Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Detection techniques for air-borne isocyanates based on fluorescent derivatizing agents
S.
Selvakumar
 *,
Subbaraj
Karunakaran
*,
Subbaraj
Karunakaran
 and
V. Siva
Rama Krishna
and
V. Siva
Rama Krishna

Chemical & Mechanical Testing Labs, QC (Process & Labs), Solid Motors Propellant Complex, SDSC-SHAR, Sriharikota – 524 124, India. E-mail: selvakumar.s@shar.gov.in; kumarreka@hotmail.com; Fax: +91-8623-223013; Fax: +91-8623-225154
First published on 26th September 2022
Abstract
The high toxicity of isocyanate species in the workplace demands the development of sensitive techniques for the detection of isocyanates to protect workers from adverse health effects. To detect airborne isocyanate molecules in a workplace, several analytical techniques such as colorimetry, amperometry, capillary zone electrophoresis, high-performance liquid chromatography and fluorescence are reported in the literature. Among them, fluorescent probes due to their high sensitivity and selectivity grabbed special attention for the determination of the isocyanate group. This review primarily focused on fluorescent derivatizing agents (with amine and hydroxyl groups) available for the determination of isocyanates in the literature and also discussed about fluorescent polymers in non-derivatization mode for isocyanate estimation, which is less explored.
Environmental significanceIsocyanates are a group of highly reactive, low molecular weight organic compounds, which are widely used in the automobile industry, and the manufacture of flexible and rigid foams, and coatings such as paints and varnishes. Although industrially important, organic isocyanates are significantly toxic and exposure to these chemicals causes respiratory-related diseases such as asthma, pulmonary emphysema and bronchitis. For example, the Bhopal gas tragedy, where over 500 thousand people were exposed and eight thousand or more people died within a short time of exposure and thousands of victims were partially injured, having severe health side effects due to the leakage of methyl isocyanate from the pesticide industry at Bhopal, India in 1984. Due to the severe physiological adverse effect of inhalation of isocyanates, regulatory organizations such as the National Institute for Occupational Safety and Health, Occupational Safety and Health Administration and American Conference of Governmental Industrial Hygienists have set occupational exposure limits for these compounds at 5 ppb (Time-Weighted Average TWA) for a full work shift and 20 ppb (TWA) for a short-range exposure limit. However, the estimation of isocyanate concentration in the working atmosphere is critical to protect workers from adverse health effects due to isocyanate's high reactivity, volatile nature and instability. To detect airborne isocyanate vapours in a workplace, several analytical techniques such as colorimetry, amperometry, capillary zone electrophoresis, high-performance liquid chromatography and fluorescence have been reported in the literature. Among them, fluorescent probes due to their high sensitivity and selectivity grabbed special attention for the determination of the isocyanate group. This review is an attempt to give a complete insight into fluorescent reagents for isocyanate detection with a chromatographic separation mode. |
I. Introduction
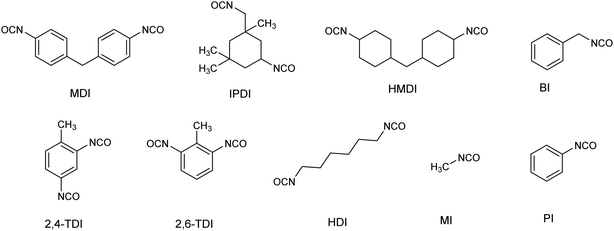
Organic isocyanates are a class of chemicals having the –NCO group attached to an organic moiety. Even though Wurtz synthesized the first organic isocyanate in 1849, the growth of the significant industrial and technical usage of isocyanates did not occur until 1937, when Bayer prepared polyurethane products from a reaction between toluene diisocyanate and polyfunctional alcohols. During the past three decades, various organic isocyanates have been widely used as building blocks to produce vital polymeric products such as polyurethane foams, synthetic rubbers, insulation materials, surface coatings, binders, and sealants.1–7 In the class of isocyanates, monoisocyanates (having one –NCO group) including methyl isocyanate (MI), phenyl isocyanate (PI) and benzyl isocyanate (BI) are mainly used as components for the production of agricultural and pharmaceutical products such as herbicides and pesticides.8–10 Diisocyanates (having two –NCO groups) including methylene bis(phenyl isocyanate) (MDI), toluene diisocyanate (TDI), hexamethylene diisocyanate (HDI), naphthalene diisocyanate (NDI), methylene bis-cyclohexyl isocyanate (HMDI) and isophorone diisocyanate (IPDI) are predominantly used as a curing agent during the production of several polymeric materials in the polymer industries. Particularly, TDI and IPDI are commonly used as curing agents during the production of HTPB based composite solid rocket motor propellant.11–13 The chemical structures and important physicochemical properties of selected organic isocyanates are shown in Fig. 1 and Table 1.| Isocyanates | M. wt | VP (mmHg) | Volatility | Form | PEL TWAd (ppb) |
|---|---|---|---|---|---|
| a VP – vapour pressure. b VP – at 20 °C. c VP – at 25 °C. d PEL – permissible exposure limit and TWA – 8 h work shift (OSHA). | |||||
| MI | 57.05 | 348b | Very high | Highly volatile liquid | 2 |
| HDI | 168.2 | 0.05c | High | Liquid | 5 |
| TDI | 174.2 | 0.01c | High | Liquid (mixture of 2,4 and 2,6-isomers) | 5 |
| IPDI | 222.3 | 0.0003b | Moderate | Liquid | 5 |
| MDI | 250.25 | 0.000005c | Very low | Low melting point solid | 5 |
Even though industrially important, organic isocyanates are significantly toxic and exposure to these chemicals may irritate the skin, mucous membranes, eyes, and respiratory tract.14–16 For example, the Bhopal gas tragedy, where over 500 thousand people were exposed and eight thousand or more people died within a short time of exposure and thousands of victims were partially injured, having severe health side effects due to the leakage of MI from the pesticide industry at Bhopal, India in 1984.17–19 The main adverse health effect associated with isocyanate exposure is respiratory-related diseases such as asthma, pulmonary emphysema and bronchitis.20–33 Due to the severe physiological adverse effect of inhalation of isocyanates, regulatory organizations such as the National Institute for Occupational Safety and Health (NIOSH), Occupational Safety and Health Administration (OSHA) and American Conference of Governmental Industrial Hygienists (ACGIH) have set occupational exposure limits (OELs) for these compounds at 5 ppb (Time-Weighted Average TWA) for a full work shift and 20 ppb (TWA) for a short-range exposure limit.34–36 However, the estimation of isocyanate concentration in the working atmosphere is critical due to its high reactivity, volatile nature and instability. Hence, various research activities have been carried out to develop rapid and sensitive analytical techniques for the detection of airborne organic isocyanates.
The most commonly used methods to detect and determine airborne isocyanates are the derivatization of isocyanates with an amine or hydroxyl agent at the time of sample collection and the analysis of the resulting derivatives by using various analytical techniques such as colorimetry, gas chromatography (GC), high-performance liquid chromatography (HPLC), capillary zone electrophoresis (CZE), proton transfer reaction mass spectrometry (PTR-MS) and fluorescence.37–39 Due to the high reactivity of isocyanates towards nucleophiles, the derivatization technique is found to be the best route for the detection and determination of isocyanates. The purpose of derivatization is to stabilize isocyanates and enhance their detection ability.40 Marcali et al. reported a colorimetric technique to determine derivatized isocyanates, which involves hydrolysis of isocyanates to amines, followed by derivatization such as diazotization.41,42 Though this technique is less expensive, it suffers from low sensitivity and is not suitable for aliphatic isocyanates as diazotization is specific only to aromatic isocyanates.43 Schanche et al. developed a detection technique by GC for TDI but the detection for a broader range of isocyanates could not be further explored.44 HPLC with amperometry and UV detection is a widely used technique for the quantification of derivatized isocyanates. However, the use of a non-selective UV detector and unstable amperometry detector result in low sensitivity and therefore is not a reliable approach.45
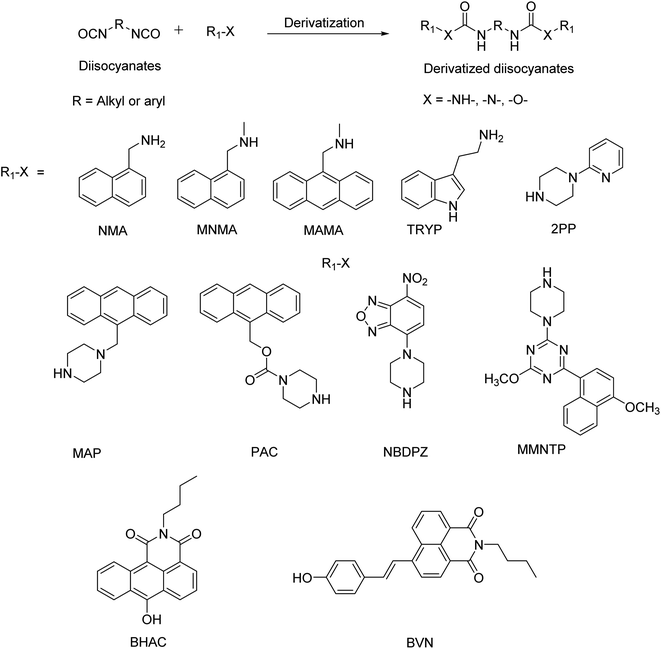
Rudzinski et al.46 used the CZE technique to determine HDI and its oligomers. Although this technique has five-fold sensitivity for HDI compared to that of HPLC, the repeatability of results was not achievable when there is a small variation in pH. Also, the capillary was found to be degraded within a short period of its usage with some of the derivatizing agents.46 Even though PTR-MS has good sensitivity towards the determination of isocyanates, the instrumental setup is highly expensive.47 Though the UV-Vis spectrophotometry technique is simple and available at any common laboratory, this technique exhibits low sensitivity for the determination of isocyanates.48 Though various derivatizing agents are available in the literature for the detection of airborne isocyanates, HPLC with the fluorescent detection technique is found to be more accurate and convenient for operation due to its high sensitivity and selectivity. Fluorescent probes based on small organic molecules, polymers and nanomaterials have become important tools in the field of cell imaging, tumour diagnosis, drug delivery and sensors because of their extreme sensitivity and excellent specificity.49–51 The Occupational Safety and Health Administration (OSHA) and National Institute for Occupational Safety and Health (NIOSH) were also using fluorescence derivatizing agents for the detection of isocyanates due to their high stability and high sensitivity. Specifically, tryptamine-NIOSH 5522, 1-(2-pyridyl)-piperazine (2PP) – OSHA 42/47, and 1-(9-anthracenylmethyl) piperazine (MAP)-NIOSH are widely used as per the NIOSH manual of analytical methods.52–55Table 2 summarizes the widely used fluorescent agents for the determination of isocyanates reported in the literature. The scheme of derivatization with fluorescent reagents is shown in Fig. 2. This review is an attempt to give a complete insight into fluorescent reagents for isocyanate detection with a chromatographic separation mode.
| Derivatization reagent | Detection mode | Detection limit | Isocyanates studied | Ref. |
|---|---|---|---|---|
| NMA | LC-fluorescence, LC-UV-Vis | 0.25 μg m−3 (10 ppt) per 20 L air sample/≈ 0.5 ng (3 ppt) per 10 μL sample injection volume | HDI, IPDI, Desmodur N | 57 |
| MNMA | LC-fluorescence, LC-UV-Vis | 0.001–0.015 mg m−3/0.14–0.44 ppb per 1 L air sample and 50 μL sample injection volume | TDI, MDI, HDI, IPDI, Mondur P, PI, Mondur TD-80, Multrathane M, Mondur HX, Desmodur N75 | 58 |
| MAMA | LC-fluorescence, LC-UV-Vis | 1 × 10−4 mg m−3 per 15 L air sample and 100 μL sample injection volume | 2,4 and 2,6 TDI, HDI, MDI | 59 |
| TRYP | LC-fluorescence, LC-ECD | 0.0005 ppm per 120 L air sample | MI, TDI, HDI, PMPPI, Mondur MR, Mondur CB-75 | 62 |
| 2PP | UV-Vis, fluorimeter | 64–67 mmol L−1 | 2,4 and 2,6 TDI, HDI, MDI | 65 |
| MAP | LC-fluorescence, LC-UV-Vis, LC-ECD | 31–124 μmol L−1 | 2,4-TDI, HDI, MDI, PI, BI | 67 |
| PAC | LC-fluorescence, LC-UV-Vis | 21–83 nmol mL−1 | 2,4-TDI, HDI, MDI, PI, BI | 69 |
| NBDPZ | LC-fluorescence, LC-UV-Vis, LC-MS–MS | 5–9 nmol L−1 | 2,4 and 2,6 TDI, HDI, MDI, MI, PI, ethyl, propyl, pentyl, hexyl and benzyl isocyanates | 70 |
| MMNTP | LC-fluorescence, LC-UV-Vis | 6.7–7.7 pmol per 30 L air sample and 20 μL sample injection volume | 2,4 and 2,6-TDI, HDI, MDI, PI | 72 |
| BHAC | Fluorimeter | 96 nM for CEI | TDI, IPDI, HDI, MDI, PI, CEI | 73 |
| BVN | Fluorimeter | 56 nM | EI | 74 |
II. Fluorescent reagents based on derivatization mode
II.a. Amine based
An amine-based derivatization technique with an N-(4-nitrobenzyl)-N-propylamine (NNNP) reagent followed by liquid chromatographic separation of the resulting urea derivatives for the detection of isocyanate species was developed by Dunlap et al.55 Later Hardy et al.56 explored liquid chromatographic separation using a UV detector with commercially available 1-(2-pyridyl) piperazine (2PP) as a derivatizing agent and found that the resulting urea derivatives with 2PP have significantly higher molar absorptivity than those derived from the NNNP reagent. Further, Levine et al.57 prepared a fluorescent reagent 1-naphthalenemethylamine (NMA), which form its fluorescent derivatives with isocyanates. These derivatives were analysed using reverse-phase isocratic LC with a fluorescence detector using an excitation wavelength of 216 nm (λex). Notably, this technique could able to detect 0.5 ng of aliphatic Desmodur N-[hexamethylene diisocyanate-biuret trimer (HDI-BT)] which was equivalent to 3 ppt in a 20 L air sample. This technique is found to be fifty times more sensitive than a previously reported NNNP based technique55 as NMA is found to react faster than NNNP with isocyanates. However, it was reported that NMA as a primary amine can cause side reactions after derivatization, which can affect the sensitivity of the analysis. To overcome the limitations of NMA, Kormos et al.58 synthesized methylated NMA, a secondary amine, with N-methyl-1-naphthalenemethylamine (MNMA) as a fluorescent reagent and this reagent was found to be versatile and chromatographic separation (LC) was also further improved. MNMA readily forms fluorescent active urea derivatives and exhibits higher sensitivity than NMA due to its fast reactivity with both aliphatic and aromatic isocyanates. However, it was reported that MNMA exhibits an impurity peak (oxidized product of MNMA) at an elution time of 1.6 min and the peak intensity increases gradually with time and exposure of light.Following the same path, C. Sango et al.59 developed an improved HPLC method with 9-(N-methylaminomethyl)-anthracene (MAMA) as a fluorescent and UV detection reagent for the detection and estimation of isocyanates. The MAMA-based urea derivative exhibits fluorescence as well as strong UV-absorption with high molar absorptivity at 254 nm. The sensitivity of this method was found to be ten to twenty-fold better than that of previously reported NNNP.55 By using MAMA, four different isocyanates 2,4-TDI, 2,6-TDI, HDI, and MDI were separated by both fluorescent and UV detectors with a detection limit of 1 × 10−4 mg m−3 in a 15 liter air sample.59 The total reactive isocyanate group (TRIG) is the total concentration of the isocyanate group, regardless of the molecule (monomer or oligomer or prepolymer) to which the isocyanate group is attached. TRIG was also evaluated by using MAMA for a series of 11 model isocyanate compounds including aliphatic, aromatic, mono-, and polyisocyanates. Derivatized products of these isocyanates were estimated by using reversed-phase HPLC with different detection modes i.e., fluorescence with λex-245 nm and λem-414 nm and UV absorbance at 245 and 370 nm. All these detection modes showed statistically significant differences for isocyanates when examined by the analysis of the variance and response factor. Here, the response factor is taken as the ratio between the integrated peak area and corresponding concentration. Fluorescence detection exhibits a higher response factor than UV detection at 245 nm and 370 nm.60
Rando et al. continued the work further to determine the airborne total reactive isocyanate group (TRIG), which is essential to protect the working atmosphere by proper quantification of isocyanate leaching during polyurethane formation.61 It was demonstrated that the MAMA reagent was successfully able to quantify TRIG using reverse-phase HPLC with fluorescence and UV detection modes in the products of polyurethane and oligomer precursors such as Lupranate M20S (40% MDI monomer & 60% MDI oligomer), CMDI (58–68% MDI monomer & remaining MDI oligomer), poly[methylene (polyphenyl isocyanate)] (PMPPI-oligomeric form of MDI), Desmodur N100 (HDI homopolymer, HDI-BT & 1.6% HDI), Imron paint activator192S (33% HDI oligomer) and Rexthane (TDI monomer and prepolymer). TRIG estimation was successfully achieved by using MAMA in all six different oligomers or prepolymer precursors using a UV response ratio of λ-245/370 nm and quantitative recoveries have been observed in comparison with a reference titrimetric procedure. However, one of the disadvantages of this technique is the presence of phosgene, which causes significant positive interference in the determination of TRIG using the MAMA reagent since phosgene also reacts readily with MAMA.61
Wu et al.62 investigated tryptamine 3-(2-aminoethyl) indole (TRYP) as the derivatizing agent for isocyanate determination with fluorescence (λex = 275 nm and λem = 320 nm) and amperometry detectors. Both fluorescence and amperometric oxidation of tryptamine are induced by the conjugated π-system of the indolyl moiety, which is located two carbon atoms away from the derivatising site of the amino group. Hence, the p-orbital (or orbitals) of isocyanates after derivatisation would not overlap with the indolyl π-system and hence the electronic structure of the π-system is not perturbed and hence the quantum efficiency of TRYP remains unchanged. The tryptamine derivatizing agent was specifically used to determine methyl isocyanate (MI) and the derivatized product (TRYP-MI) was characterized by 1H-NMR, which indicates the presence of a signature doublet peak of –CH3 at 2.8–3.3 ppm. Amperometry measurements of TRYP-MI in comparison with the existing 1-(2-methoxyphenyl) piperazine (MPP-MI) yield almost comparable results.63 However, LC based fluorescence detection of TRYP-MI was 30 times more sensitive than that of MPP-MI and found to detect less than 1 ng of TRYP-MI. Further, a stability study indicates no apparent loss of TRYP observed even after 35 days under the experimental conditions.62 As a continuation of previous work, Wu et al.64 studied TRYP derivatization with TDI, MDI, HDI and PMPPI and characterized it by using IR spectra which exhibit an IR band at around 1610 cm−1, which represents characteristic carbonyl stretching of the urea functional group. All the derivatized products formed were quantified using fluorescence and amperometry techniques efficiently. Further Wu et al.65 also studied the competitive derivatization rate of TRYP with other amine-based derivatizing agents like N-methyltryptamine (NMTP), PP, MPP and NNNP. The results indicate that a much smaller amount of TRYP or MPP is required than that of NNNP to efficiently derivatize an equivalent amount of isocyanate, which is beneficial in the HPLC system if TRYP/MPP were to be used.
Salthammer et al.66 developed a versatile fluorescent reagent 1-(2-pyridyl)-piperazine (2PP) for the determination of isocyanates. Urea derivatives of 2PP exhibit a high molar absorption coefficient and fluorescence quantum yield of 0.14–0.21 at 20 °C and display a small variation of the solvatochromic effect on absorption and fluorescence spectra. The effects of heat and light on the spectroscopic properties of these products were also studied. It is observed that these derivatives are stable in methanolic solution for more than 200 hours under dark conditions but found to be highly sensitive to temperature. Specifically, MDI-2PP and HDI-2PP exhibit a decreased quantum yield of about 10% for the temperature rise from 20 °C to 30 °C. It was shown that the absorption of 2,6-TDI-2PP is slightly blue-shifted in non-polar n-hexane and fluorescence spectra show a slight bathochromic shift because of the increased solvent polarity. This effect is stronger for only 2PP (356 nm-n-hexane; 382 nm-acetonitrile) but less pronounced for the urea derivative 2,6-TDI-2PP (355 nm-n-hexane; 369 nm-acetonitrile). Also, 2PP urea derivatives were compared with derivatives with similar structured 2-pyridinamine for variation in solvent polarity. The addition of a small amount of ethanol to 2-pyridinamine in isooctane results in notable variation in the shape and intensity of spectra due to hydrogen bond formation, and such an effect is not observed in 2PP due to the steric hindrance originating from the piperazine ring.66
A novel fluorescent derivatizing agent, 1-(9-anthracenylmethyl) piperazine (MAP) was synthesized by Streicher et al.67 to detect and estimate non-monomeric isocyanates. The effectiveness of MAP towards isocyanate derivatization was studied and compared with that of existing three different fluorescent agents MPP, MAMA and TRYP. The relative reactivity of phenyl isocyanate with MAP and MPP is almost comparable since the reactive piperazine group remains the same. It was observed that MAP reacts 4 times and 3.3 times faster than TRYP and MAMA, respectively. It was also reported that the fluorescence intensity of the derivatives of mono-isocyanates (BI and PI) of MAP and MAMA was higher than that of the derivatives of di-isocyanates (HDI, TDI, and MDI). This may be due to the intramolecular quenching in the diisocyanate ureas of MAP and MAMA. However, such an inference was not observed in the case of TRYP urea. The urea derivatives of MPP and MAP with five different isocyanates are subsequently analysed by HPLC with UV and EC detection. The average UV response of MAP urea was 4.8 times greater than that of MPP urea, but the EC response was slightly less than that of MPP urea. The replacement of the secondary amine group present in MAMA with piperazine results in MAP, which is highly reactive and more sensitive than MAMA towards isocyanates.67
Wang et al.68 described a new strategy for fluorometric determination of methyl isocyanate (MI) in air, which involves the degradation of MI to corresponding methylamine. Methylamine thus obtained reacts with acetylacetone and formaldehyde at a pH of 5.6 (sodium acetate buffer solution) to form a fluorescent compound, N-methyl-2,6-dimethyl-3,5-diacetyl-1,4-dihydropyridine. A fluorescence study was carried out at λex-404 nm and λem-474 nm. The effect of pH over the formation of a fluorescent compound was studied by varying pH from 2 to 10. It is observed that the fluorescence intensity was found to be maximum at λem-474 nm at pH 5–6 and low at pH 2–4. Also, a heating time of ∼16 min was found to be optimum to get maximum fluorescence intensity. However, heating beyond 16 min causes degradation of the compound, while a low heating time results in low fluorescence intensity as the time is not sufficient for the reaction to yield the fluorescence compound. The presence of foreign ions (Na+, K+, Mg2+, Ca2+, Cu2+, Ni2+, F−, Cl−, Br− and NO3−) was studied and found that foreign ions significantly cause positive interference in the determination of MI.68 Though this technique is highly sensitive, it is not further explored for the study of different isocyanates, since the formation of fluorescent compounds highly depends on pH, temperature, and foreign ions.
Roh et al.69 synthesized 9-anthracenylmethyl 1-piperazinecarboxylate (PAC) and developed a technique for the quantification of non-monomeric isocyanates with a single analyte peak in HPLC, which can overcome various limitations of traditional methods. In this technique, isocyanate species were derivatized with 9-anthracenylmethyl 1-piperazinecarboxylate (PAC) and subjected to a cleavage reaction with sodium thiomethoxide. The cleavage product 9-anthracenylmethyl methyl sulfide (AMMS) was estimated by HPLC as a single analyte peak. Using this strategy, all non-monomeric isocyanates are converted to a single analyte and the problem of variable response factors using different species is thus prevented. Hence by using this technique, the authors successfully separated a single AMMS analyte peak in HPLC and proposed a technique for the quantification of the total isocyanate group in polymeric isocyanates also. PAC was efficiently used to derivatize BI, PI, HDI, TDI, and MDI followed by a cleavage reaction and a single analyte peak as well as urea derivatives of PAC were separated by HPLC using a fluorescence/UV detector. This technique can also be applicable for the estimation of the surface bound free isocyanate group in the polyurethane. The relative kinetics of the reaction of phenyl isocyanate with PAC and MAP were studied and it was found that PAC is less reactive than MAP with a relative rate factor of 12.6 for MAP/PAC. The PAC usage was limited because the cleavage product of AMMS is not exactly specific for isocyanate-PAC derivatives,69 since AMMS can also be originated from the degradation of PAC or reaction product of PAC with solvent impurities.
Vogel et al.70,71 described a versatile method for the determination of mono- and di-isocyanates in air samples with UV-visible and fluorescence detection with a new reagent 4-nitro-7-piperazino-2,1,3-benzoxadiazole (NBDPZ). The urea derivatives of NBDPZ were separated by employing reverse phase HPLC and exhibited the red-shifted absorption maximum in UV and fluorescence detectors (UV/Vis – λmax-480 nm; fluorescence-λex-470 nm and λem-535 nm). NBDPZ displays increased selectivity compared with MAMA and MPP. The rate of reaction of NBDPZ with 2,6-TDI was found to be ≈ 3 times faster than that of MAMA-2,6-TDI. Also, the determination of isocyanates in the gas phase using impinge sampling methods with NBDPZ was performed and it was found that the aromatic isocyanate recoveries were in the range of 79–95%. On the other hand, aliphatic propyl isocyanate recovery was significantly lower because of the low reactivity towards NBDPZ compared to aromatic isocyanates. The LOD of MDI using NBDPZ in fluorescence detection mode was found to be 5–9 nmol L−1 for the individual isocyanate derivatives.70 Further, NBDPZ coated octadecyl modified silica (ODS)-filled glass tubes were used for the estimation of isocyanates and found that the 2,4-TDI recovery was in the range of 86–99%.70 To investigate the practical application of the NBDPZ test tube method for the determination of isocyanates, leaching resulting from polyurethane foams (PUF) based on MDI was studied. The non-heated PUF results in no MDI emission, whereas the thermally treated samples revealed a significant release of residual monomeric MDI. Despite numerous advantages and the applicability of NBDPZ for isocyanate detection, the stability of NBDPZ in the solution is very low. NBDPZ in solution under refrigerated conditions over two weeks exhibits a decreased peak area from 100% to 80% of its initial value. Further, the peak area is found to be decreased down to 41% at room temperature in the dark and 27% at RT under daylight. However, storage of the solid in the refrigerator yielded no degradation of NBDPZ for six months.70
