Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Hydrogen liquefaction: a review of the fundamental physics, engineering practice and future opportunities
Saif ZS.
Al Ghafri
 ab,
Stephanie
Munro
a,
Umberto
Cardella
c,
Thomas
Funke
d,
William
Notardonato
e,
J. P. Martin
Trusler
f,
Jacob
Leachman
g,
Roland
Span
ab,
Stephanie
Munro
a,
Umberto
Cardella
c,
Thomas
Funke
d,
William
Notardonato
e,
J. P. Martin
Trusler
f,
Jacob
Leachman
g,
Roland
Span
 h,
Shoji
Kamiya
i,
Garth
Pearce
h,
Shoji
Kamiya
i,
Garth
Pearce
 j,
Adam
Swanger
k,
Elma Dorador
Rodriguez
a,
Paul
Bajada
a,
Fuyu
Jiao
a,
Kun
Peng
a,
Arman
Siahvashi
a,
Michael L.
Johns
j,
Adam
Swanger
k,
Elma Dorador
Rodriguez
a,
Paul
Bajada
a,
Fuyu
Jiao
a,
Kun
Peng
a,
Arman
Siahvashi
a,
Michael L.
Johns
 ab and
Eric F.
May
ab and
Eric F.
May
 *ab
*ab
aFluid Science and Resources Division, Department of Chemical Engineering, University of Western Australia, Crawley, WA 6009, Australia. E-mail: eric.may@uwa.edu.au
bFuture Energy Exports Cooperative Research Centre, 35 Stirling Hwy, Crawley, WA 6009, Australia
cHS Kempten, 87435 Kempten (Allgäu)
dFormFactor GmbH, Süss Straße 1, Registergericht Dresden HRB 3021, 01561 Thiendorf, Germany
eMitaVista, the Space Life Science Lab Suite 201C, 505 Odyssey Way, Exploration Park, FL 32953, USA
fDepartment of Chemical Engineering, Imperial College London, South Kensington Campus, London SW7 2AZ, UK
gHydrogen Properties for Energy Research (HYPER) Laboratory, School of Mechanical and Material Engineering, Washington State University, USA
hLehrstuhl für Thermodynamik, Ruhr-Universität Bochum, D-44780 Bochum, Germany
iKawasaki Heavy Industries, Ltd, 1-1, Kawasaki-cho, Akashi-City, 673-8666, Japan
jSchool of Mechanical and Manufacturing Engineering, The University of New South Wales, Sydney, NSW 2052, Australia
kNASA Kennedy Space Center, Cryogenics Test Laboratory, UB-G, KSC, FL 32899NASA, USA
First published on 21st April 2022
Abstract
Hydrogen is emerging as one of the most promising energy carriers for a decarbonised global energy system. Transportation and storage of hydrogen are critical to its large-scale adoption and to these ends liquid hydrogen is being widely considered. The liquefaction and storage processes must, however, be both safe and efficient for liquid hydrogen to be viable as an energy carrier. Identifying the most promising liquefaction processes and associated transport and storage technologies is therefore crucial; these need to be considered in terms of a range of interconnected parameters ranging from energy consumption and appropriate materials usage to considerations of unique liquid-hydrogen physics (in the form of ortho–para hydrogen conversion) and boil-off gas handling. This study presents the current state of liquid hydrogen technology across the entire value chain whilst detailing both the relevant underpinning science (e.g. the quantum behaviour of hydrogen at cryogenic temperatures) and current liquefaction process routes including relevant unit operation design and efficiency. Cognisant of the challenges associated with a projected hydrogen liquefaction plant capacity scale-up from the current 32 tonnes per day to greater than 100 tonnes per day to meet projected hydrogen demand, this study also reflects on the next-generation of liquid-hydrogen technologies and the scientific research and development priorities needed to enable them.
Broader contextThe global trade of fossil fuels amounts to over 3200 million tonnes of coal and oil and more than 850 billion cubic metres of gas each year. Average global atmospheric carbon dioxide levels have exceeded 410 parts per million and are higher than at any point in the past 800![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 years. Amidst growing pressure for countries to decarbonise their economies, future energy trading will increasingly include low- and zero-emissions production. There is a growing international consensus that hydrogen will play a key role in the world's transition to a sustainable energy future. Transportation of hydrogen over long distances will likely require both liquefaction and intermediate storage. However, hydrogen liquefaction has not yet been deployed at a scale necessary to supply projected liquid hydrogen demand. As a fluid that is not well understood at cryogenic temperatures and high pressures, liquid hydrogen presents a variety of technical, economic, and commercial challenges. This paper identifies the key research challenges that must be addressed over the course of the next decade in each of these areas. 000 years. Amidst growing pressure for countries to decarbonise their economies, future energy trading will increasingly include low- and zero-emissions production. There is a growing international consensus that hydrogen will play a key role in the world's transition to a sustainable energy future. Transportation of hydrogen over long distances will likely require both liquefaction and intermediate storage. However, hydrogen liquefaction has not yet been deployed at a scale necessary to supply projected liquid hydrogen demand. As a fluid that is not well understood at cryogenic temperatures and high pressures, liquid hydrogen presents a variety of technical, economic, and commercial challenges. This paper identifies the key research challenges that must be addressed over the course of the next decade in each of these areas.
|
1 Introduction
There is increasing consensus that hydrogen will be essential for a global transition to a sustainable energy economy. This transition is becoming increasingly important in light of both ambitious international climate goals and record-breaking carbon dioxide levels realised in 2019.1–5 Hydrogen can both enable the decarbonisation of sectors that are energy-intensive, including long-haul transport, industrial chemicals, and mineral processing,6–8 and be paired with renewables to store energy and overcome the challenges of intermittency.9 It can also be used as an energy carrier to export renewable energy from regions of abundant renewable resources to nations with limited energy resources.2Such a transition will, however, require the development of Hydrogen value chains that are both sustainable and substantial in scale. Consequently, hydrogen road maps have been outlined for regions and countries such as the United States,10 European Union,11,12 Japan,13,14 Australia,15 Germany16 and the United Kingdom.17Fig. 1 shows a block diagram representing the hydrogen value chain comprising four primary stages, labelled here as (1) resources, (2) production, (3) storage & transport, and (4) utilisation. Each of these stages can be realised using one of multiple possible options resulting, therefore, in a relatively large number of pathways by which the complete hydrogen value chain can be traversed. In addition to technical merit and cost, the selection of an option within a given stage of the value chain is influenced by both previous and subsequent stages. These inter-stage interactions mean that the hydrogen value chain can be considerably more complicated than other comparable energy value chains.18–20
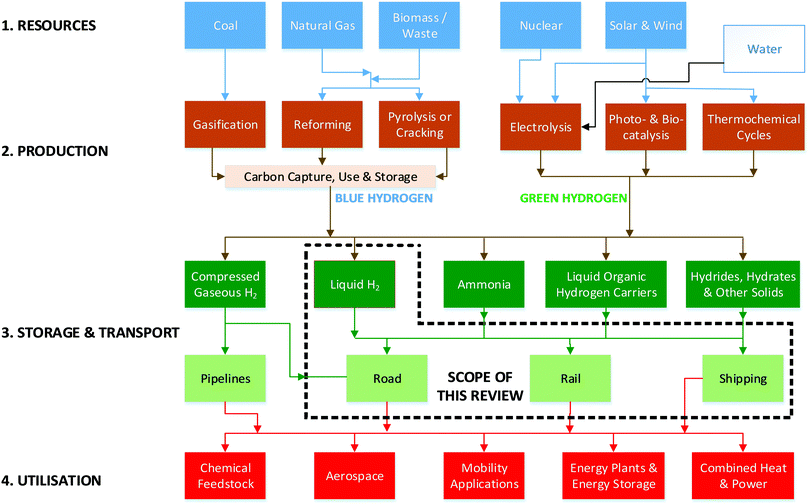 | ||
| Fig. 1 The hydrogen value chain and its relation to the focus of this review into hydrogen liquefaction. | ||
Primary energy sources are either carbon-based (natural gas, coal, biomass) or non-carbon based (wind, solar, nuclear). Water is another resource that can be critical to the first stage of the hydrogen value chain, particularly if the primary energy is produced in electrical form. These resources are then converted into hydrogen-containing materials through a variety of processes21–56 during the production stage. Production of H2 today is dominated by the use of fossil fuels57 because this is the cheapest way of manufacturing, for example, the fertiliser needed for food production. However, this does not account for the latent cost associated with the substantial emissions of CO2 resulting from such H2 production.
Table 1 presents comparative data for the current costs of producing H2 with low or no CO2 emissions. Pairing hydrogen produced from fossil fuels with carbon capture and storage (CCS)5 (to produce so-called blue hydrogen) can increase the associated cost by up to 54% while reducing CO2 emissions.37 However, the scale of CCS†![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 needed for blue H2 production technologies could represent a significant constraint on the role it can play within decarbonised energy supply chains. Green hydrogen production technologies38–56 (from renewable energy sources together with electrolysis, solar thermochemical splitting and biochemical processes) also face significant challenges as they are still limited in scale,‡
23 needed for blue H2 production technologies could represent a significant constraint on the role it can play within decarbonised energy supply chains. Green hydrogen production technologies38–56 (from renewable energy sources together with electrolysis, solar thermochemical splitting and biochemical processes) also face significant challenges as they are still limited in scale,‡![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 58 and their energy consumption is not yet cost-competitive. Nevertheless, the improving efficiencies and cost-reductions occurring in renewable energy generation and green hydrogen production suggest a cost curve trajectory that may bring them into line with blue hydrogen in the coming decade.59
58 and their energy consumption is not yet cost-competitive. Nevertheless, the improving efficiencies and cost-reductions occurring in renewable energy generation and green hydrogen production suggest a cost curve trajectory that may bring them into line with blue hydrogen in the coming decade.59
| Production process | Ref. | Feedstock | Power req. [kWh kgH2−1] | H2 capacity [TPD] | Capex [M$] | Opexa [M$ per year−1] | Cost [US$ per kgH2] | Capacity factorb |
|---|---|---|---|---|---|---|---|---|
| a Variable costs excluded. b Capacity factor of the hydrogen production method. Capacity factor is the unitless ratio of an actual electrical energy output over a given period of time to the maximum possible electrical energy output over that period. For Solar PV and Wind electrolysis, the operation of the electrolyser is dependent on the renewable energy source and as such, the system has a similar capacity factor. For SMR and CG with CCS, the capacity factor is affected by maintenance requirements. c Feedstock cost ranges from: 5.76–10.5 US$ per GJ. d Feedstock cost: 1.15–2.16 US$ per GJ. e Studies were conducted 5 years apart, 2015 and 2020. Variance in costs and capacity factor are attributed to significant developments within the solar photovoltaic industry made during that time. f Renewable energy with feedstock cost included in plant capital. g Shaner et al. estimates yearly Opex for electrolyser to be 3.2% of the Capex. Neither study states other Opex values. h Assuming the electrolyser is off-grid and powered solely by the renewable energy source. This value is location dependent. If connecting to the grid is possible, the electrolysers may operate at their maximum capacity, increasing the capacity factor to 97%. However, as most grids are currently fossil-fuel derived, CO2 emissions would be emitted. i Data retrieved from conceptual study investigating near, mid and long-term production costs associated with a 50 tonnes per day (TPD) standalone wind-hydrogen system. Stated costs is the range between the near and long-term scenarios for when the system is not connected to the grid. | ||||||||
| SMR with CCSc | 25, 37, 60 and 61 | Natural gas | 44–50 | 210–341 | 226–463 | 16.1 | 1.63–1.99 | 90–95% |
| CG with CCSd | 37, 61 and 62 | Coal | 47.2 | 277–500 | 546–677 | 27.6 | 1.63–2.26 | 85% |
| Solar PV-Electrolysise | 46 and 47 | Water | 55 | 10 | 134–260f | 4.30–8.32g | 6.22–12.1 | 20–31%h |
| Wind-Electrolysisi | 48 and 49 | Water | 44.7–53.4 | 50 | 185–500f | 9.13–25.3 | 2.37–5.69 | 41–55%h |
Storage and Transport is crucial to the establishment of a hydrogen value chain. The focus of this review, liquid hydrogen, is one of many technologies63–82 likely to play a significant role in the international trade of hydrogen. While the production stage usually generates gaseous H2, storing and transporting the associated energy over long time scales and distances requires the implementation of a physical or chemical conversion process within the 3rd stage of the value chain to achieve a viable energy density. The choice of which hydrogen vector to select to this end is strongly coupled to the mode of transport best-suited, both technically and from a cost perspective, to the intended end-use of the hydrogen with distance and the infrastructure available at both ends of the supply-chain being additional considerations.§
The final stage of the hydrogen value chain is utilisation, which is currently dominated by chemical feedstock83 (e.g. fertilisers, oil-refining, plastics, semi-conductors) and aerospace applications (rocket fuel).84–86 However, while global hydrogen production is now approximately 75 million tonnes per year,87 annual global demand is projected to reach 621 million tonnes, with the majority being used in the transportation sector.88 Certain hydrogen vectors are chemically incompatible with particular utilisation options, and thus the cost of converting them back to a compatible form must be factored in to any analysis used for vector selection. The very-high purity requirements of H2 fuel cell vehicles are similar to those needed and achieved during the hydrogen liquefaction process. For this reason, liquid hydrogen is expected to play a significant role within the supply chains needed to meet projected global demand.
Liquid hydrogen's role in the value chain
While liquid hydrogen's strengths as a vector include purity, end-use versatility, and ease of gasification, it also suffers from disadvantages relative to other possible vectors. Table 2 presents a summary of key properties of the primary hydrogen vectors available for storage and transport. These properties, together with an analysis of the costs associated with each stage of the liquid hydrogen supply chain, help with assessments of (i) applications for which it is most suited relative to other possible vectors, and (ii) technology gaps that need to be addressed to improve economic viability.| CGH2 | CGH2 | LH2 | NH3 | MeOH | LOHC | LNG | ||
|---|---|---|---|---|---|---|---|---|
| Ref. 37, 89 and 90 | Ref. 89–91 | Ref. 37, 89 and 92–110 | Ref. 60, 98 and 111–115 | Ref. 92 and 116–118 | Ref. 92, 98, 111 and 119–121 | Ref. 122 and 123 | ||
| a CGH2 – compressed hydrogen gas, LH2 – liquid hydrogen, NH3 – ammonia, MeOH – methanol, LOHC – methylcyclohexane. b Volumetric density is obtained using reference thermodynamic models implemented in REFPROP 10. c Excluding LNG, specific energy refers to amount of hydrogen available, in kWh equivalent terms, within one unit of carrier. LHV of hydrogen used: 120 MJ kg−1 and LHV of LNG [methane] used: 50 MJ kg−1 to calculate values. Molecular mass taken from the National Centre for Biotechnology Information. d Includes current industrial hydrogen liquefaction plant technology and conceptual studies for a range of plant capacities, from 5 TPD to >50 TPD. 6 kWh kg−1 has been demonstrated in conceptual studies for plant capacities > 50 TPD. The specific energy consumption of liquefiers operated in the USA is stated to range between 12.5 and 15 kWh kgH2−1 for capacities between 5.4 and 32 TPD. e Value calculated from the energy requirements for ammonia synthesis only. f Electricity consumption to synthesise methanol from CO2. This value includes energy requirement for water electrolysis to produce hydrogen. The study evaluates 2 different scenarios, transporting CO2 and placing the CO2 recovery facility nearby to the electrolyser. g Required energy to hydrogenate toluene. h Based on the energy efficiencies on various natural gas liquefaction cycles. i Includes catalytic steam reforming of methanol: 6.7 kWh kgH2−1 and methanol electrolysis: 15.4–32.4 kWh kgH2−1. The methanol electrolysis study referenced contains experimental data. Energy consumption is expected to decrease as the technology develops, hence the best case is stated. j Range varies due to the difference in enthalpies of dehydrogenation listed in the studies. k Percentage of end product consumed, either hydrogen or LNG. Calculated by total required energy to produce carrier [kWh kgH2−1] over the specific energy of the end product. Hydrogen specific energy: 33.3 kWh kg−1, LNG specific energy: 15.3 kWh kg−1. l LCOP – levelized cost of end product. Does not include hydrogen feed costs or transportation. m Includes Capex, Opex for compression and storage. Modelling assumes storage capacity is charged and discharged on a daily basis from tanks of 100 m3 capacity. Stated cost is base case for the levelized cost of hydrogen. With key actions and improvements in technology, best case range is 0.17–0.21 US$ per kg. n Cost to compress and refuel 750 bar storage tank. o Includes a range of values taken from conceptual studies. Base-case and best-case cost scenarios are included. p Includes the cost associated with hydrogenation and dehydrogenation. q Price range includes small- and large-scale methanol plants. Small scale – 350 TPD [MeOH], large scale > 1000 TPD [MeOH]. r Includes LOHC production, hydrogenation and dehydrogenation costs. s Study quantitively assessed various natural gas liquefaction processes. Costs include production, maintenance and amortized capital costs, excludes feed natural gas costs. The data was converted from $ per GJ to $ per kg using the LHV of LNG [methane, 50 MJ kg−1]. | ||||||||
| Vector properties | p/MPa | 35 | 70 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| T/K | 298 | 298 | 20.3 | 240.1 | 298 | 240.1 | 111.5 | |
| Volumetric densityb | [kg m−3] | 23.3 | 39.2 | 70.9 | 682 | 786 | 769 | 423 |
| Gravimetric hydrogen content | [wt%] | 100 | 100 | 100 | 17.8 | 12.5 | 6.16 | — |
| Volumetric H2 density | [kgH2 m−3] | 23.2 | 39.2 | 70.9 | 121 | 99 | 47.3 | — |
| Specific energy [mass]c | [kWh kgcarrier−1] | 33.3 | 33.3 | 33.3 | 5.93 | 4.20 | 2.05 | 15.3 |
| Specific energy [volume]c | [kWh Lcarrier−1] | 0.78 | 1.31 | 2.36 | 4.04 | 3.30 | 1.58 | 6.67 |
| Energy to produce carrier | [kWh kgcarrier−1] | 1.67–4.4 | 6.7 | 6–15d | 2–4e | 2.06–2.83f | 0.04–0.07g | 0.33–0.7h |
| Energy to produce carrier | [kWh kgH2−1] | 1.67–4.4 | 6.7 | 6–15d | 11.2–22.5 | 10.9–15f | 0.967 | — |
| Energy for dehydrogenation | [kWh kgH2−1] | — | — | — | 7.94 | 6.7–15.4 i | 9.7–11.2j | — |
| End product consumedk | [%] | 5–13.2 | 20 | 18.2–45.5 | 57.4–90.4 | 52.8–91.2 | 32–36.5 | 2.2–4.6 |
| LCOPl | [US$ per kg] | 0.22–0.28m | 2.83n | 0.5–3o | 1–2.17p | 2–4.17q | 0.58–1.56r | 0.03–0.21s |
An additional advantage of liquid hydrogen over other possible vectors include its relatively high volumetric hydrogen density (80% greater than gaseous H2 compressed to 70 MPa, and 50% greater than methylcyclohexane). The energy costs of producing ammonia and methanol per unit mass of hydrogen are comparable with liquid hydrogen yet the latter has no energy cost associated with dehydrogenation, which is required for fuel cell applications. For direct combustion, dealing with the greenhouse gases and other pollutants that can be emitted by liquid vectors (CO2 for methanol, NOx and N2O for ammonia) can also be a significant issue.
Nevertheless, liquid hydrogen is characterised by several appreciable limitations and challenges that restrict its current use. These include:
• Economics: hydrogen liquefaction is an energy-intensive process. Current processes have specific energy consumptions (SEC¶) of between (11.9 and 15) kWh kgLH2−1 which is (35 to 45)% of the lower heating value of hydrogen.124–126 This contributes significantly to the current specific liquefaction cost (SLC||) range of (2.5–3.0) US$ per kgLH2.124–127
• Cryogenic loss: boil-off loss associated with the storage, transportation and handling of liquid hydrogen can consume up to 40% of its available combustion energy.83 For example, the NASA Space Shuttle program carried out from 1977 to 2011 purchased over 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 tonnes of liquid hydrogen, of which 54.6% was used on-board; the rest was lost during storage, loading, or replenishment.
500 tonnes of liquid hydrogen, of which 54.6% was used on-board; the rest was lost during storage, loading, or replenishment.
• Safety: liquid hydrogen is not a common global shipping commodity. The lack of safety standards and regulation around hydrogen-based processes (especially at large-scale) could impede the establishment of liquid hydrogen supply chains.128
• Scale: currently, the largest single liquefier has a capacity of 32 tonnes per day (TPD), and the total global capacity is 350 tonnes per day. By 2050, the Hydrogen Council has estimated that 10% of total hydrogen demand, or 0.17 million tonnes per day, could be transported by sea.129 To ship even a modest fraction of this amount as liquid will require a substantial scale-up of liquefaction capacity. Achieving this goal will likely help mitigate the challenges of energy cost and economics.57 However, the technical challenges of scaling-up the necessary equipment (compressors, turbines and coldboxes) items are significant.
This review details the current state of knowledge, technology, and industrial practice relevant to the liquid hydrogen supply chain. Its objectives are to (i) provide an overview of the main challenges associated with producing and storing liquid hydrogen, and (ii) identify the primary opportunities for improving upon the four limitations detailed above: economics, cryogenic loss, safety and scale. To achieve these objectives the review starts by detailing the properties of molecular hydrogen that are relevant to liquefaction (Section 2), with a focus on the role that the spin isomers ortho- and para-hydrogen have on the thermodynamics and kinetics of liquefaction. Strengths and deficiencies in the models available for engineering design are identified together with knowledge and data gaps that if addressed would likely lead to process improvements. In Section 3, current approaches to hydrogen liquefaction are summarised before conceptual designs expected to deliver the needed efficiency improvements are reviewed. The storage and transport of liquid hydrogen is covered in Section 4, with a focus on the prediction and minimisation of boil-off losses. The specific challenges associated with safety and scale-up of the liquid hydrogen supply chain are considered in Section 5. Finally, a summative list of research and development priorities is presented.
2 Fundamental properties of molecular hydrogen
2.1 Mixture of ortho- & para-hydrogen
Molecular hydrogen occurs in two isomeric forms, ortho-hydrogen and para-hydrogen, differentiated by the nuclear spin state of the protons in each hydrogen atom. Fig. 2 shows a schematic representation of these two nuclear spin isomers, together with a curve showing the fraction of para-hydrogen in an ensemble of H2 molecules at equilibrium as a function of temperature. For a given temperature, the equilibrium ratio of ortho- to para-hydrogen concentrations (c(eq)OH2 and c(eq)pH2, respectively) is given by:130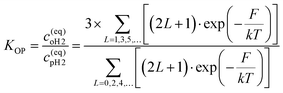 | (1) |
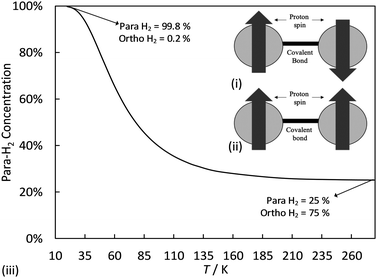 | ||
| Fig. 2 (i) para- and (ii) ortho-hydrogen spin isomers. (iii) para-Hydrogen content of hydrogen at equilibrium as a function of temperature. | ||
Here L is the molecule's rotational quantum number, with even values associated with para-hydrogen and odd values with ortho-hydrogen; k is the Boltzmann constant, T is the hydrogen temperature and F is the energy of the rotational state, which is given by eqn (2).130
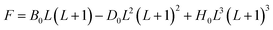 | (2) |
With decreasing temperature, the probability of a molecule being in a para-hydrogen state increases because of its lower energy, and consequently the equilibrium ortho–para ratio approaches zero. Intermolecular forces between two ortho-hydrogen molecules are slightly stronger than those between two para-hydrogen molecules because of the former's larger total nuclear spin. The ortho–para ratio thus affects the magnetic, optical, volumetric and thermal properties of the hydrogen.131
Accordingly, thermodynamic descriptions of hydrogen's properties at equilibrium should ideally represent the substance as a mixture with a temperature dependent composition. However at temperatures where a liquid phase can exist (<33 K), the substance may be well-approximated as pure para-hydrogen, while at ambient temperatures and above a pseudo-pure substance known as normal hydrogen consisting of 75% ortho-hydrogen and 25% para-hydrogen provides an excellent representation of the fluid.
When normal hydrogen is cooled appreciably, care must be taken with both the modelling approach and any physical handling of the substance. Quantum mechanical selection rules related to the conservation of molecular angular momentum mean that the transition from ortho- to para-hydrogen is forbidden and cannot occur spontaneously without an external interaction, such as a molecular collision in the presence of an inhomogeneous magnetic field as discussed below. In the absence of a suitable catalyst to facilitate the conversion, if normal hydrogen is rapidly cooled from ambient to cryogenic temperatures the time required before the equilibrium composition is reached can be of the order of days or weeks. Moreover, the conversion from normal to para-hydrogen is exothermic, releasing 525 kJ kg−1 of heat, which is larger than the enthalpy of vaporisation at liquid hydrogen's normal boiling point (448 kJ kg−1).131–133
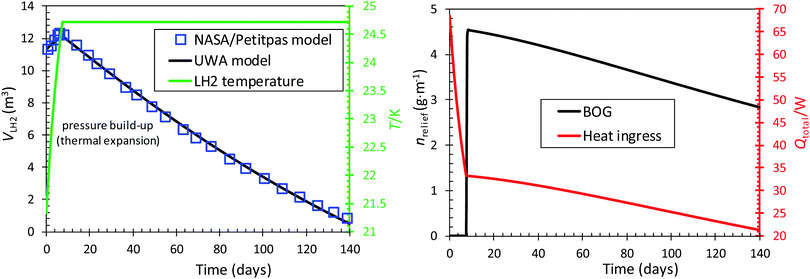
Thus, the effective and efficient conversion of ortho- to para-hydrogen is extremely important to industrial-scale applications of hydrogen liquefaction and storage. If insufficient time is allowed for the kinetics of the ortho–para conversion reaction to occur, normal hydrogen that is liquefied too rapidly will generate excessive amounts of boil-off gas. Excessive amounts of boil-off gas may cause over-pressurisation of the cryogenic storage tank, leading to serious safety issues. Even if the pressure build up is not rapid, the slow transformation of ortho- to para-hydrogen is one of the barriers to long-term liquid hydrogen storage given that the heat of conversion can evaporate more than 70% of the stored liquid hydrogen.133
Consequently, so-called catalyst materials are integrated into the construction of industrial hydrogen liquefaction processes. In the presence of such catalysts the kinetics are approximately first order with full conversion achieved in minutes.134–136 The principle cost to their use is an increased pressure drop across the liquefier.
2.2 Thermodynamic property descriptions for hydrogen liquefaction
| T c/K | p c/MPa | ρ c/kmol m−3 | |
|---|---|---|---|
| p-H2 | 32.938 | 1.2858 | 15.538 |
| o-H2 | 33.22 | 1.31065 | 15.445 |
| n-H2 | 33.145 | 1.2964 | 15.508 |
Leachman et al. developed a reference equation of state (EOS) for para-, ortho- and normal hydrogen, valid from the triple point temperature of each fluid (≈14 K) to 1000 K at pressures to 2000 MPa. Within this EOS, the reduced Helmholtz energy of each fluid is represented by a function that contains approximately 30 terms that are either polynomial, exponential, Gaussian or logarithmic functions of the reduced density and inverse reduced temperature. Each of the 30 terms has between one and seven adjustable parameters that were determined via non-linear least squares regression to the primary experimental data sets.
