. Wang, L. Chen and G. J. Snyder, Nature, 2011, 473, 66–69 CrossRef CAS PubMed.
- B. Qin, D. Wang, X. Liu, Y. Qin, J. F. Dong, J. Luo, J. W. Li, W. Liu, G. Tan, X. Tang, J. F. Li, J. He and L. D. Zhao, Science, 2021, 373, 556–561 CrossRef CAS PubMed.
- K. Biswas, J. He, I. D. Blum, C. I. Wu, T. P. Hogan, D. N. Seidman, V. P. Dravid and M. G. Kanatzidis, Nature, 2012, 489, 414–418 CrossRef CAS PubMed.
- B. Jiang, Y. Yu, J. Cui, X. Liu, L. Xie, J. Liao, Q. Zhang, Y. Huang, S. Ning, B. Jia, B. Zhu, S. Bai, L. Chen, S. J. Pennycook and J. He, Science, 2021, 371, 830–834 CrossRef CAS PubMed.
- J. Mao, G. Chen and Z. Ren, Nat. Mater., 2021, 20, 454–461 CrossRef CAS PubMed.
- Z. Bu, X. Zhang, Y. Hu, Z. Chen, S. Lin, W. Li and Y. Pei, Energy Environ. Sci., 2021, 14, 6506–6513 RSC.
- P. Ying, R. He, J. Mao, Q. Zhang, H. Reith, J. Sui, Z. Ren, K. Nielsch and G. Schierning, Nat. Commun., 2021, 12, 1121 CrossRef CAS PubMed.
- Z. Liu, N. Sato, W. Gao, K. Yubuta, N. Kawamoto, M. Mitome, K. Kurashima, Y. Owada, K. Nagase, C. H. Lee, J. Yi, K. Tsuchiya and T. Mori, Joule, 2021, 5, 1196–1208 CrossRef CAS.
- X. Zhang, Z. Bu, S. Lin, Z. Chen, W. Li and Y. Pei, Joule, 2020, 4, 986–1003 CrossRef CAS.
- M. Papapetrou, G. Kosmadakis, A. Cipollina, U. La Commare and G. Micale, Appl. Therm. Eng., 2018, 138, 207–216 CrossRef.
- T. Xing, Q. Song, P. Qiu, Q. Zhang, M. Gu, X. Xia, J. Liao, X. Shi and L. Chen, Energy Environ. Sci., 2021, 14, 995–1003 RSC.
- C. Zhou, Y. K. Lee, Y. Yu, S. Byun, Z. Z. Luo, H. Lee, B. Ge, Y. L. Lee, X. Chen, J. Y. Lee, O. Cojocaru-Mirédin, H. Chang, J. Im, S. P. Cho, M. Wuttig, V. P. Dravid, M. G. Kanatzidis and I. Chung, Nat. Mater., 2021, 20, 1378–1384 CrossRef CAS.
- Y. Tang, Z. M. Gibbs, L. A. Agapito, G. Li, H.-S. Kim, M. B. Nardelli, S. Curtarolo and G. J. Snyder, Nat. Mater., 2015, 14, 1223–1228 CrossRef CAS.
- H. Liu, X. Shi, F. Xu, L. Zhang, W. Zhang, L. Chen, Q. Li, C. Uher, T. Day and G. J. Snyder, Nat. Mater., 2012, 11, 422–425 CrossRef CAS PubMed.
- W. G. Zeier, J. Schmitt, G. Hautier, U. Aydemir, Z. M. Gibbs, C. Felser and G. J. Snyder, Nat. Rev. Mater., 2016, 1, 16032 CrossRef CAS.
- C. S. R. Matthes, D. F. Woerner, T. J. Hendricks, J. P. Fleurial, K. I. Oxnevad, C. D. Barklay and J. F. Zakrajsek, in 2018 IEEE Aerospace Conference, IEEE, Big Sky; MT, 2018, pp. 1–9 Search PubMed.
- W. D. Liu, D. Z. Wang, Q. Liu, W. Zhou, Z. Shao and Z. G. Chen, Adv. Energy Mater., 2020, 10, 2000367 CrossRef CAS.
- H. Tamaki, H. K. Sato and T. Kanno, Adv. Mater., 2016, 28, 10182–10187 CrossRef CAS PubMed.
- J. Zhang, L. Song, S. H. Pedersen, H. Yin, L. T. Hung and B. B. Iversen, Nat. Commun., 2017, 8, 13901 CrossRef CAS PubMed.