Werlich et al.72 developed a technique with a newly synthesized derivatizing reagent 4-methoxy-6-(4-methoxy-1-naphthyl)-1,3,5-triazine-2-(1-piperazine) (MMNTP) for isocyanate determination. MMNTP was successfully used to derivatize five different isocyanates (PI, HDI, 2,4-TDI, 2,6-TDI, and MDI) and all five isocyanates separated by the HPLC method using UV (λmax-325 nm) and fluorescence detection mode (λex-325 nm and λem-405 nm). The MMNTP reacts faster with aromatic isocyanates, but aliphatic isocyanates require a minimum of 2 h incubation. Also, Werlich et al. demonstrated that the MMNTP reagent is found to have good spectroscopic properties with low detection levels in the range of pmol for both UV and fluorescence detection.72 The MMNTP reagent was used for an air sampling system for the real sample DESMODUR T80. The technique involves a glass tube filled with MMNTP coated chromosorb as a backup and a sampling layer with a pump flow of 1 L min−1. Using this technique, the analyte peaks are efficiently separated in the sampling layer along with additional peaks, which originate from the impurities of diisocyanates of the technical mixture.72
II.b. Hydroxyl based
Gao et al.73 reported the first hydroxyl-based fluorescent reagent N-(n-butyl)-6-hydroxy-anthracene carboxamide (BHAC) for direct detection of isocyanates in the literature. This ratio-metric fluorescent reagent with dual fluorescence intensity is highly efficient compared to the single fluorescent intensity probe. The authors also demonstrated sensing of BHAC coated test paper, which can detect six different commercially available aromatic and aliphatic isocyanates. Further, Chen et al.74 developed a single-fluorophore-based ratio-metric fluorescent reagent N-buty-4-(4-vinyl phenol)-1,8-naphthalimide (BVN) to detect gaseous isocyanates quantitatively based on the intramolecular charge transfer (ICT) mechanism. Isocyanate group interaction with BVN by using frontier molecular orbital (FMO) theory was studied. Also, proton NMR study exhibits the incorporation of the ethyl group from ethyl isocyanate in the BVN.74 However, the combination of chromatographic separation with fluorescence detection has not been explored yet, which can further enhance the sensitivity.III. Based on non-derivatization mode
Although the derivatization of isocyanates is considered instantaneous, some of the isocyanate derivatizations take several hours to complete. In this time lag, isocyanate undergoes evaporation in the working atmosphere. According to OSHA and NIOSH methods, approximately 5% of isocyanates are lost due to the slow reaction. To overcome these issues, Ghosh et al. reported a conjugated polymer-based technique, which does not involve derivatization. A conjugated polymer was synthesized using pentiptycene and tetraphenylethylene units linked by acetylene and used for direct detection of isocyanates in air.75 It was reported that the conjugated polymer could be able to detect eight industrially available aliphatic and aromatic isocyanates with a detection limit of parts per trillion level (ppt), which is even lower than its occupational exposure limit. The isocyanate detection depends on its vapour pressure. The detection of isocyanates by this polymer is mainly due to the transfer of excited energy to the electron-deficient di-isocyanates.75IV. Conclusion
Various fluorescent derivatizing agents with analytical separation have been summarized and discussed for the detection and quantification of isocyanates. Especially amine-based fluorescent reagents TRYP, MAMA, 2PP, MAP, NBDPZ and MMNTP display high sensitivity towards isocyanates. The relative reactivity of these reagents towards isocyanates is well discussed. Further MAMA and TRYP were specifically used to determine TRIG in the polyurethane precursors and oligomers apart from monomeric isocyanates. Also, TRYP, 2PP, and MAP are also found to be used in the NIOSH manual of analytical methods for isocyanate quantification due to their high sensitivity and stability. On the other hand, hydroxyl-based reagents BHAC and BVN are equivocally efficient as amine-based fluorescent reagents and used to estimate different isocyanates using a fluorimeter. Chromatographic separation with fluorescence detection needs to be further explored in such cases. A conjugated polymer was used for the detection of isocyanates with high selectivity and sensitivity. Such a polymer can be effectively used to determine an ultra-low concentration of isocyanates in non-derivatization mode. In conclusion, still there is scope or a need for the development of different materials, based on polymers or nanomaterials for efficient determination and implementation in the practical application of airborne isocyanate determination.Abbreviations
| PTR-MS | Proton transfer reaction mass spectrometry |
| HPLC | High-performance liquid chromatography |
| MDI | Methylene bis(phenyl isocyanate) |
| TDI | Toluene diisocyanate |
| HDI | Hexamethylene diisocyanate |
| NDI | Naphthalene diisocyanate |
| HMDI | Methylene bis-cyclohexyl isocyanate |
| IPDI | Isophorone diisocyanate |
| HTPB | Lydroxyl terminated polybutadiene |
| MI | Methyl isocyanate |
| NIOSH | National Institute for Occupational Safety and Health |
| OSHA | Occupational Safety and Health Administration |
| ACGIH | American Conference of Governmental Industrial Hygienists |
| OELs | Occupational exposure limits |
| TWA | Time-weighted average |
| TRIG | Total reactive isocyanate group |
| CZE | Capillary zone electrophoresis |
| NNNP | N-(4-Nitrobenzyl)-N-propylamine |
| 2PP | 1-(2-Pyridyl) piperazine |
| NMA | 1-Naphthalenemethylamine |
| HDI-BT | Hexamethylene diisocyanate-biuret trimer |
| MNMA | N-Methyl-1-naphthalenemethylamine |
| MAMA | 9-(N-Methy1aminomethyl)-anthracene |
| TRYP | 3-(2-Aminoethyl) indole |
| PMPPI | Poly[methylene(polyphenyl isocyanate)] |
| MPP | 1-(2-Methoxyphenyl) piperazine |
| MAP | 1-(9-Anthracenylmethyl) piperazine |
| BI | Benzyl isocyanate |
| PI | Phenyl isocyanate |
| PAC | 9-Anthracenylmethyl 1-piperazinecarboxylate |
| AMMS | 9-Anthracenylmethyl methyl sulfide |
| NBDPZ | 4-Nitro-7-piperazino-2,1,3-benzoxadiazole |
| ODS | Octadecyl modified silica |
| PUF | Polyurethanes |
| MMNTP | 4-Methoxy-6-(4-methoxy-1-naphthyl)-1,3,5-triazine-2-(1-piperazine) |
| BHAC | N-(n-butyl)-6-hydroxy-anthracene carboxamide |
| BVN | N-Buty-4-(4-vinyl phenol)-1,8-naphthalimide |
| ICT | Intramolecular charge transfer |
| FMO | Frontier molecular orbital |
Conflicts of interest
There are no conflicts to declare.References
- S. M. Mitchell, K. A. N. Sachinthani, R. Pulukkody and E. B. Pentzer, Erratum: 100th anniversary of macromolecular science viewpoint: Polymerization of cumulated bonds: Isocyanates, allenes, and ketenes as monomers, ACS Macro Lett., 2020, 9(9), 1217, DOI:10.1021/acsmacrolett.0c00552.
- L. Hojabri, X. Kong and S. S. Narine, Novel long chain unsaturated diisocyanate from fatty acid: Synthesis, characterization, and application in bio-based polyurethane, J. Polym. Sci., Part A: Polym. Chem., 2010, 48(15), 3302–3310, DOI:10.1002/pola.24114.
- E. Sharmin and F. Zafar, Polyurethane: An Introduction, in: Polyurethane, 2012, DOI:10.5772/51663.