With this degree of flexibility, the Leachman EOS for para-hydrogen has an estimated expanded uncertainty (95% confidence interval) of 0.1% in density at temperatures up to 250 K and pressures to 40 MPa. Calculated heat capacities, speeds-of-sound and vapour pressures for para-hydrogen have estimated uncertainties of 1%, 0.5% and 0.1%, respectively at pressures below 100 MPa. The reference EOS for normal hydrogen has a similar performance over the same ranges and conditions: in the range (250 to 450) K at pressures up to 300 MPa, densities have an estimated uncertainty of 0.04%.
These small uncertainties make the reference EOS of Leachman et al. more than sufficient for the purpose of designing and optimising hydrogen liquefaction processes, assuming that the ortho–para ratio is known or controlled adequately at each point. Several low-cost or free software packages, including REFPROP 10,89 TREND 4142 and ThermoFAST Web,143 contain implementations of the reference EOS enabling calculations of the enthalpy changes, volumes and vapour–liquid equilibrium conditions needed to size and evaluate the performance of various liquefaction cycles. However, most commercial process simulation software packages do not typically use reference EOS, at least by default, because (i) their high-degree of non-linearity can make the solution of mass and energy balance equations impractically slow; (ii) there is often a need to also consider mixtures at some (early) stage within the process simulation; and (iii) hydrogen is often treated as a pure fluid (normal) with no consideration of the temperature-dependent ortho–para ratio. Consequently, commercial process simulation software packages tend to utilise cubic equations of state, such as the Peng–Robinson EOS,144 to represent pure hydrogen. Cubic EOS typically utilise three fluid-specific parameters based on the substance's critical point and normal boiling point. They then rely on a corresponding-states approach145,146 to calculate thermodynamic properties for the pure fluid and its mixtures over a wide-range of conditions. However, as discussed by Rowland et al.,147 the corresponding states approach tends to fail for fluids whose critical properties are influenced by quantum phenomena such as hydrogen, helium and neon.148,149
When the intermolecular separation is similar to the molecule's de Broglie wavelength, λB, quantum effects can influence the fluid's properties significantly: for liquid hydrogen at its normal boiling point, H2 has a de Broglie wavelength around 0.27 nm and an intermolecular separation of 0.66 nm. Thus the liquefaction of hydrogen provides a rare example of quantum mechanics impacting an industrial process in two separate ways: conversion between the ortho- and para- quantum states, and the wave-like properties of H2 molecules at low temperatures.
It is possible to correct the Peng–Robinson model of H2 for the effects of its de Broglie wavelength by modifying the intermolecular pair potential using the method of Feynman and Hibbs.150 Essentially, the correction makes the co-volume (size) parameter b in the cubic EOS temperature dependent, which in turn leads to a more robust mathematical representation of the pure fluid's vapour pressure curve. Aasen et al.151 applied this method to develop accurate quantum-corrected cubic equations of state for hydrogen, helium, neon, deuterium and their mixtures. With no new fitting parameters, significant improvements were achieved in the ability of the cubic equation to represent density, heat capacities and enthalpy changes at saturation for normal hydrogen (no consideration is given to ortho–para conversion). Perhaps even more significantly, given that the primary utility of cubic models is the description of vapour–liquid equilibrium (VLE) in mixtures, was the ability of the quantum-corrected Peng–Robinson EOS to represent the experimental VLE data for helium-neon and hydrogen-helium. This could be an important tool in the development and simulation of next-generation liquefaction processes that achieve higher efficiencies through the use of “quantum refrigerant mixtures” with varying ratios of (He + Ne + H2).
The first reason relates to the pre-treatment processes where impurities must be separated from the hydrogen prior to liquefaction to avoid solids forming in the cryogenic heat exchangers. The nature of the likely impurities depends upon the source of the hydrogen: if it is produced by SMR, then CH4, H2O, CO and CO2 should be considered, while if it is produced by electrolysis then H2O, O2, N2 and Ar might need to be removed before or during liquefaction. The state-of-the-art for describing the thermodynamic properties of such mixtures is the GERG-2008 EOS,152 which provides a framework for calculating the Helmholtz energy of mixtures containing up to 21 components, including normal H2 and the seven impurities listed above. However, the GERG-2008 EOS was developed for natural gas mixtures, with the primary focus being CH4 dominant systems with H2 considered only as an impurity. Furthermore the priority of the original GERG-2008 EOS was accurate calculations at pipeline conditions, with less weighting given to cryogenic temperatures (leading to the development of EOS-LNG153 in 2019).
To help address the resulting deficiencies of property calculations for multi-component hydrogen dominant mixtures, Beckmüller et al.154 have developed new Helmholtz EOS for binary mixtures of H2 + CH4, H2 + N2 H2 + CO2 and H2 + CO that can be used within the GERG-2008 framework. They replaced the pure-fluid model for H2 used in the original GERG-2008 model with the reference EOS of Leachman et al. for normal hydrogen, and also developed a new binary-specific departure functions to represent the available mixture data. The most significant of the improvements resulting from the new models of Beckmüller et al. is the accurate representation of the available low temperature VLE data, particularly for H2 + CO2 at T ≤ 296 K, H2 + CH4 at T ≤ 140 K, H2 + N2 at T ≤ 110 K, and H2 + CO at T ≤ 95 K; for these systems phase boundary predictions around or above 10 MPa made using the original GERG-2008 EOS are in substantial error. While only normal hydrogen is considered, the new Helmholtz models are valid over the same ranges of temperature and pressure as the original GERG-2008 (60 K ≤ T ≤ 700 K, p ≤ 70 MPa).
The second reason that fluid mixture property predictions are needed for hydrogen liquefaction process design is the selection and optimisation of mixed refrigerants (MRs). Conceptual studies have found that using MRs containing between two and five components can significantly improve the liquefaction cycle efficiency as detailed below. Common MR compositions can include hydrogen, nitrogen, neon, helium, and hydrocarbons ranging from methane to butane.155 The aforementioned quantum-corrected cubic EOS by Aasen et al.151 are one option for use in liquefaction simulations; these should provide reasonably accurate estimates of the cryogenic refrigerant mixture VLE properties. However, the changes in refrigerant enthalpies central to liquefaction cycle design are not so well represented by cubic EOS. Multi-parameter mixture Helmholtz energy models are the most accurate option for such calculations and should be used where available. Tkaczuk et al.156 have reported accurate Helmholtz energy EOS models with binary-specific functions for helium and neon, helium and argon and neon and argon mixtures. For refrigerant mixtures that include hydrogen with the noble gases, an extension of the work by Beckmüller et al. is underway; however, this approach will be limited by the use of normal rather than equilibrium hydrogen within the GERG-2008 framework. While this may not be problematic for predictions of phase equilibria and density it is an issue for calculations of the caloric properties needed for energy balances.
2.3 Transport properties and experimental data needs
The thermodynamic models for hydrogen are roughly one order of magnitude less accurate than those available for other fluids like methane or nitrogen, primarily because of the significantly more limited experimental data.157 Jacobsen et al.158 reviewed the experimental thermodynamic data available for normal and para-hydrogen and suggested a range of priority measurements needed to advance hydrogen property predictions at conditions of industrial interest. To improve the simulation of liquefaction processes, Jacobsen et al.158 recommended new measurements of density, speed of sound and heat capacity for normal hydrogen in the gaseous and liquid phases be acquired at temperatures below 50 K, while such measurements for para-hydrogen be conducted above 100 K because this would help resolve the contributions due to ortho-hydrogen. To acquire data with sufficiently low uncertainty, state-of-the-art experimental techniques for thermodynamic property measurements should be utilised, together with the ability to monitor the ortho–para ratio as a function of time (e.g. using Raman spectroscopy).159For hydrogen-rich mixtures, new data are required to improve upon and extend the approach of Beckmüller et al.154 For the four binaries they considered, the greatest data need identified was single-phase density data, especially for the normal H2 + CO system. However, particularly for the temperature range (30 to 150) K, (ternary) mixture measurements of equilibrium hydrogen with various other compounds likely to be present as either impurities in hydrogen production or components in mixed refrigerants should be prioritised. For mixed refrigerant candidates, enthalpy, heat capacity and/or speed of sound data should be acquired in addition to vapour–liquid equilibrium measurements, while for likely impurity compounds, the focus should be on solubility measurements as a function of temperature and concentration.
Both the experimental data situation and, consequently, the accuracy of predictive models are worse for the transport properties of hydrogen and its mixtures. Models for the viscosity and thermal conductivity of hydrogen are needed to estimate, respectively, pressure drops and heat transfer coefficients in various unit operations within the liquefaction process (e.g. cryogenic heat exchangers). Advances in fundamental theory160 have produced ab initio calculations of dilute gas transport properties for para-, ortho- and normal hydrogen from (20 to 2000) K161 that are as or more accurate than available measurements;162 however, these are only relevant for calculations involving low density gases. At higher densities, a reference viscosity model only exists for normal hydrogen163 in part because only one definitive data set for the viscosity of para-hydrogen exists.164
Muzny et al.163 suggest that para-hydrogen's viscosity is essentially equivalent to that of normal hydrogen provided the density is the same; for a given temperature, this correction can be facilitated using the Leachman et al. equations of state. The reference viscosity correlation for normal hydrogen has an estimated uncertainty of around 4% over the range (14 to 1000) K at pressures to 200 MPa, except in the critical region (where it is worse) and at pressures around 0.1 MPa (where it is better).
The thermal conductivity of para-hydrogen is nearly 30% larger than that of normal hydrogen at 140 K because of the heat capacity difference between the ortho and para spin isomers; this results from the higher rotational energy levels of the former.165 Assael et al.166 have developed reference correlations for the thermal conductivity of both normal and para-hydrogen, valid from the triple point to 1000 K and pressures to 100 MPa. Both correlations utilise the theoretical calculations of Mehl et al.161 for the dilute gas contribution. Critical enhancement contributions to thermal conductivity are more significant and wide-ranging (≈15 K from the critical temperature) than they are for viscosity, and these are explicitly modelled for both normal and para-hydrogen. Thirteen data sets were considered primary for normal hydrogen, although only three extend below 273 K, and only one was measured below 77 K. For para-hydrogen, the data situation is worse with only two primary data sets, both measured by Roder167,168 covering (17 to 153) K and (99 to 274) K, respectively. Assael et al. estimated the relative combined expanded uncertainties of these correlations as follows: normal hydrogen – 4% for temperatures above 100 K at pressures to 100 MPa, and 7% from the triple point to 100 K at pressures to 12 MPa, except in the critical region; para-hydrogen – 4% from the triple point to 300 K at pressures to 20 MPa, except in the critical region, and 6% at temperatures above 400 K. Differences in thermal conductivity have been used as a basis for measurements of the ortho–para ratio in a sample of hydrogen, particularly in the temperature range (50 to 250) K.
2.4 Conversion of ortho-hydrogen to para-hydrogen
In the absence of a heterogeneous catalyst, the ortho–para conversion occurs via a homogeneous auto-catalytic mechanism. In general, the conversion requires the presence of an inhomogeneous magnetic field (a magnetic field gradient), which exerts a torque upon the H2 molecule inducing the required ‘spin-flip’. Given that para-hydrogen is non-magnetic, while ortho-hydrogen has a net local magnetic field, the rate of homogeneous ortho-to-para conversion is dictated by collisions between ortho-hydrogen molecules. The resulting transition rates are therefore dependent on the concentration and temperature of the ortho-hydrogen molecules present, and follow a second-order rate equation:169,170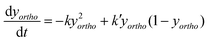 | (3) |
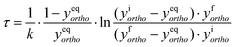 | (4) |
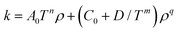 | (5) |
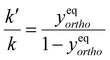 | (6) |
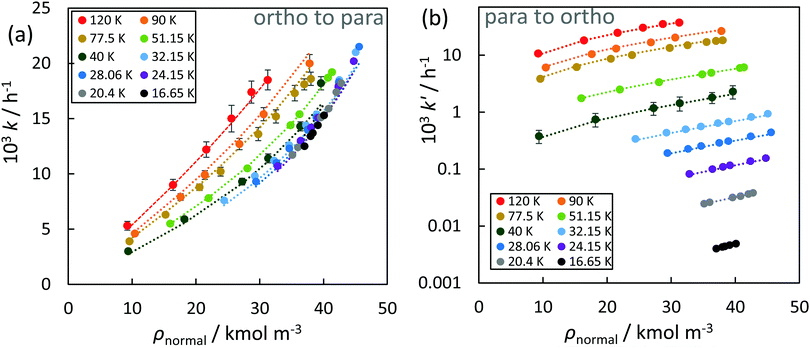 | ||
| Fig. 3 Autocatalytic homogenous ortho-to-para (a) & para-to-ortho (b) conversion rate constants as a function of normal hydrogen molar density systems. The dashed curves correspond to values calculated with the empirical correlation shown in eqn (5) and (6). | ||
Such a slow conversion rate is unviable for industrial liquefaction processes, and thus heterogeneous catalyst materials are used to ensure that the ortho–para conversion occurs sufficiently quickly as the hydrogen is cooled down to 20 K. Solid catalysts convert the spin-isomers via one of several mechanisms; at the low temperatures relevant to hydrogen liquefaction, non-dissociative mechanisms where adsorbed hydrogen remains in its molecular form are of primary relevance. The two catalytic ortho–para conversion mechanisms most relevant to liquefaction processes are (i) spin-conversion at paramagnetic surfaces, and (ii) spin-conversion at magnetically ordered surfaces. The former involves the interaction of hydrogen spin isomers with dilute paramagnetic species, such as Cr2O3, dispersed on high surface area materials such as alumina Al2O3. Chapin et al.171 found that the time constants of the ortho–para conversion reaction for H2 at 77 K and pressures to 10 MPa increased by an order of magnitude in the presence of about 0.1 wt% Cr2O3 on Al2O3. Misono and Selwood172 showed how the application of external magnetic fields up to 0.8 T could further accelerate the conversion rate achieved with 0.003 wt% Cr2O3 on Al2O3 by a factor between 40 and 90 at 298 K and 173 K, respectively. Spectron Gas Control Systems previously offered a paramagnetic surface catalyst OXISORB® based on CrO for commercial applications. However since chromium has been banned for use in multiple jurisdictions as a result of safety considerations, it is no longer commercially available.
IONEX® is a commercially available catalyst used widely by industry for ortho–para conversion that functions via the mechanism of spin conversion at magnetically-ordered surfaces. Supplied by Molecular Products,173 IONEX® is a porous particulate catalyst composed of Fe2O3 with a surface area around 216 m2 g−1 and particle (mesh) sizes between 0.3 and 0.6 mm.134 Activation to remove adsorbed water is required prior to use via heating to 110–150 °C under vacuum or dry H2 at 1 bar for one day. Exposure to trace impurities during operation can result in catalyst poisoning (H2S, mercaptans) or gradual deactivation (N2, H2O, COx, NOx).174 Such impurities are, generally removed upstream by adsorption as they might otherwise freeze-out and cause a blockage.
Generally the kinetics of the ortho–para conversion in the presence of a heterogeneous catalyst are adequately described by first order kinetics of the form:175–180
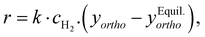 | (7) |
A few estimates of the inventory of catalyst required to convert a given amount of hydrogen are available in the open literature. Karlsson134 used 37.5 kg of IONEX® catalyst to convert 24 kg of normal hydrogen in a flow loop at 20 K to 99.8% para-hydrogen within 4.75 minutes. Zhuzhgov et al.174 estimated that approximately 65 L (or 80 kg) of an Fe2O3 catalyst (like IONEX®) is required to convert 100 kg h−1 of normal hydrogen to 99% para-hydrogen at 21 K in a continuous flow reactor. They tabulate average rate constants which allow calculation of the catalyst volume (or mass) required in a fixed bed reactor to treat a given feed flow of hydrogen to a specified outlet concentration of para-hydrogen. These average volume rate constants, which range from 0.24 mol s−1 L−1 at 78 K for Co(OH)3 to 2.5 mol s−1 L−1 at 22 K for a NiO on Al2O3 catalyst, assumed first order kinetics and accounted for mass transfer limitations (and thus particle size) using the approach of Wakao et al.181 Assuming a fixed bed containing 65 L of an Fe2O3 catalyst at 21 K with a void fraction of 0.38, Zhuzhgov et al.174 determined that a residence time of 4 s (liquid) and 400 s (gas) was required for effectively full conversion. This is a rate up to five orders of magnitude greater than that of the homogenous self-conversion reaction based on the data shown in Fig. 3. It is in fact likely that the ortho–para conversion kinetics in many practical scenarios are limited by heat transfer in terms of the removal of the heat of conversion.
Several research efforts focussed on improving ortho–para conversion efficiencies have been reported. One approach is to focus on alternative catalyst materials or properties. For example, Hutchinson179 and Wilhelmsen et al.182 found that a nickel oxide–silica catalyst doubled the catalytic activity of iron(III) oxide and reduced the cooling power consumption of the heat exchanger by 9%.182 Reducing the pressure drop associated with catalyst use within the liquefaction process is also an area of opportunity.183 Park et al.184 studied the pressure drop in a catalyst-packed heat exchanger using a cylinder filled with commercial IONEX® catalyst. It was found that the pressure drop is almost linearly dependent on space velocity (up to 1 bar pressure drop at velocity of 5 m s−1) and approximately five times lower than that predicted using the Ergun equation. Reducing such catalyst-induced pressure drops may prove to be a crucial feature of increased liquefaction efficiency.
Spectroscopic mechanisms of inducing the ortho–para conversion might also be worth considering. According to eqn (2), the smallest energy difference between the para- and ortho-states is approximately 2B0 which corresponds to electromagnetic radiation with a frequency of 3.6 THz and a wavelength of 0.083 mm. It might then be possible to use the Purcell effect,185 which is an enhancement of a molecule's spontaneous emission rate by its environment, to accelerate the conversion of ortho- to para-hydrogen by incorporating the H2 molecules within a cavity of length-scale 83 microns. In typical catalyst packings, many such micro-cavities are formed within the porous material. However, the cavities need to be electromagnetically resonant, and consideration would need to be given to the comparatively rapid diffusive motion of the gaseous hydrogen molecules and the associated effects such as Doppler line-width broadening.
3 Hydrogen liquefaction
3.1 Fundamentals of hydrogen liquefaction
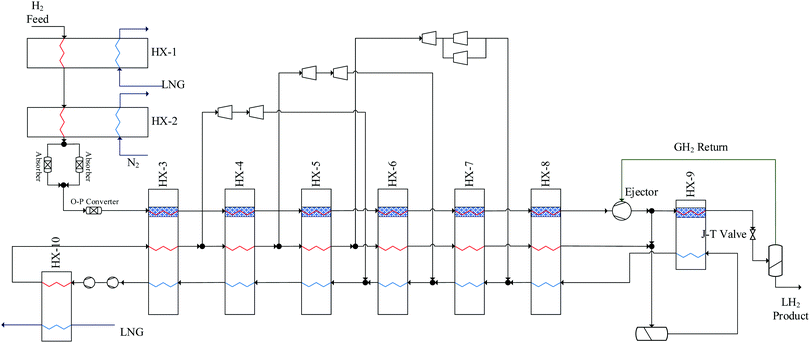
A generic hydrogen liquefaction process block diagram is shown in Fig. 4. Several recent publications cover hydrogen liquefaction methods131,186–190 so only a brief summary of the fundamentals is provided. If the hydrogen feed to the process is provided at comparatively low pressure, the first step of the process is pre-compression.190 The hydrogen then undergoes an optional pre-cooling stage to 80 K and an adsorption stage to remove impurities that may freeze out during cryogenic liquefaction.126 The hydrogen is then cooled to temperatures less than 30 K,191 using a closed-loop cryogenic refrigeration cycle. This cycle involves continuous or batch catalytic conversion of ortho to para-hydrogen.192 The final step is typically adiabatic expansion, which either takes the form of Joule–Thompson or turbine expansion.131 LH2 is usually stored at 20–23 K (0.1–0.2 MPa) with a para-hydrogen fraction greater than 98%.192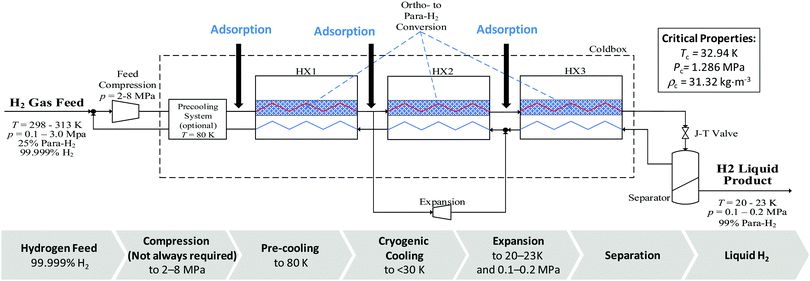 | ||
| Fig. 4 Simplified schematic diagram of the hydrogen liquefaction process based on simple Claude cycle. Critical properties of para-hydrogen are shown in this schematic. | ||
| w = W/ṁ | (8) |
The second law of thermodynamics establishes that there exists a minimum possible SEC, related to the specific enthalpy change Δh and the associated specific entropy change Δs of the hydrogen as follows:
| wideal = Δh − T0Δs | (9) |
• Inlet and outlet hydrogen pressure: 0.101 MPa;
• Inlet hydrogen temperature: 303 K;
• Ambient temperature: 298 K, and
• Inlet and outlet para-hydrogen mole fractions: 25% and 99.8%.
Exergy efficiency is a quantitative measure of the process efficiency and relates the actual SEC to the theoretical minimum value from eqn (9):201
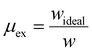 | (10) |
These studies have mostly been undertaken using commercial process simulation tools which permit detailed analysis of steady-state plant operation and the estimation of capital and operating costs for different design scenarios. For example, Cardella et al. optimized plant design using UniSim simulations incorporating a kinetic model for ortho–para conversion.210 Yin and Ju,186 Asadnia and Mehrpooya200 and Ansarinasab et al.187 all used Aspen HYSYS simulations to explore process optimisation and the economics of hydrogen liquefaction. Yang et al.,211 also using HYSYS, modelled the combined process of hydrogen production by steam methane reforming and liquefaction. Generally, the choice of simulation platform is less important than the fidelity of the models used to represent the physical properties of the hydrogen and refrigerant fluids, and the performance of key items of process equipment such as heat exchangers, turbines and compressors.
Valenti et al. assessed the influence of thermodynamic modelling on hydrogen liquefaction simulation outcomes of the Claude cycle.212 The study focused on the EOS for para-hydrogen and calculations of the heat capacity of equilibrium hydrogen (or para-hydrogen). The study found the following:
• Different EOS and heat capacity calculation methods yield significant differences in entropy (up to 11%) and exergy values (up to 13%).
• Adopting para-hydrogen models for the simulation of equilibrium hydrogen liquefaction may lead to errors greater than 10%.
• Densities calculated from the Peng–Robinson and Helmholtz-energy EOS models differed by up to 10%, with the largest differences found at low temperatures.
Collectively, heat capacity and density calculations had a major effect on the predicted cooling curves and are crucial for accurate and robust simulation of hydrogen liquefaction and subsequent equipment design.212 Furthermore, uncertainties in density at high pressure and/or liquid states result in uncertainties in volumetric flows. Erroneous volumetric flow predictions can lead to, for example, a mismatch of blade angles in turbo machinery, lowering efficiencies and, potentially leading to mis-predictions of operational range.
To demonstrate further the impact of thermodynamic property uncertainty on overall SEC, we have simulated a hydrogen liquefaction cryogenic process (hydrogen capacity: 5 TPD, T: 104 K to 20 K, p: 3 MPa) employing a basic Brayton refrigeration cycle with pure gaseous helium as the refrigerant (T: 104 K to 19 K, p: 8 MPa). The simulation determines the helium minimum flow rate needed to provide the required cooling, which significantly influences total compression power and thus SEC. Two different EOS have been applied for helium: the PR EOS213 and the reference Helmholtz EOS of Ortiz-Vega et al.214,215 The helium mass flow rate and SEC calculated using the two different EOS were 91 TPD vs 110 TPD (21% difference) and 5.5 kWh kg−1vs. 6.8 kWh kg−1 (23% difference), respectively. These significant deviations are mainly caused by the different temperature drop determinations (18.4 K vs 16.6 K) of helium across the vapour expander (isentropic efficiency: 78%), as obtained by the two EOS.
3.2 Industrial liquefaction processes and technology
The SEC of existing industrial hydrogen liquefiers ranges from (10 to 15) kWh kgLH2−1 or (35 to 45) % of the lower heating value of hydrogen,124–126 with exergy efficiencies ranging from 20–30%. This high energy demand is partially due to the relatively small size (capacity) of the existing liquefiers (less than approximately 32 TPD per liquefaction train), the design of which has focussed on lower capital cost (CAPEX) rather than high efficiency. This adds considerably to the cost and carbon intensity of the end-use hydrogen.216 More than fifty industrial hydrogen liquefaction plants have been constructed since 1952, with more than fifteen industrial plants constructed or proposed since 2000, as shown in Table 4. Recently-constructed plants are based on modified Claude cycles and use catalyst-packed heat exchangers (for continuous ortho–para conversion). Commercial catalysts are used in both the pre-cooling stage (where LN2 is often used as the refrigerant) and the cryogenic stages before the final expansion. Only a small number of existing liquefiers are discussed in detail in the literature: examples include the Linde hydrogen liquefier at Leuna217 and Praxair large liquefaction plants218 in the USA.| Location | Operator | Capacity [TPD] | Constructed | Additional information |
|---|---|---|---|---|
| *Source did not state a value.a Production capacity: 3000 L hour−1 per unit.b Sources state US tons per day, values have been converted to metric tonnes per day.c Construction was set to begin in 2020, source does not state an on-stream date. | ||||
| Kimitsu, Japan222 | Nippon Steel Corporation | 0.2 | 2004 | Pilot plant, separate H2 from coke oven gas |
| Saggonda, India223,224 | Andhra sugars | 1.2 | 2004 | Constructed by Linde |
| Osaka, Japan189,225 | Iwatani [hydro-edge] | 10 | 2006 | Total capacity split between two unitsa |
| Leuna, Germany217 | Linde | 5 | 2008 | |
| Chiba, Japan104,225 | Iwatani | 5a | 2009 | Constructed by Linde |
| Yamaguchi, Japan104,225 | Iwatani and Tokuyama | 5a | 2013 | Constructed by Linde |
| Akashi, Japan104,226 | Kawasaki Heavy Industries | 5 | 2014 | Japan's first domestically produced facility |
| Yamaguchi, Japan227 | Iwatani and Tokuyama | 10 | 2017 | Capacity of existing plant doubleda |
| Port of Hastings, Australia228 | HESC | 0.25 | 2020 | Australia's first H2 liquefaction facility |
| Las Vegas, USA56,229 | Air liquide | 27.2b | 2020c | Located in Apex Industrial Park |
| Leuna, Germany230 | Linde | 10 | 2021 | Capacity of existing plant doubled |
| La Porte, USA231 | Air products | 27.2b | 2021 | |
| La Porte, USA232 | Praxair | 27.2b | 2021 | Praxair's 5th H2 liquefaction plant |
| California, USA233 | Air products | * | 2021 | |
| Ulsan, Korea234 | Hyosung and Linde | 13 | 2022 | Constructed by Linde |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm.192,217 The reported SEC for the Leuna plant is 11.9 kWh kgLH2−1 and the exergy efficiency is 23.6%.131,217
000 rpm.192,217 The reported SEC for the Leuna plant is 11.9 kWh kgLH2−1 and the exergy efficiency is 23.6%.131,217
3.3 Conceptual liquefaction processes and technology
Over 25 theoretical hydrogen liquefaction processes have been developed conceptually since 1987.200 The capacity of these conceptual plants ranges from (5 to 864) TPD. The SEC range of conceptual hydrogen liquefaction plants is 4–14 kWh kgLH2−1. The objective of these conceptual models is to demonstrate theoretically how the efficiency of the liquefaction process can be optimised. Conceptual models do not in general fully consider total cost, operability or technical readiness.220 Large-scale hydrogen liquefaction methods and different configurations of hydrogen liquefaction cycles were reviewed by Aasadnia and Mehrpooya.131 Three of these conceptual designs proposed for large-scale hydrogen liquefaction process pants plants are summarised below.Other reported conceptual designs include the WE-NET process190,210 with a nitrogen pre-cooling stage and a SEC of 8.72 kWh kgLH2−1. This is a complex liquefaction process where a large supporting nitrogen liquefaction system is required to liquefy gaseous nitrogen after its use in the pre-cooling process. Krasae-in238 proposed a 100 TPD liquefier based on four cascaded hydrogen Brayton refrigeration cycles and a pre-cooling stage (to 80 K) using a five component refrigerant mixture. Cardella notes that, owing to the large number of refrigeration cycles, the likely viability of this process for industrial applications is low.126 Quack proposed a conceptual design239 for a 170 TPD liquefier based on propane pre-cooling and helium-neon Brayton refrigeration cycles. Challenges have been identified in determining the true efficiency of the process, owing to the high energy requirements of propane refrigeration. SINTEF reported a modified version of the process model189,240 proposed by Quack where pre-cooling of the hydrogen stream is achieved by an MR comprising C1–C5, ethylene, nitrogen, neon, and R14. Valenti and Macchi proposed a conceptual design216 for a 864 TPD liquefier based on four cascaded Brayton refrigeration cycles using a helium refrigerant. However, this is a complex process requiring a large number of compressor stages (hence higher CAPEX) due to the low molar mass of helium.
3.4 Technical gap between actual and conceptual units
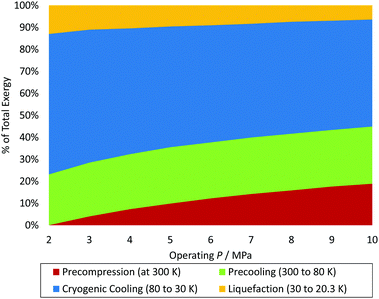
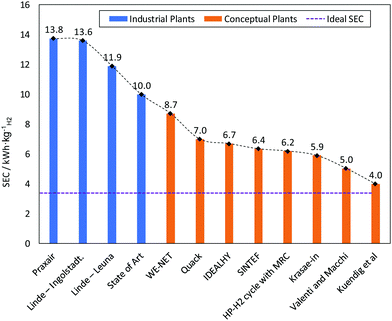
Conceptual plants are designed to provide lower SEC and higher exergy efficiency, as summarised in Fig. 11.126 However, limited consideration is given to total installed and operational costs or the technical maturity and ability to scale-up the component unit operations. This is particular true with respect to selection of the appropriate compressor type and the size of the required cold box.220 Current designs for approved future hydrogen liquefiers do not exceed the single liquefaction train capacity of approximately 30 TPD. Conceptual plants designed to be larger than 50 TPD thus help identify the required knowledge gaps and technological advances required for hydrogen liquefaction production at these elevated scales with improved SEC and exergy efficiency.A selection of potentially feasible conceptual hydrogen liquefaction processes are detailed and compared in Table 5. The following criteria were used to screen proposed processes for inclusion in Table 5:
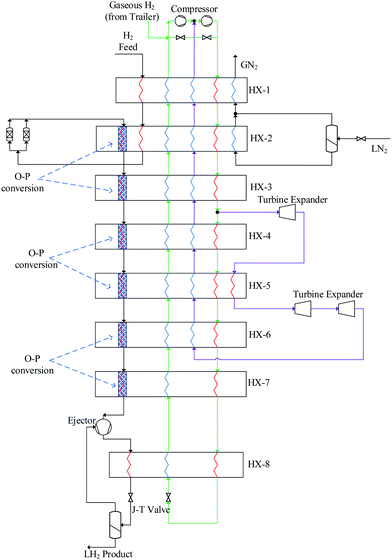
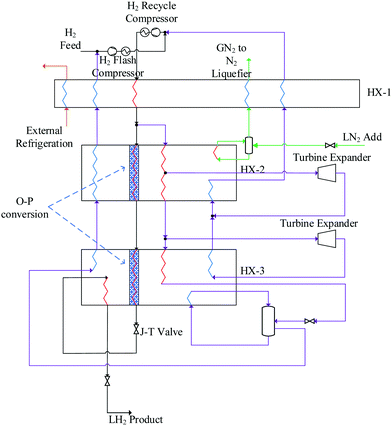
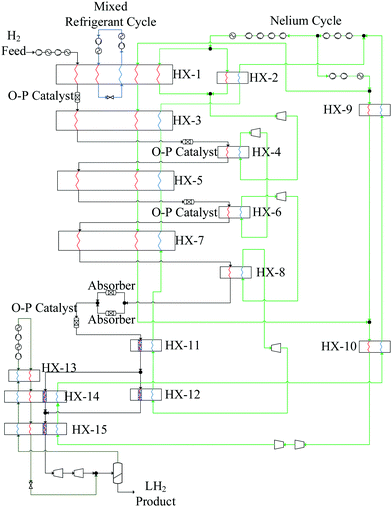
| Project | Linde – Leuna, 2007127,217,218 | Praxair – USA125,189,218 | IDEALHY, 2013192,221 | Berstad et al, 2010127 | Krasae-in, 2014238 | QUACK, 2002127,239 | WE-NET, 2004190,210 | HP-H2, 2017210 |
|---|---|---|---|---|---|---|---|---|
| Maturitya | Established | Established | Conceptual | Conceptual | Conceptual | Conceptual | Conceptual | Conceptual |
| *The study did not provide data for this value.a Conceptual – presented in research, not yet proven.b Modified pre-cooled Claude cycle with 3 heat exchangers. The first: nitrogen gas [GN2] and an external refrigeration system, second: liquid nitrogen [LN2] and H2 recycle stream, third: H2 recycle stream. Established – process has been implemented at scale.c Four cascaded hydrogen Joule–Brayton cycles pre-cooled with a multi-component refrigerant.d The largest capacity plant is a double-train liquefier.e Includes auxiliary plant energy requirements.f Dead-state temperature modified from 288.15 K to 300 K.g para-hydrogen percentage composition.h Main compressor stage efficiency assumed where source has not specifically stated a value.i Unknown category of efficiency.j Isentropic efficiency.k Multi-component mixture contains five components: hydrogen, nitrogen, methane, ethane and butane.l Two vapour compression refrigeration cycles used, propane to cool to 220 K and MR to cool to 73 K.m Liquid nitrogen at 0.12 MPa.n 15 stages required for low pressure and 25 required for high pressure.o Isothermal efficiency.p Polytropic efficiency.q J–T – Joule–Thomson valve.r J–T valves produce a SEC and an exergy efficiency of 6.5 kWh kg−1 and 44.7%, respectively. Liquid expanders produce a SEC and an exergy efficiency of 6.15 kWh kg−1 and 47.1%, respectively. | ||||||||
| Liquefaction cycle | LN2 pre-cooled Claude | N2 pre-cooled Claudeb | MR pre-cooled Brayton | MR Brayton | MR pre-cooled Braytonc | MR Brayton | LN2 pre-cooled Claude | MR pre-cooled Clauded |
| Capacity [TPD] | 5 | 20–36e | 50 | 86 | 100 | 170 | 300 | 100 |
| SEC [kWh kgLH2−1]f | 11.9 | 12.5–15 | 6.7 | 6.15–6.51 | 5.91 | 6.93 | 8.53 | 6.2 |
| Exergy efficiency | 23.6% | 19–24% | 32% | 44.7–47.1% | 39.1% | 56.8% | 46% | 43% |
| Hydrogen feed | ||||||||
| Pressure [MPa] | 2.4 | * | 2 | 2.1 | 2.1 | 0.1 | 0.1 | 2.5 |
| Temperature [K] | 313 | * | 293 | 310 | 298 | 300 | 300 | 303 |
| para-H2g | * | 25% | 25% | 25% | 25% | 25% | 25% | 25% |
| Compression | ||||||||
| Pressure [MPa] | — | * | 8 | 8 | — | 8 | 3.04 | — |
| Compressor type | — | * | Piston [2-stage] | * | — | Piston | Centrifugal | — |
| Efficiency h | — | * | 85%i | 85%j | — | 85% j | 80%i | — |
| Pre-cooling | ||||||||
| Refrigerant | Nitrogen | Nitrogen | Multi-componentk | Propane/MR | Multi-componentk | Propane/MRl | Nitrogen | MR |
| Refrigerant cycle | LN2 evaporation | GN2 & LN2 evaporationb | MR closed loop | 3 stage MR | 2 stage MR | Vapour compression | LN2 evaporationm | MRCn |
| Temperature [K] | 80 | 80 | 130 | 75 | 80 | 220/73l | 80 | 100 |
| Cryogenic cooling | ||||||||
| Refrigerant | Hydrogen | Hydrogen | Helium/Neon | Helium/Neon | Hydrogen | Helium/Neon | Hydrogen | Hydrogen |
| Refrigeration cycle | Claude | Claude | Brayton | Brayton | Brayton | Brayton | Claude | Claude |
| Temperature [K] | * | * | 26.8 | 26.5 | 20 | 25 | * | * |
| Compressor type | Piston | * | Turbo | Turbo | * | Turbo | Centrifugal | Piston |
| Compressor stages | 2 × 2 | * | 6 | 15 | 8 | 8 | 15/25o | 2p |
| Efficiency | 65–70%q | * | 85%i | 85%j | 90%j | 85% | 80% i | 76–85% |
| Expander type | Turbo | * | Turbo | Turbo | * | Turbo | Centrifugal | Turbo |
| No. of expanders | 3 | * | 5 | 2 | 4 | 6 | 2 | * |
| Expander efficiency | >85%j | * | 80%i | 90%j | * | 90%j | 85%i | 78–88%j |
| O–P conversion | Continuous | Continuous | Batch and continuous | Continuous | 6 stages | Continuous | Partially continuous | Continuous |
| Expansion | ||||||||
| Expansion device | J–Tr | J–Tr | Expander | J–Tr/Expanders | Expander | Expander & J–Tr | 2 stage J–Tr | Expander |
| Isentropic efficiency | N/A | N/A | 80%i | N/A/ 85% | * | 85% | N/A | 78–88% |
| Liquid H2 product | ||||||||
| Pressure [MPa] | 0.13 | 0.1 | 0.2 | 0.1 | 0.13 | 0.1 | 0.1 | 0.2 |
| Temperature [K] | 21 | 20.2 | 22.8 | 20.2 | 19.5 | 20.2 | 20.4 | 22.8t |
| para-H2g | >95% | >95% | >98% | >98.5% | 95% | > 99% | >95% | >98% |
• Scale ≥50 TPD,
• SEC between 4 and 10 kWh kgLH2−1,
• Exergy efficiency >23%, and
• Inclusion of novel but attainable technologies.
The key equipment needed for these most prospective large-scale conceptual are as follows:
From Table 5, it is evident that piston and turbo/centrifugal compressors are widely considered in conceptual processes. Whilst piston compressors are state-of-the-art for industrial liquefiers, they are expensive and limited in attainable flow rate and efficiency.126 Turbo-compressors generally have higher efficiency and greater throughput than piston compressors and are more suitable for large scale liquefaction. However, turbo-compressors are only really feasible for use with gases with molar masses above approximately 6 g mol−1.192 For pure hydrogen, a large number of turbo-compressor stages are required to reach the desired pressure as shown by Quack81 and Valenti and Macchi,216,239 and thus would incur higher capital expenditure. For example, the compression of hydrogen gas from atmospheric pressure to 8 MPa using a turbo-compressor would require at least 24 stages.202 Turbo-compressors are better suited to installation on the refrigerant side of the liquefaction process, as the refrigerant typically has a higher molar mass than hydrogen.239 There is no evidence of turbo compressors currently being used in industrial hydrogen liquefaction plants.192
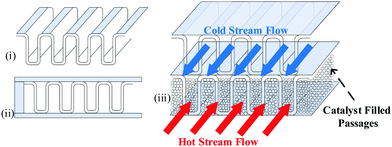 | ||
| Fig. 12 Illustration of the (i) fins, (ii) empty channels and (iii) catalyst-filled channels in a plate-fin heat exchanger with cold and warm streams flowing through. | ||
A larger heat exchange surface area and volume can deliver higher exergy efficiency but imposes higher capital expenses.245,246 The overall heat exchanger size is, however, limited primarily by the size of the accommodating coldbox and, to a lesser extent, the design pressure. Conceptual plants designed for large scale may need to address potential challenges with respect to process fluid pressure drop through the catalyst packings.183,184,246,247 Linde reported that the maximum size for LH2 industrial heat exchangers is approximately 8.2 m × 3.4 m × 1.5 m, with a core volume of 15 to 30 m3 and a specific surface of (500 to 2000) m2 m−3.242 These reported maximum feasible geometrical dimensions represent a potential limitation to up-scaling of efficient hydrogen liquefaction processes.175 However, PFHE remain the preferred technology for large-scaled hydrogen liquefaction. Skaugen et al.246 considered the use of spiral-wound heat exchangers for LH2 production with a capacity of 125 TPD. The lower surface density area and heat transfer coefficients in the spiral-wound heat exchangers meant their required weights are 2 to 14 times higher than those of the plate-fin heat exchangers.
Recently-built industrial hydrogen liquefiers and most conceptual processes in the literature employ continuous catalytic ortho–para conversion. A more thermodynamically efficient conversion that maintains the ortho–para ratio close to its equilibrium value can be achieved in such continuous catalytic conversion arrangements because both sensible and conversion heat are removed concurrently in the heat exchanger, resulting in higher exergy efficiency.248
Key limitations regarding heat exchanger design are excessive pressure drop through the catalyst bed and the ability to model quantitatively conversion kinetics while incorporating relevant heat and mass transfer limitations. Some conceptual processes use multiple stages of batch conversion to mimic continuous conversion largely because of the difficulty in quantifying the conversion kinetics.
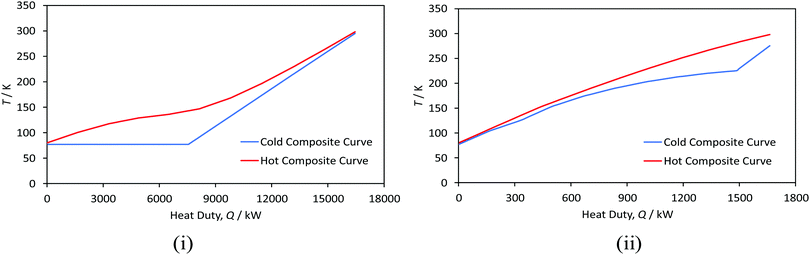
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 249), the efficiency advantage of liquid nitrogen pre-cooling decreases.250 For example, Van Hoecke et al.251 estimated that 30% of the energy required for the liquefaction of hydrogen is used to produce liquid nitrogen. A challenge with using nitrogen is, however, the large temperature differential between its cooling curve and that of the pre-cooled hydrogen gas which limits heat recovery.240 Conceptual processes have accordingly explored the use of helium, neon, LNG235 and mixed refrigerants127,200,210,238 in both pre-cooling and cryogenic stages.252 Mixed refrigerants (MRs) are advantageous because the evaporation curve can be designed to closely match the cooling curve of the hydrogen gas by altering the refrigerant mixture's composition (Fig. 13).155 The resulting minimisation of temperature difference improves thermodynamic efficiency.155 Mixtures may also have better heat transfer properties and compression features than pure refrigerants.200 Common MR compositions include hydrogen, nitrogen, neon, helium, and hydrocarbons ranging from methane to butane.155 However, achieving the desired hydrogen effluent temperature of 80 K in one stage, and the potential freeze-out of the heaviest components in the refrigerants are limitations.202 Thus conceptual processes have proposed a higher pre-cooling temperature, for example, 130 K,221 with multi-stage cascaded removal of components based on temperature.127 While implemented in LNG liquefaction, this can be challenging given the need to control and optimise the composition of the MRs throughout the cooling process.202 Improved thermodynamic modelling of novel mixed refrigerants is needed to compare their performance with simple conventional refrigerants. Based on LNG experience, research into the optimal blending of these fluids and methods aimed at preventing the refrigerant mixture components from segregating is required.253
249), the efficiency advantage of liquid nitrogen pre-cooling decreases.250 For example, Van Hoecke et al.251 estimated that 30% of the energy required for the liquefaction of hydrogen is used to produce liquid nitrogen. A challenge with using nitrogen is, however, the large temperature differential between its cooling curve and that of the pre-cooled hydrogen gas which limits heat recovery.240 Conceptual processes have accordingly explored the use of helium, neon, LNG235 and mixed refrigerants127,200,210,238 in both pre-cooling and cryogenic stages.252 Mixed refrigerants (MRs) are advantageous because the evaporation curve can be designed to closely match the cooling curve of the hydrogen gas by altering the refrigerant mixture's composition (Fig. 13).155 The resulting minimisation of temperature difference improves thermodynamic efficiency.155 Mixtures may also have better heat transfer properties and compression features than pure refrigerants.200 Common MR compositions include hydrogen, nitrogen, neon, helium, and hydrocarbons ranging from methane to butane.155 However, achieving the desired hydrogen effluent temperature of 80 K in one stage, and the potential freeze-out of the heaviest components in the refrigerants are limitations.202 Thus conceptual processes have proposed a higher pre-cooling temperature, for example, 130 K,221 with multi-stage cascaded removal of components based on temperature.127 While implemented in LNG liquefaction, this can be challenging given the need to control and optimise the composition of the MRs throughout the cooling process.202 Improved thermodynamic modelling of novel mixed refrigerants is needed to compare their performance with simple conventional refrigerants. Based on LNG experience, research into the optimal blending of these fluids and methods aimed at preventing the refrigerant mixture components from segregating is required.253
Oil-bearing and gas-bearing turbine technologies are considered state-of-the-art for hydrogen liquefiers.126,256 Oil-bearing systems require a continuous oil supply, oil pumps, and additional infrastructure for safe shutdown in the case of cycle failure.192 This additional equipment and power requirement increases their capital and operational expenditure above those of gas-bearing turbines. Gas-bearing turbines eliminate the risk of oil contaminating the hydrogen (because they use a gas film in the bearings), have higher isentropic efficiencies than oil-bearing turbines, reduced footprint, and provide greater turbine reliability.126,256
Most of the conceptual processes identified in Table 5 utilise a turbine expander both on the hydrogen and refrigeration side; however little detail as to the type to be deployed is provided. Valenti and Macchi estimated power recovery from expanders in their helium Brayton cycle to be greater than 1 kWh kgLH2−1.216 There is certainly potential to use large oil-free turbines in large-scale liquefiers;257 however, turbine expanders are both complex and attract a higher capital expenditure.104,258 Alternatively, exposing hydrogen to external magnetic fields can cause a temperature change and potentially liquefy it.259–261 Consequently, recent studies have considered the use of magnetic refrigeration as a final cooling stage in place of expansion to liquefy the hydrogen.262,263
3.5 Cost gap between existing and conceptual plants
Energy use accounts for around 30% of the total liquefaction cost of current liquefiers. These are dependent mainly on the liquefier efficiency189 which is in turn highly dependent on the energy consumption of the selected liquefaction process and electricity price. Liquefaction capacity also plays a major role in the cost breakdown. While existing liquefiers produce liquid hydrogen at (2.5 and 3) US$ per kgLH2, some conceptual designs are projected to produce liquid hydrogen below 1 US$ per kgLH2. Literature liquefaction cost estimates, as shown in Table 6 and Fig. 14, provide insight into plausible target costs. The Asia Pacific Energy Research Centre (APERC) report includes a projected hydrogen liquefaction price between (0.5 and 0.8) US$ per kgH2.94 This cost is much smaller than current liquefiers and is based on the IDEALHY system which estimates an energy consumption of approximately 6.4 kWh kgH2−1.| Study/report | Year | Country of study | Liquefaction cycle | Scale [TPD] | Liquefaction units/Individual capacity | SEC a [kWh kg−1] | Electricity cost b [$ per MW h] | Liquefaction cost b [$ per kgH2] | Status | |
|---|---|---|---|---|---|---|---|---|---|---|
a SEC – specific energy consumption.
b Average exchange rate over the last 6 months prior to submission: 0.67 USD = 1 AUD, 1.12 USD = 1 EUR, 1 USD = 107.2 JPY.
c Asia-Pacific Economic Cooperation is a grouping of 21 member countries including Australia, Canada, China, Japan, New Zealand, and the USA.
d Utility electricity cost in the USA by 2030.
e Based on IDEALHY conceptual design.
f Includes the levelized cost of compressing and transporting via pipeline the gas after production to the liquefaction facilities.
g Cost for at-scale production and transportation for selected transport routes.
h Assumed levelized cost of electricity by 2030.
i LH2 imported into Europe.
j Electricity generated through windmills with a total capacity of 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 MW.
k Cost calculated with an interest rate of 0.5 and 5%, respectively.
l Liquefaction plant is based on WE-NET conceptual design.
m Electricity generated through windmills with a total capacity of 60 500 MW.
k Cost calculated with an interest rate of 0.5 and 5%, respectively.
l Liquefaction plant is based on WE-NET conceptual design.
m Electricity generated through windmills with a total capacity of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 MW.
n Projected case.
o Kawasaki CO2 free hydrogen supply chain concept.
p Base case.
q 6.76 kWh kg−1, including auxiliary energy requirements.
r Prices account for seasonal changes in hydrogen cavern storage due to higher electricity prices.
s DOE – Department of Energy. Data from national laboratory models, Hydrogen Delivery Scenario Analysis Model [HDSAM] and Hydrogen Analysis [H2A].
t Supply chain developed for urban California market.
u Overall levelized cost is $2.75 per kg H2, which includes recurring costs. The capital contribution for a plant of this capacity is $1.41 per kg H2.
v Cost assumed to be the same that the levelized cost of electricity of renewables at Chile and Australia as analysed in the study.
w Cost calculated for a 2030 scenario. 400 MW.
n Projected case.
o Kawasaki CO2 free hydrogen supply chain concept.
p Base case.
q 6.76 kWh kg−1, including auxiliary energy requirements.
r Prices account for seasonal changes in hydrogen cavern storage due to higher electricity prices.
s DOE – Department of Energy. Data from national laboratory models, Hydrogen Delivery Scenario Analysis Model [HDSAM] and Hydrogen Analysis [H2A].
t Supply chain developed for urban California market.
u Overall levelized cost is $2.75 per kg H2, which includes recurring costs. The capital contribution for a plant of this capacity is $1.41 per kg H2.
v Cost assumed to be the same that the levelized cost of electricity of renewables at Chile and Australia as analysed in the study.
w Cost calculated for a 2030 scenario.
|
||||||||||
| APERC94 | 2018 | APECc | 800 | 16 × 50 TPD | 6.4 | 68d | 0.53–0.78 | Conceptual (2030) | ||
| Heuser et al.95 | 2019 | ARG | MR pre-cooled Braytone | 50 | 6.78 | 41 | 0.64f | Conceptual (2018) | ||
| Hydrogen Council96 | 2021 | SAU | 9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300g 300g |
13–37h | 0.7–1.0w | Conceptual (2030) | ||||
| Li et al.97 | 2020 | USA | 27–30 | 12 | 50–120 | 0.7–2.0w | Conceptual (2030) | |||
| Wijayanta et al.98 | 2019 | AUS | 822 | 15 | 0.76w | Conceptual (2030) | ||||
| Teichnmann et al.99 | 2012 | EURi | 2822 | 7 | 56 | 0.82 | Conceptual (2030) | |||
| Ishimoto et al.100 | 2020 | NOR | 500 | 1 × 500 TPD | 6.46 | 42.56 | 0.95–1.38 | Conceptual (2030) | ||
| Watanabe et al.101 | 2010 | ARG | LN2 pre-cooled Claudel | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
55 × 300 TPD | 8.72l | 54.5–77.3jk | 0.97–1.20 jk | Conceptual (2010) | |
| Watanabe et al.101 | 2010 | ARG | LN2 pre-cooled Claudel | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
322 × 51 TPD | 8.72l | 72.2–100km | 1.03–1.40km | Conceptual (2010) | |
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n n |
2018 | AUS | 210 | 7.88 | 26.80 | 1.07–1.30 | Conceptual (2025) | |||
KHI102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) o o |
2015 | AUS | 770 | 11 | 39.76 | 1.1 | Conceptual (2030) | |||
| Nyberg103 | 2021 | NOR | 176.2 | 7.5 | 46.26 | 1.68 | Conceptual (2030) | |||
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p p |
2018 | AUS | 50 | 9.05 | 40.20 | 1.72–2.10 | Conceptual (2025) | |||
| IDEALHY104–106 | 2013 | DEU | MR pre-cooled Brayton | 50 | 6.4q | 112 | 1.93 | Conceptual (2013) | ||
| Raab et al.107 | 2021 | AUS | 676.5 | 3 × 225.5 TPD | 7 | 88.14 | 1.97 | Conceptual (2020) | ||
| Reuß et al.108 | 2017 | DEU | MR pre-cooled Braytone | 50 | 6.78 | 67.2 | 2.12–2.22r | Conceptual (2015) | ||
DOE109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s s |
2019 | USAt | LN2 pre-cooled Claude | 27 | 11.5 | 42.68 | 2.75u | Established (2018) | ||
| European Commission110 | 2020 | EURi | 27 | 11.5 | 30–50v | 2.76 | Established (2019) | |||
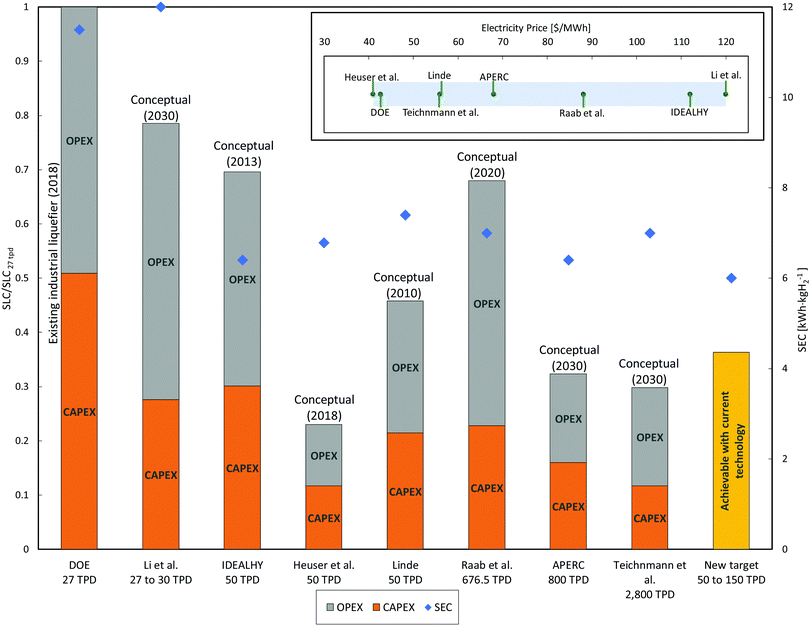 | ||
| Fig. 14 Specific energy consumption (SEC) and specific liquefaction costs (SLC) relative to a reference 27 TPD LH2 process ($2.75 per kg H2) and the various conceptual processes estimated at different electricity costs. | ||
To date, such efficiency has not yet been achieved in commercial liquefiers. In comparison, Kawasaki Heavy Industries has stated a liquefaction price of approximately 9.8 JP¥ per Nm3 (1.1 US$ per kgH2).102 Wijayanta et al.98 projected a smaller liquefaction cost by 2030 of approximately 7.3 JP¥ per Nm3 (0.76 US$ per kgH2). Other cost estimates are detailed in Table 6. However, none of the cost estimates can be achieved using existing liquefier designs, capacities and technologies. For reference, Connelly et al.109 estimated a liquefaction cost of 2.75 US$ per kgLH2 for a 27 TPD plant in California (USA), which is based on current commercially available technology used in existing industrial liquefiers. As shown in Fig. 14, specific liquefaction costs generally decrease with increasing plant capacity; however, this depends largely on the electricity cost which varied between (40 and 120) US$ per MW h across these studies. This observation is consistent with the work published by Cardella et al. who found that SLC decreased by almost 60% with a 50 TPD plant or by 67% with a 100 TPD plant, compared with a 5 TPD plant.93,264
3.6 Development potential of large-scale LH2 plants
From the above review of industrial and conceptual hydrogen liquefaction plants, key challenges were identified across the liquefaction process; these are summarised in Table 7. Obviously, for a new generation of large-scale plants (>100 TPD), there is a need to increase efficiency while managing overall total cost – a SEC target of 6 kWh kg−1 and a SLC less than 1 US$ per kg should be achievable.| Stage | Overall | Compression | Pre-cooling |
|---|---|---|---|
| Challenges | • Increase efficiency, decrease energy consumption and cost | • High energy demand | • High energy demand of producing and recycling LN2 |
| • Modular limitations | • Large number of compression stages required | • Limited industry examples of LNG or MR used for H2 cooling | |
| • Integration of renewable energy | • Flow rate limits of piston compressors | • MR thermodynamics not well understood | |
| • Translation of conceptual models to industry | • No evidence of turbo-compressors use in industrial LH2 plants | • Lack of knowledge on costs and environmental consequences for N2, LNG and MR cycles | |
| • Thermodynamic model accuracy for process design and simulation | |||
| • Hydrogen embrittlement and leakage |
| Stage | Cryogenic Cooling | Catalysts and OP conversion | Expansion |
|---|---|---|---|
| Challenges | • Freeze out of impurities from H2 | • Accurate OP ratio measurement | • Maximising work recovery |
| • Optimising heat exchanger sizes | • Optimizing residence time of H2 in the heat exchanger | • J–T expansion inefficiency | |
| • Accuracy of thermodynamic models | • Limited conversion rate data for different catalysts | • Large-scale oil-free and efficient turbine expanders | |
| • Catalyst longevity | • Optimising flow rates for different expansion technologies | ||
| • Pressure drop in heat exchangers |
A specific power requirement range between (6 and 8) kWh kg−1 is a plausible target, as shown in Fig. 14 by various conceptual studies, for scaled-up liquefiers without the need for novel technologies. However, this should be obtained via identifying rational and economically-viable means for improving efficiency which necessitates more advanced and integrated process designs that can increase exergy efficiency and thus reduce specific power requirements.237 Different design features should be considered in comparison to traditional large-scale hydrogen liquefaction processes, such as:
• Use of liquefaction cycles with new cryogenic refrigerant mixtures of helium/neon/hydrogen to enable the use of turbo compressors, which are generally well suited and scalable to very high capacities. This, and the potential for higher compressor efficiency, could be important elements when scaling-up liquefaction plants.