- K. Imasato, S. D. Kang and G. J. Snyder, Energy Environ. Sci., 2019, 12, 965–971 RSC.
- J. Mao, H. Zhu, Z. Ding, Z. Liu, G. A. Gamage, G. Chen and Z. Ren, Science, 2019, 365, 495–498 CrossRef CAS PubMed.
- Y. Pan, M. Yao, X. Hong, Y. Zhu, F. Fan, K. Imasato, Y. He, C. Hess, J. Fink, J. Yang, B. Büchner, C. Fu, G. J. Snyder and C. Felser, Energy Environ. Sci., 2020, 13, 1717–1724 RSC.
- X. Hu, P. Jood, M. Ohta, M. Kunii, K. Nagase, H. Nishiate, M. G. Kanatzidis and A. Yamamoto, Energy Environ. Sci., 2016, 9, 517–529 RSC.
- B. Jiang, X. Liu, Q. Wang, J. Cui, B. Jia, Y. Zhu, J. Feng, Y. Qiu, M. Gu, Z. Ge and J. He, Energy Environ. Sci., 2020, 13, 579–591 RSC.
- Q. Zhang, Z. Zhou, M. Dylla, M. T. Agne, Y. Pei, L. Wang, Y. Tang, J. Liao, J. Li and S. Bai, Nano Energy, 2017, 41, 501–510 CrossRef CAS.
- G. Nie, W. Li, J. Guo, A. Yamamoto, K. Kimura, X. Zhang, E. B. Isaacs, V. Dravid, C. Wolverton, M. G. Kanatzidis and S. Priya, Nano Energy, 2019, 66, 104193 CrossRef CAS.
- J. Chu, J. Huang, R. Liu, J. Liao, X. Xia, Q. Zhang, C. Wang, M. Gu, S. Bai, X. Shi and L. Chen, Nat. Commun., 2020, 11, 2723 CrossRef CAS PubMed.
- I. Aoyama, H. Kaibe, L. Rauscher, T. Kanda, M. Mukoujima, S. Sano and T. Tsuji, Jpn. J. Appl. Phys., 2005, 44, 4275 CrossRef CAS.
- K. Bartholomé, B. Balke, D. Zuckermann, M. Köhne, M. Müller, K. Tarantik and J. König, J. Electron. Mater., 2014, 43, 1775–1781 CrossRef.
- J. Yu, Y. Xing, C. Hu, Z. Huang, Q. Qiu, C. Wang, K. Xia, Z. Wang, S. Bai, X. Zhao, L. Chen and T. Zhu, Adv. Energy Mater., 2020, 10, 2000888 CrossRef CAS.
- Y. Xing, R. Liu, J. Liao, C. Wang, Q. Zhang, Q. Song, X. Xia, T. Zhu, S. Bai and L. Chen, Joule, 2020, 4, 2475–2483 CrossRef CAS.
- P. Qiu, T. Mao, Z. Huang, X. Xia, J. Liao, M. T. Agne, M. Gu, Q. Zhang, D. Ren, S. Bai, X. Shi, G. J. Snyder and L. Chen, Joule, 2019, 3, 1538–1548 CrossRef CAS.
- P. Jood, M. Ohta, A. Yamamoto and M. G. Kanatzidis, Joule, 2018, 2, 1339–1355 CrossRef CAS.
- C. Fu, S. Bai, Y. Liu, Y. Tang, L. Chen, X. Zhao and T. Zhu, Nat. Commun., 2015, 6, 8144 CrossRef PubMed.
- #ChemistryAdvent #IYPT2019 Day 23, https://www.compoundchem.com/2019advent/day23/, (accessed January 6, 2022).
- J. Zhang, L. Song, A. Mamakhel, M. R. V. Jørgensen and B. B. Iversen, Chem. Mater., 2017, 29, 5371–5383 CrossRef CAS.
- F. Zhang, C. Chen, H. Yao, F. Bai, L. Yin, X. Li, S. Li, W. Xue, Y. Wang, F. Cao, X. Liu, J. Sui and Q. Zhang, Adv. Funct. Mater., 2020, 30, 1906143 CrossRef CAS.
- J. Liang, H. Yang, C. Liu, L. Miao, J. Chen, S. Zhu, Z. Xie, W. Xu, X. Wang, J. Wang, B. Peng and K. Koumoto, ACS Appl. Mater. Interfaces, 2020, 12, 21799–21807 CrossRef CAS PubMed.
