Ferrocene-based P-chiral amidophosphinate: stereoselective synthesis and X-ray structural study†
Abstract
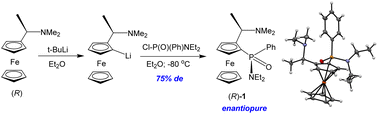
Racemic and enantiopure ferrocene-based P-chiral amidophosphinates have been simply and stereoselectively synthesized by ortho-lithiation of rac- or (R)-Ugi's amine and further reaction with amidochlorophenylphosphinate Cl–P(O)(Ph)NEt2. This is the first example of an asymmetric reaction of ortho-lithiated Ugi's amine with tetracoordinated phosphorus(V) chlorides. The structures of rac- and (R)-Ugi's amine ferrocenyl(phenyl)phosphinic acid N,N-diethylamide have been extensively studied experimentally (NMR, X-ray analysis, electrochemistry). The CV first peak refers to the oxidation of the amine fragment, which is clearly seen when (R)-Ugi's amine ferrocenyl(phenyl)phosphinic acid N,N-diethylamide reacts with anhydrous acid. The addition of two equivalents of CF3COOH leads to the protonation of nitrogen atoms, and a classical reversible wave of oxidation of Fe(II) to Fe(III) is observed.


 Please wait while we load your content...
Please wait while we load your content...