• Similarly, use of MR/LNG in the pre-cooled cycle instead of nitrogen to potentially cool down the hydrogen to around (80–110) K. Although some consideration is still required with respect to potential freeze-out and optimized composition, this can be largely implemented today. This can be accompanied by the use of expanders to increase efficiency.
• In addition use of, high-efficiency turbo-compressors on the refrigeration side, replacement of the J–T valve at the liquefaction stage by an expansion gas-bearing turbine (to minimize vapor fraction after expansion) whilst also ensuring high feed gas pressures between (1.5 and 3) MPa.
4 Liquid hydrogen storage and transportation
Rising demand for liquid hydrogen in new markets located a long-distance from the production site presents a new set of challenges in terms of transport and storage of this cryogenic fluid. These challenges stem largely from the boil-off losses caused by various heat sources leaking into the liquid, particularly over long periods of time. The severity of the problem was demonstrated in the Space Shuttle program managed by NASA, where over 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 tonnes of liquid hydrogen were purchased, of which 45.4% was lost during storage, loading, or replenishing.
500 tonnes of liquid hydrogen were purchased, of which 45.4% was lost during storage, loading, or replenishing.
4.1 Liquid hydrogen storage
Spherical tanks are used for storing larger volumes132 because they provide a minimum surface-to-volume ratio, and a more uniform distribution of stresses and strains. NASA operates the largest current storage vessel (3800 m3) for liquid hydrogen at Kennedy Space Centre, FL, USA with a storage capacity of 263 tonnes (if stored at 22 K and 0.15 MPa).265 NASA has more recently announced the construction of a 4732 m3, 327 tonne liquid hydrogen tank.86 In 2015 the US Department of Energy reported the price for a 3500 m3 liquid hydrogen tank was US$6.6 million (with a long-term target price of US$3.3 million).266
Kawasaki Heavy Industries completed the construction of its LH2 receiving terminal in the Port of Kobe in July 2020.267 The terminal features a 2500 m3 double-shell spherical storage tank with an outer diameter of approximately 19 m.267 The tank contains vacuum perlite insulation and is designed for a boil-off rate of less than 0.1% per day.267 Linde provides a variety of liquid hydrogen storage tank designs for industrial applications, fuel stations and bulk storage. These range in size from (12 to 300) m3, with a boil-off rate of <0.95% per day, depending mainly on the insulation materials used (vacuum-perlite or multi-layer insulation).268 Linde's large spherical tanks have an inner volume of 1100–2300 m3 with a storage capacity of 70–145 tonnes LH2 and a boil-off rate of <0.1% per day.268
In applications where weight is an issue, tank designs must also minimise tank weight. Tanks can, for example, exceed 50% of the dry weight of a space vehicle.270 Typical vessel designs are double-walled, vacuum-jacketed fluid containers.271,272 However, tanks may also use single walls with constant or variable thicknesses, although these are primarily employed at end-use points such as for rocket propellant tanks, and not for long term storage. High-vacuum insulation (<10−2 Pa) is commonly used for small-sized tanks (40 m3) and low-vacuum insulation (<1 Pa) is used for large tanks.102 The space between the double-walls serves as an insulation layer to minimise heat transfer to the liquid hydrogen in the inner vessel – traditionally perlite is used in this capacity. Advantages and disadvantages of common insulation methods are set out in Table 8.
| Insulation method | Advantages | Disadvantages |
|---|---|---|
| a CTE – coefficient of thermal expansion. b Fluid infiltration leads to increased thermal conductivity, potential loss of structural wall integrity. c These insulation methods are situated between walls. d The balance between the structural and thermal properties can be altered and optimized for the application. e Multilayer insulation is available in graded form to improve thermal properties and to reduce the density, but at a higher cost. f Method must be used in conjunction with an accompanying insulation material in order to achieve proper thermal protection. | ||
| Foam – outside | + Currently in use, well established | − Short term storage applications due to high thermal conductivity |
| + Low cost, easy to implement | − Low resistance to thermal radiation | |
| + Lightweight and low density | − Potential damage from environmental hazards and CTE mismatchinga | |
| + Provides good thermal resistance under non-vacuum conditions | − Degradation over time if exposed to the environment | |
| Foam – inside | + Low cost | − Larger structural tank wall required, resulting in increased mass |
| + Structural wall may be not exposed to cryogenic conditions | − Difficult to seal from cryogenic fluidb | |
| + Reduced CTEa mismatch issues because of composite constituents, therefore reduced microcracking | ||
| Vacuumcf | + Convection heat transfer suppressed or eliminated well established | − Heavier tank walls required |
| − Costly to implement and maintain | ||
| − Loss of vacuum failure scenario | ||
| Aerogelc (Bulk fill or blanket) | + Extremely low thermal conductivity and densityd | − Limited mechanical properties |
| + Provides excellent thermal resistance under non-vacuum conditions/moderate resistance under vacuum | − Not well established for large vessels | |
| Perlitec (Bulk fill) | + Low cost, well established | − Vacuum required to achieve high performance |
| + Low density | − Compaction can happen with certain tank geometries under thermal cycling and/or dynamic loads | |
| + Provides moderate thermal resistance under non-vacuum conditions/good resistance under vacuum | ||
| Glass Bubblesc (Bulk fill) | + Very low density | − Vacuum required to achieve high performance |
| + Simplified installation due to flowability | − Not well established for large vessels | |
| + Provides good thermal resistance under non-vacuum conditions/excellent resistance under vacuum | ||
| Multilayer insulationc (Blanket) | + Low density and radiation heat transfere | − High vacuum required |
| + Provides moderate thermal resistance under non-vacuum conditions/superior resistance under high vacuum | − Costly to implement and maintain | |
| − Near-catastrophic failure upon loss of vacuum | ||
| + Well established | − Difficult to execute for certain tank geometries | |
A number of composite wall materials have also been investigated, including graphite/epoxy composites and composite vessels both with and without liners.273 Challenges in the application of these materials include significant thermal residual stress in laminates due to coefficient of thermal expansion (CTE) mismatch between fibres and resin, and the embrittlement of resins under cryogenic and thermal cycling conditions.270 Research is still ongoing into the permeability and micro-cracking of these materials.92
Glass bubble insulation consisting of hollow glass spheres has been proposed. Field demonstration tests at NASA's Kennedy and Stennis Space Centres in 2015 found that boil-off losses were reduced by as much as 46% through the use of this glass bubble insulation in the field, compared to the use of perlite. Aerogel insulation may be feasible for short-term storage.273 Liquid hydrogen storage vessels manufactured with a liquid nitrogen shield have been proposed. Another design concept is to use the cold hydrogen boil-off vapor to shield the stored liquid hydrogen, resulting in a warmer gas exiting the tank.274
At the large scales planned for the future (>50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3), the biggest issue associated with liquid hydrogen storage is likely to be how to appropriately insulate the vessels. Traditional vacuum-jacketing might not be feasible at this scale. Novel insulation system schemes are needed that don’t require a self-supported outer jacket, but which still meet the thermal performance requirements associated with managing boil-off gases, preventing air liquefaction (if exposed to the environment), gas purge requirements, and which do not degrade over time.
000 m3), the biggest issue associated with liquid hydrogen storage is likely to be how to appropriately insulate the vessels. Traditional vacuum-jacketing might not be feasible at this scale. Novel insulation system schemes are needed that don’t require a self-supported outer jacket, but which still meet the thermal performance requirements associated with managing boil-off gases, preventing air liquefaction (if exposed to the environment), gas purge requirements, and which do not degrade over time.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 tank.271 Additionally, heat ingress causes the vapor temperature to increase faster than that of the liquid due to the vapor's higher thermal diffusivity, resulting in heat conduction across the vapor–liquid interface and thus a temperature gradient in the top layer of the liquid phase known as thermal stratification.277–285
000 m3 tank.271 Additionally, heat ingress causes the vapor temperature to increase faster than that of the liquid due to the vapor's higher thermal diffusivity, resulting in heat conduction across the vapor–liquid interface and thus a temperature gradient in the top layer of the liquid phase known as thermal stratification.277–285
BOG generation occurs at LH2 plants and exporting terminal for several additional reasons. These include BOG generation due to depressurisation (flashing), heat ingress into transfer pipes, heat added by equipment such as pumps, and cooling down of LH2 carrying vessels. For future large-scale LH2 storage and transport applications involving land-based tanks and sea-borne vessels, the management of such BOG is crucial from both an economic and safety perspective.
Petitpas276 modified a MATLAB code previously provided by NASA, to estimate the boil-off losses along the entire LH2 pathway from liquefaction to dispensing. During LH2 storage, Petitpas found that some temperature gradients may exist across the vessel (thermal stratification). This tendency of the stored liquid hydrogen to thermally stratify in a layer near the liquid-vapor interface causes challenges in propellant utilisation in large liquid-hydrogen fuelled rocket vehicles where pump cavitation is likely to occur which could result in the destruction of the flight vehicle.278Fig. 16 shows a comparison between calculations made by their code for a 12.5 m3 storage vertical tank (90% fill, with initial and final (relief) pressure of 0.137 and 0.31 MPa, respectively) and the super-heated vapour BOG model developed recently at UWA,286–288 originally for simulating industrial-scale LNG storage. This super-heated vapour model, implemented in the freely-available software package BoilFAST which includes options for LH2 and NH3 storage,289 considers the vapor and liquid phase temperatures to be independent but assumes they are spatially uniform; the liquid phase is assumed to be saturated, while the vapor can become superheated. For each phase, mass and energy balances are solved iteratively to determine phase amount and composition, boil-off rate, and vapor temperature. The BoilFAST results for the 12.5 m3 LH2 storage tank were in excellent agreement with those calculated by Petitpas and provided additional predictions such as BOG relief rate. On average, the daily BOG amount was calculated by both methods to be 5 kg (less than 0.5% volume loss per day).
Many studies290–303 have investigated possible ways to reduce boil-off losses and suggested that top fill (top spray) is probably the most effective way to reduce transfer losses, although more understanding of the underlying physics is needed. NASA have demonstrated the operation of an Integrated Refrigeration and Storage (IRAS)294 system allowing temperature control of the stored liquid hydrogen. This system employs an integrated heat exchanger together with a cryogenic refrigeration system that uses helium as the working fluid. Features of this system include zero boil-off and densified slush hydrogen production (mixture of liquid and solid hydrogen), which is detailed in Section 4.3.
4.2 Liquid hydrogen transport
In addition to selecting suitable materials for tank design, and minimising boil-off gas during transportation,92,304 another key consideration in liquid hydrogen transport is sloshing.304 This phenomenon is the movement of the liquid in the vessel due to the motion of the carrier, and it can lead to significant boil-off. Baffles may be installed in the tank to mitigate the effect.305 The LNG industry has conducted several studies into sloshing and how to minimise it,306–308 although generally it is avoided in practice by requiring minimum fill levels for vessels before they commence transport. This approach may not be sufficient for LH2 transport, given the higher rate of boil-off.To date, transportation of liquid hydrogen occurs predominantly by truck, with capacities up to approximately 60 m3 (≈4150 kg).271 The transportation of hydrogen by truck is primarily limited by high boil-off losses and volume capacity, rather than by weight. Safe road transport of liquid hydrogen can be achieved with existing technology; however, this is not at a scale comparable to the existing petroleum fuel distribution network.309 There exist few examples of liquid hydrogen transported by rail or aircraft.271 However these methods of LH2 transportation are limited by high energy use, boil-off losses and/or total capacity.
Transporting liquid hydrogen by marine vessels has historically been limited to fuel supply for the USA and French space programs.310 In November 2016, the International Maritime Organisation provided endorsement for the transport of liquefied hydrogen in bulk by sea between Australia and Japan.311 The Hydrogen Energy Supply Chain Project (HESC), which intends to transport liquid hydrogen from the Port of Hastings in Victoria, Australia, to Kobe, Japan, will be the first commercial project to pilot liquid hydrogen maritime transport.311 The project will inform future amendments to the IGC Code (International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk), including the potential to allow liquid hydrogen to be carried in bulk under the code without the requirement for special agreements.311
In December 2019, Kawasaki Heavy Industries launched their liquid hydrogen tanker, designed to carry 1250 m3 of hydrogen from Australia to Japan. The liquid hydrogen vessel is cylindrical and designed with a vacuum-insulated double-walled structure.267 The support structure uses glass-fibre-reinforced plastic to minimise heat transfer.267 A boil-off rate of less than 0.4% per day was reported, with BOG being re-stored without venting to atmosphere.267 In October 2020, Kawasaki Heavy Industries conducted a successful world-first sea trial of the liquefied hydrogen carrier SUISO FRONTIER. In December 2021, this ship departed from Japan bound for Australia. Conceptual approaches to BOG management have also investigated its use as a fuel, re-liquefying it or burning it.312
4.3 Densification and slush hydrogen
The use of liquid hydrogen densification and slush hydrogen are prospective technological approaches for addressing the challenges of storing and transporting LH2. Reducing the temperature of liquid hydrogen to 13.8 K can increase its density by 8.8% (77.0 kg m−3).86 Cooling further leads to the creation of slush hydrogen, a mixture of liquid and solid hydrogen at the triple point (13.8 K for para hydrogen). The mixture can have a density up to 22% higher than liquid hydrogen at its bubble point.86 This can offer potential reductions in size and mass of storage systems and transport vessels, and/or enable the transport of larger quantities in a given volume.297 Densification also allows for longer storage before boil-off, and increases cooling capacity when used as refrigerant.86 Traditionally, slush hydrogen has only been investigated for use in space missions.313–316 Slush hydrogen is produced via three key methods: (1) the spray method where liquid hydrogen is expanded, thus solidifying it, and mixed with liquid hydrogen; (2) the freeze–thaw method with a Dewar and vacuum pump; and (3) the Augur method (which uses helium refrigeration).317,318 The production cost of slush hydrogen is obviously greater than the liquefaction costs of hydrogen.3195 LH2 supply chains: prospects and challenges
Hydrogen has long been identified as a likely central component of future energy systems. In 1874, Jules Verne described the use of hydrogen and oxygen derived from water as fuels that would replace coal.320 Waves of enthusiasm for hydrogen have occurred several times in the last 50 years, particularly in the 1970s and early 2000s, largely in response to concerns about energy security. However, the cost of making and using hydrogen at those times ultimately meant these waves of enthusiasm did not lead to the envisaged wide-scale use of hydrogen for energy applications.Todays’ wave of enthusiasm for hydrogen is qualitatively different to those of the last 50 years in at least three ways. It is not only motivated by energy security but also the need to decarbonise the world's energy system. Additionally, the cost of producing renewable energy has decreased substantially, and CCS technologies are also more mature. Finally, the cost of using hydrogen to generate electricity has also dropped dramatically with the development, for example, of fuel cell technologies. These differences provide a basis for optimism regarding the current prospects for hydrogen's ascendance to a prominent and central component of global energy supply chains.
However, if hydrogen is to meet this expectation, a number of significant challenges will need to be overcome. Within the broader context of the hydrogen value chain (Fig. 1) these challenges can generally be classified as relating to cost (e.g. $2 per kgH2 is equivalent to $16.7 per GJ or more than 4 times the average 2021 Henry Hub natural gas price321), scale (e.g. the capacity to construct or convert national-scale infrastructure for hydrogen distribution), and the maintenance of social license (e.g. managing use conflicts over scarce waterresources). Challenges specific to the LH2 supply chain may similarly be placed in one or more of the four categories identified in the introduction: economics, cryogenic losses, scale and safety. These categories are clearly inter-related with, for example, safety requirements and the magnitude of cryogenic losses associated with liquid hydrogen storage inherently impacting its economics. Section 4 considered the underlying issues and emerging technical solutions relating to cryogenic losses of LH2. This section reviews the challenges and prospects associated with the other categories, by first covering the safety of liquid hydrogen supply chains and then considering their economics and scale-up. Comparisons with liquid ammonia, the primary alternative hydrogen vector to LH2, are then reviewed before a summary is presented of the priority research and development areas needed to advance liquid hydrogen supply chains.
5.1 Safety of liquid hydrogen supply chains
Ensuring the safe operation of hydrogen value chains is a challenge that relates to both cost and the maintenance of social licence. General awareness of the Hindenburg disaster after more than 80 years is a stark example of how easily energy technologies can be substantially tarnished in the mind of the public, even if the accident's root cause had little to do with hydrogen per se.322 The development and application of effective and efficient engineering standards are the central tool to address the challenge of hydrogen safety.There are several factors that give rise to safety risks inherent to the hydrogen value chain in general and liquid hydrogen in particular. Hydrogen gas has a strong propensity to leak due to its small molecular size and high diffusivity. A number of NASA launches have been halted due to hydrogen leaks, mostly in umbilicals.86 Hydrogen can also cause embrittlement of many materials, resulting in cracking and catastrophic failure of metals significantly below the yield stress. Furthermore, hydrogen has a high propensity to ignite due to its wide flammability range (4 to 74 vol% in air) and very low ignition energy (0.017 mJ).251 It has been shown to spontaneously ignite on sudden release from pressurised containers, although the mechanism is not fully understood.323 Hydrogen flames burn with a hot but near-invisible flame, making them difficult to detect.323
For systems producing or handling liquid hydrogen, the materials of construction must be both resistant to hydrogen embrittlement and suitable for use at cryogenic temperatures. Consideration must also be given to the expansion and contraction caused by the changes from ambient to liquid hydrogen temperatures.323 Liquid hydrogen can cause other gases (such as air and nitrogen) to condense and solidify – this can cause blockages and failures in equipment. As a result, storage tanks should be kept under positive pressure to prevent air ingress and purges of equipment should be followed by refilling with hydrogen, or replacement with helium.323
Liquid hydrogen and the associated boil-off gas can produce severe burns upon contact with the skin and delicate human tissue such as the eyes.323 Until the hydrogen vaporises as it warms, it will accumulate as a substance denser than air. This produces a considerable fire and explosion risk. This has resulted in vent line explosions in the past.86 In the situation that liquid hydrogen comes into contact with another liquid at a temperature above hydrogen's boiling point, there is a risk of a rapid phase transition explosion.323 This phenomenon has been observed for spills of LNG on water, but is not well understood for liquid hydrogen.323
NASA has observed significant degradation in performance of cryogenic hydrogen storage tanks due to issues with insulation systems.86 For example, a perlite void resulted in the venting of over 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 gallons per day of hydrogen.86 It was unclear what may have caused the void to form. Vacuum leaks have also posed a significant problem as evidenced by increased boil-off. These leaks resulted in the solidification of air in the storage tank annulus, which subsequently liquefied (as LH2 was removed from the tank) and cooled the vacuum jacket.86 This phenomenon decreased the tank wall temperature below its ductility limits, thereby cracking the vacuum jacket;86 it could have been prevented by draining the tank more slowly whilst heating the outer vessel with water.86
000 gallons per day of hydrogen.86 It was unclear what may have caused the void to form. Vacuum leaks have also posed a significant problem as evidenced by increased boil-off. These leaks resulted in the solidification of air in the storage tank annulus, which subsequently liquefied (as LH2 was removed from the tank) and cooled the vacuum jacket.86 This phenomenon decreased the tank wall temperature below its ductility limits, thereby cracking the vacuum jacket;86 it could have been prevented by draining the tank more slowly whilst heating the outer vessel with water.86
PRESLHY324 is currently conducting experimental work in hydrogen release and mixing, ignition, and combustion with a view to providing enhanced recommendations for safe design and operations of liquid hydrogen technologies.324–326 Kawasaki Heavy Industries in collaboration with TEN, JAXA, JARI, and the University of Tokyo have conducted experiments into the safety of liquid hydrogen storage including diffusion behaviour and heat leaks through cryogenic tank supporting structures.267 These tests will help inform the development and strengthening of engineering standards that further increase the safety of planned LH2 supply chains.
International standards for liquid hydrogen and maritime regulation. International hydrogen standards already exist and continue to be developed327 both by the International Organisation for Standardization (ISO) and the International Electrotechnical Commission (IEC). A number of international technical committees now are responsible for drafting standards in specific fields.328 Relevant standards for hydrogen liquefaction and storage include ISO/TC:22329 (road vehicles), ISO/TC 197330 (hydrogen technologies), and IEC/TC 105331 (fuel cell technology).
Most hydrogen-specific transport and storage standards are, however, still in development and targeted primarily at the utilisation stage of the value chain. Long-standing LNG and Liquefied Petroleum Gas (LPG) storage and transport standards may have the potential to cover relevant areas for the export of liquid hydrogen; however, this will require considerable review.332,333 Whilst the behaviour of LNG and LPG during transport is well understood, liquid hydrogen presents a unique set of challenges, particularly in the understanding of stratification, sloshing, boil-off gas, and pressure build-up. The National Fire Protection Association (NFPA) released a safety code which provides fundamental safeguards for the generation, installation, storage, piping, use, and handling of hydrogen in compressed gas (GH2) form or cryogenic liquid (LH2) form.334
Standards that govern the handling of liquid hydrogen at the point of use include ISO 13984 (Liquid hydrogen – land vehicle fuelling system interface) and ISO 13985 (Liquid hydrogen – land vehicle fuel tanks).329 Standards that govern safety regarding liquid hydrogen and associated infrastructure include ISO/TR 15916 (Basic considerations for the safety of hydrogen systems), ISO 26142 (Hydrogen detection apparatus – stationary applications), and IEC EN 60079-10, 14, 17, and 19 (Electrical apparatus for explosive gas atmospheres: classification of hazardous areas, inspection and maintenance, and repair). There are also eleven ISO standards for materials testing, six of which cover hydrogen embrittlement.335 Specific guidelines and recommendations have been developed by projects funded through the Fuel Cells and Hydrogen Joint Undertaking (FCH JU) (a public-private partnership) including336 HYPER, HyApproval, HyIndoor, HyFacts, HyResponse and HySEA. The transport of liquefied gases by sea is covered by the International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (IGC Code),337 which is a mandatory code under The International Convention for the Safety of Life at Sea (SOLAS Convention). The International Code of the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (IGC code) does not currently allow for the transportation of liquid hydrogen.311
The current state of standards thus requires first movers to negotiate regulation and permit requirements that are unclear, or not yet adapted for hydrogen use. Risk analysis toolkits, such as HyRAM,338 have the potential to enable industry and standard development organisations to take a performance-based engineering approach to regulation.
While the development of standards will help with addressing this safety challenge, a gap in the general public's understanding of hydrogen has been identified.339 Over fifty articles have studied public perceptions of hydrogen across a broad range of applications.339 Currently, liquid hydrogen is not prominent in the public perception as it is mainly used in industrial applications. However, emerging applications such as its storage and use in domestic refuelling stations, may change this, particularly if an incident occurs where public safety is put at risk.
Several publications studying perceptions of hydrogen have found that public attitudes appear to be generally neutral.339–345 These studies found that the most frequently identified concerns with the use of hydrogen technologies were safety and cost.339 A majority of participants identified that they would only be willing to pay for the use of hydrogen technologies if the costs were comparable or less than those of conventional technologies, even if there were clear environmental benefits.339 Thus even once the challenge of safety has been adequately addressed, the growth prospects for liquid hydrogen supply chains will be acutely dependent on how well the challenges of economics and scale can be addressed.