- J. O. Akindoyo, M. D. H. Beg, S. Ghazali, M. R. Islam, N. Jeyaratnam and A. R. Yuvaraj, Polyurethane types, synthesis and applications-a review, RSC Adv., 2016, 6, 114453–114482, 10.1039/c6ra14525f.
- G. A. Skarja and K. A. Woodhouse, Structure–property relationships of degradable polyurethane elastomers containing an amino acid-based chain extender, J. Appl. Polym. Sci., 2000, 75(12), 1522–1534, DOI:10.1002/(SICI)1097-4628(20000321)75:12<1522::AID-APP11>3.0.CO;2-A.
- M. S. Gaikwad, V. v. Gite, P. P. Mahulikar, D. G. Hundiwale and O. S. Yemul, Eco-friendly polyurethane coatings from cottonseed and karanja oil, Prog. Org. Coat., 2015, 86, 164–172, DOI:10.1016/j.porgcoat.2015.05.014.
- G. H. Kulkarni, R. H. Naik, S. K. Tandel and S. Rajappa, Contra-thermodynamic trans-esterification of carbamates by counter-attack strategy: A viable non-phosgene, non-mic route to carbamate pesticides, Tetrahedron, 1991, 47(7), 1249–1256, DOI:10.1016/S0040-4020(01)86381-X.
- J. Mráz, P. Šimek, D. Chvalová, H. Nohová and P. Šmigolová, Studies on the methyl isocyanate adducts with globin, Chem.-Biol. Interact., 2004, 148(1–2), 1–10, DOI:10.1016/j.cbi.2003.06.003.
- C. S. Senthilkumar, N. K. Sah and N. Ganesh, Methyl Isocyanate and Carcinogenesis: Bridgeable Gaps in Scientific Knowledge, Asian Pac. J. Cancer Prev., 2012, 13(6), 2429–2432, DOI:10.7314/APJCP.2012.13.6.2429.
- T. Matsukawa, K. Yokoyama and H. Itoh, Ocular irritation from product of pesticide degradation among workers in a seed warehouse, Ind. Health, 2015, 53(1), 95–99, DOI:10.2486/indhealth.2014-0147.
- K. Gnanaprakash and S. R. Chakravarthy, Effect of curing agent on the plateau burning mechanism of solid propellant sandwiches, J. Propul. Power, 2018, 34(6), 1442–1454, DOI:10.2514/1.B36978.
- V. N. Krishnamurthy and S. Thomas, ISRO Polyl – The versatile binder for composite solid propellants for launch vehicles and missiles, Def. Sci. J., 1987, 37(1), 29–37, DOI:10.14429/dsj.37.5889.
- A. K. Mahanta, I. Dharmsaktu and P. K. Pattnayak, Rheological behaviour of HTPB-based composite propellant: Effect of temperature and pot life on casting rate, Def. Sci. J., 2007, 57(4), 435–442, DOI:10.14429/dsj.57.1791.
- Q. Liu and A. v. Wisnewski, Recent developments in diisocyanate asthma, Ann. Allergy Asthma Immunol., 2003, 90(5), 35–41, DOI:10.1016/S1081-1206(10)61647-X.
- X. Baur, W. Marek, J. Ammon, A. B. Czuppon, B. Marczynski, M. Raulf-Heimsoth, H. Roemmelt and G. Fruhmann, Respiratory and other hazards of isocyanates, Int. Arch. Occup. Environ. Health, 1994, 66(3), 141–152, DOI:10.1007/BF00380772.
- J. D. Schroeter, J. S. Kimbell, B. Asgharian, E. W. Tewksbury, M. Sochaski, M. L. Foster, D. C. Dorman, B. A. Wong and M. E. Andersen, Inhalation dosimetry of hexamethylene diisocyanate vapor in the rat and human respiratory tracts, Inhalation Toxicol., 2013, 25(3), 168–177, DOI:10.3109/08958378.2013.768314.
- R. Varma and D. R. Varma, The Bhopal disaster of 1984, Bull. Sci. Technol. Soc., 2005, 25(1), 37–45, DOI:10.1177/0270467604273822.
- E. Broughton, The Bhopal disaster and its aftermath: A review, Environ. Health, 2005, 4, 6, DOI:10.1186/1476-069X-4-6.
- P. K. Gupta, Pesticide exposure – Indian scene, Toxicology, 2004, 198(1–3), 83–90, DOI:10.1016/j.tox.2004.01.021.
- S. M. Tarlo and G. M. Liss, Diisocyanate-induced asthma: Diagnosis, prognosis, and effects of medical surveillance measures, Appl. Occup. Environ. Hyg., 2002, 17(12), 902–908, DOI:10.1080/10473220290107101.
- K. M. Bodner, C. J. Burns, N. M. Randolph and E. J. Salazar, A longitudinal study of respiratory health of toluene diisocyanate production workers, J. Occup. Environ. Med., 2001, 43(10), 890–897, DOI:10.1097/00043764-200110000-00008.
- Y. S. Shin, M. A. Kim, L. D. Pham and H. S. Park, Cells and mediators in diisocyanate-induced occupational asthma, Curr. Opin. Allergy Clin. Immunol., 2013, 13(2), 125–131, DOI:10.1097/ACI.0b013e32835e0322.
- R. L. Prueitt, H. N. Lynch, K. Zu, L. Shi and J. E. Goodman, Dermal exposure to toluene diisocyanate and respiratory cancer risk, Environ. Int., 2017, 109, 181–192, DOI:10.1016/j.envint.2017.09.017.
- B. Marczynski, A. B. Czuppon, G. H. Schreiber, W. Marek and X. Baur, DNA double-strand breaks and apoptosis after in vitro exposure to toluene diisocyanate, Toxicol. in Vitro, 1993, 7(4), 531–535, DOI:10.1016/0887-2333(93)90060-I.
- M. Littorin, S. Hou, K. Broberg, J. Björk, S. Fät, G. Abdoulaye, M. Kalemba, C. Ryk and S. Skerfving, Influence of polymorphic metabolic enzymes on biotransformation and effects of diphenylmethane diisocyanate, Int. Arch. Occup. Environ. Health, 2008, 81, 429–441, DOI:10.1007/s00420-007-0232-x.
- D. Bello, S. R. Woskie, R. P. Streicher, Y. Liu, M. H. Stowe, E. A. Eisen, M. J. Ellenbecker, J. Sparer, F. Youngs, M. R. Cullen and C. A. Redlich, Polyisocyanates in occupational environments: A critical review of exposure limits and metrics, Am. J. Ind. Med., 2004, 46(5), 480–491, DOI:10.1002/ajim.20076.
- P. Grandjean and J. Lintrup, Life-threatening pulmonary reaction to car paint containing a prepolymerized isocyanate, Scand. J. Work Environ. Health, 1981, 7(4), 310–311, DOI:10.5271/sjweh.2543.
- P. Séguin, A. Allard, A. Cartier and J. L. Malo, Prevalence of occupational asthma in spray painters exposed to several types of isocyanates, including polymethylene polyphenylisocyanate, J. Occup. Med., 1987, 29(4), 340–344 Search PubMed , http://www.jstor.org/stable/45013810.
