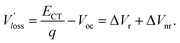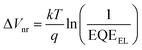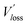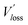Inhibiting excessive molecular aggregation to achieve highly efficient and stabilized organic solar cells by introducing a star-shaped nitrogen heterocyclic-ring acceptor†
Xunfan
Liao‡
 a,
Qian
Xie‡
ab,
Yaxiao
Guo‡
c,
Qiannan
He
b,
Zeng
Chen
d,
Na
Yu
e,
Peipei
Zhu
a,
Yongjie
Cui
ae,
Zaifei
Ma
a,
Qian
Xie‡
ab,
Yaxiao
Guo‡
c,
Qiannan
He
b,
Zeng
Chen
d,
Na
Yu
e,
Peipei
Zhu
a,
Yongjie
Cui
ae,
Zaifei
Ma
 e,
Xiaobao
Xu
e,
Xiaobao
Xu
 f,
Haiming
Zhu
d and
Yiwang
Chen
f,
Haiming
Zhu
d and
Yiwang
Chen
 *ab
*ab
aInstitute of Advanced Scientific Research (iASR), Key Laboratory of Functional Small Molecules for Ministry of Education, Jiangxi Normal University, 99 Ziyang Avenue, Nanchang 330022, China. E-mail: ywchen@ncu.edu.cn
bCollege of Chemistry/Institute of Polymers and Energy Chemistry (IPEC), Nanchang University, 999 Xuefu Avenue, Nanchang 330031, China
cState Key Laboratory of Separation Membranes and Membrane Processes, School of Chemistry, Tiangong University, Tianjin 300387, China
dCenter for Chemistry of High-Performance & Novel Materials, Department of Chemistry, Zhejiang University, Hangzhou 310027, China
eState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Center for Advanced Low-dimension Materials, College of Materials Science and Engineering, Donghua University, Shanghai, 201620, P. R. China
fSchool of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China
First published on 3rd December 2021
Abstract
Inhibiting excessive aggregation and migration of active layer molecules is essential to improve the stability and performance of organic solar cells (OSCs). Herein, the star-shaped nitrogen heterocyclic-ring acceptor TF1 featuring a unique 3D skeleton was elaborately selected as the third component for a PM6:Y6 system to suppress the extensive aggregation of Y6, and further improve the device stability and performance of OSCs. TF1 possesses a complementary absorption spectrum and a higher lowest unoccupied molecular orbital (LUMO) level compared to Y6, as well as excellent miscibility with Y6. Thus, the incorporation of TF1 in the ternary film gives rise to the enhanced short circuit current density (Jsc), elevated energy of charge transfer state (ECT) and reduced voltage loss in ternary OSCs. Meanwhile, it is found that incorporation of TF1 into the PM6:Y6 blend will also increase the exciton lifetime and diffusion distance, facilitate exciton dissociation and collection, reduce trap-assisted recombination, and accelerate the hole transfer rate. As a result, compared to the binary devices, the ternary OSCs with 10% TF1 contents achieved simultaneously improved power conversion efficiency (16.67%), open-circuit voltage (0.870 V), Jsc (25.63 mA cm−2), fill factor (74.79%) and stability, which are among the best results reported in the literature to date. Moreover, a superior efficiency approaching 17% of ternary OSCs was achieved by adopting the pseudo-planar heterojunction (PPHJ) structure. This work not only puts forward an effective strategy to further improve the efficiency and stability of OSCs, but also systematically revealed the inner working mechanism of star-shaped small molecules in ternary OSCs.
Broader contextThe ternary strategy via incorporating a third component to a binary blend film has been proved to be a promising strategy to improve the performance of organic solar cells (OSCs). However, the selection of the efficient third component is always a challenging task. Compared to the linear conjugated molecules with a similar structure to the host donor or acceptor as the third component, the star-shaped molecules with 3D geometric structure may be a better choice. The star-shaped molecules not only feature the advantages of high electron mobility and isotropic charge transfer properties, but also can effectively inhibit excessive aggregation of strong crystalline molecules to further improve device performance and stability. Herein, a star-shaped acceptor TF1 with 3D molecular structure was utilized and incorporated into the PM6:Y6 system to fabricate ternary OSCs. The star-shaped TF1 was proved to effectively suppress the excessive aggregation of Y6, and more efficient exciton dissociation and charge collection was found in the pseudo-planar heterojunction (PPHJ) structure ternary OSC, ultimately contributing to a more stable OSC with an outstanding PCE approaching 17% with better stability. This work indicates that star-shaped molecules are a promising choice for further improving the performance and stability of OSCs. |
Introduction
As a kind of green renewable energy, solar energy is becoming an effective strategy to solve the energy shortage problems and environmental pollution problems.1–3 Due to a series of merits including light weight, solution processability, mechanical flexibility and large-area roll-to-roll fabrication, organic solar cells (OSCs) have become a research hotspot in the field of solar energy utilization.4–10 Owing to the emergence of star non-fullerene acceptor (NFA) Y6 and its derivatives, the power conversion efficiency (PCE) of single-junction OSCs has exceeded 18%, and the great development potential of OSCs is like an iceberg under the sea.11–14 Encouraged by this, a lot of research work has been focused on the improvement of acceptor structure and the design of matching donor materials to further improve the PCE of OSCs, so as to break through the dream PCE above 20%.15–22 At the same time, more and more attention has been paid to device stability. It has been confirmed that the improved charge transfer efficiency in the PM6:Y6 system is due to the unique 3D stacking method of Y6, while serious molecular excessive aggregation often exists in its blend film.23–25 In addition, the improvement of morphology stability is also critical in the long-term storage process. Therefore, it is urgent to find a method that not only can improve the PCE of OSCs, but also maintain long-term stability of the device morphology.The ternary strategy via incorporating a third component to a binary OSC system has been proved to be a promising strategy to improve the performance of OSCs, due to the enhanced complementary absorption spectrum, cascade-like energy level structure and optimized blend film morphology.26–31 However, the selection of the efficient third component is always a challenging task. In the previous exploration of ternary OSCs based on the PM6:Y6 system, linear conjugated molecules with a similar structure to the host donor or host acceptor are usually selected as the third component.32–36 For example, Chen's group added the BTP-M (Y6 derivative) into the PM6:Y6 system as the third component, the similar structure of BTP-M and Y6 ensured good compatibility between the two acceptors and formed an alloy acceptor, resulting in an increased higher lowest unoccupied molecular orbital (LUMO) level of the acceptor and higher open-circuit voltage (Voc) of the device.37 Li's group introduced liquid crystal small molecule BTR into the PM6:Y6 system, benefiting from the similar structure but different crystallinity between BTR and PM6, and the more orderly molecular stacking had been found in a ternary blend film, so as to achieve the goal of optimizing the morphology of the active layer.38 Although these studies have brought improvements in device performance, the related research studies are still lacking in solving the problems caused by the excessive aggregation of Y6 and the long-term stability of the device morphology. Interestingly, the star-shaped acceptor molecules with a rigid planar skeleton and strong electron-donating ability come into our sight. The star-shaped acceptor molecules not only have the advantages of high absorption coefficient and tunable energy levels similar to linear NAFs, but also feature high electron mobility and isotropic charge transfer properties due to their unique 3D molecular structure.39–41 Furthermore, the 3D geometric structure of star-shaped molecules can effectively inhibit excessive aggregation of molecules. Therefore, the star-shaped acceptor molecule is believed to be a possible effective option to solve the excessive aggregation of molecules and the poor long-term stability of the morphology.