- X. Mo, J. Liao, G. Yuan, S. Zhu, X. Lei, L. Huang, Q. Zhang, C. Wang and Z. Ren, J. Magnesium Alloys, 2022, 10, 1024–1032 CrossRef CAS.
- T. Kanno, H. Tamaki, H. K. Sato, S. D. Kang, S. Ohno, K. Imasato, J. J. Kuo, G. J. Snyder and Y. Miyazaki, Appl. Phys. Lett., 2018, 112, 033903 CrossRef.
- J. J. Kuo, S. D. Kang, K. Imasato, H. Tamaki, S. Ohno, T. Kanno and G. J. Snyder, Energy Environ. Sci., 2018, 11, 429–434 RSC.
- L. Yin, C. Chen, F. Zhang, X. Li, F. Bai, Z. Zhang, X. Wang, J. Mao, F. Cao, X. Chen, J. Sui, X. Liu and Q. Zhang, Acta Mater., 2020, 198, 25–34 CrossRef CAS.
- J. Yang, G. Li, H. Zhu, N. Chen, T. Lu, J. Gao, L. Guo, J. Xiang, P. Sun, Y. Yao, R. Yang and H. Zhao, Joule, 2021, S2542435121005341 Search PubMed.
- L. Yin, Master thesis, School of Materials Science and Engineering, and Institute of Materials Genome & Big Data, Harbin Institute of Technology, 2019, https://cdmd.cnki.com.cn/Article/CDMD-10213-1019690487.htm (accessed in June 2022) Search PubMed.
- Q. H. Zhang, X. Y. Huang, S. Q. Bai, X. Shi, C. Uher and L. D. Chen, Adv. Eng. Mater., 2016, 18, 194–213 CrossRef CAS.
- W. Liu and S. Bai, J. Materiomics, 2019, 5, 321–336 CrossRef.
- J. Sui, J. Li, J. He, Y. L. Pei, D. Berardan, H. Wu, N. Dragoe, W. Cai and L. D. Zhao, Energy Environ. Sci., 2013, 6, 2916 RSC.
- J. Lin, X. Li, G. Qiao, Z. Wang, J. Carrete, Y. Ren, L. Ma, Y. Fei, B. Yang, L. Lei and J. Li, J. Am. Chem. Soc., 2014, 136, 1497–1504 CrossRef CAS PubMed.
- Y. He, T. Day, T. Zhang, H. Liu, X. Shi, L. Chen and G. J. Snyder, Adv. Mater., 2014, 26, 3974–3978 CrossRef CAS PubMed.
- K. Zhao, A. B. Blichfeld, H. Chen, Q. Song, T. Zhang, C. Zhu, D. Ren, R. Hanus, P. Qiu, B. B. Iversen, F. Xu, G. J. Snyder, X. Shi and L. Chen, Chem. Mater., 2017, 29, 6367–6377 CrossRef CAS.
- Z. Ren, J. Shuai, J. Mao, Q. Zhu, S. Song, Y. Ni and S. Chen, Acta Mater., 2018, 143, 265–271 CrossRef CAS.
- H. Zhu, R. He, J. Mao, Q. Zhu, C. Li, J. Sun, W. Ren, Y. Wang, Z. Liu, Z. Tang, A. Sotnikov, Z. Wang, D. Broido, D. J. Singh, G. Chen, K. Nielsch and Z. Ren, Nat. Commun., 2018, 9, 2497 CrossRef PubMed.
- J. Shuai, H. Geng, Y. Lan, Z. Zhu, C. Wang, Z. Liu, J. Bao, C.-W. Chu, J. Sui and Z. Ren, Proc. Natl. Acad. Sci. U. S. A., 2016, 113, E4125–E4132 CrossRef CAS PubMed.
- Q. Zhang, J. Liao, Y. Tang, M. Gu, C. Ming, P. Qiu, S. Bai, X. Shi, C. Uher and L. Chen, Energy Environ. Sci., 2017, 10, 956–963 RSC.
- M. T. Agne, K. Imasato, S. Anand, K. Lee, S. K. Bux, A. Zevalkink, A. J. E. Rettie, D. Y. Chung, M. G. Kanatzidis and G. J. Snyder, Mater. Today Phys., 2018, 6, 83–88 CrossRef.
- X. Lu, Q. Zhang, J. Liao, H. Chen, Y. Fan, J. Xing, S. Gu, J. Huang, J. Ma and J. Wang, Adv. Energy Mater., 2020, 10, 1902986 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ee01038k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |