Open Access Article
Open Access ArticleHighly efficient sorption and luminescence sensing of oxoanionic species by 8-connected alkyl-amino functionalized Zr4+ MOFs†
Anastasia D.
Pournara
a,
Dimitrios A.
Evangelou
a,
Christina
Roukounaki
c,
Evangelos K.
Andreou
b,
Gerasimos S.
Armatas
 b,
Theodore
Lazarides
b,
Theodore
Lazarides
 c and
Manolis J.
Manos
c and
Manolis J.
Manos
 *ad
*ad
aDepartment of Chemistry, University of Ioannina, GR-45110 Ioannina, Greece. E-mail: emanos@uoi.gr
bDepartment of Materials Science and Technology, University of Crete, GR-71003 Heraklion, Greece
cLaboratory of Inorganic Chemistry, Department of Chemistry, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece
dInstitute of Materials Science and Computing, University Research Center of Ioannina, GR-45110, Ioannina, Greece
First published on 27th October 2022
Abstract
In the present study we provide the sorption properties of four 8-connected Zr4+ MOFs with the general formula H16[Zr6O16(RNH-BDC)4]·solvent (RNH-BDC2− = 2-alkyl-amine-terephthalate; R = ethyl-, ET-MOF; R = propyl-, PROP-MOF; R = isobutyl-, SBUT-MOF; R = n-butyl, BUT-MOF) towards toxic Cr(VI) and radionuclide-related ReO4− oxoanions. These MOFs represent superior sorbents for the removal of oxoanionic species, in terms of kinetics, sorption isotherms, selectivity and regeneration/reusability. The excellent sorption capability of the MOFs is due to a combination of surface and intra-framework sorption phenomena. The latter process proceeds via replacement of terminal water/hydroxyl ligands from the Zr6 clusters and subsequent binding of oxonanions to the Zr4+ centers, a fact that was proved via Rietveld PXRD analysis for the anion-loaded BUT-MOF. Importantly, BUT-MOF demonstrated an exceptional sorption capacity for Cr2O72− (505 mg g−1) and was further utilized in a sorption column in the form of MOF/calcium alginate beads, displaying remarkable removal efficiency towards industrial (chrome-plating) wastewater. Furthermore, the luminescence Cr(VI) sensing properties of BUT-MOF were explored in detail, presenting high sensitivity (detection limits as low as 9 ppb) and selectivity for these species against various competitive anions.
Introduction
Water represents the largest natural resource and the source of life in every sense of the word, as it is the basis of the existence of most living organisms. However, the widespread growth of modern industry results in the extensive pollution of aquatic environments, which in turn poses a threat for humans and other living species. A certain number of inorganic pollutants, present in the form of oxoanions, are recorded as priority pollutants in the lists of the Environment Protection Agency (EPA) and the World Health Organization (WHO).1,2 Compared to the cationic forms, which can be easily removed by several methods, including sorption or precipitation, the oxoanionic forms display higher solubility and environmental mobility, and can be transferred freely in the environment. A representative example of oxoanionic species is hexavalent chromium Cr(VI) which exists in the form of dichromate (Cr2O72−), hydrogen chromate (HCrO4−), or chromate (CrO42−) depending on the pH values of the effluents. Hexavalent chromium is released in the environment through various industrial activities (electroplating, leather tanning etc.) and is highly toxic, with well-known carcinogenic and mutagenic properties.3,4 TcO4− is also classified as an emergent oxoanionic contaminant since it is radioactive with long half-life (t1/2 = 2.13 × 105 years) and high environmental mobility.5Over the past decades, a variety of methods have dealt with the problem of the removal of these highly toxic oxoanions from aqueous media, including sorption, biological treatments, chemical oxidation/reduction, membrane filtration, and so on.6–10 However, the greater part of these technologies suffers from disadvantages and the development of novel techniques and materials has been the subject of intensive study. Among the conventional techniques, the eco-friendly, simply designed, low-cost, and highly efficient sorption, seems to be the most promising one.
Metal–organic frameworks (MOFs), an important class of porous materials, which are built from organic linkers and metal ions through coordination bonds, are considered as par excellence innovative materials for the sorption of contaminants from aqueous solutions and wastewater.11–14 MOFs combine significantly larger surface areas than conventional sorbent materials and structural features, such as appropriate functional groups and ion-binding sites, and not surprisingly they are considered as the next generation sorbents.