5.2 Cost and scale-up of liquid hydrogen supply chains
Projections of annual global hydrogen demand in 2050 have reached as high as 621 million tonnes.129 Near term growth in the liquid hydrogen market is projected to be 5.66% annually in the five years to 2024.346 For demand to reach these levels, the levelised cost of hydrogen (LCOH) associated with the entire value chain will need to decrease. Most analyses of the pathways by which this cost reduction can be achieved focus on scale up of the process to meet the larger demand. Fig. 17 and Table 9 present a summary of literature studies which have analysed the LCOH of possible liquid hydrogen supply chains, with scales ranging from (27 to 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400) tonnes per day: the world's current largest single liquefier has a capacity of 32 TPD.
400) tonnes per day: the world's current largest single liquefier has a capacity of 32 TPD.
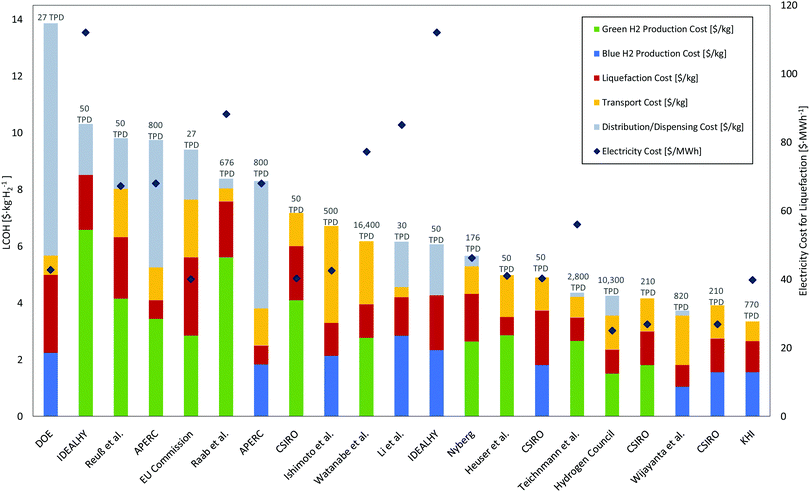 | ||
| Fig. 17 Levelised cost of hydrogen (LCOH) for proposed liquid hydrogen value chains found in literature. | ||
| Study/report | Scale [TPD] | Green H2 Production Costa [$ per kgH2] | Blue H2 Production Costa [$ per kgH2] | Liquefaction Costa [$ per kgH2] | LH2 Transport mode | Transport Costa [$ per kgH2] | Distribution Costa [$ per kgH2] | LCOHa,b [$ per kgH2] |
|---|---|---|---|---|---|---|---|---|
a
Average exchange rate over the last 6 months: 0.67 USD = 1 AUD, 1.12 USD = 1 EUR, 1 USD = 107.2 JPY. b Supply cost might include distribution and/or regasification cost, according to data available. c Asia-Pacific Economic Cooperation is a grouping of 21 member countries including Australia, Canada, China, Japan, New Zealand, and the USA. d Hydrogen produced from hydro and solar PV, respectively. e For a large (1200 N m3 h−1 ≈ 2.6 TPD) and small (300 N m3 h−1 ≈ 0.65 TPD) refuelling station, respectively. f Hydrogen produced through steam reforming and coal gasification, respectively. In both cases, carbon is captured and stored. g Hydrogen produced via electrolysis powered by wind. h Includes the levelized cost of compressing and transporting via pipeline the gas after production to the liquefaction facilities. i Includes the cost of storing LH2 before shipping, based on a maritime route from Patagonia to Japan. j Cost for at-scale production and transportation for selected transport routes. k Cost for hydrogen produced in 2030. l Transportation via ship from Saudi Arabia to Rotterdam. m Only considers liquid hydrogen distribution with a truck for 300 km, including boil-off losses, port storage cost, and hydrogen refuelling station operating costs. n Hydrogen produced through steam reforming and carbon is captured and stored. o Cost for hydrogen obtained from coal gasification and CCS. p Local distribution/transport. q LH2 imported into Europe. r For electrolysis with an electricity price of 0.02 EUR per kWh (22.4 USD per MW h) and 0.05 EUR per kWh (56 USD per MW h, respectively). s Transport of hydrogen via maritime for a 1000 km and 5000 km trip. t Distribution of LH2via truck for a 20 and 50 km trip, respectively. u Maritime transportation from Norway to the Netherlands and Norway to Japan, respectively. v Electricity generated through windmills with a total capacity of 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 MW. w Cost calculated with an interest rate of 0.5 and 5%, respectively. x Includes loading, shipping and unloading at the port. y Electricity generated through windmills with a total capacity of 60 500 MW. w Cost calculated with an interest rate of 0.5 and 5%, respectively. x Includes loading, shipping and unloading at the port. y Electricity generated through windmills with a total capacity of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 MW. z Projected case. aa Electrolysis with PEM electrolyser and alkaline electrolyser, respectively. ab Cost calculated for a truck that travels 166 400 MW. z Projected case. aa Electrolysis with PEM electrolyser and alkaline electrolyser, respectively. ab Cost calculated for a truck that travels 166![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 330 km per annum as per CSIRO's modelling with a cost of 0.62 USD per km. ac Kawasaki CO2 free hydrogen supply chain concept. ad For a trip from Australia to Japan. ae Hydrogen transported via ships with a capacity of 10 330 km per annum as per CSIRO's modelling with a cost of 0.62 USD per km. ac Kawasaki CO2 free hydrogen supply chain concept. ad For a trip from Australia to Japan. ae Hydrogen transported via ships with a capacity of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 840 tons of LH2 in a 9000 km trip, including a loading base. af Maritime transport within Norway and from Norway to the Netherlands, respectively. ag Includes buffer storage costs. ah Base case. ai Electrolysis with alkaline electrolyser and PEM electrolyser, respectively. aj Internal cost of hydrogen produced from wind power by electrolysis. ak Internal cost for hydrogen delivery and refuelling for a 100 km round trip transported by road. al Assumed a cost of 5 EUR kg−1 H2−1. am Cost for regasification only (in Japan). an Hydrogen produced via electrolysis. ao Prices account for seasonal changes in hydrogen cavern storage due to higher electricity prices. ap Cost for delivery via a trailer with a capacity of 4300 kg. aq Cost for a refuelling station with a design capacity of 850 kg day−1 (0.85 TPD). ar DOE – Department of Energy. Data from national laboratory models, Hydrogen Delivery Scenario Analysis Model [HDSAM] and Hydrogen Analysis [H2A]. as Supply chain developed for urban California market. at Hydrogen produced through steam reforming. au Overall levelized cost is $2.75 per kg H2, which includes recurring costs. The capital contribution for a plant of this capacity is $1.41 per kg H2. av Hydrogen delivered via trucks, stations are 350 kg day−1 (0.35 TPD) and dispensing at 700-bar for the light-duty vehicle market. aw Terminal profited cost adds 0.39 $ per kg. ax Hydrogen produced in Chile and the North West of Australia, respectively. ay Transport via ship from Chile to Rotterdam and Australia to Rotterdam, respectively. az Cost for large refuelling stations (larger than 20 MW h day−1 ≈ 0.6 TPD) and small refuelling stations (smaller than 20 MW h day−1 ≈ 0.6 TPD), respectively. 840 tons of LH2 in a 9000 km trip, including a loading base. af Maritime transport within Norway and from Norway to the Netherlands, respectively. ag Includes buffer storage costs. ah Base case. ai Electrolysis with alkaline electrolyser and PEM electrolyser, respectively. aj Internal cost of hydrogen produced from wind power by electrolysis. ak Internal cost for hydrogen delivery and refuelling for a 100 km round trip transported by road. al Assumed a cost of 5 EUR kg−1 H2−1. am Cost for regasification only (in Japan). an Hydrogen produced via electrolysis. ao Prices account for seasonal changes in hydrogen cavern storage due to higher electricity prices. ap Cost for delivery via a trailer with a capacity of 4300 kg. aq Cost for a refuelling station with a design capacity of 850 kg day−1 (0.85 TPD). ar DOE – Department of Energy. Data from national laboratory models, Hydrogen Delivery Scenario Analysis Model [HDSAM] and Hydrogen Analysis [H2A]. as Supply chain developed for urban California market. at Hydrogen produced through steam reforming. au Overall levelized cost is $2.75 per kg H2, which includes recurring costs. The capital contribution for a plant of this capacity is $1.41 per kg H2. av Hydrogen delivered via trucks, stations are 350 kg day−1 (0.35 TPD) and dispensing at 700-bar for the light-duty vehicle market. aw Terminal profited cost adds 0.39 $ per kg. ax Hydrogen produced in Chile and the North West of Australia, respectively. ay Transport via ship from Chile to Rotterdam and Australia to Rotterdam, respectively. az Cost for large refuelling stations (larger than 20 MW h day−1 ≈ 0.6 TPD) and small refuelling stations (smaller than 20 MW h day−1 ≈ 0.6 TPD), respectively. |
||||||||
| APERC94 | 800 | 2.55–4.33d | 0.53–0.78 | Road | 1.07–1.23 | 2.3–6.7e refuelling station (2.6–0.65 TPD) | 6.45–13.04 | |
| APERC94 | 800 | 1.22–2.44f | 0.53–0.78 | Road | 1.23–1.40 | 2.3–6.7e refuelling station (2.6–0.65 TPD) | 5.28–11.32 | |
| Heuser et al.95 | 50 | 2.86g | 0.64h | Maritime (21![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 km)i 400 km)i |
1.47 | 4.97 | ||
Hydrogen Council96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
9000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300j 300j |
1.5k | 0.7–1.0 | Maritime (8700 km)l | 1.0–1.4 | 0.7m,k distribution | 3.9–4.6k | |
Li et al.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
27–30 | 2.5–3.2n,k | 0.7–2.0 | Road (200–500 km) | 0.2–0.5k | 0.9–2.3k refuelling station (3–1 TPD) | 4.3–8.02k | |
Wijayanta et al.98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) k k |
822 | 1.04o,k | 0.76k | Maritime (9000 km)ad | 1.76k | 0.16p distribution | 3.72k | |
| Teichnmann et al.99 | 2822 | 1.87–3.45r | 0.82 | Maritime (1000–5000 km)s | 0.37–1.09 | 0.13–0.15t distribution | 3.19–5.51 | |
| Ishimoto et al.100 | 500 | 2.13 | 0.95–1.38 | Maritime (2539–23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407 km)u 407 km)u |
2.54–4.28 | 5.62–7.79 | ||
| Watanabe et al.101 | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 400 |
1.97v–3.56y | 0.97v–1.40y | Maritime (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km) 000 km) |
1.85–2.60x | 4.79–7.56w | ||
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
210 | 1.53–2.08aa | 1.07–1.30 | Road | 1.17ab | 3.77–4.55 | ||
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) z z |
210 | 1.26–1.84f | 1.07–1.30 | Road | 1.17ab | 3.5–4.31 | ||
KHI102![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ac ac |
770 | 1.55o | 1.1 | Maritime (9000 km)ad | 0.69ad,ae | 3.34 | ||
| Nyberg103 | 176.2 | 2.63 | 1.68 | Maritime (200–500 km)af | 0.28–1.68ag | 0.37 regasification | 4.96–6.36 | |
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ah ah |
50 | 3.20–4.98ai | 1.72–2.10 | Road | 1.17ab | 6.1–8.25 | ||
CSIRO37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ah ah |
50 | 1.52–2.10 | 1.72–2.10 | Road | 1.17ab | 4.41–5.37 | ||
| IDEALHY104–106 | 50 | 3.11–10.04aj | 1.93 | 1.80ak refuelling station (0.34 TPD) | 6.84–13.77 | |||
| IDEALHY104–106 | 50 | 1.83–2.84f | 1.93 | 1.80ak refuelling station (0.34 TPD) | 5.56–6.57 | |||
| Raab et al.107 | 676.5 | 5.6al | 1.97 | Maritime (9250 km)ad | 0.46 | 0.34am regasification | 8.37 | |
| Reuß et al.108 | 50 | 4.14an | 2.12–2.22ao | Road (250 km) | 1.71ap | 1.79a refuelling station (0.85 TPD)q | 9.76–10.86 | |
DOE109![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ar ar |
27 | 2.24at | 2.75au | Road (80 km) | 0.68 | 8.18av refuelling station (0.35 TPD) | 14.24aw | |
| European Commission110 | 27 | 1.61–4.07ax | 2.76 | Maritime (14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 267–20 267–20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 972 km)ay 972 km)ay |
1.64–2.43 | 1.19–2.35az refuelling station (>0.6 TPD and <0.6 TPD) | 7.2–8.85 | |
Fig. 17 shows how the LCOH of the liquid hydrogen supply chain was split by each study across four cost components: production, liquefaction, transport and distribution. The scale of the supply chain and the electricity cost assumed for the liquefaction process are also indicated. It is clear that there is significant variation between studies in the contribution of each cost component to the overall LCOH, which can make it difficult to compare different analyses or identify any trends associated with scale. The most variable component across the literature studies is the distribution cost, which ranges from 0 for several studies where it was not considered at all to 8.2 US$ per kgH2. This extreme latter value comes from a 2019 study reported by the DOE,109 where the distribution cost included the construction of 79 refuelling stations, each dispensing 350 kg day−1, as well as other costs such as taxes, insurance, licensing and permits. Reuß et al.108 have observed that the significant variation of costs for different refuelling stations is caused by site location, station design and capacity. Li et al.97 assessed the cost of various station designs, sizes and configurations. Large-scale hydrogen refuelling stations with a supply capacity of 1000 kg day−1 or more have distribution costs in the range (0.9 to 2.3) US$ per kgH2.
The second most variable component in the LCOH of prospective LH2 supply chains considered in the literature is the production cost. The comparisons shown in Fig. 17 indicate whether blue or green hydrogen production was assumed; in several studies two different LCOH values were reported with the only difference being the method of production. For example, the APERC347 conducted a study to calculate the total cost of hydrogen provision in Japan in 2030. Japan has set a target landed price of 3.3 US$ per kgH2 by 2030 and a longer term target of 2.2 US$ per kgH2).348 The study considered different international scenarios with an estimated hydrogen production cost from fossil fuel coupled with carbon capture and storage up to 2.8 US$ per kgH2, and from renewable energy up to 6.6 US$ per kgH2.94 The study compared the cost for the liquid hydrogen supply chain in Japan with conventional fuels used directly for electricity generation or in refuelling stations for fuelling FCVs. The APERC study estimated an imported (delivery) cost between (2.5 and 6.8) US$ per kgH2 when used for power generation and a total dispensing cost between (4.5 and 13) US$ per kgH2 when used in refuelling stations.94 This study concluded that imported hydrogen produced from fossil fuels with CCS can be competitive with power generation from oil by 2030 and with the cost of petrol in Japan if large capacity refuelling stations are utilised.
More recently, Longden et al.349 compared the costs and carbon emissions associated with different modes of hydrogen production. Distributions of costs were collated from 97 estimates across 16 studies that considered both green and blue hydrogen production technologies with various levels of carbon capture and storage. They reported a median cost of hydrogen production from renewables of 3.64 US$ per kgH2, with a range from (2.13 to 7.79) US$ per kgH2 resulting from the assumed capital cost ($500–2500 per kW), renewable electricity price ($10 to $85 per MW h) and/or electroyser capacity factor (26 to 48%).
The median cost of hydrogen produced by natural gas SMR with at least 90% CCS was 2.09 US$ per kgH2, with a range from (1.21 to 2.93) US$ per kgH2. At this level of CCS, the emissions intensity of using the blue hydrogen as a fuel was estimated to be about 22 kgCO2-e GJ−1, which is about one third that of using the natural gas directly.
The liquefaction and transport cost components have comparatively smaller variances than those of production and distribution and, based on the averages across the studies considered in Table 9, are the third and fourth largest contributors to the LCOH of the liquid hydrogen supply chains, respectively. The liquefaction cost component has an average of (1.45 ± 0.66) US$ per kgH2, where the error bound denotes the standard deviation for the studies listed. The transport cost component of the liquid hydrogen supply chains varies slightly more, depending on the distance considered by the supply chain with an average of (1.32 ± 0.71) US$ per kgH2 across all studies. Supply chains that involve maritime transport over distances of 1000 km or more had average transport costs of 0.14 US$ per kgH2 per 1000 km, while over shorter distances (80 to 500 km) transport costs increased to 4.78 US$ per kgH2 per 1000 km.
Liquefaction costs for the various studies depended on both the energy (electricity) price assumed and the scale of the process. Fig. 18 shows the estimated liquefaction cost component for each study in Table 9 against the scale of the LH2 supply chains. The scales considered cover a range from just below the largest current LH2 train in operation to conceptual trains 1000-times as large, with capacities similar to current mega-scale LNG trains. While there is considerable scatter in the estimated liquefaction costs, increasing the supply chain capacity above 100 TPD is expected to bring this component of the LCOH down to around 1 US$ per kgH2.
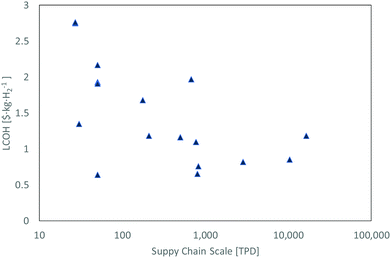 | ||
| Fig. 18 Liquefaction cost component of the LCOH studies shown in Fig. 17 as a function of the supply chain scale. | ||
At this stage, it is unclear whether increasing the scale of LH2 production trains beyond 100 TPD would lead to further reductions in unit liquefaction costs.350–352 It may be that the modularisation of unit operations at a standard size can help lower manufacturing costs by more than the economy of scale benefits that might be achieved in single trains with larger capacities. In either case, up-scaling the equipment required for larger capacity liquefaction trains presents specific technical challenges as discussed in Sections 3 and 4. These include equipment size, process efficiency, cost, safety, boil-off losses and management and insulation methods for large scale storage tanks.
A reduction in liquefaction cost to around 1 US$ per kgH2 or below will nevertheless be essential to the establishment of economically viable, wide-spread liquid hydrogen supply chains. Together with the anticipated reductions in the cost of producing clean hydrogen to 2 US$ per kgH2 or below, this should enable liquid hydrogen supply chains that service fuel cell vehicle (FCV) refuelling networks even with relatively high distribution costs. For example, a report published by the California Energy Commission,353 estimated the LCOH of hydrogen produced from renewables and used for FCVs in California will decline from around 16 US$ per kgH2 at present to a midpoint of 6 US$ per kgH2 by 2025, and to below 5 US$ per kgH2 by 2050, which is close to the 4 US$ per kgH2 long-term target established by the U.S. Department of Energy.
5.3 Comparison with ammonia
Ammonia is an alternative carrier to liquid hydrogen for storage and transport applications, which in contrast already has international supply chains established. To provide context for the above discussion of prospective liquid hydrogen supply chains, this section considers the use of ammonia as a transport vector in terms of energy consumption, cost and emissions.Using the conventional Haber Bosch (HB) process, the production of ammonia from H2 and N2 consumes power in the range of (2–4) kWh kgNH3−1.60,74,112,354–356 However, as indicated in Table 2, on a hydrogen mass basis this is equivalent to (11.2–22.5) kWh kgH2−1, which is comparable to or larger than the SEC currently required for hydrogen liquefaction of (11.9–15) kWh kgLH2−1. The primary advantage of ammonia relative to liquid hydrogen is its ease of storage and transport with minimal loss. However, if at the point of end use ammonia must be converted back to H2 (e.g. prior to use in a fuel cell), a further 7.94 kWh kgH2−1 must be used assuming a cracker efficiency of 76% in the best case scenario.60 In such cases the storage and transport advantages of using NH3 are greatly ameliorated, with between (57.4 and 90.4)% of the hydrogen stored in the vector being consumed to produce the necessary energy for conversion and (re-)cracking.
Instead of cracking, it is possible to use ammonia as an energy carrier by direct combustion357 (engine or gas turbine) or, potentially, in fuel cells.358 The latter technology is still pre-commercial, while the former must deal with challenges such as unwanted NOX emissions, CO2 tolerance and the relatively low flammability of ammonia.357 These challenges have to some extent been addressed through research and development with some demonstration-scale (10–40 kW) turbines and engines. Nevertheless, further research, development and up-scaling is required for these technologies given that the climate impact of ammonia combustion by-products can be multiple times worse than those of CO2.359
While ammonia production, storage and transport are very mature technologies, the associated costs are still important considerations when assessing its use for energy applications (as opposed e.g. to food production). Location and plant capacity are two factors influencing ammonia production cost, which range from 0.21–0.40 US$ per kgNH3 in Western Europe to as little as 0.14 US$ per kgNH3 on the US Gulf Coast.114 This is collectively equivalent to around (0.8–2.2) US$ per kgH2, considering the content of hydrogen in 1 kg of ammonia. Decomposition back to H2 adds approximately 0.67 US$ per kgH260 giving a total cost of (1.5–2.9) US$ for each kg of hydrogen transported as NH3.37,98,113–115 This is comparable with the sum of the liquefaction and transport costs averaged across all the LH2 studies considered in Table 9 (2.8 ± 0.7) US$ per kgH2.
Finally the widespread use of ammonia for energy applications has safety challenges potentially comparable to those faced by liquid hydrogen. These include increased rates of corrosion and material wear for containers storing both gaseous and liquid ammonia; high toxicity to biological systems and ammonia's capacity to rapidly dehydrate living tissue; its propensity to form secondary fine (≤2.5 μm) particulate matter upon combustion; and disruption of natural nitrogen deposition cycles.359 Thus while industry has an established track record of safely storing and transporting NH3 at commercial scales, significant handling and containment challenges remain if it is to be used on an even larger scale for energy applications.
Wijayanta et al.98 predicted an imported cost, including hydrogen production from SMR, of ammonia from Australia to Japan by 2030 of around 0.44 US$ per kgNH3 (2.5 US$ per kgH2) if ammonia can be used directly, and a total cost of 0.563 US$ per kgNH3 (3.2 US$ per kgH2), when ammonia is decomposed back to hydrogen. They concluded that when highly pure H2 is needed (such as for fuel cell vehicles), liquid hydrogen is more promising than ammonia and other hydrogen storage methods. Ishimoto et al.360 conducted a hydrogen export value chain analysis over distances ranging from (2500 to 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 407) km for both LH2 and ammonia. The hydrogen was produced by Autothermal Reforming at the same location for both vectors and the ammonia was cracked back to H2 at the destination. Using conservative costs for currently available technologies, Ishimoto et al.360 found that the LH2 supply chain costs ranged from (4.8 to 6.8 US$ per kgH2), which were generally below those of NH3 (6.1 to 6.6 US$ per kgH2), except for the longest distance supply chain. Moreover, the carbon emissions intensity of the LH2 supply chains were (5 to 7.2) times lower than those using NH3. Similarly, an analysis by the EU Science Hub – Joint Research Centre361 found that LH2 resulted in lower hydrogen delivery cost than NH3 for supply chain distances up to 22
407) km for both LH2 and ammonia. The hydrogen was produced by Autothermal Reforming at the same location for both vectors and the ammonia was cracked back to H2 at the destination. Using conservative costs for currently available technologies, Ishimoto et al.360 found that the LH2 supply chain costs ranged from (4.8 to 6.8 US$ per kgH2), which were generally below those of NH3 (6.1 to 6.6 US$ per kgH2), except for the longest distance supply chain. Moreover, the carbon emissions intensity of the LH2 supply chains were (5 to 7.2) times lower than those using NH3. Similarly, an analysis by the EU Science Hub – Joint Research Centre361 found that LH2 resulted in lower hydrogen delivery cost than NH3 for supply chain distances up to 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 km. Their analysis found that LH2 also had a supply chain cost below that of either pipelines or liquid organic hydrogen carriers for distances between (3000 and 17
000 km. Their analysis found that LH2 also had a supply chain cost below that of either pipelines or liquid organic hydrogen carriers for distances between (3000 and 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) km.
000) km.
5.4 Research, development & demonstration priorities for liquid hydrogen supply chains
The ammonia comparison helps illustrate that liquid hydrogen supply chains have reasonably good prospects relative to (and in conjunction with) those based on alternative vectors. However, practical solutions to multiple challenges in the four categories of economics, cryogenic losses, safety and scale presented in this paper will be required if large-scale LH2 supply chains are to be established. To address this array of challenges, significant levels of research, development and demonstration will be needed. Table 10 presents a summary of these needs, with a brief discussion of each of the main categories – Liquefaction, Storage & Transport, and Fundamentals – given in the text below. The areas and topics listed in Table 10 are based on the literature review presented in this paper and discussions with industry-based subject matter experts, which provided guidance on prioritisation. Accordingly, the list is non-exhaustive; rather it reflects an informed, yet subjective perspective of technical and engineering needs based on current knowledge.| Area | Sub area | R&D opportunities | Potential impact |
|---|---|---|---|
| a OP – ortho–para. b MR – mixed refrigerant. c EOS – equation of state. | |||
| Liquefaction | Process design | • Cold box modularity and system integration | • Enable construction of >100 TPD liquefiers with SEC between (6 and 8) kWh and SLC between (1 and 2) USD kgH2−1 |
| • Liquefier capacity and arrangement optimisation [e.g. 10 × 10 TPD vs. 1 × 100 TPD] | |||
| • Optimisation of MRb and pre-cooling fluids (techno-economic & environmental) | |||
| • Large-scale Brayton cycles optimised with robust, accurate dynamic process simulations | |||
| • Investigate use of electric-drive compressors powered by variable renewable energy profiles | |||
| Equipment design | • Improved heat exchanger designs with integrated catalysts for OPa conversion. | • Increased equipment efficiency and suitability for large-scale liquefiers, thus enabling construction of >100 TPD liquefiers | |
| • Large-scale oil-free turbines and/or large, economical electrochemical compressors for H2 | • Improved operational efficiency with reduced design margin requirements | ||
| • Construction with hydrogen-proof materials and effective cryogenic seals to prevent leakage | |||
| • Optimise trace impurity removal processes (e.g. integration with OP conversion process) | |||
| • Robust sensors for monitoring blockage risk in cryogenic heat exchangers | |||
| Ortho- to para-H2 conversion | • New OP catalysts with improved efficiencies, characterised over wide temperature range | • Reduced pressure drop in the heat exchangers | |
| • Catalyst performance (kinetics, heat & mass transfer) within dynamic process simulations | • Cheaper and/or more efficient heat exchangers | ||
| • Develop catalytic coatings & incorporate catalysts into novel heat exchangers (vortex tubes) | • Better monitoring of OP conversion performance allowing real time optimisation | ||
| • Robust sensors for monitoring OP ratio in process streams over wide temperature range | |||
| Storage & transport | Tank design & operation | • Vacuum panels, surface coatings, tank wall channels, 3D printed tanks for better insulation | • Safer storage with reduced boil-off losses |
| • Convert from spherical to cylindrical or conformal tank designs | • Enable construction of large-scale storage tanks for more cost-effective transport | ||
| • Efficiently integrate cryo-compressed and slush H2 storage technologies | • Improve storage capacity of LH2 | ||
| • Address deficiencies & exploit strengths of existing insulation materials & methods (see Table 8) | |||
| • Better models for predicting thermal stratification, interfacial heat transfer & resulting boil-off | |||
| Shipping & custody transfer | • Leak-free umbilicals and disconnects with good thermal insulation | • Improve safety and minimize boil-off losses | |
| • Improved design & operability of cryogenic hoses, venting systems and flow meters | • Reliable & safe export infrastructure | ||
| • Design large storage tanks with wide ranges of operating pressure to accommodate boil-off | • Minimise energy wasted during transfer operations | ||
| • Optimise ship ballast & sloshing protection requirements to account for low density cargo | |||
| • Develop operational procedures to keep empty ship tanks cool (or minimise cool-down time) | |||
| Safety | • Develop standards for leak-free operations & avoiding material compatibility problems | • Minimise risk of containment failures | |
| • Leak detection systems with appropriate spatial extent & temporal resolution | • Reduce risk of hydrogen fires or explosions | ||
| • Improve detection and develop procedures for stopping “invisible” fires | • Provide pathways for safe workforce expansion and public utilisation | ||
| • Long-term cyclic (thermal, pressure) testing of materials & systems in LH2 supply chain | |||
| Fundamentals | Fluids | • EOSc based on new (enthalpy) data for fluid mixtures containing H2 with varying OP ratios | • Helps with liquefaction opportunities listed above for process design and equipment design categories |
| • EOS based on new (enthalpy) data for cryogenic MR fluids | • Helps with storage & transport opportunities listed above for tank design and operation category | ||
| • Data & models for predicting solubilities, freeze-out kinetics & deposition risk of impurities in H2 | |||
| • Data & models for predicting impurity solubility, freeze-out & deposition risk in refrigerants | |||
| • Improved data & models for transport properties of cryogenic hydrogen & MR fluids | |||
| Catalysts & materials | • Cheaper catalysts with reaction kinetics including heat & mass transfer limitations characterised (e.g. particle size effects) over wide ranges of temperature, pressure and initial OP ratio. | • Helps with liquefaction opportunities listed above for equipment design and OP conversion categories | |
| • Cryogenic adsorption capacities & kinetics of media used for trace impurity removal | • Helps with storage & transport opportunities listed above for safety and tank design & operation categories. | ||
| • High-strength, low cost containment materials compatible with H2 & cryogenic temperatures | |||
| • Improved thermal insulation materials with long lifetimes & tolerance for cycling | |||
| Sensors | • Robust by-line or on-line measurements of OP ratio capable of operating in live plant | • Helps with liquefaction opportunities listed above for equipment design and OP conversion categories | |
| • Robust by-line or on-line measurements of impurity freeze-out or deposition risk | • Helps with storage & transport opportunities listed above for safety category | ||
| • Effective sensors for hydrogen leaks and/or fires with flexible spatial & temporal resolutions | |||
• The design and fabrication of larger coldboxes (e.g. on site), or the viability of hosting cryogenic equipment (e.g. heat exchangers) in multiple smaller coldboxes. Work on optimal modular arrangements of coldboxes to insulate different parts of the system while keeping capital costs down is also needed.
