Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Synthesis and anti-microbial activity of a new series of bis(diphosphine) rhenium(V) dioxo complexes†
Saul M.
Cooper
 ,
Christina
Siakalli
,
Christina
Siakalli
 ,
Andrew J. P.
White
,
Andrew J. P.
White
 ,
Angelo
Frei
,
Angelo
Frei
 ,
Philip W.
Miller
,
Philip W.
Miller
 * and
Nicholas J.
Long
* and
Nicholas J.
Long
 *
*
Department of Chemistry, Imperial College London, Molecular Sciences Research Hub, 82 Wood Lane, White City Campus, London, W12 0BZ, UK. E-mail: philip.miller@imperial.ac.uk; n.long@imperial.ac.uk
First published on 3rd August 2022
Abstract
Rhenium-based metallodrugs have recently been highlighted as promising candidates for new antibiotics to combat multi-drug resistant (MDR) pathogens. A new class of rhenium(V) dioxo complexes were prepared from readily accessible diphosphine ligands, and have been shown to possess potent activity against Staphylococcus aureus (S. aureus) and Candida albicans (C. albicans) alongside low human cell toxicity.
Antibiotic resistance presents one of the gravest current threats to global health and food security.1,2 Bacterial resistance to many drugs derived from typical structural classes of organic molecules deployed as first-line antibiotics has led to the routine use of second- or third-line alternatives to combat common infections.3–5 Thus a rise in so-called multi-drug resistance (MDR) pathogens, including methicillin-resistant Staphylococcus aureus (MRSA), has been highlighted by the WHO as a serious global health issue.6,7 Consequently, there is an urgent need to derive novel classes of medicinal compounds with the potential to combat these strains effectively.8,9
Metal-based compounds have recently been highlighted as a promising alternative to traditional organic antibiotic classes.10 Their successful application as drugs in other fields of medicine, including oncology,11–14 and in neurodegenerative diseases,15 is well documented. However, to date, the application of metal complexes as antibiotics has been somewhat limited despite the advantages they offer: the potential for novel mechanisms of action which could avoid multi-drug resistant pathways;16 and their ability to more readily explore 3-dimensional structure–activity space (the so-called ‘escape from flatland’).10,17,18 The potential of metal-based antibiotics has recently been reviewed by Frei et al.19
Over the last decade a range of transition metal complexes have been explored as potential antibiotics, including those derived from ruthenium,20–22 and others.19 Rhenium complexes, despite being of significant contemporary interest in anti-cancer applications,23–25 have been comparatively underexplored in this regard.
Metzler-Nolte, Bandow and co-workers have investigated a trimetallic peptide nucleic acid-derived complex containing a rhenium tricarbonyl unit chelated with a dipicolylamine derivative.26 This unit was subsequently shown to be indispensable in the activity of the complex against MRSA (MIC = 2 μM), and is the first evidence for a rhenium-dependent antibacterial mechanism of action.27 Frei et al. have published the light-mediated activity of rhenium(I) bisquinoline complexes, which were shown to also possess a second light-independent mechanism of action.28 Further Re(I) tricarbonyl complexes have been investigated in which the metal is coordinated with N-heterocyclic ligands,29,30 or various diimines.31,32 In a recent study, Zobi and co-workers identified two non-toxic cationic Re(I) tricarbonyl complexes bearing lipophilic diimine ligands as potent inhibitors of MRSA.33 Such studies illustrate the efficacy of rhenium complexes in this regard, albeit with the all the complexes tested bearing a Re(I) carbonyl unit.25 The antibacterial application of rhenium complexes in which the metal is in a different oxidation state to Re(I) are far rarer. To our knowledge, there are no examples of antimicrobial studies explicitly using Re(V) complexes, despite the prevalence of such complexes as isostructural analogues to Tc(V) used extensively in diagnostic nuclear medicine applications.34