- J. Nielsen, C. Sango, G. Winroth, T. Hallberg and S. Skerfving, Systemic reactions associated with polyisocyanate exposure, Scand. J. Work Environ. Health, 1985, 11(1), 51–54, DOI:10.5271/sjweh.2253.
- W. E. Rudzinski, B. Dahlquist, S. A. Svejda, A. Richardson and T. Thomas, Sampling and analysis of isocyanates in spray-painting operations, Am. Ind. Hyg. Assoc. J., 1995, 56(3), 284–289, DOI:10.1080/15428119591017132.
- O. Vandenplas, A. Cartier, J. Lesage, G. Perrault, L. C. Grammer and J. L. Malo, Occupational asthma caused by a polymer but not the monomer of toluene diisocyanate (TDI), J. Allergy Clin. Immunol., 1992, 89(6), 1183–1188, DOI:10.1016/0091-6749(92)90303-J.
- O. Vandenplas, A. Cartier, J. Lesage, Y. Cloutier, G. Perreault, L. C. Grammer, M. A. Shaughnessy and J. L. Malo, Prepolymers of hexamethylene diisocyanate as a cause of occupational asthma, J. Allergy Clin. Immunol., 1993, 91(4), 850–861, DOI:10.1016/0091-6749(93)90342-D.
- B. Scholten, L. Kenny, R. C. Duca, A. Pronk, T. Santonen, K. S. Galea, M. Loh, K. Huumonen, A. Sleeuwenhoek, M. Creta, L. Godderis and K. Jones, Biomonitoring for Occupational Exposure to Diisocyanates: A Systematic Review, Ann. Work Exposures Health, 2020, 64(6), 569–585, DOI:10.1093/annweh/wxaa038.
- C. J. Sennbro, M. Littorin, H. Tinnerberg and B. A. G. Jönsson, Upper reference limits for biomarkers of exposure to aromatic diisocyanates, Int. Arch. Occup. Environ. Health, 2005, 78, 541–546, DOI:10.1007/s00420-005-0619-5.
- J. A. Hathaway, D. M. Molenaar, L. D. Cassidy, T. M. Feeley and B. J. Cummings, Cross-sectional survey of workers exposed to aliphatic diisocyanates using detailed respiratory medical history and questions regarding accidental skin and respiratory exposures, J. Occup. Environ. Med., 2014, 56(1), 52–57, DOI:10.1097/JOM.0000000000000019.
- R. J. Rando and H. G. Poovey, Development and application of a dichotomous vapor/aerosol sampler for HDI-derived total reactive isocyanate group, Am. Ind. Hyg. Assoc. J., 1999, 60(6), 737–746, DOI:10.1080/00028899908984496.
- P. A. Goldberg, R. F. Walker, P. A. Ellwood and H. L. Hardy, Determination of trace atmospheric isocyanate concentrations by reversed-phase high-performance liquid chromatography using 1-(2-pyridyl)piperazine reagent, J. Chromatogr. A, 1981, 212(1), 93–104, DOI:10.1016/S0021-9673(00)80550-6.
- R. P. Streicher, C. M. Reh, R. J. Key-Schwartz, P. C. Schlecht, M. Ellen, C. Paula and F. O’Connor, Determination of airborne isocyanate exposure: Considerations in method selection, Am. Ind. Hyg. Assoc. J., 2000, 61(4), 544–556, DOI:10.1080/15298660008984567.
- M. L. Henriks-Eckerman, J. Valimaa and C. Rosenberg, Determination of airborne methyl isocyanate as dibutylamine or 1-(2-methoxyphenyl)piperazine derivatives by liquid and gas chromatography, Analyst, 2000, 125, 1949–1954, 10.1039/b005388k.
- H. Henneken, M. Vogel and U. Karst, Determination of airborne isocyanates, Anal. Bioanal. Chem., 2007, 387, 219–236, DOI:10.1007/s00216-006-0901-8.
- K. Marcali, Microdetermination of Toluenecliisocyanates in Atmosphere, Anal. Chem., 1957, 29, 552–558, DOI:10.1021/ac50162a039.
- K. E. Grim and A. L. Linch, Recent Isocyanate-in-Air Analysis Studies, Am. Ind. Hyg. Assoc. J., 1964, 25, 285–290, DOI:10.1080/00028896409342589.
- C. J. Purnell and R. F. Walker, Methods for the determination of atmospheric organic isocyanates a review, Analyst, 1985, 110(8), 893–905, 10.1039/AN9851000893.
- G. W. Schanche and E. R. Hermann, Micrograms of TDI by Chromatography, Am. Ind. Hyg. Assoc. J., 1974, 35(1), 47–52, DOI:10.1080/0002889748507005.
- W. E. Rudzinski, Determination of hexamethylene-based isocyanates in spray-painting operations Part 1. Evaluation of a polyurethane foam sponge sampler, Analyst, 1998, 123, 2079–2083, 10.1039/a803942i.
- W. E. Rudzinski, J. Yin, E. England and G. Carlton, Determination of hexamethylene diisocyanate-based isocyanates in spray-painting operations. Part 2. Comparison of high performance liquid chromatography with capillary zone electrophoresis, Analyst, 1999, 124, 119–123, 10.1039/a806351f.
- D. Gylestam, D. Karlsson, M. Dalene and G. Skarping, Determination of Gas Phase Isocyanates Using Proton Transfer Reaction Mass Spectrometry, Anal. Chem. Lett., 2011, 1(4), 261–271, DOI:10.1080/22297928.2011.10648228.
- J. Bartos and M. Pesez, Spectrophotometric and fluorimetric determination of amines, Pure Appl. Chem., 1984, 56(4), 467–477, DOI:10.1351/pac198456040467.
- M. Gao, F. Yu, C. Lv, J. Choo and L. Chen, Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy, Chem. Soc. Rev., 2017, 46, 2237–2271, 10.1039/c6cs00908e.
- X. Song, S. Bai, N. He, R. Wang, Y. Xing, C. Lv and F. Yu, Real-Time Evaluation of Hydrogen Peroxide Injuries in Pulmonary Fibrosis Mice Models with a Mitochondria-Targeted Near-Infrared Fluorescent Probe, ACS Sens., 2021, 6(3), 1228–1239, DOI:10.1021/acssensors.0c02519.
- F. Yu, P. Li, B. Wang and K. Han, Reversible near-infrared fluorescent probe introducing tellurium to mimetic glutathione peroxidase for monitoring the redox cycles between peroxynitrite and glutathione in vivo, J. Am. Chem. Soc., 2013, 135(20), 7674–7680, DOI:10.1021/ja401360a.
- W. S. Wu, R. S. Szklar and R. Smith, Application of tryptamine as a derivatizing agent for the determination of airborne isocyanates. Part 7. Selection of impinger solvents and the evaluation against dimethyl sulfoxide used in US NIOSH regulatory method 5522, Analyst, 1997, 122, 321–323, 10.1039/a606136b.