Besides sorption, which leads the research on the environmental applications of MOFs, the detection–determination of contaminants is equally important.15–17 MOFs with efficient fluorescence and capability to sorb ionic species are very promising materials for luminescence sensing applications. To date, relatively few examples of MOFs can combine both sorption and luminescence detection operations.18
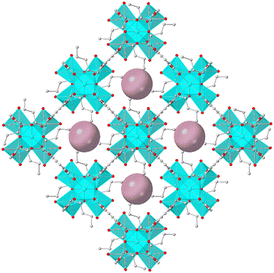
Recently, we reported four new microporous Zr4+ MOFs with the general formula H16[Zr6O16(RNH-BDC)4]·solvent (RNH-BDC2− = 2-alkyl-amine-terephthalate; R = ethyl-, ET-MOF; R = propyl-, PROP-MOF; R = isobutyl-, SBUT-MOF; R = n-butyl, BUT-MOF) (Fig. 1). These MOFs exhibited an 8-connected framework and displayed relatively large BET surface areas up to 832 m2 g−1.19 These materials have shown excellent Se(IV/VI) and SeCN− sorption properties, with fast and selective capture of these anionic species under a variety of conditions. The highly efficient sorption properties of these materials arise from a combination of external surface and intra-framework sorption processes, with the latter been facilitated by the presence of labile-terminal OH−/H2O ligands easily replaced by the Se anionic species.19
Herein, we extend our studies towards the sorption of the toxic Cr(IV) and perrhenate (ReO4−) species, with the latter be considered as a model for the radioactive TcO4− anion. The alkyl-amino functionalized MOFs exhibit some of the highest reported sorption capacities for Cr(VI) (up to 505 mg Cr2O72− per g, 187 mg CrO42− per g) and particularly high selectivity vs. common competitive anions. Furthermore, we fabricated a sorption column with BUT-MOF/calcium alginate beads as stationary phase, which displayed efficient performance for the sorption of Cr(VI) from industrial waste samples, along with noticeable recyclability. In addition, the alkyl-amino functionalized MOFs showed highly efficient sorption properties towards ReO4−, revealing exceptional sorption capacities (up to 960 mg ReO4− per g) and excellent performance over a broad pH range, including concentrated HNO3 solutions simulating acidic nuclear waste.20 Besides the excellent sorption properties, BUT-MOF displays selective luminescence sensing properties towards Cr(VI) showing particularly low detection limits, well-below the maximum permissible level of Cr(VI) in drinking water. Overall, the present work emphasizes on the multifunctionality of microporous Zr4+ MOFs with low net-connectivity, showing capability for rapid and efficient capture of multiple anionic pollutants as well as selective luminescence-based detection of such species in aqueous media.
Results and discussion
Batch sorption studies
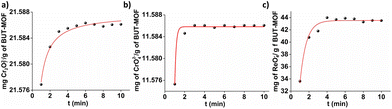
Our previous reported study revealed that alkyl-amino functionalized MOFs and especially BUT-MOF are very efficient sorbents for the decontamination of wastewater from Se species (including highly toxic SeCN− anions).19 At the current study, we examine the ability of these MOFs to uptake several hazardous oxoanions, including Cr2O72−, CrO42− and ReO4−. The removal of Cr(VI) oxoanions from aqueous resources is crucial due to their considerable toxicity, whereas the capture of ReO4− is of great interest given that it can be utilized as a model for TcO4− sorption investigations.3–5 In the following we present detailed batch Cr2O72−, CrO42− and ReO4− sorption studies as well as column Cr2O72− sorption data for BUT-MOF. Prior the sorption investigations, the MOF was activated via treatment with a HCl acid solution (ESI†).We have also conducted time dependent sorption experiments of BUT-MOF towards ReO4− anions. In Fig. 2c is presented the variation of sorbed ReO4− on BUT-MOF as a function of reaction time, with the initial ReO4− concentration being 50 ppm. The kinetic investigations indicated that the capture of ReO4− by BUT-MOF was particularly fast and comparable to the uptake of chromium(VI) anions. The equilibrium was achieved at the 4th min of contact. Lagergren's first order equation was the best model for fitting the ReO4− kinetic data (Table S1†).