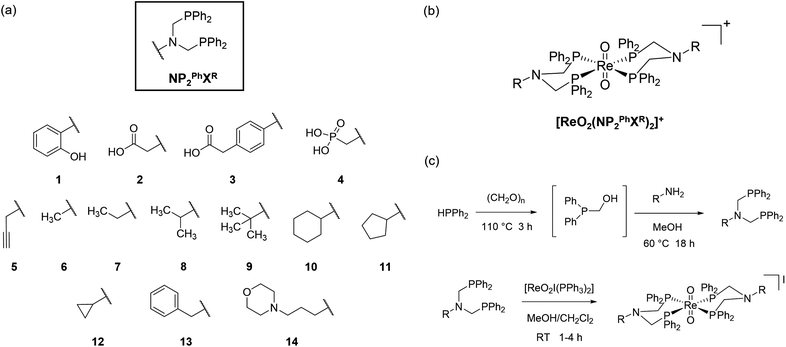
Our interest in the development of new phosphine-based chelators for the development of Tc(V) radiopharmaceuticals led us to explore the coordination chemistry of a class of N-centred N-functionalised diphosphine ligands.35 A range of such ligands (NP2PhXR, X = functional group, R = alkyl, aryl linker) can be generated through a single-step phosphorus-based Mannich reaction (Fig. 1(a)).36 This reaction has been employed by our group, and others, for the facile synthesis of numerous hetero-functionalised di- (and tri-) phosphines from parent primary amines or ammonia.37–40 Upon addition to a solution of a Re(V) precursor, the rapid formation of monocationic bis(diphosphine) complexes containing a trans-dioxo unit {ReVO2}+ was observed (Fig. 1(b)).
Here, we report the synthesis and characterisation of fourteen novel {ReVO2}+ complexes bearing N-functionalised diphosphines and their subsequent antimicrobial activity against Gram-positive and Gram-negative bacteria, and the C. albicans fungal strain. These easily accessible {ReVO2}+ complexes have been shown to exhibit a range of antimicrobial/antifungal activities and toxicities dependent on the chemical nature of the group introduced through the primary amine, and are promising candidates for further studies. The antimicrobial inhibition study was facilitated through the Community for Open Antimicrobial Drug Discovery (CO-ADD).41,42
A series of NP2PhXR ligands were synthesised through a phosphorus-based Mannich reaction from HPPh2 and paraformaldehyde, followed by addition of a primary amine (or hydrochloride salt thereof) (Fig. 1(c)).43 The range of ligands was selected from published derivatives with both acidic and alkyl substituents, alongside new derivatives to complement known species by further varying their lipophilicity. Fig. 1(a) depicts all the ligands synthesised for this study. Novel ligands synthesised for this study include the phosphonic acid derivative NP2PhPO3H2Me (4), the propargyl-functionalised derivative NP2PhPrg (5), the ethyl- derivative NP2PhEt (7), the cyclopentyl- and cyclopropyl-derivatives NP2PhCyp (11) and NP2PhCypr (12), and the morpholino-functionalised derivative NP2PhMorPr (14). Other derivatives are known but have been prepared here using our modified synthetic route (see ESI†). An alternative synthesis for the novel ligand 5 was conducted through isolation of the dihydroxymethylphosphonium chloride salt [Ph2P(CH2OH)2]Cl, and subsequent reaction with the primary amine in the presence of DIPEA.44 Ligands 1–4, and 12 were isolated as off-white solids following precipitation from CH2Cl2 with hexane. Ligands 5–11, 13 and 14 could not be isolated as easily-handled solids but instead formed oily residues upon this step. Consequently, these ligands were re-dissolved in MeOH (10 mL) and stored under N2 at −10 °C. Such solutions of the NP2PhXR derivatives were shown to be stable towards oxidation and decomposition for upwards of a year by 31P{1H} NMR spectroscopy (see ESI†) or could be used directly in complexation reactions.
The commercially-available Re(V) precursor [ReO2I(PPh3)2] was selected for the synthesis of the {ReVO2}+ complexes due to the presence of a dioxo unit in the coordination sphere of the metal. Four phosphine donors are known to favour formation of the {ReVO2}+ unit over mono-oxo variants.45 Addition of two equivalents of the ligand in MeOH to a solution of [ReO2I(PPh3)2] in CH2Cl2 at room temperature resulted in a rapid colour change from violet to brown to orange over the course of less than an hour. The reaction mixture was left stirring for several hours to ensure complete conversion. Removal of the displaced PPh3 was achieved through trituration of the crude residue with hexane. Silica column chromatography (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 v
5 v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v DCM
v DCM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH) then yielded the pure monocationic [ReO2(NP2PhR)2]+ complexes in a range of yields (32–94%).
MeOH) then yielded the pure monocationic [ReO2(NP2PhR)2]+ complexes in a range of yields (32–94%).
These Re(V) complexes are pseudo-octahedral diamagnetic low-spin d6 complexes and can be effectively characterised by 1H, 31P{1H} and 13C{1H} NMR spectroscopy. Notable consistent features in the 1H NMR spectrum include a singlet for the methylene environment of the bridging ligand arms, with negligible splitting due to small 2JPH coupling constants. This is contrary to the case of mono-oxo analogues in which complicated second-order lineshapes are observed through the presence of magnetically inequivalent hydrogen environments.35 The 31P{1H} NMR spectra contain lone singlet resonances corresponding to the four equivalent phosphorus atoms of the centrosymmetric complexes, with a higher frequency chemical shift (∼12 ppm) relative to the free ligand. The molecular ion peak of each complex was observed by high-resolution mass spectrometry with minimal fragmentation. The 13C{1H} NMR spectra were successfully obtained for all complexes.
Confirmation of the structures of complexes Re-1, Re-3 and Re-4 was achieved by single crystal X-ray diffraction despite appreciable disorder arising from the location of iodide counterions and solvent molecules in the crystallised lattice. Metathesis reactions to exchange the iodide counterion for tetrafluoroborate did not lead to an improvement in this regard. Nevertheless, the structure of the cation could be determined unambiguously and confirmed the centrosymmetric nature of the {ReVO2(NP2PhR)2}+ molecular ion, and the pseudo-chair conformations of the six-membered chelate rings (see ESI†).
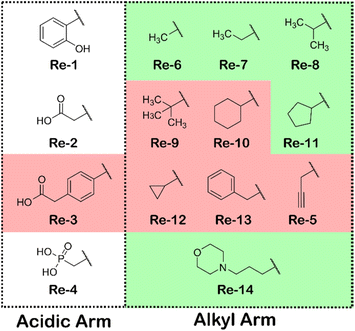
Complexes Re-1–Re-14 were tested in high-throughput bacterial broth microdilution minimal inhibition concentration (MIC) assays against a range of Gram-positive and Gram-negative bacteria and the fungal strain C. albicans according to the CO-ADD protocol (see ESI†). Re-2–Re-7 were initially screened at 32 μg mL−1 for %-inhibition, before being tested again in a dose-responsive manner to determine more accurate MICs. Re-4 was neglected in this second screening due to minimal %-inhibition in the first instance. Re-1 and Re-8–Re-14 were directly screened by the dose–response method. Table 1 displays the MICs measured to a minimum of 0.25 μg mL−1 against S. aureus and the fungal strain C. albicans. Whereas none of the complexes showed appreciable activity against Gram-negative bacterial strains (see ESI†), a range of complexes displayed significant activity towards the inhibition of both Gram-positive S. aureus and C. albicans without displaying cytotoxicity towards human embryonic kidney cells (HEK-293) (or haemolysis) namely; Re-6, Re-7, Re-8, Re-11 and Re-14 (Fig. 2). Some general observations about how the nature of the amine component affects the antimicrobial