- S. Aubin, E. M. Hamdi, A. Joly, P. Sarazin, J. Lesage, L. Breau, M. Spence and S. Gagné, On site comparison of the OSHA 42, Asset EZ4-NCO, Iso-Chek, DAN and CIP10 methods for measuring toluene diisocyanate (TDI) at a polyurethane foam factory, J. Occup. Environ. Hyg., 2020, 17(5), 207–219, DOI:10.1080/15459624.2020.1731518.
- D. Bello, R. P. Streicher and S. R. Woskie, Evaluation of the NIOSH draft method 5525 for determination of the total reactive isocyanate group (TRIG) for aliphatic isocyanates in autobody repair shops, J. Environ. Monit., 2002, 4, 351–360, 10.1039/b110613a.
- K. L. Dunlap, R. L. Sandridge and J. Keller, Determination of Isocyanates in Working Atmospheres by High Speed Liquid Chromatography, Anal. Chem., 1976, 48(3), 497–499, DOI:10.1021/ac60367a043.
- H. L. Hardy and R. F. Walker, Novel reagent for the determination of atmospheric isocyanate monomer concentrations, Analyst, 1979, 104, 890–891, 10.1039/an9790400890.
- S. P. Levine, J. H. Hoggatt, E. Chladek, G. Jungclaus and J. L. Gerlock, Determination of Aliphatic Isocyanates in Air by a Liquid Chromatographic-Fluorescence Technique, Anal. Chem., 1979, 51(8), 1106–1109, DOI:10.1021/ac50044a003.
- L. H. Kormos, R. L. Sandridge and J. Keller, Determination of Isocyanates in Air by Liquid Chromatography with Fluorescence Detection, Anal. Chem., 1981, 53(7), 1122–1125, DOI:10.1021/ac00230a046.
- C. Sangö and E. Zimerson, A new reagent for determination of isocyanates in working atmospheres by hplc using uv or fluorescence detection, J. Liq. Chromatogr., 1980, 3(7), 971–990, DOI:10.1080/01483918008060208.
- R. J. Rando, H. G. Poovey, J. J. Lefante and F. R. Esmundo, Evaluation of 9-methylamino-methylanthracene as a Chemical Label for Total Reactive Isocyanate Group: A Comparison of mono- and di-isocyanate Monomers, J. Liq. Chromatogr., 1993, 16(18), 3977–3996, DOI:10.1080/10826079308019681.
- R. J. Rando, H. G. Poovey and R. A. Gibson, Evaluation of 9-methylamino-methylanthracene as a chemical label for total reactive isocyanate group: Application to isocyanate oligomers, polyurethane precursors, and phosgene, J. Liq. Chromatogr., 1995, 18(14), 2743–2763, DOI:10.1080/10826079508009322.
- W. S. Wu, M. A. Nazar, V. S. Gaind and L. Calovini, Application of tryptamine as a derivatising agent for airborne isocyanates determination part 1. Model of derivatisation of methyl isocyanate characterised by fluorescence and amperometric detection in high-performance liquid chromatography, Analyst, 1987, 112(6), 863–866, 10.1039/AN9871200863.
- D. A. Bagon, C. J. Warwick and R. Brown, Evaluation of Total Isocyanate-in-Air Method Using 1-(2-Methoxyphenyl)piperazine and HPLC, Am. Ind. Hyg. Assoc. J., 1984, 45(1), 39–43, DOI:10.1080/15298668491399334.
- W. S. Wu, R. E. Stoyanoff, R. S. Szklar, V. S. Gaind and M. Rakanovic, Application of tryptamine as a derivatising agent for airborne isocyanate determination: Part 3. Evaluation of total isocyanates analysis by high-performance liquid chromatography with fluorescence and amperometric detection, Analyst, 1990, 115, 801–807, 10.1039/AN9901500801.
- W. S. Wu, R. E. Stoyanoff and V. S. Gaind, Application of tryptamine as a derivatizing agent for airborne isocyanate determination. Part 4. Evaluation of major high-performance liquid chromatographic methods regarding airborne isocyanate determination with specific investigation of the competitive rate of derivatization, Analyst, 1991, 116, 21–25, 10.1039/AN9911600021.
- T. Salthammer, C. Wismach and H. Miertzsch, Absorption and fluorescence of 1-(2-pyridyl)-piperazine and four diisocyanate derivatives in solution, J. Photochem. Photobiol., A, 1997, 107(1–3), 159–164, DOI:10.1016/S1010-6030(96)04610-2.
- R. P. Streicher, J. E. Arnold, M. K. Ernst and C. v. Cooper, Development of a novel derivatization reagent for the sampling and analysis of total isocyanate group in air and comparison of its performance with that of several established reagents, Am. Ind. Hyg. Assoc. J., 1996, 57(10), 905–913, DOI:10.1080/15428119691014413.
- H. Y. Wang, L. D. Liu and J. C. Ren, Determination of methyl isocyanate in air by fluorimetry, Analyst, 1999, 124, 1327–1330, 10.1039/a903014j.
- Y. M. Roh, R. P. Streicher and M. K. Ernst, Development of a new approach for total isocyanate determination using the reagent 9-anthracenylmethyl 1-piperazinecarboxylate, Analyst, 2000, 125, 1691–1696, 10.1039/b003655m.
- M. Vogel and U. Karst, 4-Nitro-7-piperazino-2,1,3-benzoxadiazole as a reagent for monitoring of airborne isocyanates by liquid chromatography, Anal. Chem., 2002, 74(24), 6418–6426, DOI:10.1021/ac0260488.
- H. Henneken, R. Lindahl, A. Östin, M. Vogel, J. O. Levin and U. Karst, Diffusive sampling of methyl isocyanate using 4-nitro-7-piperazinobenzo-2-oxa-1,3-diazole (NBDPZ) as derivatizing agent, J. Environ. Monit., 2003, 5, 100–105, 10.1039/b209816b.
- S. Werlich, H. Stockhorst, U. Witting and N. Binding, MMNTP – A new tailor-made modular derivatization agent for the selective determination of isocyanates and diisocyanates, Analyst, 2004, 129, 364–370, 10.1039/b309221f.
- Z. Gao, B. Han, K. Chen, J. Sun and X. Hou, A novel single-fluorophore-based ratiometric fluorescent probe for direct detection of isocyanates in air, Chem. Commun., 2017, 53, 6231–6234, 10.1039/c7cc02269g.
- K. Chen, W. Chen, J. Sun, M. Bai, Z. Gao and X. Hou, A novel ratiometric fluorescent probe for quantitative detection of isocyanates in air, Tetrahedron, 2020, 76, 131547, DOI:10.1016/j.tet.2020.131547.
- K. R. Ghosh, S. K. Saha, J. P. Gao and Z. Y. Wang, Direct detection of ultralow trace amounts of isocyanates in air using a fluorescent conjugated polymer, Chem. Commun., 2014, 50, 716–718, 10.1039/c3cc47934j.
| This journal is © The Royal Society of Chemistry 2022 |