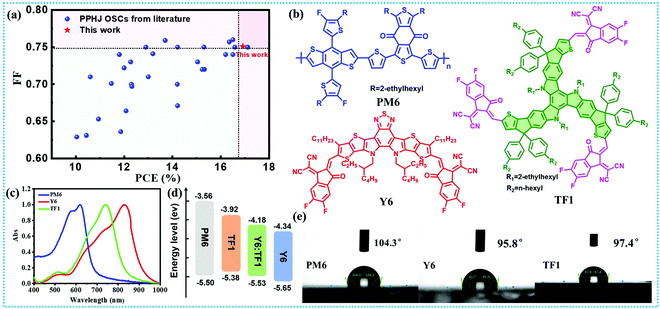
In this work, a star-shaped acceptor TF1 with 3D molecular structure was selected as the third component to add into the PM6:Y6 system. The absorption spectra of a TF1 neat film are complementary to the absorption profile of binary PM6:Y6, which is beneficial for increasing the short-circuit current density (Jsc). Besides, the LUMO level of TF1 is much higher than Y6, providing a guarantee to obtain a higher Voc. Moreover, the star-shaped acceptor TF1 possesses large steric hindrance due to its 3D structural skeleton, while exhibiting excellent miscibility with Y6, which can effectively inhibit the excessive aggregation of Y6 during the film formation process. Owing to the above advantages, the optimized ternary OSCs obtained an improved PCE of 16.67% with an increased Voc (0.870 V), which was much higher than that of the binary device (PCE = 15.62% and Voc = 0.845 V). More importantly, a pseudo-planar heterojunction structure (PPHJ) based ternary OSC with a superior PCE approaching 17% and better stability was achieved by adopting layer-by-layer spin-coating. To the best of our knowledge, the PCE of 17% is one of the highest values reported for PPHJ devices to date (Fig. 1a and Table S1, ESI†). For the first time, we used a combination of femtosecond transient absorption (fs-TA) spectroscopy and fluorescence lifetime imaging system to confirm the more efficient dissociation and longer diffusion length of an exciton in the PPHJ ternary film, and this also explain why the PPHJ device exhibited the best photovoltaic performance. The presented results in this work suggest that inhibiting excessive molecular aggregation by star shape molecules is one of the efficient strategies to realizing high efficiency and stabilized OSCs.
Results and discussion
The chemical structures of PM6, Y6 and TF1 are presented in Fig. 1b, and the synthetic details and characterizations of TF1 can be found in our previously published work.42 The UV-vis absorption spectra for PM6, Y6 and TF1 neat films feature a panchromatic absorption from 400–1000 nm, as shown in Fig. 1c, and such complementary absorption spectra are in favor of achieving high Jsc. Additionally, the energy levels of PM6, Y6 and TF1 were measured using cyclic voltammetry (CV) and the corresponding diagram was shown in Fig. 1d. The highest occupied molecular orbital (HOMO)/LUMO levels of Y6 and TF1 were calculated to be −4.34/−5.65 and −3.92/−5.38 eV. The higher LUMO level of TF1 compared to Y6 will contribute to a higher Voc of the ternary OSCs. Fig. S1a (ESI†) shows the molecular electrostatic potential (ESP) distribution maps of Y6 and TF1. The average ESP values of the surface area distribution and each atom were also analyzed, as shown in Fig. S1b and c (ESI†). From an overall perspective, the Y6 and TF1 possess positive ESP values on their main backbone, whereas the connection region between the intermediate core and terminal unit of Y6 possess negative ESP values. Thus, Y6 and TF1 tend to have dispersed aggregation under electrostatic interaction. Further quantitative structural characteristic parameters show that the molecular polarity index (MPI) of Y6 and TF1 are close, indicating that they have good compatibility with similar polarity (Table S2, ESI†). In order to deeply investigate the relationship between the two acceptors, several relevant tests were performed. Firstly, the compatibility between PM6, TF1 and Y6 was carefully explored. As we all know, the good compatibility between the two acceptors caused by the tiny intermolecular distance was believed to be the indispensable condition for forming an alloyed acceptor.43,44 Thus, the water contact angles (WCAs) of PM6, TF1 and Y6 films were measured, as shown in Fig. 1e and Table S3 (ESI†). The surface energy values of PM6, TF1 and Y6 films were calculated to be 20.32, 24.66 and 25.65 mN m−1, respectively, based on the WCAs value of the corresponding films. The similar surface energy of Y6 and TF1 indicate the good compatibility between these two acceptors. In addition, the compatibility was also evaluated by the interfacial energy between two different materials, which was defined by the following equation based on the surface energy of pure films:| γX–Y = γX + γy − 2 (γX × γY)0.5e[−β(γX−γY)2] |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 (the weight ratio is Y6
TF1 (the weight ratio is Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 = 1.1
TF1 = 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), as depicted in Fig. S2 and Table S4 (ESI†). Compared to the lower LUMO level of Y6 (−4.34 eV), the LUMO level of Y6:TF1 was calculated to be −4.18 eV, slightly lower than that of TF1 (−3.92 eV), indicating that the alloyed state should be formed between Y6 and TF1, and the higher LUMO of Y6:TF1 will be beneficial to increase Voc in ternary devices.
0.1), as depicted in Fig. S2 and Table S4 (ESI†). Compared to the lower LUMO level of Y6 (−4.34 eV), the LUMO level of Y6:TF1 was calculated to be −4.18 eV, slightly lower than that of TF1 (−3.92 eV), indicating that the alloyed state should be formed between Y6 and TF1, and the higher LUMO of Y6:TF1 will be beneficial to increase Voc in ternary devices.