properties of the complexes can be drawn from this data. The compounds Re-1–Re-4, with acidic functionalities, show reduced activity towards both S. aureus and C. albicans but also minimal toxicity. On the other hand, complexes with neutral components and overall monocationic charge exhibit greater activity towards both S. aureus and C. albicans but also a larger range of toxicity. Experiments with ruthenium complexes have shown the correlation between higher positive charge and enhanced antimicrobial activity.20 Of particular note is the high activity and low toxicity of Re-14 which contains a terminal morpholine unit. Morpholine units have been shown to promote subcellular lysosome localisation in Re(I) tricarbonyl complexes.46
| Cmpd | S. aureus | C. albicans | Toxicityc | Haeme |
|---|---|---|---|---|
| ATCC 43300 | ATCC 90028 | CC50d | HC10f | |
| a Vancomycin. b Fluconazole. c Against Human Embryonic Kidney Cells ATCC CRL 1573. d Concentration at 50% cytotoxicity. e Haemolysis against Human Red Blood Cells. f Concentration at 10% haemolysis. g Active. h Active and non-toxic. | ||||
| Re-1 | >32 | >32 | >32 | 4.878 |
| Re-2 | 32 | >32 | >32 | >32 |
| Re-3 | 16 | >32 | 27.55 | >32 |
| Re-4 | n.d. | n.d. | n.d. | — |
| Re-5 | 4 | 16 | 6.724 | 0.952 |
| Re-6 | 2 | 2 | >32 | >32 |
| Re-7 | ≤0.25 | ≤0.25 | >32 | >32 |
| Re-8 | ≤0.25 | ≤0.25 | >32 | >32 |
| Re-9 | ≤0.25 | ≤0.25 | ≤0.25 | >32 |
| Re-10 | ≤0.25 | ≤0.25 | ≤0.25 | >32 |
| Re-11 | ≤0.25 | ≤0.25 | >32 | >32 |
| Re-12 | ≤0.25 | ≤0.25 | ≤0.25 | >32 |
| Re-13 | ≤0.25 | ≤0.25 | ≤0.25 | >32 |
| Re-14 | ≤0.25 | 8 | >32 | >32 |
| Van | 1 | — | — | — |
| FCZ | — | 2 | — | — |
We present a new class of Re(V) complex, obtained through a facile synthetic route, which are the first examples of Re(V) complexes with appreciable anti-microbial activity. The preliminary data presented here are highly encouraging towards further exploration of this class of compound, including elucidation of a mechanism of action. Additionally, a particular advantage of rhenium-based antibiotics over other transition metals are their potential to form isostructural technetium-99m (99mTc) derivatives for tandem whole-body SPECT imaging of infection. This could be used to obtain biodistribution and pharmacokinetic data for promising candidates of this class due to the ease of access of the [99mTc]-{TcVO2}+ core.47 Further work in our group is focused on the synthesis of [ReVO2(NP2PhR)2]+ derivatives bearing charged groups on the amine component to further elucidate the relationship between overall complex charge and anti-microbial activity. Such an approach should also help to improve the aqueous solubility of these complexes for further application in imaging and therapy.
Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors thank the EPSRC Centre for Doctoral Training in Medical Imaging (EP/L015226/1) (SMC), the EPSRC programme for Next Generation Molecular Imaging and Therapy with Radionuclides (EP/S019901/1, “MITHRAS”) (AF) and the Department of Chemistry Dr Allan Samuel UROP bursary (CS) for funding. NJL is grateful for a Royal Society Wolfson Research Merit Award.Notes and references
- F. Prestinaci, P. Pezzotti and A. Pantosti, Pathog. Global Health, 2015, 109, 309–318 CrossRef PubMed.
- M. Woolhouse, C. Waugh, M. R. Perry and H. Nair, J Glob. Health, 2016, 6, 1–5 CrossRef PubMed.
- C. A. Arias and B. E. Murray, Nat. Rev. Microbiol., 2012, 10, 266–278 CrossRef CAS PubMed.
- C. Kallberg, C. Ardal, H. S. Blix, E. Klein, E. M. Martinez, M. Lindbæk, K. Outterson, J. A. Røttingen and R. Laxminarayan, PLoS One, 2018, 13, 1–19 CrossRef PubMed.
- G. Mancuso, A. Midiri, E. Gerace and C. Biondo, Pathogens, 2021, 10, 1–14 CrossRef PubMed.
- World Health Organization, Global Shortage of Innovative Antibiotics Fuels Emergence and Spread of Drug-Resistance, 2021 Search PubMed.