• The design of large oil-free turbines for H2 expansion and the use of turbo-compressors on the working-fluid side of the liquefaction process needs further research regarding their suitability for use in large-scale operations. Owing to the difficulties in compressing hydrogen, research into heavier compressing gases (e.g. MRs) used in the pre-cooling and refrigeration cycles should accompany this work. Additionally, the potential of using the electrochemical compression of hydrogen for large-scale operations should be explored given its established high efficiencies (95% for Carnot cycle up to 1 MPa).362,363
• Robust optimisation of large-scale liquefaction processes based on Brayton cycles through the use of new mixed-refrigerants, pre-cooling stages, process integration, efficient catalysts, and pressure drop reductions across and between unit operations. Such an optimisation will require robust, accurate and computationally efficient simulation platform for >100 TPD LH2 plant capable of describing several inherently dynamic (non-steady state) processes in a variety of configurations. Such simulations should use reference thermodynamic and transport properties models for hydrogen and mixed refrigerants, and an accurate kinetic model for ortho–para conversion, as recommended in the Fundamentals priority list below.
• The design of improved ortho- to para-hydrogen conversion reactors that optimise the rate of conversion and heat removal (and hence the management of potential heat spots) as well as the pressure drop experienced by the hydrogen during continuous operation. Currently, the amount of catalyst required for a given flowrate or quantity is specified based on proprietary kinetic data. The development of new catalysts that are easier to integrate into heat exchanger designs and/or work efficiently with trace removal systems (adsorbers) could help reduce both the capital cost (e.g. lower equipment counts) or operational cost (pressure drop) of liquefaction processes. The integration of new catalyst materials into vortex tube heat exchangers and/or the development of catalytic coatings are promising avenues for further research. Realising any of these opportunities would require a thorough quantification of reaction kinetics (Fundamentals) and their efficient inclusion into reactor models and process simulations recommended above. The development of efficient, standard approaches for the by-line or on-line measurement of para-hydrogen content would facilitate the validation of these conversion technologies as well as the real-time optimisation of plant operation.
• Demonstrate innovative insulation schemes and materials to minimise boil-off losses for future large-scale storage tanks and transfer pipelines. As detailed in Table 8, each existing method of insulation has strengths and weaknesses; mechanisms for overcoming the latter and exploiting the former should be investigated, potentially through combinations of different techniques and materials.
• Develop a comprehensive dynamic model able to reliably estimate boil-off rates, thermal stratification, pressurisation and flow dynamics, across a wide range of tank geometries and scales, both during static storage and custody transfer (loading) operations. This model should be validated against data acquired using a range of facilities with instrumentation sufficient to capture the underlying relevant multi-scale phenomena. The ability to describe the impact of sloshing, (auto) ortho–para conversion, superheated vapour phases with non-equilibrium ortho–para ratios, and boil-off return and re-liquefaction facilities will be important features of such a dynamic model.
• Explore the use of slush hydrogen technologies to simultaneously reduce boil-off rates and increase volumetric energy density. While implementing the additional refrigeration systems required will increase both capital and operational costs, these may be sufficiently offset by efficiencies achieved through system integration and economies of scale.
• Avoiding hydrogen leaks is one of the most difficult but important challenges that must be overcome to ensure sustained safe operations. While the development of standards and operational practice will help mitigate risks associated with de-pressurisation, fire and explosion, technologies that help eliminate and/or correct for human error will be just as important. Intelligent, automated and high-resolution systems for detecting and suppressing leaks or fires across a range of environments (liquefaction plants, ships, domestic fuelling stations) are needed. Additionally, innovations that are potentially “low-tech” in comparison, such as leak-free, hydrogen compatible connectors for cryogenic hoses will likely be of significant value.
To reduce the engineering margins applied within LH2 supply chains, smaller uncertainties are needed in the models used for process design. This provides a motivation to re-visit and extend the underlying database of thermodynamic and kinetic properties. Moreover, measurement technology has advanced significantly since a large fraction of the original data were acquired. Developing and applying new experimental techniques to reduce the uncertainty and extend the range of property data, both in the laboratory and in the plant via new sensors, will assist the establishment of more efficient, large-scale liquid hydrogen supply chains. Research priorities within the Fundamentals category include:
• Thermophysical property data for hydrogen and its mixtures are needed at temperatures from 20 K to 300 K and pressures from ambient to 8 MPa. As detailed in Section 2, a relatively large body of experimental data exists for the density, thermal conductivity and viscosity of hydrogen. However, their coverage of the conditions relevant to liquefaction are in some cases inadequate, and many of the data sets have significant scatter with few of the original articles providing a sufficiently detailed uncertainty analysis. New data should cover wider ranges of ortho–para ratios, particularly in the supercritical region (30 K to 100 K); however, it is essential that the ortho–para ratio for each data point be measured or well-defined. Importantly, data that provide more direct information about the fluid mixture's enthalpy (e.g. heat capacities, sound speeds, vaporisation) would enable significant improvements upon the Helmholtz EOS of Beckmüller et al.154 which only considers normal hydrogen. These more accurate and wider-ranging thermophysical property data would then enable correspondingly improved reference Helmholtz equations of state and transport property models for hydrogen mixtures that are central to process simulations.
• Similarly, new thermophysical property data and improved models are needed for a range of prospective mixed refrigerant fluids likely to be central to the development of hydrogen liquefaction cycles with SECs in the range (6 and 8) kWh kgH2−1. Given their prospective use in both the pre-cooling and cryogenic sections of the plant, there are many mixtures that could help improve overall cycle efficiency (e.g. methane + iso-pentane at temperatures as low as 98 K364). Again, measurements that provide information about the fluid mixture's enthalpy as a function (T,p) and composition would be of significant value to refrigerant design.
• A more universal kinetic expression for catalyst assisted ortho–para hydrogen conversion is required. Fundamental experiments that characterise reaction kinetics, including particle size effects and other heat and mass transfer limitations, over wide ranges of temperature, pressure and initial ortho–para ratio will be necessary to validate such an expression. Data on the reverse reaction rate should also be acquired, and a wider range of catalyst materials and morphologies should be investigated to identify potential methods of reducing the cost of conversion. Techniques for accurately and rapidly monitoring ortho–para ratios developed for the laboratory should be considered as the basis for improved sensor technologies that can operate in liquefaction plant environments.
• At the cryogenic temperatures encountered in hydrogen liquefaction plants, trace concentrations of impurities can freeze-out and lead to heat exchanger blockages and even plant shutdowns. This risk is similar to that present in the liquefaction of natural gas365–370 where the concentrations of H2O, C6H6 and CO2 impurities are controlled to below 0.1, 4 and 50 ppm, respectively. Significant effort has been invested into measuring and modelling the thermodynamic and kinetic aspects of the freeze-out phenomena. However, this problem is likely to be more severe for hydrogen liquefaction given both the additional impurity compounds that may freeze out (N2, O2, CH4, Ar), the even lower (ppb) solubilities in H2 at cryogenic temperatures, and the catalyst-packed plate and fin heat exchangers used. While existing plants employ adsorbers to remove these impurities, this may not be sufficient for larger plants (>50 TPD), particularly if allowable tolerances on impurity concentrations do not have a robust basis. Accordingly, the void of solubility and freeze-out kinetic data for relevant impurities in cryogenic H2 should be addressed through laboratory measurements, leading to the development of predictive engineering models. The efficacy of adsorbent materials and processes to remove these impurities to the requisite levels should also be investigated further to identify possible opportunities for optimising cost and performance. Finally, the adaptation of similar sensor technologies developed to provide real-time information on the freeze-out risk of impurities in LNG production371 should be considered.
6 Conclusions and outlook
Global demand for hydrogen is projected to rise from 75 million tonnes in 2019 to 621 million tonnes in 2050. Hydrogen liquefaction is likely to be a key technology enabling the growth of hydrogen transport and storage needed to meet projected global demand. Whilst it is one several technologies available, including ammonia and liquid organic carriers, LH2 offers several advantages including being an immediately pure product at the point of use. However, multiple challenges exist to the growth of liquid hydrogen, which can be placed within the broad categories of economics, cryogenic losses, safety and scale. By reviewing the current status of hydrogen liquefaction, from the fundamental physics to current engineering practice, this paper has sought to highlight the key barriers and identify potential solutions needed if LH2 is to have a substantial role in the world's future energy value chain.Two key targets for future liquid hydrogen supply chains are (i) reducing the specific liquefaction cost to around (1–2) US$ per kgH2 and (ii) lowering the specific energy consumption of the liquefaction process to between (6 and 8) kWh kgH2−1. These targets should be achievable if liquefaction train capacities can be increased to around 100 TPD or larger. Currently, global liquefaction capacity is around 350 TPD with a further 96 TPD planned; the capacity of the largest single train is 32 TPD. Current commercial processes have SECs from (11.9 to 15) kWh kgH2−1 (or 35 to 45% of the stored energy content), and SLCs around (2.5–3) US$ per kgH2, which is larger than the current cost of producing blue hydrogen. Achieving these targets will therefore require significant advances in the design and operation of hydrogen liquefaction facilities.
Hydrogen liquefaction plants that achieve these targets will likely use mixed-refrigerants in both the pre-cooling and cryogenic stages of a Brayton cycle, integrated and modular cold box configurations, high-efficiency turbo compressors on the refrigerant-side, and oil-free expanders for the H2 side. Ortho–para conversion will be more efficient using catalysts with better characterised kinetics integrated into heat exchanger designs that reduce the associated pressure drop. Potentially, LH2 storage and transport systems might utilise densification and slush hydrogen technologies to minimise boil-off losses and increase storage capacity. Improved models for quantitative predictions of thermal stratification, interfacial heat transfer, tank geometry, pressure and the resulting LH2 boil off rate in large storage tanks will be essential. Finally, if large-scale liquid hydrogen supply chains are to become a reality, addressing the challenge of safety will be vital through a combination of standards development and improved technologies for automatically detecting and eliminating hydrogen leaks and fires.
To help reach these goals, this paper first summarised the current state-of-the-art for knowledge and technology across the LH2 supply chain, and then presented a list of research, development and demonstration priorities in Table 10. While not exhaustive, over forty opportunities and topics are listed covering liquefaction (e.g. process & equipment design, ortho–para conversion), storage & transport (e.g. tank design, shipping & custody transfer, safety), and fundamentals (e.g. fluids, catalysts, sensors). Addressing this range of research, and development opportunities will require a concerted and collaborative effort by both industry and academia, with significant investments of expertise and new infrastructure for laboratory measurements, pilot-scale demonstrations and ultimately industrial-scale deployment.
Abbreviations
| Al2O3 | Aluminium oxide |
| APEC | Asia Pacific Economic Cooperation |
| APERC | Asia Pacific Energy Research Centre |
| Ar | Argon |
| AUD | Australian dollar |
| BOG | Boil-off gas |
| CAPEX | Capital expenditures |
| CCS | Carbon capture and storage |
| CG | Coal gasification |
| CGH2 | Compressed hydrogen gas |
| CH4 | Methane |
| CO | Carbon monoxide |
| Co(OH)3 | Cobalt hydroxide |
| CO2 | Carbon dioxide |
| COX | Oxide of carbon |
| Cr2O3 | Chromium oxide |
| CrO | Chromium oxide |
| CSIRO | Commonwealth Scientific and Industrial Research Organisation |
| CTE | Coefficient of thermal expansion |
| DOE | US Department of Energy |
| EOS | Equations of state |
| EU | European union |
| EUR | Euros |
| FCH JU | Fuel cells and hydrogen joint undertaking |
| FCV | Fuel cell vehicle |
| Fe2O3 | Iron(III) oxide |
| GJ | Gigajoule |
| GN2 | Nitrogen gas |
| GPD | British pound |
| H2 | Hydrogen |
| H2O | Water |
| He | Helium |
| HESC | Hydrogen energy supply chain project |
| IDEALHY | Integrated design for efficient advanced liquefaction of hydrogen |
| IEC | International electrotechnical commission |
| IRAS | Integrated refrigeration and storage |
| ISO | International organization for standardization |
| JP¥ | Japanese Yen |
| J–T | Joule–Thomson |
| K | Kelvin |
| kg | Kilogram |
| KHI | Kawasaki heavy industries |
| kJ | Kilojoule |
| kWh | Kilowatt-hour |
| LCOH | Levelised cost of hydrogen |
| LH2 | Liquid hydrogen |
| LHV | Lower heating value |
| LN2 | Liquid nitrogen |
| LNG | Liquefied natural gas |
| LOHC | Liquid organic hydrogen carriers |
| m3 | Cubic meter |
| MeOH | Methanol |
| meV | Millielectronvolt |
| MJ | Megajoule |
| mm | Millimetres |
| MPa | Megapascal |
| MRs | Mixed refrigerants |
| Mtpa | Million tonnes per annum |
| MW | Megawatt |
| N2 | Nitrogen |
| N2O | Nitrous oxide |
| NASA | US National Aeronautics and Space Administration |
| Ne | Neon |
| NFPA | National fire protection association |
| NH3 | Ammonia |
| NiO | Nickel(II) oxide |
| Nm3 | Normal cubic meter |
| NOX | Nitrogen oxides |
| O2 | Oxygen |
| OP | ortho–para |
| OPEX | Operating expenses |
| PFHE | Plate-fin heat exchangers |
| P net | Net power consumption |
| ppb | Parts per billion |
| PV | Photovoltaic |
| rpm | Revolutions per minute |
| SEC | Specific energy consumption |
| SLC | Specific liquefaction cost |
| SMR | Steam methane reforming |
| THz | Terahertz |
| TPD | Tons per day |
| USA | United States of America |
| USD | United States dollar |
| VLE | Vapour–liquid equilibrium |
| wt% | Percentage by weight |
Author contributions
Saif ZS. Al Ghafri: management, literature review, data analysis and writing original draft. Stephanie Munro: literature review and writing original draft. Umberto Cardella: particular section contribution: industrial liquefiers. Thomas Funke: particular section contribution: industrial liquefiers. William Notardonato: particular section contribution: NASA and storage tanks. J.P. Martin Trusler: particular section contribution: thermodynamics and EOS. Jacob Leachman: particular section contribution: thermodynamics and EOS. Roland Span: particular section contribution: thermodynamics and EOS. Shoji Kamiya: particular section contribution: storage tanks and BOG. Garth Pearce: particular section contribution: tanks design and materials. Adam Swanger: particular section contribution: NASA storage tanks and BOG. Elma Dorador Rodriquez: particular section contribution: liquid hydrogen cost analysis. Paul Bajada: particular section contribution: hydrogen production methods. Fuyu Jiao: particular section contribution: fundamentals of hydrogen liquefaction processes. Kun Peng: particular section contribution: ammonia. Arman Siahvashi: particular section contribution: solid freeze-out and impurities. Michael L. Johns: OP conversion, writing, reviewing and editing. Eric F. May: supervision, thermodynamics, writing, reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the support of the Australian Research Council through FT180100572 and IC150100019, the DAAD, and the Future Energy Exports CRC (CRCXXI000008), whose activities are funded by the Australian Government's Cooperative Research Centre Program. This is FEnEx CRC Document 2022/RP2-FNX-003. We also thank Dr Neil Robinson for helpful discussions particularly regarding the catalytic mechanisms involved with spin isomer conversion.References
- M. Bastos-Neto, C. Patzschke, M. Lange, J. Möllmer, A. Moeller, S. Fichtner, C. Schrage, D. Lässig, J. Lincke, R. Staudt, H. Krautscheid and R. Gläser, Energy Environ. Sci., 2012, 5, 8294–8303 RSC.
- I. Staffell, D. Scamman, A. Velazquez Abad, P. Balcombe, P. E. Dodds, P. Ekins, N. Shah and K. R. Ward, Energy Environ. Sci., 2019, 12, 463–491 RSC.
- M. Z. Jacobson, M. A. Delucchi, Z. A. F. Bauer, S. C. Goodman, W. E. Chapman, M. A. Cameron, C. Bozonnat, L. Chobadi, H. A. Clonts, P. Enevoldsen, J. R. Erwin, S. N. Fobi, O. K. Goldstrom, E. M. Hennessy, J. Liu, J. Lo, C. B. Meyer, S. B. Morris, K. R. Moy, P. L. O'Neill, I. Petkov, S. Redfern, R. Schucker, M. A. Sontag, J. Wang, E. Weiner and A. S. Yachanin, Joule, 2017, 1, 108–121 CrossRef.
- I. Capellán-Pérez, I. Arto, J. M. Polanco-Martínez, M. González-Eguino and M. B. Neumann, Energy Environ. Sci., 2016, 9, 2482–2496 RSC.
- N. MacDowell, N. Florin, A. Buchard, J. Hallett, A. Galindo, G. Jackson, C. S. Adjiman, C. K. Williams, N. Shah and P. Fennell, Energy Environ. Sci., 2010, 3, 1645–1669 RSC.
- A. Sartbaeva, V. L. Kuznetsov, S. A. Wells and P. P. Edwards, Energy Environ. Sci., 2008, 1, 79–85 RSC.
- B. Vogel, T. Feck, J.-U. Grooß and M. Riese, Energy Environ. Sci., 2012, 5, 6445–6452 RSC.
- B. Parkinson, P. Balcombe, J. F. Speirs, A. D. Hawkes and K. Hellgardt, Energy Environ. Sci., 2019, 12, 19–40 RSC.
- T. M. Gür, Energy Environ. Sci., 2018, 11, 2696–2767 RSC.
- United States Department of Energy (DOE), Hydrogen posture plan, 2006, https://www.hydrogen.energy.gov/pdfs/hydrogen_posture_plan_dec06.pdf.
- European Union, Hydrogen Roadmap Europe, 2019, Report 978-92-9246-331-1, 2019.
- European Commission. Communication from the commission to the European parliament, the European economic and social committee and the committee of the regions. A hydrogen strategy for a climate-neutral Europe, 2020, https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf.
- Ministry of Economy, Trade and Industry. Summary of the strategic road map for hydrogen and fuel cells, 2014, http:// www.meti.go.jp/english/press/2014/pdf/<?pdb_no 0624?>0624<?pdb END?>_04a.pdf.
- N. Behling, M. C. Williams and S. Managi, Econ. Anal. Policy, 2015, 48, 204–221 CrossRef.
- COAG Energy Council. Australia's National Hydrogen Strategy, 2019, https://www.industry.gov.au/sites/default/files/2019-11/australias-national-hydrogen-strategy.pdf.
- Federal Ministry for Economic Affairs and Energy: The National Hydrogen Strategy, 2020, https://www.bmwi.de/Redaktion/EN/Publikationen/Energie/the-national-hydrogen-strategy.html.
- Department of Energy and Climate Change. UK starts work on public-private roadmap for hydrogen fuel cells DOI:10.1016/S1464-2859(16)30061-X.
- J. Zhang, H. Meerman, R. Benders and A. Faaij, Energy, 2021, 224, 120049 CrossRef CAS.
- A. McFarlan, Sustainable Energy Technol. Assess., 2020, 42, 100821 CrossRef.
- K. Sapkota, A. O. Oni and A. Kumar, J. Nat. Gas Sci. Eng., 2018, 52, 401–409 CrossRef.
- S. Shiva Kumar and V. Himabindu, Mater. Sci. Energy Technol., 2019, 2, 442–454 Search PubMed.
- M. Sankir and N. D. Sankir, Hydrogen Production Technologies, Wiley, Somerset, United States, 2017 Search PubMed.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- S. Cloete, M. N. Khan and S. Amini, Int. J. Hydrogen Energy, 2019, 44, 3492–3510 CrossRef CAS.
- G. Collodi, G. Azzaro, N. Ferrari and S. Santos, Energy Procedia, 2017, 114, 2690–2712 CrossRef CAS.
- C.-C. Cormos, Int. J. Hydrogen Energy, 2012, 37, 5637–5648 CrossRef CAS.
- B. Angelo, D. Francesco, T. Jianhua and T. N. Vezirolu, Hydrogen Production, Separation and Purification for Energy, Institution of Engineering and Technology, 2017 Search PubMed.
- I. M. Karp, Energy Technol. Resource Saving, 2020, 2, 4–13 CrossRef.
- J. Chi and H. Yu, Chin. J. Catal., 2018, 39, 390–394 CrossRef CAS.
- A. Ugurlu and S. Oztuna, Int. J. Hydrogen Energy, 2020, 45, 35269–35280 CrossRef CAS.
- M. Minutillo, A. Perna and A. Sorce, Appl. Energy, 2020, 277, 115452 CrossRef CAS.
- N. Sunny, N. Mac Dowell and N. Shah, Energy Environ. Sci., 2020, 13, 4204–4224 RSC.
- R. Carapellucci and L. Giordano, J. Power Sources, 2020, 469, 228391 CrossRef CAS.
- G. Locatelli, S. Boarin, A. Fiordaliso and M. E. Ricotti, Energy, 2018, 148, 494–505 CrossRef CAS.
- H. Tebibel, International Conference on Wind Energy and Applications in Algeria (ICWEAA), 2018, DOI:10.1109/ICWEAA.2018.8605079.
- Global CCS Institute, Global Status of CCS, 2019, https://www.globalccsinstitute.com/wp-content/uploads/2019/12/GCC_GLOBAL_STATUS_REPORT_2019.pdf.
- T. M. Bruce, S. Hayward, J. Schmidt, E. Munnings, C. Palfreyman and D. Hartley, National Hydrogen Roadmap, CSIRO, Australia, 2018 Search PubMed.
- A. L. Hoskins, S. L. Millican, C. E. Czernik, I. Alshankiti, J. C. Netter, T. J. Wendelin, C. B. Musgrave and A. W. Weimer, Appl. Energy, 2019, 249, 368–376 CrossRef CAS.
- R. R. Bhosale, Fuel, 2020, 277, 118160 CrossRef CAS.
- S. Sadeghi and S. Ghandehariun, Int. J. Hydrogen Energy, 2020, 45, 28426–28436 CrossRef CAS.
- C. Acar, I. Dincer and G. F. Naterer, Int. J. Energy Res., 2016, 40, 1449–1473 CrossRef CAS.
- H. Wang, J. Xu, L. Sheng, X. Liu, Y. Lu and W. Li, Int. J. Energy Res., 2018, 42, 3442–3453 CrossRef CAS.
- B. Pandey, Y. K. Prajapati and P. N. Sheth, Int. J. Hydrogen Energy, 2019, 44, 25384–25415 CrossRef CAS.
- J. Baeyens, H. Zhang, J. Nie, L. Appels, R. Dewil, R. Ansart and Y. Deng, Renewable Sustainable Energy Rev., 2020, 131, 110023 CrossRef CAS.
- F. Safari and I. Dincer, Energy Convers. Manage., 2020, 205, 112182 CrossRef CAS.
- M. Shaner, H. Atwater, N. Lewis and E. McFarland, Energy Environ. Sci., 2016, 9 Search PubMed.
- A. Grimm, W. A. de Jong and G. J. Kramer, Int. J. Hydrogen Energy, 2020, 45, 22545–22555 CrossRef CAS.
- J. R. Bartels, M. B. Pate and N. K. Olson, Int. J. Hydrogen Energy, 2010, 35, 8371–8384 CrossRef CAS.
- J. I. Levene, Economic analysis of hydrogen production from wind, National Renewable Energy Laboratory, Golden, CO, 2005 Search PubMed.
- International Energy Agency (IEA), Electrolyser capacity installed by year, 2010-2018, https://www.iea.org/data-and-statistics/charts/electrolyser-capacity-installed-by-year-2010-2018, (accessed December, 2019).
- A. Buttler and H. Spliethoff, Renewable Sustainable Energy Rev., 2018, 82, 2440–2454 CrossRef CAS.
- O. Schmidt, A. Gambhir, I. Staffell, A. Hawkes, J. Nelson and S. Few, Int. J. Hydrogen Energy, 2017, 42, 30470–30492 CrossRef CAS.
- M. Götz, J. Lefebvre, F. Mörs, A. McDaniel Koch, F. Graf, S. Bajohr, R. Reimert and T. Kolb, Renewable Energy, 2016, 85, 1371–1390 CrossRef.
- C. Zhang and L. D. Anadon, Environ. Sci. Technol., 2013, 47, 14459–14467 CrossRef CAS PubMed.
- J. A. Turner, Science, 2004, 305, 972 CrossRef CAS PubMed.
- Y. Kuang, M. J. Kenney, Y. Meng, W.-H. Hung, Y. Liu, J. E. Huang, R. Prasanna, P. Li, Y. Li, L. Wang, M.-C. Lin, M. D. McGehee, X. Sun and H. Dai, Proc. Natl. Acad. Sci. U. S. A., 2019, 116, 6624 CrossRef CAS PubMed.
- International Energy Agency (IEA), The Future of Hydrogen, https://www.iea.org/reports/the-future-of-hydrogen.
- Air Liquid, Inauguration of the world's largest PEM electrolyze, https://www.airliquide.com/magazine/energy-transition/inauguration-worlds-largest-pem-electrolyzer.
- R. J. Detz, J. N. H. Reek and B. C. C. van der Zwaan, Energy Environ. Sci., 2018, 11, 1653–1669 RSC.