To explore the effect of star-shaped NFAs TF1 as the third component on device performance, the binary (PM6:Y6) and ternary (PM6:Y6:TF1 and PM6/Y6:TF1) devices with the conventional structure of indium tin oxide (ITO)/PEDOT:PSS/Active Layer/PDINO/Al was fabricated. The total D/A weight ratio of all devices is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2, and various ratios of Y6
1.2, and various ratios of Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 were set to 1.15
TF1 were set to 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05, 1.1
0.05, 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, 1.05
0.1, 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 and 1.0
0.15 and 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
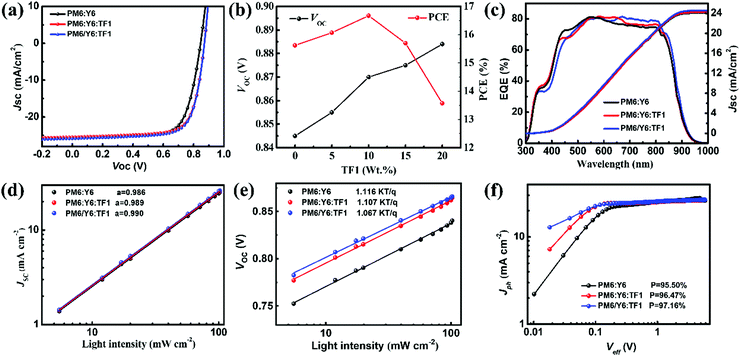
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2. Detailed fabrication procedures for the devices are provided in the ESI†. The current density–voltage (J–V) curves of the optimized binary and ternary OSCs are displayed in Fig. 2a, and the detailed photovoltaic parameters are summarized in Table 1. The binary device achieved a PCE of 15.62%, with a Voc of 0.845 V, a Jsc of 25.04 mA cm−2 and a FF of 74.84%, which are consistent with the reported results. The best PCE of the ternary device (PM6
0.2. Detailed fabrication procedures for the devices are provided in the ESI†. The current density–voltage (J–V) curves of the optimized binary and ternary OSCs are displayed in Fig. 2a, and the detailed photovoltaic parameters are summarized in Table 1. The binary device achieved a PCE of 15.62%, with a Voc of 0.845 V, a Jsc of 25.04 mA cm−2 and a FF of 74.84%, which are consistent with the reported results. The best PCE of the ternary device (PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6
Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 = 1
TF1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, wt%) was improved to 16.67% with a simultaneous enhancement of Voc, Jsc, and FF, where Voc increased to 0.870 V, Jsc increased to 25.63 mA cm−2, and FF increased to 74.79%. It is observed that with the increase of TF1 content, the Voc of a ternary device increases gradually, which is consistent with the expected results, while the Jsc and FF appeared to have a same tendency of increase first and then decrease. The change trend of PCE and Voc with TF1 content was shown in Fig. 2b, and more in-depth analysis will be mentioned later. As to the change trend of Jsc and FF, it was speculated that a small amount of TF1 can promote the proper phase separation in the active layer. However, when the mass ratio of TF1 increases to 1
0.1, wt%) was improved to 16.67% with a simultaneous enhancement of Voc, Jsc, and FF, where Voc increased to 0.870 V, Jsc increased to 25.63 mA cm−2, and FF increased to 74.79%. It is observed that with the increase of TF1 content, the Voc of a ternary device increases gradually, which is consistent with the expected results, while the Jsc and FF appeared to have a same tendency of increase first and then decrease. The change trend of PCE and Voc with TF1 content was shown in Fig. 2b, and more in-depth analysis will be mentioned later. As to the change trend of Jsc and FF, it was speculated that a small amount of TF1 can promote the proper phase separation in the active layer. However, when the mass ratio of TF1 increases to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05
1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15, the HOMO offset between donor and acceptor is too small, which is not conducive to exciton dissociation, thus resulting in decreased Jsc and FF. In order to further improve the device performance, the pseudo-planar heterojunction structure (PPHJ) was adopted to fabricate the ternary device (PM6/Y6
0.15, the HOMO offset between donor and acceptor is too small, which is not conducive to exciton dissociation, thus resulting in decreased Jsc and FF. In order to further improve the device performance, the pseudo-planar heterojunction structure (PPHJ) was adopted to fabricate the ternary device (PM6/Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 = 1
TF1 = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1
1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, wt%), which has been reported in our previous work.47,48 Compared to the bulk heterojunction (BHJ) ternary device, a higher PCE of 16.91% was obtained in the PPHJ ternary device, ascribed to the improvement of Jsc and FF.
0.1, wt%), which has been reported in our previous work.47,48 Compared to the bulk heterojunction (BHJ) ternary device, a higher PCE of 16.91% was obtained in the PPHJ ternary device, ascribed to the improvement of Jsc and FF.
PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 TF1 |
V OC (V) | J SC (mA cm−2) | FF (%) | PCEb (%) |
|---|---|---|---|---|
| a The devices were fabricated with a pseudoplanar heterojunction structure. b The values in parentheses stand for the average values over 20 devices. | ||||
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.845 ± 0.003 | 25.04 ± 0.25 | 73.84 ± 1.12 | 15.62 (15.58 ± 0.13) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.15 1.15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.05 0.05 |
0.855 ± 0.002 | 25.48 ± 0.33 | 73.79 ± 1.15 | 16.07 (15.92 ± 0.25) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 0.1 |
0.870 ± 0.002 | 25.63 ± 0.26 | 74.79 ± 1.03 | 16.67 (16.55 ± 0.17) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.05 1.05![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.15 0.15 |
0.875 ± 0.003 | 24.68 ± 0.28 | 72.73 ± 1.11 | 15.70 (15.49 ± 0.21) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2 0.2 |
0.884 ± 0.005 | 23.22 ± 0.27 | 66.13 ± 1.10 | 13.57 (13.33 ± 0.18) |
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 1.2 |
0.951 ± 0.003 | 4.76 ± 0.22 | 48.89 ± 1.06 | 2.21 (2.16 ± 0.11) |
| PM6/Y6:TF1a | 0.870 ± 0.002 | 25.89 ± 0.23 | 75.08 ± 1.13 | 16.91 (16.85 ± 0.16) |
The improved Jsc was confirmed by external quantum efficiency (EQE) measurements, as shown in Fig. 2c. It can be found that all the EQE spectra of binary and ternary devices exhibited a similar EQE spectra from 300–900 nm, and the EQE value from 600 to 800 nm was clearly enhanced in the BHJ ternary devices and PPHJ ternary devices. The enhancement of EQE value in ternary devices may be due to the strong absorption of TF1 in that wavelength range, or result from the optimized morphology which was caused by the addition of TF1. The integrated Jsc agreed well with the values measured from the J–V curves.
In order to reveal the internal photodynamic mechanism of the improvement of the photovoltaic performance of the devices caused by the introduction of the star-shaped NFAs TF1, the characteristics of exciton dissociation, recombination and charge transfer in the active layer were further studied. First of all, the J–V curves under various light intensity (Plight) were measured to investigate the charge recombination behavior. The relationship between Jsc and Plight can be described as Jsc ∝ Pαlight, where α is the recombination parameter.49 As shown in Fig. 2d, the α of binary, BHJ ternary and PPHJ ternary devices was calculated to be 0.986, 0.989 and 0.990, implying a weaker bimolecular recombination and more efficienct charge extract existed in ternary devices. Furthermore, the dependence of Voc under different light intensity (Plight) was also performed to explore the trap-assisted recombination in different devices, where the relationship was defined as Voc ∝ n(κT/q) ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Plight (κ is Boltzmann's constant, T is absolute temperature, and q is elementary charge).50 Compared to the slope of 1.157 × kT/q for the binary device, the slopes of BHJ ternary and PPHJ ternary devices were calculated to be 1.107 × kT/q and 1.067 × kT/q, as shown in Fig. 2e, indicating the trap-assisted recombination in ternary devices were further suppressed by introduction of the star-shaped NFAs TF1. Additionally, the photocurrent density (Jph) versus effective voltage (Jph–Veff) curves of binary and ternary devices were used to probe the characterizations of exciton dissociation and charge collection, as shown in Fig. 2f. The Jph was defined by Jph = JL − JD, JL (in the light) and JD (in the dark), and Veff (Veff = V0 − Vbias), V0 (Jph = 0), and Vbias is the applied external voltage bias. When Veff > 2 V, the Jph will reach saturation (Jsat), and the exciton dissociation efficiency (ηdiss) could be determined by the value of Jph/Jsat. The ηdiss of binary, BHJ ternary and PPHJ ternary devices were calculated to be 95.50%, 96.47% and 97.16%, respectively, suggesting the introduction of the star-shaped NFAs TF1 in the PM6:Y6 blend facilitates efficient charge dissociation and collection. Moreover, the steady-state photoluminescence (PL) spectra were also carried out to evaluate the charge transfer in the blend films, as shown in Fig. S3 (ESI†), and the results were consistent with the conclusion of the experiments mentioned above.
