Structure-controlled graphene electrocatalysts for high-performance H2O2 production†
Abstract
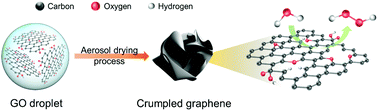
Metal-free carbon materials have emerged as cost-effective and high-performance catalysts for the production of hydrogen peroxide (H2O2) through the two-electron oxygen reduction reaction (ORR). Here, we show that 3D crumpled graphene with controlled oxygen and defect configurations significantly improves the electrocatalytic production of H2O2. The crumpled graphene electrocatalyst with optimal defect structures and oxygen functional groups exhibits outstanding H2O2 selectivity of 92–100% in a wide potential window of 0.05–0.7 V vs. reversible hydrogen electrode (RHE) and a high mass activity of 158 A g−1 at 0.65 V vs. RHE in alkaline media. In addition, the crumpled graphene catalyst showed an excellent H2O2 production rate of 473.9 mmol gcat−1 h−1 and stability over 46 h at 0.4 V vs. RHE. Moreover, density functional theory calculations revealed the role of the functional groups and defect sites in the two-electron ORR pathway through the scaling relation between OOH and O adsorption strengths. These results establish a structure-mechanism-performance relationship of functionalized carbon catalysts for the effective production of H2O2.
- This article is part of the themed collection: Energy & Environmental Science Recent HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