- S. Giddey, S. P. S. Badwal, C. Munnings and M. Dolan, ACS Sustainable Chem. Eng., 2017, 5, 10231–10239 CrossRef CAS.
- P. Nikolaidis and A. Poullikkas, Renewable Sustainable Energy Rev., 2017, 67, 597–611 CrossRef CAS.
- M. Liszka, T. Malik and G. Manfrida, Energy, 2012, 45, 142–150 CrossRef CAS.
- F. Dawood, M. Anda and G. M. Shafiullah, Int. J. Hydrogen Energy, 2020, 45, 3847–3869 CrossRef CAS.
- R. S. El-Emam and H. Özcan, J. Cleaner Prod., 2019, 220, 593–609 CrossRef CAS.
- C. Acar and I. Dincer, J. Cleaner Prod., 2019, 218, 835–849 CrossRef CAS.
- A. X. Y. Mah, W. S. Ho, C. P. C. Bong, M. H. Hassim, P. Y. Liew, U. A. Asli, M. J. Kamaruddin and N. G. Chemmangattuvalappil, Int. J. Hydrogen Energy, 2019, 44, 5661–5675 CrossRef CAS.
- M. Kaur and K. Pal, J. Energy Storage, 2019, 23, 234–249 CrossRef.
- J. O. Abe, A. P. I. Popoola, E. Ajenifuja and O. M. Popoola, Int. J. Hydrogen Energy, 2019, 44, 15072–15086 CrossRef CAS.
- A. Finkel, Griffith Rev., 2019, 64, 134–142 Search PubMed.
- R. Moradi and K. M. Groth, Int. J. Hydrogen Energy, 2019, 44, 12254–12269 CrossRef CAS.
- M. H. McCay, S. Shafiee and T. M. Letcher, Future Energy (Third Edition), Elsevier, 2020, pp. 475–493 DOI:10.1016/B978-0-08-102886-5.00022-0.
- Q.-L. Zhu and Q. Xu, Energy Environ. Sci., 2015, 8, 478–512 RSC.
- M. Niermann, S. Drünert, M. Kaltschmitt and K. Bonhoff, Energy Environ. Sci., 2019, 12, 290–307 RSC.
- C. Smith, A. K. Hill and L. Torrente-Murciano, Energy Environ. Sci., 2020, 13, 331–344 RSC.
- C. Weidenthaler and M. Felderhoff, Energy Environ. Sci., 2011, 4, 2495–2502 RSC.
- Z. Yanxing, G. Maoqiong, Z. Yuan, D. Xueqiang and S. Jun, Int. J. Hydrogen Energy, 2019, 44, 16833–16840 CrossRef.
- S. Alavi and J. A. Ripmeester, Mol. Simul., 2017, 43, 808–820 CrossRef CAS.
- Q. Lai, M. Paskevicius, D. A. Sheppard, C. E. Buckley, A. W. Thornton, M. R. Hill, Q. Gu, J. Mao, Z. Huang, H. K. Liu, Z. Guo, A. Banerjee, S. Chakraborty, R. Ahuja and K.-F. Aguey-Zinsou, ChemSusChem, 2015, 8, 2789–2825 CrossRef CAS PubMed.
- J. Bellosta von Colbe, J.-R. Ares, J. Barale, M. Baricco, C. Buckley, G. Capurso, N. Gallandat, D. M. Grant, M. N. Guzik, I. Jacob, E. H. Jensen, T. Jensen, J. Jepsen, T. Klassen, M. V. Lototskyy, K. Manickam, A. Montone, J. Puszkiel, S. Sartori, D. A. Sheppard, A. Stuart, G. Walker, C. J. Webb, H. Yang, V. Yartys, A. Züttel and M. Dornheim, Int. J. Hydrogen Energy, 2019, 44, 7780–7808 CrossRef CAS.
- V. A. Yartys, M. V. Lototskyy, E. Akiba, R. Albert, V. E. Antonov, J. R. Ares, M. Baricco, N. Bourgeois, C. E. Buckley, J. M. Bellosta von Colbe, J. C. Crivello, F. Cuevas, R. V. Denys, M. Dornheim, M. Felderhoff, D. M. Grant, B. C. Hauback, T. D. Humphries, I. Jacob, T. R. Jensen, P. E. de Jongh, J. M. Joubert, M. A. Kuzovnikov, M. Latroche, M. Paskevicius, L. Pasquini, L. Popilevsky, V. M. Skripnyuk, E. Rabkin, M. V. Sofianos, A. Stuart, G. Walker, H. Wang, C. J. Webb and M. Zhu, Int. J. Hydrogen Energy, 2019, 44, 7809–7859 CrossRef CAS.
- M. Hirscher, V. A. Yartys, M. Baricco, J. Bellosta von Colbe, D. Blanchard, R. C. Bowman, D. P. Broom, C. E. Buckley, F. Chang, P. Chen, Y. W. Cho, J.-C. Crivello, F. Cuevas, W. I. F. David, P. E. de Jongh, R. V. Denys, M. Dornheim, M. Felderhoff, Y. Filinchuk, G. E. Froudakis, D. M. Grant, E. M. Gray, B. C. Hauback, T. He, T. D. Humphries, T. R. Jensen, S. Kim, Y. Kojima, M. Latroche, H.-W. Li, M. V. Lototskyy, J. W. Makepeace, K. T. Møller, L. Naheed, P. Ngene, D. Noréus, M. M. Nygård, S.-I. Orimo, M. Paskevicius, L. Pasquini, D. B. Ravnsbæk, M. Veronica Sofianos, T. J. Udovic, T. Vegge, G. S. Walker, C. J. Webb, C. Weidenthaler and C. Zlotea, J. Alloys Compd., 2020, 827, 153548 CrossRef CAS.
- D. A. Sheppard and C. E. Buckley, Int. J. Hydrogen Energy, 2019, 44, 9143–9163 CrossRef CAS.
- S. A. Sherif, N. Zeytinoglu and T. N. Veziroǧlu, Int. J. Hydrogen Energy, 1997, 22, 683–688 CrossRef CAS.
- J. Sloop and A. Silverstein, Liquid Hydrogen as a Propulsion Fuel, https://history.nasa.gov/SP-4404/contents.htm, (accessed December, 2019).
- The NASA History Series. Taming Liquid Hydrogen: The Centaur Upper Stage Rocket 1958-2002, https://history.nasa.gov/SP-4230.pdf.
- W. Notardonato, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- International Energy Agency (IEA), Energy Technology Perspectives 2020, Paris, France, 2020.
- Y. Ishimoto, A. Kurosawa, M. Sasakura and K. Sakata, Int. J. Hydrogen Energy, 2017, 42, 13357–13367 CrossRef CAS.
- E. W. Lemmon, I. H. Bell, M. L. Huber and M. O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, version 10.0, 2018.
- J. Zheng, X. Liu, P. Xu, P. Liu, Y. Zhao and J. Yang, Int. J. Hydrogen Energy, 2012, 37, 1048–1057 CrossRef CAS.
- T. Q. Hua, R. K. Ahluwalia, J. K. Peng, M. Kromer, S. Lasher, K. McKenney, K. Law and J. Sinha, Int. J. Hydrogen Energy, 2011, 36, 3037–3049 CrossRef CAS.
- J. Andersson and S. Grönkvist, Int. J. Hydrogen Energy, 2019, 44, 11901–11919 CrossRef CAS.
- U. Cardella, L. Decker and H. Klein, Int. J. Hydrogen Energy, 2017, 42, 13329–13338 CrossRef CAS.
- K. Sichao, K. Kimura and H. Ishida, Perspectives on Hydrogen in the APEC Region, Asia Pacific Energy Research Centre, Tokyo, Japan, 2018 Search PubMed.
- P.-M. Heuser, D. S. Ryberg, T. Grube, M. Robinius and D. Stolten, Int. J. Hydrogen Energy, 2019, 44, 12733–12747 CrossRef CAS.
- Hydrogen Council, Hydrogen Insights 2021 - A Perspective on Hydrogen Investment, Deployment and Cost Competitiveness, Hydrogen Council, EU, 2021.
- X. J. Li, J. D. Allen, J. A. Stager and A. Y. Ku, Clean Energy, 2020, 4, 26–47 CrossRef.
- A. T. Wijayanta, T. Oda, C. W. Purnomo, T. Kashiwagi and M. Aziz, Int. J. Hydrogen Energy, 2019, 44, 15026–15044 CrossRef CAS.
- D. Teichmann, W. Arlt and P. Wasserscheid, Int. J. Hydrogen Energy, 2012, 37, 18118–18132 CrossRef CAS.
- Y. Ishimoto, M. Voldsund, P. Nekså, S. Roussanaly, D. Berstad and S. O. Gardarsdottir, Int. J. Hydrogen Energy, 2020, 45, 32865–32883 CrossRef CAS.
- T. Watanabe, K. Murata, S. Kamiya and K. I. Ota, Proc. 18th World Hydrog. Energy Conf., 2010, 78, 547–557 Search PubMed.
- S. Kamiya, M. Nishimura and E. Harada, Phys. Proc., 2015, 67, 11–19 CrossRef CAS.
- T. Nyberg, Master, Lund University, 2021.
- T. Funke, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- N. D. Mortimer, C. Hatto, O. Mwabonje and J. H. R. Rix, Hydrogen Production and Utilisation Report IDEALHY, EU, 2013 Search PubMed.
- K. Stolzenburg and R. Mubbala, Hydrogen Liquefaction Report IDEALHY, EU, 2013 Search PubMed.
- M. Raab, S. Maier and R.-U. Dietrich, Int. J. Hydrogen Energy, 2021, 46, 11956–11968 CrossRef CAS.
- M. Reuß, T. Grube, M. Robinius, P. Preuster, P. Wasserscheid and D. Stolten, Appl. Energy, 2017, 200, 290–302 CrossRef.
- Elizabeth Connelly, Michael Penev, Amgad Elgowainy and C. Hunter, Current Status of Hydrogen Liquefaction Costs, DOE Hydrogen and Fuel Cells Program Record, 2019 Search PubMed.
- J. Cihlar, A. Villar Lejarreta, A. Wang, F. Melgar, J. Jens and P. Rio, Hydrogen generation in Europe: Overview of costs and key benefits, European Comission, Luxembourg, 2020.
- S. y. Obara, Energy, 2019, 174, 848–860 CrossRef CAS.
- K. H. R. Rouwenhorst, A. G. J. Van der Ham, G. Mul and S. R. A. Kersten, Renewable Sustainable Energy Rev., 2019, 114, 109339 CrossRef CAS.
- G. Hochman, A. S. Goldman, F. A. Felder, J. M. Mayer, A. J. M. Miller, P. L. Holland, L. A. Goldman, P. Manocha, Z. Song and S. Aleti, ACS Sustainable Chem. Eng., 2020, 8, 8938–8948 CrossRef CAS.
- S. Ecuity, Engie, Siemens, Ammonia to Green Hydrogen Project Feasibility Study, 2019.
- International Energy Agency (IEA), Hydrogen production costs using natural gas in selected regions, 2018, https://www.iea.org/reports/the-future-of-hydrogen, (accessed December, 2019).
- A. Boretti, Int. J. Hydrogen Energy, 2013, 38, 1806–1812 CrossRef CAS.
- S. Tuomi, A. Santasalo-Aarnio, P. Kanninen and T. Kallio, J. Power Sources, 2013, 229, 32–35 CrossRef CAS.
- R. Ahluwalia, D. Papadias, J. Peng and H. Roh, U.S. DOE Hydrogen and Fuel Cells Program 2019 Annual Merit Review and Peer Evaluation Meeting, 2019 Search PubMed.
- C. Wulf and P. Zapp, Int. J. Hydrogen Energy, 2018, 43, 11884–11895 CrossRef CAS.
- S. Bano, P. Siluvai Antony, V. Jangde and R. B. Biniwale, J. Cleaner Prod., 2018, 183, 988–997 CrossRef CAS.
- A. Ozawa, Y. Kudoh, N. Kitagawa and R. Muramatsu, Int. J. Hydrogen Energy, 2019, 44, 11219–11232 CrossRef CAS.
- I. Kuz’menko, V. Peredelskii and A. Dovbish, Chem. Petroleum Eng., 2010, 46 Search PubMed.
- J. Zhang, H. Meerman, R. Benders and A. Faaij, Appl. Therm. Eng., 2020, 166, 114736 CrossRef.
- K. Ohlig and L. Decker, AIP Conf. Proc., 2013, 1573 Search PubMed.
- Raymond Drnevich, Hydrogen Delivery Liquefaction & Compression, 2003, https://www1.eere.energy.gov/hydrogenandfuelcells/pdfs/liquefaction_comp_pres_praxair.pdf.
- U. Cardella, Doktor-Ingenieurs, Universität München, 2018.
- D. Berstad, P. Nekså and J. Stang, Int. J. Hydrogen Energy, 2010, 35, 4512–4523 CrossRef CAS.
- F. Ustolin, N. Paltrinieri and F. Berto, Int. J. Hydrogen Energy, 2020, 45, 23809–23840 CrossRef CAS.
- Hydrogen Scaling Up, prepared by Hydrogen Council, 2017, https://hydrogencouncil.com/wp-content/uploads/2017/11/Hydrogen-Scaling-up_Hydrogen-Council_2017.compressed.pdf.
- L. Barrón-Palos, R. Alarcon, S. Balascuta, C. Blessinger, J. D. Bowman, T. E. Chupp, S. Covrig, C. B. Crawford, M. Dabaghyan, J. Dadras, M. Dawkins, W. Fox, M. T. Gericke, R. C. Gillis, B. Lauss, M. B. Leuschner, B. Lozowski, R. Mahurin, M. Mason, J. Mei, H. Nann, S. I. Penttilä, W. D. Ramsay, A. Salas-Bacci, S. Santra, P. N. Seo, M. Sharma, T. Smith, W. M. Snow, W. S. Wilburn and V. Yuan, Nucl. Instr. Methods Phys. Res. Sect. A: Accelerators, Spectrometers, Detectors Associated Equipment, 2011, 659, 579–586 CrossRef.
- M. Aasadnia and M. Mehrpooya, Appl. Energy, 2018, 212, 57–83 CrossRef CAS.
- H. You, J. Ahn, S. Jeong and D. Chang, Ships Offshore Struct., 2018, 13, 79–85 CrossRef.
- S. Gursu, M. Lordgooei, S. A. Sherif and T. N. Veziroǧlu, Int. J. Hydrogen Energy, 1992, 17, 227–236 CrossRef CAS.
- E. Karlsson, Master Thesis, Lund University, 2017.
- B. Burkholder, Honours Thesis, Oberlin College and Conservatory, 2009.
- J. White, Development of High-Activity Para- to Ortho-Hydrogen Conversion Catalysts, 1989 Search PubMed.
- D. White, A. S. Friedman and H. L. Johnston, J. Am. Chem. Soc., 1950, 72, 3927–3930 CrossRef CAS.
- D. White, A. S. Friedman and H. L. Johnston, J. Am. Chem. Soc., 1950, 72, 3565–3568 CrossRef CAS.
- R. D. Goodwin, D. E. Diller, H. M. Roder and L. A. Weber, Cryogenics, 1961, 2, 81–83 CrossRef CAS.
- H. M. Roder, D. E. Diller, L. A. Weber and R. D. Goodwin, Cryogenics, 1963, 3, 16–22 CrossRef CAS.
- J. W. Leachman, R. T. Jacobsen, S. G. Penoncello and E. W. Lemmon, J. Phys. Chem. Ref. Data, 2009, 38, 721–748 CrossRef CAS.
- R. Span, R. Beckmüller, T. Eckermann, S. H. S. Herrig, A. Jäger, T. Neumann, S. Pohl, B. Semrau and M. Thol, TREND. Thermodynamic Reference and Engineering Data 4.0.
- X. Yang, D. Rowland, C. C. Sampson, P. E. Falloon and E. F. May, Fuel, 2022, 314, 123033 CrossRef CAS.
- D.-Y. Peng and D. B. Robinson, Ind. Eng. Chem. Fundamentals, 1976, 15, 59–64 CrossRef CAS.
- R. H. Newton, Ind. Eng. Chem., 1935, 27, 302–306 CrossRef CAS.
- T. W. Leland and P. S. Chappelear, Ind. Eng. Chem., 1968, 60, 15–43 CrossRef CAS.
- D. Rowland, T. J. Hughes and E. F. May, J. Chem. Eng. Data, 2017, 62, 2799–2811 CrossRef CAS.
- R. D. Gunn, P. L. Chueh and J. M. Prausnitz, AIChE J., 1966, 12, 937–941 CrossRef CAS.
- P. L. Chueh and J. M. Prausnitz, Ind. Eng. Chem. Fundamentals, 1967, 6, 492–498 CrossRef CAS.
- R. P. Feynman, A. R. Hibbs and D. F. Stye, Quantum Mechanics and Path Integrals, McGraw-Hill, New York, Emended edn, 2005 Search PubMed.
- A. Aasen, M. Hammer, S. Lasala, J.-N. Jaubert and Ø. Wilhelmsen, Fluid Phase Equilib., 2020, 524, 112790 CrossRef CAS.
- O. Kunz and W. Wagner, J. Chem. Eng. Data, 2012, 57, 3032–3091 CrossRef CAS.
- T. Monika, R. Markus, F. M. Eric, W. L. Eric and S. Roland, J. Phys. Chem. Ref. Data, 2019, 48, 033102 CrossRef.
- R. Beckmüller, M. Thol, I. H. Bell, E. W. Lemmon and R. Span, J. Phys. Chem. Ref. Data, 2021, 50, 013102 CrossRef.
- S. Krasae-in, Philosophy doctorate, Norwegian University of Science and Technology, 2013.
- J. Tkaczuk, I. H. Bell, E. W. Lemmon, N. Luchier and F. Millet, J. Phys. Chem. Ref. Data, 2020, 49, 023101 CrossRef CAS.
- R. Span, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- R. T. Jacobsen, J. W. Leachman, S. G. Penoncello and E. W. Lemmon, Int. J. Thermophys., 2007, 28, 758–772 CrossRef CAS.
- J. Leachman, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- K. Patkowski, W. Cencek, P. Jankowski, K. Szalewicz, J. B. Mehl, G. Garberoglio and A. H. Harvey, J. Chem. Phys., 2008, 129, 094304 CrossRef PubMed.
- J. Mehl, M. Huber and A. Harvey, Int. J. Thermophys., 2010, 31, 740–755 CrossRef CAS.
- E. F. May, R. F. Berg and M. R. Moldover, Int. J. Thermophys., 2007, 28, 25 CrossRef.
- C. Muzny, M. Huber and A. Kazakov, J. Chem. Eng. Data, 2013, 58, 969–979 CrossRef CAS.
- D. E. Diller, J. Chem. Phys., 1965, 42, 2089–2100 CrossRef CAS.
- D. Zhou, G. G. Ihas and N. S. Sullivan, J. Low Temp. Phys., 2004, 134, 401–406 CrossRef CAS.
- M. J. Assael, J. A. M. Assael, M. L. Huber, R. A. Perkins and Y. Takata, J. Phys. Chem. Ref. Data, 2011, 40, 033101 CrossRef.
- H. M. Roder and D. E. Diller, J. Chem. Phys., 1970, 52, 5928–5949 CrossRef CAS.
- H. M. Roder, Experimental thermal conductivity values for hydrogen, methane, ethane and propane [microform]/H. M. Roder; prepared for NASA-Lewis Research Center, U.S. Dept. of Commerce, National Bureau of Standards; Order from National Technical Information Service, Washington, D.C., Springfield, Va, 1984.
- Y. Y. Milenko, R. M. Sibileva and M. A. Strzhemechny, J. Low Temp. Phys., 1997, 107, 77–92 CrossRef CAS.
- G. Petitpas, S. M. Aceves, M. J. Matthews and J. R. Smith, Int. J. Hydrogen Energy, 2014, 39, 6533–6547 CrossRef CAS.
- D. S. Chapin and H. L. Johnston, J. Am. Chem. Soc., 1957, 79, 2406–2412 CrossRef CAS.
- M. Misono and P. W. Selwood, J. Am. Chem. Soc., 1968, 90, 2977–2978 CrossRef CAS.
- IONEX Type OP Catalyst - Molecular Products, https://www.molecularproducts.com/products/ionex-type-op-catalyst.
- A. V. Zhuzhgov, O. Krivoruchko, L. Isupova, O. Martyanov and V. Parmon, Catal. Ind., 2018, 10, 9–19 CrossRef.
- P. J. Donaubauer, U. Cardella, L. Decker and H. Klein, Chem. Eng. Technol., 2019, 42, 669–679 CrossRef CAS.
- H. L. Hutchinson, Masters thesis, University of Colorado Boulder, 1966.
- H. L. Hutchinson, P. L. Barrick and L. F. Brown, in Advances in Cryogenic Engineering, ed. K. D. Timmerhaus, Springer US, Boston, MA, 1965, pp. 190–196 Search PubMed.
- H. L. Hutchinson, P. L. Barrick and L. F. Brown, Chem. Eng. Progress, Sym. Ser., 1967, 63, 18–30 CAS.
- H. L. Hutchinson, L. F. Brown and P. L. Barrick, in Advances in Cryogenic Engineering: Proceeding of the 1970 Cryogenic Engineering Conference The University of Colorado Boulder, Colorado June 17–17, 1970, ed. K. D. Timmerhaus, Springer US, Boston, MA, 1971, pp. 96–103 DOI:10.1007/978-1-4757-0244-6_12.
- D. H. Weitzel, C. Van Valin and J. W. Draper, in Advances in Cryogenic Engineering, Springer, 1960, pp. 73–84 Search PubMed.
- N. Wakao, P. W. Selwood and J. M. Smith, AIChE J., 1962, 8, 478–481 CrossRef CAS.
- Ø. Wilhelmsen, D. Berstad, A. Aasen, P. Nekså and G. Skaugen, Int. J. Hydrogen Energy, 2018, 43, 5033–5047 CrossRef.
- J. Essler and H. Ch, AIP Conf. Proc., 2010, 1218, 305–310 CrossRef CAS.
- J. Park, H. Lim, G. H. Rhee and S. W. Karng, Int. J. Heat Mass Transfer, 2021, 170, 121007 CrossRef CAS.
- F. Reif and E. M. Purcell, Phys. Rev., 1953, 91, 631–641 CrossRef CAS.
- L. Yin and Y. Ju, Front. Energy, 2019, 14, 530–544 CrossRef.
- H. Ansarinasab, M. Mehrpooya and M. Sadeghzadeh, J. Cleaner Prod., 2020, 210, 530–541 CrossRef.
- A. Alekseev, in Hydrogen Science and Engineering, 2 Volume Set: Materials, Processes, Systems and Technology, eds. D. Stolten and B. Emonts, Wiley, Germany, 2016 Search PubMed.
- S. Krasae-in, J. H. Stang and P. Neksa, Int. J. Hydrogen Energy, 2010, 35, 4524–4533 CrossRef CAS.
- G. Valenti, in Compendium of Hydrogen Energy: Hydrogen Storage, Distribution and Infrastructure, eds. R. Gupta, A. Basile and T. Nejat Veziroglu, Woodhead Publishing,Cambridge, 2016, vol. 2 Search PubMed.
- A. Léon, in Hydrogen Technology: Mobile and Portable Applications, ed. A. Léon, Springer, Berlin, 2008, p. 98 Search PubMed.
- J. Essler, C. Haberstroh, H. Quack, H. Walnum, D. Berstad, P. Nekså, J. Stang, M. Börsch, F. Holdener, L. Fecker and P. Treite, presented in part at the Report on Technology Overview and Barriers to Energy- and Cost-Efficient Large Scale Hydrogen Liquefaction, 2012.
- R. W. Moore Jr, AIChE J., 1972, 18, 1086 CrossRef.
- R. F. Barron, Advances in Cryogenic Engineering: A Collection of Invited Papers and Contributed Papers Presented at National Technical Meetings During 1970 and 1971, Springer US, Boston, MA, 1972, pp. 20–36 DOI:10.1007/978-1-4684-7826-6_3.
- C. Windmeier and R. F. Barron, Cryogenic Technology, Ullmann’s Encyclopedia of Industrial Chemistry, 2013, 1–71 Search PubMed.
- M. Mukhopadhyay, Fundamentals of Cryogenic Engineering, PHI Learning, 2010 Search PubMed.
- G. Walker, Cryocoolers: Part 1: Fundamentals, Springer US, 1983 Search PubMed.
- G. Walker, Cryocoolers: Part 2: Applications, Springer US, 1st edn, 1983 Search PubMed.
- H. Chang, K. Ryu and J. Baik, Cryogenics, 2018, 91, 68–76 CrossRef CAS.
- M. Aasadnia and M. Mehrpooya, Int. J. Hydrogen Energy, 2017, 42, 15564–15585 CrossRef.
- T. Kotas, The Exergy Method of Thermal Plant Analysis, Butterworths, Essex, 1985 Search PubMed.
- H. Walnum, D. Berstad, M. Drescher, P. Nekså, H. Quack, C. Haberstroh and J. Essler, presented in part at the 12th Cryogenics 2012 - IIR Conference, Dresden, 2012.
- F. Dauber and R. Span, Appl. Energy, 2012, 97, 822–827 CrossRef CAS.
- E. C. Carlson, Chem. Eng. Prog., 1996, 92, 35–46 CAS.
- A. H. Harvey and A. Laesecke, Chem. Eng. Prog., 2002, 98, 34–41 CAS.
- C. L. Rhodes, J. Chem. Eng. Data, 1996, 41, 947–950 CrossRef CAS.
- G. Soave, M. Barolo and A. Bertucco, Fluid Phase Equilib., 1993, 91, 87–100 CrossRef CAS.
- G. Soave, Chem. Eng. Sci., 1972, 27, 1197–1203 CrossRef CAS.
- K. E. Starling, Fluid Thermodynamic Properties of Light Petroleum Systems, Books on Demand, 1973 Search PubMed.
- U. Cardella, L. Decker, J. Sundberg and H. Klein, Int. J. Hydrogen Energy, 2017, 42, 12339–12354 CrossRef CAS.
- J.-H. Yang, Y. Yoon, M. Ryu, S.-K. An, J. Shin and C.-J. Lee, Appl. Energy, 2019, 255, 113840 CrossRef CAS.
- G. Valenti, E. Macchi and S. Brioschi, Int. J. Hydrogen Energy, 2012, 37, 10779–10788 CrossRef CAS.
- D.-Y. Peng and D. B. Robinson, Ind. Eng. Chem. Fundamentals, 1976, 15, 59–64 CrossRef CAS.
- D. O. Ortiz-Vega, K. R. Hall, J. C. Holste, V. D. Arp, A. H. Harvey and E. W. Lemmon, J. Phys. Chem. Ref. Data, 2018 Search PubMed , to be submitted.
- I. H. B. Eric, W. Lemmon, Marcia L. Huber and Mark O. McLinden, REFPROP 10.0: NIST Standard Reference Database 23, 2018 Search PubMed.
- G. Valenti and E. Macchi, Int. J. Hydrogen Energy, 2008, 33, 3116–3121 CrossRef CAS.
- M. Bracha and L. Decker, Grosstechnische Wasserstoffverflüssigung in Leuna, Deutsche Kälte-Klima-Tagung, 2008 Search PubMed.
- J. Essler, C. Haberstroh, H. Quack, H. Walnum, D. Berstad, P. Nekså, J. Stang, M. Börsch, F. Holdener, L. Decker and P. Treite, Report on technology overview and barriers to energy- and cost-efficient large-scale hydrogen liquefaction, Fuel Cells and Hydrogen Joint Undertaking (FCH JU), Europe, 2012 Search PubMed.