Plight (κ is Boltzmann's constant, T is absolute temperature, and q is elementary charge).50 Compared to the slope of 1.157 × kT/q for the binary device, the slopes of BHJ ternary and PPHJ ternary devices were calculated to be 1.107 × kT/q and 1.067 × kT/q, as shown in Fig. 2e, indicating the trap-assisted recombination in ternary devices were further suppressed by introduction of the star-shaped NFAs TF1. Additionally, the photocurrent density (Jph) versus effective voltage (Jph–Veff) curves of binary and ternary devices were used to probe the characterizations of exciton dissociation and charge collection, as shown in Fig. 2f. The Jph was defined by Jph = JL − JD, JL (in the light) and JD (in the dark), and Veff (Veff = V0 − Vbias), V0 (Jph = 0), and Vbias is the applied external voltage bias. When Veff > 2 V, the Jph will reach saturation (Jsat), and the exciton dissociation efficiency (ηdiss) could be determined by the value of Jph/Jsat. The ηdiss of binary, BHJ ternary and PPHJ ternary devices were calculated to be 95.50%, 96.47% and 97.16%, respectively, suggesting the introduction of the star-shaped NFAs TF1 in the PM6:Y6 blend facilitates efficient charge dissociation and collection. Moreover, the steady-state photoluminescence (PL) spectra were also carried out to evaluate the charge transfer in the blend films, as shown in Fig. S3 (ESI†), and the results were consistent with the conclusion of the experiments mentioned above.
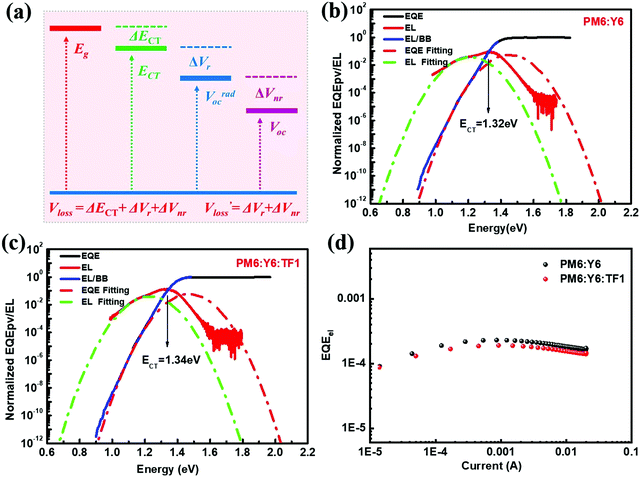
We then explored the impact of the introduced star-shaped acceptor TF1 on voltage loss (Vloss) in our BHJ devices. Due to the higher lying LUMO energies in TF1 with regard to Y6, we speculate that Vloss could be modulated in the ternary devices. We first quantified the optical bandgaps (Egap) of the blend films based on the cross point between the electroluminescence (EL) and absorption spectra of the blends. The Egap of the binary and ternary blends are both 1.40 eV. The total Vloss can be divided into three parts, including ΔECT, ΔVr and ΔVnr (Fig. 3a). ΔECT, also known as the energetic driving force (DF), is defined as the energetic difference between the charge transfer (CT) state and the local excited state of the donor or the acceptor material (S1). The 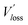 of OSCs can be determined according to the equation:
of OSCs can be determined according to the equation:
where k is the Boltzmann constant and T is the absolute temperature. It was noted that the EQEEL slightly changed after introducing the TF1 into the PM6:Y6 systems. Thus, the ΔVnr for the ternary OSC still kept a very low value of 0.219 V. Then, the total Vloss for the binary and ternary device was 0.57 and 0.55 V, respectively, illustrating why higher Voc was achieved in the PM6:Y6:TF1 devices. This result has certain guiding significance for our future investigations, indicating that we can adopt star-shape acceptors with higher LUMO energies to control the film morphology and to improve device performance without sacrificing Voc.
To gain insight into the difference in morphological characteristics after introducing the star-shaped NFAs TF1, the morphology of the blend films was carefully investigated by atomic force microscopy (AFM). As shown in Fig. S4 (ESI†), the root-mean-square (RMS) roughness values of the PM6 and Y6 neat films were measured as 0.99 and 1.93 nm. After adding TF1, the roughness of the PM6:TF1 and Y6:TF1 films increased appropriately to 1.21 and 2.87 nm, respectively. It can be easily found that the same tendency appeared in the blend films of the binary and ternary devices. The PM6:Y6 blend film showed a uniform and smooth surface morphology with a roughness value of 1.05 nm, while the roughness value of the PM6:Y6:TF1 film also increased slightly to 1.80 nm. According to our previous speculation, the excessive accumulation of Y6 in the blend film is effectively suppressed due to the star-shaped structure of TF1, and as the TF1 has good compatibility with PM6 and Y6, which means the roughness of the blend films after adding the TF1 should be much smaller. However, the roughness of both the pure film and blend films increases slightly after adding TF1 according to the actual test results. The increase in roughness may be due to the more concave and convex films causing by the dispersion effect of TF1.
As the surface morphology of the film cannot reflect the aggregation characteristics inside the active layer, grazing incidence wide angle X-ray scattering (GIWAXS) measurements were adopted to gain a deep understanding of the crystallinity and molecular orientation of the pure film and blend films, especially the changes brought about by the introduction of star-shaped NFAs TF1. The 2D GIWAXS images and 1D X-ray profiles were displayed in Fig. 4, while the crystal coherence length (CCL) of the (010) peaks in the out-of-plane (OOP) direction were calculated and are presented in Table 3. The neat Y6 film exhibited a preferred face-on orientation with a strong π–π stacking peak at q ≈ 1.717 Å−1 (d = 3.659 Å) in the OOP direction, and the CCL value of the (010) peak in the OOP direction was calculated to be 27.762 Å. As to the in-plane (IP) direction of the neat Y6 film, there are two (100) peaks located at 0.280 Å−1 (d = 22.440 Å) and 0.411 Å−1 (d = 15.288 Å), consistent with previous reports. Interestingly, although the Y6:TF1 film had similar diffraction peaks in the IP and OOP directions with the Y6 pure film, the CCL value of the Y6:TF1 film was relatively reduced to 25.182 Å, and a larger π–π stacking distance (qz = 1.715 Å−1, d = 3.664 Å; qz = 1.78 Å−1, d = 3.53 Å) were also observed. The reduced CCL and larger π–π stacking distance suggesting the addition of star-shaped NFAs TF1 can disperse Y6 agglomerates and reduce the excessive aggregation of Y6.