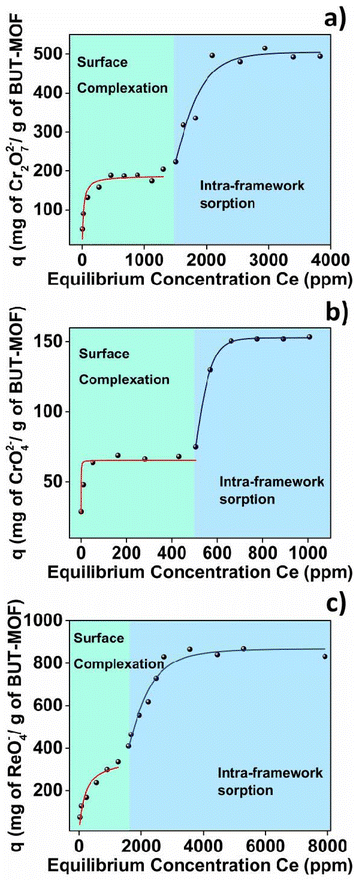 | ||
| Fig. 3 (a) Cr2O72−, (b) CrO42− and (c) ReO4− isotherm sorption data for BUT-MOF fitting with the various models. Red: Langmuir model; blue: Langmuir–Freundlich model. | ||
The excellent performance of BUT-MOF in ReO4− uptake impelled us further to investigate the sorption isotherms under acidic and basic conditions. Interestingly, sorption isotherms conducted in HNO3 solutions (1 M) revealed that BUT-MOF retains its excellent efficiency to remove ReO4− even under such extreme acidic conditions, achieving maximum sorption capacity as high as 776 mg g−1, which is relatively close to that obtained at neutral solutions (Fig. S1; Table S5†). Should be pointed out here that HNO3 solutions simulate acidic nuclear waste and subsequently BUT-MOF could be an attractive superior sorbent for nuclear waste remediation processes.20 Isotherm study conducted in pH ∼ 9 revealed a decrease in the removal of ReO4− by BUT-MOF with maximum sorption capacity at 504 mg g−1 (Fig. S2; Table S5†). The reduced ReO4− sorption capacity of BUT-MOF in the alkaline environment can be explained by the fact that under such conditions the amine groups of the MOF may not be protonated and the surface sorption is less effective. Thus, there is a decrease in the surface sorption capability of BUT-MOF, leading to a smaller ReO4− sorption capacity under alkaline conditions. The sorption capacity of the other MOFs (ET-, PROP-, and SBUT-MOF) towards Cr(VI) anions was also investigated in detail (Fig. S3–S5†).
The Cr(VI) sorption isotherms of these MOFs consist of two components, with maximum sorption capacities in the range of 217–451 mg Cr2O72− and 139–187 mg CrO42− per g of MOF (Tables S2 and S3†). The ReO4− sorption isotherms of PROP- and SBUT-MOFs also show the two-step profile fitted with Langmuir (first step) and Langmuir–Freundlich model (second step), whereas ET-MOF displays a one-step isotherm profile fitted only with Freundlich model. The maximum sorption capacities were found between 640 and 960 mg ReO4− per g (Fig. S3–S5; Table S4†).
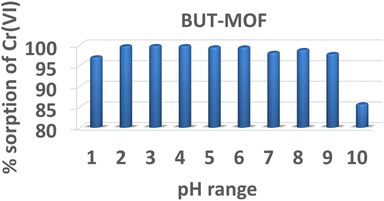
As seen from Fig. S9† optimal uptake of ReO4− occurred at pH from 3 to 8 with removal percentages at the range of 86–92%. At higher pH values, a small decrease in removal efficiency by BUT-MOF was observed at pH ∼ 9 (removal percentage 74%), while decreases sharply at pH ∼ 10 (18%). Likewise, at lower pH values a slight loss of removal performance occurs at pH ∼ 2 with removal percentage of 66% which is further reduced as low as 30% at pH ∼ 1.