- M. Bracha, G. Lorenz, A. Patzelt and M. Wanner, Int. J. Hydrogen Energy, 1994, 19, 53–59 CrossRef CAS.
- U. Cardella, L. Decker and H. Klein, presented in part at the Materials Science and Engineering, 2017.
- K. Stolzenburg, D. Berstad, L. Decker, A. Elliott, C. Haberstroh, C. Hatto, H. Klaus, N. D. Mortimer, R. Mubbala, O. Mwabonje, P. Nekså, H. Quack, J. H. R. Rix, I. Seemann and H. T. Walnum, presented in part at the Energie Symposium, Germany, 2013.
- Production of Liquefied Hydrogen Sourced by COG, Nippon Steel Corporation, 2004.
- V. M. Medisetty, R. Kumar, M. H. Ahmadi, D.-V. N. Vo, A. A. V. Ochoa and R. Solanki, Chem. Eng. Technol., 2020, 43, 613–624 CrossRef CAS.
- Linde. Liquefaction for highest density - Hydrogen solutions, https://www.linde-kryotechnik.ch/wp-content/uploads/2016/10/hydrogen_solutions_eng.pdf.
- Iwatani's Third Liquid Hydrogen Plant in Japan Completed, http://www.iwatani.co.jp/eng/newsrelease/detail_50.html.
- Accommodating the Energy Supply Needs of the Next Era: Kawasaki's Hydrogen Liquefaction System, https://global.kawasaki.com/en/stories/articles/vol57/.
- Production Capacity of Liquid Hydrogen Doubled, http://www.iwatani.co.jp/eng/newsrelease/detail_66.html.
- A. Bauer, T. Mayer, M. Semmel, M. A. Guerrero Morales and J. Wind, Int. J. Hydrogen Energy, 2019, 44, 6795–6812 CrossRef CAS.
- North Las Vegas lands $150 million liquid hydrogen plant, 2019, https://lasvegassun.com/news/2019/nov/11/north-las-vegas-lands-150-million-liquid-hydrogen/.
- L. Decker, Latest Global Trend in Liquid Hydrogen Production,https://www.sintef.no/globalassets/project/hyper/presentations-day-1/day1_1430_decker_latest-global-trend-in-liquid-hydrogen-production_linde.pdf/.
- Air Products’ New World-Scale Liquid Hydrogen Plant is Onstream at Its La Porte, Texas Facility, 2021, https://www.airproducts.com/news-center/2021/10/1007-air-products-new-liquid-hydrogen-plant-onstream-at-laporte-texas-facility.
- Praxair to Build New Liquid Hydrogen Plant in La Porte Texas, 2018, https://investors.linde.com/-/media/linde/investors/documents/investors-archive/praxair-archive/news-releases/2018/praxairtobuildnewliquidhydrogenplantinlaportetexas11718final.pdf.
- Air Products to Build Second Liquid Hydrogen Production Facility in California, 2019, http://www.airproducts.ca/Company/news-center/2019/01/0107-air-products-to-build-second-liquid-hydrogen-productions-facility-in-california.aspx.
- M. Marray, Linde, Hyosung to build world's largest liquid hydrogen plant in South Korea, 2020, https://www.theasset.com/asia-connect/40347/linde-hyosung-to-build-worlds-largest-liquid-hydrogen-plant-in-south-korea.
- A. Kuendig, K. Loehlein, G. J. Kramer and J. Huijsmans, Proceedings of the 16th world hydrogen energy conference, 2006, pp. 3326–3333.
- H. Shigekiyo, Air Products Liquid Hydrogen Technologies, Japan Ship Technology Research Association, Japan, 2015 Search PubMed.
- D. Berstad, G. Skaugen and Ø. Wilhelmsen, Int. J. Hydrogen Energy, 2021, 46, 8014–8029 CrossRef CAS.
- S. Krasae-in, Int. J. Hydrogen Energy, 2014, 39, 7015–7029 CrossRef CAS.
- H. Quack, Adv. Cryog. Eng., 2002, 613, 255–263 CAS.
- J. Eckroll, Master of Energy and Environmental Engineering, Norwegian University of Science and Technology, 2017 Search PubMed.
- A. Witkowski, A. Rusin, M. Majkut and K. Stolecka, Energy, 2017, 141, 2508–2518 CrossRef.
- Linde Group. Aluminium plate-fin heat exchangers, http://www.linde-engineering.com.
- S. Krasae-in, J. Stang and P. Nekså, Int. J. Hydrogen Energy, 2010, 35, 12531–12544 CrossRef CAS.
- D. Popov, K. Fikiin, B. Stankov, A. Alvarez, M. Youbi-Idrissi, A. Damas, J. Evans and T. Brown, Appl. Therm. Eng., 2019, 153, 275–290 CrossRef.
- R. Hånde and Ø. Wilhelmsen, Int. J. Hydrogen Energy, 2019, 44, 15045–15055 CrossRef.
- G. Skaugen, D. Berstad and Ø. Wilhelmsen, Int. J. Hydrogen Energy, 2020, 45, 6663–6679 CrossRef CAS.
- T. Kim, B.-I. Choi, Y.-S. Han and K. H. Do, Trans. Korean Hydrogen New Energy Soc., 2020, 31, 8 CrossRef.
- J. Meagher, Master of Science, State University of New York, 2008 Search PubMed.
- C. Knowlen, A. Mattick, A. Bruckner and A. Hertzberg, SAE Technical Papers, 1998 DOI:10.4271/981898.
- M. Green, AIP Conf. Proc., 2008, 985, 872–878 CrossRef CAS.
- L. Van Hoecke, L. Laffineur, R. Campe, P. Perreault, S. W. Verbruggen and S. Lenaerts, Energy Environ. Sci., 2021, 14, 815–843 RSC.
- R. Bruno, P. Bevilacqua and N. Arcuri, in Advances in Natural Gas Emerging Technologies, eds. H. Al-Megren and R. Altamimi, IntechOpen, Croatia, 2017, p. 3 Search PubMed.
- E. May, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- E. Brown, Expansion Engines for Hydrogen Liquefiers, 1959 Search PubMed.
- M. Boyce, in Gas Turbine Engineering Handbook, ed. M. Boyce, Gulf Professional Publishing, USA, 3rd edn, 2006, pp. 336–337 Search PubMed.
- K. Ohlig and S. Bischoff, Am. Inst. Phys. Conf. Ser., 2012, 57, 814 Search PubMed.
- J. Y. Chen, X. M. Li, X. B. Dong, C. Lv and J. H. Wu, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 755, 012033 CAS.
- M. Trusler, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- H. Zhang, R. Gimaev, B. Kovalev, K. Kamilov, V. Zverev and A. Tishin, Phys. B, 2019, 558, 65–73 CrossRef CAS.
- T. Feng, R. Chen and R. V. Ihnfeldt, Int. J. Refrig., 2020, 119 DOI:10.1016/j.ijrefrig.2020.06.032.
- R. Li, Energy Technol., 2019, 7, 1801070 CrossRef.
- T. Numazawa, K. Kamiya, T. Utaki and K. Matsumoto, Cryogenics, 2014, 62, 185–192 CrossRef CAS.
- K. Kamiya, H. Takahashi, T. Numazawa, H. Nozawa and T. Yanagitani, presented in part at the International Cryocooler Conference, Boulder, 2014.
- U. Cardella, L. Decker and H. Klein, presented in part at the Cryogenic Engineering Conference 2017, Madison, USA, 2017.
- T. Vanessa, L. Sebastian and S. Detlef, Hydrogen Science and Engineering: Materials, Processes, Systems and Technology, 2016, pp. 659–690 DOI:10.1002/9783527674268.ch27.
- DOE Technical Targets for Hydrogen Delivery, https://www.energy.gov/eere/fuelcells/doe-technical-targets-hydrogen-delivery, (accessed 2 January, 2020).
- S. Kamiya, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- L. Decker, Liquid Hydrogen Distribution Technology, https://www.sintef.no/globalassets/project/hyper/presentations-day-2/day2_1105_decker_liquid-hydrogen-distribution-technology_linde.pdf/.
- S. Mital, J. Gyekenyesi, S. Arnold, R. Sullivan, J. Manderscheid and P. Murthy, Review of Current State of the Art and Key Design Issues With Potential Solutions for Liquid Hydrogen Cryogenic Storage Tank Structures for Aircraft Applications, National Aeronautics and Space Administration, Cleveland, Ohio, 2006 Search PubMed.
- G. Pearce, presented in part at the Hydrogen Liquefaction & Storage Symposium, Perth, 2019.
- K. Verfondern, Forschungszentrum Jülich GmbH, Institut für Energieforschung Sicherheitsforschung und Reaktortechnik. Safety Considerations on Liquid Hydrogen, 2007, http://juser.fz-juelich.de/record/1311/files/Energie%26Umwelt_10.pdf.
- M. Klell, Handbook of Hydrogen Storage, Storage of Hydrogen in the Pure Form, 2010, ch. 1 Search PubMed.
- R. Sullivan, J. Palko, R. Tornabene, B. Bednarcyk, L. Powers, S. Mital, L. Smith, X. Wange and J. Hunter, Engineering Analysis Studies for Preliminary Design of Lightweight Cryogenic Hydrogen Tanks in UAV Applications, National Aeronautics and Space Administration, Cleveland, Ohio, 2006.
- P. M. Adam, Doctor of philosophy, Washington State University, 2017.
- A. Abe, M. Nakamura, I. Sato, H. Uetani and T. Fujitani, Int. J. Hydrogen Energy, 1998, 23, 115–121 CrossRef CAS.
- G. Petitpas, Boil-off losses along the LH2 pathway, Lawrence Livermore National Laboratory, 2018 Search PubMed.
- M. Kang, J. Kim, H. You and D. Chang, Exp. Therm. Fluid Sci., 2018, 96, 371–382 CrossRef CAS.
- S. Gursu, S. A. Sherif, T. N. Veziroglu and J. W. Sheffield, J. Energy Resources Technol., 1993, 115, 221–227 CrossRef.
- S. W. Choi, W. I. Lee and H. S. Kim, Numerical Heat Transfer, Part A: Appl., 2017, 71, 402–422 CrossRef CAS.
- J. L. Ferrín and L. J. Pérez-Pérez, Comput. Chem. Eng., 2020, 138, 106840 CrossRef.
- J.-J. Ren, J.-Y. Shi, P. Liu, M.-S. Bi and K. Jia, Int. J. Hydrogen Energy, 2013, 38, 4017–4023 CrossRef CAS.
- A. Saleem, S. Farooq, I. A. Karimi and R. Banerjee, Ind. Eng. Chem. Res., 2020, 59, 14126–14144 CrossRef CAS.
- J. Q. Shi, C. Beduz and R. G. Scurlock, Cryogenics, 1993, 33, 1116–1124 CrossRef CAS.
- A. Mawire, Appl. Energy, 2013, 108, 459–465 CrossRef.
- N. K. Ghaddar, A. M. Al-Marafie and A. Al-Kandari, Appl. Energy, 1989, 32, 225–239 CrossRef.
- F. Perez, S. Z. S. Al Ghafri, L. Gallagher, A. Siahvashi, Y. Ryu, S. Kim, S. G. Kim, M. L. Johns and E. F. May, Energy, 2021, 222, 119853 CrossRef CAS.
- S. Z. S. Al Ghafri, A. Swanger, K. H. Park, V. Jusko, Y. Ryu, S. Kim, S. G. Kim, D. Zhang, Y. Seo, M. L. Johns and E. F. May, Acta Astronautica, 2022, 190, 444–454 CrossRef CAS.
- S. Z. S. Al Ghafri, F. Perez, K. H. Park, L. Gallagher, L. Warr, A. Stroda, A. Siahvashi, Y. Ryu, S. Kim, S. G. Kim, Y. Seo, M. L. Johns and E. F. May, Appl. Therm. Eng., 2021, 189, 116735 CrossRef CAS.
- V. Jusko, S. Z. S. Al Ghafri and E. F. May, BoilFAST v1.0.0, https://www.fsr.ecm.uwa.edu.au/software/boilfast/.
- M. Moran and T. E. D. Nyland, 28th Joint Propulsion Conference and Exhibit, American Institute of Aeronautics and Astronautics, 1992 DOI:10.2514/6.1992-3063.
- M. E. Moran, T. W. Nyland and S. L. Driscoll, in Advances in Cryogenic Engineering, ed. R. W. Fast, Springer US, Boston, MA, 1991, DOI:10.1007/978-1-4615-33.
- D. Chato, 29th Joint Propulsion Conference and Exhibit, American Institute of Aeronautics and Astronautics, 1993 DOI:10.2514/6.1993-1967.
- W. Taylor and D. Chato, in 26th Thermophysics Conference, 1991, DOI:10.2514/6.1991-1379.
- W. U. Notardonato, A. M. Swanger, J. E. Fesmire, K. M. Jumper, W. L. Johnson and T. M. Tomsik, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 278, 012012 Search PubMed.
- W. U. Notardonato, A. M. Swanger, J. E. Fesmire, K. M. Jumper, W. L. Johnson and T. M. Tomsik, Cryogenics, 2017, 88, 147–155 CrossRef CAS PubMed.
- W. U. Notardonato, W. L. Johnson, A. M. Swanger and T. Tomsik, IOP Conf. Ser.: Mater. Sci. Eng., 2015, 101, 012081 Search PubMed.
- A. M. Swanger, W. U. Notardonato, J. E. Fesmire, K. M. Jumper, W. L. Johnson and T. M. Tomsik, IOP Conf. Ser.: Mater. Sci. Eng., 2017, 278, 012013 Search PubMed.
- Y. Kim, C. Lee, J. Park, M. Seo and S. Jeong, Cryogenics, 2016, 74, 123–130 CrossRef CAS.
- L. Wang, Y. Li, C. Li and Z. Zhao, Cryogenics, 2013, 57, 63–73 CrossRef CAS.
- L. Wang, Y. Li, F. Zhang and Y. Ma, Cryogenics, 2015, 72, 161–171 CrossRef CAS.
- L. Jin, C. Park, H. Cho, C. Lee and S. Jeong, Cryogenics, 2016, 79, 96–105 CrossRef CAS.
- K. J. Kim, L. Jin, Y. Kim and S. Jeong, presented in part at the The 27th International Ocean and Polar Engineering Conference, San Francisco, California, USA, 2017/7/31/, 2017.
- Y. Ma, Y. Li, K. Zhu, Y. Wang, L. Wang and H. Tan, Int. J. Hydrogen Energy, 2017, 42, 8264–8277 CrossRef CAS.
- K. Maekawa, M. Takeda, Y. Miyake and H. Kumakura, Sensors, 2018, 18, 3694 CrossRef PubMed.
- Z. Zhongqi, J. Wenbing and H. Yonghua, Cryogenics, 2018, 96, 116–124 CrossRef.
- F. Pistani and K. Thiagarajan, Ocean Eng., 2012, 52, 60–74 CrossRef.
- A. Rafiee, F. Pistani and K. Thiagarajan, Comput. Mech., 2011, 47, 65–75 CrossRef.
- K. P. Thiagarajan, D. Rakshit and N. Repalle, Ocean Eng., 2011, 38, 498–508 CrossRef.
- L. Decker, Liquid Hydrogen Distribution Technology- HYPER Closing Seminar, https://www.sintef.no/globalassets/project/hyper/presentations-day-2/day2_1105_decker_liquid-hydrogen-distribution-technology_linde.pdf/.
- J. Kelley and E. Laumann, Hydrogen Tomorrow: Demands & Technology Requirements, NASA, California, 1975 Search PubMed.
- Australia and Japan develop safety standards for shipping liquid hydrogen, Australian Maritime Safety Authority, Australia, 2017.
- H. Lee, Y. Shao, S. Lee, G. Roh, K. Chun and H. Kang, Int. J. Hydrogen Energy, 2019, 44, 15056–15071 CrossRef CAS.
- A. Friedlander, R. Zubrin and T. Hardy, Benefits of Slush Hydrogen for Space Missions, National Aeronautics and Space Administration, Cleveland, Ohio, 1991 Search PubMed.
- F. Ma, P. Zhang and X. J. Shi, Int. J. Heat Mass Transfer, 2017, 110, 482–495 CrossRef CAS.
- N. Frischauf, in Compendium of Hydrogen Energy, eds. M. Ball, A. Basile and T. N. Veziroğlu, Woodhead Publishing, Oxford, 2016, pp. 87–107 DOI:10.1016/B978-1-78242-364-5.00005-1.
- M. T. Scelzo, L. Peveroni and J.-M. Buchlin, AIAA Aviation 2020 Forum, American Institute of Aeronautics and Astronautics, 2020 DOI:10.2514/6.2020-3037.
- K. Ohira, AIP Conf. Proc., 2004, 710, 27–34 CrossRef CAS.
- K. Ohira, in Compendium of Hydrogen Energy, eds. R. B. Gupta, A. Basile and T. N. Veziroğlu, Woodhead Publishing, 2016, pp. 53–90 DOI:10.1016/B978-1-78242-362-1.00003-1.
- S. Gursu, S. Sheriff, T. Veziroglu and J. Sheffield, Int. J. Hydrogen Energy, 1994, 19, 491–496 CrossRef CAS.
- J. Verne, Mysterious island, Watermill Press, Mahwah, N.J., 1983 Search PubMed.
- International Energy Agency (IEA), Henry Hub Natural Gas Spot Price, https://www.eia.gov/dnav/ng/hist/rngwhhdm.htm.
- N. Ferguson, Doom: The Politics of Catastrophe, Penguin Press, 2021 Search PubMed.
- D. Pritchards and W. Rattigan, Hazards of liquid hydrogen position paper, Health and Safety Executive, 2010.
- T. Jordan, S. Jallais, L. Bernard, A. Venetsanos, S. Coldrick, M. Kuznetsov and D. Cirrone, presented in part at the International Conference on Hydrogen Safety, 2019.
- K. Lyons, C. Proust, D. Cirrone, P. Hooker, M. Kuznetsov and S. Coldrick, Theory and analysis of ignition with specific conditions related to cryogenic hydrogen, https://hysafe.info/wp-content/uploads/sites/3/2020/02/200127-WP4_D4_1_Theory-of-cryogenic-hydrogen-V1.2.pdf.
- Prenormative Research for Safe Use of Liquid Hydrogen (PRESLHY), https://preslhy.eu/.
- S. Pique, B. Weinberger, V. De-Dianous and B. Debray, Int. J. Hydrogen Energy, 2017, 42, 7429–7439 CrossRef CAS.
- G. Hinds, Fuel Cells Bull., 2017, 2017, 14–15 CrossRef.
- Y. Yang, G. Wang, S. Zhang, L. Zhang and L. Lin, E3S Web Conf., 2019, 118, 03032 CrossRef CAS.
- J.-W. Kim, T. H. Lee and J.-W. Choi, Trans. of the Korean Hydrogen and New Energy Society, 2016, 27, 245–255 CrossRef.
- A. V. Tchouvelev, S. P. de Oliveira and N. P. Neves, in Science and Engineering of Hydrogen-Based Energy Technologies, ed. P. E. V. de Miranda, Academic Press, 2019, pp. 303–356 DOI:10.1016/B978-0-12-814251-6.00006-X.
- S. Tomevska, Hydrogen Technologies Standards Discussion Paper, Standards Australia, Australia, 2018 Search PubMed.
- S. Tomevska, Hydrogen Standards Forum Outcomes Report, Standards Australia, Australia, 2018 Search PubMed.
- National Fire Protection Association. Hydrogen Technologies Code, https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=2.
- Y. Yang, G. Wang, S. Zhang, L. Zhang and L. Lin, E3S Web Conf., 2019, 118, 03042 CrossRef CAS.
- Safety Planning for Hydrogen and Fuel Cell Projects, European Hydrogen Safety Panel, Brussels, 2019.
- International Maritime Organization. International Code for the Construction and Equipment of Ships Carrying Liquefied Gases in Bulk (IGC Code), https://www.imo.org/en/OurWork/Environment/Pages/IGCCode.aspx.
- Hydrogen Risk Assessment Model (HyRAM), https://energy.sandia.gov/programs/sustainable-transportation/hydrogen/hydrogen-safety-codes-and-standards/hydrogen-risk-assessment-model-hyram/, (accessed January, 2019).
- P. Ashworth and V. Lambert, The Australian public's perception of hydrogen for energy, S. o. C. Engineering, The University of Queensland, Queensland, Australia, 2018 Search PubMed.
- L. Garrity, presented in part at the Hydrogen and Fuel Cell Futures Conference, Perth, Australia, 12th–15th September 2004, 2004.
- T. O’Garra, Comparative Analysis of the Impact of the Hydrogen Bus Trials on Public Awareness, Attitudes and Preferences: a Comparative Study of Four Cities, Imperial College, London, 2005 Search PubMed.
- G. Thesen and O. Langhelle, Int. J. Hydrogen Energy, 2008, 33, 5859–5867 CrossRef CAS.
- R. Zimmer and J. Welke, Int. J. Hydrogen Energy, 2012, 37, 17502–17508 CrossRef CAS.
- Australian Department of the Environment and Energy, Solar PV and batteries, https://www.energy.gov.au/households/solar-pv-and-batteries, (accessed January, 2019).
- K. Itaoka, A. Saito and K. Sasaki, Int. J. Hydrogen Energy, 2017, 42, 7290–7296 CrossRef CAS.
- Global Liquid Hydrogen Market Report 2019-2024, Business Wire, Dublin, 2019.
- Asia Pacific Energy Research Centre (APERC), https://aperc.or.jp/.
- Hydrogen for Australia's Future – A Briefing Paper for the COAG Energy Council, prepared by the Hydrogen Strategy Group, 2018, http://www.coagenergycouncil.gov.au/publications/hydrogen-australias-future.
- T. Longden, F. J. Beck, F. Jotzo, R. Andrews and M. Prasad, Appl. Energy, 2022, 306, 118145 CrossRef CAS.
- K. Buckland, Why Asia's biggest economies are backing hydrogen fuel cell cars, The Japan Times, 2019.
- Germany launches world's first hydrogen-powered train, 2019, https://www.theguardian.com/environment/2018/sep/17/germany-launches-worlds-first-hydrogen-powered-train.
- ACLNGF, Hydrogen liquefaction and storage symposium: outcomes report. September 2019., https://lngfutures.edu.au/hydrogen-liquefaction-and-storage-symposium-outcomes-report-september-2019/.
- J. G. Reed, E. E. Dailey, B. P. Shaffer, B. A. Lane, R. J. Flores, A. A. Fong and G. S. Samuelsen, Final Project Report - Roadmap for the Deployment and Buildout of Renewable Hydrogen Production Plants in California, California Energy Commission, 2020 Search PubMed.
- J. R. Bartels, A feasibility study of implementing an Ammonia Economy, Iowa State University Digital Repository, 2008 Search PubMed.
- European Fertilizer Manufacturers Association, 2000.
- F. R. D. Velázquez, J. Rodríguez and F. Galindo, Nitrogen+ Syngas, 2013.
- H. Kobayashi, A. Hayakawa, K. D. Kunkuma, A. Somarathne and E. C. Okafor, Proc. Combust. Inst., 2019, 37, 109–133 CrossRef CAS.
- G. Jeerh, M. Zhang and S. Tao, J. Mater. Chem. A, 2021, 9, 727–752 RSC.
- A. Valera-Medina, F. Amer-Hatem, A. K. Azad, I. C. Dedoussi, M. de Joannon, R. X. Fernandes, P. Glarborg, H. Hashemi, X. He, S. Mashruk, J. McGowan, C. Mounaim-Rouselle, A. Ortiz-Prado, A. Ortiz-Valera, I. Rossetti, B. Shu, M. Yehia, H. Xiao and M. Costa, Energy Fuels, 2021, 35, 6964–7029 CrossRef CAS.
- SINTEF, Presentations from the HYPER closing seminar, https://www.sintef.no/projectweb/hyper/presentations-from-the-hyper-closeing-seminar/.
- EU Science Hub – Joint Research Centre, Assessment of Hydrogen Delivery Options, https://ec.europa.eu/jrc/sites/default/files/jrc124206_assessment_of_hydrogen_delivery_options.pdf.
- R. Maha, T. Marine, D. Florence, D. Jonathan and C. Marian, Chin. J. Catal., 2020, 41, 756–769 CrossRef.
- R. Maha, T. Marine, D. Florence, D. Jonathan and C. Marian, Chin. J. Catal., 2020, 41, 770–782 CrossRef.
- M. S. Sadaghiani, A. Siahvashi, B. W. E. Norris, S. Z. S. Al Ghafri, A. Arami-Niya and E. F. May, Energy, 2022, 250, 123789 CrossRef.
- A. Siahvashi, S. Z. S. Al Ghafri and E. F. May, Fluid Phase Equilib., 2020, 519, 112609 CrossRef CAS.
- A. Siahvashi, PhD, The University of Western Australia, 2019 Search PubMed.
- A. Siahvashi, S. Z. S. Al Ghafri, T. J. Hughes, B. F. Graham, S. H. Huang and E. F. May, J. Exp. Therm. Fluid Sci., 2019, 105, 47–57 CrossRef CAS.
- A. Siahvashi, S. Z. S. Al Ghafri, J. H. Oakley, T. J. Hughes, B. F. Graham and E. F. May, J. Chem. Eng. Data, 2017, 62, 2896–2910 CrossRef CAS.
- C. C. Sampson, P. J. Metaxas, A. Siahvashi, P. L. Stanwix, B. F. Graham, M. L. Johns and E. F. May, Chem. Eng. J., 2021, 407, 127086 CrossRef CAS.
- J. P. Kohn and K. D. Luks, GPA Research Report RR-22: Solubility of Hydrocarbons in Cryogenic LNG and NGL Mixtures, Gas Processors Association, 1976 Search PubMed.
- M. Hopkins, A. Siahvashi, X. Yang, M. Richter, P. Stanwix and E. May, Fuel Process. Technol., 2021, 219, 106878 CrossRef CAS.
Footnotes |
| † Global capture and storage capacity of projects currently operating or under construction is around 40 million tonnes per annum (Mtpa). |
| ‡ The largest PEM electrolyser currently in use has a capacity of 20 MW. This is equivalent to a hydrogen production capacity of 8 TPD, assuming a power consumption of 66 kWh kgH2−1. |
| § Recently, BMW Group developed cryo-compressed hydrogen storage technology, involves storing gaseous hydrogen at low temperature on board the vehicle at a pressure of up to 35 MPa. This offers 50% more hydrogen storage capacity than the 70 MPa storage tanks. Liquid hydrogen is typically stored in insulated tankers at near atmospheric pressure. However, heat ingress is hard to avoid, owing to the temperature gradient between the liquid hydrogen and the external environment. This causes liquid hydrogen to evaporate to enter the vapor phase. This evaporated gas is usually called boil-off gas (BOG). |
| ¶ Specific energy consumption is defined as the actual specific work that is required by the hydrogen liquefaction process. SEC (kWh kgLH2−1) = (Pnet/mLH2). Net power consumption, Pnet, is defined as the difference between the total power consumption and total power recovered (by the turbine expanders). |
| || Specific liquefaction cost is defined as the total cost to produce 1 kg of liquid hydrogen. This include CAPEX, OPEX and Operation & Maintenance (OP) cost. |
| This journal is © The Royal Society of Chemistry 2022 |