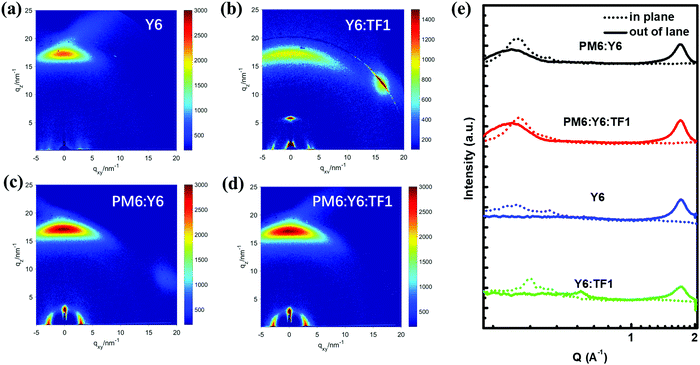 | ||
Fig. 4 2D GIWAXS patterns of (a) Y6, (b) Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1 (1.1 TF1 (1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1), (c) PM6 0.1), (c) PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 (1 Y6 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2), (d) PM6 1.2), (d) PM6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Y6 Y6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TF1(1 TF1(1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 1.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1) and (e) line cuts of the corresponding films. 0.1) and (e) line cuts of the corresponding films. | ||
| Substance | q z (Å−1) | d-Spacing (Å) | FWHM (Å−1) | Coherence length (Å) |
|---|---|---|---|---|
| PM6:Y6 | 1.707 | 3.681 | 2.182 | 25.912 |
| PM6:Y6:TF1 | 1.705 | 3.685 | 2.270 | 24.911 |
| Y6 | 1.717 | 3.659 | 2.037 | 27.762 |
| Y6:TF1 | 1.715 | 3.664 | 2.249 | 25.182 |
A similar tendency also appeared in the comparison of the PM6:Y6 and PM6:Y6:TF1 systems. For the PM6:Y6 blend film, a strong (010) peak at 1.707 Å−1 (d = 3.681 Å) with a CCL value of 25.912 Å can be easily found in the OOP direction. After adding TF1, the corresponding diffraction peak intensity of the ternary blend was reduced as we speculated, and a stronger (010) peak at 1.705 Å−1 (d = 3.685 Å) with a decreased CCL value of 24.911 Å was observed in the OOP direction. Summarizing the results of AFM and GIWAXS, the unique star-shaped molecular structure of TF1 helps to inhibit the excessive aggregation of a certain component during the film formation process, which has important guiding significance for optimizing the morphology of the active layer. Such as in this work, the suppression of the excessive aggregation of Y6 in the film after adding TF1, the PM6:Y6:TF1 film formed a more suitable phase separation size, facilitating charge dissociation and transfer in the ternary devices (vide infra). In addition, more evidence for the dispersion of TF1 was found. As presented in Fig. S5 (ESI†), the blue-shifted absorption spectra and the decreased melting peak temperature of blend films of Y6:TF1 can be easily observed, suggesting the aggregation of Y6 had been weakened.
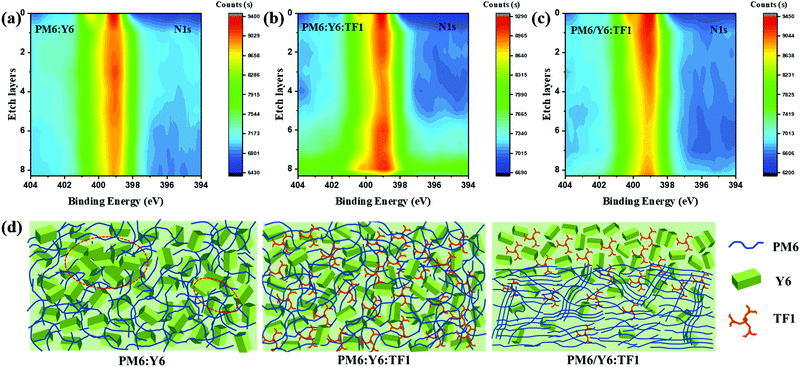
To gain further insight into the internal structure and vertical phase characteristics of the BHJ and PPHJ films, especially the precise role of TF1 in ternary devices, dynamic X-ray photoelectron spectroscopy (DXPS) measurements were obtained. As shown in Fig. 5a–c, the binding energies of the N 1s peak of the acceptors is around 399.1 eV and the brightness of the color represents the strength of the peak signal. Since N is the unique element in Y6 and TF1, we can track the acceptors distribution via the strength of the N 1s peak signal. As for the active layer of PM:Y6 (Fig. 5a), it can be seen that the N 1s peak signal in the bottom is a little weaker than that in the top, indicating strong aggregation of Y6 exists in the active layer and they tend to distribute on the upper layer. However, the acceptor distribution in the active layer of PM6:Y6:TF1 changed significantly, where a stronger signal was observed in the bottom layer, indicating that the distribution of the acceptor components (Y6 and TF1) at the bottom of the active layer increased after the third component TF1 was incorporated into the PM6:Y6 system. The obvious change in acceptor distribution may be caused by the better compatibility of PM6:TF1 than PM6:Y6. Furthermore, the N 1s peak signal in the active layer of PM/Y6:TF1 showed a different trend where a stronger signal appeared in the etch layer from 0 to 5, and then the signal gradually weakens with the increase of depth. The unique change in the trend of the N 1s peak signal in the active layer of PM/Y6:TF1 indicates that the acceptors were enriched on the surface of the active layer, and gradually decreased as the depth increased. In other words, vertical phase separation was observed in the active layer with the PPHJ structure, and this phenomenon was considered to be one of the key factors to obtain higher charge separation and transfer efficiency in the PPHJ structure ternary devices. It is worth pointing out that as the star-shaped acceptor TF1 also contains nitrogen atoms, it can be judged that the distribution range of TF1 is similar as that of the host acceptor Y6 due to their excellent compatibility. This also implies that TF1 can still play the role in the ternary PPHJ structure to effectively inhibit the excessive aggregation of Y6 molecules. Based on the above results, we can probably infer the internal microstructure of these three active layer films, as shown in Fig. 5d. In comparison to the PM6:Y6 blend, it can be easily found that the excessive aggregation of Y6 has been inhibited in the PM6:Y6:TF1 ternary blend, which facilitates weaker bimolecular recombination in the ternary devices. Moreover, obvious vertical phase separation is considered to exist in the active layer of PM6/Y6:TF1, where the acceptors were enriched on the surface of the active layer, and gradually decreased as the depth increased. This vertical phase separation is conductive to charge separation and transport, and thereby leads to higher JSC and FF. The improved charge transport was confirmed by measuring the hole and electron mobilities (μh and μe) of the three different blend films through a space charge limited current method (SCLC). The details are provided in Fig. S6 and Table S5 (ESI†). As we expected, the device based on the PM6/Y6:TF1 blend exhibited the highest μh (2.24 × 10−4 cm2 V−1 s−1) and μe (3.04 × 10−4 cm2 V−1 s−1) with the best balanced μe/μh ratio, which is much better than that of the BHJ ternary device and binary device.