- Antibiotic Resistance Collaborators, Lancet, 2022, 399, 629–655 CrossRef.
- V. Cattoir and B. Felden, J. Infect. Dis., 2019, 220, 350–360 CrossRef PubMed.
- World Health Organization, Antibacterial Agents in Clinical and Pre-clinical Development: An Overview and Analysis, 2021 Search PubMed.
- A. Frei, J. Zuegg, A. G. Elliott, M. Baker, S. Braese, C. Brown, F. Chen, C. G. Dowson, G. Dujardin, N. Jung, A. P. King, A. M. Mansour, M. Massi, J. Moat, H. A. Mohamed, A. K. Renfrew, P. J. Rutledge, P. J. Sadler, M. H. Todd, C. E. Willans, J. J. Wilson, M. A. Cooper and M. A. T. Blaskovich, Chem. Sci., 2020, 11, 2627–2639 RSC.
- L. Zeng, P. Gupta, Y. Chen, E. Wang, L. Ji, H. Chao and Z. S. Chen, Chem. Soc. Rev., 2017, 46, 5771–5804 RSC.
- R. G. Kenny and C. J. Marmion, Chem. Rev., 2019, 119, 1058–1137 CrossRef CAS PubMed.
- S. Monro, K. L. Colón, H. Yin, J. Roque, P. Konda, S. Gujar, R. P. Thummel, L. Lilge, C. G. Cameron and S. A. McFarland, Chem. Rev., 2019, 119, 797–828 CrossRef CAS PubMed.
- L. Gourdon, K. Cariou and G. Gasser, Chem. Soc. Rev., 2022, 51, 1167–1195 RSC.
- T. A. Sales, I. G. Prandi, A. A. de Castro, D. H. S. Leal, E. F. F. da Cunha, K. Kuca and T. C. Ramalho, Int. J. Mol. Sci., 2019, 20, 1829–1862 CrossRef CAS PubMed.
- G. Gasser and N. Metzler-Nolte, Curr. Opin. Chem. Biol., 2012, 16, 84–91 CrossRef CAS PubMed.
- C. N. Morrison, K. E. Prosser, R. W. Stokes, A. Cordes, N. Metzler-Nolte and S. M. Cohen, Chem. Sci., 2020, 11, 1216–1225 RSC.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- A. Frei, Antibiotics, 2020, 9, 90–114 CrossRef CAS PubMed.
- F. Li, J. G. Collins and F. R. Keene, Chem. Soc. Rev., 2015, 44, 2529–2542 RSC.
- D. K. Weber, M. A. Sani, M. T. Downton, F. Separovic, F. R. Keene and J. G. Collins, J. Am. Chem. Soc., 2016, 138, 15267–15277 CrossRef CAS PubMed.
- K. L. Smitten, H. M. Southam, J. B. De La Serna, M. R. Gill, P. J. Jarman, C. G. W. Smythe, R. K. Poole and J. A. Thomas, ACS Nano, 2019, 13, 5133–5146 CrossRef CAS PubMed.
- A. Leonidova and G. Gasser, ACS Chem. Biol., 2014, 9, 2180–2193 CrossRef CAS PubMed.
- C. C. Konkankit, S. C. Marker, K. M. Knopf and J. J. Wilson, Dalton Trans., 2018, 47, 9934–9974 RSC.
- K. Schindler and F. Zobi, Molecules, 2022, 27, 539–564 CrossRef CAS PubMed.
- M. Wenzel, M. Patra, C. H. R. Senges, I. Ott, J. J. Stepanek, A. Pinto, P. Prochnow, C. Vuong, S. Langklotz, N. Metzler-Nolte and J. E. Bandow, ACS Chem. Biol., 2013, 8, 1442–1450 CrossRef CAS PubMed.
- M. Patra, M. Wenzel, P. Prochnow, V. Pierroz, G. Gasser, J. E. Bandow and N. Metzler-Nolte, Chem. Sci., 2015, 6, 214–224 RSC.