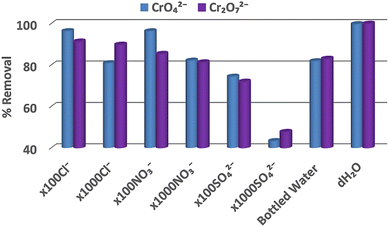 | ||
| Fig. 5 Cr2O72− (0.1 mM) and CrO42− (0.1 mM) sorption data for BUT-MOF in the presence of various competitive anions. | ||
Interestingly, BUT-MOF retains its sorptive ability in the presence of 100-fold excess of Cl− or NO3− (removal percentages >90% and >85%, respectively), while the MOF displays only a slight loss in solutions containing 1000-fold excess of these anions (removal percentages >80%). Interestingly, in solutions containing 100-fold excess of SO42−, which is a strong competitor for Cr(VI) species,22 Cr(VI) removal percentages are retained relatively high (72 and 75% for Cr2O72− and CrO42− respectively). Even when 1000-fold excess of SO42− was used, the Cr(VI) sorption efficiencies were appreciable (47% and 44% for Cr2O72− and CrO42−, respectively). In addition, we performed antagonistic experiments with ReO4− and the selected anions (Fig. S10†). The removal percentages for ReO4− in presence of high concentrations of competitive species were not as high as in the case of Cr(VI). Nevertheless, BUT-MOF was still able to remove at least a third of the initial ReO4− content (initial Re concentration = 50 ppm) in the presence of 100-fold excess of the competitive anions. As a last step at this study, we performed sorption experiments with bottled water solutions intentionally contaminated with Cr(VI) species or ReO4− (Fig. 5 & S10–S12†). These samples contained a variety of competitive anions including Cl−, NO3−, SO42− and HCO3− (and several cations) in large excess (up to 2.4 × 103-fold) in relation to the oxoanionic species (Table S6†). Similar to the sorption studies in the presence of competitive anions, BUT-MOF was more efficient to remove Cr(VI) (80–83% removal) from bottled water samples than ReO4− (36% removal). The selectivity of the other MOF analogues for Cr(VI) and ReO4− was found similar to that of BUT-MOF (Fig. S10–S12†). Presumably, the bivalent Cr(VI) species are expected to interact more strongly than the monoanionic ReO4− with the positively-charged surface of BUT-MOF (zeta potential = +24.2 ± 5.0 mV),19 thus leading to the observed higher selectivity of BUT-MOF for the Cr(VI) oxoanions. As revealed by the sorption isotherm studies discussed above, relatively low initial concentrations of anions, as those used for the selectivity tests (0.1–0.2 mM) favor mainly surface binding of the oxoanions to MOF particles and under such conditions the driving force for sorption is electrostatic interactions.
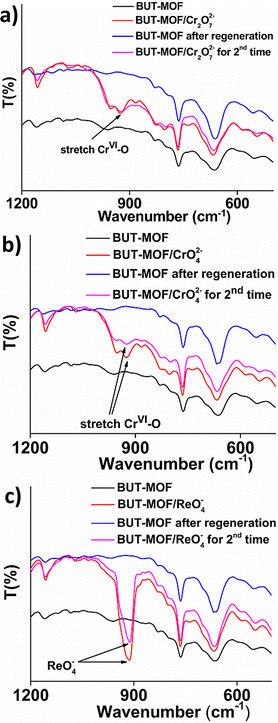
The regenerated MOF was then reloaded with Cr2O72−, CrO42− or ReO4− anions. IR data (Fig. 6) confirm the sorption of the anions by the MOF, which demonstrates the reusability of BUT-MOF as sorbent for oxoanions. The ability of BUT-MOF to be regenerated and reused is further proved by the column sorption experiments, discussed in detail below.
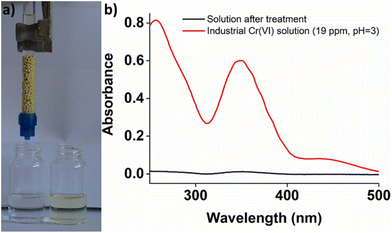
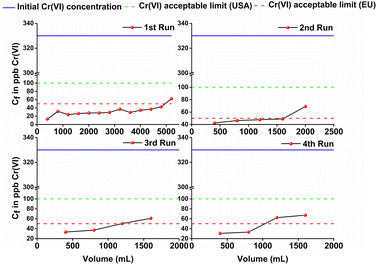
Then, the column, operated in up-flow mode (flow rate 2.5 mL min−1) (Fig. S14†), was utilized for the treatment of industrial (chrome-plating) wastewater, containing 330 ppb of Cr(VI). Samples of 400 ml were passed twice through the column, in order to achieve concentrations of the effluents less than 50 ppb (EU-defined upper limit of Cr(VI) in drinking water).25 For practical applications, two or more columns filled with BUT-MOF/CA beads should be utilized for the decontamination of large amounts of wastewater. This is a common practice in wastewater treatment applications, where multicolumn technology is applied to enhance the ion removal efficiencies.26 In the first run 5.2 L of Cr(VI) solution were passed through the column, till reaching a concentration above the acceptable limit of 50 ppb in drinking water (Fig. 8).
However, even after passing such high quantity of wastewater sample, the concentration of Cr(VI) was well below the acceptable limit of 100 ppb defined by U.S.-EPA.27BUT-MOF/CA can be easily regenerated by washing the column with 1 M HCl solution. After the regeneration process, a decrease in removal efficiency was observed as a concentration >50 ppb was found when 2 L solution passed through the column. The removal efficiency was further reduced in the next cycles (Fig. 8). Nevertheless, the performance of the BUT-MOF/CA column is considered very efficient, since a relatively large amount (∼9 L) of industrial wastewater sample can be purified (i.e. contain Cr(VI) in concentration less than 50 ppb) with only 4 runs of the column. These data are very encouraging for BUT-MOF which seems to be a promising sorbent for applications under real-world conditions.