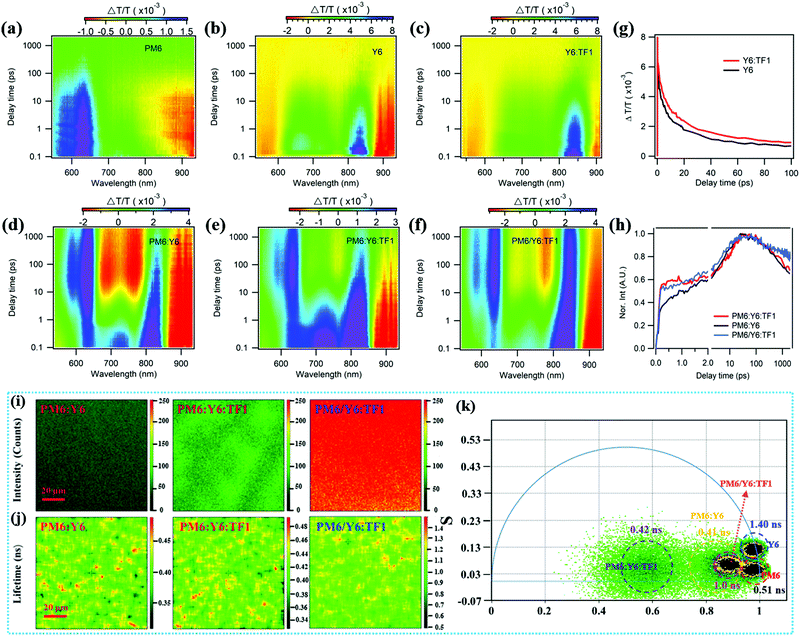
We further confirmed the charge transfer process and investigated the change in excited state dynamics after adding the third component TF1 via femtosecond transient absorption (fs-TA) spectroscopy measurements. Fig. 6a and Fig. S7 (ESI†) show the 2D color plots and TA spectra at selected decay times for the donor PM6. Here, we used a pump laser of 750 nm to only excite the acceptors. The 2D color plots and TA spectra at selected delay times for pure Y6, Y6:TF1, PM6:Y6, PM6:Y6:TF1 and PM6/Y6:TF1 under 750 nm excitation are shown in Fig. 6b–f and Fig. S7 (ESI†), respectively. In Fig. 6g, the ground state bleach (GSB) lifetime of the Y6:TF1 blend is longer than that of the pure Y6 probe at ∼840 nm, suggesting a longer exciton lifetime in the Y6:TF1 blend which may favor charge generation, probably owing to the reduced Y6 over-aggregation with the addition of the star-shaped molecule TF1. Moreover, we also observed the hole transfer process in the D/A blend films where a new kind of bleach peaks at 550–675 nm appeared ascribed to the signal of PM6. The rising kinetics of PM6 GSB in the binary and ternary blends extracted in Fig. 6h and Table S6 (ESI†) directly reflect the hole transfer process with a biexponential function:
I = A1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ1) + A2 exp(−t/τ1) + A2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ2) exp(−t/τ2) |
The emissive PL photon counts in the films were recorded to analyze the emission distribution of a certain micro area and gain information of the surface traps. As we can see from Fig. 6i and Fig. S8 (ESI†), the films have obviously smooth surfaces and homogeneous PL. For blend films, with the additives of TF1, the PM6:Y6:TF1 film had an enhanced PL emission in comparison with PM6:Y6, while PM6/Y6:TF1 exhibited the strongest PL intensity, which could be ascribed to the suppression of trap-assisted recombination caused by the reduced molecule aggregation and the synergy of the PM6/Y6:TF1 interface. It is worth noting excitation light mainly excites the molecules at the film surface. The extremely strong PL intensity in PM6/Y6:TF1 is mainly attributed to Y6 molecules, which also indicates that the PPHJ structure was formed by the LBL method and Y6 were abundant in the cathode. To further clarify the effect of additives, the time-resolved confocal imaging system composed of fluorescence lifetime imaging microscopy (FLIM) and phasor plots was conducted to investigate the carrier dynamics in the PM6:Y6, PM6:Y6:TF1 and PM6/Y6:TF1 films. Different from the traditional time-resolved photo luminescence (TRPL), confocal time-resolved imaging systems can acquire a lifetime distribution of films within a scale of 100 × 100 μm, along with a fine resolution. As shown in Fig. 6j, the carrier lifetime in PM6:Y6 and PM6:Y6:TF1 was estimated to be 0.41 ns and 0.42 ns by single-exponential fitting. This result indicates that the TF1 additive slightly increased the carrier lifetime in the bulk-junction film. It is deduced that the enhancement of PCE in the device with PM6:Y6:TF1 could result from the more effective excitons. In the PM6/Y6:TF1 planar junction film, the average carrier lifetime mainly from the Y6 layer significantly increased to 1.0 ns. Combined with the performance of the device containing PM6/Y6:TF1, it is reasonable to conclude a much longer diffusion length of exciton in the PPHJ ternary film related to the BHJ film, which has been reported in many other studies.54 These changes can also be observed from the phasor plots. This plot was obtained by transforming the phase (Φ) and modulation (m) measurements in the frequency domain to phase space (G, S), for Gω = mωcos(Φω) and Sω = mωsin(Φω), in which ω is the modulation frequency. Their relationship is presented as a semicircle, centering at (0.5, 0) with a radius of 0.5. The raw carrier lifetime data are located on the 2D phasor plot with lifetime increasing anticlockwise, from (1, 0) being zero to (0, 0) being infinite. As can be seen from Fig. 6k, the lifetime of PM6:Y6 has a shorter lifetime and a large lifetime distribution, which shows multi-component lifetime species induced by molecules aggregation in the blend film. Luckily, after adding TF1 molecules, the lifetime dataset of PM6/Y6:TF1 moved towards the semicircle curve and had a narrower distribution, indicating less recombination channels and reduced defects owing to less molecular aggregation by the incorporation of TF1. By using a combination of fs-TA spectroscopy and fluorescence lifetime imaging system, the longer exciton lifetime and longer diffusion distance was confirmed in PPHJ ternary OSCs, contributing to more exciton dissociation and charge collection, and ultimately improving the device performance.
As mentioned above, the excessive aggregation of molecules was effectively inhibited during the blend film formation process due to the star-shaped structure of TF1, and whether the stability of the active layer can also be effectively improved during long-term storage has aroused our interest. Thus, we compared the storage stability of the devices based on PM6:Y6, PM6:Y6:TF1 and PM6/Y6:TF1 to explore the stability difference between with or without the addition of TF1, as shown in Fig. S9 (ESI†). All of the binary and ternary devices displayed good stability in five days by retaining over 90% of their initial PCE values. A more significant difference in stability can be found after 27 days. The ternary devices exhibited better stability with a slower decrease in PCE by retaining 70% of their initial value, while the binary devices lost 35% of their initial PCE value. In addition, the photovoltaic parameters (Jsc, Voc and FF) of the three types of devices versus time were also contrasting, and the change in tendency of Jsc and FF agreed well with that of device performance, while the Voc almost had no change. The results suggest that the device stability had been improved by introducing the star-shaped structure molecule of TF1, and to a certain extent, it also proved the important role of the star-shaped molecules to effectively improve the stability of the active layer by inhibiting excessive molecular aggregation in the devices during the long-term storage, which had important guiding significance for improving the stability of the device. Furthermore, it was also found that the PPHJ ternary device has excellent stability compared with the BHJ ternary device, which was consistent with our previous research results.