- A. Frei, M. Amado, M. A. Cooper and M. A. T. Blaskovich, Chem. – Eur. J., 2020, 26, 2852–2858 CrossRef CAS PubMed.
- A. Noor, G. S. Hu, S. V. Kumar, J. E. M. Lewis, B. M. Paterson, C. Schieber, P. S. Donnelly, H. J. L. Brooks, K. C. Gordon, S. C. Moratti and J. D. Crowley, Organometallics, 2014, 33, 7031–7043 CrossRef CAS.
- D. Siegmund, N. Lorenz, Y. Gothe, C. Spies, B. Geissler, P. Prochnow, P. Nuernberger, J. E. Bandow and N. Metzler-Nolte, Dalton Trans., 2017, 46, 15269–15279 RSC.
- S. V. Kumar, W. K. C. Lo, H. J. L. Brooks, L. R. Hanton and J. D. Crowley, Aust. J. Chem., 2016, 69, 489–498 CrossRef CAS.
- S. N. Sovari, S. Vojnovic, S. S. Bogojevic, A. Crochet, A. Pavic, J. Nikodinovic-Runic and F. Zobi, Eur. J. Med. Chem., 2020, 205, 112533–112549 CrossRef CAS PubMed.
- S. N. Sovari, N. Radakovic, P. Roch, A. Crochet, A. Pavic and F. Zobi, Eur. J. Med. Chem., 2021, 226, 113858–113871 CrossRef CAS PubMed.
- M. D. Bartholomä, A. S. Louie, J. F. Valliant and J. Zubieta, Chem. Rev., 2010, 110, 2903–2920 CrossRef.
- S. M. Cooper, A. J. P. White, T. R. Eykyn, M. T. Ma, P. W. Miller and N. J. Long, Inorg. Chem., 2022, 61, 8000–8014 CrossRef CAS PubMed.
- G. Märkl and G. Yu Jin, Tetrahedron Lett., 1981, 22, 1105–1108 CrossRef.
- J. T. Bays, N. Priyadarshani, M. S. Jeletic, E. B. Hulley, D. L. Miller, J. C. Linehan and W. J. Shaw, ACS Catal., 2014, 4, 3663–3670 CrossRef CAS.
- A. Phanopoulos, A. J. P. White, N. J. Long and P. W. Miller, Dalton Trans., 2016, 45, 5536–5548 RSC.
- B. Cao, M. R. J. Elsegood, N. Lastra-Calvo and M. B. Smith, J. Organomet. Chem., 2017, 853, 159–167 CrossRef CAS.
- E. S. Wiedner, A. M. Appel, S. Raugei, W. J. Shaw and R. M. Bullock, Chem. Rev., 2022, 122, 12427–12474 CrossRef CAS PubMed.
- M. A. Cooper, Nat. Rev. Drug Discovery, 2015, 14, 587–588 CrossRef CAS PubMed.
- M. A. T. Blaskovich, J. Zuegg, A. G. Elliott and M. A. Cooper, ACS Infect. Dis., 2016, 1, 285–287 CrossRef PubMed.
- S. E. Durran, M. R. J. Elsegood, N. Hawkins, M. B. Smith and S. Talib, Tetrahedron Lett., 2003, 44, 5255–5257 CrossRef CAS.
- D. Moiseev, B. R. James, B. O. Patrick and T. Q. Hu, Inorg. Chem., 2006, 45, 2917–2924 CrossRef CAS.
- U. Abram, in Comprehensive Coordination Chemistry II, 2003, pp. 271–402 Search PubMed.
- J. Shum, P. Z. Zhang, L. C. C. Lee and K. K. W. Lo, Chempluschem, 2020, 85, 1374–1378 CrossRef CAS PubMed.
- I. N. Hungnes, F. Al-Salemee, P. J. Gawne, T. Eykyn, R. A. Atkinson, S. Y. A. Terry, F. Clarke, P. J. Blower, P. G. Pringle and M. T. Ma, Dalton Trans., 2021, 50, 16156–16165 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2165137–2165139. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt02157a |
| This journal is © The Royal Society of Chemistry 2022 |