Solid state characterization of the ion-loaded MOFs-mechanism of sorption
The successful binding of the anions on BUT-MOF was confirmed via several characterization techniques including IR, EDS and XPS data. IR spectra include characteristic peaks at 923 and 916 cm−1 attributed to Cr(VI) and Re(VII) oxoanions respectively (Fig. 6). EDS and XPS confirmed the presence of Cr and Re in the Cr(VI) and Re(VII)-loaded MOFs (Fig. S15–S17 and Fig. S18–S20†). However, the loaded materials contained no Cl, in contrast to the initial BUT-MOF sample (Fig. S15–S21†). Thus, besides the intra-framework binding of the oxo-anionic species discussed below, the sorption process also involves exchange of extra-framework Cl− anions by Cr(VI) or Re(VII) oxoanions. Furthermore, EDS and XPS data revealed (a) no Re and Cr and (b) presence of Cl for MOF samples after regeneration (Fig. S22–S25†). In addition, high resolution Re 4f and Cr 2p core-level photoelectron spectra for the loaded materials showed binding energies consistent with Re(VII) and Cr(VI) oxidation states respectively (Fig. S26†).BET surface area measurements revealed that the surface area of BUT-MOF is dramatically decreased after the sorption process. This confirms the findings from the isotherm study, that sorption of the anions occurs not only on the surface, via exchange of Cl− and electrostatic interactions of the inserted oxoanions with the positively charged surface of the MOF (see above), but also in the interior of the structure. Specifically, the BET surface area of the pristine BUT-MOF was reduced from 609 m2 g−1 to 139, 197, and 370 m2 g−1 after the sorption of Cr2O72−, CrO42− and ReO4−, respectively (Fig. S27†). We should also note that the pore sizes of BUT-MOF (5.5–8.5 Å)19 are large enough to allow diffusion of Cr(VI) and Re(VII) species into the MOF's structure, considering that the radii of hydrated Cr(VI) and Re(VII) oxoanions are 2.4–3.2 Å.33
As reported in our previous publication,19 the terminal OH−/H2O ligands in the Zr6 units of BUT-MOF can be replaced by oxoanionic species. Thus, the intra-framework sorption of CrO42− by BUT-MOF may be described with following equation
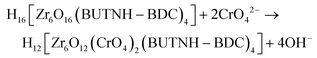 | (1) |
According to eqn (1), four terminal OH− ligands are replaced by 2 CrO42− anions. This is in good agreement with the intra-framework CrO42− sorption capacity of BUT-MOF corresponding to the insertion of ∼1.8 moles of CrO42− per formula unit of the MOF (see above).
Similarly, the intra-framework capture of ReO4− can be described as follows:
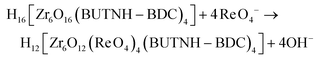 | (2) |
The replacement of 4 OH− by 4 ReO4− is in good accordance with the sorption results indicating capture of ∼4.8 mol of ReO4− per formula unit of BUT-MOF.
The intra-framework capture of dichromate anions is likely to be described with the following equation:
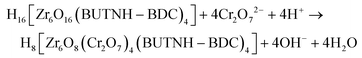 | (3) |
According to eqn (3), four dichromate anions replace 4 OH− and 4 H2O from the Zr6 building unit and the excess of negative charge due to the insertion of 4 Cr2O72− is compensated by H+ ions provided by the acidic solution (dichromate sorption proceeds at pH ∼ 3). This is in relatively good agreement with the observed intra-framework sorption capacity indicating insertion of 3.45 moles of dichromate per Zr6 cluster of BUT-MOF. The relatively small deviations of the experimental intra-framework sorption capacities from the ideal values are within the errors (10–15%) in the determination of the sorption capacities from the isotherm data.
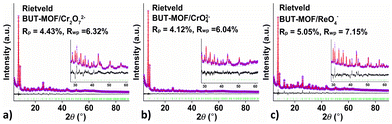
PXRD patterns, unit cell indexing and Le Bail (structureless) refinement indicate that the structure of the MOF is retained after the sorption of oxoanions (Fig. 9 & S28–S30†). The high crystallinity of the ion-loaded BUT-MOF allowed us to solve and refine the structures of these materials via PXRD methods. Thus, we built structural models involving 2 CrO42−, 4 ReO4− and 4 Cr2O72− per formula unit of BUT-MOF, with the anions initially placed in random positions in the pores. After optimization (simulating annealing) of the initial structural models, chromate and dichromate anions were found to be connected to Zr6 clusters as bridging ligands, whereas ReO4− anions were found to ligate to Zr4+ metal ions in a monodentate coordination mode (Fig. 10 & S31–S33†). These coordination modes for Cr(VI) and Re(VIII)-oxoanions are in agreement with those reported for MOR-2, another example of a Zr4+-terephthalate MOF with 8-connected structure.22,32 Rietveld refinements were satisfactory indicating the accuracy of the proposed structural models (Fig. 9 and ESI†).