Conclusion
In conclusion, high-performance ternary OSCs were constructed by incorporating a star-shaped nitrogen heterocyclic-ring acceptor TF1 as the third component into the PM6:Y6 matrix. TF1 exhibits a high LUMO level, complementary absorption spectra and excellent miscibility with Y6. Through the reduced CCL and larger π–π stacking distance in the PM6:Y6:TF1 ternary devices, the 3D geometric structure of star-shaped TF1 was proved to effectively inhibit excessive aggregation of the host acceptor Y6. Besides, for the first time by using a combination of fs-TA spectroscopy and a fluorescence lifetime imaging system, a longer exciton lifetime and longer diffusion distance was found in the PPHJ structure ternary OSCs, which result in more exciton dissociation and charge collection. Owing to the above advantages, the optimized PM6:Y6:TF1 ternary BHJ OSCs obtained the best PCE of 16.67% with an increased Voc (0.870 V), and a superior PCE approaching 17% was achieved in PPHJ ternary OSCs. It is worth noting that the PCE of 17% is among the best values reported for PPHJ devices to date. Moreover, the ternary devices also demonstrated more excellent device stability. Our work indicates that star-shaped molecules are a promising choice for further improving the performance and stability of OSCs.Author contributions
X. L., Q. X. and Y. G. conceived the idea. X. L., Q. H and Q. X. carried out the materials selection, device fabrication and characterization. Q. H and Q. X. and P. Z. carried out the Vis-UV, CV and AFM measurements. Y. C. performed the DFT calculations. The voltage loss characterization was carried out by Z. M. and N. Y., the femtosecond transient absorption was carried out by Z. C. and H. Z., and the fluorescence lifetime imaging system was performed by X. X. All the authors discussed the results and contributed to the writing of the manuscript. X. L. and Y. C. supervised the project.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
X. L., Q. X. and Y. G. contributed equally to this work. This work was financially supported by the National Natural Science Foundation of China (NSFC) (51973032, 21905043, 51833004, 52073056, and 52172146), the Natural Science Foundation of Jiangxi Province (20212ACB203005), “Double Thousand Plan” Science and Technology Innovation High-end Talent Project of Jiangxi Province (jxsq2019101051), and the National Key Research and Development Program of China (2017YFA0207700).References
- G. Li, R. Zhu and Y. Yang, Nat. Photonics, 2012, 6, 153–161 CrossRef CAS.
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS PubMed.
- P. Cheng, G. Li, X. Zhan and Y. Yang, Nat. Photonics, 2018, 12, 131–142 CrossRef CAS.
- A. Armin, W. Li, O. J. Sandberg, Z. Xiao, L. Ding, J. Nelson, D. Neher, K. Vandewal, S. Shoaee, H. A. T. Wang, T. Heumueller, C. Brabec and P. Meredith, Adv. Energy Mater., 2021, 11, 2003570 CrossRef CAS.
- S. Dong, T. Jia, K. Zhang, J. Jing and F. Huang, Joule, 2020, 4, 2004–2016 CrossRef CAS.
- Y. Liu, J. Zhao, Z. Li, C. Mu, W. Ma, H. Hu, K. Jiang, H. Lin, H. Ade and H. Yan, Nat. Commun., 2014, 5, 1–8 Search PubMed.
- F. Zhao, S. Dai, Y. Wu, Q. Zhang, J. Wang, L. Jiang, Q. Ling, Z. Wei, W. Ma, W. You, C. Wang and X. Zhan, Adv. Mater., 2017, 29, 1700144 CrossRef PubMed.
- K. Li, Y. S. Wu, X. M. Li, H. B. Fu and C. L. Zhan, Sci. China: Chem., 2020, 63, 490 CrossRef CAS.
- Q. He, W. Sheng, M. Zhang, G. Xu, P. Zhu, H. Zhang, Z. Yao, F. Gao, F. Liu, X. Liao and Y. Chen, Adv. Energy Mater., 2021, 11, 2003390 CrossRef CAS.
- X. Liao, Q. He, G. Zhou, X. Xia, P. Zhu, Z. Xing, H. Zhu, Z. Yao, X. Lu and Y. Chen, Chem. Mater., 2021, 33, 430–440 CrossRef CAS.
- J. Yuan, Y. Zhang, L. Zhou, G. Zhang, H.-L. Yip, T.-K. Lau, X. Lu, C. Zhu, H. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. Li and Y. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- Y. Cui, H. Yao, J. Zhang, K. Xian, T. Zhang, L. Hong, Y. Wang, Y. Xu, K. Ma, C. An, C. He, Z. Wei, F. Gao and J. Hou, Adv. Mater., 2020, 32, 1908205 CrossRef CAS PubMed.
- X. Liao, Y. Cui, X. Shi, Z. Yao, H. Zhao, Y. An, P. Zhu, Y. Guo, X. Fei, L. Zuo, K. Gao, F. Lin, Q. Xie, L. Chen, W. Ma, Y. Chen and A. K.-Y. Jen, Mater. Chem. Front., 2020, 4, 1507–1518 RSC.
- Q. Liu, Y. Jiang, K. Jin, J. Qin, J. Xu, W. Li, J. Xiong, J. Liu, Z. Xiao, K. Sun, S. Yang, X. Zhang and L. Ding, Sci. Bull., 2020, 65, 1792–1795 CrossRef.
- Q. Kang, Z. Zheng, Y. Zu, Q. Liao, P. Bi, S. Zhang, Y. Yang, B. Xu and J. Hou, Joule, 2021, 5, 646–658 CrossRef CAS.
- Z. Wang, Z. Peng, Z. Xiao, D. Seyitliyev, K. Gundogdu, L. Ding and H. Ade, Adv. Mater., 2020, 32, 2005386 CrossRef CAS PubMed.
- J. Wan, Z. Chen, L. Zeng, X. Liao, Q. He, S. Liu, P. Zhu, H. Zhu and Y. Chen, J. Energy Chem., 2021, 65, 133–140 CrossRef.
- T. Liu, R. Ma, Z. Luo, Y. Guo, G. Zhang, Y. Xiao, T. Yang, Y. Chen, G. Li, Y. Yi, X. Lu, H. Yan and B. Tang, Energy Environ. Sci., 2020, 13, 2115–2123 RSC.
- R. Ma, Y. Tao, Y. Chen, T. Liu, Z. Luo, Y. Guo, Y. Xiao, J. Fang, G. Zhang, X. Li, X. Guo, Y. Yi, M. Zhang, X. Lu, Y. Li and H. Yan, Sci. China: Chem., 2021, 64, 581–589 CrossRef CAS.
- F. Zhao, H. Zhang, R. Zhang, J. Yuan, D. He, Y. Zou and F. Gao, Adv. Energy Mater., 2020, 10, 2002746 CrossRef CAS.
- L. Zhou, L. Meng, J. Zhang, C. Zhu, S. Qin, I. Angunawela, Y. Wan, H. Ade and Y. Li, Adv. Funct. Mater., 2021, 2109271, DOI:10.1002/adfm.202109271.
- J. Zhang, F. Bai, I. Angunawela, X. Xu, S. Luo, C. Li, G. Chai, H. Yu, Y. Chen, H. Hu, Z. Ma, H. Ade and H. Yan, Adv. Energy Mater., 2021, 2102596, DOI:10.1002/aenm.202102596.
- L. Zhu, M. Zhang, G. Zhou, T. Hao, J. Xu, J. Wang, C. Qiu, N. Prine, J. Ali, W. Feng, X. Gu, Z. Ma, Z. Tang, H. Zhu, L. Ying, Y. Zhang and F. Liu, Adv. Energy Mater., 2020, 10, 1904234 CrossRef CAS.
- G. Zhang, X.-K. Chen, J. Xiao, P. C. Y. Chow, M. Ren, G. Kupgan, X. Jiao, C. C. S. Chan, X. Du, R. Xia, Z. Chen, J. Yuan, Y. Zhang, S. Zhang, Y. Liu, Y. Zou, H. Yan, K.-S. Wong, V. Coropceanu, N. Li, C. J. Brabec, J.-L. Bredas, H.-L. Yip and Y. Cao, Nat. Commun., 2020, 11, 1–10 Search PubMed.