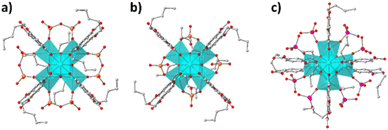 | ||
| Fig. 10 The coordination mode of (a) Cr2O72−, (b) CrO42− and (c) ReO4− (Cr atoms shown as orange balls; Re atoms shown as purple balls) in the structures of BUT-MOF/Cr2O72−, BUT-MOF/CrO42− and BUT-MOF/ReO4−, respectively, as determined via Rietveld refinement (details are discussed in ESI†). H atoms were omitted for clarity. | ||
Photophysical properties and luminescence sensing
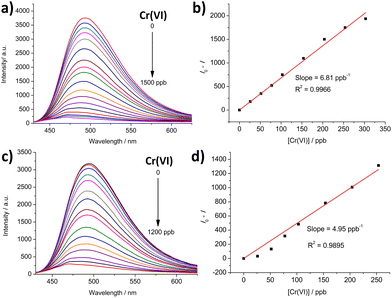
Solid BUT-MOF shows a strong cyan fluorescence under UV light irradiation (Fig. S34†). In agreement to our previous studies on a related functionalized Zr4+ MOF,22 an aqueous suspension of BUT-MOF at pH = 3 (0.1 mg mL−1) displays a broad fluorescence profile with maximum at ca. 490 nm upon excitation at 400 nm. The fluorescence is attributed to the radiative deactivation of the lowest ligand-based singlet excited state, which bears significant charge transfer character since it involves a shift of electron density from the amino group to the aromatic core.22A fluorescence titration where aliquots of a 10−4 M aqueous K2CrO7 solution were added to a suspension of acid activated BUT-MOF leads to strong emission quenching reaching 95% at the end of the titration (Fig. 11a). The calibration curve shows good linearity in the concentration range 0–300 ppb and a linear fit affords limit of detection (LOD) and quantification (LOQ) values of 9.0 and 30 ppb (Fig. 11b), respectively. Both values are well below the acceptable levels of Cr(VI) in water according to EU (50 ppb) and U.S.-EPA (100 ppb) guidelines.25,27,34 A Stern–Volmer analysis of the initial linear part of the titration curve yields a Stern–Volmer constant of 3.2 × 105 M−1 (Fig. S35†). At higher concentrations the Stern–Volmer plot shows an upwards curvature and fits better to a second-degree polynomial. This behavior is consistent with the presence of more than one quenching mechanisms possibly involving collisional (dynamic) quenching in combination with quenching through complex formation (static quenching).35 As shown in Table S10,† the sensing performance of BUT-MOF is comparable to those of the best performing MOF materials found in the recent literature.
Control experiments with 100-fold larger concentrations (10−2 M) of NO3−, Cl− and SO42− anions show that the material displays only minimal changes in emission intensity (Fig. S36–S38†). Thus, these ions do not induce an emission quenching effect. In agreement with the batch sorption studies (vide supra), the presence of a 100-fold excess of Cl− and NO3− only moderately raised the LOD and LOQ values to ca. 15 and 50 ppb, respectively (Fig. S39 and S40†). However, when a titration experiment is carried out in the presence of 10-fold excess (10−3 M) of SO42− anions, as shown in Fig. S41a,† the material displays saturation behaviour much earlier in the titration showing a final quenching percentage of ca. 50%. However, interestingly, at the initial stages of the titration the material displays comparable quenching behaviour as that observed in the experiments which are carried out in the absence of SO42− anions, as shown by the calibration curve gradient (Fig. S41b†) corresponding to LOD and LOQ values of 15 and 51 ppb, respectively. This observation may be interpreted by noting that SO42− anions are effective competitors to Cr(VI) species (vide supra) albeit without inducing appreciable quenching effects to BUT-MOF (Fig. S38†). Therefore, the initial quenching shown by BUT-MOF is due to its interaction with Cr(VI) species but as the material is exposed to increasing amounts of SO42−, the latter block the binding sites, thus preventing further interaction with Cr(VI) anions. In addition, titrations where distilled water is replaced with bottled water containing Cl− (7.53 ppm), SO42− (12.7 ppm) and NO3− (8.94 ppm), show a final quenching percentage of 92% and LOD and LOQ values of 12 and 40 ppb (Fig. 11c and d). The values are not much higher than those observed in the absence of competitive species and still lower than the acceptable levels of Cr(VI) in drinking water.
As in our previous work on the related MOR-2 material,22 we attribute the quenching behaviour of BUT-MOF to energy and/or electron transfer from the photoexcited ligand towards the material's Zr(IV)6 units where Cr(VI) species are attached (vide supra).36
Conclusions
In summary, this work emphasizes the capability of Zr4+ MOFs with low net-connectivity and easily replaceable terminal OH−/H2O ligands to be utilized as efficient sorbents towards toxic and radionuclide-related oxoanions as well as their promising luminescent sensing properties towards Cr(VI). Importantly, BUT-MOF and analogues seem to be the most promising sorbents for dichromate anions among reported MOF-based materials, as they combine exceptional maximum sorption capacities (up to 505 mg g−1), quite fast equilibrium sorption times (3 min), limited interference from competitive anions and reusability. Towards real applications, BUT-MOF/calcium alginate beads were successfully utilized in a column showing capability for decontamination of relatively large amounts of industrial waste samples. The alkyl-amino functionalized MOFs were also found quite efficient for sorption of CrO42− and ReO4− oxoanions from aqueous media, thus indicating these MOFs as truly multifunctional anion sorbents. Last but not least, the detailed photophysical investigations revealed that BUT-MOF constitutes also a powerful luminescent sensor capable of detecting Cr(VI) in concentrations well-below the acceptable limit in drinking water, even in the presence of several competitive species in large excess.Author contributions
A. D. Pournara: Investigation, formal analysis, writing – original draft, D. A. Evangelou; Investigation, formal analysis, writing – original draft, C. Roukounaki: Investigation, formal analysis, E. K. Andreou: Investigation, formal analysis, G. S. Armatas: Investigation, formal analysis, writing – review and editing, T. G. Lazarides: Investigation, formal analysis, writing – review and editing, Manolis J. Manos: Conceptualization, investigation, writing – review and editing, funding acquisition, project administration, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research project was supported by the Hellenic Foundation for Research and Innovation (H.F.R.I.) under the “1st Call for H.F.R.I. Research Projects to support Faculty Members & Researchers and the Procurement of high-cost research equipment grant” (Project Number: 348).References
- L. Keith and W. Telliard, Environ. Sci. Technol., 1979, 13, 416–423 CrossRef.
- N. Graham, Urban Water, 1999, 1, 183 Search PubMed.
- P. A. Terry, Chemosphere, 2004, 57, 541–546 CrossRef CAS.
- B. M. Weckhuysen, I. E. Wachs and R. A. Schoonheydt, Chem. Rev., 1996, 96, 3327–3349 CrossRef CAS PubMed.
- D. Banerjee, D. Kim, M. J. Schweiger, A. A. Kruger and P. K. Thallapally, Chem. Soc. Rev., 2016, 45, 2724–27396 RSC.
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marĩas and A. M. Mayes, Nature, 2008, 452, 301–310 CrossRef CAS.
- B. Hu, Y. Ai, J. Jin, T. Hayat, A. Alsaedi, L. Zhuang and X. Wang, Biochar, 2020, 2, 47–64 CrossRef.
- Y. Choi, M. S. Koo, A. D. Bokare, D. H. Kim, D. W. Bahnemann and W. Choi, Environ. Sci. Technol., 2017, 51, 3973–3981 CrossRef CAS.
- G. Chen, J. Feng, W. Wang, Y. Yin and H. Liu, Water Res., 2017, 108, 383–390 CrossRef CAS PubMed.
- S. Mishra, A. K. Singh and J. K. Singh, J. Membr. Sci., 2020, 593, 117422 CrossRef.
- M. Eddaoudi, D. B. Moler, H. Li, B. Chen, T. M. Reineke, M. O'Keeffe and O. M. Yaghi, Acc. Chem. Res., 2001, 34, 319–330 CrossRef PubMed.
- G. Férey, Chem. Soc. Rev., 2008, 37, 191–214 RSC.
- S. Horike, S. Shimomura and S. Kitagawa, Nat. Chem., 2009, 1, 695–704 CrossRef CAS PubMed.
- P. Kumar, A. Pournara, K. H. Kim, V. Bansal, S. Rapti and M. J. Manos, Prog. Mater. Sci., 2017, 86, 25–74 CrossRef CAS.
- B. Parmar, K. K. Bisht, Y. Rachuri and E. Suresh, Inorg. Chem. Front., 2020, 7, 1082–1107 RSC.