- F. Lin, K. Jiang, W. Kaminsky, Z. Zhu and A. K.-Y. Jen, J. Am. Chem. Soc., 2020, 142, 15246–15251 CrossRef CAS PubMed.
- Y. Lin, M. I. Nugraha, Y. Firdaus, A. D. Scaccabarozzi, F. Anies, A.-H. Emwas, E. Yengel, X. Zheng, J. I. Liu, W. Wahyudi, E. Yarali, H. Faber, O. M. Bakr, L. Tsetseris, M. Heeney and T. D. Anthopoulos, ACS Energy Lett, 2020, 5, 3663–3671 CrossRef CAS.
- Y. Xu, Y. Cui, H. Yao, T. Zhang, J. Zhang, L. Ma, J. Wang, Z. Wei and J. Hou, Adv. Mater., 2021, 33, 2101090 CrossRef CAS PubMed.
- X. Chen, D. Wang, Z. Wang, Y. Li, H. Zhu, X. Lu, W. Chen, H. Qiu and Q. Zhang, Chem. Eng. J., 2021, 424, 130397 CrossRef CAS.
- K. Yu, W. Song, Y. Li, Z. Chen, J. Ge, D. Yang, J. Zhang, L. Xie, C. Liu and Z. Ge, Small Struct., 2021, 2, 2100099 CrossRef.
- L. Zhan, S. Li, X. Xia, Y. Li, X. Lu, L. Zuo, M. Shi and H. Chen, Adv. Mater., 2021, 33, 2007231 CrossRef CAS PubMed.
- H. Chen, R. Zhang, X. Chen, G. Zeng, L. Kobera, S. Abbrent, B. Zhang, W. Chen, G. Xu, J. Oh, S.-H. Kang, S. Chen, C. Yang, J. Brus, J. Hou, F. Gao, Y. Li and Y. Li, Nat. Energy, 2021, 6, 1045–1053 CrossRef.
- Q. Ma, Z. Jia, L. Meng, J. Zhang, H. Zhang, W. Huang, J. Yuan, F. Gao, Y. Wan, Z. Zhang and Y. Li, Nano Energy, 2020, 78, 105272 CrossRef CAS.
- R. Yu, H. Yao, Y. Cui, L. Hong, C. He and J. Hou, Adv. Mater., 2019, 31, 1902302 CrossRef PubMed.
- J. Wang, C. Han, F. Bi, D. Huang, Y. Wu, Y. Li, S. Wen, L. Han, C. Yang, X. Bao and J. Chu, Energy Environ. Sci., 2021, 14, 5968–5978 RSC.
- W. Peng, Y. Lin, S. Y. Jeong, Z. Genene, A. Magomedov, H. Y. Woo, C. Chen, W. Wahyudi, Q. Tao, J. Deng, Y. Han, V. Getautis, W. Zhu, T. D. Anthopoulos and E. Wang, Nano Energy, 2021, 106681, DOI:10.1016/j.nanoen.2021.106681.
- N. Gasparini, S. H. K. Paleti, J. Bertrandie, G. Cai, G. Zhang, A. Wadsworth, X. Lu, H.-L. Yip, I. McCulloch and D. Baran, ACS Energy Lett., 2020, 5, 1371–1379 CrossRef CAS.
- L. Zhan, S. Li, T.-K. Lau, Y. Cui, X. Lu, M. Shi, C.-Z. Li, H. Li, J. Hou and H. Chen, Energy Environ. Sci., 2020, 13, 635–645 RSC.
- C. Yan, H. Tang, R. Ma, M. Zhang, T. Liu, J. Lv, J. Huang, Y. Yang, T. Xu, Z. Kan, H. Yan, F. Liu, S. Lu and G. Li, Adv. Sci., 2020, 7, 2000149 CrossRef CAS PubMed.
- Y.-Q. Pan and G.-Y. Sun, ChemSusChem, 2019, 12, 4570–4600 CrossRef CAS PubMed.
- X. Wu, W. Wang, H. Hang, H. Li, Y. Chen, Q. Xu, H. Tong and L. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 28115–28124 CrossRef CAS PubMed.
- D. Liu, T. Wang, Z. Chang, N. Zheng, Z. Xie and Y. Liu, J. Mater. Chem. A, 2021, 9, 2319–2324 RSC.
- Z. Yao, X. Liao, Y. Guo, H. Zhao, Y. Guo, F. Zhang, L. Zhang, Z. Zhu, L. Kloo, W. Ma, Y. Chen and L. Sun, Chem. Mater., 2019, 31, 8810–8819 CrossRef CAS.
- V. Gupta, A. K. Kyaw, D. H. Wang, S. Chand, G. C. Bazan and A. J. Heeger, Sci. Rep., 2013, 3, 1–6 Search PubMed.
- K.-H. Kim, H. Kang, H. J. Kim, P. S. Kim, S. C. Yoon and B. J. Kim, Chem. Mater., 2012, 24, 2373–2381 CrossRef CAS.
- A. D. de Zerio and C. Müller, Adv. Energy Mater., 2018, 8, 1702741 CrossRef.
- Q. An, J. Wang, W. Gao, X. Ma, Z. Hu, J. Gao, C. Xu, M. Hao, X. Zhang, C. Yang and F. Zhang, Sci. Bull., 2020, 65, 538–545 CrossRef CAS.
- J. Wan, L. Zhang, Q. He, S. Liu, B. Huang, L. Hu, W. Zhou and Y. Chen, Adv. Funct. Mater., 2020, 30, 1909760 CrossRef CAS.
- S. Liu, D. Chen, X. Hu, Z. Xing, J. Wan, L. Zhang, L. Tan, W. Zhou and Y. Chen, Adv. Funct. Mater., 2020, 30, 2003223 CrossRef CAS.
- J.-S. Huang, T. Goh, X. Li, M. Y. Sfeir, E. A. Bielinski, S. Tomasulo, M. L. Lee, N. Hazari and A. D. Taylor, Nat. Photonics, 2013, 7, 479–485 CrossRef CAS.
- S. Honda, H. Ohkita, H. Benten and S. Ito, Adv. Energy Mater., 2011, 1, 588–598 CrossRef CAS.
- Z. Tang, J. Wang, A. Melianas, Y. Wu, R. Kroon, W. Li, W. Ma, M. R. Andersson, Z. Ma, W. Cai, W. Tress and O. Inganäs, J. Mater. Chem. A, 2018, 6, 12574–12581 RSC.
- Z. Jia, Z. Chen, X. Chen, L. Bai, H. Zhu and Y. M. Yang, J. Phys. Chem. Lett., 2021, 12, 151–156 CrossRef CAS PubMed.
- Z. Chen, X. Chen, B. Qiu, G. Zhou, Z. Jia, W. Tao, Y. Li, Y. M. Yang and H. Zhu, J. Phys. Chem. Lett., 2020, 11, 3226–3233 CrossRef CAS PubMed.
- J. Yuan, X. Ling, D. Yang, F. Li, S. Zhou, J. Shi, Y. Qian, J. Hu, Y. Sun, Y. Yang, X. Gao, S. Duhm, Q. Zhang and W. Ma, Joule, 2018, 2, 2450–2463 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d1ee02858h |
| ‡ Xunfan Liao, Qian Xie and Yaxia Guo contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2022 |