- B. Parmar, Y. Rachuri, K. K. Bisht, R. Laiya and E. Suresh, Inorg. Chem., 2017, 56, 2627–2638 CrossRef CAS PubMed.
- Y. Rachuri, B. Parmar, K. K. Bisht and E. Suresh, Cryst. Growth Des., 2017, 17, 1363–1372 CrossRef CAS.
- S. A. Diamantis, A. Margariti, A. D. Pournara, G. S. Papaefstathiou, M. J. Manos and T. Lazarides, Inorg. Chem. Front., 2018, 5, 1493–1511 RSC.
- A. D. Pournara, S. Rapti, A. Valmas, I. Margiolaki, E. Andreou, G. S. Armatas, A. C. Tsipis, J. C. Plakatouras, D. L. Giokas and M. J. Manos, J. Mater. Chem. A, 2021, 9, 3379–3387 RSC.
- J. D. Law, R. S. Herbst and T. A. Todd, Sep. Sci. Technol., 2002, 37, 1353–1373 CrossRef CAS.
- Y. Georgiou, S. Rapti, A. Mavrogiorgou, G. Armatas, M. J. Manos, M. Louloudi and Y. Deligiannakis, Sci. Rep., 2020, 10, 9358 CrossRef CAS PubMed.
- S. Rapti, D. Sarma, S. A. Diamantis, E. Skliri, G. S. Armatas, A. C. Tsipis, Y. S. Hassan, M. Alkordi, C. D. Malliakas, M. G. Kanatzidis, T. Lazarides, J. C. Plakatouras and M. J. Manos, J. Mater. Chem. A, 2017, 5, 14707–14719 RSC.
- S. Rapti, A. Pournara, D. Sarma, I. T. Papadas, G. S. Armatas, A. C. Tsipis, T. Lazarides, M. G. Kanatzidis and M. J. Manos, Chem. Sci., 2016, 7, 2427–2436 RSC.
- S. Rapti, A. Pournara, D. Sarma, I. T. Papadas, G. S. Armatas, Y. S. Hassan, M. H. Alkordi, M. G. Kanatzidis and M. J. Manos, Inorg. Chem. Front., 2016, 3, 635–644 RSC.
- European Comission. Council Directive 98/83/EC on the Quality of Water in-tented for Human Consumption, http://eurlex.europa.eu/legalcontent/EN/TXT/?uriOCELEX:31998L0083.
- A. A. Zagorodni, Ion Exchange Materials: Properties and Applications, Elsevier, 2007, p. 297 Search PubMed.
- USA-EPA, Chromium in Drinking Water, https://water.epa.gov/drink/info/chromium/.
- H. Niu, Y. Zheng, S. Wang, S. He and Y. Cai, J. Mater. Chem. A, 2017, 5, 16600–16604 RSC.
- M. B. Luo, Y. Y. Xiong, H. Q. Wu, X. F. Feng, J. Q. Li and F. Luo, Angew. Chem., Int. Ed., 2017, 56, 16376–16379 CrossRef CAS PubMed.
- D. Evangelou, A. Pournara, C. Tziasiou, E. Andreou, G. S. Armatas and M. J. Manos, Inorg. Chem., 2022, 61, 2017–2030 CrossRef CAS PubMed.
- H. Li, Q. Li, X. He, N. Zhang, Z. Xu, Y. Wang and Y. Wang, Cryst. Growth Des., 2018, 18, 6248–6256 CrossRef CAS.
- S. Rapti, S. A. Diamantis, A. Dafnomili, A. Pournara, E. Skliri, G. S. Armatas, A. C. Tsipis, I. Spanopoulos, C. D. Malliakas, M. G. Kanatzidis, J. C. Plakatouras, F. Noli, T. Lazarides and M. J. Manos, J. Mater. Chem. A, 2018, 6, 20813–20821 RSC.
- Y. Marcus, J. Chem. Soc., Faraday Trans., 1993, 89, 713–718 RSC.
- C. Peng, H. Meng, S. Song, S. Lu and A. Lopez-Vaidivieso, Sep. Sci. Technol., 2004, 39, 1501–1517 CrossRef CAS.
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer science & business media, 2013 Search PubMed.
- D. Sun, Y. Fu, W. Liu, L. Ye, D. Wang, L. Yang, X. Fu and Z. Li, Chem. – Eur. J., 2013, 19, 14279–14285 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, details of Rietveld refinement, characterization and fluorescence titration data. CCDC 2204055–2204057. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt02848d |
| This journal is © The Royal Society of Chemistry 2022 |